Platelet Polyphosphate Signals Through NFκB to Induce Myofibroblast Differentiation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Culture
2.3. Immunofluorescence
2.4. Reverse Transcription Quantitative Polymerase Chain Reaction (RT-qPCR)
2.5. Platelet Releasates
3. Results
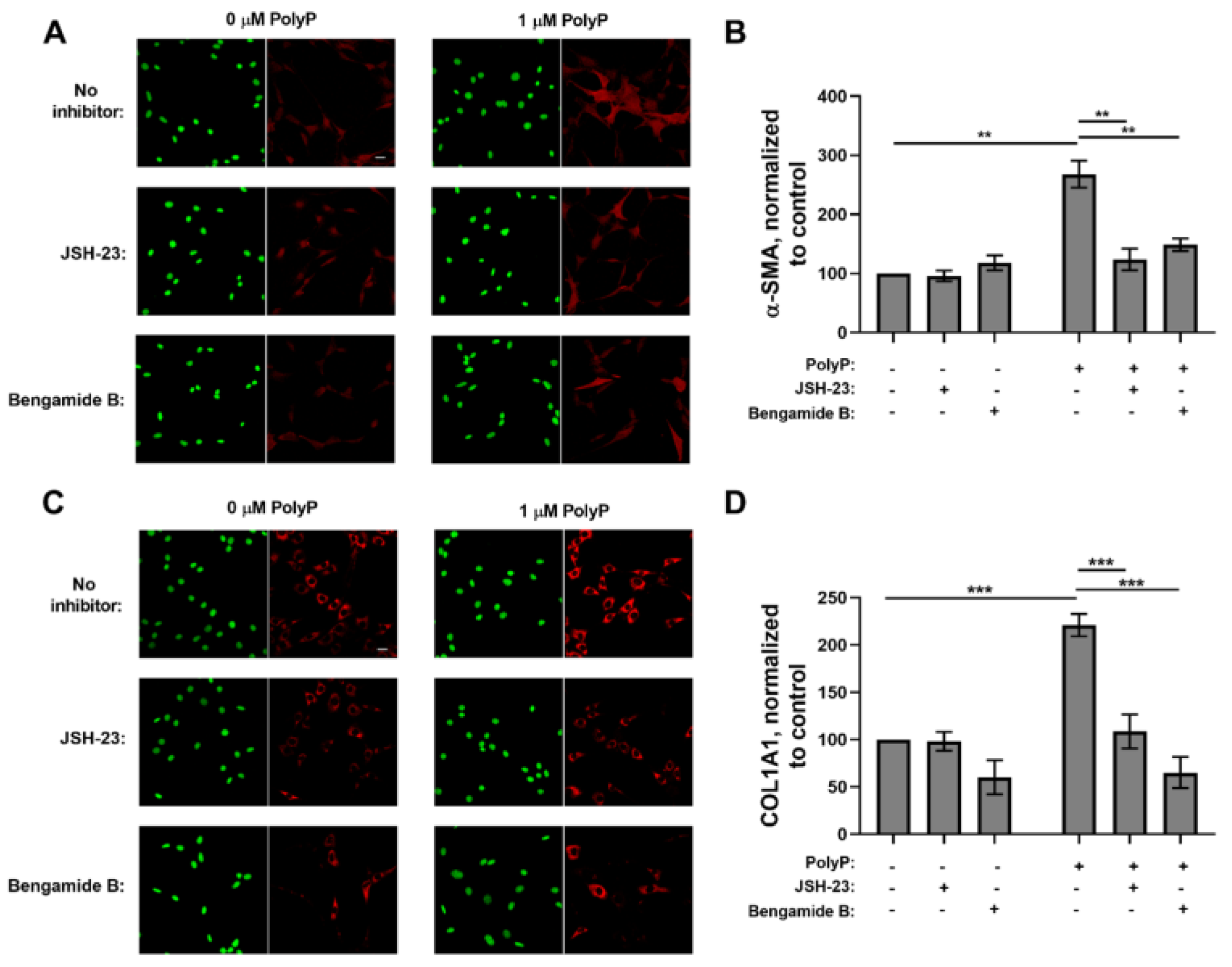
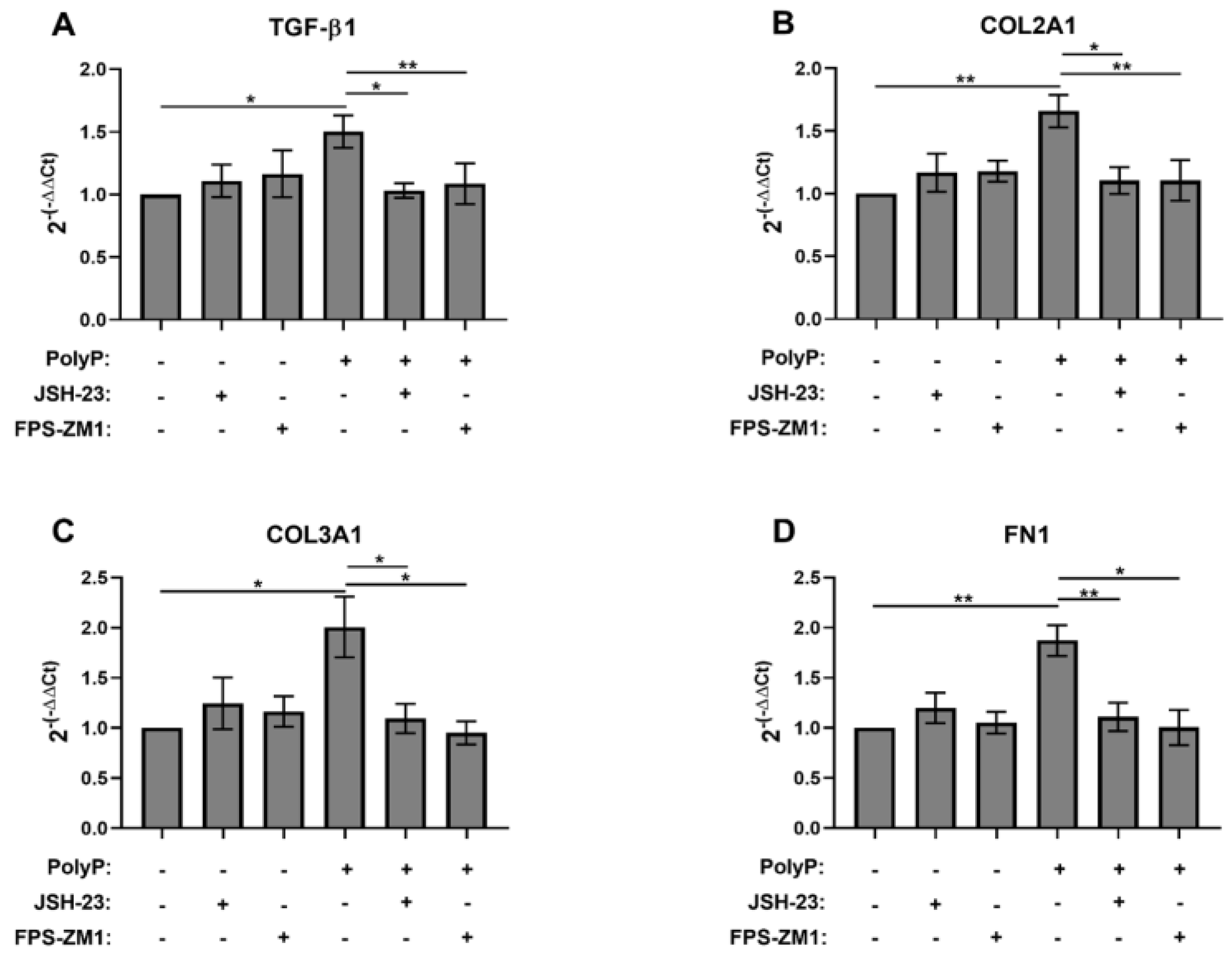
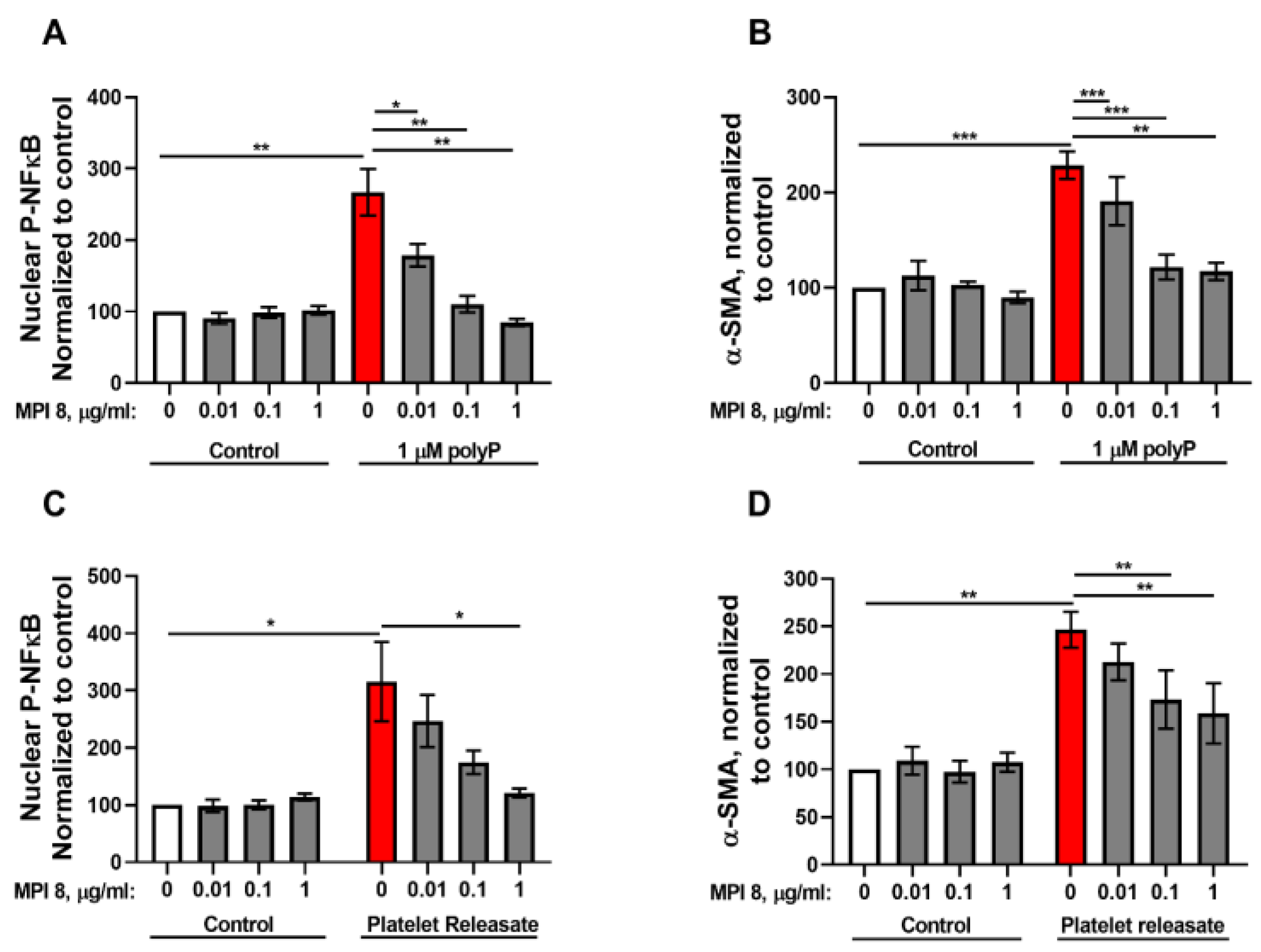
3.1. Role of NFκB in the Response of Fibroblasts to PolyP
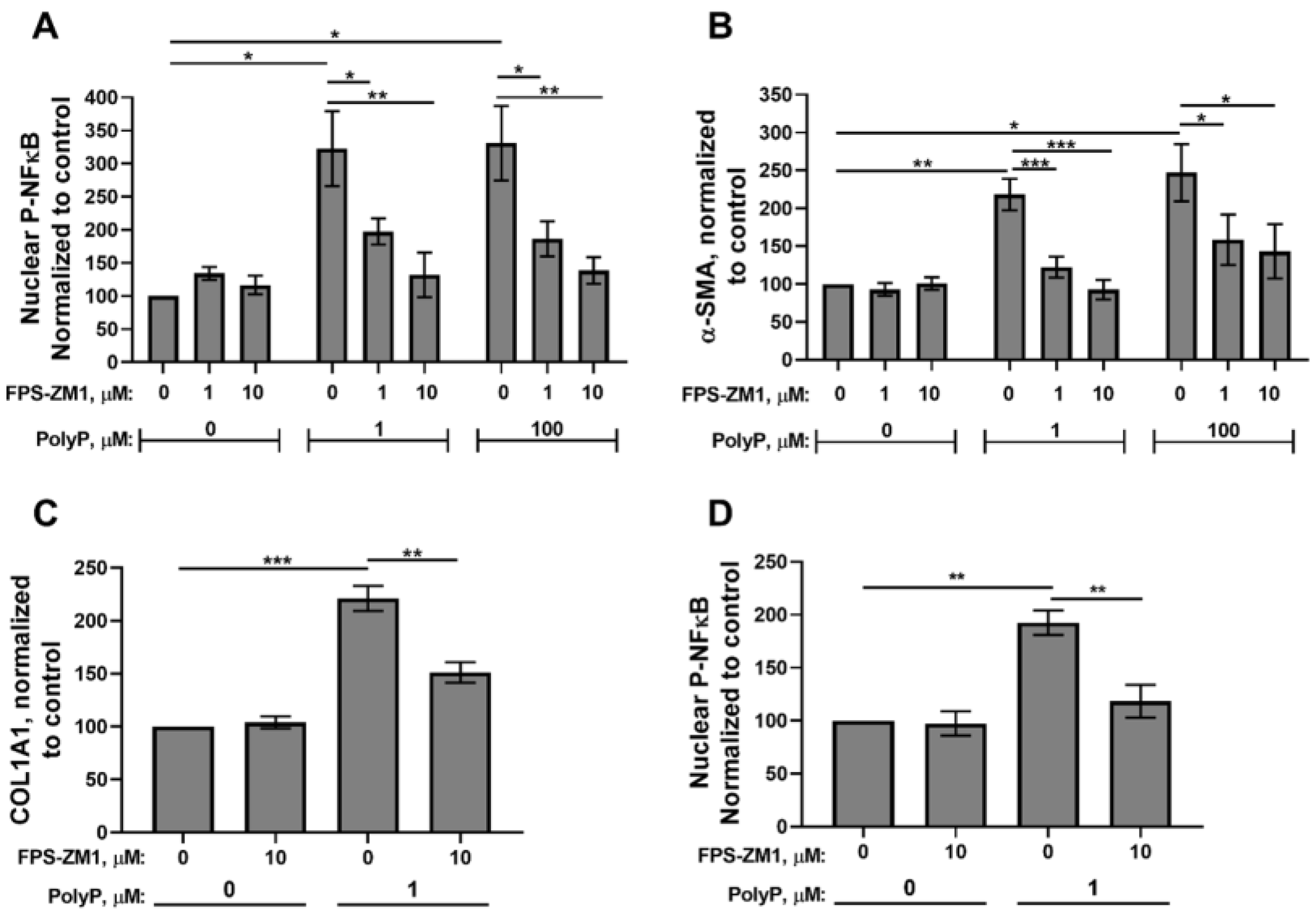
3.2. RAGE Mediates the Fibroblast Response to PolyP
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| α-SMA | alpha-smooth muscle actin |
| BCS | bovine calf serum |
| BSA | bovine serum albumin |
| COL1A1 | alpha-1chain of type I collagen |
| COL2A1 | alpha-1 chain of type II collagen |
| COL3A1 | alpha-1 chain of type III collagen |
| DMEM | Dulbecco’s modified Eagle medium |
| FN1 | fibronectin |
| HEPES | 4-(2-hydroxyethyl)piperazine-1-ethanesulfonic acid |
| NFκB | nuclear factor kappa B |
| polyP | polyphosphate |
| polyP75 | platelet-sized polyphosphate |
| polyP700 | long-chain polyphosphate |
| RAGE | receptor for advanced glycation end products (RAGE) |
| RPL13A | ribosomal protein L13a |
| RT-qPCR | reverse transcription quantitative polymerase chain reaction |
| TBS | 50 mmol/L Tris-HCl pH 7.5, 150 mmol/L NaCl |
| TGF-β1 | transforming growth factor beta 1 |
References
- Brown, M.R.; Kornberg, A. Inorganic polyphosphate in the origin and survival of species. Proc. Natl. Acad. Sci. USA 2004, 101, 16085–16087. [Google Scholar] [CrossRef] [PubMed]
- Rashid, M.H.; Rao, N.N.; Kornberg, A. Inorganic polyphosphate is required for motility of bacterial pathogens. J. Bacteriol. 2000, 182, 225–227. [Google Scholar] [CrossRef] [PubMed]
- Rashid, M.H.; Rumbaugh, K.; Passador, L.; Davies, D.G.; Hamood, A.N.; Iglewski, B.H.; Kornberg, A. Polyphosphate kinase is essential for biofilm development, quorum sensing, and virulence of Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 2000, 97, 9636–9641. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Gómez-García, M.R.; Brown, M.R.; Kornberg, A. Inorganic polyphosphate in Dictyostelium discoideum: Influence on development, sporulation, and predation. Proc. Natl. Acad. Sci. USA 2005, 102, 2731–2735. [Google Scholar] [CrossRef]
- Rao, N.N.; Gómez-García, M.R.; Kornberg, A. Inorganic polyphosphate: Essential for growth and survival. Annu. Rev. Biochem. 2009, 78, 605–647. [Google Scholar] [CrossRef]
- Nikel, P.I.; Chavarría, M.; Martínez-García, E.; Taylor, A.C.; de Lorenzo, V. Accumulation of inorganic polyphosphate enables stress endurance and catalytic vigour in Pseudomonas putida KT2440. Microb. Cell Fact. 2013, 12, 50. [Google Scholar] [CrossRef]
- Livermore, T.M.; Chubb, J.R.; Saiardi, A. Developmental accumulation of inorganic polyphosphate affects germination and energetic metabolism in Dictyostelium discoideum. Proc. Natl. Acad. Sci. USA 2016, 113, 996–1001. [Google Scholar] [CrossRef]
- Singh, M.; Tiwari, P.; Arora, G.; Agarwal, S.; Kidwai, S.; Singh, R. Establishing virulence associated polyphosphate kinase 2 as a drug target for Mycobacterium tuberculosis. Sci. Rep. 2016, 6, 26900. [Google Scholar] [CrossRef][Green Version]
- Suess, P.M.; Gomer, R.H. Extracellular polyphosphate inhibits proliferation in an autocrine negative feedback loop in Dictyostelium discoideum. J. Biol. Chem. 2016, 291, 20260–20269. [Google Scholar] [CrossRef]
- Kulakovskaya, T. Inorganic polyphosphates and heavy metal resistance in microorganisms. World J. Microbiol. Biotechnol. 2018, 34, 139. [Google Scholar] [CrossRef]
- Ruiz, F.A.; Lea, C.R.; Oldfield, E.; Docampo, R. Human platelet dense granules contain polyphosphate and are similar to acidocalcisomes of bacteria and unicellular eukaryotes. J. Biol. Chem. 2004, 279, 44250–44257. [Google Scholar] [CrossRef]
- Moreno-Sanchez, D.; Hernandez-Ruiz, L.; Ruiz, F.A.; Docampo, R. Polyphosphate is a novel pro-inflammatory regulator of mast cells and is located in acidocalcisomes. J. Biol. Chem. 2012, 287, 28435–28444. [Google Scholar] [CrossRef]
- Arredondo, C.; Cefaliello, C.; Dyrda, A.; Jury, N.; Martinez, P.; Díaz, I.; Amaro, A.; Tran, H.; Morales, D.; Pertusa, M.; et al. Excessive release of inorganic polyphosphate by ALS/FTD astrocytes causes non-cell-autonomous toxicity to motoneurons. Neuron 2022, 110, 1656–1670.e12. [Google Scholar] [CrossRef]
- Smith, S.A.; Mutch, N.J.; Baskar, D.; Rohloff, P.; Docampo, R.; Morrissey, J.H. Polyphosphate modulates blood coagulation and fibrinolysis. Proc. Natl. Acad. Sci. USA 2006, 103, 903–908. [Google Scholar] [CrossRef]
- Usui, Y.; Uematsu, T.; Uchihashi, T.; Takahashi, M.; Takahashi, M.; Ishizuka, M.; Doto, R.; Tanaka, H.; Komazaki, Y.; Osawa, M.; et al. Inorganic polyphosphate induces osteoblastic differentiation. J. Dent. Res. 2010, 89, 504–509. [Google Scholar] [CrossRef]
- Hassanian, S.M.; Dinarvand, P.; Smith, S.A.; Rezaie, A.R. Inorganic polyphosphate elicits proinflammatory responses through activation of the mammalian target of rapamycin complexes 1 and 2 in vascular endothelial cells. J. Thromb. Haemost. 2015, 13, 860–871. [Google Scholar] [CrossRef] [PubMed]
- Chrysanthopoulou, A.; Kambas, K.; Stakos, D.; Mitroulis, I.; Mitsios, A.; Vidali, V.; Angelidou, I.; Bochenek, M.; Arelaki, S.; Arampatzioglou, A.; et al. Interferon lambda1/IL-29 and inorganic polyphosphate are novel regulators of neutrophil-driven thromboinflammation. J. Pathol. 2017, 243, 111–122. [Google Scholar] [CrossRef]
- Müller, W.E.G.; Relkovic, D.; Ackermann, M.; Wang, S.; Neufurth, M.; Paravic Radicevic, A.; Ushijima, H.; Schröder, H.C.; Wang, X. Enhancement of wound healing in normal and diabetic mice by topical application of amorphous polyphosphate. Superior effect of a host–guest composite material composed of collagen (host) and polyphosphate (guest). Polymers 2017, 9, 300. [Google Scholar] [CrossRef] [PubMed]
- Müller, W.E.G.; Ackermann, M.; Wang, S.; Neufurth, M.; Muñoz-Espí, R.; Feng, Q.; Schröder, H.C.; Wang, X. Inorganic polyphosphate induces accelerated tube formation of HUVEC endothelial cells. Cell. Mol. Life Sci. 2018, 75, 21–32. [Google Scholar] [CrossRef]
- Suess, P.M.; Chinea, L.E.; Pilling, D.; Gomer, R.H. Extracellular polyphosphate promotes macrophage and fibrocyte differentiation, inhibits leukocyte proliferation, and acts as a chemotactic agent for neutrophils. J. Immunol. 2019, 203, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Carney, B.C.; Simbulan-Rosenthal, C.M.; Gaur, A.; Browne, B.J.; Moghe, M.; Crooke, E.; Moffatt, L.T.; Shupp, J.W.; Rosenthal, D.S. Inorganic polyphosphate in platelet rich plasma accelerates re-epithelialization in vitro and in vivo. Regen. Ther. 2020, 15, 138–148. [Google Scholar] [CrossRef] [PubMed]
- Rijal, R.; Cadena, L.A.; Smith, M.R.; Carr, J.F.; Gomer, R.H. Polyphosphate is an extracellular signal that can facilitate bacterial survival in eukaryotic cells. Proc. Natl. Acad. Sci. USA 2020, 117, 31923–31934. [Google Scholar] [CrossRef]
- Suess, P.M.; Smith, S.A.; Morrissey, J.H. Platelet polyphosphate induces fibroblast chemotaxis and myofibroblast differentiation. J. Thromb. Haemost. 2020, 18, 3043–3052. [Google Scholar] [CrossRef]
- Bochaton-Piallat, M.L.; Gabbiani, G.; Hinz, B. The myofibroblast in wound healing and fibrosis: Answered and unanswered questions. F1000Research 2016, 5, F1000 Faculty Rev-752. [Google Scholar] [CrossRef] [PubMed]
- Hinz, B. Myofibroblasts. Exp. Eye Res. 2016, 142, 56–70. [Google Scholar] [CrossRef]
- Powers, J.G.; Higham, C.; Broussard, K.; Phillips, T.J. Wound healing and treating wounds: Chronic wound care and management. J. Am. Acad. Dermatol. 2016, 74, 607–625, quiz 625–606. [Google Scholar] [CrossRef] [PubMed]
- Bowers, S.; Franco, E. Chronic wounds: Evaluation and management. Am. Fam. Physician 2020, 101, 159–166. [Google Scholar]
- Henderson, N.C.; Rieder, F.; Wynn, T.A. Fibrosis: From mechanisms to medicines. Nature 2020, 587, 555–566. [Google Scholar] [CrossRef]
- La, C.C.; Smith, S.A.; Vappala, S.; Adili, R.; Luke, C.E.; Abbina, S.; Luo, H.D.; Chafeeva, I.; Drayton, M.; Creagh, L.A.; et al. Smart thrombosis inhibitors without bleeding side effects via charge tunable ligand design. Nat. Commun. 2023, 14, 2177. [Google Scholar] [CrossRef]
- Smith, S.A.; Baker, C.J.; Gajsiewicz, J.M.; Morrissey, J.H. Silica particles contribute to the procoagulant activity of DNA and polyphosphate isolated using commercial kits. Blood 2017, 130, 88–91. [Google Scholar] [CrossRef]
- Smith, S.A.; Wang, Y.; Morrissey, J.H. DNA ladders can be used to size polyphosphate resolved by polyacrylamide gel electrophoresis. Electrophoresis 2018, 39, 2454–2459. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Bae, J.S.; Lee, W.; Rezaie, A.R. Polyphosphate elicits pro-inflammatory responses that are counteracted by activated protein C in both cellular and animal models. J. Thromb. Haemost. 2012, 10, 1145–1151. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.A.; Choi, S.H.; Davis-Harrison, R.; Huyck, J.; Boettcher, J.; Rienstra, C.M.; Morrissey, J.H. Polyphosphate exerts differential effects on blood clotting, depending on polymer size. Blood 2010, 116, 4353–4359. [Google Scholar] [CrossRef]
- Wang, Y.; Ivanov, I.; Smith, S.A.; Gailani, D.; Morrissey, J.H. Polyphosphate, Zn2+ and high molecular weight kininogen modulate individual reactions of the contact pathway of blood clotting. J. Thromb. Haemost. 2019, 17, 2131–2140. [Google Scholar] [CrossRef] [PubMed]
- Jainchill, J.L.; Aaronson, S.A.; Todaro, G.J. Murine sarcoma and leukemia viruses: Assay using clonal lines of contact-inhibited mouse cells. J. Virol. 1969, 4, 549–553. [Google Scholar] [CrossRef]
- Hershberger, R.P.; Culp, L.A. Cell-type-specific expression of alternatively spliced human fibronectin IIICS mRNAs. Mol. Cell. Biol. 1990, 10, 662–671. [Google Scholar] [CrossRef] [PubMed]
- Watson, M.R.; Wallace, K.; Gieling, R.G.; Manas, D.M.; Jaffray, E.; Hay, R.T.; Mann, D.A.; Oakley, F. NF-kappaB is a critical regulator of the survival of rodent and human hepatic myofibroblasts. J. Hepatol. 2008, 48, 589–597. [Google Scholar] [CrossRef]
- Pan, H.W.; Cui, Y.H.; Zeng, J.W. NF-kappaB mediates the survival of corneal myofibroblast induced by angiotensin II. Investig. Ophthalmol. Vis. Sci. 2014, 55, 4220–4228. [Google Scholar] [CrossRef]
- Shin, H.M.; Kim, M.H.; Kim, B.H.; Jung, S.H.; Kim, Y.S.; Park, H.J.; Hong, J.T.; Min, K.R.; Kim, Y. Inhibitory action of novel aromatic diamine compound on lipopolysaccharide-induced nuclear translocation of NF-κB without affecting IκB degradation. FEBS Lett. 2004, 571, 50–54. [Google Scholar] [CrossRef]
- Johnson, T.A.; Sohn, J.; Vaske, Y.M.; White, K.N.; Cohen, T.L.; Vervoort, H.C.; Tenney, K.; Valeriote, F.A.; Bjeldanes, L.F.; Crews, P. Myxobacteria versus sponge-derived alkaloids: The bengamide family identified as potent immune modulating agents by scrutiny of LC-MS/ELSD libraries. Bioorg. Med. Chem. 2012, 20, 4348–4355. [Google Scholar] [CrossRef]
- Dinarvand, P.; Hassanian, S.M.; Qureshi, S.H.; Manithody, C.; Eissenberg, J.C.; Yang, L.; Rezaie, A.R. Polyphosphate amplifies proinflammatory responses of nuclear proteins through interaction with receptor for advanced glycation end products and P2Y1 purinergic receptor. Blood 2014, 123, 935–945. [Google Scholar] [CrossRef]
- Holmström, K.M.; Marina, N.; Baev, A.Y.; Wood, N.W.; Gourine, A.V.; Abramov, A.Y. Signalling properties of inorganic polyphosphate in the mammalian brain. Nat. Commun. 2013, 4, 1362. [Google Scholar] [CrossRef] [PubMed]
- Deane, R.; Singh, I.; Sagare, A.P.; Bell, R.D.; Ross, N.T.; LaRue, B.; Love, R.; Perry, S.; Paquette, N.; Deane, R.J.; et al. A multimodal RAGE-specific inhibitor reduces amyloid β-mediated brain disorder in a mouse model of Alzheimer disease. J. Clin. Investig. 2012, 122, 1377–1392. [Google Scholar] [CrossRef] [PubMed]
- Tarbit, E.; Singh, I.; Peart, J.N.; Rose’Meyer, R.B. Biomarkers for the identification of cardiac fibroblast and myofibroblast cells. Heart Fail. Rev. 2019, 24, 1–15. [Google Scholar] [CrossRef]
- Vallée, A.; Lecarpentier, Y. TGF-beta in fibrosis by acting as a conductor for contractile properties of myofibroblasts. Cell Biosci. 2019, 9, 98. [Google Scholar] [CrossRef] [PubMed]
- Shenoi, R.A.; Kalathottukaren, M.T.; Travers, R.J.; Lai, B.F.L.; Creagh, A.L.; Lange, D.; Yu, K.; Weinhart, M.; Chew, B.H.; Du, C.; et al. Affinity-based design of a synthetic universal reversal agent for heparin anticoagulants. Sci. Transl. Med. 2014, 6, 260ra150. [Google Scholar] [CrossRef]
- Kalathottukaren, M.T.; Creagh, A.L.; Abbina, S.; Lu, G.; Karbarz, M.J.; Pandey, A.; Conley, P.B.; Kizhakkedathu, J.N.; Haynes, C. Comparison of reversal activity and mechanism of action of UHRA, andexanet, and PER977 on heparin and oral FXa inhibitors. Blood Adv. 2018, 2, 2104–2114. [Google Scholar] [CrossRef]
- Kalathottukaren, M.T.; Abraham, L.; Kapopara, P.R.; Lai, B.F.; Shenoi, R.A.; Rosell, F.I.; Conway, E.M.; Pryzdial, E.L.; Morrissey, J.H.; Haynes, C.A.; et al. Alteration of blood clotting and lung damage by protamine are avoided using the heparin and polyphosphate inhibitor UHRA. Blood 2017, 129, 1368–1379. [Google Scholar] [CrossRef]
- Mafi, A.; Abbina, S.; Kalathottukaren, M.T.; Morrissey, J.H.; Haynes, C.; Kizhakkedathu, J.N.; Pfaendtner, J.; Chou, K.C. Design of polyphosphate inhibitors: A molecular dynamics investigation on polyethylene glycol-linked cationic binding groups. Biomacromolecules 2018, 19, 1358–1367. [Google Scholar] [CrossRef]
- Travers, R.J.; Shenoi, R.A.; Kalathottukaren, M.T.; Kizhakkedathu, J.N.; Morrissey, J.H. Nontoxic polyphosphate inhibitors reduce thrombosis while sparing hemostasis. Blood 2014, 124, 3183–3190. [Google Scholar] [CrossRef]
- Xu, A.; Deng, F.; Chen, Y.; Kong, Y.; Pan, L.; Liao, Q.; Rao, Z.; Xie, L.; Yao, C.; Li, S.; et al. NF-κB pathway activation during endothelial-to-mesenchymal transition in a rat model of doxorubicin-induced cardiotoxicity. Biomed. Pharmacother. 2020, 130, 110525. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.M.; Zhang, W.; Wang, L.P.; Li, G.R.; Deng, X.L. Advanced glycation end products promote proliferation of cardiac fibroblasts by upregulation of KCa3.1 channels. Pflügers Arch. 2012, 464, 613–621. [Google Scholar] [CrossRef]
- Liang, B.; Zhou, Z.; Yang, Z.; Liu, J.; Zhang, L.; He, J.; Li, H.; Huang, Y.; Yang, Q.; Xian, S.; et al. AGEs-RAGE axis mediates myocardial fibrosis via activation of cardiac fibroblasts induced by autophagy in heart failure. Exp. Physiol. 2022, 107, 879–891. [Google Scholar] [CrossRef] [PubMed]
- Golebiewska, E.M.; Poole, A.W. Platelet secretion: From haemostasis to wound healing and beyond. Blood Rev. 2014, 29, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Nurden, A.T.; Nurden, P.; Sanchez, M.; Andia, I.; Anitua, E. Platelets and wound healing. Front. Biosci. 2008, 13, 3532–3548. [Google Scholar] [CrossRef]
- Darby, I.A.; Zakuan, N.; Billet, F.; Desmouliere, A. The myofibroblast, a key cell in normal and pathological tissue repair. Cell. Mol. Life Sci. 2016, 73, 1145–1157. [Google Scholar] [CrossRef]
- Malara, A.; Abbonante, V.; Zingariello, M.; Migliaccio, A.; Balduini, A. Megakaryocyte contribution to bone marrow fibrosis: Many arrows in the quiver. Mediterr. J. Hematol. Infect. Dis. 2018, 10, e2018068. [Google Scholar] [CrossRef]
- Papadantonakis, N.; Matsuura, S.; Ravid, K. Megakaryocyte pathology and bone marrow fibrosis: The lysyl oxidase connection. Blood 2012, 120, 1774–1781. [Google Scholar] [CrossRef]
- Crooks, M.G.; Fahim, A.; Naseem, K.M.; Morice, A.H.; Hart, S.P. Increased platelet reactivity in idiopathic pulmonary fibrosis is mediated by a plasma factor. PLoS ONE 2014, 9, e111347. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suess, P.M.; La, C.C.; Vappala, S.; Kizhakkedathu, J.N.; Morrissey, J.H. Platelet Polyphosphate Signals Through NFκB to Induce Myofibroblast Differentiation. Biomolecules 2025, 15, 1441. https://doi.org/10.3390/biom15101441
Suess PM, La CC, Vappala S, Kizhakkedathu JN, Morrissey JH. Platelet Polyphosphate Signals Through NFκB to Induce Myofibroblast Differentiation. Biomolecules. 2025; 15(10):1441. https://doi.org/10.3390/biom15101441
Chicago/Turabian StyleSuess, Patrick M., Chanel C. La, Sreeparna Vappala, Jayachandran N. Kizhakkedathu, and James H. Morrissey. 2025. "Platelet Polyphosphate Signals Through NFκB to Induce Myofibroblast Differentiation" Biomolecules 15, no. 10: 1441. https://doi.org/10.3390/biom15101441
APA StyleSuess, P. M., La, C. C., Vappala, S., Kizhakkedathu, J. N., & Morrissey, J. H. (2025). Platelet Polyphosphate Signals Through NFκB to Induce Myofibroblast Differentiation. Biomolecules, 15(10), 1441. https://doi.org/10.3390/biom15101441





