1. Introduction
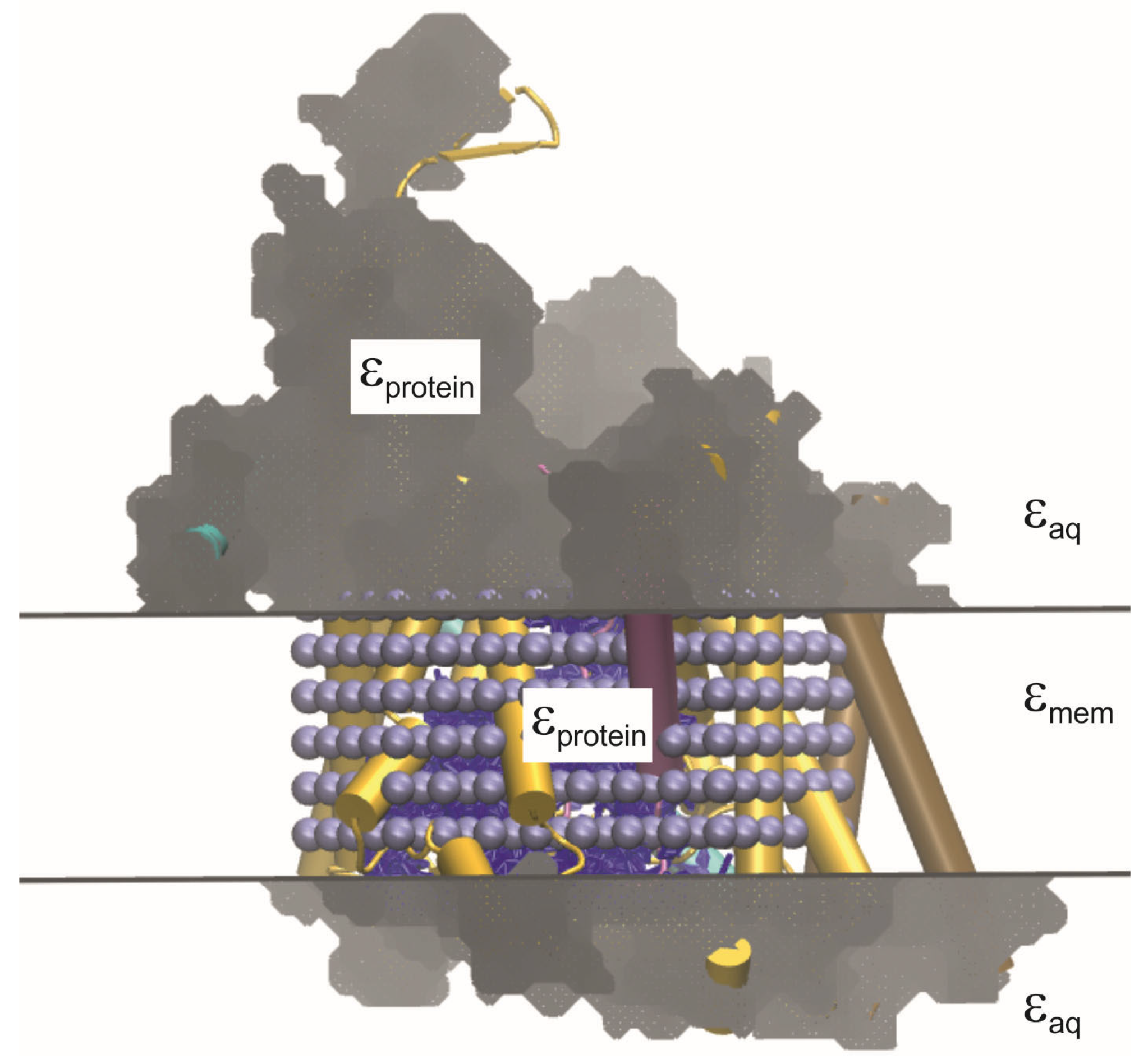
In nature, membranes composed of lipid bilayers are used by both pro- and eukaryotic cells to contain their interior structures and protect them from the environment. The tail-to-tail arrangement of the lipids in such membranes gives the structure a hydrophobic core, while the polar head groups point towards the aqueous exterior (see
Figure 1a). This provides an efficient barrier to polar and charged (or simply very large) substances on either side, which require the help of channel proteins embedded in the membrane in order to cross [
1]. The transportation of proteins or polypeptide chains—which are charged, polar, and very large—is facilitated by specialized channel proteins called translocons.
The Sec (from secretion system) complex is a universally conserved, heterotrimeric membrane protein that is involved in protein transport across, and insertion into, the plasma membrane. In prokaryotes, it is made up of subunits SecY, SecE, and SecG, while the analogous complexes found in eukaryotes and archaea are known as Sec61αβγ and SecYEβ, respectively [
2,
3,
4,
5]. The main subunit of SecYEG is SecY, whose ten transmembrane helices (TM) form two halves of a clamshell-like structure. It has a roughly hourglass shape, perpendicular to the membrane plane, with a ring of six hydrophobic amino acids—called the pore ring (PR)—whereby four of the hydrophobic groups define the constriction. For the insertion of proteins into the membrane, the translocon can open its lateral gate (LG), formed by helices TM2b and TM7 [
6]. In the resting state, the central pore is blocked by a short helix, TM2a, termed the plug, which moves to the side in the active state [
5,
7].
Figure 1b shows a cartoon representation of the main features of the channel. Of the other two subunits, only SecE is essential for function by wrapping around the central unit and holding the two halves together. SecG is non-essential for cell viability. The full structure of the heterotrimeric complex is shown in
Figure 1a.
Figure 1.
(
a) Side view of SecYEG embedded in a lipid bilayer in the open state with the cytoplasmic side at the top and the periplasmic side at the bottom. Subunits SecY, SecE, and SecG are shown in yellow, cyan, and brown, respectively, with the exception of the lateral gate helices (TM2b, TM7), which are highlighted in orange. The pore ring (represented by four isoleucine residues) as well as the plug domain are colored red, while the membrane lipids are light gray. Figure made with VMD using the active state version of the
T. thermophilus channel [
5]. (
b) Cartoon representation of the SecY subunit highlighting its primary features. The hourglass shape of the channel interior and the hydrophobic pore ring can be seen in the center, while the lateral gate is shown on the left and on the right. The importance of the plug domain in sealing the pore is emphasized on the right (Figure adapted from [
2]).
Figure 1.
(
a) Side view of SecYEG embedded in a lipid bilayer in the open state with the cytoplasmic side at the top and the periplasmic side at the bottom. Subunits SecY, SecE, and SecG are shown in yellow, cyan, and brown, respectively, with the exception of the lateral gate helices (TM2b, TM7), which are highlighted in orange. The pore ring (represented by four isoleucine residues) as well as the plug domain are colored red, while the membrane lipids are light gray. Figure made with VMD using the active state version of the
T. thermophilus channel [
5]. (
b) Cartoon representation of the SecY subunit highlighting its primary features. The hourglass shape of the channel interior and the hydrophobic pore ring can be seen in the center, while the lateral gate is shown on the left and on the right. The importance of the plug domain in sealing the pore is emphasized on the right (Figure adapted from [
2]).
In order to activate the channel for translocation or insertion, it must dock to either a ribosome, in what is known as co-translational translocation, or the motor protein SecA for post-translational translocation. In the former, the protein is translocated as it emerges from the ribosome; in the latter, the protein is translated first and is then targeted to the translocon via a signal recognition particle and receptor complex [
2]. Once the protein is in the active state, however, the question arises as to what the driving forces are for the subsequent translocation. For the insertion of largely hydrophobic helices into the membrane, it has been shown that the free energy gained by the partitioning into the lipid membrane causes a pulling force on the peptide chain still in the channel [
8]. Regarding the translocation through the membrane, extensive research, both experimentally and theoretically, has been performed to uncover the physical mechanisms [
2,
4,
5,
9,
10]. It appears that there is a kind of consensus on a general ‘‘rough’’ model of activation, which considers a series of steps: (i) association of the channel with the protein to be translocated and ATP-bound SecA, (ii) opening of the LG by the signal sequence (SS) of the transported protein (via the movement of TM7 and TM2b), and (iii) plug relocation. However, details of the physical mechanism underlying activation and operation of the SecY machinery are still poorly understood. Recently, we tried to wrap the available information into a working model based on molecular dynamics (MD) simulations [
5]. We came to the conclusion that binding of SecA (in the ATP-bound state) and the SS leads to a transmembrane helix rearrangement that weakens contacts inside the hydrophobic core of SecYEG and provides a driving force for plug opening. The conformational transitions are enabled by a delicate interplay between hydrophobic forces on one side, and a combination of forces on the other side that we termed PEES. This abbreviation stands for the proton motive force, external forces (due to binding with the translocation partners), entropic effects (e.g., due to conformational changes in the protein), and solvent-induced forces (e.g., due to bound water). In the open state, SecYEG still provides a barrier for bulky residues, meaning translocation is very likely not just a diffusion process, but pulling forces are required. In this context, it has been hypothesized earlier that the proton motive force plays a significant role in translocation [
10].
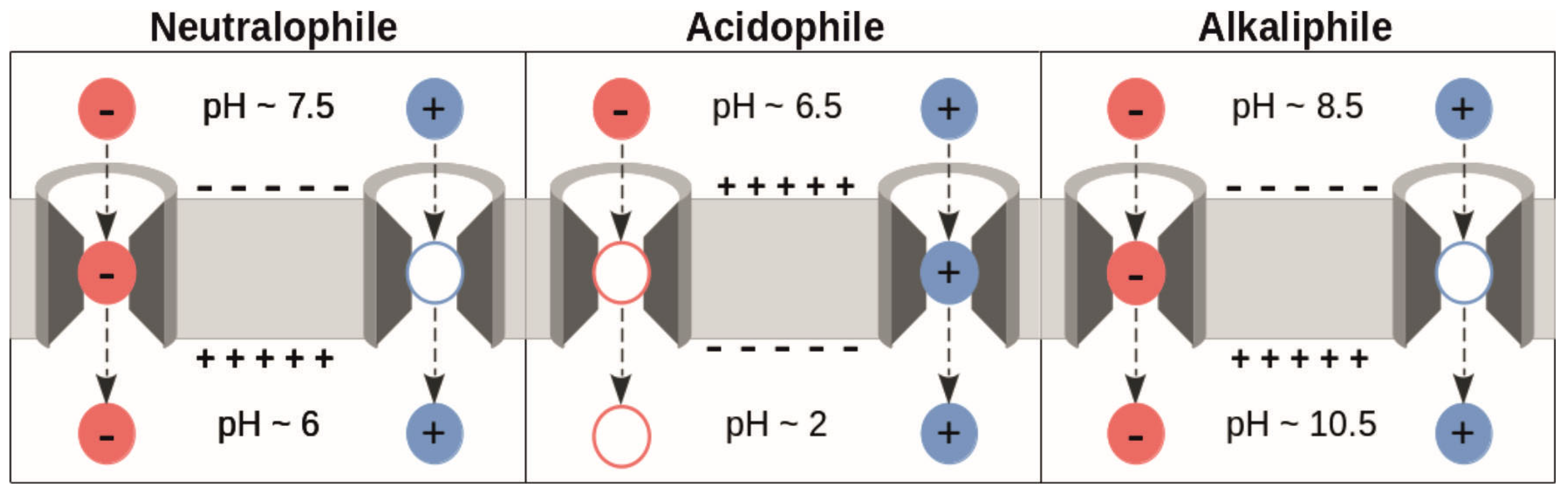
The proton motive force (PMF) is defined as the sum of the transmembrane potential and the transmembrane proton gradient, and it is an essential source of energy for cellular processes [
11]. In
Escherichia coli cells, the transmembrane potential is about 100–150 mV (negative inside), and has been shown to influence the translocation of model peptides containing presumably negatively charged residues [
12]. However, as proteins can contain both acidic and basic amino acids—and generally have similar amounts of both—this means that, given the
values of those residues at physiological pH values, it is questionable that there is a net force supporting translocation [
10]. Since
values of titratable groups depend on the electrostatic environment in which they are located, it is possible that shifts in
values may cause a net charge of the substrate due to different charge states in acids and bases [
10]. If the electrostatic interactions in the translocon stabilize negative charges, to compensate for them existing in an area of lower permittivity, while not doing the same for positively charged residues, there would be a net force acting in the direction of translocation.
Additionally, while the above considerations are based on the transmembrane potential and pH values of neutralophilic bacteria, the mechanism could still work in the direction of translocation in extremophilic bacteria [
10], if the
shifts experienced by acids and bases lead to the protonation states shown in
Figure 2. Additionally, the electrostatic interactions with titratable residues in SecYEG could stabilize one charge over another in the different bacteria, as the specific protonation state of the translocon is pH dependent, too. However, there must be an alternative driving force involved in protein translocation in eukaryotic cells, as Sec61 is located in the endoplasmic reticulum (ER) membrane which does not have an appreciable transmembrane potential [
10,
13].
The goal of the present work is to computationally determine the titration behavior of amino acids during their translocation across SecYEG in order to gain insight into the driving forces behind this process.
3. Results
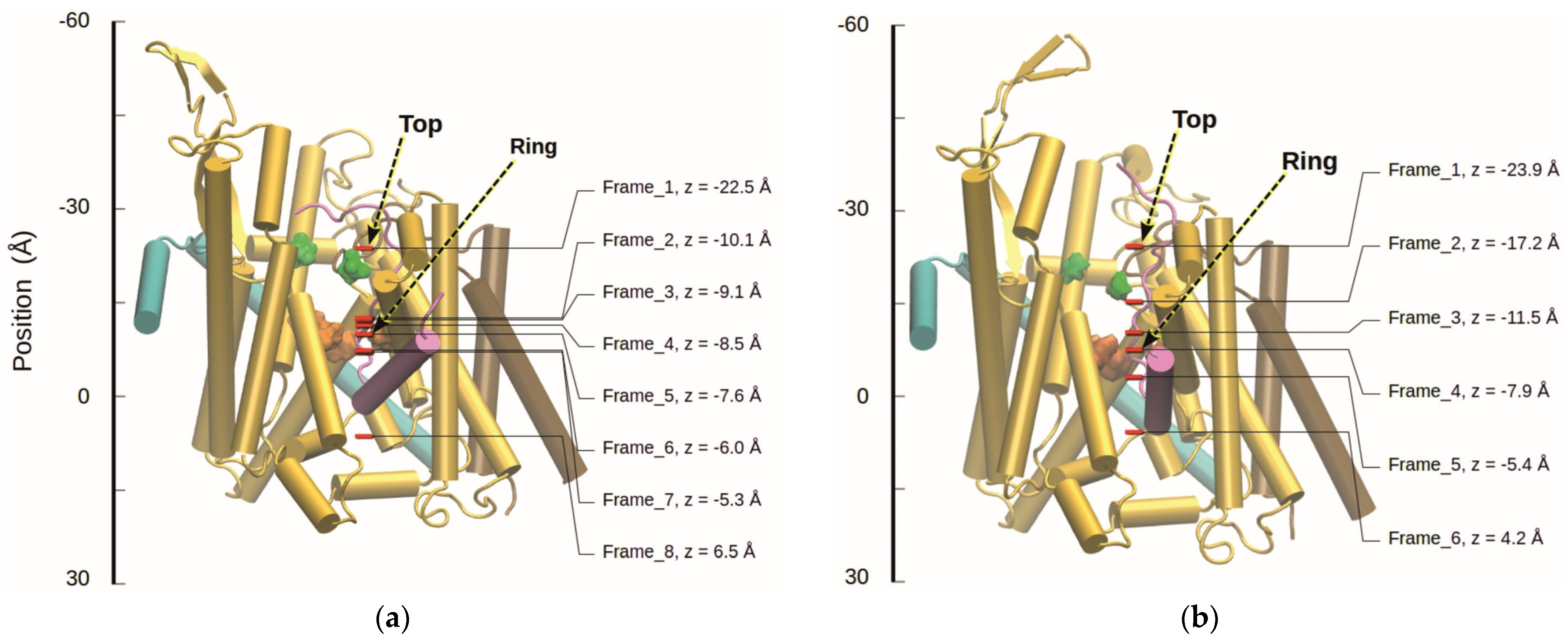
Following an SMD simulation of the translocation, the
shift in peptide residue 31 as a result of its position was investigated by performing continuum electrostatics calculations and MC titrations with the residue at selected positions in the channel. For this purpose, snapshots were taken from the SMD trajectory and used as starting structures for MD simulations with positional constraints on the C
α-atom of the residue. The positions chosen can be seen in
Figure 5.
In
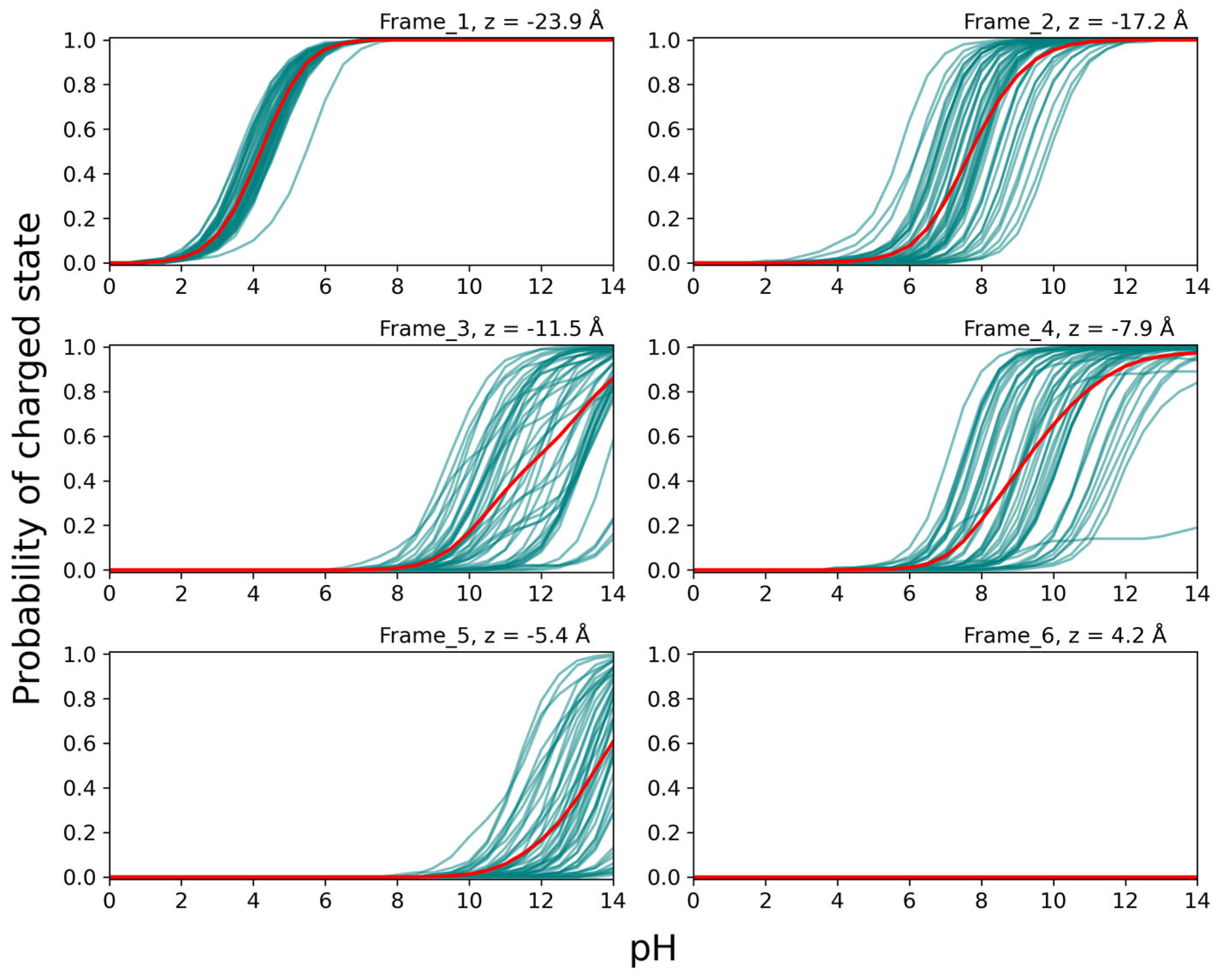
Figure 8, the titration curves for Lys at position 31 for structures taken from MD simulations with Lys 31 in the LYS protonation form (cf.
Figure 7) are shown for the eight different positions in the SecYEG channel. Titrations were performed on 50 structures for each frame. A clear trend can be seen in the average probability of the charged state decreasing as the residue moves farther into the channel (frames 1 to 5), and increasing again once it has passed the PR (frames 6 to 8). The form of some individual titration curves deviates strongly from the standard Henderson-Hasselbalch (HH) form, indicating strong coupling of Lys 31 to other titratable groups above and below the PR. The titration curves for frames 3 and 4 can be divided into two groups corresponding to fully charged (i.e., protonation probability near 1) or fully uncharged Lys 31 at physiological pH, as indicated for frame 3 in
Figure 8. This binary behavior is due to strong interactions with Asp G34 on SecG, which is less than 5 Å away in this frame (measured from the NZ atom of LYS to the CD atom of Asp). This is confirmed by the fact that in the frame 3 structures, Asp G34 shows the same binary titration behavior and protonation state as LYS, meaning when LYS 31 is charged, Asp G34 is charged, too, and when LYS 31 is uncharged, so is Asp G34. The Asp residue is located on the cytosolic loop of SecG at the top of the channel, and is thus free to interact with the peptide in multiple positions. The wide spread of the individual titration curves in other frames also shows the strong dependence of the residue’s protonation probability on its orientation, as the C
α-atom of the residue is constrained, and so variations are due to thermal fluctuations of the side chain.
In
Figure 9, the titration curves for Glu at position 31 for structures taken from MD simulations with Glu 31 in the GLU protonation form (cf.
Figure 7) are shown for the six different positions in the SecYEG channel. For the titration curves of the GLU residue (
Figure 9), a general transition to the uncharged state can be seen as the residue travels further down the channel, with the exception of the small increase from frame 3 to 4. Unlike LYS 31, GLU 31 does not return to the charged state once it has passed the PR. This may be due to residues Glu 68 and Glu 195 located at the bottom of the channel—which remain almost entirely charged in frames 4–6—though this was not tested explicitly by fixing their protonation states, as was done for two Asp residues (see below). Additionally, it appears that the protonation state of GLU 31 is less dependent on its orientation, as the individual titration curves have a much smaller spread compared to LYS 31. This observation might be related to the fact that the Glu chain is not only shorter, but also less flexible than that of Lys.
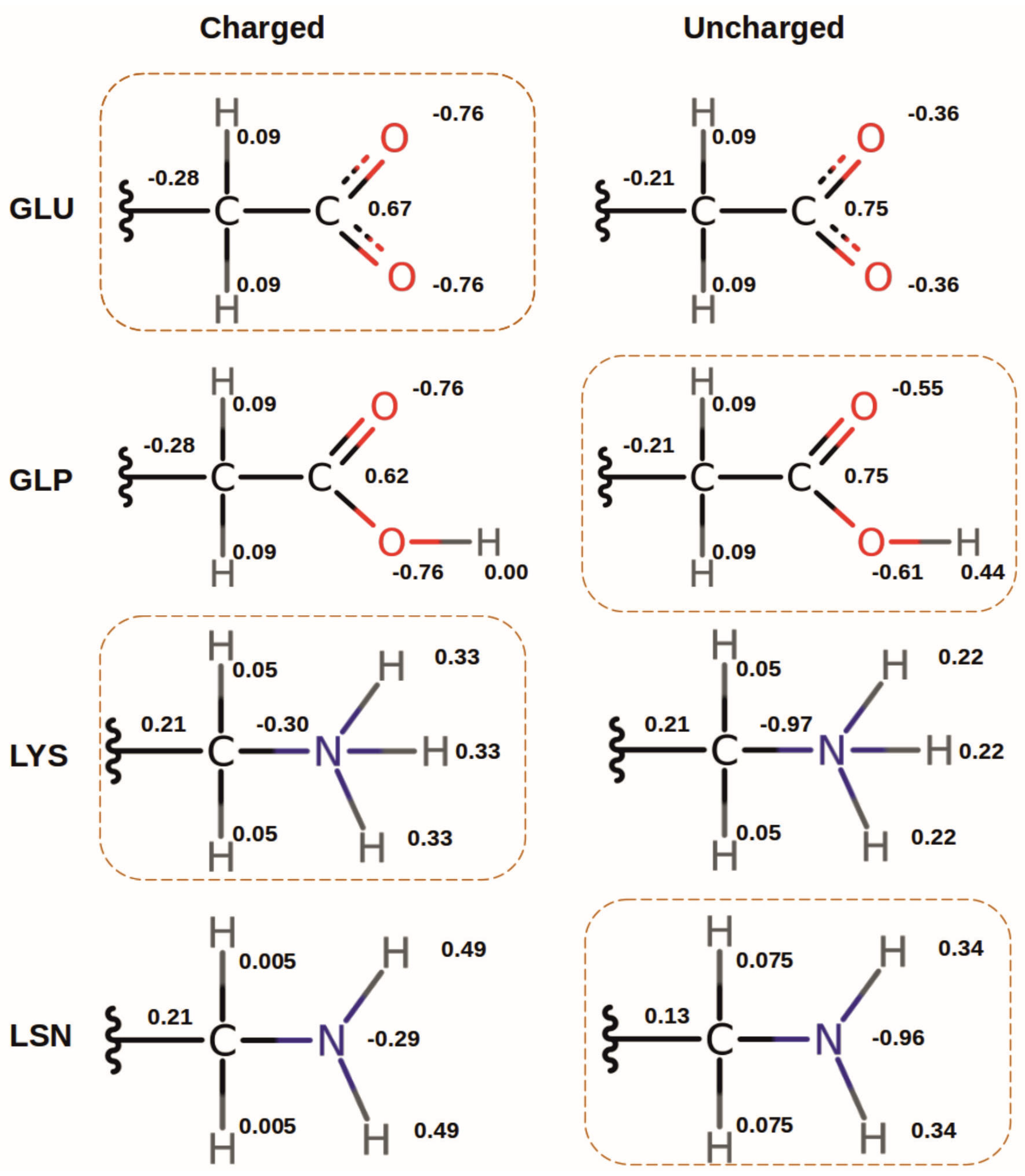
In order to determine the bias in protonation probability introduced by the choice of the protonation form (cf.
Section 2.3.5) used for the MD simulation, we performed additional simulations with the alternative protonation forms GLP and LSN. These simulations were done only for two frames in each case referred to as “top” (frame 1, where residue 31 is located in the cytoplasmic entrance in the channel) and “ring” (frame 5 for LSN and frame 4 for GLP, where residue 31 is located in the PR). The average titration curves of the 50 snapshots from the top and ring frames were then compared for the two protonation forms. Additionally, the effect of other titratable residues on the
shift in a translocating peptide was investigated by fixing Asp G34 on SecG and Asp Y410 on SecY in different combinations of protonation states and titrating peptide residue 31. To determine the effects of positive and negative charges, one run was done with all Asp and Glu residues (except the test residue) fixed in their uncharged states (“pos_only” curves), and another fixing Arg and Lys residues (except the test residue) in their uncharged states (“neg_only” curves). The net effect of these titratable residues was determined by fixing them all in the uncharged state (“none” curves), so that any
shift must be due to the dielectric environment and interaction with non-titrating charges. All titration curve averages are overlaid for comparison in
Figure 10.
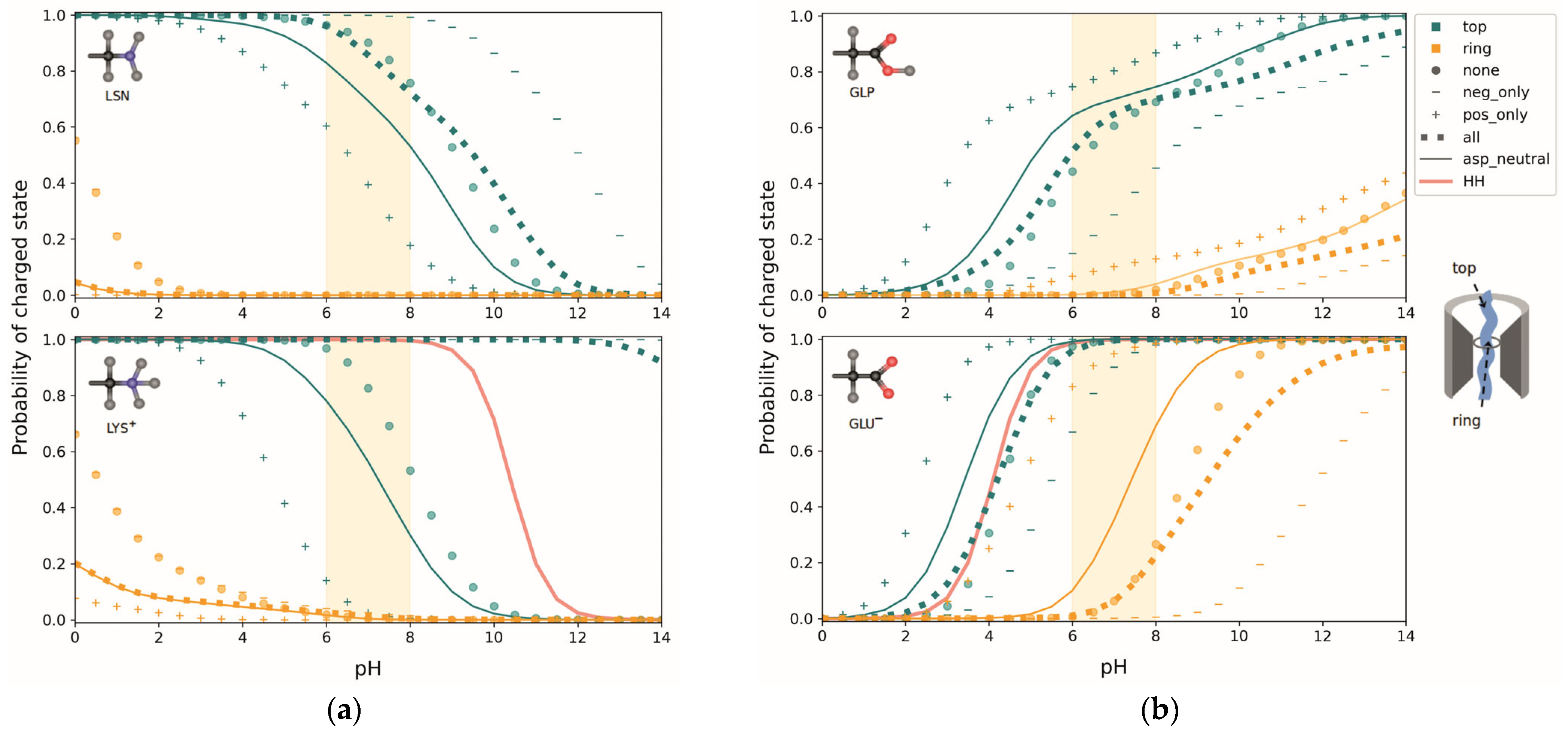
The averaged titration curves show a largely monotonic behavior, so that an effective
value of the titrating group may be defined by the pH for which the probability of the charged state is 0.5. For both LYS and LSN, a shift in the
to lower values is apparent in the ring frame compared to the top frame (
Figure 10a)—meaning a higher probability for residue 31 being uncharged in the PR at physiological pH. In fact, in this pH range, the probability of Lys being charged in the PR is essentially zero. However, when the deprotonated and uncharged form LSN is used in the MD simulation, the
in both the top and the ring frames is lower compared to LYS, indicating a bias towards the uncharged state. Possibly titrations on structures obtained from MD simulations tend to be biased towards the protonation states of the protonation forms chosen.
The largest changes in the top frames is seen when Asp G34 is fixed in the uncharged state, resulting in a
shift from >14 to about 7 for the LYS curves (
Figure 10a, bottom), while the corresponding shift in the LSN curves is only about 1 pH unit (
Figure 10a, top graph). The change in charge state of Asp Y410 does not result in significant shifts. This finding can be explained by the fact that Asp G34 is spatially very close to residue 31 in the top frame. Therefore, there are strong stabilizing interactions between the anionic Asp and cationic Lys, preventing the latter from giving up its proton. As LSN is uncharged during the MD simulation, there are no such interactions and the charge state of Asp G34 has a smaller effect. The influence of Asp G34 on the charge state of Lys 31 is thus dependent on the standard charge states of the protonation forms used in the MD simulations, and is likely exaggerated in the LYS frames. Labeled graphs for the different Asp states can be found in
Supplementary Material SM4, Figures S1 and S2.
Switching off the remaining negative charges (“pos_only”) in the top frames lowers the
of LYS by another 4 pH units and LSN by about 2, while switching off all positive charges (“neg_only”) has virtually no effect on LYS, but increases the
of LSN by 2 pH units. The combined effect of all titratable residues on LSN is negligible, with the “none” and “all” states resulting in nearly identical curves. In the LYS case, the interaction with Asp G34 dominates, resulting in an ∼7 pH unit shift between the “all” and “none” states. Any effects on Lys in the PR are negligible as it is almost entirely uncharged independent of any other titratable residues. What is noticeable, however, is that for both LYS and LSN, the “neg_only” state is identical to the “none” state, showing that negatively charged groups have no significant interactions with positively charged peptide residues when in the PR.
Similar to Lys, Glu experiences a shift towards the uncharged state as it moves through the pore (
Figure 10b), as well as a difference in the protonation probabilities between the deprotonated and protonated form used for the MD simulation. Again, the frames using the protonated form (GLP) show a bias towards the protonated state, seen by an increase in
. Additionally, the GLP residue’s protonation probability seems to be more dependent on its orientation than that of GLU, as is evident from the “double- humped” shape of the average titration curves resulting from a large spread in the curves of individual structures (not shown for GLP). In all cases, though, the “all” and “none” curves are very similar, meaning that the combined effect of titratable residues on the
of Glu is very small. The presence of only positive or negative charges would result in a significant shift, as is shown by the curves in the “pos_only” and “neg_only” states. Apparently, the effects of positively and negatively charged groups cancel each other. This is an interesting finding as there are 1.7 times more basic amino acids in SecYEG than acidic ones. The position of Glu in the channel allows for a more balanced interaction with basic and acidic groups, including a strong influence of Asp G34. Labeled graphs for the different Asp states can be found in
Supplementary Material SM4, Figures S3 and S4. Overall, the
of Glu, like that of Lys, appears to be dictated by the dielectric properties of its environment, interaction with non-titrating charges, and its distance from the dielectric interface—rather than interactions with ionizable residues in the channel.
We have also tested the effect of the precise spatial dependence of
, i.e., the position of the interface between high and low dielectric constant in the channel (
Figure 11). This was done only for the ring frames, where residue 31 is in the PR. For the first test (
Figure 11a), where neither a membrane slab nor dummy atoms, but an explicit membrane was used, the SES probe (sphere of radius 1.4 Å; cf.
Section 2.3.4.) was able to travel all the way through the pore—causing the residue to be surrounded by a medium of
. In the test visualized in
Figure 11b, there are also no dummy atoms, but an implicit membrane in the form of a slab with
replacing the explicit lipid bilayer (cf.
Figure 6). This resulted in the positioning of a 2/80 interface almost at the position of the test residue. Additionally, due to the algorithms in TAPBS, the low permittivity area of the membrane slab was also defined in the pore region. In both of these setups, the test residue was in contact with the
interface and thus remained charged. The final panel (
Figure 11c) shows the setup generally used in this project: the same implicit membrane as in (b) but with the addition of uncharged dummy atoms in the channel to impede the entry of the SES probe. This procedure results in the entire protein, including the PR area, being assigned
, and holds the
interface off from protruding almost to the PR. This was the only case in which the test residue changed its protonation state. Thus, if the channel water is modeled as having bulk properties, the peptide residue 31 is not neutralized.
4. Discussion
The goal of the present work was to determine the probability of being charged of a test residue in a translocating peptide as it travels through the SecYEG translocon. The simulations clearly suggest that both, Lys and Glu as test residues, are charge-neutralized when they pass the PR, i.e., the constriction in the hydrophobic interior of the translocon. This result argues against a discrimination of positively and negatively charged residues that would suggest the electric component of the PMF directly contributes to the driving force behind peptide translocation in neutralophilic bacteria as previously hypothesized [
10]. However, the simulation methods involve a number of approximations that must be discussed, and the results should be confronted with recent experimental studies on the translocation of charged residues [
12,
56].
PB-based electrostatic methods to determine
values are usually applied to X-ray structures of proteins—and probably work best in this context. In the case of active SecYEG, we had neither X-ray nor cryo-EM structures of the translocon in different stages of the translocation process with a sufficiently high resolution at our disposal. So, we decided to create structures of the translocating peptide by employing MD simulations. These simulations were built upon an open
T. thermophilus structure that was constructed in previous work [
5,
26] from the closed channel [
24] by taking into account information about the opening from an active-state chimera structure [
25]. The different stages of translocation were created using an SMD protocol [
35] and the test residue fixed at its backbone by a harmonic constraint. One possible way to proceed from there would have been an energy minimization of the respective stages of translocation and PB computations based upon these minimized structures. However, due to the flexibility of the test residue’s side chain, it probably would have been difficult to find a single, representative conformation. Therefore, we decided to perform MD simulations to create an ensemble of structures and to perform PB/MC titrations on each structure. It should be noted that the translocation process is slow (in the range of milliseconds per residue), so that the transported peptide can in fact sample a large number of conformations in each stage of the translocation.
Since the used structures were taken randomly (i.e., with equal weight) from the second half of a 10 ns
simulation, the structural ensembles may be considered as representing roughly isothermal-isobaric ensembles. Then, the ensemble-averaged protonation probabilities are obtained by just averaging the individual titration curves as shown in
Figure 8 and
Figure 9. Clearly, the results may be improved by increasing the number of structures and the length of the
trajectories. There is always a compromise between accuracy and simulation time. A problem that cannot be solved within this framework is the bias of the MD simulations due to the explicit protonation form of the titratable residue used in these simulations. Note that any standard MD simulation is limited by this problem. Therefore, it is advisable to perform tests with different protonation forms to assess errors. In the present case, we obtain the same basic result for each protonation form: The probability of the test residue being charged is close to zero when the residue is in the PR.
A critical—and much debated [
20,
45,
57]—aspect of PB computations is the choice of the dielectric constant of the protein. We shall not discuss here the applicability of the macroscopic concepts of medium electrodynamics to the length scale of biomolecules, as this is—to our knowledge—an unsolved fundamental problem. Instead, we work with the widely accepted presumption that PB-based electrostatics provides a meaningful physical model of proteins. The next problem is that
is actually the real part of a complex function that, for analytical purposes, can be extended to the complex plane [
46,
58,
59]. The value that we use in the computations is the zero-frequency limit, i.e., the static dielectric constant. To understand why it can be used in computations on MD snapshots, one has to note that our MD simulations do not actually serve to study dynamics, but to provide a sampling of structural variations. Each snapshot then corresponds to a static structure, and we are left with two questions: (i) What is the appropriate value of
? And: (ii) What about the water in the channel?
Ad (i): As discussed by Gilson and Honig [
45], dipolar groups in a protein play a dual role: They act as sources of the protein’s permanent electrostatic field (represented here by APCs) and also, to the extent that they are free to rotate, contribute to enhance the protein’s dielectric constant over that of electronic polarization (
). When we compute on a given snapshot, the APCs represent the permanent electrostatic field in that particular conformation. However, we also have to take into account possible reorientations of polar groups that may take place on the timescale of the equilibration of de/protonation reactions starting from that protein conformation. We think that the moderately high value of
corresponding to protein powders with a low water content [
45,
46] is suitable for this purpose, particularly for the inner region of a membrane protein.
Ad (ii): The channel in SecYEG, through which the peptide is translocated, contains a lot of water even in the presence of the peptide. Clearly, for a PB/MC computation, the explicit water has to be removed to avoid biasing of the MC sampling; the water must be represented implicitly by a dielectric continuum, and the question arises as to what value of
is appropriate here. The algorithms in TABPS assign to each cavity, which is able to accommodate a sphere of radius 1.4 Å (corresponding to a water molecule) a value of
. Such an assignment is also made to a significant part of the SecYEG channel so that the test residue 31 still is in contact with a highly polarizable environment when being positioned at the PR (
Figure 11a). From a molecular point of view, the high dielectric constant means that the water molecules in this region are free to rotate, so that they basically behave like bulk water. However, it is questionable that water in the channel behaves like that.
Earlier numerical simulations suggest that confined water should have a significantly smaller dielectric constant than bulk water [
60,
61,
62]. This prediction was confirmed experimentally by Fumagalli et al. [
63] for liquid water within nanochannels. Seyedi and Matyushov [
64] computed the dielectric constant of the hydration shell of cytochrome
c based on MD simulations and found a value of
at room temperature. Therefore, we think that assigning such a low dielectric constant to the whole interior of SecYEG, including the water-filled regions, is physically realistic. This assumption is crucial, as we found that only with such a low dielectric constant, is it possible to achieve charge neutralization of the test residue. So, we conclude that the low dipolar susceptibility of water confined in the channel during the translocation process critically contributes to the preference of the titratable residues Lys and Glu being transported in the uncharged state. This behavior simply reflects the energetic cost of transferring a charge from an aqueous environment to a less polar one, which according to the approximation provided by Born’s formula for ions
where
is the radius of the ion, does not depend on the sign of the charge
. The same is true for the more elaborate computations based on the LPBE. Thus, if the dielectric properties of the interior of SecYEG determine the protonation state changes, no discrimination between acids and bases is to be expected. The only exception could be arginine (Arg) due to its high reference
(see below).
Previous MD simulations [
65], performed on the basis of a SecYE structure from the hyperthermopile archaeon
Pyrococcus furiosus [
66], revealed an anomalous behavior of water inside the channel. The rotational relaxation time of water molecules (defined as the time required for the dipole autocorrelation function to decay to
), which is 1 ps in bulk water, was found to increase to 20–30 ps inside SecY and even above the observation time near the center of SecY. It should be noted that the structure used for these simulations is highly distorted and it is unclear which state of the translocon it represents. Nonetheless, the simulations can be taken as a hint that the rotational dynamics of channel-bound water is retarded, which may be related to a decreased dielectric constant. Another important result from these simulations is that the water molecules are highly oriented inside SecYE. This finding, together with the retarded reorientation, hints at a problem: Oriented water molecules can stabilize the charged state of a titratable residue by virtue of their dipole. Such behavior is not covered by a dielectric continuum model with a low dielectric constant. Rather, one would have to put explicit water molecules with non-zero APCs in the model. However, explicit water molecules are prohibitive in PB/MC titrations as they bias the MC sampling as mentioned above. A solution to this problem would be a constant-pH MD simulation [
67] employing a non-equilibrium MD/MC approach [
68] or λ-dynamics [
69]. However, these methods are expensive, are outside the scope of the present project, and have their own problems [
67]. Nevertheless, our conclusion that Lys and Glu are charge-neutralized during translocation (
Figure 12) has to be drawn subject to the proviso that our continuum model mimics the behavior of channel water well enough.
In their extensive study on the rate-limiting transport of positively charged residues through the Sec-machinery, Allen et al. [
56] showed that translocation is retarded by Arg residues in the peptide, while no such retardation is observed for Lys. Allen et al. explain the different behavior of Lys by a deprotonation, which is principally in agreement with our findings. To substantiate their proposal, Allen et al. [
56] performed an in silico
analysis based on MD snapshots (by successively substituting each peptide residue with Lys), but they employed the PROPKA3.1 program [
70]. While the
computation in PROPKA is structure-based, it is rather empirical than physics-based. In particular, the long-range electrostatic interaction of a titratable group with background charges is computed only within a radius of 6 Å, and coupled sites are not treated with MC, but by employing empirical rules [
70,
71]. Also, it remains unclear how the channel water is included in the computations performed by Allen et al. [
56]. Another problem is that for each position in the channel, a different peptide was investigated. Remarkably, though, Allen et al. found a
decrease for Lys inside the channel down to about 6.5. While this
shift is probably not sufficient to ensure deprotonation at physiological pH, it qualitatively points in the same direction as our simulations.
We do not know how Arg at position 31 in the peptide would behave under our simulation conditions. On one hand, it has a high reference
value of 13.8 [
72], and Arg is generally considered to stay protonated even when buried inside the protein [
73]. On the other hand, it could be deprotonated if it experiences a
shift in the same order of magnitude as Lys in our simulations. In our opinion, the significance of positively charged residues in a translocating peptide for barriers to transport is not clear. Allen et al. [
56] found a major barrier in the free energy profile for translocation (based on the coarse-grained MARTINI 2.2 force field [
74,
75]) that is dominated by the positively charged Lys residue at the PR and significantly reduced by de-charging the Lys. In our previous simulation of the Lys-peptide [
5] (based on the all-atom CHARMM36m force field), we found several barriers that are rather determined by bulky amino acids than a single charged residue—although we cannot exclude that de-charging Lys would alter the free energy profile. We believe that the role of net charges for the translocation barrier is overestimated. The different behavior of Arg compared to Lys could also result from the bulkiness of the guanidino group. Further simulations are necessary to clarify this point.
In line with this argumentation is the finding of Allen et al. [
56] that the stimulation of translocation by the PMF does not significantly depend on the (presumed) net charge of the peptide. On the other hand, there is a stimulation of translocation by the PMF. We have shown in our previous work [
5] that the transmembrane potential, which is part of the PMF, affects the orientation of transmembrane α-helices and, in this way, can contribute to an opening of the channel. Thus, the effect of the PMF is more complicated than simply dragging negatively charged residues through the PR, meaning that it is not required that a Glu residue in the peptide remains charged.
Ismail et al. [
12] found evidence of a force acting on a translocating peptide containing an Asp
5 segment (5D stretch), which they ascribed to the transmembrane potential—based on experiments with the uncoupler indole. According to our understanding of these results, the force is exerted on the 5D stretch while it is located in the cytoplasmic vestibule of the translocon. Based on our simulations, we speculate that the 5D stretch is still highly charged in this position, although not necessarily fully charged because of the mutual electrostatic interaction of the five Asp residues. It remains to be clarified, how these charges—in conjunction with the transmembrane potential—facilitate translocation of the 5D stretch.
In the present work, we concentrated on the course of the translocating residue from the cytoplasmic vestibule of SecYEG to the PR. However, some frames also cover the periplasmic region below the PR (
Figure 5,
Figure 8, and
Figure 9). Here, we made the interesting observation that Lys and Glu behave differently: Lys is recharged, while Glu stays uncharged. In the light of a simple model of how the transmembrane potential acts on charged residues, this finding is counterintuitive, since a positive charge at the periplasmic side is not favored. The focus of our work was on the behavior of the charged groups when they enter the PR region; we investigated only one frame, in which the residue has passed the PR (frame 8 for Lys and frame 6 for Glu, cf.
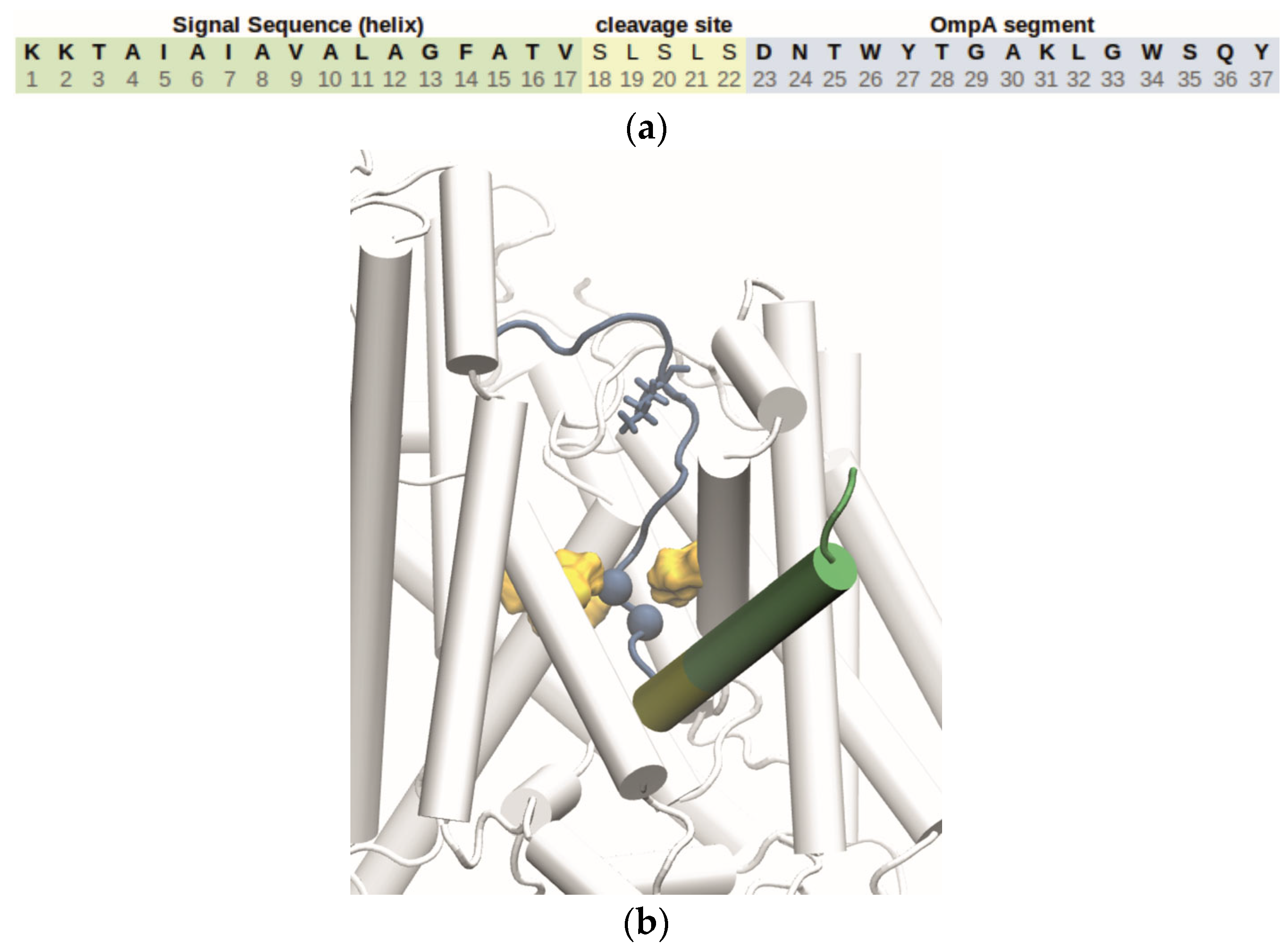
Figure 5). More simulations are required before the titration behavior of the peptide at the periplasmic side of the membrane and the role of the PMF in the later stages of the translocation can be adequately discussed. Those investigations should also consider the charge state of Asp 23, which is located next to the cleavage site in the peptide (
Figure 4a) and could influence the translocation process by interacting with the transmembrane potential in the periplasmic region.
It remains a challenge to directly include the protein structural flexibility in
calculations. Our work has confirmed that fixing the protonation form during an MD simulation causes a bias. Therefore, protonation state changes should be accounted for during the simulation of the dynamics. This goal can be achieved with the constant-pH MD methods mentioned above [
67,
68,
69], which could also solve the problem of explicit water.