Baxdrostat: A Next-Generation Aldosterone Synthase Inhibitor Offering New Hope in Resistant Hypertension
Abstract
1. Introduction
2. Blood Pressure Measurement
2.1. Office Blood Pressure Measurement
2.2. HBPM—Home Blood Pressure Monitoring
2.3. ABPM—Ambulatory Blood Pressure Monitoring
3. Classification of Elevated Blood Pressure
| Non-Elevated BP | Elevated BP | Hypertension | |
|---|---|---|---|
| Office BP (mmHg) | <120/70 | 120/70–<140/90 | ≥140/90 |
| Home BP (mmHg) | <120/70 | 120/70–<135/85 | ≥135/85 |
| Daytime ABPM (mmHg) | <120/70 | 120/70–<135/85 | ≥135/85 |
| 24 h ABPM (mmHg) | <115/65 | 115/65–<130/80 | ≥130/80 |
4. Complications
4.1. Stroke
4.2. Myocardial Infarction
4.3. Heart Failure
4.4. Chronic Kidney Disease
5. Treatment
5.1. Non-Pharmacological
- -
- Reducing salt intake;
- -
- Increasing physical activity;
- -
- Losing weight;
- -
- Increasing potassium intake;
- -
- Reducing alcohol consumption;
- -
- Quitting smoking [8].
5.2. Pharmacological
6. The Role of Aldosterone and Cortisol in Hypertension
6.1. Characteristics of Aldosterone
6.2. Mechanisms Leading to Hypertension Related to Aldosterone Activity
6.2.1. Sodium and Water Retention
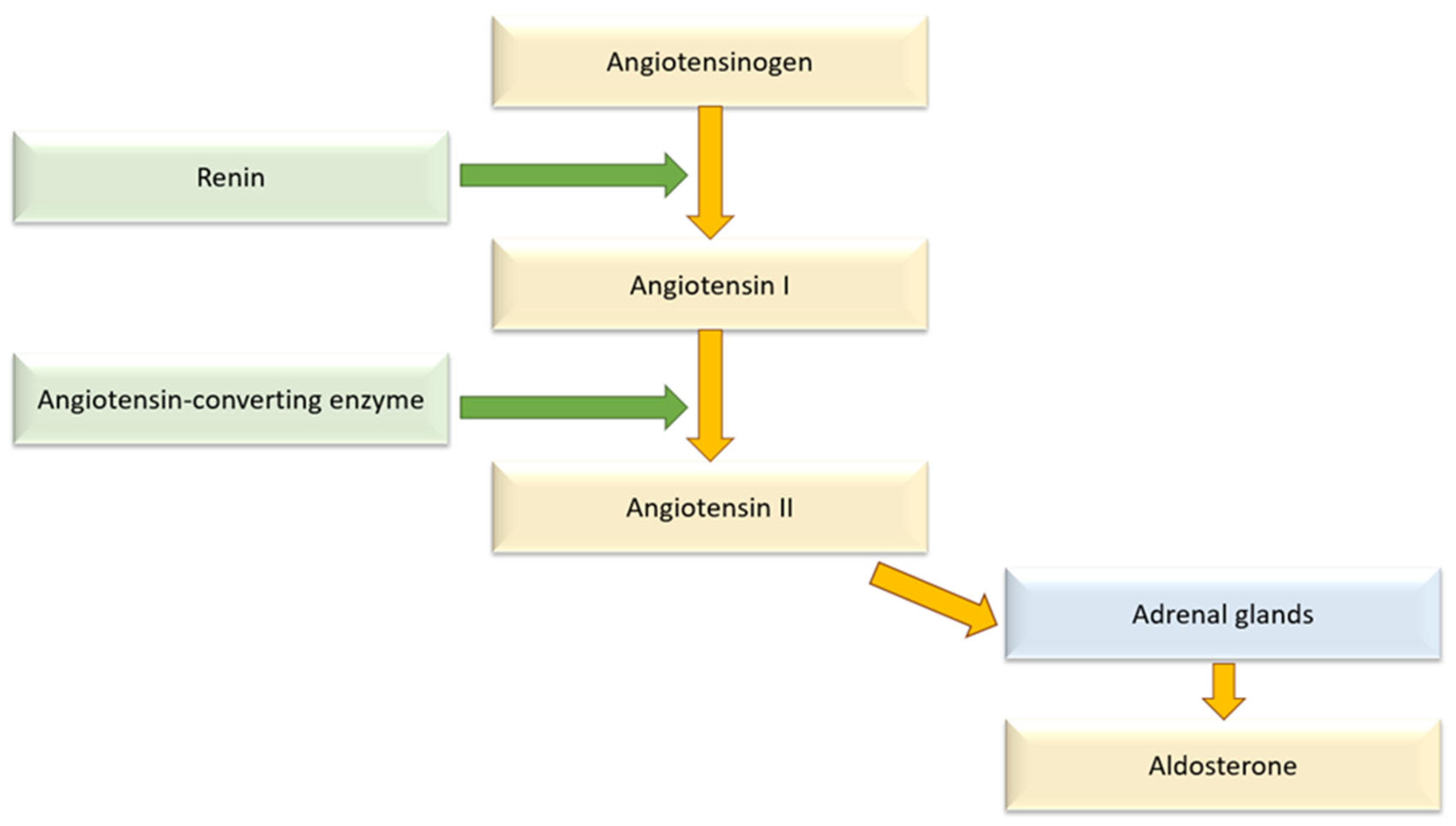
6.2.2. RAAS Activation
6.2.3. Remodeling of Vessels in Cardiovascular System
6.2.4. Hypokalemic Nephropathy
6.3. Characteristics of Cortisol
6.4. Mechanisms Leading to Hypertension Related to Cortisol Activity
6.4.1. Nitric Oxide System
6.4.2. Erythropoietin
6.4.3. Sodium and Water Retention
6.5. Adrenal Steroid Modulation in the Management of Hypertension
6.5.1. Mineralocorticoid Receptor Antagonists
6.5.2. Aldosterone Synthase Inhibitors
6.5.3. Glucocorticoid Receptor Antagonists
7. Baxdrostat
7.1. Characteristics of Baxdrostat
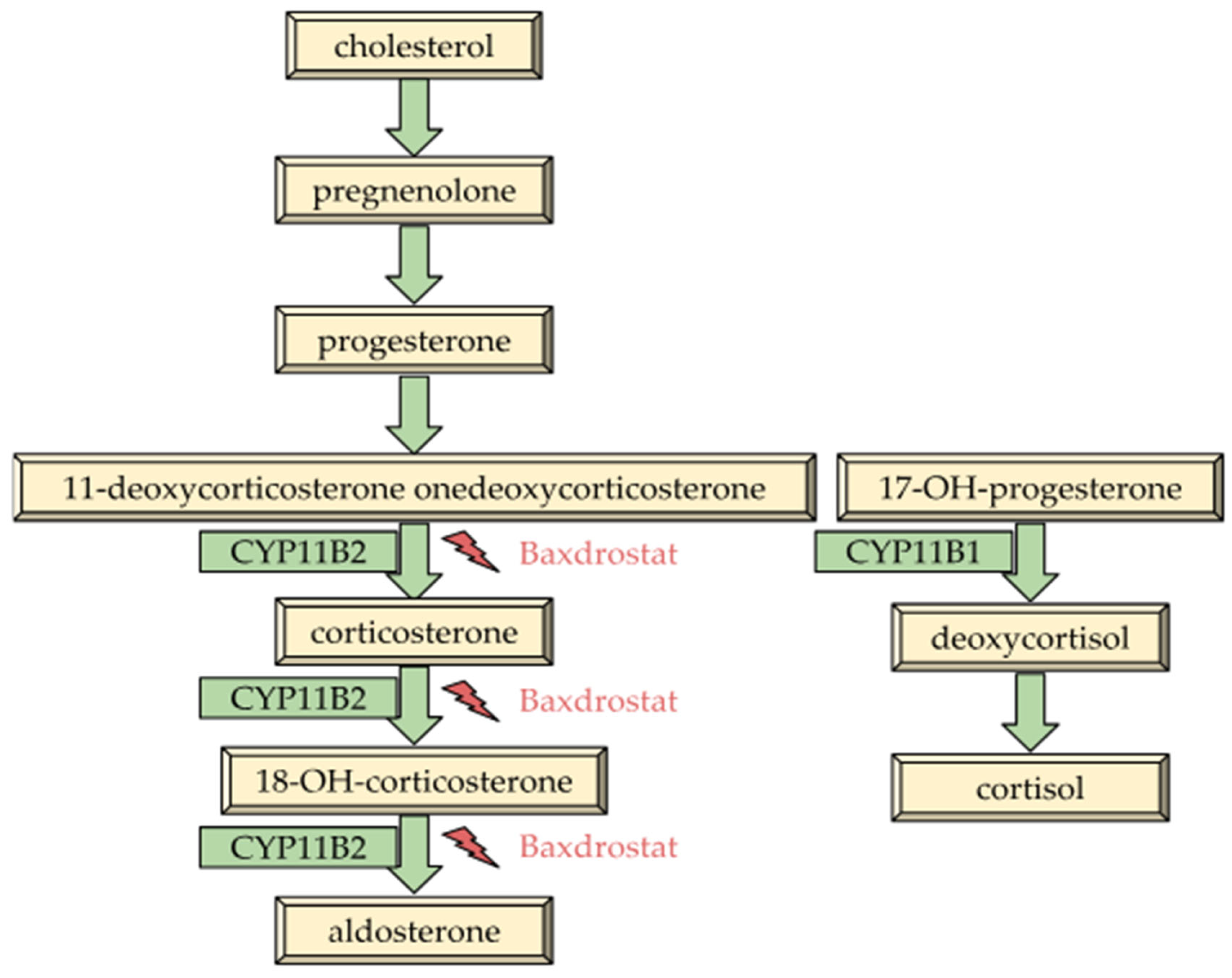
7.2. Pharmacokinetics and Efficacy of Baxdrostat
| The BrigHTN trial [95] | |||
| dose | SBP [mmHg] | DBP [mmHg] | |
| placebo | −9.4 | −9.2 | |
| baxdrostat | 0.5 mg | −12.1 | −8.6 |
| 1 mg | −17.5 | −11.8 | |
| 2 mg | −20.3 | −14.3 | |
| the HALO trial [104] | |||
| dose | SBP [mmHg] | DBP [mmHg] | |
| placebo | −16.6 | −5.9 | |
| baxdrostat | 0.5 mg | −17.0 | −5.8 |
| 1 mg | −16.0 | −5.0 | |
| 2 mg | −19.8 | −5.4 | |
7.3. Adverse Effects
7.4. Interactions Between Baxdrostat and Other Medications
7.5. Possible Concerns About Baxdrostat
7.6. Role of Baxdrostat in Specific Patient Populations
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Burlacu, A.; Kuwabara, M.; Brinza, C.; Kanbay, M. Key Updates to the 2024 ESC Hypertension Guidelines and Future Perspectives. Medicina 2025, 61, 193. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Boateng, E.B.; Ampofo, A.G. A glimpse into the future: Modelling global prevalence of hypertension. BMC Public Health 2023, 23, 1906. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yusuf, S.; Joseph, P.; Rangarajan, S.; Islam, S.; Mente, A.; Hystad, P.; Brauer, M.; Kutty, V.R.; Gupta, R.; Wielgosz, A.; et al. Modifiable risk factors, cardiovascular disease, and mortality in 155,722 individuals from 21 high-income, middle-income, and low-income countries (PURE): A prospective cohort study. Lancet 2020, 395, 795–808, Erratum in Lancet 2020, 395, 784. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- GBD 2017 Causes of Death Collaborators. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1736–1788, Erratum in Lancet 2019, 393, e44. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sliwa, K.; Stewart, S.; Gersh, B.J. Hypertension: A global perspective. Circulation 2011, 123, 2892–2896. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.M. When and how to use ambulatory blood pressure monitoring and home blood pressure monitoring for managing hypertension. Clin. Hypertens. 2024, 30, 10. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Myers, M.G. The great myth of office blood pressure measurement. J. Hypertens. 2012, 30, 1894–1898. [Google Scholar] [CrossRef] [PubMed]
- McEvoy, J.W.; McCarthy, C.P.; Bruno, R.M.; Brouwers, S.; Canavan, M.D.; Ceconi, C.; Christodorescu, R.M.; Daskalopoulou, S.S.; Ferro, C.J.; Gerdts, E.; et al. ESC Scientific Document Group. 2024 ESC Guidelines for the management of elevated blood pressure and hypertension. Eur. Heart J. 2024, 45, 3912–4018, Erratum in Eur. Heart J. 2025, 46, 1300. [Google Scholar] [CrossRef] [PubMed]
- Myers, M.G.; Godwin, M.; Dawes, M.; Kiss, A.; Tobe, S.W.; Grant, F.C.; Kaczorowski, J. Conventional versus automated measurement of blood pressure in primary care patients with systolic hypertension: Randomised parallel design controlled trial. BMJ 2011, 342, d286. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vischer, A.S.; Burkard, T. Principles of Blood Pressure Measurement—Current Techniques, Office vs Ambulatory Blood Pressure Measurement. Adv. Exp. Med. Biol. 2017, 956, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Verberk, W.J.; Kroon, A.A.; Jongen-Vancraybex, H.A.; de Leeuw, P.W. The applicability of home blood pressure measurement in clinical practice: A review of literature. Vasc. Health Risk Manag. 2007, 3, 959–966. [Google Scholar] [PubMed]
- Liyanage-Don, N.; Fung, D.; Phillips, E.; Kronish, I.M. Implementing Home Blood Pressure Monitoring into Clinical Practice. Curr. Hypertens. Rep. 2019, 21, 14. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Mandizadza, O.O.; Ji, C. Home blood pressure monitoring: Technology, digitisation and future development. BMJ Support. Palliat. Care 2025, 15, 436–443. [Google Scholar] [CrossRef] [PubMed]
- Bradley, C.K.; Choi, E.; Abdalla, M.; Mizuno, H.; Lam, M.; Cepeda, M.; Sangapalaarachchi, D.; Liu, J.; Muntner, P.; Kario, K.; et al. Use of Different Blood Pressure Thresholds to Reduce the Number of Home Blood Pressure Monitoring Days Needed for Detecting Hypertension. Hypertension 2023, 80, 2169–2177. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Muntner, P.; Shimbo, D.; Carey, R.M.; Charleston, J.B.; Gaillard, T.; Misra, S.; Myers, M.G.; Ogedegbe, G.; Schwartz, J.E.; Townsend, R.R.; et al. Measurement of Blood Pressure in Humans: A Scientific Statement from the American Heart Association. Hypertension 2019, 73, e35–e66. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lozano, R.; Naghavi, M.; Foreman, K.; Lim, S.; Shibuya, K.; Aboyans, V.; Abraham, J.; Adair, T.; Aggarwal, R.; Ahn, S.Y.; et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2095–2128, Erratum in Eur. Heart J. 2025, 46, 1300. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wajngarten, M.; Silva, G.S. Hypertension and Stroke: Update on Treatment. Eur. Cardiol. 2019, 14, 111–115. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kuklina, E.V.; Tong, X.; George, M.G.; Bansil, P. Epidemiology and prevention of stroke: A worldwide perspective. Expert Rev. Neurother. 2012, 12, 199–208. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Webb, A.J.S.; Werring, D.J. New Insights into Cerebrovascular Pathophysiology and Hypertension. Stroke 2022, 53, 1054–1064. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gorelick, P.B.; Whelton, P.K.; Sorond, F.; Carey, R.M. Blood Pressure Management in Stroke. Hypertension 2020, 76, 1688–1695. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Law, M.R.; Morris, J.K.; Wald, N.J. Use of blood pressure lowering drugs in the prevention of cardiovascular disease: Meta-analysis of 147 randomised trials in the context of expectations from prospective epidemiological studies. BMJ 2009, 338, b1665. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pedrinelli, R.; Ballo, P.; Fiorentini, C.; Denti, S.; Galderisi, M.; Ganau, A.; Germanò, G.; Innelli, P.; Paini, A.; Perlini, S.; et al. Hypertension and acute myocardial infarction: An overview. J. Cardiovasc. Med. 2012, 13, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Picariello, C.; Lazzeri, C.; Attanà, P.; Chiostri, M.; Gensini, G.F.; Valente, S. The impact of hypertension on patients with acute coronary syndromes. Int. J. Hypertens. 2011, 2011, 563657. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gallo, G.; Savoia, C. Hypertension and Heart Failure: From Pathophysiology to Treatment. Int. J. Mol. Sci. 2024, 25, 6661. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Messerli, F.H.; Rimoldi, S.F.; Bangalore, S. The Transition From Hypertension to Heart Failure: Contemporary Update. JACC Heart Fail. 2017, 5, 543–551, Erratum in JACC Heart Fail. 2017, 5, 948. [Google Scholar] [CrossRef] [PubMed]
- Baffour, P.K.; Jahangiry, L.; Jain, S.; Sen, A.; Aune, D. Blood pressure, hypertension, and the risk of heart failure: A systematic review and meta-analysis of cohort studies. Eur. J. Prev. Cardiol. 2024, 31, 529–556. [Google Scholar] [CrossRef] [PubMed]
- Whelton, P.K.; Carey, R.M.; Mancia, G.; Kreutz, R.; Bundy, J.D.; Williams, B. Harmonization of the American College of Cardiology/American Heart Association and European Society of Cardiology/European Society of Hypertension Blood Pressure/Hypertension Guidelines: Comparisons, Reflections, and Recommendations. J. Am. Coll. Cardiol. 2022, 80, 1192–1201. [Google Scholar] [CrossRef] [PubMed]
- Ettehad, D.; Emdin, C.A.; Kiran, A.; Anderson, S.G.; Callender, T.; Emberson, J.; Chalmers, J.; Rodgers, A.; Rahimi, K. Blood pressure lowering for prevention of cardiovascular disease and death: A systematic review and meta-analysis. Lancet 2016, 387, 957–967. [Google Scholar] [CrossRef] [PubMed]
- Hao, X.M.; Liu, Y.; Hailaiti, D.; Gong, Y.; Zhang, X.D.; Yue, B.N.; Liu, J.P.; Wu, X.L.; Yang, K.Z.; Wang, J.; et al. Mechanisms of inflammation modulation by different immune cells in hypertensive nephropathy. Front. Immunol. 2024, 15, 1333170. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Costantino, V.V.; Gil Lorenzo, A.F.; Bocanegra, V.; Vallés, P.G. Molecular Mechanisms of Hypertensive Nephropathy: Renoprotective Effect of Losartan Through Hsp70. Cells 2021, 10, 3146. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lin, L.; Ren, J.; Wang, C.; Mei, M.; Zheng, L.; Yang, J. A set of urinary peptides can predict early renal damage in primary hypertension. J. Hypertens. 2023, 41, 1653–1660. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Arzaghi, H.; Ma, Z.; Roye, Y.; Musah, S. Epigenetics of Hypertensive Nephropathy. Biomedicines 2024, 12, 2622. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Seccia, T.M.; Caroccia, B.; Calò, L.A. Hypertensive nephropathy. Moving from classic to emerging pathogenetic mechanisms. J. Hypertens. 2017, 35, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Liu, M.; Fang, Z. Combined Therapy of Hypertensive Nephropathy with Breviscapine Injection and Antihypertensive Drugs: A Systematic Review and a Meta-Analysis. Evid. Based Complement. Altern. Med. 2018, 2018, 2958717. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti Rosei, E.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; de Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension. J. Hypertens. 2018, 36, 1953–2041, Erratum in J. Hypertens. 2019, 37, 226. [Google Scholar] [CrossRef] [PubMed]
- Lackland, D.T.; Egan, B.M. Dietary salt restriction and blood pressure in clinical trials. Curr. Hypertens. Rep. 2007, 9, 314–319. [Google Scholar] [CrossRef] [PubMed]
- Dybiec, J.; Krzemińska, J.; Radzioch, E.; Szlagor, M.; Wronka, M.; Młynarska, E.; Rysz, J.; Franczyk, B. Advances in the Pathogenesis and Treatment of Resistant Hypertension. Int. J. Mol. Sci. 2023, 24, 12911. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Filippou, C.; Tatakis, F.; Polyzos, D.; Manta, E.; Thomopoulos, C.; Nihoyannopoulos, P.; Tousoulis, D.; Tsioufis, K. Overview of salt restriction in the Dietary Approaches to Stop Hypertension (DASH) and the Mediterranean diet for blood pressure reduction. Rev. Cardiovasc. Med. 2022, 23, 36. [Google Scholar] [CrossRef] [PubMed]
- Cutler, J.A.; Follmann, D.; Allender, P.S. Randomized trials of sodium reduction: An overview. Am. J. Clin. Nutr. 1997, 65, 643S–651S. [Google Scholar] [CrossRef] [PubMed]
- Hooper, L.; Bartlett, C.; Davey Smith, G.; Ebrahim, S. Systematic review of long term effects of advice to reduce dietary salt in adults. BMJ 2002, 325, 628. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shalaeva, E.V.; Messerli, F.H. What is resistant arterial hypertension? Blood Press. 2023, 32, 2185457. [Google Scholar] [CrossRef] [PubMed]
- Ruilope, L.M.; Rodríguez-Sánchez, E.; Navarro-García, J.A.; Segura, J.; Órtiz, A.; Lucia, A.; Ruiz-Hurtado, G. Resistant hypertension: New insights and therapeutic perspectives. Eur. Heart J. Cardiovasc. Pharmacother. 2020, 6, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Samadian, F.; Dalili, N.; Jamalian, A. Lifestyle Modifications to Prevent and Control Hypertension. Iran. J. Kidney Dis. 2016, 10, 237–263. [Google Scholar] [PubMed]
- Egan, B.M. Treatment Resistant Hypertension. Ethn. Dis. 2015, 25, 495–498. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Neter, J.E.; Stam, B.E.; Kok, F.J.; Grobbee, D.E.; Geleijnse, J.M. Influence of weight reduction on blood pressure: A meta-analysis of randomized controlled trials. Hypertension 2003, 42, 878–884. [Google Scholar] [CrossRef] [PubMed]
- Brunström, M.; Carlberg, B. Association of Blood Pressure Lowering with Mortality and Cardiovascular Disease Across Blood Pressure Levels: A Systematic Review and Meta-analysis. JAMA Intern. Med. 2018, 178, 28–36. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Whelton, P.K.; Carey, R.M.; Aronow, W.S.; Casey, D.E.; Collins, K.J.; Dennison Himmelfarb, C.; DePalma, S.M.; Gidding, S.; Jamerson, K.A.; Jones, D.W.; et al. Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2018, 71, e127–e248, Erratum in J. Am. Coll. Cardiol. 2018, 71, 2275–2279. [Google Scholar] [CrossRef] [PubMed]
- Heidari, B.; Avenatti, E.; Nasir, K. Pharmacotherapy for Essential Hypertension: A Brief Review. Methodist Debakey Cardiovasc. J. 2022, 18, 5–16. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Unger, T.; Borghi, C.; Charchar, F.; Khan, N.A.; Poulter, N.R.; Prabhakaran, D.; Ramirez, A.; Schlaich, M.; Stergiou, G.S.; Tomaszewski, M.; et al. 2020 International Society of Hypertension global hypertension practice guidelines. J. Hypertens. 2020, 38, 982–1004. [Google Scholar] [CrossRef] [PubMed]
- Catena, C.; Colussi, G.; Marzano, L.; Sechi, L.A. Aldosterone and the heart: From basic research to clinical evidence. Horm. Metab. Res. 2012, 44, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Grundy, H.M.; Simpson, S.A.; Tait, J.F. Isolation of a highly active mineralocorticoid from beef adrenal extract. Natures 1952, 169, 795–796. [Google Scholar] [CrossRef]
- Hattangady, N.G.; Olala, L.O.; Bollag, W.B.; Rainey, W.E. Acute and chronic regulation of aldosterone production. Mol. Cell. Endocrinol. 2012, 350, 151–162. [Google Scholar] [CrossRef]
- Farkash, Y.; Timberg, R.; Orly, J. Preparation of antiserum to rat cytochrome P-450 cholesterol side chain cleavage, and its use for ultrastructural localization of the immunoreactive enzyme by protein A-gold technique. Endocrinology 1986, 118, 1353–1365. [Google Scholar] [CrossRef] [PubMed]
- Ishimura, K.; Fujita, H. Light and electron microscopic immunohistochemistry of the localization of adrenal steroidogenic enzymes. Microsc. Res. Tech. 1997, 36, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Ichimura, T.; Yamamura, H.; Sasamoto, K.; Tominaga, Y.; Taoka, M.; Kakiuchi, K.; Shinkawa, T.; Takahashi, N.; Shimada, S.; Isobe, T. 14-3-3 proteins modulate the expression of epithelial Na+ channels by phosphorylation-dependent interaction with Nedd4-2 ubiquitin ligase. J. Biol. Chem. 2005, 280, 13187–13194. [Google Scholar] [CrossRef] [PubMed]
- Verrey, F. Transcriptional control of sodium transport in tight epithelial by adrenal steroids. J. Membr. Biol. 1995, 144, 93–110. [Google Scholar] [CrossRef] [PubMed]
- Scott, J.H.; Menouar, M.A.; Dunn, R.J. Physiology, Aldosterone. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar] [PubMed]
- Tomaschitz, A.; Pilz, S.; Ritz, E.; Obermayer-Pietsch, B.; Pieber, T.R. Aldosterone and arterial hypertension. Nat. Rev. Endocrinol. 2010, 6, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Briet, M.; Schiffrin, E.L. Vascular actions of aldosterone. J. Vasc. Res. 2013, 50, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Brown, N.J. Contribution of aldosterone to cardiovascular and renal inflammation and fibrosis. Nat. Rev. Nephrol. 2013, 9, 459–469. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nguyen, T.; Do, H.; Pham, T.; Vu, L.T.; Zuin, M.; Rigatelli, G. Left ventricular dysfunction causing ischemia in patients with patent coronary arteries. Perfusion 2018, 33, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Jia, G.; Habibi, J.; Aroor, A.R.; Hill, M.A.; Yang, Y.; Whaley-Connell, A.; Jaisser, F.; Sowers, J.R. Epithelial Sodium Channel in Aldosterone-Induced Endothelium Stiffness and Aortic Dysfunction. Hypertension 2018, 72, 731–738. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yalamanchili, H.B.; Calp-Inal, S.; Zhou, X.J.; Choudhury, D. Hypokalemic Nephropathy. Kidney Int. Rep. 2018, 3, 1482–1488. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chan, S.; Debono, M. Replication of cortisol circadian rhythm: New advances in hydrocortisone replacement therapy. Ther. Adv. Endocrinol. Metab. 2010, 1, 129–138. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Heim, C.; Ehlert, U.; Hellhammer, D.H. The potential role of hypocortisolism in the pathophysiology of stress-related bodily disorders. Psychoneuroendocrinology 2000, 25, 1–35. [Google Scholar] [CrossRef] [PubMed]
- Jankord, R.; Herman, J.P. Limbic regulation of hypothalamo-pituitary-adrenocortical function during acute and chronic stress. Ann. N.Y. Acad. Sci. 2008, 1148, 64–73. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- McEwen, B.S. Central effects of stress hormones in health and disease: Understanding the protective and damaging effects of stress and stress mediators. Eur. J. Pharmacol. 2008, 583, 174–185. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ramamoorthy, S.; Cidlowski, J.A. Corticosteroids: Mechanisms of Action in Health and Disease. Rheum. Dis. Clin. N. Am. 2016, 42, 15–31. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wallerath, T.; Witte, K.; Schafer, S.C.; Schwarz, P.M.; Prellwitz, W.; Wohlfart, P.; Kleinet, H.; Lehr, H.-A.; Lemmer, B.; Forstermann, U. Down regulation of the expression of endothelial NO synthase is likely to contribute to glucocorticoid-mediated hypertension. Proc. Natl. Acad. Sci. USA 1999, 96, 13357–13362. [Google Scholar] [CrossRef]
- Turner, S.W.; Wen, C.H.; Li, M.; Whitworth, J.A. l-Arginine prevents corticotropin-induced increases in blood pressure in the rat. Hypertension 1996, 27, 184–188. [Google Scholar] [CrossRef]
- Wang, J.; Brown, M.A.; Tarn, S.H.; Chan, M.C.; Whitworth, J.A. The effects of diet on measurement of nitric oxide metabolites. Clin. Exp. Pharmacol. Physiol. 1997, 24, 418–420. [Google Scholar] [CrossRef]
- Mangos, G.J.; Walker, B.R.; Kelly, J.J.; Lawson, J.A.; Webb, D.J.; Whitworth, J.A. Cortisol inhibits cholinergic vasodilation in the human forearm. Am. J. Hypertens. 2000, 13, 1155–1160. [Google Scholar] [CrossRef]
- Langenfeld, M.R.W.; Veelken, R.; Schobel, H.P.; Freidrich, A.; Schmieder, R.E. Is endogenous erythropoietin a pathogenetic factor in the development of essential hypertension? Nephrol. Dial. Transplant. 1997, 12, 1155–1160. [Google Scholar] [CrossRef]
- Kelly, J.J.; Martin, A.; Whitworth, J.A. Role of erythropoietin in cortisol induced hypertension. J. Hum. Hypertens. 2000, 14, 195–198. [Google Scholar] [CrossRef]
- Vaziri, N.D.; Zhou, X.J.; Naqvi, F.; Smith, J.; Oveisi, F.; Wang, Z.Q.; Purdy, R.E. Role of nitric oxide resistance in erythropoietin-induced hypertension in rats with chronic renal failure. Am. J. Physiol. 1996, 271, E113–E122. [Google Scholar] [CrossRef]
- Panarelli, M.; Holloway, C.D.; Fraser, R.; Connell, J.M.C.; Ingram, M.; Anderson, N.H.; Kenyon, C.J. Glucocorticoid receptor polymorphism, skin vasoconstriction, and other metabolic intermediate phenotypes in normal human subjects. J. Clin. Endocrinol. Metab. 1998, 83, 1846–1852. [Google Scholar] [CrossRef]
- Whitworth, J.A.; Kelly, J.J. Evidence that high dose cortisol-induced Na+ retention in man is not mediated by the mineralocorticoid receptor. J. Endocrinol. Investig. 1995, 18, 586–591. [Google Scholar] [CrossRef]
- Montrella-Waybill, M.; Clore, J.N.; Schoolwerth, A.C.; Watlington, C.O. Evidence that high dose cortisol induced Na+ retention in man is not mediated by the mineralocorticoid receptor. J. Clin. Endocrinol. Metab. 1991, 72, 1060–1066. [Google Scholar] [CrossRef] [PubMed]
- Yugar-Toledo, J.C.; Modolo, R.; de Faria, A.P.; Moreno, H. Managing resistant hypertension: Focus on mineralocorticoid-receptor antagonists. Vasc. Health Risk Manag. 2017, 13, 403–411. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Marzano, L.; Merlo, M.; Martinelli, N.; Pizzolo, F.; Friso, S. Efficacy and Safety of Aldosterone Synthase Inhibitors for Hypertension: A Meta-Analysis of Randomized Controlled Trials and Systematic Review. Hypertension 2025, 82, e47–e56. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, R.; Nadeem, H.; Sattar, M.A.; Rehan, M.; Sheikh, D.N.; Jawed, S.; Akram, A. Efficacy and safety of aldosterone synthase inhibitors in hypertension: A systematic review and meta- analysis. Curr. Probl. Cardiol. 2024, 49, 102875. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Sanchez, C.E.; Gomez-Sanchez, E.P. Aldosterone Synthase Inhibitors and the Treatment of Essential Hypertension. J. Clin. Endocrinol. Metab. 2023, 108, e638–e639. [Google Scholar] [CrossRef]
- Agarwal, R.; Kolkhof, P.; Bakris, G.; Bauersachs, J.; Haller, H.; Wada, T.; Zannad, F. Steroidal and non-steroidal mineralocorticoid receptor antagonists in cardiorenal medicine. Eur. Heart J. 2021, 42, 152–161. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pandey, A.K.; Bhatt, D.L.; Cosentino, F.; Marx, N.; Rotstein, O.; Pitt, B.; Pandey, A.; Butler, J.; Verma, S. Non-steroidal mineralocorticoid receptor antagonists in cardiorenal disease. Eur. Heart J. 2022, 43, 2931–2945, Erratum in Eur. Heart J. 2022, 43, 4391. [Google Scholar] [CrossRef] [PubMed]
- Kallistratos, M.S.; Pittaras, A.; Theodoulidis, I.; Grassos, C.; Poulimenos, L.E.; Manolis, A.J. Adverse Effects of Mineralocorticoid Receptor Antagonist Administration. Curr. Pharm. Des. 2018, 24, 5537–5541. [Google Scholar] [CrossRef] [PubMed]
- Fleseriu, M.; Pivonello, R.; Newell-Price, J.; Gadelha, M.R.; Biller, B.M.K.; Auchus, R.J.; Feelders, R.A.; Shimatsu, A.; Witek, P.; Bex, M.; et al. Osilodrostat improves blood pressure and glycemic control in patients with Cushing’s disease: A pooled analysis of LINC 3 and LINC 4 studies. Pituitary 2025, 28, 22, Erratum in Pituitary 2025, 28, 62. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nagendra, L.; Dutta, D.; Raizada, N.; Surana, V.; Selvan, C.; Bhattacharya, S. Efficacy and Safety of Osilodrostat in Managing Cushing’s Syndrome: A Systematic Review and Meta-Analysis. Indian J. Endocrinol. Metab. 2024, 28, 232–238. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Verma, S.; Pandey, A.; Pandey, A.K.; Butler, J.; Lee, J.S.; Teoh, H.; Mazer, C.D.; Kosiborod, M.N.; Cosentino, F.; Anker, S.D.; et al. Aldosterone and aldosterone synthase inhibitors in cardiorenal disease. Am. J. Physiol. Heart Circ. Physiol. 2024, 326, H670–H688. [Google Scholar] [CrossRef] [PubMed]
- Laffin, L.J.; Kopjar, B.; Melgaard, C.; Wolski, K.; Ibbitson, J.; Bhikam, S.; Weir, M.R.; Ofili, E.O.; Mehra, R.; Luther, J.M.; et al. Advance-HTN Investigators. Lorundrostat Efficacy and Safety in Patients with Uncontrolled Hypertension. N. Engl. J. Med. 2025, 392, 1813–1823. [Google Scholar] [CrossRef] [PubMed]
- Pivonello, R.; Ferrigno, R.; De Martino, M.C.; Simeoli, C.; Di Paola, N.; Pivonello, C.; Barba, L.; Negri, M.; De Angelis, C.; Colao, A. Medical Treatment of Cushing’s Disease: An Overview of the Current and Recent Clinical Trials. Front. Endocrinol. 2020, 11, 648. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chang, A.Y.; Mirfakhraee, S.; King, E.E.; Mercado, J.U.; Donegan, D.M.; Yuen, K.C. Mifepristone as Bridge or Adjunct Therapy in the Management of Challenging Cushing Disease Cases. Clin. Med. Insights Endocrinol. Diabetes 2021, 14, 1179551421994102. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dogra, S.; Shah, S.; Gitzel, L.; Pusukur, B.; Sood, A.; Vyas, A.V.; Gupta, R. Baxdrostat: A Novel Aldosterone Synthase Inhibitor for Treatment Resistant Hypertension. Curr. Probl. Cardiol. 2023, 48, 101918. [Google Scholar] [CrossRef] [PubMed]
- Nardoianni, G.; Pala, B.; Scoccia, A.; Volpe, M.; Barbato, E.; Tocci, G. Systematic Review Article: New Drug Strategies for Treating Resistant Hypertension-the Importance of a Mechanistic, Personalized Approach. High Blood Press. Cardiovasc. Prev. 2024, 31, 99–112. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Freeman, M.W.; Halvorsen, Y.D.; Marshall, W.; Pater, M.; Isaacsohn, J.; Pearce, C.; Murphy, B.; Alp, N.; Srivastava, A.; Bhatt, D.L.; et al. Investigators. Phase 2 Trial of Baxdrostat for Treatment-Resistant Hypertension. N. Engl. J. Med. 2023, 388, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Feldman, R.D.; Sanjanwala, R.; Padwal, R.; Leung, A.A. Revising the Roles of Aldosterone in Vascular Physiology and Pathophysiology: From Electocortin to Baxdrostat. Can. J. Cardiol. 2023, 39, 1808–1815. [Google Scholar] [CrossRef] [PubMed]
- Byrd, J.B.; Auchus, R.J.; White, P.C. Aldosterone Synthase Promoter Polymorphism and Cardiovascular Phenotypes in a Large, Multiethnic Population-Based Study. J. Invest. Med. 2015, 63, 862–866. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Paikray, E.; Mohapatra, S. Can baxdrostat revamp resistant hypertension status in India? Indian J. Pharmacol. 2024, 56, 293–294. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Freeman, M.W.; Bond, M.; Murphy, B.; Hui, J.; Isaacsohn, J. Results from a phase 1, randomized, double-blind, multiple ascending dose study characterizing the pharmacokinetics and demonstrating the safety and selectivity of the aldosterone synthase inhibitor baxdrostat in healthy volunteers. Hypertens. Res. 2023, 46, 108–118. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Azzam, O.; Nejad, S.H.; Carnagarin, R.; Nolde, J.M.; Galindo-Kiuchi, M.; Schlaich, M.P. Taming resistant hypertension: The promise of novel pharmacologic approaches and renal denervation. Br. J. Pharmacol. 2024, 181, 319–339. [Google Scholar] [CrossRef] [PubMed]
- Bogman, K.; Schwab, D.; Delporte, M.L.; Palermo, G.; Amrein, K.; Mohr, S.; De Vera Mudry, M.C.; Brown, M.J.; Ferber, P. Preclinical and Early Clinical Profile of a Highly Selective and Potent Oral Inhibitor of Aldosterone Synthase (CYP11B2). Hypertension 2017, 69, 189–196. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Oelkers, W. Adrenal insufficiency. New Engl. J. Med. 1996, 335, 1206–1212. [Google Scholar] [CrossRef] [PubMed]
- Wagner, R.L.; White, P.F. Etomidate inhibits adrenocortical function in surgical patients. Anesthesiology 1984, 61, 647–651. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, D. HALO Trial. In AHA Congress; The American College of Cardiology: Washington, DC, USA, 2023; Available online: https://www.acc.org/Latest-in-Cardiology/Clinical-Trials/2023/03/01/23/34/halo (accessed on 27 June 2025).
- Freeman, M.W.; Halvorsen, Y.D.; Bond, M.; Murphy, B.; Isaacsohn, J. Results from a Phase 1 Study Assessing the Pharmacokinetics of the Aldosterone Synthase Inhibitor Baxdrostat in Participants with Varying Degrees of Renal Function. Clin. Pharmacol. Drug Dev. 2024, 13, 410–418. [Google Scholar] [CrossRef] [PubMed]
- Götzinger, F.; Kunz, M.; Lauder, L.; Böhm, M.; Mahfoud, F. New ways of mitigating aldosterone in cardiorenal disease. Eur. Heart J. Cardiovasc. Pharmacother. 2024, 10, 557–565. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Sanchez, E.P. Brain mineralocorticoid receptors in cognition and cardiovascular homeostasis. Steroids 2014, 91, 20–31. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Freeman, M.W.; Bond, M.; Murphy, B.; Hui, J.; Isaacsohn, J. Results from a Randomized, Open-Label, Crossover Study Evaluating the Effect of the Aldosterone Synthase Inhibitor Baxdrostat on the Pharmacokinetics of Metformin in Healthy Human Subjects. Am. J. Cardiovasc. Drugs 2023, 23, 277–286. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yonezawa, A.; Inui, K. Importance of the multidrug and toxin extrusion MATE/SLC47A family to pharmacokinetics, pharmacodynamics/toxicodynamics and pharmacogenomics. Br. J. Pharmacol. 2011, 164, 1817–1825. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- AstraZeneca. A Phase III Study to Investigate the Efficacy and Safety of Baxdrostat in Combination with Dapagliflozin on CKD Progression in Participants with CKD and High Blood Pressure. NCT06268873. Available online: https://clinicaltrials.gov/study/NCT06268873?rank=1 (accessed on 27 June 2025).
- A Study to Investigate the Pharmacokinetics of Baxdrostat When Given Alone and in Combination with Itraconazole in Healthy Participants. Available online: https://www.astrazenecaclinicaltrials.com/study/D6970C00005/ (accessed on 27 June 2025).
- Sydorchuk, L.; Dzhuryak, V.; Sydorchuk, A.; Levytska, S.; Petrynych, V.; Knut, R.; Kshanovska, A.; Iftoda, O.; Tkachuk, O.; Kyfiak, P.; et al. The cytochrome 11B2 aldosterone synthase gene rs1799998 single nucleotide polymorphism determines elevated aldosterone, higher blood pressure, and reduced glomerular filtration, especially in diabetic female patients. Endocr Regul. 2020, 54, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Flack, J.M.; Azizi, M.; Brown, J.M.; Dwyer, J.P.; Jones, E.S.W.; Lihn, A.S.; Liu, L.; Olsson, D.S.; Perl, S.; Shibata, H.; et al. Baxdrostat for uncontrolled and resistant hypertension: Rationale and design of the Phase 3 clinical trials BaxHTN, BaxAsia, and Bax24. Hypertens Res. 2025. [CrossRef] [PubMed]
- AstraZeneca. A Study to Investigate the Efficacy and Safety of Baxdrostat in Participants With Uncontrolled Hypertension on Two or More Medications Including Participants With Resistant Hypertension (BaxAsia). NCT06344104. Available online: https://clinicaltrials.gov/study/NCT06344104 (accessed on 24 September 2025).
- Flack, J.M.; Azizi, M.; Brown, J.M.; Dwyer, J.P.; Fronczek, J.; Jones, E.S.W.; Olsson, D.S.; Perl, S.; Shibata, H.; Wang, J.G.; et al. Efficacy and Safety of Baxdrostat in Uncontrolled and Resistant Hypertension. N. Engl. J. Med. 2025, 30. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Forzano, I.; Mone, P.; Varzideh, F.; Jankauskas, S.S.; Kansakar, U.; De Luca, A.; Santulli, G. The selective aldosterone synthase inhibitor Baxdrostat significantly lowers blood pressure in patients with resistant hypertension. Front Endocrinol. (Lausanne) 2022, 13, 1097968. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Turcu, A.F.; Freeman, M.W.; Bancos, I.; Ben-Shlomo, A.; Hamidi, O.; Hamrahian, A.H.; Huang, W.; Kirschner, L.S.; Sam, R.; Mallappa, A.; et al. Phase 2a Study of Baxdrostat in Primary Aldosteronism. N Engl. J. Med. 2025, 393, 515–518. [Google Scholar] [CrossRef] [PubMed]
- Mazzieri, A.; Timio, F.; Patera, F.; Trepiccione, F.; Bonomini, M.; Reboldi, G. Aldosterone Synthase Inhibitors for Cardiorenal Protection: Ready for Prime Time? Kidney Blood Press Res. 2024, 49, 1041–1056. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rasmussen, A.A.; Nordestgaard, K.L.; Simonsen, U.; Buus, N.H. Blood Pressure-Lowering Effects of Aldosterone Synthase Inhibitors-A Systematic Review. Basic Clin. Pharmacol. Toxicol. 2025, 137, e70080. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Młynarska, E.; Czarnik, W.; Dzieża, N.; Jędraszak, W.; Majchrowicz, G.; Prusinowski, F.; Stabrawa, M.; Rysz, J.; Franczyk, B. Baxdrostat: A Next-Generation Aldosterone Synthase Inhibitor Offering New Hope in Resistant Hypertension. Biomolecules 2025, 15, 1439. https://doi.org/10.3390/biom15101439
Młynarska E, Czarnik W, Dzieża N, Jędraszak W, Majchrowicz G, Prusinowski F, Stabrawa M, Rysz J, Franczyk B. Baxdrostat: A Next-Generation Aldosterone Synthase Inhibitor Offering New Hope in Resistant Hypertension. Biomolecules. 2025; 15(10):1439. https://doi.org/10.3390/biom15101439
Chicago/Turabian StyleMłynarska, Ewelina, Witold Czarnik, Natasza Dzieża, Weronika Jędraszak, Gabriela Majchrowicz, Filip Prusinowski, Magdalena Stabrawa, Jacek Rysz, and Beata Franczyk. 2025. "Baxdrostat: A Next-Generation Aldosterone Synthase Inhibitor Offering New Hope in Resistant Hypertension" Biomolecules 15, no. 10: 1439. https://doi.org/10.3390/biom15101439
APA StyleMłynarska, E., Czarnik, W., Dzieża, N., Jędraszak, W., Majchrowicz, G., Prusinowski, F., Stabrawa, M., Rysz, J., & Franczyk, B. (2025). Baxdrostat: A Next-Generation Aldosterone Synthase Inhibitor Offering New Hope in Resistant Hypertension. Biomolecules, 15(10), 1439. https://doi.org/10.3390/biom15101439








