Abstract
In recent years, the scientific community has increasingly delved into the study of the interaction between the nervous system and tumors, revealing that the nervous system not only regulates bodily functions under physiological conditions, but also assumes a vital part in the emergence and progression of tumors. Research has demonstrated that the extensive neural network directly regulates tumor progression and can influence tumors by modulating the tumor microenvironment and immune system. Moreover, tumors induce neural networks to provide favorable conditions for their proliferation and metastasis. In the above process, neurotransmitters play a vital role. They directly act or bind to their receptor, activating various classical signaling pathways, among which are PI3K/AKT, MEK/ERK, and WNT/β-catenin, to facilitate tumor advancement. Therefore, this study systematically reviews the regulatory mechanisms of neurotransmitters and their receptors in the advancement of cancer, along with the utilization of targeted drugs. At the same time, we also analyzed that targeting specific receptor subtypes may produce more significant therapeutic effects in different types of cancer. Additionally, this research further explores the limitations of neurotransmitter-based drugs currently used in clinical cancer treatment. In summary, the field of cancer neuroscience is rapidly advancing, constantly revealing the regulatory effects of neurotransmitters on tumor progression and their specific molecular mechanisms, providing broad application prospects for future clinical therapy.
1. Introduction
Cancer has long been a formidable challenge in the history of medical science, and its emergence and progression are intimately associated with the dysfunction of multiple systems [1]. Cancer treatment has garnered extensive attention. Despite traditional therapeutic approaches like surgical resection, radiotherapy, chemotherapy, and interventional therapy have achieved certain results, the recurrence, metastasis, drug resistance, and tumor heterogeneity of cancer, a highly complex disease, remain key factors affecting treatment outcomes. The nervous system, being the core system exerting a dominant function within the body, assumes a significant role in regulating tumor advancement and influencing cancer metastasis. Consequently, the field of cancer neuroscience has garnered considerable attention [2]. Although it is still in its infancy as an emerging research field, it is developing rapidly.
In the nervous system, signal transmission typically relies on neurotransmitters, especially those involved in rapid and precise complex signaling. As research deepens, more evidence suggests that neurotransmitters regulate cancer progression through multiple mechanisms [3,4,5]. Therefore, exploring the impact of neurotransmitters on cancer progression is beneficial for us to understand the nature of cancer from a new perspective and may bring entirely new strategies for cancer treatment. In this review, we outline the molecular mechanisms as well as the clinical applications of different types of neurotransmitters in the advancement of cancer, explore the potential of neurotransmitters as biomarkers, and emphasize therapeutic strategies of targeted drugs in cancer treatment. Through this research perspective, we hope to provide innovative theoretical and practical guidance for the field of cancer therapy.
2. The Biological Basis of Neurotransmitters
Neurotransmitters are a type of chemical substance responsible for signal transmission in the nervous system. As a chemical messenger, they rapidly transmit signals between neurons and between neurons and effector cells [6,7]. The classic pathway for neurotransmitter transmission is orthodromic conduction, which means that when presynaptic neurons are excited and transmitted to the axon terminals, the presynaptic membrane depolarizes, opens voltage-gated calcium ion channels, and the calcium influx facilitates the fusion of synaptic vesicles containing neurotransmitters with the presynaptic membrane. Neurotransmitters are discharged into the synaptic cleft and attach to the corresponding receptors on the postsynaptic membrane, thereby transmitting nerve impulses to the next neuron or effector [8]. Classical neurotransmitters primarily utilize the orthodromic conduction pathways to transmit signals, including acetylcholine, norepinephrine, dopamine, serotonin, glutamate and gamma-aminobutyric acid (GABA). Next is the antidromic conduction [9], which refers to the process of transmission from postsynaptic neurons to presynaptic neurons. This mode of communication is crucial for regulating synaptic plasticity, signal feedback, neuroprotection and repair, as well as the correct formation of neural circuits. The molecules that use the above mode for signal transduction are called retrograde messengers. There are mainly NO, CO, and endogenous cannabinoids. In addition, there is also a special class of high molecular weight bioactive substances called neuropeptides, which are mostly composed of 5–31 amino acid residues [10]. They can act as neurotransmitters or neuroregulatory factors, participating in various functions of the nervous system. Neuropeptides are not typical neurotransmitters, but like neurotransmitters, they act on receptors after release, and then regulate the release and function of neurotransmitters. Compared with classical neurotransmitters, neuropeptides exert broader and long-term physiological regulatory effects [11]. Different neurotransmitters require specific enzymes to catalyze their synthesis process. For example, monoamine neurotransmitters such as dopamine are converted from amino acids (such as tyrosine) [12], while amino acid neurotransmitters such as GABA and glutamate are directly derived from amino acid metabolism [13]. The site of neurotransmitter synthesis is often located in the cell body or axon end of neurons, and stored in vesicles for release.
Recent investigations have demonstrated that neurotransmitters not only play a significant role in the nervous system, but may also be implicated in the emergence, advancement, and metastasis of cancer [14,15]. Neurotransmitters interact with tumor cells and immune cells to be involved in the modulation of cancer-related biological procedures like angiogenesis, immune escape, and cell proliferation [16]. Studies have discovered that neurotransmitters assume a dual role in cancer progression, and the promotion or inhibition of tumor progression depends on specific neurotransmitter types, receptor expression, and microenvironment factors [17]. For example, neurotransmitters such as norepinephrine accelerate angiogenesis to provide a good metastatic environment for tumor cells and promote tumor spread and metastasis [18]. However, neurotransmitters such as dopamine show inhibitory effects in some cancers, inhibiting tumor growth by slowing angiogenesis or activating the immune response [19]. Therefore, in the next section, we describe the functions and molecular mechanisms of different neurotransmitters in regulating cancer progression, as shown in Figure 1. We systematically discuss their potential as therapeutic agents. This provides more ideas for the clinic to improve the survival and prognosis of patients.
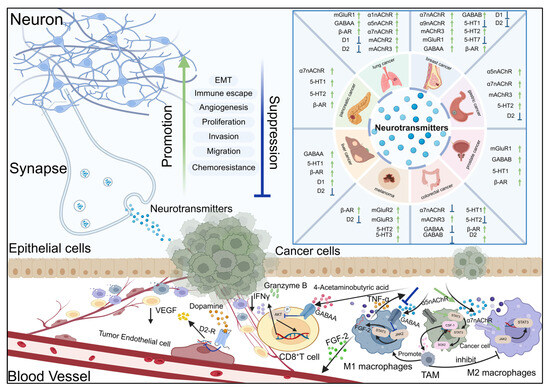
Figure 1.
Neurotransmitter substances regulate signal transduction and the development of cancer by activating their receptors.
The signal interaction between neurons and epithelial cells, vascular endothelial cells, and immune cells. The figure lists the expression and activation status of key neurotransmitter receptors (including nAChR subtypes α1, α5, α7, α9, mAChR2/3, GABAA, GABAB, D1 and D2 dopamine receptors, β - AR, 5-HT1/2/3/7, mGluR1/2/3) in tumor tissues. These biological functions include epithelial–mesenchymal transition (EMT), angiogenesis, immune escape, proliferation, invasion, migration, chemoresistance, and synaptic plasticity. The green arrow (↑) indicates that receptor activation promotes biological function. The blue arrow (↓) indicates the inhibition of biological function after receptor activation. After neurons release neurotransmitters and act on tumor cells, they affect different cell types (neurons, epithelial cells, endothelial cells, immune cells, and tumor cells) through ligand receptor interactions, forming a microenvironmental signaling network that jointly regulates tumor progression and nervous system function.
3. The Role of Major Neurotransmitters in Cancer
3.1. Classic Neurotransmitters
3.1.1. Acetylcholine
Acetylcholine (Ach) is an ester formed by choline and acetic acid, containing quaternary ammonium ions and showing strong alkalinity. It is the chemical transmitter of cholinergic nerves and is mainly synthesized in nerve endings [20]. The synthetic pathway is that acetyl coenzyme A transfers the acetyl group to choline under the catalysis of choline acetyltransferase (ChAT). The synthesized Ach regulates various physiological processes by activating the corresponding receptors, and finally is inactivated through the hydrolysis, diffusion, and reuptake pathways of cholinesterase. ACh receptors are divided into two categories, including nicotinic acetylcholine receptors (nAChRs) and muscarinic acetylcholine receptors (mAChRs) [21]. nAChRs are a ligand-gated ion channel receptor constituted by diverse α and β subunits, including α1-10, β1-4, and α4β2, α3β4, and α6β2β3. These diverse subunit combinations result in multiple nAChRs subtypes [22]. This receptor holds a significant position in neural signal transmission, cognitive function, and the regulation of the autonomic nervous system [23]. nAChRs are not only widely present in the nervous system, but also expressed in certain non-neural tissues (like immune cells and epithelial cells). mAChRs are a G protein-coupled receptor, currently divided into five pharmacological subtypes [24], all of which signal through G protein mediation. However, the M1, M3, and M5 subtypes trigger the activation of phospholipase C (PLC) on the plasma membrane through Gq/11 proteins, hydrolyzing 4,5-bisphosphatidylinositol (PIP2) into inositol trisphosphate (IP3) and diacylglycerol (DG), stimulating the release of Ca2+ from the endoplasmic reticulum and increasing intracellular Ca2+ concentration and protein kinase C (PKC) activation. Thus, it participates in mediating cell signal transduction. M2 and M4 inhibit adenylyl cyclase (AC) through Gi protein, thereby reducing the generation of cyclic adenosine monophosphate (cAMP), and then regulating cell activities and affecting physiological functions [25]. M1 receptors are predominantly located in the cerebral cortex, hippocampus, striatum, as well as the thalamus, and are closely related to cognitive function, learning, and memory; M2 receptors are mainly distributed in the heart and are capable of regulating heart rate, and they are also distributed in nerves and smooth muscles; M3 receptors are widely distributed in exocrine glands and intestinal smooth muscles, responsible for gland secretion and smooth muscle contraction; M4 receptors are distributed in many brain regions, but its role in the striatum is particularly prominent, and it negatively feedback regulates the release of dopamine; M5 receptors are less distributed, mainly located in the ventral tegmental area and substantia nigra of the midbrain, and exert a function in modulating dopamine neuron activity as well as vascular dilation [26]. With the in-depth discussion of cancer neuroscience, studies have revealed that acetylcholine is intimately associated with the progression of various cancers. It regulates tumor progression by activating different receptor subtypes, as shown in Figure 2.
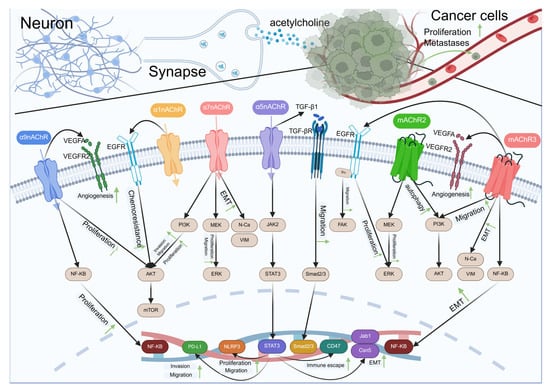
Figure 2.
Acetylcholine-type neurotransmitters regulate cancer development via receptors.
Schematic representation of the crosstalk between neurons and cancer cells mediated by acetylcholine and various signaling pathways. Neurons release acetylcholine that interacts with α7nAChR, α3nAChR, and α5nAChR receptors on the synaptic membrane. These interactions trigger downstream signaling pathways involving VEGFR2, EGFR, TGF-βR, and mAChR2/3, leading to cellular responses such as angiogenesis, chemoresistance, proliferation, and migration. In cancer cells, acetylcholine binding to mAChR3 also influences proliferation and metastasis through PI3K/AKT and NF-κB pathways. The diagram illustrates the complex interplay between neuronal signaling and cancer progression, highlighting potential therapeutic targets for cancer treatment.
Based on the latest report of the World Health Organization International Agency for Research on Cancer, lung cancer holds the position as the malignancy with the greatest mortality rate globally, featuring a mortality rate of 18.7%, and smoking constitutes a significant risk factor for lung cancer [27]. After nicotine enters the human blood circulation through the lungs, it can directly pass through the blood–brain barrier and exerting an effect on nAChRs [28]. Studies have found that nicotine is capable of upregulating HIF-1α to enhance the Warburg effect of lung cancer cells via nAChRs receptors [29]. In non-small cell lung cancer, the combination of ACh and α7nAchR activates the MEK/ERK signal and promotes the EMT development of lung cancer cells [30]. Many studies have additionally discovered that the activation of nAChRs significantly promotes the proliferation, invasion, migration, angiogenesis, chemoresistance, and immune escape of various cancer cells [31], as shown in Table 1.

Table 1.
Regulatory mechanisms of nicotinic acetylcholine receptors in cancer progression.
This indicates that research on α5 and α7nAChR (especially lung cancer) has more opportunities to serve as a novel target for cancer therapy. In gastric cancer, the combination of Ach and mAChRM3 can activate the overexpression of nerve growth factor (NGF) in the gastric epithelium, and NGF targets the TrkA receptor to increase synaptic growth, regulate mucosal innervation, and promote tumorigenesis [56]. For patients suffering from non-small cell lung cancer, despite the fact that EGFR–tyrosine kinase inhibitors (EGFR-TKI) can be employed for treatment, the emergence of drug resistance significantly influences survival and prognosis. Researchers found that Ach mediates the development of chemoresistance by inducing the activation of the WNT signaling pathway, and blocking the Ach/mAchRM3 signal significantly reduces tumor recurrence [57]. In addition, mAChRs, including mAchRM3, also promote the proliferation, invasion, and migration of other tumors, as shown in Table 2.

Table 2.
The regulatory mechanisms of mAChRs in cancer progression.
Not all mAChRs can cooperate with tumor progression. Studies have found that in colon cancer, the elevated expression level of mAChRM1 is capable of suppressing the development of colon cancer; however, the precise mechanism remains unknown. Augmenting the expression of mAChRM1 might be capable of suppressing the tumor-promoting effect of other mAChRs subtypes, which may be an effective target for the therapy of colon cancer. Clinical investigations have found that the expression of mAChRM3 is extremely low in well-differentiated tumors and normal tissues, but as the degree of differentiation decreases, the expression level of mAChRM3 also continues to increase, indicating that mAChRM3 is closely related to the malignancy of the tumor [75]. This also suggests that compared with mAChRM3, mAChRM1 might exert a more crucial function in the initial phase of gastric cancer. Consequently, investigations on mAChRM1 could be more beneficial for the therapy of patients suffering from early gastric cancer.
3.1.2. Glutamate
Glutamate is an excitatory neurotransmitter that can be formed by adding amino groups to α-ketoglutarate under the action of transaminases, or by deamination of glutamine by glutaminase [76,77]. Upon depolarization of nerve terminals, vesicular glutamate is released into the synaptic cleft, where it binds to specific receptors to enhance neuronal excitability. Glutamate receptors are broadly classified into ionotropic (iGluRs) and metabotropic (mGluRs) types. iGluRs include AMPA, KA, and NMDA receptors, which differ in structure, function, and activation kinetics [78]. AMPA receptors are homo- or heterotetramers (GluA1–4) widely expressed in the cerebral cortex, limbic system, and thalamus. They mediate fast excitatory postsynaptic currents, support rapid synaptic transmission, and are essential for learning and memory. KA receptors are multimers composed of five subunits, including Gluk1-3 with low affinity binding sites and GluK4 and Gluk5 with high affinity binding sites. KA receptors are extensively dispersed in the central and peripheral nervous systems, featuring an activation rate between AMPA and NMDA, a faster response, and a stronger regulatory effect. KA receptors participate in certain specific synaptic activities and contribute to synaptic plasticity by modulating the release of neurotransmitters. NMDA receptors are heteromeric complexes (GluN1, GluN2, GluN3) broadly localized in the central nervous system [79]. Their activation requires both membrane depolarization (to relieve Mg2+ blockade) and binding of glutamate and glycine. Unlike AMPA/KA receptors, NMDA receptor opening significantly increases Ca2+ permeability, leading to slow, sustained excitatory postsynaptic potentials and serving as a major source of intracellular Ca2+ signaling. However, excessive NMDA receptor activation may cause Ca2+ overload, contributing to neurodegenerative pathology. In recent years, investigations have continuously found that glutamate signals are involved in the progression of diverse cancers. mGluRs belong to G protein-coupled receptors [80], and can be classified into three types of mGluRs based on their sequence similarity, agonist potency order, and intracellular signal transduction mechanism. The first group of receptors (mGlu1/5) is coupled to Gαq protein, and stimulates IP3 receptor to trigger Ca2+ release through a series of pathways. The second group of receptors (mGlu2/3) and the third group of receptors (mGlu4/6/7/8) are coupled to Gαi proteins, and receptor activation inhibits AC, thereby reducing intracellular cAMP levels.
In recent years, the research within the domain of cancer neuroscience on glutamate receptors has mainly focused on mGluRs (especially mGluR1), which have a significant role in cancer cell proliferation, invasion, migration, and angiogenesis compared to iGluRs [81,82], as shown in Figure 3 and Table 3.
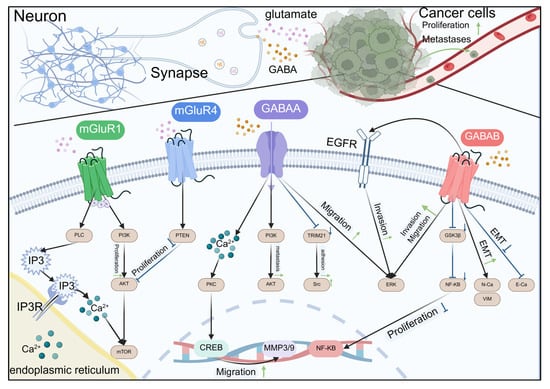
Figure 3.
Amino acid neurotransmitters regulate cancer development through receptors.

Table 3.
Regulatory mechanisms of mGluRs in cancer development.
Schematic representation of the intricate crosstalk between glutamate and GABA signaling in neurons and their influence on cancer cell behaviors. Neurons release glutamate and GABA that interact with mGluR1, mGluR4, GABAA, and GABAB receptors on the synaptic membrane. These interactions trigger downstream signaling pathways involving PI3K, AKT, PLC, PKC, CREB, GSK3β, NF-κB, and ERK, leading to cellular responses such as proliferation, migration, and invasion. In cancer cells, glutamate binding to mGluR1 and mGluR4 also influences proliferation and migration through PI3K/AKT pathways. GABA binding to GABAA and GABAB receptors modulates cell migration and invasion through PKC and GSK3β pathways, respectively. The diagram illustrates the complex interplay between neuronal signaling and cancer progression, highlighting potential therapeutic targets for cancer treatment.
Lung cancer is prone to brain metastasis, and the mortality rate after brain metastasis is very high, and the survival time of patients is very short, with a natural average survival time of only 1-2 months. The latest study found that lung cancer cells rely on the activation of mGluR1 signals in the brain microenvironment. This is because astrocytes secrete glutamate, which induces the mGluR1 signal in cancer cells through the WNT-5a/PRICKLE1/RE1 silent transcription factor REST axis, and then mGluR1 interacts with the epidermal growth factor receptor (EGFR) of lung cancer cells in a glutamate-dependent manner. When EGFR is stabilized by mGluR1, it enhances the migration of cancer cells, thereby accelerating the spread of cancer in brain tissue [87]. Although there are few studies on iGluRs in tumors, NMDA receptors have shown tumor-promoting effects in many cancers. Therefore, in tumor research, focusing on the regulation of NMDA receptors and mGluR1 on tumors and their mechanisms may be more helpful in developing new therapeutic strategies and biomarkers, thereby improving the efficacy of cancer diagnosis and therapy.
3.1.3. Gamma-Aminobutyric Acid
Gamma-aminobutyric acid (GABA) is an inhibitory amino acid released by nerve tissue and widely present in the nervous system. GABA is generated by the decarboxylation of glutamate, stored in neurons, and released and degraded into succinic acid, which enters the tricarboxylic acid cycle, or is reuptaken by transporters to terminate the effect [88]. GABA receptors are mainly divided into two categories, GABAA and GABAB, according to their responsiveness to specific agonists and antagonists. GABAA receptors are ligand-gated ion channels that cause postsynaptic inhibition through the Cl− channel, hyperpolarizing the membrane potential of neurons and reducing the excitability of neurons [89]. GABAA receptors are predominantly situated on the postsynaptic membrane, where they mediate fast inhibitory synaptic signaling within the central nervous system. GABAB receptors are a G protein-coupled receptor that inhibits AC by activating Gi/o-type G proteins and reduces intracellular cAMP levels [90]. In addition, GABAB receptors inhibit neurotransmission by regulating K+ and Ca2+ channels. In the presynaptic membrane, GABAB receptors reduce Ca2+ influx and regulate the release of neurotransmitters and neuropeptides; in the postsynaptic membrane, it is mainly coupled with inward rectifying K+ channels to mediate chronic inhibitory postsynaptic potentials. GABA is mainly distributed in the mammalian brain and serves as the primary inhibitory neurotransmitter in the brain, affecting about half of the central neurons. It is also distributed in trace amounts in other organs such as the liver, kidneys, and blood vessels.
High-grade gliomas and neural networks engage in bidirectional interactions, mutually influencing each other. Neuronal activity can increase the growth of gliomas, while gliomas also cause an increase in neuronal excitability. Therefore, the abundant GABA in the brain holds great significance for glioma research. In glioma cells, GABA is metabolized to gamma-hydroxybutyric acid (GHB), which enhances tumor cells’ energy metabolism and maintains glioma stem cell characteristics, thereby activating specific signaling pathways to promote tumor stem cell proliferation and survival [91]. Research has indicated that GABA is involved in multiple cancers, influencing cancer cell proliferation, invasion, and migration, as shown in Figure 3 and Table 4.

Table 4.
Regulatory mechanisms of GABA receptors in cancer progression.
Various types of GABA receptors exert distinct functions in diverse cancers. In pediatric medulloblastoma (SHH-MB), after the expression of GABAA receptors is induced by agonists, it can inhibit the PKA-cAMP response element-binding protein (CREB)-Gli1 signaling pathway mediated by cAMP in SHH-MB, thereby suppressing the proliferation of tumor cells. In vivo experiments also discovered that oral administration of the GABAA receptor agonist moxidectin can notably restrain the growth of SHH-MB tumors [106], so the GABAA receptor could potentially serve as an efficacious therapeutic target. In non-small cell lung cancer, an elevated level of GABAB receptors is correlated with an unfavorable prognosis for patients. GABA inhibits GSK-3β activity by activating GABAB receptors, thereby enhancing β-catenin signaling, promoting tumor cell proliferation, and reducing CD8+ T cell infiltration. These findings imply that GABAA and GABAB receptors, as part of the GABAergic system, may act antagonistically in various cancers, just as mAchRM1 and mAchRM3 in the cholinergic system. This also reflects the high complexity between the nerve and the tumor, so it is still a major challenge in future research to clarify the specific functions and mechanisms of different receptors in different cancers.
3.1.4. Norepinephrine and Epinephrine
Norepinephrine (NE) and Epinephrine (E) are catecholamine hormones, which are synthesized from tyrosine through a series of enzymatic reactions. NE is mainly secreted in sympathetic nerve endings and the central nervous system; it functions as a neurotransmitter to convey signals across nerve synapses. Additionally, a minor quantity is released by the adrenal medulla; E is predominantly secreted by the adrenal medulla, and a small amount exists in sympathetic nerve endings. It is primarily secreted as a hormone into the bloodstream, exerting a broad spectrum of effects. Both act on adrenergic α and β receptors, but have different affinities for different receptors [107]. NE mainly acts on α1 and α2 receptors, and also has a strong affinity for β1 receptors, However, its impact on β2 receptors is weak. Consequently, it mainly affects vasoconstriction and increases blood pressure, while having a weak effect on the bronchus and metabolism. E binds strongly to α1, α2, β1, and β2 receptors, particularly β2 receptors, leading to significant dilation of the heart and bronchus [108,109]. Studies have found that NE and E, as catecholamine stress hormones, not only participate in the regulation of the cardiovascular system, but also exert a crucial function in the cancer progression, as shown in Figure 4.
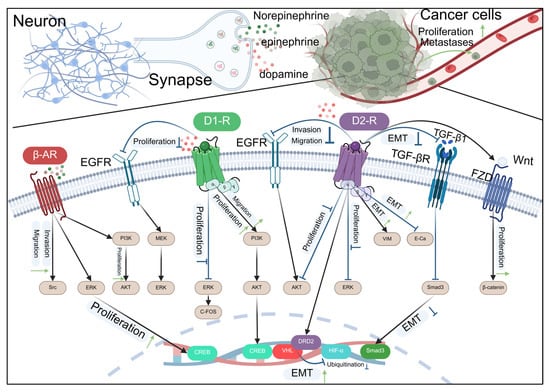
Figure 4.
Catecholamine-type neurotransmitters regulate the development of cancer through receptors.
This diagram illustrates the complex interactions between neurotransmitters released by neurons and signaling pathways in cancer cells. Norepinephrine, epinephrine, and dopamine, released at the synapse, can influence cancer cell behavior by interacting with various receptors. The β-adrenergic receptor (β-AR) and epidermal growth factor receptor (EGFR) pathways are activated, leading to downstream signaling through PI3K, MEK, and ERK, which promote cell proliferation and invasion. The dopamine D1 and D2 receptors also modulate cell proliferation and migration. The D2 receptor pathway involves the activation of ERK and AKT, which can lead to the inhibition of epithelial–mesenchymal transition (EMT) through the downregulation of DRD2, VHL, and HIF-α, and the upregulation of Smad3. The TGF-β receptor (TGF-βR) pathway, when activated, can induce EMT, promoting cell migration and invasion. The Wnt/FZD pathway also contributes to EMT and cell proliferation. The diagram highlights the potential therapeutic targets for inhibiting cancer cell proliferation and metastasis by modulating these signaling pathways.
It can be known from epidemiological investigations that chronic disease invasion [110,111,112,113] and long-term stress [114,115] are risk factors for cancer. Long-term stress induces hypothalamic–pituitary–adrenal axis activation, increasing cortisol levels, which indirectly promotes the release of catecholamines, forming a stress cascade effect [116]. Under chronic stress, the sympathetic nerve is constantly stimulated, and NE and E remain at high levels for a long time, driving cancer occurrence and development. The pancreas is richly innervated by sympathetic nerve fibres, and stress appears to be particularly associated with the advancement of pancreatic cancer. Studies have found that anti-stress drug treatment can decelerate the advancement of pancreatic cancer. The β-adrenergic receptor agonist isoproterenol is capable of directly facilitating the development of pancreatic tumors, whereas the non-selective β-blocker propranolol inhibits tumor growth [117]. In primary liver cancer, chronic stress promotes increased NE release and sustained activation of β-adrenergic receptors. Studies indicate that this signaling enhances HepG2 liver cancer cell proliferation via the ERK1/2/CREB axis [118] and confers survival advantages under anchorage-independent conditions—a key mechanism in metastasis [119]. E primarily signals through the β2-adrenergic receptor (β2-AR), coordinating tumor-promoting processes via downstream effectors including cAMP, PI3K/AKT, and MAPK/ERK pathways, which collectively regulate cancer cell metabolism, proliferation, and survival [120,121,122]. However, under acute stress, β2-adrenergic receptor (β2-AR) activation is transient due to rapid receptor desensitization and internalization, limiting sustained downstream signaling. Among adrenergic receptors, β2-AR plays a particularly critical role in cancer progression, largely due to its frequent overexpression in tumors and ability to coordinate multiple pro-tumorigenic processes. Upon activation, it drives key oncogenic pathways supporting proliferation, survival, and angiogenesis. It also facilitates immune evasion by suppressing the activity of T and NK cells [123,124,125] and is implicated in inflammation and angiogenesis [126]. Therefore, thoroughly exploring the mechanisms by which the β2 receptor influences cancer progression is crucial for developing targeted therapies against the β2 receptor and improving clinical management of patient.
3.1.5. Dopamine
Dopamine (DA) is a catecholamine neurotransmitter, an intermediate product produced by tyrosine in the metabolic process through dihydroxyphenylalanine, and it is the precursor of NE and E synthesis. The DA system, being a crucial reward system within the human body, exerts a significant regulatory function in behaviors like emotion, learning, cognition, reward, and social interaction [127,128,129]. Dopamine receptors are categorized into two groups based on their G protein coupling and signaling properties: D1-like receptors (D1 and D5) and D2-like receptors (D2, D3, and D4) [130]. D1-like receptors are excitatory DA receptors that activate AC through the αs subunit of G proteins and enhance the excitability of downstream neurons. D2-like receptors are inhibitory DA receptors, mainly coupled with inhibitory G protein Gi/o, and inhibit AC activity and cAMP production after activating Gi/o protein, reducing neuronal excitability. It has been demonstrated by studies that the activation of dopamine receptors exerts an intricate influence on tumor proliferation, apoptosis, angiogenesis, and immune regulation, as shown in Figure 4 and Table 5.

Table 5.
Regulatory mechanisms of dopamine receptors in the progression of cancer.
This may be related to dopamine concentration [152,153], receptor subtype [154,155], tumor microenvironment [156,157,158], tumor type, and activated signaling pathways [157].
Generally, D2-like receptors, particularly the D2 receptor, function as tumor suppressors in many cancers by inhibiting cAMP and cyclin expression. The D2 receptor can suppress tumor growth by inducing apoptosis via p53 and caspases [159], inhibiting VEGF-mediated angiogenesis [9], and promoting anti-tumor M1 macrophage polarization [132]. However, its role is context-dependent, as it can also promote glioblastoma progression via the ERK/GSK3β/β-catenin pathway [148]. Therefore, when studying the regulation of tumor progression by dopamine receptors, the D2 receptor and different types of downstream signaling pathways may be prospective targets for the therapy of cancer. In addition, there has been an increasing amount of research on D1 receptors, and it plays different functions in different cancers. In cholangiocarcinoma, the D1 receptor can inhibit tumor growth. Inhibition of the D1 receptor increases the expression of intracellular endogenous WNT7B, thereby driving the expansion of stem cell-like populations and contributing to cholangiocarcinoma initiation and progression. Targeting the D1 receptor feedback signal could serve as a promising treatment approach for cholangiocarcinoma [145]. In contrast, in hepatocellular carcinoma, elevated DA and D1 receptor expression drives tumor proliferation and metastasis through the cAMP/PI3K/AKT/CREB pathway [139]. Therefore, in the anti-tumor treatment strategy targeting dopamine receptors, the precise selection of specific dopamine receptor subtypes and their corresponding regulators has important research and application value for the efficacy and safety of future cancer treatment.
3.1.6. 5-Hydroxytryptamine
5-hydroxytryptamine (5-HT), or serotonin, is an indole ethylamine compound that cannot cross the blood–brain barrier or enter cells directly. Its synthesis is restricted to neurons. 5-HT receptors can be categorized into two principal groups, namely the ligand-gated ion channel receptor family and the G protein-coupled receptor family [160,161]. The ligand-gated ion channel receptor family includes 5-HT3A, 5-HT3B, and 5-HT3C. The G protein-coupled receptor family is categorized into three groups: receptors that couple with Gi/o proteins to inhibit AC (for instance: 5-HA1A, 5-HT1B, 5-HT1C, 5-HT1D, 5-HT1E, 5-HT1F, and 5-HT5A); receptors that couple with Gq proteins to activate PLC (such as: 5-HT2A, 5-HT2B, 5-HT2C); and receptors that couple with Gs proteins to activate AC (such as: 5-HT4, 5-HT6, and 5-HT7). 5-HT has a low content in the brain, yet it is extensively implicated in numerous physiological and pathological regulatory procedures [162]. Including food intake, body temperature regulation, sleep and wakefulness, learning and memory, drug addiction, pain, gastrointestinal diseases, and mental illness [163]. With the continuous exploration of the field of tumor neuroscience, there is evidence that 5-HT can directly promote cancer progression, or affect tumor cells proliferation, angiogenesis, invasion, and migration through various 5-HT receptors [164,165], as shown in Figure 5.
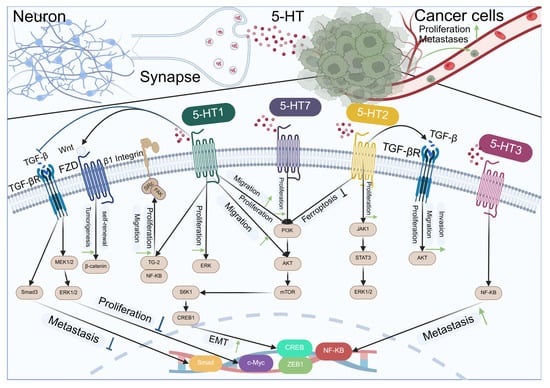
Figure 5.
Indoleamine neurotransmitters regulate the development of cancer through receptors.
Schematic representation of the interactions between serotonin receptors (5-HT1 to 5-HT7) and their downstream signaling pathways in neurons and cancer cells. In neurons (left side), the activation of 5-HT1 receptors leads to the inhibition of proliferation and metastasis through the Wnt/β-catenin pathway, while 5-HT2 receptors promote proliferation via the ERK pathway. In cancer cells (right side), 5-HT2 and 5-HT3 receptors enhance proliferation and metastasis through the PI3K/AKT/mTOR pathway. Additionally, 5-HT7 receptors promote proliferation and migration in cancer cells via the JAK1/STAT3 pathway. The diagram also illustrates the role of 5-HT receptors in ferroptosis inhibition and the epithelial–mesenchymal transition (EMT) process, which is associated with metastasis. Key signaling molecules and pathways are indicated, including TGF-β, FZD, β1 Integrin, MEK1/2, ERK1/2, PI3K, AKT, mTOR, JAK1, STAT3, NF-κB, and the transcription factors CREB, CREB1, c-Myc, and ZEB1.
About 90% of 5-HT in peripheral tissues is synthesized in the intestine, mainly produced by enterochromaffin cells and intestinal cells in the intestine, which convert tryptophan into 5-HT and secrete it into the intestinal lumen and blood [166]. 5-HT levels within the gut lumen significantly influence the development of gastrointestinal malignancies. Research indicates that 5-HT modulates both immune functions and microbiota populations by regulating the intestinal microenvironment under physiological conditions, thereby regulating cancer progression [167]; 5-HT has been shown to exacerbate intestinal inflammation, which is a significant contributor to the occurrence of colon cancer. In contrast to normal colorectal epithelial cells, the levels of 5-HT and tryptophan hydroxylase 1 in cancer cells, animal models, and colon cancer patients are significantly up-regulated, and high levels of 5-HT enhance the stimulation of the NLRP3 inflammasome through the 5-HT3A receptor, promoting the progression of colitis-related colorectal cancer [168]. Facilitated by the serotonin transporter, 5-HT is internalized into cells, triggering the RHOA/ROCK/YAP signaling pathway, which drives the progression of colon cancer [169]. In addition, 5-HT contributes to gastric cancer occurrence by up-regulating the expression of the stemness marker LGR5 [170]. Additionally, it influences tumor progression through immune modulation, such as accelerating pancreatic cancer growth by enhancing PD-L1 levels and inhibiting CD8+ T cells infiltration [171]. 5-HT plays a significant role not only in gastrointestinal cancers but also influences other cancer types. For example, in non-small cell lung cancer (NSCLC), 5-HT triggers the c-Myc/SLC6A4 signaling pathway, enhances 5-HT reuptake in A549 lung cancer cells and establishes a positive feedback mechanism that promotes tumor metastasis [172]. Studies have shown that 5-HT not only directly acts on the development process of a variety of tumors, but also regulates tumor progression by binding to its receptors and activating downstream signals [173,174], as shown in Table 6.

Table 6.
Regulatory mechanisms of 5-HT receptors in cancer progression.
Therefore, when discussing the role of 5-HT in tumor cells and its regulatory role in cancer progression, it is essential to consider its multifaceted effects. These include direct effects on tumor metabolism, heterogeneity, and the immune microenvironment, as well as the complex regulatory mechanisms that arise from its receptor binding and subsequent influence on tumor behavior. Due to the elevated level of 5-HT in the digestive system activates a variety of signaling pathways, digestive system tumors should also be the focus of attention. These findings reveal that different signaling pathways activated by 5-HT may lead to different outcomes of tumor progression, indicating the multifaceted nature of 5-HT in tumor biology and providing a new perspective for cancer treatment strategies targeting different targets.
3.1.7. Retrograde Messenger
Retrograde messengers are crucial in the nervous system for enabling signal transmission from postsynaptic to presynaptic neurons, opposing the role of conventional neurotransmitters. These atypical neurotransmitters can feedback and regulate the activity of presynaptic neurons, and have important effects on synaptic plasticity, learning and memory, and the coordination and network balance between neurons [192]. Based on their functional mechanisms, retrograde messengers can be categorized into three types: the first is the transmembrane diffusible retrograde messengers, such as gaseous retrograde messengers NO, CO [193] and lipid molecule endogenous cannabinoids [194], which are the focus of current research; the second is the retrograde messengers that are released by exocytosis and act on presynaptic receptors, such as nerve growth factor, brain-derived neurotrophic factor [195], etc.; the third is those molecules that realize signal transmission through the direct interaction of postsynaptic molecules with presynaptic molecules.
3.1.8. The Dual Role of NO
NO, a gaseous molecule with free radical characteristics, serves critical physiological functions across various systems, including the central nervous system. It is primarily produced from L-arginine through the catalysis of nitric oxide synthase (NOS) and interacts with soluble guanylyl cyclase (sGC). Under the activation of NO, sGC facilitates the transformation of guanosine triphosphate (GTP) into cyclic guanosine monophosphate (cGMP), initiating downstream signaling cascades involving protein kinase G (PKG) and phosphodiesterase (PDE) [196]. The functions of NO in the body are diverse and dynamic. It can interact with superoxide to form peroxynitrite, a potent oxidant, which induces cellular DNA damage. It can also directly react with DNA, causing DNA strand breaks, base mismatches, and cross-links. The accumulation of these DNA damages is one of the key factors for cell carcinogenesis [197]. Research indicates that NO significantly influences tumor progression, metastasis, angiogenesis, and chemoresistance [198,199], and shows dual roles of promoting and suppressing tumors in most cancers [200]. This dual role may stem from its concentration-dependent influence on cellular signaling pathways. In a variety of cancers, high concentrations of NO can induce apoptosis of cancer cells, while low concentrations of NO may promote tumor growth [201]. This dual role of NO suggests that we need to consider individual biological characteristics comprehensively when treating cancer to develop individualized treatment plans. In cancer treatment, precise control of the concentration and release rate of NO may exert better anti-tumor effects. In addition, post-surgical NO level monitoring can serve as a diagnostic and prognostic tool for cancer patients.
3.1.9. Therapeutic Potential and Challenges of CO
CO, as an endogenous signaling molecule, also plays a vital role in biological organisms. Like NO, its primary target is sGC, which it activates to drive the conversion of GTP to cGMP. This process regulates the PKG pathway, influencing vascular dilation, stimulating phosphoprotein phosphorylation, enhancing presynaptic terminal aggregation, and ultimately modulating neurotransmitter release. Although the high affinity of high concentrations of CO with hemoglobin may cause serious health problems, at a certain concentration, carbon monoxide-releasing molecules (CORMs) have shown significant therapeutic potential in a variety of cancer models [202]. CO influences tumor behavior by suppressing cancer cell growth, inducing apoptosis, inhibiting angiogenesis, reducing drug resistance, and limiting invasion and metastasis [203,204,205,206,207]. For example, in melanoma, CO induces immunometabolic reprogramming by activating PERK-regulated protective autophagy, enhancing anti-tumor T cells activity [208]. Within the domain of breast cancer therapeutics, the ROS-activated CO prodrug can release CO at a controllable release rate, significantly inhibiting the growth of breast tumors. This research initially demonstrated both in laboratory and living systems that the speed of CO liberation significantly influences its ability to inhibit cell growth, establishing a connection between the liberation rate and growth suppression effectiveness [209]. Therapeutic intervention for pulmonary malignancies through transformation of transient reactive oxygen species (ROS) into sustained carbon monoxide delivery demonstrated significant efficacy in controlling tumor development, preventing disease relapse, and suppressing metastatic dissemination, ultimately improving patient outcomes. This transformation approach additionally improves treatment efficacy by overcoming neoplastic resistance mechanisms against reactive oxygen species-based interventions. As a gaseous signaling molecule, recent studies have found that it shows great potential in cancer treatment. The clinical application of CO mainly focuses on its combination with other treatment methods. The combination not only enhances the therapeutic effect but also reduces the dose of single treatment to reduce the toxic side effects of CO. Advancing understanding of carbon monoxide’s pharmacological mechanisms has facilitated significant progress in prodrug development and controlled-release technologies, resulting in more cost-effective cancer therapeutic approaches that offer enhanced clinical and economic benefits. However, despite the progress in laboratory research, CO still seriously affects the human blood system and nervous system, and the clinical application of CO still requires more preclinical studies and clinical trials to ensure its effectiveness and safety in clinical applications. In conclusion, although CO has great potential in treating cancer, its side effects on the body cannot be ignored. The development of tumor-selective carbon monoxide delivery systems with minimized systemic toxicity and optimized therapeutic efficacy represents a crucial focus for advancing CO-based anticancer strategies.
3.1.10. Complex Regulation by Endocannabinoids
Endocannabinoids are important diffusible retrograde signaling molecules in the body, mainly including N-arachidonoylethanolamine (AEA) and 2-arachidonoylglycerol (2-AG). Cerebral endocannabinoid signaling is initiated through type I metabotropic glutamate receptor stimulation, triggering elevated intracellular calcium levels in postsynaptic neuronal compartments. PLC is activated, which in turn generates DAG, and finally 2-AG is generated. At the same time, Ca2+ acts on phospholipids, activates acyl transferase, cleaves to generate N-arachidonoyl phosphatidylethanolamine, and is finally cleaved by phosphodiesterase to generate AEA. Following their synaptic release, these endogenous cannabinoids exert their biological effects through presynaptic CB1 and CB2 receptor modulation [210]. CB1 receptors are predominantly localized in neural tissues, and after binding to endocannabinoids, they reduce the release of neurotransmitters by activating Gi/Go proteins and inhibiting the AC-cAMP-PKA pathway. In contrast, CB2 receptors have a lower homology with CB1 receptors, about 44% [211]. They are primarily expressed in peripheral tissues (e.g., spleen, immune cells, and tonsils) and regulate signal transduction through the inhibition of AC and N-type Ca2+ channels, similar to CB1 receptors [212]. in short, endocannabinoids regulate the release of neurotransmitters by affecting Ca2+ signaling and phospholipid metabolism, and affect neural signal transmission through CB1 and CB2 receptors [213]. CB1/2 receptors primarily exert anti-tumor effects during cancer progression. Their activation suppresses cancer cell proliferation, promotes apoptosis, and inhibits angiogenesis by modulating multiple signaling pathways [214]. The activation of CB1 receptors inhibits nerve growth factor (NGF)-induced proliferation of breast cancer cells in breast cancer [215]. Additionally, CB1 agonists ACPA and ACEA significantly suppress MDA-MB-231 breast cancer cells proliferation and induce apoptosis [216]. In bladder cancer, the CB2 agonist JWH0151 inhibits tumor growth by blocking the AKT signaling pathway [217]. However, CB1/2 receptors activation may also promote cancer progression through other signaling pathways. For example, 2-AG activates the Fyn/ERK/AP-1 signaling pathway in JB6 P+ tumor-sensitive epidermal cells through CB1/2 receptors, and this is intimately associated with tumor proliferation, invasion, metastasis, and chemoresistance [218]. Compared with CB1 receptors, CB2 receptors primarily affect tumor progression through immune regulation. For example, after AEA activates CB2 receptors, it promotes tumor progression by inhibiting the JAK1/STAT1/3 signal of tumor-killing T cells and suppressing anti-tumor immunity [219]. To sum up, CB1/CB2 receptors play a role in tumor development, but whether their activity promotes or inhibits tumor growth may vary depending on the environment. Given the anti-tumor effects of CB1/CB2 receptors in various cancers, targeting these receptors holds significant therapeutic potential. However, the central nervous system activity of CB1 receptors needs to be carefully considered, including its potential side effects such as drug addiction, tolerance, and metabolic disorders, and the immune regulation mediated by CB2 receptors cannot be ignored. Therefore, the combined application strategy of CB1/2 receptors may reduce the drug dose, reduce the side effects of single-target treatment, and may produce a synergistic effect and enhance the therapeutic effect.
3.1.11. Neuropeptide
Neuropeptides are short-chain amino acid-based bioactive molecules involved in multiple physiological functions, including pain modulation, metabolic control, hormonal balance, and emotional management [220]. Different from classical neurotransmitters, neuropeptides are initially produced in neuronal cell bodies as large precursor proteins, which are subsequently transported to nerve endings via axonal transport and processed post-translationally to generate their active forms. These neuropeptides are stored in large dense-core vesicles (LDCVs), and release depends on calcium ions and voltage-gated calcium channels. This process typically requires intense stimulation, resulting in prolonged release, extended duration of action, and broad physiological effects [221]. Neuropeptides regulate tumor cell proliferation, invasion, angiogenesis, tumor microenvironment, and immune escape by modulating various signaling pathways, including MAPK, cAMP, and PI3K/AKT, mediating cancer progression.
3.1.12. Neuropeptide Y (NPY) in Tumor Progression and Therapy
NPY is strongly linked to cancer progression, influencing tumor cell growth, invasion, migration, and angiogenesis through interactions with its receptors [222]. For instance, in breast cancer, upregulation of Y1 and Y5 receptors correlates with enhanced tumor cell proliferation and migration [223], while their antagonists demonstrate significant tumor-suppressive effects [224]. The Y5 receptor also promotes tumor angiogenesis by stimulating VEGF secretion [225]. In prostate cancer, NPY inhibits the apoptosis of tumor cells through NF-κB, mediating the chemotherapy resistance of the tumor [226]. In hepatocellular carcinoma, dipeptidyl peptidase 4-mediated cleavage of NPY amplifies Y5 receptor signaling, contributing to tumor progression [227]. The overexpression of NPY and its receptors in a variety of cancers provides a new research direction for targeted therapy. Studies have shown that DOX-P18, an NPY analog delivered via nanocarriers, achieves precise tumor targeting and shows potential in inhibiting growth and metastasis in triple-negative breast cancer (TNBC) [228]. Therefore, targeting NPY and its receptors shows significant promise for advancing cancer therapeutics, with potential applications in both research and clinical applications.
3.1.13. Substance P Mediated Oncogenic Signaling
Substance P, a tachykinin family member encoded by the preprotachykinin A gene, significantly influences cancer progression by binding to its specific receptor, neurokinin-1 receptor (NK-1R) [229]. In breast cancer, substance P interacts with NK-1R, inducing apoptosis in a subset of NK-1R-high cancer cells. The released single-stranded RNA (ssRNA) subsequently activates adjacent tumor TLR7, facilitating metastasis [230]. Substance P also enhances cancer cell invasion and migration through the activation of NF-κB, MAPK, and other signaling pathways [231]. Therefore, substance P regulates multiple signaling pathways related to cell survival, drug efflux, tumor microenvironment, and oxidative stress by activating the receptor NK-1, mediating resistance demonstrated by cancer cells against chemotherapeutic agents [232,233].
Emerging evidence indicates that, alongside NPY and substance P, additional neuropeptides—including growth hormone-releasing peptide (GHRH) [234,235], corticotropin-releasing hormone (CRH) [236,237], and calcitonin gene-related peptide (CGRP) [238,239]—play a role in tumorigenesis. These peptides influence cancer initiation and progression through interactions with their corresponding receptors. To sum up, the regulation of cancer progression by neuropeptides shows a considerable level of intricacy and diversity, which is related to its biosynthesis, receptor activation, signal transduction, and impact on the tumor microenvironment. In-depth exploration of the regulatory mechanism of neuropeptides on cancer not only enhances our understanding of tumor biology but also provides a scientific basis for creating novel therapeutic approaches. Therapeutic strategies focusing on neuropeptides and their receptors may revolutionize cancer treatment, offering enhanced clinical benefits and a higher quality of life for patients.
4. Conclusions, Challenges, and Future Directions
During the innervation of tumors, neurotransmitters, as key signal transduction molecules, and their receptors are widely involved in cancer progression. Therefore, drugs based on neurotransmitters, receptors, or their related pathways are gradually receiving attention, and some neuromodulatory drugs have been explored or applied in cancer treatment. Research has identified several nAChR blockers, including α-bungarotoxin, α-cyclophosphamide, and the glycoprotein of the rabies virus, that effectively suppress nicotine-driven growth, enhance cell death, and reduce the movement of A549 lung cancer cells. These findings offer a novel approach to lung cancer therapy. In addition, mAChRs antagonists, such as Darifenacin and Atropine, also exhibit broad-spectrum anti-tumor activity across multiple cancer types. Darifenacin, a selective mAChRs antagonist, is clinically used to treat overactive bladder, whereas Atropine, a non-selective M receptor inhibitor, is widely employed to alleviate smooth muscle spasms, reduce glandular secretion, and manage bradyarrhythmias. Research indicates that both agents suppress tumor cell proliferation, invasion, and migration by targeting multiple mAChR subtypes. Propranolol, a non-selective β-adrenergic receptor antagonist targeting both β1 and β2 receptors, is widely utilized in adjuvant cancer therapy due to its ability to suppress angiogenesis, tumor cell proliferation, and invasion across various cancer types. Additionally, selective β1 blockers like metoprolol and atenolol, as well as non-selective agents such as carvedilol, have demonstrated potential in inhibiting tumor growth in lung and breast cancers. Among DA receptors, the D2 receptor has always been the focus of attention of researchers, and it plays different regulatory roles in the progression of different cancers. Two D2 receptor agonists, bromocriptine and cabergoline, suppress tumor progression through hormonal modulation and inhibition of angiogenesis associated with cancer development. Chlorpromazine, haloperidol, and sulpiride, as D2 receptor antagonists, also show anti-cancer potential by blocking the D2 receptor. In the study of 5-HT receptors, the study of 5-HT2A receptors is expected to be applied to the clinical treatment of cancer earlier. For example, the drugs clozapine and ritanserin, which are used in the clinical treatment of schizophrenia, can reduce the release of VEGF, promote the polarization of tumor-associated macrophages from the M2 to the M1 phenotype within the tumor microenvironment, thereby enhancing the anti-cancer immune response by antagonizing 5-HT2A receptors. In cancer treatment, 5-HT3 receptor antagonists such as ondansetron and granisetron are primarily used to manage chemotherapy-induced nausea and vomiting. By reducing overstimulation of the gastrointestinal tract and the brainstem vomiting center, these drugs significantly enhance patients’ quality of life, although they do not directly target cancer. High concentrations of NO and CO, while toxic to the central nervous and respiratory systems, demonstrate considerable promise for applications in cancer therapy. These gas prodrugs are precisely delivered to the tumor site through oral administration or nanoparticle technology to release appropriate concentrations of NO and CO, thereby inducing tumor cell cycle arrest, promoting apoptosis, and enhancing the sensitivity of tumor cells to radiotherapy and chemotherapy, significantly improving the therapeutic effect of tumors. Therefore, precise targeting of different neurotransmitters, receptors, and their signaling pathways provides a new perspective for cancer treatment.
Although neurotransmitters show great potential in cancer treatment, translating their research results into clinical applications still faces major challenges. First, the broad presence of neurotransmitters and their receptors throughout the body means that targeting them with agonists or antagonists could impact normal tissues, causing serious side effects. For example, β-blockers act on the entire sympathetic nervous system, and while acting on tumor cells, they may also affect the heart and blood pressure. Therefore, improving the targeting of drugs is an urgent problem to be solved to avoid the impact of neurotransmitter drugs on normal physiological functions. Secondly, neurotransmitters have multiple receptor subtypes, and their mechanism of action is complex and diverse, which increases the difficulty of treating specific subtypes. In addition, long-term use of neurotransmitter receptor antagonists may lead to tumor resistance, a major contributor to poor prognosis in advanced cancer patients. Therefore, how to avoid or reduce resistance is also an important challenge. While basic research shows the great potential of neurotransmitter drugs in the treatment of cancer, their efficacy remains insufficiently validated in human trials, with limited clinical evidence suggesting a prolonged timeline for clinical translation.
Recent studies have increasingly focused on the role of neurotransmitter–tumor microenvironment interactions. Neurotransmitters influence both tumor cells and vascular endothelial cells within the microenvironment, while also directly modulating immune cells to impact cancer progression. Furthermore, the interaction between the nervous system and tumors involves bidirectional communication, rather than unidirectional regulation. Although the specific molecular mechanism has not been fully elucidated, in-depth exploration of the neuro-tumor axis in future research will help us reveal the complex mutual regulatory network between the nerve and cancer, which holds great significance for the advancement of cancer treatment strategies. At the same time, in current clinical practice, neurotransmitter-targeted drugs are mainly used to relieve postoperative nausea and pain symptoms in cancer patients, or to enhance the sensitivity of patients to radiotherapy and chemotherapy, while the vast majority of drugs that directly act on tumors are still in the development and clinical trial stage. These drugs usually lack tumor specificity and may still cause serious side effects due to the non-specificity of the drug and individual differences in the human body at an effective dose. Therefore, in-depth research on neurotransmitter types and their receptor subtypes is crucial for the development of more efficient and precise neurotransmitter regulatory drugs, which are expected to directly act on tumors, thereby improving the effect of cancer treatment. In conclusion, the regulation of cancer progression by neurotransmitters represents a critical area of tumor neuroscience research, offering not only profound insights into tumor biology but also promising potential for clinical translation.
Author Contributions
X.Z.: Conceptualization, Literature review, Writing—Original Draft. J.C.: Literature collection, writing—reviewing and editing. Y.Z. and C.L.: Literature collection, Data Curation. Y.J.: Supervision, Writing—Review & Editing. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by Gansu Province Joint Research Fund (Grant Number 24JRRA817).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
All the figures in this review were created with http://BioRender.com. accessed on 29 September 2025.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Swanton, C.; Bernard, E.; Abbosh, C.; Andre, F.; Auwerx, J.; Balmain, A.; Bar-Sagi, D.; Bernards, R.; Bullman, S.; DeGregori, J.; et al. Embracing cancer complexity: Hallmarks of systemic disease. Cell 2024, 187, 1589–1616. [Google Scholar] [CrossRef]
- Mancusi, R.; Monje, M. The neuroscience of cancer. Nature 2023, 618, 467–479. [Google Scholar] [CrossRef]
- Li, J.; Che, M.J.; Zhang, B.; Zhao, K.W.; Wan, C.; Yang, K.Y. The association between the neuroendocrine system and the tumor immune microenvironment: Emerging directions for cancer immunotherapy. BBA-Rev. Cancer 2023, 1878, 189007. [Google Scholar] [CrossRef]
- Chen, L.; Huang, S.; Wu, X.; He, W.; Song, M. Serotonin signalling in cancer: Emerging mechanisms and therapeutic opportunities. Clin. Transl. Med. 2024, 14, e1750. [Google Scholar] [CrossRef]
- Schledwitz, A.; Sundel, M.H.; Alizadeh, M.; Hu, S.; Xie, G.; Raufman, J.P. Differential Actions of Muscarinic Receptor Subtypes in Gastric, Pancreatic, and Colon Cancer. Int. J. Mol. Sci. 2021, 22, 13153. [Google Scholar] [CrossRef]
- Nimgampalle, M.; Chakravarthy, H.; Sharma, S.; Shree, S.; Bhat, A.R.; Pradeepkiran, J.A.; Devanathan, V. Neurotransmitter systems in the etiology of major neurological disorders: Emerging insights and therapeutic implications. Ageing Res. Rev. 2023, 89, 101994. [Google Scholar] [CrossRef] [PubMed]
- Rizo, J. Molecular Mechanisms Underlying Neurotransmitter Release. Annu. Rev. Biophys. 2022, 51, 377–408. [Google Scholar] [CrossRef]
- Sudhof, T.C. Neurotransmitter release: The last millisecond in the life of a synaptic vesicle. Neuron 2013, 80, 675–690. [Google Scholar] [CrossRef] [PubMed]
- Chakroborty, D.; Sarkar, C.; Basu, B.; Dasgupta, P.S.; Basu, S. Catecholamines regulate tumor angiogenesis. Cancer Res. 2009, 69, 3727–3730. [Google Scholar] [CrossRef]
- Abid, M.S.R.; Mousavi, S.; Checco, J.W. Identifying Receptors for Neuropeptides and Peptide Hormones: Challenges and Recent Progress. ACS Chem. Biol. 2021, 16, 251–263. [Google Scholar] [CrossRef] [PubMed]
- Kaczynska, K.; Zajac, D.; Wojciechowski, P.; Kogut, E.; Szereda-Przestaszewska, M. Neuropeptides and breathing in health and disease. Pulm. Pharmacol. Ther. 2018, 48, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Teleanu, R.I.; Niculescu, A.G.; Roza, E.; Vladacenco, O.; Grumezescu, A.M.; Teleanu, D.M. Neurotransmitters-Key Factors in Neurological and Neurodegenerative Disorders of the Central Nervous System. Int. J. Mol. Sci. 2022, 23, 5954. [Google Scholar] [CrossRef]
- Sears, S.M.; Hewett, S.J. Influence of glutamate and GABA transport on brain excitatory/inhibitory balance. Exp. Biol. Med. 2021, 246, 1069–1083. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.H.; Hu, L.P.; Wang, X.; Li, J.; Zhang, Z.G. Neurotransmitters: Emerging targets in cancer. Oncogene 2020, 39, 503–515. [Google Scholar] [CrossRef]
- Shi, Y.M.; Luo, J.M.; Wang, X.Q.; Zhang, Y.Q.; Zhu, H.; Su, D.S.; Yu, W.F.; Tian, J. Emerging Trends on the Correlation Between Neurotransmitters and Tumor Progression in the Last 20 Years: A Bibliometric Analysis CiteSpace. Front. Oncol. 2022, 12, 800499. [Google Scholar] [CrossRef]
- Zahalka, A.H.; Frenette, P.S. Nerves in cancer. Nat. Rev. Cancer 2020, 20, 143–157. [Google Scholar] [CrossRef]
- Silverman, D.A.; Martinez, V.K.; Dougherty, P.M.; Myers, J.N.; Calin, G.A.; Amit, M. Cancer-Associated Neurogenesis and Nerve-Cancer Cross-talk. Cancer Res. 2021, 81, 1431–1440. [Google Scholar] [CrossRef]
- Zahalka, A.H.; Arnal-Estape, A.; Maryanovich, M.; Nakahara, F.; Cruz, C.D.; Finley, L.W.S.; Frenette, P.S. Adrenergic nerves activate an angio-metabolic switch in prostate cancer. Science 2017, 358, 321–326. [Google Scholar] [CrossRef]
- Mendoza-Torreblanca, J.G.; Cardenas-Rodriguez, N.; Carro-Rodriguez, J.; Contreras-Garcia, I.J.; Garciadiego-Cazares, D.; Ortega-Cuellar, D.; Martinez-Lopez, V.; Alfaro-Rodriguez, A.; Evia-Ramirez, A.N.; Ignacio-Mejia, I.; et al. Antiangiogenic Effect of Dopamine and Dopaminergic Agonists as an Adjuvant Therapeutic Option in the Treatment of Cancer, Endometriosis, and Osteoarthritis. Int. J. Mol. Sci. 2023, 24, 10199. [Google Scholar] [CrossRef]
- Prado, M.A.; Reis, R.A.; Prado, V.F.; de Mello, M.C.; Gomez, M.V.; de Mello, F.G. Regulation of acetylcholine synthesis and storage. Neurochem. Int. 2002, 41, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Colangelo, C.; Shichkova, P.; Keller, D.; Markram, H.; Ramaswamy, S. Cellular, Synaptic and Network Effects of Acetylcholine in the Neocortex. Front. Neural Circuits 2019, 13, 24. [Google Scholar] [CrossRef]
- Hogg, R.C.; Raggenbass, M.; Bertrand, D. Nicotinic acetylcholine receptors: From structure to brain function. Rev. Physiol. Biochem. Pharmacol. 2003, 147, 1–46. [Google Scholar] [CrossRef]
- Terry, A.V., Jr.; Jones, K.; Bertrand, D. Nicotinic acetylcholine receptors in neurological and psychiatric diseases. Pharmacol. Res. 2023, 191, 106764. [Google Scholar] [CrossRef]
- Fredriksson, R.; Lagerstrom, M.C.; Lundin, L.G.; Schioth, H.B. The G-protein-coupled receptors in the human genome form five main families. Phylogenetic analysis, paralogon groups, and fingerprints. Mol. Pharmacol. 2003, 63, 1256–1272. [Google Scholar] [CrossRef]
- Kruse, A.C.; Kobilka, B.K.; Gautam, D.; Sexton, P.M.; Christopoulos, A.; Wess, J. Muscarinic acetylcholine receptors: Novel opportunities for drug development. Nat. Rev. Drug Discov. 2014, 13, 549–560. [Google Scholar] [CrossRef]
- Hulme, E.C.; Birdsall, N.J.; Buckley, N.J. Muscarinic receptor subtypes. Annu. Rev. Pharmacol. Toxicol. 1990, 30, 633–673. [Google Scholar] [CrossRef]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Improgo, M.R.; Scofield, M.D.; Tapper, A.R.; Gardner, P.D. The nicotinic acetylcholine receptor CHRNA5/A3/B4 gene cluster: Dual role in nicotine addiction and lung cancer. Prog. Neurobiol. 2010, 92, 212–226. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.K.; Lin, P.C.; Huang, T.T.; Hung, H.Y.; Huang, T.W.; Huang, E.Y.K. Nicotine activates HIF-1α and regulates acid extruders through the nicotinic acetylcholine receptor to promote the Warburg effect in non-small cell lung cancer cells. Eur. J. Pharmacol. 2023, 950, 175778. [Google Scholar] [CrossRef]
- Zhang, C.; Ding, X.P.; Zhao, Q.N.; Yang, X.J.; An, S.M.; Wang, H.; Xu, L.; Zhu, L.; Chen, H.Z. Role of alpha7-nicotinic acetylcholine receptor in nicotine-induced invasion and epithelial-to-mesenchymal transition in human non-small cell lung cancer cells. Oncotarget 2016, 7, 59199–59208. [Google Scholar] [CrossRef] [PubMed]
- Friedman, J.R.; Richbart, S.D.; Merritt, J.C.; Brown, K.C.; Nolan, N.A.; Akers, A.T.; Lau, J.K.; Robateau, Z.R.; Miles, S.L.; Dasgupta, P. Acetylcholine signaling system in progression of lung cancers. Pharmacol. Ther. 2019, 194, 222–254. [Google Scholar] [CrossRef]
- Li, H.; Wang, S.; Takayama, K.; Harada, T.; Okamoto, I.; Iwama, E.; Fujii, A.; Ota, K.; Hidaka, N.; Kawano, Y.; et al. Nicotine induces resistance to erlotinib via cross-talk between alpha 1 nAChR and EGFR in the non-small cell lung cancer xenograft model. Lung Cancer 2015, 88, 1–8. [Google Scholar] [CrossRef]
- Chen, X.; Jia, Y.; Zhang, Y.; Zhou, D.; Sun, H.; Ma, X. alpha5-nAChR contributes to epithelial-mesenchymal transition and metastasis by regulating Jab1/Csn5 signalling in lung cancer. J. Cell Mol. Med. 2020, 24, 2497–2506. [Google Scholar] [CrossRef]
- Zhu, P.; Kang, G.; Jiao, Y.; Gui, C.; Fan, H.; Li, X.; Jia, Y.; Zhang, L.; Ma, X. The alpha5-nAChR/PD-L1 axis facilitates lung adenocarcinoma cell migration and invasion. Hum. Cell 2022, 35, 1207–1218. [Google Scholar] [CrossRef]
- Ding, K.; Jiang, X.; Ni, J.; Zhang, C.; Li, A.; Zhou, J. JWA inhibits nicotine-induced lung cancer stemness and progression through CHRNA5/AKT-mediated JWA/SP1/CD44 axis. Ecotoxicol. Environ. Saf. 2023, 259, 115043. [Google Scholar] [CrossRef]
- Kang, G.; Jiao, Y.; Pan, P.; Fan, H.; Li, Q.; Li, X.; Li, J.; Wang, Y.; Jia, Y.; Wang, J.; et al. alpha5-nAChR/STAT3/CD47 axis contributed to nicotine-related lung adenocarcinoma progression and immune escape. Carcinogenesis 2023, 44, 773–784. [Google Scholar] [CrossRef]
- Kang, G.; Song, H.; Bo, L.; Liu, Q.; Li, Q.; Li, J.; Pan, P.; Wang, J.; Jia, Y.; Sun, H.; et al. Nicotine promotes M2 macrophage polarization through alpha5-nAChR/SOX2/CSF-1 axis in lung adenocarcinoma. Cancer Immunol. Immunother. 2024, 74, 11. [Google Scholar] [CrossRef]
- Zhang, Q.; Jia, Y.; Pan, P.; Zhang, X.; Jia, Y.; Zhu, P.; Chen, X.; Jiao, Y.; Kang, G.; Zhang, L.; et al. alpha5-nAChR associated with Ly6E modulates cell migration via TGF-beta1/Smad signaling in non-small cell lung cancer. Carcinogenesis 2022, 43, 393–404. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.L.; Hsu, Y.F.; Liu, C.H.; Kuo, Y.L.; Chen, Y.C.; Yeh, Y.C.; Ho, H.L.; Wu, Y.C.; Chou, T.Y.; Wu, C.W. Low-Dose Nicotine Activates EGFR Signaling via alpha5-nAChR and Promotes Lung Adenocarcinoma Progression. Int. J. Mol. Sci. 2020, 21, 6829. [Google Scholar] [CrossRef]
- Jia, Y.; Zhang, Q.; Liu, Z.; Pan, P.; Jia, Y.; Zhu, P.; Jiao, Y.; Kang, G.; Ma, X. The role of alpha5-nicotinic acetylcholine receptor/NLRP3 signaling pathway in lung adenocarcinoma cell proliferation and migration. Toxicology 2022, 469, 153120. [Google Scholar] [CrossRef]
- Witayateeraporn, W.; Arunrungvichian, K.; Pothongsrisit, S.; Doungchawee, J.; Vajragupta, O.; Pongrakhananon, V. alpha7-Nicotinic acetylcholine receptor antagonist QND7 suppresses non-small cell lung cancer cell proliferation and migration via inhibition of Akt/mTOR signaling. Biochem. Biophys. Res. Commun. 2020, 521, 977–983. [Google Scholar] [CrossRef]
- Bai, S.; Wen, W.; Hou, X.; Wu, J.; Yi, L.; Zhi, Y.; Lv, Y.; Tan, X.; Liu, L.; Wang, P.; et al. Inhibitory effect of sinomenine on lung cancer cells via negative regulation of alpha7 nicotinic acetylcholine receptor. J. Leukoc. Biol. 2021, 109, 843–852. [Google Scholar] [CrossRef]
- Joukhan, A.; Kononenko, V.; Bele, T.; Sollner Dolenc, M.; Peigneur, S.; Pinheiro-Junior, E.L.; Tytgat, J.; Turk, T.; Krizaj, I.; Drobne, D. Attenuation of Nicotine Effects on A549 Lung Cancer Cells by Synthetic alpha7 nAChR Antagonists APS7-2 and APS8-2. Mar. Drugs 2024, 22, 147. [Google Scholar] [CrossRef]
- Jia, Y.; Sun, H.; Wu, H.; Zhang, H.; Zhang, X.; Xiao, D.; Ma, X.; Wang, Y. Nicotine Inhibits Cisplatin-Induced Apoptosis via Regulating alpha5-nAChR/AKT Signaling in Human Gastric Cancer Cells. PLoS ONE 2016, 11, e0149120. [Google Scholar] [CrossRef] [PubMed]
- Bu, X.; Zhang, A.; Chen, Z.; Zhang, X.; Zhang, R.; Yin, C.; Zhang, J.; Zhang, Y.; Yan, Y. Migration of gastric cancer is suppressed by recombinant Newcastle disease virus (rL-RVG) via regulating alpha7-nicotinic acetylcholine receptors/ERK- EMT. BMC Cancer 2019, 19, 976. [Google Scholar] [CrossRef]
- Chen, P.C.; Lee, W.Y.; Ling, H.H.; Cheng, C.H.; Chen, K.C.; Lin, C.W. Activation of fibroblasts by nicotine promotes the epithelial-mesenchymal transition and motility of breast cancer cells. J. Cell Physiol. 2018, 233, 4972–4980. [Google Scholar] [CrossRef]
- Guo, X.; He, L.; Xu, W.; Wang, W.; Feng, X.; Fu, Y.; Zhang, X.; Ding, R.B.; Qi, X.; Bao, J.; et al. alphaO-Conotoxin GeXIVA [1,2] Suppresses In Vivo Tumor Growth of Triple-Negative Breast Cancer by Inhibiting AKT-mTOR, STAT3 and NF-kappaB Signaling Mediated Proliferation and Inducing Apoptosis. Mar. Drugs 2024, 22, 252. [Google Scholar] [CrossRef]
- Sun, Z.; Zhangsun, M.; Dong, S.; Liu, Y.; Qian, J.; Zhangsun, D.; Luo, S. Differential Expression of Nicotine Acetylcholine Receptors Associates with Human Breast Cancer and Mediates Antitumor Activity of alphaO-Conotoxin GeXIVA. Mar. Drugs 2020, 18, 61. [Google Scholar] [CrossRef]
- Sun, Z.; Bao, J.; Zhangsun, M.; Dong, S.; Zhangsun, D.; Luo, S. alphaO-Conotoxin GeXIVA Inhibits the Growth of Breast Cancer Cells via Interaction with alpha9 Nicotine Acetylcholine Receptors. Mar. Drugs 2020, 18, 195. [Google Scholar] [CrossRef] [PubMed]
- Ochirbat, S.; Kan, T.C.; Hsu, C.C.; Huang, T.H.; Chuang, K.H.; Chen, M.; Cheng, C.C.; Chang, C.C.; Rahayu, S.; Chang, J. The angiogenic role of the alpha 9-nicotinic acetylcholine receptor in triple-negative breast cancers. Angiogenesis 2024, 27, 827–843. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, T.; Friedman, T.; Vadgama, J.; Singh, V.; Tucker, A.; Collazo, J.; Sinha, S.; Hikim, A.S.; Singh, R.; Pervin, S. Nicotine Synergizes with High-Fat Diet to Induce an Anti-Inflammatory Microenvironment to Promote Breast Tumor Growth. Mediators Inflamm. 2020, 2020, 5239419. [Google Scholar] [CrossRef]
- Xiang, T.; Yu, F.; Fei, R.; Qian, J.; Chen, W. CHRNA7 inhibits cell invasion and metastasis of LoVo human colorectal cancer cells through PI3K/Akt signaling. Oncol. Rep. 2016, 35, 999–1005. [Google Scholar] [CrossRef]
- Fei, R.; Zhang, Y.; Wang, S.; Xiang, T.; Chen, W. alpha7 nicotinic acetylcholine receptor in tumor-associated macrophages inhibits colorectal cancer metastasis through the JAK2/STAT3 signaling pathway. Oncol. Rep. 2017, 38, 2619–2628. [Google Scholar] [CrossRef]
- Nimmakayala, R.K.; Seshacharyulu, P.; Lakshmanan, I.; Rachagani, S.; Chugh, S.; Karmakar, S.; Rauth, S.; Vengoji, R.; Atri, P.; Talmon, G.A.; et al. Cigarette Smoke Induces Stem Cell Features of Pancreatic Cancer Cells via PAF1. Gastroenterology 2018, 155, 892–908.e6. [Google Scholar] [CrossRef]
- Chen, S.; Kang, X.; Liu, G.; Zhang, B.; Hu, X.; Feng, Y. α7-Nicotinic Acetylcholine Receptor Promotes Cholangiocarcinoma Progression and Epithelial-Mesenchymal Transition Process. Dig. Dis. Sci. 2019, 64, 2843–2853. [Google Scholar] [CrossRef] [PubMed]
- Hayakawa, Y.; Sakitani, K.; Konishi, M.; Asfaha, S.; Niikura, R.; Tomita, H.; Renz, B.W.; Tailor, Y.; Macchini, M.; Middelhoff, M.; et al. Nerve Growth Factor Promotes Gastric Tumorigenesis through Aberrant Cholinergic Signaling. Cancer Cell 2017, 31, 21–34. [Google Scholar] [CrossRef]
- Nie, M.; Chen, N.; Pang, H.; Jiang, T.; Jiang, W.; Tian, P.; Yao, L.; Chen, Y.; DeBerardinis, R.J.; Li, W.; et al. Targeting acetylcholine signaling modulates persistent drug tolerance in EGFR-mutant lung cancer and impedes tumor relapse. J. Clin. Investig. 2022, 132, e160152. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Gu, X.; Zhang, C.; Lu, Q.; Chen, H.; Xu, L. Blocking M2 muscarinic receptor signaling inhibits tumor growth and reverses epithelial-mesenchymal transition (EMT) in non-small cell lung cancer (NSCLC). Cancer Biol. Ther. 2015, 16, 634–643. [Google Scholar] [CrossRef]
- Zhao, Q.; Yue, J.; Zhang, C.; Gu, X.; Chen, H.; Xu, L. Inactivation of M2 AChR/NF-kappaB signaling axis reverses epithelial-mesenchymal transition (EMT) and suppresses migration and invasion in non-small cell lung cancer (NSCLC). Oncotarget 2015, 6, 29335–29346. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chang, C.H.; Chen, M.C.; Chiu, T.H.; Li, Y.H.; Yu, W.C.; Liao, W.L.; Oner, M.; Yu, C.R.; Wu, C.C.; Yang, T.Y.; et al. Arecoline Promotes Migration of A549 Lung Cancer Cells through Activating the EGFR/Src/FAK Pathway. Toxins 2019, 11, 185. [Google Scholar] [CrossRef]
- Xu, R.; Shang, C.; Zhao, J.; Han, Y.; Liu, J.; Chen, K.; Shi, W. Activation of M3 muscarinic receptor by acetylcholine promotes non-small cell lung cancer cell proliferation and invasion via EGFR/PI3K/AKT pathway. Tumour Biol. 2015, 36, 4091–4100. [Google Scholar] [CrossRef]
- Wang, L.; Zhi, X.; Zhang, Q.; Wei, S.; Li, Z.; Zhou, J.; Jiang, J.; Zhu, Y.; Yang, L.; Xu, H.; et al. Muscarinic receptor M3 mediates cell proliferation induced by acetylcholine and contributes to apoptosis in gastric cancer. Tumour Biol. 2016, 37, 2105–2117. [Google Scholar] [CrossRef]
- Yu, H.; Xia, H.; Tang, Q.; Xu, H.; Wei, G.; Chen, Y.; Dai, X.; Gong, Q.; Bi, F. Acetylcholine acts through M3 muscarinic receptor to activate the EGFR signaling and promotes gastric cancer cell proliferation. Sci. Rep. 2017, 7, 40802. [Google Scholar] [CrossRef]
- Yang, T.; He, W.; Cui, F.; Xia, J.; Zhou, R.; Wu, Z.; Zhao, Y.; Shi, M. MACC1 mediates acetylcholine-induced invasion and migration by human gastric cancer cells. Oncotarget 2016, 7, 18085–18094. [Google Scholar] [CrossRef]
- Pelegrina, L.T.; Lombardi, M.G.; Fiszman, G.L.; Azar, M.E.; Morgado, C.C.; Sales, M.E. Immunoglobulin g from breast cancer patients regulates MCF-7 cells migration and MMP-9 activity by stimulating muscarinic acetylcholine receptors. J. Clin. Immunol. 2013, 33, 427–435. [Google Scholar] [CrossRef]
- Lombardi, M.G.; Negroni, M.P.; Pelegrina, L.T.; Castro, M.E.; Fiszman, G.L.; Azar, M.E.; Morgado, C.C.; Sales, M.E. Autoantibodies against muscarinic receptors in breast cancer: Their role in tumor angiogenesis. PLoS ONE 2013, 8, e57572. [Google Scholar] [CrossRef]
- Ahmed, E.A.; Alkuwayti, M.A.; Ibrahim, H.M. Atropine Is a Suppressor of Epithelial-Mesenchymal Transition (EMT) That Reduces Stemness in Drug-Resistant Breast Cancer Cells. Int. J. Mol. Sci. 2022, 23, 9849. [Google Scholar] [CrossRef]
- Cholpraipimolrat, W.; Suriyo, T.; Rangkadilok, N.; Nookabkaew, S.; Satayavivad, J. Hijiki and sodium arsenite stimulate growth of human colorectal adenocarcinoma cells through ERK1/2 activation. Food Chem. Toxicol. 2017, 110, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.S.; Liu, Z.; Vasamsetti, B.M.; Cho, N.J. The ERK1/2 and mTORC1 Signaling Pathways Are Involved in the Muscarinic Acetylcholine Receptor-Mediated Proliferation of SNU-407 Colon Cancer Cells. J. Cell Biochem. 2016, 117, 2854–2863. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Cho, N.J. Muscarinic acetylcholine receptors mediate eIF4B phosphorylation in SNU-407 colon cancer cells. Biochem. Biophys. Res. Commun. 2016, 480, 450–454. [Google Scholar] [CrossRef]
- Feng, Y.; Hu, X.; Liu, G.; Lu, L.; Zhao, W.; Shen, F.; Ma, K.; Sun, C.; Zhu, C.; Zhang, B. M3 muscarinic acetylcholine receptors regulate epithelial-mesenchymal transition, perineural invasion, and migration/metastasis in cholangiocarcinoma through the AKT pathway. Cancer Cell Int. 2018, 18, 173. [Google Scholar] [CrossRef]
- Amonyingcharoen, S.; Suriyo, T.; Thiantanawat, A.; Watcharasit, P.; Satayavivad, J. Taurolithocholic acid promotes intrahepatic cholangiocarcinoma cell growth via muscarinic acetylcholine receptor and EGFR/ERK1/2 signaling pathway. Int. J. Oncol. 2015, 46, 2317–2326. [Google Scholar] [CrossRef]
- Guerriero, C.; Manfredelli, M.; Matera, C.; Iuzzolino, A.; Conti, L.; Dallanoce, C.; De Amici, M.; Trisciuoglio, D.; Tata, A.M. M2 Muscarinic Receptor Stimulation Induces Autophagy in Human Glioblastoma Cancer Stem Cells via mTOR Complex-1 Inhibition. Cancers 2023, 16, 25. [Google Scholar] [CrossRef]
- Taggi, M.; Kovacevic, A.; Capponi, C.; Falcinelli, M.; Cacciamani, V.; Vicini, E.; Canipari, R.; Tata, A.M. The activation of M2 muscarinic receptor inhibits cell growth and survival in human epithelial ovarian carcinoma. J. Cell Biochem. 2022, 123, 1440–1453. [Google Scholar] [CrossRef] [PubMed]
- Mehedinteanu, A.M.; Mirea, C.S.; Stovicek, P.O.; Schenker, M.; Stancu, M.I.; Ciurea, A.M.; Streba, L.; Istrate-Ofiteru, A.M.; Sas, T.N.; Vere, C.C. Expression of M3 muscarinic acetylcholine receptors in gastric cancer. Rom. J. Morphol. Embryol. 2021, 62, 1001–1010. [Google Scholar] [CrossRef] [PubMed]
- Walker, M.C.; van der Donk, W.A. The many roles of glutamate in metabolism. J. Ind. Microbiol. Biotechnol. 2016, 43, 419–430. [Google Scholar] [CrossRef]
- Andersen, J.V.; Markussen, K.H.; Jakobsen, E.; Schousboe, A.; Waagepetersen, H.S.; Rosenberg, P.A.; Aldana, B.I. Glutamate metabolism and recycling at the excitatory synapse in health and neurodegeneration. Neuropharmacology 2021, 196, 108719. [Google Scholar] [CrossRef] [PubMed]
- Hansen, K.B.; Wollmuth, L.P.; Bowie, D.; Furukawa, H.; Menniti, F.S.; Sobolevsky, A.I.; Swanson, G.T.; Swanger, S.A.; Greger, I.H.; Nakagawa, T.; et al. Structure, Function, and Pharmacology of Glutamate Receptor Ion Channels. Pharmacol. Rev. 2021, 73, 298–487. [Google Scholar] [CrossRef]
- Hansen, K.B.; Yi, F.; Perszyk, R.E.; Furukawa, H.; Wollmuth, L.P.; Gibb, A.J.; Traynelis, S.F. Structure, function, and allosteric modulation of NMDA receptors. J. Gen. Physiol. 2018, 150, 1081–1105. [Google Scholar] [CrossRef]
- Niswender, C.M.; Conn, P.J. Metabotropic glutamate receptors: Physiology, pharmacology, and disease. Annu. Rev. Pharmacol. Toxicol. 2010, 50, 295–322. [Google Scholar] [CrossRef]
- Yu, L.J.; Wall, B.A.; Wangari-Talbot, J.; Chen, S. Metabotropic glutamate receptors in cancer. Neuropharmacology 2017, 115, 193–202. [Google Scholar] [CrossRef]
- Eddy, K.; Eddin, M.N.; Fateeva, A.; Pompili, S.V.B.; Shah, R.; Doshi, S.; Chen, S. Implications of a Neuronal Receptor Family, Metabotropic Glutamate Receptors, in Cancer Development and Progression. Cells 2022, 11, 2857. [Google Scholar] [CrossRef]
- Speyer, C.L.; Hachem, A.H.; Assi, A.A.; Johnson, J.S.; DeVries, J.A.; Gorski, D.H. Metabotropic glutamate receptor-1 as a novel target for the antiangiogenic treatment of breast cancer. PLoS ONE 2014, 9, e88830. [Google Scholar] [CrossRef]
- Kaittanis, C.; Andreou, C.; Hieronymus, H.; Mao, N.; Foss, C.A.; Eiber, M.; Weirich, G.; Panchal, P.; Gopalan, A.; Zurita, J.; et al. Prostate-specific membrane antigen cleavage of vitamin B9 stimulates oncogenic signaling through metabotropic glutamate receptors. J. Exp. Med. 2018, 215, 159–175. [Google Scholar] [CrossRef]
- Morikawa, N.; Tachibana, M.; Ago, Y.; Goda, H.; Sakurai, F.; Mizuguchi, H. LY341495, an mGluR2/3 Antagonist, Regulates the Immunosuppressive Function of Myeloid-Derived Suppressor Cells and Inhibits Melanoma Tumor Growth. Biol. Pharm. Bull. 2018, 41, 1866–1869. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, Y.; Wang, K.; Zhu, K.; Zheng, X.; Wang, L.; Luan, Y.; Wang, X.; Lu, H.; Wu, K.; et al. Activation of type 4 metabotropic glutamate receptor promotes cell apoptosis and inhibits proliferation in bladder cancer. J. Cell Physiol. 2019, 234, 2741–2755. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, K.; Ichinose, T.; Kadokawa, R.; Mizutani, R.; Iwabuchi, S.; Togi, S.; Ura, H.; Tange, S.; Shinjo, K.; Nakayama, J.; et al. Astrocyte-induced mGluR1 activates human lung cancer brain metastasis via glutamate-dependent stabilization of EGFR. Dev. Cell 2024, 59, 579–594.e6. [Google Scholar] [CrossRef] [PubMed]
- Koh, W.; Kwak, H.; Cheong, E.; Lee, C.J. GABA tone regulation and its cognitive functions in the brain. Nat. Rev. Neurosci. 2023, 24, 523–539. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Zou, G.; Zhu, R.; Kong, C.; Miao, C.; Zhang, M.; Li, J.; Xiong, W.; Wang, C. Structural basis of GABARAP-mediated GABA(A) receptor trafficking and functions on GABAergic synaptic transmission. Nat. Commun. 2021, 12, 297. [Google Scholar] [CrossRef]
- Kerr, D.I.; Ong, J. GABAB receptors. Pharmacol. Ther. 1995, 67, 187–246. [Google Scholar] [CrossRef]
- El-Habr, E.A.; Dubois, L.G.; Burel-Vandenbos, F.; Bogeas, A.; Lipecka, J.; Turchi, L.; Lejeune, F.X.; Coehlo, P.L.; Yamaki, T.; Wittmann, B.M.; et al. A driver role for GABA metabolism in controlling stem and proliferative cell state through GHB production in glioma. Acta Neuropathol. 2017, 133, 645–660. [Google Scholar] [CrossRef]
- Dong, Y.; Wang, G.; Nie, D.; Xu, Y.; Bai, X.; Lu, C.; Jian, F.; Wang, H.; Zheng, X. Tumor-derived GABA promotes lung cancer progression by influencing TAMs polarization and neovascularization. Int. Immunopharmacol. 2024, 126, 111217. [Google Scholar] [CrossRef]
- Cui, C.; Zhang, D.; Sun, K.; Zhu, Y.; Xu, J.; Kang, Y.; Zhang, G.; Cai, Y.; Mao, S.; Long, R.; et al. Propofol maintains Th17/Treg cell balance in elderly patients undergoing lung cancer surgery through GABAA receptor. BMC Immunol. 2022, 23, 58. [Google Scholar] [CrossRef]
- Liu, Q.; Sheng, Z.; Cheng, C.; Zheng, H.; Lanuti, M.; Liu, R.; Wang, P.; Shen, Y.; Xie, Z. Anesthetic Propofol Promotes Tumor Metastasis in Lungs via GABA(A) R-Dependent TRIM21 Modulation of Src Expression. Adv. Sci. 2021, 8, e2102079. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Xu, X.; Liu, D.; Gao, J.; Gao, Y.; Wu, X.; Sheng, H.; Li, Q.; Mi, J. The delta subunit of the GABA(A) receptor is necessary for the GPT2-promoted breast cancer metastasis. Theranostics 2023, 13, 1355–1369. [Google Scholar] [CrossRef]
- Gumireddy, K.; Li, A.; Kossenkov, A.V.; Sakurai, M.; Yan, J.; Li, Y.; Xu, H.; Wang, J.; Zhang, P.J.; Zhang, L.; et al. The mRNA-edited form of GABRA3 suppresses GABRA3-mediated Akt activation and breast cancer metastasis. Nat. Commun. 2016, 7, 10715. [Google Scholar] [CrossRef] [PubMed]
- Bundy, J.; Shaw, J.; Hammel, M.; Nguyen, J.; Robbins, C.; Mercier, I.; Suryanarayanan, A. Role of beta3 subunit of the GABA type A receptor in triple negative breast cancer proliferation, migration, and cell cycle progression. Cell Cycle 2024, 23, 448–465. [Google Scholar] [CrossRef] [PubMed]
- Sizemore, G.M.; Sizemore, S.T.; Seachrist, D.D.; Keri, R.A. GABA(A) receptor pi (GABRP) stimulates basal-like breast cancer cell migration through activation of extracellular-regulated kinase 1/2 (ERK1/2). J. Biol. Chem. 2014, 289, 24102–24113. [Google Scholar] [CrossRef]
- Zhang, D.; Li, X.; Yao, Z.; Wei, C.; Ning, N.; Li, J. GABAergic signaling facilitates breast cancer metastasis by promoting ERK1/2-dependent phosphorylation. Cancer Lett. 2014, 348, 100–108. [Google Scholar] [CrossRef]
- Miao, Y.; Zhang, Y.; Wan, H.; Chen, L.; Wang, F. GABA-receptor agonist, propofol inhibits invasion of colon carcinoma cells. Biomed. Pharmacother. 2010, 64, 583–588. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, H.; Sun, Z.; Chen, W.; Miao, C. GABAB receptor inhibits tumor progression and epithelial-mesenchymal transition via the regulation of Hippo/YAP1 pathway in colorectal cancer. Int. J. Biol. Sci. 2021, 17, 1953–1962. [Google Scholar] [CrossRef]
- Shu, Q.; Liu, J.; Liu, X.; Zhao, S.; Li, H.; Tan, Y.; Xu, J. GABAB R/GSK-3beta/NF-kappaB signaling pathway regulates the proliferation of colorectal cancer cells. Cancer Med. 2016, 5, 1259–1267. [Google Scholar] [CrossRef]
- An, J.; Seok, H.; Ha, E.M. GABA-producing Lactobacillus plantarum inhibits metastatic properties and induces apoptosis of 5-FU-resistant colorectal cancer cells via GABA(B) receptor signaling. J. Microbiol. 2021, 59, 202–216. [Google Scholar] [CrossRef]
- Xia, S.; He, C.; Zhu, Y.; Wang, S.; Li, H.; Zhang, Z.; Jiang, X.; Liu, J. GABA(B)R-Induced EGFR Transactivation Promotes Migration of Human Prostate Cancer Cells. Mol. Pharmacol. 2017, 92, 265–277. [Google Scholar] [CrossRef]
- Zhou, X.; Chen, Z.; Yu, Y.; Li, M.; Cao, Y.; Prochownik, E.V.; Li, Y. Increases in 4-Acetaminobutyric Acid Generated by Phosphomevalonate Kinase Suppress CD8(+) T Cell Activation and Allow Tumor Immune Escape. Adv. Sci. 2024, 11, e2403629. [Google Scholar] [CrossRef]
- Kaushik, I.; Srivastava, S.K. GABA(A) receptor agonist suppresses pediatric medulloblastoma progression by inhibiting PKA-Gli1 signaling axis. Mol. Ther. 2022, 30, 2584–2602. [Google Scholar] [CrossRef] [PubMed]
- Strosberg, A.D. Structure, function, and regulation of adrenergic receptors. Protein Sci. 1993, 2, 1198–1209. [Google Scholar] [CrossRef] [PubMed]
- Verberne, A.J.; Korim, W.S.; Sabetghadam, A.; Llewellyn-Smith, I.J. Adrenaline: Insights into its metabolic roles in hypoglycaemia and diabetes. Br. J. Pharmacol. 2016, 173, 1425–1437. [Google Scholar] [CrossRef] [PubMed]
- Alexander, S.P.; Davenport, A.P.; Kelly, E.; Marrion, N.; Peters, J.A.; Benson, H.E.; Faccenda, E.; Pawson, A.J.; Sharman, J.L.; Southan, C.; et al. The Concise Guide to PHARMACOLOGY 2015/16: G protein-coupled receptors. Br. J. Pharmacol. 2015, 172, 5744–5869. [Google Scholar] [CrossRef]
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W.; et al. Chronic inflammation in the etiology of disease across the life span. Nat. Med. 2019, 25, 1822–1832. [Google Scholar] [CrossRef]
- Huang, W.C.; Donin, N.M.; Levey, A.S.; Campbell, S.C. Chronic Kidney Disease and Kidney Cancer Surgery: New Perspectives. J. Urol. 2020, 203, 475–485. [Google Scholar] [CrossRef] [PubMed]
- Wilcox, N.S.; Amit, U.; Reibel, J.B.; Berlin, E.; Howell, K.; Ky, B. Cardiovascular disease and cancer: Shared risk factors and mechanisms. Nat. Rev. Cardiol. 2024, 21, 617–631. [Google Scholar] [CrossRef]
- Tu, H.; Wen, C.P.; Tsai, S.P.; Chow, W.H.; Wen, C.; Ye, Y.; Zhao, H.; Tsai, M.K.; Huang, M.; Dinney, C.P.; et al. Cancer risk associated with chronic diseases and disease markers: Prospective cohort study. BMJ 2018, 360, k134. [Google Scholar] [CrossRef] [PubMed]
- Eckerling, A.; Ricon-Becker, I.; Sorski, L.; Sandbank, E.; Ben-Eliyahu, S. Stress and cancer: Mechanisms, significance and future directions. Nat. Rev. Cancer 2021, 21, 767–785. [Google Scholar] [CrossRef]
- Forte, P.; Abate, V.; Bolognini, I.; Mazzoni, O.; Quagliariello, V.; Maurea, N.; Di Bonito, D.; Quarata, E.; Migliaccio, G.; Petrillo, M.; et al. Mindfulness-based stress reduction in cancer patients: Impact on overall survival, quality of life and risk factor. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 8190–8197. [Google Scholar] [CrossRef]
- Smith, S.M.; Vale, W.W. The role of the hypothalamic-pituitary-adrenal axis in neuroendocrine responses to stress. Dialogues Clin. Neurosci. 2006, 8, 383–395. [Google Scholar] [CrossRef]
- Kim-Fuchs, C.; Le, C.P.; Pimentel, M.A.; Shackleford, D.; Ferrari, D.; Angst, E.; Hollande, F.; Sloan, E.K. Chronic stress accelerates pancreatic cancer growth and invasion: A critical role for beta-adrenergic signaling in the pancreatic microenvironment. Brain Behav. Immun. 2014, 40, 40–47. [Google Scholar] [CrossRef]
- Lin, X.; He, J.; Liu, F.; Li, L.; Sun, L.; Niu, L.; Xi, H.; Zhan, Y.; Liu, X.; Hu, P. beta-adrenergic receptor activation promotes the proliferation of HepG2 cells via the ERK1/2/CREB pathways. Oncol. Lett. 2023, 26, 519. [Google Scholar] [CrossRef]
- Reavis, H.D.; Gysler, S.M.; McKenney, G.B.; Knarr, M.; Lusk, H.J.; Rawat, P.; Rendulich, H.S.; Mitchell, M.A.; Berger, D.S.; Moon, J.S.; et al. Norepinephrine induces anoikis resistance in high-grade serous ovarian cancer precursor cells. JCI Insight 2024, 9, e170961. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.H.; Park, H.J.; Park, S.H.; Choi, Y.H.; Chi, G.Y. beta2-Adrenergic Receptor Signaling Pathway Stimulates the Migration and Invasion of Cancer Cells via Src Activation. Molecules 2022, 27, 5940. [Google Scholar] [CrossRef]
- Mohammadpour, H.; MacDonald, C.R.; McCarthy, P.L.; Abrams, S.I.; Repasky, E.A. beta2-adrenergic receptor signaling regulates metabolic pathways critical to myeloid-derived suppressor cell function within the TME. Cell Rep. 2021, 37, 109883. [Google Scholar] [CrossRef]
- Xu, Y.; He, Z.; Rao, Z.; Li, Z.; Hu, Y.; Zhang, Z.; Zhou, J.; Zhou, T.; Wang, H. The role of beta2-AR/PI3K/AKT pathway in the proliferation, migration and invasion of THLE-2 cells induced by nicotine. Toxicology 2024, 508, 153924. [Google Scholar] [CrossRef]
- Zhao, X.; Li, F.; Cheng, C.; Bi, M.; Li, J.; Cong, J.; Wang, X. Social isolation promotes tumor immune evasion via beta2-adrenergic receptor. Brain Behav. Immun. 2024, 123, 607–618. [Google Scholar] [CrossRef] [PubMed]
- Daher, C.; Vimeux, L.; Stoeva, R.; Peranzoni, E.; Bismuth, G.; Wieduwild, E.; Lucas, B.; Donnadieu, E.; Bercovici, N.; Trautmann, A.; et al. Blockade of beta-Adrenergic Receptors Improves CD8(+) T-cell Priming and Cancer Vaccine Efficacy. Cancer Immunol. Res. 2019, 7, 1849–1863. [Google Scholar] [CrossRef]
- Sun, Z.; Hou, D.; Liu, S.; Fu, W.; Wang, J.; Liang, Z. Norepinephrine inhibits the cytotoxicity of NK92-MI cells via the beta2-adrenoceptor/cAMP/PKA/p-CREB signaling pathway. Mol. Med. Rep. 2018, 17, 8530–8535. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Li, J.; Yu, J.; Wang, Y.; Wang, Z.; He, Z.; Ouyang, D.; Liu, H.; Wang, Y. Tumor microenvironment adrenergic nerves blockade liposomes for cancer therapy. J. Control Release 2022, 351, 656–666. [Google Scholar] [CrossRef]
- Garritsen, O.; van Battum, E.Y.; Grossouw, L.M.; Pasterkamp, R.J. Development, wiring and function of dopamine neuron subtypes. Nat. Rev. Neurosci. 2023, 24, 134–152. [Google Scholar] [CrossRef] [PubMed]
- Speranza, L.; di Porzio, U.; Viggiano, D.; de Donato, A.; Volpicelli, F. Dopamine: The Neuromodulator of Long-Term Synaptic Plasticity, Reward and Movement Control. Cells 2021, 10, 735. [Google Scholar] [CrossRef]
- Cutando, L.; Puighermanal, E.; Castell, L.; Tarot, P.; Belle, M.; Bertaso, F.; Arango-Lievano, M.; Ango, F.; Rubinstein, M.; Quintana, A.; et al. Cerebellar dopamine D2 receptors regulate social behaviors. Nat. Neurosci. 2022, 25, 900–911. [Google Scholar] [CrossRef]
- Xu, P.; Huang, S.; Krumm, B.E.; Zhuang, Y.; Mao, C.; Zhang, Y.; Wang, Y.; Huang, X.P.; Liu, Y.F.; He, X.; et al. Structural genomics of the human dopamine receptor system. Cell Res. 2023, 33, 604–616. [Google Scholar] [CrossRef]
- Yong, L.; Yao, Y.; Chen, G.S.; Yan, X.X.; Guo, Y.C.; Han, M.Y.; Xue, J.S.; Jian, W.Z.; Zhou, T.Y. QAP14 suppresses breast cancer stemness and metastasis via activation of dopamine D1 receptor. Acta Pharmacol. Sin. 2022, 43, 1001–1012. [Google Scholar] [CrossRef]
- Tan, Y.; Sun, R.; Liu, L.; Yang, D.; Xiang, Q.; Li, L.; Tang, J.; Qiu, Z.; Peng, W.; Wang, Y.; et al. Tumor suppressor DRD2 facilitates M1 macrophages and restricts NF-kappaB signaling to trigger pyroptosis in breast cancer. Theranostics 2021, 11, 5214–5231. [Google Scholar] [CrossRef]
- Zhang, S.; Zhong, M.; Zhu, H.; You, Q.; Yuan, H.; Li, Y. Hypomethylation of DRD2 promotes breast cancer through the FLNA-ERK pathway. Cancer Genet. 2023, 278–279, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Li, L.; Chen, J.; Chen, H.; Cui, B.; Feng, Y.; Zhang, P.; Zhang, Q.; Xia, Y.; Luo, M. Thioridazine hydrochloride: An antipsychotic agent that inhibits tumor growth and lung metastasis in triple-negative breast cancer via inducing G0/G1 arrest and apoptosis. Cell Cycle 2020, 19, 3521–3533. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Yang, J.; Zhang, Y.; Han, J.; Yang, Y.; Zhao, Z.; Dai, X.; Wang, H.; Ding, X.; Liu, Y.; et al. Psychologic Stress Drives Progression of Malignant Tumors via DRD2/HIF1alpha Signaling. Cancer Res. 2021, 81, 5353–5365. [Google Scholar] [CrossRef] [PubMed]
- Grant, C.E.; Flis, A.L.; Toulabi, L.; Zingone, A.; Rossi, E.; Aploks, K.; Sheppard, H.; Ryan, B.M. DRD1 suppresses cell proliferation and reduces EGFR activation and PD-L1 expression in NSCLC. Mol. Oncol. 2024, 18, 1631–1648. [Google Scholar] [CrossRef]
- Hoeppner, L.H.; Wang, Y.; Sharma, A.; Javeed, N.; Van Keulen, V.P.; Wang, E.; Yang, P.; Roden, A.C.; Peikert, T.; Molina, J.R.; et al. Dopamine D2 receptor agonists inhibit lung cancer progression by reducing angiogenesis and tumor infiltrating myeloid derived suppressor cells. Mol. Oncol. 2015, 9, 270–281. [Google Scholar] [CrossRef]
- Roy, S.; Lu, K.; Nayak, M.K.; Bhuniya, A.; Ghosh, T.; Kundu, S.; Ghosh, S.; Baral, R.; Dasgupta, P.S.; Basu, S. Activation of D2 Dopamine Receptors in CD133+ve Cancer Stem Cells in Non-small Cell Lung Carcinoma Inhibits Proliferation, Clonogenic Ability, and Invasiveness of These Cells. J. Biol. Chem. 2017, 292, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Pan, J.; Chen, Y.; Xing, W.; Li, Q.; Wang, D.; Zhou, X.; Xie, J.; Miao, C.; Yuan, Y.; et al. Increased dopamine and its receptor dopamine receptor D1 promote tumor growth in human hepatocellular carcinoma. Cancer. Commun. 2020, 40, 694–710. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.B.; Zhang, B.H.; Zhang, K.Z.; Meng, X.T.; Jia, Q.A.; Zhang, Q.B.; Bu, Y.; Zhu, X.D.; Ma, D.N.; Ye, B.G.; et al. Moderate swimming suppressed the growth and metastasis of the transplanted liver cancer in mice model: With reference to nervous system. Oncogene 2016, 35, 4122–4131. [Google Scholar] [CrossRef]
- Hartwell, H.J.; Petrosky, K.Y.; Fox, J.G.; Horseman, N.D.; Rogers, A.B. Prolactin prevents hepatocellular carcinoma by restricting innate immune activation of c-Myc in mice. Proc. Natl. Acad. Sci. USA 2014, 111, 11455–11460. [Google Scholar] [CrossRef]
- Huang, H.; Wu, K.; Ma, J.; Du, Y.; Cao, C.; Nie, Y. Dopamine D2 receptor suppresses gastric cancer cell invasion and migration via inhibition of EGFR/AKT/MMP-13 pathway. Int. Immunopharmacol. 2016, 39, 113–120. [Google Scholar] [CrossRef]
- Sim, S.J.; Jang, J.H.; Choi, J.S.; Chun, K.S. Domperidone, a Dopamine Receptor D2 Antagonist, Induces Apoptosis by Inhibiting the ERK/STAT3-Mediated Pathway in Human Colon Cancer HCT116 Cells. Biomol. Ther. 2024, 32, 568–576. [Google Scholar] [CrossRef]
- Ren, Y.; Tao, J.; Jiang, Z.; Guo, D.; Tang, J. Pimozide suppresses colorectal cancer via inhibition of Wnt/beta-catenin signaling pathway. Life Sci. 2018, 209, 267–273. [Google Scholar] [CrossRef]
- Yogo, A.; Masui, T.; Takaishi, S.; Masuo, K.; Chen, R.; Kasai, Y.; Nagai, K.; Anazawa, T.; Watanabe, S.; Sakamoto, S.; et al. Inhibition of dopamine receptor D1 signaling promotes human bile duct cancer progression via WNT signaling. Cancer Sci. 2023, 114, 1324–1336. [Google Scholar] [CrossRef] [PubMed]
- Basu, S.; Sarkar, C.; Chakroborty, D.; Nagy, J.; Mitra, R.B.; Dasgupta, P.S.; Mukhopadhyay, D. Ablation of peripheral dopaminergic nerves stimulates malignant tumor growth by inducing vascular permeability factor/vascular endothelial growth factor-mediated angiogenesis. Cancer Res. 2004, 64, 5551–5555. [Google Scholar] [CrossRef]
- Jeon, H.M.; Oh, Y.T.; Shin, Y.J.; Chang, N.; Kim, D.; Woo, D.; Yeup, Y.; Joo, K.M.; Jo, H.; Yang, H.; et al. Dopamine receptor D2 regulates glioblastoma survival and death through MET and death receptor 4/5. Neoplasia 2023, 39, 100894. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.; Wang, K.; Qi, J.; Zhang, Y.; Wang, X.; Zhang, L.; Zhou, Y.; Gu, L.; Yu, R.; et al. Chronic stress accelerates glioblastoma progression via DRD2/ERK/beta-catenin axis and Dopamine/ERK/TH positive feedback loop. J. Exp. Clin. Cancer Res. 2023, 42, 161. [Google Scholar] [CrossRef]
- Caragher, S.P.; Shireman, J.M.; Huang, M.; Miska, J.; Atashi, F.; Baisiwala, S.; Hong Park, C.; Saathoff, M.R.; Warnke, L.; Xiao, T.; et al. Activation of Dopamine Receptor 2 Prompts Transcriptomic and Metabolic Plasticity in Glioblastoma. J. Neurosci. 2019, 39, 1982–1993. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, W.; Wang, F.; Wu, Q.; Li, W.; Zhong, X.; Tian, K.; Zeng, T.; Gao, L.; Liu, Y.; et al. Paired related homeobox 1 transactivates dopamine D2 receptor to maintain propagation and tumorigenicity of glioma-initiating cells. J. Mol. Cell Biol. 2017, 9, 302–314. [Google Scholar] [CrossRef] [PubMed]
- Marisetty, A.L.; Lu, L.; Veo, B.L.; Liu, B.; Coarfa, C.; Kamal, M.M.; Kassem, D.H.; Irshad, K.; Lu, Y.; Gumin, J.; et al. REST-DRD2 mechanism impacts glioblastoma stem cell-mediated tumorigenesis. Neuro Oncol. 2019, 21, 775–785. [Google Scholar] [CrossRef]
- Chakroborty, D.; Sarkar, C.; Mitra, R.B.; Banerjee, S.; Dasgupta, P.S.; Basu, S. Depleted dopamine in gastric cancer tissues: Dopamine treatment retards growth of gastric cancer by inhibiting angiogenesis. Clin. Cancer Res. 2004, 10, 4349–4356. [Google Scholar] [CrossRef]
- Coufal, M.; Invernizzi, P.; Gaudio, E.; Bernuzzi, F.; Frampton, G.A.; Onori, P.; Franchitto, A.; Carpino, G.; Ramirez, J.C.; Alvaro, D.; et al. Increased local dopamine secretion has growth-promoting effects in cholangiocarcinoma. Int. J. Cancer 2010, 126, 2112–2122. [Google Scholar] [CrossRef]
- Wang, Z.; Wen, P.; Hu, B.; Cao, S.; Shi, X.; Guo, W.; Zhang, S. Dopamine and dopamine receptor D1 as a novel favourable biomarker for hepatocellular carcinoma. Cancer Cell Int. 2021, 21, 586. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Z.B.; Luo, C.; Mao, X.Y.; Li, X.; Yin, J.Y.; Zhang, W.; Zhou, H.H.; Liu, Z.Q. The Prospective Value of Dopamine Receptors on Bio-Behavior of Tumor. J. Cancer 2019, 10, 1622–1632. [Google Scholar] [CrossRef]
- Cervantes-Villagrana, R.D.; Albores-Garcia, D.; Cervantes-Villagrana, A.R.; Garcia-Acevez, S.J. Tumor-induced neurogenesis and immune evasion as targets of innovative anti-cancer therapies. Signal Transduct. Target. Ther. 2020, 5, 99. [Google Scholar] [CrossRef]
- Grant, C.E.; Flis, A.L.; Ryan, B.M. Understanding the role of dopamine in cancer: Past, present and future. Carcinogenesis 2022, 43, 517–527. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Q.; Liao, Q.; Zhao, Y. Potential Roles of Peripheral Dopamine in Tumor Immunity. J. Cancer 2017, 8, 2966–2973. [Google Scholar] [CrossRef] [PubMed]
- Akbarian, F.; Dadkhah, F.; Campbell, A.; Asadi, F.; Ahangari, G. Characterization of Dopamine Receptor Associated Drugs on the Proliferation and Apoptosis of Prostate Cancer Cell Lines. Anticancer. Agents Med. Chem. 2021, 21, 1160–1171. [Google Scholar] [CrossRef] [PubMed]
- Saxena, P.R. Serotonin receptors: Subtypes, functional responses and therapeutic relevance. Pharmacol. Ther. 1995, 66, 339–368. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, A.W.; Peroutka, S.J. 5-Hydroxytryptamine receptor “families”. FASEB J. 1989, 3, 2242–2249. [Google Scholar] [CrossRef]
- Okaty, B.W.; Commons, K.G.; Dymecki, S.M. Embracing diversity in the 5-HT neuronal system. Nat. Rev. Neurosci. 2019, 20, 397–424. [Google Scholar] [CrossRef] [PubMed]
- De Deurwaerdere, P.; Di Giovanni, G. Serotonin in Health and Disease. Int. J. Mol. Sci. 2020, 21, 3500. [Google Scholar] [CrossRef] [PubMed]
- Karmakar, S.; Lal, G. Role of serotonin receptor signaling in cancer cells and anti-tumor immunity. Theranostics 2021, 11, 5296–5312. [Google Scholar] [CrossRef]
- Ye, D.; Xu, H.; Tang, Q.; Xia, H.; Zhang, C.; Bi, F. The role of 5-HT metabolism in cancer. Biochim. Biophys. Acta Rev. Cancer 2021, 1876, 188618. [Google Scholar] [CrossRef]
- Sanger, G.J. 5-hydroxytryptamine and the gastrointestinal tract: Where next? Trends Pharmacol. Sci. 2008, 29, 465–471. [Google Scholar] [CrossRef]
- Cheng, P.; Shen, P.; Shan, Y.; Yang, Y.; Deng, R.; Chen, W.; Lu, Y.; Wei, Z. Gut Microbiota-Mediated Modulation of Cancer Progression and Therapy Efficacy. Front. Cell Dev. Biol. 2021, 9, 626045. [Google Scholar] [CrossRef]
- Li, T.; Fu, B.; Zhang, X.; Zhou, Y.; Yang, M.; Cao, M.; Chen, Y.; Tan, Y.; Hu, R. Overproduction of Gastrointestinal 5-HT Promotes Colitis-Associated Colorectal Cancer Progression via Enhancing NLRP3 Inflammasome Activation. Cancer Immunol. Res. 2021, 9, 1008–1023. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Qu, T.; Yang, J.; Dai, Q. Serotonin acts through YAP to promote cell proliferation: Mechanism and implication in colorectal cancer progression. Cell Commun. Signal 2023, 21, 75. [Google Scholar] [CrossRef]
- Niu, Q.; Li, L.; Zhang, C.; Qi, C.; He, Q.; Zhu, Y. Expression of 5-HT Relates to Stem Cell Marker LGR5 in Patients with Gastritis and Gastric Cancer. Dig. Dis. Sci. 2023, 68, 1864–1872. [Google Scholar] [CrossRef]
- Schneider, M.A.; Heeb, L.; Beffinger, M.M.; Pantelyushin, S.; Linecker, M.; Roth, L.; Lehmann, K.; Ungethum, U.; Kobold, S.; Graf, R.; et al. Attenuation of peripheral serotonin inhibits tumor growth and enhances immune checkpoint blockade therapy in murine tumor models. Sci. Transl. Med. 2021, 13, eabc8188. [Google Scholar] [CrossRef]
- Tu, Y.; Yao, S.; Chen, Q.; Li, W.; Song, Y.; Zhang, P. 5-Hydroxytryptamine activates a 5-HT/c-Myc/SLC6A4 signaling loop in non-small cell lung cancer. Biochim. Biophys. Acta Gen. Subj. 2022, 1866, 130093. [Google Scholar] [CrossRef]
- Dizeyi, N.; Hedlund, P.; Bjartell, A.; Tinzl, M.; Austild-Tasken, K.; Abrahamsson, P.A. Serotonin activates MAP kinase and PI3K/Akt signaling pathways in prostate cancer cell lines. Urol. Oncol. 2011, 29, 436–445. [Google Scholar] [CrossRef] [PubMed]
- Corvino, A.; Fiorino, F.; Severino, B.; Saccone, I.; Frecentese, F.; Perissutti, E.; Di Vaio, P.; Santagada, V.; Caliendo, G.; Magli, E. The Role of 5-HT1A Receptor in Cancer as a New Opportunity in Medicinal Chemistry. Curr. Med. Chem. 2018, 25, 3214–3227. [Google Scholar] [CrossRef] [PubMed]
- Zhu, P.; Lu, T.; Chen, Z.; Liu, B.; Fan, D.; Li, C.; Wu, J.; He, L.; Zhu, X.; Du, Y.; et al. 5-hydroxytryptamine produced by enteric serotonergic neurons initiates colorectal cancer stem cell self-renewal and tumorigenesis. Neuron 2022, 110, 2268–2282.e4. [Google Scholar] [CrossRef] [PubMed]
- Koh, S.J.; Kim, J.M.; Kim, I.K.; Kim, N.; Jung, H.C.; Song, I.S.; Kim, J.S. Fluoxetine inhibits NF-kappaB signaling in intestinal epithelial cells and ameliorates experimental colitis and colitis-associated colon cancer in mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2011, 301, G9–G19. [Google Scholar] [CrossRef]
- Pan, J.; Xu, Y.; Song, H.; Zhou, X.; Yao, Z.; Ji, G. Extracts of Zuo Jin Wan, a traditional Chinese medicine, phenocopies 5-HTR1D antagonist in attenuating Wnt/beta-catenin signaling in colorectal cancer cells. BMC Complement. Altern. Med. 2017, 17, 506. [Google Scholar] [CrossRef]
- Mao, L.; Xin, F.; Ren, J.; Xu, S.; Huang, H.; Zha, X.; Wen, X.; Gu, G.; Yang, G.; Cheng, Y.; et al. 5-HT2B-mediated serotonin activation in enterocytes suppresses colitis-associated cancer initiation and promotes cancer progression. Theranostics 2022, 12, 3928–3945. [Google Scholar] [CrossRef]
- Li, T.; Wei, L.; Zhang, X.; Fu, B.; Zhou, Y.; Yang, M.; Cao, M.; Chen, Y.; Tan, Y.; Shi, Y.; et al. Serotonin Receptor HTR2B Facilitates Colorectal Cancer Metastasis via CREB1-ZEB1 Axis-Mediated Epithelial-Mesenchymal Transition. Mol. Cancer Res. 2024, 22, 538–554. [Google Scholar] [CrossRef]
- Lee, J.Y.; Park, S.; Park, E.J.; Pagire, H.S.; Pagire, S.H.; Choi, B.W.; Park, M.; Fang, S.; Ahn, J.H.; Oh, C.M. Inhibition of HTR2B-mediated serotonin signaling in colorectal cancer suppresses tumor growth through ERK signaling. Biomed. Pharmacother. 2024, 179, 117428. [Google Scholar] [CrossRef]
- Gurbuz, N.; Ashour, A.A.; Alpay, S.N.; Ozpolat, B. Down-regulation of 5-HT1B and 5-HT1D receptors inhibits proliferation, clonogenicity and invasion of human pancreatic cancer cells. PLoS ONE 2014, 9, e105245. [Google Scholar] [CrossRef]
- Wu, W.; Li, Q.; Zhu, Z.; Li, C.; Lu, P.; Zhou, X.; Huang, Y.; Liu, Y.; Wang, M.; Gong, J. HTR1D functions as a key target of HOXA10-AS/miR-340-3p axis to promote the malignant outcome of pancreatic cancer via PI3K-AKT signaling pathway. Int. J. Biol. Sci. 2022, 18, 3777–3794. [Google Scholar] [CrossRef]
- Jiang, S.H.; Li, J.; Dong, F.Y.; Yang, J.Y.; Liu, D.J.; Yang, X.M.; Wang, Y.H.; Yang, M.W.; Fu, X.L.; Zhang, X.X.; et al. Increased Serotonin Signaling Contributes to the Warburg Effect in Pancreatic Tumor Cells Under Metabolic Stress and Promotes Growth of Pancreatic Tumors in Mice. Gastroenterology 2017, 153, 277–291.e19. [Google Scholar] [CrossRef]
- Zuo, X.; Chen, Z.; Cai, J.; Gao, W.; Zhang, Y.; Han, G.; Pu, L.; Wu, Z.; You, W.; Qin, J.; et al. 5-Hydroxytryptamine Receptor 1D Aggravates Hepatocellular Carcinoma Progression Through FoxO6 in AKT-Dependent and Independent Manners. Hepatology 2019, 69, 2031–2047. [Google Scholar] [CrossRef]
- Tu, R.H.; Wu, S.Z.; Huang, Z.N.; Zhong, Q.; Ye, Y.H.; Zheng, C.H.; Xie, J.W.; Wang, J.B.; Lin, J.X.; Chen, Q.Y.; et al. Neurotransmitter Receptor HTR2B Regulates Lipid Metabolism to Inhibit Ferroptosis in Gastric Cancer. Cancer Res. 2023, 83, 3868–3885. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Sun, H.; Liu, Y.; Li, X.; Xu, B.; Li, L.; Jin, W. HTR1A Inhibits the Progression of Triple-Negative Breast Cancer via TGF-beta Canonical and Noncanonical Pathways. Adv. Sci. 2022, 9, e2105672. [Google Scholar] [CrossRef]
- Sola-Penna, M.; Paixao, L.P.; Branco, J.R.; Ochioni, A.C.; Albanese, J.M.; Mundim, D.M.; Baptista-de-Souza, D.; Figueiredo, C.P.; Coelho, W.S.; Marcondes, M.C.; et al. Serotonin activates glycolysis and mitochondria biogenesis in human breast cancer cells through activation of the Jak1/STAT3/ERK1/2 and adenylate cyclase/PKA, respectively. Br. J. Cancer 2020, 122, 194–208. [Google Scholar] [CrossRef] [PubMed]
- Cinar, V.; Hamurcu, Z.; Guler, A.; Nurdinov, N.; Ozpolat, B. Serotonin 5-HT7 receptor is a biomarker poor prognostic factor and induces proliferation of triple-negative breast cancer cells through FOXM1. Breast Cancer 2022, 29, 1106–1120. [Google Scholar] [CrossRef] [PubMed]
- Gautam, J.; Banskota, S.; Regmi, S.C.; Ahn, S.; Jeon, Y.H.; Jeong, H.; Kim, S.J.; Nam, T.G.; Jeong, B.S.; Kim, J.A. Tryptophan hydroxylase 1 and 5-HT(7) receptor preferentially expressed in triple-negative breast cancer promote cancer progression through autocrine serotonin signaling. Mol. Cancer 2016, 15, 75. [Google Scholar] [CrossRef]
- Zhou, J.; Geng, K.K.; Ping, F.F.; Gao, Y.Y.; Liu, L.; Feng, B.N. Cross-talk between 5-hydroxytryptamine and substance P in the melanogensis and apoptosis of B16F10 melanoma cells. Eur. J. Pharmacol. 2016, 775, 106–112. [Google Scholar] [CrossRef]
- Barzegar-Fallah, A.; Alimoradi, H.; Dunlop, J.L.; Torbati, E.; Baird, S.K. Serotonin type-3 receptor antagonists selectively kill melanoma cells through classical apoptosis, microtubule depolymerisation, ERK activation, and NF-kappaB downregulation. Cell Biol. Toxicol. 2023, 39, 1119–1135. [Google Scholar] [CrossRef]
- Fazeli, M.S. Synaptic plasticity: On the trail of the retrograde messenger. Trends Neurosci. 1992, 15, 115–117. [Google Scholar] [CrossRef]
- Haley, J.E. Gases as neurotransmitters. Essays Biochem. 1998, 33, 79–91. [Google Scholar] [CrossRef]
- Dudok, B.; Fan, L.Z.; Farrell, J.S.; Malhotra, S.; Homidan, J.; Kim, D.K.; Wenardy, C.; Ramakrishnan, C.; Li, Y.; Deisseroth, K.; et al. Retrograde endocannabinoid signaling at inhibitory synapses in vivo. Science 2024, 383, 967–970. [Google Scholar] [CrossRef] [PubMed]
- Getz, A.M.; Janes, T.A.; Visser, F.; Zaidi, W.; Syed, N.I. Neurotrophic factors and target-specific retrograde signaling interactions define the specificity of classical and neuropeptide cotransmitter release at identified Lymnaea synapses. Sci. Rep. 2020, 10, 13526. [Google Scholar] [CrossRef]
- Friebe, A.; Sandner, P.; Schmidtko, A. cGMP: A unique 2nd messenger molecule—Recent developments in cGMP research and development. Naunyn Schmiedebergs Arch. Pharmacol. 2020, 393, 287–302. [Google Scholar] [CrossRef]
- Ducrocq, C.; Blanchard, B.; Pignatelli, B.; Ohshima, H. Peroxynitrite: An endogenous oxidizing and nitrating agent. Cell Mol. Life Sci. 1999, 55, 1068–1077. [Google Scholar] [CrossRef] [PubMed]
- Somasundaram, V.; Basudhar, D.; Bharadwaj, G.; No, J.H.; Ridnour, L.A.; Cheng, R.Y.S.; Fujita, M.; Thomas, D.D.; Anderson, S.K.; McVicar, D.W.; et al. Molecular Mechanisms of Nitric Oxide in Cancer Progression, Signal Transduction, and Metabolism. Antioxid. Redox Signal 2019, 30, 1124–1143. [Google Scholar] [CrossRef]
- Thomas, D.D.; Wink, D.A. NOS2 as an Emergent Player in Progression of Cancer. Antioxid. Redox Signal 2017, 26, 963–965. [Google Scholar] [CrossRef]
- Reddy, T.P.; Glynn, S.A.; Billiar, T.R.; Wink, D.A.; Chang, J.C. Targeting Nitric Oxide: Say NO to Metastasis. Clin. Cancer Res. 2023, 29, 1855–1868. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Patino, R.; Avalos-Navarro, G.; Figuera, L.E.; Varela-Hernandez, J.J.; Bautista-Herrera, L.A.; Munoz-Valle, J.F.; Gallegos-Arreola, M.P. Influence of nitric oxide signaling mechanisms in cancer. Int. J. Immunopathol. Pharmacol. 2022, 36, 3946320221135454. [Google Scholar] [CrossRef]
- Motterlini, R.; Otterbein, L.E. The therapeutic potential of carbon monoxide. Nat. Rev. Drug Discov. 2010, 9, 728–743. [Google Scholar] [CrossRef] [PubMed]
- Tien Vo, T.T.; Vo, Q.C.; Tuan, V.P.; Wee, Y.; Cheng, H.C.; Lee, I.T. The potentials of carbon monoxide-releasing molecules in cancer treatment: An outlook from ROS biology and medicine. Redox Biol. 2021, 46, 102124. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, G.; Chen, X.; Chen, Z.; Tan, A.Y.; Lin, A.; Zhang, C.; Torres, L.K.; Bajrami, S.; Zhang, T.; et al. Low-dose carbon monoxide suppresses metastatic progression of disseminated cancer cells. Cancer Lett. 2022, 546, 215831. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Yang, Q.; Yong, J.; Fang, X.; Yang, Z.; Liu, Z.; Jiang, X.; Miao, W.; Li, X. Mitochondria targeted nanoparticles to generate oxygen and responsive-release of carbon monoxide for enhanced photogas therapy of cancer. Biomater. Sci. 2021, 9, 2709–2720. [Google Scholar] [CrossRef]
- Bi, J.; Witt, E.; McGovern, M.K.; Cafi, A.B.; Rosenstock, L.L.; Pearson, A.B.; Brown, T.J.; Karasic, T.B.; Absler, L.C.; Machkanti, S.; et al. Oral Carbon Monoxide Enhances Autophagy Modulation in Prostate, Pancreatic, and Lung Cancers. Adv. Sci. 2024, 11, e2308346. [Google Scholar] [CrossRef]
- Cui, Q.; Liang, X.L.; Wang, J.Q.; Zhang, J.Y.; Chen, Z.S. Therapeutic implication of carbon monoxide in drug resistant cancers. Biochem. Pharmacol. 2022, 201, 115061. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, P.; Parikh, R.Y.; Choi, S.; Tran, D.; Gooz, M.; Hedley, Z.T.; Kim, D.S.; Pytel, D.; Kang, I.; Nadig, S.N.; et al. Carbon Monoxide Activates PERK-Regulated Autophagy to Induce Immunometabolic Reprogramming and Boost Antitumor T-cell Function. Cancer Res. 2022, 82, 1969–1990. [Google Scholar] [CrossRef]
- Li, Z.; Wang, Y.; Liu, M.; Pan, Y.; Ni, Z.; Min, Q.; Wang, B.; Ke, H.; Ji, X. Reactive Oxygen Species-Activated Metal-Free Carbon Monoxide Prodrugs for Targeted Cancer Treatment. J. Med. Chem. 2023, 66, 14583–14596. [Google Scholar] [CrossRef]
- Mechoulam, R.; Parker, L.A. The endocannabinoid system and the brain. Annu. Rev. Psychol. 2013, 64, 21–47. [Google Scholar] [CrossRef]
- Munro, S.; Thomas, K.L.; Abu-Shaar, M. Molecular characterization of a peripheral receptor for cannabinoids. Nature 1993, 365, 61–65. [Google Scholar] [CrossRef]
- Lutz, B. Neurobiology of cannabinoid receptor signaling. Dialogues Clin. Neurosci. 2020, 22, 207–222. [Google Scholar] [CrossRef]
- Cristino, L.; Bisogno, T.; Di Marzo, V. Cannabinoids and the expanded endocannabinoid system in neurological disorders. Nat. Rev. Neurol. 2020, 16, 9–29. [Google Scholar] [CrossRef] [PubMed]
- Chakravarti, B.; Ravi, J.; Ganju, R.K. Cannabinoids as therapeutic agents in cancer: Current status and future implications. Oncotarget 2014, 5, 5852–5872. [Google Scholar] [CrossRef] [PubMed]
- Melck, D.; De Petrocellis, L.; Orlando, P.; Bisogno, T.; Laezza, C.; Bifulco, M.; Di Marzo, V. Suppression of nerve growth factor Trk receptors and prolactin receptors by endocannabinoids leads to inhibition of human breast and prostate cancer cell proliferation. Endocrinology 2000, 141, 118–126. [Google Scholar] [CrossRef]
- Turgut, N.H.; Armagan, G.; Kasapligil, G.; Erdogan, M.A. Anti-cancer effects of selective cannabinoid agonists in pancreatic and breast cancer cells. Bratisl. Lek. Listy 2022, 123, 813–821. [Google Scholar] [CrossRef]
- Bettiga, A.; Aureli, M.; Colciago, G.; Murdica, V.; Moschini, M.; Luciano, R.; Canals, D.; Hannun, Y.; Hedlund, P.; Lavorgna, G.; et al. Bladder cancer cell growth and motility implicate cannabinoid 2 receptor-mediated modifications of sphingolipids metabolism. Sci. Rep. 2017, 7, 42157. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; He, Z.; Chen, N.; Cho, Y.Y.; Zhu, F.; Lu, C.; Ma, W.Y.; Bode, A.M.; Dong, Z. 2-Arachidonoylglycerol stimulates activator protein-1-dependent transcriptional activity and enhances epidermal growth factor-induced cell transformation in JB6 P+ cells. J. Biol. Chem. 2005, 280, 26735–26742. [Google Scholar] [CrossRef]
- Xiong, X.; Chen, S.; Shen, J.; You, H.; Yang, H.; Yan, C.; Fang, Z.; Zhang, J.; Cai, X.; Dong, X.; et al. Cannabis suppresses antitumor immunity by inhibiting JAK/STAT signaling in T cells through CNR2. Signal Transduct. Target. Ther. 2022, 7, 99. [Google Scholar] [CrossRef]
- Marder, E. Neuromodulation of neuronal circuits: Back to the future. Neuron 2012, 76, 1–11. [Google Scholar] [CrossRef]
- van den Pol, A.N. Neuropeptide transmission in brain circuits. Neuron 2012, 76, 98–115. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, M.L.; Rodriguez, F.D.; Covenas, R. Neuropeptide Y Peptide Family and Cancer: Antitumor Therapeutic Strategies. Int. J. Mol. Sci. 2023, 24, 9962. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.T.; Li, Y.Z.; Sun, X.Q.; Chen, Q.Q.; Huang, S.F.; Lin, S.; Cai, S.Q. Update on the Role of Neuropeptide Y and Other Related Factors in Breast Cancer and Osteoporosis. Front. Endocrinol. 2021, 12, 705499. [Google Scholar] [CrossRef] [PubMed]
- Pascetta, S.A.; Kirsh, S.M.; Cameron, M.; Uniacke, J. Pharmacological inhibition of neuropeptide Y receptors Y1 and Y5 reduces hypoxic breast cancer migration, proliferation, and signaling. BMC Cancer 2023, 23, 494. [Google Scholar] [CrossRef]
- Medeiros, P.J.; Jackson, D.N. Neuropeptide Y Y5-receptor activation on breast cancer cells acts as a paracrine system that stimulates VEGF expression and secretion to promote angiogenesis. Peptides 2013, 48, 106–113. [Google Scholar] [CrossRef]
- Ding, Y.; Lee, M.; Gao, Y.; Bu, P.; Coarfa, C.; Miles, B.; Sreekumar, A.; Creighton, C.J.; Ayala, G. Neuropeptide Y nerve paracrine regulation of prostate cancer oncogenesis and therapy resistance. Prostate 2021, 81, 58–71. [Google Scholar] [CrossRef]
- Dietrich, P.; Wormser, L.; Fritz, V.; Seitz, T.; De Maria, M.; Schambony, A.; Kremer, A.E.; Gunther, C.; Itzel, T.; Thasler, W.E.; et al. Molecular crosstalk between Y5 receptor and neuropeptide Y drives liver cancer. J. Clin. Investig. 2020, 130, 2509–2526. [Google Scholar] [CrossRef]
- Cao, Y.; Ge, X.; Zhu, X.; Han, Y.; Wang, P.; Akakuru, O.U.; Wu, A.; Li, J. Transformable Neuropeptide Prodrug with Tumor Microenvironment Responsiveness for Tumor Growth and Metastasis Inhibition of Triple-Negative Breast Cancer. Adv. Sci. 2023, 10, e2300545. [Google Scholar] [CrossRef]
- Ye, Y.; Xie, T.; Amit, M. Targeting the Nerve-Cancer Circuit. Cancer Res. 2023, 83, 2445–2447. [Google Scholar] [CrossRef]
- Padmanaban, V.; Keller, I.; Seltzer, E.S.; Ostendorf, B.N.; Kerner, Z.; Tavazoie, S.F. Neuronal substance P drives metastasis through an extracellular RNA-TLR7 axis. Nature 2024, 633, 207–215. [Google Scholar] [CrossRef]
- Munoz, M.; Covenas, R.; Esteban, F.; Redondo, M. The substance P/NK-1 receptor system: NK-1 receptor antagonists as anti-cancer drugs. J. Biosci. 2015, 40, 441–463. [Google Scholar] [CrossRef]
- Covenas, R.; Munoz, M. Involvement of the Substance P/Neurokinin-1 Receptor System in Cancer. Cancers 2022, 14, 3539. [Google Scholar] [CrossRef]
- Bosnjak, S.M.; Gralla, R.J.; Schwartzberg, L. Prevention of chemotherapy-induced nausea: The role of neurokinin-1 (NK(1)) receptor antagonists. Support. Care Cancer 2017, 25, 1661–1671. [Google Scholar] [CrossRef]
- Gesmundo, I.; Pedrolli, F.; Cai, R.; Sha, W.; Schally, A.V.; Granata, R. Growth hormone-releasing hormone and cancer. Rev. Endocr. Metab. Disord. 2024, 26, 443–456. [Google Scholar] [CrossRef] [PubMed]
- Mehrabadi, S.; Velayati, M.; Zafari, N.; Hassanian, S.M.; Mobarhan, M.G.; Ferns, G.; Khazaei, M.; Avan, A. Growth-hormone-releasing Hormone as a Prognostic Biomarker and Therapeutic Target in Gastrointestinal Cancer. Curr. Cancer Drug Targets 2023, 23, 346–353. [Google Scholar] [CrossRef]
- Xiong, S.Y.; Wen, H.Z.; Dai, L.M.; Lou, Y.X.; Wang, Z.Q.; Yi, Y.L.; Yan, X.J.; Wu, Y.R.; Sun, W.; Chen, P.H.; et al. A brain-tumor neural circuit controls breast cancer progression in mice. J. Clin. Investig. 2023, 133, e167725. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Li, S. Role of CRH in colitis and colitis-associated cancer: A combinative result of central and peripheral effects? Front. Endocrinol. 2024, 15, 1363748. [Google Scholar] [CrossRef] [PubMed]
- Balood, M.; Ahmadi, M.; Eichwald, T.; Ahmadi, A.; Majdoubi, A.; Roversi, K.; Roversi, K.; Lucido, C.T.; Restaino, A.C.; Huang, S.; et al. Nociceptor neurons affect cancer immunosurveillance. Nature 2022, 611, 405–412. [Google Scholar] [CrossRef]
- Zhang, Y.; Lin, C.; Liu, Z.; Sun, Y.; Chen, M.; Guo, Y.; Liu, W.; Zhang, C.; Chen, W.; Sun, J.; et al. Cancer cells co-opt nociceptive nerves to thrive in nutrient-poor environments and upon nutrient-starvation therapies. Cell Metab. 2022, 34, 1999–2017.e1910. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).