Field-Gated Anion Transport in Nanoparticle Superlattices Controlled by Charge Density and Ion Geometry: Insights from Molecular Dynamics Simulations
Abstract
1. Introduction
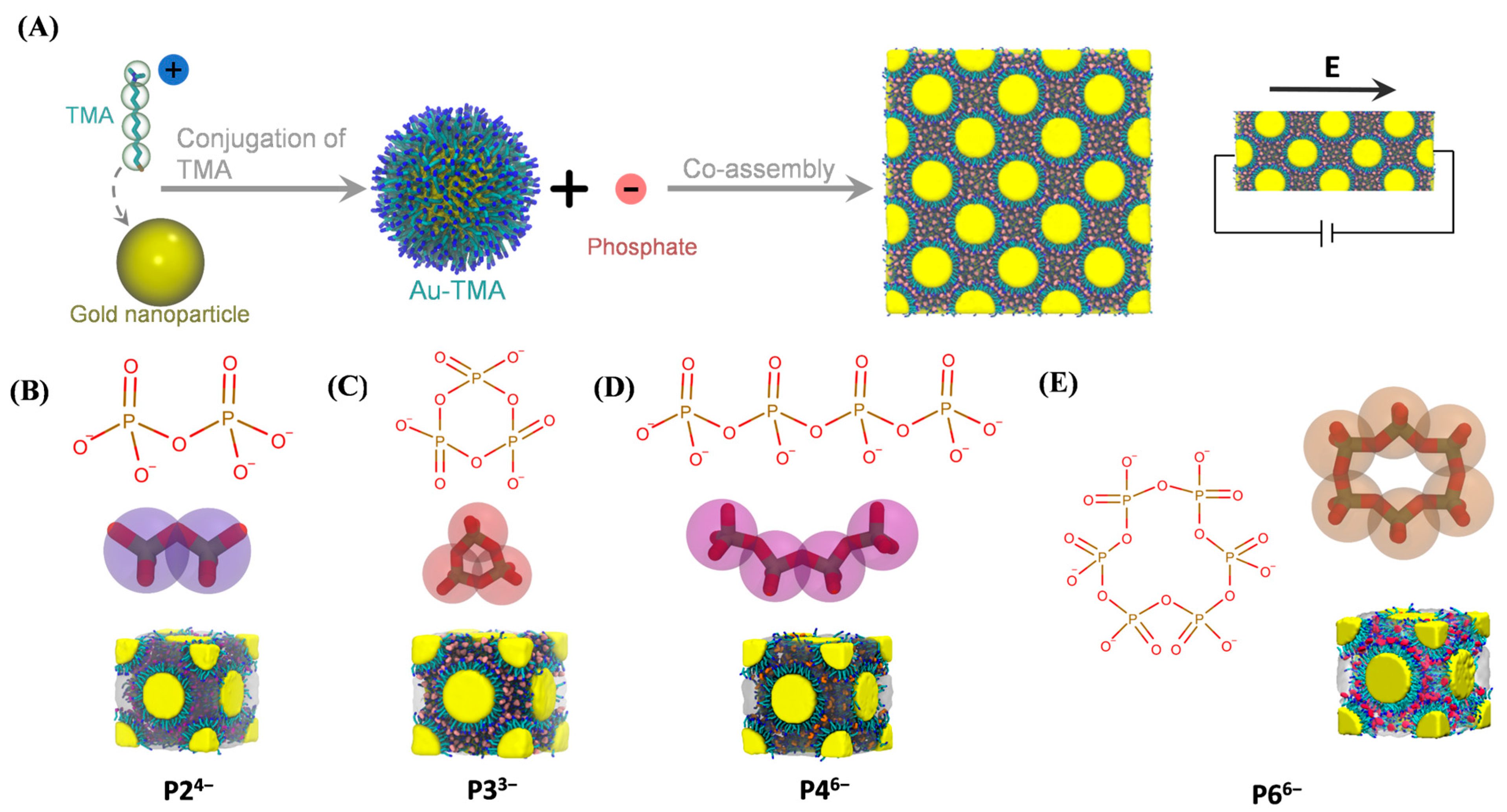
2. Models and Methods
3. Results and Discussion
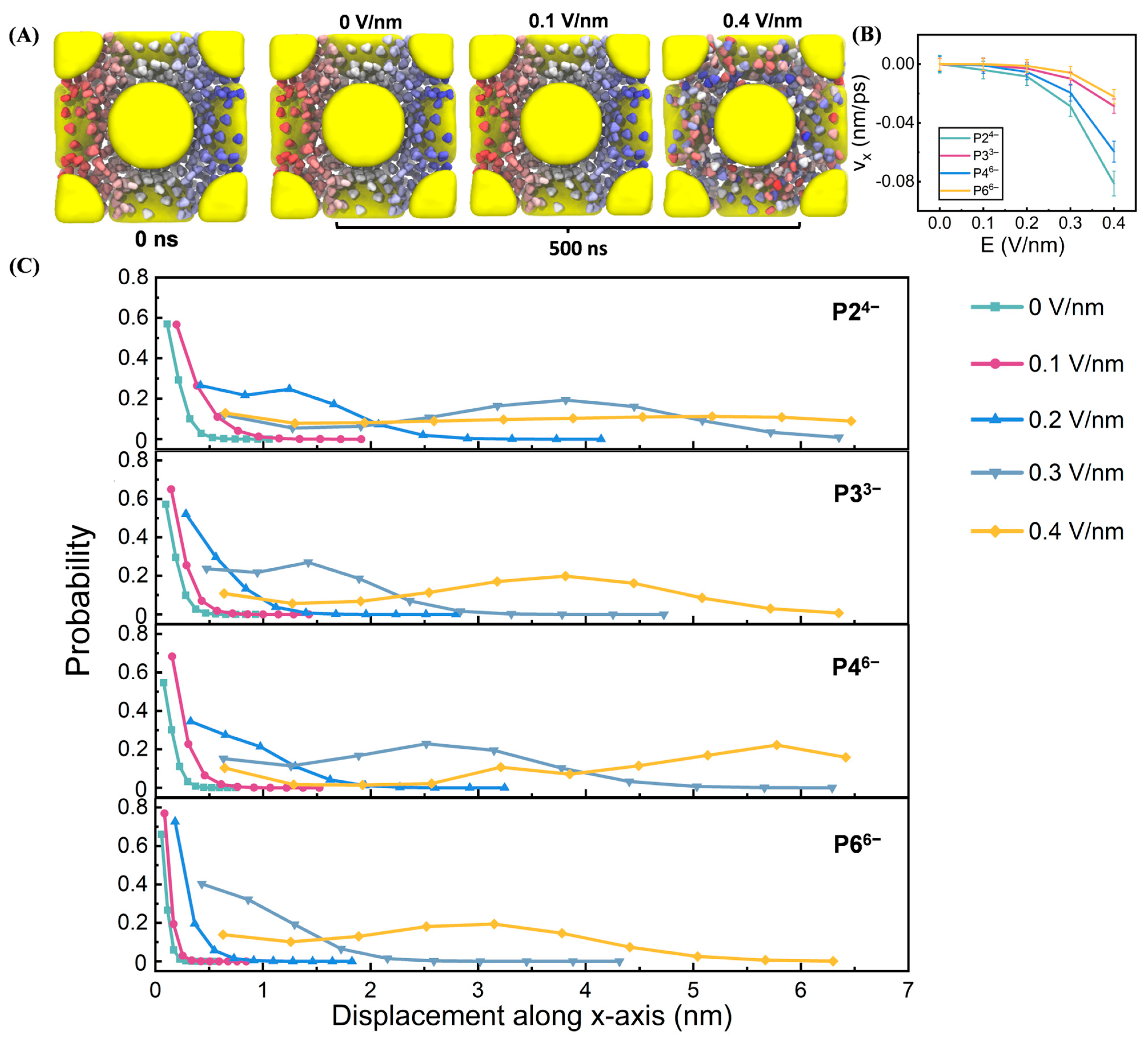
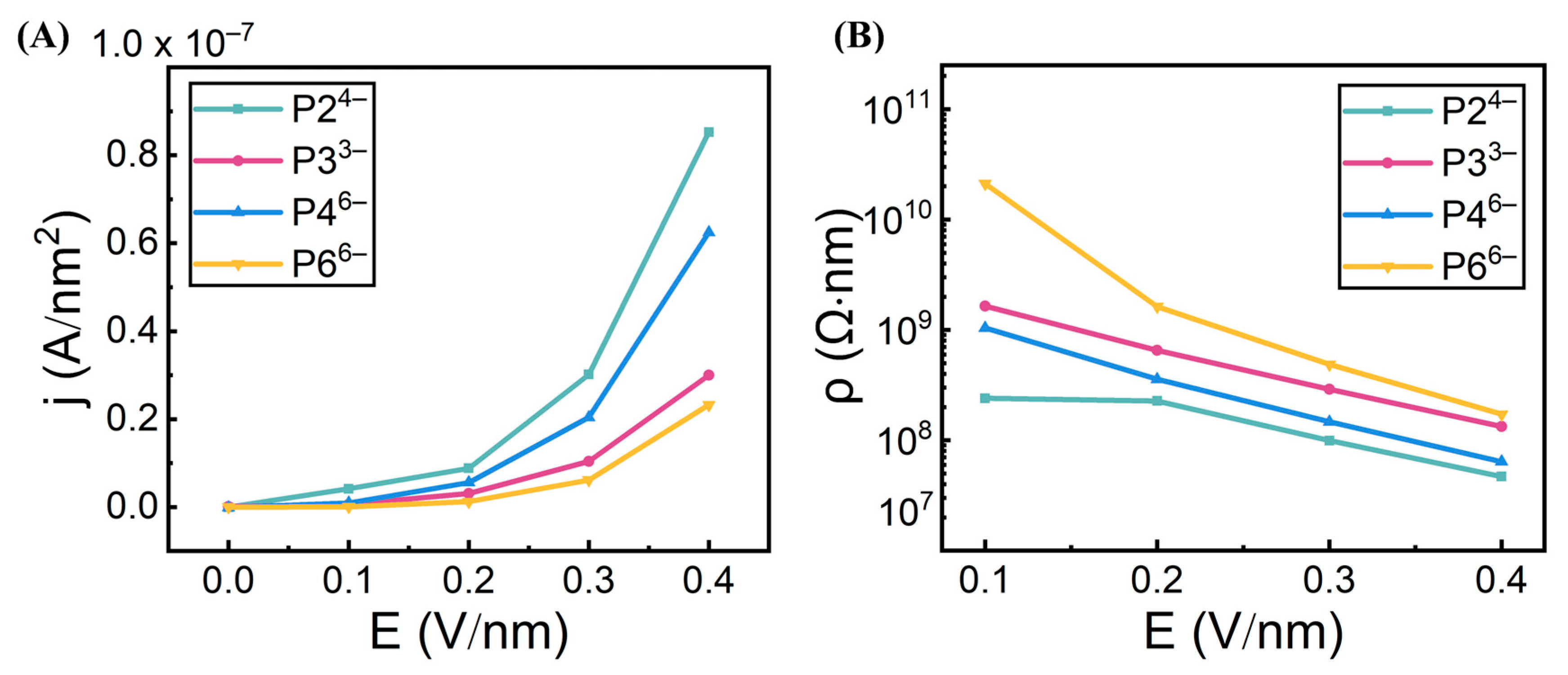
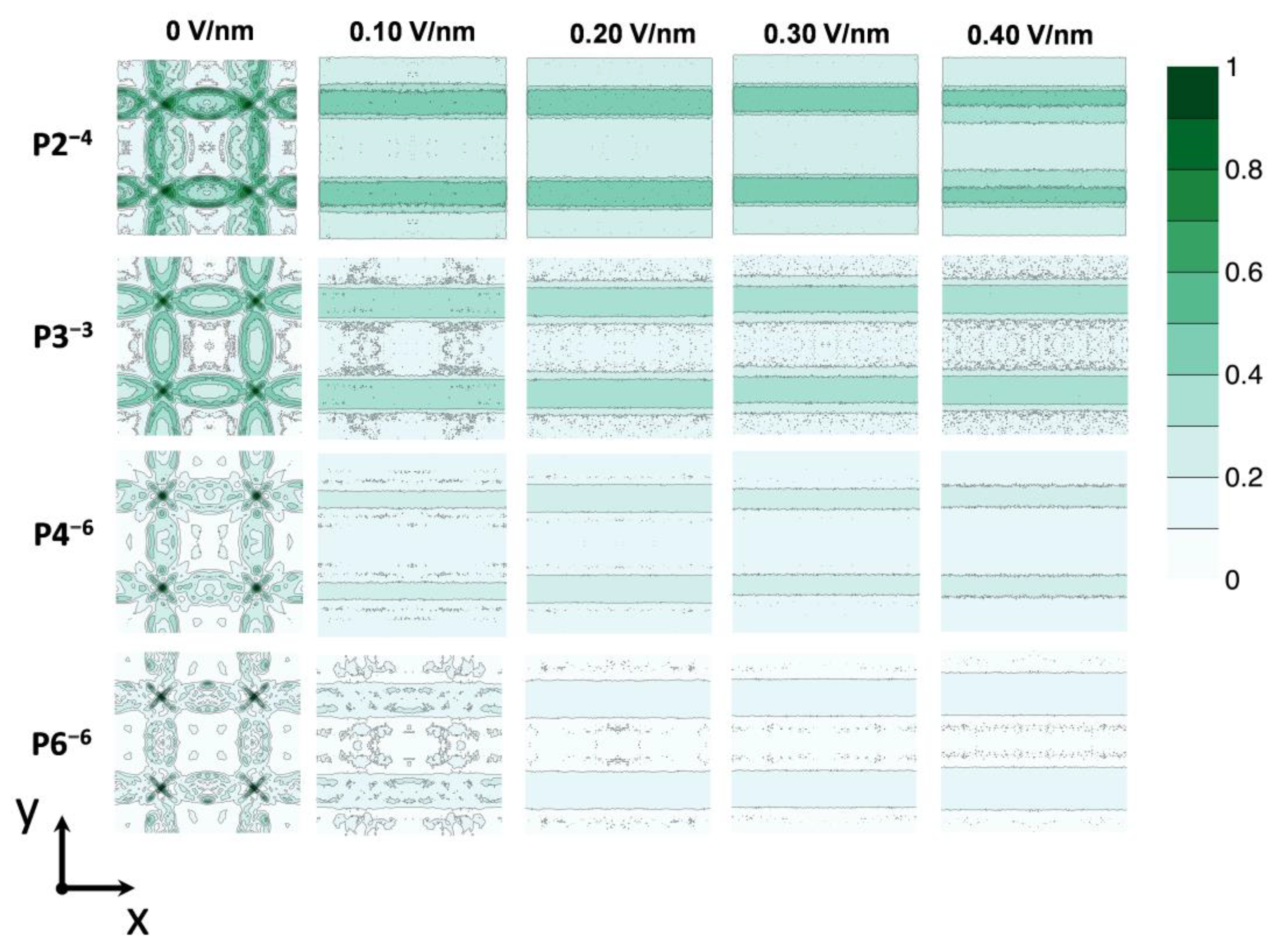
3.1. Electric Field-Dependent Conductivity
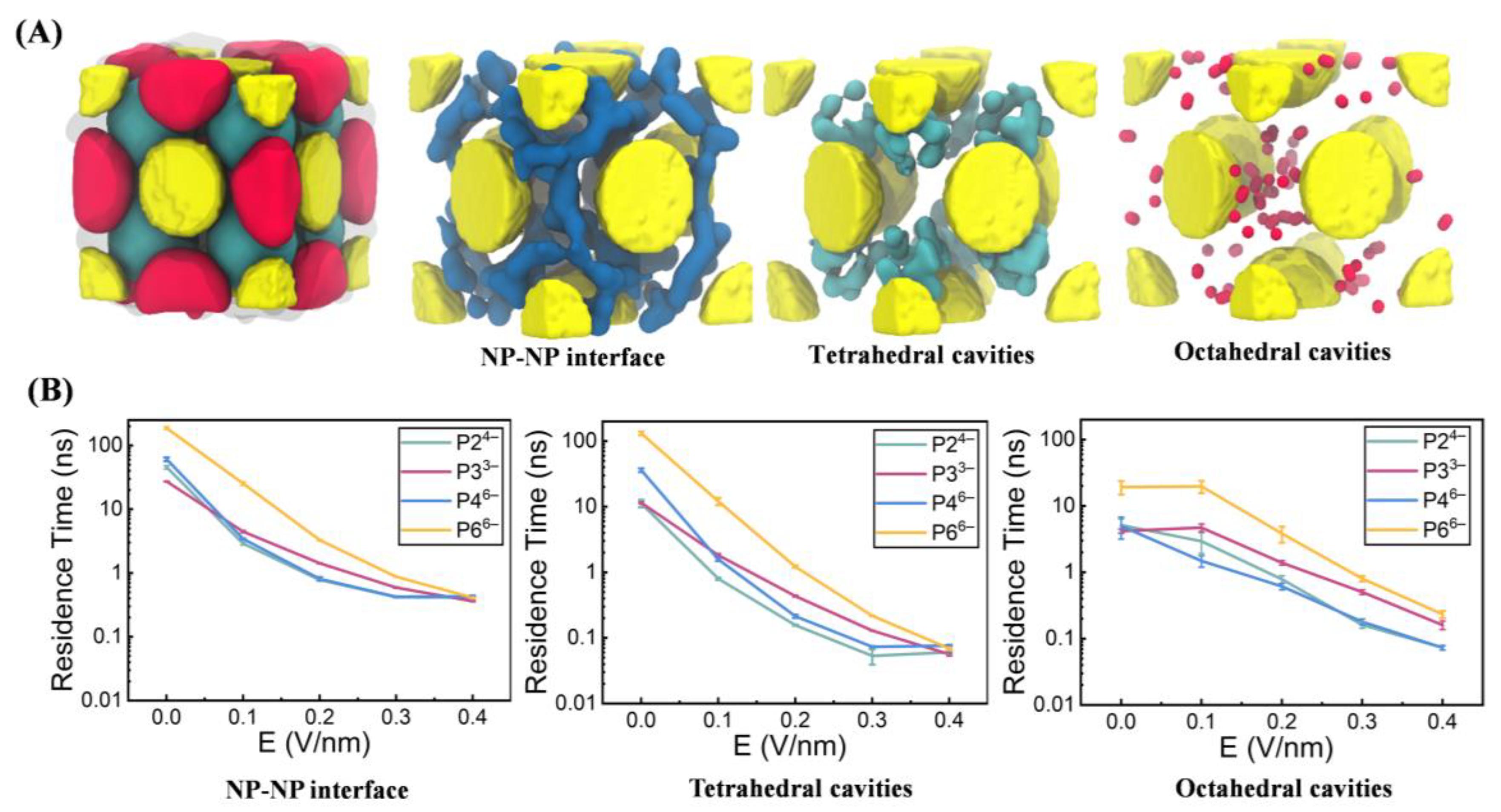
3.2. Compartment-Specific Anion Dynamics
3.3. Field-Driven Anion Redistribution
4. Broader Implications and Future Prospects
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Santos, P.J.; Gabrys, P.A.; Zornberg, L.Z.; Lee, M.S.; Macfarlane, R.J. Macroscopic materials assembled from nanoparticle superlattices. Nature 2021, 591, 586–591. [Google Scholar] [CrossRef]
- Li, D.; Chen, Q.; Chun, J.; Fichthorn, K.; De Yoreo, J.; Zheng, H. Nanoparticle Assembly and Oriented Attachment: Correlating Controlling Factors to the Resulting Structures. Chem. Rev. 2023, 123, 3127–3159. [Google Scholar] [CrossRef]
- Li, Y.; Stec, G.J.; Kim, H.K.; Thapa, S.; Zheng, S.-L.; McClelland, A.; Mason, J.A. Self-assembly of chiroptical ionic co-crystals from silver nanoclusters and organic macrocycles. Nat. Chem. 2025, 17, 169–176. [Google Scholar] [CrossRef]
- Zhou, Y.; Shaukat, A.; Seitsonen, J.; Rigoni, C.; Timonen, J.V.I.; Kostiainen, M.A. Protein Cage Directed Assembly of Binary Nanoparticle Superlattices. Adv. Sci. 2024, 11, 2408416. [Google Scholar] [CrossRef]
- Rütten, M.; Lang, L.; Wagler, H.; Lach, M.; Mucke, N.; Laugks, U.; Seuring, C.; Keller, T.F.; Stierle, A.; Ginn, H.M.; et al. Assembly of Differently Sized Supercharged Protein Nanocages into Superlattices for Construction of Binary Nanoparticle–Protein Materials. ACS Nano 2024, 18, 25325–25336. [Google Scholar] [CrossRef]
- Lu, B.; Vegso, K.; Micky, S.; Ritz, C.; Bodik, M.; Fedoryshyn, Y.M.; Siffalovic, P.; Stemmer, A. Tunable Subnanometer Gaps in Self-Assembled Monolayer Gold Nanoparticle Superlattices Enabling Strong Plasmonic Field Confinement. ACS Nano 2023, 17, 12774–12787. [Google Scholar] [CrossRef] [PubMed]
- Qi, F.; Jeong, K.-J.; Gong, J.; Tang, Z. Modulation of Nano-superstructures and Their Optical Properties. Acc. Chem. Res. 2022, 55, 2425–2438. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; LaCour, R.A.; Cai, Y.-Y.; Xu, J.; Rosen, D.J.; Zhang, Y.; Kagan, C.R.; Glotzer, S.C.; Murray, C.B. Self-Assembly of Atomically Aligned Nanoparticle Superlattices from Pt–Fe3O4 Heterodimer Nanoparticles. J. Am. Chem. Soc. 2023, 145, 6280–6288. [Google Scholar] [CrossRef]
- Si, K.J.; Chen, Y.; Shi, Q.; Cheng, W. Nanoparticle Superlattices: The Roles of Soft Ligands. Adv. Sci. 2018, 5, 1700179. [Google Scholar] [CrossRef] [PubMed]
- Nayak, B.P.; Zhang, H.; Bu, W.; Ocko, B.M.; Travesset, A.; Vaknin, D.; Mallapragada, S.K.; Wang, W. Ionic-like Superlattices by Charged Nanoparticles: A Step Toward Photonics Applications. ACS Appl. Nano Mater. 2024, 7, 3220–3228. [Google Scholar] [CrossRef]
- Boong, S.K.; Chong, C.; Lee, J.-K.; Ang, Z.Z.; Li, H.; Lee, H.K. Superlattice-based Plasmonic Catalysis: Concentrating Light at the Nanoscale to Drive Efficient Nitrogen-to-Ammonia Fixation at Ambient Conditions. Angew. Chem. Int. Ed. 2023, 62, e202216562. [Google Scholar] [CrossRef]
- Wang, J.; Peled, T.S.; Klajn, R. Photocleavable Anionic Glues for Light-Responsive Nanoparticle Aggregates. J. Am. Chem. Soc. 2023, 145, 4098–4108. [Google Scholar] [CrossRef]
- Bian, T.; Gardin, A.; Gemen, J.; Houben, L.; Perego, C.; Lee, B.; Elad, N.; Chu, Z.; Pavan, G.M.; Klajn, R. Electrostatic co-assembly of nanoparticles with oppositely charged small molecules into static and dynamic superstructures. Nat. Chem. 2021, 13, 940–949. [Google Scholar] [CrossRef]
- Thrasher, C.J.; Jia, F.; Yee, D.W.; Kubiak, J.M.; Wang, Y.; Lee, M.S.; Onoda, M.; Hart, A.J.; Macfarlane, R.J. Rationally Designing the Supramolecular Interfaces of Nanoparticle Superlattices with Multivalent Polymers. J. Am. Chem. Soc. 2024, 146, 11532–11541. [Google Scholar] [CrossRef] [PubMed]
- Coropceanu, I.; Janke, E.M.; Portner, J.; Haubold, D.; Nguyen, T.D.; Das, A.; Tanner, C.P.N.; Utterback, J.K.; Teitelbaum, S.W.; Hudson, M.H.; et al. Self-assembly of nanocrystals into strongly electronically coupled all-inorganic supercrystals. Science 2022, 375, 1422–1426. [Google Scholar] [CrossRef] [PubMed]
- Lionello, C.; Perego, C.; Gardin, A.; Klajn, R.; Pavan, G.M. Supramolecular Semiconductivity through Emerging Ionic Gates in Ion–Nanoparticle Superlattices. ACS Nano 2023, 17, 275–287. [Google Scholar] [CrossRef]
- Bonoli, S.F.; Missoni, L.L.; Perez Sirkin, Y.A.; Tagliazucchi, M. Nonohmic Ionic Conductivity and Bulk Current Rectification in Nanoparticle Superlattices. J. Phys. Chem. C 2024, 128, 10723–10732. [Google Scholar] [CrossRef]
- Huang, J.; Buratto, D.; Zhou, R. Probing the electrostatic aggregation of nanoparticles with oppositely charged molecular ions. Aggregate 2022, 4, e324. [Google Scholar] [CrossRef]
- Souza, P.C.T.; Alessandri, R.; Barnoud, J.; Thallmair, S.; Faustino, I.; Grünewald, F.; Patmanidis, I.; Abdizadeh, H.; Bruininks, B.M.H.; Wassenaar, T.A.; et al. Martini 3: A general purpose force field for coarse-grained molecular dynamics. Nat. Methods 2021, 18, 382–388. [Google Scholar] [CrossRef]
- Marrink, S.J.; Monticelli, L.; Melo, M.N.; Alessandri, R.; Tieleman, D.P.; Souza, P.C.T. Two decades of Martini: Better beads, broader scope. WIREs Comput. Mol. Sci. 2022, 13, e1620. [Google Scholar] [CrossRef]
- Grunewald, F.; Souza, P.C.T.; Marrink, S.J. The martini 3 ecosystem for coarse-grained simulations. Biophys. J. 2023, 122, 419a–420a. [Google Scholar] [CrossRef]
- Vanommeslaeghe, K.; Hatcher, E.; Acharya, C.; Kundu, S.; Zhong, S.; Shim, J.; Darian, E.; Guvench, O.; Lopes, P.; Vorobyov, I.; et al. CHARMM General Force Field: A Force Field for Drug-Like Molecules Compatible with the CHARMM All-Atom Additive Biological Force Fields. J. Comput. Chem. 2010, 31, 671–690. [Google Scholar] [CrossRef] [PubMed]
- Vanommeslaeghe, K.; MacKerell, A.D., Jr. Automation of the CHARMM General Force Field (CGenFF) I: Bond Perception and Atom Typing. J. Chem. Inf. Model. 2012, 52, 3144–3154. [Google Scholar] [CrossRef]
- Hess, B.; Kutzner, C.; van der Spoel, D.; Lindahl, E. GROMACS 4: Algorithms for highly efficient, load-balanced, and scalable molecular simulation. J. Chem. Theory Comput. 2008, 4, 435–447. [Google Scholar] [CrossRef]
- Bussi, G.; Donadio, D.; Parrinello, M. Canonical sampling through velocity rescaling. J. Chem. Phys. 2007, 126, 014101. [Google Scholar] [CrossRef]
- Parrinello, M.; Rahman, A. Polymorphic transitions in single crystals: A new molecular dynamics method. J. Appl. Phys. 1981, 52, 7182–7190. [Google Scholar] [CrossRef]
- Darden, T.; York, D.; Pedersen, L. Particle mesh Ewald: An N⋅log(N) method for Ewald sums in large systems. J. Chem. Phys. 1993, 98, 10089–10092. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. Model. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Wu, C.; Liu, S.; Zhang, S.; Yang, Z. Molcontroller: A VMD Graphical User Interface Featuring Molecule Manipulation. J. Chem. Inf. Model. 2020, 60, 5126–5131. [Google Scholar] [CrossRef]
- Naughton, F.B.; Alibay, I.; Barnoud, J.; Barreto-Ojeda, E.; Beckstein, O.; Bouysset, C.; Cohen, O.; Gowers, R.J.; MacDermott-Opeskin, H.; Matta, M.; et al. MDAnalysis 2.0 and beyond: Fast and interoperable, community driven simulation analysis. Biophys. J. 2022, 121, 272a–273a. [Google Scholar] [CrossRef]
- Michaud-Agrawal, N.; Denning, E.J.; Woolf, T.B.; Beckstein, O. MDAnalysis: A toolkit for the analysis of molecular dynamics simulations. J. Comput. Chem. 2011, 32, 2319–2327. [Google Scholar] [CrossRef] [PubMed]
- Schoch, R.B.; Han, J.; Renaud, P. Transport Phenomena in Nanofluidics. Rev. Mod. Phys. 2008, 80, 839–883. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, Y.; Huang, J.; Yang, Z.; Jiang, Y.; Zhou, R. Field-Gated Anion Transport in Nanoparticle Superlattices Controlled by Charge Density and Ion Geometry: Insights from Molecular Dynamics Simulations. Biomolecules 2025, 15, 1427. https://doi.org/10.3390/biom15101427
Su Y, Huang J, Yang Z, Jiang Y, Zhou R. Field-Gated Anion Transport in Nanoparticle Superlattices Controlled by Charge Density and Ion Geometry: Insights from Molecular Dynamics Simulations. Biomolecules. 2025; 15(10):1427. https://doi.org/10.3390/biom15101427
Chicago/Turabian StyleSu, Yuexin, Jianxiang Huang, Zaixing Yang, Yangwei Jiang, and Ruhong Zhou. 2025. "Field-Gated Anion Transport in Nanoparticle Superlattices Controlled by Charge Density and Ion Geometry: Insights from Molecular Dynamics Simulations" Biomolecules 15, no. 10: 1427. https://doi.org/10.3390/biom15101427
APA StyleSu, Y., Huang, J., Yang, Z., Jiang, Y., & Zhou, R. (2025). Field-Gated Anion Transport in Nanoparticle Superlattices Controlled by Charge Density and Ion Geometry: Insights from Molecular Dynamics Simulations. Biomolecules, 15(10), 1427. https://doi.org/10.3390/biom15101427





