Inhibition of the RAC/PAK Signaling Axis Enhances the Potency of MAPK Cascade Inhibitors Against Uveal Melanoma
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Reagents
2.2. Western Blotting and Antibodies
2.3. Rac1 Activity Assay
2.4. Cell Number Comparison in Treated Cultures
2.5. EdU-Incorporation Assay
2.6. Quantitative Reverse Transcription PCR (qRT-PCR)
2.7. Animal Experimentation
2.8. Comparison of Intracellular GTP Content
2.9. Statistical Methods
3. Results
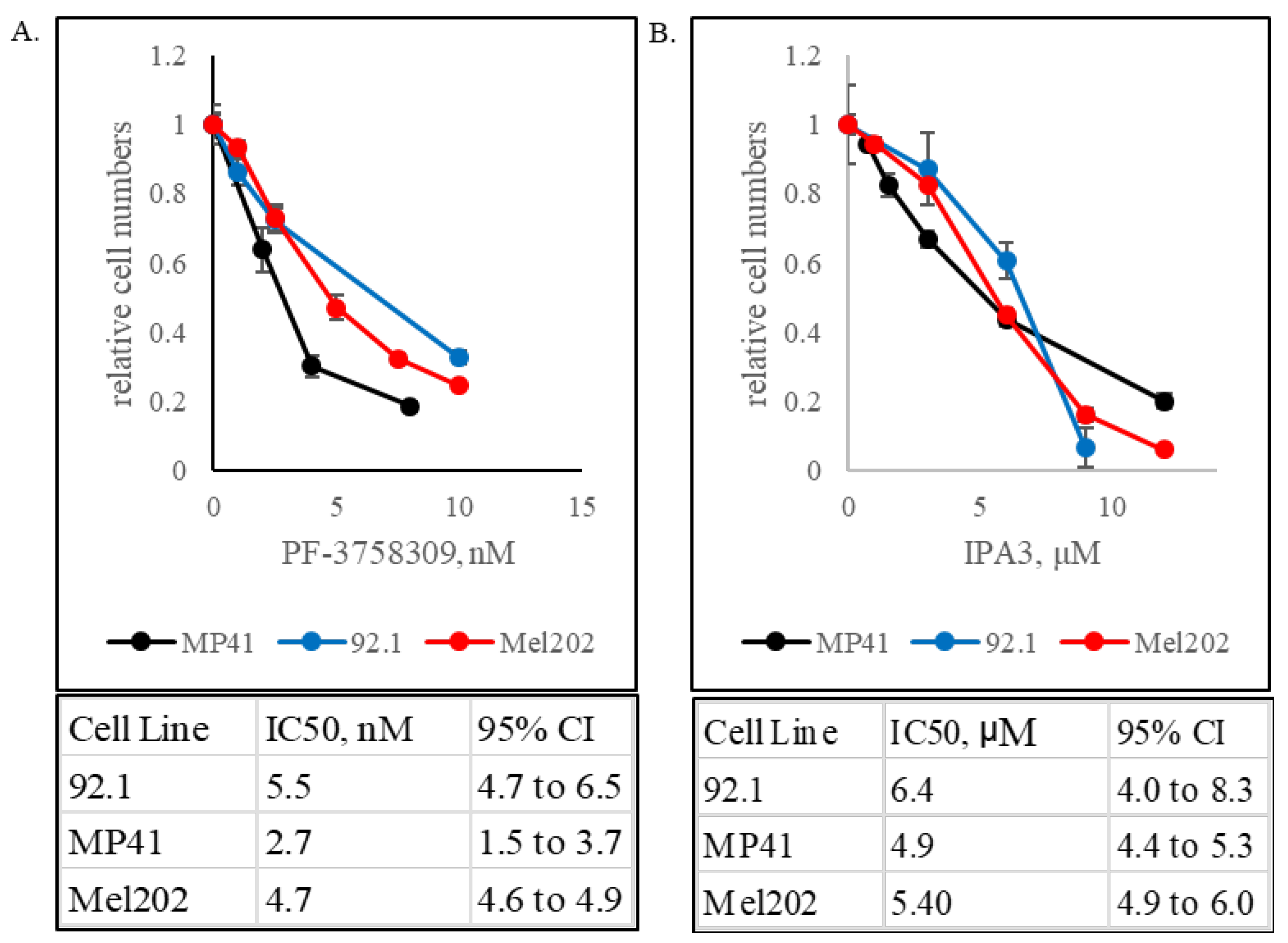
3.1. GNAQ- and GNA11-Mutant Uveal Melanomas Are Vulnerable to PAK Inhibition
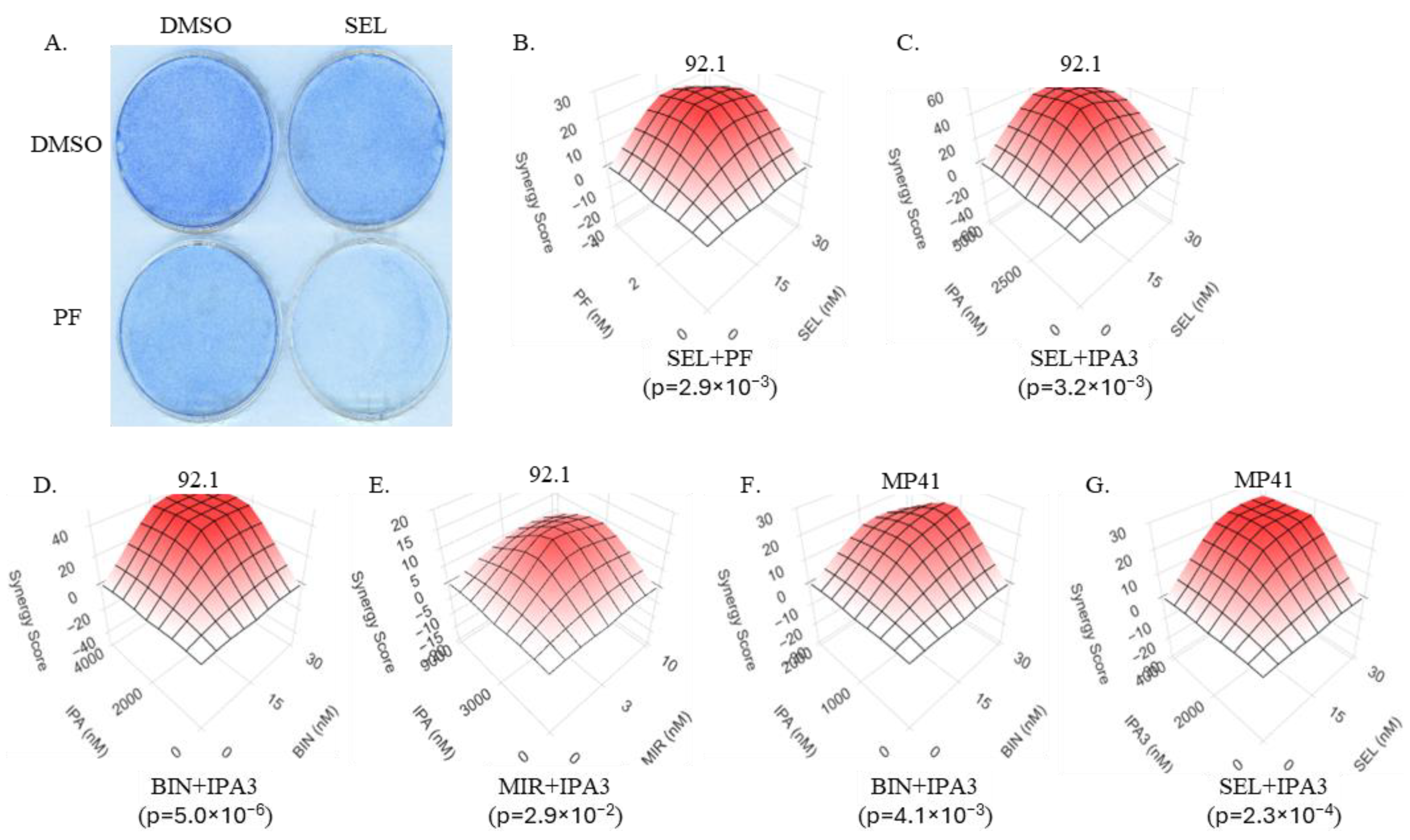
3.2. Inhibitors of the RAC/PAK Signaling Axis Synergize with the MEK Inhibitors in Uveal Melanoma Cells
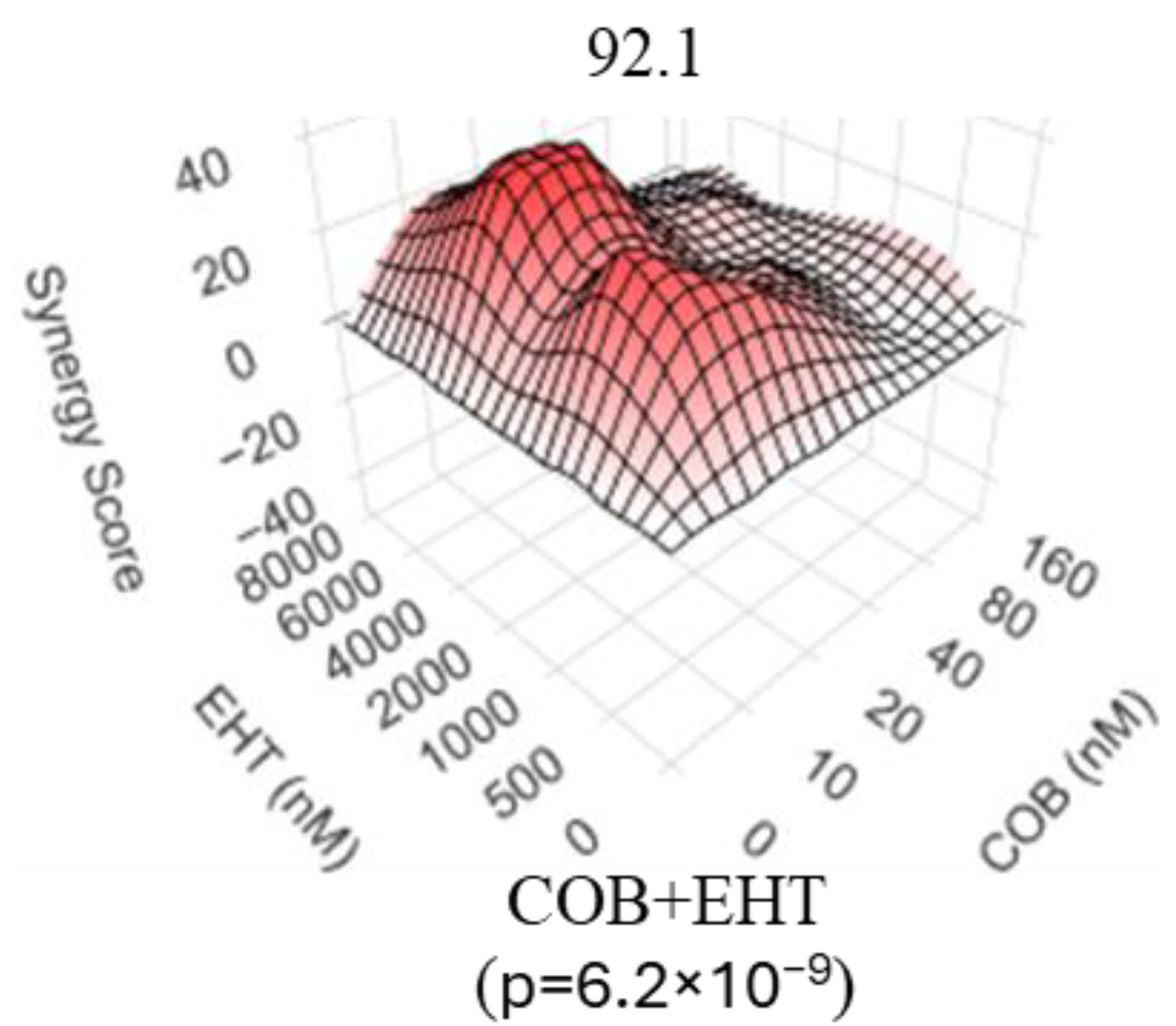
3.3. Inhibitors of GTP Biosynthesis Synergize with MEK Inhibitors in Uveal Melanoma Cells
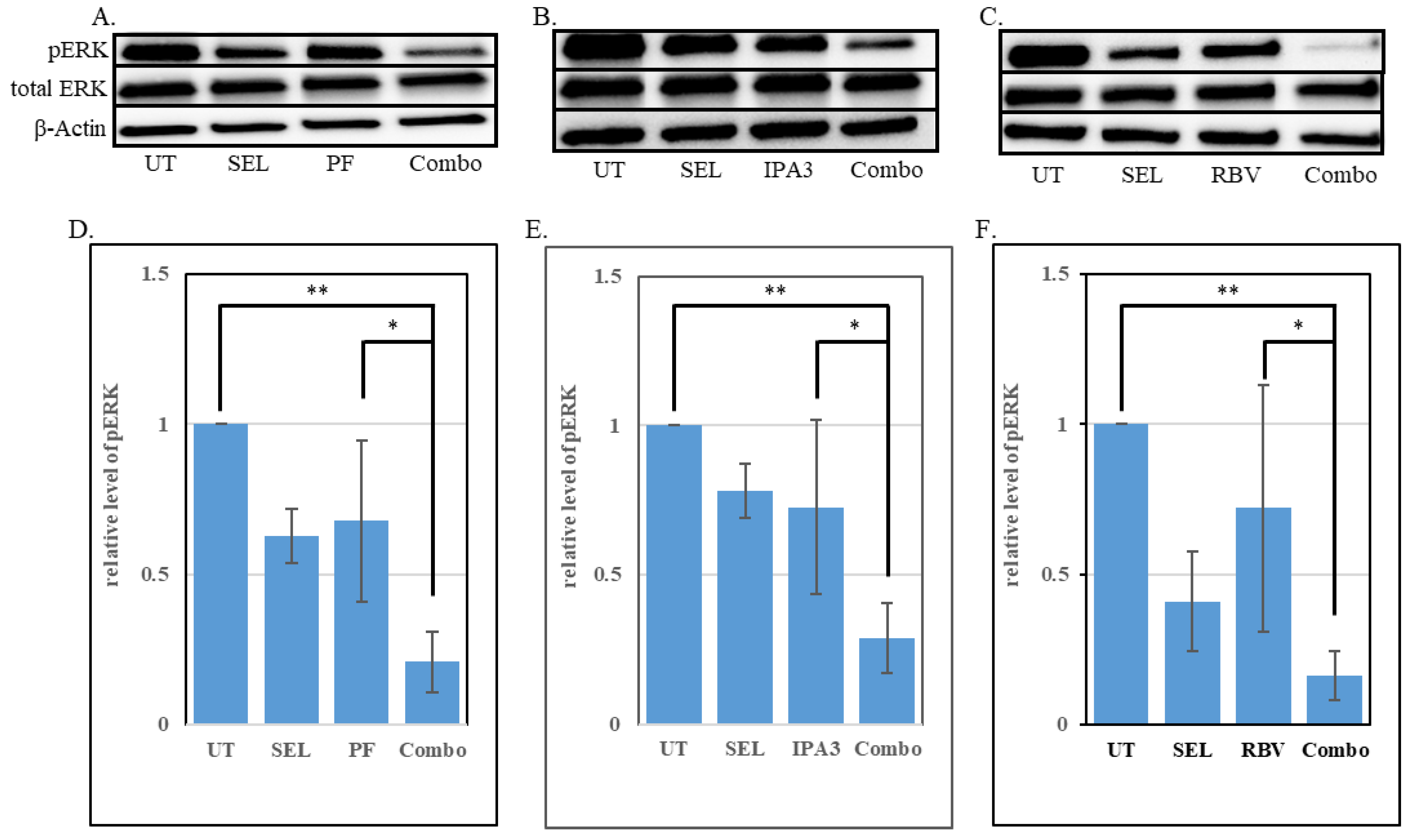
3.4. Enhanced Suppression of the MAPK Signaling Cascade by Combined Treatments
3.5. An IMPDHi and MEKi Combination Reduces the Expression of EGR1 in Uveal Melanoma Cells
3.6. An IMPDH Inhibitor Enhances the Potency of a MEK Inhibitor Against Uveal Melanoma In Vivo
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BIN | Binimetinib. |
| COB | Cobimetinib. |
| EHT | EHT1864. |
| GPCR | G-protein coupled receptor. |
| IMPDHi | Inhibitor of inosine monophosphate dehydrogenase (IMPDH). |
| MAPK | Mitogen-activated protein kinase |
| MEKi | Inhibitor of mitogen-activated protein kinase kinase (MEK). |
| MIR | Mirdametinib |
| MIZ | Mizoribine. |
| PAKi | Inhibitor of p21-activated protein kinase (PAK). |
| PF | PF-3758309. |
| RACi | Inhibitor of Ras-related C3 botulinum toxin substrate (RAC). |
| RBV | Ribavirin. |
| SEL | Selumetinib. |
| UM | Uveal melanoma |
References
- Rodrigues, M.; Koning, L.; Coupland, S.E.; Jochemsen, A.G.; Marais, R.; Stern, M.H.; Valente, A.; Barnhill, R.; Cassoux, N.; Evans, A.; et al. So Close, yet so Far: Discrepancies between Uveal and Other Melanomas. A Position Paper from UM Cure 2020. Cancers 2019, 11, 1032. [Google Scholar] [CrossRef]
- Blum, E.S.; Yang, J.; Komatsubara, K.M.; Carvajal, R.D. Clinical Management of Uveal and Conjunctival Melanoma. Oncology 2016, 30, 29–32, 34–43, 48. [Google Scholar] [PubMed]
- Rao, P.K.; Barker, C.; Coit, D.G.; Joseph, R.W.; Materin, M.; Rengan, R.; Sosman, J.; Thompson, J.A.; Albertini, M.R.; Boland, G.; et al. NCCN Guidelines Insights: Uveal Melanoma, Version 1.2019. J. Natl. Compr. Canc. Netw. 2020, 18, 120–131. [Google Scholar] [PubMed]
- Carvajal, R.D.; Sacco, J.J.; Jager, M.J.; Eschelman, D.J.; Olofsson Bagge, R.; Harbour, J.W.; Chieng, N.D.; Patel, S.P.; Joshua, A.M.; Piperno-Neumann, S. Advances in the clinical management of uveal melanoma. Nat. Rev. Clin. Oncol. 2023, 20, 99–115. [Google Scholar] [CrossRef]
- Chen, Y.N.; Wang, Y.N.; Chen, M.X.; Zhang, K.; Chen, R.T.; Fang, R.; Wang, H.; Zhang, H.H.; Huang, Y.N.; Feng, Y.; et al. Machine learning models for outcome prediction of Chinese uveal melanoma patients: A 15-year follow-up study. Cancer Commun. 2022, 42, 273–276. [Google Scholar] [CrossRef]
- Diener-West, M.; Reynolds, S.M.; Agugliaro, D.J.; Caldwell, R.; Cumming, K.; Earle, J.D.; Hawkins, B.S.; Hayman, J.A.; Jaiyesimi, I.; Jampol, L.M.; et al. Development of metastatic disease after enrollment in the COMS trials for treatment of choroidal melanoma: Collaborative Ocular Melanoma Study Group Report No. 26. Arch. Ophthalmol. 2005, 123, 1639–1643. [Google Scholar]
- Larribere, L.; Utikal, J. Update on GNA Alterations in Cancer: Implications for Uveal Melanoma Treatment. Cancers 2020, 12, 1524. [Google Scholar] [CrossRef]
- Johnson, D.B.; Daniels, A.B. Continued Poor Survival in Metastatic Uveal Melanoma: Implications for Molecular Prognostication, Surveillance Imaging, Adjuvant Therapy, and Clinical Trials. JAMA Ophthalmol. 2018, 136, 986–988. [Google Scholar] [CrossRef]
- Nathan, P.; Hassel, J.C.; Rutkowski, P.; Baurain, J.F.; Butler, M.O.; Schlaak, M.; Sullivan, R.J.; Ochsenreither, S.; Dummer, R.; Kirkwood, J.M.; et al. Overall Survival Benefit with Tebentafusp in Metastatic Uveal Melanoma. N. Engl. J. Med. 2021, 385, 1196–1206. [Google Scholar] [CrossRef]
- Howlett, S.; Carter, T.J.; Shaw, H.M.; Nathan, P.D. Tebentafusp: A first-in-class treatment for metastatic uveal melanoma. Ther. Adv. Med. Oncol. 2023, 15, 17588359231160140. [Google Scholar] [CrossRef]
- Wootten, D.; Christopoulos, A.; Marti-Solano, M.; Babu, M.M.; Sexton, P.M. Mechanisms of signalling and biased agonism in G protein-coupled receptors. Nat. Rev. Mol. Cell Biol. 2018, 19, 638–653. [Google Scholar] [CrossRef]
- Silva-Rodriguez, P.; Fernandez-Diaz, D.; Bande, M.; Pardo, M.; Loidi, L.; Blanco-Teijeiro, M.J. GNAQ and GNA11 Genes: A Comprehensive Review on Oncogenesis, Prognosis and Therapeutic Opportunities in Uveal Melanoma. Cancers 2022, 14, 3066. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Weng, L.; Bastian, B.C.; Chen, X. Functional characterization of uveal melanoma oncogenes. Oncogene 2021, 40, 806–820. [Google Scholar] [CrossRef] [PubMed]
- Carvajal, R.D.; Sosman, J.A.; Quevedo, J.F.; Milhem, M.M.; Joshua, A.M.; Kudchadkar, R.R.; Linette, G.P.; Gajewski, T.F.; Lutzky, J.; Lawson, D.H.; et al. Effect of selumetinib vs chemotherapy on progression-free survival in uveal melanoma: A randomized clinical trial. JAMA 2014, 311, 2397–2405. [Google Scholar] [CrossRef]
- Carvajal, R.D.; Piperno-Neumann, S.; Kapiteijn, E.; Chapman, P.B.; Frank, S.; Joshua, A.M.; Piulats, J.M.; Wolter, P.; Cocquyt, V.; Chmielowski, B.; et al. Selumetinib in Combination With Dacarbazine in Patients With Metastatic Uveal Melanoma: A Phase III, Multicenter, Randomized Trial (SUMIT). J. Clin. Oncol. 2018, 36, 1232–1239. [Google Scholar] [CrossRef]
- Steeb, T.; Wessely, A.; Ruzicka, T.; Heppt, M.V.; Berking, C. How to MEK the best of uveal melanoma: A systematic review on the efficacy and safety of MEK inhibitors in metastatic or unresectable uveal melanoma. Eur. J. Cancer 2018, 103, 41–51. [Google Scholar] [CrossRef]
- Sacco, J.J.; Jackson, R.; Corrie, P.; Danson, S.; Evans, T.R.J.; Ochsenreither, S.; Kumar, S.; Goodman, A.; Larkin, J.; Karydis, I.; et al. A three-arm randomised phase II study of the MEK inhibitor selumetinib alone or in combination with paclitaxel in metastatic uveal melanoma. Eur. J. Cancer 2024, 202, 114009. [Google Scholar] [CrossRef]
- Yang, H.; Zhou, X.; Fu, D.; Le, C.; Wang, J.; Zhou, Q.; Liu, X.; Yuan, Y.; Ding, K.; Xiao, Q. Targeting RAS mutants in malignancies: Successes, failures, and reasons for hope. Cancer Commun. 2023, 43, 42–74. [Google Scholar] [CrossRef]
- Lu, H.; Liu, S.; Zhang, G.; Bin, W.; Zhu, Y.; Frederick, D.T.; Hu, Y.; Zhong, W.; Randell, S.; Sadek, N.; et al. PAK signalling drives acquired drug resistance to MAPK inhibitors in BRAF-mutant melanomas. Nature 2017, 550, 133–136. [Google Scholar] [CrossRef]
- Singhal, R.; Kandel, E.S. The response to PAK1 inhibitor IPA3 distinguishes between cancer cells with mutations in BRAF and Ras oncogenes. Oncotarget 2012, 3, 700–708. [Google Scholar] [CrossRef]
- Babagana, M.; Johnson, S.; Slabodkin, H.; Bshara, W.; Morrison, C.; Kandel, E.S. P21-activated kinase 1 regulates resistance to BRAF inhibition in human cancer cells. Mol. Carcinog. 2017, 56, 1515–1525. [Google Scholar] [CrossRef]
- Beeser, A.; Jaffer, Z.M.; Hofmann, C.; Chernoff, J. Role of group A p21-activated kinases in activation of extracellular-regulated kinase by growth factors. J. Biol. Chem. 2005, 280, 36609–36615. [Google Scholar] [CrossRef] [PubMed]
- Eblen, S.T.; Slack, J.K.; Weber, M.J.; Catling, A.D. Rac-PAK signaling stimulates extracellular signal-regulated kinase (ERK) activation by regulating formation of MEK1-ERK complexes. Mol. Cell Biol. 2002, 22, 6023–6033. [Google Scholar] [CrossRef] [PubMed]
- Frost, J.A.; Xu, S.; Hutchison, M.R.; Marcus, S.; Cobb, M.H. Actions of Rho family small G proteins and p21-activated protein kinases on mitogen-activated protein kinase family members. Mol. Cell Biol. 1996, 16, 3707–3713. [Google Scholar] [CrossRef]
- Tran, N.H.; Wu, X.; Frost, J.A. B-Raf and Raf-1 are regulated by distinct autoregulatory mechanisms. J. Biol. Chem. 2005, 280, 16244–16253. [Google Scholar] [CrossRef]
- Chow, H.Y.; Jubb, A.M.; Koch, J.N.; Jaffer, Z.M.; Stepanova, D.; Campbell, D.A.; Duron, S.G.; O’Farrell, M.; Cai, K.Q.; Klein-Szanto, A.J.; et al. p21-Activated kinase 1 is required for efficient tumor formation and progression in a Ras-mediated skin cancer model. Cancer Res. 2012, 72, 5966–5975. [Google Scholar] [CrossRef]
- Nheu, T.; He, H.; Hirokawa, Y.; Walker, F.; Wood, J.; Maruta, H. PAK is essential for RAS-induced upregulation of cyclin D1 during the G1 to S transition. Cell Cycle 2004, 3, 71–74. [Google Scholar] [CrossRef]
- Qiu, R.G.; Chen, J.; Kirn, D.; McCormick, F.; Symons, M. An essential role for Rac in Ras transformation. Nature 1995, 374, 457–459. [Google Scholar] [CrossRef]
- Tang, Y.; Yu, J.; Field, J. Signals from the Ras, Rac, and Rho GTPases converge on the Pak protein kinase in Rat-1 fibroblasts. Mol. Cell Biol. 1999, 19, 1881–1891. [Google Scholar] [CrossRef]
- Chen, X.; Wu, Q.; Depeille, P.; Chen, P.; Thornton, S.; Kalirai, H.; Coupland, S.E.; Roose, J.P.; Bastian, B.C. RasGRP3 Mediates MAPK Pathway Activation in GNAQ Mutant Uveal Melanoma. Cancer Cell 2017, 31, 685–696.e6. [Google Scholar] [CrossRef]
- Moore, A.R.; Ran, L.; Guan, Y.; Sher, J.J.; Hitchman, T.D.; Zhang, J.Q.; Hwang, C.; Walzak, E.G.; Shoushtari, A.N.; Monette, S.; et al. GNA11 Q209L Mouse Model Reveals RasGRP3 as an Essential Signaling Node in Uveal Melanoma. Cell Rep. 2018, 22, 2455–2468. [Google Scholar] [CrossRef] [PubMed]
- Vaque, J.P.; Dorsam, R.T.; Feng, X.; Iglesias-Bartolome, R.; Forsthoefel, D.J.; Chen, Q.; Debant, A.; Seeger, M.A.; Ksander, B.R.; Teramoto, H.; et al. A genome-wide RNAi screen reveals a Trio-regulated Rho GTPase circuitry transducing mitogenic signals initiated by G protein-coupled receptors. Mol. Cell 2013, 49, 94–108. [Google Scholar] [CrossRef] [PubMed]
- Pavey, S.; Zuidervaart, W.; van Nieuwpoort, F.; Packer, L.; Jager, M.; Gruis, N.; Hayward, N. Increased p21-activated kinase-1 expression is associated with invasive potential in uveal melanoma. Melanoma Res. 2006, 16, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Kichina, J.V.; Maslov, A.; Kandel, E.S. PAK1 and Therapy Resistance in Melanoma. Cells 2023, 12, 2373. [Google Scholar] [CrossRef]
- Verbik, D.J.; Murray, T.G.; Tran, J.M.; Ksander, B.R. Melanomas that develop within the eye inhibit lymphocyte proliferation. Int. J. Cancer 1997, 73, 470–478. [Google Scholar] [CrossRef]
- De Waard-Siebinga, I.; Blom, D.J.; Griffioen, M.; Schrier, P.I.; Hoogendoorn, E.; Beverstock, G.; Danen, E.H.; Jager, M.J. Establishment and characterization of an uveal-melanoma cell line. Int. J. Cancer 1995, 62, 155–161. [Google Scholar] [CrossRef]
- Amirouchene-Angelozzi, N.; Nemati, F.; Gentien, D.; Nicolas, A.; Dumont, A.; Carita, G.; Camonis, J.; Desjardins, L.; Cassoux, N.; Piperno-Neumann, S.; et al. Establishment of novel cell lines recapitulating the genetic landscape of uveal melanoma and preclinical validation of mTOR as a therapeutic target. Mol. Oncol. 2014, 8, 1508–1520. [Google Scholar] [CrossRef]
- Griewank, K.G.; Yu, X.; Khalili, J.; Sozen, M.M.; Stempke-Hale, K.; Bernatchez, C.; Wardell, S.; Bastian, B.C.; Woodman, S.E. Genetic and molecular characterization of uveal melanoma cell lines. Pigment. Cell Melanoma Res. 2012, 25, 182–187. [Google Scholar] [CrossRef]
- Hayflick, L.; Moorhead, P.S. The serial cultivation of human diploid cell strains. Exp. Cell Res. 1961, 25, 585–621. [Google Scholar] [CrossRef]
- Milyavsky, M.; Shats, I.; Erez, N.; Tang, X.; Senderovich, S.; Meerson, A.; Tabach, Y.; Goldfinger, N.; Ginsberg, D.; Harris, C.C.; et al. Prolonged culture of telomerase-immortalized human fibroblasts leads to a premalignant phenotype. Cancer Res. 2003, 63, 7147–7157. [Google Scholar]
- Babagana, M.; Brown, L.R.; Slabodkin, H.Z.; Kichina, J.V.; Kandel, E.S. Proteotoxic Stress as an Exploitable Vulnerability in Cells with Hyperactive AKT. Int. J. Mol. Sci. 2021, 22, 11376. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Euhus, D.M.; Hudd, C.; LaRegina, M.C.; Johnson, F.E. Tumor measurement in the nude mouse. J. Surg. Oncol. 1986, 31, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Bianchi-Smiraglia, A.; Rana, M.S.; Foley, C.E.; Paul, L.M.; Lipchick, B.C.; Moparthy, S.; Moparthy, K.; Fink, E.E.; Bagati, A.; Hurley, E.; et al. Internally ratiometric fluorescent sensors for evaluation of intracellular GTP levels and distribution. Nat. Methods 2017, 14, 1003–1009. [Google Scholar] [CrossRef]
- da Silva Fernandes, T.; Gillard, B.M.; Dai, T.; Martin, J.C.; Chaudhry, K.A.; Dugas, S.M.; Fisher, A.A.; Sharma, P.; Wu, R.; Attwood, K.M.; et al. Inosine monophosphate dehydrogenase 2 (IMPDH2) modulates response to therapy and chemo-resistance in triple negative breast cancer. Sci. Rep. 2025, 15, 1061. [Google Scholar] [CrossRef]
- Zheng, S.; Wang, W.; Aldahdooh, J.; Malyutina, A.; Shadbahr, T.; Tanoli, Z.; Pessia, A.; Tang, J. SynergyFinder Plus: Toward Better Interpretation and Annotation of Drug Combination Screening Datasets. Genom. Proteom. Bioinform. 2022, 20, 587–596. [Google Scholar] [CrossRef]
- Tang, Z.; Li, C.; Kang, B.; Gao, G.; Li, C.; Zhang, Z. GEPIA: A web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Res. 2017, 45, W98–W102. [Google Scholar] [CrossRef]
- Van Raamsdonk, C.D.; Bezrookove, V.; Green, G.; Bauer, J.; Gaugler, L.; O’Brien, J.M.; Simpson, E.M.; Barsh, G.S.; Bastian, B.C. Frequent somatic mutations of GNAQ in uveal melanoma and blue naevi. Nature 2009, 457, 599–602. [Google Scholar] [CrossRef]
- Van Raamsdonk, C.D.; Griewank, K.G.; Crosby, M.B.; Garrido, M.C.; Vemula, S.; Wiesner, T.; Obenauf, A.C.; Wackernagel, W.; Green, G.; Bouvier, N.; et al. Mutations in GNA11 in uveal melanoma. N. Engl. J. Med. 2010, 363, 2191–2199. [Google Scholar] [CrossRef]
- Jiang, H.; Galtes, D.; Wang, J.; Rockman, H.A. G protein-coupled receptor signaling: Transducers and effectors. Am. J. Physiol. Cell Physiol. 2022, 323, C731–C748. [Google Scholar] [CrossRef]
- Tang, Y.; Chen, Z.; Ambrose, D.; Liu, J.; Gibbs, J.B.; Chernoff, J.; Field, J. Kinase-deficient Pak1 mutants inhibit Ras transformation of Rat-1 fibroblasts. Mol. Cell Biol. 1997, 17, 4454–4464. [Google Scholar] [CrossRef] [PubMed]
- Khosravi-Far, R.; Solski, P.A.; Clark, G.J.; Kinch, M.S.; Der, C.J. Activation of Rac1, RhoA, and mitogen-activated protein kinases is required for Ras transformation. Mol. Cell Biol. 1995, 15, 6443–6453. [Google Scholar] [CrossRef] [PubMed]
- Smalley, K.S.; Weber, J.S. Taming the wild-types: Targeting PAK1 in melanomas that lack BRAF mutations. J. Natl. Cancer Inst. 2013, 105, 591–592. [Google Scholar] [CrossRef]
- Deacon, S.W.; Beeser, A.; Fukui, J.A.; Rennefahrt, U.E.; Myers, C.; Chernoff, J.; Peterson, J.R. An isoform-selective, small-molecule inhibitor targets the autoregulatory mechanism of p21-activated kinase. Chem. Biol. 2008, 15, 322–331. [Google Scholar] [CrossRef]
- Semenova, G.; Chernoff, J. Targeting PAK1. Biochem. Soc. Trans. 2017, 45, 79–88. [Google Scholar] [CrossRef]
- Ong, C.C.; Jubb, A.M.; Jakubiak, D.; Zhou, W.; Rudolph, J.; Haverty, P.M.; Kowanetz, M.; Yan, Y.; Tremayne, J.; Lisle, R.; et al. P21-activated kinase 1 (PAK1) as a therapeutic target in BRAF wild-type melanoma. J. Natl. Cancer Inst. 2013, 105, 606–607. [Google Scholar] [CrossRef]
- Zynda, E.R.; Maloy, M.H.; Kandel, E.S. The role of PAK1 in the sensitivity of kidney epithelial cells to ischemia-like conditions. Cell Cycle 2019, 18, 596–604. [Google Scholar] [CrossRef]
- Khan, S.; Patel, S.P.; Shoushtari, A.N.; Ambrosini, G.; Cremers, S.; Lee, S.; Franks, L.; Singh-Kandah, S.; Hernandez, S.; Sender, N.; et al. Intermittent MEK inhibition for the treatment of metastatic uveal melanoma. Front. Oncol. 2022, 12, 975643. [Google Scholar] [CrossRef]
- Shutes, A.; Onesto, C.; Picard, V.; Leblond, B.; Schweighoffer, F.; Der, C.J. Specificity and mechanism of action of EHT 1864, a novel small molecule inhibitor of Rac family small GTPases. J. Biol. Chem. 2007, 282, 35666–35678. [Google Scholar] [CrossRef]
- Kichina, J.V.; Goc, A.; Al-Husein, B.; Somanath, P.R.; Kandel, E.S. PAK1 as a therapeutic target. Expert Opin. Ther. Targets 2010, 14, 703–725. [Google Scholar] [CrossRef]
- Wawrzyniak, J.A.; Bianchi-Smiraglia, A.; Bshara, W.; Mannava, S.; Ackroyd, J.; Bagati, A.; Omilian, A.R.; Im, M.; Fedtsova, N.; Miecznikowski, J.C.; et al. A purine nucleotide biosynthesis enzyme guanosine monophosphate reductase is a suppressor of melanoma invasion. Cell Rep. 2013, 5, 493–507. [Google Scholar] [CrossRef] [PubMed]
- Bianchi-Smiraglia, A.; Wolff, D.W.; Marston, D.J.; Deng, Z.; Han, Z.; Moparthy, S.; Wombacher, R.M.; Mussell, A.L.; Shen, S.; Chen, J.; et al. Regulation of local GTP availability controls RAC1 activity and cell invasion. Nat. Commun. 2021, 12, 6091. [Google Scholar] [CrossRef] [PubMed]
- Loustaud-Ratti, V.; Debette-Gratien, M.; Jacques, J.; Alain, S.; Marquet, P.; Sautereau, D.; Rousseau, A.; Carrier, P. Ribavirin: Past, present and future. World J. Hepatol. 2016, 8, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Yokota, S. Mizoribine: Mode of action and effects in clinical use. Pediatr. Int. 2002, 44, 196–198. [Google Scholar] [CrossRef]
- Van Allen, E.M.; Wagle, N.; Sucker, A.; Treacy, D.J.; Johannessen, C.M.; Goetz, E.M.; Place, C.S.; Taylor-Weiner, A.; Whittaker, S.; Kryukov, G.V.; et al. The genetic landscape of clinical resistance to RAF inhibition in metastatic melanoma. Cancer Discov. 2014, 4, 94–109. [Google Scholar] [CrossRef]
- Flaherty, K.T.; Infante, J.R.; Daud, A.; Gonzalez, R.; Kefford, R.F.; Sosman, J.; Hamid, O.; Schuchter, L.; Cebon, J.; Ibrahim, N.; et al. Combined BRAF and MEK inhibition in melanoma with BRAF V600 mutations. N. Engl. J. Med. 2012, 367, 1694–1703. [Google Scholar] [CrossRef]
- Corcoran, R.B.; Andre, T.; Atreya, C.E.; Schellens, J.H.M.; Yoshino, T.; Bendell, J.C.; Hollebecque, A.; McRee, A.J.; Siena, S.; Middleton, G.; et al. Combined BRAF, EGFR, and MEK Inhibition in Patients with BRAF(V600E)-Mutant Colorectal Cancer. Cancer Discov. 2018, 8, 428–443. [Google Scholar] [CrossRef]
- Bollag, G.; Hirth, P.; Tsai, J.; Zhang, J.; Ibrahim, P.N.; Cho, H.; Spevak, W.; Zhang, C.; Zhang, Y.; Habets, G.; et al. Clinical efficacy of a RAF inhibitor needs broad target blockade in BRAF-mutant melanoma. Nature 2010, 467, 596–599. [Google Scholar] [CrossRef]
- Babagana, M.; Kichina, J.V.; Slabodkin, H.; Johnson, S.; Maslov, A.; Brown, L.; Attwood, K.; Nikiforov, M.A.; Kandel, E.S. The role of polo-like kinase 3 in the response of BRAF-mutant cells to targeted anticancer therapies. Mol. Carcinog. 2020, 59, 5–14. [Google Scholar] [CrossRef]
- Ullah, R.; Yin, Q.; Snell, A.H.; Wan, L. RAF-MEK-ERK pathway in cancer evolution and treatment. Semin. Cancer Biol. 2022, 85, 123–154. [Google Scholar] [CrossRef]
- Chehrehasa, F.; Meedeniya, A.C.; Dwyer, P.; Abrahamsen, G.; Mackay-Sim, A. EdU, a new thymidine analogue for labelling proliferating cells in the nervous system. J. Neurosci. Methods 2009, 177, 122–130. [Google Scholar] [CrossRef]
- Wang, B.; Guo, H.; Yu, H.; Chen, Y.; Xu, H.; Zhao, G. The Role of the Transcription Factor EGR1 in Cancer. Front. Oncol. 2021, 11, 642547. [Google Scholar] [CrossRef] [PubMed]
- Miley, D.R.; Andrews-Pfannkoch, C.M.; Pulido, J.S.; Erickson, S.A.; Vile, R.G.; Fautsch, M.P.; Marmorstein, A.D.; Dalvin, L.A. Direct early growth response-1 knockdown decreases melanoma viability independent of mitogen-activated extracellular signal-related kinase inhibition. Melanoma Res. 2023, 33, 482–491. [Google Scholar] [CrossRef] [PubMed]
- Sriramareddy, S.N.; Faiao-Flores, F.; Emmons, M.F.; Saha, B.; Chellappan, S.; Wyatt, C.; Smalley, I.; Licht, J.D.; Durante, M.A.; Harbour, J.W.; et al. HDAC11 activity contributes to MEK inhibitor escape in uveal melanoma. Cancer Gene Ther. 2022, 29, 1840–1846. [Google Scholar] [CrossRef] [PubMed]
- Dalvin, L.A.; Andrews-Pfannkoch, C.; Pulido, J.S.; Miley, D.R.; Adams, S. The role of early growth response-1 in uveal melanoma viability and treatment resistance to MEK inhibition. Investig. Ophthalmol. Vis. Sci. 2022, 63, 1462. [Google Scholar]
- Yu, J.; Zhuang, A.; Gu, X.; Hua, Y.; Yang, L.; Ge, S.; Ruan, J.; Chai, P.; Jia, R.; Fan, X. Nuclear PD-L1 promotes EGR1-mediated angiogenesis and accelerates tumorigenesis. Cell Discov. 2023, 9, 33. [Google Scholar] [CrossRef]
- Meir, T.; Dror, R.; Yu, X.; Qian, J.; Simon, I.; Pe’er, J.; Chowers, I. Molecular characteristics of liver metastases from uveal melanoma. Invest. Ophthalmol. Vis. Sci. 2007, 48, 4890–4896. [Google Scholar] [CrossRef]
- Richards, J.R.; Yoo, J.H.; Shin, D.; Odelberg, S.J. Mouse models of uveal melanoma: Strengths, weaknesses, and future directions. Pigment. Cell Melanoma Res. 2020, 33, 264–278. [Google Scholar] [CrossRef]
- Bailly, C.; Beignet, J.; Loirand, G.; Sauzeau, V. Rac1 as a therapeutic anticancer target: Promises and limitations. Biochem. Pharmacol. 2022, 203, 115180. [Google Scholar] [CrossRef]
- Somanath, P.R.; Chernoff, J.; Cummings, B.S.; Prasad, S.M.; Homan, H.D. Targeting P21-Activated Kinase-1 for Metastatic Prostate Cancer. Cancers 2023, 15, 2236. [Google Scholar] [CrossRef] [PubMed]
- Al-Azayzih, A.; Missaoui, W.N.; Cummings, B.S.; Somanath, P.R. Liposome-mediated delivery of the p21 activated kinase-1 (PAK-1) inhibitor IPA-3 limits prostate tumor growth in vivo. Nanomedicine 2016, 12, 1231–1239. [Google Scholar] [CrossRef] [PubMed]
- Kciuk, M.; Gielecinska, A.; Budzinska, A.; Mojzych, M.; Kontek, R. Metastasis and MAPK Pathways. Int. J. Mol. Sci. 2022, 23, 3847. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.; Meng, Q.; Xu, J.; Wang, W.; Zhang, B.; Liu, J.; Liang, C.; Hua, J.; Zhao, Y.; Yu, X.; et al. Signaling pathways in cancer-associated fibroblasts: Recent advances and future perspectives. Cancer Commun. 2023, 43, 3–41. [Google Scholar] [CrossRef]
- Austin, R.K.; Trefts, P.E.; Hintz, M.; Connor, J.D.; Kagnoff, M.F. Sensitive radioimmunoassay for the broad-spectrum antiviral agent ribavirin. Antimicrob. Agents Chemother. 1983, 24, 696–701. [Google Scholar] [CrossRef]
- Endres, C.J.; Moss, A.M.; Govindarajan, R.; Choi, D.S.; Unadkat, J.D. The role of nucleoside transporters in the erythrocyte disposition and oral absorption of ribavirin in the wild-type and equilibrative nucleoside transporter 1-/- mice. J. Pharmacol. Exp. Ther. 2009, 331, 287–296. [Google Scholar] [CrossRef]
- Gilbert, B.E.; Wyde, P.R. Pharmacokinetics of ribavirin aerosol in mice. Antimicrob. Agents Chemother. 1988, 32, 117–121. [Google Scholar] [CrossRef]
- Langhans, B.; Nischalke, H.D.; Arndt, S.; Braunschweiger, I.; Nattermann, J.; Sauerbruch, T.; Spengler, U. Ribavirin exerts differential effects on functions of Cd4+ Th1, Th2, and regulatory T cell clones in hepatitis C. PLoS ONE 2012, 7, e42094. [Google Scholar] [CrossRef]
- Nakatsuka, K.; Atsukawa, M.; Shimizu, M.; Takahashi, H.; Kawamoto, C. Ribavirin contributes to eradicate hepatitis C virus through polarization of T helper 1/2 cell balance into T helper 1 dominance. World J. Hepatol. 2015, 7, 2590–2596. [Google Scholar] [CrossRef]
- Phadke, M.S.; Chen, Z.; Li, J.; Mohamed, E.; Davies, M.A.; Smalley, I.; Duckett, D.R.; Palve, V.; Czerniecki, B.J.; Forsyth, P.A.; et al. Targeted Therapy Given after Anti-PD-1 Leads to Prolonged Responses in Mouse Melanoma Models through Sustained Antitumor Immunity. Cancer Immunol. Res. 2021, 9, 554–567. [Google Scholar] [CrossRef]
- Tian, J.; Chen, J.H.; Chao, S.X.; Pelka, K.; Giannakis, M.; Hess, J.; Burke, K.; Jorgji, V.; Sindurakar, P.; Braverman, J.; et al. Combined PD-1, BRAF and MEK inhibition in BRAF(V600E) colorectal cancer: A phase 2 trial. Nat. Med. 2023, 29, 458–466. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, S.; Yang, Z.; Algazi, A.P.; Lomeli, S.H.; Wang, Y.; Othus, M.; Hong, A.; Wang, X.; Randolph, C.E.; et al. Anti-PD-1/L1 lead-in before MAPK inhibitor combination maximizes antitumor immunity and efficacy. Cancer Cell 2021, 39, 1375–1387.E6. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maslov, A.A.; Trageser, N.H.; Kichina, J.V.; Elamir, H.; Gardner, E.; Teaman, F.; Vishwanath, V.; Dugas, S.M.; Heid, J.; Maslov, A.Y.; et al. Inhibition of the RAC/PAK Signaling Axis Enhances the Potency of MAPK Cascade Inhibitors Against Uveal Melanoma. Biomolecules 2025, 15, 1425. https://doi.org/10.3390/biom15101425
Maslov AA, Trageser NH, Kichina JV, Elamir H, Gardner E, Teaman F, Vishwanath V, Dugas SM, Heid J, Maslov AY, et al. Inhibition of the RAC/PAK Signaling Axis Enhances the Potency of MAPK Cascade Inhibitors Against Uveal Melanoma. Biomolecules. 2025; 15(10):1425. https://doi.org/10.3390/biom15101425
Chicago/Turabian StyleMaslov, Alexei A., Nicholas H. Trageser, Julia V. Kichina, Haya Elamir, Evelyn Gardner, Frances Teaman, Vera Vishwanath, Scott M. Dugas, Johanna Heid, Alexander Y. Maslov, and et al. 2025. "Inhibition of the RAC/PAK Signaling Axis Enhances the Potency of MAPK Cascade Inhibitors Against Uveal Melanoma" Biomolecules 15, no. 10: 1425. https://doi.org/10.3390/biom15101425
APA StyleMaslov, A. A., Trageser, N. H., Kichina, J. V., Elamir, H., Gardner, E., Teaman, F., Vishwanath, V., Dugas, S. M., Heid, J., Maslov, A. Y., Withers, H. G., Bianchi-Smiraglia, A., Leonova, K. I., Nikiforov, M. A., & Kandel, E. S. (2025). Inhibition of the RAC/PAK Signaling Axis Enhances the Potency of MAPK Cascade Inhibitors Against Uveal Melanoma. Biomolecules, 15(10), 1425. https://doi.org/10.3390/biom15101425





