Glucocorticoids Induce an Opposite Metabolic Switch in Human Monocytes Contingent upon Their Polarization
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation of Human Monocytes
2.2. Cell Culture Experiments
2.3. Flow Cytometry
2.4. Seahorse Experiments
2.5. Lactate Assay
2.6. SCENITH Assay
2.7. Quantitative RT-PCR
2.8. Statistical Analysis
3. Results
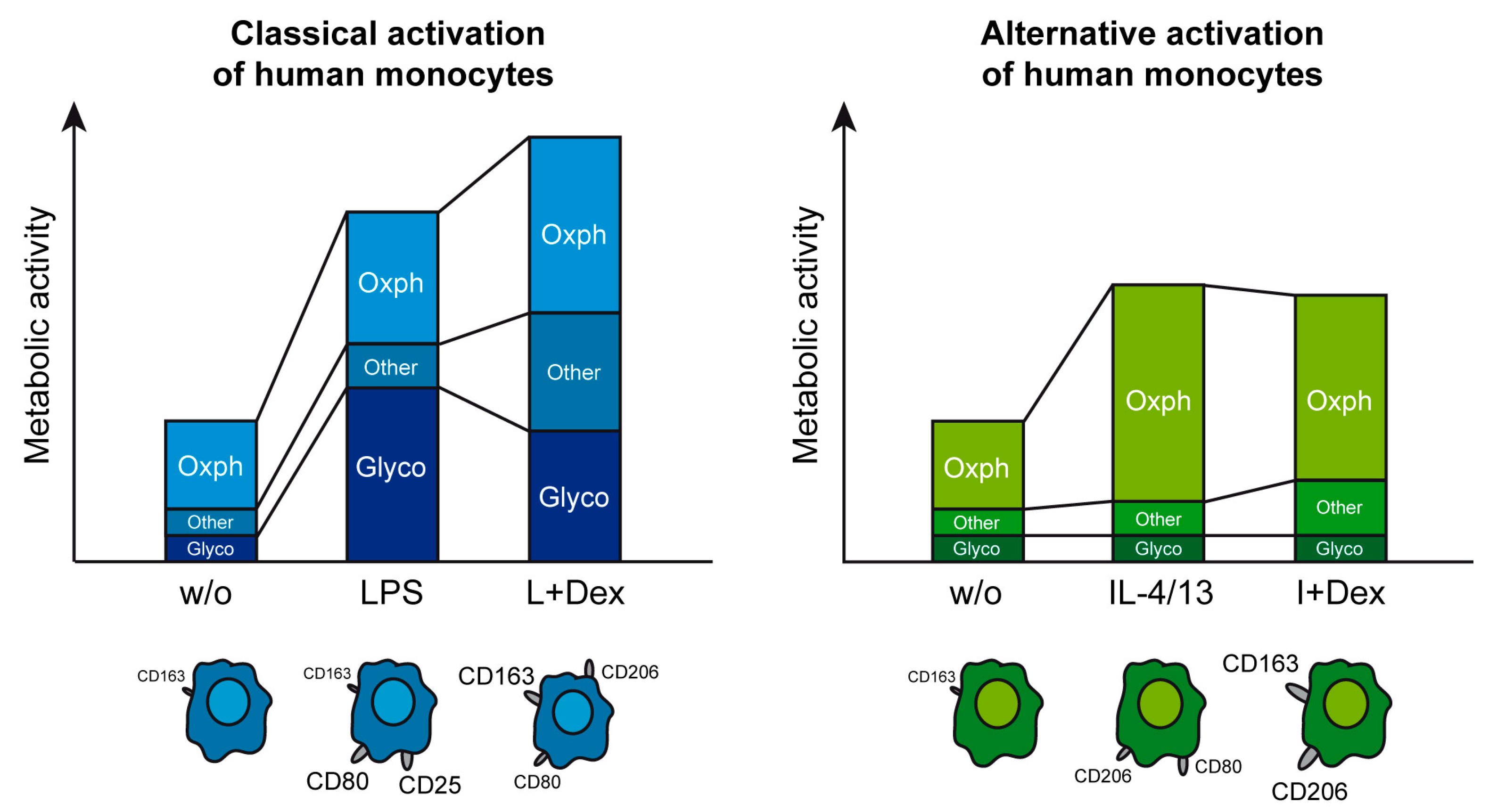
3.1. Classical and Alternative Activation of Human Monocytes
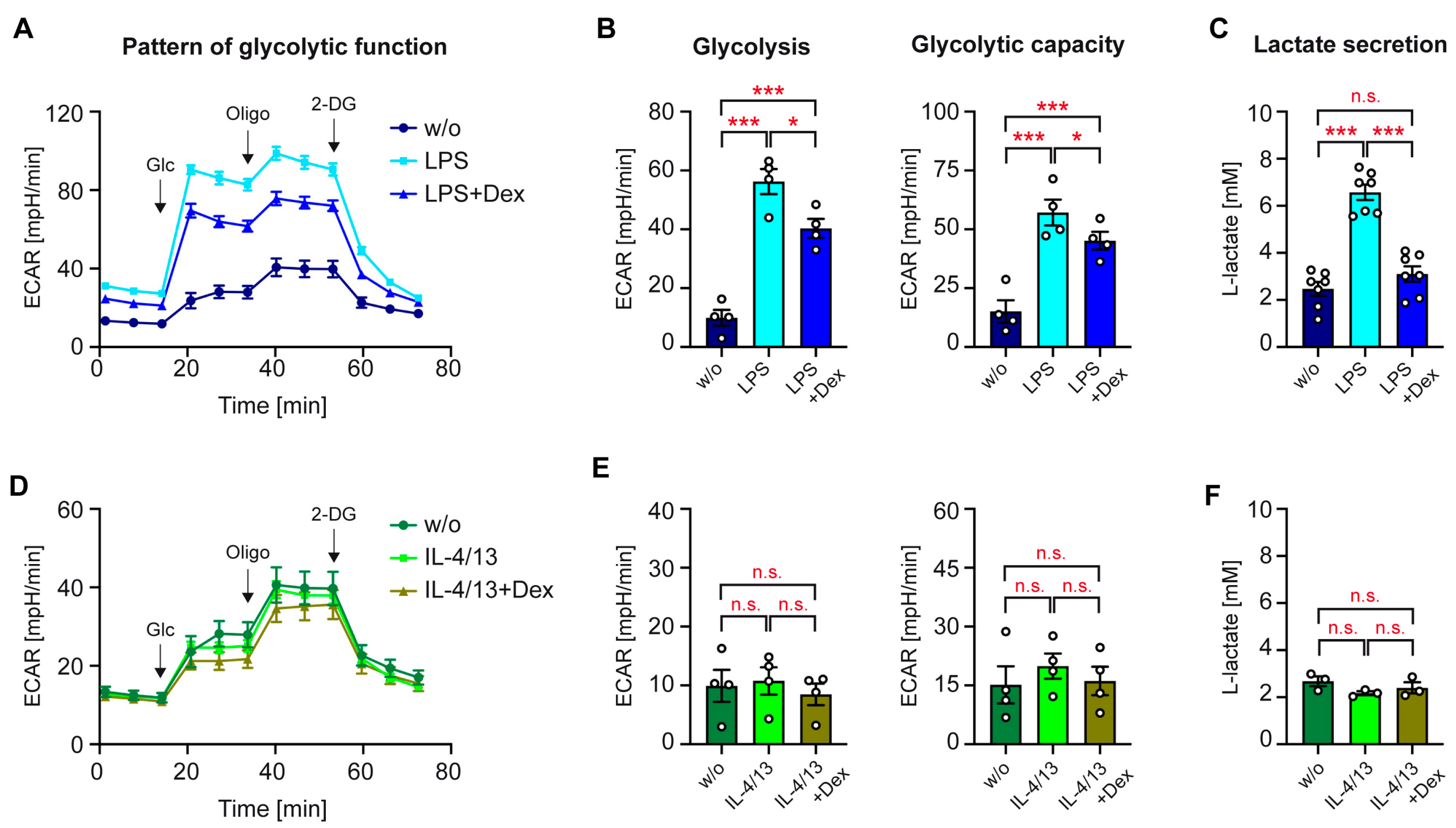
3.2. Glycolytic Function of Activated Human Monocytes
3.3. Transcriptional Control of the Glycolytic Pathway
3.4. GCs Control Glycolysis in Monocytes via mTORC1

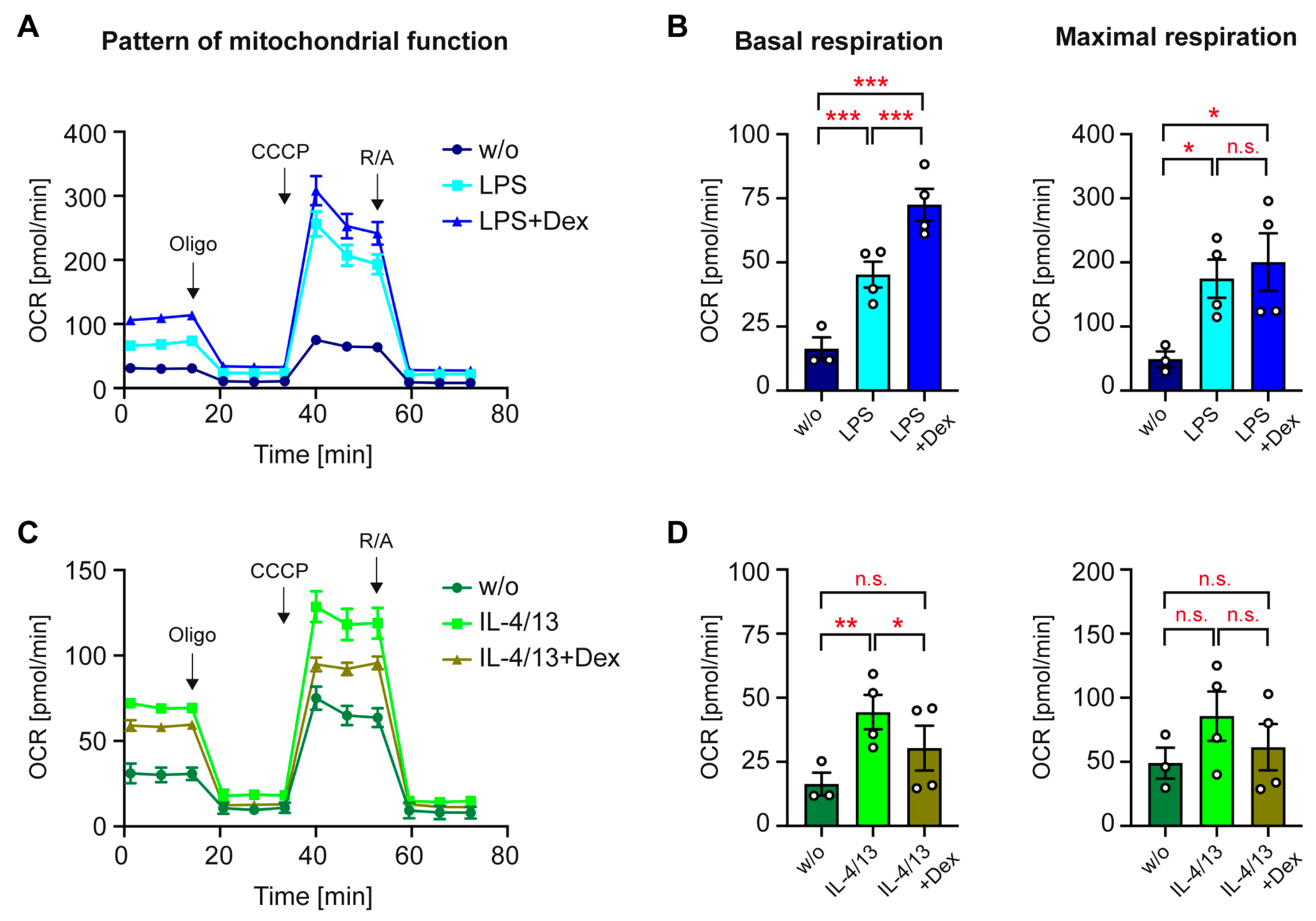
3.5. Mitochondrial Function of Activated Human Monocytes
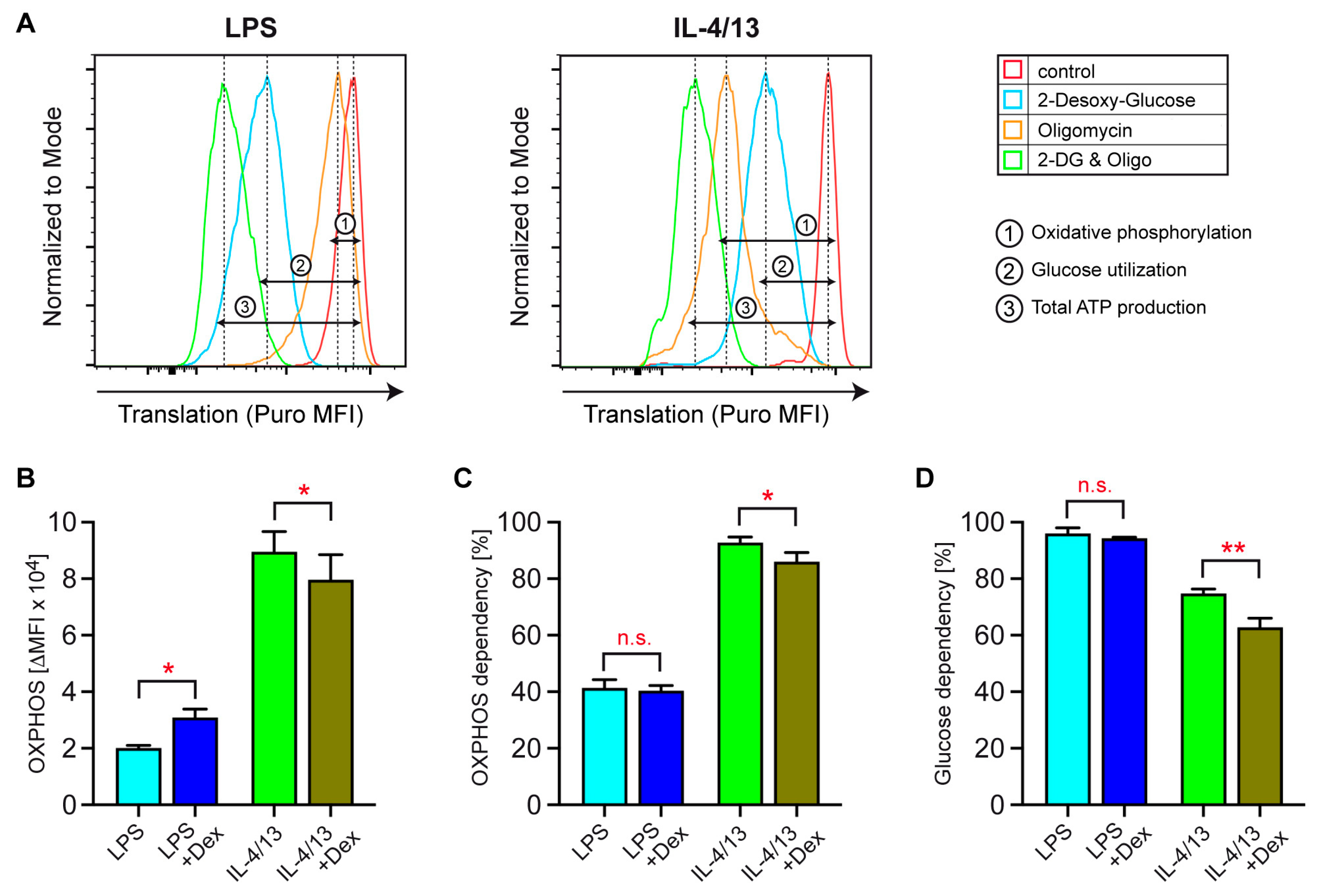
3.6. Dependency of Activated Human Monocytes on OXPHOS and Glucose
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 2-DG | 2-desoxy-D-glucose |
| ECAR | extracellular acidification rate |
| GC | glucocorticoid |
| GR | glucocorticoid receptor |
| Dex | dexamethasone |
| OCR | oxygen consumption rate |
| OXPHOS | oxidative phosphorylation |
| SCENITH | single-cell energetic metabolism by profiling translation inhibition |
References
- Reichardt, S.D.; Amouret, A.; Muzzi, C.; Vettorazzi, S.; Tuckermann, J.P.; Lühder, F.; Reichardt, H.M. The Role of Glucocorticoids in Inflammatory Diseases. Cells 2021, 10, 2921. [Google Scholar] [CrossRef]
- Escoter-Torres, L.; Caratti, G.; Mechtidou, A.; Tuckermann, J.; Uhlenhaut, N.H.; Vettorazzi, S. Fighting the Fire: Mechanisms of Inflammatory Gene Regulation by the Glucocorticoid Receptor. Front. Immunol. 2019, 10, 1859. [Google Scholar] [CrossRef]
- Crabtree, G.R.; Gillis, S.; Smith, K.A.; Munck, A. Glucocorticoids and immune responses. Arthritis Rheum. 1979, 22, 1246–1256. [Google Scholar] [CrossRef]
- Mildner, A.; Kim, K.W.; Yona, S. Unravelling monocyte functions: From the guardians of health to the regulators of disease. Discov. Immunol. 2024, 3, kyae014. [Google Scholar] [CrossRef]
- Gordon, S. Alternative activation of macrophages. Nat. Rev. Immunol. 2003, 3, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Martinez, F.O.; Sica, A.; Mantovani, A.; Locati, M. Macrophage activation and polarization. Front. Biosci. 2008, 13, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Nanni, L.; Novakovic, B.; Megchelenbrink, W.; Kuznetsova, T.; Stunnenberg, H.G.; Ceri, S.; Logie, C. Extensive epigenomic integration of the glucocorticoid response in primary human monocytes and in vitro derived macrophages. Sci. Rep. 2019, 9, 2772. [Google Scholar] [CrossRef] [PubMed]
- Mills, C.D. Anatomy of a discovery: m1 and m2 macrophages. Front. Immunol. 2015, 6, 212. [Google Scholar] [CrossRef]
- Mosser, D.M.; Edwards, J.P. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 2008, 8, 958–969. [Google Scholar] [CrossRef]
- Stein, M.; Keshav, S.; Harris, N.; Gordon, S. Interleukin 4 potently enhances murine macrophage mannose receptor activity: A marker of alternative immunologic macrophage activation. J. Exp. Med. 1992, 176, 287–292. [Google Scholar] [CrossRef]
- Hesse, M.; Modolell, M.; La Flamme, A.C.; Schito, M.; Fuentes, J.M.; Cheever, A.W.; Pearce, E.J.; Wynn, T.A. Differential regulation of nitric oxide synthase-2 and arginase-1 by type 1/type 2 cytokines in vivo: Granulomatous pathology is shaped by the pattern of L-arginine metabolism. J. Immunol. 2001, 167, 6533–6544. [Google Scholar] [CrossRef]
- Austermann, J.; Roth, J.; Barczyk-Kahlert, K. The Good and the Bad: Monocytes’ and Macrophages’ Diverse Functions in Inflammation. Cells 2022, 11, 1979. [Google Scholar] [CrossRef] [PubMed]
- Heideveld, E.; Hampton-O’Neil, L.A.; Cross, S.J.; van Alphen, F.P.J.; van den Biggelaar, M.; Toye, A.M.; van den Akker, E. Glucocorticoids induce differentiation of monocytes towards macrophages that share functional and phenotypical aspects with erythroblastic island macrophages. Haematologica 2018, 103, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Buck, M.D.; Sowell, R.T.; Kaech, S.M.; Pearce, E.L. Metabolic Instruction of Immunity. Cell 2017, 169, 570–586. [Google Scholar] [CrossRef] [PubMed]
- Viola, A.; Munari, F.; Sanchez-Rodriguez, R.; Scolaro, T.; Castegna, A. The Metabolic Signature of Macrophage Responses. Front. Immunol. 2019, 10, 1462. [Google Scholar] [CrossRef]
- Freemerman, A.J.; Johnson, A.R.; Sacks, G.N.; Milner, J.J.; Kirk, E.L.; Troester, M.A.; Macintyre, A.N.; Goraksha-Hicks, P.; Rathmell, J.C.; Makowski, L. Metabolic reprogramming of macrophages: Glucose transporter 1 (GLUT1)-mediated glucose metabolism drives a proinflammatory phenotype. J. Biol. Chem. 2014, 289, 7884–7896. [Google Scholar] [CrossRef]
- Jha, A.K.; Huang, S.C.; Sergushichev, A.; Lampropoulou, V.; Ivanova, Y.; Loginicheva, E.; Chmielewski, K.; Stewart, K.M.; Ashall, J.; Everts, B.; et al. Network integration of parallel metabolic and transcriptional data reveals metabolic modules that regulate macrophage polarization. Immunity 2015, 42, 419–430. [Google Scholar] [CrossRef]
- Wang, T.; Liu, H.; Lian, G.; Zhang, S.Y.; Wang, X.; Jiang, C. HIF1alpha-Induced Glycolysis Metabolism Is Essential to the Activation of Inflammatory Macrophages. Mediat. Inflamm. 2017, 2017, 9029327. [Google Scholar] [CrossRef]
- Aki, T.; Funakoshi, T.; Noritake, K.; Unuma, K.; Uemura, K. Extracellular glucose is crucially involved in the fate decision of LPS-stimulated RAW264.7 murine macrophage cells. Sci. Rep. 2020, 10, 10581. [Google Scholar] [CrossRef]
- Cheng, S.C.; Quintin, J.; Cramer, R.A.; Shepardson, K.M.; Saeed, S.; Kumar, V.; Giamarellos-Bourboulis, E.J.; Martens, J.H.; Rao, N.A.; Aghajanirefah, A.; et al. mTOR- and HIF-1alpha-mediated aerobic glycolysis as metabolic basis for trained immunity. Science 2014, 345, 1250684. [Google Scholar] [CrossRef]
- Tan, Z.; Xie, N.; Cui, H.; Moellering, D.R.; Abraham, E.; Thannickal, V.J.; Liu, G. Pyruvate dehydrogenase kinase 1 participates in macrophage polarization via regulating glucose metabolism. J. Immunol. 2015, 194, 6082–6089. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Zhang, S.; Vuckovic, I.; Jeon, R.; Lerman, A.; Folmes, C.D.; Dzeja, P.P.; Herrmann, J. Glycolytic Stimulation Is Not a Requirement for M2 Macrophage Differentiation. Cell Metab. 2018, 28, 463–475.e4. [Google Scholar] [CrossRef] [PubMed]
- Bishop, E.L.; Gudgeon, N.; Dimeloe, S. Control of T Cell Metabolism by Cytokines and Hormones. Front. Immunol. 2021, 12, 653605. [Google Scholar] [CrossRef] [PubMed]
- Meyer-Heemsoth, L.; Mitschke, K.; Bier, J.; Schutz, K.; Villunger, A.; Legler, T.J.; Weber, M.S.; Luhder, F.; Reichardt, H.M. T Cell Energy Metabolism Is a Target of Glucocorticoids in Mice, Healthy Humans, and MS Patients. Cells 2023, 12, 450. [Google Scholar] [CrossRef]
- Auger, J.P.; Zimmermann, M.; Faas, M.; Stifel, U.; Chambers, D.; Krishnacoumar, B.; Taudte, R.V.; Grund, C.; Erdmann, G.; Scholtysek, C.; et al. Metabolic rewiring promotes anti-inflammatory effects of glucocorticoids. Nature 2024, 629, 184–192. [Google Scholar] [CrossRef]
- Clayton, S.A.; Lockwood, C.; O’Neil, J.D.; Daley, K.K.; Hain, S.; Abdelmottaleb, D.; Bolimowska, O.O.; Tennant, D.A.; Clark, A.R. The glucocorticoid dexamethasone inhibits HIF-1alpha stabilization and metabolic reprogramming in lipopolysaccharide-stimulated primary macrophages. Discov. Immunol. 2023, 2, kyad027. [Google Scholar] [CrossRef]
- Stifel, U.; Wolfschmitt, E.M.; Vogt, J.; Wachter, U.; Vettorazzi, S.; Tews, D.; Hogg, M.; Zink, F.; Koll, N.M.; Winning, S.; et al. Glucocorticoids coordinate macrophage metabolism through the regulation of the tricarboxylic acid cycle. Mol. Metab. 2022, 57, 101424. [Google Scholar] [CrossRef]
- Ball, A.B.; Jones, A.E.; Nguyen, K.B.; Rios, A.; Marx, N.; Hsieh, W.Y.; Yang, K.; Desousa, B.R.; Kim, K.K.O.; Veliova, M.; et al. Pro-inflammatory macrophage activation does not require inhibition of oxidative phosphorylation. EMBO Rep. 2025, 26, 982–1002. [Google Scholar] [CrossRef]
- Reichardt, S.D.; Lühder, F.; Wiegers, G.J.; Reichardt, H.M. A flow cytometric approach to study glucocorticoid receptor expression in immune cell subpopulations of genetically engineered mice. Immunol. Lett. 2021, 233, 68–79. [Google Scholar] [CrossRef]
- Fischer, H.J.; Sie, C.; Schumann, E.; Witte, A.K.; Dressel, R.; van den Brandt, J.; Reichardt, H.M. The Insulin Receptor Plays a Critical Role in T Cell Function and Adaptive Immunity. J. Immunol. 2017, 198, 1910–1920. [Google Scholar] [CrossRef]
- Arguello, R.J.; Combes, A.J.; Char, R.; Gigan, J.P.; Baaziz, A.I.; Bousiquot, E.; Camosseto, V.; Samad, B.; Tsui, J.; Yan, P.; et al. SCENITH: A Flow Cytometry-Based Method to Functionally Profile Energy Metabolism with Single-Cell Resolution. Cell Metab. 2020, 32, 1063–1075.e7. [Google Scholar] [CrossRef] [PubMed]
- Powell, J.D.; Pollizzi, K.N.; Heikamp, E.B.; Horton, M.R. Regulation of immune responses by mTOR. Annu. Rev. Immunol. 2012, 30, 39–68. [Google Scholar] [CrossRef] [PubMed]
- Dickensheets, H.L.; Venkataraman, C.; Schindler, U.; Donnelly, R.P. Interferons inhibit activation of STAT6 by interleukin 4 in human monocytes by inducing SOCS-1 gene expression. Proc. Natl. Acad. Sci. U S A 1999, 96, 10800–10805. [Google Scholar] [CrossRef] [PubMed]
- Rahal, O.M.; Wolfe, A.R.; Mandal, P.K.; Larson, R.; Tin, S.; Jimenez, C.; Zhang, D.; Horton, J.; Reuben, J.M.; McMurray, J.S.; et al. Blocking Interleukin (IL)4- and IL13-Mediated Phosphorylation of STAT6 (Tyr641) Decreases M2 Polarization of Macrophages and Protects Against Macrophage-Mediated Radioresistance of Inflammatory Breast Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2018, 100, 1034–1043. [Google Scholar] [CrossRef]
- Kruse, N.J.; Rowe, D.W.; Fujimoto, W.Y.; Bornstein, P. Inhibitory effects of glucocorticoids on collagen synthesis by mouse sponge granulomas and granuloma fibroblasts in culture. Biochim. Biophys. Acta 1978, 540, 101–116. [Google Scholar] [CrossRef]
- Abraham, S.M.; Lawrence, T.; Kleiman, A.; Warden, P.; Medghalchi, M.; Tuckermann, J.; Saklatvala, J.; Clark, A.R. Antiinflammatory effects of dexamethasone are partly dependent on induction of dual specificity phosphatase 1. J. Exp. Med. 2006, 203, 1883–1889. [Google Scholar] [CrossRef]
- Müller, N.; Fischer, H.J.; Tischner, D.; van den Brandt, J.; Reichardt, H.M. Glucocorticoids induce effector T cell depolarization via ERM proteins, thereby impeding migration and APC conjugation. J. Immunol. 2013, 190, 4360–4370. [Google Scholar] [CrossRef]
- Jeong, J.Y.; Jeoung, N.H.; Park, K.G.; Lee, I.K. Transcriptional regulation of pyruvate dehydrogenase kinase. Diabetes Metab. J. 2012, 36, 328–335. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peruzzi, E.; Heidenreich, S.; Klaus, L.; Boshnakovska, A.; Amouret, A.; Legler, T.; Reichardt, S.D.; Lühder, F.; Reichardt, H.M. Glucocorticoids Induce an Opposite Metabolic Switch in Human Monocytes Contingent upon Their Polarization. Biomolecules 2025, 15, 1422. https://doi.org/10.3390/biom15101422
Peruzzi E, Heidenreich S, Klaus L, Boshnakovska A, Amouret A, Legler T, Reichardt SD, Lühder F, Reichardt HM. Glucocorticoids Induce an Opposite Metabolic Switch in Human Monocytes Contingent upon Their Polarization. Biomolecules. 2025; 15(10):1422. https://doi.org/10.3390/biom15101422
Chicago/Turabian StylePeruzzi, Elisa, Sophia Heidenreich, Lucas Klaus, Angela Boshnakovska, Agathe Amouret, Tobias Legler, Sybille D. Reichardt, Fred Lühder, and Holger M. Reichardt. 2025. "Glucocorticoids Induce an Opposite Metabolic Switch in Human Monocytes Contingent upon Their Polarization" Biomolecules 15, no. 10: 1422. https://doi.org/10.3390/biom15101422
APA StylePeruzzi, E., Heidenreich, S., Klaus, L., Boshnakovska, A., Amouret, A., Legler, T., Reichardt, S. D., Lühder, F., & Reichardt, H. M. (2025). Glucocorticoids Induce an Opposite Metabolic Switch in Human Monocytes Contingent upon Their Polarization. Biomolecules, 15(10), 1422. https://doi.org/10.3390/biom15101422





