Curcumin Alleviates Doxorubicin-Induced Cardiotoxicity by Modulating Apelin Expression
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Blood Pressure Measurement
2.3. ECG Measurement
2.4. Sample Collection
2.5. Biochemical Analyses in Heart Tissue
2.6. Quantification of Ctn, Apelin, Tnf, Il-1β
2.7. RT-qPCR for mRNA Expression of Apelin and Nf-Κb
2.8. Histopathological Analysis
2.9. Immunohistochemical Analysis
2.10. Statistical Analysis
3. Results
3.1. Electrocardiogram (ECG)
3.2. Biochemical Analyses
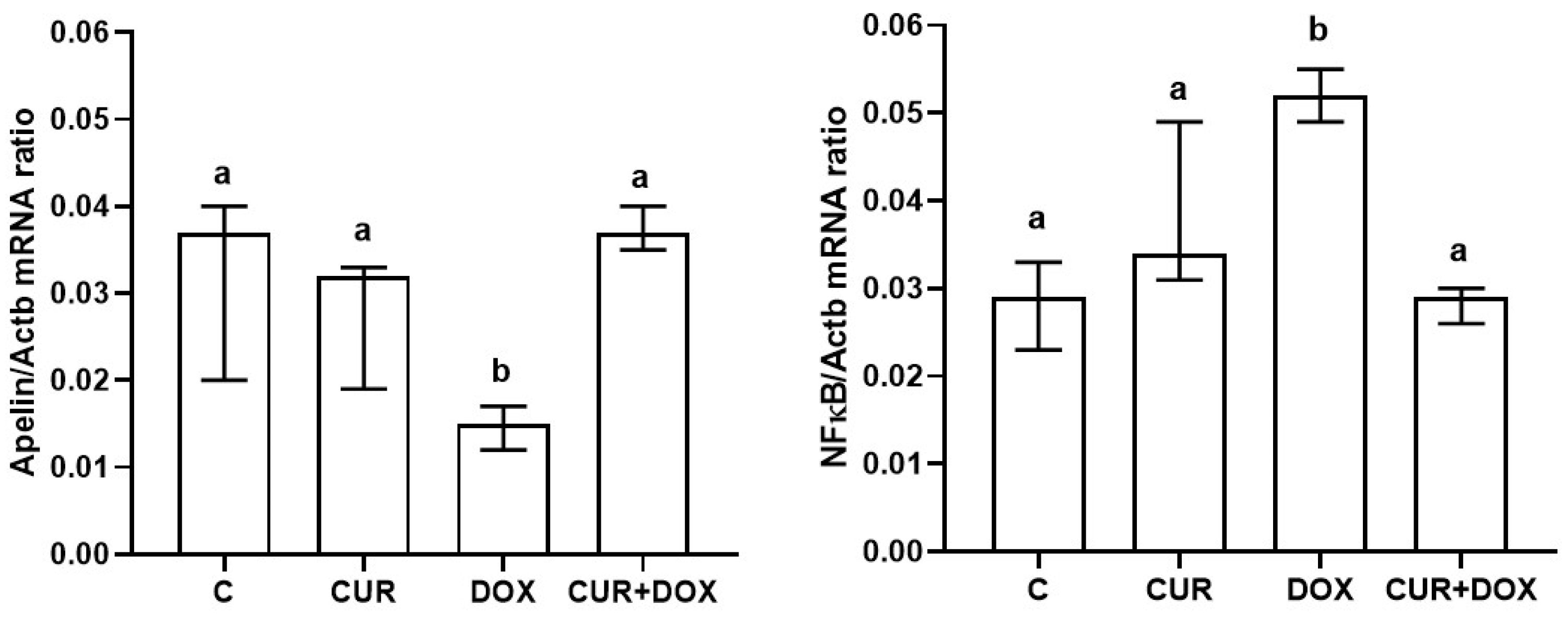
3.3. Apelin and NF-κB Gene Expression
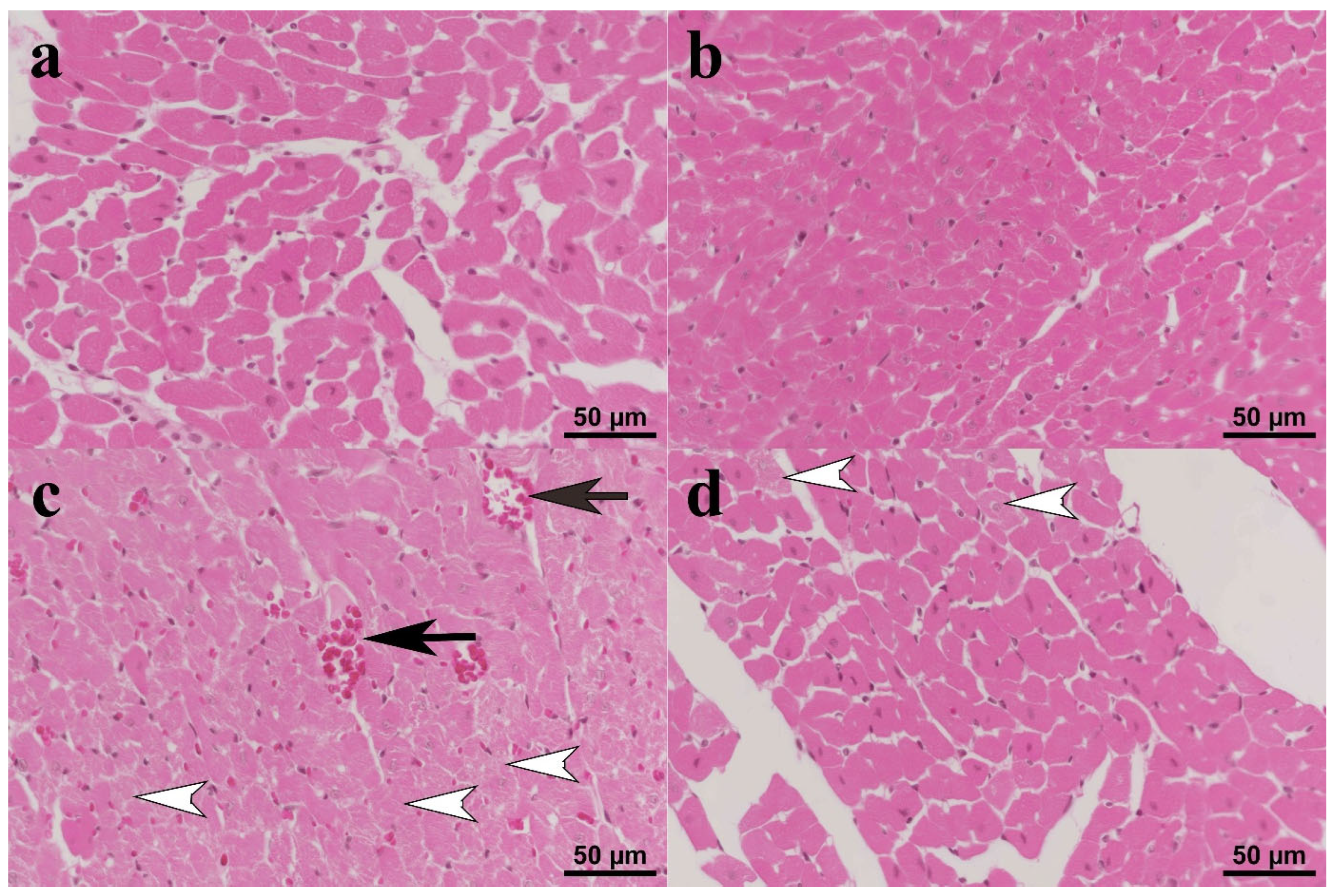
3.4. Histopathology
3.5. Immunohistochemistry
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Dox | Doxorubicin |
| MDA | Malondialdehyde |
| GSH | Glutathione |
| SOD | Superoxide dismutase |
| CAT | Catalase |
| GPx | Glutathione peroxidase |
| ROS | Reactive oxygen species |
| TNF-α | Tumor necrosis factor alpha |
| cTn | Troponin |
| NF-κB | Nuclear Factor Kappa B |
| ECG | Electrocardiography |
| iNOS | Inducible nitric oxide synthase |
| NO | Nitric oxide |
References
- Lyon, A.R.; Lopez-Fernandez, T.; Couch, L.S.; Asteggiano, R.; Aznar, M.C.; Bergler-Klein, J.; Boriani, G.; Cardinale, D.; Cordoba, R.; Cosyns, B.; et al. 2022 ESC Guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS). Eur. Heart J. Cardiovasc. Imaging 2022, 23, e333–e465. [Google Scholar] [CrossRef]
- Takemura, G.; Fujiwara, H. Doxorubicin-induced cardiomyopathy from the cardiotoxic mechanisms to management. Prog. Cardiovasc. Dis. 2007, 49, 330–352. [Google Scholar] [CrossRef] [PubMed]
- Radeva, L.; Yoncheva, K. Doxorubicin Toxicity and Recent Approaches to Alleviating Its Adverse Effects with Focus on Oxidative Stress. Molecules 2025, 30, 3311. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shati, A.A.; Eid, R.A.; El-Kott, A.F.; Alqahtani, Y.A.; Shatoor, A.S.; Ahmed Zaki, M.S. Curcumin attenuates doxorubicin-induced cardiotoxicity via suppressing oxidative Stress, preventing inflammation and apoptosis: Ultrastructural and computational approaches. Heliyon 2024, 10, e27164. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Octavia, Y.; Tocchetti, C.G.; Gabrielson, K.L.; Janssens, S.; Crijns, H.J.; Moens, A.L. Doxorubicin-induced cardiomyopathy: From molecular mechanisms to therapeutic strategies. J. Mol. Cell. Cardiol. 2012, 52, 1213–1225. [Google Scholar] [CrossRef] [PubMed]
- Pecoraro, M.; Del Pizzo, M.; Marzocco, S.; Sorrentino, R.; Ciccarelli, M.; Iaccarino, G.; Pinto, A.; Popolo, A. Inflammatory mediators in a short-time mouse model of doxorubicin-induced cardiotoxicity. Toxicol. Appl. Pharmacol. 2016, 293, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Lin, S.; Wu, W.; Tan, H.; Wang, Z.; Cheng, C.; Lu, L.; Zhang, X. Ghrelin prevents doxorubicin-induced cardiotoxicity through TNF-alpha/NF-kappaB pathways and mitochondrial protective mechanisms. Toxicology 2008, 247, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.K.; Cheng, R.; Nguyen, T.; Fan, T.; Kariyawasam, A.P.; Liu, Y.; Osmond, D.H.; George, S.R.; O’Dowd, B.F. Characterization of apelin, the ligand for the APJ receptor. J. Neurochem. 2000, 74, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Szokodi, I.; Tavi, P.; Földes, G.; Voutilainen-Myllylä, S.; Ilves, M.; Tokola, H.; Pikkarainen, S.; Piuhola, J.; Rysä, J.; Tóth, M.; et al. Apelin, the novel endogenous ligand of the orphan receptor APJ, regulates cardiac contractility. Circ. Res. 2002, 91, 434–440. [Google Scholar] [CrossRef] [PubMed]
- Pitkin, S.L.; Maguire, J.J.; Kuc, R.E.; Davenport, A.P. Modulation of the apelin/APJ system in heart failure and atherosclerosis in man. Br. J. Pharmacol. 2010, 160, 1785–1795. [Google Scholar] [CrossRef]
- Capelli, I.; Aiello, V.; Gasperoni, L.; La Manna, G. Apelin-13 alleviate inflammatory reaction of ischemia reperfusion in rat kidney transplantation via NF-kappa B signaling pathway. bioRxiv 2024. [Google Scholar] [CrossRef]
- Arruda, F.S.; Tome, F.D.; Milhomem, A.C.; Franco, P.I.R.; Justino, A.B.; Franco, R.R.; Campos, E.C.; Espindola, F.S.; Soave, D.F.; Celes, M.R.N. Curcumin Attenuates Doxorubicin-Induced Cardiac Oxidative Stress and Increases Survival in Mice. Pharmaceutics 2024, 16, 1105. [Google Scholar] [CrossRef]
- Jafarinezhad, Z.; Rafati, A.; Ketabchi, F.; Noorafshan, A.; Karbalay-Doust, S. Cardioprotective effects of curcumin and carvacrol in doxorubicin-treated rats: Stereological study. Food Sci. Nutr. 2019, 7, 3581–3588. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Benzer, F.; Kandemir, F.M.; Ozkaraca, M.; Kucukler, S.; Caglayan, C. Curcumin ameliorates doxorubicin-induced cardiotoxicity by abrogation of inflammation, apoptosis, oxidative DNA damage, and protein oxidation in rats. J. Biochem. Mol. Toxicol. 2018, 32, e22030. [Google Scholar] [CrossRef] [PubMed]
- Morton, A.B.; Mor Huertas, A.; Hinkley, J.M.; Ichinoseki-Sekine, N.; Christou, D.D.; Smuder, A.J. Mitochondrial accumulation of doxorubicin in cardiac and diaphragm muscle following exercise preconditioning. Mitochondrion 2019, 45, 52–62. [Google Scholar] [CrossRef]
- Mihara, M.; Uchiyama, M. Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal. Biochem. 1978, 86, 271–278. [Google Scholar] [CrossRef]
- Ellman, G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Oberley, L.W.; Li, Y. A simple method for clinical assay of superoxide dismutase. Clin. Chem. 1988, 34, 497–500. [Google Scholar] [CrossRef]
- Lück, H. Catalase. In Methods of Enzymatic Analysis; Bergmeyer, H.-U., Ed.; Academic Press: Cambridge, MA, USA, 1965; pp. 885–894. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Wettersten, N.; Maisel, A. Role of Cardiac Troponin Levels in Acute Heart Failure. Card. Fail. Rev. 2015, 1, 102–106. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Elberry, A.A.; Abdel-Naim, A.B.; Abdel-Sattar, E.A.; Nagy, A.A.; Mosli, H.A.; Mohamadin, A.M.; Ashour, O.M. Cranberry (Vaccinium macrocarpon) protects against doxorubicin-induced cardiotoxicity in rats. Food Chem. Toxicol. 2010, 48, 1178–1184. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Zhang, X.; Wei, W.; Zhang, N.; Wu, H.; Ma, Z.; Li, L.; Deng, W.; Tang, Q. Matrine attenuates oxidative stress and cardiomyocyte apoptosis in doxorubicin-induced cardiotoxicity via maintaining AMPKα/UCP2 pathway. Acta Pharm. Sin. B 2019, 9, 690–701. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Beinart, R.; Zhang, Y.; Lima, J.A.; Bluemke, D.A.; Soliman, E.Z.; Heckbert, S.R.; Post, W.S.; Guallar, E.; Nazarian, S. The QT interval is associated with incident cardiovascular events: The MESA study. J. Am. Coll. Cardiol. 2014, 64, 2111–2119. [Google Scholar] [CrossRef] [PubMed]
- Davey, P.P.; Barlow, C.; Hart, G. Prolongation of the QT interval in heart failure occurs at low but not at high heart rates. Clin. Sci. 2000, 98, 603–610. [Google Scholar] [CrossRef]
- Volkova, M.; Russell, R., 3rd. Anthracycline cardiotoxicity: Prevalence, pathogenesis and treatment. Curr. Cardiol. Rev. 2011, 7, 214–220. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chatterjee, K.; Zhang, J.; Honbo, N.; Karliner, J.S. Doxorubicin cardiomyopathy. Cardiology 2010, 115, 155–162. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Benjanuwattra, J.; Siri-Angkul, N.; Chattipakorn, S.C.; Chattipakorn, N. Doxorubicin and its proarrhythmic effects: A comprehensive review of the evidence from experimental and clinical studies. Pharmacol. Res. 2020, 151, 104542. [Google Scholar] [CrossRef] [PubMed]
- Kwatra, M.; Kumar, V.; Jangra, A.; Mishra, M.; Ahmed, S.; Ghosh, P.; Vohora, D.; Khanam, R. Ameliorative effect of naringin against doxorubicin-induced acute cardiac toxicity in rats. Pharm. Biol. 2016, 54, 637–647. [Google Scholar] [CrossRef] [PubMed]
- Dursun, N.; Taşkın, E.; Oztürk, F. Protection against adriamycin-induced cardiomyopathy by carnosine in rats: Role of endogenous antioxidants. Biol. Trace Elem. Res. 2011, 143, 412–424. [Google Scholar] [CrossRef] [PubMed]
- Warpe, V.S.; Mali, V.R.; Arulmozhi, S.; Bodhankar, S.L.; Mahadik, K.R. Cardioprotective effect of ellagic acid on doxorubicin induced cardiotoxicity in wistar rats. J. Acute Med. 2015, 5, 1–8. [Google Scholar] [CrossRef]
- Mantawy, E.M.; El-Bakly, W.M.; Esmat, A.; Badr, A.M.; El-Demerdash, E. Chrysin alleviates acute doxorubicin cardiotoxicity in rats via suppression of oxidative stress, inflammation and apoptosis. Eur. J. Pharmacol. 2014, 728, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Banerjee, S.K.; Maulik, M.; Dinda, A.K.; Talwar, K.K.; Maulik, S.K. Protection against acute adriamycin-induced cardiotoxicity by garlic: Role of endogenous antioxidants and inhibition of TNF-alpha expression. BMC Pharmacol. 2003, 3, 16. [Google Scholar] [CrossRef] [PubMed] [PubMed Central][Green Version]
- Zhu, J.; Zhang, J.; Zhang, L.; Du, R.; Xiang, D.; Wu, M.; Zhang, R.; Han, W. Interleukin-1 signaling mediates acute doxorubicin-induced cardiotoxicity. Biomed. Pharmacother. 2011, 65, 481–485. [Google Scholar] [CrossRef] [PubMed]
- Abdelatty, A.; Ahmed, M.S.; Abdel-Kareem, M.A.; Dmerdash, M.; Mady, R.; Saad, A.S.; Albrakati, A.; Elmahallawy, E.K.; Elsawak, A.; Abdo, W. Acute and Delayed Doxorubicin-Induced Myocardiotoxicity Associated with Elevation of Cardiac Biomarkers, Depletion of Cellular Antioxidant Enzymes, and Several Histopathological and Ultrastructural Changes. Life 2021, 11, 880. [Google Scholar] [CrossRef] [PubMed]
- Bhutani, V.; Varzideh, F.; Wilson, S.; Kansakar, U.; Jankauskas, S.S.; Santulli, G. Doxorubicin-Induced Cardiotoxicity: A Comprehensive Update. J. Cardiovasc. Dev. Dis. 2025, 12, 207. [Google Scholar] [CrossRef]
- Dulf, P.L.; Mocan, M.; Coada, C.A.; Dulf, D.V.; Moldovan, R.; Baldea, I.; Farcas, A.D.; Blendea, D.; Filip, A.G. Doxorubicin-induced acute cardiotoxicity is associated with increased oxidative stress, autophagy, and inflammation in a murine model. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2023, 396, 1105–1115. [Google Scholar] [CrossRef]
- Pereira, G.C.; Silva, A.M.; Diogo, C.V.; Carvalho, F.S.; Monteiro, P.; Oliveira, P.J. Drug-induced cardiac mitochondrial toxicity and protection: From doxorubicin to carvedilol. Curr. Pharm. Des. 2011, 17, 2113–2129. [Google Scholar] [CrossRef] [PubMed]
- Winterbourn, C.C. Toxicity of iron and hydrogen peroxide: The Fenton reaction. Toxicol. Lett. 1995, 82–83, 969–974. [Google Scholar] [CrossRef] [PubMed]
- Cao, G.; Giovanoni, M.; Prior, R.L. Antioxidant capacity in different tissues of young and old rats. Proc. Soc. Exp. Biol. Med. 1996, 211, 359–365. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Luo, Y.; Qiao, Y.; Zhang, Z.; Yin, D.; Yao, J.; You, J.; He, M. Curcumin attenuates doxorubicin-induced cardiotoxicity via suppressing oxidative stress and preventing mitochondrial dysfunction mediated by 14-3-3γ. Food Funct. 2018, 9, 4404–4418. [Google Scholar] [CrossRef] [PubMed]
- Klymenko, O.O.; Drevytska, T.I.; Gonchar, O.O.; Tarasova, K.V.; Nosar, V.I.; Dosenko, V.Y.; Mankovska, I.M. Curcumin exerts protective effects against doxorubicin-induced cardiotoxicity. Ukr. Biochem. J. 2025, 97, 25. [Google Scholar] [CrossRef]
- Adachi, T.; Nagae, T.; Ito, Y.; Hirano, K.; Sugiura, M. Relation between cardiotoxic effect of adriamycin and superoxide anion radical. J. Pharmacobiodyn. 1983, 6, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Sesarman, A.; Muntean, D.; Abrudan, B.; Tefas, L.; Sylvester, B.; Licarete, E.; Rauca, V.; Luput, L.; Patras, L.; Banciu, M.; et al. Improved pharmacokinetics and reduced side effects of doxorubicin therapy by liposomal co-encapsulation with curcumin. J. Liposome Res. 2021, 31, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Aktan, F. iNOS-mediated nitric oxide production and its regulation. Life Sci. 2004, 75, 639–653. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yao, L.; Wu, X.; Guo, Q.; Sun, S.; Li, J.; Shi, G.; Caldwell, R.B.; Caldwell, R.W.; Chen, Y. Protection against Doxorubicin-Induced Cardiotoxicity through Modulating iNOS/ARG 2 Balance by Electroacupuncture at PC6. Oxid. Med. Cell. Longev. 2021, 6628957. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- De Boo, S.; Kopecka, J.; Brusa, D.; Gazzano, E.; Matera, L.; Ghigo, D.; Bosia, A.; Riganti, C. iNOS activity is necessary for the cytotoxic and immunogenic effects of doxorubicin in human colon cancer cells. Mol. Cancer 2009, 8, 108. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kim, J.Y.; Jung, H.; Ahn, S. The relationship between nuclear factor (NF)-κB family gene expression and prognosis in triple-negative breast cancer (TNBC) patients receiving adjuvant doxorubicin treatment. Sci. Rep. 2016, 6, 31804. [Google Scholar] [CrossRef]
- Guo, Q.; Jin, Y.; Chen, X.; Ye, X.; Shen, X.; Lin, M.; Zeng, C.; Zhou, T.; Zhang, J. NF-κB in biology and targeted therapy: New insights and translational implications. Signal Transduct. Target. Ther. 2024, 9, 53. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, S.; Kotamraju, S.; Konorev, E.; Kalivendi, S.; Joseph, J.; Kalyanaraman, B. Activation of nuclear factor-kappaB during doxorubicin-induced apoptosis in endothelial cells and myocytes is pro-apoptotic: The role of hydrogen peroxide. Biochem. J. 2002, 367, 729–740. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Guo, R.M.; Xu, W.M.; Lin, J.C.; Mo, L.Q.; Hua, X.X.; Chen, P.X.; Wu, K.; Zheng, D.D.; Feng, J.Q. Activation of the p38 MAPK/NF-κB pathway contributes to doxorubicin-induced inflammation and cytotoxicity in H9c2 cardiac cells. Mol. Med. Rep. 2013, 8, 603–608. [Google Scholar] [CrossRef] [PubMed]
- Kleinz, M.J.; Skepper, J.N.; Davenport, A.P. Immunocytochemical localisation of the apelin receptor, APJ, to human cardiomyocytes, vascular smooth muscle and endothelial cells. Regul. Pept. 2005, 126, 233–240. [Google Scholar] [CrossRef]
- Matusik, K.; Kamińska, K.; Sobiborowicz-Sadowska, A.; Borzuta, H.; Buczma, K.; Cudnoch-Jędrzejewska, A. The significance of the apelinergic system in doxorubicin-induced cardiotoxicity. Heart Fail. Rev. 2024, 29, 969–988. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hamada, J.; Baasanjav, A.; Ono, N.; Murata, K.; Kako, K.; Ishida, J.; Fukamizu, A. Possible involvement of downregulation of the apelin-APJ system in doxorubicin-induced cardiotoxicity. Am. J. Physiol. Heart Circ. Physiol. 2015, 308, H931–H941. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Xing, G.; Fu, T.; Ma, Y.; Wang, Q.; Zhang, S.; Chang, X.; Tong, Y. Role of mitochondria in doxorubicin-mediated cardiotoxicity: From molecular mechanisms to therapeutic strategies. Int. J. Med. Sci. 2024, 21, 809–816. [Google Scholar] [CrossRef]
- Dai, T.; Ramirez-Correa, G.; Gao, W.D. Apelin increases contractility in failing cardiac muscle. Eur. J. Pharmacol. 2006, 553, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Du, J.F.; Wu, F. Apelin decreases the SR Ca2+ content but enhances the amplitude of [Ca2+]i transient and contractions during twitches in isolated rat cardiac myocytes. Am. J. Physiol. Heart Circ. Physiol. 2008, 294, H2540–H2546. [Google Scholar] [CrossRef] [PubMed]
- Urosevic, M.; Nikolic, L.; Gajic, I.; Nikolic, V.; Dinic, A.; Miljkovic, V. Curcumin: Biological Activities and Modern Pharmaceutical Forms. Antibiotics 2022, 11, 135. [Google Scholar] [CrossRef] [PubMed]
- Pulido-Moran, M.; Moreno-Fernandez, J.; Ramirez-Tortosa, C.; Ramirez-Tortosa, M. Curcumin and Health. Molecules 2016, 21, 264. [Google Scholar] [CrossRef]
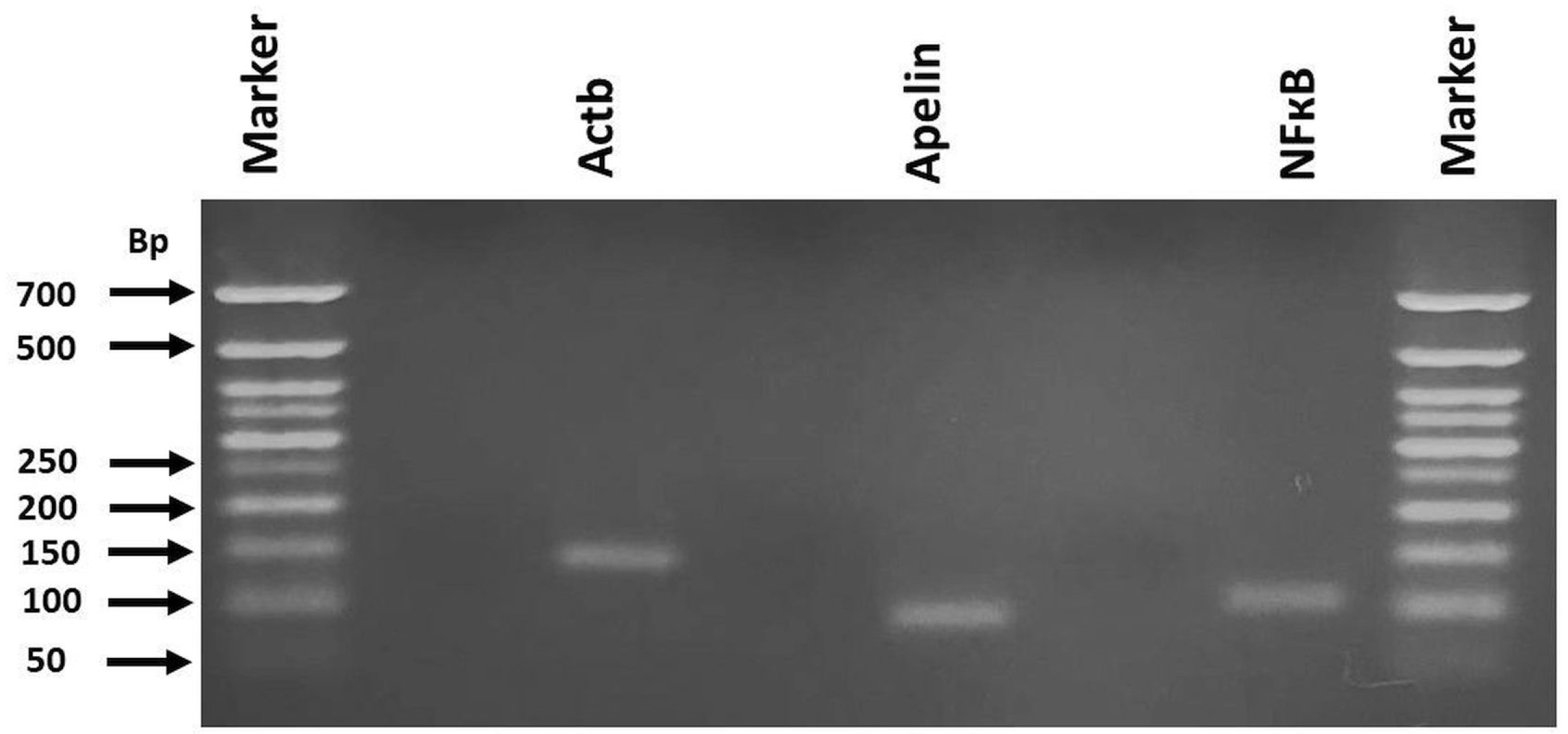


| Genes | Primer Sequences. | Gene Ref. Seq. Number | Product Size (bp) |
|---|---|---|---|
| Actb | F: 5′-ATGAGCTGCCTGACGGTCAGGT-3′ R: 5′-GTGACGTTGACATCCGTAAAGACC-3′ | NM_031144.3 | 139 |
| Apelin | F: 5′-TGCCTCTTGCCTTATTAGCCTGC-3′ R: 5′-CTTCTGTTTCTATCTCTCCTCT-3′ | NM_031612.3 | 93 |
| NF-кB | F: 5′-TTCTCCGCCCGCGCCGCAGCCA-3′ R: 5′-GCTTCCGCGCCTGCGGGCTCCCG-3′ | NM_001276711 | 103 |
| Groups | HR (Beats/Min) | SBP (mm-Hg) | DBP (mm-Hg) | MBP (mm-Hg) | PR Interval (ms) | QRS Duration (ms) | QT Interval (ms) |
|---|---|---|---|---|---|---|---|
| C | 312 (295–339) a | 76 (67–94) a | 72 (63–90) a | 74 (65–92) a | 30 (30–35) a | 51 (48–56) a | 88 (78–90) a |
| CUR | 319 (285–363) a | 89 (76–99) a | 85 (72–94) a | 87 (75–97) a | 34 (31–35) a | 46 (44–53) a | 86 (81–90) a |
| DOX | 400 (390–410) b | 117 (112–129) b | 111 (109–122) b | 115 (111–127) b | 31 (28–34) a | 54 (48–56) a | 97 (88–108) b |
| CUR+DOX | 400 (327–420) b | 115 (103–116) b | 108 (97–113) b | 111 (101–115) b | 32 (30–34) a | 48 (47–56) a | 86 (83–89) a |
| Groups | Apelin (ng/L) | IL-1β (ng/L) | TNF-α (ng/L) | Troponin (ng/L) | |||
|---|---|---|---|---|---|---|---|
| Serum | Heart | Serum | Heart | Serum | Heart | Serum | |
| C | 1794 (1556–1969) a | 4081 (4006–4219) bc | 26 (23–30) a | 51 (46–54) a | 128 (114–148) a | 197 (191–214) a | 79 (71–95) b |
| CUR | 1957 (1719–2075) a | 4194 (3969–4425) ac | 27 (18–41) a | 50 (48–53) a | 121 (115–136) a | 215 (212–226) a | 86 (63–103) b |
| DOX | 1357 (1094–1619) b | 3982 (3581–4106) b | 22 (19–25) a | 56 (50–60) a | 112 (85–145) a | 214 (201–226) a | 113 (92–106) a |
| CUR+DOX | 1931 (1681–2094) a | 4425 (4169–4794) a | 24 (20–25) a | 49 (47–52) a | 116 (91–138) a | 202 (192–209) a | 97 (93–101) ab |
| Groups | MDA (nmol/gwt) | GSH (nmol/gwt) | CuZn-SOD (U/g Protein) | CAT (K/g Protein) |
|---|---|---|---|---|
| C | 172.4 (159.5–177.5) a | 979.5 (927.3–1113.6) a | 248.1 (186.4–287.7) a | 2.3 (2–2.8) a |
| CUR | 182.9 (178.3–188.8) a | 1038.6 (979.5–1063.6) a | 273.4 (244.9–294.2) a | 2.1 (1.9–2.3) a |
| DOX | 204.5 (175.9–211.1) b | 988.6 (922.7–1118.2) a | 210.8 (152.8–253.1) b | 3.0 (2.5–3.2) b |
| CUR+DOX | 171.1 (154.1–196.3) a | 954.5 (895.5–1036.4) a | 161.2 (137.8–183.1) b | 2.9 (2.5–3.1) b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akca, B.; Disli, O.M.; Erdil, N.; Cigremis, Y.; Ozen, H.; Durhan, M.; Tunc, S.; Ozhan, O.; Ulutas, Z.; Erdil, F.A. Curcumin Alleviates Doxorubicin-Induced Cardiotoxicity by Modulating Apelin Expression. Biomolecules 2025, 15, 1416. https://doi.org/10.3390/biom15101416
Akca B, Disli OM, Erdil N, Cigremis Y, Ozen H, Durhan M, Tunc S, Ozhan O, Ulutas Z, Erdil FA. Curcumin Alleviates Doxorubicin-Induced Cardiotoxicity by Modulating Apelin Expression. Biomolecules. 2025; 15(10):1416. https://doi.org/10.3390/biom15101416
Chicago/Turabian StyleAkca, Baris, Olcay Murat Disli, Nevzat Erdil, Yilmaz Cigremis, Hasan Ozen, Merve Durhan, Selahattin Tunc, Onural Ozhan, Zeynep Ulutas, and Feray Akgul Erdil. 2025. "Curcumin Alleviates Doxorubicin-Induced Cardiotoxicity by Modulating Apelin Expression" Biomolecules 15, no. 10: 1416. https://doi.org/10.3390/biom15101416
APA StyleAkca, B., Disli, O. M., Erdil, N., Cigremis, Y., Ozen, H., Durhan, M., Tunc, S., Ozhan, O., Ulutas, Z., & Erdil, F. A. (2025). Curcumin Alleviates Doxorubicin-Induced Cardiotoxicity by Modulating Apelin Expression. Biomolecules, 15(10), 1416. https://doi.org/10.3390/biom15101416






