UHPLC-QTOF-ESI-MS/MS, SNAP-MS Identification, In Silico Prediction of Pharmacokinetic Properties of Constituents from the Stem Bark of Holarrhena floribunda (G. Don) T. Durand and Schinz (Apocynaceae)
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagent and Materials
2.2. UHPLC-QTOF-ESI-MS/MS Analyses
2.3. In Vitro Antiplasmodial Activity
Plasmodium falciparum Culture and Growth Inhibition Assay
2.4. Plant Material
Extraction and Isolation
2.5. UHPLC/MS/MS Data Processing
2.6. SwissADME, SwissTarget Prediction and In Silico Oral Toxicity
3. Results and Discussion
3.1. UHPLC-ESI-QTOF-MS/MS Identification of Compounds
3.2. Isolation of Compounds
3.3. Chemotaxonomic Significance
3.4. Biological Contribution
3.5. In Silico Pharmacokinetic Parameters, Drug-likeness Properties and Targets Prediction of Some Identified Compounds
3.6. In Silico Oral Toxicity Prediction
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| TLC | Thin Layer Chromatography |
| UHPLC | Ultra-High Performance Liquid Chromatography |
| QTOF-ESI-MS/MS | Quadrupole Time Of Flight Electrospray Ionization Tandem Mass Spectrometry |
| SNAP-MS | Structural Similarity Network Annotation Platform for Mass Spectrometry |
| ADMET | Absorption, Desorption, Metabolism, Excretion and Toxicity |
References
- WHO. World Malaria Report; WHO: Geneva, Switzerland, 2023. [Google Scholar]
- WHO. World Malaria Report: Addressing Inequity in the Global Malaria Response; WHO: Geneva, Switzerland, 2024. [Google Scholar]
- El-Moamly, A.A. How can we get malaria control back on track? BMJ 2024, 385, q1408. [Google Scholar] [CrossRef]
- Cameroon Kicks Off Malaria Vaccine Rollout. Available online: https://www.afro.who.int/countries/cameroon/news/cameroon-kicks-malaria-vaccine-rollout (accessed on 18 August 2025).
- WHO. World Malaria Report; WHO: Geneva, Switzerland, 2022. [Google Scholar]
- Gnangoran, B.N.; N’guessan, B.B.; Amoateng, P.; Dosso, K.; Yapo, A.P.; Ehile, E.E. Hypoglycaemic activity of ethanolic leaf extract and fractions of Holarrhena floribunda (Apocynaceae). J. Med. Biomed. Sci. 2012, 1, 46–54. [Google Scholar]
- Bogne, K.P.; Penlap, B.V.; Lontsi, D.; Etoa, F.X. Antibacterial activities of the extracts and conessine from Holarrhena floribunda G. Don. (Apocynaceae). Afr. J. Tradit. Complement. Altern. Med. 2008, 4, 352–356. [Google Scholar] [CrossRef] [PubMed]
- Fotie, J.; Bohle, D.S.; Leimanis, M.L.; Georges, E.; Rukunga, G.; Nkengfack, A.E. Lupeol long-chain fatty acid esters with antimalarial activity from Holarrhena floribunda. J. Nat. Prod. 2006, 69, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Hoekou, Y.P.; Tchacondo, T.; Karou, S.D.; Yerbanga, R.S.; Achoribo, E.; Da, O.; Atakpama, W.; Batawila, K. Therapeutic potentials of ethanolic extract of leaves of Holarrhena floribunda (G. Don) Dur. and schinz (Apocynaceae). Afr. J. Tradit. Complement. Altern. Med. 2017, 14, 227–233. [Google Scholar] [CrossRef]
- Nnadi, C.O.; Nwodo, N.J.; Kaiser, M.; Brun, R.; Schmidt, T.J. Steroid alkaloids from Holarrhena africana with strong activity against Trypanosoma brucei rhodesiense. Molecules 2017, 22, 1129. [Google Scholar] [CrossRef]
- Badmus, J.A.; Ekpo, O.E.; Hussein, A.A.; Meyer, M.; His, D.C. Antiproliferative and apoptosis induction potential of the methanolic leaf extract of Holarrhena floribunda (G. Don). Evid. Based Complement. Alternat. Med. 2015, 2015, 756482. [Google Scholar] [CrossRef]
- Yemoa, A.; Gbenou, J.; Affolabi, D.M.; Moudachirou, M.; Bigot, A.; Anagonou, S.; Portaels, F.; Martin, A.; Quetin-Leclercq, J. Beninese medicinal plants as a source of antimycobacterial agents: Bioguided fractionation and in vitro activity of alkaloids isolated from Holarrhena floribunda used in traditional treatment of buruli ulcer. Biomed Res. Int. 2015, 2015, 835767. [Google Scholar] [CrossRef]
- Badmus, J.A.; Okobi, E.; Rautenbach, F.; Marnewick, J.L.; Hussein, A.; Hiss, D.C. Isolation and antioxidant activity of flavonoids from Holarrhena floribunda (G.don) leaves. Acta Biochim. Pol. 2016, 63, 353–358. [Google Scholar] [CrossRef]
- Loukaci, A.; Kayser, O.; Bindseil, K.U.; Siems, K.; Frevert, J.; Abreu, M.P. New trichothecenes isolated from Holarrhena floribunda. J. Nat. Prod. 2000, 63, 52–56. [Google Scholar] [CrossRef]
- Trager, W.; Jensen, J.B. Human malaria parasites in continuous culture. J. Parasitol. 2005, 91, 484–486. [Google Scholar] [CrossRef]
- Alshammari, F. Application of computational tools for ADME and target modeling of bioactive compounds from hydroalcoholic extracts of Erodium glaucophyllum flowers. Med. Sci. 2020, 24, 4178–4183. [Google Scholar]
- Zirihi, G.N.; Grellier, P.; Guédé-Guina, F.; Bodo, B.; Mambu, L. Isolation, characterization and antiplasmodial activity of steroidal alkaloids from Funtumia elastica (Preuss) Stapf. Bioorg. Med. Chem. Lett. 2005, 15, 2637–2640. [Google Scholar] [CrossRef]
- Cheenpracha, S.; Boapun, P.; Limtharakul Née Ritthiwigrom, T.; Laphookhieo, S.; Pyne, S.G. Antimalarial and cytotoxic activities of pregnene-type steroidal alkaloids from Holarrhena pubescens roots. Nat. Prod. Res. 2017, 33, 782–788. [Google Scholar] [CrossRef]
- Zhou, L.N.; Ge, X.L.; Dong, T.T.; Gao, H.; Sun, B.H. Antibacterial steroidal alkaloids from Holarrhena antidysenteriaca. Chin. J. Nat. Med. 2017, 15, 540–545. [Google Scholar] [CrossRef]
- Tschesche, R.; Ockenfels, H. Uber, kurchi-alkaloide, V. 7α-hydroxy-conessin und holonamin, zwei neue basen aus kurchi-rinde. Chem. Ber. 1964, 97, 2316–2325. [Google Scholar] [CrossRef]
- Bhutani, K.K.; Vaid, R.M.; Ali, M.; Kapoor, R.; Soodan, S.R.; Kumar, D. Steroidal alkaloids from Holarrhena antidysenterica. Phytochemistry 1990, 29, 969–972. [Google Scholar] [CrossRef]
- Khalid, A.; Anjum, S.; Khan, M.R.; Choudhary, M.I. Kinetics and structure–activity relationship studies on pregnane-type steroidal alkaloids that inhibit cholinesterases. Bioorg. Med. Chem. 2004, 12, 1995–2003. [Google Scholar] [CrossRef]
- Kumari, G.K.; Rao, L.J.M.; Rao, K.R.; Rao, N.P.; Kaneko, K.; Mitsuhashi, H. Solanopubamides A and B, two further steroidal alkaloids from Solanum pubescens. Phytochemistry 2003, 25, 2003–2004. [Google Scholar] [CrossRef]
- Cheenpracha, S.; Jitonnom, J.; Komek, M.; Ritthiwigrom, T.; Laphookhieo, S. Acetylcholinesterase inhibitory activity and molecular docking study of steroidal alkaloids from Holarrhena pubescens barks. Steroids 2016, 108, 92–98. [Google Scholar] [CrossRef]
- Atta-Ur-Rahman; Choudhary, M.I.; Khan, M.R.; Anjum, S.; Farooq, A.; Iqbal, M.Z. New steroidal alkaloids from Sarcococca saligna. Nat. Prod. Res. 2003, 17, 235–241. [Google Scholar] [CrossRef]
- Gelaw, H. Isolation of crotepoxide from berries of Croton macrostachyus and evaluation of its anti-leishmanial activity. J. Pharmacogn. Phytochem. 2012, 1, 15–24. [Google Scholar]
- Srisurichan, S.; Pornpakakul, S. Triterpenoids from the seedpods of Holarrhena curtisii King and Gamble. Phytochem. Lett. 2015, 12, 282–286. [Google Scholar] [CrossRef]
- Zeb, M.A. Isolation and biological activity of β-Sitosterol and Stigmasterol from the roots of Indigofera heterantha. Pharm. Pharmacol. Intern. J. 2017, 5, 204–207. [Google Scholar] [CrossRef]
- Choudhary, M.I.; Ali, A.; Wahab, A.; Khan, S.; Rasheed, S.L.; Shyaula, R.A. New antiglycation and enzyme inhibitors from Parmotrema cooperi. Sci. China Chem. 2011, 54, 1926–1931. [Google Scholar] [CrossRef]
- Hong, Y.H.; Wang, S.C.; Hsu, C.; Lin, B.F.; Kuo, Y.H.; Huang, C.J. Phytoestrogenic compounds in alfalfa sprout (Medicago sativa) beyond coumestrol. J. Agric. Food Chem. 2011, 59, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Díaz, A.B.; Vera, J.; Cote, V.; Bruno-Colmenárez, I.; De Delgado, G.D. NMR elucidation and crystal structure analysis of 1-hydroxy-3,6-dimethoxy-8-methyl-9H-xanthen-9-one (lichexanthone) isolated from Vismia baccifera (Guttiferae). Bol. Latinoam. Caribe Plantas Med. Aromat. 2010, 9, 470–474. [Google Scholar]
- Egbubine, C.O.; Adeyemi, M.M.; Habila, J.D. Isolation and characterization of betulinic acid from the stem bark of Feretia canthioides Hiern and its antimalarial potential. Bull. Natl. Res. Cent. 2020, 44, 441–447. [Google Scholar] [CrossRef]
- Lunardi, I.; Peixoto, J.L.B.; Silva, C.C.D.; Shuquel, I.T.A.; Basso, E.A.; Vidotti, G.J. Triterpenic acids from Eugenia moraviana. J. Braz. Chem. Soc. 2001, 12, 180–183. [Google Scholar] [CrossRef]
- Castellano, J.M.; Ramos-Romero, S.; Perona, J.S. Oleanolic acid: Extraction, characterization and biological activity. Nutrients 2022, 14, 623. [Google Scholar] [CrossRef]
- Birhanu, G. Isolation of ursolic acid from the leaves of Ocimum lamiifolium collected from Addis Ababa Area, Ethiopia. Afr. J. Biotechnol. 2020, 19, 65–70. [Google Scholar] [CrossRef]
- Xing-Gen, T.; Xiao-Rong, Z.; Shu-Lin, P.; Xun, L.; Li-Sheng, D. Chemical constituents from the roots of Biondia hemsleyana. Chem. J. Chin. Univ. 2003, 24, 436–441. [Google Scholar]
- Baniadam, S.; Rahiminejad, M.R.; Ghannadian, M.; Saeidi, H.; Ayatollahi, A.; Aghaei, M.M. Cycloartane triterpenoids from Euphorbia Macrostegia with their cytotoxicity against MDA-MB48 and MCF-7 cancer cell lines. Iran. J. Pharm. Res. IJPR 2014, 13, 135–141. [Google Scholar]
- Prabowo, W.C.; Wirasutisna, K.R.; Insanu, M. Isolation and characterization of 3-acetyl aleuritolic acid and scopoletin from stem bark of Aleurites moluccana (L.) Willd. Int. J. Pharm. Pharm. Sci. 2013, 5, 851–853. [Google Scholar]
- Singh, N.; Kaushik, N.K.; Mohanakrishnan, D.; Tiwari, S.K.; Sahal, D. Antiplasmodial activity of medicinal plants from Chhotanagpur plateau, Jharkhand, India. J. Ethnopharmacol. 2015, 13, 152–162. [Google Scholar] [CrossRef]
- Sajjadi, S.E.; Ghanadian, M.; Haghighi, M.; Mouhebat, L. Cytotoxic effect of Cousinia verbascifolia Bunge against OVCAR-3 and HT-29 cancer cells. J. HerbMed Pharmacol. 2015, 4, 15–19. [Google Scholar]
- Dua, V.K.; Verma, G.; Singh, B.; Rajan, A.; Bagai, U.; Agarwal, D.D.; Gupta, N.; Kumar, S.; Rastogi, A. Anti-malarial property of steroidal alkaloid conessine isolated from the bark of Holarrhena antidysenterica. Malar. J. 2013, 12, 194. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissTargetPrediction: Updated data and new features for efficient prediction of protein targets of small molecules. Nucleic Acids Res. 2019, 47, W357–W364. [Google Scholar] [CrossRef] [PubMed]
- Elhag, I.Y. Chapter 12 Role of AI in ADME/Tox toward formulation optimization and delivery. In A Handbook of Artificial Intelligence in Drug Delivery; Academic Press: Cambridge, MA, USA, 2023; pp. 301–345. [Google Scholar] [CrossRef]
- Banerjee, P.; Kemmler, E.; Dunkel, M.; Preissner, R. ProTox 3.0: A webserver for the prediction of toxicity of chemicals. Nucleic Acids Res. 2024, 52, W513–W520. [Google Scholar] [CrossRef] [PubMed]


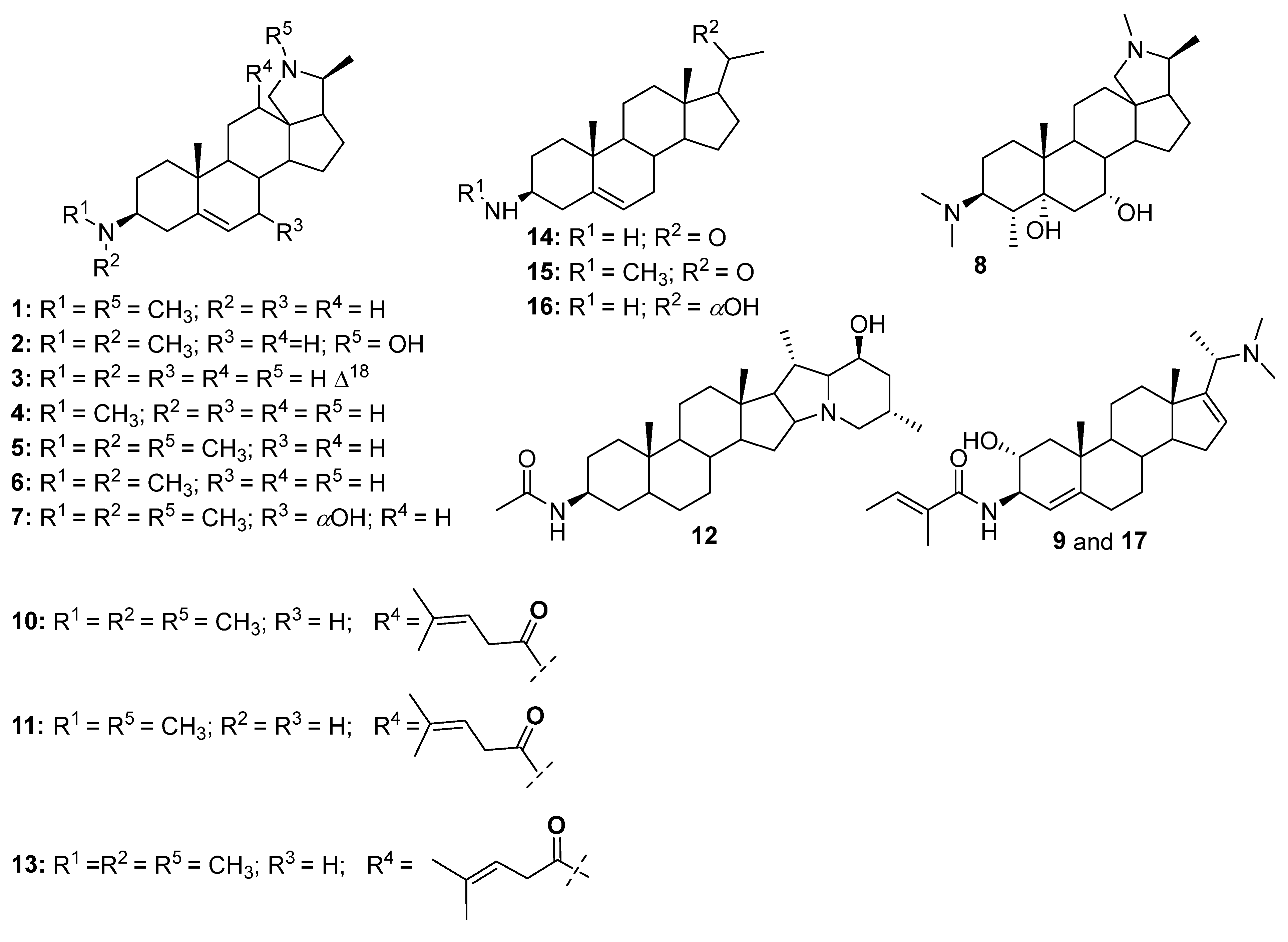

| N° | RT (min) | [M + H]+ Exp | [M + H]+ Theo | MS/MS Patterns | Names | References |
|---|---|---|---|---|---|---|
| 1 | 2.6 | 343.3113 | 343.3108 | 312, 269 | Isoconessimine (1) | [17] |
| 2 | 4.7 | 359.3059 | 359.3057 | 341, 191 | Regholarrhenine D (2) | [18] |
| 3 | 7.0 | 313.2684 | 313.2638 | / | Irheline (3) | [18] |
| 4 | 7.5 | 329.2949 | 329.2951 | 269 | Conimin/conamine (4) | [19] |
| 5 | 9.3 | 357.3275 | 357.3264 | 312, 269 | Conessine (5) | [10] |
| 6 | 12.5 | 343.3163 | 343.3108 | 298, 269 | Conessimine (6) | [17] |
| 7 | 12.8 | 373.3259 | 373.3214 | 312, 269 | 7α-hydroxyconessine (7) | [20] |
| 8 | 14.0 | 405.3402 | 405.3476 | 312, 267 | Regholarrhenine E (8) | [21] |
| 9 | 15.0 | 441.3476 | 441.3476 | 327, 267, 157 | Salignemamide D (9) | [22] |
| 10 | 15.2 | 469.3793 | 469.3789 | 455, 424, 343 | Holarrhetine (10) | [17] |
| 11 | 15.3 | 455.3653 | 455.3632 | 267, 341 | Holarrhesine (11) | [17] |
| 12 | 15.5 | 457.3781 | 457.3789 | 441, 427 | Solanopubamide B (12) | [23] |
| 13 | 16.0 | 469.3778 | 469.3789 | 455, 441, 424 | Holarrhetine isomer (13) | / |
| 14 | 18.7 | 316.2674 | 316.2635 | 161 | Holaphyllamine (14) | [24] |
| 15 | 19.2 | 318.2796 | 318.2791 | 161, 261 | Holaphyllaminol (15) | [24] |
| 16 | 19.3 | 330.2789 | 330.2791 | 261, 161, 316 | N-methylholaphyllamine (16) | [10] |
| 17 | 19.4 | 441.3476 | 441.3476 | 327, 267 | Salignemamide D isomer (17) | [25] |
| Extract/Fractions/ Compounds | IC50/Pf3D7 | IC50)/PfDd2 | RI: [IC50PfDd2/IC50Pf3D7] | CC50 on Vero Cells | CC50 on KB-3-1 |
|---|---|---|---|---|---|
| IC50 Values [Compound and Reference Drug (µM) and Extracts and Fractions (µg/mL)] | |||||
| EtOH crude extract | 14.11 ± 3.06 | 23.32 ± 2.39 | 1.65 | >500 µg/mL | ND |
| n-hexane fraction | 36.36 ± 1.59 | 43.54 ± 2.74 µg/mL | 1.19 | >500 µg/mL | ND |
| CH2Cl2 fraction | >100 | >100 | - | >500 µg/mL | ND |
| n-butanol fraction | NT | NT | - | ND | ND |
| Alkaloid fraction | 6.54 ± 0.570 | 8.27 ± 0.650 | 1.26 | 162.9 ± 3.970 µg/mL | NT |
| conessine (5) | 25.97 ± 0.43 | 28.51 ± 0.704 | 1.09 | NT | NT |
| Irheline (3) | >50 | >50 | - | NT | NT |
| Holarrhesine (11) | NT | NT | - | NT | NT |
| Holaphyllamine (14) | 49.53 ± 4.830 | 55.78 ± 9.750 µM | 1.12 | NT | 9.8 µM |
| Holaphylaminol (15) | NT | NT | - | NT | NT |
| Betulinic acid (22) | NT | NT | - | NT | NT |
| Ethylorsalinate (26) | >50 | >50 | - | NT | NT |
| Lichexanthone (27) | 33.41 ± 1.200 | 36.46 ± 1.39 | 1.09 | NT | NT |
| Chloroquine * | 0.045 ± 0.003 | 0.73 ± 0.090 | 16.22 | NT | NT |
| Artemisinin * | 0.035 ± 0.000 | 0.025 ± 0.006 | 0.71 | NT | NT |
| Cryptophycin * | - | - | - | - | 1.3 × 10−5 μM |
| Names | IC50 (µM) | References |
|---|---|---|
| Isoconessimine (1) | 3.39 ± 079 (Pf FCB1) | [17] |
| Irheline (3) | 1.2 (Pf K1) | [18] |
| Conimin (4) | 8.0 (Pf K1) | |
| Conessine (5) | 1.04 ± 014 (Pf FCB1) | [17] |
| Holarrhetine (10) | 1.13 ± 0.32 (Pf FCB1) | |
| Holarrhesine (11) | 0.97 ± 0.11 (Pf FCB1) | |
| N-methylholaphyllamine (16) | 10.6 (Pf K1) | [18] |
| Holaphyllaminol (15) | 11.7 (Pf K1) | |
| Chloroquine * | 0.13 ± 0.03 | [17] |
| Holarrhesine (11) | Irheline (3) | Holarrhetine (10) | Holaphyllaminol (15) | Holaphyllamine (14) | Conessine (5) | N-methylholaphyllamine (16) | Isoconessimine (1) | Conimin (4) | |
|---|---|---|---|---|---|---|---|---|---|
| Physicochemical Property | |||||||||
| MW (g/mol) | 454.69 | 312.49 | 468.71 | 317.51 | 315.49 | 356.59 | 329.52 | 342.56 | 328.53 |
| Pharmacokinetics | |||||||||
| GI absorption | High | High | High | High | High | High | High | High | High |
| BBB permeant | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes |
| P-gp substrate | No | No | No | Yes | No | No | No | No | No |
| CYP1A2 inhibitor | No | No | No | No | No | No | No | No | No |
| CYP2C19 inhibitor | No | No | No | No | No | No | No | No | No |
| CYP2C9 inhibitor | No | No | No | No | No | No | No | No | No |
| CYP2D6 inhibitor | No | No | No | No | No | No | No | No | No |
| CYP3A4 inhibitor | No | No | No | No | No | No | No | No | No |
| Lipophilicity | |||||||||
| Log Kp (skin permeation) | −5.24 cm/s | −6.15 cm/s | −4.99 cm/s | −5.20 cm/s | −5.41 cm/s | −5.03 cm/s | −5.14 cm/s | −5.27 cm/s | −5.52 cm/s |
| Log S (ESOL) | −5.73 | −3.60 | −6.11 | −4.44 | −4.22 | −5.04 | −4.57 | −4.66 | −4.28 |
| Solubility | 8.45 × 10−4 mg/mL; 1.86 × 10−6 mol/L | 7.88 × 10−2 mg/mL; 2.52 × 10−4 mol/L | 3.60 × 10−4 mg/mL; 7.69 × 10−7 mol/L | 1.16 × 10−2 mg/mL; 3.64 × 10−5 mol/L | 1.88 × 10−2 mg/mL; 5.96 × 10−5 mol/L | 3.25 × 10−3 mg/mL; 9.11 × 10−6 mol/L | 8.93 × 10−3 mg/mL; 2.71 × 10−5 mol/L | 7.43 × 10−3 mg/mL; 2.17 × 10−5 mol/L | 1.72 × 10−2 mg/mL; 5.24 × 10−5 mol/L |
| Class | Moderately soluble | Soluble | Poorly soluble | Moderately soluble | Moderately soluble | Moderately soluble | Moderately soluble | Moderately soluble | Moderately soluble |
| Log S (Ali) | −6.03 | −3.36 | −6.33 | −4.96 | −3.90 | −4.72 | −4.80 | −4.43 | −4.12 |
| Solubility | 4.27 × 10−4 mg/mL; 9.38 × 10−7 mol/L | 1.38 × 10−1 mg/mL; 4.40 × 10−4 mol/L | 2.19 × 10−4 mg/mL; 4.67 × 10−7 mol/L | 3.45 × 10−3 mg/mL; 1.09 × 10−5 mol/L | 3.94 × 10−2 mg/mL; 1.25 × 10−4 mol/L | 6.79 × 10−3 mg/mL; 1.90 × 10−5 mol/L | 5.21 × 10−3 mg/mL; 1.58 × 10−5 mol/L | 1.38 × 10−2 mg/mL; 4.04 × 10−5 mol/L | 2.47 × 10−2 mg/mL; 7.51 × 10−5 mol/L |
| Drug-likeness | |||||||||
| Lipinski | Yes; 1 violation: MLOGP > 4.15 | Yes; 0 violation | Yes; 1 violation: MLOGP > 4.15 | Yes; 1 violation: MLOGP > 4.15 | Yes, 0 violation | Yes; 1 violation: MLOGP > 4.15 | Yes; 1 violation: MLOGP > 4.15 | Yes; 1 violation: MLOGP > 4.15 | Yes; 1 violation: MLOGP > 4.15 |
| Ghose | No; 2 violations: MR > 130, atoms > 70 | Yes | No; 2 violations: MR > 130, atoms > 70 | Yes | Yes | Yes | Yes | Yes | Yes |
| Veber | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Egan | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Muegge | No; 1 violation: XLOGP3 > 5 | Yes | No; 1 violation: XLOGP3 > 5 | Yes | Yes | Yes | Yes | Yes | Yes |
| Medicinal Chemistry | |||||||||
| Bioavailability Score | 0.55 | 0.55 | 0.55 | 0.55 | 0.55 | 0.55 | 0.55 | 0.55 | 0.55 |
| Lead-likeness | No; 2 violations: MW > 350, XLOGP3 > 3.5 | Yes | No; 2 violations: MW > 350, XLOGP3 > 3.5 | No; 1 violation: XLOGP3 > 3.5 | No; 1 violation: XLOGP3 > 3.5 | No; 2 violations: MW > 350, XLOGP3 > 3.5 | No; 1 violation: XLOGP3 > 3.5 | No; 1 violation: XLOGP3 > 3.5 | No; 1 violation: XLOGP3 > 3.5 |
| Targets Compounds | Hepatotoxicity (SP) | Nephrotoxicity (SP) | Cardiotoxicity (SP) | Respiratory Toxicity (SP) | LD50 (mg/kg) |
|---|---|---|---|---|---|
| Conesine (5) | Inactive (0.89) | Inactive (0.94) | Inactive (0.81) | Active (0.77) | 750 |
| Holaphyllamine (14) | Inactive (0.75) | Inactive (0.92) | Inactive (0.78) | Active (0.73) | 390 |
| Lichexanthone (27) | Inactive (0.75) | active (0.61) | Active (0.53) | Active (0.75) | 3200 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Djila Possi, F.L.; Kinyok, M.J.; Mbasso Tameko, J.E.; G. Mountessou, B.Y.; Jumeta Dongmo, J.K.; Tchatat Tali, M.B.; Kene Dongmo, A.; Fekam Boyom, F.; Kezetas Bankeu, J.J.; Sewald, N.; et al. UHPLC-QTOF-ESI-MS/MS, SNAP-MS Identification, In Silico Prediction of Pharmacokinetic Properties of Constituents from the Stem Bark of Holarrhena floribunda (G. Don) T. Durand and Schinz (Apocynaceae). Biomolecules 2025, 15, 1415. https://doi.org/10.3390/biom15101415
Djila Possi FL, Kinyok MJ, Mbasso Tameko JE, G. Mountessou BY, Jumeta Dongmo JK, Tchatat Tali MB, Kene Dongmo A, Fekam Boyom F, Kezetas Bankeu JJ, Sewald N, et al. UHPLC-QTOF-ESI-MS/MS, SNAP-MS Identification, In Silico Prediction of Pharmacokinetic Properties of Constituents from the Stem Bark of Holarrhena floribunda (G. Don) T. Durand and Schinz (Apocynaceae). Biomolecules. 2025; 15(10):1415. https://doi.org/10.3390/biom15101415
Chicago/Turabian StyleDjila Possi, Franck Landry, Mc Jesus Kinyok, Joseph Eric Mbasso Tameko, Bel Youssouf G. Mountessou, Johanne Kevine Jumeta Dongmo, Mariscal Brice Tchatat Tali, Appolinaire Kene Dongmo, Fabrice Fekam Boyom, Jean Jules Kezetas Bankeu, Norbert Sewald, and et al. 2025. "UHPLC-QTOF-ESI-MS/MS, SNAP-MS Identification, In Silico Prediction of Pharmacokinetic Properties of Constituents from the Stem Bark of Holarrhena floribunda (G. Don) T. Durand and Schinz (Apocynaceae)" Biomolecules 15, no. 10: 1415. https://doi.org/10.3390/biom15101415
APA StyleDjila Possi, F. L., Kinyok, M. J., Mbasso Tameko, J. E., G. Mountessou, B. Y., Jumeta Dongmo, J. K., Tchatat Tali, M. B., Kene Dongmo, A., Fekam Boyom, F., Kezetas Bankeu, J. J., Sewald, N., Chouna, J. R., & Ndjakou Lenta, B. (2025). UHPLC-QTOF-ESI-MS/MS, SNAP-MS Identification, In Silico Prediction of Pharmacokinetic Properties of Constituents from the Stem Bark of Holarrhena floribunda (G. Don) T. Durand and Schinz (Apocynaceae). Biomolecules, 15(10), 1415. https://doi.org/10.3390/biom15101415






