GLP-1 Receptor Agonists in Heart Failure
Abstract
1. Introduction
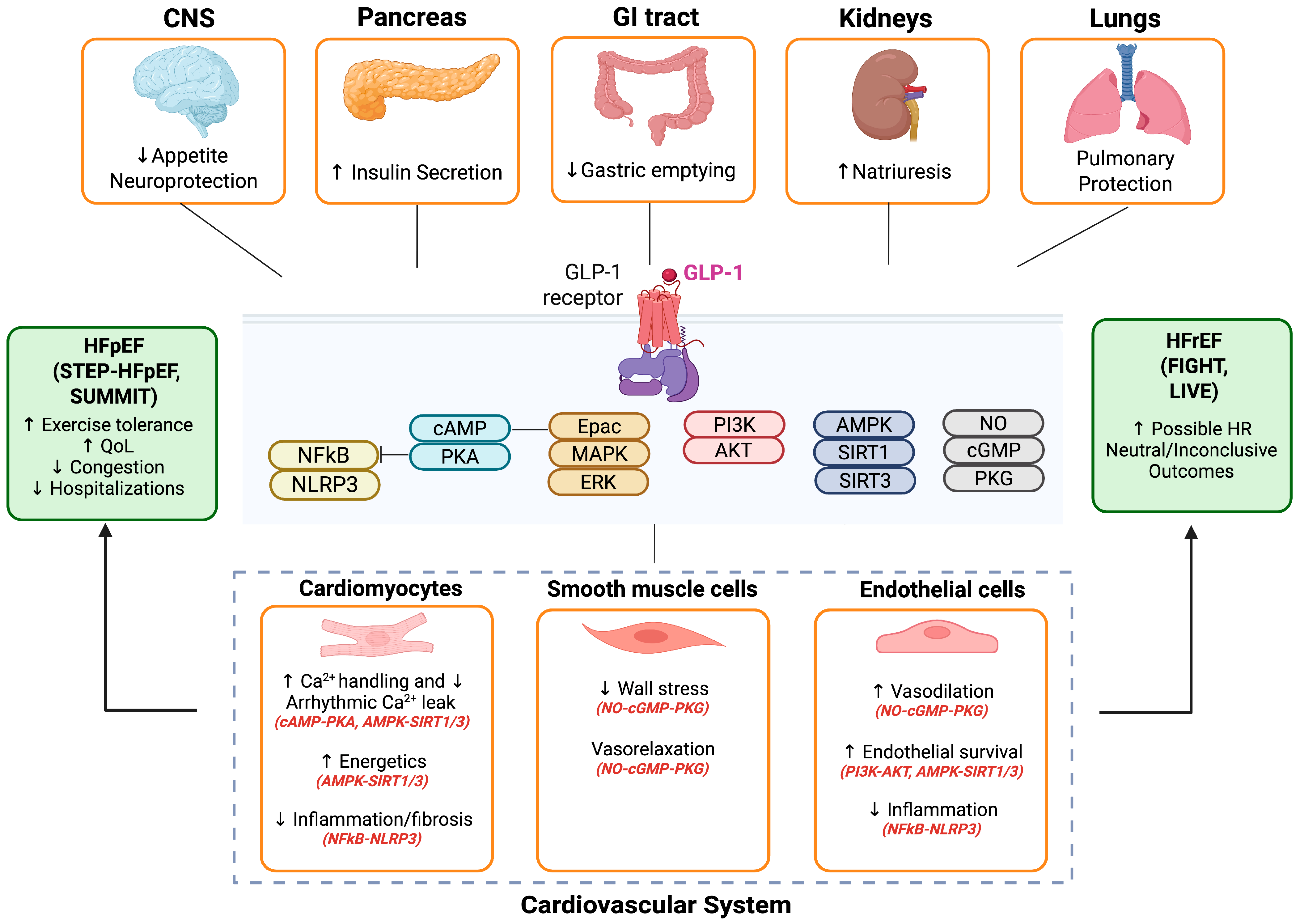
2. GLP-1 Receptor Agonists Biology in Heart Failure
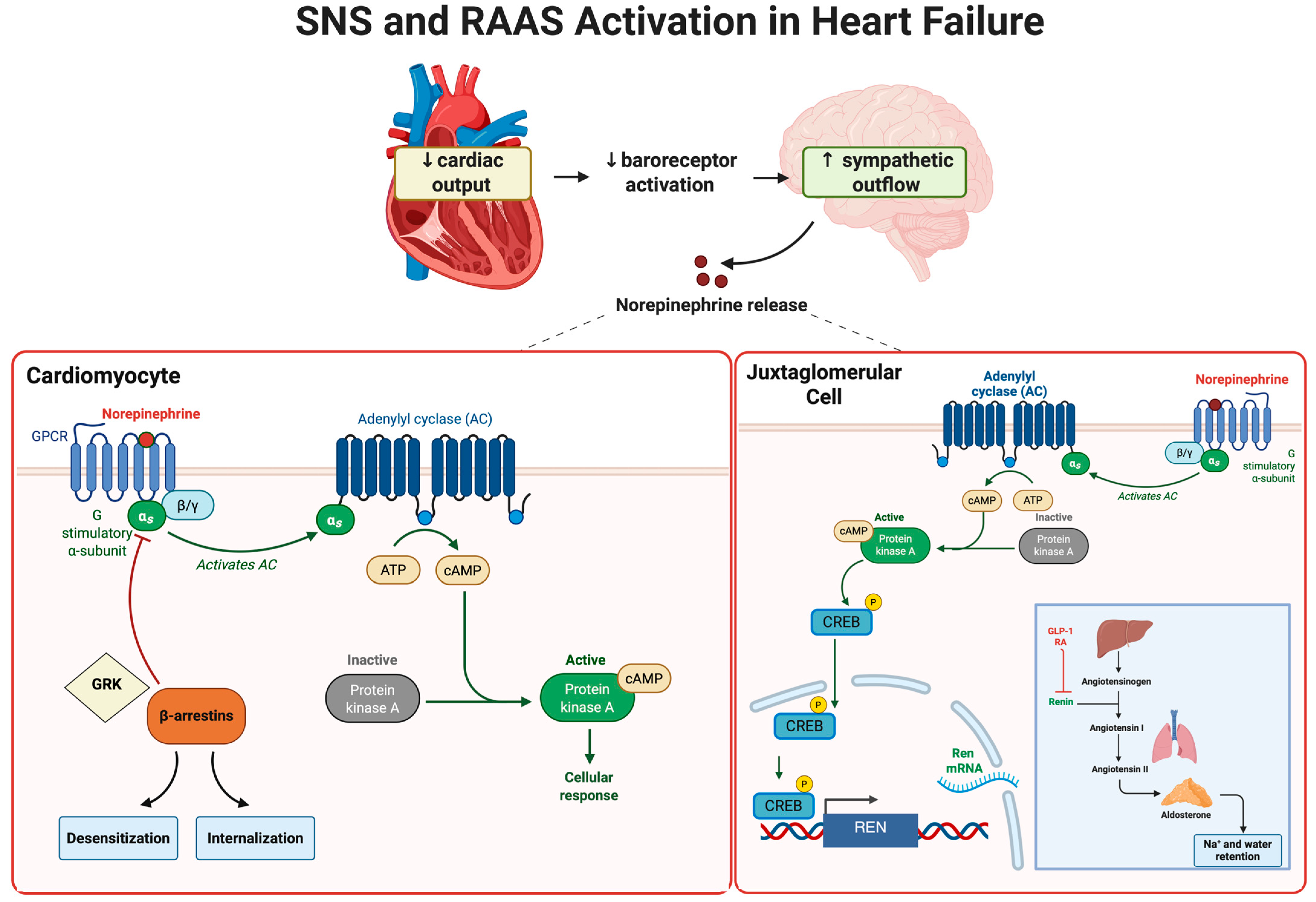
3. Sympathetic Nervous System Activation in Heart Failure
4. The Renin–Angiotensin–Aldosterone System in Heart Failure
5. Inflammation and Oxidative Stress in Heart Failure
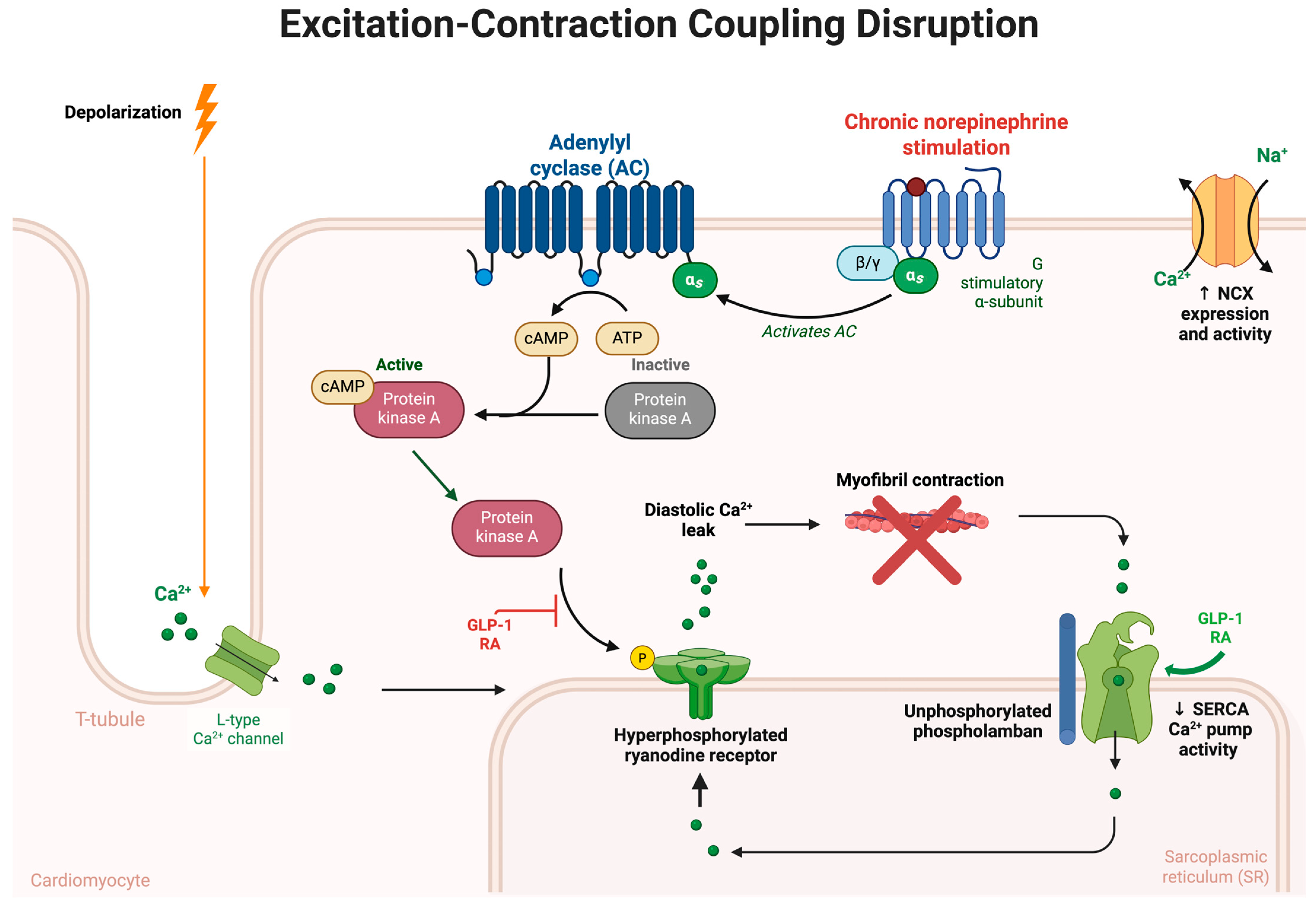
6. Calcium Handling and Excitation-Contraction Coupling in Heart Failure
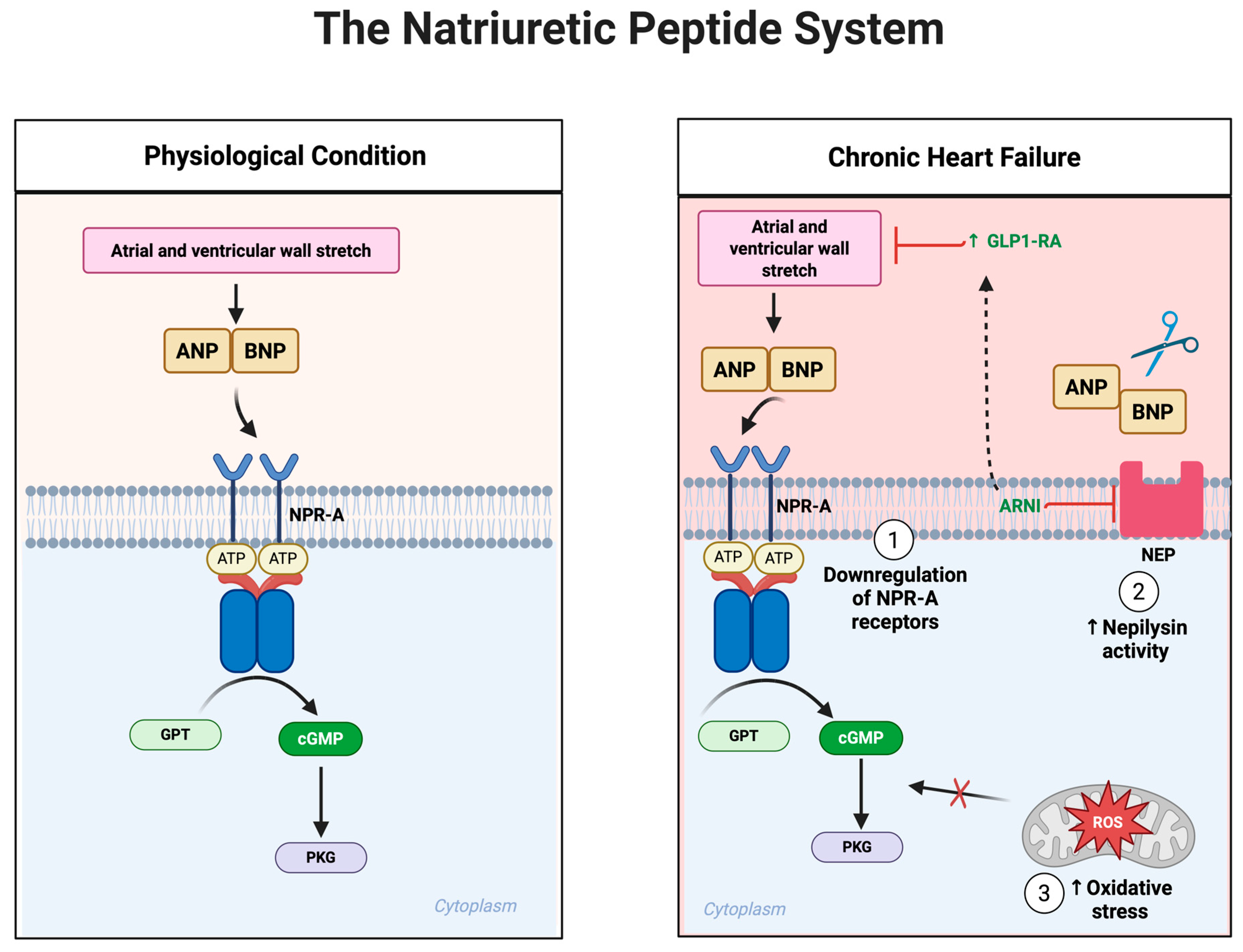
7. The Natriuretic Peptide System in Heart Failure
8. Discussion
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACE | Angiotensin-Converting Enzyme |
| AMPK | AMP-Activated Protein Kinase |
| ANP | Atrial Natriuretic Peptide |
| Ang II | Angiotensin II |
| ARNI | Angiotensin Receptor–Neprilysin Inhibitor |
| BNP | B-type Natriuretic Peptide |
| BMI | Body Mass Index |
| cAMP | Cyclic Adenosine Monophosphate |
| cGMP | Cyclic Guanosine Monophosphate |
| CRP | C-Reactive Protein |
| eNOS | Endothelial Nitric Oxide Synthase |
| GLP-1 RA | Glucagon-Like Peptide-1 Receptor Agonist |
| GIP | Glucose Dependent Insulinotropic Polypeptide |
| HF | Heart Failure |
| HFpEF | Heart Failure with preserved Ejection Fraction |
| HFrEF | Heart Failure with reduced Ejection Fraction |
| IL-1β | Interleukin-1 beta |
| IL-6 | Interleukin-6 |
| LTCC | L-Type Calcium Channel |
| MAPK | Mitogen-Activated Protein Kinase |
| NCX | Sodium–Calcium Exchanger |
| NF-κB | Nuclear Factor kappa-light-chain-enhancer of activated B cells |
| NLRP3 | NOD-like Receptor Pyrin domain-containing 3 inflammasome |
| NO | Nitric Oxide |
| NPR-A | Natriuretic Peptide Receptor A |
| NT-proBNP | N-terminal pro-B-type Natriuretic Peptide |
| NADPH | Nicotinamide Adenine Dinucleotide Phosphate Hydrogen |
| PGC-1α | PPAR Gamma Coactivator-1 Alpha |
| PKA | Protein Kinase A |
| PKCε | Protein Kinase C epsilon |
| PKG | Protein Kinase G |
| PLB | Phospholamban |
| RAAS | Renin–Angiotensin–Aldosterone System |
| ROS | Reactive Oxygen Species |
| RyR2 | Ryanodine Receptor 2 |
| SERCA2a | Sarco/Endoplasmic Reticulum Ca2+-ATPase 2a |
| SIRT1 | Sirtuin 1 |
| SIRT3 | Sirtuin 3 |
| Smad | SMAD family of signaling proteins |
| SNS | Sympathetic Nervous System |
| SR | Sarcoplasmic Reticulum |
| TGF-β | Transforming Growth Factor-beta |
| TNF-α | Tumor Necrosis Factor-alpha |
References
- Bozkurt, B.; Ahmad, T.; Alexander, K.; Baker, W.L.; Bosak, K.; Breathett, K.; Carter, S.; Drazner, M.H.; Dunlay, S.M.; Fonarow, G.C.; et al. HF STATS 2024: Heart Failure Epidemiology and Outcomes Statistics an Updated 2024 Report from the Heart Failure Society of America. J. Card. Fail. 2025, 31, 66–116. [Google Scholar] [CrossRef] [PubMed]
- Ponikowski, P.; Voors, A.; Anker, S.; Bueno, H.; Cleland, J.; Coats, A.; Falk, V.; González-Juanatey, J.; Harjola, V.; Jankowska, E. Authors/task force members. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the Special Contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2016, 37, 2129–2200. [Google Scholar]
- Powell-Wiley, T.M.; Poirier, P.; Burke, L.E.; Després, J.P.; Gordon-Larsen, P.; Lavie, C.J.; Lear, S.A.; Ndumele, C.E.; Neeland, I.J.; Sanders, P.; et al. Obesity and Cardiovascular Disease: A Scientific Statement from the American Heart Association. Circulation 2021, 143, e984–e1010. [Google Scholar] [CrossRef]
- Kenchaiah, S.; Evans, J.C.; Levy, D.; Wilson, P.W.F.; Benjamin, E.J.; Larson, M.G.; Kannel, W.B.; Vasan, R.S. Obesity and the risk of heart failure. N. Engl. J. Med. 2002, 347, 305–313. [Google Scholar] [CrossRef]
- Hu, G.; Jousilahti, P.; Antikainen, R.; Katzmarzyk, P.T.; Tuomilehto, J. joint effects of physical activity, body mass index, waist circumference, and waist-to-hip ratio on the risk of heart failure. Circulation 2010, 121, 237–244. [Google Scholar] [CrossRef]
- Pandey, A.; Patel, K.V.; Vaduganathan, M.; Sarma, S.; Haykowsky, M.J.; Berry, J.D.; Lavie, C.J. physical activity, fitness, and obesity in heart failure with preserved ejection fraction. JACC Heart Fail. 2018, 6, 975–982. [Google Scholar] [CrossRef]
- Pandey, A.; LaMonte, M.; Klein, L.; Ayers, C.; Psaty, B.M.; Eaton, C.B.; Allen, N.B.; de Lemos, J.A.; Carnethon, M.; Greenland, P.; et al. Relationship Between Physical Activity, Body Mass Index, and Risk of Heart Failure. J. Am. Coll. Cardiol. 2017, 69, 1129–1142. [Google Scholar] [CrossRef]
- Obokata, M.; Reddy, Y.N.V.; Pislaru, S.V.; Melenovsky, V.; Borlaug, B.A. Evidence Supporting the Existence of a Distinct Obese Phenotype of Heart Failure with Preserved Ejection Fraction. Circulation 2017, 136, 6–19. [Google Scholar] [CrossRef]
- Reddy, Y.N.V.; Rikhi, A.; Obokata, M.; Shah, S.J.; Lewis, G.D.; AbouEzzedine, O.F.; Dunlay, S.; McNulty, S.; Chakraborty, H.; Stevenson, L.W.; et al. Quality of life in heart failure with preserved ejection fraction: Importance of obesity, functional capacity, and physical inactivity. Eur. J. Heart Fail. 2020, 22, 1009–1018. [Google Scholar] [CrossRef] [PubMed]
- Lincoff, A.M.; Brown-Frandsen, K.; Colhoun, H.M.; Deanfield, J.; Emerson, S.S.; Esbjerg, S.; Hardt-Lindberg, S.; Hovingh, G.K.; Kahn, S.E.; Kushner, R.F.; et al. Semaglutide and Cardiovascular Outcomes in Obesity Without Diabetes. N. Engl. J. Med. 2023, 389, 2221–2232. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association Professional Practice Committee; Introduction and Methodology: Standards of Care in Diabetes—2024. Diabetes Care 2023, 47 (Suppl. S1), S1–S4.
- Margulies, K.B.; Hernandez, A.F.; Redfield, M.M.; Givertz, M.M.; Oliveira, G.H.; Cole, R.; Mann, D.L.; Whellan, D.J.; Kiernan, M.S.; Felker, G.M.; et al. Effects of Liraglutide on Clinical Stability Among Patients with Advanced Heart Failure and Reduced Ejection Fraction: A Randomized Clinical Trial. JAMA 2016, 316, 500–508. [Google Scholar] [CrossRef]
- Jorsal, A.; Kistorp, C.; Holmager, P.; Tougaard, R.S.; Nielsen, R.; Hänselmann, A.; Nilsson, B.; Møller, J.E.; Hjort, J.; Rasmussen, J.; et al. Effect of liraglutide, a glucagon-like peptide-1 analogue, on left ventricular function in stable chronic heart failure patients with and without diabetes (live)-a multicentre, double-blind, randomised, placebo-controlled trial. Eur. J. Heart Fail. 2017, 19, 69–77. [Google Scholar] [CrossRef]
- Kosiborod, M.N.; Abildstrøm, S.Z.; Borlaug, B.A.; Butler, J.; Rasmussen, S.; Davies, M.; Hovingh, G.K.; Kitzman, D.W.; Lindegaard, M.L.; Møller, D.V.; et al. Semaglutide in Patients with Heart Failure with Preserved Ejection Fraction and Obesity. N. Engl. J. Med. 2023, 389, 1069–1084. [Google Scholar] [CrossRef]
- Kosiborod, M.N.; Petrie, M.C.; Borlaug, B.A.; Butler, J.; Davies, M.J.; Hovingh, G.K.; Kitzman, D.W.; Møller, D.V.; Treppendahl, M.B.; Verma, S.; et al. Semaglutide in Patients with Obesity-Related Heart Failure and Type 2 Diabetes. N. Engl. J. Med. 2024, 390, 1394–1407. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, H.F.; Waqas, S.A.; Batool, R.M.; Salim, H.; Minhas, A.M.K.; Hasni, S.F.; Alsaid, A.; Sannino, A.; Afzal, A.M.; Khan, M.S. The Effect of GLP-1 Receptor Agonists on Cardiac Remodeling in Heart Failure Patients with Preserved and Reduced Ejection Fraction: A Systematic Review and Meta-Analysis. Heart Fail. Rev. 2025, 30, 991–1004. [Google Scholar] [CrossRef] [PubMed]
- Baggio, L.L.; Yusta, B.; E Mulvihill, E.; Cao, X.; Streutker, C.J.; Butany, J.; Cappola, T.P.; Margulies, K.B.; Drucker, D.J. GLP-1 receptor expression within the human heart. Endocrinology 2018, 159, 1570–1584. [Google Scholar] [CrossRef]
- Ma, X.; Liu, Z.; Ilyas, I.; Little, P.J.; Kamato, D.; Sahebka, A.; Chen, Z.; Luo, S.; Zheng, X.; Weng, J.; et al. GLP-1 receptor agonists (GLP-1RAS): Cardiovascular actions and therapeutic potential. Int. J. Biol. Sci. 2021, 17, 2050–2068. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Zong, Y.; Ma, Y.; Tian, Y.; Pang, Y.; Zhang, C.; Gao, J. Glucagon-like peptide-1 receptor: Mechanisms and advances in therapy. Signal Transduct. Target. Ther. 2024, 9, 234. [Google Scholar] [CrossRef]
- Hullon, D.; Subeh, G.K.; Volkova, Y.; Janiec, K.; Trach, A.; Mnevets, R. The role of glucagon-like peptide-1 receptor (GLP-1R) agonists in enhancing endothelial function: A potential avenue for improving heart failure with preserved ejection fraction (HFpEF). Cardiovasc. Diabetol. 2025, 24, 70. [Google Scholar] [CrossRef]
- Lin, K.; Wang, A.; Zhai, C.; Zhao, Y.; Hu, H.; Huang, D.; Zhai, Q.; Yan, Y.; Ge, J. Semaglutide protects against diabetes-associated cardiac inflammation via Sirt3-dependent RKIP pathway. Br. J. Pharmacol. 2025, 182, 1561–1581. [Google Scholar] [CrossRef]
- Sequeira, V.; Theisen, J.; Ermer, K.J.; Oertel, M.; Xu, A.; Weissman, D.; Ecker, K.; Dudek, J.; Fassnacht, M.; Nickel, A.; et al. Semaglutide normalizes increased cardiomyocyte calcium transients in a rat model of high fat diet-induced obesity. ESC Heart Fail. 2025, 12, 1386–1397. [Google Scholar] [CrossRef] [PubMed]
- Gliozzi, M.; Coppoletta, A.R.; Cardamone, A.; Carresi, C.; Mollace, R.; Musolino, V.; Mollace, V. Modulation of GLP-1 signalling as an innovative strategy counteracting the onset of heart failure: Potential for natural compound supplementation. Pharmacol. Res. 2025, 216, 107744. [Google Scholar] [CrossRef]
- Al-Noshokaty, T.M.; Abdelhamid, R.; Abdelmaksoud, N.M.; Khaled, A.; Hossam, M.; Ahmed, R.; Saber, T.; Khaled, S.; Elshaer, S.S.; Abulsoud, A.I. Unlocking the multifaceted roles of GLP-1: Physiological functions and therapeutic potential. Toxicol. Rep. 2025, 14, 101895. [Google Scholar] [CrossRef]
- Martins, F.L.; Bailey, M.A.; Girardi, A.C.C. Endogenous Activation of Glucagon-Like Peptide-1 Receptor Contributes to Blood Pressure Control: Role of Proximal Tubule Na(+)/H(+) Exchanger Isoform 3, Renal Angiotensin II, and Insulin Sensitivity. Hypertension 2020, 76, 839–848. [Google Scholar] [CrossRef]
- Skov, J.; Dejgaard, A.; Frøkiær, J.; Holst, J.J.; Jonassen, T.; Rittig, S.; Christiansen, J.S. Glucagon-like peptide-1 (GLP-1): Effect on kidney hemodynamics and renin-angiotensin-aldosterone system in healthy men. J. Clin. Endocrinol. Metab. 2013, 98, E664–E671. [Google Scholar] [CrossRef]
- Ma, Y.-L.; Kong, C.-Y.; Guo, Z.; Wang, M.-Y.; Wang, P.; Liu, F.-Y.; Yang, D.; Yang, Z.; Tang, Q.-Z. Semaglutide ameliorates cardiac remodeling in male mice by optimizing energy substrate utilization through the Creb5/NR4a1 axis. Nat. Commun. 2024, 15, 4757. [Google Scholar] [CrossRef] [PubMed]
- Tian, M.-Y.; Yang, J.-Q.; Hu, J.-C.; Lu, S.; Ji, Y. Semaglutide administration protects cardiomyocytes in db/db mice via energetic improvement and mitochondrial quality control. Acta Pharmacol. Sin. 2025, 46, 1250–1261. [Google Scholar] [CrossRef]
- Li, Q.; Tuo, X.; Li, B.; Deng, Z.; Qiu, Y.; Xie, H. Semaglutide attenuates excessive exercise-induced myocardial injury through inhibiting oxidative stress and inflammation in rats. Life Sci. 2020, 250, 117531. [Google Scholar] [CrossRef]
- Zhang, L.; Tian, J.; Diao, S.; Zhang, G.; Xiao, M.; Chang, D. Glp-1 receptor agonist liraglutide protects cardiomyocytes from il-1β-induced metabolic disturbance and mitochondrial dysfunction. Chem. Biol. Interact. 2020, 332, 109252. [Google Scholar] [CrossRef] [PubMed]
- Krammer, T.; Baier, M.J.; Hegner, P.; Zschiedrich, T.; Lukas, D.; Wolf, M.; Le Phu, C.; Lutz, V.; Evert, K.; Kozakov, K. Cardioprotective effects of semaglutide on isolated human ventricular myocardium. Eur. J. Heart Fail. 2025, 27, 1315–1325. [Google Scholar] [CrossRef]
- Yan, H.; Yao, W.; Li, Y.; Li, T.; Song, K.; Yan, P.; Dang, Y. Cardiometabolic modulation by semaglutide contributes to cardioprotection in rats with myocardial infarction. Drug Design. Dev. Ther. 2024, 18, 5485–5500. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Z.; Xu, X.; Zou, Y.; Zhang, M.; Chen, Y.; Zhu, W.; Han, B. Semaglutide Attenuates Myocardial Ischemia-Reperfusion Injury by Inhibiting Ferroptosis of Cardiomyocytes Via Activation of PKC-S100A9 Axis. Front. Pharmacol. 2025, 16, 1529652. [Google Scholar] [CrossRef]
- Zhu, Q.; Luo, Y.; Wen, Y.; Wang, D.; Li, J.; Fan, Z. Semaglutide inhibits ischemia/reperfusion-induced cardiomyocyte apoptosis through activating pkg/pkcε/erk1/2 pathway. Biochem. Biophys. Res. Commun. 2023, 647, 1–8. [Google Scholar] [CrossRef]
- Packer, M.; Zile, M.R.; Kramer, C.M.; Baum, S.J.; Litwin, S.E.; Menon, V.; Ge, J.; Weerakkody, G.J.; Ou, Y.; Bunck, M.C.; et al. Tirzepatide for Heart Failure with Preserved Ejection Fraction and Obesity. N. Engl. J. Med. 2025, 392, 427–437. [Google Scholar] [CrossRef] [PubMed]
- Ismaiel, A.; Scarlata, G.G.M.; Boitos, I.; Leucuta, D.-C.; Popa, S.-L.; Al Srouji, N.; Abenavoli, L.; Dumitrascu, D.L. Gastrointestinal adverse events associated with GLP-1 ra in non-diabetic patients with overweight or obesity: A systematic review and network meta-analysis. Int. J. Obes. 2025. [Google Scholar] [CrossRef]
- Zhang, D.Y.; Anderson, A.S. The sympathetic nervous system and heart failure. Cardiol. Clin. 2014, 32, 33–45, vii. [Google Scholar] [CrossRef] [PubMed]
- Triposkiadis, F.; Karayannis, G.; Giamouzis, G.; Skoularigis, J.; Louridas, G.; Butler, J. The Sympathetic Nervous System in Heart Failure. JACC 2009, 54, 1747–1762. [Google Scholar] [CrossRef] [PubMed]
- Triposkiadis, F.; Briasoulis, A.; Kitai, T.; Magouliotis, D.; Athanasiou, T.; Skoularigis, J.; Xanthopoulos, A. The sympathetic nervous system in heart failure revisited. Heart Fail. Rev. 2024, 29, 355–365. [Google Scholar] [CrossRef]
- Gronda, E.; Dusi, V.; D’Elia, E.; Iacoviello, M.; Benvenuto, E.; Vanoli, E. Sympathetic activation in heart failure. Eur. Heart J. Suppl. 2022, 24 (Suppl. E), E4–E11. [Google Scholar] [CrossRef]
- Salah, H.M.; Gupta, R.; Hicks, A.J., III.; Mahmood, K.; Haglund, N.A.; Bindra, A.S.; Antoine, S.M.; Garcia, R.; Yehya, A.; Yaranov, D.M. Baroreflex function in cardiovascular disease. J. Card. Fail. 2024, 31, 117–126. [Google Scholar] [CrossRef]
- Sturgill, S.L.; Salyer, L.G.; Shettigar, V.; Brundage, E.A.; Biesiadecki, B.J.; Ziolo, M.T. Troponin I Phosphorylation is Essential for Cardiac Reserve: Calcium Signaling and Excitation–Contraction in Cardiac, Skeletal and Smooth Muscle. J. Gen. Physiol. 2021, 154, e2021ecc2031. [Google Scholar]
- Lymperopoulos, A.; Rengo, G.; Koch, W.J. Adrenergic nervous system in heart failure: Pathophysiology and therapy. Circ. Res. 2013, 113, 739–753. [Google Scholar] [CrossRef]
- Mahmood, A.; AhMed, K.; Zhang, Y. Β-adrenergic receptor desensitization/down-regulation in heart failure: A friend or foe? Front. Cardiovasc. Med. 2022, 9, 925692. [Google Scholar] [CrossRef]
- Johnson, E.; Nguyen, L.; Albakri, J.S.; Smith, O. Mitochondrial Dysfunction and Calcium Homeostasis in Heart Failure: Exploring the Interplay Between Oxidative Stress and Cardiac Remodeling for Future Therapeutic Innovations. Curr. Probl. Cardiol. 2024, 50, 102968. [Google Scholar] [CrossRef]
- Motiejunaite, J.; Amar, L.; Vidal-Petiot, E. Adrenergic Receptors and Cardiovascular Effects of Catecholamines, Annales D’Endocrinologie; Elsevier: Amsterdam, The Netherlands, 2021; pp. 193–197. [Google Scholar]
- Perez, D.M. Current developments on the role of α1-adrenergic receptors in cognition, cardioprotection, and metabolism. Front. Cell Dev. Biol. 2021, 9, 652152. [Google Scholar] [CrossRef]
- Kanugula, A.K.; Kaur, J.; Batra, J.; Ankireddypalli, A.R.; Velagapudi, R.; Kanugula, A.K. Renin-angiotensin system: Updated understanding and role in physiological and pathophysiological states. Cureus 2023, 15, e40725. [Google Scholar] [CrossRef]
- Landstrom, A.P.; Dobrev, D.; Wehrens, X.H. Calcium signaling and cardiac arrhythmias. Circ. Res. 2017, 120, 1969–1993. [Google Scholar] [CrossRef] [PubMed]
- Lena, A.; Ebner, N.; Anker, M.S. Cardiac cachexia. Eur. Heart J. Suppl. 2019, 21 (Suppl. L), L24–L27. [Google Scholar] [CrossRef] [PubMed]
- Soto, M.E.; Pérez-Torres, I.; Rubio-Ruiz, M.E.; Manzano-Pech, L.; Guarner-Lans, V. Interconnection between cardiac cachexia and heart failure—Protective role of cardiac obesity. Cells 2022, 11, 1039. [Google Scholar] [CrossRef] [PubMed]
- Ferhatbegović, L.; Mršić, D.; Macić-Džanković, A. The benefits of GLP1 receptors in cardiovascular diseases. Front. Clin. Clin. Diabetes Healthc. 2023, 4, 1293926. [Google Scholar] [CrossRef]
- Khalid, A.; Ahmed, A.-B.; Gill, R.; Shaikh, T.; Khorsandi, J.; Kia, A. Molecular mechanisms of L-type calcium channel dysregulation in heart failure. Int. J. Mol. Sci. 2025, 26, 5738. [Google Scholar] [CrossRef]
- Dobrev, D.; Wehrens, X.H.T. Role of RYR2 phosphorylation in heart failure and arrhythmias. Circ. Res. 2014, 114, 1311–1319. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, C.; Roston, T.M.; van der Werf, C.; Sanatani, S.; Chen, S.R.W.; Wilde, A.A.M.; Krahn, A.D. RYR2-ryanodinopathies: From calcium overload to calcium deficiency. Europace 2023, 25, euad156. [Google Scholar] [CrossRef] [PubMed]
- Primeau, J.O.; Armanious, G.P.; Fisher, M.E.; Young, H.S. The sarcoendoplasmic reticulum calcium atpase. Subcell Biochem. 2018, 87, 229–258. [Google Scholar]
- Sutanto, H.; Lyon, A.; Lumens, J.; Schotten, U.; Dobrev, D.; Heijman, J. Cardiomyocyte calcium handling in health and disease: Insights from in vitro and in silico studies. Prog. Biophys. Mol. Biol. 2020, 157, 54–75. [Google Scholar] [CrossRef] [PubMed]
- Gorski, P.A.; Ceholski, D.K.; Hajjar, R.J. Altered myocardial calcium cycling and energetics in heart failure—A rational approach for disease treatment. Cell Metab. 2015, 21, 183–194. [Google Scholar] [CrossRef]
- Younce, C.W.; Burmeister, M.A.; Ayala, J.E. Exendin-4 attenuates high glucose-induced cardiomyocyte apoptosis via inhibition of endoplasmic reticulum stress and activation of serca2a. Am. J. Physiol. Cell Physiol. 2013, 304, C508–C518. [Google Scholar] [CrossRef]
- Chen, J.; Xu, S.; Zhou, W.; Wu, L.; Wang, L.; Li, W. Exendin-4 Reduces Ventricular Arrhythmia Activity and Calcium Sparks-Mediated Sarcoplasmic Reticulum Ca Leak in Rats with Heart Failure. Int. Heart J. 2020, 61, 145–152. [Google Scholar] [CrossRef]
- Lubberding, A.F.; Veedfald, S.; Achter, J.S.; Nissen, S.D.; Soattin, L.; Sorrentino, A.; Vega, E.T.; Linz, B.; Eggertsen, C.H.E.; Mulvey, J. Glucagon-like peptide-1 increases heart rate by a direct action on the sinus node. Cardiovasc. Res. 2024, 120, 1427–1441. [Google Scholar] [CrossRef]
- Nakatani, Y.; Kawabe, A.; Matsumura, M.; Aso, Y.; Yasu, T.; Banba, N.; Nakamoto, T. Effects of GLP-1 receptor agonists on heart rate and the autonomic nervous system using holter electrocardiography and power spectrum analysis of heart rate variability. Diabetes Care 2016, 39, e22–e23. [Google Scholar] [CrossRef]
- Griffioen, K.J.; Wan, R.; Okun, E.; Wang, X.; Lovett-Barr, M.R.; Li, Y.; Mughal, M.R.; Mendelowitz, D.; Mattson, M.P. GLP-1 receptor stimulation depresses heart rate variability and inhibits neurotransmission to cardiac vagal neurons. Cardiovasc. Res. 2011, 89, 72–78. [Google Scholar] [CrossRef]
- Pauza, A.G.; Thakkar, P.; Tasic, T.; Felippe, I.; Bishop, P.; Greenwood, M.P.; Rysevaite-Kyguoliene, K.; Ast, J.; Broichhagen, J.; Hodson, D.J. Glp1r attenuates sympathetic response to high glucose via carotid body inhibition. Circ. Res. 2022, 130, 694–707. [Google Scholar] [CrossRef] [PubMed]
- Baggio, L.L.; Ussher, J.R.; McLean, B.A.; Cao, X.; Kabir, M.G.; Mulvihill, E.E.; Mighiu, A.S.; Zhang, H.; Ludwig, A.; Seeley, R.J.; et al. The autonomic nervous system and cardiac GLP-1 receptors control heart rate in mice. Mol. Metab. 2017, 6, 1339–1349. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.S.; Fonarow, G.C.; McGuire, D.K.; Hernandez, A.F.; Vaduganathan, M.; Rosenstock, J.; Handelsman, Y.; Verma, S.; Anker, S.D.; McMurray, J.J. Glucagon-like peptide 1 receptor agonists and heart failure: The need for further evidence generation and practice guidelines optimization. Circulation 2020, 142, 1205–1218. [Google Scholar] [CrossRef] [PubMed]
- Manolis, A.A.; Manolis, T.A.; Manolis, A.S. Neurohumoral activation in heart failure. Int. J. Mol. Sci. 2023, 24, 15472. [Google Scholar] [CrossRef]
- Sayer, G.; Bhat, G. The renin-angiotensin-aldosterone system and heart failure. Cardiol. Clin. 2014, 32, 21–32. [Google Scholar] [CrossRef]
- Forrester, S.J.; Booz, G.W.; Sigmund, C.D.; Coffman, T.M.; Kawai, T.; Rizzo, V.; Scalia, R.; Eguchi, S. Angiotensin ii signal transduction: An update on mechanisms of physiology and pathophysiology. Physiol. Rev. 2018, 98, 1627–1738. [Google Scholar] [CrossRef]
- YTham, K.; Bernardo, B.C.; Ooi, J.Y.; Weeks, K.L.; McMullen, J.R. Pathophysiology of cardiac hypertrophy and heart failure: Signaling pathways and novel therapeutic targets. Arch. Toxicol. 2015, 89, 1401–1438. [Google Scholar] [CrossRef]
- Rao, W.; Li, D.; Zhang, Q.; Liu, T.; Gu, Z.; Huang, L.; Dai, J.; Wang, J.; Hou, X. Complex regulation of cardiac fibrosis: Insights from immune cells and signaling pathways. J. Transl. Med. 2025, 23, 242. [Google Scholar] [CrossRef]
- Bansal, S.; Lindenfeld, J.; Schrier, R.W. Sodium retention in heart failure and cirrhosis: Potential role of natriuretic doses of mineralocorticoid antagonist? Circ. Heart Fail. 2009, 2, 370–376. [Google Scholar] [CrossRef]
- Brown, N.J. Contribution of aldosterone to cardiovascular and renal inflammation and fibrosis. Nat. Rev. Nephrol. 2013, 9, 459–469. [Google Scholar] [CrossRef]
- Jia, G.; Aroor, A.R.; Hill, M.A.; Sowers, J.R. Role of renin-angiotensin-aldosterone system activation in promoting cardiovascular fibrosis and stiffness. Hypertension 2018, 72, 537–548. [Google Scholar] [CrossRef]
- Al-Hashedi, E.M.; Abdu, F.A. Aldosterone Effect on Cardiac Structure and Function. Curr. Cardiol. Rev. 2024, 20, 60–67. [Google Scholar] [CrossRef]
- Abassi, Z.; Khoury, E.E.; Karram, T.; Aronson, D. Edema formation in congestive heart failure and the underlying mechanisms. Front. Cardiovasc. Med. 2022, 9, 933215. [Google Scholar] [CrossRef]
- Khan, L.A.; Jamil, A.; Greene, S.J.; Khan, M.S.; Butler, J. Aldosterone and Potassium in Heart Failure: Overcoming this Major Impediment in Clinical Practice. Card. Fail. Rev. 2024, 10, e18. [Google Scholar] [CrossRef]
- Palmer, B.F.; Clegg, D.J. Extrarenal effects of aldosterone on potassium homeostasis. Kidney360 2022, 3, 561–568. [Google Scholar] [CrossRef]
- Rroji, M.; Spasovski, G. Transforming diabetes care: The molecular pathways through which GLP1-RAS impact the kidneys in diabetic kidney disease. Biomedicines 2024, 12, 657. [Google Scholar] [CrossRef] [PubMed]
- Hamed, K.; Alosaimi, M.N.; Ali, B.A.; Alghamdi, A.; Alkhashi, T.; Alkhaldi, S.S.; Altowarqi, N.A.; Alzahrani, H.; Alshehri, A.M.; Alkhaldi, R.K. Glucagon-like peptide-1 (GLP-1) receptor agonists: Exploring their impact on diabetes, obesity, and cardiovascular health through a comprehensive literature review. Cureus 2024, 16, 68390. [Google Scholar] [CrossRef] [PubMed]
- Wajdlich, M.; Nowicki, M. The impact of GLP-1 receptor agonist liraglutide on blood pressure profile, hydration, natriuresis in diabetic patients with severely impaired kidney function. Sci. Rep. 2024, 14, 5002. [Google Scholar] [CrossRef] [PubMed]
- Alharbi, S.H. Anti-inflammatory role of glucagon-like peptide 1 receptor agonists and its clinical implications. Ther. Adv. Endocrinol. Metab. 2024, 15, 20420188231222367. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Huang, Q.; Feng, J.; Xiao, Z.; Zhang, X.; Zhao, L. Glp-1 alleviates NLRP3 inflammasome-dependent inflammation in perivascular adipose tissue by inhibiting the NF-κB signalling pathway. J. Int. Med. Res. 2021, 49, 300060521992981. [Google Scholar] [CrossRef] [PubMed]
- Saadat, S.; Noureddini, M.; Mahjoubin-Tehran, M.; Nazemi, S.; Shojaie, L.; Aschner, M.; Maleki, B.; Abbasi-Kolli, M.; Moghadam, H.R.; Alani, B.; et al. Pivotal role of TGF-β/smad signaling in cardiac fibrosis: Non-coding rnas as effectual players. Front. Cardiovasc. Med. 2020, 7, 588347. [Google Scholar] [CrossRef]
- Mahdy, R.N.E.; Nader, M.A.; Helal, M.G.; Abu-Risha, S.E.; Abdelmageed, M.E. Protective effect of dulaglutide, a GLP1 agonist, on acetic acid-induced ulcerative colitis in rats: Involvement of GLP-1, TFF-3, and TGF-β/PI3K/NF-κB signaling pathway. Naunyn. Schmiedebergs Arch. Pharmacol. 2025, 398, 5611–5628. [Google Scholar] [CrossRef]
- Koska, J.; Sands, M.; Burciu, C.; D’Souza, K.M.; Raravikar, K.; Liu, J.; Truran, S.; Franco, D.A.; Schwartz, E.A.; Schwenke, D.C.; et al. Exenatide Protects Against Glucose- and Lipid-Induced Endothelial Dysfunction: Evidence for Direct Vasodilation Effect of GLP-1 Receptor Agonists in Humans. Diabetes 2015, 64, 2624–2635. [Google Scholar] [CrossRef] [PubMed]
- Puglisi, S.; Rossini, A.; Poli, R.; Dughera, F.; Pia, A.; Terzolo, M.; Reimondo, G. Effects of SGLT2 Inhibitors and GLP-1 Receptor Agonists on Renin-Angiotensin-Aldosterone System. Front. Endocrinol. 2021, 12, 738848. [Google Scholar] [CrossRef]
- Hartupee, J.; Mann, D.L. Neurohormonal activation in heart failure with reduced ejection fraction. Nat. Rev. Cardiol. 2017, 14, 30–38. [Google Scholar] [CrossRef]
- Van Linthout, S.; Tschöpe, C. Inflammation—Cause or Consequence of Heart Failure or Both? Curr. Heart Fail. Rep 2017, 14, 251–265. [Google Scholar] [CrossRef]
- SSchumacher, M.; Prasad, S.V.N. Tumor Necrosis Factor-α in Heart Failure: An Updated Review. Curr. Cardiol. Rep. 2018, 20, 117. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.-C. Nf-κb signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef]
- Su, J.-H.; Luo, M.-Y.; Liang, N.; Gong, S.-X.; Chen, W.; Huang, W.-Q.; Tian, Y.; Wang, A.-P. Interleukin-6: A novel target for cardio-cerebrovascular diseases. Front. Pharmacol. 2021, 12, 745061. [Google Scholar] [CrossRef]
- Murphy, S.P.; Kakkar, R.; McCarthy, C.P.; Januzzi, J.L. Inflammation in heart failure. JACC 2020, 75, 1324–1340. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Li, C.; Liu, Y.; Jin, Y.; Yu, Y.; Tan, X.; Zhang, C. The effect of macrophages and their exosomes in ischemic heart disease. Front. Immunol. 2024, 15, 1402468. [Google Scholar] [CrossRef]
- Alcaide, P.; Kallikourdis, M.; Emig, R.; Prabhu, S.D. Myocardial Inflammation in Heart Failure with Reduced and Preserved Ejection Fraction. Circ. Res. 2024, 134, 1752–1766. [Google Scholar] [CrossRef]
- Tavares, L.P.; Negreiros-Lima, G.L.; Lima, K.M.; Silva, P.M.R.E.; Pinho, V.; Teixeira, M.M.; Sousa, L.P. Blame the signaling: Role of camp for the resolution of inflammation. Pharmacol. Res. 2020, 159, 105030. [Google Scholar] [CrossRef]
- Stoicovy, R.A.; Cora, N.; Perez, A.; Nagliya, D.; Del Calvo, G.; Lopez, T.B.; Weinstein, E.C.; Borges, J.I.; Maning, J.; Lymperopoulos, A. Cyclic adenosine monophosphate critically modulates cardiac GLP-1 receptor’s anti-inflammatory effects. Inflamm. Res. 2024, 73, 2043–2056. [Google Scholar] [CrossRef]
- Mehdi, S.F.; Pusapati, S.; Anwar, M.S.; Lohana, D.; Kumar, P.; Nandula, S.A.; Nawaz, F.K.; Tracey, K.; Yang, H.; LeRoith, D.; et al. Glucagon-like peptide-1: A multi-faceted anti-inflammatory agent. Front. Immunol. 2023, 14, 1148209. [Google Scholar] [CrossRef]
- Christian, F.; Smith, E.L.; Carmody, R.J. The Regulation of NF-κB Subunits by Phosphorylation. Cells 2016, 5, 12. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Han, L.; Lu, Y.; Chen, Q. Liraglutide attenuates autoimmune myocarditis by inhibiting NLRP3 and nf-κb pathways. Sci. Rep. 2025, 15, 27274. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Chen, C.; Chen, Z.; Liu, L.; Jiang, J.; Wu, Z.; Zhao, M.; Chen, Y. Nlrp3: A novel mediator in cardiovascular disease. J. Immunol. Res. 2018, 2018, 5702103. [Google Scholar] [CrossRef]
- Kelley, N.; Jeltema, D.; Duan, Y.; He, Y. The NLRP3 Inflammasome: An Overview of Mechanisms of Activation and Regulation. Int. J. Mol. Sci. 2019, 20, 3328. [Google Scholar] [CrossRef] [PubMed]
- Lugrin, J.; Parapanov, R.; Milano, G.; Cavin, S.; Debonneville, A.; Krueger, T.; Liaudet, L. The systemic deletion of interleukin-1α reduces myocardial inflammation and attenuates ventricular remodeling in murine myocardial infarction. Sci. Rep. 2023, 13, 4006. [Google Scholar] [CrossRef]
- Li, X.; Jiang, X.; Jiang, M.; Wang, Z.F.; Zhao, T.; Cao, S.M.; Li, Q.M. GLP-1RAS inhibit the activation of the NLRP3 inflammasome signaling pathway to regulate mouse renal podocyte pyroptosis. Acta Diabetol. 2024, 61, 225–234. [Google Scholar] [CrossRef]
- Heid, M.E.; Keyel, P.A.; Kamga, C.; Shiva, S.; Watkins, S.C.; Salter, R.D. Mitochondrial reactive oxygen species induces NLRP3-dependent lysosomal damage and inflammasome activation. J. Immunol. 2013, 191, 5230–5238. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, A.; Ghosh, K.K.; Chakrabortty, S.; Gulyás, B.; Padmanabhan, P.; Ball, W.B. Mitochondrial Reactive Oxygen Species in Infection and Immunity. Biomolecules 2024, 14, 670. [Google Scholar] [CrossRef] [PubMed]
- Blevins, H.M.; Xu, Y.; Biby, S.; Zhang, S. The NLRP3 Inflammasome Pathway: A Review of Mechanisms and Inhibitors for the Treatment of Inflammatory Diseases. Front. Aging Neurosci. 2022, 14, 879021. [Google Scholar] [CrossRef]
- CKuo, Y.; Tsou, S.-H.; Kornelius, E.; Chan, K.-C.; Chang, K.-W.; Li, J.-C.; Huang, C.-N.; Lin, C.-L. The protective effects of liraglutide in reducing lipid droplets accumulation and myocardial fibrosis in diabetic cardiomyopathy. Cell. Mol. Life Sci. 2025, 82, 39. [Google Scholar] [CrossRef]
- Kondapalli, N.B.; Katari, V.; Dalal, K.; Paruchuri, S.; Thodeti, C.K. Angiotensin ii induces endothelial dysfunction and vascular remodeling by downregulating trpv4 channels. J. Mol. Cell. Cardiol. Plus 2023, 6, 100055. [Google Scholar] [CrossRef]
- Pfeiffer, E.R.; Tangney, J.R.; Omens, J.H.; McCulloch, A.D. Biomechanics of cardiac electromechanical coupling and mechanoelectric feedback. J. Biomech. Eng. 2014, 136, 021007. [Google Scholar] [CrossRef]
- Ottolia, M.; Torres, N.; Bridge, J.H.B.; Philipson, K.D.; Goldhaber, J.I. Na/ca exchange and contraction of the heart. J. Mol. Cell. Cardiol. 2013, 61, 28–33. [Google Scholar] [CrossRef]
- Papakonstantinou, I.; Tsioufis, K.; Katsi, V. Spotlight on the Mechanism of Action of Semaglutide. Curr. Issues Mol. Biol. 2024, 46, 14514–14541. [Google Scholar] [CrossRef] [PubMed]
- Monji, A.; Bando, Y.K.; Mitsui, T.; Aoyama, M.; Murohara, T. Mitochondrial Sirt3 is upregulated by glucagon-like peptide-1 receptor activation and contributes to reversal of cardiac mitochondrial remodeling induced by type 2 diabetes. Eur. Heart J. 2013, 34, 778. [Google Scholar] [CrossRef][Green Version]
- Xiang, J.; Qin, L.; Zhong, J.; Xia, N.; Liang, Y. GLP-1RA Liraglutide and Semaglutide Improves Obesity-Induced Muscle Atrophy Via SIRT1 Pathway. Diabetes Metab. Syndr. Obes. 2023, 16, 2433–2446. [Google Scholar] [CrossRef]
- Santulli, G.; Xie, W.; Reiken, S.R.; Marks, A.R. Mitochondrial calcium overload is a key determinant in heart failure. Proc. Natl. Acad. Sci. USA 2015, 112, 11389–11394. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Lv, J.; Pan, Z.; Wang, D.; Zhao, L.; Guo, X. Mitochondrial dysfunction in heart failure and its therapeutic implications. Front. Cardiovasc. Med. 2022, 9, 945142. [Google Scholar] [CrossRef] [PubMed]
- Numata, G.; Takimoto, E. Cyclic GMP and PKG Signaling in Heart Failure. Front. Pharmacol. 2022, 13, 792798. [Google Scholar] [CrossRef]
- Karakasis, P.; Patoulias, D.; Theofilis, P.; Pamporis, K.; Sagris, M.; Vlachakis, P.K.; Koufakis, T.; Antoniadis, A.P.; Fragakis, N. GLP-1 Receptor Agonists and Myocardial Perfusion: Bridging Mechanisms to Clinical Outcomes. Int. J. Mol. Sci. 2025, 26, 3050. [Google Scholar] [CrossRef]
- Fajmut, A. Molecular Mechanisms and Targets of cyclic Guanosine Monophosphate (CGMP) in Vascular Smooth Muscles. In Muscle Cell and Tissue—Novel Molecular Targets and Current Advances; Sakuma, K., Ed.; IntechOpen: Rijeka, Croatia, 2021. [Google Scholar]
- Ahmad, A.; Dempsey, S.K.; Daneva, Z.; Azam, M.; Li, N.; Li, P.-L.; Ritter, J.K. Role of Nitric Oxide in the Cardiovascular and Renal Systems. Int. J. Mol. Sci. 2018, 19, 2605. [Google Scholar] [CrossRef]
- Zhang, X.Q.; Tang, R.; Li, L.; Szucsik, A.; Javan, H.; Saegusa, N.; Spitzer, K.W.; Selzman, C.H. Cardiomyocyte-specific P65 NF-κB deletion protects the injured heart by preservation of calcium handling. Am. J. Physiol. Heart Circ. Physiol. 2013, 305, H1089–H1097. [Google Scholar] [CrossRef]
- Ang, R.; Mastitskaya, S.; Hosford, P.S.; Basalay, M.; Specterman, M.; Aziz, Q.; Li, Y.; Orini, M.; Taggart, P.; Lambiase, P.D.; et al. Modulation of Cardiac Ventricular Excitability by GLP-1 (Glucagon-Like Peptide-1). Circ. Arrhythm. Electrophysiol. 2018, 11, e006740. [Google Scholar] [CrossRef]
- Samad, M.; Malempati, S.; Restini, C.B.A. Natriuretic Peptides as Biomarkers: Narrative Review and Considerations in Cardiovascular and Respiratory Dysfunctions. Yale J. Biol. Med. 2023, 96, 137–149. [Google Scholar] [CrossRef]
- Díez, J. Chronic heart failure as a state of reduced effectiveness of the natriuretic peptide system: Implications for therapy. Eur. J. Heart Fail. 2017, 19, 167–176. [Google Scholar] [CrossRef]
- Kuwahara, K. The natriuretic peptide system in heart failure: Diagnostic and therapeutic implications. Pharmacol. Ther. 2021, 227, 107863. [Google Scholar] [CrossRef]
- Pandey, K.N. Molecular Signaling Mechanisms and Function of Natriuretic Peptide Receptor-A in the Pathophysiology of Cardiovascular Homeostasis. Front. Physiol. 2021, 12, 693099. [Google Scholar] [CrossRef]
- Tsutsui, H.; Albert, N.M.; Coats, A.J.S.; Anker, S.D.; Bayes-Genis, A.; Butler, J.; Chioncel, O.; Defilippi, C.R.; Drazner, M.H.; Felker, G.M.; et al. Natriuretic Peptides: Role in the Diagnosis and Management of Heart Failure: A scientific Statement from the Heart Failure Association of the European Society of Cardiology, Heart Failure Society of America and Japanese Heart Failure Society. Eur. J. Heart Fail. 2023, 25, 616–631. [Google Scholar] [CrossRef] [PubMed]
- Wong, P.C.; Guo, J.; Zhang, A. The Renal and Cardiovascular Effects of Natriuretic Peptides. Adv. Physiol. Educ. 2017, 41, 179–185. [Google Scholar] [CrossRef]
- Lee, N.S.; Daniels, L.B. Current understanding of the compensatory actions of cardiac natriuretic peptides in cardiac failure: A clinical perspective. Card. Fail. Rev. 2016, 2, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Hu, C.; Tian, E.; Shen, Y.; Liu, W.; Li, J. Comprehensive review on neprilysin (nep) inhibitors: Design, structure-activity relationships, and clinical applications. Front. Pharmacol. 2024, 15, 1501407. [Google Scholar] [CrossRef] [PubMed]
- Packer, M. Augmentation of glucagon-like peptide-1 receptor signalling by neprilysin inhibition: Potential implications for patients with heart failure. Eur. J. Heart Fail. 2018, 20, 973–977. [Google Scholar] [CrossRef]
- Mahtani, K.; Patel, B.; Wang, B.; Barron, A. Activation of GLP-1 receptor signalling by sacubitril/valsartan: Implications for patients with poor glycaemic control. Int. J. Cardiol. 2022, 367, 81–89. [Google Scholar] [CrossRef]
- Nicolas, D.; Patel, P.; Reed, M. Sacubitril-Valsartan. In Statpearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2025. [Google Scholar]
- Gidlöf, O. Toward a New Paradigm for Targeted Natriuretic Peptide Enhancement in Heart Failure. Front. Physiol. 2021, 12, 650124. [Google Scholar] [CrossRef]
- Mehta, A.; Marso, S.P.; Neeland, I.J. Liraglutide for weight management: A critical review of the evidence. Obes. Sci. Pract. 2017, 3, 3–14. [Google Scholar] [CrossRef]
- Bergmann, N.C.; Davies, M.J.; Lingvay, I.; Knop, F.K. Semaglutide for the treatment of overweight and obesity: A review. Diabetes Obes. Metab. 2023, 25, 18–35. [Google Scholar] [CrossRef]
- Wilding, J.P.H.; Batterham, R.L.; Calanna, S.; Davies, M.; Gaal, L.F.V.; Lingvay, I.; McGowan, B.M.; Rosenstock, J.; Tran, M.T.D.; Wadden, T.A.; et al. Once-Weekly Semaglutide in Adults with Overweight or Obesity. N. Engl. J. Med. 2021, 384, 989–1002. [Google Scholar] [CrossRef]
- Aronne, L.J.; Horn, D.B.; Roux, C.W.L.; Ho, W.; Falcon, B.L.; Valderas, E.G.; Das, S.; Lee, C.J.; Glass, L.C.; Senyucel, C.; et al. Tirzepatide as Compared with Semaglutide for the Treatment of Obesity. N. Engl. J. Med. 2025, 393, 26–36. [Google Scholar] [CrossRef]
- Jastreboff, A.M.; Aronne, L.J.; Ahmad, N.N.; Wharton, S.; Connery, L.; Alves, B.; Kiyosue, A.; Zhang, S.; Liu, B.; Bunck, M.C.; et al. Tirzepatide Once Weekly for the Treatment of Obesity. N. Engl. J. Med. 2022, 387, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Ussher, J.R.; Drucker, D.J. Glucagon-like peptide 1 receptor agonists: Cardiovascular benefits and mechanisms of action. Nat. Rev. Cardiol. 2023, 20, 463–474. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the Diagnosis and Treatment of Acute and Chronic Heart Failure: Developed by the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure of the European Society of Cardiology (ESC) with the Special Contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [PubMed]
- Talha, K.M.; Anker, S.D.; Butler, J. SGLT-2 Inhibitors in Heart Failure: A Review of Current Evidence. Int. J. Heart Fail. 2023, 5, 82–90. [Google Scholar] [CrossRef]
- Kim, M.; Platt, M.J.; Shibasaki, T.; E Quaggin, S.; Backx, P.H.; Seino, S.; A Simpson, J.; Drucker, D.J. GLP-1 receptor activation and EPAC2 link atrial natriuretic peptide secretion to control of blood pressure. Nat. Med. 2013, 19, 567–575. [Google Scholar] [CrossRef]

| Trial Name (Year) | Trial Type | Drug & Dose | Population | Sample Size | Primary Endpoint | Key Results | Follow-Up |
|---|---|---|---|---|---|---|---|
| STEP-HFpEF (2023) [14] | Randomized, double-blind, placebo-controlled | Semaglutide 2.4 mg weekly SC | HFpEF (LVEF ≥ 45%), BMI ≥ 30 kg/m2, No diabetes | N = 529 (263 semaglutide, 266 placebo) | Change in KCCQ-CSS and body weight | Improved HF symptoms, physical limitations, exercise function; Greater weight loss vs. placebo | 52 weeks |
| STEP-HFpEF DM (2024) [15] | Randomized, double- blind, placebo-controlled | Semaglutide 2.4 mg weekly SC | HFpEF, Obesity, Type 2 diabetes | N = 616 (310 semaglutide, 306 placebo) | Change in KCCQ-CSS and body weight | Larger reductions in HF symptoms and physical limitations; Greater weight loss (~6.4%) vs. placebo | 52 weeks |
| SUMMIT (2025) [35] | Randomized, double-blind, placebo-controlled, event-driven | Tirzepatide up to 15 mg weekly SC | HFpEF (LVEF ≥ 50%), BMI ≥ 30 kg/m2 | N = 731 (364 tirzepatide, 367 placebo) | Composite of CV death or worsening HF events | HR 0.62 (95% CI: 0.41–0.95); Improved KCCQ-CSS and 6MWD | Median 104 weeks |
| FIGHT (2016) [12] | Randomized, double-blind, placebo-controlled | Liraglutide up to 1.8 mg daily SC | Advanced HFrEF (LVEF ≤ 40%), Recent HF hospitalization | N = 300 (154 liraglutide, 146 placebo) | Global rank score (death, HF rehospitalization, NT-proBNP change) | No benefit demonstrated; Trend toward increased HF hospitalizations | 180 days |
| LIVE (2017) [13] | Randomized, double-blind, placebo-controlled, multicenter | Liraglutide 1.8 mg daily SC | Stable chronic HFrEF (LVEF ≤ 45%) with and without diabetes | N = 241 (122 liraglutide, 119 placebo) | Change in LVEF | No improvement in LVEF; Increased heart rate and serious cardiac adverse events | 24 weeks |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahmani, A.R.; Dhaliwal, S.K.; Pastena, P.; Kazakov, E.; Jayaseelan, K.; Kalogeropoulos, A. GLP-1 Receptor Agonists in Heart Failure. Biomolecules 2025, 15, 1403. https://doi.org/10.3390/biom15101403
Rahmani AR, Dhaliwal SK, Pastena P, Kazakov E, Jayaseelan K, Kalogeropoulos A. GLP-1 Receptor Agonists in Heart Failure. Biomolecules. 2025; 15(10):1403. https://doi.org/10.3390/biom15101403
Chicago/Turabian StyleRahmani, Ali Reza, Simrat Kaur Dhaliwal, Paola Pastena, Eliot Kazakov, Keerthana Jayaseelan, and Andreas Kalogeropoulos. 2025. "GLP-1 Receptor Agonists in Heart Failure" Biomolecules 15, no. 10: 1403. https://doi.org/10.3390/biom15101403
APA StyleRahmani, A. R., Dhaliwal, S. K., Pastena, P., Kazakov, E., Jayaseelan, K., & Kalogeropoulos, A. (2025). GLP-1 Receptor Agonists in Heart Failure. Biomolecules, 15(10), 1403. https://doi.org/10.3390/biom15101403







