Secretome from Uterine Cervical Mesenchymal Stem Cells as a Protector of Neuronal Cells Against Oxidative Stress and Inflammation
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Isolation and Characterization of hUCESC and Secretome Production
2.3. PC-12 and HMC3 Cell Culture
2.4. Oxidative Stress and Inflammation Induction
2.5. qRT-PCR
2.6. Statistical Analysis
3. Results
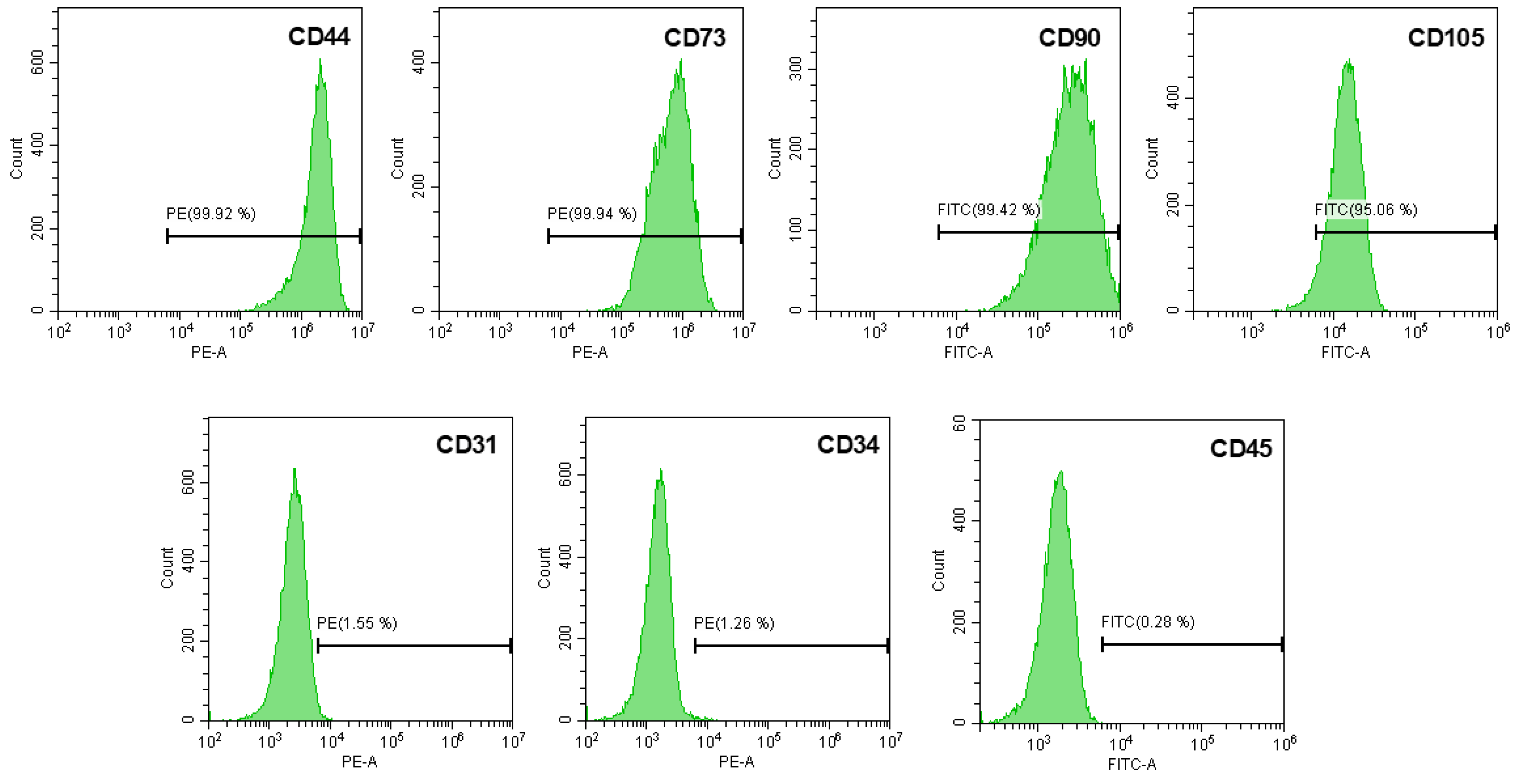
3.1. Isolation and Characterization of hUCESC
3.2. PC-12 Cell Differentiation
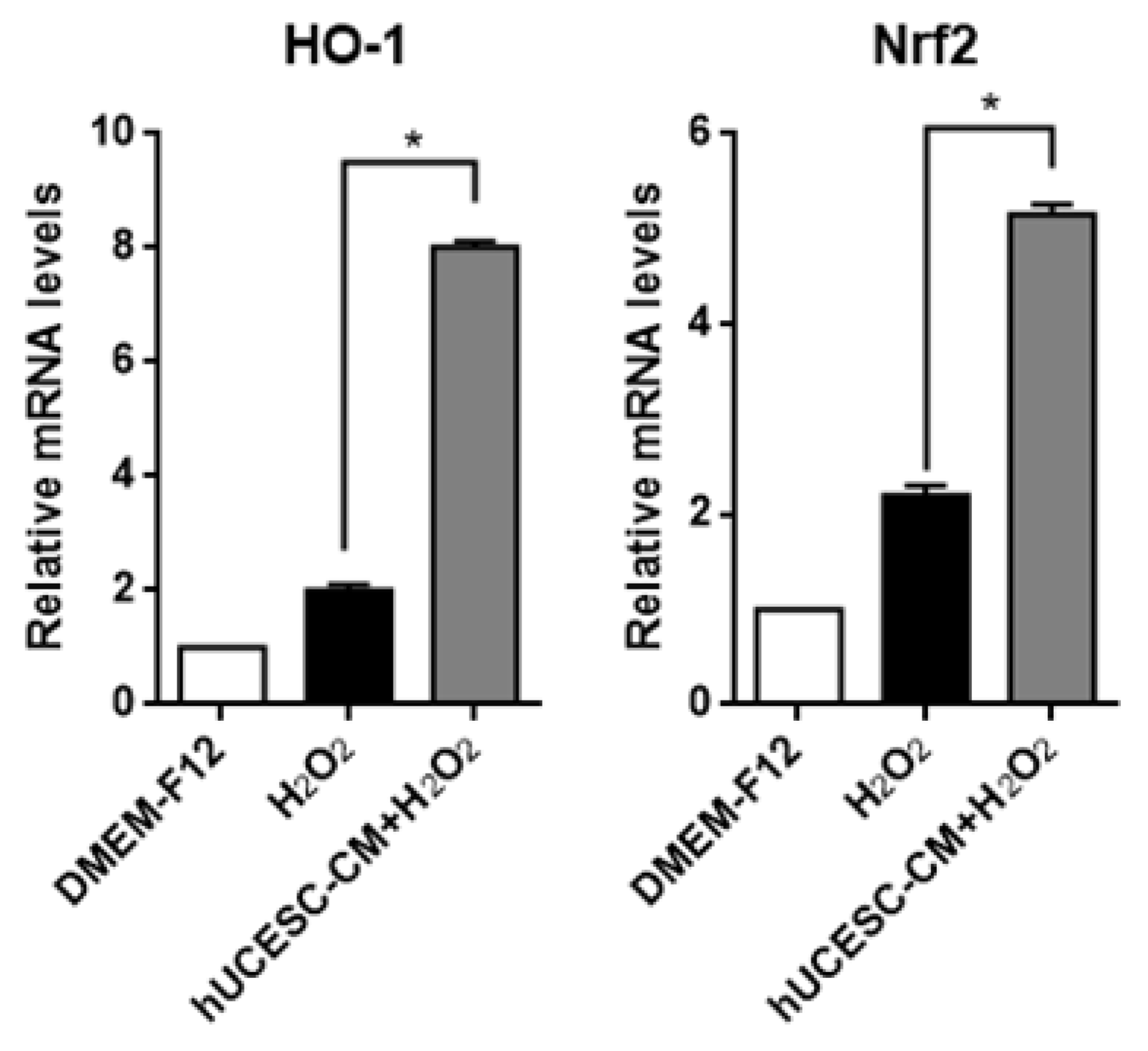
3.3. Effect of hUCESC-CM on PC-12 Oxidative Stress Injury
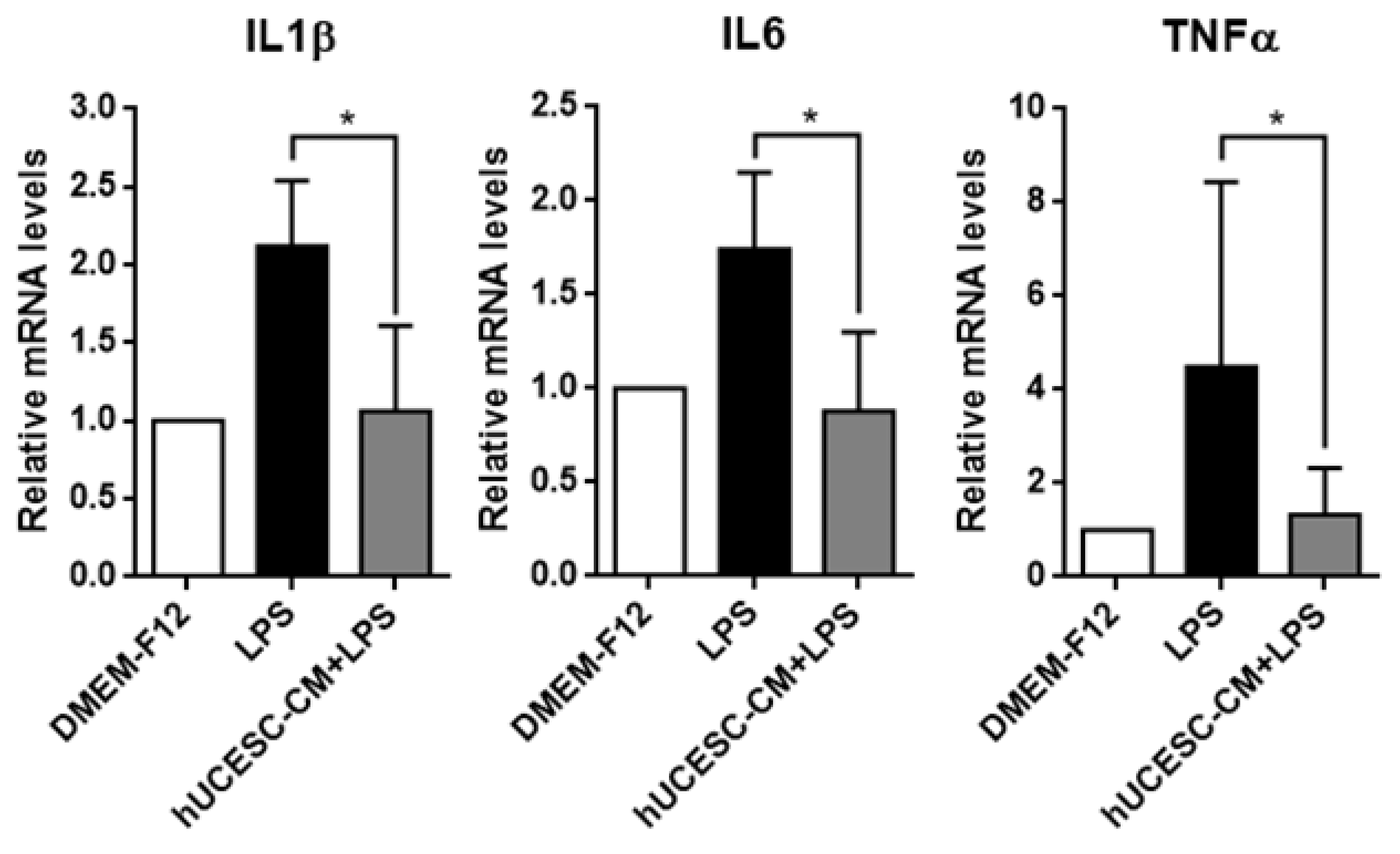
3.4. Effect of hUCESC-CM on Inflamed PC-12
3.5. Effect of hUCESC-CM on Inflamed HMC3
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kehlet, H.; Jensen, T.S.; Woolf, C.J. Persistent postsurgical pain: Risk factors and prevention. Lancet 2006, 367, 1618–1625. [Google Scholar] [CrossRef]
- Staff, N.P.; Engelstad, J.; Klein, C.J.; Amrami, K.K.; Spinner, R.J.; Dyck, P.J.; Warner, M.A.; Warner, M.E.; Dyck, P.J. Post-surgical inflammatory neuropathy. Brain 2010, 133, 2866–2880. [Google Scholar] [CrossRef]
- Woodruff, T.M.; Thundyil, J.; Tang, S.C.; Sobey, C.G.; Taylor, S.M.; Arumugam, T.V. Pathophysiology, treatment, and animal and cellular models of human ischemic stroke. Mol. Neurodegener. 2011, 6, 11. [Google Scholar] [CrossRef]
- Burton, G.J.; Jauniaux, E. Oxidative stress. Best Pract. Res. Clin. Obstet. Gynaecol. 2011, 25, 287–299. [Google Scholar] [CrossRef] [PubMed]
- Garaschuk, O.; Semchyshyn, H.M.; Lushchak, V.I. Healthy brain aging: Interplay between reactive species, inflammation and energy supply. Ageing Res. Rev. 2018, 43, 26–45. [Google Scholar] [CrossRef] [PubMed]
- Xiang, M.; Lu, Y.; Xin, L.; Gao, J.; Shang, C.; Jiang, Z.; Lin, H.; Fang, X.; Qu, Y.; Wang, Y.; et al. Role of Oxidative Stress in Reperfusion following Myocardial Ischemia and Its Treatments. Oxid. Med. Cell. Longev. 2021, 2021, 6614009. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Li, C.; Qiao, Y.; Lu, C.; Li, J.; Song, W.; Sun, J.; Zhai, X.; Niu, J.; Ren, Q.; et al. Safflower yellow B suppresses HepG2 cell injury induced by oxidative stress through the AKT/Nrf2 pathway. Int. J. Mol. Med. 2016, 37, 603–612. [Google Scholar] [CrossRef]
- Singh, A.; Kukreti, R.; Saso, L.; Kukreti, S. Oxidative Stress: A Key Modulator in Neurodegenerative Diseases. Molecules 2019, 24, 1583. [Google Scholar] [CrossRef]
- Martín-Guerrero, S.M.; Muñoz-Gámez, J.A.; Carrasco, M.C.; Salmerón, J.; Martín-Estebané, M.; Cuadros, M.A.; Navascués, J.; Martín-Oliva, D. Poly(ADP-ribose)polymerases inhibitors prevent early mitochondrial fragmentation and hepatocyte cell death induced by H2O. PLoS ONE 2017, 12, e0187130. [Google Scholar] [CrossRef]
- Wan, X.; Fujita, Y.; Chang, L.; Wei, Y.; Ma, L.; Wuyun, G.; Pu, Y.; Hammock, B.D.; Hashimoto, K. Lack of rewarding effects of a soluble epoxide hydrolase inhibitor TPPU in mice: Comparison with morphine. Neuropsychopharmacol. Rep. 2020, 40, 412–416. [Google Scholar] [CrossRef]
- Shabab, T.; Khanabdali, R.; Moghadamtousi, S.Z.; Kadir, H.A.; Mohan, G. Neuroinflammation pathways: A general review. Int. J. Neurosci. 2017, 127, 624–633. [Google Scholar] [CrossRef]
- De Virgilio, A.; Greco, A.; Fabbrini, G.; Inghilleri, M.; Rizzo, M.I.; Gallo, A.; Conte, M.; Rosato, C.; Ciniglio Appiani, M.; de Vincentiis, M. Parkinson’s disease: Autoimmunity and neuroinflammation. Autoimmun. Rev. 2016, 15, 1005–1011. [Google Scholar] [CrossRef]
- Yong, H.Y.F.; Rawji, K.S.; Ghorbani, S.; Xue, M.; Yong, V.W. The benefits of neuroinflammation for the repair of the injured central nervous system. Cell. Mol. Immunol. 2019, 16, 540–546. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Xu, J.; Cheng, Q. Proto-oncogene tyrosine-protein kinase SRC (Src) inhibition in microglia relieves neuroinflammation in neuropathic pain mouse models. Bioengineered 2021, 12, 11390–11398. [Google Scholar] [CrossRef] [PubMed]
- Tofaris, G.K.; Patterson, P.H.; Jessen, K.R.; Mirsky, R. Denervated Schwann cells attract macrophages by secretion of leukemia inhibitory factor (LIF) and monocyte chemoattractant protein-1 in a process regulated by interleukin-6 and LIF. J. Neurosci. 2002, 22, 6696–6703. [Google Scholar] [CrossRef]
- Assinck, P.; Duncan, G.J.; Hilton, B.J.; Plemel, J.R.; Tetzlaff, W. Cell transplantation therapy for spinal cord injury. Nat. Neurosci. 2017, 20, 637–647. [Google Scholar] [CrossRef] [PubMed]
- Cofano, F.; Boido, M.; Monticelli, M.; Zenga, F.; Ducati, A.; Vercelli, A.; Garbossa, D. Mesenchymal Stem Cells for Spinal Cord Injury: Current Options, Limitations, and Future of Cell Therapy. Int. J. Mol. Sci. 2019, 20, 2698. [Google Scholar] [CrossRef]
- Wang, Q.; He, H.; Xie, S.; Wei, Q.; He, C. Mesenchymal Stem Cells Transplantation for Neuropathic Pain Induced By Peripheral Nerve Injury in Animal Models: A Systematic Review. Stem Cells Dev. 2020, 29, 1420–1428. [Google Scholar] [CrossRef]
- Yi, S.; Zhang, Y.; Gu, X.; Huang, L.; Zhang, K.; Qian, T.; Gu, X. Application of stem cells in peripheral nerve regeneration. Burns Trauma. 2020, 8, tkaa002. [Google Scholar] [CrossRef]
- Maguire, G. Stem cell therapy without the cells. Commun. Integr. Biol. 2013, 6, e26631. [Google Scholar] [CrossRef]
- Beer, L.; Mildner, M.; Ankersmit, H.J. Cell secretome based drug substances in regenerative medicine: When regulatory affairs meet basic science. Ann. Transl. Med. 2017, 5, 170. [Google Scholar] [CrossRef]
- Bermudez, M.A.; Sendon-Lago, J.; Eiro, N.; Treviño, M.; Gonzalez, F.; Yebra-Pimentel, E.; Giraldez, M.J.; Macia, M.; Lamelas, M.L.; Saa, J.; et al. Corneal epithelial wound healing and bactericidal effect of conditioned medium from human uterine cervical stem cells. Investig. Ophthalmol. Vis. Sci. 2015, 56, 983–992. [Google Scholar] [CrossRef]
- Bermudez, M.A.; Sendon-Lago, J.; Seoane, S.; Eiro, N.; Gonzalez, F.; Saa, J.; Vizoso, F.; Perez-Fernandez, R. Anti-inflammatory effect of conditioned medium from human uterine cervical stem cells in uveitis. Exp. Eye Res. 2016, 149, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Eiro, N.; Sendon-Lago, J.; Cid, S.; Saa, J.; de Pablo, N.; Vega, B.; Bermudez, M.A.; Perez-Fernandez, R.; Vizoso, F.J. Conditioned Medium from Human Uterine Cervical Stem Cells Regulates Oxidative Stress and Angiogenesis of Retinal Pigment Epithelial Cells. Ophthalmic Res. 2022, 65, 556–565. [Google Scholar] [CrossRef] [PubMed]
- Eiró, N.; Sendon-Lago, J.; Seoane, S.; Bermúdez, M.A.; Lamelas, M.L.; Garcia-Caballero, T.; Schneider, J.; Perez-Fernandez, R.; Vizoso, F.J. Potential therapeutic effect of the secretome from human uterine cervical stem cells against both cancer and stromal cells compared with adipose tissue stem cells. Oncotarget 2014, 5, 10692–10708. [Google Scholar] [CrossRef] [PubMed]
- Sendon-Lago, J.; Rio, L.G.; Eiro, N.; Diaz-Rodriguez, P.; Avila, L.; Gonzalez, L.O.; Vizoso, F.J.; Perez-Fernandez, R.; Landin, M. Tailored Hydrogels as Delivery Platforms for Conditioned Medium from Mesenchymal Stem Cells in a Model of Acute Colitis in Mice. Pharmaceutics 2021, 13, 1127. [Google Scholar] [CrossRef] [PubMed]
- Sendon-Lago, J.; Seoane, S.; Martinez-Ordoñez, A.; Eiro, N.; Saa, J.; Vizoso, F.J.; Gonzalez, F.; Perez-Fernandez, R.; Bermudez, M.A. Corneal regeneration by conditioned medium of human uterine cervical stem cells is mediated by TIMP-1 and TIMP-2. Exp. Eye Res. 2019, 180, 110–121. [Google Scholar] [CrossRef]
- Westerink, R.H.; Ewing, A.G. The PC12 cell as model for neurosecretion. Acta Physiol. 2008, 192, 273–285. [Google Scholar] [CrossRef]
- Magliaro, B.C.; Saldanha, C.J. Clozapine protects PC-12 cells from death due to oxidative stress induced by hydrogen peroxide via a cell-type specific mechanism involving inhibition of extracellular signal-regulated kinase phosphorylation. Brain Res. 2009, 1283, 14–24. [Google Scholar] [CrossRef]
- Chen, B.; Yue, R.; Yang, Y.; Zeng, H.; Chang, W.; Gao, N.; Yuan, X.; Zhang, W.; Shan, L. Protective effects of (E)-2-(1-hydroxyl-4-oxocyclohexyl) ethyl caffeine against hydrogen peroxide-induced injury in PC12 cells. Neurochem. Res. 2015, 40, 531–541. [Google Scholar] [CrossRef]
- Xie, D.; Deng, T.; Zhai, Z.; Sun, T.; Xu, Y. The cellular model for Alzheimer’s disease research: PC12 cells. Front. Mol. Neurosci. 2022, 15, 1016559. [Google Scholar] [CrossRef]
- Finkel, T. Oxidant signals and oxidative stress. Curr. Opin. Cell Biol. 2003, 15, 247–254. [Google Scholar] [CrossRef]
- Mayorga-Martinez, C.C.; Sofer, Z.; Pumera, M. Binary Phosphorene Redox Behavior in Oxidoreductase Enzymatic Systems. ACS Nano 2019, 13, 13217–13224. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; An, C.; Gao, Y.; Leak, R.K.; Chen, J.; Zhang, F. Emerging roles of Nrf2 and phase II antioxidant enzymes in neuroprotection. Prog. Neurobiol. 2013, 100, 30–47. [Google Scholar] [CrossRef] [PubMed]
- Conti, P.; Lauritano, D.; Caraffa, A.; Gallenga, C.E.; Kritas, S.K.; Ronconi, G.; Martinotti, S. Microglia and mast cells generate proinflammatory cytokines in the brain and worsen inflammatory state: Suppressor effect of IL-37. Eur. J. Pharmacol. 2020, 875, 173035. [Google Scholar] [CrossRef] [PubMed]
- Subhramanyam, C.S.; Wang, C.; Hu, Q.; Dheen, S.T. Microglia-mediated neuroinflammation in neurodegenerative diseases. Semin. Cell Dev. Biol. 2019, 94, 112–120. [Google Scholar] [CrossRef]
- Dec, E.; Rana, P.; Katheria, V.; Dec, R.; Khare, M.; Nalbandian, A.; Leu, S.Y.; Radom-Aizik, S.; Llewellyn, K.; BenMohamed, L.; et al. Cytokine profiling in patients with VCP-associated disease. Clin. Transl. Sci. 2014, 7, 29–32. [Google Scholar] [CrossRef]
- Ramsey, C.P.; Tansey, M.G. A survey from 2012 of evidence for the role of neuroinflammation in neurotoxin animal models of Parkinson’s disease and potential molecular targets. Exp. Neurol. 2014, 256, 126–132. [Google Scholar] [CrossRef]
- Tułowiecka, N.; Kotlęga, D.; Bohatyrewicz, A.; Szczuko, M. Could Lipoxins Represent a New Standard in Ischemic Stroke Treatment? Int. J. Mol. Sci. 2021, 22, 4207. [Google Scholar] [CrossRef]
- Saito, K.; Suyama, K.; Nishida, K.; Sei, Y.; Basile, A.S. Early increases in TNF-alpha, IL-6 and IL-1 beta levels following transient cerebral ischemia in gerbil brain. Neurosci. Lett. 1996, 206, 149–152. [Google Scholar] [CrossRef]
- Palasz, E.; Wilkaniec, A.; Stanaszek, L.; Andrzejewska, A.; Adamczyk, A. Glia-Neurotrophic Factor Relationships: Possible Role in Pathobiology of Neuroinflammation-Related Brain Disorders. Int. J. Mol. Sci. 2023, 24, 6321. [Google Scholar] [CrossRef]
- Wu, S.Y.; Pan, B.S.; Tsai, S.F.; Chiang, Y.T.; Huang, B.M.; Mo, F.E.; Kuo, Y.M. BDNF reverses aging-related microglial activation. J. Neuroinflammation 2020, 17, 210. [Google Scholar] [CrossRef]
- Rickert, U.; Grampp, S.; Wilms, H.; Spreu, J.; Knerlich-Lukoschus, F.; Held-Feindt, J.; Lucius, R. Glial Cell Line-Derived Neurotrophic Factor Family Members Reduce Microglial Activation via Inhibiting p38MAPKs-Mediated Inflammatory Responses. J. Neurodegener. Dis. 2014, 2014, 369468. [Google Scholar] [CrossRef]
- Garcia-Contreras, M.; Thakor, A.S. Human adipose tissue-derived mesenchymal stem cells and their extracellular vesicles modulate lipopolysaccharide activated human microglia. Cell Death Discov. 2021, 7, 98. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Zhang, M.M.; Wang, M.; Jiang, Z.H.; Tan, Z.G. Bone Marrow-Derived Mesenchymal Stem Cell-Derived Exosomes Containing Gli1 Alleviate Microglial Activation and Neuronal Apoptosis In Vitro and in a Mouse Parkinson Disease Model by Direct Inhibition of Sp1 Signaling. J. Neuropathol. Exp. Neurol. 2022, 81, 522–534. [Google Scholar] [CrossRef] [PubMed]
- Vizoso, F.J.; Eiro, N.; Cid, S.; Schneider, J.; Perez-Fernandez, R. Mesenchymal Stem Cell Secretome: Toward Cell-Free Therapeutic Strategies in Regenerative Medicine. Int. J. Mol. Sci. 2017, 18, 1852. [Google Scholar] [CrossRef]
- Hong, E.G.; Ko, H.J.; Cho, Y.R.; Kim, H.J.; Ma, Z.; Yu, T.Y.; Friedline, R.H.; Kurt-Jones, E.; Finberg, R.; Fischer, M.A.; et al. Interleukin-10 prevents diet-induced insulin resistance by attenuating macrophage and cytokine response in skeletal muscle. Diabetes 2009, 58, 2525–2535. [Google Scholar] [CrossRef]
- Oh, S.K.; Jeon, S.R. Current Concept of Stem Cell Therapy for Spinal Cord Injury: A Review. Korean J. Neurotrauma 2016, 12, 40–46. [Google Scholar] [CrossRef]
- Wu, C.L.; Chen, S.D.; Yin, J.H.; Hwang, C.S.; Yang, D.I. Nuclear Factor-kappaB-Dependent Sestrin2 Induction Mediates the Antioxidant Effects of BDNF Against Mitochondrial Inhibition in Rat Cortical Neurons. Mol. Neurobiol. 2016, 53, 4126–4142. [Google Scholar] [CrossRef]
- Liu, W.; Wang, X.; O’Connor, M.; Wang, G.; Han, F. Brain-Derived Neurotrophic Factor and Its Potential Therapeutic Role in Stroke Comorbidities. Neural Plast. 2020, 2020, 1969482. [Google Scholar] [CrossRef]
- Cao, B.; Bauer, I.E.; Sharma, A.N.; Mwangi, B.; Frazier, T.; Lavagnino, L.; Zunta-Soares, G.B.; Walss-Bass, C.; Glahn, D.C.; Kapczinski, F.; et al. Reduced hippocampus volume and memory performance in bipolar disorder patients carrying the BDNF val66met met allele. J. Affect. Disord. 2016, 198, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Tanila, H. The role of BDNF in Alzheimer’s disease. Neurobiol. Dis. 2017, 97, 114–118. [Google Scholar] [CrossRef] [PubMed]
- Duman, R.S.; Deyama, S.; Fogaça, M.V. Role of BDNF in the pathophysiology and treatment of depression: Activity-dependent effects distinguish rapid-acting antidepressants. Eur. J. Neurosci. 2021, 53, 126–139. [Google Scholar] [CrossRef] [PubMed]
- Palasz, E.; Wysocka, A.; Gasiorowska, A.; Chalimoniuk, M.; Niewiadomski, W.; Niewiadomska, G. BDNF as a Promising Therapeutic Agent in Parkinson’s Disease. Int. J. Mol. Sci. 2020, 21, 1170. [Google Scholar] [CrossRef]
- Bayazit, H.; Dulgeroglu, D.; Selek, S. Brain-Derived Neurotrophic Factor and Oxidative Stress in Cannabis Dependence. Neuropsychobiology 2020, 79, 186–190. [Google Scholar] [CrossRef]
- Escudero-Cernuda, S.; Eiro, N.; Fraile, M.; Vizoso, F.J.; Fernández-Colomer, B.; Fernández-Sánchez, M.L. Limitations and challenges in the characterization of extracellular vesicles from stem cells and serum. Mikrochim. Acta 2025, 192, 311. [Google Scholar] [CrossRef]
- Eiro, N.; Fraile, M.; Escudero-Cernuda, S.; Sendon-Lago, J.; Gonzalez, L.O.; Fernandez-Sánchez, M.L.; Vizoso, F.J. Synergistic effect of human uterine cervical mesenchymal stem cell secretome and paclitaxel on triple negative breast cancer. Stem Cell Res. Ther. 2024, 15, 121. [Google Scholar] [CrossRef]
- Li, P.; Kaslan, M.; Lee, S.H.; Yao, J.; Gao, Z. Progress in Exosome Isolation Techniques. Theranostics 2017, 7, 789–804. [Google Scholar] [CrossRef]
- Blanc, L.; Vidal, M. New insights into the function of Rab GTPases in the context of exosomal secretion. Small GTPases 2018, 9, 95–106. [Google Scholar] [CrossRef]
- Marote, A.; Teixeira, F.G.; Mendes-Pinheiro, B.; Salgado, A.J. MSC-Derived Exosomes: Cell-Secreted Nanovesicles with Regenerative Potential. Front. Pharmacol. 2016, 7, 231. [Google Scholar] [CrossRef]
- Yang, B.; Chen, Y.; Shi, J. Exosome Biochemistry and Advanced Nanotechnology for Next-Generation Theranostic Platforms. Adv. Mater. 2019, 31, e1802896. [Google Scholar] [CrossRef]
- Rhim, T.; Lee, D.Y.; Lee, M. Drug delivery systems for the treatment of ischemic stroke. Pharm. Res. 2013, 30, 2429–2444. [Google Scholar] [CrossRef]
- Wang, Y.; Niu, H.; Li, L.; Han, J.; Liu, Z.; Chu, M.; Sha, X.; Zhao, J. Anti-CHAC1 exosomes for nose-to-brain delivery of miR-760-3p in cerebral ischemia/reperfusion injury mice inhibiting neuron ferroptosis. J. Nanobiotechnology 2023, 21, 109. [Google Scholar] [CrossRef]
- Kodali, M.; Castro, O.W.; Kim, D.K.; Thomas, A.; Shuai, B.; Attaluri, S.; Upadhya, R.; Gitai, D.; Madhu, L.N.; Prockop, D.J.; et al. Intranasally Administered Human MSC-Derived Extracellular Vesicles Pervasively Incorporate into Neurons and Microglia in both Intact and Status Epilepticus Injured Forebrain. Int. J. Mol. Sci. 2019, 21, 181. [Google Scholar] [CrossRef]


| Gene | Forward Primer | Reverse Primer | Annealing Temperature (°C) | Product Length (bp) |
|---|---|---|---|---|
| IL1β | 5′CACCTCTCAAGCAGAGCACAG3′ | 5′GGGTTCCATGGTGAAGTCAAC3′ | 62.1 | 79 |
| IL6 | 5′TCCTACCCCAACTTCCAATGCTC3′ | 5′TTGGATGGTCTTGGTCCTTAGCC3′ | 63.9 | 79 |
| TNFα | 5′AAATGGGCTCCCTCTCATCAGTTC3′ | 5′TCTGCTTGGTGGTTTGCTACGAC3′ | 63.5 | 111 |
| HO-1 | 5′GCCTTCCTGCTCAACATTG3′ | 5′GCGAAGAAACTCTGTCTGTGA3′ | 56.1 | 96 |
| Nrf2 | 5′CAGTGGATCTGTCAGCTACTC3′ | 5′CAAGCGACTCATGGTCATCTAC3′ | 58.9 | 122 |
| GAPDH | 5′AACCCATCACCATCTTCCAG3′ | 5′CCAGTAGACTCCACGACATAC3′ | 56.9 | 85 |
| Gene | Forward Primer | Reverse Primer | Annealing Temperature (°C) | Product Length (bp) |
|---|---|---|---|---|
| IL6 | 5′CCTTCCCTGCCCCAGTA3′ | 5′ATTCGTTCTGAAGAAGAGGTGAGTG3′ | 59.4 | 117 |
| TNFα | 5′TGCACTTTGGAGTGATCGG3′ | 5′TCAGCTTGAGGGTTTGCTAC3′ | 56.7 | 145 |
| BDNF | 5′AATGCTCACACTCCACATCC3′ | 5′CAAACATAGGTCCTTCCGTCA3′ | 56.7 | 100 |
| GDNF | 5′GGCACCTGGAGTTAATGTCC3′ | 5′CCACGACATCCCATAACTTCA3′ | 58 | 98 |
| GAPDH | 5′ACATCGCTCAGACACCATG3′ | 5′TGTAGTTGAGGTCAATGAAGGG3′ | 56.4 | 143 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mateo, J.; Suárez-Suárez, M.Á.; Fraile, M.; Piñera-Parrilla, Á.R.; Vizoso, F.J.; Eiro, N. Secretome from Uterine Cervical Mesenchymal Stem Cells as a Protector of Neuronal Cells Against Oxidative Stress and Inflammation. Biomolecules 2025, 15, 1402. https://doi.org/10.3390/biom15101402
Mateo J, Suárez-Suárez MÁ, Fraile M, Piñera-Parrilla ÁR, Vizoso FJ, Eiro N. Secretome from Uterine Cervical Mesenchymal Stem Cells as a Protector of Neuronal Cells Against Oxidative Stress and Inflammation. Biomolecules. 2025; 15(10):1402. https://doi.org/10.3390/biom15101402
Chicago/Turabian StyleMateo, Javier, Miguel Ángel Suárez-Suárez, Maria Fraile, Ángel Ramón Piñera-Parrilla, Francisco J. Vizoso, and Noemi Eiro. 2025. "Secretome from Uterine Cervical Mesenchymal Stem Cells as a Protector of Neuronal Cells Against Oxidative Stress and Inflammation" Biomolecules 15, no. 10: 1402. https://doi.org/10.3390/biom15101402
APA StyleMateo, J., Suárez-Suárez, M. Á., Fraile, M., Piñera-Parrilla, Á. R., Vizoso, F. J., & Eiro, N. (2025). Secretome from Uterine Cervical Mesenchymal Stem Cells as a Protector of Neuronal Cells Against Oxidative Stress and Inflammation. Biomolecules, 15(10), 1402. https://doi.org/10.3390/biom15101402






