Anticancer Activity of Jania rubens in HCT-116 Cells via EMT Suppression, TET Downregulation, and ROS-Mediated Cytotoxicity
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of the Extract
2.2. Cell Culture
2.3. Cell Viability Assay
2.4. Crystal Violet Assay
2.5. Cell Cycle Assay
2.6. Quantitative ROS Determination
2.7. Immunofluorescence Assay
2.8. Zymography
2.9. Analysis with Gas Chromatography-Mass Spectrometry (GC-MS)
2.10. RNA Extraction, Reverse Transcription, and qRTPCR
2.11. Statistical Analysis
3. Results
3.1. Major Bioactive Compounds Identified in Jania rubens DM Extract
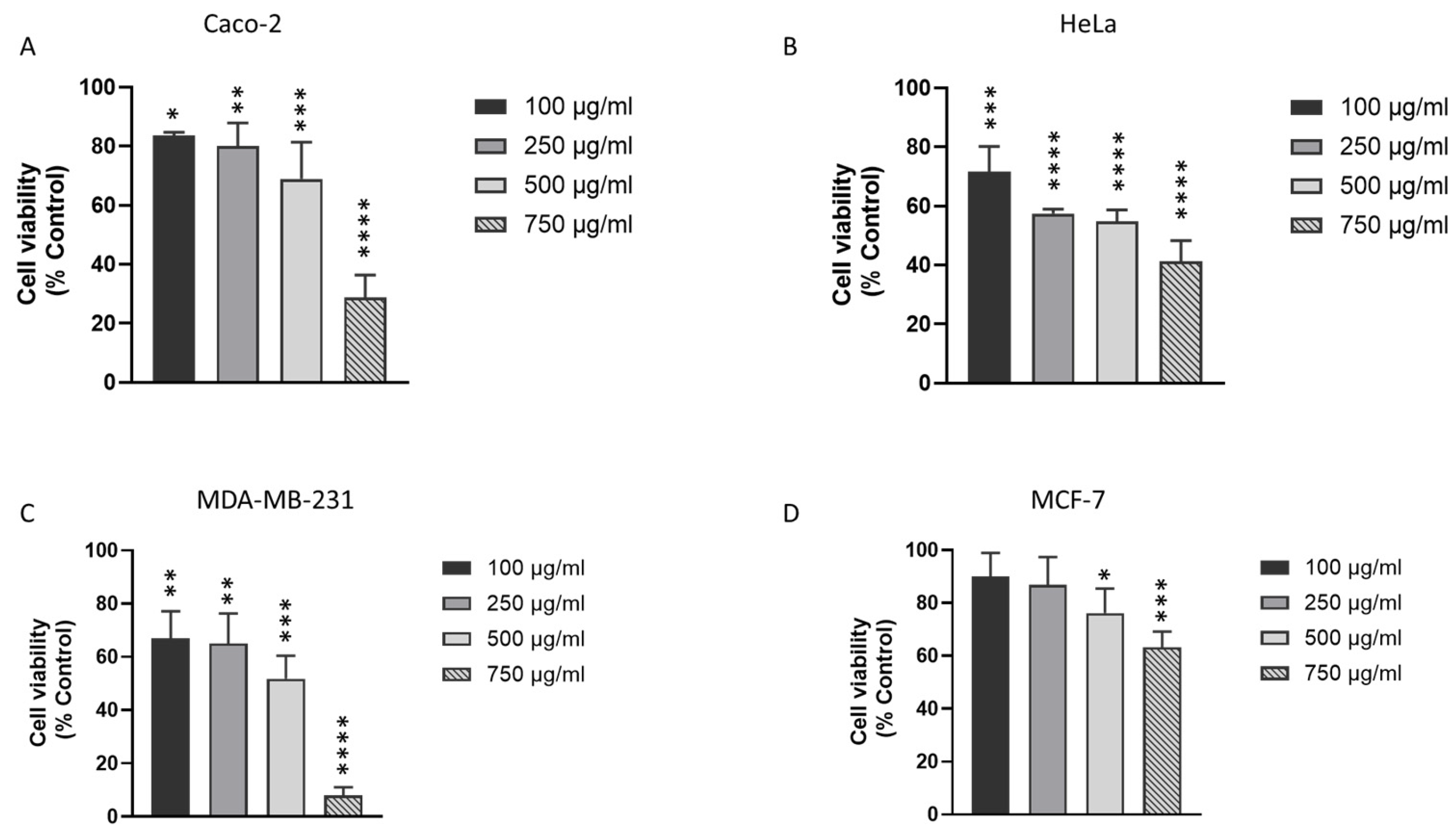
3.2. J. rubens DM Extract Decreases the Viability of Various Solid Cancer Cell Lines
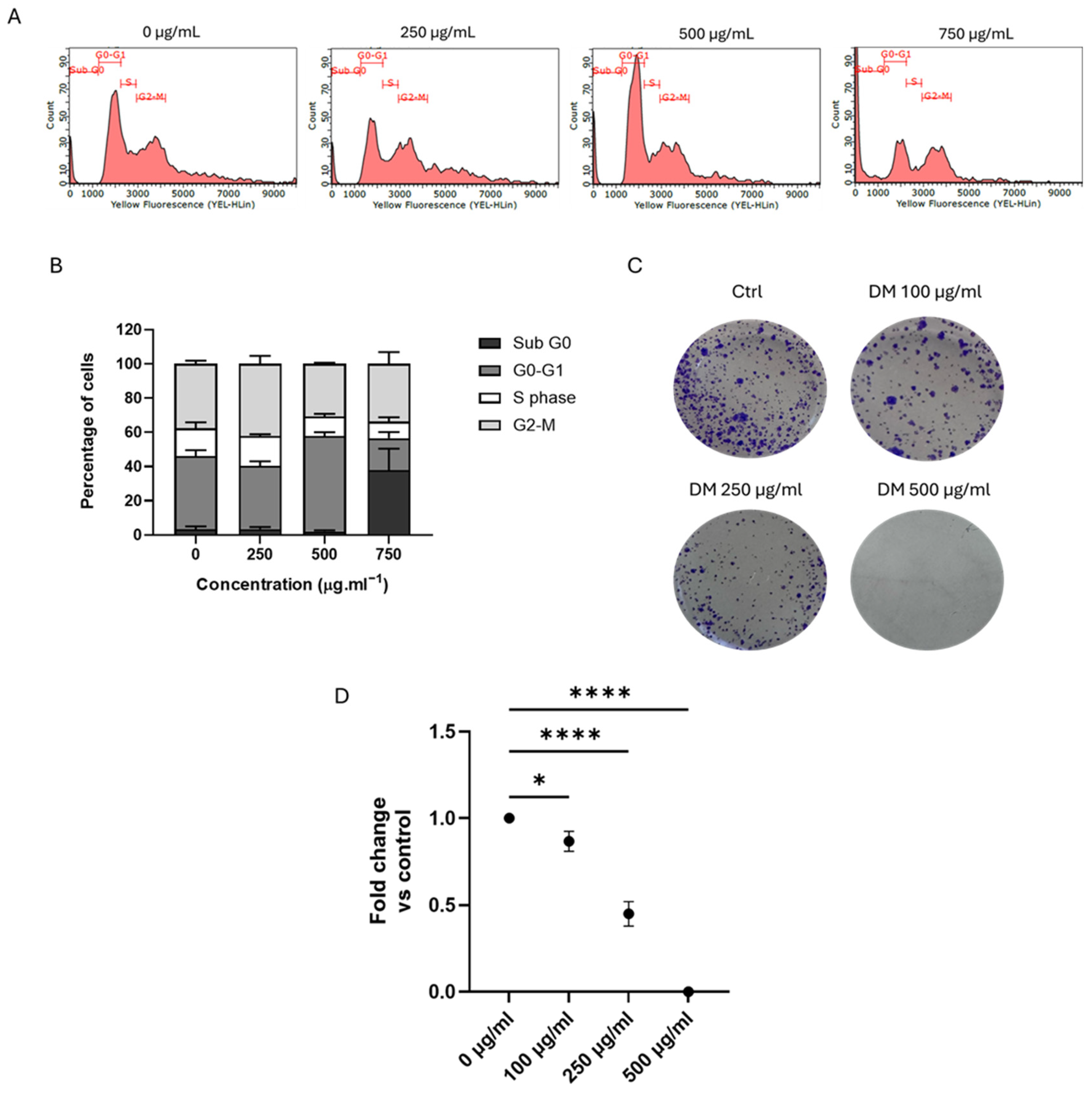
3.3. J. rubens DM Extract Induced Cell Cycle Arrest and Inhibited the Long-Term Survival of HCT-116 Cells
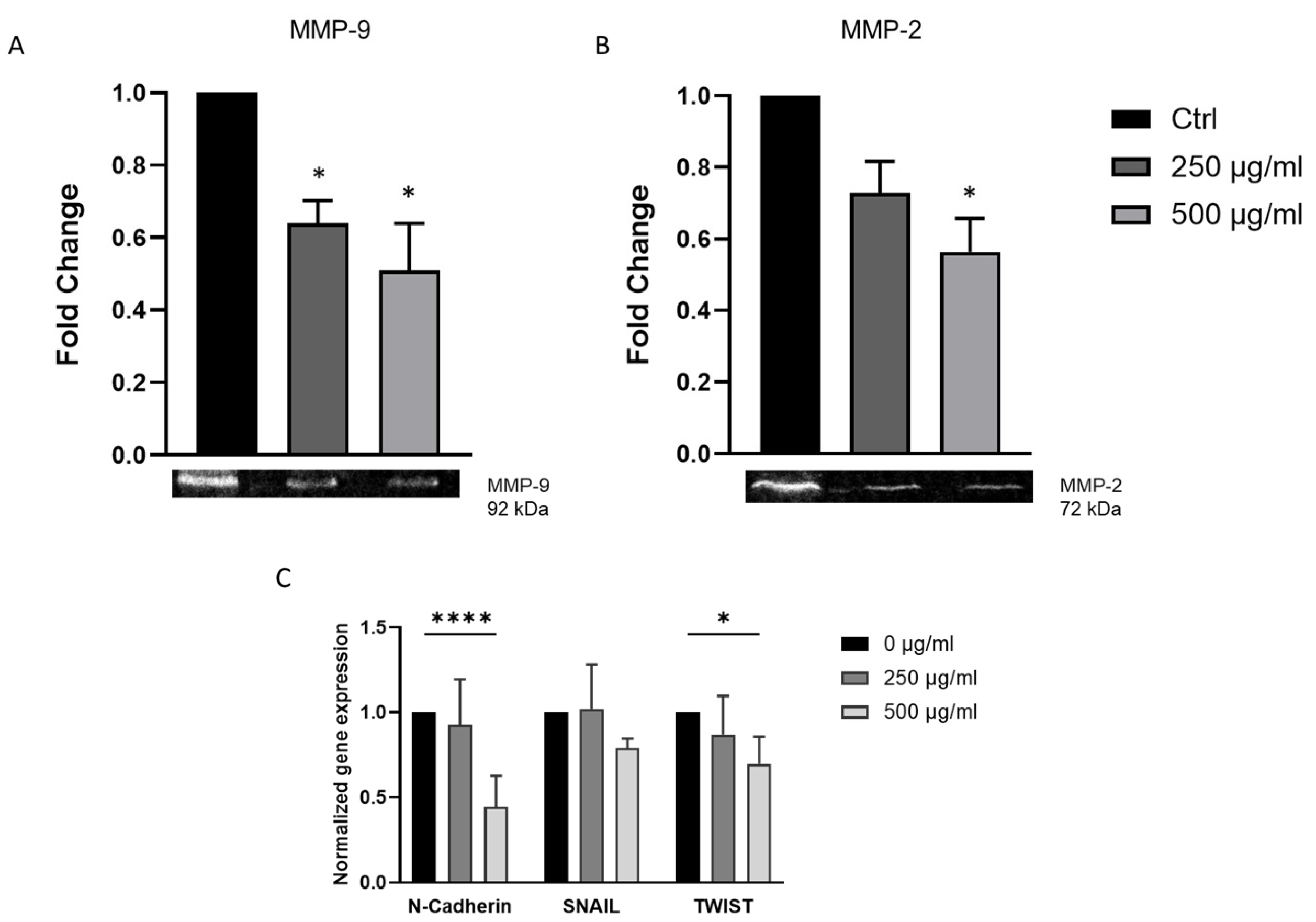
3.4. J. rubens DM Extract Downregulates EMT Markers and Inhibits Matrix Metalloproteinase Activity in HCT-116 Cells
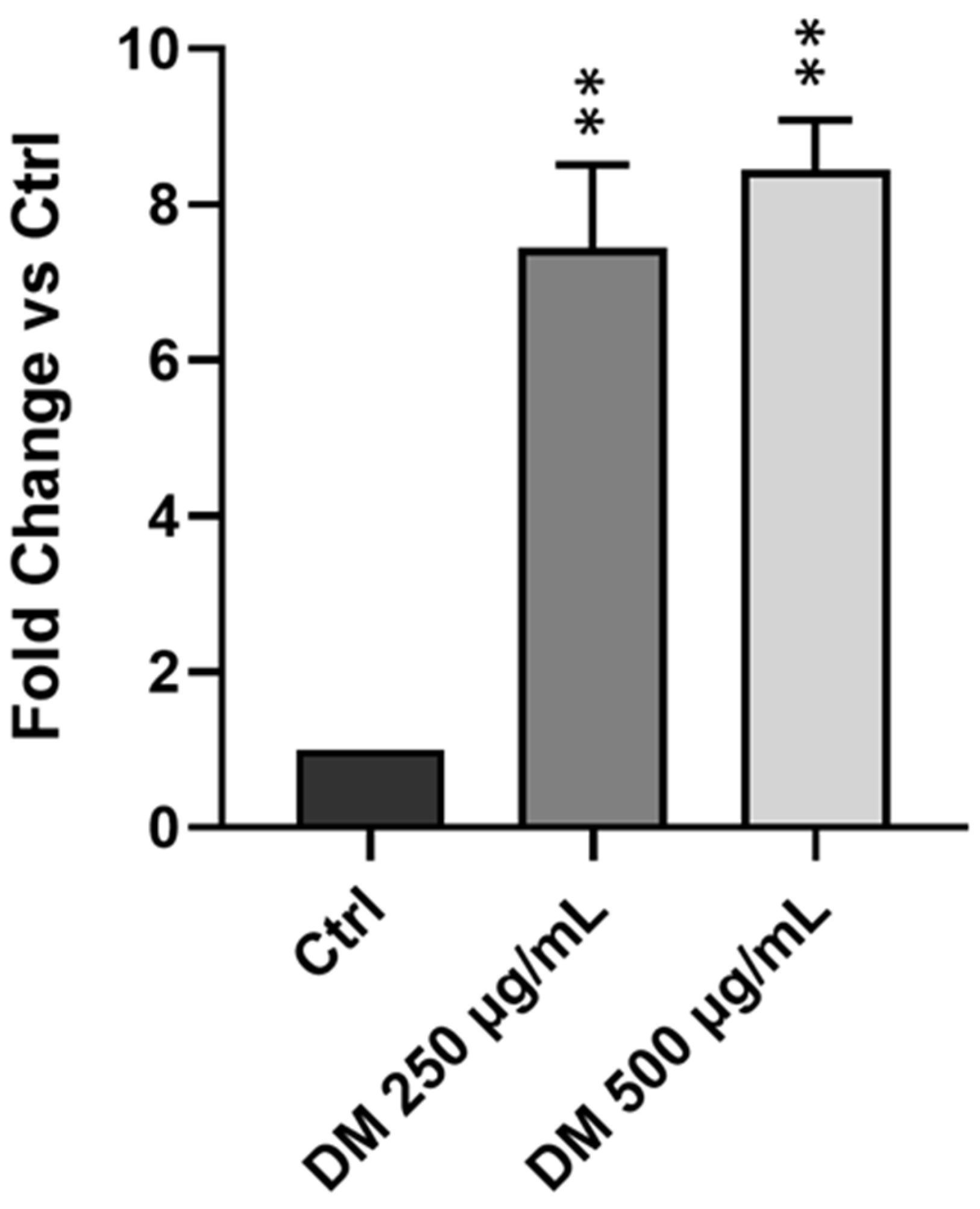
3.5. J. rubens DM Soxhlet Extract Increases ROS Production in HCT-116 Cells
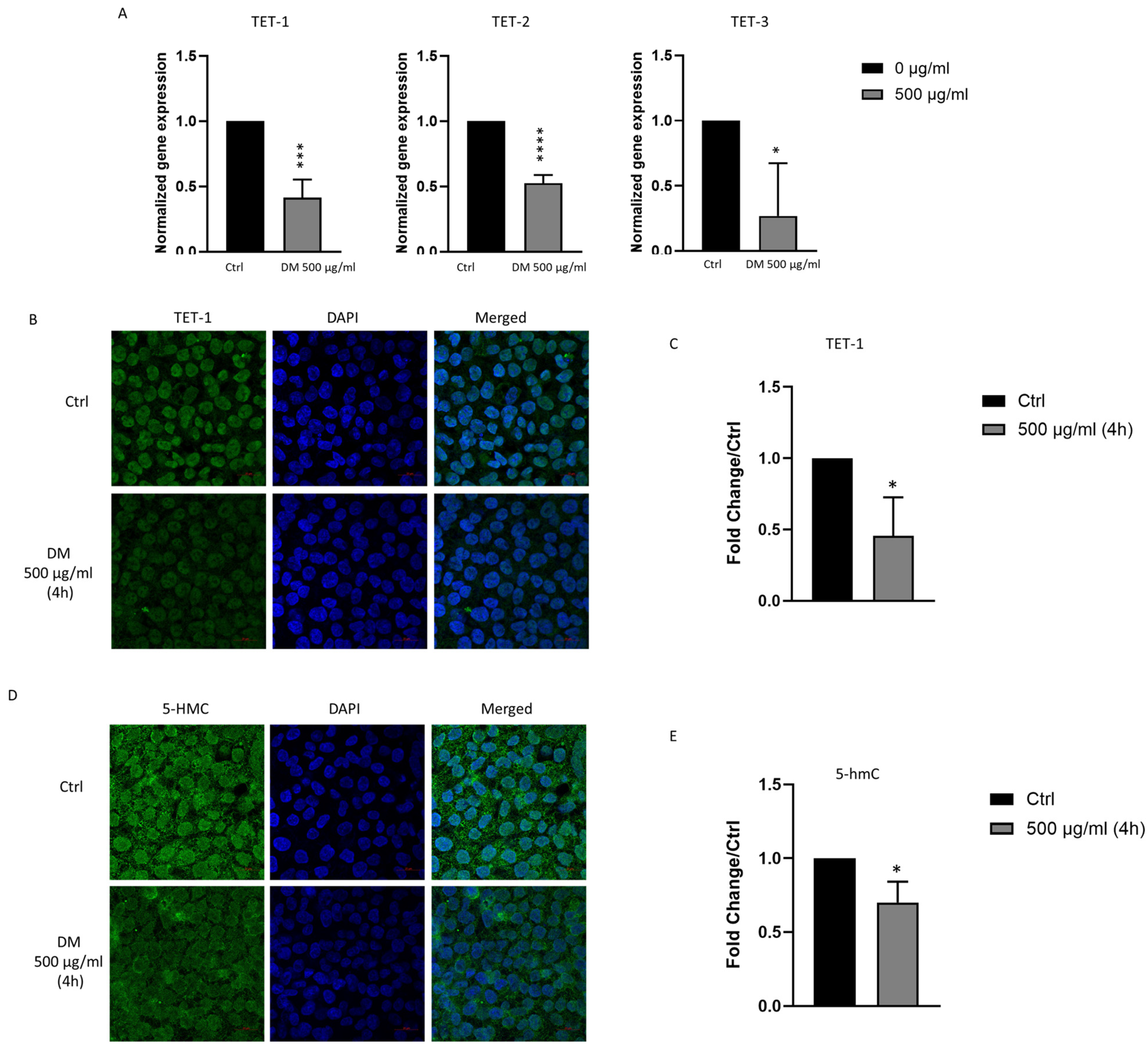
3.6. J. rubens DM Extract Decreases TET-1, TET-2, TET-3 and 5-hmC Expression
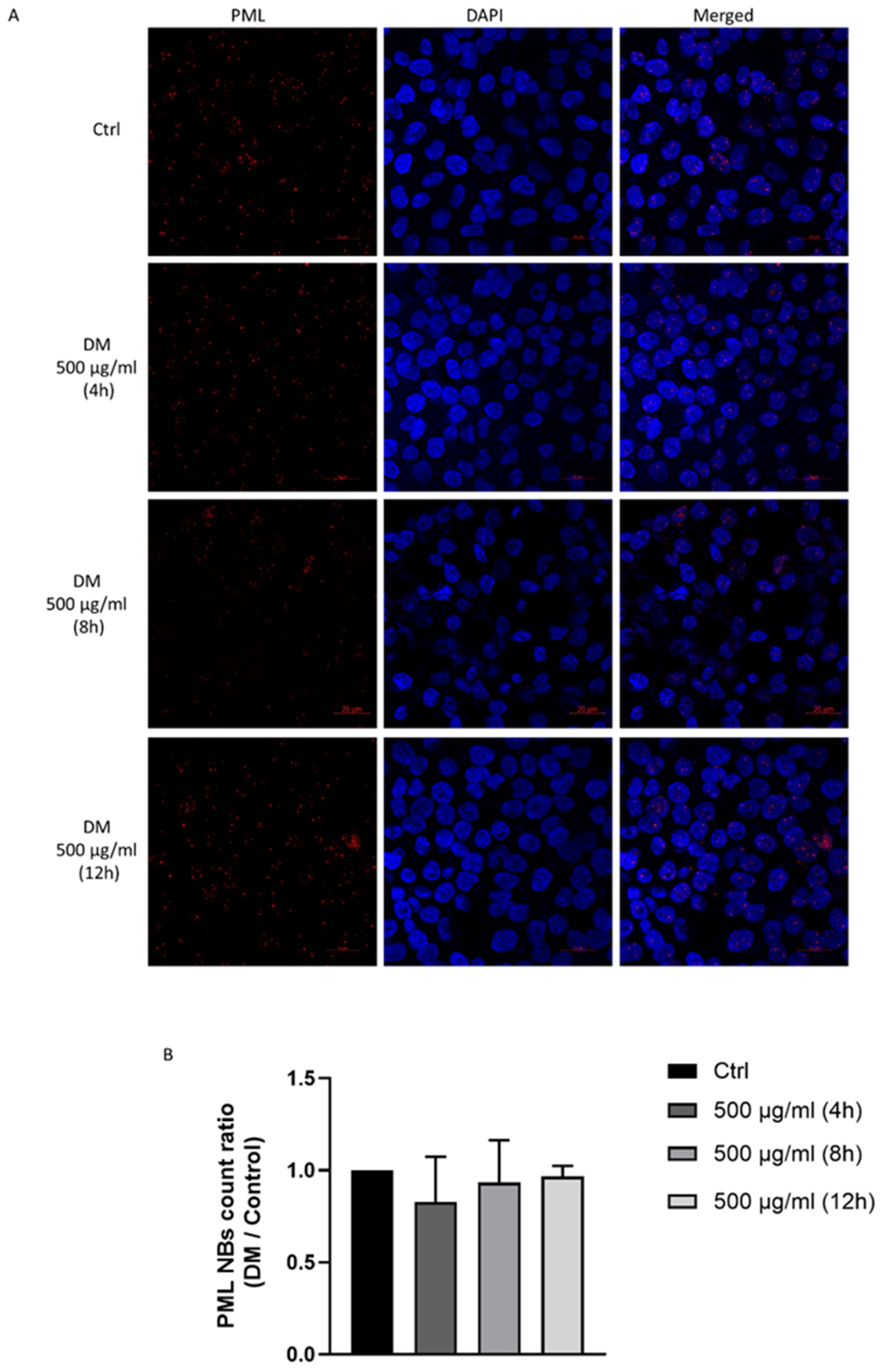
3.7. J. rubens DM Extract Does Not Affect PML Nuclear Body Biogenesis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CRC | Colorectal cancer |
| DM | Dichloromethane–methanol extract |
| EMT | Epithelial–mesenchymal transition |
| TET | Ten-Eleven Translocation enzymes |
| 5-hmC | 5-hydroxymethylcytosine |
| ROS | Reactive oxygen species |
| DHE | Dihydroethidium |
| PML | Promyelocytic Leukemia protein |
| MMP | Matrix metalloproteinase |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Morgan, E.; Arnold, M.; Gini, A.; Lorenzoni, V.; Cabasag, C.J.; Laversanne, M.; Vignat, J.; Ferlay, J.; Murphy, N.; Bray, F. Global burden of colorectal cancer in 2020 and 2040: Incidence and mortality estimates from GLOBOCAN. Gut 2023, 72, 338–344. [Google Scholar] [CrossRef]
- Charafeddine, M.A.; Olson, S.H.; Mukherji, D.; Temraz, S.N.; Abou-Alfa, G.K.; Shamseddine, A.I. Proportion of cancer in a Middle eastern country attributable to established risk factors. BMC Cancer 2017, 17, 337. [Google Scholar] [CrossRef] [PubMed]
- Salhab, H.A.; Fares, M.Y.; Khachfe, H.H.; Khachfe, H.M. Epidemiological Study of Lung Cancer Incidence in Lebanon. Medicina 2019, 55, 217. [Google Scholar] [CrossRef] [PubMed]
- Alzahrani, S.M.; Al Doghaither, H.A.; Al-Ghafari, A.B. General insight into cancer: An overview of colorectal cancer (Review). Mol. Clin. Oncol. 2021, 15, 271. [Google Scholar] [CrossRef]
- Rejhová, A.; Opattová, A.; Čumová, A.; Slíva, D.; Vodička, P. Natural compounds and combination therapy in colorectal cancer treatment. Eur. J. Med. Chem. 2018, 144, 582–594. [Google Scholar] [CrossRef]
- Köhne, C.H. Successes and limitations of Targeted Cancer Therapy in Colon Cancer. In Progress in Tumor Research; Karger: Basel, Switzerland, 2014; Volume 41, pp. 36–50. [Google Scholar] [CrossRef]
- Andrade, K.A.M.; Lauritano, C.; Romano, G.; Ianora, A. Marine Microalgae with Anti-Cancer Properties. Mar. Drugs 2018, 16, 165. [Google Scholar] [CrossRef]
- Arfin, S.; Jha, N.K.; Jha, S.K.; Kesari, K.K.; Ruokolainen, J.; Roychoudhury, S.; Rathi, B.; Kumar, D. Oxidative Stress in Cancer Cell Metabolism. Antioxidants 2021, 10, 642. [Google Scholar] [CrossRef]
- Ju, S.; Singh, M.K.; Han, S.; Ranbhise, J.; Ha, J.; Choe, W.; Yoon, K.S.; Yeo, S.G.; Kim, S.S.; Kang, I. Oxidative Stress and Cancer Therapy: Controlling Cancer Cells Using Reactive Oxygen Species. Int. J. Mol. Sci. 2024, 25, 12387. [Google Scholar] [CrossRef]
- Huang, R.; Chen, H.; Liang, J.; Li, Y.; Yang, J.; Luo, C.; Tang, Y.; Ding, Y.; Liu, X.; Yuan, Q.; et al. Dual Role of Reactive Oxygen Species and their Application in Cancer Therapy. J. Cancer 2021, 12, 5543–5561. [Google Scholar] [CrossRef]
- He, H.; Zhuo, R.; Dai, J.; Wang, X.; Huang, X.; Wang, H.; Xu, D. Chelerythrine induces apoptosis via ROS-mediated endoplasmic reticulum stress and STAT3 pathways in human renal cell carcinoma. J. Cell. Mol. Med. 2020, 24, 50–60. [Google Scholar] [CrossRef]
- Kim, B.R.; Park, S.H.; Jeong, Y.A.; Na, Y.J.; Kim, J.L.; Jo, M.J.; Jeong, S.; Yun, H.K.; Oh, S.C.; Lee, D.H. RUNX3 enhances TRAIL-induced apoptosis by upregulating DR5 in colorectal cancer. Oncogene 2019, 38, 3903–3918. [Google Scholar] [CrossRef]
- Yin, C.; Dai, X.; Huang, X.; Zhu, W.; Chen, X.; Zhou, Q.; Wang, C.; Zhao, C.; Zou, P.; Liang, G.; et al. Alantolactone promotes ER stress-mediated apoptosis by inhibition of TrxR1 in triple-negative breast cancer cell lines and in a mouse model. J. Cell. Mol. Med. 2019, 23, 2194–2206. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Liu, Y.; Peng, X.; Hua, S.; Zhou, G.; Yan, K.; Liu, Y. Synthesis, characterization, and antitumor properties of Au(i)-thiourea complexes. Metallomics 2020, 12, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Basak, D.; Punganuru, S.R.; Srivenugopal, K.S. Piperlongumine exerts cytotoxic effects against cancer cells with mutant p53 proteins at least in part by restoring the biological functions of the tumor suppressor. Int. J. Oncol. 2016, 48, 1426–1436. [Google Scholar] [CrossRef] [PubMed]
- Shao, J.; Li, M.; Guo, Z.; Jin, C.; Zhang, F.; Ou, C.; Xie, Y.; Tan, S.; Wang, Z.; Zheng, S.; et al. TPP-related mitochondrial targeting copper (II) complex induces p53-dependent apoptosis in hepatoma cells through ROS-mediated activation of Drp1. Cell Commun. Signal. 2019, 17, 149. [Google Scholar] [CrossRef]
- El Osmani, N.; Prévostel, C.; Lasorsa, L.P.; El Harakeh, M.; Radwan, Z.; Mawlawi, H.; El Sabban, M.; Shirinian, M.; Dassouki, Z. Vitamin C enhances co-localization of novel TET1 nuclear bodies with both Cajal and PML bodies in colorectal cancer cells. Epigenetics 2024, 19, 2337142. [Google Scholar] [CrossRef]
- Feng, Y.; Li, X.; Cassady, K.; Zou, Z.; Zhang, X. TET2 Function in Hematopoietic Malignancies, Immune Regulation, and DNA Repair. Front. Oncol. 2019, 9, 210. [Google Scholar] [CrossRef]
- Rahim, A.; Ruis, B.L.; Rajczewski, A.T.; Kruk, M.; Tretyakova, N.Y. TET1 Functions as a Tumor Suppressor in Lung Adenocarcinoma Through Epigenetic Remodeling and Immune Modulation. Epigenetics Chromatin 2025, 18, 53. [Google Scholar] [CrossRef]
- Cimmino, L.; Dawlaty, M.M.; Ndiaye-Lobry, D.; Yap, Y.S.; Bakogianni, S.; Yu, Y.; Bhattacharyya, S.; Shaknovich, R.; Geng, H.; Lobry, C.; et al. TET1 is a tumor suppressor of hematopoietic malignancy. Nat. Immunol. 2015, 16, 653–662. [Google Scholar] [CrossRef]
- Good, C.R.; Panjarian, S.; Kelly, A.D.; Madzo, J.; Patel, B.; Jelinek, J.; Issa, J.-P.J. TET1-Mediated Hypomethylation Activates Oncogenic Signaling in Triple-Negative Breast Cancer. Cancer Res. 2018, 78, 4126–4137. [Google Scholar] [CrossRef]
- Huang, H.; Jiang, X.; Li, Z.; Li, Y.; Song, C.X.; He, C.; Sun, M.; Chen, P.; Gurbuxani, S.; Wang, J.; et al. TET1 plays an essential oncogenic role in MLL-rearranged leukemia. Proc. Natl. Acad. Sci. USA 2013, 110, 11994–11999. [Google Scholar] [CrossRef]
- Yang, L.; Zhao, R.; Qiao, P.; Cui, J.; Chen, X.; Fan, J.; Hu, A.; Huang, S. The novel oncogenic factor TET3 combines with AHR to promote thyroid cancer lymphangiogenesis via the HIF-1α/VEGF signaling pathway. Cancer Cell Int. 2023, 23, 206. [Google Scholar] [CrossRef]
- Zhu, M.; Shi, B.; Li, C.; Xu, S. TET3 governs malignant behaviors and unfavorable prognosis of esophageal squamous cell carcinoma by activating the PI3K/AKT/GSK3β/β-catenin pathway. Open Med. 2022, 17, 1883–1895. [Google Scholar] [CrossRef] [PubMed]
- Leal, M.C.; Munro, M.H.G.; Blunt, J.W.; Puga, J.; Jesus, B.; Calado, R.; Rosa, R.; Madeira, C. Biogeography and biodiscovery hotspots of macroalgal marine natural products. Nat. Prod. Rep. 2013, 30, 1380–1390. [Google Scholar] [CrossRef] [PubMed]
- Ismail, M.M.; Alotaibi, B.S.; El-Sheekh, M.M. Therapeutic Uses of Red Macroalgae. Molecules 2020, 25, 4411. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Guzmán, M.; Rodríguez-Nogales, A.; Algieri, F.; Gálvez, J. Potential Role of Seaweed Polyphenols in Cardiovascular-Associated Disorders. Mar. Drugs 2018, 16, 250. [Google Scholar] [CrossRef]
- Ferdous, U.T.; Balia Yusof, Z.N. Insight into Potential Anticancer Activity of Algal Flavonoids: Current Status and Challenges. Molecules 2021, 26, 6844. [Google Scholar] [CrossRef]
- Ktari, L.l.; Blond, A.; Guyot, M. 16β-Hydroxy-5α-cholestane-3, 6-dione, a novel cytotoxic oxysterol from the red alga Jania rubens. Bioorg. Med. Chem. Lett. 2000, 10, 2563–2565. [Google Scholar] [CrossRef]
- Awad, N.E. Bioactive brominated diterpenes from the marine red alga Jania rubens (L.) Lamx. Phytother. Res. 2004, 18, 275–279. [Google Scholar] [CrossRef]
- Gheda, S.; El-Sheekh, M.; Abou-Zeid, A. In vitro anticancer activity of polysaccharide extracted from red alga Jania rubens against breast and colon cancer cell lines. Asian Pac. J. Trop. Med. 2018, 11, 583–589. [Google Scholar] [CrossRef]
- Rifi, M.; Radwan, Z.; AlMonla, R.; Fajloun, Z.; Sabatier, J.M.; Kouzayha, A.; El-Sabban, M.; Mawlawi, H.; Dassouki, Z. The Lebanese Red Algae Jania rubens: Promising Biomolecules against Colon Cancer Cells. Molecules 2022, 27, 6617. [Google Scholar] [CrossRef] [PubMed]
- Otero-Estévez, O.; De Chiara, L.; Rodríguez-Girondo, M.; Rodríguez-Berrocal, F.J.; Cubiella, J.; Castro, I.; Hernández, V.; Martínez-Zorzano, V.S. Serum matrix metalloproteinase-9 in colorectal cancer family-risk population screening. Sci. Rep. 2015, 5, 13030. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Tang, F.; Zhang, B.; Zhao, Y.; Feng, J.; Rao, Z. Matrix metalloproteinase-9 overexpression is closely related to poor prognosis in patients with colon cancer. World J. Surg. Oncol. 2014, 12, 24. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.; Li, H.; Zhang, Y.; Yang, H.; Guo, M.; Li, L.; Liu, T. Matrix metalloproteinase 2 promotes cell growth and invasion in colorectal cancer. Acta Biochim. Biophys. Sin. 2011, 43, 840–848. [Google Scholar] [CrossRef]
- Ibraheem, Q. The Role of Matrix Metalloproteinase-2 (MMP2) in Colorectal Cancer Progression: Correlation with Clinicopathological Features and Impact on Cellular Processes. Cureus 2024, 16, e61941. [Google Scholar] [CrossRef]
- Lee, E.; Yang, D.; Hong, J.H. Prominent Naturally Derived Oxidative-Stress-Targeting Drugs and Their Applications in Cancer Treatment. Antioxidants 2025, 14, 49. [Google Scholar] [CrossRef]
- Yu, S.; Yin, Y.; Hong, S.; Cao, S.; Huang, Y.; Chen, S.; Liu, Y.; Guan, H.; Zhang, Q.; Li, Y.; et al. TET1 is a Tumor Suppressor That Inhibits Papillary Thyroid Carcinoma Cell Migration and Invasion. Int. J. Endocrinol. 2020, 2020, 3909610. [Google Scholar] [CrossRef]
- Niwa-Kawakita, M.; Ferhi, O.; Soilihi, H.; Le Bras, M.; Lallemand-Breitenbach, V.; de Thé, H. PML is a ROS sensor activating p53 upon oxidative stress. J. Exp. Med. 2017, 214, 3197–3206. [Google Scholar] [CrossRef]
- Guo, S.; Cheng, X.; Lim, J.H.; Liu, Y.; Kao, H.Y. Control of antioxidative response by the tumor suppressor protein PML through regulating Nrf2 activity. Mol. Biol. Cell 2014, 25, 2485–2498. [Google Scholar] [CrossRef]
- Rifi, M.; Radwan, Z.; Sari-Chmayssem, N.; Kassir, R.; Fajloun, Z.; Rahman, A.A.; El-Sabban, M.; Prévostel, C.; Dassouki, Z.; Mawlawi, H. Exploring the Antineoplastic Properties of the Lebanese Jania rubens Against Colorectal Cancer. Metabolites 2025, 15, 90. [Google Scholar] [CrossRef]
- Otto, T.; Sicinski, P. Cell cycle proteins as promising targets in cancer therapy. Nat. Rev. Cancer 2017, 17, 93–115. [Google Scholar] [CrossRef] [PubMed]
- Guzi, T.J.; Paruch, K.; Dwyer, M.P.; Labroli, M.; Shanahan, F.; Davis, N.; Taricani, L.; Wiswell, D.; Seghezzi, W.; Penaflor, E.; et al. Targeting the replication checkpoint using SCH 900776, a potent and functionally selective CHK1 inhibitor identified via high content screening. Mol. Cancer Ther. 2011, 10, 591–602. [Google Scholar] [CrossRef] [PubMed]
- King, C.; Diaz, H.B.; McNeely, S.; Barnard, D.; Dempsey, J.; Blosser, W.; Beckmann, R.; Barda, D.; Marshall, M.S. LY2606368 Causes Replication Catastrophe and Antitumor Effects through CHK1-Dependent Mechanisms. Mol. Cancer Ther. 2015, 14, 2004–2013. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Zhang, Y.; Chen, S.; Kmieciak, M.; Leng, Y.; Lin, H.; Rizzo, K.A.; Dumur, C.I.; Ferreira-Gonzalez, A.; Dai, Y.; et al. A regimen combining the Wee1 inhibitor AZD1775 with HDAC inhibitors targets human acute myeloid leukemia cells harboring various genetic mutations. Leukemia 2015, 29, 807–818. [Google Scholar] [CrossRef]
- Sherr, C.J.; Beach, D.; Shapiro, G.I. Targeting CDK4 and CDK6: From Discovery to Therapy. Cancer Discov. 2016, 6, 353–367. [Google Scholar] [CrossRef]
- Agoni, L.; Basu, I.; Gupta, S.; Alfieri, A.; Gambino, A.; Goldberg, G.L.; Reddy, E.P.; Guha, C. Rigosertib is a more effective radiosensitizer than cisplatin in concurrent chemoradiation treatment of cervical carcinoma, in vitro and in vivo. Int. J. Radiat. Oncol. Biol. Phys. 2014, 88, 1180–1187. [Google Scholar] [CrossRef]
- Bailon-Moscoso, N.; Cevallos-Solorzano, G.; Romero-Benavides, J.C.; Orellana, M.I. Natural Compounds as Modulators of Cell Cycle Arrest: Application for Anticancer Chemotherapies. Curr. Genom. 2017, 18, 106–131. [Google Scholar] [CrossRef]
- Khan, H.; Alam, W.; Alsharif, K.F.; Aschner, M.; Pervez, S.; Saso, L. Alkaloids and Colon Cancer: Molecular Mechanisms and Therapeutic Implications for Cell Cycle Arrest. Molecules 2022, 27, 920. [Google Scholar] [CrossRef]
- Fu, Y.; Xie, D.; Zhu, Y.; Zhang, X.; Yue, H.; Zhu, K.; Pi, Z.; Dai, Y. Anti-colorectal cancer effects of seaweed-derived bioactive compounds. Front. Med. 2022, 9, 988507. [Google Scholar] [CrossRef]
- Arumugam, P.; Arunkumar, K.; Sivakumar, L.; Murugan, M.; Murugan, K. Anticancer effect of fucoidan on cell proliferation, cell cycle progression, genetic damage and apoptotic cell death in HepG2 cancer cells. Toxicol. Rep. 2019, 6, 556–563. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Selvaraj, B.; Lee, J.W. Anticancer effects of seaweed-derived bioactive compounds. Appl. Sci. 2021, 11, 11261. [Google Scholar] [CrossRef]
- Xie, Y.; Wang, X.; Wang, W.; Pu, N.; Liu, L. Epithelial-mesenchymal transition orchestrates tumor microenvironment: Current perceptions and challenges. J. Transl. Med. 2025, 23, 386. [Google Scholar] [CrossRef]
- Pastushenko, I.; Blanpain, C. EMT Transition States during Tumor Progression and Metastasis. Trends Cell Biol. 2019, 29, 212–226. [Google Scholar] [CrossRef]
- Vu, T.; Datta, P.K. Regulation of EMT in Colorectal Cancer: A Culprit in Metastasis. Cancers 2017, 9, 171. [Google Scholar] [CrossRef]
- Yan, X.; Yan, L.; Liu, S.; Shan, Z.; Tian, Y.; Jin, Z. N-cadherin, a novel prognostic biomarker, drives malignant progression of colorectal cancer. Mol. Med. Rep. 2015, 12, 2999–3006. [Google Scholar] [CrossRef]
- Gonzalez-Avila, G.; Sommer, B.; Mendoza-Posada, D.A.; Ramos, C.; Garcia-Hernandez, A.A.; Falfan-Valencia, R. Matrix metalloproteinases participation in the metastatic process and their diagnostic and therapeutic applications in cancer. Crit. Rev. Oncol. Hematol. 2019, 137, 57–83. [Google Scholar] [CrossRef]
- He, X.; Xue, M.; Jiang, S.; Li, W.; Yu, J.; Xiang, S. Fucoidan Promotes Apoptosis and Inhibits EMT of Breast Cancer Cells. Biol. Pharm. Bull. 2019, 42, 442–447. [Google Scholar] [CrossRef]
- Hsu, H.-Y.; Lin, T.-Y.; Hwang, P.-A.; Tseng, L.-M.; Chen, R.-H.; Tsao, S.-M.; Hsu, J. Fucoidan induces changes in the epithelial to mesenchymal transition and decreases metastasis by enhancing ubiquitin-dependent TGFβ receptor degradation in breast cancer. Carcinogenesis 2013, 34, 874–884. [Google Scholar] [CrossRef]
- Kim, R.K.; Suh, Y.; Yoo, K.C.; Cui, Y.H.; Hwang, E.; Kim, H.J.; Kang, J.S.; Kim, M.J.; Lee, Y.Y.; Lee, S.J. Phloroglucinol suppresses metastatic ability of breast cancer cells by inhibition of epithelial-mesenchymal cell transition. Cancer Sci. 2015, 106, 94–101. [Google Scholar] [CrossRef]
- Huang, Y.; Rao, A. Connections between TET proteins and aberrant DNA modification in cancer. Trends Genet. 2014, 30, 464–474. [Google Scholar] [CrossRef]
- Zhang, C.; Zheng, J.; Liu, J.; Li, Y.; Xing, G.; Zhang, S.; Chen, H.; Wang, J.; Shao, Z.; Li, Y.; et al. Pan-cancer analyses reveal the molecular and clinical characteristics of TET family members and suggests that TET3 maybe a potential therapeutic target. Front. Pharmacol. 2024, 15, 1418456. [Google Scholar] [CrossRef]
- Ehrlich, M. DNA hypomethylation in cancer cells. Epigenomics 2009, 1, 239–259. [Google Scholar] [CrossRef]
- Funaki, S.; Nakamura, T.; Nakatani, T.; Umehara, H.; Nakashima, H.; Okumura, M.; Oboki, K.; Matsumoto, K.; Saito, H.; Nakano, T. Global DNA hypomethylation coupled to cellular transformation and metastatic ability. FEBS Lett. 2015, 589, 4053–4060. [Google Scholar] [CrossRef] [PubMed]
- Hesselink, E.N.K.; Zafon, C.; Villalmanzo, N.; Iglesias, C.; van Hemel, B.M.; Hesselink, M.S.K.; Montero-Conde, C.; Buj, R.; Mauricio, D.; Peinado, M.A.; et al. Increased Global DNA Hypomethylation in Distant Metastatic and Dedifferentiated Thyroid Cancer. J. Clin. Endocrinol. Metab. 2018, 103, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Cao, D.; Sun, S.; Wang, Y. Anticancer therapeutic effect of ginsenosides through mediating reactive oxygen species. Front. Pharmacol. 2023, 14, 1215020. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, H.; Takada, K. Reactive oxygen species in cancer: Current findings and future directions. Cancer Sci. 2021, 112, 3945–3952. [Google Scholar] [CrossRef]
- Chang, H.R.; Munkhjargal, A.; Kim, M.J.; Park, S.Y.; Jung, E.; Ryu, J.H.; Yang, Y.; Lim, J.S.; Kim, Y. The functional roles of PML nuclear bodies in genome maintenance. Mutat. Res. Fundam. Mol. Mech. Mutagen. 2018, 809, 99–107. [Google Scholar] [CrossRef]
- Liebl, M.C.; Hofmann, T.G. Regulating the p53 Tumor Suppressor Network at PML Biomolecular Condensates. Cancers 2022, 14, 4549. [Google Scholar] [CrossRef]
- Wu, W.; Tan, Y.; Yin, H.; Jiang, M.; Jiang, Y.; Ma, X.; Yin, T.; Li, Y.; Zhang, H.; Cai, X.; et al. Phase separation is required for PML nuclear body biogenesis and function. FASEB J. 2023, 37, e22986. [Google Scholar] [CrossRef]
- Jackson, S.P.; Bartek, J. The DNA-damage response in human biology and disease. Nature 2009, 461, 1071–1078. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Feng, C.; Cai, X.; Hao, Y.; Gu, X.; Cai, L.; Wu, S.; Chen, J.; Liu, Z.; Xie, W.; et al. 7-Methoxyisoflavone ameliorates atopic dermatitis symptoms by regulating multiple signaling pathways and reducing chemokine production. Sci. Rep. 2022, 12, 8760. [Google Scholar] [CrossRef] [PubMed]
- Genişel, M.; Aydın, S. 7-Methoxyflavone and 7-Hydroxy-4′-nitroisoflavone confer ameliorative effects to human erythrocytes exposed to oxidative damage. East. Anatol. J. Sci. 2021, 7, 6–11. [Google Scholar]
- Walle, T. Methoxylated flavones, a superior cancer chemopreventive flavonoid subclass? Semin. Cancer Biol. 2007, 17, 354–362. [Google Scholar] [CrossRef]
- Cherukupalli, N.; Bhumireddy, S.R.; Akella, S.S.V.; Sataniya, A.; Sripadi, P.; Khareedu, V.R.; Vudem, D.R. Phytochemical profiling and in vitro anticancer activity of purified flavonoids of andrographis glandulosa. Planta Med. Int. Open 2017, 4, e24–e34. [Google Scholar] [CrossRef]
- Fatokun, A.A.; Liu, J.O.; Dawson, V.L.; Dawson, T.M. Identification through high-throughput screening of 4′-methoxyflavone and 3′,4′-dimethoxyflavone as novel neuroprotective inhibitors of parthanatos. Br. J. Pharmacol. 2013, 169, 1263–1278. [Google Scholar] [CrossRef]
- Asthana, J.; Mishra, B.; Pandey, R. 5, 7-Dihydroxy-4-Methoxyflavone a bioactive flavonoid delays amyloid beta-induced paralysis and attenuates oxidative stress in transgenic Caenorhabditis elegans. Pharmacogn. Mag. 2018, 14, S57–S64. [Google Scholar]
- Aidiel, M.; Mutalib, M.A.; Ramasamy, R.; Ramli, N.N.N.; Tang, S.G.H.; Adam, S.H. Mechanistic Insights into the Anticancer Potential of Methoxyflavones Analogs: A Review. Molecules 2025, 30, 346. [Google Scholar] [CrossRef]
- Shendge, A.K.; Chaudhuri, D.; Basu, T.; Mandal, N. A natural flavonoid, apigenin isolated from Clerodendrum viscosum leaves, induces G2/M phase cell cycle arrest and apoptosis in MCF-7 cells through the regulation of p53 and caspase-cascade pathway. Clin. Transl. Oncol. 2021, 23, 718–730. [Google Scholar] [CrossRef]
- Imani, A.; Maleki, N.; Bohlouli, S.; Kouhsoltani, M.; Sharifi, S.; Dizaj, S.M. Molecular mechanisms of anticancer effect of rutin. Phytother. Res. 2021, 35, 2500–2513. [Google Scholar] [CrossRef]
- Tang, S.M.; Deng, X.T.; Zhou, J.; Li, Q.P.; Ge, X.X.; Miao, L. Pharmacological basis and new insights of quercetin action in respect to its anti-cancer effects. Biomed. Pharmacother. 2020, 121, 109604. [Google Scholar] [CrossRef]
- Reheim, M.A.M.A.; Hafiz, I.S.A.; Reffat, H.M.; Rady, H.S.A.; Shehadi, I.A.; Rashdan, H.R.M.; Hassan, A.; Abdelmonsef, A.H. New 1,3-diphenyl-1H-pyrazol-5-ols as anti-methicillin resistant Staphylococcus aureus agents: Synthesis, antimicrobial evaluation and in silico studies. Heliyon 2024, 10, e33160. [Google Scholar] [CrossRef]
- Lusardi, M.; Spallarossa, A.; Brullo, C. Amino-Pyrazoles in Medicinal Chemistry: A Review. Int. J. Mol. Sci. 2023, 24, 7834. [Google Scholar] [CrossRef] [PubMed]
- Naim, M.J.; Alam, O.; Nawaz, F.; Alam, M.J.; Alam, P. Current status of pyrazole and its biological activities. J. Pharm. Bioallied Sci. 2016, 8, 2–17. [Google Scholar] [CrossRef] [PubMed]
- Karrouchi, K.; Radi, S.; Ramli, Y.; Taoufik, J.; Mabkhot, Y.N.; Al-Aizari, F.A.; Ansar, M. Synthesis and Pharmacological Activities of Pyrazole Derivatives: A Review. Molecules 2018, 23, 134. [Google Scholar] [CrossRef] [PubMed]
- Ramadan, M.; Aly, A.A.; El-Haleem, L.E.A.; Alshammari, M.B.; Bräse, S. Substituted Pyrazoles and Their Heteroannulated Analogs-Recent Syntheses and Biological Activities. Molecules 2021, 26, 4995. [Google Scholar] [CrossRef]
- Xu, Z.; Zhuang, Y.; Chen, Q. Current scenario of pyrazole hybrids with in vivo therapeutic potential against cancers. Eur. J. Med. Chem. 2023, 257, 115495. [Google Scholar] [CrossRef]
- Alsayari, A.; Asiri, Y.I.; Muhsinah, A.B.; Hassan, M.Z. Anticolon Cancer Properties of Pyrazole Derivatives Acting through Xanthine Oxidase Inhibition. J. Oncol. 2021, 2021, 5691982. [Google Scholar] [CrossRef]
- Patil, S.A.; Patil, R.; Patil, S.A. Recent developments in biological activities of indanones. Eur. J. Med. Chem. 2017, 138, 182–198. [Google Scholar] [CrossRef]
- Atom, R.S.; Shaikh, S.A.M.; Maharabam, K.; Khunjamayum, R.; Ahanthem, D.; Laitonjam, W.S.; Ningthoujam, R.S.; Kunwar, A. GC-MS profiling, in vitro antioxidant and antimicrobial activities of Kaempferia parviflora Wall. ex. Baker rhizome extract. Int. J. Pharm. Investig. 2022, 12, 430–437. [Google Scholar] [CrossRef]
- Kar, S.; Akhir, A.; Chopra, S.; Ohki, S.; Karanam, B.; Golakoti, N.R. Benzopyrylium salts as new anticancer, antibacterial, and antioxidant agents. Med. Chem. Res. 2021, 30, 877–885. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Lv, S.; Liu, J.; Yu, Y.; Wang, H.; Zhang, H. An Overview of Bioactive 1,3-Oxazole-Containing Alkaloids from Marine Organisms. Pharmaceuticals 2021, 14, 1274. [Google Scholar] [CrossRef] [PubMed]
- Kuang, Y.; Han, X.; Xu, M.; Yang, Q. Oxaloacetate induces apoptosis in HepG2 cells via inhibition of glycolysis. Cancer Med. 2018, 7, 1416–1429. [Google Scholar] [CrossRef] [PubMed]
- Kuş, C.; Uğurlu, E.; Özdamar, E.D.; Can-Eke, B. Synthesis and Antioxidant Properties of New Oxazole-5(4H)-one Derivatives. Turk. J. Pharm. Sci. 2017, 14, 174–178. [Google Scholar] [CrossRef]
- Singh, N.; Shreshtha, A.K.; Thakur, M.S.; Patra, S. Xanthine scaffold: Scope and potential in drug development. Heliyon 2018, 4, e00829. [Google Scholar] [CrossRef]
- Landowski, T.H.; Samulitis, B.K.; Dorr, R.T. The diaryl oxazole PC-046 is a tubulin-binding agent with experimental anti-tumor efficacy in hematologic cancers. Investig. New Drugs 2013, 31, 1616–1625. [Google Scholar] [CrossRef]
- Kulkarni, S.; Kaur, K.; Jaitak, V. Recent Developments in Oxazole Derivatives as Anticancer Agents: Review on Synthetic Strategies, Mechanism of Action and SAR Studies. Anticancer Agents Med. Chem. 2022, 22, 1859–1882. [Google Scholar] [CrossRef]
- Frolinger, T.; Herman, F.; Sharma, A.; Sims, S.; Wang, J.; Pasinetti, G.M. Epigenetic modifications by polyphenolic compounds alter gene expression in the hippocampus. Biol. Open 2018, 7, bio035196. [Google Scholar] [CrossRef]
- Roy, S.; Deka, D.; Kondaveeti, S.B.; Ayyadurai, P.; Siripragada, S.; Philip, N.; Pathak, S.; Duttaroy, A.K.; Banerjee, A. An overview of potential of natural compounds to regulate epigenetic modifications in colorectal cancer: A recent update. Epigenetics 2025, 20, 2491316. [Google Scholar] [CrossRef]
- Lee, W.J.; Shim, J.Y.; Zhu, B.T. Mechanisms for the inhibition of DNA methyltransferases by tea catechins and bioflavonoids. Mol. Pharmacol. 2005, 68, 1018–1030. [Google Scholar] [CrossRef]
- Gilbert, E.R.; Liu, D. Flavonoids influence epigenetic-modifying enzyme activity: Structure—Function relationships and the therapeutic potential for cancer. Curr. Med. Chem. 2010, 17, 1756–1768. [Google Scholar] [CrossRef]
- Fang, M.; Chen, D.; Yang, C.S. Dietary polyphenols may affect DNA methylation. J. Nutr. 2007, 137, 223s–228s. [Google Scholar] [CrossRef]
| Gene | Primer Sequence | Annealing Temperature |
|---|---|---|
| N-Cadherin | F: 5′-GGTGGAGGAGAAGACCAG-3′ R: 5′-CGGTGGGGTTGAGGATCT-3′ | 58 |
| SNAIL | F: 5′-CTTCCAGCAGCCCTACGAC-3′ R: 5′-CGGTGGGGTTGAGGATCT-3′ | 57 |
| TWIST | F: 5′-AGCTACGCCTTCTCGGTCT-3′ R: 5′-CCTTCTCTGGAAACAATGACATC-3′ | 57.5 |
| TET-1 | F: 5′-TTCGTCACTGCCAACCTTAG-3′ R: 5′-ATGCCTCTTTCACTGGGTG-3′ | 60.5 |
| TET-2 | F: 5′-CACTGCATGTTTGGACTTCTG-3′ R: 5′-TGCTCATCCTCAGGTTTTCC-3′ | 58 |
| TET-3 | F: 5′-GCCCACAAGGACCAGGATAA-3′ R: 5′-CGCAGCGATTGTCTTCCTTG-3′ | 60 |
| pic | RT | Area | Compound | Ref | CAS | Qual |
|---|---|---|---|---|---|---|
| 3 | 27.095 | 2.53 | 5-amino-1,3-diphenyl-1H pyrazol | 97544 | 005356-71-8 | 38 |
| 3 | 27.095 | 2.53 | 4′-Methoxyflavone | 113426 | 004143-74-2 | 30 |
| 5 | 30.238 | 2.29 | 1-(Anilinomethylene)-2-indanone | 97595 | 1000075-55-7 | 18 |
| 6 | 30.749 | 2.21 | 4-bromo-N-(2-chloroacetyl)pyrazole-1-carboxamide | 125768 | 1000268-17-5 | 20 |
| 11 | 34.465 | 3.18 | Oxazolo [3,2-E]xanthine 2,3-dihydro-2-hydroxymethyl-5,7-dimethyl- | 112689 | 1000285-58-3 | 30 |
| 11 | 34.465 | 3.18 | 2,3-dihydro-2-hydroxymethyl-5,7-dimethyl-4H-1-Benzopyran-4-one | 113438 | 007622-32-4 | 30 |
| 30 | 42.996 | 2.71 | 4H-1-Benzopyran-4-one, 8-methoxy-2-phenyl- | 113437 | 026964-26-1 | 22 |
| 41 | 45.922 | 2.95 | 7-Methoxyflavone | 113425 | 022395-22-8 | 30 |
| 41 | 45.922 | 2.95 | 2-[3-Methoxyphenyl]-4H-1-benzopyran-4-one | 113433 | 053906-83-5 | 27 |
| 60 | 51.093 | 1.75 | Indan-1,3-dione, 2-(1,3-dimethyl-1 H-pyrazol-4-ylmethylene)- | 113316 | 1000316-71-1 | 18 |
| 62 | 51.635 | 1.70 | 4-[5-(4-Methoxyphenyl)-2-oxazolyl] pyridine | 113289 | 096753-33-2 | 18 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radwan, Z.; Kassir, R.; Al Feghaly, F.; Zaiter, R.; Daher, M.A.; Roufayel, R.; Fajloun, Z.; Mawlawi, H.; El-Sabban, M.; Dassouki, Z. Anticancer Activity of Jania rubens in HCT-116 Cells via EMT Suppression, TET Downregulation, and ROS-Mediated Cytotoxicity. Biomolecules 2025, 15, 1361. https://doi.org/10.3390/biom15101361
Radwan Z, Kassir R, Al Feghaly F, Zaiter R, Daher MA, Roufayel R, Fajloun Z, Mawlawi H, El-Sabban M, Dassouki Z. Anticancer Activity of Jania rubens in HCT-116 Cells via EMT Suppression, TET Downregulation, and ROS-Mediated Cytotoxicity. Biomolecules. 2025; 15(10):1361. https://doi.org/10.3390/biom15101361
Chicago/Turabian StyleRadwan, Zeina, Rayan Kassir, Fouad Al Feghaly, Rouaa Zaiter, Mira Abou Daher, Rabih Roufayel, Ziad Fajloun, Hiba Mawlawi, Marwan El-Sabban, and Zeina Dassouki. 2025. "Anticancer Activity of Jania rubens in HCT-116 Cells via EMT Suppression, TET Downregulation, and ROS-Mediated Cytotoxicity" Biomolecules 15, no. 10: 1361. https://doi.org/10.3390/biom15101361
APA StyleRadwan, Z., Kassir, R., Al Feghaly, F., Zaiter, R., Daher, M. A., Roufayel, R., Fajloun, Z., Mawlawi, H., El-Sabban, M., & Dassouki, Z. (2025). Anticancer Activity of Jania rubens in HCT-116 Cells via EMT Suppression, TET Downregulation, and ROS-Mediated Cytotoxicity. Biomolecules, 15(10), 1361. https://doi.org/10.3390/biom15101361






