Applications of Isothermal Titration Calorimetry in Studying Biomimetic Nanocarriers
Abstract
1. Introduction
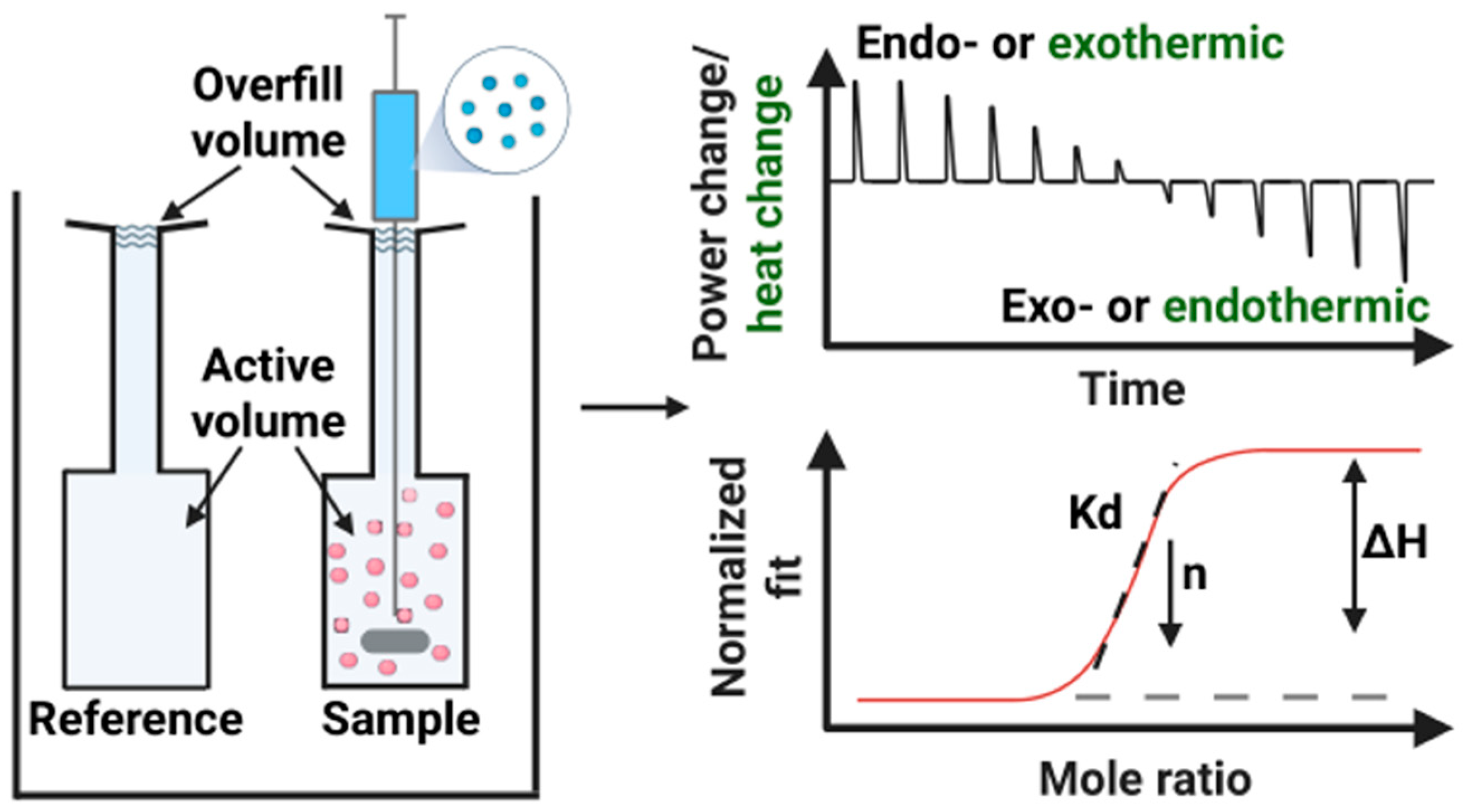
2. Isothermal Titration Calorimetry
2.1. Principle of ITC
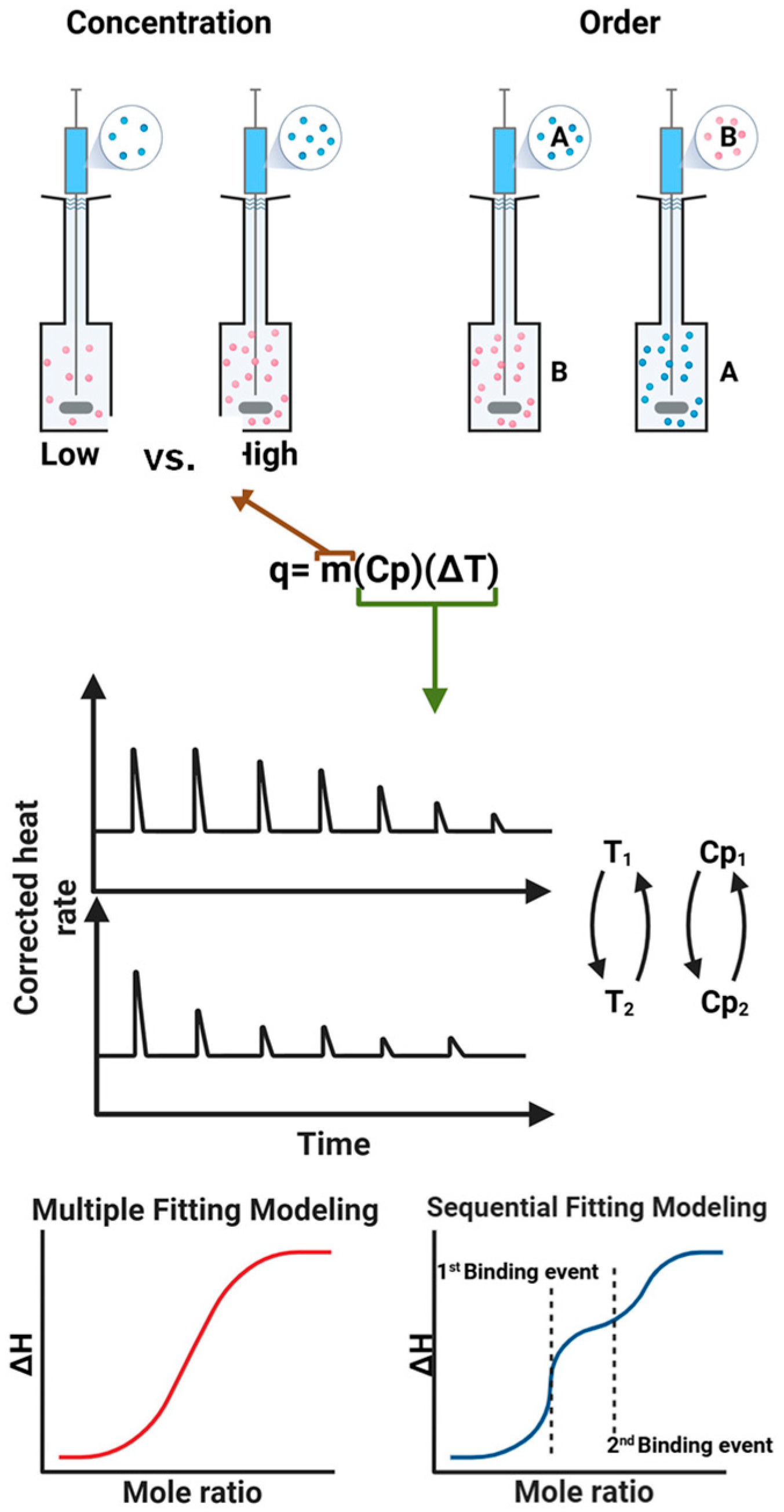
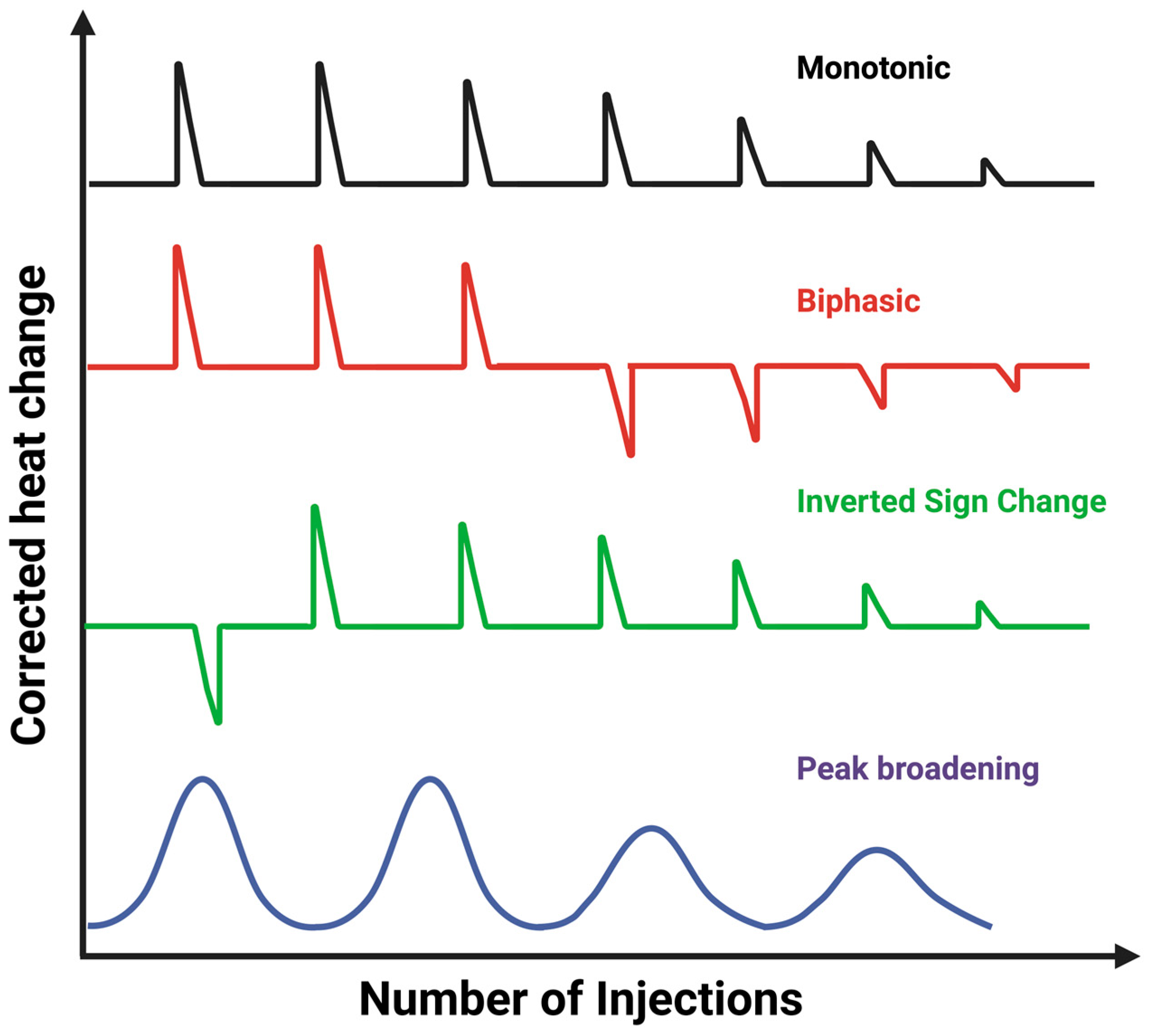
2.2. Important Parameters Affecting ITC Results
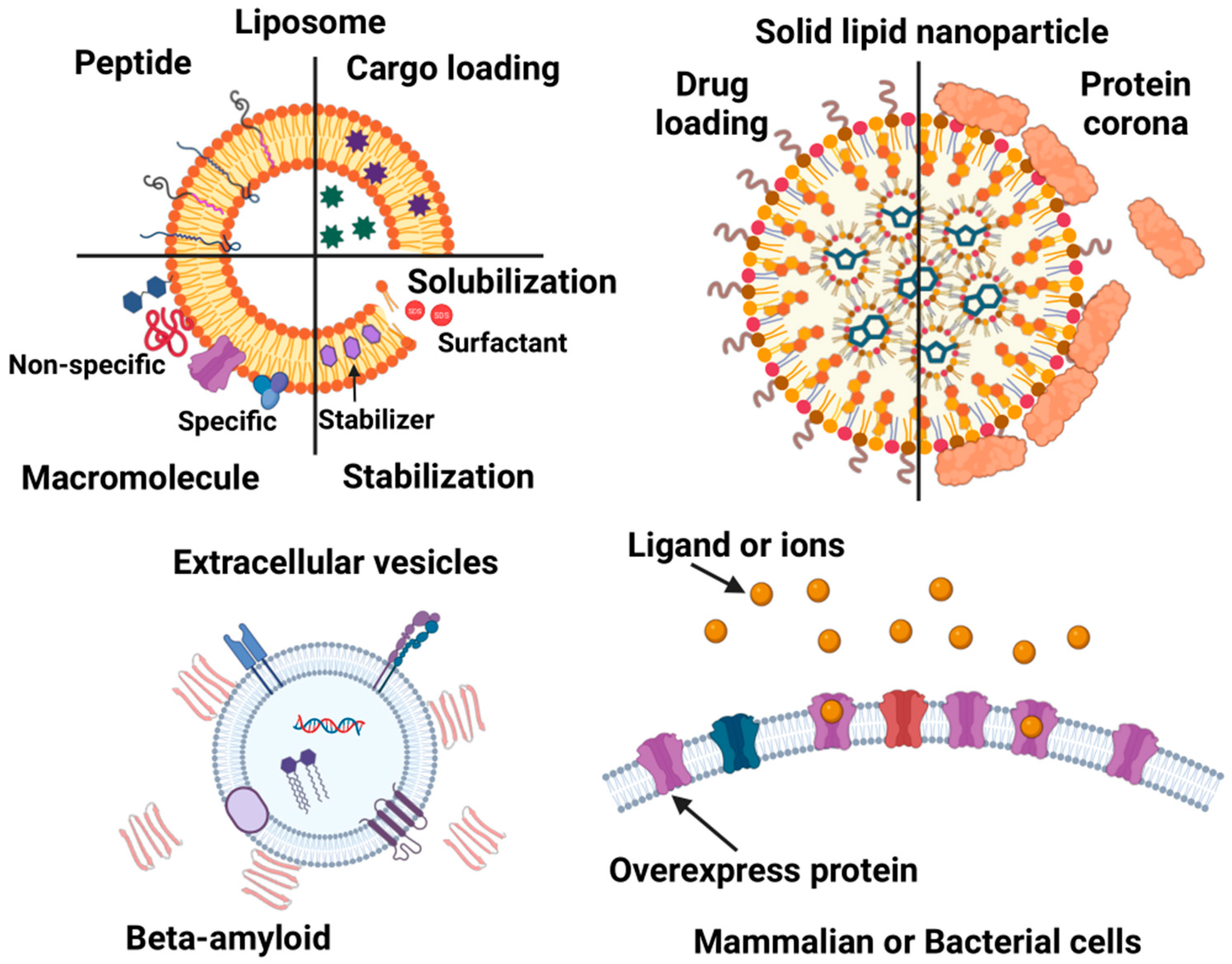
3. ITC Applications of Biomimetic Nanocarriers
3.1. ITC Applications of Solid Lipid Nanoparticles
3.2. ITC Applications in Liposomes
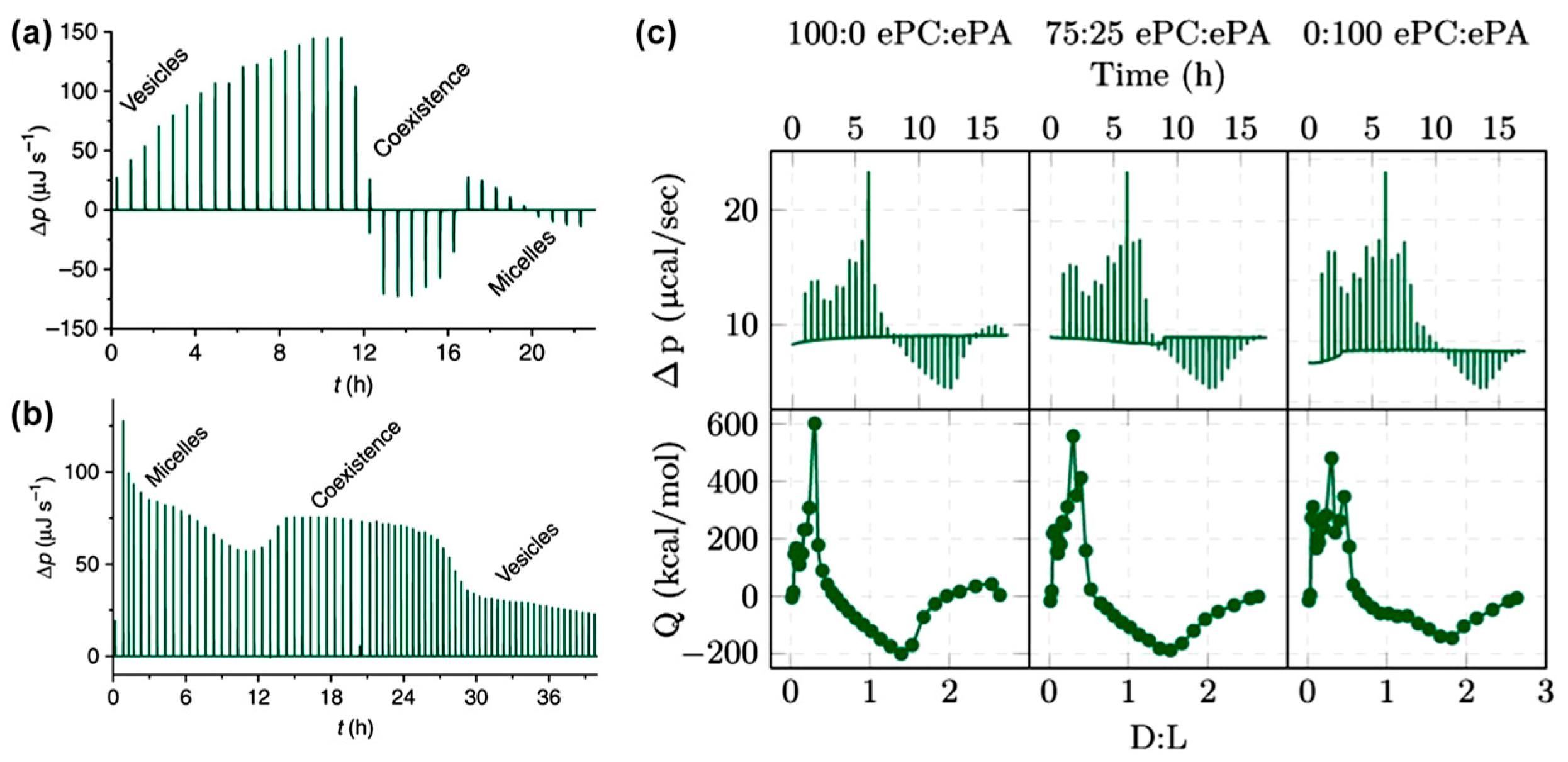
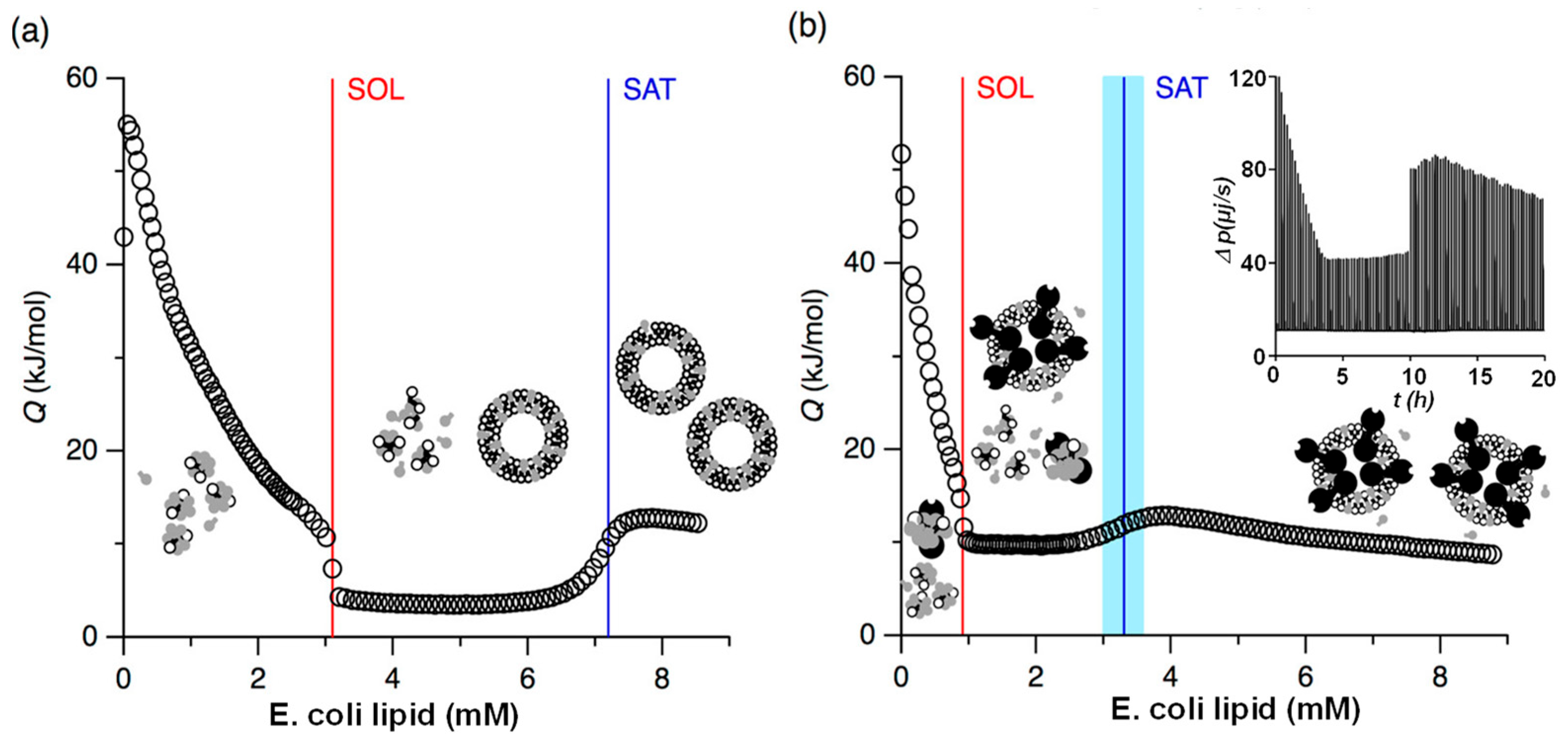
3.3. Membrane Solubilization and Stability
3.4. Cargo Loading into Liposomes
3.5. Non-Specific Interactions of Liposomes
3.6. Specific Interactions with Liposomes
3.7. ITC Applications of Cell-Derived Vesicles
3.8. ITC Applications in Native Cell Membrane and Live Cells
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ITC | Isothermal Titration Calorimetry |
| EVs | Extracellular Vesicles |
| RNA | Ribonucleic Acid |
| mRNA | Messenger RNA |
| BSA | Bovine Serum Albumin |
| HAS | Human Serum Albumin |
| DOPE | Dioleoylphosphatidylethanolamine |
| Dlin-MC3-DMA | Heptatriaconta-6,9,28,31-tetraen-19-yl 4-(dimethylamino)butanoate |
| DOPC | 1,2-dioleoyl-sn-glycero-3-phosphocholine |
| DSPE-PEG2000 | 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-poly(ethylene glycol)-2000 |
| PLGA | Poly(lactic-co-glycolic acid) |
| ApoA1 | Apolipoprotein A1 |
| PC | Phosphatidylcholine |
| PE | Phosphatidylethanolamine |
| PA | Phosphatidic Acid |
| PS | Phosphatidylserine |
| POPC | 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine |
| POPG | 1-palmitoyl-2-oleoyl-sn-glycero-3-phospho-(1’-rac-glycerol) |
| DMPC | 1,2-dimyristoyl-sn-glycero-3-phosphocholine |
| CL | Cardiolipin |
| DOTAP | 1,2-dioleoyl-3-trimethylammoniumpropane |
| ANS | 1-anilino-8-naphthalenesulfonate |
| TPB | Tetraphenylborate |
| DPPC | 1,2-dipalmitoyl-sn-glycero-3-phosphocholine |
| DSPC | 1,2-distearoyl-sn-glycero-3-phosphocholine |
| CPPs | Cell-Penetrating Peptides |
| TAT | Trans-Activator of Transcription |
| HIV-1 | Human Immunodeficiency Virus type 1 |
| NaCl | Sodium Chloride |
| Ca2+ | Calcium ion |
| CHO | Chinese Hamster Ovary cells |
| mGluR1 | Group I Metabotropic Glutamate Receptor 1 |
| D2O | Deuterium Oxide |
| H2O | Water |
| D/H | Deuterium/Hydrogen exchange |
| DHPG | 3,5-Dihydroxyphenylglycine |
| NDM-1 | New Delhi Metallo-β-lactamase 1 |
| EDTA | Ethylenediaminetetraacetic acid |
| VanX | D-alanyl-D-alanine dipeptidase |
| Aβ | Amyloid beta peptide |
References
- Sushnitha, M.; Evangelopoulos, M.; Tasciotti, E.; Taraballi, F. Cell membrane-based biomimetic nanoparticles and the immune system: Immunomodulatory interactions to therapeutic applications. Front. Bioeng. Biotechnol. 2020, 8, 627. [Google Scholar] [CrossRef]
- Motallebi, M.; Heidarizadeh, F. Introduction to biomimetic nanoparticles for biomedical applications. In Cell Membrane Surface-Engineered Nanoparticles: Biomimetic Nanomaterials for Biomedical Applications; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2024; Volume 1464, Chapter 1; pp. 1–7. [Google Scholar]
- Issaka, E.; Tornyeava, B.; Agyekum, E.A.; Enyan, M.; Okai Amu-Darko, J.N.; Chimuza, H.T. Nature-inspired solutions: A comprehensive review of biomimetic nanoparticles in nanomedicine. J. Thermoplast. Compos. Mater. 2024, 38, 1637–1672. [Google Scholar] [CrossRef]
- Vatankhah, A.; Oroojalian, F.; Hoseinzadeh Moghaddam, S.; Kesharwani, P.; Sahebkar, A. State-of-the-art review on liposomes as versatile cancer vaccine delivery systems. J. Drug Deliv. Sci. Technol. 2025, 109, 106975. [Google Scholar] [CrossRef]
- Chen, L.; Hong, W.; Ren, W.; Xu, T.; Qian, Z.; He, Z. Recent progress in targeted delivery vectors based on biomimetic nanoparticles. Signal Transduct. Target. Ther. 2021, 6, 225. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Jin, K.; Luo, M.; Wang, X.; Zhu, X.; Liu, X.; Jiang, T.; Zhang, Q.; Wang, S.; Pang, Z. Size dependency of circulation and biodistribution of biomimetic nanoparticles: Red blood cell membrane-coated nanoparticles. Cells 2019, 8, 881. [Google Scholar] [CrossRef]
- Gao, W.; Yang, X.; Lin, Z.; Gao, S.; He, B.; Mei, D.; Wang, D.; Yuan, L.; Zhang, H.; Dai, W.; et al. The use of a hydrophobic binding peptide modified lipid nanocarrier improving tumor distribution and antitumor efficacy. J. Biomed. Nanotechnol. 2016, 12, 1183–1198. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, B.; Chen, H.; Zhang, L.; Jiang, X. Application and method of surface plasmon resonance technology in the preparation and characterization of biomedical nanoparticle materials. Int. J. Nanomed. 2024, 19, 7049–7069. [Google Scholar] [CrossRef] [PubMed]
- Behan, J.A.; Xie, Z.; Wang, Y.F.; Yang, X.; Aastrup, T.; Yan, Y.; Adumeau, L.; Dawson, K.A. Quartz crystal microbalance method to measure nanoparticle-receptor interactions and evaluate nanoparticle design efficiency. JACS Au 2023, 3, 1623–1633. [Google Scholar] [CrossRef]
- Petty, R.M.; Ceresa, L.; Alexander, E.; Pham, D.; Sabnis, N.; Fudala, R.; Lacko, A.G.; Krishnamoorthy, R.R.; Gryczynski, Z.; Gryczynski, I. Fluorescence resonance energy transfer for drug loading assessment in reconstituted high-density lipoprotein manoparticles. Int. J. Mol. Sci. 2025, 26, 3276. [Google Scholar] [CrossRef] [PubMed]
- Nowak, J.S.; Czarna, A.; Grudnik, P.; Grygier, P.; Pustelny, K.; Langer, A.; Dubin, G. Microscale thermophoresis (MST) and spectral shift (SpS) in drug discovery. TrAC Trends Anal. Chem. 2024, 176, 117716. [Google Scholar] [CrossRef]
- Winiewska-Szajewska, M.; Poznański, J. Differential scanning fluorimetry followed by microscale thermophoresis and/or isothermal titration calorimetry as an efficient tool for ligand screening. Biophys. Rev. 2025, 17, 199–223. [Google Scholar] [CrossRef]
- Zhang, C.H.; Rodriguez, E.; Bi, C.; Zheng, X.W.; Suresh, D.; Suh, K.; Li, Z.; Elsebaei, F.; Hage, D.S. High performance affinity chromatography and related separation methods for the analysis of biological and pharmaceutical agents. Analyst 2018, 143, 374–391. [Google Scholar] [CrossRef]
- Ostergaard, J.; Heegaard, N.H.H. Capillary electrophoresis frontal analysis: Principles and applications for the study of drug-plasma protein binding. Electrophoresis 2003, 24, 2903–2913. [Google Scholar] [CrossRef]
- ÉEcija-Arenas, A.; Román-Pizarro, V.; Fernández-Romero, J.M. Separation and characterization of liposomes using asymmetric flow field-flow fractionation with online multi-angle light scattering detection. J. Chromatogr. A 2021, 1636, 461798. [Google Scholar] [CrossRef]
- Menéndez, M. Isothermal Titration Calorimetry: Principles and Applications. In eLS; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2020; pp. 113–127. [Google Scholar]
- Su, H.; Xu, Y. Application of ITC-Based characterization of thermodynamic and kinetic association of ligands with proteins in drug design. Front. Pharmacol. 2018, 9, 1133. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.W.; Armaou, A.; Rioux, R.M. Continuous injection isothermal titration calorimetry for in situ evaluation of thermodynamic binding properties of ligand–receptor binding models. J. Phys. Chem. B 2021, 125, 8075–8087. [Google Scholar] [CrossRef] [PubMed]
- Abian, O.; Vega, S.; Velazquez-Campoy, A. Biological calorimetry: Old friend, new insights. Biophysica 2023, 3, 21–34. [Google Scholar] [CrossRef]
- Archer, W.R.; Schulz, M.D. Isothermal titration calorimetry: Practical approaches and current applications in soft matter. Soft Matter 2020, 16, 8760–8774. [Google Scholar] [CrossRef] [PubMed]
- Lima Cavalcanti, I.D.; Xavier Junior, F.H.; Santos Magalhães, N.S.; Lira Nogueira, M.C.B. Isothermal titration calorimetry (ITC) as a promising tool in pharmaceutical nanotechnology. Int. J. Pharm. 2023, 641, 123063. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, G.; Moitessier, N.; Mittermaier, A.K. Enzyme kinetics by isothermal titration calorimetry: Allostery, inhibition, and dynamics. Front. Mol. Biosci. 2020, 7, 583826. [Google Scholar] [CrossRef]
- Hagedoorn, P.-L. Isothermal titration calorimetry in biocatalysis. Front. Catal. 2022, 2, 906668. [Google Scholar] [CrossRef]
- Prozeller, D.; Morsbach, S.; Landfester, K. Isothermal titration calorimetry as a complementary method for investigating nanoparticle–protein interactions. Nanoscale 2019, 11, 19265–19273. [Google Scholar] [CrossRef]
- Swaine, T.S.; Garcia, P.; Tang, Y.; Lewis, A.L.; Parkes, G.; Waters, L.J. Characterizing drug-polymer bead interactions using isothermal titration calorimetry. J. Pharm. Sci. 2019, 108, 1772–1778. [Google Scholar] [CrossRef]
- Wieprecht, T.; Seelig, J. Isothermal titration calorimetry for studying interactions between peptides and lipid membranes. Curr. Top. Membr. 2002, 52, 31–56. [Google Scholar]
- Rajarathnam, K.; Rösgen, J. Isothermal titration calorimetry of membrane proteins—Progress and challenges. Biochim. Biophys. Acta (BBA)—Biomembr. 2014, 1838, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Swamy, M.J.; Sankhala, R.S.; Singh, B.P. Thermodynamic Analysis of Protein–Lipid Interactions by Isothermal Titration Calorimetry. In Lipid-Protein Interactions: Methods and Protocols; Kleinschmidt, J.H., Ed.; Springer: New York, NY, USA, 2019; pp. 71–89. [Google Scholar]
- Duff, M.R., Jr.; Grubbs, J.; Howell, E.E. Isothermal titration calorimetry for measuring macromolecule-ligand affinity. J. Vis. Exp. 2011, 55, e2796. [Google Scholar] [CrossRef]
- Dunitz, J.D. Win some, lose some: Enthalpy-entropy compensation in weak intermolecular interactions. Chem. Biol. 1995, 2, 709–712. [Google Scholar] [CrossRef]
- Bronowska, A.K. Thermodynamics of Ligand-Protein Interactions: Implications for Molecular Design. In Thermodynamics—Interaction Studies—Solids, Liquids and Gases; Moreno Piraján, J.C., Ed.; IntechOpen: Rijeka, Croatia, 2011. [Google Scholar]
- Bouchemal, K. New challenges for pharmaceutical formulations and drug delivery systems characterization using isothermal titration calorimetry. Drug Discov. Today 2008, 13, 960–972. [Google Scholar] [CrossRef]
- Tellinghuisen, J. Optimizing experimental parameters in isothermal titration calorimetry. J. Phys. Chem. B 2005, 109, 20027–20035. [Google Scholar] [CrossRef]
- Sprakel, L.M.J.; Schuur, B. Improving understanding of solvent effects on intermolecular interactions in reactive liquid–liquid extraction with Isothermal Titration Calorimetry and molecular modeling. J. Ind. Eng. Chem. 2019, 72, 364–373. [Google Scholar] [CrossRef]
- Wangsakan, A.; Chinachoti, P.; McClements, D.J. Isothermal titration calorimetry study of the influence of temperature, pH and salt on maltodextrin–anionic surfactant interactions. Food Hydrocoll. 2006, 20, 461–467. [Google Scholar] [CrossRef]
- Mpye, K.L.; Gildenhuys, S.; Mosebi, S. The effects of temperature on streptavidin-biotin binding using affinity isothermal titration calorimetry. AIMS Biophys. 2020, 7, 236–247. [Google Scholar] [CrossRef]
- Erlekam, F.; Zumbansen, M.; Weber, M. Parameter estimation on multivalent ITC data sets. Sci. Rep. 2022, 12, 13402. [Google Scholar] [CrossRef] [PubMed]
- Medoš, Ž.; Bešter-Rogač, M.; Leontidis, E.; Tellinghuisen, J. Calibrating ITC instruments: Problems with weak base neutralization. Anal. Biochem. 2024, 694, 115602. [Google Scholar] [CrossRef]
- Le, V.H.; Buscaglia, R.; Chaires, J.B.; Lewis, E.A. Modeling complex equilibria in isothermal titration calorimetry experiments: Thermodynamic parameters estimation for a three-binding-site model. Anal. Biochem. 2013, 434, 233–241. [Google Scholar] [CrossRef]
- Brautigam, C.A. Fitting two- and three-site binding models to isothermal titration calorimetric data. Methods 2015, 76, 124–136. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Tian, W.; Yun, Y.; Tian, Y.; Sun, C.; Ding, R.; Chen, H. A discussion on the affecting factors of the fitting procedures’ reliability in isothermal titration calorimetry analysis. Arch. Biochem. Biophys. 2021, 713, 109045. [Google Scholar] [CrossRef]
- Kantonen, S.A.; Henriksen, N.M.; Gilson, M.K. Evaluation and minimization of uncertainty in itc binding measurements: Heat error, concentration error, saturation, and stoichiometry. Biochim. Biophys. Acta Gen. Subj. 2017, 1861, 485–498. [Google Scholar] [CrossRef]
- Baranauskienė, L.; Petrikaitė, V.; Matulienė, J.; Matulis, D. Titration calorimetry standards and the precision of isothermal titration calorimetry data. Int. J. Mol. Sci. 2009, 10, 2752–2762. [Google Scholar] [CrossRef]
- Wang, T.Y.; Bijlani, A.; Chao, E.H.P.; Johnson, P.E.; Krylov, S.N. A browser-based tool for assessing accuracy of isothermal titration calorimetry-derived parameters: K(d), ΔH°, and n. Chembiochem 2025, 26, e202500194. [Google Scholar] [CrossRef]
- Leavitt, S.; Freire, E. Direct measurement of protein binding energetics by isothermal titration calorimetry. Curr. Opin. Struct. Biol. 2001, 11, 560–566. [Google Scholar] [CrossRef] [PubMed]
- Freyer, M.W.; Lewis, E.A. Isothermal titration calorimetry: Experimental design, data analysis, and probing macromolecule/ligand binding and kinetic interactions. Methods Cell Biol. 2008, 84, 79–113. [Google Scholar] [CrossRef]
- Velazquez-Campoy, A.; Freire, E. Isothermal titration calorimetry to determine association constants for high-affinity ligands. Nat. Protoc. 2006, 1, 186–191. [Google Scholar] [CrossRef]
- Duan, Y.; Dhar, A.; Patel, C.; Khimani, M.; Neogi, S.; Sharma, P.; Siva Kumar, N.; Vekariya, R.L. A brief review on solid lipid nanoparticles: Part and parcel of contemporary drug delivery systems. RSC Adv. 2020, 10, 26777–26791. [Google Scholar] [CrossRef]
- Swetha, K.; Kotla, N.G.; Tunki, L.; Jayaraj, A.; Bhargava, S.K.; Hu, H.; Bonam, S.R.; Kurapati, R. Recent advances in the lipid nanoparticle-mediated delivery of mRNA vaccines. Vaccines 2023, 11, 658. [Google Scholar] [CrossRef] [PubMed]
- Schoenmaker, L.; Witzigmann, D.; Kulkarni, J.A.; Verbeke, R.; Kersten, G.; Jiskoot, W.; Crommelin, D.J.A. mRNA-lipid nanoparticle COVID-19 vaccines: Structure and stability. Int. J. Pharm. 2021, 601, 120586. [Google Scholar] [CrossRef]
- Huang, Z.; Ma, C.; Wu, M.; Li, X.; Lu, C.; Zhang, X.; Ma, X.; Yang, Y.; Huang, Y.; Pan, X.; et al. Exploring the drug-lipid interaction of weak-hydrophobic drug loaded solid lipid nanoparticles by isothermal titration calorimetry. J. Nanoparticle Res. 2019, 22, 3. [Google Scholar] [CrossRef]
- Brader, M.L.; Williams, S.J.; Banks, J.M.; Hui, W.H.; Zhou, Z.H.; Jin, L. Encapsulation state of messenger RNA inside lipid nanoparticles. Biophys. J. 2021, 120, 2766–2770. [Google Scholar] [CrossRef]
- Pink, D.L.; Loruthai, O.; Ziolek, R.M.; Wasutrasawat, P.; Terry, A.E.; Lawrence, M.J.; Lorenz, C.D. On the structure of solid lipid nanoparticles. Small 2019, 15, e1903156. [Google Scholar] [CrossRef] [PubMed]
- Rathee, J.; Kishore, N. Interaction of solid lipid nanoparticles with bovine serum albumin: Physicochemical mechanistic insights. Phys. Chem. Chem. Phys. 2025, 27, 5876–5888. [Google Scholar] [CrossRef] [PubMed]
- Miao, L.; Lin, J.; Huang, Y.; Li, L.; Delcassian, D.; Ge, Y.; Shi, Y.; Anderson, D.G. Synergistic lipid compositions for albumin receptor mediated delivery of mRNA to the liver. Nat. Commun. 2020, 11, 2424. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Huang, Z.; Li, Y.; Shi, J.; Fu, F.; Huang, Y.; Pan, X.; Wu, C. Impact of particle size and pH on protein corona formation of solid lipid nanoparticles: A proof-of-concept study. Acta Pharm. Sin. B 2021, 11, 1030–1046. [Google Scholar] [CrossRef]
- Frank, P.G.; Marcel, Y.L. Apolipoprotein A-I: Structure-function relationships. J. Lipid Res. 2000, 41, 853–872. [Google Scholar] [CrossRef]
- Li, M.; Jin, X.; Liu, T.; Fan, F.; Gao, F.; Chai, S.; Yang, L. Nanoparticle elasticity affects systemic circulation lifetime by modulating adsorption of apolipoprotein A-I in corona formation. Nat. Commun. 2022, 13, 4137. [Google Scholar] [CrossRef]
- Sawant, R.R.; Torchilin, V.P. Liposomes as ‘smart’ pharmaceutical nanocarriers. Soft Matter 2010, 6, 4026–4044. [Google Scholar] [CrossRef]
- Nsairat, H.; Khater, D.; Sayed, U.; Odeh, F.; Al Bawab, A.; Alshaer, W. Liposomes: Structure, composition, types, and clinical applications. Heliyon 2022, 8, e09394. [Google Scholar] [CrossRef]
- Li, S.; Wang, F.; Li, X.; Chen, J.; Zhang, X.; Wang, Y.; Liu, J. Dipole orientation matters: Longer-circulating choline phosphate than phosphocholine liposomes for enhanced tumor targeting. Acs Appl. Mater. Interfaces 2017, 9, 17736–17744. [Google Scholar] [CrossRef] [PubMed]
- Rampado, R.; Giordano, F.; Moracci, L.; Crotti, S.; Caliceti, P.; Agostini, M.; Taraballi, F. Optimization of a detergent-based protocol for membrane proteins purification from mammalian cells. J. Pharm. Biomed. Anal. 2022, 219, 114926. [Google Scholar] [CrossRef] [PubMed]
- Sideratou, Z.; Tsiourvas, D.; Paleos, C.; Tsortos, A.; Nounesis, G. Molecular recognition of complementary liposomes: The enhancing role of cholesterol. Langmuir 2000, 16, 9186–9191. [Google Scholar] [CrossRef]
- Caritá, A.; Cavalcanti, R.; Oliveira, M.; Riske, K. Solubilization of biomimetic lipid mixtures by some commonly used non-ionic detergents. Chem. Phys. Lipids 2023, 255, 105327. [Google Scholar] [CrossRef]
- Angerer, N.; Piller, P.; Semeraro, E.F.; Keller, S.; Pabst, G. Interaction of detergent with complex mimics of bacterial membranes. Biophys. Chem. 2023, 296, 107002. [Google Scholar] [CrossRef] [PubMed]
- Krylova, O.O.; Jahnke, N.; Keller, S. Membrane solubilisation and reconstitution by octylglucoside: Comparison of synthetic lipid and natural lipid extract by isothermal titration calorimetry. Biophys. Chem. 2010, 150, 105–111. [Google Scholar] [CrossRef]
- Heerklotz, H.; Seelig, J. Titration calorimetry of surfactant–membrane partitioning and membrane solubilization. Biochim. Biophys. Acta (BBA)—Biomembr. 2000, 1508, 69–85. [Google Scholar] [CrossRef]
- Heerklotz, H.; Lantzsch, G.; Binder, H.; Klose, G.; Blume, A. Application of isothermal titration calorimetry for detecting lipid membrane solubilization. Chem. Phys. Lett. 1995, 235, 517–520. [Google Scholar] [CrossRef]
- Heerklotz, H.; Tsamaloukas, A.D.; Keller, S. Monitoring detergent-mediated solubilization and reconstitution of lipid membranes by isothermal titration calorimetry. Nat. Protoc. 2009, 4, 686–697. [Google Scholar] [CrossRef]
- Clark, S.T.; Arras, M.M.L.; Sarles, S.A.; Frymier, P.D. Lipid shape determination of detergent solubilization in mixed-lipid liposomes. Colloids Surf. B Biointerfaces 2020, 187, 110609. [Google Scholar] [CrossRef]
- Yokoyama, H.; Ikeda, K.; Wakabayashi, M.; Ishihama, Y.; Nakano, M. Effects of lipid membrane curvature on lipid packing state evaluated by isothermal titration calorimetry. Langmuir 2013, 29, 857–860. [Google Scholar] [CrossRef]
- Keller, S.; Heerklotz, H.; Jahnke, N.; Blume, A. Thermodynamics of Lipid Membrane Solubilization by Sodium Dodecyl Sulfate. Biophys. J. 2006, 90, 4509–4521. [Google Scholar] [CrossRef] [PubMed]
- Lombardo, D.; Kiselev, M.A. Methods of liposomes preparation: Formation and control factors of versatile nanocarriers for biomedical and nanomedicine application. Pharmaceutics 2022, 14, 543. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Chen, W.; Tasi, L.; Yang, S. Microcalorimetric and shear studies on the effects of cholesterol on the physical stability of lipid vesicles. Colloids Surf. A Physicochem. Eng. Asp. 2000, 172, 57–67. [Google Scholar] [CrossRef]
- Wu, G.; Lee, K.Y.C. Interaction of poloxamers with liposomes: An isothermal titration calorimetry study. J. Phys. Chem. B 2009, 113, 15522–15531. [Google Scholar] [CrossRef] [PubMed]
- Solis-Gonzalez, O.A.; Ramon, A.-G.J.; Rojas-Aguilar, A. A thermodynamic study of F108 and F127 block copolymer interactions with liposomes at physiological temperature. J. Liposome Res. 2022, 32, 32–44. [Google Scholar] [CrossRef] [PubMed]
- Rai, R.; Kumar, D.; Dhule, A.A.; Rudani, B.A.; Tiwari, S. Alkanols regulate the fluidity of phospholipid bilayer in accordance to their concentration and polarity. Langmuir 2024, 40, 14057–14065. [Google Scholar] [CrossRef]
- Šturm, L.; Poklar Ulrih, N. Basic methods for preparation of liposomes and studying their interactions with different compounds, with the emphasis on polyphenols. Int. J. Mol. Sci. 2021, 22, 6547. [Google Scholar] [CrossRef]
- Roy, B.; Guha, P.; Nahak, P.; Karmakar, G.; Maity, S.; Mandal, A.; Bykov, A.G.; Akentiev, A.; Noskov, B.; Tsuchiya, K.; et al. Biophysical correlates on the composition, functionality, and structure of dendrimer−liposome aggregates. ACS Omega 2018, 3, 12235–12245. [Google Scholar] [CrossRef]
- Mertins, O.; Dimova, R. Binding of chitosan to phospholipid vesicles studied with isothermal titration calorimetry. Langmuir 2011, 27, 5506–5515. [Google Scholar] [CrossRef]
- Navon, Y.; Radavidson, H.; Putaux, J.-L.; Jean, B.; Heux, L. pH-sensitive interactions between cellulose nanocrystals and DOPC liposomes. Biomacromolecules 2017, 18, 2918–2927. [Google Scholar] [CrossRef]
- Samelo, J.; Mora, M.J.; Granero, G.E.; Moreno, M.J. Partition of amphiphilic molecules to lipid bilayers by ITC: Low-affinity solutes. ACS Omega 2017, 2, 6863–6869. [Google Scholar] [CrossRef]
- Vargas, C.; Klingler, J.; Keller, S. Membrane Partitioning and Translocation Studied by Isothermal Titration Calorimetry. In Membrane Biogenesis: Methods and Protocols; Rapaport, D., Herrmann, J.M., Eds.; Humana Press: Totowa, NJ, USA, 2013; pp. 253–271. [Google Scholar]
- Tsamaloukas, A.D.; Keller, S.; Heerklotz, H. Uptake and release protocol for assessing membrane binding and permeation by way of isothermal titration calorimetry. Nat. Protoc. 2007, 2, 695–704. [Google Scholar] [CrossRef] [PubMed]
- Osanai, H.; Ikehara, T.; Miyauchi, S.; Shimono, K.; Tamogami, J.; Nara, T.; Kamo, N. A study of the interaction of drugs with liposomes with isothermal titration calorimetry. J. Biophys. Chem. 2013, 4, 11–21. [Google Scholar] [CrossRef]
- Kaur, H.; Kishore, N. Anti-cancer and anti-microbial drug encapsulated lipid vesicles as drug delivery systems: Calorimetric and spectroscopic study. Colloids Surf. A Physicochem. Eng. Asp. 2025, 705, 135691. [Google Scholar] [CrossRef]
- Matos, C.; Lima, J.L.; Reis, S.; Lopes, A.; Bastos, M. Interaction of antiinflammatory drugs with EPC liposomes: Calorimetric study in a broad concentration range. Biophys. J. 2004, 86, 946–954. [Google Scholar] [CrossRef][Green Version]
- Nordstrom, R.; Zhu, L.; Harmark, J.; Levi-Kalisman, Y.; Koren, E.; Barenholz, Y.; Levinton, G.; Shamrakov, D. Quantitative cryo-TEM reveals new structural details of Doxil-like pegylated liposomal doxorubicin formulation. Pharmaceutics 2021, 13, 123. [Google Scholar] [CrossRef]
- Ben-Fadhel, Y.; Maherani, B.; Salmieri, S.; Lacroix, M. Preparation and characterization of natural extracts-loaded food grade nanoliposomes. LWT 2022, 154, 112781. [Google Scholar] [CrossRef]
- Zhang, M.; Meng, J.-Y.; Wang, Y.-N.; Wu, R.-G. Investigation on the interaction between Jingui Shenqi pill and DPPC liposome mimetic biofilms. J. Therm. Anal. Calorim. 2025, 150, 1731–1740. [Google Scholar] [CrossRef]
- Cordeiro, M.; Filipe, H.; dos Santos, P.; Samelo, J.; Ramalho, J.; Loura, L.; Moreno, M. Interaction of Hoechst 33342 with POPC membranes at different pH values. Molecules 2023, 28, 5640. [Google Scholar] [CrossRef]
- Boruah, J.S.; Chowdhury, D. Liposome-azobenzene nanocomposite as photo-responsive drug delivery vehicle. Appl. Nanosci. 2022, 12, 4005–4017. [Google Scholar] [CrossRef]
- Has, C.; Das, S.L. The functionality of membrane-inserting proteins and peptides: Curvature sensing, generation, and pore formation. J. Membr. Biol. 2023, 256, 343–372. [Google Scholar] [CrossRef]
- Kim, S.; Lee, J.; Lee, S.; Kim, H.; Sim, J.-Y.; Pak, B.; Kim, K.; Kim, J.I. Matching amino acids membrane preference profile to improve activity of antimicrobial peptides. Commun. Biol. 2022, 5, 1199. [Google Scholar] [CrossRef]
- Henriksen, J.R.; Andresen, T.L. Thermodynamic profiling of peptide membrane interactions by isothermal titration calorimetry: A search for pores and micelles. Biophys. J. 2011, 101, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Kumagai, P.S.; Sousa, V.K.; Donato, M.; Itri, R.; Beltramini, L.M.; Araujo, A.P.U.; Buerck, J.; Wallace, B.A.; Lopes, J.L.S. Unveiling the binding and orientation of the antimicrobial peptide Plantaricin 149 in zwitterionic and negatively charged membranes. Eur. Biophys. J. 2019, 48, 621–633. [Google Scholar] [CrossRef]
- Vieira, A.P.; de Souza, A.N.; Lima, W.G.; Brito, J.C.; Simião, D.C.; Gonçalves, L.V.; Cordeiro, L.P.; de Oliveira Scoaris, D.; Fernandes, S.O.; Resende, J.M.; et al. The synthetic peptide LyeTx I mn∆K, derived from Lycosa erythrognatha spider toxin, is active against methicillin-resistant staphylococcus aureus (MRSA) in vitro and in vivo. Antibiotics 2024, 13, 248. [Google Scholar] [CrossRef]
- Beck, K.; Nandy, J.; Hoernke, M. Membrane permeabilization can be crucially biased by a fusogenic lipid composition—Leaky fusion caused by antimicrobial peptides in model membranes. Soft Matter 2023, 19, 2919–2931. [Google Scholar] [CrossRef] [PubMed]
- Stulz, A.; Breitsamer, M.; Winter, G.; Heerklotz, H. Primary and secondary binding of exenatide to liposomes. Biophys. J. 2020, 118, 600–611. [Google Scholar] [CrossRef]
- Valkova, I.; Todorov, P.; Vitkova, V. VV-hemorphin-5 association to lipid bilayers and alterations of membrane bending rigidity. Aims Biophys. 2022, 9, 294–307. [Google Scholar] [CrossRef]
- Sonju, J.J.; Dahal, A.; Singh, S.S.; Jois, S.D. Peptide-functionalized liposomes as therapeutic and diagnostic tools for cancer treatment. J. Control Release 2021, 329, 624–644. [Google Scholar] [CrossRef]
- Sauder, R.; Seelig, J.; Ziegler, A. Thermodynamics of Lipid Interactions with Cell-Penetrating Peptides. In Cell-Penetrating Peptides: Methods and Protocols; Langel, Ü., Ed.; Humana Press: Totowa, NJ, USA, 2011; pp. 129–155. [Google Scholar]
- Ziegler, A.; Li Blatter, X.; Seelig, A.; Seelig, J. Protein transduction domains of HIV-1 and SIV TAT interact with charged lipid vesicles: Binding mechanism and thermodynamic analysis. Biochemistry 2003, 42, 9185–9194. [Google Scholar] [CrossRef]
- Takechi, Y.; Tanaka, H.; Kitayama, H.; Yoshii, H.; Tanaka, M.; Saito, H. Comparative study on the interaction of cell-penetrating polycationic polymers with lipid membranes. Chem. Phys. Lipids 2012, 165, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Cavalcanti, R.; Lira, R.; Riske, K. Membrane fusion biophysical analysis of fusogenic liposomes. Langmuir 2022, 38, 10430–10441. [Google Scholar] [CrossRef]
- Wu, S.; Jiang, P.; Zhang, X.; Mao, C.; Dai, Y.; Zhuang, H.; Pang, Y. Understanding the transepithelial transport and transbilayer diffusion of the antihypertensive peptide Asn-Cys-Trp: Insights from Caco-2 Cell monolayers and the DPPC model membrane. J. Agric. Food Chem. 2024, 72, 9828–9841. [Google Scholar] [CrossRef]
- Terakawa, M.S.; Yagi, H.; Adachi, M.; Lee, Y.-H.; Goto, Y. Small liposomes accelerate the fibrillation of amyloid β (1–40). J. Biol. Chem. 2015, 290, 815–826. [Google Scholar] [CrossRef]
- Dimitrova, M.; Matsumura, H.; Terezova, N.; Neytchev, V. Binding of globular proteins to lipid membranes studied by isothermal titration calorimetry and fluorescence. Colloids Surf. B-Biointerfaces 2002, 24, 53–61. [Google Scholar] [CrossRef]
- Anbazhagan, V.; Sankhala, R.S.; Singh, B.P.; Swamy, M.J. Isothermal titration calorimetric studies on the interaction of the major bovine seminal plasma protein, PDC-109 with phospholipid membranes. PLoS ONE 2011, 6, e25993. [Google Scholar] [CrossRef]
- Sangrà, M.; Estelrich, J.; Sabaté, R.; Espargaró, A.; Busquets, M.A. Evidence of protein adsorption in pegylated liposomes: Influence of liposomal decoration. Nanomaterials 2017, 7, 37. [Google Scholar] [CrossRef] [PubMed]
- Nele, V.; D’Aria, F.; Campani, V.; Silvestri, T.; Biondi, M.; Giancola, C.; De Rosa, G. Unravelling the role of lipid composition on liposome-protein interactions. J. Liposome Res. 2024, 34, 88–96. [Google Scholar] [CrossRef]
- Arseneault, M.; Lafleur, M. Isothermal titration calorimetric study of calcium association to lipid bilayers: Influence of the vesicle preparation and composition. Chem. Phys. Lipids 2006, 142, 84–93. [Google Scholar] [CrossRef]
- Miller, D.; Booth, P.J. The use of isothermal titration calorimetry to study multidrug transport proteins in liposomes. Methods Mol. Biol. 2010, 606, 233–245. [Google Scholar] [CrossRef] [PubMed]
- Boudker, O.; Oh, S. Isothermal titration calorimetry of ion-coupled membrane transporters. Methods 2015, 76, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Ha, D.; Yang, N.N.; Nadithe, V. Exosomes as therapeutic drug carriers and delivery vehicles across biological membranes: Current perspectives and future challenges. Acta Pharm. Sin. B 2016, 6, 287–296. [Google Scholar] [CrossRef]
- Luan, X.; Sansanaphongpricha, K.; Myers, I.; Chen, H.W.; Yuan, H.B.; Sun, D.X. Engineering exosomes as refined biological nanoplatforms for drug delivery. Acta Pharmacol. Sin. 2017, 38, 754–763. [Google Scholar] [CrossRef]
- Horibe, S.; Tanahashi, T.; Kawauchi, S.; Murakami, Y.; Rikitake, Y. Mechanism of recipient cell-dependent differences in exosome uptake. BMC Cancer 2018, 18, 47. [Google Scholar] [CrossRef]
- Narain, A.; Asawa, S.; Chhabria, V.; Patil-Sen, Y. Cell membrane coated nanoparticles: Next-generation therapeutics. Nanomedicine 2017, 12, 2677–2692. [Google Scholar] [CrossRef]
- Gao, W.W.; Zhang, L.F. Coating nanoparticles with cell membranes for targeted drug delivery. J. Drug Target. 2015, 23, 619–626. [Google Scholar] [CrossRef]
- Hessvik, N.P.; Llorente, A. Current knowledge on exosome biogenesis and release. Cell. Mol. Life Sci. 2018, 75, 193–208. [Google Scholar] [CrossRef]
- Thery, C.; Zitvogel, L.; Amigorena, S. Exosomes: Composition, biogenesis and function. Nat. Rev. Immunol. 2002, 2, 569–579. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.M.J.; Zhang, L.; Aryal, S.; Cheung, C.; Fang, R.H.; Zhang, L.F. Erythrocyte membrane-camouflaged polymeric nanoparticles as a biomimetic delivery platform. Proc. Natl. Acad. Sci. USA 2011, 108, 10980–10985. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.F.; Rao, L.; Zan, M.H.; Chen, M.; Yu, G.T.; Wei, X.Y.; Wu, Z.H.; Sun, Y.; Guo, S.S.; Zhao, X.Z.; et al. Macrophage membrane-coated iron oxide nanoparticles for enhanced photothermal tumor therapy. Nanotechnology 2018, 29, 134004. [Google Scholar] [CrossRef] [PubMed]
- Elliott, R.O.; He, M. Unlocking the power of exosomes for crossing biological barriers in drug delivery. Pharmaceutics 2021, 13, 122. [Google Scholar] [CrossRef]
- Li, R.X.; He, Y.W.; Zhang, S.Y.; Qin, J.; Wang, J.X. Cell membrane-based nanoparticles: A new biomimetic platform for tumor diagnosis and treatment. Acta Pharm. Sin. B 2018, 8, 14–22. [Google Scholar] [CrossRef]
- Kroll, A.V.; Fang, R.H.; Zhang, L.F. Biointerfacing and applications of cell membrane-coated nanoparticles. Bioconjugate Chem. 2017, 28, 23–32. [Google Scholar] [CrossRef]
- Coughlan, C.; Lindenberger, J.; Jacot, J.G.; Johnson, N.R.; Anton, P.; Bevers, S.; Welty, R.; Graner, M.W.; Potter, H. Specific binding of Alzheimer’s Aβ peptides to extracellular vesicles. Int. J. Mol. Sci. 2024, 25, 3703. [Google Scholar] [CrossRef] [PubMed]
- Jahnke, N.; Krylova, O.O.; Hoomann, T.; Vargas, C.; Fiedler, S.; Pohl, P.; Keller, S. Real-time monitoring of membrane-protein reconstitution by isothermal titration calorimetry. Anal. Chem. 2014, 86, 920–927. [Google Scholar] [CrossRef] [PubMed]
- Virtanen, V.; Green, R.J.; Karonen, M. Karonen, M. Interactions between Hydrolysable tannins and lipid vesicles from Escherichia coli with isothermal titration calorimetry. Molecules 2022, 27, 3204. [Google Scholar] [CrossRef]
- Hirakura, Y.; Sugiyama, T.; Takeda, M.; Ikeda, M.; Yoshioka, T. Deuteration as a tool in investigating the role of protons in cell signaling. Biochim. Biophys. Acta (BBA)—Gen. Subj. 2011, 1810, 218–225. [Google Scholar] [CrossRef]
- Zhang, Y.-J.; Wang, W.-M.; Oelschlaeger, P.; Chen, C.; Lei, J.-E.; Lv, M.; Yang, K.-W. Real-time monitoring of NDM-1 activity in live bacterial cells by isothermal titration calorimetry: A new approach to measure inhibition of antibiotic-resistant bacteria. ACS Infect. Dis. 2018, 4, 1671–1678. [Google Scholar] [CrossRef] [PubMed]
- Lv, M.; Zhang, Y.-J.; Zhou, F.; Ge, Y.; Zhao, M.-H.; Liu, Y.; Yang, K.-W. Real-time monitoring of D-Ala-D-Ala dipeptidase activity of VanX in living bacteria by isothermal titration calorimetry. Anal. Biochem. 2019, 578, 29–35. [Google Scholar] [CrossRef]
- Matulis, D.; Wadsö, L.; Fahmy, K. Special Issue “advances in monitoring metabolic activities of microorganisms by calorimetry”. Microorganisms 2023, 11, 1204. [Google Scholar] [CrossRef]
- Patnaik, A.; Rai, S.K.; Dhaked, R.K. Analytical techniques and molecular platforms for detection and surveillance of antimicrobial resistance: Advancements of the past decade. 3 Biotech 2025, 15, 108. [Google Scholar] [CrossRef]
- Fahmy, K. Simple growth–metabolism relations are revealed by conserved patterns of heat flow from cultured microorganisms. Microorganisms 2022, 10, 1397. [Google Scholar] [CrossRef]
- Lemos, D.; Oliveira, T.; Martins, L.; de Azevedo, V.R.; Rodrigues, M.F.; Ketzer, L.A.; Rumjanek, F.D. Isothermal microcalorimetry of tumor cells: Enhanced thermogenesis by metastatic cells. Front. Oncol. 2020, 9, 1430. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guerrero, M.; Braden, C.; Bao, Y. Applications of Isothermal Titration Calorimetry in Studying Biomimetic Nanocarriers. Biomolecules 2025, 15, 1349. https://doi.org/10.3390/biom15101349
Guerrero M, Braden C, Bao Y. Applications of Isothermal Titration Calorimetry in Studying Biomimetic Nanocarriers. Biomolecules. 2025; 15(10):1349. https://doi.org/10.3390/biom15101349
Chicago/Turabian StyleGuerrero, Martin, Colby Braden, and Yuping Bao. 2025. "Applications of Isothermal Titration Calorimetry in Studying Biomimetic Nanocarriers" Biomolecules 15, no. 10: 1349. https://doi.org/10.3390/biom15101349
APA StyleGuerrero, M., Braden, C., & Bao, Y. (2025). Applications of Isothermal Titration Calorimetry in Studying Biomimetic Nanocarriers. Biomolecules, 15(10), 1349. https://doi.org/10.3390/biom15101349








