Circadian Clock Gene Bmal1: A Molecular Bridge from AKI to CKD
Abstract
1. Introduction
2. Circadian Clock Gene Bmal1
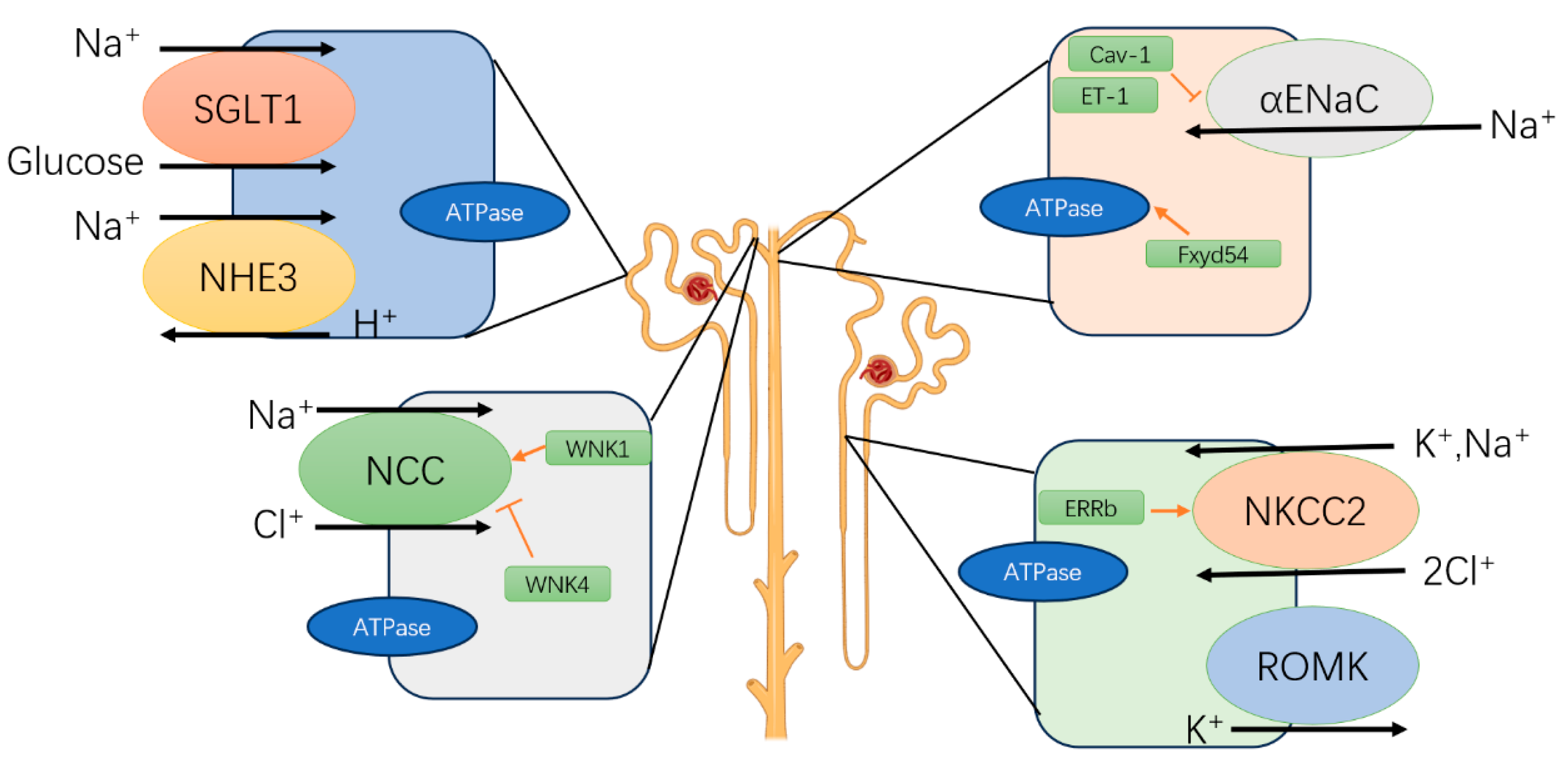
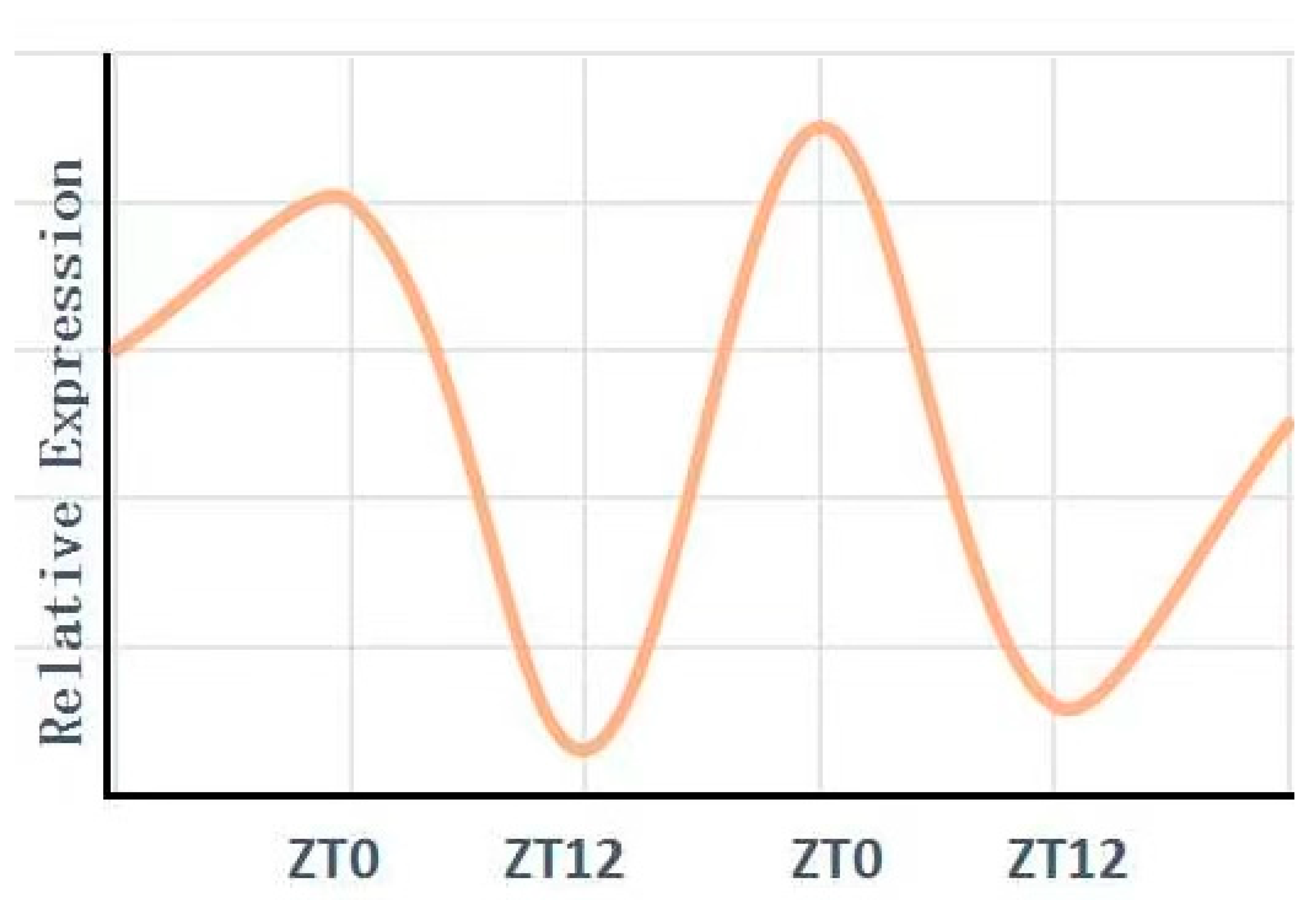
3. Circadian Rhythm in the Kidney
4. Regulatory Role and Mechanisms of Bmal1 in Cell Death During Acute Kidney Injury
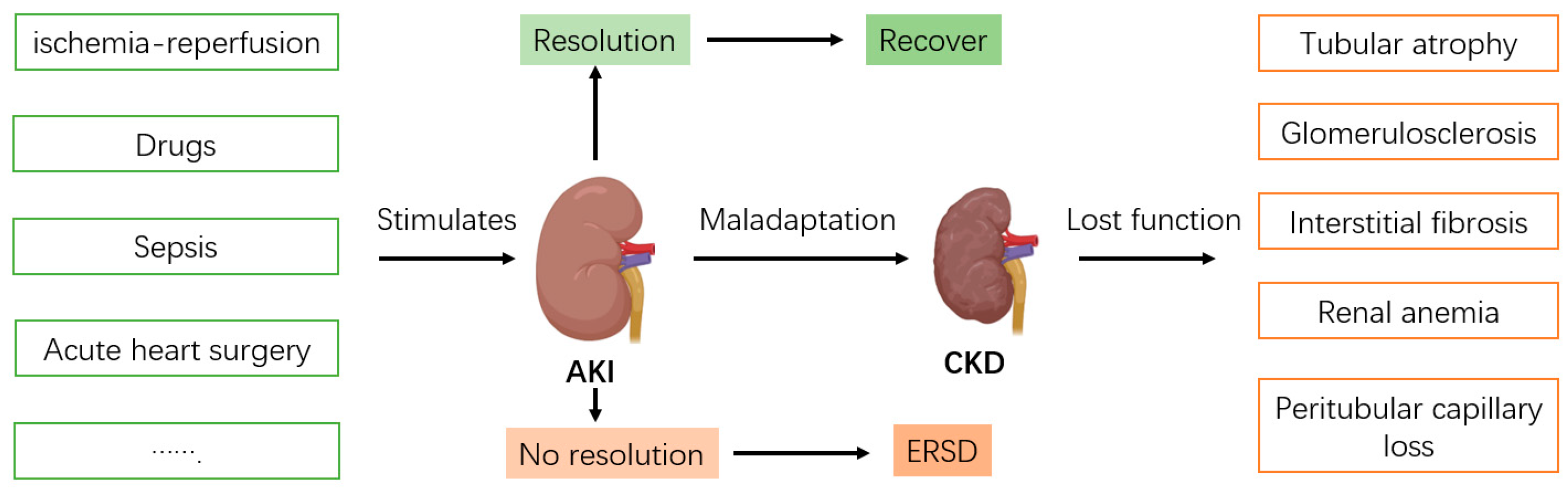
5. Regulatory Role and Mechanisms of Bmal1 in Chronic Kidney Disease
6. Therapeutic Potential for AKI and CKD Targeting Bmal1 Signaling
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vijayan, A. Tackling AKI: Prevention, timing of dialysis and follow-up. Nat. Rev. Nephrol. 2021, 17, 87–88. [Google Scholar] [CrossRef] [PubMed]
- Graversen, H.V.; Jensen, S.K.; Vestergaard, S.V.; Heide-Jørgensen, U.; Christiansen, C.F. Defining Baseline Creatinine for Identification of AKI in Population-Based Laboratory Databases: A Danish Nationwide Cohort Study. Kidney360 2021, 3, 232–241. [Google Scholar] [CrossRef] [PubMed]
- Yu, N.; Ouyang, X.; Li, J.; Gao, J.; Zeng, S.; Zhuang, H.; Jiang, M.; Pei, Y.; Jiang, X. Risk factors and renal outcomes of AKI in children with secondary steroid-resistant nephrotic syndrome. Ren. Fail. 2024, 46, 2314637. [Google Scholar] [CrossRef]
- Chen-Xu, M.; Kassam, C.; Cameron, E.; Ryba, S.; Yiu, V. Impact of electronic AKI alert/care bundle on AKI inpatient outcomes: A retrospective single-center cohort study. Ren. Fail. 2024, 46, 2313177. [Google Scholar] [CrossRef] [PubMed]
- Adiyeke, E.; Ren, Y.; Guan, Z.; Ruppert, M.M.; Rashidi, P.; Bihorac, A.; Ozrazgat-Baslanti, T. Clinical courses of acute kidney injury in hospitalized patients: A multistate analysis. Sci. Rep. 2023, 13, 17781. [Google Scholar] [CrossRef] [PubMed]
- Al-Jaghbeer, M.; Dealmeida, D.; Bilderback, A.; Ambrosino, R.; Kellum, J.A. Clinical Decision Support for In-Hospital AKI. J. Am. Soc. Nephrol. 2018, 29, 654–660. [Google Scholar] [CrossRef] [PubMed]
- Susantitaphong, P.; Cruz, D.N.; Cerda, J.; Abulfaraj, M.; Alqahtani, F.; Koulouridis, I.; Jaber, B.L.; Acute Kidney Injury Advisory Group of the American Society of Nephrology. World incidence of AKI: A meta-analysis. Clin. J. Am. Soc. Nephrol. 2013, 8, 1482–1493, Erratum in Clin. J. Am. Soc. Nephrol. 2014, 9, 1148. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Basile, D.P.; Anderson, M.D.; Sutton, T.A. Pathophysiology of acute kidney injury. Compr. Physiol. 2012, 2, 1303–1353. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, M.; Bi, R.; Su, Y.; Quan, F.; Lin, Y.; Yue, C.; Cui, X.; Zhao, Q.; Liu, S.; et al. ACSL4 deficiency confers protection against ferroptosis-mediated acute kidney injury. Redox Biol. 2022, 51, 102262. [Google Scholar] [CrossRef]
- Martin-Sanchez, D.; Ruiz-Andres, O.; Poveda, J.; Carrasco, S.; Cannata-Ortiz, P.; Sanchez-Niño, M.D.; Ortega, M.R.; Egido, J.; Linkermann, A.; Ortiz, A.; et al. Ferroptosis, but Not Necroptosis, Is Important in Nephrotoxic Folic Acid–Induced AKI. J. Am. Soc. Nephrol. 2017, 28, 218–229. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Wu, X.; Song, Z.; Sun, S.; Su, Y.; Wang, T.; Cheng, X.; Yu, Y.; Yu, C.; Chen, W.; et al. Metformin potentiates nephrotoxicity by promoting NETosis in response to renal ferroptosis. Cell Discov. 2023, 9, 104, Erratum in Cell Discov. 2024, 10, 4. https://doi.org/10.1038/s41421-023-00630-3. [Google Scholar] [CrossRef] [PubMed]
- Livingston, M.J.; Shu, S.; Fan, Y.; Li, Z.; Jiao, Q.; Yin, X.-M.; Venkatachalam, M.A.; Dong, Z. Tubular cells produce FGF2 via autophagy after acute kidney injury leading to fibroblast activation and renal fibrosis. Autophagy 2023, 19, 256–277. [Google Scholar] [CrossRef]
- Lin, Q.; Li, S.; Jiang, N.; Jin, H.; Shao, X.; Zhu, X.; Wu, J.; Zhang, M.; Zhang, Z.; Shen, J.; et al. Inhibiting NLRP3 inflammasome attenuates apoptosis in contrast-induced acute kidney injury through the upregulation of HIF1A and BNIP3-mediated mitophagy. Autophagy 2021, 17, 2975–2990. [Google Scholar] [CrossRef] [PubMed]
- Maremonti, F.; Meyer, C.; Linkermann, A. Mechanisms and Models of Kidney Tubular Necrosis and Nephron Loss. J. Am. Soc. Nephrol. 2022, 33, 472–486. [Google Scholar] [CrossRef]
- Bikbov, B.; Purcell, C.A.; Levey, A.S.; Smith, M.; Abdoli, A.; Abebe, M.; Adebayo, O.M.; Afarideh, M.; Agarwal, S.K.; Agudelo-Botero, M.; et al. Global, regional, and national burden of chronic kidney disease, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2020, 395, 709–733. [Google Scholar] [CrossRef]
- Drawz, P.; Rahman, M. Chronic Kidney Disease. Ann. Intern. Med. 2015, 162, ITC1–ITC16. [Google Scholar] [CrossRef]
- Guo, R.; Duan, J.; Pan, S.; Cheng, F.; Qiao, Y.; Feng, Q.; Liu, D.; Liu, Z. The Road from AKI to CKD: Molecular Mechanisms and Therapeutic Targets of Ferroptosis. Cell Death Dis. 2023, 14, 426. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, R.; Wang, Y.; Liu, Y.; Qiao, Y.; Li, P.; Chen, J.; Pan, S.; Feng, Q.; Liu, Z.; et al. Ferroptosis: A new insight for treatment of acute kidney injury. Front. Pharmacol. 2022, 13, 1065867. [Google Scholar] [CrossRef] [PubMed]
- Nørgård, M.; Svenningsen, P. Acute Kidney Injury by Ischemia/Reperfusion and Extracellular Vesicles. Int. J. Mol. Sci. 2023, 24, 15312. [Google Scholar] [CrossRef] [PubMed]
- Zarbock, A.; Gomez, H.; Kellum, J.A. Sepsis-induced acute kidney injury revisited. Curr. Opin. Crit. Care 2014, 20, 588–595. [Google Scholar] [CrossRef]
- Kanbay, M.; Solak, Y.; Afsar, B.; Nistor, I.; Aslan, G.; Çağlayan, O.H.; Aykanat, A.; Donciu, M.-D.; Lanaspa, M.A.; Ejaz, A.A.; et al. Serum Uric Acid and Risk for Acute Kidney Injury Following Contrast. Angiology 2017, 68, 132–144. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Ding, J. Pediatric Acute Kidney Injury to the Subsequent CKD Transition. Kidney Dis. 2021, 7, 10–13. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Takahashi, M.; Yanagita, M. Pathophysiology of AKI to CKD progression. Semin Nephrol. 2020, 40, 206–215, Erratum in Semin Nephrol. 2020, 40, 328. https://doi.org/10.1016/j.semnephrol.2020.05.001. [Google Scholar] [CrossRef] [PubMed]
- Kurzhagen, J.T.; Dellepiane, S.; Cantaluppi, V.; Rabb, H. AKI: An increasingly recognized risk factor for CKD development and progression. J. Nephrol. 2020, 33, 1171–1187. [Google Scholar] [CrossRef]
- Whitmore, D.; Sassone-Corsi, P.; Foulkes, N.S. PASting together the mammalian clock. Curr. Opin. Neurobiol. 1998, 8, 635–641. [Google Scholar] [CrossRef]
- King, D.P.; Takahashi, J.S. Molecular genetics of circadian rhythms in mammals. Annu. Rev. Neurosci. 2000, 23, 713–742. [Google Scholar] [CrossRef]
- Lowrey, P.L.; Takahashi, J.S. Genetics of the mammalian circadian system: Photic entrainment, circadian pacemaker mechanisms, and posttranslational regulation. Annu. Rev. Genet. 2000, 34, 533–562. [Google Scholar] [CrossRef]
- Loros, J.J.; Dunlap, J.C. Genetic and molecular analysis of circadian rhythms in Neurospora. Annu. Rev. Physiol. 2001, 63, 757–794. [Google Scholar] [CrossRef] [PubMed]
- Chang, D.C.; Reppert, S.M. The circadian clocks of mice and men. Neuron 2001, 29, 555–558. [Google Scholar] [CrossRef]
- Brown, S.A.; Schibler, U. Circadian rhythms: Mop up the clock! Curr. Biol. 2001, 11, R268–R270. [Google Scholar] [CrossRef]
- Ikeda, M.; Yua, W.; Hiraib, M.; Ebisawac, T.; Honmad, S.; Yoshimuraa, K.; Honmad, K.I.; Nomuraa, M. cDNA Cloning of a Novel bHLH-PAS Transcription Factor Superfamily Gene, BMAL2: Its mRNA Expression, Subcellular Distribution, and Chromosomal Localization. Biochem. Biophys. Res. Commun. 2000, 275, 493–502. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, M.; Yoshitane, H.; Du, N.-H.; Okano, T.; Fukada, Y. Preferential inhibition of BMAL2-CLOCK activity by PER2 reemphasizes its negative role and a positive role of BMAL2 in the circadian transcription. J. Biol. Chem. 2009, 284, 25149–25159. [Google Scholar] [CrossRef]
- Wang, H. Comparative genomic analysis of teleost fish bmal genes. Genetica 2008, 136, 149–161. [Google Scholar] [CrossRef]
- Dardente, H.; Fortier, E.E.; Martineau, V.; Cermakian, N. Cryptochromes impair phosphorylation of transcriptional activators in the clock: A general mechanism for circadian repression. Biochem. J. 2007, 402, 525–536. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Hida, A.; McGuinness, O.P.; Wasserman, D.H.; Yamazaki, S.; Johnson, C.H. Circadian clock gene bmal1 is not essential, functional replacement with its paralog, Bmal2. Curr. Biol. 2010, 20, 316–321. [Google Scholar] [CrossRef] [PubMed]
- Bunger, M.K.; Wilsbacher, L.D.; Moran, S.M.; Clendenin, C.; Radcliffe, L.A.; Hogenesch, J.B.; Simon, M.C.; Takahashi, J.S.; Bradfield, C.A. Mop3 is an essential component of the master circadian pacemaker in mammals. Cell 2000, 103, 1009–1017. [Google Scholar] [CrossRef] [PubMed]
- Gekakis, N.; Staknis, D.; Nguyen, H.B.; Davis, F.C.; Wilsbacher, L.D.; King, D.P.; Takahashi, J.S.; Weitz, C.J. Role of the CLOCK Protein in the Mammalian Circadian Mechanism. Science 1998, 280, 1564–1569. [Google Scholar] [CrossRef] [PubMed]
- King, D.P.; Zhao, Y.; Sangoram, A.M.; Wilsbacher, L.D.; Tanaka, M.; Antoch, M.P.; Steeves, T.D.L.; Vitaterna, M.H.; Kornhauser, J.M.; Lowrey, P.L.; et al. Positional cloning of the mouse circadian clock gene. Cell 1997, 89, 641–653. [Google Scholar] [CrossRef] [PubMed]
- Akashi, M.; Tsuchiya, Y.; Yoshino, T.; Nishida, E. Control of intracellular dynamics of mammalian period proteins by casein kinase I epsilon (CKIepsilon) and CKIdelta in cultured cells. Mol. Cell. Biol. 2002, 22, 1693–1703. [Google Scholar] [CrossRef]
- Camacho, F.; Cilio, M.; Guo, Y.; Virshup, D.; Patel, K.; Khorkova, O.; Styren, S.; Morse, B.; Yao, Z.; Keesler, G. Human casein kinase Iδ phosphorylation of human circadian clock proteins period 1 and 2. FEBS Lett. 2001, 489, 159–165. [Google Scholar] [CrossRef]
- Eide, E.J.; Vielhaber, E.L.; Hinz, W.A.; Virshup, D.M. The circadian regulatory proteins BMAL1 and cryptochromes are substrates of casein kinase Iε. J. Biol. Chem. 2002, 277, 17248–17254. [Google Scholar] [CrossRef] [PubMed]
- Etchegaray, J.-P.; Machida, K.K.; Noton, E.; Constance, C.M.; Dallmann, R.; Di Napoli, M.N.; DeBruyne, J.P.; Lambert, C.M.; Yu, E.A.; Reppert, S.M.; et al. Casein kinase 1 delta regulates the pace of the mammalian circadian clock. Mol. Cell. Biol. 2009, 29, 3853–3866. [Google Scholar] [CrossRef] [PubMed]
- Akashi, M.; Takumi, T. The orphan nuclear receptor RORα regulates circadian transcription of the mammalian core-clock Bmal1. Nat. Struct. Mol. Biol. 2005, 12, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Guillaumond, F.; Dardente, H.; Giguère, V.; Cermakian, N. Differential control of bmal1 circadian transcription by REV-ERB and ROR nuclear receptors. J. Biol. Rhythm. 2005, 20, 391–403. [Google Scholar] [CrossRef]
- Martin, R.A.; Viggars, M.R.; Esser, K.A. Metabolism and exercise: The skeletal muscle clock takes centre stage. Nat. Rev. Endocrinol. 2023, 19, 272–284. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Lin, Y.; Gao, L.; Yang, Z.; Lin, J.; Ren, S.; Li, F.; Chen, J.; Wang, Z.; Dong, Z.; et al. PPAR-γ integrates obesity and adipocyte clock through epigenetic regulation of Bmal1. Theranostics 2022, 12, 1589–1606. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, L.; Sun, L.; Jin, H.; Ren, K.; Liu, S.; Qian, Y.; Li, S.; Li, F.; Zhu, C.; et al. BMAL1 collaborates with CLOCK to directly promote DNA double-strand break repair and tumor chemoresistance. Oncogene 2023, 42, 967–979. [Google Scholar] [CrossRef]
- Wang, D.; Wang, F.; Zhang, H.; Chen, P.; Yang, M. Circadian clock protein Bmal1 accelerates acute myeloid leukemia by inhibiting ferroptosis through the EBF3/ALOX15 axis. Cancer Sci. 2023, 114, 3446–3460. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.; Xiong, W.; Zhao, X.; Fan, Y.; Guo, Y.; Garcia-Barrio, M.; Zhang, J.; Jiang, Z.; Lin, J.D.; Chen, Y.E. Bmal1 in Perivascular Adipose Tissue Regulates Resting-Phase Blood Pressure Through Transcriptional Regulation of Angiotensinogen. Circulation 2018, 138, 67–79. [Google Scholar] [CrossRef]
- Li, E.; Li, X.; Huang, J.; Xu, C.; Liang, Q.; Ren, K.; Bai, A.; Lu, C.; Qian, R.; Sun, N. BMAL1 regulates mitochondrial fission and mitophagy through mitochondrial protein BNIP3 and is critical in the development of dilated cardiomyopathy. Protein Cell 2020, 11, 661–679. [Google Scholar] [CrossRef] [PubMed]
- Patke, A.; Murphy, P.J.; Onat, O.E.; Krieger, A.C.; Özçelik, T.; Campbell, S.S.; Young, M.W. Mutation of the Human Circadian Clock Gene CRY1 in Familial Delayed Sleep Phase Disorder. Cell 2017, 169, 203–215.e13. [Google Scholar] [CrossRef] [PubMed]
- Rohman, M.S.; Emoto, N.; Nonaka, H.; Okura, R.; Nishimura, M.; Yagita, K.; Van Der Horst, G.T.; Matsuo, M.; Okamura, H.; Yokoyama, M. Circadian clock genes directly regulate expression of the Na+/H+ exchanger NHE3 in the kidney. Kidney Int. 2005, 67, 1410–1419. [Google Scholar] [CrossRef]
- Pizarro, A.; Hayer, K.; Lahens, N.F.; Hogenesch, J.B. CircaDB: A database of mammalian circadian gene expression profiles. Nucleic Acids Res. 2013, 41, D1009–D1013. [Google Scholar] [CrossRef] [PubMed]
- Krid, H.; Dorison, A.; Salhi, A.; Cheval, L.; Crambert, G. Expression profile of nuclear receptors along male mouse nephron segments reveals a link between ERRβ and thick ascending limb function. PLoS ONE 2012, 7, e34223. [Google Scholar] [CrossRef] [PubMed]
- Richards, J.; Ko, B.; All, S.; Cheng, K.-Y.; Hoover, R.S.; Gumz, M.L. A role for the circadian clock protein Per1 in the regulation of the NaCl co-transporter (NCC) and the with-no-lysine kinase (WNK) cascade in mouse distal convoluted tubule cells. J. Biol. Chem. 2014, 289, 11791–11806. [Google Scholar] [CrossRef]
- Susa, K.; Sohara, E.; Isobe, K.; Chiga, M.; Rai, T.; Sasaki, S.; Uchida, S. WNK-OSR1/SPAK-NCC signal cascade has circadian rhythm dependent on aldosterone. Biochem. Biophys. Res. Commun. 2012, 427, 743–747. [Google Scholar] [CrossRef]
- Zuber, A.M.; Centeno, G.; Pradervand, S.; Nikolaeva, S.; Maquelin, L.; Cardinaux, L.; Bonny, O.; Firsov, D. Molecular clock is involved in predictive circadian adjustment of renal function. Proc. Natl. Acad. Sci. USA 2009, 106, 16523–16528. [Google Scholar] [CrossRef] [PubMed]
- Gumz, M.L.; Stow, L.R.; Lynch, I.J.; Greenlee, M.M.; Rudin, A.; Cain, B.D.; Weaver, D.R.; Wingo, C.S. The circadian clock protein Period 1 regulates expression of the renal epithelial sodium channel in mice. J. Clin. Investig. 2009, 119, 2423–2434. [Google Scholar] [CrossRef]
- Koopman, M.G.; Koomen, G.C.M.; Krediet, R.T.; de Moor, E.A.M.; Hoek, F.J.; Arisz, L. Circadian rhythm of glomerular filtration rate in normal individuals. Clin. Sci. 1989, 77, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Pons, M.; Forpomès, O.; Espagnet, S.; Cambar, J. Relationship between circadian changes in renal hemodynamics and circadian changes in urinary glycosaminoglycan excretion in normal rats. Chrono-Int. 1996, 13, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Lahens, N.F.; Ballance, H.I.; Hughes, M.E.; Hogenesch, J.B. A circadian gene expression atlas in mammals: Implications for biology and medicine. Proc. Natl. Acad. Sci. USA 2014, 111, 16219–16224. [Google Scholar] [CrossRef] [PubMed]
- Tokonami, N.; Mordasini, D.; Pradervand, S.; Centeno, G.; Jouffe, C.; Maillard, M.; Bonny, O.; Gachon, F.; Gomez, R.A.; Sequeira-Lopez, M.L.S.; et al. Local renal circadian clocks control fluid-electrolyte homeostasis and BP. J. Am. Soc. Nephrol. 2014, 25, 1430–1439. [Google Scholar] [CrossRef] [PubMed]
- Lameire, N.H.; Bagga, A.; Cruz, D.; De Maeseneer, J.; Endre, Z.; Kellum, J.A.; Liu, K.D.; Mehta, R.L.; Pannu, N.; Van Biesen, W.; et al. Acute kidney injury: An increasing global concern. Lancet 2013, 382, 170–179. [Google Scholar] [CrossRef]
- Mehta, R.L.; Burdmann, E.A.; Cerdá, J.; Feehally, J.; Finkelstein, F.; García-García, G.; Godin, M.; Jha, V.; Lameire, N.H.; Levin, N.W.; et al. Recognition and management of acute kidney injury in the International Society of Nephrology 0by25 Global Snapshot: A multinational cross-sectional study. Lancet 2016, 387, 2017–2025, Erratum in Lancet 2016, 387, 1998. https://doi.org/10.1016/S0140-6736(16)30469-X. [Google Scholar] [CrossRef]
- Mehta, R.L.; Cerdá, J.; Burdmann, E.A.; Tonelli, M.; García-García, G.; Jha, V.; Susantitaphong, P.; Rocco, M.; Vanholder, R.; Sever, M.S.; et al. International Society of Nephrology’s 0by25 initiative for acute kidney injury (zero preventable deaths by 2025): A human rights case for nephrology. Lancet 2015, 385, 2616–2643. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Dong, G.; Liang, X.; Dong, Z. Epigenetic regulation in AKI and kidney repair: Mechanisms and therapeutic implications. Nat. Rev. Nephrol. 2019, 15, 220–239. [Google Scholar] [CrossRef]
- Linkermann, A.; Chen, G.; Dong, G.; Kunzendorf, U.; Krautwald, S.; Dong, Z. Regulated Cell Death in AKI. J. Am. Soc. Nephrol. 2014, 25, 2689–2701. [Google Scholar] [CrossRef]
- Agarwal, A.; Dong, Z.; Harris, R.; Murray, P.; Parikh, S.M.; Rosner, M.H.; Kellum, J.A.; Ronco, C. Cellular and Molecular Mechanisms of AKI. J. Am. Soc. Nephrol. 2016, 27, 1288–1299. [Google Scholar] [CrossRef] [PubMed]
- Rayego-Mateos, S.; Marquez-Expósito, L.; Rodrigues-Diez, R.; Sanz, A.B.; Guiteras, R.; Doladé, N.; Rubio-Soto, I.; Manonelles, A.; Codina, S.; Ortiz, A.; et al. Molecular Mechanisms of Kidney Injury and Repair. Int. J. Mol. Sci. 2022, 23, 1542. [Google Scholar] [CrossRef]
- Zuk, A.; Bonventre, J.V. Acute Kidney Injury. Annu. Rev. Med. 2016, 67, 293–307. [Google Scholar] [CrossRef] [PubMed]
- Venkatachalam, M.A.; Weinberg, J.M.; Kriz, W.; Bidani, A.K. Failed Tubule Recovery, AKI-CKD Transition, and Kidney Disease Progression. J. Am. Soc. Nephrol. 2015, 26, 1765–1776. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Wei, Q.; Liu, J.; Yi, M.; Liu, Y.; Liu, H.; Sun, L.; Peng, Y.; Liu, F.; Venkatachalam, M.A.; et al. AKI on CKD: Heightened injury, suppressed repair, and the underlying mechanisms. Kidney Int. 2017, 92, 1071–1083. [Google Scholar] [CrossRef]
- Chawla, L.S.; Eggers, P.W.; Star, R.A.; Kimmel, P.L. Acute kidney injury and chronic kidney disease as interconnected syndromes. N. Engl. J. Med. 2014, 371, 58–66. [Google Scholar] [CrossRef]
- Ferenbach, D.A.; Bonventre, J.V. Mechanisms of maladaptive repair after AKI leading to accelerated kidney ageing and CKD. Nat. Rev. Nephrol. 2015, 11, 264–276. [Google Scholar] [CrossRef] [PubMed]
- Mercan, M.; Şehirli, A.; Chukwunyere, U.; Abacıoğlu, N. Acute kidney injury due to COVID-19 and the circadian rhythm. Med. Hypotheses 2021, 146, 110463. [Google Scholar] [CrossRef]
- Dong, C.; Li, J.; Tang, Q.; Wang, Y.; Zeng, C.; Du, L.; Sun, Q. Denervation aggravates renal ischemia reperfusion injury via BMAL1-mediated Nrf2/ARE pathway. Arch. Biochem. Biophys. 2023, 746, 109736. [Google Scholar] [CrossRef]
- Yang, S.; Ye, Z.; Chen, W.; Wang, P.; Zhao, S.; Zhou, X.; Li, W.; Cheng, F. BMAL1 alleviates sepsis-induced AKI by inhibiting ferroptosis. Int. Immunopharmacol. 2024, 142, 113159. [Google Scholar] [CrossRef]
- Ye, P.; Li, W.; Huang, X.; Zhao, S.; Chen, W.; Xia, Y.; Yu, W.; Rao, T.; Ning, J.; Zhou, X.; et al. BMAL1 regulates mitochondrial homeostasis in renal ischaemia-reperfusion injury by mediating the SIRT1/PGC-1α axis. J. Cell. Mol. Med. 2022, 26, 1994–2009. [Google Scholar] [CrossRef] [PubMed]
- Guan, S.; Wang, Z.; Zhang, R.; Chen, S.; Bu, X.; Lu, J. 3-MCPD Induced Mitochondrial Damage of Renal Cells Via the Rhythmic Protein BMAL1 Targeting SIRT3/SOD2. J. Agric. Food Chem. 2023, 71, 14351–14364. [Google Scholar] [CrossRef]
- Yoshioka, H.; Yokota, S.; Tominaga, S.; Tsukiboshi, Y.; Suzui, M.; Shinohara, Y.; Yoshikawa, M.; Sasaki, H.; Sasaki, N.; Maeda, T.; et al. Involvement of Bmal1 and Clock in Bromobenzene Metabolite-Induced Diurnal Renal Toxicity. Biol. Pharm. Bull. 2023, 46, 824–829. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Z.; Wu, Z.; Chen, M.; Dong, D.; Yu, P.; Lu, D.; Wu, B. Involvement of REV-ERBα dysregulation and ferroptosis in aristolochic acid I-induced renal injury. Biochem. Pharmacol. 2021, 193, 114807. [Google Scholar] [CrossRef]
- Zha, M.; Tian, T.; Xu, W.; Liu, S.; Jia, J.; Wang, L.; Yan, Q.; Li, N.; Yu, J.; Huang, L. The circadian clock gene Bmal1 facilitates cisplatin-induced renal injury and hepatization. Cell Death Dis. 2020, 11, 446. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.; Atkins, R.; Coresh, J.; Cohen, E.; Collins, A.; Eckardt, K.-U.; Nahas, M.; Jaber, B.; Jadoul, M.; Levin, A.; et al. Chronic kidney disease as a global public health problem: Approaches and initiatives a position statement from Kidney Disease Improving Global Outcomes. Kidney Int. 2007, 72, 247–259. [Google Scholar] [CrossRef] [PubMed]
- Levin, A.; Stevens, P.E.; Bilous, R.W.; Coresh, J.; De Francisco, A.L.M.; De Jong, P.E.; Griffith, K.E.; Hemmelgarn, B.R.; Iseki, K.; Lamb, E.J.; et al. KDIGO clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int. Suppl. 2013, 3, 1–150. [Google Scholar] [CrossRef]
- Rebollo-Rubio, A.; Morales-Asencio, J.M.; Pons-Raventos, M.E.; Mansilla-Francisco, J.J. Review of studies on health related quality of life in patients with advanced chronic kidney disease in Spain. Nefrologi 2015, 35, 92–109. [Google Scholar] [CrossRef]
- Molina, P.; Gavela, E.; Vizcaíno, B.; Huarte, E.; Carrero, J.J. Optimizing Diet to Slow CKD Progression. Front. Med. 2021, 8, 654250. [Google Scholar] [CrossRef]
- Yamada, S.; Nakano, T. Role of Chronic Kidney Disease (CKD)–Mineral and Bone Disorder (MBD) in the Pathogenesis of Cardiovascular Disease in CKD. J. Atheroscler. Thromb. 2023, 30, 835–850. [Google Scholar] [CrossRef]
- Hain, D.; Bednarski, D.; Cahill, M.; Dix, A.; Foote, B.; Haras, M.S.; Pace, R.; Gutiérrez, O.M. Iron-Deficiency Anemia in CKD: A Narrative Review for the Kidney Care Team. Kidney Med. 2023, 5, 100677. [Google Scholar] [CrossRef]
- Crislip, G.R.; Costello, H.M.; Juffre, A.; Cheng, K.-Y.; Lynch, I.J.; Johnston, J.G.; Drucker, C.B.; Bratanatawira, P.; Agarwal, A.; Mendez, V.M.; et al. Male kidney-specific BMAL1 knockout mice are protected from K-deficient, high-salt diet-induced blood pressure increases. Am. J. Physiol. Renal. Physiol. 2023, 325, F656–F668. [Google Scholar] [CrossRef]
- Zhang, R.Y.; Mou, L.J.; Li, X.M.; Li, X.W.; Qin, Y. Effect of FCGR3A Polymorphisms on Antibody-dependent Cetuximab-mediated Cytotoxicity in A549 Cells. Zhongguo Yi Xue Ke Xue Yuan Xue Bao 2015, 37, 698–704. [Google Scholar] [CrossRef]
- Wang, X.-H.; Zhang, Z.-Z.; Ou, Y.; Ning, Z.-H.; Yang, J.-Y.; Huang, H.; Tang, H.-F.; Jiang, Z.-S.; Hu, H.-J. High-Salt Diet Inhibits the Expression of Bmal1 and Promotes Atrial Fibrosis and Vulnerability to Atrial Fibrillation in Dahl Salt-Sensitive Rats. Am. J. Hypertens. 2024, 37, 726–733. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zhao, S.; Xing, J.; Yu, W.; Rao, T.; Zhou, X.; Ruan, Y.; Li, S.; Xia, Y.; Song, T.; et al. BMAL1 inhibits renal fibrosis and renal interstitial inflammation by targeting the ERK1/2/ELK-1/Egr-1 axis. Int. Immunopharmacol. 2023, 125, 111140. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, C.; Liang, Q.; Zheng, F.; Guan, Y.; Yang, G.; Chen, L. Postnatal deletion of Bmal1 in mice protects against obstructive renal fibrosis via suppressing Gli2 transcription. FASEB J. 2021, 35, e21530. [Google Scholar] [CrossRef]
- Chen, W.D.; Yeh, J.-K.; Peng, M.-T.; Shie, S.-S.; Lin, S.-L.; Yang, C.-H.; Chen, T.-H.; Hung, K.-C.; Wang, C.-C.; Hsieh, I.-C.; et al. Circadian CLOCK Mediates Activation of Transforming Growth Factor-β Signaling and Renal Fibrosis through Cyclooxygenase 2. Am. J. Pathol. 2015, 185, 3152–3163. [Google Scholar] [CrossRef] [PubMed]
- Rey-Serra, C.; Tituaña, J.; Lin, T.; Herrero, J.I.; Miguel, V.; Barbas, C.; Meseguer, A.; Ramos, R.; Chaix, A.; Panda, S.; et al. Reciprocal regulation between the molecular clock and kidney injury. Life Sci. Alliance 2023, 6, e202201886. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Jo, S.-K.; Park, S.-J.; Yang, J.; Ko, Y.S.; Lee, H.Y.; Oh, S.W.; Cho, W.Y.; Kim, K.; Son, G.H.; et al. Role of the Circadian Clock and Effect of Time-Restricted Feeding in Adenine-Induced Chronic Kidney Disease. Lab. Invest. 2023, 103, 100008. [Google Scholar] [CrossRef]
- Xing, L.; Wu, S.; Shi, Y.; Yue, F.; Wei, L.; Russell, R.; Zhang, D. Chronic constant light exposure aggravates high fat diet-induced renal injury in rats. Front. Endocrinol. 2022, 13, 900392. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Li, S.; Ji, S.; Zhang, J.; Zheng, F.; Guan, Y.; Yang, G.; Chen, L. Proximal tubular Bmal1 protects against chronic kidney injury and renal fibrosis by maintaining of cellular metabolic homeostasis. Biochim. et Biophys. Acta BBA Mol. Basis Dis. 2023, 1869, 166572. [Google Scholar] [CrossRef]
- Ansermet, C.; Centeno, G.; Bignon, Y.; Ortiz, D.; Pradervand, S.; Garcia, A.; Menin, L.; Gachon, F.; Yoshihara, H.A.; Firsov, D. Dysfunction of the circadian clock in the kidney tubule leads to enhanced kidney gluconeogenesis and exacerbated hyperglycemia in diabetes. Kidney Int. 2022, 101, 563–573. [Google Scholar] [CrossRef]
- Crislip, G.R.; Wohlgemuth, S.E.; Wolff, C.A.; Gutierrez-Monreal, M.A.; Douglas, C.M.; Ebrahimi, E.; Cheng, K.-Y.; Masten, S.H.; Barral, D.; Bryant, A.J.; et al. Apparent Absence of BMAL1-Dependent Skeletal Muscle–Kidney Cross Talk in Mice. Biomolecules 2022, 12, 261. [Google Scholar] [CrossRef] [PubMed]
- Rangarajan, S.; Walsh, L.; Lester, W.; Perry, D.; Madan, B.; Laffan, M.; Yu, H.; Vettermann, C.; Pierce, G.F.; Wong, W.Y.; et al. AAV5–Factor VIII Gene Transfer in Severe Hemophilia A. N. Engl. J. Med. 2017, 377, 2519–2530. [Google Scholar] [CrossRef]
- Di Minno, G.; Miesbach, W.; Castaman, G.; Peyvandi, F. Next-generation strategies to improve safety and efficacy of adeno-associated virus-based gene therapy for hemophilia: Lessons from clinical trials in other gene therapies. Haematologica 2024, 109, 3879–3891. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.P.; Haasnoot, J.; ter Brake, O.; Berkhout, B.; Konstantinova, P. Inhibition of HIV-1 by multiple siRNAs expressed from a single microRNA polycistron. Nucleic Acids Res. 2008, 36, 2811–2824. [Google Scholar] [CrossRef] [PubMed]
- Yasen, A.; Herrera, R.; Rosbe, K.; Lien, K.; Tugizov, S.M. Release of HIV-1 sequestered in the vesicles of oral and genital mucosal epithelial cells by epithelial-lymphocyte interaction. PLOS Pathog. 2017, 13, e1006247. [Google Scholar] [CrossRef] [PubMed]
- Meisel, R. CRISPR-Cas9 Gene Editing for Sickle Cell Disease and β-Thalassemia. N. Engl. J. Med. 2021, 384, e91. [Google Scholar] [CrossRef] [PubMed]
- Dever, D.P.; Bak, R.O.; Reinisch, A.; Camarena, J.; Washington, G.; Nicolas, C.E.; Pavel-Dinu, M.; Saxena, N.; Wilkens, A.B.; Mantri, S.; et al. CRISPR/Cas9 β-globin gene targeting in human haematopoietic stem cells. Nature 2016, 539, 384–389. [Google Scholar] [CrossRef]
- Grupp, S.A.; Kalos, M.; Barrett, D.; Aplenc, R.; Porter, D.L.; Rheingold, S.R.; Teachey, D.T.; Chew, A.; Hauck, B.; Wright, J.F.; et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N. Engl. J. Med. 2013, 368, 1509–1518, Erratum in N. Engl. J. Med. 2016, 374, 998. https://doi.org/10.1056/NEJMx160005. [Google Scholar] [CrossRef] [PubMed]
- Kalos, M.; Levine, B.L.; Porter, D.L.; Katz, S.; Grupp, S.A.; Bagg, A.; June, C.H. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci. Transl. Med. 2011, 3, 95ra73. [Google Scholar] [CrossRef] [PubMed]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef]
- Skowronski, D.M.; De Serres, G. Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine. N. Engl. J. Med. 2021, 384, 1576–1577. [Google Scholar] [CrossRef]
- Maguire, A.M.; Simonelli, F.; Pierce, E.A.; Pugh, E.N.; Mingozzi, F.; Bennicelli, J.; Banfi, S.; Marshall, K.A.; Testa, F.; Surace, E.M.; et al. Safety and efficacy of gene transfer for Leber’s congenital amaurosis. N. Engl. J. Med. 2008, 358, 2240–2248. [Google Scholar] [CrossRef] [PubMed]
- Bainbridge, J.W.; Mehat, M.S.; Sundaram, V.; Robbie, S.J.; Barker, S.E.; Ripamonti, C.; Georgiadis, A.; Mowat, F.M.; Beattie, S.G.; Gardner, P.J.; et al. Long-term effect of gene therapy on Leber’s congenital amaurosis. N. Engl. J. Med. 2015, 372, 1887–1897. [Google Scholar] [CrossRef] [PubMed]
- Mendell, J.R.; Rodino-Klapac, L.R.; Sahenk, Z.; Roush, K.; Bird, L.; Lowes, L.P.; Alfano, L.; Gomez, A.M.; Lewis, S.; Kota, J.; et al. Eteplirsen for the treatment of Duchenne muscular dystrophy. Ann. Neurol. 2013, 74, 637–647. [Google Scholar] [CrossRef] [PubMed]
- Lim, K.R.Q.; Maruyama, R.; Yokota, T. Eteplirsen in the treatment of Duchenne muscular dystrophy. Drug Des. Dev. Ther. 2017, 11, 533–545. [Google Scholar] [CrossRef]
- Zeng, T.; Liang, L.; Deng, W.; Xie, M.; Zhao, M.; Wang, S.; Liu, J.; Yang, M. BMAL1 plays a crucial role in immune homeostasis during sepsis-induced acute lung injury. Biochem. Pharmacol. 2024, 226, 116379. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Pan, L.; Wang, F.; Yan, J.; Wang, T.; Xia, Y.; Yao, L.; Deng, K.; Zheng, Y.; Xia, X.; et al. Neural function of Bmal1: An overview. Cell Biosci. 2023, 13, 1. [Google Scholar] [CrossRef]
- Schiaffino, S.; Blaauw, B.; Dyar, K.A. The functional significance of the skeletal muscle clock: Lessons from Bmal1 knockout models. Skelet. Muscle 2016, 6, 33. [Google Scholar] [CrossRef] [PubMed]
- Fagiani, F.; Di Marino, D.; Romagnoli, A.; Travelli, C.; Voltan, D.; Mannelli, L.D.C.; Racchi, M.; Govoni, S.; Lanni, C. Molecular regulations of circadian rhythm and implications for physiology and diseases. Signal Transduct. Target. Ther. 2022, 7, 41. [Google Scholar] [CrossRef] [PubMed]


| AKI Models | Protective or Harmful | Mechanism | References |
|---|---|---|---|
| COVID-19-induced AKI model (according to retrospective clinical analysis) | Unclear | Unclear | [75] |
| Ischemia–reperfusion acute kidney injury model (according to preclinical animal models) | Protective | Nrf2/ARE pathway | [76] |
| Sepsis-induced AKI model (according to preclinical animal models) | Protective | YAP/ACSL4 pathway | [77] |
| Ischemia–reperfusion acute kidney injury model (according to preclinical animal models) | Protective | SIRT1/PGC-1α axis | [78] |
| 3-monochloro-1,2-propanediol (3-MCPD)-induced AKI model (according to preclinical animal models) | Protective | SIRT3/SOD2 pathway | [79] |
| 4-bromo-o-cresol (4-BrCA) induced AKI model (according to preclinical animal models) | Unclear | Unclear | [80] |
| AAI-induced renal injury model (according to preclinical animal models) | Unclear | Unclear | [81] |
| Cisplatin-induced acute kidney injury model (according to preclinical animal models) | Harmful | Unclear | [82] |
| Serial Number | Therapeutic Strategy | Overview | Application | Example |
|---|---|---|---|---|
| 1 | Gene Replacement Therapy | Replacing a defective or mutated gene in the patient by introducing a functional gene. | Frequently applied in the treatment of genetic diseases, such as Cystic Fibrosis and Duchenne Muscular Dystrophy. | Introducing normal genes into liver cells through adeno-associated virus (AAV) vectors for the treatment of hemophilia [101,102] |
| 2 | Gene Silencing | Using RNA interference (RNAi) techniques, such as small interfering RNA (siRNA) or antisense oligonucleotides (ASO), to suppress the expression of harmful genes. | Applied in the treatment of diseases associated with overexpression of genes, such as certain cancers and genetic eye disorders. | siRNA is used to target and suppress the gene expression of HIV, thereby stopping the virus from replicating [103,104] |
| 3 | Gene Editing | Utilizing tools like CRISPR-Cas9, TALEN, or ZFN to directly and accurately edit specific gene sequences within the genome. | Applied to correct gene mutations that cause diseases, with wide potential uses, such as in cancer, hereditary diseases, and HIV/AIDS. | The CRISPR-Cas9 technique is applied to correct the defective gene in individuals with sickle cell anemia [105,106] |
| 4 | Immunogene Therapy | Using genetic engineering to modify immune cells, enabling them to more efficiently identify and target cancer cells or pathogens. | Mainly applied in cancer therapies, such as CAR-T cell treatment. | In CAR-T cell therapy, the patient’s T cells are altered to express chimeric antigen receptors (CAR), enabling them to identify and destroy specific cancer cells [107,108] |
| 5 | Gene Vaccines | Vaccines created through genetic engineering introduce genes that encode antigens, stimulating the immune system to generate specific antibodies and T-cell responses. | Broadly used in infectious disease prevention, such as the COVID-19 mRNA vaccines, with potential applications in cancer vaccines. | The mRNA vaccines created by Pfizer and Moderna work by injecting mRNA encoding the spike (S) protein, prompting the body to produce an immune response [109,110] |
| 6 | Gene Transduction Therapy | Employing viral or non-viral vectors to introduce genes into target cells to achieve therapeutic effects. | Extensively applied in different types of gene therapy, such as gene replacement, gene silencing, and gene editing. | Employing an adenovirus vector to introduce the normal RPE65 gene into retinal cells, treating genetic retinal disorders [111,112] |
| 7 | Antisense Therapy | Antisense oligonucleotides bind to target mRNA, preventing its translation or promoting its degradation, thus suppressing the production of harmful proteins. | Applied in the treatment of amyotrophic lateral sclerosis (ALS), Duchenne muscular dystrophy, and similar diseases. | Eteplirsen is applied in the treatment of Duchenne muscular dystrophy by using antisense oligonucleotides to modify the mRNA of the DMD gene, restoring the expression of partially functional proteins [113,114] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, S.; Ye, Z.; Chen, L.; Zhou, X.; Li, W.; Cheng, F. Circadian Clock Gene Bmal1: A Molecular Bridge from AKI to CKD. Biomolecules 2025, 15, 77. https://doi.org/10.3390/biom15010077
Yang S, Ye Z, Chen L, Zhou X, Li W, Cheng F. Circadian Clock Gene Bmal1: A Molecular Bridge from AKI to CKD. Biomolecules. 2025; 15(1):77. https://doi.org/10.3390/biom15010077
Chicago/Turabian StyleYang, Songyuan, Zehua Ye, Lijia Chen, Xiangjun Zhou, Wei Li, and Fan Cheng. 2025. "Circadian Clock Gene Bmal1: A Molecular Bridge from AKI to CKD" Biomolecules 15, no. 1: 77. https://doi.org/10.3390/biom15010077
APA StyleYang, S., Ye, Z., Chen, L., Zhou, X., Li, W., & Cheng, F. (2025). Circadian Clock Gene Bmal1: A Molecular Bridge from AKI to CKD. Biomolecules, 15(1), 77. https://doi.org/10.3390/biom15010077







