Insights into Transient Dimerization of Carnitine/Acylcarnitine Carrier (SLC25A20) from Sarkosyl/PAGE, Cross-Linking Reagents, and Comparative Modelling Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Rat Liver Mitoplasts and Purification and Reconstitution of Mitochondrial CAC in Proteoliposomes
2.3. Production, Purification, and Reconstitution of the CAC WT and C-lessV Mutant
2.4. Transport Assays in Proteoliposomes
2.5. Sarkosyl/PAGE Analysis
2.6. Chemical Cross-Linking of Purified and Reconstituted CAC Protein
2.7. Western Blotting
2.8. Gel Filtration Analysis
2.9. 3D Molecular Modelling Analysis
2.10. Membrane Building and Energy Minimisation
| c-/c-CAC Dimer | m-/m-CAC Dimer | c-/m-CAC Dimer | m-/c-CAC Dimer | |
|---|---|---|---|---|
| Number of Atoms | 148,922 | 139,584 | 146,885 | 145,207 |
| Crystal type | tetragonal | tetragonal | tetragonal | tetragonal |
| System size (Å) | (A) 117.75 × (B) 117.75 × (C) 116.15 | (A) 115.91 × (B) 115.91 × (C) 112.47 | (A) 115.81 × (B) 115.81 × (C) 118.05 | (A) 115.73 × (B) 115.73 × (C) 117.16 |
| Crystal angle (degrees) | (alpha) 90 × (beta) 90 × (gamma) 90 | (alpha) 90 × (beta) 90 × (gamma) 90 | (alpha) 90 × (beta) 90 × (gamma) 90 | (alpha) 90 × (beta) 90 × (gamma) 90 |
| Number of lipids | 300 (158 POPC; 98 POPE; 44 CL) | 290 (153 POPC; 95 POPE; 42 CL) | 290 (153 POPC; 95 POPE; 42 CL) | 290 (153 POPC; 95 POPE; 42 CL) |
| Number of water molecules (TIP3P) | 31,988 | 29,307 | 31,766 | 31,208 |
| Number of K+ ions | 108 | 92 | 102 | 101 |
| Number of Cl− ions | 82 | 75 | 82 | 81 |
2.11. Interaction Energy
2.12. Other Methods
2.13. Statistical Analysis
3. Results
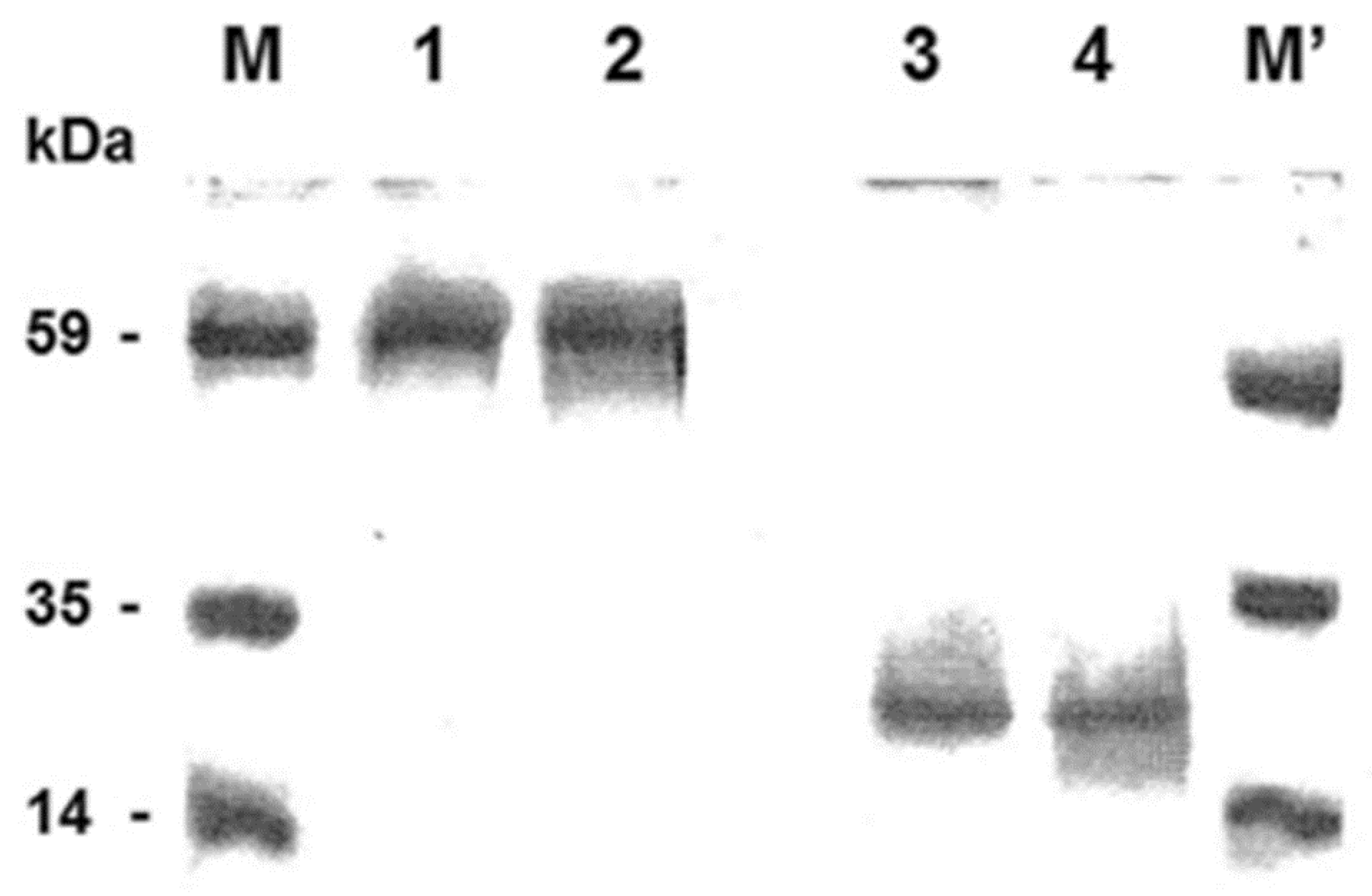
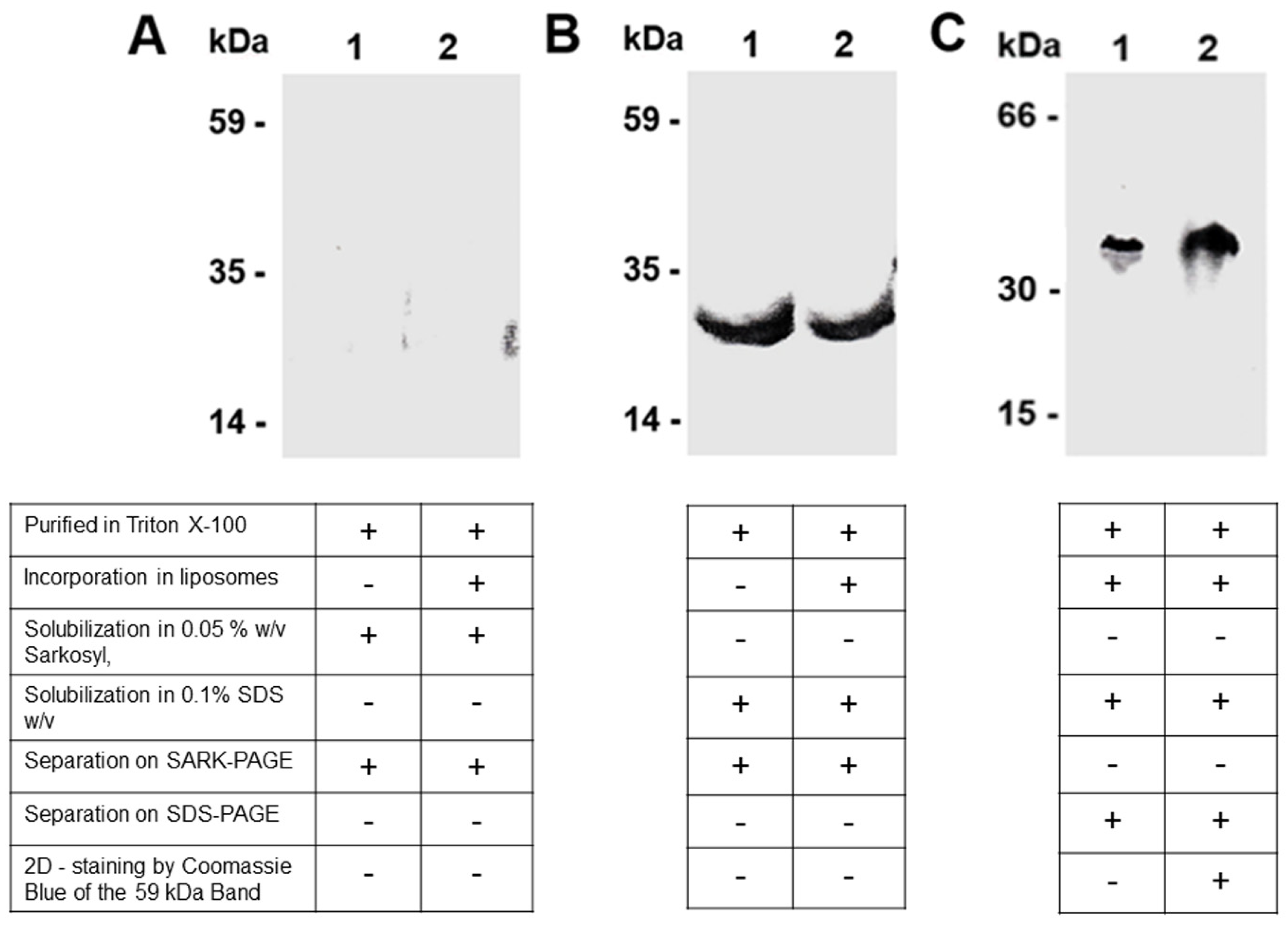
3.1. Dimer Detection of the Purified Carnitine/Acylcarnitine Carrier (CAC) by Sarkosyl/PAGE
3.2. The Detection of the Dimeric Form of the Purified and Reconstituted CAC Protein Following Western Blotting
3.3. The Detection of the Dimeric Form of the Purified and Reconstituted CAC Protein by the Chemical Cross-Linking Strategy
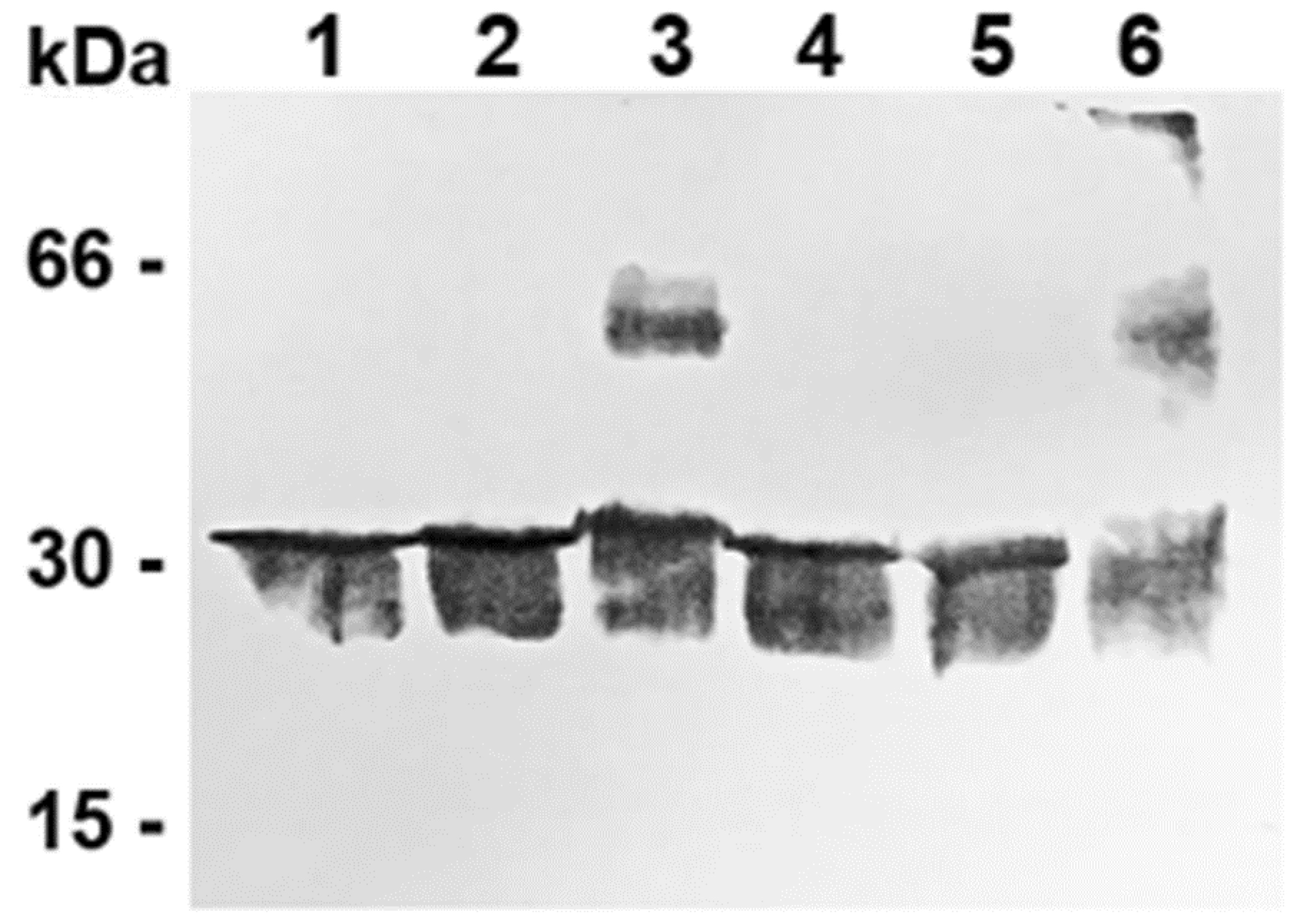
3.4. Different Ratios of Recombinant WT and C-lessV Proteins Reconstituted in Liposomes Lead to Changes in the Dimeric Structure of the CAC
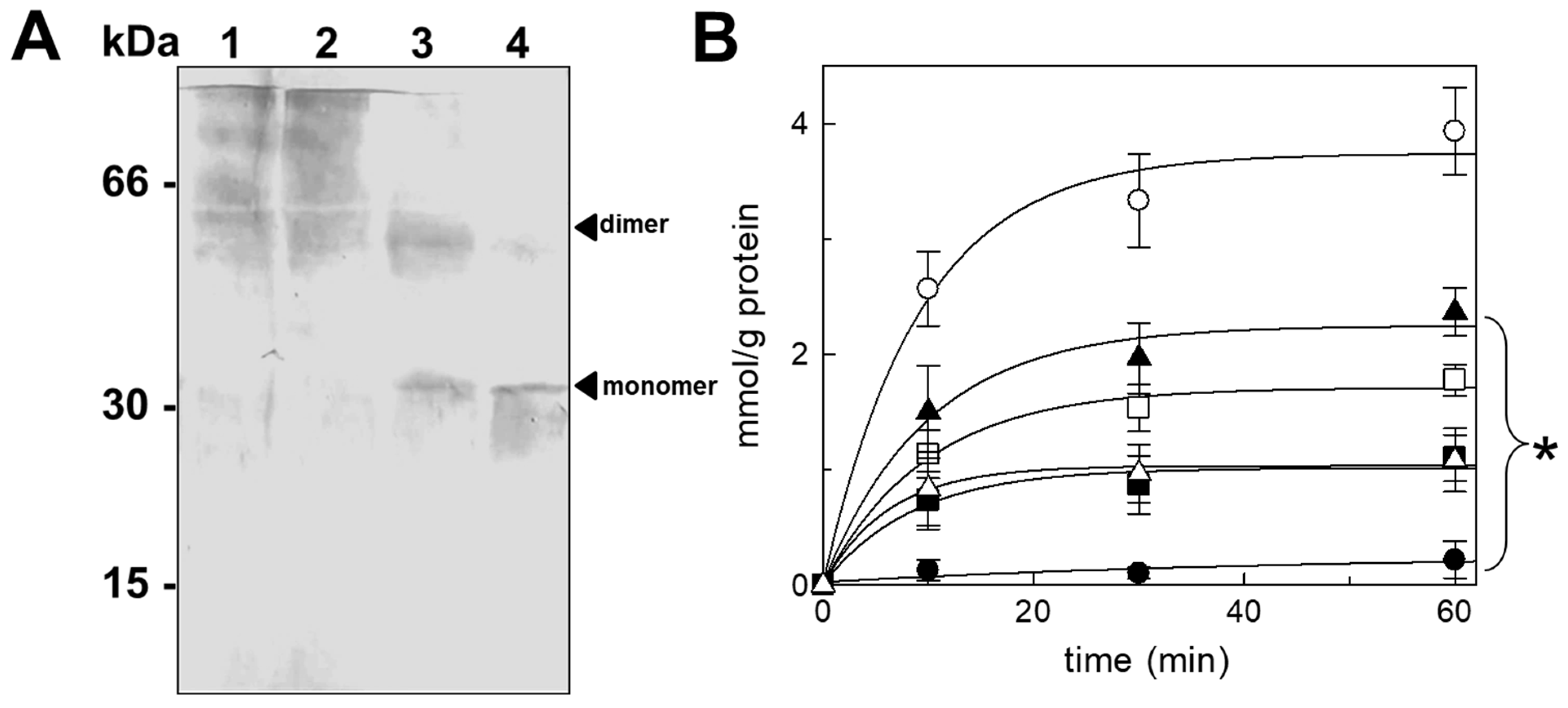
3.5. The Identification of the Dimeric Form of Purified CAC Protein by Size-Exclusion Chromatography
3.6. 3D Molecular Modelling and Energy Minimisation
| c-conf/c-conf CAC Dimer | m-conf/m-conf CAC Dimer | c-conf/m-conf CAC Dimer | m-conf/c-conf CAC Dimer | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Protein Dimer Regions | Secondary Structure Elements | Residues at the Protein–Protein Interface | Residues at the Protein–Protein Interface | Residues at the Protein–Protein Interface | Residues at the Protein–Protein Interface | ||||
| Residues involved in interactions in the CAC dimer bottom half (between matrix gate area and matrix loops) | QY41 | SJ53 | SK53 | SK53 | SJ53 | LY38 | |||
| h12 | SX53 | SY53 | TJ55 | RJ60 | PY42 | ||||
| IX56 | IY56 | IJ56 | IK56 | IX56 | IK56 | SY53 | |||
| DJ57 | DK57 | DX57 | DK57 | TY55 | |||||
| RX60 | RY60 | RJ60 | RK60 | RK60 | IY56 | ||||
| KJ61 | KK61 | KK61 | |||||||
| IJ257 | |||||||||
| DJ259 | |||||||||
| EY260 | EJ260 | EK260 | EX260 | EJ260 | EY260 | ||||
| h56-ml56b | VJ262 | VK262 | VX262 | VJ262 | |||||
| TX263 | TY263 | TJ263 | TK263 | TX263 | TJ263 | TY263 | |||
| SY264 | YJ266 | YK266 | |||||||
| KY267 | KJ267 | KK267 | KK267 | ||||||
| Residues involved in interactions in the CAC dimer upper half (between the cytosolic gate area and cytosolic loops) | H4–H5 | LX193 | |||||||
| IX196 | FJ197 | ||||||||
| FX197 | FY197 | FK197 | FX197 | FK197 | |||||
| TX198 | TY198 | TK198 | TX198 | TK198 | TJ198 | TY198 | |||
| PX199 | EJ200 | EK200 | PX199 | EJ200 | VY204 | ||||
| LY207 | VJ204 | VK204 | LX207 | ||||||
| PJ210 | PK210 | PY210 | |||||||
| RX211 | RY211 | RJ211 | RX211 | RK211 | RJ211 | RY211 | |||
| LK213 | LK213 | ||||||||
| VJ214 | VK214 | VX214 | VK214 | VJ214 | VY214 | ||||
| AJ215 | AX215 | AY215 | |||||||
| Residues involved in interactions in the CAC dimer middle regions (between PG-levels) | H5 | FX218 | FY218 | FJ218 | FK218 | FX218 | FK218 | FJ218 | FY218 |
| H6 | NK270 | NX270 | |||||||
| FK277 | IX274 | NK270 | NJ270 | NY270 | |||||
3.7. Interaction Energies
4. Discussion
4.1. The Choice of Sarkosyl in the Solubilisation and Separation of CAC Dimers
4.2. Detection of the Solubilised Purified/Recombinant CAC by Western Blot
4.3. Possible Role of Cardiolipin in Dimerization along the Solubilisation/Separation Steps
4.4. Employment of Cross-Linking Agents for Investigating CAC Dimerization
4.5. Size-Exclusion Chromatography for Determining the Molecular Weight of the Naïve Protein Purified from Rat Liver Mitochondria
4.6. Crystallographic Dimers of the ADP/ATP Carrier Used as a Template to Model the CAC Dimer
4.7. Energy Minimisation of the CAC Dimer 3D Models and Calculation of the Free Energy of Interaction at the CAC Monomer–Monomer Interface
4.8. Physiological/Structural Requirements Justifying the Existence of the CAC Dimers/Oligomers
4.9. General Considerations and Open Questions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Correction Statement
Abbreviations
References
- Palmieri, F. The Mitochondrial Transporter Family SLC25: Identification, Properties and Physiopathology. Mol. Asp. Med. 2013, 34, 465–484. [Google Scholar] [CrossRef]
- Tonazzi, A.; Giangregorio, N.; Console, L.; Palmieri, F.; Indiveri, C. The Mitochondrial Carnitine Acyl-Carnitine Carrier (Slc25a20): Molecular Mechanisms of Transport, Role in Redox Sensing and Interaction with Drugs. Biomolecules 2021, 11, 521. [Google Scholar] [CrossRef] [PubMed]
- Ramsay, R.R.; Gandour, R.D.; van der Leij, F.R. Molecular Enzymology of Carnitine Transfer and Transport. Biochim. Biophys. Acta 2001, 1546, 21–43. [Google Scholar] [CrossRef] [PubMed]
- Ijlst, L.; Ruiter, J.P.N.; Oostheim, W.; Niezen-Koning, K.E.; Palmieri, F.; Wanders, R.J.A. Identification of a Missense Mutation in a Patient with Lethal Carnitine Acyl-Carnitine Carrier Deficiency. Adv. Exp. Med. Biol. 2000, 466, 347–351. [Google Scholar]
- Zhang, L.; Hu, Y.; Xie, M.; Zhang, Y.; Cen, K.; Chen, L.; Cui, Y.; Li, H.; Wang, D. Carnitine-Acylcarnitine Translocase Deficiency Caused by SLC25A20 Gene Heterozygous Variants in Twins: A Case Report. J. Int. Med. Res. 2023, 51, 03000605231163811. [Google Scholar] [CrossRef]
- Yan, H.-M.; Hu, H.; Ahmed, A.; Feng, B.-B.; Liu, J.; Jia, Z.-J.; Wang, H. Carnitine-Acylcarnitine Translocase Deficiency with c.199-10 T>G and Novel c.1A>G Mutation: Two Case Reports and Brief Literature Review. Medicine 2017, 96, e8549. [Google Scholar] [CrossRef]
- Li, X.; Zhao, F.; Zhao, Z.; Zhao, X.; Meng, H.; Zhang, D.; Zhao, S.; Ding, M. Neonatal Sudden Death Caused by a Novel Heterozygous Mutation in SLC25A20 Gene: A Case Report and Brief Literature Review. Leg. Med. 2022, 54, 101990. [Google Scholar] [CrossRef]
- Indiveri, C.; Iacobazzi, V.; Tonazzi, A.; Giangregorio, N.; Infantino, V.; Convertini, P.; Console, L.; Palmieri, F. The Mitochondrial Carnitine/Acylcarnitine Carrier: Function, Structure and Physiopathology. Mol. Asp. Med. 2011, 32, 223–233. [Google Scholar] [CrossRef]
- Gutiérrez-Aguilar, M.; Baines, C.P. Physiological and Pathological Roles of Mitochondrial SLC25 Carriers. Biochem. J. 2013, 454, 371–386. [Google Scholar] [CrossRef]
- Monné, M.; Palmieri, F. Antiporters of the Mitochondrial Carrier Family. Curr. Top. Membr. 2014, 73, 289–320. [Google Scholar] [CrossRef]
- Indiveri, C.; Tonazzi, A.; Palmieri, F. Characterization of the Unidirectional Transport of Carnitine Catalyzed by the Reconstituted Carnitine Carrier from Rat Liver Mitochondria. Biochim. Biophys. Acta 1991, 1069, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Indiveri, C.; Tonazzi, A.; Palmieri, F. The Reconstituted Carnitine Carrier from Rat Liver Mitochondria: Evidence for a Transport Mechanism Different from That of the Other Mitochondrial Translocators. Biochim. Biophys. Acta 1994, 1189, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Cimadamore-Werthein, C.; Jaiquel Baron, S.; King, M.S.; Springett, R.; Kunji, E.R. Human Mitochondrial ADP/ATP Carrier SLC25A4 Operates with a Ping-pong Kinetic Mechanism. EMBO Rep. 2023, 24, e57127. [Google Scholar] [CrossRef] [PubMed]
- Palmieri, F.; Pierri, C.L. Structure and Function of Mitochondrial Carriers—Role of the Transmembrane Helix P and G Residues in the Gating and Transport Mechanism. FEBS Lett. 2010, 584, 1931–1939. [Google Scholar] [CrossRef]
- Klingenberg, M. Ligand-Protein Interaction in Biomembrane Carriers. The Induced Transition Fit of Transport Catalysis. Biochemistry 2005, 44, 8563–8570. [Google Scholar] [CrossRef]
- Klingenberg, M. The ADP/ATP Carrier in Mitochondrial Membranes. In The Enzymes of Biological Membranes: Membrane Transport; Plenum Publishing Corp.: New York, NY, USA; London, UK, 1976; Volume 3. [Google Scholar]
- Kunji, E.R.S.; Crichton, P.G. Mitochondrial Carriers Function as Monomers. Biochim. Biophys. Acta—Bioenerg. 2010, 1797, 817–831. [Google Scholar] [CrossRef]
- Giangregorio, N.; Pierri, C.L.; Tonazzi, A.; Incampo, G.; Tragni, V.; De Grassi, A.; Indiveri, C. Proline/Glycine Residues of the PG-Levels Guide Conformational Changes along the Transport Cycle in the Mitochondrial Carnitine/Acylcarnitine Carrier (SLC25A20). Int. J. Biol. Macromol. 2022, 221, 1453–1465. [Google Scholar] [CrossRef]
- Novack, G.V.; Galeano, P.; Castaño, E.M.; Morelli, L. Mitochondrial Supercomplexes: Physiological Organization and Dysregulation in Age-Related Neurodegenerative Disorders. Front. Endocrinol. 2020, 11, 600. [Google Scholar] [CrossRef]
- Todisco, S.; Musio, B.; Pesce, V.; Cavalluzzi, M.M.; Petrosillo, G.; La Piana, G.; Sgobba, M.N.; Schlosserová, N.; Cafferati Beltrame, L.; Di Lorenzo, R.; et al. Targeting Mitochondrial Impairment for the Treatment of Cardiovascular Diseases: From Hypertension to Ischemia-Reperfusion Injury, Searching for New Pharmacological Targets. Biochem. Pharmacol. 2023, 208, 115405. [Google Scholar] [CrossRef]
- Scorrano, L. Control of Cristae Remodelling by Opa1 and Parl: A Checkpoint for Cytochrome c Release and Apoptosis. Bio Tech. Int. 2007, 41, 1875–1883. [Google Scholar]
- Benarroch, E. What Is the Role of Organelle Membrane Contact Sites in Neurodegenerative Diseases? Neurology 2022, 99, 703–710. [Google Scholar] [CrossRef] [PubMed]
- Scorrano, L.; De Matteis, M.A.; Emr, S.; Giordano, F.; Hajnóczky, G.; Kornmann, B.; Lackner, L.L.; Levine, T.P.; Pellegrini, L.; Reinisch, K.; et al. Coming Together to Define Membrane Contact Sites. Nat. Commun. 2019, 10, 1287. [Google Scholar] [CrossRef] [PubMed]
- Ghaffari, M.H.; Alaedin, M.T.; Sadri, H.; Hofs, I.; Koch, C.; Sauerwein, H. Longitudinal Changes in Fatty Acid Metabolism and in the Mitochondrial Protein Import System in Overconditioned and Normal Conditioned Cows: A Transcriptional Study Using Microfluidic Quantitative PCR. J. Dairy. Sci. 2021, 104, 10338–10354. [Google Scholar] [CrossRef] [PubMed]
- Iacobazzi, V.; Infantino, V.; Palmieri, F. Transcriptional Regulation of the Mitochondrial Citrate and Carnitine/Acylcarnitine Transporters: Two Genes Involved in Fatty Acid Biosynthesis and β-Oxidation. Biology 2013, 2, 284–303. [Google Scholar] [CrossRef] [PubMed]
- Barbot, M.; Jans, D.C.; Schulz, C.; Denkert, N.; Kroppen, B.; Hoppert, M.; Jakobs, S.; Meinecke, M. Mic10 Oligomerizes to Bend Mitochondrial Inner Membranes at Cristae Junctions. Cell Metab. 2015, 21, 756–763. [Google Scholar] [CrossRef]
- Bigay, J.; Antonny, B. Curvature, Lipid Packing, and Electrostatics of Membrane Organelles: Defining Cellular Territories in Determining Specificity. Dev. Cell 2012, 23, 886–895. [Google Scholar] [CrossRef]
- Scorrano, L. Multiple Functions of Mitochondria-Shaping Proteins. In Mitochondrial Biology: New Perspectives: Novartis Foundation Symposium 287; John Wiley & Sons, Ltd.: Chichester, UK, 2008; ISBN 9780470725207. [Google Scholar]
- von der Malsburg, A.; Sapp, G.M.; Zuccaro, K.E.; von Appen, A.; Moss, F.R.; Kalia, R.; Bennett, J.A.; Abriata, L.A.; Dal Peraro, M.; van der Laan, M.; et al. Structural Mechanism of Mitochondrial Membrane Remodelling by Human OPA1. Nature 2023, 620, 1101–1108. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, L.; Yang, C.; Pu, S.; Guo, Z.; Wu, Q.; Zhou, Z.; Zhao, H. Mitochondrial Membrane Remodeling. Front. Bioeng. Biotechnol. 2022, 9, 786806. [Google Scholar] [CrossRef] [PubMed]
- Pfanner, N.; Warscheid, B.; Wiedemann, N. Mitochondrial Proteins: From Biogenesis to Functional Networks. Nat. Rev. Mol. Cell Biol. 2019, 20, 267–284. [Google Scholar] [CrossRef]
- Indiveri, C.; Krämer, R.; Palmieri, F. Reconstitution of the Malate/Aspartate Shuttle from Mitochondria. J. Biol. Chem. 1987, 262, 15979–15983. [Google Scholar] [CrossRef]
- Halestrap, A.P.; McStay, G.P.; Clarke, S.J. The Permeability Transition Pore Complex: Another View. Biochimie 2002, 84, 153–166. [Google Scholar] [CrossRef] [PubMed]
- Bernardi, P.; Gerle, C.; Halestrap, A.P.; Jonas, E.A.; Karch, J.; Mnatsakanyan, N.; Pavlov, E.; Sheu, S.S.; Soukas, A.A. Identity, Structure, and Function of the Mitochondrial Permeability Transition Pore: Controversies, Consensus, Recent Advances, and Future Directions. Cell Death Differ. 2023, 30, 1869–1885. [Google Scholar] [CrossRef] [PubMed]
- Thangaratnarajah, C.; Ruprecht, J.J.; Kunji, E.R. Calcium-Induced Conformational Changes of the Regulatory Domain of Human Mitochondrial Aspartate/Glutamate Carriers. Nat. Commun. 2014, 5, 5491. [Google Scholar] [CrossRef] [PubMed]
- Chorev, D.S.; Baker, L.A.; Wu, D.; Beilsten-Edmands, V.; Rouse, S.L.; Zeev-Ben-Mordehai, T.; Jiko, C.; Samsudin, F.; Gerle, C.; Khalid, S.; et al. Protein Assemblies Ejected Directly from Native Membranes Yield Complexes for Mass Spectrometry. Science 2018, 362, 829–834. [Google Scholar] [CrossRef] [PubMed]
- Kunji, E.R.S.; Ruprecht, J.J. The Mitochondrial ADP/ATP Carrier Exists and Functions as a Monomer. Biochem. Soc. Trans. 2020, 48, 1419–1432. [Google Scholar] [CrossRef]
- Hirst, J.; Kunji, E.R.S.; Walker, J.E. Comment on “Protein Assemblies Ejected Directly from Native Membranes Yield Complexes for Mass Spectrometry”. Science 2019, 366, eaaw9830. [Google Scholar] [CrossRef]
- Chorev, D.S.; Robinson, C.V. Response to Comment on “Protein Assemblies Ejected Directly from Native Membranes Yield Complexes for Mass Spectrometry”. Science 2019, 366, eaax3102. [Google Scholar] [CrossRef]
- Todisco, S.; Di Noia, M.A.; Onofrio, A.; Parisi, G.; Punzi, G.; Redavid, G.; De Grassi, A.; Pierri, C.L. Identification of New Highly Selective Inhibitors of the Human ADP/ATP Carriers by Molecular Docking and in Vitro Transport Assays. Biochem. Pharmacol. 2016, 100, 112–132. [Google Scholar] [CrossRef]
- Favre, B.; Turowski, P.; Hemmings, B.A. Differential Inhibition and Posttranslational Modification of Protein Phosphatase 1 and 2A in MCF7 Cells Treated with Calyculin-A, Okadaic Acid, and Tautomycin. J. Biol. Chem. 1997, 272, 13856–13863. [Google Scholar] [CrossRef]
- Ruprecht, J.J.; Hellawell, A.M.; Harding, M.; Crichton, P.G.; McCoy, A.J.; Kunji, E.R.S. Structures of Yeast Mitochondrial ADP/ATP Carriers Support a Domain-Based Alternating-Access Transport Mechanism. Proc. Natl. Acad. Sci. USA 2014, 111, E426–E434. [Google Scholar] [CrossRef]
- Schroers, A.; Burkovski, A.; Wohlrab, H.; Krämer, R. The Phosphate Carrier from Yeast Mitochondria. Dimerization Is a Prerequisite for Function. J. Biol. Chem. 1998, 273, 14269–14276. [Google Scholar] [CrossRef] [PubMed]
- Kotaria, R.; Mayor, J.A.; Walters, D.E.; Kaplan, R.S. Oligomeric State of Wild-Type and Cysteine-Less Yeast Mitochondrial Citrate Transport Proteins. J. Bioenerg. Biomembr. 1999, 31, 543–549. [Google Scholar] [CrossRef]
- Bisaccia, F.; Zara, V.; Capobianco, L.; Iacobazzi, V.; Mazzeo, M.; Palmieri, F. The Formation of a Disulfide Cross-Link between the Two Subunits Demonstrates the Dimeric Structure of the Mitochondrial Oxoglutarate Carrier. Biochim. Biophys. Acta 1996, 1292, 281–288. [Google Scholar] [CrossRef]
- Ardalan, A.; Sowlati-Hashjin, S.; Uwumarenogie, S.O.; Fish, M.; Mitchell, J.; Karttunen, M.; Smith, M.D.; Jelokhani-Niaraki, M. Functional Oligomeric Forms of Uncoupling Protein 2: Strong Evidence for Asymmetry in Protein and Lipid Bilayer Systems. J. Phys. Chem. B 2021, 125, 169–183. [Google Scholar] [CrossRef] [PubMed]
- Wohlrab, H. Transport Proteins (Carriers) of Mitochondria. IUBMB Life 2009, 61, 40–46. [Google Scholar] [CrossRef]
- Wieckowski, M.R.M.R.; Giorgi, C.; Lebiedzinska, M.; Duszynski, J.; Pinton, P. Isolation of Mitochondria-Associated Membranes and Mitochondria from Animal Tissues and Cells. Nat. Protoc. 2009, 4, 1582–1590. [Google Scholar] [CrossRef] [PubMed]
- INDIVERI, C.; Tonazzi, A.; PALMIERI, F. Identification and Purification of the Ornithine/Citrulline Carrier from Rat Liver Mitochondira. Eur. J. Biochem. 1992, 207, 449–454. [Google Scholar] [CrossRef]
- Indiveri, C.; Tonazzi, A.; Giangregorio, N.; Palmieri, F. Probing the Active Site of the Reconstituted Carnitine Carrier from Rat Liver Mitochondria with Sulfhydryl Reagents: A Cysteine Residue Is Localized in or Near the Substrate Binding Site. Eur. J. Biochem. 1995, 228, 271–278. [Google Scholar] [CrossRef]
- Indiveri, C.; Tonazzi, A.; Palmieri, F. Identification and Purification of the Carnitine Carrier from Rat Liver Mitochondria. Biochim. Biophys. Acta 1990, 1020, 81–86. [Google Scholar] [CrossRef]
- Indiveri, C.; Iacobazzi, V.; Giangregorio, N.; Palmieri, F.; Giangregorio, N.; Tonazzi, A.; Indiveri, C.; Palmieri, F. The Mitochondrial Carnitine Carrier Protein: CDNA Cloning, Primary Structure and Comparison with Other Mitochondrial Transport Proteins Probing the Active Site of the Reconstituted Carnitine Carrier from Rat Liver Mitochondria with Sulfhydryl Reagents Con. Biochem. J. 1997, 321 Pt 3, 713–719. [Google Scholar] [CrossRef]
- Console, L.; Giangregorio, N.; Cellamare, S.; Bolognino, I.; Palasciano, M.; Indiveri, C.; Incampo, G.; Campana, S.; Tonazzi, A. Human Mitochondrial Carnitine Acylcarnitine Carrier: Molecular Target of Dietary Bioactive Polyphenols from Sweet Cherry (Prunus avium L.). Chem. Biol. Interact. 2019, 307, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Giangregorio, N.; Tonazzi, A.; Console, L.; Indiveri, C.; Palmieri, F. Site-Directed Mutagenesis of Charged Amino Acids of the Human Mitochondrial Carnitine/Acylcarnitine Carrier: Insight into the Molecular Mechanism of Transport. Biochim. Biophys. Acta 2010, 1797, 839–845. [Google Scholar] [CrossRef] [PubMed]
- Indiveri, C.; Giangregorio, N.; Iacobazzi, V.; Palmieri, F. Site-Directed Mutagenesis and Chemical Modification of the Six Native Cysteine Residues of the Rat Mitochondrial Carnitine Carrier: Implications for the Role of Cysteine-136. Biochemistry 2002, 41, 8649–8656. [Google Scholar] [CrossRef]
- Palmieri, F.; Indiveri, C.; Bisaccia, F.; Iacobazzi, V. Mitochondrial Metabolite Carrier Proteins: Purification, Reconstitution, and Transport Studies. Methods Enzym. 1995, 260, 349–369. [Google Scholar] [CrossRef]
- Huang, L.; Kou, X.; Zheng, W.; Xiao, X.; Li, C.; Liu, M.; Liu, Y.; Jiang, L. 05SAR-PAGE: Separation of Protein Dimerization and Modification Using a Gel with 0.05% Sarkosyl. Anal. Chim. Acta 2020, 1101, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Thibeault, J.; Patrick, J.; Martin, A.; Ortiz-Perez, B.; Hill, S.; Zhang, S.; Xia, K.; Colón, W. Sarkosyl: A Milder Detergent than SDS for Identifying Proteins with Moderately High Hyperstability Using Gel Electrophoresis. Anal. Biochem. 2019, 571, 21–24. [Google Scholar] [CrossRef]
- Tao, H.; Liu, W.; Simmons, B.N.; Harris, H.K.; Cox, T.C.; Massiah, M.A. Purifying Natively Folded Proteins from Inclusion Bodies Using Sarkosyl, Triton X-100, and CHAPS. Biotechniques 2010, 48, 61–64. [Google Scholar] [CrossRef]
- Huang, L.; Tong, Q.; Chen, L.; Zhao, W.; Zhang, Z.; Chai, Z.; Yang, J.; Li, C.; Liu, M.; Jiang, L. An Efficient Method for Detecting Membrane Protein Oligomerization and Complex Using 05SAR-PAGE. Electrophoresis 2024, 45, 1450–1454. [Google Scholar] [CrossRef] [PubMed]
- Tseng, T.S.; Wang, S.H.; Chang, T.W.; Wei, H.M.; Wang, Y.J.; Tsai, K.C.; Liao, Y.D.; Chen, C. Sarkosyl-Induced Helical Structure of an Antimicrobial Peptide GW-Q6 Plays an Essential Role in the Binding of Surface Receptor OprI in Pseudomonas Aeruginosa. PLoS ONE 2016, 11, e0164597. [Google Scholar] [CrossRef]
- Console, L.; Giangregorio, N.; Indiveri, C.; Tonazzi, A. Carnitine/Acylcarnitine Translocase and Carnitine Palmitoyltransferase 2 Form a Complex in the Inner Mitochondrial Membrane. Mol. Cell. Biochem. 2014, 394, 307–314. [Google Scholar] [CrossRef]
- Tonazzi, A.; Mantovani, C.; Colella, M.; Terenghi, G.; Indiveri, C. Localization of Mitochondrial Carnitine/Acylcarnitine Translocase in Sensory Neurons from Rat Dorsal Root Ganglia. Neurochem. Res. 2013, 38, 2535–2541. [Google Scholar] [CrossRef] [PubMed]
- Ruprecht, J.J.; King, M.S.; Zö, T.; Crichton, P.G.; Steyaert, J.; Kunji, E.R.S.; Aleksandrova, A.A.; Pardon, E. The Molecular Mechanism of Transport by the Mitochondrial ADP/ATP Carrier Correspondence The Molecular Mechanism of Transport by the Mitochondrial ADP/ATP Carrier. Cell 2019, 176, 435–447. [Google Scholar] [CrossRef] [PubMed]
- Pierri, C.L.; Bossis, F.; Punzi, G.; De Grassi, A.; Cetrone, M.; Parisi, G.; Tricarico, D. Molecular Modeling of Antibodies for the Treatment of TNFα-Related Immunological Diseases. Pharmacol. Res. Perspect. 2016, 4, e00197. [Google Scholar] [CrossRef] [PubMed]
- Tragni, V.; Preziusi, F.; Laera, L.; Onofrio, A.; Mercurio, I.; Todisco, S.; Volpicella, M.; De Grassi, A.; Pierri, C.L. Modeling SARS-CoV-2 Spike/ACE2 Protein–Protein Interactions for Predicting the Binding Affinity of New Spike Variants for ACE2, and Novel ACE2 Structurally Related Human Protein Targets, for COVID-19 Handling in the 3PM Context. EPMA J. 2022, 13, 149–175. [Google Scholar] [CrossRef] [PubMed]
- Trisolini, L.; Gambacorta, N.; Gorgoglione, R.; Montaruli, M.; Laera, L.; Colella, F.; Volpicella, M.; De Grassi, A.; Pierri, C.L. FAD/NADH Dependent Oxidoreductases: From Different Amino Acid Sequences to Similar Protein Shapes for Playing an Ancient Function. J. Clin. Med. 2019, 8, 2117. [Google Scholar] [CrossRef]
- Funai, K.; Summers, S.A.; Rutter, J. Reign in the Membrane: How Common Lipids Govern Mitochondrial Function. Curr. Opin. Cell Biol. 2020, 63, 162–173. [Google Scholar] [CrossRef]
- Huang, J.; Rauscher, S.; Nawrocki, G.; Ran, T.; Feig, M.; De Groot, B.L.; Grubmüller, H.; MacKerell, A.D. CHARMM36m: An Improved Force Field for Folded and Intrinsically Disordered Proteins. Nat. Methods 2016, 14, 71–73. [Google Scholar] [CrossRef]
- Bitencourt-Ferreira, G.; de Azevedo, W.F. Molecular Dynamics Simulations with NAMD2. In Methods in Molecular Biology; Humana: New York, NY, USA, 2019. [Google Scholar]
- Li, Y.; Liu, J.; Gumbart, J.C. Preparing Membrane Proteins for Simulation Using CHARMM-GUI. In Methods in Molecular Biology; Humana: New York, NY, USA, 2021. [Google Scholar]
- Bossis, F.; De Grassi, A.; Palese, L.L.L.; Pierri, C.L.L. Prediction of High- and Low-Affinity Quinol-Analogue-Binding Sites in the Aa3 and Bo3 Terminal Oxidases from Bacillus Subtilis and Escherichia coli. Biochem. J. 2014, 461, 305–314. [Google Scholar] [CrossRef]
- Schymkowitz, J.; Borg, J.; Stricher, F.; Nys, R.; Rousseau, F.; Serrano, L. The FoldX Web Server: An Online Force Field. Nucleic Acids Res. 2005, 33, W382–W388. [Google Scholar] [CrossRef]
- Van Durme, J.; Delgado, J.; Stricher, F.; Serrano, L.; Schymkowitz, J.; Rousseau, F. A Graphical Interface for the FoldX Forcefield. Bioinformatics 2011, 27, 1711–1712. [Google Scholar] [CrossRef]
- Giangregorio, N.; Console, L.; Tonazzi, A.; Palmieri, F.; Indiveri, C. Identification of Amino Acid Residues Underlying the Antiport Mechanism of the Mitochondrial Carnitine/Acylcarnitine Carrier by Site-Directed Mutagenesis and Chemical Labeling. Biochemistry 2014, 53, 6924–6933. [Google Scholar] [CrossRef] [PubMed]
- Wittig, I.; Beckhaus, T.; Wumaier, Z.; Karas, M.; Schägger, H. Mass Estimation of Native Proteins by Blue Native Electrophoresis: Principles and Practical Hints. Mol. Cell. Proteom. 2010, 9, 2149–2161. [Google Scholar] [CrossRef] [PubMed]
- Chadda, R.; Robertson, J.L. Measuring Membrane Protein Dimerization Equilibrium in Lipid Bilayers by Single-Molecule Fluorescence Microscopy. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 2016. [Google Scholar]
- Yernool, D.; Boudker, O.; Folta-Stogniew, E.; Gouaux, E. Trimeric Subunit Stoichiometry of the Glutamate Transporters from Bacillus Caldotenax and Bacillus Stearothermophilus. Biochemistry 2003, 42, 12981–12988. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Gerber, S.; Korkhov, V.M.; Mireku, S.; Bucher, M.; Locher, K.P.; Zenobi, R. On the Efficiency of NHS Ester Cross-Linkers for Stabilizing Integral Membrane Protein Complexes. J. Am. Soc. Mass. Spectrom. 2015, 26, 493–498. [Google Scholar] [CrossRef]
- Kalathiya, U.; Padariya, M.; Faktor, J.; Coyaud, E.; Alfaro, J.A.; Fahraeus, R.; Hupp, T.R.; Goodlett, D.R. Interfaces with Structure Dynamics of the Workhorses from Cells Revealed through Cross-linking Mass Spectrometry (Clms). Biomolecules 2021, 11, 382. [Google Scholar] [CrossRef]
- Pierri, C.L.; Palmieri, F.; De Grassi, A. Single-Nucleotide Evolution Quantifies the Importance of Each Site along the Structure of Mitochondrial Carriers. Cell Mol. Life Sci. 2014, 71, 349–364. [Google Scholar] [CrossRef]
- Sapozhnikov, Y.; Patel, J.S.; Ytreberg, F.M.; Miller, C.R. Statistical Modeling to Quantify the Uncertainty of FoldX-Predicted Protein Folding and Binding Stability. BMC Bioinform. 2023, 24, 426. [Google Scholar] [CrossRef]
- Cogliati, S.; Frezza, C.; Soriano, M.E.; Varanita, T.; Quintana-Cabrera, R.; Corrado, M.; Cipolat, S.; Costa, V.; Casarin, A.; Gomes, L.C.; et al. Mitochondrial Cristae Shape Determines Respiratory Chain Supercomplexes Assembly and Respiratory Efficiency. Cell 2013, 155, 160–171. [Google Scholar] [CrossRef] [PubMed]
- Chaban, Y.; Boekema, E.J.; Dudkina, N.V. Structures of Mitochondrial Oxidative Phosphorylation Supercomplexes and Mechanisms for Their Stabilisation. Biochim. Biophys. Acta—Bioenerg. 2014, 1837, 418–426. [Google Scholar] [CrossRef]
- Nakamura, S.; Matsui, A.; Akabane, S.; Tamura, Y.; Hatano, A.; Miyano, Y.; Omote, H.; Kajikawa, M.; Maenaka, K.; Moriyama, Y.; et al. The Mitochondrial Inner Membrane Protein LETM1 Modulates Cristae Organization through Its LETM Domain. Commun. Biol. 2020, 3, 99. [Google Scholar] [CrossRef]
- Restrepo, D.; Cronise, B.L.; Snyder, R.B.; Knauf, P.A. A Novel Method to Differentiate between Ping-Pong and Simultaneous Exchange Kinetics and Its Application to the Anion Exchanger of the HL60 Cell. J. Gen. Physiol. 1992, 100, 825–846. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Wang, C.; Zhou, Q.; An, J.; Hildebrandt, E.; Aleksandrov, L.A.; Kappes, J.C.; DeLucas, L.J.; Riordan, J.R.; Urbatsch, I.L.; et al. Membrane Protein Stability Can Be Compromised by Detergent Interactions with the Extramembranous Soluble Domains. Protein Sci. 2014, 23, 769–789. [Google Scholar] [CrossRef] [PubMed]
- Bamber, L.; Harding, M.; Butler, P.J.G.; Kunji, E.R.S.; On, F.; Bamber, L.; Harding, M.; Butler, P.J.G.; Kunji, E.R.S. Yeast Mitochondrial ADP/ATP Carriers Are Monomeric in Detergents. Proc. Natl. Acad. Sci. USA 2006, 103, 4–5. [Google Scholar] [CrossRef] [PubMed]
- Bamber, L.; Slotboom, D.-J.J.; Kunji, E.R.S. Yeast Mitochondrial ADP/ATP Carriers Are Monomeric in Detergents as Demonstrated by Differential Affinity Purification. J. Mol. Biol. 2007, 371, 388–395. [Google Scholar] [CrossRef]
- Kunji, E.R.S.; Harding, M.; Butler, P.J.G.; Akamine, P. Determination of the Molecular Mass and Dimensions of Membrane Proteins by Size Exclusion Chromatography. Methods 2008, 46, 62–72. [Google Scholar] [CrossRef]
- Amoedo, N.D.; Punzi, G.; Obre, E.; Lacombe, D.; De Grassi, A.; Pierri, C.L.; Rossignol, R. AGC1/2, the Mitochondrial Aspartate-Glutamate Carriers. Biochim. Biophys. Acta 2016, 1863, 2394–2412. [Google Scholar] [CrossRef]
- Scalise, M.; Pochini, L.; Panni, S.; Pingitore, P.; Hedfalk, K.; Indiveri, C. Transport Mechanism and Regulatory Properties of the Human Amino Acid Transporter ASCT2 (SLC1A5). Amino Acids 2014, 46, 2463–2475. [Google Scholar] [CrossRef] [PubMed]
- Heuberger, E.H.M.L.; Veenhoff, L.M.; Duurkens, R.H.; Friesen, R.H.E.; Poolman, B. Oligomeric State of Membrane Transport Proteins Analyzed with Blue Native Electrophoresis and Analytical Ultracentrifugation. J. Mol. Biol. 2002, 317, 591–600. [Google Scholar] [CrossRef]
- Lencina, A.M.; Gennis, R.B.; Schurig-Briccio, L.A. The Oligomeric State of the Caldivirga Maquilingensis Type III Sulfide:Quinone Oxidoreductase Is Required for Membrane Binding. Biochim. Biophys. Acta—Bioenerg. 2020, 1861, 148132. [Google Scholar] [CrossRef]
- Hwang, J.; Park, K.; Lee, G.Y.; Yoon, B.Y.; Kim, H.; Roh, S.H.; Lee, B.C.; Kim, K.; Lim, H.H. Transmembrane Topology and Oligomeric Nature of an Astrocytic Membrane Protein, MLC1. Open Biol. 2021, 11, 210103. [Google Scholar] [CrossRef]
- Pebay-Peyroula, E.; Dahout-Gonzalez, C.; Kahn, R.; Trézéguet, V.; Lauquin, G.J.-M.; Brandolin, G. Structure of Mitochondrial ADP/ATP Carrier in Complex with Carboxyatractyloside. Nature 2003, 426, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Duncan, A.L.; Ruprecht, J.J.; Kunji, E.R.S.; Robinson, A.J. Cardiolipin Dynamics and Binding to Conserved Residues in the Mitochondrial ADP/ATP Carrier. Biochim. Biophys. Acta—Biomembr. 2018, 1860, 1035–1045. [Google Scholar] [CrossRef] [PubMed]
- Wilson, B.A.; Ramanathan, A.; Lopez, C.F. Cardiolipin-Dependent Properties of Model Mitochondrial Membranes from Molecular Simulations. Biophys. J. 2019, 117, 429–444. [Google Scholar] [CrossRef] [PubMed]
- Klingenberg, M. Cardiolipin and Mitochondrial Carriers. Biochim. Biophys. Acta 2009, 1788, 2048–2058. [Google Scholar] [CrossRef]
- Giangregorio, N.; Tonazzi, A.; Indiveri, C.; Palmieri, F. Conformation-Dependent Accessibility of Cys-136 and Cys-155 of the Mitochondrial Rat Carnitine/Acylcarnitine Carrier to Membrane-Impermeable SH Reagents. Biochim. Biophys. Acta 2007, 1767, 1331–1339. [Google Scholar] [CrossRef] [PubMed]
- Tonazzi, A.; Giangregorio, N.; Indiveri, C.; Palmieri, F. Identification by Site-Directed Mutagenesis and Chemical Modification of Three Vicinal Cysteine Residues in Rat Mitochondrial Carnitine/Acylcarnitine Transporter. J. Biol. Chem. 2005, 280, 19607–19612. [Google Scholar] [CrossRef]
- Indiveri, C.; Iacobazzi, V.; Giangregorio, N.; Palmieri, F. Bacterial Overexpression, Purification, and Reconstitution of the Carnitine/Acylcarnitine Carrier from Rat Liver Mitochondria. Biochem. Biophys. Res. Commun. 1998, 249, 589–594. [Google Scholar] [CrossRef]
- Nury, H.; Dahout-Gonzalez, C.; Trézéguet, V.; Lauquin, G.; Brandolin, G.; Pebay-Peyroula, E. Structural Basis for Lipid-Mediated Interactions between Mitochondrial ADP/ATP Carrier Monomers. FEBS Lett. 2005, 579, 6031–6036. [Google Scholar] [CrossRef]
- Lee, Y.; Willers, C.; Kunji, E.R.S.; Crichton, P.G. Uncoupling Protein 1 Binds One Nucleotide per Monomer and Is Stabilized by Tightly Bound Cardiolipin. Proc. Natl. Acad. Sci. USA 2015, 112, 6973–6978. [Google Scholar] [CrossRef]
- Chang, Y.N.; Jaumann, E.A.; Reichel, K.; Hartmann, J.; Oliver, D.; Hummer, G.; Joseph, B.; Geertsma, E.R. Structural Basis for Functional Interactions in Dimers of SLC26 Transporters. Nat. Commun. 2019, 10, 2032. [Google Scholar] [CrossRef]
- Pyle, E.; Kalli, A.C.; Amillis, S.; Hall, Z.; Lau, A.M.; Hanyaloglu, A.C.; Diallinas, G.; Byrne, B.; Politis, A. Structural Lipids Enable the Formation of Functional Oligomers of the Eukaryotic Purine Symporter UapA. Cell Chem. Biol. 2018, 25, 840–848. [Google Scholar] [CrossRef] [PubMed]
- Gupta, K.; Donlan, J.A.C.; Hopper, J.T.S.; Uzdavinys, P.; Landreh, M.; Struwe, W.B.; Drew, D.; Baldwin, A.J.; Stansfeld, P.J.; Robinson, C.V. The Role of Interfacial Lipids in Stabilizing Membrane Protein Oligomers. Nature 2017, 541, 421–424. [Google Scholar] [CrossRef] [PubMed]
- Crichton, P.G.; Lee, Y.; Ruprecht, J.J.; Cerson, E.; Thangaratnarajah, C.; King, M.S.; Kunji, E.R. Trends in Thermostability Provide Information on the Nature of Substrate, Inhibitor, and Lipid Interactions with Mitochondrial Carriers. J. Biol. Chem. 2015, 290, 8206–8217. [Google Scholar] [CrossRef] [PubMed]
- Chadda, R.; Bernhardt, N.; Kelley, E.G.; Teixeira, S.C.M.; Griffith, K.; Gil-Ley, A.; Öztürk, T.N.; Hughes, L.E.; Forsythe, A.; Krishnamani, V.; et al. Membrane Transporter Dimerization Driven by Differential Lipid Solvation Energetics of Dissociated and Associated States. eLife 2021, 10, e63288. [Google Scholar] [CrossRef]
- Tonazzi, A.; Giangregorio, N.; Console, L.; Indiveri, C. Mitochondrial Carnitine/Acylcarnitine Translocase: Insights in Structure/Function Relationships. Basis for Drug Therapy and Side Effects Prediction. Mini-Rev. Med. Chem. 2015, 15, 396–405. [Google Scholar] [CrossRef]
- Yi, Q.; Yao, S.; Ma, B.; Cang, X. The Effects of Cardiolipin on the Structural Dynamics of the Mitochondrial ADP/ATP Carrier in Its Cytosol-Open State. J. Lipid Res. 2022, 63, 100227. [Google Scholar] [CrossRef]
- Veenhoff, L.M.; Heuberger, E.H.M.L.; Poolman, B. Quaternary Structure and Function of Transport Proteins. Trends Biochem. Sci. 2002, 27, 242–249. [Google Scholar] [CrossRef]
- Vinothkumar, K.R.; Raunser, S.; Jung, H.; Kühlbrandt, W. Oligomeric Structure of the Carnitine Transporter CaiT from Escherichia coli. J. Biol. Chem. 2006, 281, 4795–4801. [Google Scholar] [CrossRef]
- Yu, X.; Yang, G.; Yan, C.; Baylon, J.L.; Jiang, J.; Fan, H.; Lu, G.; Hasegawa, K.; Okumura, H.; Wang, T.; et al. Dimeric Structure of the Uracil:Proton Symporter UraA Provides Mechanistic Insights into the SLC4/23/26 Transporters. Cell Res. 2017, 27, 1020–1033. [Google Scholar] [CrossRef]
- Tonazzi, A.; Console, L.; Giangregorio, N.; Indiveri, C.; Palmieri, F. Identification by Site-Directed Mutagenesis of a Hydrophobic Binding Site of the Mitochondrial Carnitine/Acylcarnitine Carrier Involved in the Interaction with Acyl Groups. Biochim. Biophys. Acta—Bioenerg. 2012, 1817, 697–704. [Google Scholar] [CrossRef]
- Tonazzi, A.; Giangregorio, N.; Indiveri, C.; Palmieri, F. Site-Directed Mutagenesis of the His Residues of the Rat Mitochondrial Carnitine/Acylcarnitine Carrier: Implications for the Role of His-29 in the Transport Pathway. Biochim. Biophys. Acta 2009, 1787, 1009–1015. [Google Scholar] [CrossRef] [PubMed]
- Ota, N.; Brett, T.J.; Murphy, T.L.; Fremont, D.H.; Murphy, K.M. N-Domain-Dependent Nonphosphorylated STAT4 Dimers Required for Cytokine-Driven Activation. Nat. Immunol. 2004, 5, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Hellberg, K.; Grimsrud, P.A.; Kruse, A.C.; Banaszak, L.J.; Ohlendorf, D.H.; Bernlohr, D.A. X-ray Crystallographic Analysis of Adipocyte Fatty Acid Binding Protein (AP2) Modified with 4-Hydroxy-2-Nonenal. Protein Sci. 2010, 19, 1480–1489. [Google Scholar] [CrossRef]
- Belviso, B.D.; Galliani, A.; Lasorsa, A.; Mirabelli, V.; Caliandro, R.; Arnesano, F.; Natile, G. Oxaliplatin Binding to Human Copper Chaperone Atox1 and Protein Dimerization. Inorg. Chem. 2016, 55, 6563–6573. [Google Scholar] [CrossRef]
- Pietropaolo, A.; Pierri, C.L.; Palmieri, F.; Klingenberg, M. The Switching Mechanism of the Mitochondrial ADP/ATP Carrier Explored by Free-Energy Landscapes. Biochim. Biophys. Acta 2016, 1857, 772–781. [Google Scholar] [CrossRef] [PubMed]
- Dourado, D.F.A.R.; Flores, S.C. Modeling and Fitting Protein-Protein Complexes to Predict Change of Binding Energy. Sci. Rep. 2016, 6, 25406. [Google Scholar] [CrossRef]
- Nooren, I.M.A.; Thornton, J.M. Diversity of Protein-Protein Interactions. EMBO J. 2003, 22, 3486–3492. [Google Scholar] [CrossRef]
- Wohlrab, H. Homodimeric Intrinsic Membrane Proteins. Identification and Modulation of Interactions between Mitochondrial Transporter (Carrier) Subunits. Biochem. Biophys. Res. Commun. 2010, 393, 746–750. [Google Scholar] [CrossRef][Green Version]
- Postis, V.; De Marcos Lousa, C.; Arnou, B.; Lauquin, G.J.-M.M.; Trézéguet, V. Subunits of the Yeast Mitochondrial ADP/ATP Carrier: Cooperation within the Dimer. Biochemistry 2005, 44, 14732–14740. [Google Scholar] [CrossRef]
- Huang, S.G.; Odoy, S.; Klingenberg, M. Chimers of Two Fused ADP/ATP Carrier Monomers Indicate a Single Channel for ADP/ATP Transport. Arch. Biochem. Biophys. 2001, 394, 67–75. [Google Scholar] [CrossRef]
- Trézéguet, V.; Le Saux, A.; David, C.; Gourdet, C.; Fiore, C.; Dianoux, A.; Brandolin, G.; Lauquin, G.J.; Nadtochiy, S.M.; Tompkins, A.J.; et al. A Covalent Tandem Dimer of the Mitochondrial ADP/ATP Carrier Is Functional in Vivo Different Mechanisms of Mitochondrial Proton Leak in Ischaemia/Reperfusion Injury and Preconditioning: Implications for Pathology and Cardioprotection. Biochim. Biophys. Acta 2000, 1457, 81–93. [Google Scholar] [CrossRef] [PubMed]
- Ressl, S.; Terwisscha Van Scheltinga, A.C.; Vonrhein, C.; Ott, V.; Ziegler, C. Molecular Basis of Transport and Regulation in the Na+/Betaine Symporter BetP. Nature 2009, 458, 47–52. [Google Scholar] [CrossRef] [PubMed]
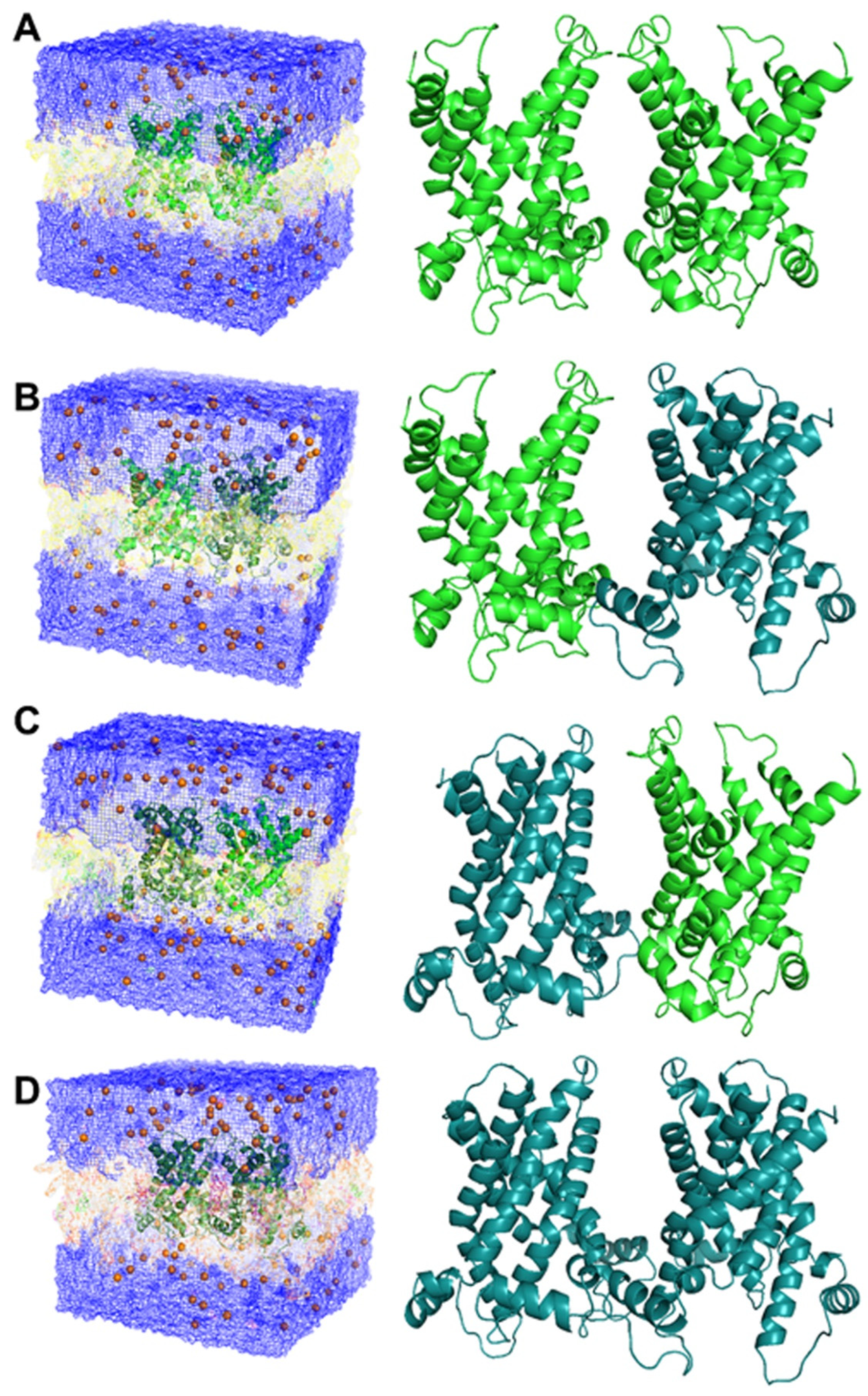
| Dimeric CAC in c-conf Post Minimisation | Dimeric CAC in m-conf Post Minimisation | Dimeric CAC c.conf./m.conf.dx | Dimeric CAC m.conf./c.conf.dx | |
|---|---|---|---|---|
| Group A | X (CAC c.conf) | J (CAC m.conf) | X (CAC c.conf) | J (CAC m.conf) |
| Group B | Y (CAC c.conf) | K (CAC m.conf) | K (CAC m.conf) | Y (CAC c.conf) |
| IntraclashesGroup1 | 26.8128 | 20.1334 | 18.5575 | 20.7697 |
| IntraclashesGroup2 | 22.4227 | 26.3712 | 22.0363 | 31.8141 |
| Interaction Energy | −5.65407 | −6.70847 | −3.5527 | −8.81348 |
| Backbone Hbond | −2.06262 | −1.21455 | −1.00236 | −3.08866 |
| Sidechain Hbond | −9.33346 | −5.00837 | −1.84149 | −5.87783 |
| Van der Waals | −6.28856 | −8.61397 | −4.49641 | −7.60667 |
| Electrostatics | 0.611469 | −0.828307 | −0.358759 | 0.0574812 |
| Solvation Polar | 8.2012 | 9.68964 | 5.52538 | 9.71022 |
| Solvation Hydrophobic | −6.85538 | −12.8032 | −6.35007 | −9.63725 |
| Van der Waals clashes | 0.315786 | 0.139778 | 0.0171242 | 0.28503 |
| Entropy sidechain | 8.61905 | 9.46367 | 3.50595 | 6.42998 |
| Entropy mainchain | 1.71522 | 2.48441 | 1.27416 | 1.33332 |
| Cis_bond | 4.44 × 10−16 | 1.48 × 10−1 | 0.211593 | 1.11 × 10−16 |
| Torsional clash | 0.00915503 | 0.0934752 | 0.00694907 | 0.139602 |
| Backbone clash | 0.666715 | 1.79593 | 1.1584 | 3.18061 |
| Helix dipole | −0.613325 | 0.335984 | −0.0318095 | −0.42639 |
| Electrostatic kon | 0.0273986 | −0.594598 | −0.0129609 | −0.132315 |
| Entropy Complex | 2.384 | 2.384 | 2.384 | 2.384 |
| Number of Residues | 580 | 580 | 580 | 580 |
| Interface Residues | 24 | 37 | 29 | 29 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giangregorio, N.; Tonazzi, A.; Pierri, C.L.; Indiveri, C. Insights into Transient Dimerization of Carnitine/Acylcarnitine Carrier (SLC25A20) from Sarkosyl/PAGE, Cross-Linking Reagents, and Comparative Modelling Analysis. Biomolecules 2024, 14, 1158. https://doi.org/10.3390/biom14091158
Giangregorio N, Tonazzi A, Pierri CL, Indiveri C. Insights into Transient Dimerization of Carnitine/Acylcarnitine Carrier (SLC25A20) from Sarkosyl/PAGE, Cross-Linking Reagents, and Comparative Modelling Analysis. Biomolecules. 2024; 14(9):1158. https://doi.org/10.3390/biom14091158
Chicago/Turabian StyleGiangregorio, Nicola, Annamaria Tonazzi, Ciro Leonardo Pierri, and Cesare Indiveri. 2024. "Insights into Transient Dimerization of Carnitine/Acylcarnitine Carrier (SLC25A20) from Sarkosyl/PAGE, Cross-Linking Reagents, and Comparative Modelling Analysis" Biomolecules 14, no. 9: 1158. https://doi.org/10.3390/biom14091158
APA StyleGiangregorio, N., Tonazzi, A., Pierri, C. L., & Indiveri, C. (2024). Insights into Transient Dimerization of Carnitine/Acylcarnitine Carrier (SLC25A20) from Sarkosyl/PAGE, Cross-Linking Reagents, and Comparative Modelling Analysis. Biomolecules, 14(9), 1158. https://doi.org/10.3390/biom14091158








