Preclinical Evaluation of a Novel Series of Polyfluorinated Thalidomide Analogs in Drug-Resistant Multiple Myeloma
Abstract
1. Introduction
2. Materials and Methods
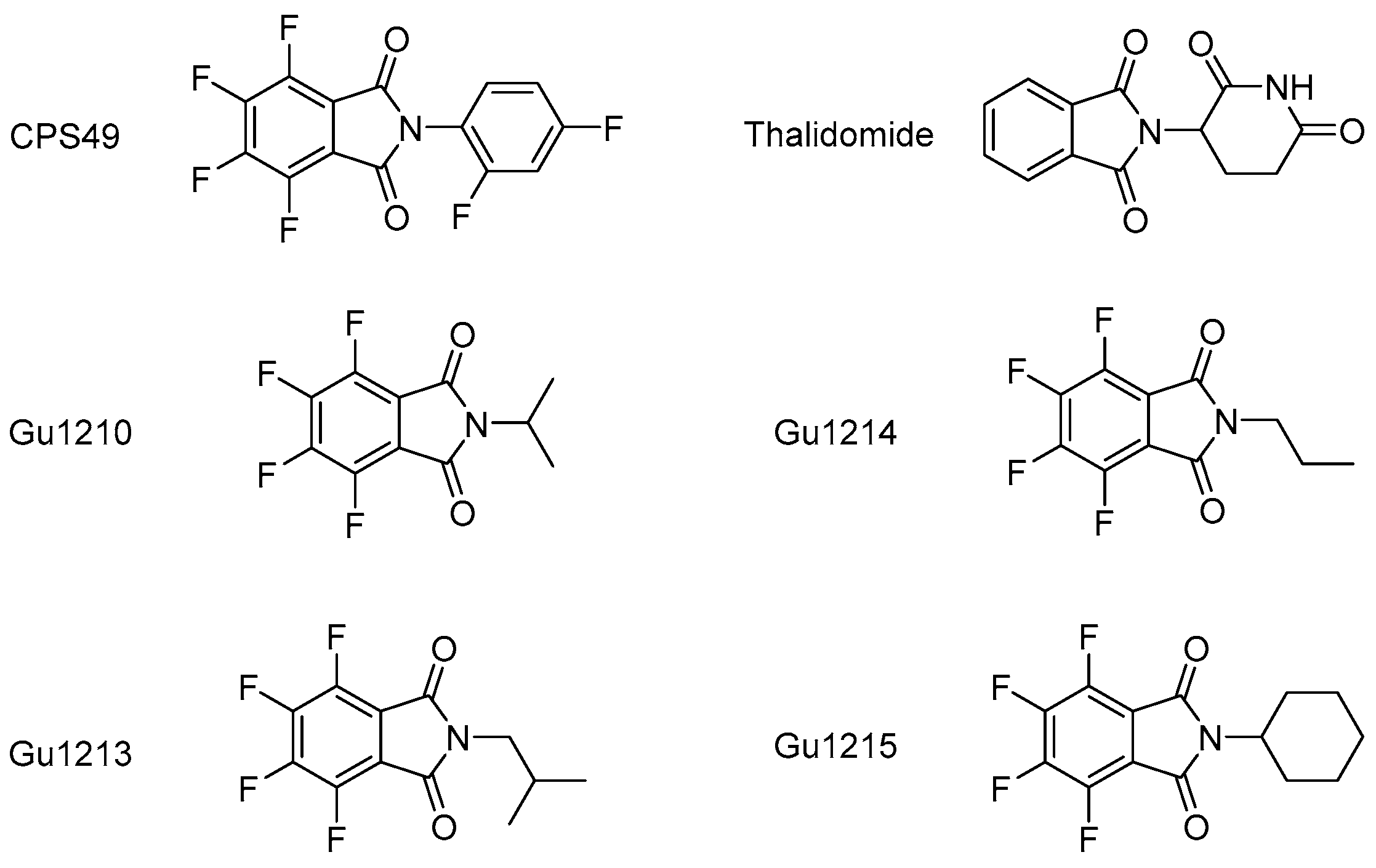
2.1. Thalidomide Analogs
2.2. Cell Lines and Reagents
2.3. Cell Proliferation Assay
2.4. Three-Dimensional Tumor Spheroid Assay
2.5. THP-1 Inflammatory Assay
2.6. Endothelial Cell Tube Formation Assay (Lattice Assay)
2.7. Human Saphenous Vein (HSV) Angiogenesis Assay
2.8. Western Blot Analysis
2.9. Statistics
3. Results
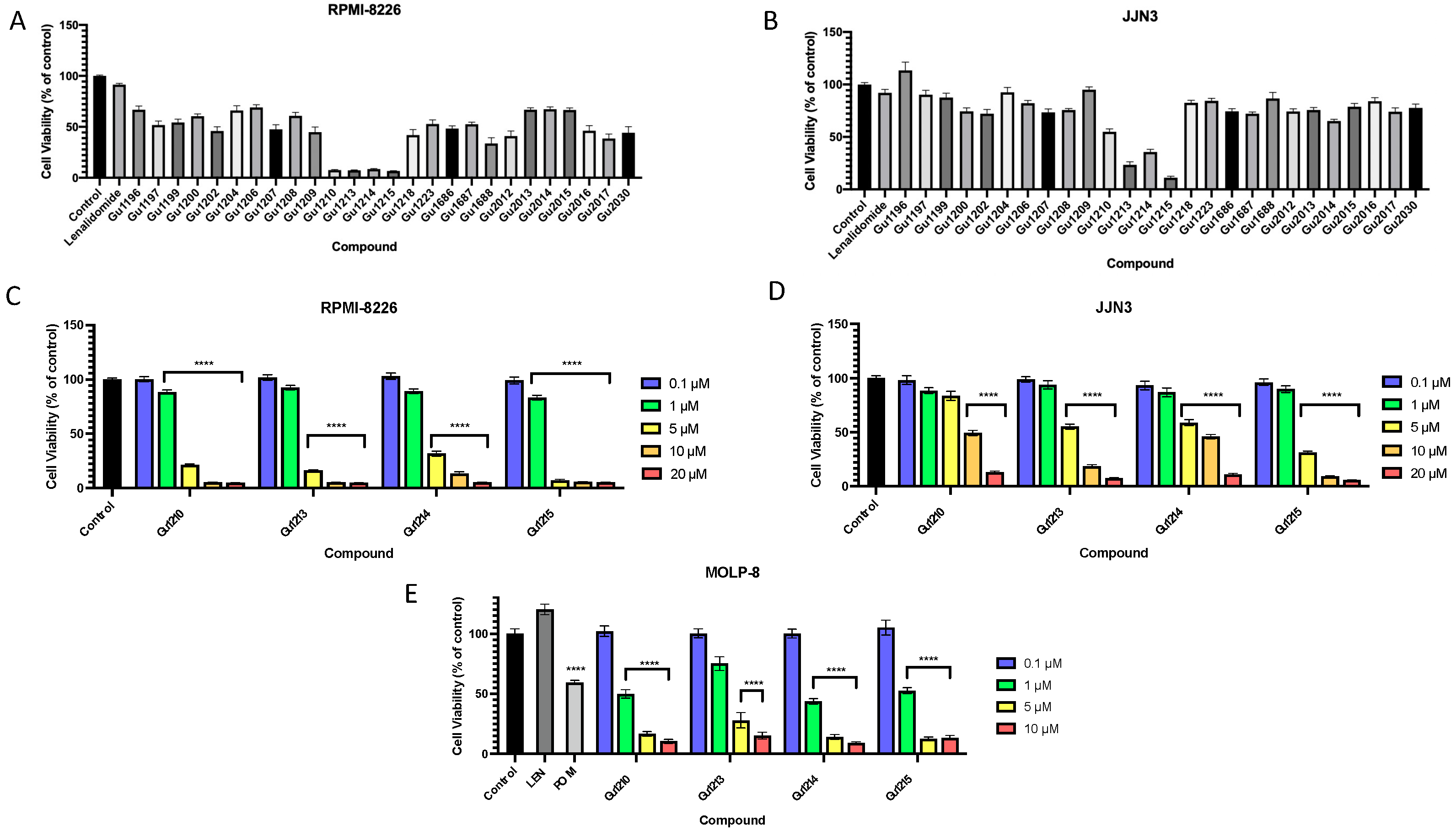
3.1. Effects of Thalidomide Analogs on In Vitro Proliferation of Intrinsically IMiD-Resistant MM Cells
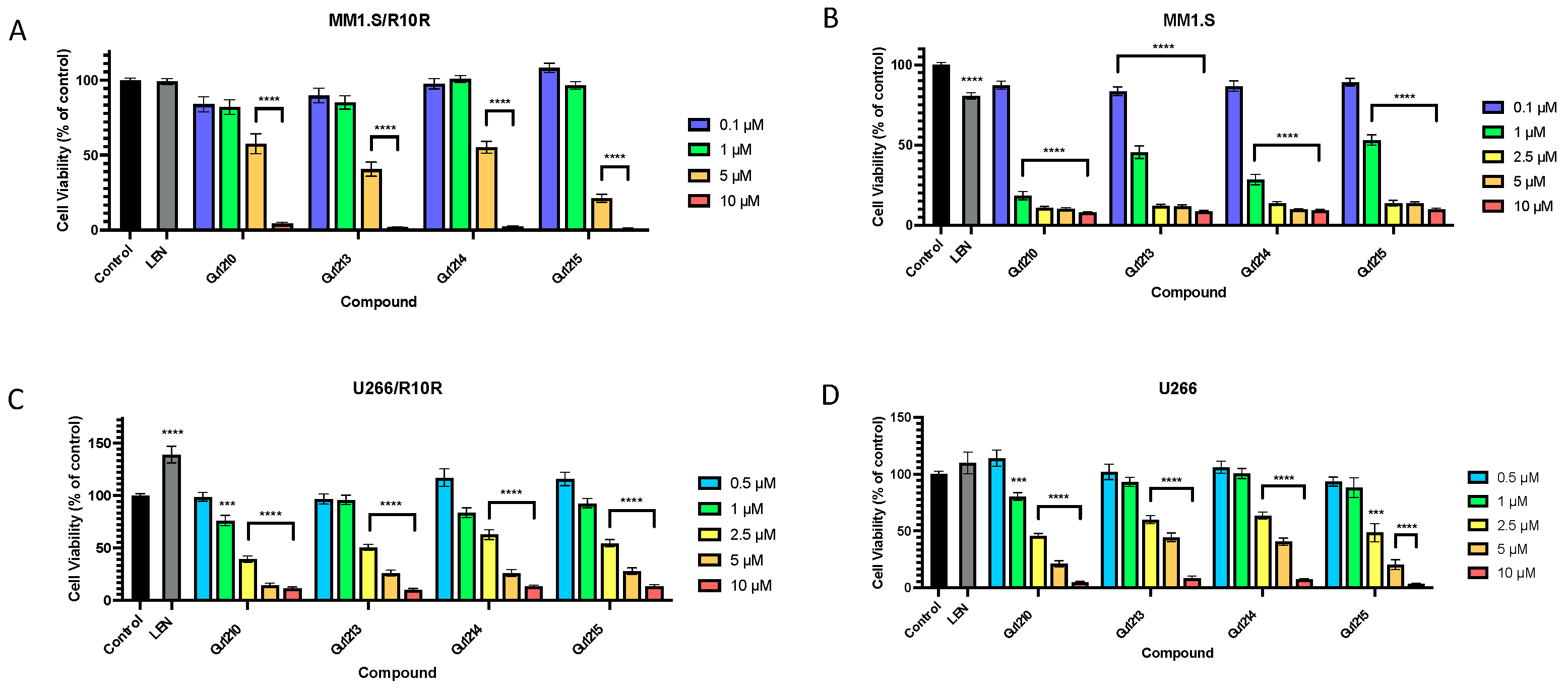
3.2. Effects of Thalidomide Analogs on In Vitro Proliferation of MM Cells with Acquired IMiD Resistance
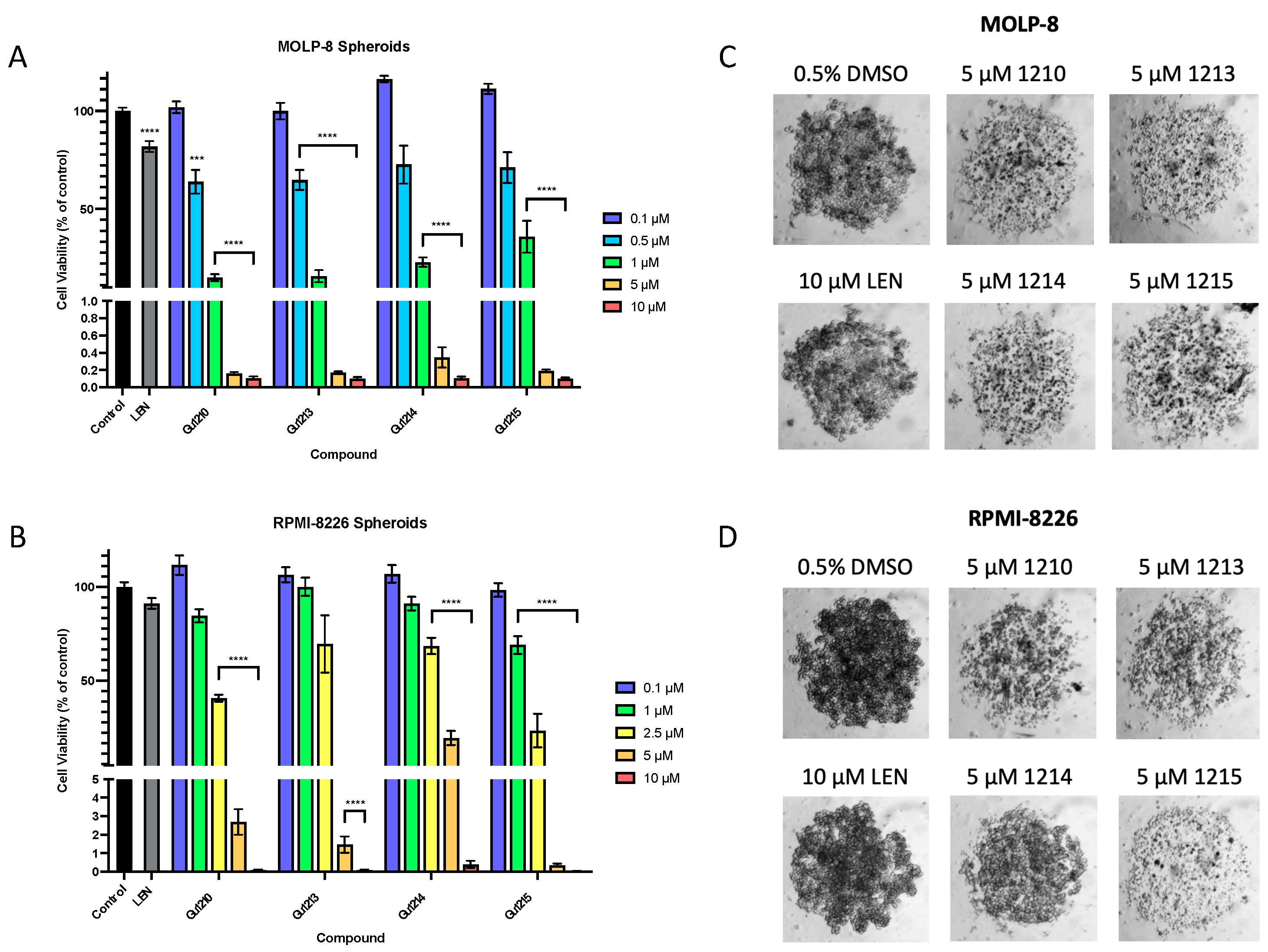
3.3. Effects of Thalidomide Analogs on 3D Myeloma Spheroid Growth
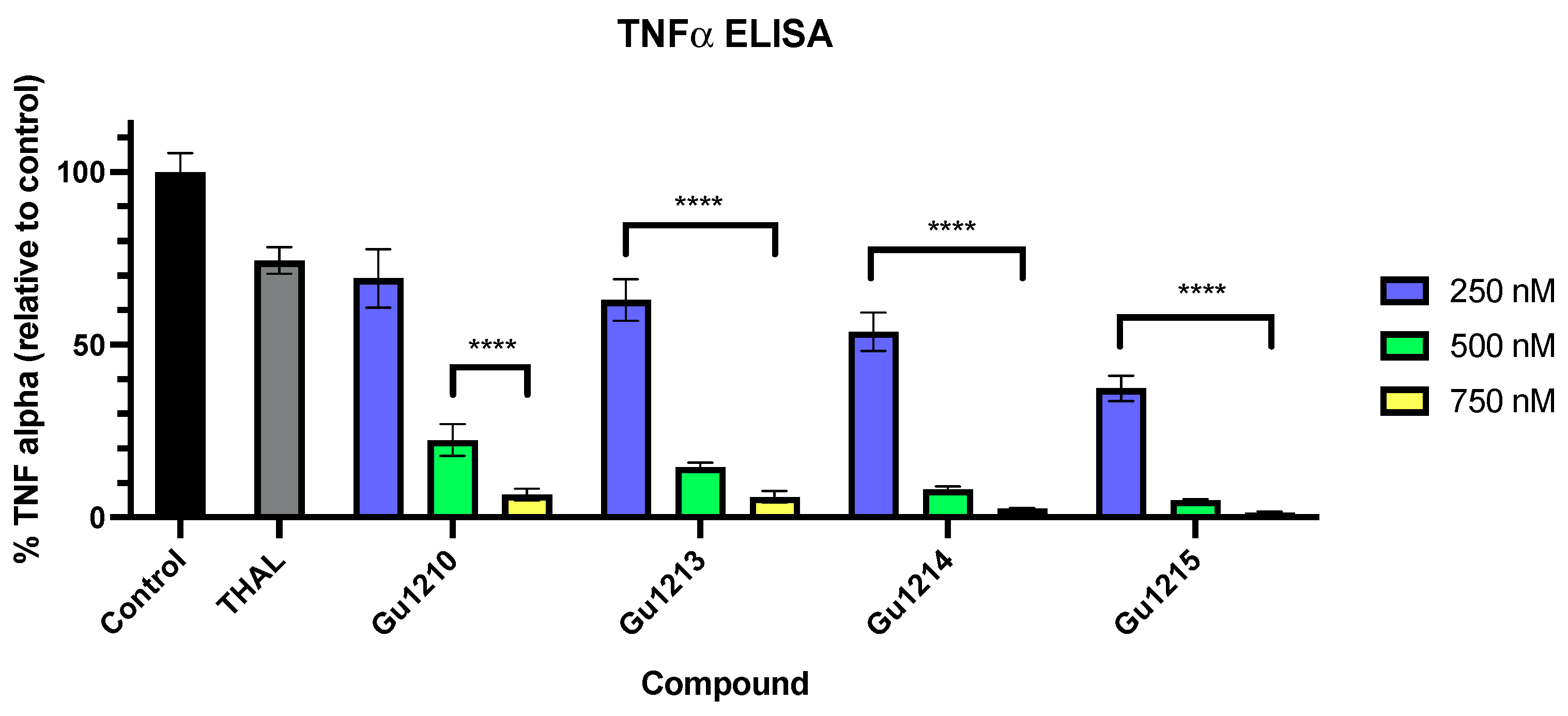
3.4. In Vitro Inflammatory Response to Thalidomide Analogs
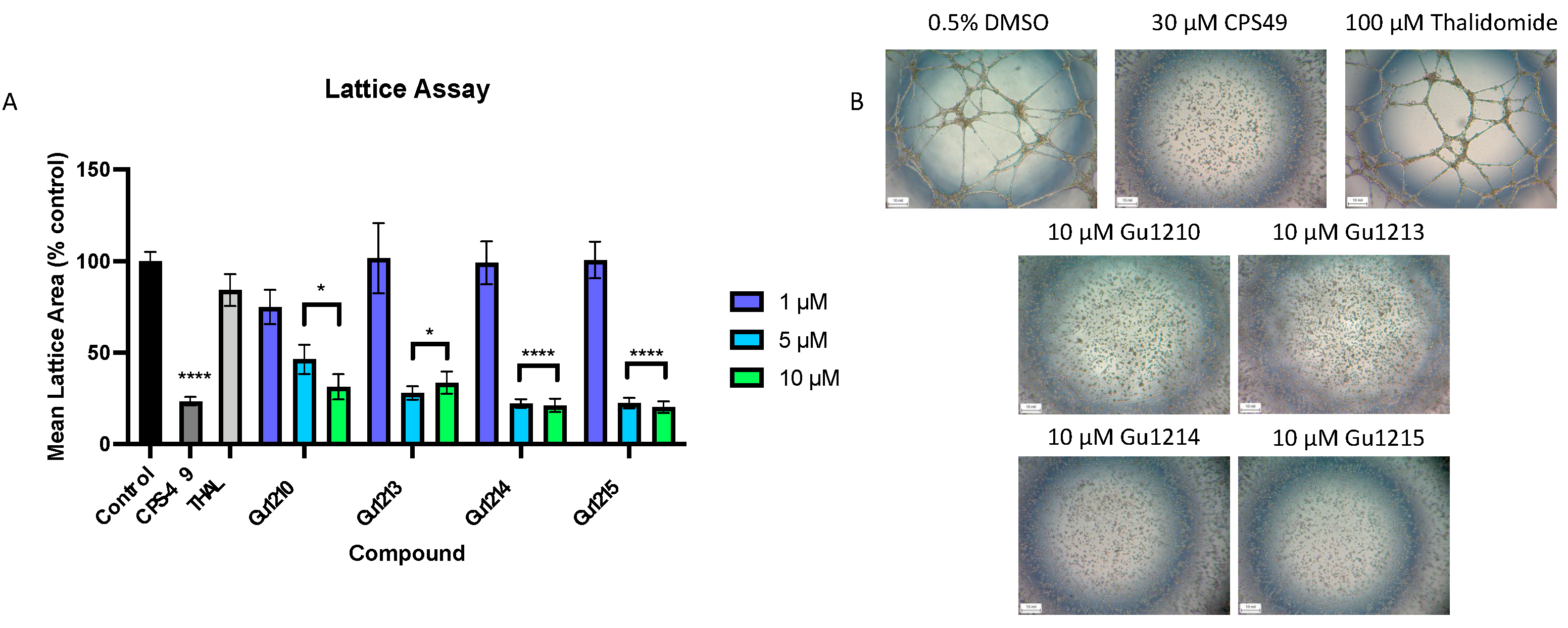
3.5. Effects of Thalidomide Analogs on Endothelial Tube Formation
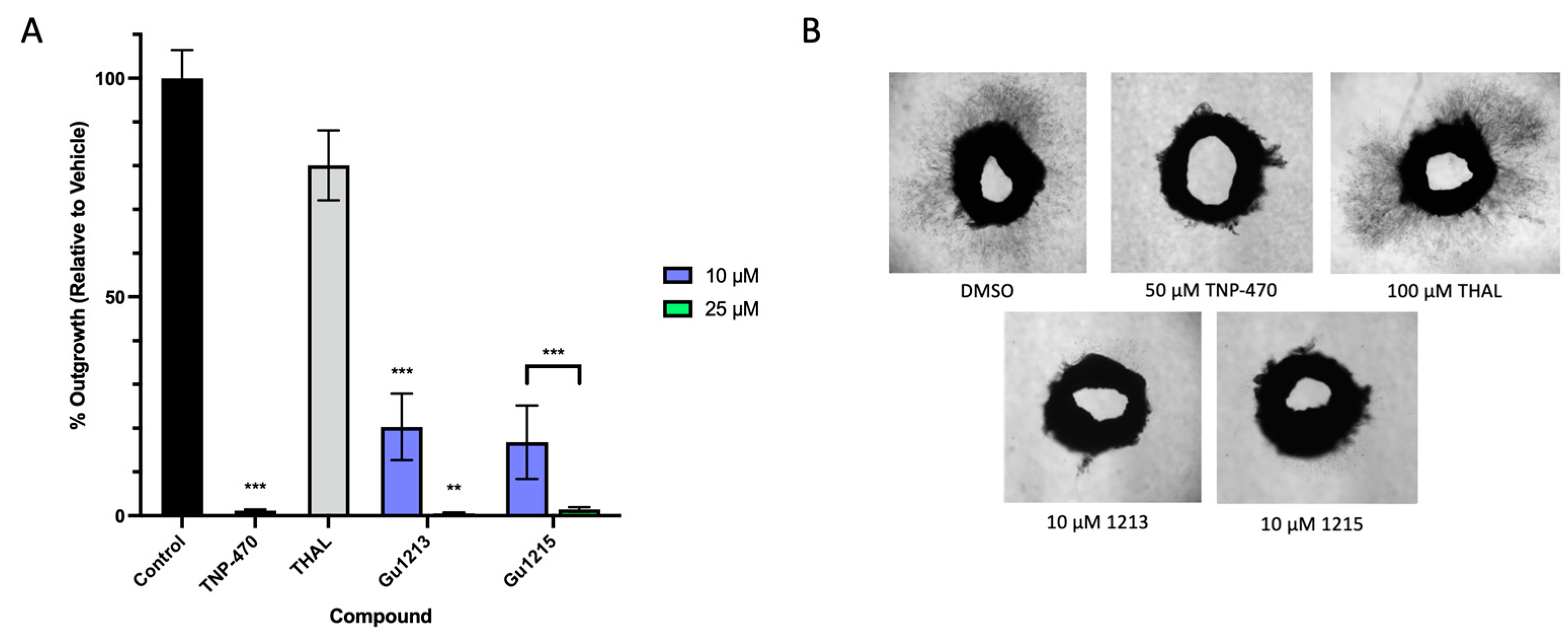
3.6. Anti-Angiogenic Effects of Thalidomide Analogs in the Ex Vivo Human Saphenous Vein Model
3.7. Effects of Treatment with Lead Thalidomide Analog on the Expression of Proteins Downstream of CRBN
4. Discussion
5. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer Statistics, 2024. CA Cancer J. Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef]
- SEER Cancer Stat Facts: Myeloma. Available online: https://seer.cancer.gov/statfacts/html/mulmy.html (accessed on 1 February 2024).
- van de Donk, N.; Pawlyn, C.; Yong, K.L. Multiple myeloma. Lancet 2021, 397, 410–427. [Google Scholar] [CrossRef]
- Davis, L.N.; Sherbenou, D.W. Emerging Therapeutic Strategies to Overcome Drug Resistance in Multiple Myeloma. Cancers 2021, 13, 1686. [Google Scholar] [CrossRef]
- Pinto, V.; Bergantim, R.; Caires, H.R.; Seca, H.; Guimaraes, J.E.; Vasconcelos, M.H. Multiple Myeloma: Available Therapies and Causes of Drug Resistance. Cancers 2020, 12, 407. [Google Scholar] [CrossRef]
- Kumar, S.K.; Lee, J.H.; Lahuerta, J.J.; Morgan, G.; Richardson, P.G.; Crowley, J.; Haessler, J.; Feather, J.; Hoering, A.; Moreau, P.; et al. Risk of progression and survival in multiple myeloma relapsing after therapy with IMiDs and bortezomib: A multicenter international myeloma working group study. Leukemia 2012, 26, 149–157. [Google Scholar] [CrossRef]
- Giuliani, N.; Storti, P.; Bolzoni, M.; Palma, B.D.; Bonomini, S. Angiogenesis and multiple myeloma. Cancer Microenviron. 2011, 4, 325–337. [Google Scholar] [CrossRef]
- Ribatti, D.; Vacca, A. New Insights in Anti-Angiogenesis in Multiple Myeloma. Int. J. Mol. Sci. 2018, 19, 2031. [Google Scholar] [CrossRef]
- Lamanuzzi, A.; Saltarella, I.; Frassanito, M.A.; Ribatti, D.; Melaccio, A.; Desantis, V.; Solimando, A.G.; Ria, R.; Vacca, A. Thrombopoietin Promotes Angiogenesis and Disease Progression in Patients with Multiple Myeloma. Am. J. Pathol. 2021, 191, 748–758. [Google Scholar] [CrossRef]
- Musolino, C.; Allegra, A.; Innao, V.; Allegra, A.G.; Pioggia, G.; Gangemi, S. Inflammatory and Anti-Inflammatory Equilibrium, Proliferative and Antiproliferative Balance: The Role of Cytokines in Multiple Myeloma. Mediat. Inflamm. 2017, 2017, 1852517. [Google Scholar] [CrossRef]
- Corral, L.G.; Haslett, P.A.; Muller, G.W.; Chen, R.; Wong, L.M.; Ocampo, C.J.; Patterson, R.T.; Stirling, D.I.; Kaplan, G. Differential cytokine modulation and T cell activation by two distinct classes of thalidomide analogues that are potent inhibitors of TNF-alpha. J. Immunol. 1999, 163, 380–386. [Google Scholar] [CrossRef]
- D’Amato, R.J.; Loughnan, M.S.; Flynn, E.; Folkman, J. Thalidomide is an inhibitor of angiogenesis. Proc. Natl. Acad. Sci. USA 1994, 91, 4082–4085. [Google Scholar] [CrossRef]
- De Luisi, A.; Ferrucci, A.; Coluccia, A.M.; Ria, R.; Moschetta, M.; de Luca, E.; Pieroni, L.; Maffia, M.; Urbani, A.; Di Pietro, G.; et al. Lenalidomide restrains motility and overangiogenic potential of bone marrow endothelial cells in patients with active multiple myeloma. Clin. Cancer Res. 2011, 17, 1935–1946. [Google Scholar] [CrossRef]
- Lacy, M.Q.; Hayman, S.R.; Gertz, M.A.; Dispenzieri, A.; Buadi, F.; Kumar, S.; Greipp, P.R.; Lust, J.A.; Russell, S.J.; Dingli, D.; et al. Pomalidomide (CC4047) plus low-dose dexamethasone as therapy for relapsed multiple myeloma. J. Clin. Oncol. 2009, 27, 5008–5014. [Google Scholar] [CrossRef]
- Rajkumar, S.V.; Hayman, S.R.; Lacy, M.Q.; Dispenzieri, A.; Geyer, S.M.; Kabat, B.; Zeldenrust, S.R.; Kumar, S.; Greipp, P.R.; Fonseca, R.; et al. Combination therapy with lenalidomide plus dexamethasone (Rev/Dex) for newly diagnosed myeloma. Blood 2005, 106, 4050–4053. [Google Scholar] [CrossRef]
- Ito, T.; Ando, H.; Suzuki, T.; Ogura, T.; Hotta, K.; Imamura, Y.; Yamaguchi, Y.; Handa, H. Identification of a primary target of thalidomide teratogenicity. Science 2010, 327, 1345–1350. [Google Scholar] [CrossRef]
- Krönke, J.; Udeshi, N.D.; Narla, A.; Grauman, P.; Hurst, S.N.; McConkey, M.; Svinkina, T.; Heckl, D.; Comer, E.; Li, X.; et al. Lenalidomide causes selective degradation of IKZF1 and IKZF3 in multiple myeloma cells. Science 2014, 343, 301–305. [Google Scholar] [CrossRef]
- Lu, G.; Middleton, R.E.; Sun, H.; Naniong, M.; Ott, C.J.; Mitsiades, C.S.; Wong, K.K.; Bradner, J.E.; Kaelin, W.G., Jr. The myeloma drug lenalidomide promotes the cereblon-dependent destruction of Ikaros proteins. Science 2014, 343, 305–309. [Google Scholar] [CrossRef]
- Zhu, Y.X.; Braggio, E.; Shi, C.X.; Bruins, L.A.; Schmidt, J.E.; Van Wier, S.; Chang, X.B.; Bjorklund, C.C.; Fonseca, R.; Bergsagel, P.L.; et al. Cereblon expression is required for the antimyeloma activity of lenalidomide and pomalidomide. Blood 2011, 118, 4771–4779. [Google Scholar] [CrossRef]
- Gooding, S.; Ansari-Pour, N.; Towfic, F.; Ortiz Estevez, M.; Chamberlain, P.P.; Tsai, K.T.; Flynt, E.; Hirst, M.; Rozelle, D.; Dhiman, P.; et al. Multiple cereblon genetic changes are associated with acquired resistance to lenalidomide or pomalidomide in multiple myeloma. Blood 2021, 137, 232–237. [Google Scholar] [CrossRef]
- Sperling, A.S.; Burgess, M.; Keshishian, H.; Gasser, J.A.; Bhatt, S.; Jan, M.; Slabicki, M.; Sellar, R.S.; Fink, E.C.; Miller, P.G.; et al. Patterns of substrate affinity, competition, and degradation kinetics underlie biological activity of thalidomide analogs. Blood 2019, 134, 160–170. [Google Scholar] [CrossRef]
- Eichner, R.; Heider, M.; Fernandez-Saiz, V.; van Bebber, F.; Garz, A.K.; Lemeer, S.; Rudelius, M.; Targosz, B.S.; Jacobs, L.; Knorn, A.M.; et al. Immunomodulatory drugs disrupt the cereblon-CD147-MCT1 axis to exert antitumor activity and teratogenicity. Nat. Med. 2016, 22, 735–743. [Google Scholar] [CrossRef]
- Beedie, S.L.; Peer, C.J.; Pisle, S.; Gardner, E.R.; Mahony, C.; Barnett, S.; Ambrozak, A.; Gütschow, M.; Chau, C.H.; Vargesson, N.; et al. Anticancer Properties of a Novel Class of Tetrafluorinated Thalidomide Analogues. Mol. Cancer Ther. 2015, 14, 2228–2237. [Google Scholar] [CrossRef]
- Ng, S.S.; Gütschow, M.; Weiss, M.; Hauschildt, S.; Teubert, U.; Hecker, T.K.; Luzzio, F.A.; Kruger, E.A.; Eger, K.; Figg, W.D. Antiangiogenic activity of N-substituted and tetrafluorinated thalidomide analogues. Cancer Res. 2003, 63, 3189–3194. [Google Scholar]
- Ng, S.S.; MacPherson, G.R.; Gütschow, M.; Eger, K.; Figg, W.D. Antitumor effects of thalidomide analogs in human prostate cancer xenografts implanted in immunodeficient mice. Clin. Cancer Res. 2004, 10, 4192–4197. [Google Scholar] [CrossRef][Green Version]
- Lepper, E.R.; Ng, S.S.; Gütschow, M.; Weiss, M.; Hauschildt, S.; Hecker, T.K.; Luzzio, F.A.; Eger, K.; Figg, W.D. Comparative molecular field analysis and comparative molecular similarity indices analysis of thalidomide analogues as angiogenesis inhibitors. J. Med. Chem. 2004, 47, 2219–2227. [Google Scholar] [CrossRef]
- Steinebach, C.; Ambrozak, A.; Dosa, S.; Beedie, S.L.; Strope, J.D.; Schnakenburg, G.; Figg, W.D.; Gütschow, M. Synthesis, Structural Characterization, and Antiangiogenic Activity of Polyfluorinated Benzamides. ChemMedChem 2018, 13, 2080–2089. [Google Scholar] [CrossRef]
- Bjorklund, C.C.; Ma, W.; Wang, Z.Q.; Davis, R.E.; Kuhn, D.J.; Kornblau, S.M.; Wang, M.; Shah, J.J.; Orlowski, R.Z. Evidence of a role for activation of Wnt/beta-catenin signaling in the resistance of plasma cells to lenalidomide. J. Biol. Chem. 2011, 286, 11009–11020. [Google Scholar] [CrossRef]
- Selby, M.; Delosh, R.; Laudeman, J.; Ogle, C.; Reinhart, R.; Silvers, T.; Lawrence, S.; Kinders, R.; Parchment, R.; Teicher, B.A.; et al. 3D Models of the NCI60 Cell Lines for Screening Oncology Compounds. SLAS Discov. 2017, 22, 473–483. [Google Scholar] [CrossRef]
- Takashiba, S.; Van Dyke, T.E.; Amar, S.; Murayama, Y.; Soskolne, A.W.; Shapira, L. Differentiation of monocytes to macrophages primes cells for lipopolysaccharide stimulation via accumulation of cytoplasmic nuclear factor kappaB. Infect. Immun. 1999, 67, 5573–5578. [Google Scholar] [CrossRef]
- Thakurta, A.; Gandhi, A.K.; Waldman, M.F.; Bjorklund, C.; Ning, Y.; Mendy, D.; Schafer, P.; Lopez-Girona, A.; Lentzsch, S.; A Schey, S.; et al. Absence of mutations in cereblon (CRBN) and DNA damage-binding protein 1 (DDB1) genes and significance for IMiD therapy. Leukemia 1129. [Google Scholar] [CrossRef]
- Dimopoulos, K.; Sogaard Helbo, A.; Fibiger Munch-Petersen, H.; Sjo, L.; Christensen, J.; Sommer Kristensen, L.; Asmar, F.; Hermansen, N.E.U.; O’Connel, C.; Gimsing, P.; et al. Dual inhibition of DNMTs and EZH2 can overcome both intrinsic and acquired resistance of myeloma cells to IMiDs in a cereblon-independent manner. Mol. Oncol. 2018, 12, 180–195. [Google Scholar] [CrossRef]
- Kruger, E.A.; Duray, P.H.; Price, D.K.; Pluda, J.M.; Figg, W.D. Approaches to preclinical screening of antiangiogenic agents. Semin Oncol. 2001, 28, 570–576. [Google Scholar] [CrossRef]
- Therapontos, C.; Erskine, L.; Gardner, E.R.; Figg, W.D.; Vargesson, N. Thalidomide induces limb defects by preventing angiogenic outgrowth during early limb formation. Proc. Natl. Acad. Sci. USA 2009, 106, 8573–8578. [Google Scholar] [CrossRef]
- El-Aarag, B.Y.; Kasai, T.; Zahran, M.A.; Zakhary, N.I.; Shigehiro, T.; Sekhar, S.C.; Agwa, H.S.; Mizutani, A.; Murakami, H.; Kakuta, H.; et al. In vitro anti-proliferative and anti-angiogenic activities of thalidomide dithiocarbamate analogs. Int. Immunopharmacol. 2014, 21, 283–292. [Google Scholar] [CrossRef]
- Gütschow, M.; Hecker, T.; Thiele, A.; Hauschildt, S.; Eger, K. Aza analogues of thalidomide: Synthesis and evaluation as inhibitors of tumor necrosis factor-alpha production in vitro. Bioorg. Med. Chem. 2001, 9, 1059–1065. [Google Scholar] [CrossRef]
- Chamberlain, P.P.; Lopez-Girona, A.; Miller, K.; Carmel, G.; Pagarigan, B.; Chie-Leon, B.; Rychak, E.; Corral, L.G.; Ren, Y.J.; Wang, M.; et al. Structure of the human Cereblon-DDB1-lenalidomide complex reveals basis for responsiveness to thalidomide analogs. Nat. Struct. Mol. Biol. 2014, 21, 803–809. [Google Scholar] [CrossRef]
- Fischer, E.S.; Bohm, K.; Lydeard, J.R.; Yang, H.; Stadler, M.B.; Cavadini, S.; Nagel, J.; Serluca, F.; Acker, V.; Lingaraju, G.M.; et al. Structure of the DDB1-CRBN E3 ubiquitin ligase in complex with thalidomide. Nature 2014, 512, 49–53. [Google Scholar] [CrossRef]
- Beedie, S.L.; Huang, P.A.; Harris, E.M.; Strope, J.D.; Mahony, C.; Chau, C.H.; Vargesson, N.; Figg, W.D. Role of cereblon in angiogenesis and in mediating the antiangiogenic activity of immunomodulatory drugs. FASEB J. 2020, 34, 11395–11404. [Google Scholar] [CrossRef]
- Peach, M.L.; Beedie, S.L.; Chau, C.H.; Collins, M.K.; Markolovic, S.; Luo, W.; Tweedie, D.; Steinebach, C.; Greig, N.H.; Gütschow, M.; et al. Antiangiogenic Activity and in Silico Cereblon Binding Analysis of Novel Thalidomide Analogs. Molecules 2020, 25, 5683. [Google Scholar] [CrossRef]
- Heim, C.; Maiwald, S.; Steinebach, C.; Collins, M.K.; Strope, J.; Chau, C.H.; Figg, W.D.; Gütschow, M.; Hartmann, M.D. On the correlation of cereblon binding, fluorination and antiangiogenic properties of immunomodulatory drugs. Biochem. Biophys. Res. Commun. 2021, 534, 67–72. [Google Scholar] [CrossRef]
- Boichenko, I.; Bar, K.; Deiss, S.; Heim, C.; Albrecht, R.; Lupas, A.N.; Hernandez Alvarez, B.; Hartmann, M.D. Chemical Ligand Space of Cereblon. ACS Omega 2018, 3, 11163–11171. [Google Scholar] [CrossRef]
- Lopez-Girona, A.; Mendy, D.; Ito, T.; Miller, K.; Gandhi, A.K.; Kang, J.; Karasawa, S.; Carmel, G.; Jackson, P.; Abbasian, M.; et al. Cereblon is a direct protein target for immunomodulatory and antiproliferative activities of lenalidomide and pomalidomide. Leukemia 2012, 26, 2326–2335. [Google Scholar] [CrossRef]
- Zhu, Y.X.; Shi, C.X.; Bruins, L.A.; Wang, X.; Riggs, D.L.; Porter, B.; Ahmann, J.M.; de Campos, C.B.; Braggio, E.; Bergsagel, P.L.; et al. Identification of lenalidomide resistance pathways in myeloma and targeted resensitization using cereblon replacement, inhibition of STAT3 or targeting of IRF4. Blood Cancer J. 2019, 9, 19. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barton, B.E.; Collins, M.K.; Chau, C.H.; Choo-Wosoba, H.; Venzon, D.J.; Steinebach, C.; Garchitorena, K.M.; Shah, B.; Sarin, E.L.; Gütschow, M.; et al. Preclinical Evaluation of a Novel Series of Polyfluorinated Thalidomide Analogs in Drug-Resistant Multiple Myeloma. Biomolecules 2024, 14, 725. https://doi.org/10.3390/biom14060725
Barton BE, Collins MK, Chau CH, Choo-Wosoba H, Venzon DJ, Steinebach C, Garchitorena KM, Shah B, Sarin EL, Gütschow M, et al. Preclinical Evaluation of a Novel Series of Polyfluorinated Thalidomide Analogs in Drug-Resistant Multiple Myeloma. Biomolecules. 2024; 14(6):725. https://doi.org/10.3390/biom14060725
Chicago/Turabian StyleBarton, Blaire E., Matthew K. Collins, Cindy H. Chau, Hyoyoung Choo-Wosoba, David J. Venzon, Christian Steinebach, Kathleen M. Garchitorena, Bhruga Shah, Eric L. Sarin, Michael Gütschow, and et al. 2024. "Preclinical Evaluation of a Novel Series of Polyfluorinated Thalidomide Analogs in Drug-Resistant Multiple Myeloma" Biomolecules 14, no. 6: 725. https://doi.org/10.3390/biom14060725
APA StyleBarton, B. E., Collins, M. K., Chau, C. H., Choo-Wosoba, H., Venzon, D. J., Steinebach, C., Garchitorena, K. M., Shah, B., Sarin, E. L., Gütschow, M., & Figg, W. D. (2024). Preclinical Evaluation of a Novel Series of Polyfluorinated Thalidomide Analogs in Drug-Resistant Multiple Myeloma. Biomolecules, 14(6), 725. https://doi.org/10.3390/biom14060725







