Comprehensive Review of Olea europaea: A Holistic Exploration into Its Botanical Marvels, Phytochemical Riches, Therapeutic Potentials, and Safety Profile
Abstract
1. Introduction
2. Investigative Methodology
3. Results and Discussion
3.1. Taxonomy, Botany, Morphology, and Ecology Description of Olive
3.2. Geographical Location of Olea europaea L., in the World
3.3. Worldwide Naming of Olive Varieties
3.4. Phytochemistry Characteristics
3.5. Biological Properties
3.5.1. Antibacterial Activity
| Part Used | Extract | Strains | Method | Key Results | Reference |
|---|---|---|---|---|---|
| Seed | Vegetal extract | GP Staphylococcus aureus Enterococcus faecalis Listeria innocua Listeria monocytogenes Bacillus cereus Streptococcus mutans Bacillus subtilis Staphylococcus epidermidis Clostridium sporogenes Bacillus subtilis subsp. Spizizenii Bacillus subtilis Streptococcus sobrinus Streptococcus ralis Staphylococcus epedermidis, Propionibacterium acnes Lactobacillus plantarum GN Escherichia coli Salmonella typhimurium Aeromonas hydrophila Agrobacterium tumefaciens Pseudomonas aeruginosa Acinetobacter baumannii Shigella. sonnei Proteus mirabilis Citrobacter freundii Salmonella. enteritidis Pseudomonas fluorescens Brochotrix thermosphacta Pseudomonas fragi Pseudomonas putida Salmonella enterica Enterobacter cloacae Klebsiella pneumoniae Salmonella Enteritidis Pseudomonas vulgaris Morganella Morganii Haemophilus influenzae Yersinia enterolitica Salmonella enterica subsp. heindelberg | Disc diffusion method Minimum inhibitory concentration Minimum bactericidal concentration Broth micro-dilution assay Live/dead bacterial staining assay Bacterial inhibition assays Bacterial motility assays | MIC = 100–200 µg/mL | [104] |
| Fruit | Vegetable oil | Ø = 5–18 mm | [81,116] | ||
| Vegetal extract | Ø = 13–18.5 mg/mL MIC = 12.5–25 mg/mL MBC = 25–50 mg/mL | [102,105] | |||
| Leaves | Essential oil | Ø = 9–29 mm MIC = 0.625–5 mg/mL | [6,96,115,117] | ||
| Vegetal extract | Ø = 1–20 mm MIC = 0.60–25 mg/mL MBC = 0.70–12.5 mg/mL | [109,110,118,119,120,121,122,123,124,125,126,127] |
3.5.2. Antifungal Activity
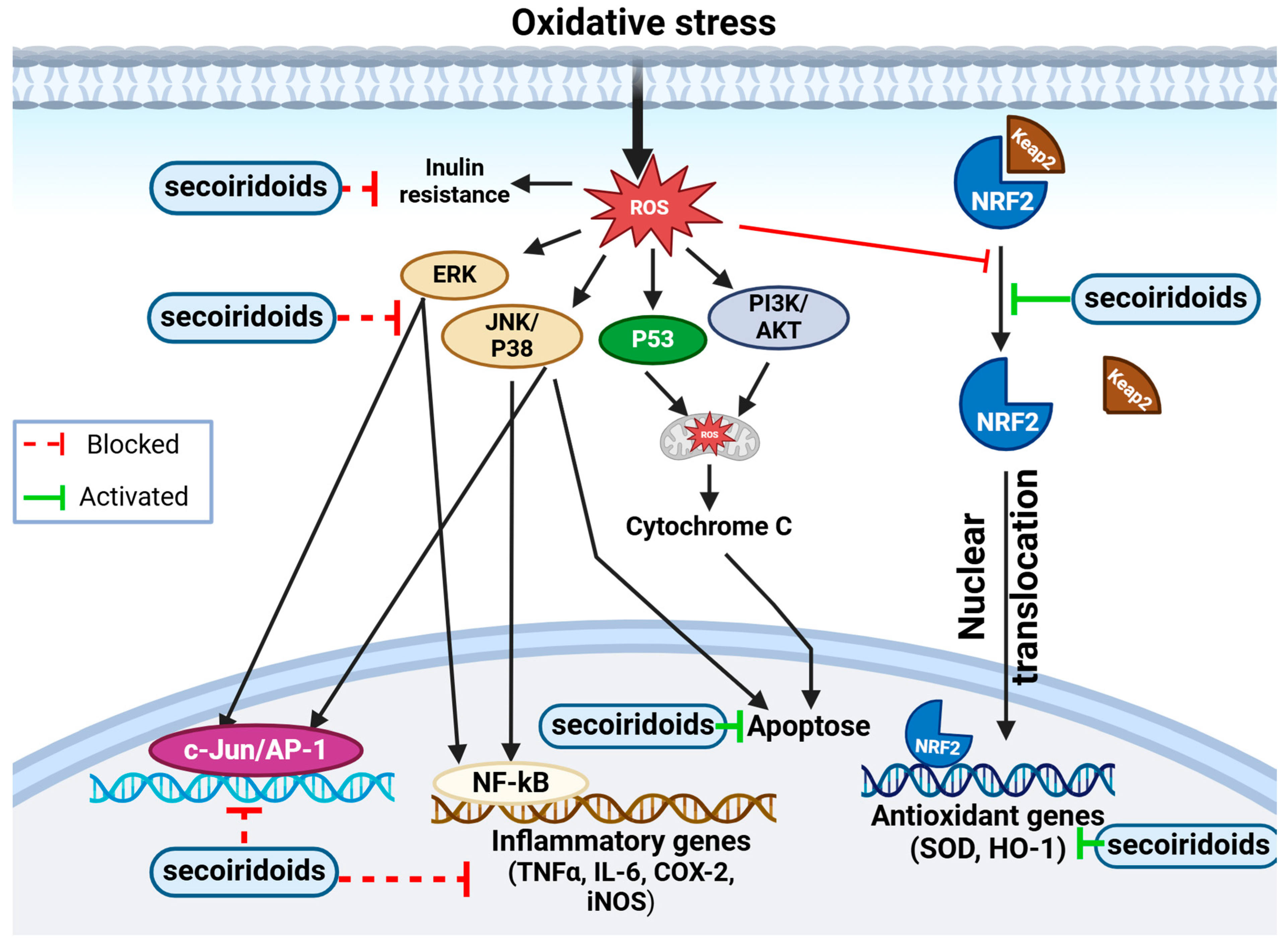
3.5.3. Antioxidant Activity
3.5.4. Antidiabetic Activity
| Part Used | Extract | Method In Vitro | Key Results | Reference |
|---|---|---|---|---|
| Buds | Hydroalcoholic extract | Pancreatic lipase activity inhibition (PLI) The inhibition of α-amylase (IAM) The inhibition of α-glucosidase Glucose uptake using the yeast cells assay Determination of surface GLUT4myc L6-GLUT4myc cell line | IC50 = 1.27 ± 0.04 mg/mL IC50 = 0.1269 ± 0.023 mg/mL | [183] |
| Seed | Aqueous extract | IC50 = 0.3194 mg/mL | [184] | |
| Fruit | Ethyl acetate extracts | IC50 = 0.00531 ± 0.003 mg/mL IC50 = 0.0547 ± 0.001 mg/mL | [191] | |
| Extra virgin olive oil | IC50 = 0.06 ± 0.008 mg/mL | [186] | ||
| Leaves | Hydroalcoholic extract | IC50 = 150 µM | [101] | |
| Ethanolic extract | IC50 = 0.037 mg/mL | [189] | ||
| Aqueous extract | IC50 = 0.014 ± 0.041 µg/mL | [17] | ||
| IC50 = 0.2 mg/mL | [192] | |||
| Methanolic extract | IC50 = 43.47 µM EC50 = 47.12 µM | [193] |
| Part Used | Extract | Dose | Method In Vivo | Key Results | Reference |
|---|---|---|---|---|---|
| Seed | Methanolic extract | 750 mg/kg body weight | Alloxan-induced diabetes in Wistar albino rats Swiss albino mice (aged 3–4 weeks) of both sex Wistar albino male rats (streptozotocin) Sprague–Dawley male rats (streptozotocin) | The treatment of seed MeOH extracts was seen to have a significant hypoglycemic impact and to have reversed weight loss in diabetic rats. | [184] |
| Fruit | Vegetable oil | 150 mg/kg body weight | The blood sugar levels of the group 2 alloxan-induced diabetic rats exhibited a gradual decline, eventually stabilizing within the normal range of 4.9–5.5 mg/dl. | [185] | |
| Leaves | 2%/kg body weight | Low-density lipoprotein, total cholesterol, triglycerides, and serum glucose levels were all lowered. High-density lipoprotein levels rose as a result. It reduced atherogenic index and atherogenic coefficient. | [187] | ||
| Aqueous extract | 200–400 mg/kg body weight | The application of both low and high doses of olive leaf extract effectively ameliorated the identified physiological, molecular, and histopathological changes. | [195] | ||
| 1–3%/kg body weight | Lowered blood glucose levels, regulated biochemical parameters including LDL, HDL, TG, and insulin secretion. OLP functions by inhibiting AS160, thereby causing a reduction in blood glucose levels. | [188] | |||
| 100 mg/kg body weight | Oral treatment with olive extract controlled biochemical parameters to mimic normal rat models and contributed to a considerable reduction in blood glucose levels in the diabetic rat group after 4 weeks when compared with control diabetic rats. | [190] | |||
| Methanolic extract | 25 and 50 mg/kg body weight | It demonstrated a strong regulation of biochemical parameters, and this effect was even more pronounced when coupled with an antidiabetic drug. | [196] | ||
| 25 mg/kg body weight | The flavonoid extracted from olive leaves had a positive effect on the blood glucose level, showing a significant reduction of 49.59% compared to the normal control group. | [16] |
3.5.5. Anticancer Activity
3.5.6. Toxicology Investigation
4. Conclusions and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bracci, T.; Busconi, M.; Fogher, C.; Sebastiani, L. Molecular studies in olive (Olea europaea L.): Overview on DNA markers applications and recent advances in genome analysis. Plant Cell Rep. 2011, 30, 449–462. [Google Scholar] [CrossRef] [PubMed]
- Ghanbari, R.; Anwar, F.; Alkharfy, K.M.; Gilani, A.-H.; Saari, N. Valuable Nutrients and Functional Bioactives in Different Parts of Olive (Olea europaea L.)—A Review. Int. J. Mol. Sci. 2012, 13, 3291–3340. [Google Scholar] [CrossRef] [PubMed]
- Spennemann, D.H.R.; Allen, L.R. Feral olives (Olea europaea) as future woody weeds in Australia: A review. Aust. J. Exp. Agric. 2000, 40, 889–901. [Google Scholar] [CrossRef]
- Waisel, Y.; Geller-Bernstein, C.; Keynan, N.; Arad, G. Antigenicity of the pollen proteins of various cultivars of Olea europaea. Allergy 1996, 51, 819–821. [Google Scholar] [CrossRef] [PubMed]
- Zighed, A.; Derradji, L.; Hadef, Y.; Zighed, A.; Derradji, L.; Hadef, Y. Determination of polyphenolic components by High Performance Liquid Chromatography (HPLC) and evaluation of the antioxidant activity of leaves of Olea europaea L. var. sylvestris (Miller) Lehr. GSC Biol. Pharm. Sci. 2022, 20, 095–099. [Google Scholar] [CrossRef]
- Boukhebti, H.; Chaker, A.; Lograda, T.; Messaoud, R. Pharmacology & Toxicology. Int. J. Pharmacol. Toxicol. 2015, 5, 42–46. [Google Scholar]
- Farag, R.S.; Abdel-Latif, M.S.; Abd El Baky, H.H.; Tawfeek, L.S. Phytochemical screening and antioxidant activity of some medicinal plants’ crude juices. Biotechnol. Rep. 2020, 28, e00536. [Google Scholar] [CrossRef] [PubMed]
- Hannachi, H.; Elfalleh, W.; Laajel, M.; Ennajeh, I.; Mechlouch, R.F.; Nagaz, K. Chemical Profiles and Antioxidant Activities of Leaf, Pulp, and Stone of Cultivated and Wild Olive Trees (Olea europaea L.). Int. J. Fruit Sci. 2020, 20, 350–370. [Google Scholar] [CrossRef]
- Talhaoui, N.; Gómez-Caravaca, A.M.; León, L.; De la Rosa, R.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Determination of phenolic compounds of ‘Sikitita’ olive leaves by HPLC-DAD-TOF-MS. Comparison with its parents ‘Arbequina’ and ‘Picual’ olive leaves. LWT—Food Sci. Technol. 2014, 58, 28–34. [Google Scholar] [CrossRef]
- Ben-Amor, I.; Musarra-Pizzo, M.; Smeriglio, A.; D’Arrigo, M.; Pennisi, R.; Attia, H.; Gargouri, B.; Trombetta, D.; Mandalari, G.; Sciortino, M.T. Phytochemical Characterization of Olea europaea Leaf Extracts and Assessment of Their Anti-Microbial and Anti-HSV-1 Activity. Viruses 2021, 13, 1085. [Google Scholar] [CrossRef]
- Qabaha, K.; AL-Rimawi, F.; Qasem, A.; Naser, S.A. Oleuropein Is Responsible for the Major Anti-Inflammatory Effects of Olive Leaf Extract. J. Med. Food 2018, 21, 302–305. [Google Scholar] [CrossRef] [PubMed]
- Ramata-Stunda, A.; Petriņa, Z.; Valkovska, V.; Borodušķis, M.; Gibnere, L.; Gurkovska, E.; Nikolajeva, V. Synergistic Effect of Polyphenol-Rich Complex of Plant and Green Propolis Extracts with Antibiotics against Respiratory Infections Causing Bacteria. Antibiotics 2022, 11, 160. [Google Scholar] [CrossRef] [PubMed]
- Shialy, Z.; Zarrin, M.; Sadeghi Nejad, B.; Yusef Naanaie, S. In vitro antifungal properties of Pistacia atlantica and olive extracts on different fungal species. Curr. Med. Mycol. 2015, 1, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Slobodníková, L.; Fialová, S.; Rendeková, K.; Kováč, J.; Mučaji, P. Antibiofilm Activity of Plant Polyphenols. Molecules 2016, 21, 1717. [Google Scholar] [CrossRef] [PubMed]
- Sheweita, S.A.; Mashaly, S.; Newairy, A.A.; Abdou, H.M.; Eweda, S.M. Changes in Oxidative Stress and Antioxidant Enzyme Activities in Streptozotocin-Induced Diabetes Mellitus in Rats: Role of Alhagi maurorum Extracts. Oxid. Med. Cell. Longev. 2016, 2016, e5264064. [Google Scholar] [CrossRef] [PubMed]
- Mechchate, H.; Es-Safi, I.; Bourhia, M.; Kyrylchuk, A.; El Moussaoui, A.; Conte, R.; Ullah, R.; Ezzeldin, E.; Mostafa, G.A.; Grafov, A.; et al. In-Vivo Antidiabetic Activity and In-Silico Mode of Action of LC/MS-MS Identified Flavonoids in Oleaster Leaves. Molecules 2020, 25, 5073. [Google Scholar] [CrossRef] [PubMed]
- Mansour, H.M.M.; Zeitoun, A.A.; Abd-Rabou, H.S.; El Enshasy, H.A.; Dailin, D.J.; Zeitoun, M.A.A.; El-Sohaimy, S.A. Antioxidant and Anti-Diabetic Properties of Olive (Olea europaea) Leaf Extracts: In Vitro and In Vivo Evaluation. Antioxidants 2023, 12, 1275. [Google Scholar] [CrossRef] [PubMed]
- Qidwai, A.; Pandey, M.; Kumar, R.; Dikshit, A. Comprehensive evaluation of pharmacological properties of Olea europaea L. for Cosmeceuticals prospects. Clin. Phytoscience 2017, 3, 12. [Google Scholar] [CrossRef]
- Tunç, Y.; Yaman, M.; Keçe, Y.M.; Yilmaz, K.U.; Yildiz, E.; Güneş, A. Characterization of Olive (Olea Europaea L.) Cultivars; Colour Properties, Biochemical Contents, Antioxidant Activity and Nutrient Contents. Genet. Resour. Crop Evol. 2024. [Google Scholar] [CrossRef]
- Wang, B.; Qu, J.; Luo, S.; Feng, S.; Li, T.; Yuan, M.; Huang, Y.; Liao, J.; Yang, R.; Ding, C. Optimization of Ultrasound-Assisted Extraction of Flavonoids from Olive (Olea europaea) Leaves, and Evaluation of Their Antioxidant and Anticancer Activities. Molecules 2018, 23, 2513. [Google Scholar] [CrossRef]
- Widyaningrum, N.; Hussaana, A.; Puspitasari, N. Invitro Antioxidant and Cytotoxic Potential of Ficus carica L. and Olea europeae L. against Cervical Cancer. Sains Med. 2020, 11, 14. [Google Scholar] [CrossRef]
- Guex, C.G.; Reginato, F.Z.; Figueredo, K.C.; da Silva, A.R.H.; Pires, F.B.; Jesus, R.d.S.; Lhamas, C.L.; Lopes, G.H.H.; Bauermann, L.d.F. Safety assessment of ethanolic extract of Olea europaea L. leaves after acute and subacute administration to Wistar rats. Regul. Toxicol. Pharmacol. 2018, 95, 395–399. [Google Scholar] [CrossRef] [PubMed]
- Besnard, G.; Henry, P.; Wille, L.; Cooke, D.; Chapuis, E. On the origin of the invasive olives (Olea europaea L., Oleaceae). Heredity 2007, 99, 608–619. [Google Scholar] [CrossRef] [PubMed]
- Hashmi, M.A.; Khan, A.; Hanif, M.; Farooq, U.; Perveen, S. Traditional Uses, Phytochemistry, and Pharmacology of Olea europaea (Olive). Evid. Based Complement. Altern. Med. 2015, 2015, e541591. [Google Scholar] [CrossRef] [PubMed]
- Tsantili, E.; Evangelou, E.; Kiritsakis, A. Botanical characteristics of olive trees: Cultivation and growth conditions—Defense mechanisms to various stressors and effects on olive growth and functional compounds. In Olives and Olive Oil as Functional Foods, 1st ed.; Shahidi, F., Kiritsakis, A., Eds.; Wiley: Hoboken, NJ, USA, 2017; pp. 13–33. [Google Scholar] [CrossRef]
- Muzzalupo, I. Olive Germplasm: Italian Catalogue of Olive Varieties; BoD—Books on Demand: Norderstedt, Germany, 2012. [Google Scholar]
- Therios, I.N. Olives; CABI: Wallingford, UK, 2009. [Google Scholar]
- The Olive Tree. International Olive Council. Available online: https://www.internationaloliveoil.org/olive-world/olive-tree/ (accessed on 7 June 2024).
- Gixhari, B.; Hodaj, B.; Gjeloshi, A.; Ismaili, H. Olive in the story and art in Albania. In Proceedings of the International Conference “Adriatic Olive Grove: Risk Prevention, Sustainability, Learning”, Corfu, Greece, 19–20 June 2014. [Google Scholar]
- Haddad, B.; Gristina, A.S.; Mercati, F.; Saadi, A.E.; Aiter, N.; Martorana, A.; Sharaf, A.; Carimi, F. Molecular Analysis of the Official Algerian Olive Collection Highlighted a Hotspot of Biodiversity in the Central Mediterranean Basin. Genes 2020, 11, 303. [Google Scholar] [CrossRef]
- Trentacoste, E.R.; Banco, A.P.; Piccoli, P.N.; Monasterio, R.P. Olive oil characterization of cv. ‘Arauco’ harvested at different times in areas with early frost in Mendoza, Argentina. J. Sci. Food Agric. 2020, 100, 953–960. [Google Scholar] [CrossRef] [PubMed]
- Contreras, R.C.; Aguayo, F.; Guerra, A.; Tapia, F.; Porcile, V. Genetic characterization of centennial olive trees from northern Chile: The case of extra virgin olive oil from huasco in the process of designation of origin. Chil. J. Agric. Anim. Sci. 2018, 34, 126–139. [Google Scholar] [CrossRef]
- Emmanouilidou, M.G.; Kyriacou, M.C.; Trujillo, I. Characterization and Identification of Indigenous Olive Germplasm from Cyprus Using Morphological and Simple Sequence Repeat Markers. HortScience 2018, 53, 1306–1313. [Google Scholar] [CrossRef]
- Klepo, T.; Benčić, Đ.; Liber, Z.; Belaj, A.; Strikić, F.; Kević, N.; Šatović, Z. Revealing the Diversity and Complex Relationships of Croatian Olive Germplasm. Int. J. Mol. Sci. 2024, 25, 3170. [Google Scholar] [CrossRef]
- Mohamed, A.A.H.; Nagaty, M.A.; El-Baghdady, M.M.S.; Radwan, K.H. Morphological and molecular characterization of some olive (Olea europaea) cultivars in El-Arish, Egypt. J. Biosci. Appl. Res. 2017, 3, 237–251. [Google Scholar] [CrossRef]
- Khadari, B.; El Bakkali, A.; Essalouh, L.; Tollon, C.; Pinatel, C.; Besnard, G. Cultivated Olive Diversification at Local and Regional Scales: Evidence from the Genetic Characterization of French Genetic Resources. Front. Plant Sci. 2019, 10, 1593. [Google Scholar] [CrossRef]
- Roubos, K.; Moustakas, M.; Aravanopoulos, F.A. Molecular identification of Greek olive (Olea europaea) cultivars based on microsatellite loci. Genet. Mol. Res. 2010, 9, 1865–1876. [Google Scholar] [CrossRef]
- Goldental-Cohen, S.; Biton, I.; Many, Y.; Ben-Sason, S.; Zemach, H.; Avidan, B.; Ben-Ari, G. Green Olive Browning Differ Between Cultivars. Front. Plant Sci. 2019, 10, 1260. [Google Scholar] [CrossRef]
- Cicatelli, A.; Fortunati, T.; De Feis, I.; Castiglione, S. Oil composition and genetic biodiversity of ancient and new olive (Olea europea L.) varieties and accessions of southern Italy. Plant Sci. 2013, 210, 82–92. [Google Scholar] [CrossRef]
- Al Ganideh, S.F.; Good, L.K. Nothing Tastes as Local: Jordanians’ Perceptions of Buying Domestic Olive Oil. J. Food Prod. Mark. 2016, 22, 168–190. [Google Scholar] [CrossRef]
- El Riachy, M.; Moubarak, P.; Al Hawi, G.; Geha, M.; Mushantaf, W.; Estephan, N.; Skaff, W. Fatty Acid and Phenolic Profiles of Virgin Olive Oils from Local and European Varieties Planted in Lebanon. Plants 2023, 12, 2681. [Google Scholar] [CrossRef]
- Bajoub, A.; Medina-Rodríguez, S.; Olmo-García, L.; Ajal, E.A.; Monasterio, R.P.; Hanine, H.; Fernández-Gutiérrez, A.; Carrasco-Pancorbo, A. In-Depth Two-Year Study of Phenolic Profile Variability among Olive Oils from Autochthonous and Mediterranean Varieties in Morocco, as Revealed by a LC-MS Chemometric Profiling Approach. Int. J. Mol. Sci. 2017, 18, 52. [Google Scholar] [CrossRef]
- Abuamsha, R.; Abueid, M.; Hajjeh, H.; Salman, M. Evaluation of the Incidence and Severity of Olive Leaf Spot caused by Spilocaea oleagina in different olive cultivars in Palestine. J. Agric. Environ. Int. Dev. JAEID 2013, 107, 201–212. [Google Scholar] [CrossRef]
- Seabra, R.M.; Andrade, P.B.; Valentão, P.; Faria, M.; Paice, A.G.; Oliveira, M.B.P.P. Chapter 20—Phenolic Profiles of Portuguese Olives: Cultivar and Geographics. In Olives and Olive Oil in Health and Disease Prevention; Preedy, V.R., Watson, R.R., Eds.; Academic Press: San Diego, CA, USA, 2010; pp. 177–186. [Google Scholar] [CrossRef]
- Arenas-Castro, S.; Gonçalves, J.F.; Moreno, M.; Villar, R. Projected climate changes are expected to decrease the suitability and production of olive varieties in southern Spain. Sci. Total Environ. 2020, 709, 136161. [Google Scholar] [CrossRef]
- Fabbri, A.; Baldoni, L.; Caruso, T.; Famiani, F. The Olive: Botany and Production; CABI: Wallingford, UK, 2023. [Google Scholar]
- Saddoud Debbabi, O.; Rahmani Mnasri, S.; Ben Amar, F.; Ben Naceur, M.; Montemurro, C.; Miazzi, M.M. Applications of Microsatellite Markers for the Characterization of Olive Genetic Resources of Tunisia. Genes 2021, 12, 286. [Google Scholar] [CrossRef]
- Dıraman, H. Characterization by chemometry of the most important domestic and foreign olive cultivars from the National Olive Collection Orchard of Turkey. Grasas Aceites 2010, 61, 341–351. [Google Scholar] [CrossRef][Green Version]
- Jerman, T.; Trebše, P.; Mozetič Vodopivec, B. Ultrasound-assisted solid liquid extraction (USLE) of olive fruit (Olea europaea) phenolic compounds. Food Chem. 2010, 123, 175–182. [Google Scholar] [CrossRef]
- Xie, P.; Cecchi, L.; Bellumori, M.; Balli, D.; Giovannelli, L.; Huang, L.; Mulinacci, N. Phenolic Compounds and Triterpenes in Different Olive Tissues and Olive Oil By-Products, and Cytotoxicity on Human Colorectal Cancer Cells: The Case of Frantoio, Moraiolo and Leccino Cultivars (Olea europaea L.). Foods 2021, 10, 2823. [Google Scholar] [CrossRef]
- Ammar, S.; Contreras, M.d.M.; Gargouri, B.; Segura-Carretero, A.; Bouaziz, M. RP-HPLC-DAD-ESI-QTOF-MS based metabolic profiling of the potential Olea europaea by-product “wood” and its comparison with leaf counterpart. Phytochem. Anal. 2017, 28, 217–229. [Google Scholar] [CrossRef]
- Khlif, I.; Jellali, K.; Michel, T.; Halabalaki, M.; Skaltsounis, A.L.; Allouche, N. Characteristics, Phytochemical Analysis and Biological Activities of Extracts from Tunisian Chetoui Olea europaea Variety. J. Chem. 2015, 2015, e418731. [Google Scholar] [CrossRef]
- Alves, E.; Rey, F.; Melo, T.; Barros, M.P.; Domingues, P.; Domingues, R. Bioprospecting Bioactive Polar Lipids from Olive (Olea europaea cv. Galega vulgar) Fruit Seeds: LC-HR-MS/MS Fingerprinting and Sub-Geographic Comparison. Foods 2022, 11, 951. [Google Scholar] [CrossRef]
- Bouarroudj, K.; Tamendjari, A.; Larbat, R. Quality, composition and antioxidant activity of Algerian wild olive (Olea europaea L. subsp. Oleaster) oil. Ind. Crops Prod. 2016, 83, 484–491. [Google Scholar] [CrossRef]
- Angelis, A.; Antoniadi, L.; Stathopoulos, P.; Halabalaki, M.; Skaltsounis, L.A. Oleocanthalic and Oleaceinic acids: New compounds from Extra Virgin Olive Oil (EVOO). Phytochem. Lett. 2018, 26, 190–194. [Google Scholar] [CrossRef]
- Pérez, A.G.; León, L.; Pascual, M.; de la Rosa, R.; Belaj, A.; Sanz, C. Analysis of Olive (Olea europaea L.) Genetic Resources in Relation to the Content of Vitamin E in Virgin Olive Oil. Antioxidants 2019, 8, 242. [Google Scholar] [CrossRef]
- Cecchi, L.; Piazzini, V.; D’Ambrosio, M.; Luceri, C.; Rocco, F.; Innocenti, M.; Vanti, G.; Mulinacci, N.; Bergonzi, M.C. Formulation of a Phenol-Rich Extract from Unripe Olives (Olea europaea L.) in Microemulsion to Improve Its Solubility and Intestinal Permeability. Molecules 2020, 25, 3198. [Google Scholar] [CrossRef]
- Fernández-Poyatos, M.d.P.; Ruiz-Medina, A.; Llorent-Martínez, E.J. Phytochemical profile and mineral content of Royal variety olive fruits. Influence of the ripening stage. J. Food Compos. Anal. 2021, 95, 103671. [Google Scholar] [CrossRef]
- Xie, P.; Huang, L.; Zhang, C.; Zhang, Y. Phenolic compositions, and antioxidant performance of olive leaf and fruit (Olea europaea L.) extracts and their structure–activity relationships. J. Funct. Foods 2015, 16, 460–471. [Google Scholar] [CrossRef]
- Jurišić Grubešić, R.; Nazlić, M.; Miletić, T.; Vuko, E.; Vuletić, N.; Ljubenkov, I.; Dunkić, V. Antioxidant Capacity of Free Volatile Compounds from Olea europaea L. cv. Oblica Leaves Depending on the Vegetation Stage. Antioxidants 2021, 10, 1832. [Google Scholar] [CrossRef]
- Tlili, A.; Bouziane, M.; Flamini, G.; Hadj Mahammed, M. Volatiles Variation of Two Major Cultivars of Olea europaea L. Cultivated in Mediterranean and Arid Regions of Algeria. Rec. Nat. Prod. 2021, 16, 34–45. [Google Scholar] [CrossRef]
- Brahmi, F.; Mechri, B.; Dhibi, M.; Hammami, M. Variations in phenolic compounds and antiradical scavenging activity of Olea europaea leaves and fruits extracts collected in two different seasons. Ind. Crops Prod. 2013, 49, 256–264. [Google Scholar] [CrossRef]
- Benincasa, C.; Romano, E.; Pellegrino, M.; Perri, E. Characterization of Phenolic Profiles of Italian Single Cultivar Olive Leaves (Olea europaea L.) by Mass Spectrometry. Mass Spectrom. Purif. Tech. 2018, 4, 124. [Google Scholar] [CrossRef]
- Aljeddani, G.S. Evaluation of Phytochemical, Antimicrobial, and Antioxidant Properties of Wild versus Cultivated Olive Leaves. Nat. Sci. 2022, 14, 448–461. [Google Scholar] [CrossRef]
- Charisiadis, P.; Kontogianni, V.G.; Tsiafoulis, C.G.; Tzakos, A.G.; Gerothanassis, I.P. Determination of Polyphenolic Phytochemicals using Highly Deshielded –OH 1H-NMR Signals. Phytochem. Anal. 2016, 28, 159–170. [Google Scholar] [CrossRef] [PubMed]
- Cukrov, M.; Žurga, P.; Germek, V.M.; Brkljača, M.; Ban, D.; Lukić, I.; Ban, S.G.; Pasković, I. Effect of Olive (Olea europaea L.) Variety on Leaf Biophenolic Profile. Agric. Conspec. Sci. 2021, 86, 277–282. [Google Scholar]
- Dini, I.; Graziani, G.; Fedele, F.L.; Sicari, A.; Vinale, F.; Castaldo, L.; Ritieni, A. Effects of Trichoderma Biostimulation on the Phenolic Profile of Extra-Virgin Olive Oil and Olive Oil By-Products. Antioxidants 2020, 9, 284. [Google Scholar] [CrossRef]
- Edziri, H.; Jaziri, R.; Chehab, H.; Verschaeve, L.; Flamini, G.; Boujnah, D.; Hammami, M.; Aouni, M.; Mastouri, M. A comparative study on chemical composition, antibiofilm and biological activities of leaves extracts of four Tunisian olive cultivars. Heliyon 2019, 5, e01604. [Google Scholar] [CrossRef] [PubMed]
- Essafi, H.; Trabelsi, N.; Benincasa, C.; Tamaalli, A.; Perri, E.; Zarrouk, M. Phytochemical profile, antioxidant and antiproliferative activities of olive leaf extracts from autochthonous Tunisian cultivars. Acta Aliment. 2019, 48, 384–390. [Google Scholar] [CrossRef]
- Orak, H.H.; Karamać, M.; Amarowicz, R.; Orak, A.; Penkacik, K. Genotype-Related Differences in the Phenolic Compound Profile and Antioxidant Activity of Extracts from Olive (Olea europaea L.) Leaves. Molecules 2019, 24, 1130. [Google Scholar] [CrossRef] [PubMed]
- Majumder, D.; Debnath, M.; Libin Kumar, K.V.; Nath, P.; Debnath, R.; Sarkar, C.; Prasad, G.B.K.S.; Verma, Y.K.; Maiti, D. Metabolic profiling and investigations on crude extract of Olea europaea L. leaves as a potential therapeutic agent against skin cancer. J. Funct. Foods 2019, 58, 266–274. [Google Scholar] [CrossRef]
- Nicolì, F.; Negro, C.; Vergine, M.; Aprile, A.; Nutricati, E.; Sabella, E.; Miceli, A.; Luvisi, A.; De Bellis, L. Evaluation of Phytochemical and Antioxidant Properties of 15 Italian Olea europaea L. Cultivar Leaves. Molecules 2019, 24, 1998. [Google Scholar] [CrossRef] [PubMed]
- Omar, S.H.; Kerr, P.G.; Scott, C.J.; Hamlin, A.S.; Obied, H.K. Olive (Olea europaea L.) Biophenols: A Nutriceutical against Oxidative Stress in SH-SY5Y Cells. Molecules 2017, 22, 1858. [Google Scholar] [CrossRef]
- Gagour, J.; Hallouch, O.; Asbbane, A.; Bijla, L.; Laknifli, A.; Lee, L.-H.; Zengin, G.; Bouyahya, A.; Sakar, E.H.; Gharby, S. A Review of Recent Progresses on Olive Oil Chemical Profiling, Extraction Technology, Shelf-Life, and Quality Control. Chem. Biodivers. 2024, 21, e202301697. [Google Scholar] [CrossRef] [PubMed]
- Sarikurkcu, C.; Locatelli, M.; Tartaglia, A.; Ferrone, V.; Juszczak, A.M.; Ozer, M.S.; Tepe, B.; Tomczyk, M. Enzyme and Biological Activities of the Water Extracts from the Plants Aesculus hippocastanum, Olea europaea and Hypericum perforatum That Are Used as Folk Remedies in Turkey. Molecules 2020, 25, 1202. [Google Scholar] [CrossRef]
- Termentzi, A.; Halabalaki, M.; Skaltsounis, A.L. 6—From Drupes to Olive Oil: An Exploration of Olive Key Metabolites. In Olive and Olive Oil Bioactive Constituents; Boskou, D., Ed.; AOCS Press: Champaign, IL, USA, 2015; pp. 147–177. [Google Scholar] [CrossRef]
- Damtoft, S.; Franzyk, H.; Jensen, S.R. Excelsioside, a secoiridoid glucoside from Fraxinus excelsior. Phytochemistry 1992, 31, 4197–4201. [Google Scholar] [CrossRef]
- Cecchi, L.; Migliorini, M.; Cherubini, C.; Innocenti, M.; Mulinacci, N. Whole Lyophilized Olives as Sources of Unexpectedly High Amounts of Secoiridoids: The Case of Three Tuscan Cultivars. J. Agric. Food Chem. 2015, 63, 1175–1185. [Google Scholar] [CrossRef]
- Šarolić, M.; Gugić, M.; Tuberoso, C.I.G.; Jerković, I.; Šuste, M.; Marijanović, Z.; Kuś, P.M. Volatile Profile, Phytochemicals and Antioxidant Activity of Virgin Olive Oils from Croatian Autochthonous Varieties Mašnjača and Krvavica in Comparison with Italian Variety Leccino. Molecules 2014, 19, 881–895. [Google Scholar] [CrossRef]
- Waqar Ahmad, N.A.; Afridi, M.S.; Rahman, H.; Adnan, M.; Ullah, N.; Muhammad, U.; Ilyas, M.; Khan, H. Phytochemical profile, antimicrobial potential and GC-MS analysis of wild variety of Olea europaea (Olive) cultivated in Pakistan. Pure Appl. Biol. PAB 2017, 6, 337–345. [Google Scholar]
- Nazzaro, F.; Fratianni, F.; Cozzolino, R.; Martignetti, A.; Malorni, L.; De Feo, V.; Cruz, A.G.; d’Acierno, A. Antibacterial Activity of Three Extra Virgin Olive Oils of the Campania Region, Southern Italy, Related to Their Polyphenol Content and Composition. Microorganisms 2019, 7, 321. [Google Scholar] [CrossRef]
- Alagna, F.; Geu-Flores, F.; Kries, H.; Panara, F.; Baldoni, L.; O’Connor, S.E.; Osbourn, A. Identification and Characterization of the Iridoid Synthase Involved in Oleuropein Biosynthesis in Olive (Olea europaea) Fruits*. J. Biol. Chem. 2016, 291, 5542–5554. [Google Scholar] [CrossRef]
- Maalej, A.; Bouallagui, Z.; Hadrich, F.; Isoda, H.; Sayadi, S. Assessment of Olea europaea L. fruit extracts: Phytochemical characterization and anticancer pathway investigation. Biomed. Pharmacother. 2017, 90, 179–186. [Google Scholar] [CrossRef]
- Hannachi, H.; Elfalleh, W.; Marzouk, S. Oil, protein, antioxidants and free radical scavenging activity of stone from wild olive trees (Olea europaea L.). Pak. J. Pharm. Sci. 2013, 26, 503–510. [Google Scholar] [PubMed]
- Montealegre, C.; Sánchez-Hernández, L.; Crego, A.L.; Marina, M.L. Determination and Characterization of Glycerophospholipids in Olive Fruit and Oil by Nonaqueous Capillary Electrophoresis with Electrospray-Mass Spectrometric Detection. J. Agric. Food Chem. 2013, 61, 1823–1832. [Google Scholar] [CrossRef]
- He, M.; Ding, N.-Z. Plant Unsaturated Fatty Acids: Multiple Roles in Stress Response. Front. Plant Sci. 2020, 11, 562785. [Google Scholar] [CrossRef]
- Alves, E.; Melo, T.; Rey, F.; Moreira, A.S.P.; Domingues, P.; Domingues, M.R. Polar lipid profiling of olive oils as a useful tool in helping to decipher their unique fingerprint. LWT 2016, 74, 371–377. [Google Scholar] [CrossRef]
- Silva, S.; Gomes, L.; Leitão, F.; Bronze, M.; Coelho, A.V.; Boas, L.V. Secoiridoids in olive seed: Characterization of nüzhenide and 11-methyl oleosides by liquid chromatography with diode array and mass spectrometry. Grasas Aceites 2010, 61, 157–164. [Google Scholar] [CrossRef]
- Cano-Lamadrid, M.; Lipan, L.; Hernández, F.; Martínez, J.J.; Legua, P.; Carbonell-Barrachina, Á.A.; Melgarejo, P. Quality Parameters, Volatile Composition, and Sensory Profiles of Highly Endangered Spanish Citrus Fruits. J. Food Qual. 2018, 2018, e3475461. [Google Scholar] [CrossRef]
- Abu-Izneid, T.; Rauf, A.; Shariati, M.A.; Khalil, A.A.; Imran, M.; Rebezov, M.; Uddin, M.d.S.; Mahomoodally, M.F.; Rengasamy, K.R.R. Sesquiterpenes and their derivatives-natural anticancer compounds: An update. Pharmacol. Res. 2020, 161, 105165. [Google Scholar] [CrossRef]
- Belščak-Cvitanović, A.; Durgo, K.; Bušić, A.; Franekić, J.; Komes, D. Phytochemical Attributes of Four Conventionally Extracted Medicinal Plants and Cytotoxic Evaluation of Their Extracts on Human Laryngeal Carcinoma (HEp2) Cells. J. Med. Food 2014, 17, 206–217. [Google Scholar] [CrossRef]
- El-Darier, S.; Abdel-Rahman, A.; Saad, T. Differential Responses of Hordeum vulgare L. to Allelochemicals of Some Olea europaea L. Cultivars. Catrina Int. J. Environ. Sci. 2018, 17, 91–101. [Google Scholar] [CrossRef]
- Badawy, M.; Faraj, H. Chemical Composition and Activity of Bark and Leaf Extracts of Pinus Halepensis and Olea europaea Grown in Al-Jabel Al-Akhdar Region, Libya against some Plant Phytopathogens. J. Appl. Biotechnol. Bioeng. 2019, 3, 331–342. [Google Scholar] [CrossRef]
- Dobrinčić, A.; Repajić, M.; Garofulić, I.E.; Tuđen, L.; Dragović-Uzelac, V.; Levaj, B. Comparison of Different Extraction Methods for the Recovery of Olive Leaves Polyphenols. Processes 2020, 8, 1008. [Google Scholar] [CrossRef]
- Mechri, B.; Tekaya, M.; Hammami, M.; Chehab, H. Effects of drought stress on phenolic accumulation in greenhouse-grown olive trees (Olea europaea). Biochem. Syst. Ecol. 2020, 92, 104112. [Google Scholar] [CrossRef]
- Šikić Pogačar, M.; Klančnik, A.; Bucar, F.; Langerholc, T.; Smole Možina, S. Anti-adhesion activity of thyme (Thymus vulgaris L.) extract, thyme post-distillation waste, and olive (Olea europea L.) leaf extract against Campylobacter jejuni on polystyrene and intestine epithelial cells. J. Sci. Food Agric. 2016, 96, 2723–2730. [Google Scholar] [CrossRef]
- Rashed, S.A.; Saad, T.I.; El-Darier, S.M. Potential aptitude of four olive cultivars as anticancer and antioxidant agents: Oleuropein content. Rend. Lincei Sci. Fis. Nat. 2022, 33, 195–203. [Google Scholar] [CrossRef]
- Ghosia, L.; Khan, A.; Tila, H.; Hussain, A.; Khan, A. Evaluation of Plants Extracts for Proximate Chemical Composition, Antimicrobial and Antifungal Activities. Am.-Eurasian J. Agric. Environ. Sci. 2014, 14, 964–970. [Google Scholar]
- Celik, H.; Nadaroglu, H.; Senol, M. Evaluation of antioxidant, antiradicalic and antimicrobial activities of olive pits (Olea europaea L.). Bulg. J. Agric. Sci. 2014, 20, 1392–1400. [Google Scholar]
- Coccia, A.; Bastianelli, D.; Mosca, L.; Monticolo, R.; Panuccio, I.; Carbone, A.; Calogero, A.; Lendaro, E. Extra Virgin Olive Oil Phenols Suppress Migration and Invasion of T24 Human Bladder Cancer Cells through Modulation of Matrix Metalloproteinase-2. Nutr. Cancer 2014, 66, 946–954. [Google Scholar] [CrossRef]
- Hadrich, F.; Bouallagui, Z.; Junkyu, H.; Isoda, H.; Sayadi, S. The α-Glucosidase and α-Amylase Enzyme Inhibitory of Hydroxytyrosol and Oleuropein. J. Oleo Sci. 2015, 64, 835–843. [Google Scholar] [CrossRef]
- Guo, L.; Sun, Q.; Gong, S.; Bi, X.; Jiang, W.; Xue, W.; Fei, P. Antimicrobial Activity and Action Approach of the Olive Oil Polyphenol Extract against Listeria monocytogenes. Front. Microbiol. 2019, 10, 1586. [Google Scholar] [CrossRef]
- Lood, R.; Waldetoft, K.W.; Nordenfelt, P. Localization-triggered bacterial pathogenesis. Future Microbiol. 2015, 10, 1659–1668. [Google Scholar] [CrossRef]
- Kadir, B.; Zehra, K.F.; Abdullah, A.M.; Selami, G.; Yakup, Y. Antioxidant and antithrombotic properties of fruit, leaf, and seed extracts of the halhali olive (Olea europaea L.) native to the hatay region in turkey. Foods Raw Mater. 2023, 11, 84–93. [Google Scholar]
- Hanene, G.; Aouadhi, C.; Hamrouni, S.; Mnif, W. Antibacterial, antifungal and antioxidant activities of tunisian Olea europaea ssp. oleaster fruit pulp and its essential fatty acids. Int. J. Pharm. Pharm. Sci 2014, 7, 52–55. [Google Scholar]
- Chen, M.; Zhao, Z.; Meng, H.; Yu, S. The antibiotic activity and mechanisms of sugar beet (Beta vulgaris) molasses polyphenols against selected food-borne pathogens. LWT—Food Sci. Technol. 2017, 82, 354–360. [Google Scholar] [CrossRef]
- Dias, M.I.; Barros, L.; Barreira, J.C.M.; Alves, M.J.; Barracosa, P.; Ferreira, I.C.F.R. Phenolic profile and bioactivity of cardoon (Cynara cardunculus L.) inflorescence parts: Selecting the best genotype for food applications. Food Chem. 2018, 268, 196–202. [Google Scholar] [CrossRef]
- Wang, L.-H.; Zhang, Z.-H.; Zeng, X.-A.; Gong, D.-M.; Wang, M.-S. Combination of microbiological, spectroscopic and molecular docking techniques to study the antibacterial mechanism of thymol against Staphylococcus aureus: Membrane damage and genomic DNA binding. Anal. Bioanal. Chem. 2017, 409, 1615–1625. [Google Scholar] [CrossRef]
- Abdullah, B.A.; Saeed, H.S.M.; Al-Taii, N.A. Antimicrobial activity and evaluation of genetic effects of olive leaves using molecular techniques. J. Entomol. Zool. Stud. 2017, 6, 1493–1495. [Google Scholar]
- Alsulaymani, F.A.; Elmhdwi, M.; Gaber, S. In Vitro Antioxidant and Antibacterial Activity of Olive Leave Extract. J. Pharm. Appl. Chem. 2021, 7, 41–47. [Google Scholar] [CrossRef]
- De la Ossa, J.G.; El Kadri, H.; Gutierrez-Merino, J.; Wantock, T.; Harle, T.; Seggiani, M.; Danti, S.; Di Stefano, R.; Velliou, E. Combined Antimicrobial Effect of Bio-Waste Olive Leaf Extract and Remote Cold Atmospheric Plasma Effluent. Molecules 2021, 26, 1890. [Google Scholar] [CrossRef]
- Himour, S.; Yahia, A.; Belattar, H. Oleuropein and Antibacterial Activities of Olea europaea L. Leaf Extract. Eur. Sci. J. ESJ 2017, 13, 342. [Google Scholar] [CrossRef]
- Da Rosa, G.S.; Martiny, T.R.; Dotto, G.L.; Vanga, S.K.; Parrine, D.; Gariepy, Y.; Lefsrud, M.; Raghavan, V. Eco-friendly extraction for the recovery of bioactive compounds from Brazilian olive leaves. Sustain. Mater. Technol. 2021, 28, e00276. [Google Scholar] [CrossRef]
- Ahmed, A.M.; Rabii, N.S.; Garbaj, A.M.; Abolghait, S.K. Antibacterial Effect of Olive (Olea europaea L.) Leaves Extract in Raw Peeled Undeveined Shrimp (Penaeus semisulcatus). Int. J. Vet. Sci. Med. 2014, 2, 53–56. [Google Scholar] [CrossRef]
- Vural, N.; Akay, M.A. Chemical compounds, antioxidant properties and antimicrobial activity of olive leaves derived volatile oil in West Anatolia. J. Turk. Chem. Soc. Sect. Chem. 2021, 8, 511–518. [Google Scholar] [CrossRef]
- El Yamani, M.; Sakar, E.H.; Boussakouran, A.; Benali, T.; Rharrabti, Y. Antibacterial and antioxidant potentials of phenolic extracts from olive mill wastewater and their use to enhance the stability of olive oil. Riv. Ital. Delle Sostanze Grasse 2020, 97, 31–42. [Google Scholar]
- Guo, L.; Gong, S.; Wang, Y.; Sun, Q.; Duo, K.; Fei, P. Antibacterial Activity of Olive Oil Polyphenol Extract against Salmonella Typhimurium and Staphylococcus aureus: Possible Mechanisms. Foodborne Pathog. Dis. 2020, 17, 396–403. [Google Scholar] [CrossRef]
- Ahmad, W.; Khan, H.; Safeer, M.; Khan, S.; Junaid, M.; Mehmood, A.; Siddiqueafridi, M.; Rafi, A.; Khan, A.; Ayaz, U.; et al. Comparative antimicrobial activities of wild and cultivated varieties of Olea europaea different leaves extracts in Pakistan. Bull. Env. Pharmacol. Life Sci. 2020, 9, 73–82. [Google Scholar]
- Elnahas, R.A.; Elwakil, B.H.; Elshewemi, S.S.; Olama, Z.A. Egyptian Olea europaea leaves bioactive extract: Antibacterial and wound healing activity in normal and diabetic rats. J. Tradit. Complement. Med. 2021, 11, 427–434. [Google Scholar] [CrossRef]
- Benali, T.; Habbadi, K.; Khabbach, A.; Marmouzi, I.; Zengin, G.; Bouyahya, A.; Chamkhi, I.; Chtibi, H.; Aanniz, T.; Achbani, E.H.; et al. GC–MS Analysis, Antioxidant and Antimicrobial Activities of Achillea odorata Subsp. Pectinata and Ruta montana Essential Oils and Their Potential Use as Food Preservatives. Foods 2020, 9, 668. [Google Scholar] [CrossRef]
- Testa, B.; Lombardi, S.J.; Macciola, E.; Succi, M.; Tremonte, P.; Iorizzo, M. Efficacy of olive leaf extract (Olea europaea L. cv Gentile di Larino) in marinated anchovies (Engraulis encrasicolus, L.) process. Heliyon 2019, 5, e01727. [Google Scholar] [CrossRef]
- Dada, E.O. Antibacterial Activities of Olea europaea Leaf Extract on Some Bacteria Isolated from a Refused Dump Site in Akure, Nigeria. J. Biol. 2013, 1, 118–124. [Google Scholar]
- Ghomari, O.; Sounni, F.; Massaoudi, Y.; Ghanam, J.; Drissi Kaitouni, L.B.; Merzouki, M.; Benlemlih, M. Phenolic profile (HPLC-UV) of olive leaves according to extraction procedure and assessment of antibacterial activity. Biotechnol. Rep. 2019, 23, e00347. [Google Scholar] [CrossRef]
- Golestannejad, Z.; Khozeimeh, F.; Abtahi, R.; Zarei, Z.; Sadeghalbanaei, L.; Sadeghian, R. Inhibitory effects of ethanolic, methanolic, and hydroalcoholic extracts of olive (Olea europaea) leaf on growth, acid production, and adhesion of Streptococcus mutans. Dent. Res. J. 2020, 17, 179–185. [Google Scholar]
- Karygianni, L.; Cecere, M.; Skaltsounis, A.L.; Argyropoulou, A.; Hellwig, E.; Aligiannis, N.; Wittmer, A.; Al-Ahmad, A. High-Level Antimicrobial Efficacy of Representative Mediterranean Natural Plant Extracts against Oral Microorganisms. BioMed Res. Int. 2014, 2014, e839019. [Google Scholar] [CrossRef]
- Lass-Flörl, C.; Kanj, S.S.; Govender, N.P.; Thompson, G.R.; Ostrosky- Zeichner, L.; Govrins, M.A. Invasive Candidiasis. Nat. Rev. Dis. Primers 2024, 10, 1–18. [Google Scholar] [CrossRef]
- Noor, S. Synergistic Effect of the Methanolic Extract of Lemongrass and Some Antibiotics to Treat Urinary Tract Bacteria. J. Biosci. Med. 2016, 4, 48–58. [Google Scholar] [CrossRef][Green Version]
- Bodey, G.P.; Mardani, M.; Hanna, H.A.; Boktour, M.; Abbas, J.; Girgawy, E.; Hachem, R.Y.; Kontoyiannis, D.P.; Raad, I.I. The epidemiology of Candida glabrata and Candida albicans fungemia in immunocompromised patients with cancer. Am. J. Med. 2002, 112, 380–385. [Google Scholar] [CrossRef]
- Brandt, C.; Makarewicz, O.; Fischer, T.; Stein, C.; Pfeifer, Y.; Werner, G.; Pletz, M.W. The bigger picture: The history of antibiotics and antimicrobial resistance displayed by scientometric data. Int. J. Antimicrob. Agents 2014, 44, 424–430. [Google Scholar] [CrossRef]
- Llor, C.; Bjerrum, L. Antimicrobial resistance: Risk associated with antibiotic overuse and initiatives to reduce the problem. Ther. Adv. Drug Saf. 2014, 5, 229–241. [Google Scholar] [CrossRef]
- Khalil, M.M.H.; Ismail, E.H.; El-Baghdady, K.Z.; Mohamed, D. Green synthesis of silver nanoparticles using olive leaf extract and its antibacterial activity. Arab. J. Chem. 2014, 7, 1131–1139. [Google Scholar] [CrossRef]
- Brahmi, F.; Flamini, G.; Issaoui, M.; Dhibi, M.; Dabbou, S.; Mastouri, M.; Hammami, M. Chemical composition and biological activities of volatile fractions from three Tunisian cultivars of olive leaves. Med. Chem. Res. 2012, 21, 2863–2872. [Google Scholar] [CrossRef]
- Upadhyay, R.K. Evaluation of antibacterial and antifungal activities of olive (Olea europaea) essential oil. Int. J. Green Pharm. IJGP 2014, 8, 180. [Google Scholar]
- Alimosazadeh, P.; Khan, M.S.A.; Sanei, S.J. Phytochemical and anticandidal efficacy of Olea europaea leaf extract from different cultivars and seasonal variation. J. Herbmed. Pharmacol. 2023, 12, 505–511. [Google Scholar] [CrossRef]
- Bawadekji, A.; Imran, M.; Randahawa, M.A. Antimicrobial Effects of the Water Immiscible Solvent Extracts of Olive Tree Leaves. J. Pure Appl. Microbiol. 2019, 13, 2189–2194. [Google Scholar] [CrossRef]
- Nasrollahi, Z.; Abolhasannezhad, M. Evaluation of the antifungal activity of olive leaf aqueous extracts against Candida albicans PTCC-5027. Curr. Med. Mycol. 2015, 1, 37–39. [Google Scholar] [CrossRef]
- Omar, H.S.; Abd El-Rahman, S.N.; AlGhannam, S.M.; Reyad, N.E.-H.A.; Sedeek, M.S. Antifungal Evaluation and Molecular Docking Studies of Olea europaea Leaf Extract, Thymus vulgaris and Boswellia carteri Essential Oil as Prospective Fungal Inhibitor Candidates. Molecules 2021, 26, 6118. [Google Scholar] [CrossRef]
- Wang, B.; Shen, S.; Qu, J.; Xu, Z.; Feng, S.; Chen, T.; Ding, C. Optimizing Total Phenolic and Oleuropein of Chinese Olive (Olea europaea) Leaves for Enhancement of the Phenols Content and Antioxidant Activity. Agronomy 2021, 11, 686. [Google Scholar] [CrossRef]
- Ilias, F.; Kholkhal, W.; Gaouar, N.; Bekhechi, C.; Bekkara, F. Antibacterial and antifungal Activities of olive (Olea europaea L.) from Algeria. J. Microbiol. Biotech Res. 2011, 1, 69–73. [Google Scholar]
- Najee, H.B.; Alkurjia, D.; Almahdawy, O.; Kamerzan, C.; Marutescu, L.; Gheorghe, I.; Popa, M.; Chifiriuc, M.C.; Lazăr, V. Antimicrobial Activity of Olea europaea Fatty Oil against Multi-Drug Resistant and Biofilm Forming Microorganisms. Not. Sci. Biol. 2018, 10, 498–502. [Google Scholar] [CrossRef][Green Version]
- Janakat, S.; Al-Nabulsi, A.A.R.; Allehdan, S.; Olaimat, A.N.; Holley, R.A. Antimicrobial activity of amurca (olive oil lees) extract against selected foodborne pathogens. Food Sci. Technol. 2015, 35, 259–265. [Google Scholar] [CrossRef]
- Umai, D.; Vikranth, A.; Meenambiga, S.S. A study on the green synthesis of silver nanoparticles from Olea europaea and its activity against oral pathogens. Mater. Today Proc. 2021, 44, 3647–3651. [Google Scholar] [CrossRef]
- Suwan, T.; Khongkhunthian, S.; Okonogi, S. Green synthesis and inhibitory effects against oral pathogens of silver nanoparticles mediated by rice extracts. Drug Discov. Ther. 2018, 12, 189–196. [Google Scholar] [CrossRef]
- Fleming, D.; Rumbaugh, K.P. Approaches to Dispersing Medical Biofilms. Microorganisms 2017, 5, 15. [Google Scholar] [CrossRef]
- Mohanta, Y.K.; Biswas, K.; Jena, S.K.; Hashem, A.; Abd_Allah, E.F.; Mohanta, T.K. Anti-biofilm and Antibacterial Activities of Silver Nanoparticles Synthesized by the Reducing Activity of Phytoconstituents Present in the Indian Medicinal Plants. Front. Microbiol. 2020, 11, 1143. [Google Scholar] [CrossRef]
- Hussain, M.A.; Khan, M.Q.; Ali, I.; Dar, M.E.U.I.; Habib, T. 08. Antifungal potential of different parts of Olea europaea and Olea cuspidata growing in Azad Jammu and Kashmir. Pure Appl. Biol. PAB 2021, 4, 204–216. [Google Scholar] [CrossRef]
- Di Pietro, M.; Filardo, S.; Mattioli, R.; Francioso, A.; Raponi, G.; Mosca, L.; Sessa, R. Extra Virgin Olive Oil-Based Green Formulations with Promising Antimicrobial Activity against Drug-Resistant Isolates. Front. Pharmacol. 2022, 13, 885735. [Google Scholar] [CrossRef]
- Kleef, F.; Salman, M. Antifungal Effect of Ambrosia artemisiifolia L. Extract and Chemical Fungicide against Spilocaea oleagina Causing Olive Leaf Spot. Arab. J. Sci. Eng. 2022, 47, 113–117. [Google Scholar] [CrossRef]
- Zorić, N.; Kopjar, N.; Kraljić, K.; Oršolić, N.; Tomić, S.; Kosalec, I. Olive leaf extract activity against Candida albicans and C. dubliniensis—The in vitro viability study. Acta Pharm. 2016, 66, 411–431. [Google Scholar] [CrossRef]
- Laaboudi, W. Eco-extraction of phenolic compounds from Moroccan olive fruits and leaves and their potential use as antimicrobial agents. Eur. J. Sci. Res. 2015, 132, 255–265. [Google Scholar]
- Halliwell, B. Oxygen and nitrogen are pro-carcinogens. Damage to DNA by reactive oxygen, chlorine and nitrogen species: Measurement, mechanism and the effects of nutrition. Mutat. Res. 1999, 443, 37–52. [Google Scholar] [CrossRef]
- Jakubczyk, K.; Dec, K.; Kałduńska, J.; Kawczuga, D.; Kochman, J.; Janda, K. Reactive oxygen species—Sources, functions, oxidative damage. Pol. Merkur. Lek. Organ Pol. Tow. Lek. 2020, 48, 124–127. [Google Scholar]
- Anjaneyulu, E.; Reddy, P.S.; Sunita, M.S.; Kishor, P.B.K.; Meriga, B. Salt tolerance and activity of antioxidative enzymes of transgenic finger millet overexpressing a vacuolar H+-pyrophosphatase gene (SbVPPase) from Sorghum bicolor. J. Plant Physiol. 2014, 171, 789–798. [Google Scholar] [CrossRef]
- Hahn, A.; Zuryn, S. Mitochondrial Genome (mtDNA) Mutations that Generate Reactive Oxygen Species. Antioxidants 2019, 8, 392. [Google Scholar] [CrossRef]
- Yadav, D.K.; Kumar, S.; Choi, E.-H.; Chaudhary, S.; Kim, M.-H. Molecular dynamic simulations of oxidized skin lipid bilayer and permeability of reactive oxygen species. Sci. Rep. 2019, 9, 4496. [Google Scholar] [CrossRef]
- Höhn, A.; Tramutola, A.; Cascella, R. Proteostasis Failure in Neurodegenerative Diseases: Focus on Oxidative Stress. Oxid. Med. Cell. Longev. 2020, 2020, 5497046. [Google Scholar] [CrossRef]
- Kao, Y.-C.; Ho, P.-C.; Tu, Y.-K.; Jou, I.-M.; Tsai, K.-J. Lipids and Alzheimer’s Disease. Int. J. Mol. Sci. 2020, 21, 1505. [Google Scholar] [CrossRef]
- Raghav, K.; Bailey, A.M.; Loree, J.M.; Kopetz, S.; Holla, V.; Yap, T.A.; Wang, F.; Chen, K.; Salgia, R.; Hong, D. Untying the gordion knot of targeting MET in cancer. Cancer Treat. Rev. 2018, 66, 95–103. [Google Scholar] [CrossRef]
- Cadenas, S. Mitochondrial uncoupling, ROS generation and cardioprotection. Biochim. Biophys. Acta Bioenerg. 2018, 1859, 940–950. [Google Scholar] [CrossRef]
- Zaïri, A.; Nouir, S.; Zarrouk, A.; Haddad, H.; Khélifa, A.; Achour, L. Phytochemical Profile, Cytotoxic, Antioxidant, and Allelopathic Potentials of Aqueous Leaf Extracts of Olea europaea. Food Sci. Nutr. 2020, 8, 4805–4813. [Google Scholar] [CrossRef]
- Dağdelen, A. Identifying Antioxidant and Antimicrobial Activities of the Phenolic Extracts and Mineral Contents of Virgin Olive Oils (Olea europaea L. cv. Edincik Su) from Different Regions in Turkey. J. Chem. 2016, 2016, e9589763. [Google Scholar] [CrossRef]
- Yuan, J.-J.; Wang, C.-Z.; Ye, J.-Z.; Tao, R.; Zhang, Y.-S. Enzymatic Hydrolysis of Oleuropein from Olea europea (Olive) Leaf Extract and Antioxidant Activities. Molecules 2015, 20, 2903–2921. [Google Scholar] [CrossRef]
- Lfitat, A.; Zejli, H.; Bousselham, A.; Atki, Y.E.; Lyoussi, B.; Gourch, A.; Abdellaoui, A. Comparative Evaluation of Argania spinosa and Olea europaea Leaf Phenolic Compounds and their Antioxidant Activity. Botanica 2020, 26, 76–87. [Google Scholar] [CrossRef]
- De Melo, M.P. Antioxidant actions of olive leaf extract (Olea europaea L.) on reactive species scavengers. J. Anal. Pharm. Res. 2020, 9, 68–71. [Google Scholar] [CrossRef]
- Pennisi, R.; Ben Amor, I.; Gargouri, B.; Attia, H.; Zaabi, R.; Chira, A.B.; Saoudi, M.; Piperno, A.; Trischitta, P.; Tamburello, M.P.; et al. Analysis of Antioxidant and Antiviral Effects of Olive (Olea europaea L.) Leaf Extracts and Pure Compound Using Cancer Cell Model. Biomolecules 2023, 13, 238. [Google Scholar] [CrossRef]
- Zun-Qiu, W.; Gui-Zhou, Y.; Qing-Ping, Z.; You-Jun, J.; Kai-Yu, T.; Hua-Ping, C.; Ze-Shen, Y.; Qian-Ming, H. Purification, Dynamic Changes and Antioxidant Activities of Oleuropein in Olive (Olea europaea L.) Leaves. J. Food Biochem. 2015, 39, 566–574. [Google Scholar] [CrossRef]
- Fernández-Poyatos, M.P.; Ruiz-Medina, A.; Llorent-Martínez, E.J. Phytochemical profile, mineral content, and antioxidant activity of Olea europaea L. cv. Cornezuelo table olives. Influence of in vitro simulated gastrointestinal digestion. Food Chem. 2019, 297, 124933. [Google Scholar] [CrossRef]
- Tamasi, G.; Baratto, M.C.; Bonechi, C.; Byelyakova, A.; Pardini, A.; Donati, A.; Leone, G.; Consumi, M.; Lamponi, S.; Magnani, A.; et al. Chemical characterization and antioxidant properties of products and by-products from Olea europaea L. Food Sci. Nutr. 2019, 7, 2907–2920. [Google Scholar] [CrossRef]
- Borjan, D.; Leitgeb, M.; Knez, Ž.; Hrnčič, M.K. Microbiological and Antioxidant Activity of Phenolic Compounds in Olive Leaf Extract. Molecules 2020, 25, 5946. [Google Scholar] [CrossRef]
- Blasi, F.; Urbani, E.; Simonetti, M.; Chiesi, C.; Cossignani, L. Seasonal variations in antioxidant compounds of Olea europaea leaves collected from different Italian cultivars. J. Appl. Bot. Food Qual. 2016, 89, 202–207. [Google Scholar] [CrossRef]
- Calvo, M.M.; Martín-Diana, A.B.; Rico, D.; López-Caballero, M.E.; Martínez-Álvarez, O. Antioxidant, Antihypertensive, Hypoglycaemic and Nootropic Activity of a Polyphenolic Extract from the Halophyte Ice Plant (Mesembryanthemum crystallinum). Foods 2022, 11, 1581. [Google Scholar] [CrossRef]
- Lins, P.G.; Marina Piccoli Pugine, S.; Scatolini, A.M.; De Melo, M.P. In vitro antioxidant activity of olive leaf extract (Olea europaea L.) and its protective effect on oxidative damage in human erythrocytes. Heliyon 2018, 4, e00805. [Google Scholar] [CrossRef]
- Ayoub, L.; Hassan, F.; Hamid, S.; Abdelhamid, Z.; Souad, A. Phytochemical screening, antioxidant activity and inhibitory potential of Ficus carica and Olea europaea leaves. Bioinformation 2019, 15, 226–232. [Google Scholar] [CrossRef]
- Monteleone, J.I.; Sperlinga, E.; Siracusa, L.; Spagna, G.; Parafati, L.; Todaro, A.; Palmeri, R. Water as a Solvent of Election for Obtaining Oleuropein-Rich Extracts from Olive (Olea europaea) Leaves. Agronomy 2021, 11, 465. [Google Scholar] [CrossRef]
- Zhao, H.; Avena-Bustillos, R.J.; Wang, S.C. Extraction, Purification and In Vitro Antioxidant Activity Evaluation of Phenolic Compounds in California Olive Pomace. Foods 2022, 11, 174. [Google Scholar] [CrossRef]
- Ribas, J.C.R.; Lazzari, A.; Gonzalez, L.B.F.; da Silva, C.M.; Adamuchio, L.G.; Cuquel, F.L.; Sakurada, R.; Pintro, P.T.M. Bioactive compounds and antioxidant activity of leaves from olive trees grown in Paraná, Brazil. Pesqui. Agropecuária Bras. 2023, 58, e03025. [Google Scholar] [CrossRef]
- Romero-Márquez, J.M.; Navarro-Hortal, M.D.; Forbes-Hernández, T.Y.; Varela-López, A.; Puentes, J.G.; Pino-García, R.D.; Sánchez-González, C.; Elio, I.; Battino, M.; García, R.; et al. Exploring the Antioxidant, Neuroprotective, and Anti-Inflammatory Potential of Olive Leaf Extracts from Spain, Portugal, Greece, and Italy. Antioxidants 2023, 12, 1538. [Google Scholar] [CrossRef]
- Cho, N.H.; Shaw, J.E.; Karuranga, S.; Huang, Y.; da Rocha Fernandes, J.D.; Ohlrogge, A.W.; Malanda, B. IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res. Clin. Pract. 2018, 138, 271–281. [Google Scholar] [CrossRef]
- Park, M.-K.; Jung, U.; Roh, C. Fucoidan from Marine Brown Algae Inhibits Lipid Accumulation. Mar. Drugs 2011, 9, 1359–1367. [Google Scholar] [CrossRef]
- Thomas, N.J.; Jones, S.E.; Weedon, M.N.; Shields, B.M.; Oram, R.A.; Hattersley, A.T. Frequency and phenotype of type 1 diabetes in the first six decades of life: A cross-sectional, genetically stratified survival analysis from UK Biobank. Lancet Diabetes Endocrinol. 2018, 6, 122–129. [Google Scholar] [CrossRef]
- Priscilla, D.H.; Jayakumar, M.; Thirumurugan, K. Flavanone naringenin: An effective antihyperglycemic and antihyperlipidemic nutraceutical agent on high fat diet fed streptozotocin induced type 2 diabetic rats. J. Funct. Foods 2015, 14, 363–373. [Google Scholar] [CrossRef]
- Sundaram, R.; Shanthi, P.; Sachdanandam, P. Effect of tangeretin, a polymethoxylated flavone on glucose metabolism in streptozotocin-induced diabetic rats. Phytomedicine 2014, 21, 793–799. [Google Scholar] [CrossRef]
- Perri, M.R.; Marrelli, M.; Statti, G.; Conforti, F. Olea europaea bud extracts: Inhibitory effects on pancreatic lipase and α-amylase activities of different cultivars from Calabria region (Italy). Plant Biosyst.—Int. J. Deal. Asp. Plant Biol. 2022, 156, 338–344. [Google Scholar] [CrossRef]
- Akhtar, M.F.; Ashraf, K.M.; Saleem, A.; Sharif, A.; Zubair, H.M.; Anwar, F. Antidiabetic Potential and Antioxidant Activity of Olea europaea subsp. Cuspidata (Indian Olive) Seed Extracts. Evid. Based Complement. Altern. Med. 2022, 2022, e5164985. [Google Scholar] [CrossRef]
- Zakari, A.; Umar, H.; Auwal, I. Hypoglycemic Effect of Olive Oil (Olea europea) on Alloxan-Induced Diabetic Albino Rats. Niger. J. Chem. Res. 2023, 28, 051–058. [Google Scholar] [CrossRef]
- Figueiredo-González, M.; Reboredo-Rodríguez, P.; González-Barreiro, C.; Carrasco-Pancorbo, A.; Cancho-Grande, B.; Simal-Gándara, J. The involvement of phenolic-rich extracts from Galician autochthonous extra-virgin olive oils against the α-glucosidase and α-amylase inhibition. Food Res. Int. 2019, 116, 447–454. [Google Scholar] [CrossRef]
- Abdelkarem, H.M.; El-Sherif, M.A.; Gomma, S.B.; Kassem, S.S.; Abdelkader, M.M. Olive Leaf Powder Modulate Insulin Production and Circulating Adipokines in Streptozotocin Induced Diabetic Rats. J. Diet. Suppl. 2022, 19, 550–565. [Google Scholar] [CrossRef]
- Al-Shudiefat, A.A.-R.; Alturk, H.; Al-Ameer, H.J.; Zihlif, M.; Alenazy, M. Olive Leaf Extract of Olea europaea Reduces Blood Glucose Level through Inhibition of AS160 in Diabetic Rats. Appl. Sci. 2023, 13, 5939. [Google Scholar] [CrossRef]
- Chigurupati, S.; Alharbi, F.S.; Almahmoud, S.; Aldubayan, M.; Almoshari, Y.; Vijayabalan, S.; Bhatia, S.; Chinnam, S.; Venugopal, V. Molecular docking of phenolic compounds and screening of antioxidant and antidiabetic potential of Olea europaea L. Ethanolic leaves extract. Arab. J. Chem. 2021, 14, 103422. [Google Scholar] [CrossRef]
- Laaboudi, W.; Ghanam, J.; Ghoumari, O.; Sounni, F.; Merzouki, M.; Benlemlih, M. Hypoglycemic and hypolipidemic effects of phenolic olive tree extract in streptozotocin diabetic rats. Int. J. Pharm. Pharm. Sci. 2016, 8, 287. [Google Scholar] [CrossRef]
- Dekdouk, N.; Malafronte, N.; Russo, D.; Faraone, I.; De Tommasi, N.; Ameddah, S.; Severino, L.; Milella, L. Phenolic Compounds from Olea europaea L. Possess Antioxidant Activity and Inhibit Carbohydrate Metabolizing Enzymes In Vitro. Evid. Based Complement. Altern. Med. 2015, 2015, e684925. [Google Scholar] [CrossRef]
- Palmeri, R.; Parafati, L.; Trippa, D.; Siracusa, L.; Arena, E.; Restuccia, C.; Fallico, B. Addition of Olive Leaf Extract (OLE) for Producing Fortified Fresh Pasteurized Milk with an Extended Shelf Life. Antioxidants 2019, 8, 255. [Google Scholar] [CrossRef]
- Rauf, A.; Rashid, U.; Shah, Z.A.; Rehman, G.; Bashir, K.; Jamil, J.; Iftikhar; Rahman, A.; Alsahammari, A.; Alharbi, M.; et al. Anti-Inflammatory and Anti-Diabetic Activity of Ferruginan, a Natural Compound from Olea ferruginea. Processes 2023, 11, 545. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, J.; Xin, X.; Zhu, S.; Niu, E.; Wu, Q.; Li, T.; Liu, D. Changes in Phytochemical Profiles and Biological Activity of Olive Leaves Treated by Two Drying Methods. Front. Nutr. 2022, 9, 854680. [Google Scholar] [CrossRef]
- Al-Attar, A.M.; Alsalmi, F.A. Effect of Olea europaea leaves extract on streptozotocin induced diabetes in male albino rats. Saudi J. Biol. Sci. 2019, 26, 118–128. [Google Scholar] [CrossRef]
- Mechchate, H.; Ouedrhiri, W.; Es-safi, I.; Amaghnouje, A.; Jawhari, F.Z.; Bousta, D. Optimization of a New Antihyperglycemic Formulation Using a Mixture of Linum usitatissimum L., Coriandrum sativum L., and Olea europaea var. sylvestris Flavonoids: A Mixture Design Approach. Biologics 2021, 1, 154–163. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Zhong, L.; Li, Y.; Xiong, L.; Wang, W.; Wu, M.; Yuan, T.; Yang, W.; Tian, C.; Miao, Z.; Wang, T.; et al. Small molecules in targeted cancer therapy: Advances, challenges, and future perspectives. Signal Transduct. Target. Ther. 2021, 6, 201. [Google Scholar] [CrossRef]
- Quirantes-Piné, R.; Zurek, G.; Barrajón-Catalán, E.; Bäßmann, C.; Micol, V.; Segura-Carretero, A.; Fernández-Gutiérrez, A. A metabolite-profiling approach to assess the uptake and metabolism of phenolic compounds from olive leaves in SKBR3 cells by HPLC–ESI-QTOF-MS. J. Pharm. Biomed. Anal. 2013, 72, 121–126. [Google Scholar] [CrossRef]
- Ruzzolini, J.; Peppicelli, S.; Andreucci, E.; Bianchini, F.; Scardigli, A.; Romani, A.; La Marca, G.; Nediani, C.; Calorini, L. Oleuropein, the Main Polyphenol of Olea europaea Leaf Extract, Has an Anti-Cancer Effect on Human BRAF Melanoma Cells and Potentiates the Cytotoxicity of Current Chemotherapies. Nutrients 2018, 10, 1950. [Google Scholar] [CrossRef]
- Casaburi, I.; Puoci, F.; Chimento, A.; Sirianni, R.; Ruggiero, C.; Avena, P.; Pezzi, V. Potential of olive oil phenols as chemopreventive and therapeutic agents against cancer: A review of in vitro studies. Mol. Nutr. Food Res. 2013, 57, 71–83. [Google Scholar] [CrossRef]
- Carpi, S.; Polini, B.; Manera, C.; Digiacomo, M.; Salsano, J.E.; Macchia, M.; Scoditti, E.; Nieri, P. miRNA Modulation and Antitumor Activity by the Extra-Virgin Olive Oil Polyphenol Oleacein in Human Melanoma Cells. Front. Pharmacol. 2020, 11, 574317. [Google Scholar] [CrossRef]
- Boss, A.; Bishop, K.S.; Marlow, G.; Barnett, M.P.G.; Ferguson, L.R. Evidence to Support the Anti-Cancer Effect of Olive Leaf Extract and Future Directions. Nutrients 2016, 8, 513. [Google Scholar] [CrossRef]
- Quero, J.; Ballesteros, L.F.; Ferreira-Santos, P.; Velderrain-Rodriguez, G.R.; Rocha, C.M.R.; Pereira, R.N.; Teixeira, J.A.; Martin-Belloso, O.; Osada, J.; Rodríguez-Yoldi, M.J. Unveiling the Antioxidant Therapeutic Functionality of Sustainable Olive Pomace Active Ingredients. Antioxidants 2022, 11, 828. [Google Scholar] [CrossRef]
- Albogami, S.; Hassan, A.M. Assessment of the Efficacy of Olive Leaf (Olea europaea L.) Extracts in the Treatment of Colorectal Cancer and Prostate Cancer Using In Vitro Cell Models. Molecules 2021, 26, 4069. [Google Scholar] [CrossRef]
- Uğuz, A.C.; Rocha-Pimienta, J.; Martillanes, S.; Garrido, M.; Espino, J.; Delgado-Adámez, J. Chlorophyll Pigments of Olive Leaves and Green Tea Extracts Differentially Affect Their Antioxidant and Anticancer Properties. Molecules 2023, 28, 2779. [Google Scholar] [CrossRef]
- Shah, Z.A.; Mujawah, A.A.H.; Ullah, I.; Rauf, A.; Rashid, U.; Khalil, A.A.; Shah, S.M.M.; Pervaiz, A.; Shaheen, F.; Al-Awthan, Y.S.; et al. Antioxidant and Cytotoxic Activity of a New Ferruginan A from Olea ferruginea: In Vitro and In Silico Studies. Oxid. Med. Cell. Longev. 2022, 2022, e8519250. [Google Scholar] [CrossRef]
- Samet, I.; Han, J.; Jlaiel, L.; Sayadi, S.; Isoda, H. Olive (Olea europaea) Leaf Extract Induces Apoptosis and Monocyte/Macrophage Differentiation in Human Chronic Myelogenous Leukemia K562 Cells: Insight into the Underlying Mechanism. Oxid. Med. Cell. Longev. 2014, 2014, e927619. [Google Scholar] [CrossRef]
- Morsy, F.S.; Abdel-Aziz, M.E. Efficiency of olive (Olea europaea L.) leaf extract as antioxidant and anticancer agents. J. Agroaliment. Process. Technol. 2014, 20, 46–53. [Google Scholar]
- Essafi Rhouma, H.; Trabelsi, N.; Chimento, A.; Benincasa, C.; Tamaalli, A.; Perri, E.; Zarrouk, M.; Pezzi, V. Olea Europaea L. Flowers as a New Promising Anticancer Natural Product: Phenolic Composition, Antiproliferative Activity and Apoptosis Induction. Nat. Prod. Res. 2021, 35, 1836–1839. [Google Scholar] [CrossRef]
- Alzandi, A.A.; Taher, E.A.; Al-Sagheer, N.A.; Al-Khulaidi, A.W.; Azizi, M.; Naguib, D.M. Phytochemical components, antioxidant and anticancer activity of 18 major medicinal plants in Albaha region, Saudi Arabia. Biocatal. Agric. Biotechnol. 2021, 34, 102020. [Google Scholar] [CrossRef]
- Goldsmith, C.D.; Vuong, Q.V.; Sadeqzadeh, E.; Stathopoulos, C.E.; Roach, P.D.; Scarlett, C.J. Phytochemical Properties and Anti-Proliferative Activity of Olea europaea L. Leaf Extracts against Pancreatic Cancer Cells. Molecules 2015, 20, 12992–13004. [Google Scholar] [CrossRef]
- Najibullah, S.N.M.; Ahamad, J.; Sultana, S.; Uthirapathy, S. Potential anticancer activity of chemically characterized extract of Olea europaea (Olive) leaves. Emir. J. Food Agric. 2023, 35, 890–896. [Google Scholar] [CrossRef]
- Mutlu, M.; Tunca, B.; Aksoy, S.A.; Tekin, C.; Cecener, G.; Egeli, U. Olea europaea leaf extract decreases tumour size by affecting the LncRNA expression status in glioblastoma 3D cell cultures. Eur. J. Integr. Med. 2021, 45, 101345. [Google Scholar] [CrossRef]
- Fontana, G.; Bruno, M.; Notarbartolo, M.; Labbozzetta, M.; Poma, P.; Spinella, A.; Rosselli, S. Cytotoxicity of oleanolic and ursolic acid derivatives toward hepatocellular carcinoma and evaluation of NF-κB involvement. Bioorganic Chem. 2019, 90, 103054. [Google Scholar] [CrossRef]
- Clewell, A.E.; Béres, E.; Vértesi, A.; Glávits, R.; Hirka, G.; Endres, J.R.; Murbach, T.S.; Szakonyiné, I.P. A Comprehensive Toxicological Safety Assessment of an Extract of Olea europaea L. Leaves (BonoliveTM). Int. J. Toxicol. 2016, 35, 208–221. [Google Scholar] [CrossRef]

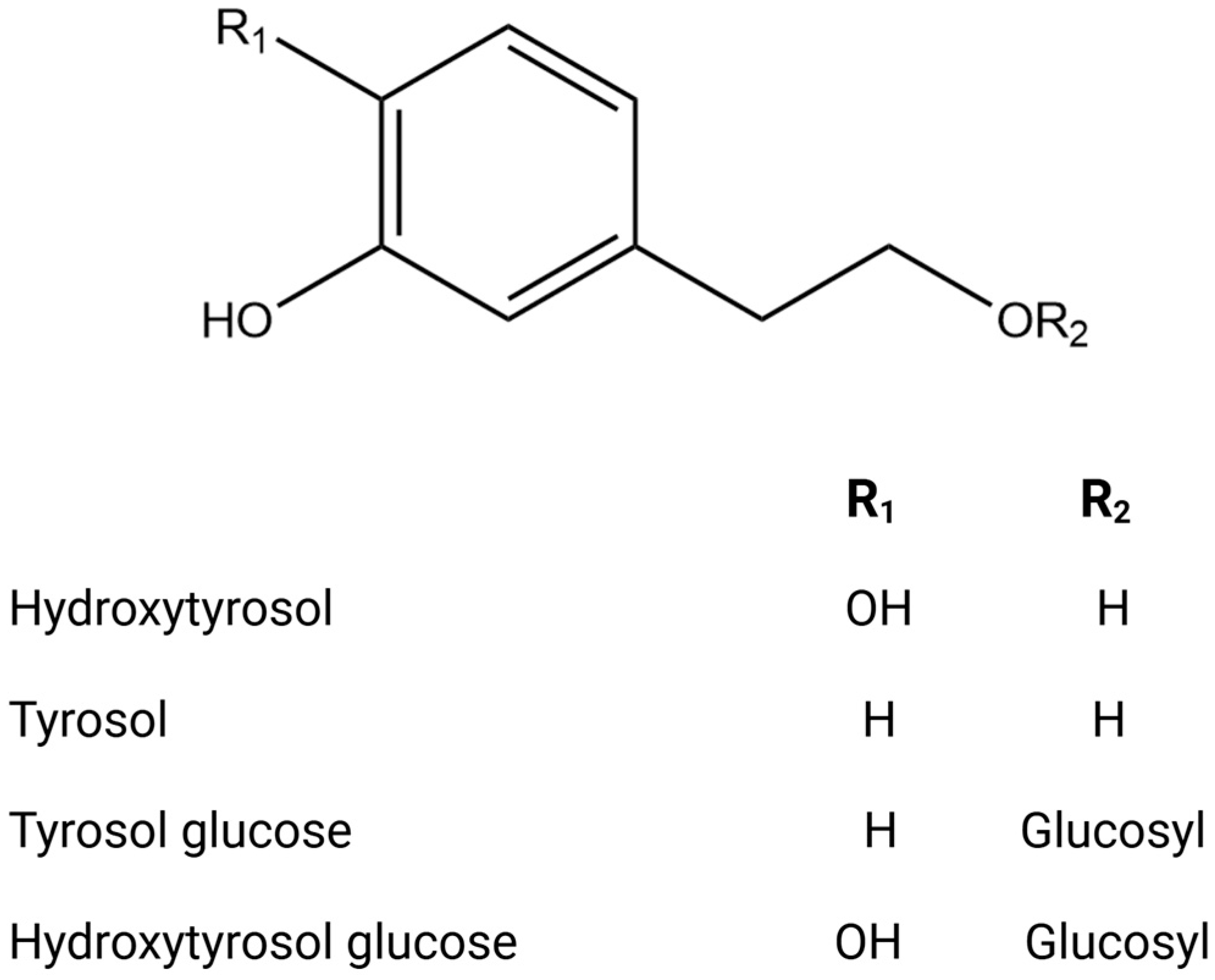
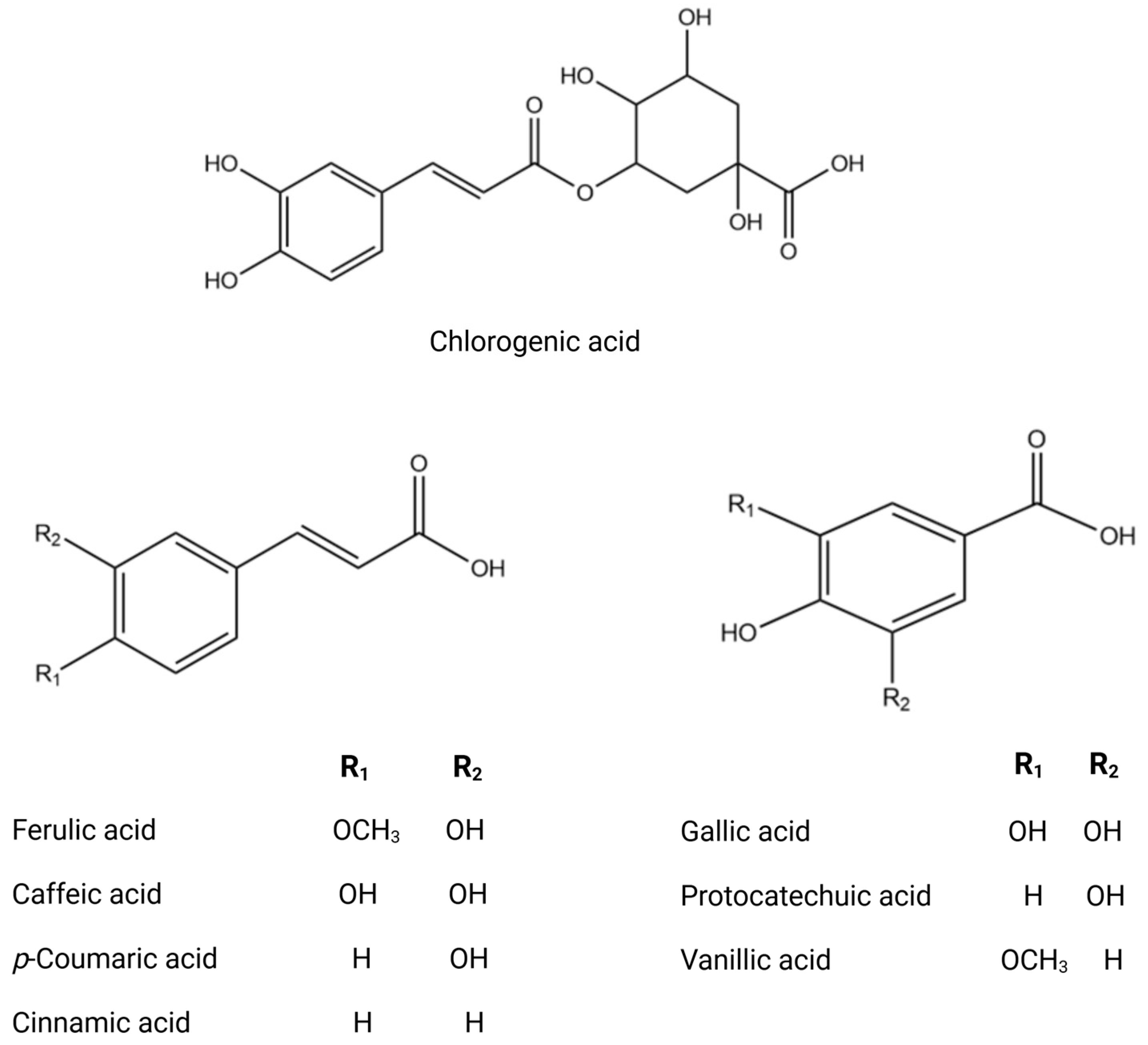
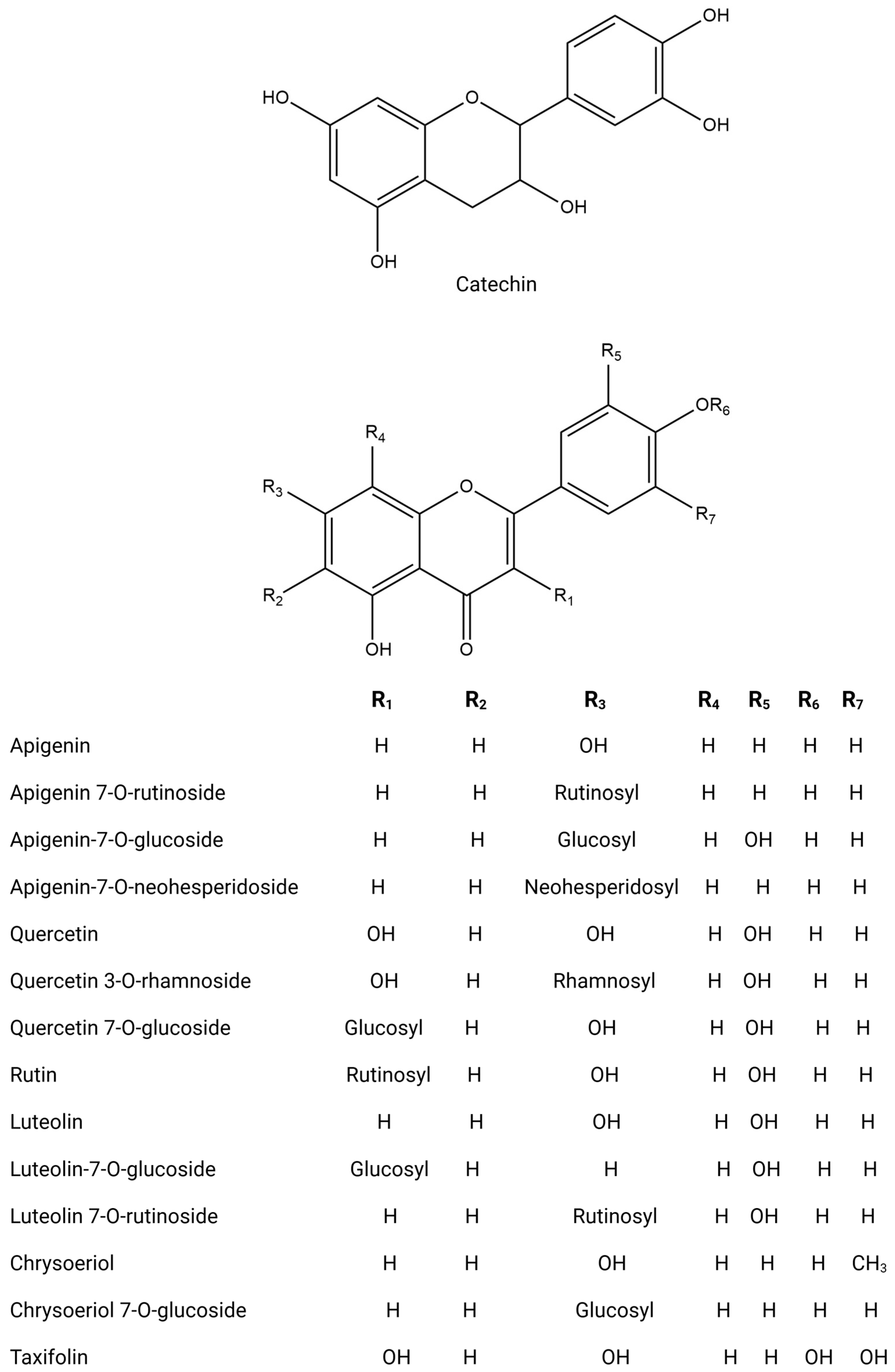
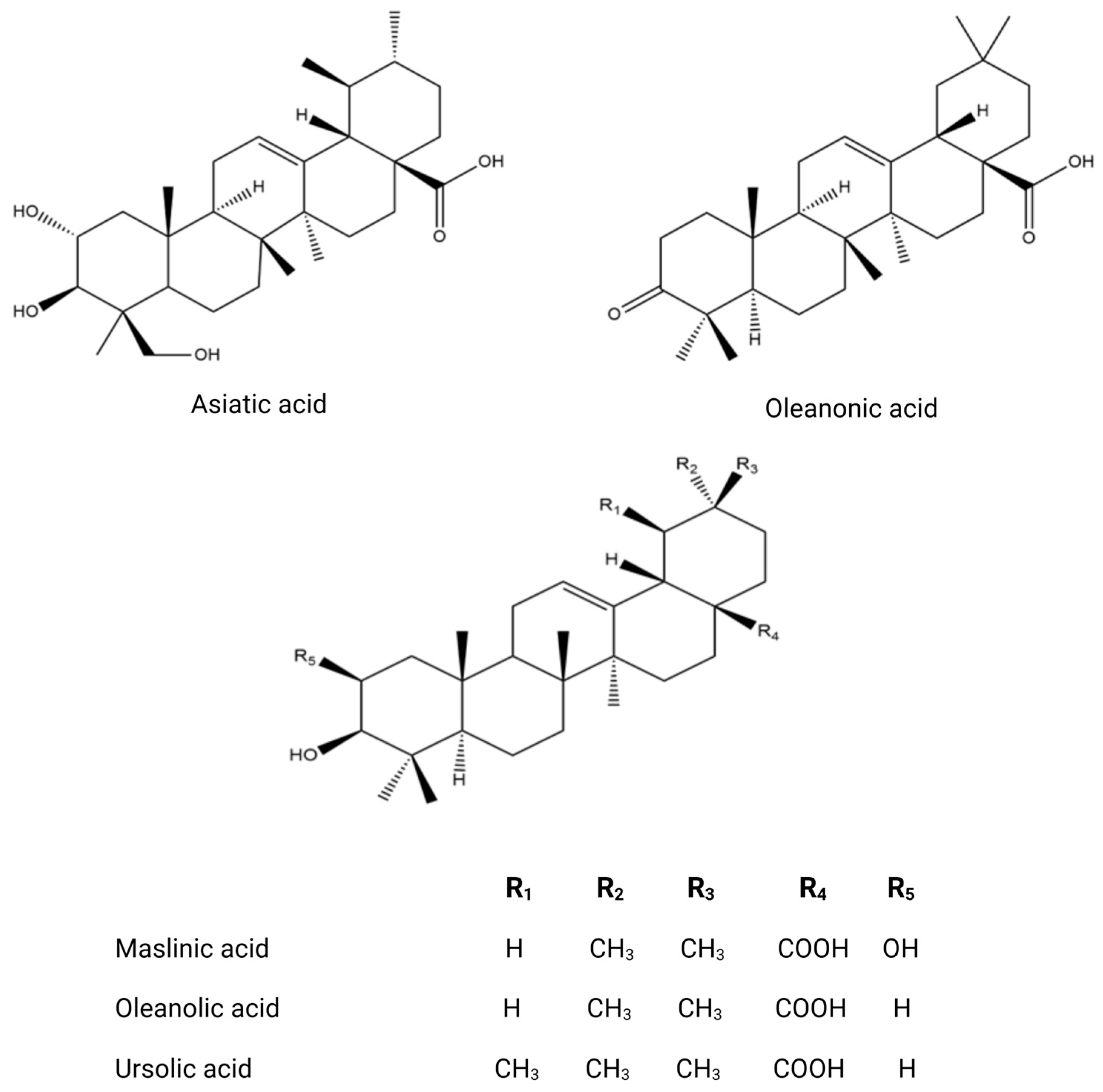
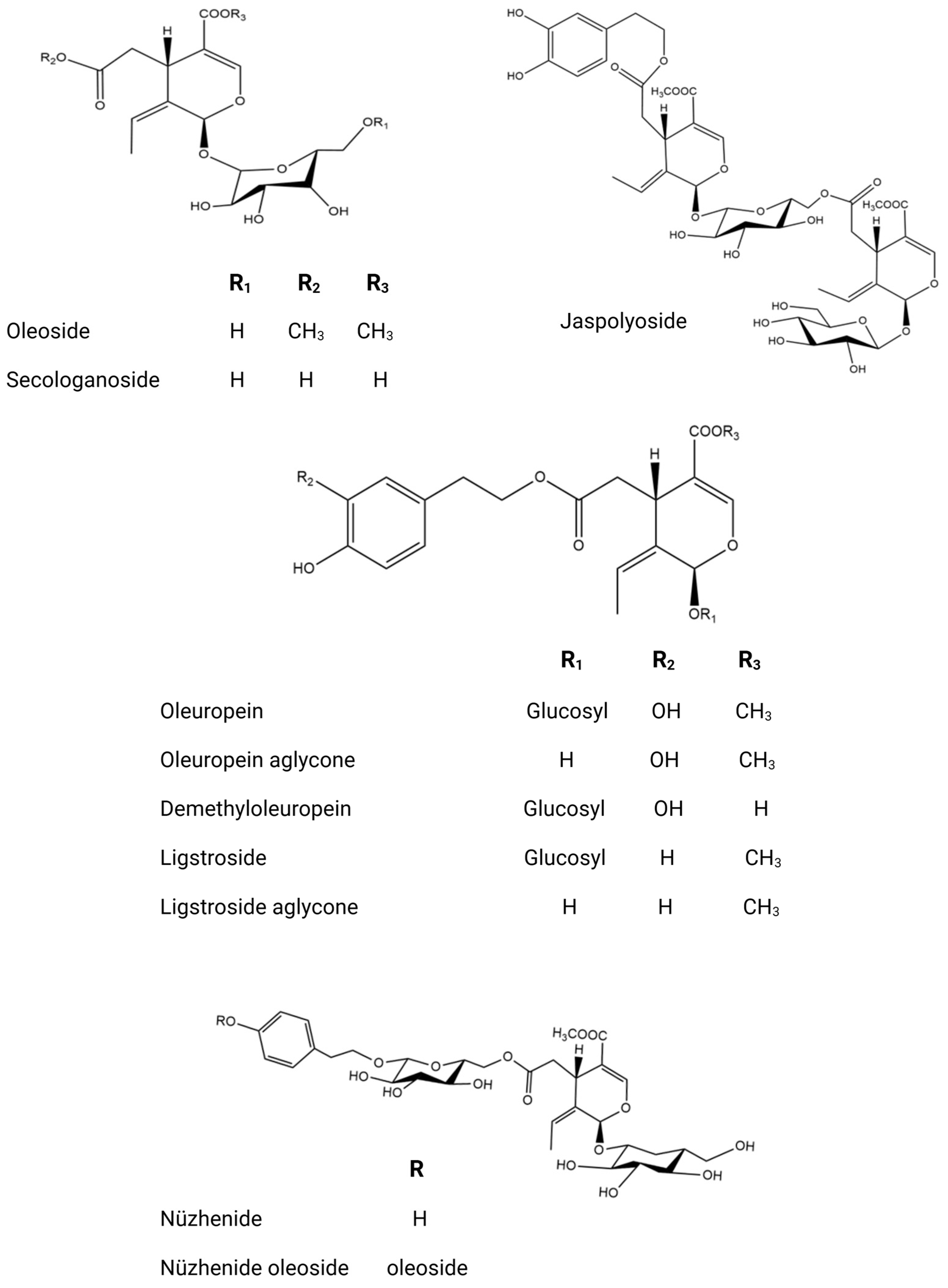
| Country | Olive Variety | Reference |
|---|---|---|
| Albania | Kalinjot | [29] |
| Algeria | Blanquette de Guelma, Azeradj, Sigoise | [30] |
| Argentina | Arauco | [31] |
| Chile | Azapa | [32] |
| Cyprus | Ladoelia, Kato Drys | [33] |
| Croatia | Oblica, Crnica, Karbonaca | [34] |
| Egypt | Toffahi, Koroneiki, Aggezi Shami | [35] |
| France | Grossane, Aglandau, Picholine, Tanche | [36] |
| Greece | Koutsourelia, Kalamón, Megaritiki, Mastoidis | [37] |
| Israel | Kadesh, Barnea, Merhavia | [38] |
| Italy | Leccino, Pisciottana, Frantoiana, Salella, Ravece, Cilentana, Carolea | [39] |
| Jordan | Rasi’i | [40] |
| Lebanon | Soury, Maurino, Baladi | [41] |
| Morocco | Picholine Marocaine, Menara, Haouzia, Arbequina | [42] |
| Palestine | Nabali Baladi, Yunani, Barouni | [43] |
| Portugal | Maçanilha Algarvia, Cordovil de Serpa, Galega, Carrasquenha, Redondal | [44] |
| Spain | Lechín de Sevilla, Manzanilla de Sevilla, Picudo, Nevadillo negro, Hojiblanca | [45] |
| Syria | Sorani, Doebli | [46] |
| Tunisia | Gerboui, Chétoui, Chemlali, Oueslati | [47] |
| Turkey | Memeli, Çelebi, Gemlik Memecik, Erkence, Çekiste | [48] |
| Part Used | Plant Extract | Method | Chemical Composition | Reference |
|---|---|---|---|---|
| Branch | Vegetal extract | HPLC-DAD-MS | Phenolic compounds Oleuropein; comselogoside; flavanonols; taxifolin; hydroxytyrosol; verbascoside; 1-acetoxypinoresinol glucoside; eriodictyol; esculetin Triterpenoids Oleanolic acid, ursolic acid, maslinic acid; and erythrodiol | [50] |
| Stems | Vegetal extract | RP-HPLC-DAD-ESI-QTOF-MS and -MS/MS | Phenolic compounds Phenolic acids Chlorogenic acid Gallic acid Dihydroxybenzoic acid hexoside Phenolic aldehydes Vanillin Phenylethanoids Hydroxytyrosol Hydroxytyrosol-hexoside II Tyrosol Verbascoside Coumarins Aesculetin Flavonoids Taxifolin Luteolin di-O-hexoside Apigenin 6–8-di-C-glucoside Quercetin Quercetin 3-O-rutinoside Luteolin 7-O-glucoside Luteolin-7-O-rutinoside Chrysoeriol 7-O-glucoside Apigenin 7-O-rutinoside Chrysoeriol β-Hydroxyverbascoside Verbascoside Calceolarioside Iridoids and derivatives Loganic acid glucoside Loganic acid Loganin 7-Deoxyloganic acid Secoiridoids and derivatives Oleuropein Acyclodihydroelenolic acid hexoside Hydroxyoleuropein Oleuropein hexoside Dihydro oleuropein Fraxamoside | [51,52] |
| Seed | Vegetable oil | Gas Chromatography Bradford Assay Flame photometer LC-HR-MS/MS | Protein Albumin, Globulin, Prolamin and Glutelin Mineral K, Na, P, Mg2+ Ca2+ Fatty Acid Monounsaturated FA Palmitoleic acid C16:1, oleic acid C18:1, eicosenoic acid C20:1, behenic acid C22:1 Polyunsaturated FA Linolenic acid C18:3, Linoleic acid C18:2 Saturated FA Palmitic acid C16:0, stearic acid C18:0, eicosenoic acid C20:0 and lignoceric acid C24:0 Polar fatty lipid Phospholipid Glycolipid Sphingolipid Acyl sterol glycoside | [8,53] |
| Vegetal extract | HPLC-DAD-MS | Phenolic compounds Oleuropein Oleoside 11-methyl ester Ligstroside oleoside Nüzhenide Nüzhenide 11-methyl oleoside | [50,52] | |
| Fruit | Vegetable oil | GC-MS UHPLC-HRMS HPLC-DAD-UV HPLC-MS/MS | Fatty acids Tridecanoic acid Myristic acid Palmitic acid Palmitoleic acid Margaric acid Heptadecenoic acid Stearic acid Oleic acid Linoleic acid Linolenic acid Arachidic acid Eicosenoic acid Tocopherols δ-tocopherol γ-tocopherol α-tocopherol β-tocopherol Carotenoids β-carotene Volatile compounds Isoprene Pent-1-en-3-one Pentan-3-one (Z)-Hex-3-enal (E)-Pent-2-enal Hexanal (E)-Hex-2-enal trans-β-Ocimene 3-Ethyloct-1,5-diene α-Copaene Phenolic compounds Hydroxytyrosol Tyrosol Vanillic acid Verbascoside Rutin p-Coumaric acid Eriodictyol Catechin Naringenin Quercetin Oleuropein Oleocanthalic acid Oleaceinic acid Dadzein Caffeic acid Gallic acid Pinoresinol Luteolin Apigenin | [54,55,56] |
| Vegetal extract | HPLC-DAD-UV ICP-MS HPLC-MS/MS | Phenolic compounds Tyrosol Hydroxytyrosol Vanillic acid Gallic acid Caffeic acid Chlorogenic acid Vanillin p-coumaric acid Verbascoside Rutin Apigenin-7-β-D-glucose Luteolin-7-β-D-glucose Luteolin Apigenin Secoiridoids Secologanoside Oleoside-11-methylester Oleuropein aglycone Dihydrooleuropein Oleuropein glucoside 6′-β-hexopyranosyloleoside Oleuropein Ligstroside Mineral As, Al, Ba, Fe, K, Mg, Mn, Ti, Zn, Na, Ni, P, Si, Ca, Cu, Cd, | [57,58,59] | |
| Leaves | Essential oil | GC-MS | Monoterpene hydrocarbons α-Pinene α-Thujene β-Thujone Myrcene Oxygenated monoterpenes Linalool Borneol Terpinen-4-ol Hydrocarbons α-Cubebene α-Copaene Sesquiterpene β-Cubebene β-Elemene β-Caryophyllene (Z)-β-Farnesene α-Humulene Germacrene D β-Copaene β-Bisabolene δ-Cadinene Oxygenated sesquiterpenes Caryophyllene oxide α-Cadinol Spathulenol Phenolic compounds Tricosane Tetracosane Hydrocarbons Heptacosane Heneicosane Docosane Pentacosane Eugenol Myristicin Hexacosane Alcohols n-Decanol n-Dodecanol Acids Hexadecanoic acid Oleic acid Ketones α-Ionone (E)-β-Damascenone β-Ionone Esters Linalyl acetate Endo-Fenchyl acetate | [60,61] |
| Vegetal extract | HPLC-ESI-TOF and IT/MS LC-MS/MS GC-MS HPLC-DAD-TOF-MS HPLC–TOF-HRMS UHPLC/MS | Phenolic compounds Hydroxytyrosol Tyrosol Tyrosol glucoside Hydroxytyrosol glucoside Verbascoside Phenolic acid 3-Hydroxybenzoic Acid p-hydroxybenzoic acid Hydroxyphenylacetic acid 4-Hydroxybenzoic acid Gallic acid Protocatechuic acid Chlorogenic acid Vanillic acid Ferulic acid Salycilic acid Benzoic acid p-Coumaric acid Cinnamic acid Syringic acid Gallocatechin Flavonoid Apigenin Catechin Luteolin Rutin Taxifolin Hesperetin Quercetin Diosmetin Aromadendrine Kampferol Eriodictyol Flavan 3-ols Hyperoside Quercetin 7-O-glucoside Quercetin 3-O-rhamnoside Luteolin-7-O-glucoside Luteolin 7-O-rutinoside Apigenin-7-O-glucoside Apigenin-7-O-neohesperidoside Lignans Syringaresinol Pinoresinol Acetoxypinoresinol Triterpenoids Ursolic acid Maslinic acid Oleanolic acid Asiatic acid Corosolic acid Oleanonic acid Secoiridoids and related derivatives Ligstroside Oleuropein Secologanoside Loganoside Elenolic acid Oleacein Methoxyoleuropein Demethyloleuropein Hydroxyoleuropein Oleuropein aglycone Ligstroside aglycone | [10,62,63,64,65,66,67,68,69,70,71,72,73,74,75] |
| Part Used | Extract | Strains | Method | Key Results | Reference |
|---|---|---|---|---|---|
| Seed | Vegetal extract | Candida albicans Candida glabrata Candida dubliniensis Candida parapsilosis Candida kreusei Aspergillus fumigatus Aspergillus niger Aspergillus flavus Alternaria alternata Botrytis cinerea Fusarium moniliform Mauginiella scaettae Magnaporthe grisea Penicillium digitatum Trichothecium roseum Saccharomyces cerevisiae | Disc diffusion Micro-dilution Trypan blue exclusion method Fluorescent dye exclusion method | Ethyl acetate extract Ø = 10–35 mm Methanolic extract Ø = 9–19 mm | [146] |
| Fruit | Vegetable oil | MIC = 100–400 µg/mL MFC = 808–1260 µg/mL | [140,147,148] | ||
| Vegetal extract | Aqueous extract Ø = 7–13 mm | [142] | |||
| Leaves | Essential oil | ZOI = 7–17 mm MIC = 250–1250 µg/mL | [115] | ||
| Vegetal extract | Aqueous extract Ø = 16–19 mm Ethanolic extract Ø = 12.5–25 mm MIC* = 4687 µg/mL MIC* = 6250 µg/mL | [13,134,135,136,149,150] |
| Part Used | Extract | Method | Key Results | Reference |
|---|---|---|---|---|
| Seed | Vegetal extract | Radical scavenging activity 2,2-Diphenyl-1-Picrylhydrazyl (DPPH) 6-sulfonic acid (ABTS) Ferric reducing antioxidant power (FRAP) β-Carotene bleaching assay (BCB) H2O2 scavenging activity Metal ion chelating activity (EDTA) Nitric oxide scavenging activity (NO) Reducing capacity of Fe3+/Fe2+ conversion | DPPH/IC50 = 5.25 µg/mL Fe3+/Fe2+ IC50 = 20.24 µg/mL EDTA/IC50 = 17.37 µg/mL H2O2/IC50 = 30.50 µg/mL | [96] |
| Fruit | Vegetable oil | DPPH = 207.00 mg GAE/kg ABTS = 1.806 ± 0.042 mmol Trolox/kg β-Carotene = 65.41% of inhibition | [160,161] | |
| Vegetal extract | DPPH/IC50 = 9.47 µg/mL Fe3+/Fe2+ IC50 = 77.25 µg/mL EDTA/IC50 = 24.76 µg/mL H2O2/IC50 = 36.39 µg/mL | [96] | ||
| Leaves | Vegetal extract | DPPH/IC50 = 0.034–1.38 mg/mL EDTA/IC50 = 0.155 mg/mL FRAP/IC50 = 0.0753–1.018 mg/mL ABTS/IC50 = 0.0187–1.647 mg/mL NO/IC50 = 0.15–1.00 mg/mL | [19,119,162,163,164,165,166] |
| Parts Used | Extracts | Cell Line | In Vitro Model | Key Results | Reference |
|---|---|---|---|---|---|
| Seed | Methanolic | Human colorectal cell (HCT-116) Human melanoma cells (501 Mel) Colon cancer cell line (HT29) Prostate cancer cell line (PC3) Breast cancer cell line (MCF-7) Malignant mesothelioma cell line (REN) Hepatocellular cancer cell line (HEPG-2) Human promyelocytic leukemia cells (HL-60) Human glioblastoma cell line (T98G) Human lung cancer cell lines (A549) Pancreatic cancer cell line (AsPC-1) Larynx cancer cell line (HEP2) Luminal A breast cancer Cell line (MCF7) Human chronic leukemia cell line (K562) | MTT and MTS assay Cell cycle in-cell ELISA Kit Cell death detection ELISA+ The RNeasy mini kit Resazurin assay Agarose gel electrophoresis Assessment of cell growth inhibition WST assay for cell viability | IC50 = 875.5 µg/mL | [210] |
| Fruit | Methanolic | IC50 = 154.3 µg/mL | |||
| Vegetable oil | IC50 = 81.9 ± 6.9 mM IC50 = 19.1 ± 5.8 mM | [202] | |||
| Ethanolic | IC50 = 1000 µg/mL IC50 = 600 µg/mL | [83] | |||
| Leave | Aqueous | IC50 = 203.1 µg/mL IC50 = 236.6 µg/mL | [205] | ||
| IC50 = 15.5 ± 0.40 µg/mL IC50 = 10.2 ± 0.45 µg/mL IC50 = 9.9 ± 0.15 µg/mL IC50 = 10.4 ± 0.16 µg/mL IC50 = 8.6 ± 0.13 µg/mL | [211] | ||||
| IC50 = 100 µg/mL | [212] | ||||
| IC50 = 21.91 ± 1.8 μg/mL | [213] | ||||
| Ethanolic | IC50 = 200 µg/mL | [214] | |||
| IC50 = 100 µM IC50 = 40.8 μM IC50 = 52 µM | [215] | ||||
| Methanolic | IC50 = 21.5 mg GAE/L | [209] | |||
| EC50 = 0.26 mg/mL | [20] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elhrech, H.; Aguerd, O.; El Kourchi, C.; Gallo, M.; Naviglio, D.; Chamkhi, I.; Bouyahya, A. Comprehensive Review of Olea europaea: A Holistic Exploration into Its Botanical Marvels, Phytochemical Riches, Therapeutic Potentials, and Safety Profile. Biomolecules 2024, 14, 722. https://doi.org/10.3390/biom14060722
Elhrech H, Aguerd O, El Kourchi C, Gallo M, Naviglio D, Chamkhi I, Bouyahya A. Comprehensive Review of Olea europaea: A Holistic Exploration into Its Botanical Marvels, Phytochemical Riches, Therapeutic Potentials, and Safety Profile. Biomolecules. 2024; 14(6):722. https://doi.org/10.3390/biom14060722
Chicago/Turabian StyleElhrech, Hamza, Oumayma Aguerd, Chaimae El Kourchi, Monica Gallo, Daniele Naviglio, Imane Chamkhi, and Abdelhakim Bouyahya. 2024. "Comprehensive Review of Olea europaea: A Holistic Exploration into Its Botanical Marvels, Phytochemical Riches, Therapeutic Potentials, and Safety Profile" Biomolecules 14, no. 6: 722. https://doi.org/10.3390/biom14060722
APA StyleElhrech, H., Aguerd, O., El Kourchi, C., Gallo, M., Naviglio, D., Chamkhi, I., & Bouyahya, A. (2024). Comprehensive Review of Olea europaea: A Holistic Exploration into Its Botanical Marvels, Phytochemical Riches, Therapeutic Potentials, and Safety Profile. Biomolecules, 14(6), 722. https://doi.org/10.3390/biom14060722


_Kwok.png)






