When Chromatin Decondensation Affects Nuclear γH2AX Foci Pattern and Kinetics and Biases the Assessment of DNA Double-Strand Breaks by Immunofluorescence
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines
2.2. Chemicals
2.3. Irradiation
2.4. Immunofluorescence
2.5. Images and Statistical Analysis
3. Results
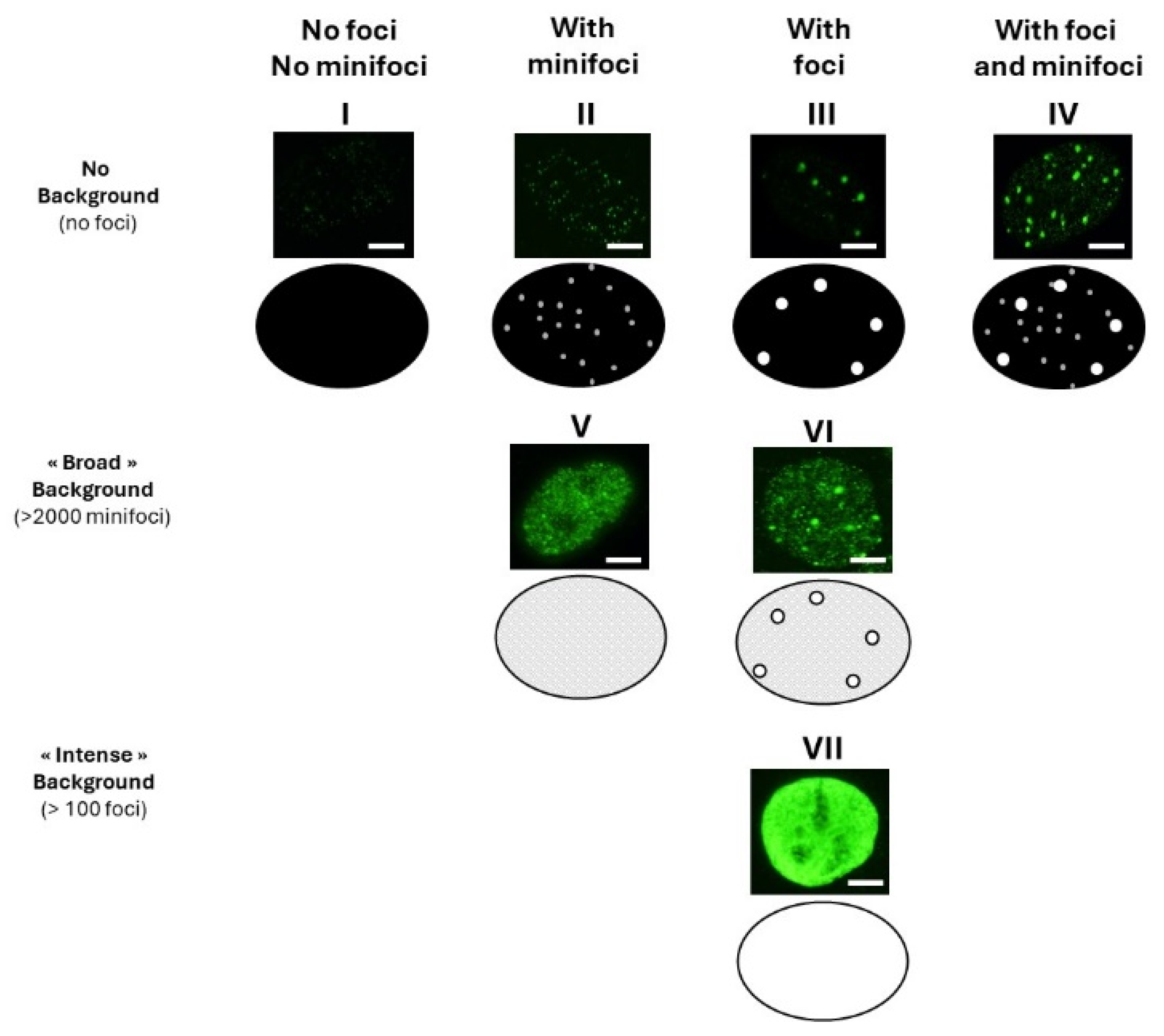
3.1. Current Observations with γH2AX Images: Seven Combinations of γH2AX Foci and Minifoci
- -
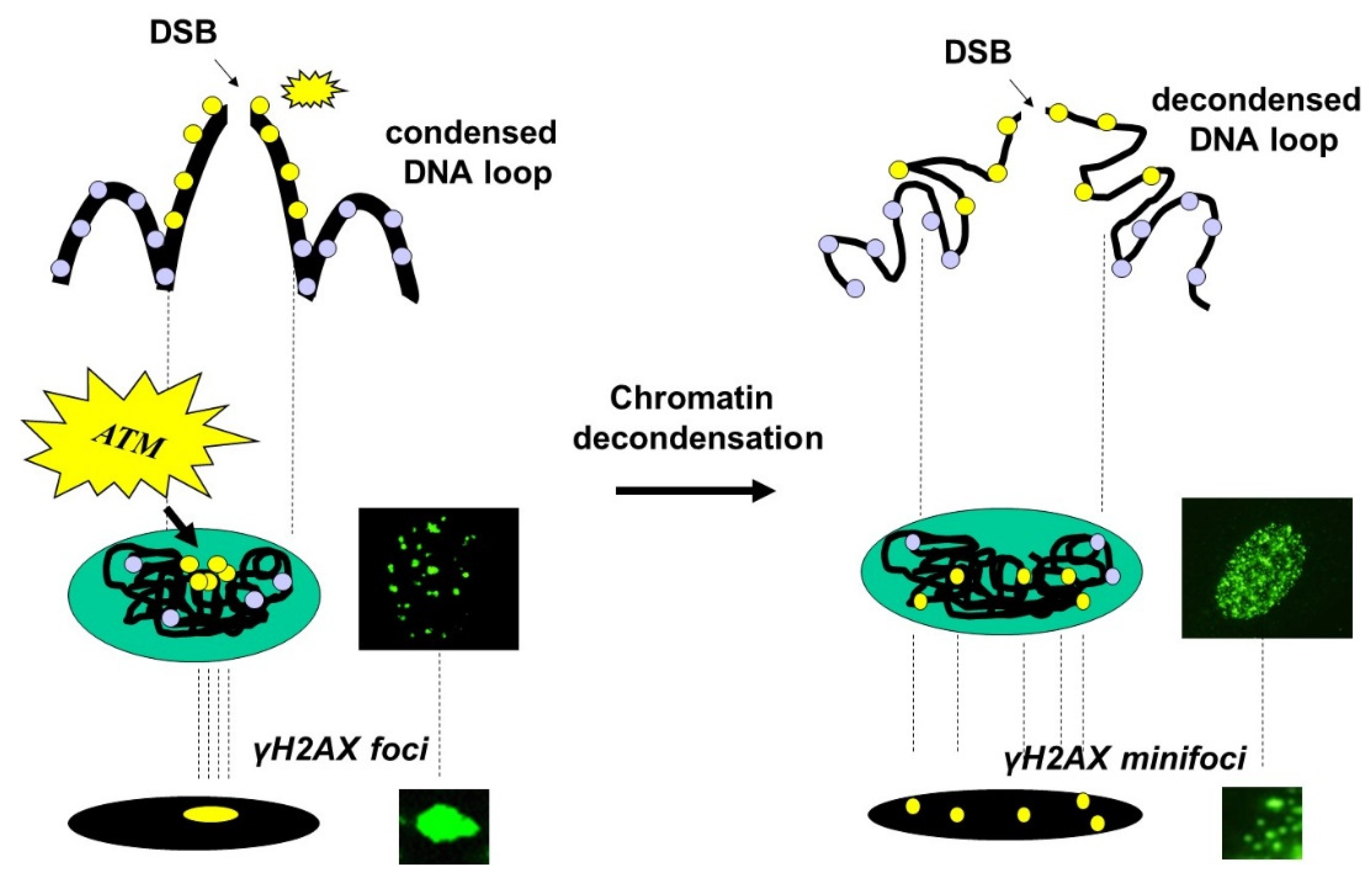
- Some γH2AX foci showed a very intense immunofluorescence signal. The shape of these foci was not systematically round, and their average surface was found to be 5 ± 3 µm2 (from more than 10,000 different nuclei analyzed). When a large number (>100 foci) of these foci were present per cell, nucleus staining appeared uniform and intense.
- -
- Some γH2AX foci were about 10 to 20 times smaller than the previous ones and with a lower signal intensity, which made them difficult to detect and score. Their average surface was found to be 0.3 ± 0.1 µm2 (from more than 10,000 different nuclei analyzed). In this study, we called them “γH2AX minifoci”. When a very large number (>2000) of these minifoci were present, nucleus staining appeared sheepy and relatively intense (systematically less intense than a signal from the γH2AX foci described above).
- -
- If the two types of foci were absent from the immunofluorescence image, the background was dark (i.e., signal intensity was nil) (Figure 1).
- -
- Nuclei with no background: neither γH2AX minifoci nor foci were observed (pattern I; e.g., non-irradiated fibroblasts from apparently healthy donors) or presence of γH2AX minifoci only (pattern II; e.g., non-irradiated fibroblasts from aging syndromes), or presence of γH2AX foci only (pattern III; e.g., non-irradiated fibroblasts from aging syndromes), or else presence of both γH2AX foci and minifoci (pattern IV; e.g., fibroblasts aging syndromes irradiated at low (mGy) doses).
- -
- Nuclei with numerous γH2AX minifoci (>2000 minifoci) forming a sheepy background: no γH2AX foci were observed (pattern V; e.g., non-irradiated fibroblasts from apparently healthy donors in S phase), or some γH2AX foci were observed (pattern VI; e.g., fibroblasts exposed to 30 µM CuSO4).
- -
- Nuclei with numerous γH2AX foci (>100 foci) forming an intense background (pattern VII; e.g., fibroblasts exposed to doses higher than 4 Gy).
- -
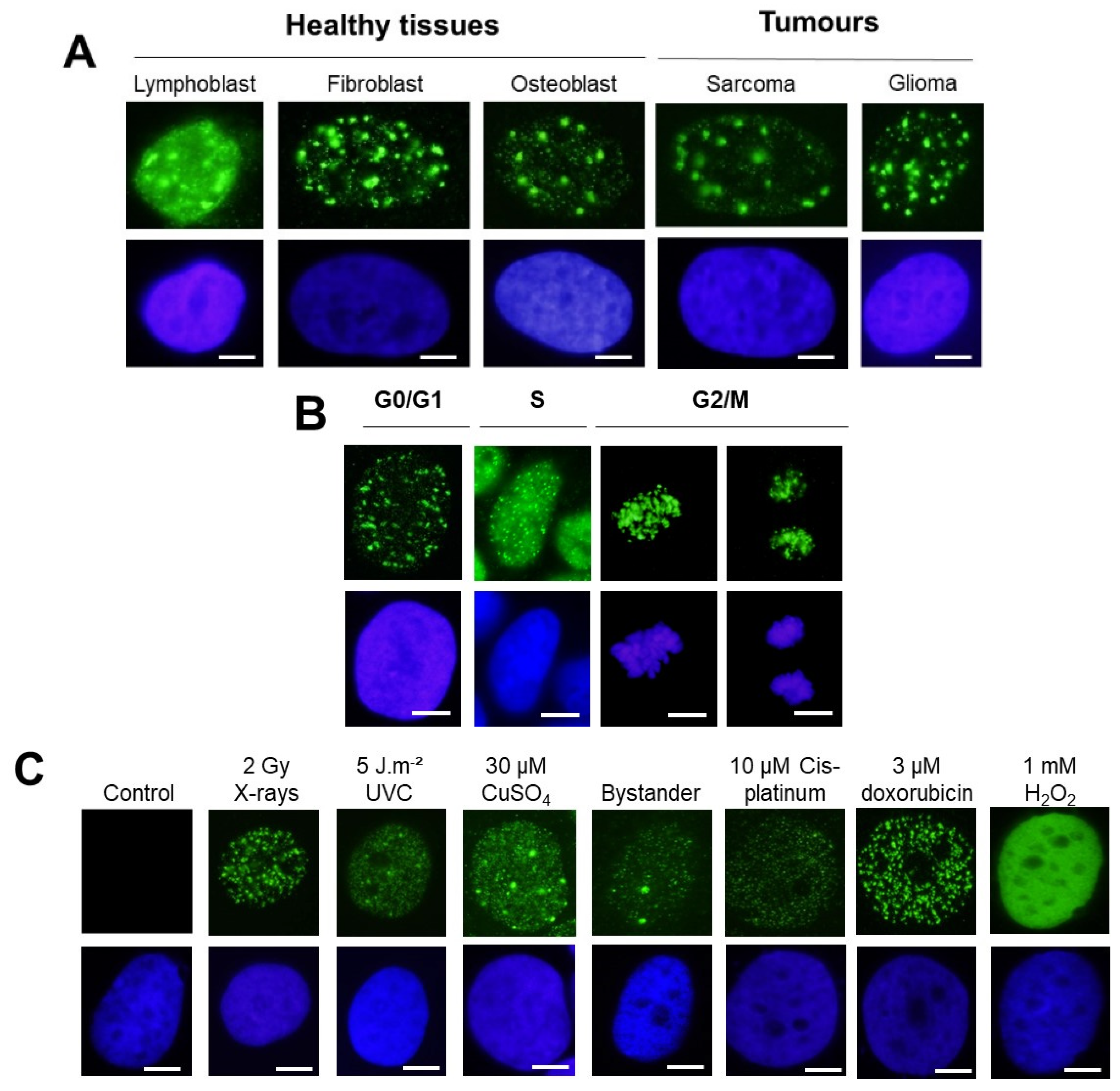
- With regard to the cell types: untransformed skin fibroblasts and astrocytes or even lymphoblasts generally elicited both γH2AX minifoci and foci. This statement was also relevant for tumor cells. However, the γH2AX minifoci were generally observed spontaneously in cells showing genomic instability and/or radiosensitivity and also shortly after exposure to stress (Figure 2A).
- -
- With regard to the cell cycle: The G1 cells showed both γH2AX minifoci and γH2AX foci, as described above. The S phase cells showed a large number of γH2AX minifoci. In the G2/M phase, the focus shape became more concentrated on chromatin and on chromosomes. In mitosis, the background was completely dark (Figure 2B).
- -
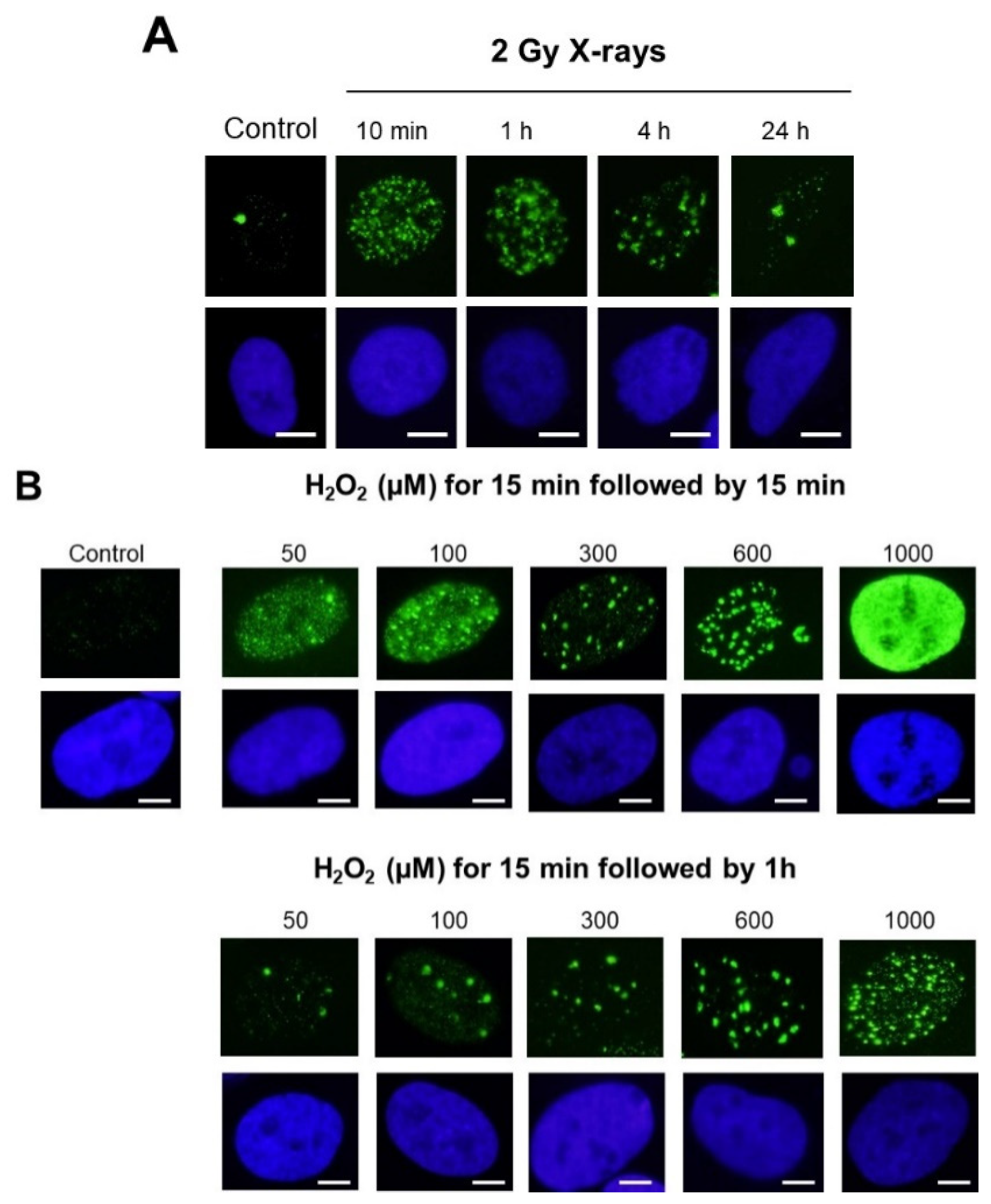
- With regard to the stress types: while 2 Gy-X-ray-irradiated fibroblasts showed a mixture of γH2AX foci and minifoci, fibroblasts exposed to UV, metal or bystander fibroblasts showed a majority of γH2AX minifoci and some rare γH2AX foci [28,31,32]. However, if UV dose or drug concentration increased drastically, the number of γH2AX foci increased, while the number of γH2AX minifoci decreased, at a rate depending on the nature of the stress induced. Interestingly, cisplatinum, which induces DNA adducts whose repair essentially involves SSBs, showed γH2AX minifoci [34], while bleomycin, a well-documented DSB inducer, elicited mostly γH2AX foci but very few γH2AX minifoci [35] (Figure 2C). Oxygen peroxide (H2O2) produces SSBs at low concentrations, and some DSBs appeared at high concentrations, leading to the γH2AX signal saturation (pattern VII) (Figure 2C). Altogether, these examples suggested a potential link between SSBs and DSB occurrence on one side and γH2AX minifocus and focus appearance on the other side.
3.2. Apparent Analogies between SSBs and DSBs vs. γH2AX Minifoci and Foci
- -
- regarding a dark background without any foci (pattern I), the induction of SSBs appeared with γH2AX minifoci under the condition that few DSBs are already present (patterns II and V) or that SSBs are so numerous that some DSBs are induced (patterns IV or VI). If these conditions are not met, the signal remains dark (pattern I). If repair is allowed, with the SSBs being repaired faster than DSBs, γH2AX minifoci disappeared faster than γH2AX foci.
- -
- regarding a dark background, the induction of DSBs was systematically associated with γH2AX foci (patterns III or VII). An increase of DSBs does not appear to be necessarily associated with SSBs: an increase in the number of γH2AX foci is not necessarily associated with an occurrence of γH2AX minifoci, except if the DSB repair involved SSBs such as with a recombination-like pathway [5,11,15,16,17,18]. In this case, the presence of minifoci can accompany each γH2AX foci (Table 2). Again, the analogy established above between the DSBs and SSBs and the γH2AX foci and minifoci, respectively, remains relevant with such treatments.
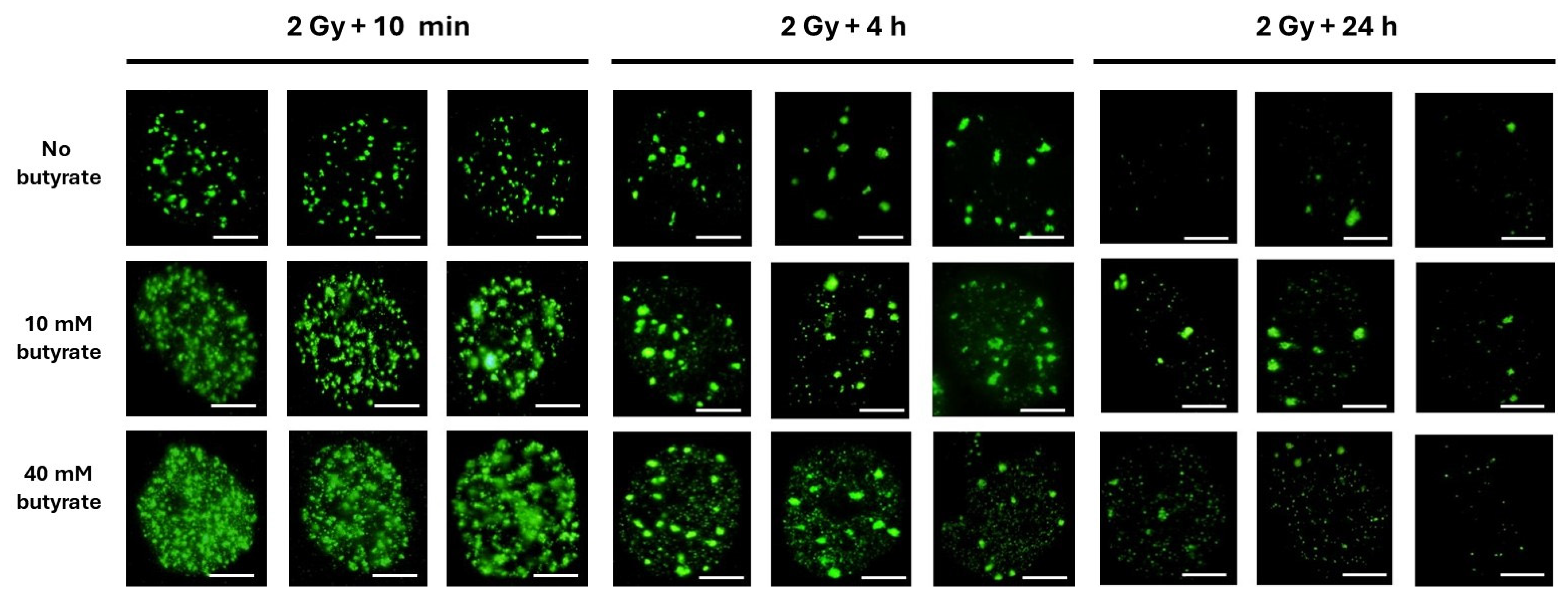
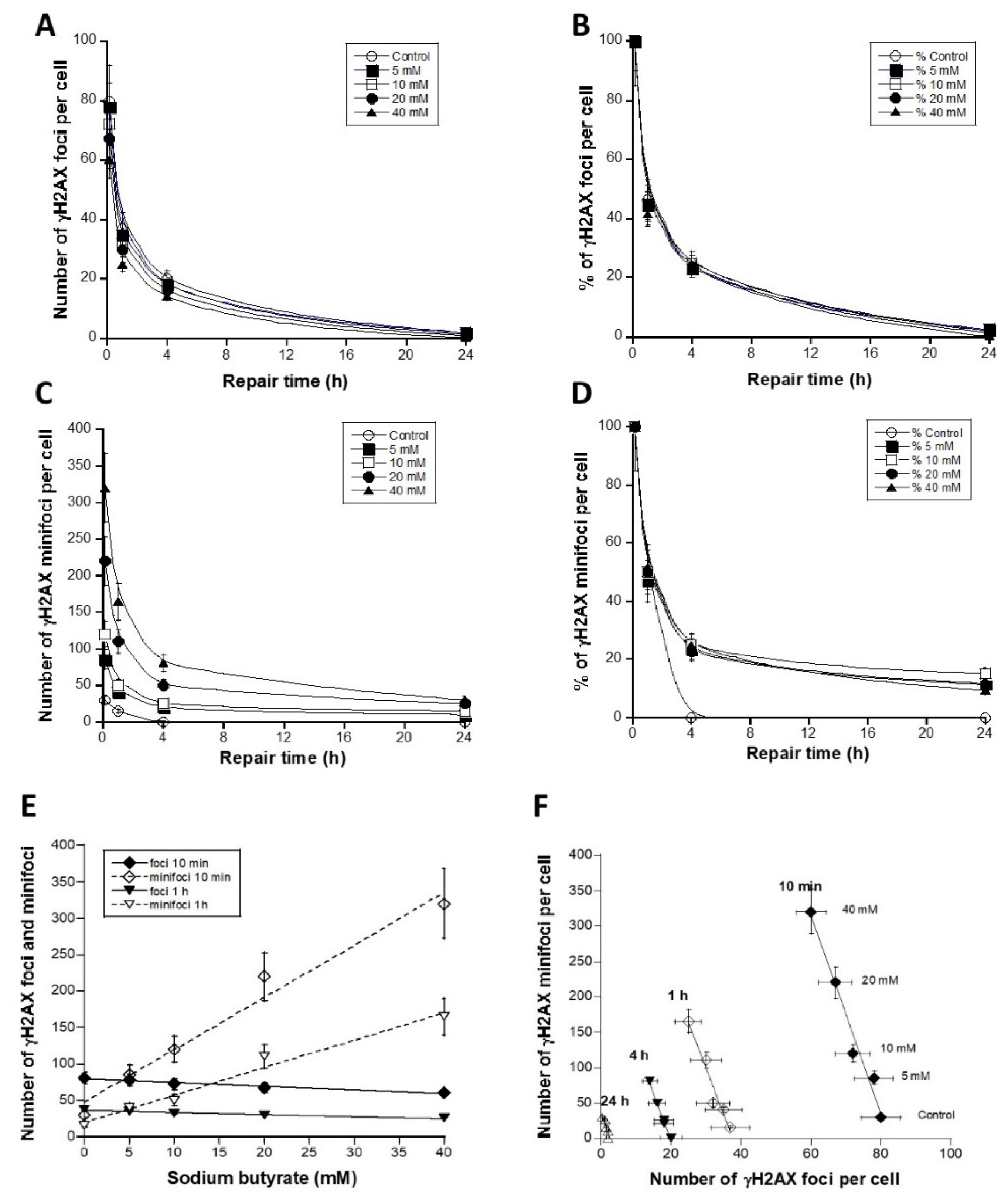
3.3. The Use of Sodium Butyrate to Decondense Chromatin
4. Discussion
4.1. Evidence of the Variety of γH2AX Foci Patterns
4.2. The Christmas Lights Model
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bennett, B.T.; Bewersdorf, J.; Knight, K.L. Immunofluorescence imaging of DNA damage response proteins: Optimizing protocols for super-resolution microscopy. Methods 2009, 48, 63–71. [Google Scholar] [CrossRef]
- Lisby, M.; Barlow, J.H.; Burgess, R.C.; Rothstein, R. Choreography of the DNA damage response: Spatiotemporal relationships among checkpoint and repair proteins. Cell 2004, 118, 699–713. [Google Scholar] [CrossRef]
- Bekker-Jensen, S.; Mailand, N. Assembly and function of DNA double-strand break repair foci in mammalian cells. DNA Repair. 2010, 9, 1219–1228. [Google Scholar] [CrossRef]
- Costes, S.V.; Chiolo, I.; Pluth, J.M.; Barcellos-Hoff, M.H.; Jakob, B. Spatiotemporal characterization of ionizing radiation induced DNA damage foci and their relation to chromatin organization. Mutat. Res. 2010, 704, 78–87. [Google Scholar] [CrossRef]
- Abend, M.; Badie, C.; Quintens, R.; Kriehuber, R.; Manning, G.; Macaeva, E.; Njima, M.; Oskamp, D.; Strunz, S.; Moertl, S.; et al. Examining Radiation-Induced In Vivo and In Vitro Gene Expression Changes of the Peripheral Blood in Different Laboratories for Biodosimetry Purposes: First RENEB Gene Expression Study. Radiat. Res. 2016, 185, 109–123. [Google Scholar] [CrossRef]
- Rothkamm, K.; Lobrich, M. Evidence for a lack of DNA double-strand break repair in human cells exposed to very low x-ray doses. Proc. Natl. Acad. Sci. USA 2003, 100, 5057–5062. [Google Scholar] [CrossRef]
- Belyaev, I.Y. Radiation-induced DNA repair foci: Spatio-temporal aspects of formation, application for assessment of radiosensitivity and biological dosimetry. Mutat. Res. 2010, 704, 132–141. [Google Scholar] [CrossRef]
- Foray, N.; Marot, D.; Gabriel, A.; Randrianarison, V.; Carr, A.M.; Perricaudet, M.; Ashworth, A.; Jeggo, P. A subset of ATM- and ATR-dependent phosphorylation events requires the BRCA1 protein. EMBO J. 2003, 22, 2860–2871. [Google Scholar] [CrossRef]
- Burma, S.; Chen, B.P.; Murphy, M.; Kurimasa, A.; Chen, D.J. ATM phosphorylates histone H2AX in response to DNA double-strand breaks. J. Biol. Chem. 2001, 276, 42462–42467. [Google Scholar] [CrossRef]
- Polo, S.E.; Jackson, S.P. Dynamics of DNA damage response proteins at DNA breaks: A focus on protein modifications. Genes. Dev. 2011, 25, 409–433. [Google Scholar] [CrossRef]
- Joubert, A.; Zimmerman, K.M.; Bencokova, Z.; Gastaldo, J.; Rénier, W.; Chavaudra, N.; Favaudon, V.; Arlett, C.; Foray, N. DNA double-strand break repair defects in syndromes associated with acute radiation response: At least two different assays to predict intrinsic radiosensitivity? Int. J. Radiat. Biol. 2008, 84, 107–125. [Google Scholar] [CrossRef]
- Berthel, E.; Ferlazzo, M.C.D.; Bourguignon, M.; Foray, N. What does the History of Research on the Repair of DNA Double—Strand Breaks Tell Us?—A Comprehensive Review of Human Radiosensitivity. Int. J. Mol. Sci. 2019, 20, 5339. [Google Scholar] [CrossRef]
- Kinner, A.; Wu, W.; Staudt, C.; Iliakis, G. Gamma-H2AX in recognition and signaling of DNA double-strand breaks in the context of chromatin. Nucleic Acids Res. 2008, 36, 5678–5694. [Google Scholar] [CrossRef]
- Marti, T.M.; Hefner, E.; Feeney, L.; Natale, V.; Cleaver, J.E. H2AX phosphorylation within the G1 phase after UV irradiation depends on nucleotide excision repair and not DNA double-strand breaks. Proc. Natl. Acad. Sci. USA 2006, 103, 9891–9896. [Google Scholar] [CrossRef]
- Foray, N.; Bourguignon, M.; Hamada, N. Individual response to ionizing radiation. Mutat. Res. Rev. 2016, 770, 369–386. [Google Scholar]
- Le Reun, E.; Bodgi, L.; Granzotto, A.; Sonzogni, L.; Ferlazzo, M.L.; Al-Choboq, J.; El-Nachef, L.; Restier-Verlet, J.; Berthel, E.; Devic, C.; et al. Quantitative correlations between radiosensitivity biomarkers show that the ATM protein kinase is strongly involved in the radiotoxicities observed after radiotherapy. Int. J. Mol. Sci. 2022, 23, 10434. [Google Scholar] [CrossRef]
- Kopp, B.; Khoury, L.; Audebert, M. Validation of the gammaH2AX biomarker for genotoxicity assessment: A review. Arch. Toxicol. 2019, 93, 2103–2114. [Google Scholar] [CrossRef]
- Lee, Y.; Wang, Q.; Seong, K.M.; Turner, H.C. High-Throughput gamma-H2AX Assay Using Imaging Flow Cytometry. Methods Mol. Biol. 2023, 2635, 123–134. [Google Scholar]
- Lobrich, M.; Rief, N.; Kuhne, M.; Heckmann, M.; Fleckenstein, J.; Rube, C.; Uder, M. In vivo formation and repair of DNA double-strand breaks after computed tomography examinations. Proc. Natl. Acad. Sci. USA 2005, 102, 8984–8989. [Google Scholar] [CrossRef]
- Firsanov, D.V.; Solovjeva, L.V.; Svetlova, M.P. H2AX phosphorylation at the sites of DNA double-strand breaks in cultivated mammalian cells and tissues. Clin. Epigenetics 2011, 2, 283–297. [Google Scholar] [CrossRef]
- Bewersdorf, J.; Bennett, B.T.; Knight, K.L. H2AX chromatin structures and their response to DNA damage revealed by 4Pi microscopy. Proc. Natl. Acad. Sci. USA 2006, 103, 18137–18142. [Google Scholar] [CrossRef]
- Rogakou, E.P.; Pilch, D.R.; Orr, A.H.; Ivanova, V.S.; Bonner, W.M. DNA double-stranded breaks induce histone H2AX phosphorylation on serine 139. J. Biol. Chem. 1998, 273, 5858–5868. [Google Scholar] [CrossRef]
- Savic, V.; Yin, B.; Maas, N.L.; Bredemeyer, A.L.; Carpenter, A.C.; Helmink, B.A.; Yang-Iott, K.S.; Sleckman, B.P.; Bassing, C.H. Formation of dynamic gamma-H2AX domains along broken DNA strands is distinctly regulated by ATM and MDC1 and dependent upon H2AX densities in chromatin. Mol. Cell 2009, 34, 298–310. [Google Scholar] [CrossRef]
- Natale, F.; Rapp, A.; Yu, W.; Maiser, A.; Harz, H.; Scholl, A.; Grulich, S.; Anton, T.; Horl, D.; Chen, W.; et al. Identification of the elementary structural units of the DNA damage response. Nat. Commun. 2017, 8, 15760. [Google Scholar] [CrossRef]
- Rybak, P.; Hoang, A.; Bujnowicz, L.; Bernas, T.; Berniak, K.; Zarebski, M.; Darzynkiewicz, Z.; Dobrucki, J. Low level phosphorylation of histone H2AX on serine 139 (gammaH2AX) is not associated with DNA double-strand breaks. Oncotarget 2016, 7, 49574–49587. [Google Scholar] [CrossRef]
- McManus, K.J.; Hendzel, M.J. ATM-dependent DNA damage-independent mitotic phosphorylation of H2AX in normally growing mammalian cells. Mol. Biol. Cell 2005, 16, 5013–5025. [Google Scholar] [CrossRef] [PubMed]
- Ismail, I.H.; Hendzel, M.J. The g-H2A.X: Is it just a surrogate marker of double-strand breaks or much more? Environ. Mol. Mutagen. 2008, 49, 73–82. [Google Scholar] [CrossRef]
- Viau, M.; Sonzogni, L.; Ferlazzo, M.L.; Berthel, E.; Pereira, S.; Bodgi, L.; Granzotto, A.; Devic, C.; Fervers, B.; Charlet, L.; et al. DNA Double-Strand Breaks Induced in Human Cells by Twelve Metallic Species: Quantitative Inter-Comparisons and Influence of the ATM Protein. Biomolecules 2021, 11, 1462. [Google Scholar] [CrossRef]
- Foray, N.; Priestley, A.; Alsbeih, G.; Badie, C.; Capulas, E.P.; Arlett, C.F.; Malaise, E.P. Hypersensitivity of ataxia telangiectasia fibroblasts to ionizing radiation is associated with a repair deficiency of DNA double-strand breaks. Int. J. Radiat. Biol. 1997, 72, 271–283. [Google Scholar]
- Granzotto, A.; Benadjaoud, M.A.; Vogin, G.; Devic, C.; Ferlazzo, M.L.; Bodgi, L.; Pereira, S.; Sonzogni, L.; Forcheron, F.; Viau, M.; et al. Influence of Nucleoshuttling of the ATM Protein in the Healthy Tissues Response to Radiation Therapy: Toward a Molecular Classification of Human Radiosensitivity. Int. J. Radiat. Oncol. Biol. Phys. 2016, 94, 450–460. [Google Scholar] [CrossRef] [PubMed]
- Restier-Verlet, J.; Joubert, A.; Ferlazzo, M.L.; Granzotto, A.; Sonzogni, L.; Al-Choboq, J.; El Nachef, L.; Le Reun, E.; Bourguignon, M.; Foray, N. X-rays-Induced Bystander Effect Consists in the Formation of DNA Breaks in a Calcium-Dependent Manner: Influence of the Experimental Procedure and the Individual Factor. Biomolecules 2023, 13, 542. [Google Scholar] [CrossRef]
- Foray, N.; Charvet, A.M.; Duchemin, D.; Favaudon, V.; Lavalette, D. The repair rate of radiation-induced DNA damage: A stochastic interpretation based on the gamma function. J. Theor. Biol. 2005, 236, 448–458. [Google Scholar] [CrossRef]
- Ferlazzo, M.; Berthel, E.; Granzotto, A.; Devic, C.; Sonzogni, L.; Bachelet, J.T.; Pereira, S.; Bourguignon, M.; Sarasin, A.; Mezzina, M.; et al. Some mutations in the xeroderma pigmentosum D gene may lead to moderate but significant radiosensitivity associated with a delayed radiation-induced ATM nuclear localization. Int. J. Radiat. Biol. 2020, 96, 394–410. [Google Scholar] [CrossRef]
- Gastaldo, J.; Bencokova, Z.; Massart, C.; Joubert, A.; Balosso, J.; Charvet, A.M.; Foray, N. Specific molecular and cellular events induced by irradiated X-ray photoactivatable drugs raise the problem of co-toxicities: Particular consequences for anti-cancer synchrotron therapy. J. Synchrotron Radiat. 2011, 18 Pt 3, 456–463. [Google Scholar] [CrossRef] [PubMed]
- Tounekti, O.; Kenani, A.; Foray, N.; Orlowski, S.; Mir, L.M. The ratio of single- to double-strand DNA breaks and their absolute values determine cell death pathway. Br. J. Cancer 2001, 84, 1272–1279. [Google Scholar] [CrossRef] [PubMed]
- Ye, B.; Hou, N.; Xiao, L.; Xu, Y.; Xu, H.; Li, F. Dynamic monitoring of oxidative DNA double-strand break and repair in cardiomyocytes. Cardiovasc. Pathol. 2016, 25, 93–100. [Google Scholar] [CrossRef]
- Ito, T. Role of histone modification in chromatin dynamics. J. Biochem. 2007, 141, 609–614. [Google Scholar] [CrossRef]
- Reczek, P.R.; Weissman, D.; Huvos, P.E.; Fasman, G.D. Sodium butyrate induced structural changes in HeLa cell chromatin. Biochemistry 1982, 21, 993–1002. [Google Scholar] [CrossRef] [PubMed]
- Kitzis, A.; Tichonicky, L.; Defer, N.; Kruh, J. Effect of sodium butyrate on chromatin structure. Biochem. Biophys. Res. Commun. 1980, 93, 833–841. [Google Scholar] [CrossRef]
- Renier, W.; Joubert, A.; Bencokova, Z.; Gastaldo, J.; Massart, C.; Foray, N. Consequences of the bleed-through phenomenon in immunofluorescence of proteins forming radiation-induced nuclear foci. Int. J. Radiat. Biol. 2007, 83, 543–549. [Google Scholar]
- Markova, E.; Schultz, N.; Belyaev, I.Y. Kinetics and dose-response of residual 53BP1/gamma-H2AX foci: Co-localization, relationship with DSB repair and clonogenic survival. Int. J. Radiat. Biol. 2007, 83, 319–329. [Google Scholar] [CrossRef] [PubMed]
| Cell Lines | Nature of the Tissue | Radiosensitivity | Collection |
|---|---|---|---|
| Healthy tissue cells lines | |||
| MRC5 | Untransformed skin fibroblast | Apparently healthy patient | ECACC |
| 1BR3 | Untransformed skin fibroblast | Apparently healthy patient | ECACC |
| 144BR | Untransformed skin fibroblast | Apparently healthy patient (same as LB173) | ECACC |
| LB173 | Epstein–Barr virus (EBV)-transformed lymphoblastoid cells | Apparently healthy patient (same as 144BR) | ECACC |
| HA | Fetal cortex astrocytes | Sciencell (#1800) | |
| Tumor cell lines | |||
| U2OS | Osteosarcoma | ATCC (#HTB-96) | |
| U118 | Glioblastoma | ATCC (#HTB-15) | |
| SSB-Induced | DSB-Induced | γH2AX Minifoci | γH2AX Foci | |
|---|---|---|---|---|
| Cell type | ||||
| Lymphoblasts | x | x | x | x |
| Fibroblasts | x | x | x | x |
| Osteoblasts | x | x | x | x |
| Sarcomas | x | x | x | x |
| Gliomas | x | x | x | x |
| Cell cycle phase | ||||
| G0/G1 | x | x | I, II, V | I, III, IV, VI, VII |
| S | x | II, V | ||
| G2/M | x | x | x | |
| Stress type | ||||
| Control | 0 | 0 | I | I |
| X-rays | ||||
| UVC | x | x a | II, IV, V, VI | III, IV, VI, VII |
| CuSO4 | x | x a | II, IV, V, VI | III, IV, VI, VII |
| Bystander | x | x a | II, IV, V, VI | III, IV, VI |
| Cisplatinum | x | II, V, VI, VII | ||
| Doxorubicin | x | III, VII | ||
| H2O2 | x | x a | II, IV, V, VI | III, IV, VI, VII |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Granzotto, A.; El Nachef, L.; Restier-Verlet, J.; Sonzogni, L.; Al-Choboq, J.; Bourguignon, M.; Foray, N. When Chromatin Decondensation Affects Nuclear γH2AX Foci Pattern and Kinetics and Biases the Assessment of DNA Double-Strand Breaks by Immunofluorescence. Biomolecules 2024, 14, 703. https://doi.org/10.3390/biom14060703
Granzotto A, El Nachef L, Restier-Verlet J, Sonzogni L, Al-Choboq J, Bourguignon M, Foray N. When Chromatin Decondensation Affects Nuclear γH2AX Foci Pattern and Kinetics and Biases the Assessment of DNA Double-Strand Breaks by Immunofluorescence. Biomolecules. 2024; 14(6):703. https://doi.org/10.3390/biom14060703
Chicago/Turabian StyleGranzotto, Adeline, Laura El Nachef, Juliette Restier-Verlet, Laurène Sonzogni, Joëlle Al-Choboq, Michel Bourguignon, and Nicolas Foray. 2024. "When Chromatin Decondensation Affects Nuclear γH2AX Foci Pattern and Kinetics and Biases the Assessment of DNA Double-Strand Breaks by Immunofluorescence" Biomolecules 14, no. 6: 703. https://doi.org/10.3390/biom14060703
APA StyleGranzotto, A., El Nachef, L., Restier-Verlet, J., Sonzogni, L., Al-Choboq, J., Bourguignon, M., & Foray, N. (2024). When Chromatin Decondensation Affects Nuclear γH2AX Foci Pattern and Kinetics and Biases the Assessment of DNA Double-Strand Breaks by Immunofluorescence. Biomolecules, 14(6), 703. https://doi.org/10.3390/biom14060703






