Slow Sulfide Donor GYY4137 Increased the Sensitivity of Two Breast Cancer Cell Lines to Paclitaxel by Different Mechanisms
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Cultivation and Treatments
2.2. Cell Viability Determination Using AquaBluer and IC50 Determination
2.3. Apoptosis Determination Using Annexin V-FLUOS
2.4. Cell Migration Assay
2.5. MTT Assay
2.6. Intracellular pH (pHi) Measurement
2.7. Determination of Oxidative Stress
2.8. Pyruvate Assay
2.9. Lactate Assay
2.10. Western Blot Analysis
2.11. Immunofluorescence
2.12. Determination of Angiogenesis Using CAM
2.13. Statistical Significance
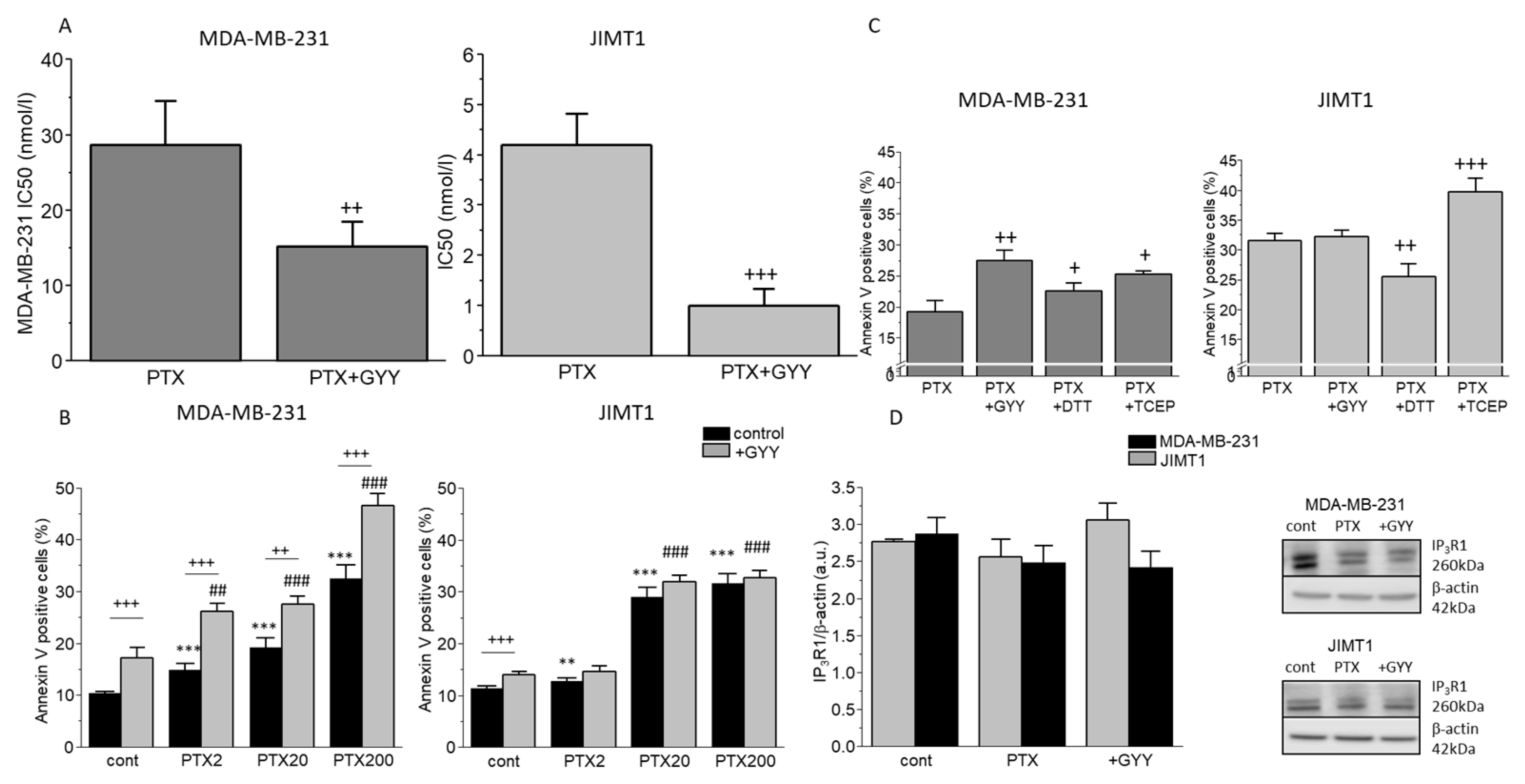
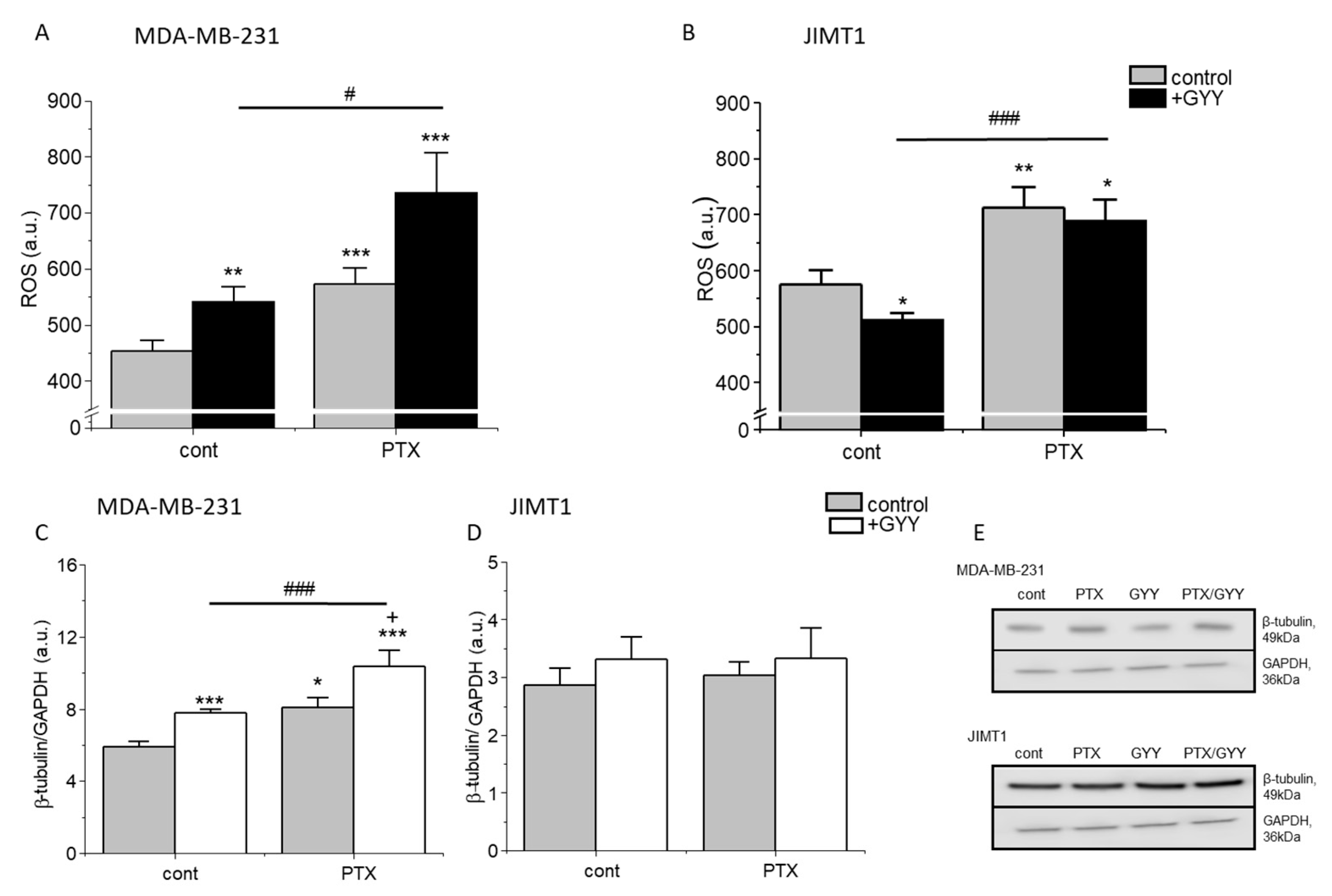
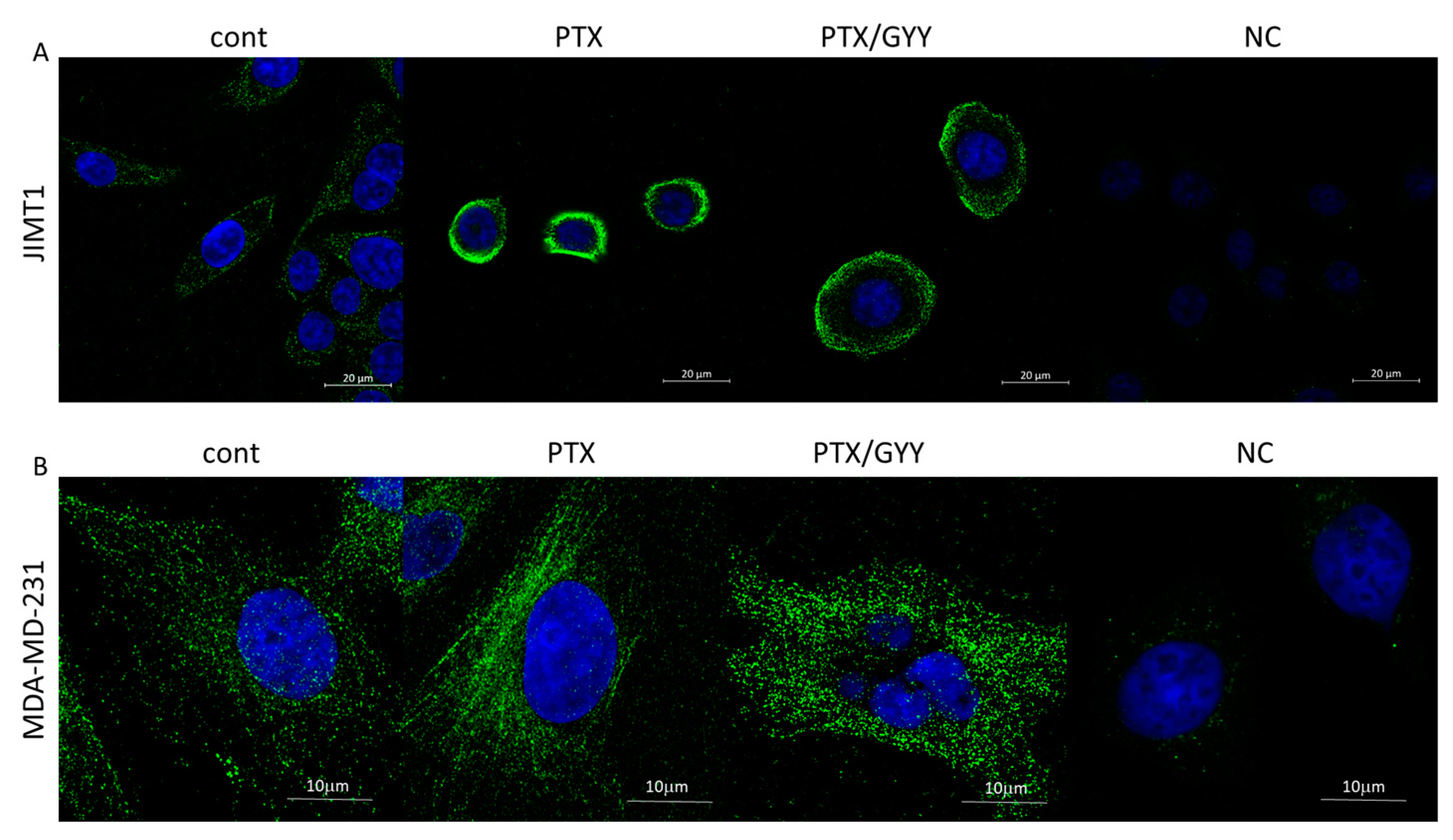
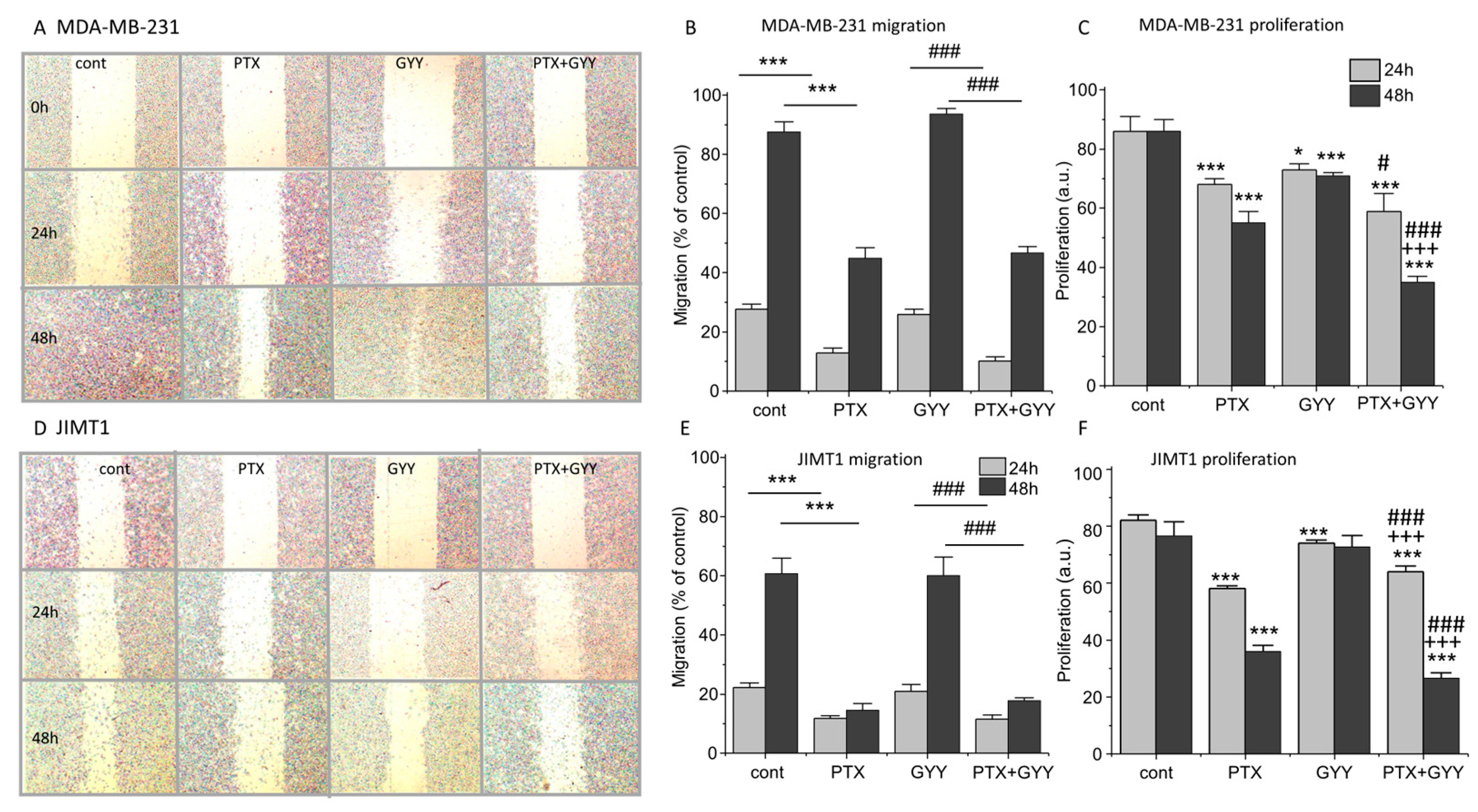
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ER | estrogen receptor |
| PR | progesterone receptor |
| HER2 | herceptin receptor type 2 |
| PTX | paclitaxel |
| H2S | hydrogen sulfide |
| TCEP | tris(2-carboxyethyl)phosphine |
| DTT | dithiothreitol |
| GYY4137 | slow sulfide donor, morpholin-4-ium 4-methoxyphenyl(morpholino) phosphinodithioate |
| NCX1 | sodium–calcium exchanger 1 |
| STS | sodium thiosulfate |
| JIMT1 | a model of breast cancer with amplified HER2 |
| MDA-MB-231 | a model of triple-negative breast cancer |
References
- Dewi, C.; Fristiohady, A.; Amalia, R.; Khairul Ikram, N.K.; Ibrahim, S.; Muchtaridi, M. Signaling Pathways and Natural Compounds in Triple-Negative Breast Cancer Cell Line. Molecules 2022, 27, 3661. [Google Scholar] [CrossRef]
- Chiu, H.W.; Lin, H.Y.; Tseng, I.J.; Lin, Y.F. OTUD7B upregulation predicts a poor response to paclitaxel in patients with triple-negative breast cancer. Oncotarget 2018, 9, 553–565. [Google Scholar] [CrossRef]
- Rugo, H.S.; Umanzor, G.A.; Barrios, F.J.; Vasallo, R.H.; Chivalan, M.A.; Bejarano, S.; Ramírez, J.R.; Fein, L.; Kowalyszyn, R.D.; Kramer, E.D.; et al. Open-Label, Randomized, Multicenter, Phase III Study Comparing Oral Paclitaxel Plus Encequidar Versus Intravenous Paclitaxel in Patients With Metastatic Breast Cancer. J. Clin. Oncol. 2023, 41, 65–74. [Google Scholar] [CrossRef]
- Gao, D.; Asghar, S.; Ye, J.; Zhang, M.; Hu, R.; Wang, Y.; Huang, L.; Yuan, C.; Chen, Z.; Xiao, Y. Dual-targeted enzyme-sensitive hyaluronic acid nanogels loading paclitaxel for the therapy of breast cancer. Carbohydr. Polym. 2022, 294, 119785. [Google Scholar] [CrossRef]
- Attia, Y.M.; El-Kersh, D.M.; Ammar, R.A.; Adel, A.; Khalil, A.; Walid, H.; Eskander, K.; Hamdy, M.; Reda, N.; Mohsen, N.E.; et al. Inhibition of aldehyde dehydrogenase-1 and p-glycoprotein-mediated multidrug resistance by curcumin and vitamin D3 increases sensitivity to paclitaxel in breast cancer. Chem. Biol. Interact. 2020, 315, 10886. [Google Scholar] [CrossRef]
- Mohammadhosseinpour, S.; Weaver, A.; Sudhakaran, M.; Ho, L.-C.; Le, T.; Doseff, A.I.; Medina-Bolivar, F. Arachidin-1, a Prenylated Stilbenoid from Peanut, Enhances the Anticancer Effects of Paclitaxel in Triple-Negative Breast Cancer Cells. Cancers 2023, 15, 399. [Google Scholar] [CrossRef]
- Abu Samaan, T.M.; Samec, M.; Liskova, A.; Kubatka, P.; Büsselberg, D. Paclitaxel’s Mechanistic and Clinical Effects on Breast Cancer. Biomolecules 2019, 9, 789. [Google Scholar] [CrossRef]
- Yang, Y.H.; Mao, J.W.; Tan, X.L. Research progress on the source, production, and anti-cancer mechanisms of paclitaxel. Chin. J. Nat. Med. 2020, 18, 890–897. [Google Scholar] [CrossRef]
- Kajsik, M.; Chovancova, B.; Liskova, V.; Babula, P.; Krizanova, O. Slow sulfide donor GYY4137 potentiates effect of paclitaxel on colorectal carcinoma cells. Eur. J. Pharmacol. 2022, 922, 174875. [Google Scholar] [CrossRef]
- Mathai, J.C.; Missner, A.; Kügler, P.; Saparov, S.M.; Zeidel, M.L.; Lee, J.K.; Pohl, P. No facilitator required for membrane transport of hydrogen sulfide. Proc. Natl. Acad. Sci. USA 2009, 106, 16633–16638. [Google Scholar] [CrossRef]
- Nagy, P.; Palinkas, Z.; Nagy, A.; Budai, B.; Toth, I.; Vasas, A. Chemical aspects of hydrogen sulfide measurements in physiological samples. Biochim. Biophys. Acta 2014, 1840, 876–891. [Google Scholar] [CrossRef]
- Vitvitsky, V.; Kabil, O.; Banerjee, R. High turnover rates for hydrogen sulfide allow for rapid regulation of its tissue concentrations. Antioxid. Redox Signal. 2012, 17, 22–31. [Google Scholar] [CrossRef]
- Reis, A.K.C.A.; Stern, A.; Monteiro, H.P. S-nitrosothiols and H2S donors: Potential chemo-therapeutic agents in cancer. Redox Biol. 2019, 27, 101190. [Google Scholar] [CrossRef]
- Wu, D.; Hu, Q.; Zhu, Y. Therapeutic application of hydrogen sulfide donors: The potential and challenges. Front. Med. 2016, 10, 18–27. [Google Scholar] [CrossRef]
- Wu, D.; Li, J.; Zhang, Q.; Tian, W.; Zhong, P.; Liu, Z.; Wang, H.; Wang, H.; Ji, A.; Li, Y. Exogenous Hydrogen Sulfide Regulates the Growth of Human Thyroid Carcinoma Cells. Oxidative Med. Cell. Longev. 2019, 2019, 6927298. [Google Scholar] [CrossRef]
- Nadkarni, D.V. Conjugations to Endogenous Cysteine Residues. Methods Mol. Biol. 2020, 2078, 37–49. [Google Scholar]
- Yang, D.; Wei, X.; Zhang, Z.; Chen, X.; Zhu, R.; Oh, Y.; Gu, N. Tris (2-chloroethyl) phosphate (TCEP) induces obesity and hepatic steatosis via FXR-mediated lipid accumulation in mice: Long-term exposure as a potential risk for metabolic diseases. Chem. Biol. Interact. 2022, 363, 110027. [Google Scholar] [CrossRef]
- Valabrega, G.; Capellero, S.; Cavalloni, G.; Zaccarello, G.; Petrelli, A.; Migliardi, G.; Milani, A.; Peraldo-Neia, C.; Gammaitoni, L.; Sapino, A.; et al. HER2-positive breast cancer cells resistant to trastuzumab and lapatinib lose reliance upon HER2 and are sensitive to the multitargeted kinase inhibitor sorafenib. Breast Cancer Res. Treat. 2011, 130, 29–40. [Google Scholar] [CrossRef]
- Rezuchova, I.; Hudecova, S.; Soltysova, A.; Matuskova, M.; Durinikova, E.; Chovancova, B.; Zuzcak, M.; Cihova, M.; Burikova, M.; Penesova, A.; et al. Type 3 inositol 1,4,5-trisphosphate receptor has antiapoptotic and proliferative role in cancer cells. Cell Death Dis. 2019, 10, 186. [Google Scholar] [CrossRef]
- Ta, N.; Li, C.; Fang, Y.; Liu, H.; Lin, B.; Jin, H.; Tian, L.; Zhang, H.; Zhang, W.; Xi, Z. Toxicity of TDCPP and TCEP on PC12 cell: Changes in CAMKII, GAP43, tubulin and NF-H gene and protein levels. Toxicol. Lett. 2014, 227, 164–171. [Google Scholar] [CrossRef]
- Pastorek, M.; Simko, V.; Takacova, M.; Barathova, M.; Bartosova, M.; Hunakova, L.; Sedlakova, O.; Hudecova, S.; Krizanova, O.; Dequiedt, F.; et al. Sulforaphane reduces molecular response to hypoxia in ovarian tumor cells independently of their resistance to chemotherapy. Int. J. Oncol. 2015, 47, 51–60. [Google Scholar] [CrossRef]
- Chovancova, B.; Liskova, V.; Miklikova, S.; Hudecova, S.; Babula, P.; Penesova, A.; Sevcikova, A.; Durinikova, E.; Novakova, M.; Matuskova, M.; et al. Calcium signaling affects migration and proliferation differently in individual cancer cells due to nifedipine treatment. Biochem. Pharmacol. 2020, 171, 113695. [Google Scholar] [CrossRef]
- Lau, D.H.; Xue, L.; Young, L.J.; Burke, P.A.; Cheung, A.T. Paclitaxel (Taxol): An inhibitor of angiogenesis in a highly vascularized transgenic breast cancer. Cancer Biother. Radiopharm. 1999, 14, 31 – 36. [Google Scholar] [CrossRef]
- Hunter, C.P. Epidemiology, stage at diagnosis, and tumor biology of breast carcinoma in multiracial and multiethnic populations. Cancer 2000, 88, 1193–1202. [Google Scholar] [CrossRef]
- Sakach, E.; O’Regan, R.; Meisel, J.; Li, X. Molecular classification of triple negative breast cancer and the emergence of targeted therapies. Clin. Breast Cancer 2021, 21, 509–520. [Google Scholar] [CrossRef]
- Zhao, S.; Zuo, W.-J.; Shao, Z.-M.; Jiang, Y.-Z. Molecular subtypes and precision treatment of triple-negative breast cancer. Ann. Transl. Med. 2020, 8, 499. [Google Scholar] [CrossRef]
- Dong, Q.; Yang, B.; Han, J.-G.; Zhang, M.-M.; Liu, W.; Zhang, X.; Yu, H.-L.; Liu, Z.-G.; Zhang, S.-H.; Li, T.; et al. A novel hydrogen sulfide-releasing donor, HA-ADT, suppresses the growth of human breast cancer cells through inhibiting the PI3K/AKT/mTOR and Ras/Raf/MEK/ERK signaling pathways. Cancer Lett. 2019, 455, 60–72. [Google Scholar] [CrossRef]
- Nakayama, S.; Torikoshi, Y.; Takahashi, T.; Yoshida, T.; Sudo, T.; Matsushima, T.; Kawasaki, Y.; Katayama, A.; Gohda, K.; Hortobagyi, G.N.; et al. Prediction of paclitaxel sensitivity by CDK1 and CDK2 activity in human breast cancer cells. Breast Cancer Res. 2009, 11, R12. [Google Scholar] [CrossRef]
- Jeansonne, D.P.; Koh, G.Y.; Zhang, F.; Kirk-Ballard, H.; Wolff, L.; Liu, D.; Eilertsen, K.; Liu, Z. Paclitaxel-induced apoptosis is blocked by camptothecin in human breast and pancreatic cancer cells. Oncol. Rep. 2011, 25, 1473–1480. [Google Scholar]
- Haghnavaz, N.; Asghari, F.; Elieh Ali Komi, D.; Shanehbandi, D.; Baradaran, B.; Kazemi, T. HER2 positivity may confer resistance to therapy with paclitaxel in breast cancer cell lines. Artif. Cells Nanomed. Biotechnol. 2018, 46, 518–523. [Google Scholar] [CrossRef]
- Markova, J.; Hudecova, S.; Soltysova, A.; Sirova, M.; Csaderova, L.; Lencesova, L.; Ondrias, K.; Krizanova, O. Sodium/calcium exchanger is upregulated by sulfide signaling, forms complex with the β1 and β3 but not β2 adrenergic receptors, and induces apoptosis. Pflügers Arch. Eur. J. Physiol. 2014, 466, 1329–1342. [Google Scholar] [CrossRef]
- Bae, J.; Kumazoe, M.; Yamashita, S.; Tachibana, H. Hydrogen sulphide donors selectively potentiate a green tea polyphenol EGCG-induced apoptosis of multiple myeloma cells. Sci. Rep. 2017, 7, 6665. [Google Scholar] [CrossRef]
- Tian, Y.; Ge, Z.; Xu, M.; Ge, X.; Zhao, M.; Ding, F.; Yin, J.; Wang, X.; You, Y.; Shi, Z.; et al. Diallyl trisulfide sensitizes radiation therapy on glioblastoma through directly targeting thioredoxin 1. Free. Radic. Biol. Med. 2022, 189, 157–168. [Google Scholar] [CrossRef]
- Henklewska, M.; Pawlak, A.; Pruchnik, H.; Obminska-Mrukowicz, B. Complex of Platinum(II) with Tris(2-carboxyethyl)phosphine Induces Apoptosis in Canine Lymphoma/Leukemia Cell Lines. Anticancer Res. 2017, 37, 539–546. [Google Scholar] [CrossRef]
- Perillo, B.; Di Donato, M.; Pezone, A.; Di Zazzo, E.; Giovannelli, P.; Galasso, G.; Castoria, G.; Migliaccio, A. ROS in cancer therapy: The bright side of the moon. Exp. Mol. Med. 2020, 52, 192–203. [Google Scholar] [CrossRef]
- Lencesova, L.; Hudecova, S.; Csaderova, L.; Markova, J.; Soltysova, A.; Pastorek, M.; Sedlak, J.; Wood, M.E.; Whiteman, M.; Ondrias, K.; et al. Sulphide signalling potentiates apoptosis through the up-regulation of IP3 receptor types 1 and 2. Acta Physiol. 2013, 208, 350–361. [Google Scholar] [CrossRef]
- Pal, S.; Sharma, A.; Mathew, S.P.; Jaganathan, B.G. Targeting cancer-specific metabolic pathways for developing novel cancer therapeutics. Front. Immunol. 2022, 13, 955476. [Google Scholar] [CrossRef]
- Zhou, S.; Liu, J.; Wan, A.; Zhang, Y.; Qi, X. Epigenetic regulation of diverse cell death modalities in cancer: A focus on pyroptosis, ferroptosis, cuproptosis, and disulfidptosis. J. Hematol. Oncol. 2024, 17, 22. [Google Scholar] [CrossRef]
- Schell, J.C.; Olson, K.A.; Jiang, L.; Hawkins, A.J.; Van Vranken, J.G.; Xie, J.; Egnatchik, R.A.; Earl, E.G.; DeBerardinis, R.J.; Rutter, J. A role for the mitochondrial pyruvate carrier as a repressor of the Warburg effect and colon cancer cell growth. Mol. Cell 2014, 56, 400–413. [Google Scholar] [CrossRef]
- Untereiner, A.A.; Olah, G.; Modis, K.; Hellmich, M.R.; Szabo, C. H2S-induced S-sulfhydration of lactate dehydrogenase a (LDHA) stimulates cellular bioenergetics in HCT116 colon cancer cells. Biochem. Pharmacol. 2017, 136, 86–98. [Google Scholar] [CrossRef]
- Görlach, A.; Bertram, K.; Hudecova, S.; Krizanova, O. Calcium and ROS: A mutual interplay. Redox Biol. 2015, 6, 260–271. [Google Scholar] [CrossRef]
- Zhang, Y.; Tang, Y.; Tang, X.; Wang, Y.; Zhang, Z.; Yang, H. Paclitaxel Induces the Apoptosis of Prostate Cancer Cells via ROS-Mediated HIF-1α Expression. Molecules 2022, 27, 7183. [Google Scholar] [CrossRef]
- Wedmann, R.; Bertlein, S.; Macinkovic, I.; Böltz, S.; Miljkovic, J.L.; Muñoz, L.E.; Herrmann, M.; Filipovic, M.R. Working with "H2S": Facts and apparent artifacts. Nitric Oxide 2014, 41, 85–96. [Google Scholar] [CrossRef]
- Xie, Z.Z.; Liu, Y.; Bian, J.S. Hydrogen Sulfide and Cellular Redox Homeostasis. Oxidative Med. Cell. Longev. 2016, 2016, 6043038. [Google Scholar] [CrossRef]
- Hu, J.; Xu, Z.; Liao, D.; Jiang, Y.; Pu, H.; Wu, Z.; Xu, X.; Zhao, Z.; Liu, J.; Lu, X.; et al. An H2 S-BMP6 Dual-Loading System with Regulating Yap/Taz and Jun Pathway for Synergistic Critical Limb Ischemia Salvaging Therapy. Adv. Healthc. Mater. 2023, 2, e2301316. [Google Scholar] [CrossRef]
- Murphy, B.; Bhattacharya, R.; Mukherjee, P. Hydrogen sulfide signaling in mitochondria and disease. FASEB J. 2019, 33, 13098–13125. [Google Scholar] [CrossRef]
- Chang, Y.C.; Fong, Y.; Tsai, E.-M.; Chang, Y.-G.; Chou, H.L.; Wu, C.-Y.; Teng, Y.-N.; Liu, T.-C.; Yuan, S.-S.; Chiu, C.-C. Exogenous C8-Ceramide Induces Apoptosis by Overproduction of ROS and the Switch of Superoxide Dismutases SOD1 to SOD2 in Human Lung Cancer Cells. Int. J. Mol. Sci. 2018, 19, 3010. [Google Scholar] [CrossRef]
- de Souza Grinevicius, V.M.A.; Kviecinski, M.R.; Santos Mota, N.S.R.; Ourique, F.; Porfirio Will Castro, L.S.E.; Andreguetti, R.R.; Gomes Correia, J.F.; Filho, D.W.; Pich, C.T.; Pedrosa, R.C. Piper nigrum ethanolic extract rich in piperamides causes ROS overproduction, oxidative damage in DNA leading to cell cycle arrest and apoptosis in cancer cells. J. Ethnopharmacol. 2016, 189, 139–147. [Google Scholar] [CrossRef]
- Yang, C.H.; Horwitz, S.B. Taxol®: The First Microtubule Stabilizing Agent. Int. J. Mol. Sci. 2017, 18, 1733. [Google Scholar] [CrossRef]
- Li, J.; Chen, S.; Wang, X.; Shi, C.; Liu, H.; Yang, J.; Shi, W.; Guo, J.; Jia, H. Hydrogen Sulfide Disturbs Actin Polymerization via S-Sulfhydration Resulting in Stunted Root Hair Growth. Plant Physiol. 2018, 178, 936–949. [Google Scholar] [CrossRef]
- Mustafa, A.K.; Gadalla, M.M.; Sen, N.; Kim, S.; Mu, W.; Gazi, S.K.; Barrow, R.K.; Yang, G.; Wang, R.; Snyder, S.H. H2S signals through protein S-sulfhydration. Sci. Signal. 2009, 2, ra72. [Google Scholar] [CrossRef]
- Madurga, A.; Mižíková, I.; Ruiz-Camp, J.; Vadász, I.; Herold, S.; Mayer, K.; Fehrenbach, H.; Seeger, W.; Morty, R.E. Systemic hydrogen sulfide administration partially restores normal alveolarization in an experimental animal model of bronchopulmonary dysplasia. Am. J. Physiol. Lung Cell. Mol. Physiol. 2014, 306, L684–L697. [Google Scholar] [CrossRef]
- Zheng, D.; Dong, S.; Li, T.; Yang, F.; Yu, X.; Wu, J.; Zhong, X.; Zhao, Y.; Wang, L.; Xu, C.; et al. Exogenous Hydrogen Sulfide Attenuates Cardiac Fibrosis Through Reactive Oxygen Species Signal Pathways in Experimental Diabetes Mellitus Models. Cell Physiol. Biochem. 2015, 36, 917–929. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, S.; Zhu, J.; Guo, S.; Yue, T.; Xu, H.; Hu, J.; Huang, Z.; Chen, Z.; Wang, P.; et al. Overexpression of CBS/H2S inhibits proliferation and metastasis of colon cancer cells through downregulation of CD44. Cancer Cell Int. 2022, 22, 85. [Google Scholar] [CrossRef]
- Jang, H.; Oh, M.; Kim, Y.; Choi, I.; Yang, H.S.; Ryu, W.; Lee, S.; Yoon, B. Hydrogen sulfide treatment induces angiogenesis after cerebral ischemia. J. Neurosci. Res. 2014, 92, 1520–1528. [Google Scholar] [CrossRef]
- Polhemus, D.J.; Kondo, K.; Bhushan, S.; Bir, S.C.; Kevil, C.G.; Murohara, T.; Lefer, D.J.; Calvert, J.W. Hydrogen sulfide attenuates cardiac dysfunction after heart failure via induction of angiogenesis. Circ. Heart Fail. 2013, 6, 1077–1086. [Google Scholar] [CrossRef]
- Papapetropoulos, A.; Pyriochou, A.; Altaany, Z.; Yang, G.; Marazioti, A.; Zhou, Z.; Jeschke, M.G.; Branski, L.K.; Herndon, D.N.; Wang, R.; et al. Hydrogen sulfide is an endogenous stimulator of angiogenesis. Proc. Natl. Acad. Sci. USA 2009, 106, 21972–21977. [Google Scholar] [CrossRef]
- Macabrey, D.; Joniová, J.; Gasser, Q.; Bechelli, C.; Longchamp, A.; Urfer, S.; Lambelet, M.; Fu, C.-Y.; Schwarz, G.; Wagnières, G.; et al. Sodium thiosulfate, a source of hydrogen sulfide, stimulates endothelial cell proliferation and neovascularization. Front. Cardiovasc. Med. 2022, 9, 965965. [Google Scholar] [CrossRef]
- Ausprunk, D.H.; Knighton, D.R.; Folkman, J. Vascularization of normal and neoplastic tissues grafted to the chick chorioallantois. Am. J. Pathol. 1975, 79, 597–618. [Google Scholar]
- Kuri, P.M.; Pion, E.; Mahl, L.; Kainz, P.; Schwarz, S.; Brochhausen, C.; Aung, T.; Haerteis, S. Deep learning-based image analysis for the quantification of tumor-induced angiogenesis in the 3D in vivo tumor model—Establishment and addition to Laser Speckle Contrast Imaging (LSCI). Cells 2022, 11, 2321. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liskova, V.; Chovancova, B.; Galvankova, K.; Klena, L.; Matyasova, K.; Babula, P.; Grman, M.; Rezuchova, I.; Bartosova, M.; Krizanova, O. Slow Sulfide Donor GYY4137 Increased the Sensitivity of Two Breast Cancer Cell Lines to Paclitaxel by Different Mechanisms. Biomolecules 2024, 14, 651. https://doi.org/10.3390/biom14060651
Liskova V, Chovancova B, Galvankova K, Klena L, Matyasova K, Babula P, Grman M, Rezuchova I, Bartosova M, Krizanova O. Slow Sulfide Donor GYY4137 Increased the Sensitivity of Two Breast Cancer Cell Lines to Paclitaxel by Different Mechanisms. Biomolecules. 2024; 14(6):651. https://doi.org/10.3390/biom14060651
Chicago/Turabian StyleLiskova, Veronika, Barbora Chovancova, Kristina Galvankova, Ladislav Klena, Katarina Matyasova, Petr Babula, Marian Grman, Ingeborg Rezuchova, Maria Bartosova, and Olga Krizanova. 2024. "Slow Sulfide Donor GYY4137 Increased the Sensitivity of Two Breast Cancer Cell Lines to Paclitaxel by Different Mechanisms" Biomolecules 14, no. 6: 651. https://doi.org/10.3390/biom14060651
APA StyleLiskova, V., Chovancova, B., Galvankova, K., Klena, L., Matyasova, K., Babula, P., Grman, M., Rezuchova, I., Bartosova, M., & Krizanova, O. (2024). Slow Sulfide Donor GYY4137 Increased the Sensitivity of Two Breast Cancer Cell Lines to Paclitaxel by Different Mechanisms. Biomolecules, 14(6), 651. https://doi.org/10.3390/biom14060651






