Investigation of Uterine Fluid Extracellular Vesicles’ Proteomic Profiles Provides Novel Diagnostic Biomarkers of Bovine Endometritis
Abstract
1. Introduction
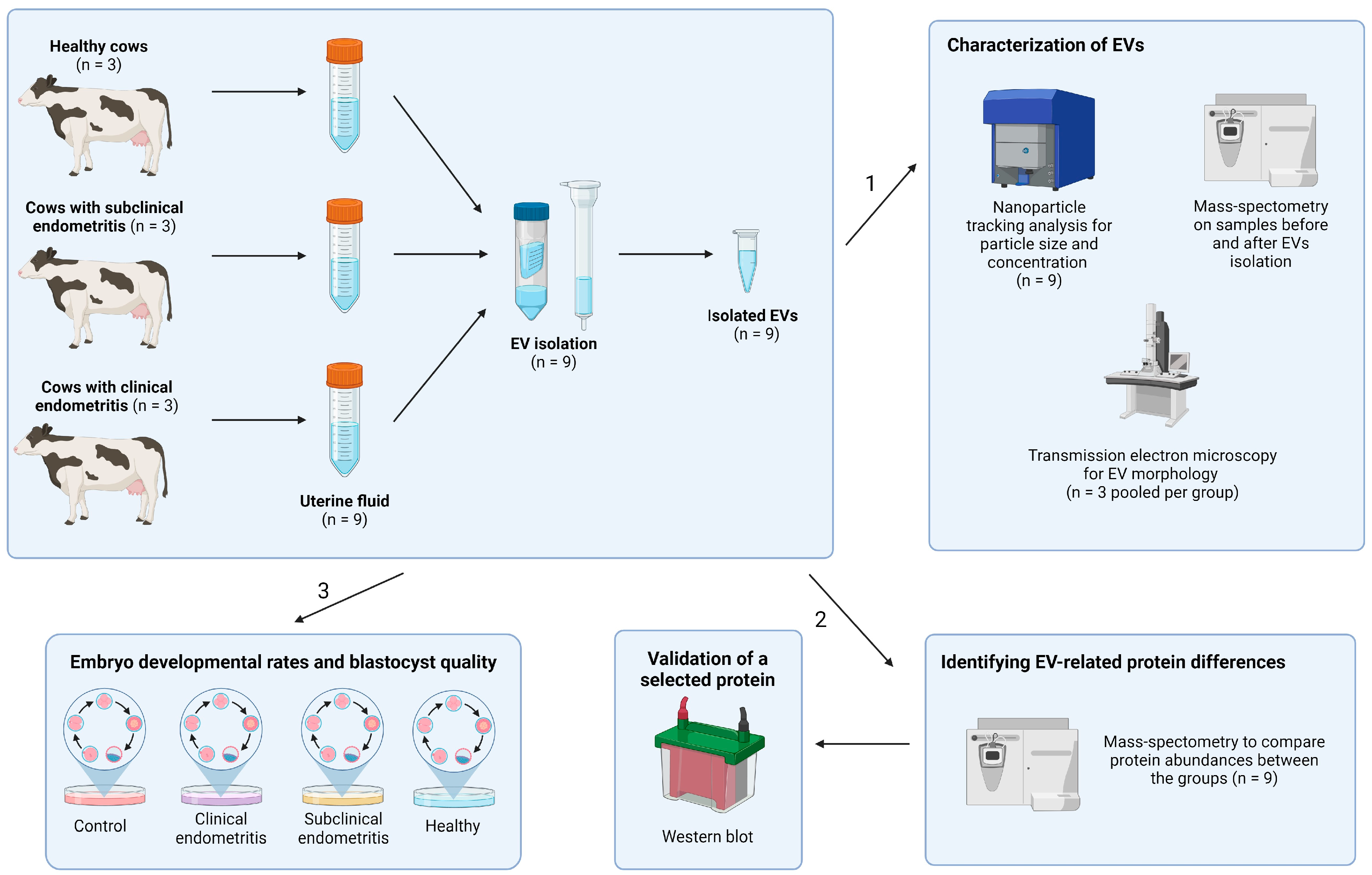
2. Materials and Methods
2.1. Selection of Cows
2.2. Collection of UF for EV Enrichment
2.3. Sample Preparation and Storage
2.4. UF-EV Enrichment
2.5. Characterization of UF-EVs
2.6. Analysis of UF-EV Total Proteomic Profile
2.7. Validation with Western Blot Analysis
2.8. In Vitro Production of Embryos
2.8.1. Embryo Culturing
2.8.2. Supplementation of UF-EVs to Embryo Cultures
2.9. Experimental Design and Data Analysis
3. Results
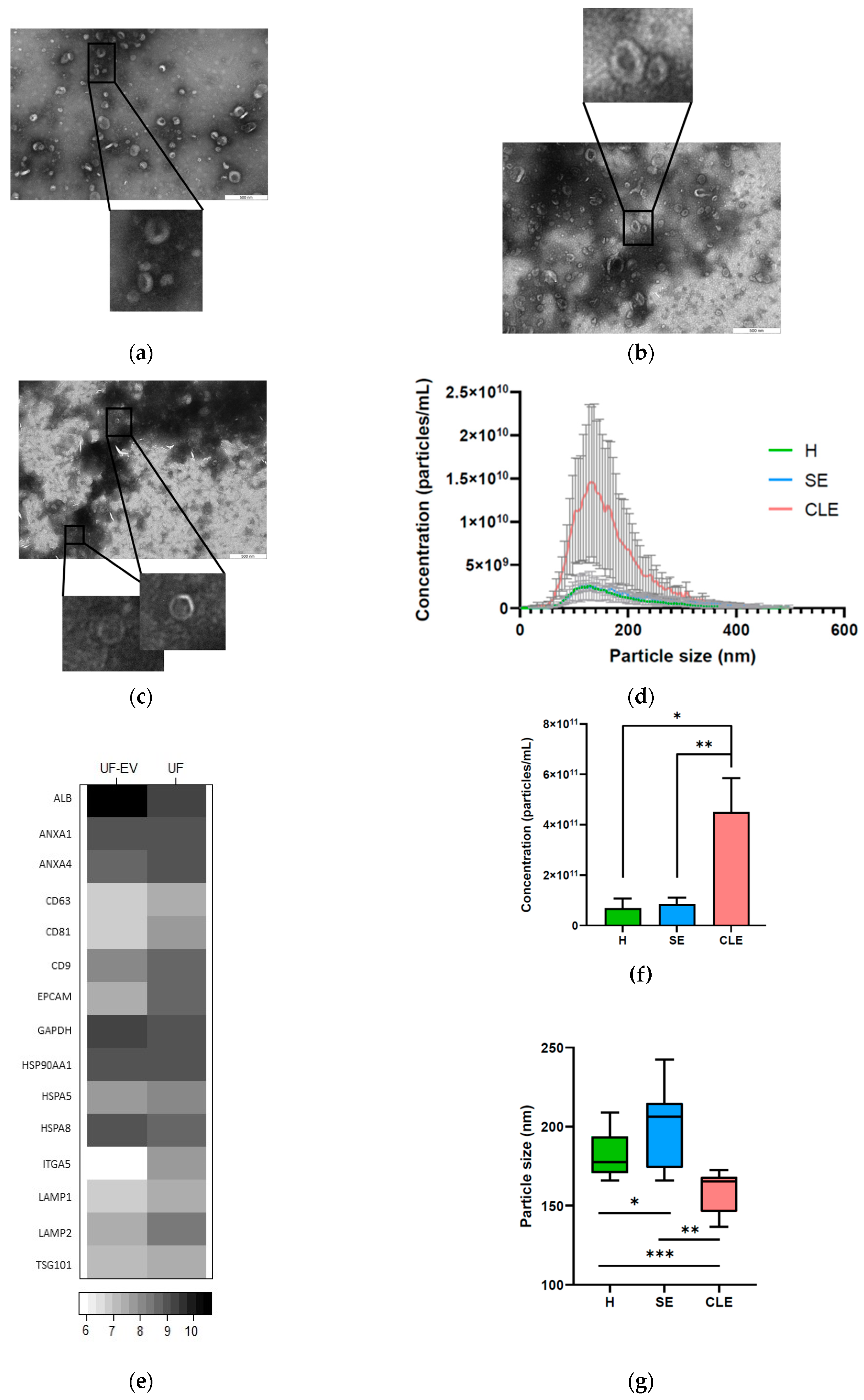
3.1. Characterization of UF-EVs
3.2. EV Particle Concentrations and Sizes Were Different in Different Endometritis Types
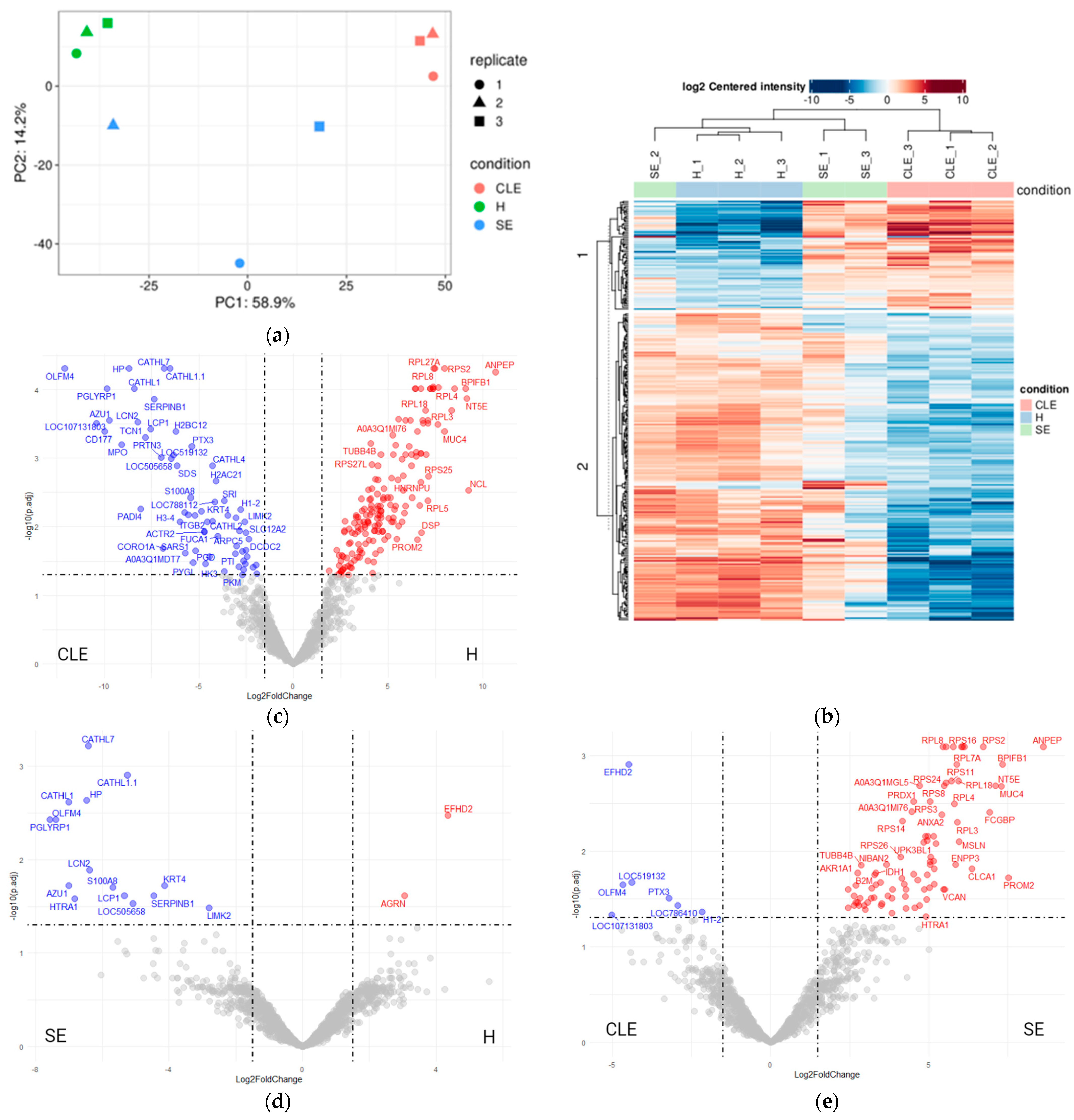
3.3. UF-EV Proteomic Profiles Were Varied between Different Endometritis Forms
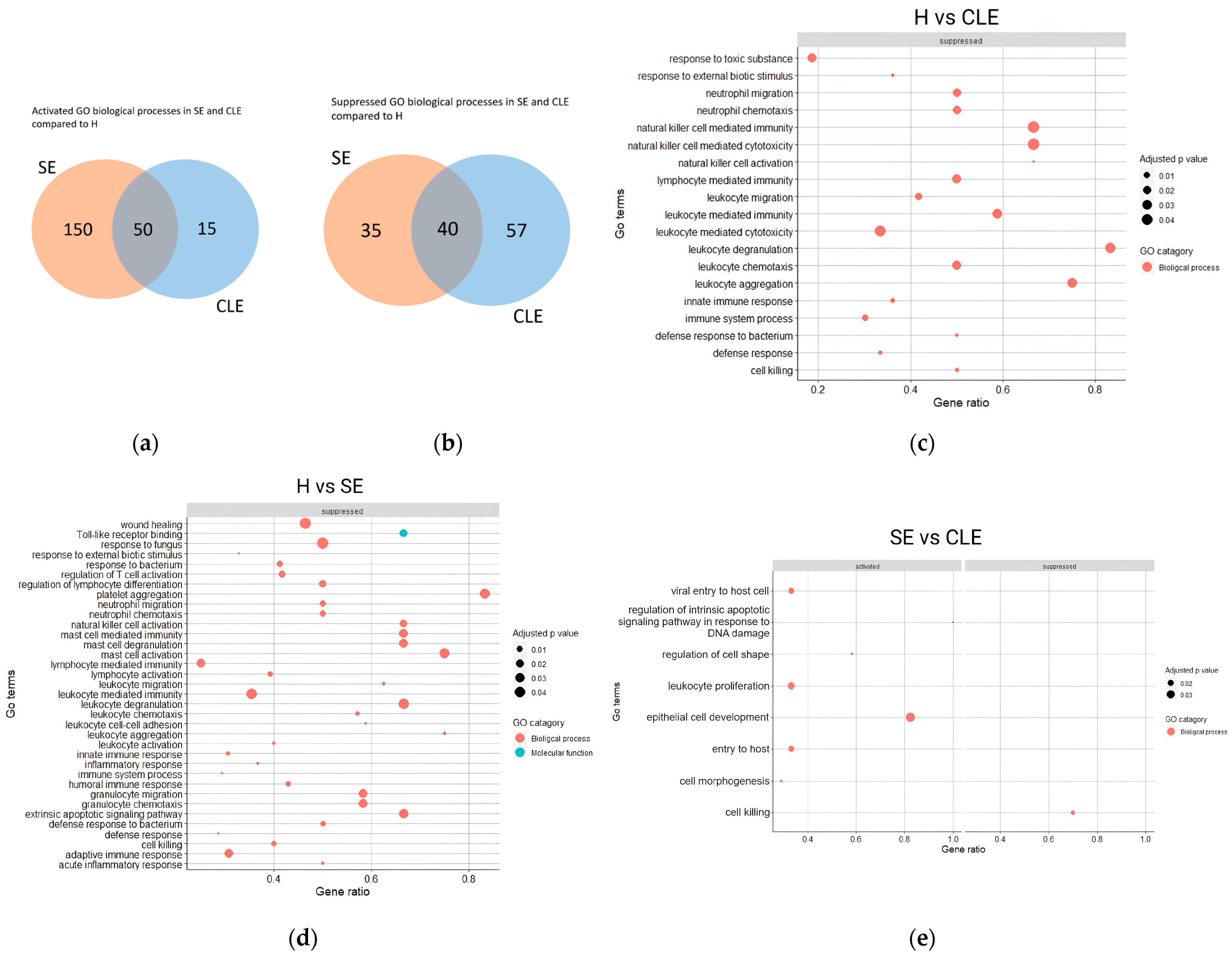
3.4. The Varied Enrichment of Inflammatory Pathways in the Different Endometritis Forms
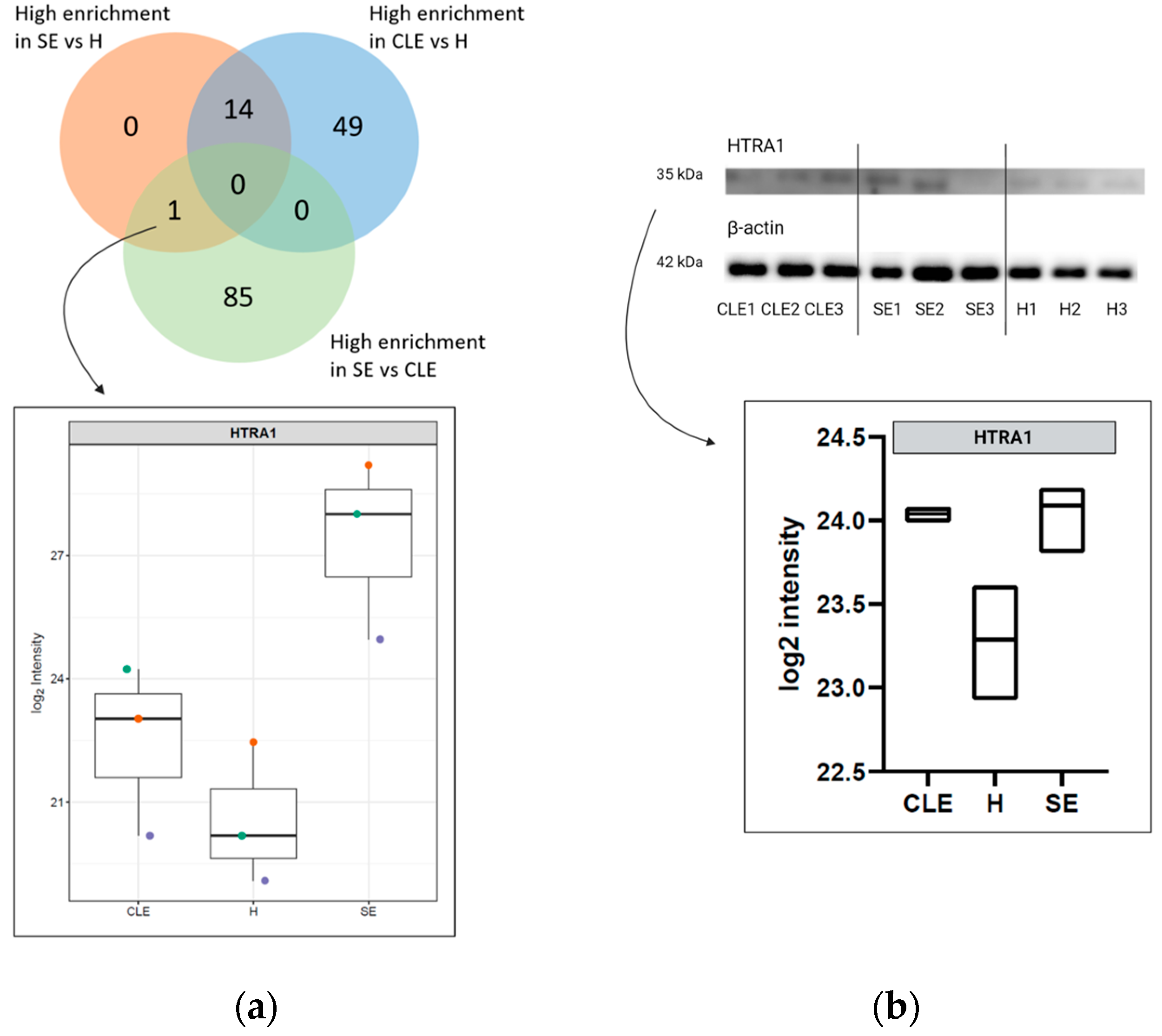
3.5. HTRA1 Protein Can Be a Potential Biomarker for SE
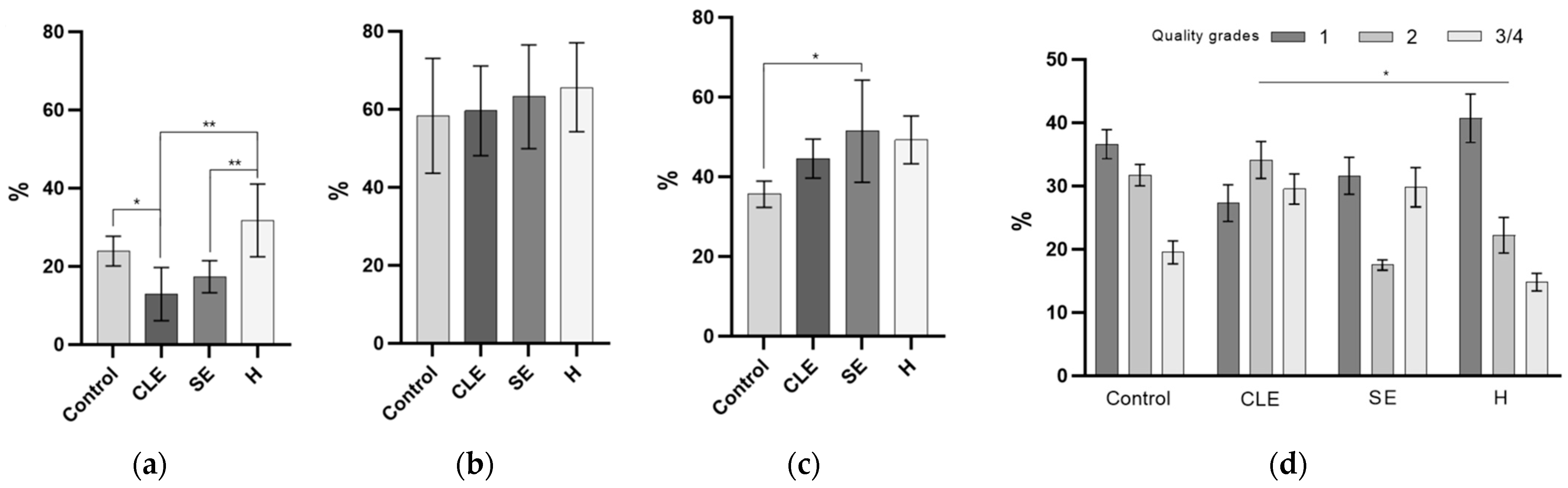
3.6. The Impact of Endometritis UF-EV on Embryo Development
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, I.H.; Jeong, J.K. Risk factors limiting first service conception rate in dairy cows and their economic impact. Asian-Australas. J. Anim. Sci. 2019, 32, 519–526. [Google Scholar] [PubMed]
- Yanga, D.S.; Jaja, I.F. Culling and mortality of dairy cows: Why it happens and how it can be mitigated. F1000Research 2022, 10, 1014. [Google Scholar] [CrossRef] [PubMed]
- Guadagnin, A.R.; Cardoso, F.C. Association of dry matter intake, milk yield, and days to first ovulation with cytological endometritis in Holstein cows. J. Dairy Sci. 2023, 106, 7240–7265. [Google Scholar] [CrossRef] [PubMed]
- LeBlanc, S.J.; Duffield, T.F.; Leslie, K.E.; Bateman, K.G.; Keefe, G.P.; Walton, J.S.; Johnson, W.H. Defining and diagnosing postpartum clinical endometritis and its impact on reproductive performance in dairy cows. J. Dairy Sci. 2002, 85, 2223–2236. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, I.M.; Lewis, G.S.; LeBlanc, S.; Gilbert, R.O. Defining postpartum uterine disease in cattle. Theriogenology 2006, 65, 1516–1530. [Google Scholar] [CrossRef] [PubMed]
- Pascottini, O.B.; Hostens, M.; Dini, P.; Vandepitte, J.; Ducatelle, R.; Opsomer, G. Comparison between cytology and histopathology to evaluate subclinical endometritis in dairy cows. Theriogenology 2016, 86, 1550–1556. [Google Scholar] [CrossRef] [PubMed]
- Wagener, K.; Gabler, C.; Drillich, M. A review of the ongoing discussion about definition, diagnosis and pathomechanism of subclinical endometritis in dairy cows. Theriogenology 2017, 94, 21–30. [Google Scholar] [CrossRef]
- Pascottini, O.B.; LeBlanc, S.J.; Gnemi, G.; Leroy, J.L.M.R.; Opsomer, G. Genesis of clinical and subclinical endometritis in dairy cows. Reproduction 2023, 166, R15–R24. [Google Scholar] [CrossRef]
- Pirtea, P.; Cicinelli, E.; De Nola, R.; de Ziegler, D.; Ayoubi, J.M. Endometrial causes of recurrent pregnancy losses: Endometriosis, adenomyosis, and chronic endometritis. Fertil. Steril. 2021, 115, 546–560. [Google Scholar] [CrossRef]
- Sheldon, I.M.; Dobson, H. Postpartum uterine health in cattle. Anim. Reprod. Sci. 2004, 82–83, 295–306. [Google Scholar] [CrossRef]
- Sheldon, I.M.; Williams, E.J.; Miller, A.N.A.; Nash, D.M.; Herath, S. Uterine diseases in cattle after parturition. Vet. J. Lond. Engl. 1997 2008, 176, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Pothmann, H.; Müller, J.; Pothmann, I.; Tichy, A.; Drillich, M. Reproducibility of endometrial cytology using cytobrush technique and agreement for the diagnosis of subclinical endometritis between five predefined endometrial sites. Reprod. Domest. Anim. 2019, 54, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Lundahl, S.; Garmo, R.T.; Gillund, P.; Klem, T.B.; Waldmann, A.; Krogenæs, A.K. Prevalence, risk factors, and effects on fertility of cytological endometritis at the time of insemination in Norwegian Red cows. J. Dairy Sci. 2021, 104, 6961–6974. [Google Scholar] [CrossRef] [PubMed]
- Melcher, Y.; Prunner, I.; Drillich, M. Degree of variation and reproducibility of different methods for the diagnosis of subclinical endometritis. Theriogenology 2014, 82, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Druker, S.A.; Sicsic, R.; van Straten, M.; Goshen, T.; Kedmi, M.; Raz, T. Cytological endometritis diagnosis in primiparous versus multiparous dairy cows. J. Dairy Sci. 2022, 105, 665–683. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Sethi, A. Endometritis-Diagnosis, Treatment and its impact on fertility-A Scoping Review. JBRA Assist. Reprod. 2022, 26, 538–546. [Google Scholar] [CrossRef] [PubMed]
- Simon, C.; Greening, D.W.; Bolumar, D.; Balaguer, N.; Salamonsen, L.A.; Vilella, F. Extracellular Vesicles in Human Reproduction in Health and Disease. Endocr. Rev. 2018, 39, 292–332. [Google Scholar] [CrossRef]
- Li, K.; Lin, Y.; Luo, Y.; Xiong, X.; Wang, L.; Durante, K.; Li, J.; Zhou, F.; Guo, Y.; Chen, S.; et al. A signature of saliva-derived exosomal small RNAs as predicting biomarker for esophageal carcinoma: A multicenter prospective study. Mol. Cancer 2022, 21, 21. [Google Scholar] [CrossRef]
- Araki, Y.; Asano, N.; Yamamoto, N.; Hayashi, K.; Takeuchi, A.; Miwa, S.; Igarashi, K.; Higuchi, T.; Abe, K.; Taniguchi, Y.; et al. A validation study for the utility of serum microRNA as a diagnostic and prognostic marker in patients with osteosarcoma. Oncol. Lett. 2023, 25, 222. [Google Scholar] [CrossRef]
- You, Y.; Muraoka, S.; Jedrychowski, M.P.; Hu, J.; McQuade, A.K.; Young-Pearse, T.; Aslebagh, R.; Shaffer, S.A.; Gygi, S.P.; Blurton-Jones, M.; et al. Human neural cell type-specific extracellular vesicle proteome defines disease-related molecules associated with activated astrocytes in Alzheimer’s disease brain. J. Extracell. Vesicles 2022, 11, e12183. [Google Scholar] [CrossRef]
- Lyu, L.; Zhang, X.; Li, C.; Yang, T.; Wang, J.; Pan, L.; Jia, H.; Li, Z.; Sun, Q.; Yue, L.; et al. Small RNA Profiles of Serum Exosomes Derived From Individuals With Latent and Active Tuberculosis. Front. Microbiol. 2019, 10, 1174. [Google Scholar] [CrossRef] [PubMed]
- Zahoor, M.A.; Yao, X.D.; Henrick, B.M.; Verschoor, C.P.; Abimiku, A.; Osawe, S.; Rosenthal, K.L. Expression profiling of human milk derived exosomal microRNAs and their targets in HIV-1 infected mothers. Sci. Rep. 2020, 10, 12931. [Google Scholar] [CrossRef]
- Chaparro, A.; Gaedechens, D.; Ramírez, V.; Zuñiga, E.; Kusanovic, J.P.; Inostroza, C.; Varas-Godoy, M.; Silva, K.; Salomon, C.; Rice, G.; et al. Placental biomarkers and angiogenic factors in oral fluids of patients with preeclampsia. Prenat. Diagn. 2016, 36, 476–482. [Google Scholar] [CrossRef]
- Sammar, M.; Dragovic, R.; Meiri, H.; Vatish, M.; Sharabi-Nov, A.; Sargent, I.; Redman, C.; Tannetta, D. Reduced placental protein 13 (PP13) in placental derived syncytiotrophoblast extracellular vesicles in preeclampsia–A novel tool to study the impaired cargo transmission of the placenta to the maternal organs. Placenta 2018, 66, 17–25. [Google Scholar] [CrossRef]
- Lv, Y.; Lu, C.; Ji, X.; Miao, Z.; Long, W.; Ding, H.; Lv, M. Roles of microRNAs in preeclampsia. J. Cell. Physiol. 2019, 234, 1052–1061. [Google Scholar] [CrossRef]
- Almughlliq, F.B.; Koh, Y.Q.; Peiris, H.N.; Vaswani, K.; McDougall, S.; Graham, E.M.; Burke, C.R.; Arachchige, B.J.; Reed, S.; Mitchell, M.D. Proteomic content of circulating exosomes in dairy cows with or without uterine infection. Theriogenology 2018, 114, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, R.; Takashima, S.; Wakihara, Y.; Kamatari, Y.O.; Kitamura, Y.; Shimizu, K.; Okada, A.; Inoshima, Y. Comparing microRNA in milk small extracellular vesicles among healthy cattle and cattle at high risk for bovine leukemia virus transmission. J. Dairy Sci. 2022, 105, 5370–5380. [Google Scholar] [CrossRef]
- Tsukada, F.; Takashima, S.; Wakihara, Y.; Kamatari, Y.O.; Shimizu, K.; Okada, A.; Inoshima, Y. Characterization of miRNAs in Milk Small Extracellular Vesicles from Enzootic Bovine Leukosis Cattle. Int. J. Mol. Sci. 2022, 23, 10782. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yao, X.; Xie, T.; Chang, Z.; Guo, Y.; Ni, H. Exosome-derived uterine miR-218 isolated from cows with endometritis regulates the release of cytokines and chemokines. Microb. Biotechnol. 2020, 13, 1103–1117. [Google Scholar] [CrossRef]
- Jiang, X.; Li, Z.; Chang, X.; Lian, Z.; Wang, A.; Lin, P.; Chen, H.; Zhou, D.; Tang, K.; Jin, Y. A Comparative Proteomic Analysis to Explore the Influencing Factors on Endometritis Using LC-MS/MS. Int. J. Mol. Sci. 2023, 24, 10018. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Eguchi, A.; Tamai, Y.; Fukuda, S.; Tempaku, M.; Izuoka, K.; Iwasa, M.; Takei, Y.; Togashi, K. Protein Composition of Circulating Extracellular Vesicles Immediately Changed by Particular Short Time of High-Intensity Interval Training Exercise. Front. Physiol. 2021, 12, 693007. [Google Scholar] [CrossRef] [PubMed]
- Kingsmore, S.F. Multiplexed protein measurement: Technologies and applications of protein and antibody arrays. Nat. Rev. Drug Discov. 2006, 5, 310–320. [Google Scholar] [CrossRef] [PubMed]
- Elsik, C.G.; Tellam, R.L.; Worley, K.C. The Genome Sequence of Taurine Cattle: A window to ruminant biology and evolution. Science 2009, 324, 522–528. [Google Scholar] [PubMed]
- Malhi, P.S.; Adams, G.P.; Singh, J. Bovine Model for the Study of Reproductive Aging in Women: Follicular, Luteal, and Endocrine Characteristics1. Biol. Reprod. 2005, 73, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Wenz, J.R.; Moore, D.A.; Kasimanickam, R. Factors associated with the rectal temperature of Holstein dairy cows during the first 10 days in milk. J. Dairy Sci. 2011, 94, 1864–1872. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, P.T.; Munksgaard, L.; Tøgersen, F.A. Evaluation of a Lameness Scoring System for Dairy Cows. J. Dairy Sci. 2008, 91, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Roche, J.; Friggens, N.; Kay, J.; Fisher, M.W.; Stafford, K.; Berry, D. Invited review: Body condition score and its association with dairy cow productivity, health, and welfare. J. Dairy Sci. 2009, 92, 5769–5801. [Google Scholar] [CrossRef] [PubMed]
- Waldmann, A.; Ropstad, E.; Landsverk, K.; Sørensen, K.; Sølverød, L.; Dahl, E. Level and distribution of progesterone in bovine milk in relation to storage in the mammary gland. Anim. Reprod. Sci. 1999, 56, 79–91. [Google Scholar] [CrossRef] [PubMed]
- Waldmann, A. Enzyme immunoassay (EIA) for milk progesterone using a monoclonal antibody. Anim. Reprod. Sci. 1993, 34, 19–30. [Google Scholar] [CrossRef]
- Waldmann, A. Monoclonal antibodies to progesterone: Characterization and selection for enzyme immunoassay in bovine milk. Hybridoma 1999, 18, 289–296. [Google Scholar] [CrossRef]
- Williams, E.J.; Fischer, D.P.; Pfeiffer, D.U.; England, G.C.; Noakes, D.E.; Dobson, H.; Sheldon, I.M. Clinical evaluation of postpartum vaginal mucus reflects uterine bacterial infection and the immune response in cattle. Theriogenology 2005, 63, 102–117. [Google Scholar] [CrossRef] [PubMed]
- Valdmann, M.; Kurykin, J.; Waldmann, A. Individual and Combined Effects of Diseases and Cytological Endometritis on Reproductive Performance and Culling of Dairy Cows: Preliminary Results. Animals 2022, 12, 2913. [Google Scholar] [CrossRef] [PubMed]
- Piibor, J.; Waldmann, A.; Dissanayake, K.; Andronowska, A.; Ivask, M.; Prasadani, M.; Kavak, A.; Kodithuwakku, S.; Fazeli, A. Uterine fluid extracellular vesicles proteome is altered during the oestrous cycle. Mol. Cell. Proteomics 2023, 22, 100642. [Google Scholar] [CrossRef] [PubMed]
- Welsh, J.A.; Goberdhan, D.C.I.; O’Driscoll, L.; Buzas, E.I.; Blenkiron, C.; Bussolati, B.; Cai, H.; Di Vizio, D.; Driedonks, T.A.P.; Erdbrügger, U.; et al. Minimal information for studies of extracellular vesicles (MISEV2023): From basic to advanced approaches. J. Extracell. Vesicles 2024, 13, e12404. [Google Scholar] [CrossRef] [PubMed]
- Bó, G.A.; Mapletoft, R. Evaluation and classification of bovine embryos. Anim. Reprod. 2013, 10, 344–348. [Google Scholar]
- Shah, A.D.; Goode, R.J.A.; Huang, C.; Powell, D.R.; Schittenhelm, R.B. LFQ-Analyst: An Easy-To-Use Interactive Web Platform to Analyze and Visualize Label-Free Proteomics Data Preprocessed with MaxQuant. J. Proteome Res. 2020, 19, 204–211. [Google Scholar] [CrossRef] [PubMed]
- ExoCarta: Exosome Markers. 2023. Available online: http://exocarta.org/exosome_markers_new (accessed on 19 November 2023).
- Pascottini, O.B.; Aurich, C.; England, G.; Grahofer, A. General and comparative aspects of endometritis in domestic species: A review. Reprod. Domest. Anim. 2023, 58, 49–71. [Google Scholar] [CrossRef] [PubMed]
- Freeman, D.W.; Noren Hooten, N.; Eitan, E.; Green, J.; Mode, N.A.; Bodogai, M.; Zhang, Y.; Lehrmann, E.; Zonderman, A.B.; Biragyn, A.; et al. Altered Extracellular Vesicle Concentration, Cargo, and Function in Diabetes. Diabetes 2018, 67, 2377–2388. [Google Scholar] [CrossRef] [PubMed]
- Lashin, H.M.S.; Nadkarni, S.; Oggero, S.; Jones, H.R.; Knight, J.C.; Hinds, C.J.; Perretti, M. Microvesicle Subsets in Sepsis Due to Community Acquired Pneumonia Compared to Faecal Peritonitis. Shock 2018, 49, 393. [Google Scholar] [CrossRef]
- O’Dea, K.P.; Porter, J.R.; Tirlapur, N.; Katbeh, U.; Singh, S.; Handy, J.M.; Takata, M. Circulating Microvesicles Are Elevated Acutely following Major Burns Injury and Associated with Clinical Severity. PLoS ONE 2016, 11, e0167801. [Google Scholar] [CrossRef]
- Bonjoch, L.; Casas, V.; Carrascal, M.; Closa, D. Involvement of exosomes in lung inflammation associated with experimental acute pancreatitis. J. Pathol. 2016, 240, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Verma, V.K.; Li, H.; Wang, R.; Hirsova, P.; Mushref, M.; Liu, Y.; Cao, S.; Contreras, P.C.; Malhi, H.; Kamath, P.S.; et al. Alcohol stimulates macrophage activation through caspase-dependent hepatocyte derived release of CD40L containing extracellular vesicles. J. Hepatol. 2016, 64, 651–660. [Google Scholar] [CrossRef] [PubMed]
- Longobardi, A.; Nicsanu, R.; Bellini, S.; Squitti, R.; Catania, M.; Tiraboschi, P.; Saraceno, C.; Ferrari, C.; Zanardini, R.; Binetti, G.; et al. Cerebrospinal Fluid EV Concentration and Size Are Altered in Alzheimer’s Disease and Dementia with Lewy Bodies. Cells 2022, 11, 462. [Google Scholar] [CrossRef] [PubMed]
- Pulliam, L.; Sun, B.; Mustapic, M.; Chawla, S.; Kapogiannis, D. Plasma neuronal exosomes serve as biomarkers of cognitive impairment in HIV infection and Alzheimer’s Disease. J. Neurovirol. 2019, 25, 702–709. [Google Scholar] [CrossRef] [PubMed]
- Delabranche, X.; Boisramé-Helms, J.; Asfar, P.; Berger, A.; Mootien, Y.; Lavigne, T.; Grunebaum, L.; Lanza, F.; Gachet, C.; Freyssinet, J.M.; et al. Microparticles are new biomarkers of septic shock-induced disseminated intravascular coagulopathy. Intensive Care Med. 2013, 39, 1695–1703. [Google Scholar] [CrossRef] [PubMed]
- Baxter, A.A.; Phan, T.K.; Hanssen, E.; Liem, M.; Hulett, M.D.; Mathivanan, S.; Poon, I.K.H. Analysis of extracellular vesicles generated from monocytes under conditions of lytic cell death. Sci. Rep. 2019, 9, 7538. [Google Scholar] [CrossRef] [PubMed]
- Distler, J.H.W.; Huber, L.C.; Hueber, A.J.; Reich, C.F., 3rd; Gay, S.; Distler, O.; Pisetsky, D.S. The release of microparticles by apoptotic cells and their effects on macrophages. Apoptosis 2005, 10, 731–741. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xu, Q.; Zhang, W.; Yang, Q.; Guo, Z.; Li, C.; Zhang, Z.; Dong, Q.; Sun, H.; Zhang, C.; et al. A highly-parallelized and low-sample-size chip for simultaneous detection of protein and nucleic acid biomarkers in hepatocellular carcinoma. Sens. Actuators B Chem. 2023, 392, 134112. [Google Scholar] [CrossRef]
- Pepe, M.S.; Li, C.I.; Feng, Z. Improving the Quality of Biomarker Discovery Research: The Right Samples and Enough of Them. Cancer Epidemiol. Biomark. Prev. Publ. Am. Assoc. Cancer Res. Cosponsored Am. Soc. Prev. Oncol. 2015, 24, 944. [Google Scholar] [CrossRef]
- Buzzaccarini, G.; Vitagliano, A.; Andrisani, A.; Santarsiero, C.M.; Cicinelli, R.; Nardelli, C.; Ambrosini, G.; Cicinelli, E. Chronic endometritis and altered embryo implantation: A unified pathophysiological theory from a literature systematic review. J. Assist. Reprod. Genet. 2020, 37, 2897–2911. [Google Scholar] [CrossRef]
- Di Pietro, C.; Cicinelli, E.; Guglielmino, M.R.; Ragusa, M.; Farina, M.; Palumbo, M.A.; Cianci, A. Altered Transcriptional Regulation of Cytokines, Growth Factors, and Apoptotic Proteins in the Endometrium of Infertile Women with Chronic Endometritis. Am. J. Reprod. Immunol. 2013, 69, 509–517. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhang, H.; Chen, Z.Q.; Zhang, W.; Liu, X.M.; Fang, J.Y.; Liu, F.J.; Kwak-Kim, J. Endometrial TGF-β, IL-10, IL-17 and autophagy are dysregulated in women with recurrent implantation failure with chronic endometritis. Reprod. Biol. Endocrinol. 2019, 17, 2. [Google Scholar] [CrossRef] [PubMed]
- Bradford, B.J.; Swartz, T.H. Review: Following the smoke signals: Inflammatory signaling in metabolic homeostasis and homeorhesis in dairy cattle. Animal 2020, 14, s144–s154. [Google Scholar] [CrossRef] [PubMed]
- Esposito, G.; Irons, P.C.; Webb, E.C.; Chapwanya, A. Interactions between negative energy balance, metabolic diseases, uterine health and immune response in transition dairy cows. Anim. Reprod. Sci. 2014, 144, 60–71. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Xuan, Y.; Zhang, X.; Liu, Y.; Yang, S.; Yang, K. Immune cell metabolism and metabolic reprogramming. Mol. Biol. Rep. 2022, 49, 9783–9795. [Google Scholar] [CrossRef] [PubMed]
- Iyer, A.; Brown, L.; Whitehead, J.P.; Prins, J.B.; Fairlie, D.P. Nutrient and immune sensing are obligate pathways in metabolism, immunity, and disease. FASEB J. 2015, 29, 3612–3625. [Google Scholar] [CrossRef] [PubMed]
- Madoz, L.V.; Giuliodori, M.J.; Migliorisi, A.L.; Jaureguiberry, M.; de la Sota, R.L. Endometrial cytology, biopsy, and bacteriology for the diagnosis of subclinical endometritis in grazing dairy cows. J. Dairy Sci. 2014, 97, 195–201. [Google Scholar] [CrossRef]
- Pascottini, O.B.; Van Schyndel, S.J.; Spricigo, J.F.W.; Rousseau, J.; Weese, J.S.; LeBlanc, S.J. Dynamics of uterine microbiota in postpartum dairy cows with clinical or subclinical endometritis. Sci. Rep. 2020, 10, 12353. [Google Scholar] [CrossRef]
- Jiang, X.; Li, Z.; Chang, X.; Huang, C.; Qiu, R.; Wang, A.; Lin, P.; Tang, K.; Chen, H.; Zhou, D.; et al. Proteomic analysis of uterine lavage fluid of dairy cows at different time after delivery by mass spectrometry. Theriogenology 2023, 207, 31–48. [Google Scholar] [CrossRef]
- Runyon, S.T.; Zhang, Y.; Appleton, B.A.; Sazinsky, S.L.; Wu, P.; Pan, B.; Wiesmann, C.; Skelton, N.J.; Sidhu, S.S. Structural and functional analysis of the PDZ domains of human HtrA1 and HtrA3. Protein Sci. Publ. Protein Soc. 2007, 16, 2454–2471. [Google Scholar] [CrossRef]
- Risør, M.W.; Poulsen, E.T.; Thomsen, L.R.; Dyrlund, T.F.; Nielsen, T.A.; Nielsen, N.C.; Sanggaard, K.W.; Enghild, J.J. The Autolysis of Human HtrA1 Is Governed by the Redox State of Its N-Terminal Domain. Biochemistry 2014, 53, 3851–3857. [Google Scholar] [CrossRef]
- Oka, C.; Saleh, R.; Bessho, Y.; Reza, H.M. Interplay between HTRA1 and classical signalling pathways in organogenesis and diseases. Saudi J. Biol. Sci. 2022, 29, 1919–1927. [Google Scholar] [CrossRef]
- Globus, O.; Evron, T.; Caspi, M.; Siman-Tov, R.; Rosin-Arbesfeld, R. High-Temperature Requirement A1 (Htra1)—A Novel Regulator of Canonical Wnt Signaling. Sci. Rep. 2017, 7, 17995. [Google Scholar] [CrossRef]
- Hu, S.-I.; Carozza, M.; Klein, M.; Nantermet, P.; Luk, D.; Crowl, R.M. Human HtrA, an Evolutionarily Conserved Serine Protease Identified as a Differentially Expressed Gene Product in Osteoarthritic Cartilage*. J. Biol. Chem. 1998, 273, 34406–34412. [Google Scholar] [CrossRef]
- Dogru, S.; Dai, Z.; Alba, G.M.; Simone, N.J.; Albro, M.B. Computational and experimental characterizations of the spatiotemporal activity and functional role of TGF-β in the synovial joint. J. Biomech. 2023, 156, 111673. [Google Scholar] [CrossRef]
- van Beuningen, H.M.; Glansbeek, H.L.; van der Kraan, P.M.; van den Berg, W.B. Osteoarthritis-like changes in the murine knee joint resulting from intra-articular transforming growth factor-β injections. Osteoarthr. Cartil. 2000, 8, 25–33. [Google Scholar] [CrossRef]
- Masuda, Y.; Hasebe, R.; Kuromi, Y.; Matsuo, M.; Hishinuma, M.; Ohbayashi, T.; Nishimura, R. Three-dimensional morphology of bovine blastocysts hatched against lipopolysaccharide exposure in vitro. Reprod. Biol. 2024, 24, 100843. [Google Scholar] [CrossRef]
- Williams, C.L.; Teeling, J.L.; Perry, V.H.; Fleming, T.P. Mouse maternal systemic inflammation at the zygote stage causes blunted cytokine responsiveness in lipopolysaccharide-challenged adult offspring. BMC Biol. 2011, 9, 49. [Google Scholar] [CrossRef]
- Alarcon, V.B.; Marikawa, Y. Trophectoderm formation: Regulation of morphogenesis and gene expressions by RHO, ROCK, cell polarity, and HIPPO signaling. Reproduction 2022, 164, R75–R86. [Google Scholar] [CrossRef]
- Tóth, E.Á.; Turiák, L.; Visnovitz, T.; Cserép, C.; Mázló, A.; Sódar, B.W.; Försönits, A.I.; Petővári, G.; Sebestyén, A.; Komlósi, Z.; et al. Formation of a protein corona on the surface of extracellular vesicles in blood plasma. J. Extracell. Vesicles 2021, 10, e12140. [Google Scholar] [CrossRef]
- Perez-Riverol, Y.; Bai, J.; Bandla, C.; García-Seisdedos, D.; Hewapathirana, S.; Kamatchinathan, S.; Kundu, D.J.; Prakash, A.; Frericks-Zipper, A.; Eisenacher, M.; et al. The PRIDE database resources in 2022: A hub for mass spectrometry-based proteomics evidences. Nucleic Acids Res. 2021, 50, D543–D552. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piibor, J.; Waldmann, A.; Prasadani, M.; Kavak, A.; Andronowska, A.; Klein, C.; Kodithuwakku, S.; Fazeli, A. Investigation of Uterine Fluid Extracellular Vesicles’ Proteomic Profiles Provides Novel Diagnostic Biomarkers of Bovine Endometritis. Biomolecules 2024, 14, 626. https://doi.org/10.3390/biom14060626
Piibor J, Waldmann A, Prasadani M, Kavak A, Andronowska A, Klein C, Kodithuwakku S, Fazeli A. Investigation of Uterine Fluid Extracellular Vesicles’ Proteomic Profiles Provides Novel Diagnostic Biomarkers of Bovine Endometritis. Biomolecules. 2024; 14(6):626. https://doi.org/10.3390/biom14060626
Chicago/Turabian StylePiibor, Johanna, Andres Waldmann, Madhusha Prasadani, Ants Kavak, Aneta Andronowska, Claudia Klein, Suranga Kodithuwakku, and Alireza Fazeli. 2024. "Investigation of Uterine Fluid Extracellular Vesicles’ Proteomic Profiles Provides Novel Diagnostic Biomarkers of Bovine Endometritis" Biomolecules 14, no. 6: 626. https://doi.org/10.3390/biom14060626
APA StylePiibor, J., Waldmann, A., Prasadani, M., Kavak, A., Andronowska, A., Klein, C., Kodithuwakku, S., & Fazeli, A. (2024). Investigation of Uterine Fluid Extracellular Vesicles’ Proteomic Profiles Provides Novel Diagnostic Biomarkers of Bovine Endometritis. Biomolecules, 14(6), 626. https://doi.org/10.3390/biom14060626





