Recent Research Progress on Polyamidoamine-Engineered Hydrogels for Biomedical Applications
Abstract
1. Introduction
2. PAMAM-Crosslinked Hydrogels for Drug Delivery
2.1. Click Chemistry Reaction
2.2. Aza-Michael Addition
2.3. Schiff Base Reactions
2.4. Amidation Reaction
2.5. Other Reaction Methods
3. PAMAM-Crosslinked Hydrogels for Tissue Engineering
3.1. Bacterial Infection Treatment
3.2. Bone Tissue Engineering
3.3. Cartilage Tissue Engineering
3.4. Wound Healing
3.5. Hemostasis
4. PAMAM-Crosslinked Hydrogels for Other Applications
4.1. Tumor Photothermal Therapy (PTT)
4.2. Biofabrication
4.3. Smart PAMAM-Engineered Hydrogels
5. PAMAM-Engineered Nano-Hydrogels
6. Conclusions and Outlook
7. Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Mignani, S.; Shi, X.; Zablocka, M.; Majoral, J.-P. Dendritic macromolecular architectures: Dendrimer-based polyion complex micelles. Biomacromolecules 2021, 22, 262–274. [Google Scholar] [CrossRef] [PubMed]
- Yousefi, M.; Narmani, A.; Jafari, S.M. Dendrimers as efficient nanocarriers for the protection and delivery of bioactive phytochemicals. Adv. Colloid Interface Sci. 2020, 278, 102125. [Google Scholar] [CrossRef] [PubMed]
- Svenson, S.; Tomalia, D.A. Dendrimers in biomedical applications—Reflections on the field. Adv. Drug Deliv. Rev. 2012, 64, 102–115. [Google Scholar] [CrossRef]
- Li, Z.; Hu, J.; Yang, L.; Zhang, X.; Liu, X.; Wang, Z.; Li, Y. Integrated POSS-dendrimer nanohybrid materials: Current status and future perspective. Nanoscale 2020, 12, 11395–11415. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, H.; Pandey, M.; Mohgan, R.; Jong, J.S.J.; David, R.N.; Ngan, W.Y.; Chin, T.L.; Ting, S.; Kesharwani, P.; Gorain, B. Dendrimer-based delivery of macromolecules for the treatment of brain tumor. Biomater. Adv. 2022, 141, 213118. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, M.; Li, Y.; Cong, H.; Yu, B.; Shen, Y. Research status of dendrimer micelles in tumor therapy for drug delivery. Small 2023, 19, 2304006. [Google Scholar] [CrossRef] [PubMed]
- Tsukamoto, T.; Imaoka, T.; Yamamoto, K. Unique functions and applications of rigid dendrimers featuring radial aromatic chains. Acc. Chem. Res. 2021, 54, 4486–4497. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Shen, M.; Rodrigues, J.; Mignani, S.; Majoral, J.-P.; Shi, X. Superstructured poly(amidoamine) dendrimer-based nanoconstructs as platforms for cancer nanomedicine: A concise review. Coord. Chem. Rev. 2020, 421, 213463. [Google Scholar] [CrossRef]
- Chowdhury, S.; Toth, I.; Stephenson, R.J. Dendrimers in vaccine delivery: Recent progress and advances. Biomaterials 2022, 280, 121303. [Google Scholar] [CrossRef]
- Bruña, S.; Perles, J.; Cuadrado, I. A convergent growth approach to electroactive ferrocene rich carbosilane-and siloxane-based dendrons, dendrimers, and dendronized polymers. Dalton Trans. 2023, 52, 5663–5679. [Google Scholar] [CrossRef]
- Zhang, H.; Zhu, J.; He, J.; Qiu, F.; Zhang, H.; Yang, Y.; Lee, H.; Chang, T. Easy synthesis of dendrimer-like polymers through a divergent iterative “end-grafting” method. Polym. Chem. 2013, 4, 830–839. [Google Scholar] [CrossRef]
- Walter, M.V.; Malkoch, M.J.C.S.R. Simplifying the synthesis of dendrimers: Accelerated approaches. Chem. Soc. Rev. 2012, 41, 4593–4609. [Google Scholar] [CrossRef]
- Zhu, H.; Wu, X.; Liu, R.; Zhao, Y.; Sun, L. ECM-inspired hydrogels with ADSCs encapsulation for rheumatoid arthritis treatment. Adv. Sci. 2023, 10, 2206253. [Google Scholar] [CrossRef] [PubMed]
- Recio-Ruiz, J.; Carloni, R.; Ranganathan, S.; Muñoz-Moreno, L.; Carmena, M.J.; Ottaviani, M.F.; de la Mata, F.J.; García-Gallego, S. Amphiphilic dendritic hydrogels with carbosilane nanodomains: Preparation and characterization as drug delivery systems. Chem. Mater. 2023, 35, 2797–2807. [Google Scholar] [CrossRef]
- Namata, F.; Sanz del Olmo, N.; Wågberg, L.; Malkoch, M. High water content physically cross-linked hybrid hydrogels based on polyester dendrimers and cellulose nanofibrils: A comprehensive study. Chem. Mater. 2023, 35, 8561–8573. [Google Scholar] [CrossRef]
- Ferri-Angulo, D.; Yousefi-Mashouf, H.; Michel, M.; Mcleer, A.; Orgéas, L.; Bailly, L.; Sohier, J. Versatile fiber-reinforced hydrogels to mimic the microstructure and mechanics of human vocal-fold upper layers. ACTA Biomater. 2023, 172, 92–105. [Google Scholar] [CrossRef] [PubMed]
- Gill, K.; Mei, X.; Gillies, E.R. Self-immolative dendron hydrogels. Chem. Commun. 2021, 57, 11072–11075. [Google Scholar] [CrossRef] [PubMed]
- Bi, F.; Zhang, J.; Wei, Z.; Yu, D.; Zheng, S.; Wang, J.; Li, H.; Hua, Z.; Zhang, H.; Yang, G. Dynamic Glycopeptide dendrimers: Synthesis and their controllable self-assembly into varied Glyco-nanostructures for the biomimicry of Glycans. Biomacromolecules 2021, 23, 128–139. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Sahebkar, A.; Kesharwani, P. Poly (propylene imine) dendrimer as an emerging polymeric nanocarrier for anticancer drug and gene delivery. Eur. Polym. J. 2021, 158, 110683. [Google Scholar] [CrossRef]
- Uppuluri, S.; Keinath, S.E.; Tomalia, D.A.; Dvornic, P.R. Rheology of dendrimers. I. Newtonian flow behavior of medium and highly concentrated solutions of polyamidoamine (PAMAM) dendrimers in ethylenediamine (EDA) solvent. Macromolecules 1998, 31, 4498–4510. [Google Scholar] [CrossRef]
- Li, S.J.; Duan, G.G.; Zhang, G.Y.; Yang, H.Q.; Hou, H.Q.; Dai, Y.Q.; Sun, Y.M.; Jiang, S.H. Electrospun nanofiber nonwovens and sponges towards practical applications of waterproofing, thermal insulation, and electromagnetic shielding/absorption. Mater. Today Nano 2024, 25, 100452. [Google Scholar] [CrossRef]
- Maiti, P.K.; Çaǧın, T.; Wang, G.; Goddard, W.A. Structure of PAMAM dendrimers: Generations 1 through 11. Macromolecules 2004, 37, 6236–6254. [Google Scholar] [CrossRef]
- Huang, B.; Wan, Q.; Li, T.; Yu, L.; Du, W.; Calhoun, C.; Leong, K.W.; Qiang, L. Polycationic PAMAM ameliorates obesity-associated chronic inflammation and focal adiposity. Biomaterials 2023, 293, 121850. [Google Scholar] [CrossRef] [PubMed]
- Joubert, F.; Munson, M.J.; Sabirsh, A.; England, R.M.; Hemmerling, M.; Alexander, C.; Ashford, M.B. Precise and systematic end group chemistry modifications on PAMAM and poly(l-lysine) dendrimers to improve cytosolic delivery of mRNA. J. Control. Release 2023, 356, 580–594. [Google Scholar] [CrossRef] [PubMed]
- Dannert, C.; Mardal, I.; Lale, R.; Stokke, B.T.; Dias, R.S. DNA condensation by peptide-conjugated PAMAM dendrimers. influence of peptide charge. ACS Omega 2023, 8, 44624–44636. [Google Scholar] [CrossRef] [PubMed]
- Katzur, V.; Eichler, M.; Deigele, E.; Stage, C.; Karageorgiev, P.; Geis-Gerstorfer, J.; Schmalz, G.; Ruhl, S.; Rupp, F.; Müller, R. Surface-immobilized PAMAM-dendrimers modified with cationic or anionic terminal functions: Physicochemical surface properties and conformational changes after application of liquid interface stress. J. Colloid. Interf. Sci. 2012, 366, 179–190. [Google Scholar] [CrossRef] [PubMed]
- Sadekar, S.; Ghandehari, H. Transepithelial transport and toxicity of PAMAM dendrimers: Implications for oral drug delivery. Adv. Drug Deliver. Rev. 2012, 64, 571–588. [Google Scholar] [CrossRef] [PubMed]
- Luong, D.; Kesharwani, P.; Deshmukh, R.; Amin, M.C.I.M.; Gupta, U.; Greish, K.; Iyer, A.K. PEGylated PAMAM dendrimers: Enhancing efficacy and mitigating toxicity for effective anticancer drug and gene delivery. ACTA Biomater. 2016, 43, 14–29. [Google Scholar] [CrossRef] [PubMed]
- Jeong, W.-j.; Bu, J.; Mickel, P.; Han, Y.; Rawding, P.A.; Wang, J.; Kang, H.; Hong, H.; Král, P.; Hong, S.J.B. Dendrimer-peptide conjugates for effective blockade of the interactions between SARS-CoV-2 spike protein and human ACE2 receptor. Biomacromolecules 2022, 24, 141–149. [Google Scholar] [CrossRef]
- Kaur, J.; Mishra, V.; Singh, S.K.; Gulati, M.; Kapoor, B.; Chellappan, D.K.; Gupta, G.; Dureja, H.; Anand, K.; Dua, K. Harnessing amphiphilic polymeric micelles for diagnostic and therapeutic applications: Breakthroughs and bottlenecks. J. Control. Release 2021, 334, 64–95. [Google Scholar] [CrossRef]
- Zhang, Z.; Fu, H.; Li, Z.; Huang, J.; Xu, Z.; Lai, Y.; Qian, X.; Zhang, S. Hydrogel materials for sustainable water resources harvesting & treatment: Synthesis, mechanism and applications. Chem. Eng. J. 2022, 439, 135756. [Google Scholar]
- Córdoba, A.; Durán, B.; Bonardd, S.; Diaz Diaz, D.; Leiva, A.; Saldías, C. In situ synthesis and immobilization of CuO nanoparticles in alginate-poly(amido amine) nanogels for photocatalytic applications. Mater. Lett. X 2022, 14, 100148. [Google Scholar] [CrossRef]
- Tibbitt, M.W.; Anseth, K.S. Hydrogels as extracellular matrix mimics for 3D cell culture. Biotechnol. Bioeng. 2009, 103, 655–663. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.D.; Patel, D.K.; Lim, K.-T. Functional cellulose-based hydrogels as extracellular matrices for tissue engineering. J. Biol. Eng. 2019, 13, 55. [Google Scholar] [CrossRef]
- Muir, V.G.; Burdick, J.A. Chemically modified biopolymers for the formation of biomedical hydrogels. Chem. Rev. 2020, 121, 10908–10949. [Google Scholar] [CrossRef] [PubMed]
- Fan, P.; Fan, H.; Wang, S. From emerging modalities to advanced applications of hydrogel piezoelectrics based on chitosan, gelatin and related biological macromolecules: A review. Int. J. Biol. Macromol. 2024, 262, 129691. [Google Scholar] [CrossRef]
- Li, J.; Lv, Y.; Chen, Z.; Zhao, J.; Wang, S. Citric acid loaded hydrogel-coated stent for dissolving pancreatic duct calculi. Gels 2024, 10, 125. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, Y.; Yan, M.; Hu, G.; Li, Z.; He, W.; Wang, X.; Abulimit, A.; Li, R. Research progress on the application of inkjet printing technology combined with hydrogels. Appl. Mater. Today 2024, 36, 102036. [Google Scholar] [CrossRef]
- Xiang, T.; Guo, Q.; Jia, L.; Yin, T.; Huang, W.; Zhang, X.; Zhou, S. Multifunctional hydrogels for the healing of diabetic wounds. Adv. Healthc. Mater. 2024, 13, 2301885. [Google Scholar] [CrossRef]
- Ma, Y.; Duan, X.; Huang, J. DNA hydrogels as functional materials and their biomedical applications. Adv. Funct. Mater. 2024, 34, 2309070. [Google Scholar] [CrossRef]
- Jiang, Y.; Chen, Y.; Feng, W.; Zhong, X.; Yu, D.; Wang, W. Rapid gelation of polyacrylic acids-like hydrogel via the dopamine acrylamide-Fe3+ system and its formation mechanism. Chem. Eng. J. 2024, 484, 149460. [Google Scholar] [CrossRef]
- Nele, V.; Wojciechowski, J.P.; Armstrong, J.P.; Stevens, M.M. Tailoring gelation mechanisms for advanced hydrogel applications. Adv. Funct. Mater. 2020, 30, 2002759. [Google Scholar] [CrossRef]
- Xu, Z.; Liu, G.; Zheng, L.; Wu, J. A polyphenol-modified chitosan hybrid hydrogel with enhanced antimicrobial and antioxidant activities for rapid healing of diabetic wounds. Nano Res. 2023, 16, 905–916. [Google Scholar] [CrossRef]
- Ding, X.; Fan, L.; Wang, L.; Zhou, M.; Wang, Y.; Zhao, Y. Designing self-healing hydrogels for biomedical applications. Mater. Horiz. 2023, 10, 3929–3947. [Google Scholar] [CrossRef]
- Cheng, Q.; Hao, A.; Xing, P. Stimulus-responsive luminescent hydrogels: Design and applications. Adv. Colloid Interface Sci. 2020, 286, 102301. [Google Scholar] [CrossRef]
- Zhao, Y.; Song, S.; Ren, X.; Zhang, J.; Lin, Q.; Zhao, Y. Supramolecular adhesive hydrogels for tissue engineering applications. Chem. Rev. 2022, 122, 5604–5640. [Google Scholar] [CrossRef] [PubMed]
- Pourshahrestani, S.; Zeimaran, E.; Kadri, N.A.; Mutlu, N.; Boccaccini, A.R. Polymeric hydrogel systems as emerging biomaterial platforms to enable hemostasis and wound healing. Adv. Healthc. Mater. 2020, 9, 2000905. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; He, J.; Guo, B. Functional hydrogels as wound dressing to enhance wound healing. ACS Nano 2021, 15, 12687–12722. [Google Scholar] [CrossRef] [PubMed]
- Aswathy, S.; Narendrakumar, U.; Manjubala, I. Commercial hydrogels for biomedical applications. Heliyon 2020, 6, e03719. [Google Scholar] [CrossRef]
- Kasai, R.D.; Radhika, D.; Archana, S.; Shanavaz, H.; Koutavarapu, R.; Lee, D.-Y.; Shim, J. A review on hydrogels classification and recent developments in biomedical applications. Int. J. Polym. Mater. 2023, 72, 1059–1069. [Google Scholar] [CrossRef]
- Peters, J.T.; Wechsler, M.E.; Peppas, N.A. Advanced biomedical hydrogels: Molecular architecture and its impact on medical applications. Regen. Biomater. 2021, 8, rbab060. [Google Scholar] [CrossRef] [PubMed]
- Su, M.; Ruan, L.; Dong, X.; Tian, S.; Lang, W.; Wu, M.; Chen, Y.; Lv, Q.; Lei, L. Current state of knowledge on intelligent-response biological and other macromolecular hydrogels in biomedical engineering: A review. Int. J. Biol. Macromol. 2023, 227, 472–492. [Google Scholar] [CrossRef] [PubMed]
- Kaga, S.; Arslan, M.; Sanyal, R.; Sanyal, A. Dendrimers and dendrons as versatile building blocks for the fabrication of functional hydrogels. Molecules 2016, 21, 497. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, S.; Shi, W.; Liu, H. Thermo-/pH-dual-sensitive PEG/PAMAM nanogel: Reaction dynamics and plugging application of CO2 channeling. Gels 2022, 8, 683. [Google Scholar] [CrossRef] [PubMed]
- Mekuria, S.L.; Ouyang, Z.; Song, C.; Rodrigues, J.; Shen, M.; Shi, X. Dendrimer-based nanogels for cancer nanomedicine applications. Bioconjugate Chem. 2021, 33, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Pistone, A.; Iannazzo, D.; Celesti, C.; Scolaro, C.; Giofré, S.V.; Romeo, R.; Visco, A. Chitosan/PAMAM/hydroxyapatite engineered drug release hydrogels with tunable rheological properties. Polymers 2020, 12, 754. [Google Scholar] [CrossRef] [PubMed]
- Holden, C.A.; Tyagi, P.; Thakur, A.; Kadam, R.; Jadhav, G.; Kompella, U.B.; Yang, H. Polyamidoamine dendrimer hydrogel for enhanced delivery of antiglaucoma drugs. Nanomed-Nanotechnol. Biol. Biomed. 2012, 8, 776–783. [Google Scholar] [CrossRef] [PubMed]
- Desai, P.N.; Yuan, Q.; Yang, H. Synthesis and characterization of photocurable polyamidoamine dendrimer hydrogels as a versatile platform for tissue engineering and drug delivery. Biomacromolecules 2010, 11, 666–673. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Cooper, R.C.; Wang, J.; Yeudall, W.A.; Yang, H. Synthesis and application of injectable bioorthogonal dendrimer hydrogels for local drug delivery. ACS Biomater. Sci. Eng. 2017, 3, 1641–1653. [Google Scholar] [CrossRef] [PubMed]
- Kegley, Z.; Makay, M.; Rogers, J.; Phelps, K.; Malcom, C.; Hellmig, D.; Kroninger, A.; Bi, X. Polyamidoamine dendrimer-polyethylene glycol hydrogel for solubility enhancement and sustained release of diflunisal. J. Sol-Gel Sci. Technol. 2022, 104, 160–168. [Google Scholar] [CrossRef]
- Bi, X.; Watts, D.B.; Dorman, I.; Kirk, C.M.; Thomas, M.; Singleton, I.; Malcom, C.; Barnes, T.; Carter, C.; Liang, A. Polyamidoamine dendrimer-mediated hydrogel for solubility enhancement and anti-cancer drug delivery. J. Biomater. Appl. 2023, 38, 733–742. [Google Scholar] [CrossRef] [PubMed]
- Soiberman, U.; Kambhampati, S.P.; Wu, T.; Mishra, M.K.; Oh, Y.; Sharma, R.; Wang, J.; Al Towerki, A.E.; Yiu, S.; Stark, W.J.; et al. Subconjunctival injectable dendrimer-dexamethasone gel for the treatment of corneal inflammation. Biomaterials 2017, 125, 38–53. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; He, H.; Cooper, R.C.; Gui, Q.; Yang, H. Drug-conjugated dendrimer hydrogel enables sustained drug release via a self-cleaving mechanism. Mol. Pharmaceutics 2019, 16, 1874–1880. [Google Scholar] [CrossRef]
- Wang, J.; Williamson, G.S.; Lancina III, M.G.; Yang, H. Mildly cross-linked dendrimer hydrogel prepared via aza-Michael addition reaction for topical brimonidine delivery. J. Biomed. Nanotechnol. 2017, 13, 1089–1096. [Google Scholar] [CrossRef]
- Wang, J.; He, H.; Cooper, R.C.; Yang, H. In situ-forming polyamidoamine dendrimer hydrogels with tunable properties prepared via Aza-Michael addition reaction. ACS Appl. Mater. Interfaces 2017, 9, 10494–10503. [Google Scholar] [CrossRef]
- Wang, J.; Cooper, R.C.; He, H.; Li, B.; Yang, H. Polyamidoamine dendrimer microgels: Hierarchical arrangement of dendrimers into micrometer domains with expanded structural features for programmable drug delivery and release. Macromolecules 2018, 51, 6111–6118. [Google Scholar] [CrossRef]
- Magalhães, T.M.; Guerra, R.C.; San Gil, R.A.d.S.; Valente, A.P.; Simão, R.A.; Soares, B.G.; Mendes, T.d.C.; Pyrrho, A.d.S.; Sousa, V.P.d.; Rodrigues-Furtado, V.L. PAMAM dendrimer hydrogel film-biocompatible material to an efficient dermal delivery of drugs. J. Nanopart. Res. 2017, 19, 277. [Google Scholar]
- Buwalda, S.J.; Bethry, A.; Hunger, S.; Kandoussi, S.; Coudane, J.; Nottelet, B. Ultrafast in situ forming poly(ethylene glycol)-poly(amido amine) hydrogels with tunable drug release properties via controllable degradation rates. Eur. J. Pharm. Biopharm. 2019, 139, 232–239. [Google Scholar]
- Tong, N.-A.N.; Nguyen, T.H.; Nguyen, D.H.; Nguyen, C.K.; Tran, N.Q. Preparation of the cationic dendrimer-based hydrogels for controlled heparin release. J. Macromol. Sci. Part A Pure Appl. Chem. 2015, 52, 830–837. [Google Scholar] [CrossRef]
- Bekhradnia, S.; Zhu, K.; Knudsen, K.D.; Sande, S.A.; Nyström, B. Structure, swelling, and drug release of thermoresponsive poly (amidoamine) dendrimer–poly(N-isopropylacrylamide) hydrogels. J. Mater. Sci. 2014, 49, 6102–6110. [Google Scholar] [CrossRef]
- Krishna, P.R.; Sreeshailam, A.; Srinivas, R. Recent advances and applications in asymmetric aza-Michael addition chemistry. Tetrahedron 2009, 65, 9657–9672. [Google Scholar] [CrossRef]
- Pommier, Y. Topoisomerase I inhibitors: Camptothecins and beyond. Nat. Rev. Cancer 2006, 6, 789–802. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, K.; Wang, J.; Yuan, Y.; Wu, H. High-tough hydrogels formed via Schiff base reaction between PAMAM dendrimer and Tetra-PEG and their potential as dual-function delivery systems. Mater. Today Commun. 2022, 30, 103019. [Google Scholar] [CrossRef]
- Wong, L.C.; Poh, J.H.; Tan, W.T.; Khor, B.-K.; Murugaiyah, V.; Leh, C.P.; Goh, C.F. Cellulose hydrogel development from unbleached oil palm biomass pulps for dermal drug delivery. Int. J. Biol. Macromol. 2023, 224, 483–495. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Liu, S.; Yang, X.; Du, S.; Tang, W.; Cao, W.; Zhou, J.; Gong, X.; Xing, X. Low-drug resistance carbon quantum dots decorated injectable self-healing hydrogel with potent antibiofilm property and cutaneous wound healing. Chem. Eng. J. 2021, 403, 126387. [Google Scholar] [CrossRef]
- Modi, S.; Inwati, G.K.; Gacem, A.; Saquib Abullais, S.; Prajapati, R.; Yadav, V.K.; Syed, R.; Alqahtani, M.S.; Yadav, K.K.; Islam, S. Nanostructured antibiotics and their emerging medicinal applications: An overview of nanoantibiotics. Antibiotics 2022, 11, 708. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Hu, C.; Shao, L. The antimicrobial activity of nanoparticles: Present situation and prospects for the future. Int. J. Nanomed. 2017, 12, 1227–1249. [Google Scholar] [CrossRef] [PubMed]
- Anaya-López, J.L.; López-Meza, J.E.; Ochoa-Zarzosa, A. Bacterial resistance to cationic antimicrobial peptides. Crit. Rev. Microbiol. 2013, 39, 180–195. [Google Scholar] [CrossRef]
- Cheng, Y.; Qu, H.; Ma, M.; Xu, Z.; Xu, P.; Fang, Y.; Xu, T. Polyamidoamine (PAMAM) dendrimers as biocompatible carriers of quinolone antimicrobials: An in vitro study. Eur. J. Med. Chem. 2007, 42, 1032–1038. [Google Scholar] [CrossRef]
- Holmes, A.M.; Heylings, J.R.; Wan, K.-W.; Moss, G.P. Antimicrobial efficacy and mechanism of action of poly(amidoamine) (PAMAM) dendrimers against opportunistic pathogens. Int. J. Antimicrob. Agents 2019, 53, 500–507. [Google Scholar] [CrossRef]
- Dai, T.; Wang, C.; Wang, Y.; Xu, W.; Hu, J.; Cheng, Y. A nanocomposite hydrogel with potent and broad-spectrum antibacterial activity. ACS Appl. Mater. Interfaces 2018, 10, 15163–15173. [Google Scholar] [CrossRef] [PubMed]
- He, G.; Zhu, C.; Ye, S.; Cai, W.; Yin, Y.; Zheng, H.; Yi, Y. Preparation and properties of novel hydrogel based on chitosan modified by poly(amidoamine) dendrimer. Int. J. Biol. Macromol. 2016, 91, 828–837. [Google Scholar] [CrossRef] [PubMed]
- He, G.; Kong, Y.; Zheng, H.; Ke, W.; Chen, X.; Yin, Y.; Yi, Y. Preparation and properties of poly(amidoamine) dendrimer/quaternary ammonium chitosan hydrogels. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2018, 33, 736–743. [Google Scholar] [CrossRef]
- Qin, Y.; Li, P.; Guo, Z. Cationic chitosan derivatives as potential antifungals: A review of structural optimization and applications. Carbohydr. Polym. 2020, 236, 116002. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Sheikh, A.; Abourehab, M.A.S.; Kesharwani, P. Amelioration of full-thickness wound using hesperidin loaded dendrimer-based hydrogel bandages. Biosensors 2022, 12, 462. [Google Scholar] [CrossRef]
- Shariatinia, Z. Pharmaceutical applications of chitosan. Adv. Colloid Interface Sci. 2019, 263, 131–194. [Google Scholar] [CrossRef]
- Laubach, M.; Hildebrand, F.; Suresh, S.; Wagels, M.; Kobbe, P.; Gilbert, F.; Kneser, U.; Holzapfel, B.M.; Hutmacher, D.W. The concept of scaffold-guided bone regeneration for the treatment of long bone defects: Current clinical application and future perspective. J. Funct. Biomater. 2023, 14, 341. [Google Scholar] [CrossRef]
- Wang, W.; Yeung, K.W. Bone grafts and biomaterials substitutes for bone defect repair: A review. Bioact. Mater. 2017, 2, 224–247. [Google Scholar] [CrossRef]
- Kurian, A.G.; Mandakhbayar, N.; Singh, R.K.; Lee, J.-H.; Jin, G.; Kim, H.-W. Multifunctional dendrimer@nanoceria engineered GelMA hydrogel accelerates bone regeneration through orchestrated cellular responses. Mater. Today Bio 2023, 20, 100664. [Google Scholar] [CrossRef]
- Ravichandran, A.; Meinert, C.; Bas, O.; Hutmacher, D.W.; Bock, N. Engineering a 3D bone marrow adipose composite tissue loading model suitable for studying mechanobiological questions. Mater. Sci. Eng. C 2021, 128, 112313. [Google Scholar] [CrossRef]
- Song, Y.; Zhang, Y.; Qu, Q.; Zhang, X.; Lu, T.; Xu, J.; Ma, W.; Zhu, M.; Huang, C.; Xiong, R. Biomaterials based on hyaluronic acid, collagen and peptides for three-dimensional cell culture and their application in stem cell differentiation. Int. J. Biol. Macromol. 2023, 226, 14–36. [Google Scholar] [CrossRef]
- Bi, X.; Maturavongsadit, P.; Tan, Y.; Watts, M.; Bi, E.; Kegley, Z.; Morton, S.; Lu, L.; Wang, Q.; Liang, A. Polyamidoamine dendrimer-PEG hydrogel and its mechanical property on differentiation of mesenchymal stem cells. Bio-Med. Mater. Eng. 2019, 30, 111–123. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, Q.; Zhang, H.; Yang, S.; Jia, X. A novel poly(amido amine)-dendrimer-based hydrogel as a mimic for the extracellular matrix. Adv. Mater. 2014, 26, 4163–4167. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, Q.; Luo, Y.; Xu, Z.; Zhang, H.; Yang, S.; Wei, Y.; Jia, X. A high stiffness bio-inspired hydrogel from the combination of a poly(amido amine) dendrimer with DOPA. Chem. Commun. 2015, 51, 16786–16789. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Chen, Q.; Deng, C.; Xu, B.; Zhang, Z.; Yang, Y.; Lu, T. Exquisite design of injectable hydrogels in cartilage repair. Theranostics 2020, 10, 9843–9864. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Chen, G.; Xu, X.; Abdou, P.; Jiang, Q.; Shi, D.; Gu, Z. Advances of injectable hydrogel-based scaffolds for cartilage regeneration. Regen. Biomater. 2019, 6, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Wang, X.; Li, Y.; Ren, M.; He, P.; Wang, L.; Xu, J.; Yang, S.; Ji, P. Dendrimer-modified gelatin methacrylate hydrogels carrying adipose-derived stromal/stem cells promote cartilage regeneration. Stem Cell Res. Ther. 2022, 13, 26. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zeng, Y.; Chen, Z.; Yu, Y.; Wang, H.; Lu, X.; Zhao, J.; Wang, S. Chitosan-based multifunctional hydrogel for sequential wound inflammation elimination, infection inhibition, and wound healing. Int. J. Biol. Macromol. 2023, 235, 123847. [Google Scholar] [CrossRef]
- Su, L.; Zheng, J.; Wang, Y.; Zhang, W.; Hu, D. Emerging progress on the mechanism and technology in wound repair. Biomed. Pharmacother. 2019, 117, 109191. [Google Scholar] [CrossRef]
- De Mik, S.; Stubenrouch, F.; Legemate, D.; Balm, R.; Ubbink, D.; DISCOVAR Study Group; Balm, R.; Becquemin, J.; Blankensteijn, J.; de Borst, G. Delphi study to reach international consensus among vascular surgeons on major arterial vascular surgical complications. World J. Surg. 2019, 43, 2328–2336. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, M.; Liu, Q.; Liu, G.; Wang, S.; Li, J. Recent advances in the medical applications of hemostatic materials. Theranostics 2023, 13, 161–196. [Google Scholar] [CrossRef] [PubMed]
- Muñoz Taboada, G.; Dosta, P.; Edelman, E.R.; Artzi, N. Sprayable hydrogel for instant sealing of vascular anastomosis. Adv. Mater. 2022, 34, 2203087. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Zhao, X.; Hu, T.; Chen, B.; Yin, Z.; Ma, P.X.; Guo, B. Adhesive hemostatic conducting injectable composite hydrogels with sustained drug release and photothermal antibacterial activity to promote full-thickness skin regeneration during wound healing. Small 2019, 15, 1900046. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Li, Z.; Huang, Y.; Yu, R.; Guo, B. Dual-dynamic-bond cross-linked antibacterial adhesive hydrogel sealants with on-demand removability for post-wound-closure and infected wound healing. ACS Nano 2021, 15, 7078–7093. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, J.; Song, L.; Zhou, Y.; Zhao, J.; Hou, X.; Yuan, X. Injectable PAMAM/ODex double-crosslinked hydrogels with high mechanical strength. Biomed. Mater. 2016, 12, 015012. [Google Scholar] [CrossRef] [PubMed]
- Tomalia, D.; Baker, H.; Dewald, J. A new class of polymers: Starburst-dendritic macromolecules. Polym. J. 2002, 34, 132–147. [Google Scholar] [CrossRef]
- Piao, Y.; Wu, T.; Chen, B. One-step synthesis of graphene oxide–polyamidoamine dendrimer nanocomposite hydrogels by self-assembly. Ind. Eng. Chem. Res. 2016, 55, 6113–6121. [Google Scholar] [CrossRef]
- Li, Z.; Ding, Y.; Liu, J.; Wang, J.; Mo, F.; Wang, Y.; Chen-Mayfield, T.-J.; Sondel, P.M.; Hong, S.; Hu, Q. Depletion of tumor associated macrophages enhances local and systemic platelet-mediated anti-PD-1 delivery for post-surgery tumor recurrence treatment. Nat. Commun. 2022, 13, 1845. [Google Scholar] [CrossRef]
- Wang, C.; Wang, X.; Dong, K.; Luo, J.; Zhang, Q.; Cheng, Y. Injectable and responsively degradable hydrogel for personalized photothermal therapy. Biomaterials 2016, 104, 129–137. [Google Scholar] [CrossRef]
- Li, L.; Wang, C.; Huang, Q.; Xiao, J.; Zhang, Q.; Cheng, Y. A degradable hydrogel formed by dendrimer-encapsulated platinum nanoparticles and oxidized dextran for repeated photothermal cancer therapy. J. Mater. Chem. B 2018, 6, 2474–2480. [Google Scholar] [CrossRef]
- Conde, J.; Oliva, N.; Atilano, M.; Song, H.S.; Artzi, N. Self-assembled RNA-triple-helix hydrogel scaffold for microRNA modulation in the tumour microenvironment. Nat. Mater. 2016, 15, 353–363. [Google Scholar] [CrossRef] [PubMed]
- Kort-Mascort, J.; Flores-Torres, S.; Peza-Chavez, O.; Jang, J.H.; Pardo, L.A.; Tran, S.D.; Kinsella, J. Decellularized ECM hydrogels: Prior use considerations, applications, and opportunities in tissue engineering and biofabrication. Biomater. Sci. 2023, 11, 400–431. [Google Scholar] [CrossRef] [PubMed]
- Daly, A.C. Granular hydrogels in biofabrication—Recent advances and future perspectives. Adv. Healthc. Mater. 2023, 2023, 2301388. [Google Scholar] [CrossRef] [PubMed]
- Bi, X.; Liang, A.; Tan, Y.; Maturavongsadit, P.; Higginbothem, A.; Gado, T.; Gramling, A.; Bahn, H.; Wang, Q. Thiol-ene crosslinking polyamidoamine dendrimer-hyaluronic acid hydrogel system for biomedical applications. J. Biomater. Sci. Polym. Ed. 2016, 27, 743–757. [Google Scholar] [CrossRef] [PubMed]
- Bi, X.; Amie Luckanagul, J.; Allen, A.; Ramaboli, M.; Campbell, E.; West, D.; Maturavongsadit, P.; Brummett, K.; Wang, Q. Synthesis of PAMAM dendrimer-based fast cross-linking hydrogel for biofabrication. J. Biomater. Sci. Polym. Ed. 2015, 26, 669–682. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Su, X. Multifunctional smart hydrogels: Potential in tissue engineering and cancer therapy. J. Mater. Chem. B 2018, 6, 4714–4730. [Google Scholar] [CrossRef] [PubMed]
- El-Husseiny, H.M.; Mady, E.A.; Hamabe, L.; Abugomaa, A.; Shimada, K.; Yoshida, T.; Tanaka, T.; Yokoi, A.; Elbadawy, M.; Tanaka, R. Smart/stimuli-responsive hydrogels: Cutting-edge platforms for tissue engineering and other biomedical applications. Mater. Today Bio 2022, 13, 100186. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, C.Q.; Li, J.; Yang, J.Z. Demulsification of crude oil emulsion using polyamidoamine dendrimers. Sep. Sci. Technol. 2007, 42, 2111–2120. [Google Scholar] [CrossRef]
- Liu, X.; Qu, G.; Yu, Q.; Zhang, N.; Wang, L.; Wang, J. Synthesis of poly(ethylene glycol) grafted polyamidoamine dendrimer hydrogels and their temperature and pH sensitive properties. Polym. Sci. Ser. B 2020, 62, 400–410. [Google Scholar]
- Bharathan Jeneena, K.; Vivek, B. Peripherally modified poly (amido amine) nanocomposite hydrogel with stimuli-responsive self-healing, high tensile strength, and selective superadsorption poperties. ChemistrySelect 2023, 8, e202303066. [Google Scholar] [CrossRef]
- Duan, Q.-Y.; Zhu, Y.-X.; Jia, H.-R.; Wang, S.-H.; Wu, F.-G. Nanogels: Synthesis, properties, and recent biomedical applications. Prog. Mater. Sci. 2023, 139, 101167. [Google Scholar] [CrossRef]
- Mohammadi, M.; Arabi, L.; Alibolandi, M. Doxorubicin-loaded composite nanogels for cancer treatment. J. Control. Release 2020, 328, 171–191. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, B.; Kompella, U.B.; Yang, H. Dendrimer and dendrimer gel-derived drug delivery systems: Breaking bottlenecks of topical administration of glaucoma medications. MedComm-Biomater. Appl. 2023, 2, e30. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Tyagi, P.; Kadam, R.S.; Holden, C.A.; Kompella, U.B. Hybrid dendrimer hydrogel/PLGA nanoparticle platform sustains drug delivery for one week and antiglaucoma effects for four days following one-time topical administration. ACS Nano 2012, 6, 7595–7606. [Google Scholar] [CrossRef] [PubMed]
- Daly, A.C.; Riley, L.; Segura, T.; Burdick, J.A. Hydrogel microparticles for biomedical applications. Nat. Rev. Mater. 2020, 5, 20–43. [Google Scholar] [CrossRef]
- Wang, Y.; Luo, Y.; Zhao, Q.; Wang, Z.; Xu, Z.; Jia, X. An enzyme-responsive nanogel carrier based on PAMAM dendrimers for drug delivery. ACS Appl. Mater. Interfaces 2016, 8, 19899–19906. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Xiao, T.; Zhang, C.; Wang, Z.; Li, G.; Chen, J.; Ouyang, Z.; Wang, H.; Shi, X.; Shen, M. Multifunctional low-generation dendrimer nanogels as an emerging probe for tumor-specific CT/MR dual-modal imaging. Biomacromolecules 2023, 24, 967–976. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Zhan, M.; Zhang, C.; Wang, Z.; Sun, H.; Tao, Y.; Shi, Q.; He, M.; Wang, H.; Rodrigues, J.; et al. Redox-responsive dendrimer nanogels enable ultrasound-enhanced chemoimmunotherapy of pancreatic cancer via endoplasmic reticulum stress amplification and macrophage polarization. Adv. Sci. 2023, 10, 2301759. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, B.; Huang, D.; Norat, P.; Grannonico, M.; Cooper, R.C.; Gui, Q.; Nam Chow, W.; Liu, X.; Yang, H. Nano-in-Nano dendrimer gel particles for efficient topical delivery of antiglaucoma drugs into the eye. Chem. Eng. J. 2021, 425, 130498. [Google Scholar] [CrossRef]
- Ferruti, P.; Bianchi, S.; Ranucci, E.; Chiellini, F.; Piras, A. Novel agmatine-containing poly(amidoamine) hydrogels as scaffolds for tissue engineering. Biomacromolecules 2005, 6, 2229–2235. [Google Scholar] [CrossRef]
- Fenili, F.; Manfredi, A.; Ranucci, E.; Ferruti, P. Poly(amidoamine) Hydrogels as Scaffolds for Cell Culturing and Conduits for Peripheral Nerve Regeneration. Int. J. Polym. Sci. 2011, 2011, 161749. [Google Scholar] [CrossRef]

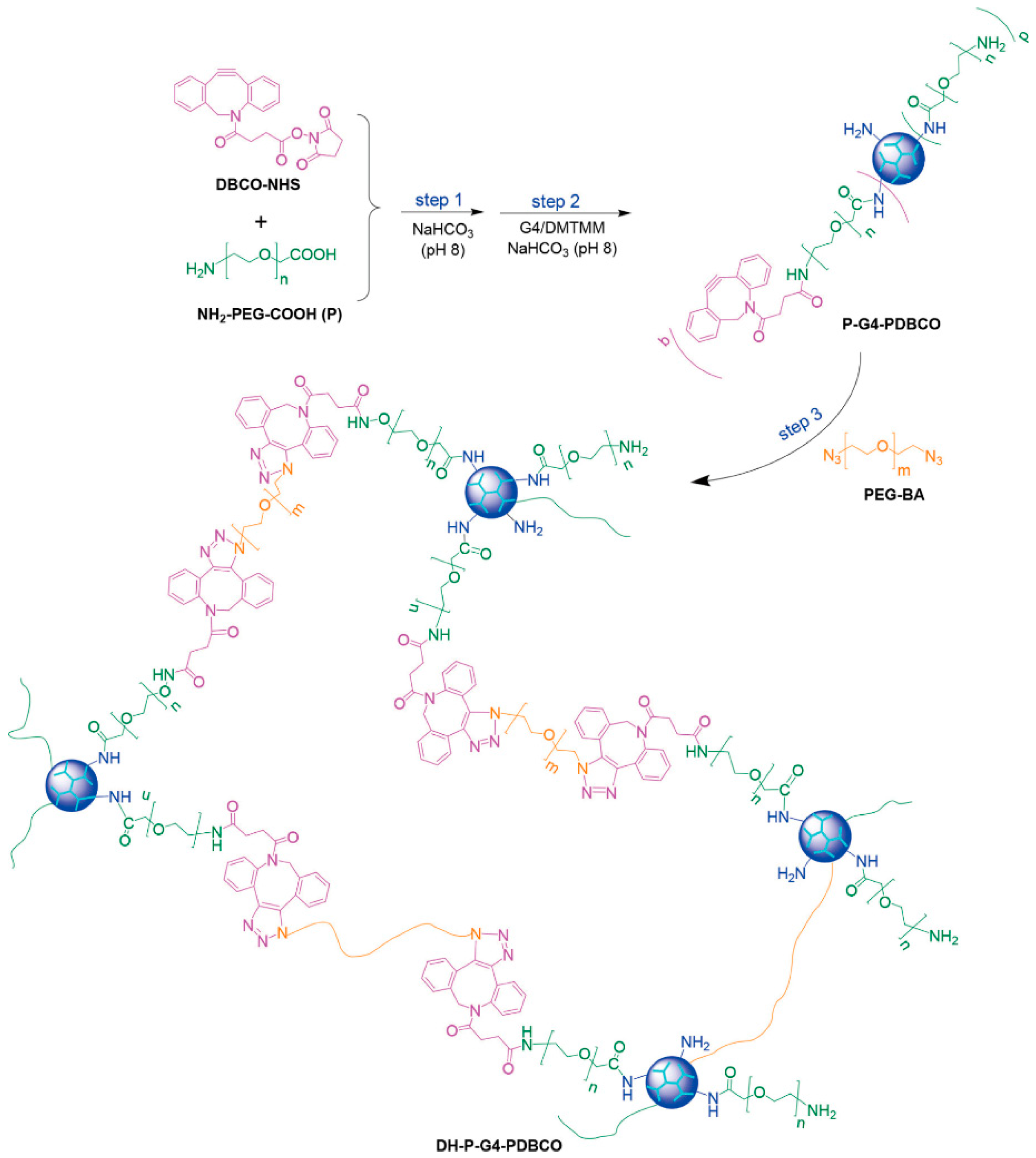

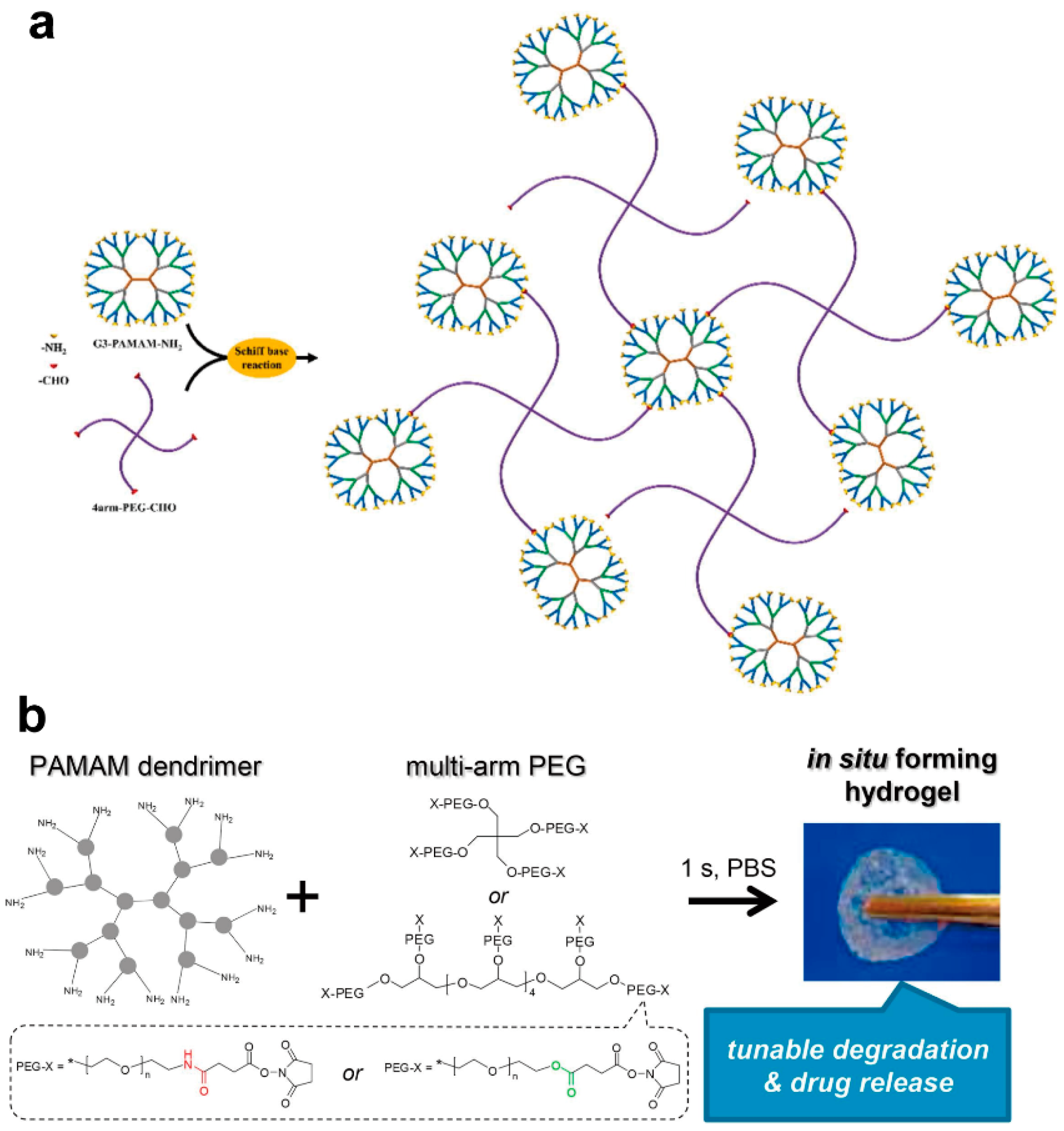

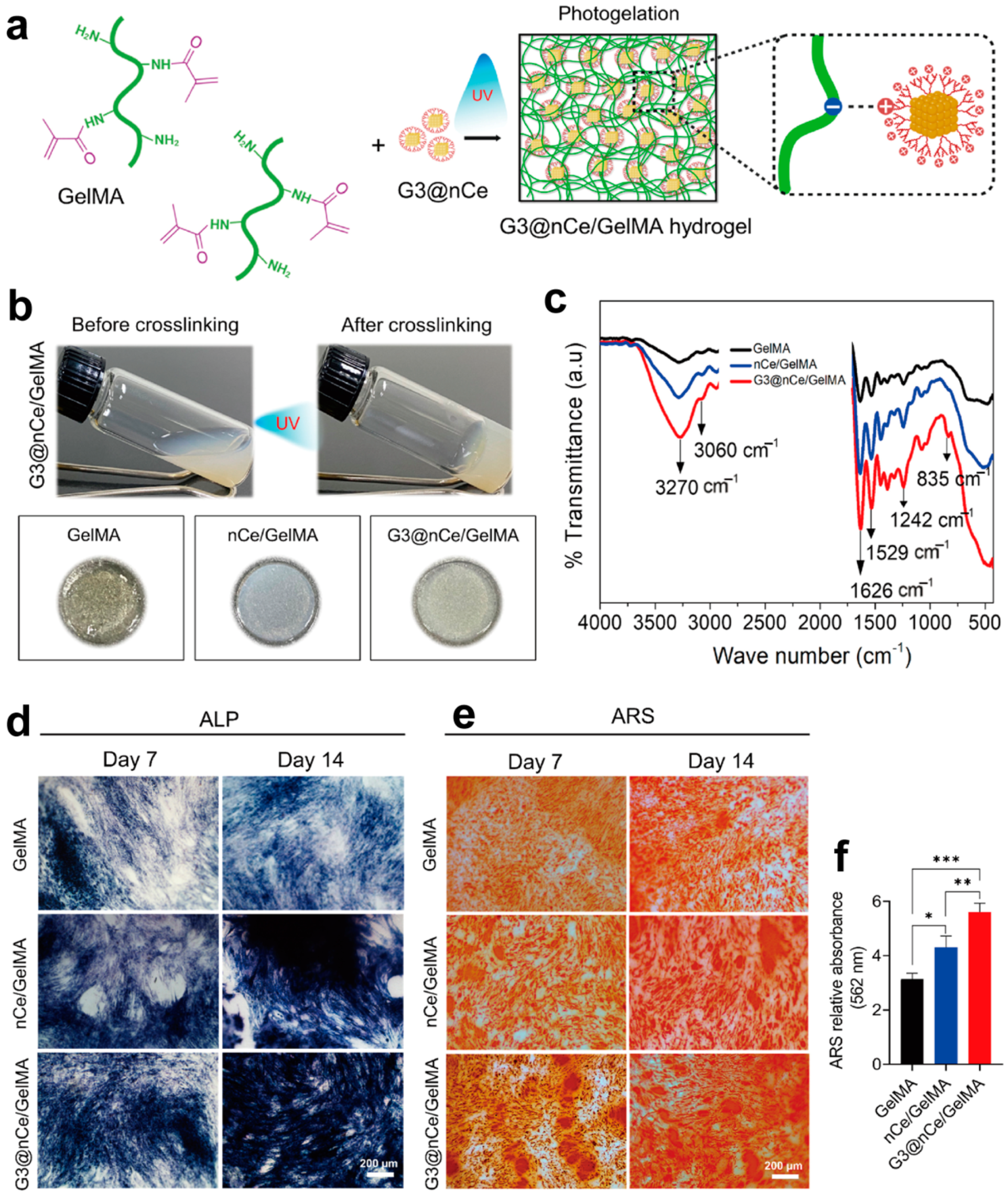
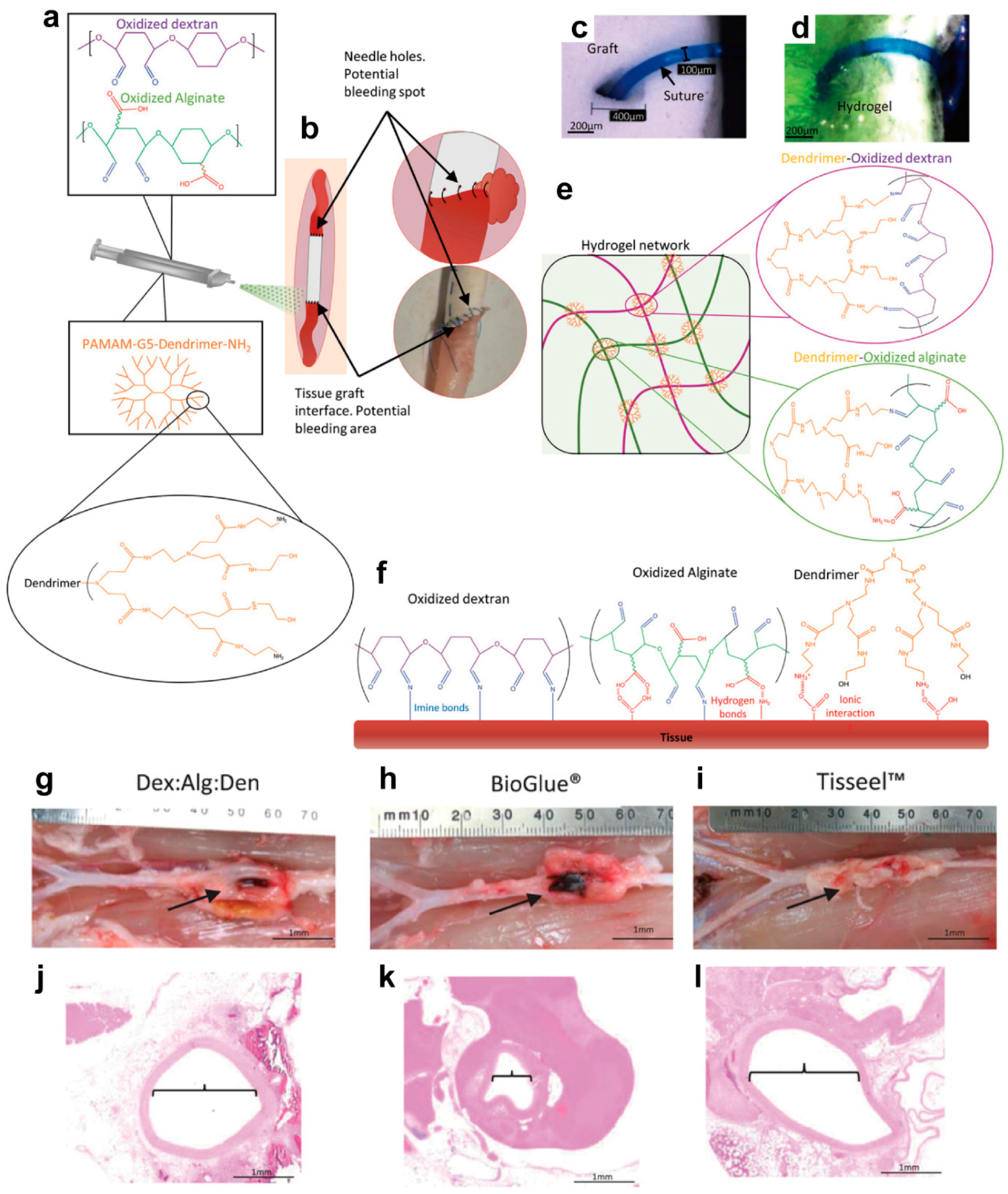

| Precursors | Drug | Drug Loading Method | Hydrogel Formation Method | Ref. |
|---|---|---|---|---|
| Polyethylene glycol bisazide, G4-functionalized dibenzocyclooctyne | 5-fluorouracil | One-step strategy | Click chemistry reaction | [59] |
| Vinyl-sulfone-functionalized G5, thiolated polyethylene glycol (PEG) | Diflunisal | Two-step strategy | [60] | |
| Vinyl-sulfone-functionalized G5, thiolated PEG | Silibinin, methotrexate, and camptothecin | [61] | ||
| G4-pentenoic acid conjugates, thiolated hyaluronic acid | Dexamethasone | [62] | ||
| G3, PEG diacrylate (PEG-DA) | Camptothecin (CPT) | Aza-Michael addition | [63] | |
| G5, PEG-DA | Brimonidine tartrate | One-step strategy | [64] | |
| G5, PEG-DA | 5-fluorouracil | [65] | ||
| G5, PEG-DA | CPT | Two-step strategy | Aza-Michael addition and inverse microemulsion method | [66] |
| G4, glutaraldehyde | Ketoprofen | Schiff base reaction | [67] | |
| G2, multi-armed PEG with N-succinimidyl ester end groups | Fluorescein isothiocyanate-dextran | One-step strategy | Amidation reaction | [68] |
| Tyramine-conjugated tetronic and p-hydroxyphenyl-acetic-acid-functionalized G3 | Heparin | Enzymatic reaction | [69] | |
| N-isopropy-lacrylamide, N,N′-methylenebis(acrylamide), and G6 | Paracetamol | Direct binding to the hydrogel matrix | Radical polymerization | [70] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, L.; Li, Z.; Yang, B.; Jia, X.; Wang, S. Recent Research Progress on Polyamidoamine-Engineered Hydrogels for Biomedical Applications. Biomolecules 2024, 14, 620. https://doi.org/10.3390/biom14060620
Liu L, Li Z, Yang B, Jia X, Wang S. Recent Research Progress on Polyamidoamine-Engineered Hydrogels for Biomedical Applications. Biomolecules. 2024; 14(6):620. https://doi.org/10.3390/biom14060620
Chicago/Turabian StyleLiu, Li, Zhiling Li, Baiyan Yang, Xiaoqing Jia, and Shige Wang. 2024. "Recent Research Progress on Polyamidoamine-Engineered Hydrogels for Biomedical Applications" Biomolecules 14, no. 6: 620. https://doi.org/10.3390/biom14060620
APA StyleLiu, L., Li, Z., Yang, B., Jia, X., & Wang, S. (2024). Recent Research Progress on Polyamidoamine-Engineered Hydrogels for Biomedical Applications. Biomolecules, 14(6), 620. https://doi.org/10.3390/biom14060620







