Gypenoside XVII Reduces Synaptic Glutamate Release and Protects against Excitotoxic Injury in Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Drugs
2.2. Animals
2.3. Synaptosomes
2.4. Evaluation of Glutamate Release and FM1-43 Release in Rat Cortical Synaptosomes
2.5. KA-Induced Excitotoxicity Animal Model and Drug Treatment
2.6. Histological Analysis of Neuronal Degeneration by Fluoro-Jade B (FJB) Staining
2.7. High-Performance Liquid Chromatography (HPLC) Assay of Glutamate and γ-Aminobutyric Acid (GABA) Concentrations in the Cortex
2.8. Cerebral Blood Flow Monitoring by Laser Speckle Imaging System
2.9. Protein Isolation and Western Blotting
2.10. Statistical Analysis
3. Results
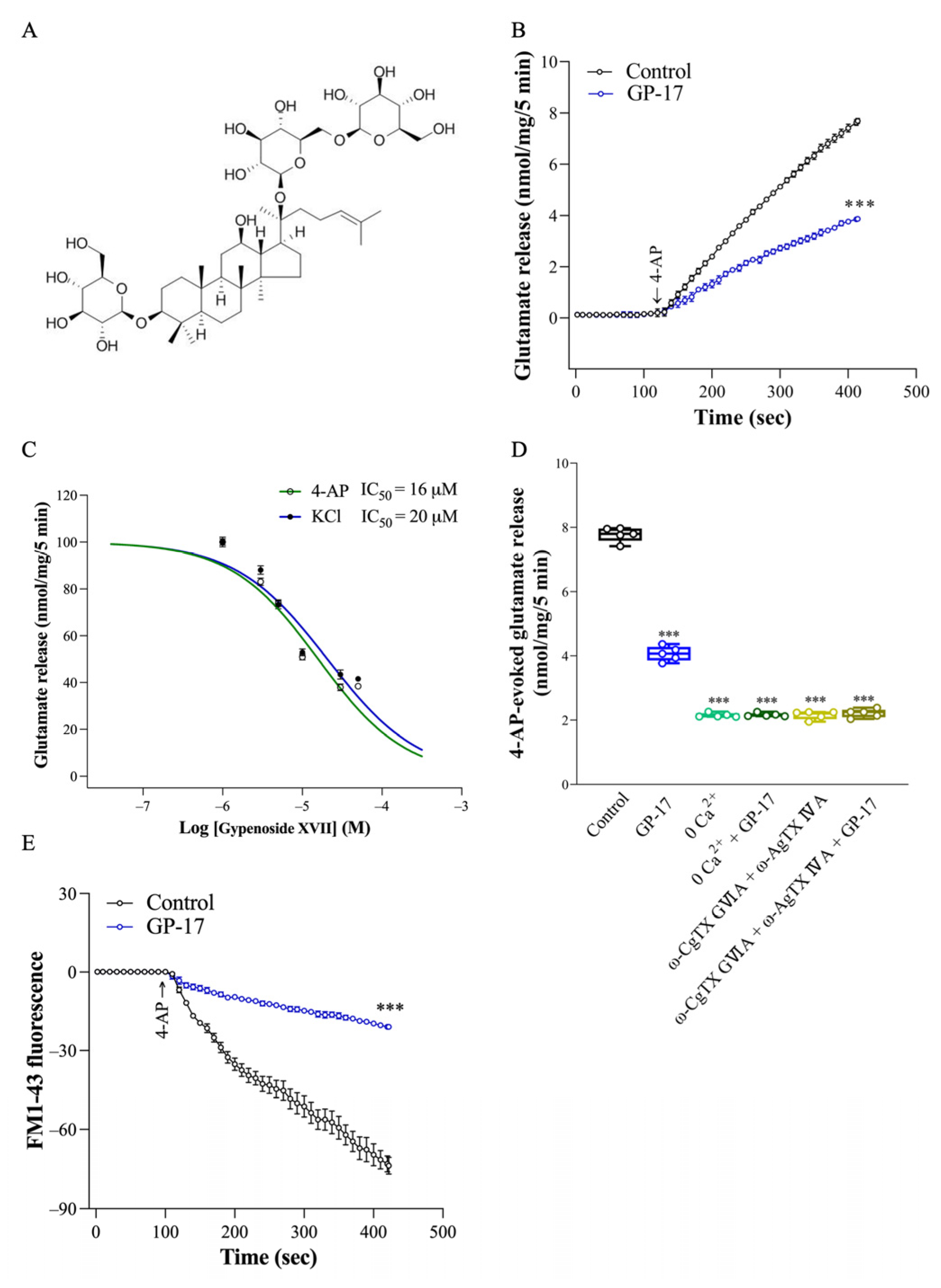
3.1. GP-17 Reduces Ca2+-Dependent Glutamate Release Evoked by 4-AP in Rat Cerebral Cortex Glutamatergic Nerve Terminals
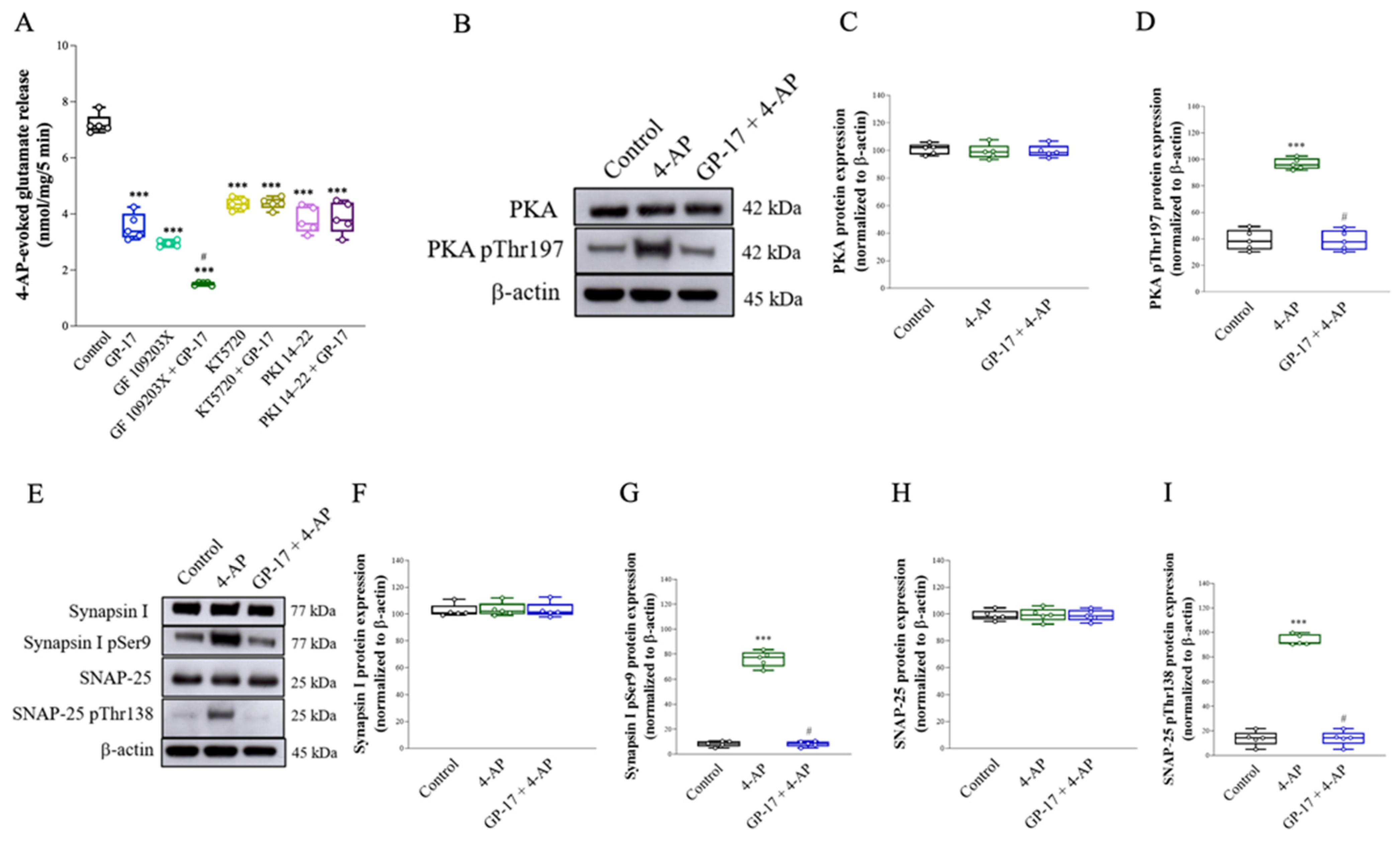
3.2. Suppression of the Protein Kinase A Pathway Is Involved in the GP-17-Mediated Inhibition of Glutamate Release from the Cerebrocortical Synaptosomes of Rats
3.3. GP-17 Prevents KA-Induced Excitotoxicity in Rats
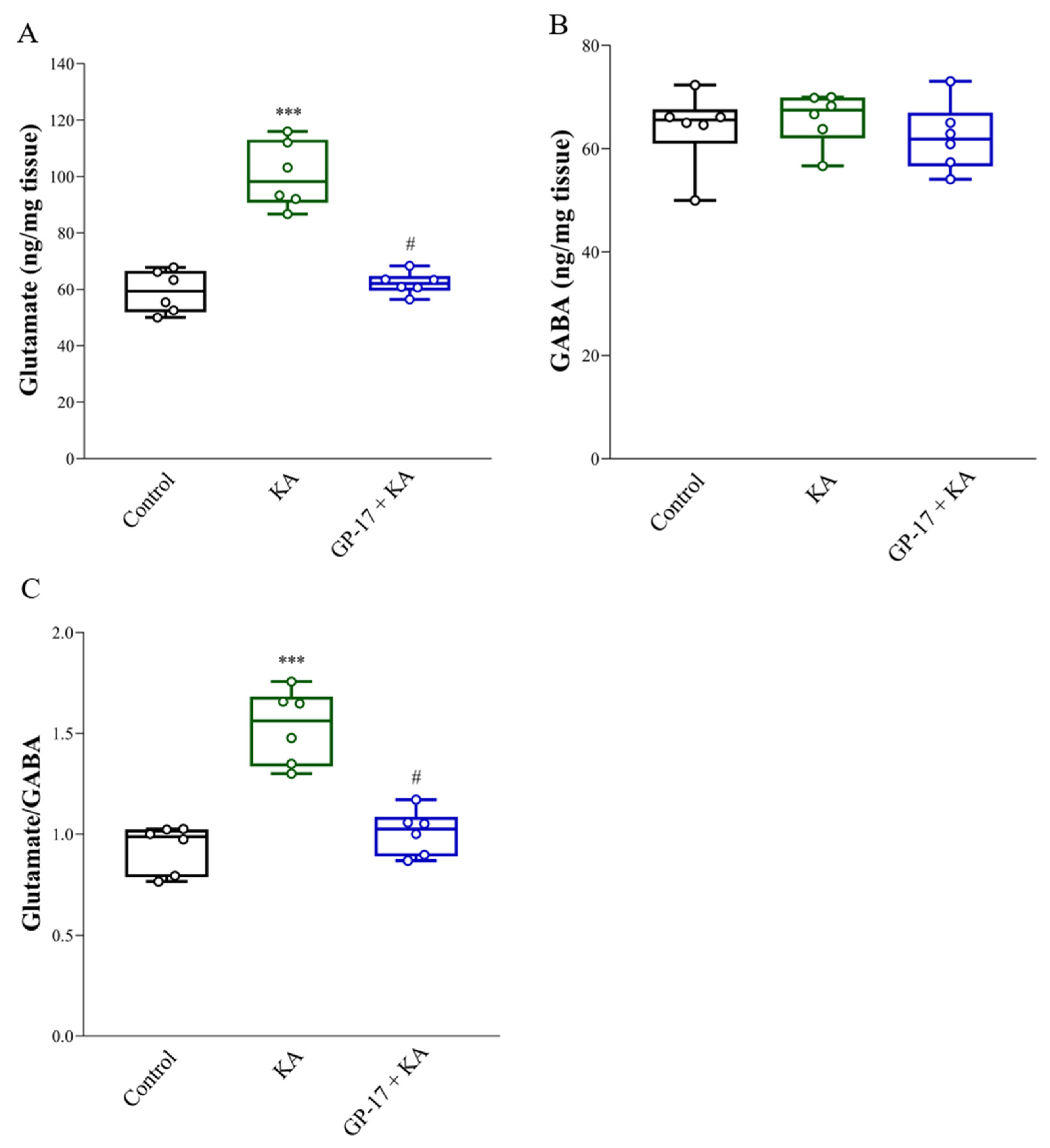
3.4. GP-17 Decreases Glutamate Concentrations in the Cortex of KA-Treated Rats
3.5. GP-17 Decreases the Protein Levels of SNAT1, Glutaminase and VGLUT1 but Increases the Protein Level of GDH in the Cortex of KA-Treated Rats
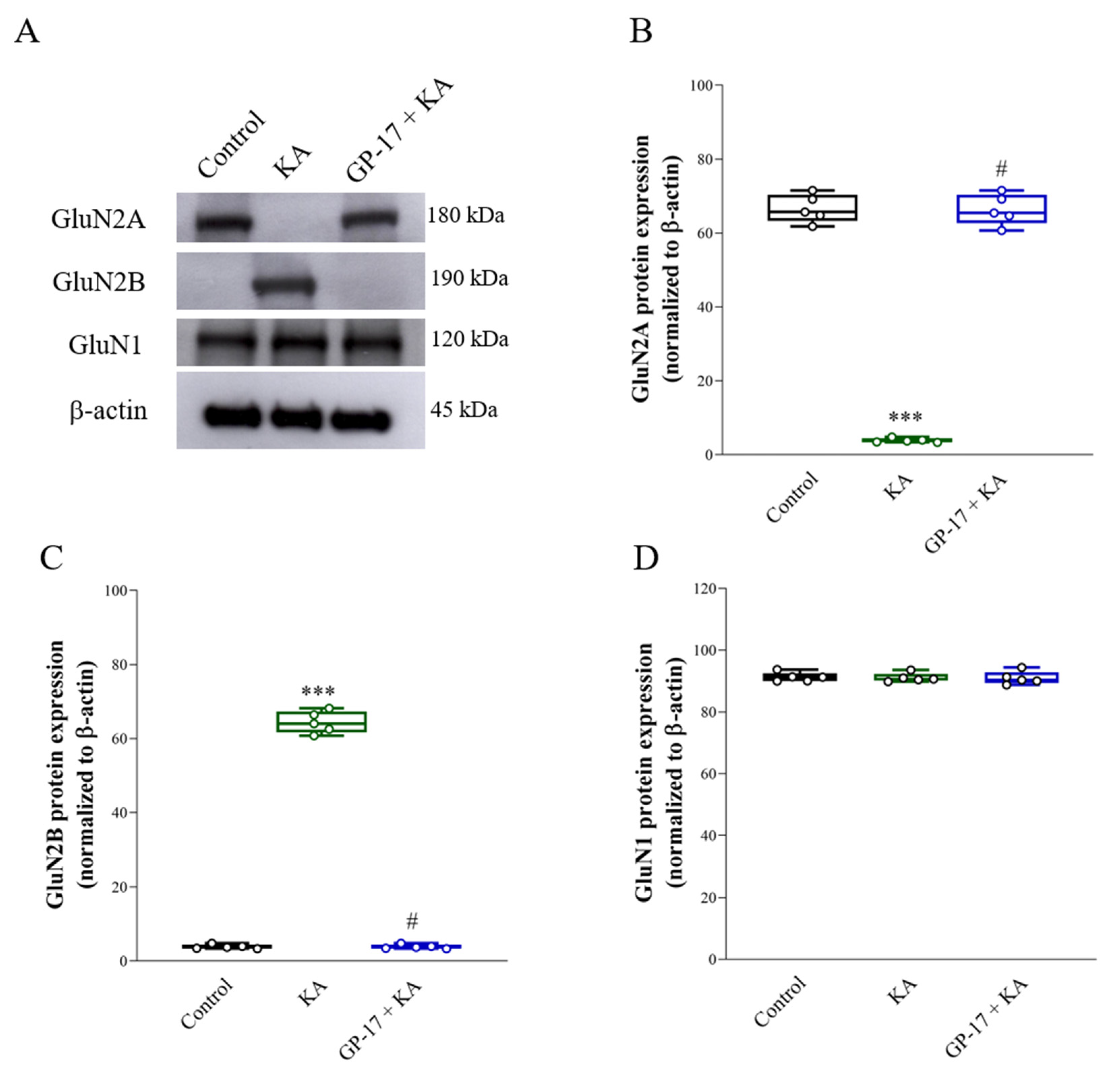
3.6. GP-17 Alters the Protein Expression of the N-methyl-D-aspartate (NMDA) Receptor Subunits GluN2A and GluN2B in the Cortex of KA-Treated Rats
3.7. GP-17 Prevents Decreases in Cerebral Blood Flow and ArgII Expression in KA-Treated Rats
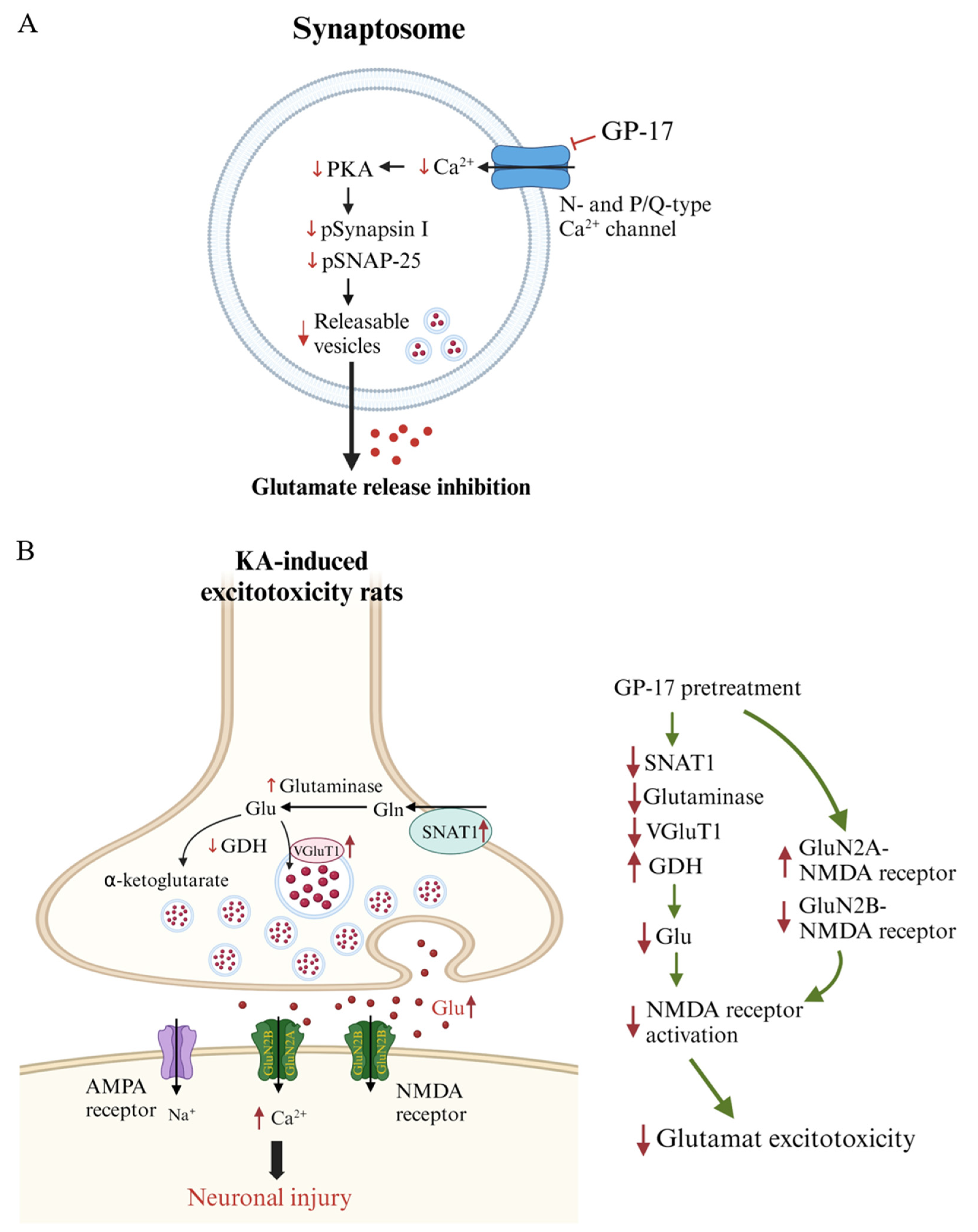
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhou, Y.; Danbolt, N.C. Glutamate as a neurotransmitter in the healthy brain. J. Neural Transm. 2014, 121, 799–817. [Google Scholar] [CrossRef] [PubMed]
- Olloquequi, J.; Cornejo-Cordova, E.; Verdaguer, E.; Soriano, F.X.; Binvignat, O.; Auladell, C.; Camins, A. Excitotoxicity in the pathogenesis of neurological and psychiatric disorders: Therapeutic implications. J. Psychopharmacol. 2018, 32, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Binvignat, O.; Olloquequi, J. Excitotoxicity as a target against neurodegenerative processes. Curr. Pharm. Des. 2020, 26, 1251–1262. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.X.; Wang, Y.; Qin, Z.H. Molecular mechanisms of excitotoxicity and their relevance to pathogenesis of neurodegenerative diseases. Acta Pharmacol. Sin. 2009, 30, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Lai, T.W.; Zhang, S.; Wang, Y.T. Excitotoxicity and stroke: Identifying novel targets for neuroprotection. Prog. Neurobiol. 2014, 115, 157–188. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Liu, N.; Chen, J.; Zheng, P.; Niu, J.; Tang, S.; Peng, X.; Wu, J.; Yu, J.; Ma, L. Neuropharmacological insight into preventive intervention in posttraumatic epilepsy based on regulating glutamate homeostasis. CNS Neurosci. Ther. 2023, 29, 2430–2444. [Google Scholar] [CrossRef] [PubMed]
- Pomierny, B.; Krzyzanowska, W.; Skorkowska, A.; Jurczyk, J.; Bystrowska, B.; Budziszewska, B.; Pera, J. Inhibition of vesicular glutamate transporters (VGLUTs) with Chicago Sky Blue 6B before focal cerebral ischemia offers neuroprotection. Mol. Neurobiol. 2023, 60, 3130–3146. [Google Scholar] [CrossRef] [PubMed]
- Borbely, E.; Simon, M.; Fuchs, E.; Wiborg, O.; Czeh, B.; Helyes, Z. Novel drug developmental strategies for treatment-resistant depression. Br. J. Pharmacol. 2022, 179, 1146–1186. [Google Scholar] [CrossRef]
- Hasan, S.; Khatri, N.; Rahman, Z.N.; Menezes, A.A.; Martini, J.; Shehjar, F.; Mujeeb, N.; Shah, Z.A. Neuroprotective potential of flavonoids in brain disorders. Brain Sci. 2023, 13, 1258. [Google Scholar] [CrossRef]
- Zhou, J.C.; Li, H.L.; Zhou, Y.; Li, X.T.; Yang, Z.Y.; Tohda, C.; Komatsu, K.; Piao, X.H.; Ge, Y.W. The roles of natural triterpenoid saponins against Alzheimer’s disease. Phytother. Res. 2023, 37, 5017–5040. [Google Scholar] [CrossRef]
- Zhang, M.M.; Huo, G.M.; Cheng, J.; Zhang, Q.P.; Li, N.Z.; Guo, M.X.; Liu, Q.; Xu, G.H.; Zhu, J.X.; Li, C.F.; et al. Gypenoside XVII, an active ingredient from gynostemma pentaphyllum, inhibits C3aR-associated synaptic pruning in stressed mice. Nutrients 2022, 14, 2418. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yu, Y.; Zhang, H.; Li, L.; Wang, J.; Su, S.; Zhang, Y.; Song, L.; Zhou, K. Gypenoside XVII attenuates renal ischemia-reperfusion injury by inhibiting endoplasmic reticulum stress and NLRP3 inflammasome-triggered pyroptosis. Eur. J. Pharmacol. 2024, 962, 176187. [Google Scholar] [CrossRef] [PubMed]
- Su, S.; Wang, J.; Wang, J.; Yu, R.; Sun, L.; Zhang, Y.; Song, L.; Pu, W.; Tang, Y.; Yu, Y.; et al. Cardioprotective effects of gypenoside XVII against ischemia/reperfusion injury: Role of endoplasmic reticulum stress, autophagy, and mitochondrial fusion fission balance. Phytother. Res. 2022, 36, 2982–2998. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Wang, M.; Chen, R.; Sun, X.; Sun, G.; Sun, X. Gypenoside XVII protects against myocardial ischemia and reperfusion injury by inhibiting ER stress-induced mitochondrial injury. J. Ginseng Res. 2021, 45, 642–653. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.Y.; Zhou, C.L.; Zeng, M.Y. Gypenoside XVII inhibits ox-LDL-induced macrophage inflammatory responses and promotes cholesterol efflux through activating the miR-182-5p/HDAC9 signaling pathway. J. Ethnopharmacol. 2024, 319, 117070. [Google Scholar] [CrossRef]
- Zhou, K.; Zhang, Y.; Zhou, Y.; Xu, M.; Yu, S. Production of gypenoside XVII from ginsenoside Rb1 by enzymatic transformation and their anti-inflammatory activity in vitro and in vivo. Molecules 2023, 28, 7001. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Zhu, T.; Zhang, S.; Sun, X. Protective effects of Gypenoside XVII against cerebral ischemia/reperfusion injury via SIRT1-FOXO3A- and Hif1a-BNIP3-mediated mitochondrial autophagy. J. Transl. Med. 2022, 20, 622. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Luo, Y.; Liang, T.; Wang, M.; Zhao, J.; Sun, G.; Sun, X. Gypenoside XVII enhances lysosome biogenesis and autophagy flux and accelerates autophagic clearance of amyloid-beta through TFEB activation. J. Alzheimers Dis. 2016, 52, 1135–1150. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Wang, M.; Sun, G.; Ye, J.; Zhou, Y.; Dong, X.; Wang, T.; Lu, S.; Sun, X. Attenuation of Abeta25-35-induced parallel autophagic and apoptotic cell death by gypenoside XVII through the estrogen receptor-dependent activation of Nrf2/ARE pathways. Toxicol. Appl. Pharmacol. 2014, 279, 63–75. [Google Scholar] [CrossRef]
- Raiteri, L.; Raiteri, M. Synaptosomes still viable after 25 years of superfusion. Neurochem. Res. 2000, 25, 1265–1274. [Google Scholar] [CrossRef]
- Wang, Q.; Yu, S.; Simonyi, A.; Sun, G.Y.; Sun, A.Y. Kainic acid-mediated excitotoxicity as a model for neurodegeneration. Mol. Neurobiol. 2005, 31, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Ferkany, J.W.; Zaczek, R.; Coyle, J.T. Kainic acid stimulates excitatory amino acid neurotransmitter release at presynaptic receptors. Nature 1982, 298, 757–759. [Google Scholar] [CrossRef] [PubMed]
- Dunkley, P.R.; Jarvie, P.E.; Robinson, P.J. A rapid Percoll gradient procedure for preparation of synaptosomes. Nat. Protoc. 2008, 3, 1718–1728. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.C.; Hsieh, P.W.; Kuo, J.R.; Wang, S.J. Rosmarinic acid, a bioactive phenolic compound, inhibits glutamate release from rat cerebrocortical synaptosomes through GABAA receptor activation. Biomolecules 2021, 11, 1029. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.Y.; Lu, C.W.; Hsieh, P.W.; Chiu, K.M.; Lee, M.Y.; Wang, S.J. Natural product isoliquiritigenin activates GABAB receptors to decrease voltage-gate Ca2+ channels and glutamate release in rat cerebrocortical nerve terminals. Biomolecules 2021, 11, 1537. [Google Scholar] [CrossRef] [PubMed]
- Murthy, V.N. Optical detection of synaptic vesicle exocytosis and endocytosis. Curr. Opin. Neurobiol. 1999, 9, 314–320. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, M.L.; Rostas, J.A.; Sim, A.T. Two modes of exocytosis from synaptosomes are differentially regulated by protein phosphatase types 2A and 2B. J. Neurochem. 2003, 85, 1190–1199. [Google Scholar] [CrossRef]
- Jean, W.H.; Huang, C.T.; Hsu, J.H.; Chiu, K.M.; Lee, M.Y.; Shieh, J.S.; Lin, T.Y.; Wang, S.J. Anticonvulsive and neuroprotective effects of eupafolin in rats are associated with the inhibition of glutamate overexcitation and upregulation of the Wnt/beta-catenin signaling pathway. ACS Chem. Neurosci. 2022, 13, 1594–1603. [Google Scholar] [CrossRef]
- Friedman, L.K.; Pellegrini-Giampietro, D.E.; Sperber, E.F.; Bennett, M.V.; Moshe, S.L.; Zukin, R.S. Kainate-induced status epilepticus alters glutamate and GABAA receptor gene expression in adult rat hippocampus: An in situ hybridization study. J. Neurosci. 1994, 14, 2697–2707. [Google Scholar] [CrossRef]
- Spigolon, G.; Veronesi, C.; Bonny, C.; Vercelli, A. c-Jun N-terminal kinase signaling pathway in excitotoxic cell death following kainic acid-induced status epilepticus. Eur. J. Neurosci. 2010, 31, 1261–1272. [Google Scholar] [CrossRef] [PubMed]
- Racine, R.J. Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr. Clin. Neurophysiol. 1972, 32, 281–294. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.Y.; Lu, C.W.; Wang, S.J. Luteolin protects the hippocampus against neuron impairments induced by kainic acid in rats. Neurotoxicology 2016, 55, 48–57. [Google Scholar] [CrossRef]
- Pai, M.S.; Wang, K.C.; Yeh, K.C.; Wang, S.J. Stabilization of mitochondrial function by chlorogenic acid protects against kainic acid-induced seizures and neuronal cell death in rats. Eur. J. Pharmacol. 2023, 961, 176197. [Google Scholar] [CrossRef] [PubMed]
- Chang, A.; Chang, Y.; Wang, S.J. Rutin prevents seizures in kainic acid-treated rats: Evidence of glutamate levels, inflammation and neuronal loss modulation. Food Funct. 2022, 13, 10401–10414. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Mu, Y.; Ding, H.; Du, B.; Zhou, M.; Li, Q.; Gong, S.; Zhang, F.; Geng, D.; Wang, Y. 1alpha,25-Dihydroxyvitamin D3 Promotes Angiogenesis After Cerebral Ischemia Injury in Rats by Upregulating the TGF-beta/Smad2/3 Signaling Pathway. Front. Cardiovasc. Med. 2022, 9, 769717. [Google Scholar] [CrossRef] [PubMed]
- Yeh, K.C.; Hung, C.F.; Lee, H.L.; Hsieh, T.Y.; Wang, S.J. Soybean meal extract preserves memory ability by increasing presynaptic function and modulating gut microbiota in rats. Mol. Neurobiol. 2022, 59, 1649–1664. [Google Scholar] [CrossRef] [PubMed]
- Millan, C.; Sanchez-Prieto, J. Differential coupling of N- and P/Q-type calcium channels to glutamate exocytosis in the rat cerebral cortex. Neurosci. Lett. 2002, 330, 29–32. [Google Scholar] [CrossRef] [PubMed]
- Coffey, E.T.; Sihra, T.S.; Nicholls, D.G. Protein kinase C and the regulation of glutamate exocytosis from cerebrocortical synaptosomes. J. Biol. Chem. 1993, 268, 21060–21065. [Google Scholar] [CrossRef]
- Millan, C.; Torres, M.; Sanchez-Prieto, J. Co-activation of PKA and PKC in cerebrocortical nerve terminals synergistically facilitates glutamate release. J. Neurochem. 2003, 87, 1101–1111. [Google Scholar] [CrossRef]
- Greengard, P.; Valtorta, F.; Czernik, A.J.; Benfenati, F. Synaptic vesicle phosphoproteins and regulation of synaptic function. Science 1993, 259, 780–785. [Google Scholar] [CrossRef] [PubMed]
- Kyllo, T.; Singh, V.; Shim, H.; Latika, S.; Nguyen, H.M.; Chen, Y.J.; Terry, E.; Wulff, H.; Erickson, J.D. Riluzole and novel naphthalenyl substituted aminothiazole derivatives prevent acute neural excitotoxic injury in a rat model of temporal lobe epilepsy. Neuropharmacology 2023, 224, 109349. [Google Scholar] [CrossRef]
- Ferkany, J.W.; Coyle, J.T. Kainic acid selectively stimulates the release of endogenous excitatory acidic amino acids. J. Pharmacol. Exp. Ther. 1983, 225, 399–406. [Google Scholar]
- Mohd Sairazi, N.S.; Sirajudeen, K.N.; Asari, M.A.; Muzaimi, M.; Mummedy, S.; Sulaiman, S.A. Kainic acid-induced excitotoxicity experimental model: Protective merits of natural products and plant extracts. Evid. Based Complement. Alternat. Med. 2015, 2015, 972623. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhang, X.; Chen, X.; Wei, Y. Neuronal injuries in cerebral infarction and ischemic stroke: From mechanisms to treatment (Review). Int. J. Mol. Med. 2022, 49, 15. [Google Scholar] [CrossRef]
- Ahmad, A.S.; Shah, Z.A.; Dore, S. Protective role of arginase II in cerebral ischemia and excitotoxicity. J. Neurol. Neurosci. 2016, 7, 88. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.J.; Sihra, T.S. Opposing facilitatory and inhibitory modulation of glutamate release elicited by cAMP production in cerebrocortical nerve terminals (synaptosomes). Neuropharmacology 2003, 44, 686–697. [Google Scholar] [CrossRef]
- Nagy, G.; Reim, K.; Matti, U.; Brose, N.; Binz, T.; Rettig, J.; Neher, E.; Sorensen, J.B. Regulation of releasable vesicle pool sizes by protein kinase A-dependent phosphorylation of SNAP-25. Neuron 2004, 41, 417–429. [Google Scholar] [CrossRef]
- Ceccaldi, P.E.; Grohovaz, F.; Benfenati, F.; Chieregatti, E.; Greengard, P.; Valtorta, F. Dephosphorylated synapsin I anchors synaptic vesicles to actin cytoskeleton: An analysis by videomicroscopy. J. Cell Biol. 1995, 128, 905–912. [Google Scholar] [CrossRef]
- Menegon, A.; Bonanomi, D.; Albertinazzi, C.; Lotti, F.; Ferrari, G.; Kao, H.T.; Benfenati, F.; Baldelli, P.; Valtorta, F. Protein kinase A-mediated synapsin I phosphorylation is a central modulator of Ca2+-dependent synaptic activity. J. Neurosci. 2006, 26, 11670–11681. [Google Scholar] [CrossRef]
- Hsu, S.K.; Lu, C.W.; Chiu, K.M.; Lee, M.Y.; Lin, T.Y.; Wang, S.J. Mangiferin depresses vesicular glutamate release in synaptosomes from the rat cerebral cortex by decreasing synapsin I phosphorylation. Eur. J. Pharmacol. 2023, 950, 175772. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.W.; Lin, T.Y.; Hsieh, P.W.; Chiu, K.M.; Lee, M.Y.; Wang, S.J. Cynarin, a caffeoylquinic acid derivative in artichoke, inhibits exocytotic glutamate release from rat cortical nerve terminals (synaptosomes). Neurochem. Int. 2023, 167, 105537. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.K.; Yeh, K.C.; Pai, M.S.; Hsieh, P.W.; Wang, S.J. Ursolic acid inhibits the synaptic release of glutamate and prevents glutamate excitotoxicity in rats. Eur. J. Pharmacol. 2024, 963, 176280. [Google Scholar] [CrossRef]
- Lu, C.W.; Wu, C.C.; Chiu, K.M.; Lee, M.Y.; Lin, T.Y.; Wang, S.J. Inhibition of synaptic glutamate exocytosis and prevention of glutamate neurotoxicity by eupatilin from artemisia argyi in the rat cortex. Int. J. Mol. Sci. 2022, 23, 13406. [Google Scholar] [CrossRef] [PubMed]
- Pietrancosta, N.; Djibo, M.; Daumas, S.; El Mestikawy, S.; Erickson, J.D. Molecular, structural, functional, and pharmacological sites for vesicular glutamate transporter regulation. Mol. Neurobiol. 2020, 57, 3118–3142. [Google Scholar] [CrossRef] [PubMed]
- Chaudhry, F.A.; Schmitz, D.; Reimer, R.J.; Larsson, P.; Gray, A.T.; Nicoll, R.; Kavanaugh, M.; Edwards, R.H. Glutamine uptake by neurons: Interaction of protons with system a transporters. J. Neurosci. 2002, 22, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Daniels, R.W.; Miller, B.R.; DiAntonio, A. Increased vesicular glutamate transporter expression causes excitotoxic neurodegeneration. Neurobiol. Dis. 2011, 41, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Lynch, D.R.; Guttmann, R.P. Excitotoxicity: Perspectives based on N-methyl-D-aspartate receptor subtypes. J. Pharmacol. Exp. Ther. 2002, 300, 717–723. [Google Scholar] [CrossRef]
- Paoletti, P.; Bellone, C.; Zhou, Q. NMDA receptor subunit diversity: Impact on receptor properties, synaptic plasticity and disease. Nat. Rev. Neurosci. 2013, 14, 383–400. [Google Scholar] [CrossRef]
- Ladagu, A.D.; Olopade, F.E.; Adejare, A.; Olopade, J.O. GluN2A and GluN2B N-methyl-D-aspartate receptor (NMDARs) subunits: Their roles and therapeutic antagonists in neurological diseases. Pharmaceuticals 2023, 16, 1535. [Google Scholar] [CrossRef]
- Choo, A.M.; Geddes-Klein, D.M.; Hockenberry, A.; Scarsella, D.; Mesfin, M.N.; Singh, P.; Patel, T.P.; Meaney, D.F. NR2A and NR2B subunits differentially mediate MAP kinase signaling and mitochondrial morphology following excitotoxic insult. Neurochem. Int. 2012, 60, 506–516. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Zhu, L.; Li, T.; Tang, X.; Xiang, Y.; Han, X.; Xia, L.; Zeng, L.; Nie, J.; Huang, Y.; et al. Neuroprotective mechanisms of Lycium barbarum polysaccharides against ischemic insults by regulating NR2B and NR2A containing NMDA receptor signaling pathways. Front. Cell. Neurosci. 2017, 11, 288. [Google Scholar] [CrossRef] [PubMed]
- Saito, A.; Maier, C.M.; Narasimhan, P.; Nishi, T.; Song, Y.S.; Yu, F.; Liu, J.; Lee, Y.S.; Nito, C.; Kamada, H.; et al. Oxidative stress and neuronal death/survival signaling in cerebral ischemia. Mol. Neurobiol. 2005, 31, 105–116. [Google Scholar] [CrossRef] [PubMed]
- Dawson, V.L.; Dawson, T.M.; London, E.D.; Bredt, D.S.; Snyder, S.H. Nitric oxide mediates glutamate neurotoxicity in primary cortical cultures. Proc. Natl. Acad. Sci. USA 1991, 88, 6368–6371. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Duan, L.; Li, J.; Guo, H.; Xiong, M. Gypenoside XVII protects against spinal cord injury in mice by regulating the microRNA-21-mediated PTEN/AKT/mTOR pathway. Int. J. Mol. Med. 2021, 48, 146. [Google Scholar] [CrossRef] [PubMed]
- Lazarevic, V.; Yang, Y.; Ivanova, D.; Fejtova, A.; Svenningsson, P. Riluzole attenuates the efficacy of glutamatergic transmission by interfering with the size of the readily releasable neurotransmitter pool. Neuropharmacology 2018, 143, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Cheng, X.; Zhao, M.; Zhao, T.; Zhang, M.; Shi, Z.; Yue, X.; Geng, Y.; Gao, J.; Wang, C.; et al. Gypenoside-14 Reduces Depression via downregulation of the nuclear factor kappa B (NF-kB) signaling pathway on the lipopolysaccharide (LPS)-induced depression model. Pharmaceuticals 2023, 16, 1152. [Google Scholar] [CrossRef]
- Zhang, G.L.; Deng, J.P.; Wang, B.H.; Zhao, Z.W.; Li, J.; Gao, L.; Liu, B.L.; Xong, J.R.; Guo, X.D.; Yan, Z.Q.; et al. Gypenosides improve cognitive impairment induced by chronic cerebral hypoperfusion in rats by suppressing oxidative stress and astrocytic activation. Behav. Pharmacol. 2011, 22, 633–644. [Google Scholar] [CrossRef]



| KA 15 mg/kg | GP-17 10 mg/kg + KA 15 mg/kg | GP-17 30 mg/kg + KA 15 mg/kg | |
|---|---|---|---|
| Seizure score | 4.7 ± 0.1 | 4.3 ± 0.2 | 0.8 ± 0.3 *** |
| Latency to first seizure (min) | 84.4 ± 6.5 | 111.1 ± 13.4 | - |
| % Seizure | 20/20 (100%) | 9/10 (90%) | 0/14 (0%) |
| Mortality | 6/20 | 2/10 | 0/14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, C.-W.; Lin, T.-Y.; Chiu, K.-M.; Lee, M.-Y.; Wang, S.-J. Gypenoside XVII Reduces Synaptic Glutamate Release and Protects against Excitotoxic Injury in Rats. Biomolecules 2024, 14, 589. https://doi.org/10.3390/biom14050589
Lu C-W, Lin T-Y, Chiu K-M, Lee M-Y, Wang S-J. Gypenoside XVII Reduces Synaptic Glutamate Release and Protects against Excitotoxic Injury in Rats. Biomolecules. 2024; 14(5):589. https://doi.org/10.3390/biom14050589
Chicago/Turabian StyleLu, Cheng-Wei, Tzu-Yu Lin, Kuan-Ming Chiu, Ming-Yi Lee, and Su-Jane Wang. 2024. "Gypenoside XVII Reduces Synaptic Glutamate Release and Protects against Excitotoxic Injury in Rats" Biomolecules 14, no. 5: 589. https://doi.org/10.3390/biom14050589
APA StyleLu, C.-W., Lin, T.-Y., Chiu, K.-M., Lee, M.-Y., & Wang, S.-J. (2024). Gypenoside XVII Reduces Synaptic Glutamate Release and Protects against Excitotoxic Injury in Rats. Biomolecules, 14(5), 589. https://doi.org/10.3390/biom14050589







