Cholesterol Oxime Olesoxime Assessed as a Potential Ligand of Human Cholinesterases
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals for Cholinesterase Assays
2.2. Determination of Oxime Inhibition Potency as IC50 Value
2.3. In Vitro Reactivation Assay
2.4. In Silico Molecular Modeling
3. Results and Discussion
4. Conclusions
5. Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bordet, T.; Buisson, B.; Michaud, M.; Drouot, C.; Galéa, P.; Delaage, P.; Akentieva, N.P.; Evers, A.S.; Covey, D.F.; Ostuni, M.A.; et al. Identification and characterization of cholest-4-en-3-one, oxime (TRO19622), a novel drug candidate for amyotrophic lateral sclerosis. J. Pharmacol. Exp. Ther. 2007, 322, 709–720. [Google Scholar] [CrossRef] [PubMed]
- Calder, A.N.; Androphy, E.J.; Hodgetts, K.J. Small molecules in development for the treatment of spinal muscular atrophy. J. Med. Chem. 2016, 59, 10067–10083. [Google Scholar] [CrossRef] [PubMed]
- Weber, J.J.; Clemensson, L.E.; Schiöth, H.B.; Nguyen, H.P. Olesoxime in neurodegenerative diseases: Scrutinising a promising drug candidate. Biochem. Pharmacol. 2019, 168, 305–318. [Google Scholar] [CrossRef]
- Bordet, T.; Berna, P.; Abitbol, J.-L.; Pruss, R.M. Olesoxime (TRO19622): A novel mitochondrial-targeted neuroprotective compound. Pharmaceuticals 2010, 3, 345–368. [Google Scholar] [CrossRef]
- Lenglet, T.; Lacomblez, L.; Abitbol, J.L.; Ludolph, A.; Mora, J.S.; Robberecht, W.; Shaw, P.J.; Pruss, R.M.; Cuvier, V.; Meininger, V. A phase II-III trial of olesoxime in subjects with amyotrophic lateral sclerosis. Eur. J. Neurol. 2014, 21, 529–536. [Google Scholar] [CrossRef]
- Muntoni, F.; Bertini, E.; Comi, G.; Kirschner, J.; Lusakowska, A.; Mercuri, E.; Scoto, M.; van der Pol, W.L.; Vuillerot, C.; Burdeska, A.; et al. Long-term follow-up of patients with type 2 and non-ambulant type 3 spinal muscular atrophy (SMA) treated with olesoxime in the OLEOS trial. Neuromuscul. Disord. 2020, 30, 959–969. [Google Scholar] [CrossRef] [PubMed]
- Petrov, A.M. Oxysterols in central and peripheral synaptic communication. Adv. Exp. Med. Biol. 2024, 1440, 91–123. [Google Scholar] [CrossRef]
- Song, Y.H.; Lei, H.X.; Yu, D.; Zhu, H.; Hao, M.Z.; Cui, R.H.; Meng, X.S.; Sheng, X.H.; Zhang, L. Endogenous chemicals guard health through inhibiting ferroptotic cell death. Biofactors 2024, 50, 266–293. [Google Scholar] [CrossRef]
- Garcia-Segura, M.E.; Durainayagam, B.R.; Liggi, S.; Graça, G.; Jimenez, B.; Dehghan, A.; Tzoulaki, I.; Karaman, I.; Elliott, P.; Griffin, J.L. Pathway-based integration of multi-omics data reveals lipidomics alterations validated in an Alzheimer’s disease mouse model and risk loci carriers. J. Neurochem. 2023, 164, 57–76. [Google Scholar] [CrossRef]
- Clemens, L.E.; Weber, J.J.; Wlodkowski, T.T.; Yu-Taeger, L.; Michaud, M.; Calaminus, C.; Eckert, S.H.; Gaca, J.; Weiss, A.; Magg, J.C.D.; et al. Olesoxime suppresses calpain activation and mutant Huntingtin fragmentation in the BACHD rat. Brain 2015, 138, 3632–3653. [Google Scholar] [CrossRef]
- Gouarné, C.; Tracz, J.; Paoli, M.G.; Deluca, V.; Seimandi, M.; Tardif, G.; Xilouri, M.; Stefanis, L.; Bordet, T.; Pruss, R.M. Protective role of olesoxime against wild-type α-synuclein-induced toxicity in human neuronally differentiated SHSY-5Y cells. Br. J. Pharmacol. 2015, 172, 235–245. [Google Scholar] [CrossRef]
- Eckert, G.P.; Eckert, S.H.; Eckmann, J.; Hagl, S.; Muller, W.E.; Friedland, K. Olesoxime improves cerebral mitochondrial dysfunction and enhances Aβ levels in preclinical models of Alzheimer’s disease. Exp. Neurol. 2020, 329, 113286. [Google Scholar] [CrossRef] [PubMed]
- Maček Hrvat, N.; Kovarik, Z. Counteracting poisoning with chemical warfare nerve agents. Arh. Hig. Rada Toksikol. 2020, 71, 266–284. [Google Scholar] [CrossRef]
- Timperley, C.M.; Abdollahi, M.; Al-Amri, A.S.; Baulig, A.; Benachour, D.; Borrett, V.; Cariño, F.A.; Geist, M.; Gonzalez, D.; Kane, W.; et al. Advice on assistance and protection by the Scientific Advisory Board of the Organisation for the Prohibition of Chemical Weapons: Part 2. On preventing and treating health effects from acute, prolonged, and repeated nerve agent exposure, and the identification of medical countermeasures able to reduce or eliminate the longer term health effects of nerve agents. Toxicology 2019, 413, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Chatonnet, A.; Lockridge, O. Comparison of butyrylcholinesterase and acetylcholinesterase. Biochem. J. 1989, 260, 625–634. [Google Scholar] [CrossRef]
- Kolić, D.; Kovarik, Z. N-methyl-D-aspartate receptors: Structure, function, and role in organophosphorus compound poisoning. Biofactors 2024, 1–17. [Google Scholar] [CrossRef]
- Lorke, D.; Kalasz, H.; Petroianu, G.; Tekes, K. Entry of oximes into the brain: A review. Curr. Med. Chem. 2008, 15, 743–753. [Google Scholar] [CrossRef]
- Jaćević, V.; Dumanović, J.; Grujić-Milanović, J.; Milovanović, Z.; Amidžić, L.; Vojinović, N.; Nežić, L.; Marković, B.; Dobričić, V.; Milosavljević, P.; et al. Oxidative stress status assessment of rats’ brains injury following subacute exposure to K-oximes. Chem. Biol. Interact. 2023, 383, 110658. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.G.; Fan, L.; Huang, L.L.; Liu, H.L.; Zhou, A.M. Synthesis and evaluation of some steroidal oximes as cytotoxic agents: Structure/activity studies (I). Steroids 2009, 74, 62–72. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres, V.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Zorbaz, T.; Braïki, A.; Maraković, N.; Renou, J.; de la Mora, E.; Maček Hrvat, N.; Katalinić, M.; Silman, I.; Sussman, J.L.; Mercey, G.; et al. Potent 3-hydroxy-2-pyridine aldoxime reactivators of organophosphate-inhibited cholinesterases with predicted blood–brain barrier penetration. Chem. Eur. J. 2018, 24, 9675–9691. [Google Scholar] [CrossRef] [PubMed]
- Kovarik, Z.; Radić, Z.; Berman, H.A.; Simeon-Rudolf, V.; Reiner, E.; Taylor, P. Mutant cholinesterases possessing enhanced capacity for reactivation of their phosphonylated conjugates. Biochemistry 2004, 43, 3222–3229. [Google Scholar] [CrossRef] [PubMed]
- Brooks, B.R.; Bruccoleri, R.E.; Olafson, B.D.; States, D.J.; Swaminathan, S.; Karplus, M. CHARMM: A program for macromolecular energy, minimization, and dynamics calculations. J. Comput. Chem. 1983, 4, 187–217. [Google Scholar] [CrossRef]
- Momany, F.A.; Rone, R. Validation of the general purpose QUANTA®3.2/CHARMm® force field. J. Comput. Chem. 1992, 13, 888–900. [Google Scholar] [CrossRef]
- Dym, O.; Unger, T.; Toker, L.; Silman, I.; Sussman, J.L. Crystal Structure of Human Acetylcholinesterase; Israel Structural Proteomics Center (ISPC): Rehovot, Israel, 2015. [Google Scholar] [CrossRef]
- Ngamelue, M.N.; Homma, K.; Lockridge, O.; Asojo, O.A. Crystallization and X-ray structure of full-length recombinant human butyrylcholinesterase. Acta Crystallogr. 2007, 63, 723–727. [Google Scholar] [CrossRef] [PubMed]
- Čadež, T.; Kolić, D.; Šinko, G.; Kovarik, Z. Assessment of four organophosphorus pesticides as inhibitors of human acetylcholinesterase and butyrylcholinesterase. Sci. Rep. 2021, 11, 21486. [Google Scholar] [CrossRef]
- Kuča, K.; Cabal, J.; Kassa, J. A Comparison of the potency of newly developed oximes (K005, K027, K033, K048) and currently used oximes (pralidoxime, obidoxime, HI-6) to reactivate sarin-inhibited rat brain acetylcholinesterase by in vitro methods. J. Toxicol. Environ. Health A 2005, 68, 677–686. [Google Scholar] [CrossRef] [PubMed]
- Kovarik, Z.; Čalić, M.; Bosak, A.; Šinko, G.; Jelić, D. In vitro evaluation of aldoxime interactions with human acetylcholinesterase. Croat. Chem. Acta 2008, 81, 47–57. [Google Scholar]
- Kovarik, Z.; Kalisiak, J.; Hrvat, N.M.; Katalinić, M.; Zorbaz, T.; Žunec, S.; Green, C.; Radić, Z.; Fokin, V.V.; Sharpless, K.B.; et al. Reversal of tabun toxicity enabled by a triazole-annulated oxime library—Reactivators of acetylcholinesterase. Chem. Eur. J. 2019, 25, 4100–4114. [Google Scholar] [CrossRef]
- Horn, G.; Wille, T.; Musilek, K.; Kuča, K.; Thiermann, H.; Worek, F. Reactivation kinetics of 31 structurally different bispyridinium oximes with organophosphate-inhibited human butyrylcholinesterase. Arch. Toxicol. 2015, 89, 405–414. [Google Scholar] [CrossRef]
- Winter, M.; Wille, T.; Musilek, K.; Kuča, K.; Thiermann, H.; Worek, F. Investigation of the reactivation kinetics of a large series of bispyridinium oximes with organophosphate-inhibited human acetylcholinesterase. Toxicol. Lett. 2016, 244, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Worek, F.; Von Der Wellen, J.; Musilek, K.; Kuča, K.; Thiermann, H. Reactivation kinetics of a homologous series of bispyridinium bis-oximes with nerve agent-inhibited human acetylcholinesterase. Arch. Toxicol. 2012, 86, 1379–1386. [Google Scholar] [CrossRef]
- Katalinić, M.; Maček Hrvat, N.; Baumann, K.; Morasi Piperčić, S.; Makarić, S.; Tomić, S.; Jović, O.; Hrenar, T.; Miličević, A.; Jelić, D.; et al. A comprehensive evaluation of novel oximes in creation of butyrylcholinesterase-based nerve agent bioscavengers. Toxicol. Appl. Pharmacol. 2016, 310, 195–204. [Google Scholar] [CrossRef]
- Zorbaz, T.; Malinak, D.; Kuča, K.; Musilek, K.; Kovarik, Z. Butyrylcholinesterase inhibited by nerve agents is efficiently reactivated with chlorinated pyridinium oximes. Chem. Biol. Interact. 2019, 307, 16–20. [Google Scholar] [CrossRef]
- Zorbaz, T.; Malinak, D.; Hofmanova, T.; Maraković, N.; Žunec, S.; Hrvat, N.M.; Andrys, R.; Psotka, M.; Zandona, A.; Svobodova, J.; et al. Halogen substituents enhance oxime nucleophilicity for reactivation of cholinesterases inhibited by nerve agents. Eur. J. Med. Chem. 2022, 238, 114377. [Google Scholar] [CrossRef]
- Zandona, A.; Katalinić, M.; Šinko, G.; Radman Kastelic, A.; Primožič, I.; Kovarik, Z. Targeting organophosphorus compounds poisoning by novel quinuclidine-3 oximes: Development of butyrylcholinesterase-based bioscavengers. Arch. Toxicol. 2020, 94, 3157–3171. [Google Scholar] [CrossRef]
- Sit, R.K.; Kovarik, Z.; Maček Hrvat, N.; Zunec, S.; Green, C.; Fokin, V.V.; Barry Sharpless, K.; Radić, Z.; Taylor, P. Pharmacology, pharmacokinetics, and tissue disposition of zwitterionic hydroxyiminoacetamido alkylamines as reactivating antidotes for organophosphate exposure. J. Pharmacol. Exp. Ther. 2018, 367, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Gorecki, L.; Gerlits, O.; Kong, X.; Cheng, X.; Blumenthal, D.K.; Taylor, P.; Ballatore, C.; Kovalevsky, A.; Radić, Z. Rational design, synthesis, and evaluation of uncharged, “smart” bis-oxime antidotes of organophosphate-inhibited human acetylcholinesterase. J. Biol. Chem. 2020, 295, 4079–4092. [Google Scholar] [CrossRef] [PubMed]
- Gorecki, L.; Markova, A.; Hepnarova, V.; Zivna, N.; Junova, L.; Hrabinova, M.; Janousek, J.; Kobrlova, T.; Prchal, L.; Jun, D.; et al. Uncharged mono- and bisoximes: In search of a zwitterion to countermeasure organophosphorus intoxication. Chem. Biol. Interact. 2024, 394, 110941. [Google Scholar] [CrossRef]
- Saxena, A.; Redman, A.M.G.; Jiang, X.; Lockridge, O.; Doctor, B.P. Differences in active-site gorge dimensions of cholinesterases revealed by binding of inhibitors to human butyrylcholinesterase. Chem. Biol. Interact. 1999, 119–120, 61–69. [Google Scholar] [CrossRef]
- Pehar, V.; Kolić, D.; Zandona, A.; Šinko, G.; Katalinić, M.; Stepanić, V.; Kovarik, Z. Selected herbicides screened for toxicity and analysed as inhibitors of both cholinesterases. Chem. Biol. Interact. 2023, 379, 110506. [Google Scholar] [CrossRef] [PubMed]
- Matošević, A.; Opsenica, D.M.; Spasić, M.; Maraković, N.; Zandona, A.; Žunec, S.; Bartolić, M.; Kovarik, Z.; Bosak, A. Evaluation of 4-aminoquinoline derivatives with an N-octylamino spacer as potential multi-targeting ligands for the treatment of Alzheimer’s disease. Chem. Biol. Interact. 2023, 382, 110620. [Google Scholar] [CrossRef] [PubMed]
- Šinko, G.; Kovarik, Z.; Reiner, E.; Simeon-Rudolf, V.; Stojan, J. Mechanism of stereoselective interaction between butyrylcholinesterase and ethopropazine enantiomers. Biochimie 2011, 93, 1797–1807. [Google Scholar] [CrossRef] [PubMed]
- Mlakić, M.; Čadež, T.; Barić, D.; Puček, I.; Ratković, A.; Marinić, Ž.; Lasić, K.; Kovarik, Z.; Škorić, I. New Uncharged 2-thienostilbene oximes as reactivators of organophosphate-inhibited cholinesterases. Pharmaceuticals 2021, 14, 1147. [Google Scholar] [CrossRef] [PubMed]
- Ekström, F.; Akfur, C.; Tunemalm, A.K.; Lundberg, S. Structural changes of phenylalanine 338 and histidine 447 revealed by the crystal structures of tabun-inhibited murine acetylcholinesterase. Biochemistry 2006, 45, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Musilova, L.; Kuča, K.; Jung, Y.S.; Jun, D. In vitro oxime-assisted reactivation of paraoxon-inhibited human acetylcholinesterase and butyrylcholinesterase. Clin. Toxicol. 2009, 47, 545–550. [Google Scholar] [CrossRef] [PubMed]
- Kovarik, Z.; Katalinić, M.; Šinko, G.; Binder, J.; Holas, O.; Jung, Y.S.; Musilova, L.; Jun, D.; Kuča, K. Pseudo-catalytic scavenging: Searching for a suitable reactivator of phosphorylated butyrylcholinesterase. Chem. Biol. Interact. 2010, 187, 167–171. [Google Scholar] [CrossRef]
- Radić, Z.; Dale, T.; Kovarik, Z.; Berend, S.; Garcia, E.; Zhang, L.; Amitai, G.; Green, C.; Radić, B.; Duggan, B.M.; et al. Catalytic detoxification of nerve agent and pesticide organophosphates by butyrylcholinesterase assisted with non-pyridinium oximes. Biochem. J. 2013, 450, 231–242. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, Y.J.; Wang, J.; Ooms, T.; Rajendran, N.; Mao, L.; Jiang, X.; Lees, J.; Urban, L.; Momper, J.D.; Sepulveda, Y.; et al. Post-exposure treatment with the oxime RS194B rapidly reactivates and reverses advanced symptoms of lethal inhaled paraoxon in macaques. Toxicol. Lett. 2018, 293, 229–234. [Google Scholar] [CrossRef]
- Katalinić, M.; Zandona, A.; Ramić, A.; Zorbaz, T.; Primožič, I.; Kovarik, Z. New cinchona oximes evaluated as reactivators of acetylcholinesterase and butyrylcholinesterase inhibited by organophosphorus compounds. Molecules 2017, 22, 1234. [Google Scholar] [CrossRef]
- Shevtsova, E.F.; Maltsev, A.V.; Vinogradova, D.V.; Shevtsov, P.N.; Bachurin, S.O. Mitochondria as a promising target for developing novel agents for treating Alzheimer’s disease. Med. Res. Rev. 2021, 41, 803–827. [Google Scholar] [CrossRef] [PubMed]
- Rupprecht, R.; Papadopoulos, V.; Rammes, G.; Baghai, T.C.; Fan, J.; Akula, N.; Groyer, G.; Adams, D.; Schumacher, M. Translocator protein (18 kDa) (TSPO) as a therapeutic target for neurological and psychiatric disorders. Nat. Rev. Drug Discov. 2010, 9, 971–988. [Google Scholar] [CrossRef] [PubMed]
- Caisberger, F.; Pejchal, J.; Misik, J.; Kassa, J.; Valis, M.; Kuča, K. The benefit of combinations of oximes for the ability of antidotal treatment to counteract sarin-induced brain damage in rats. BMC Pharmacol. Toxicol. 2018, 19, 35. [Google Scholar] [CrossRef] [PubMed]
- Bohnert, S.; van den Berg, R.M.; Mikler, J.; Klaassen, S.D.; Joosen, M.J.A. Pharmacokinetics of three oximes in a guinea pig model and efficacy of combined oxime therapy. Toxicol. Lett. 2020, 324, 86–94. [Google Scholar] [CrossRef] [PubMed]
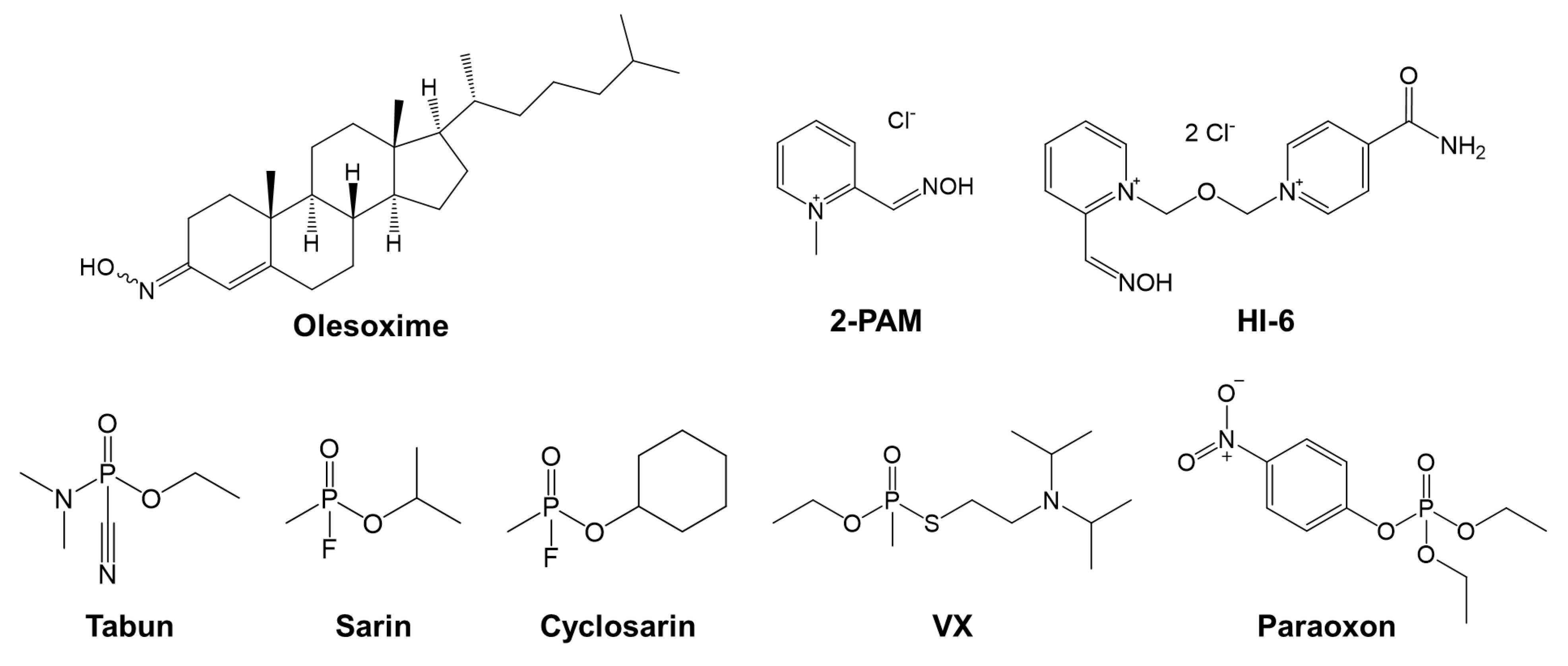
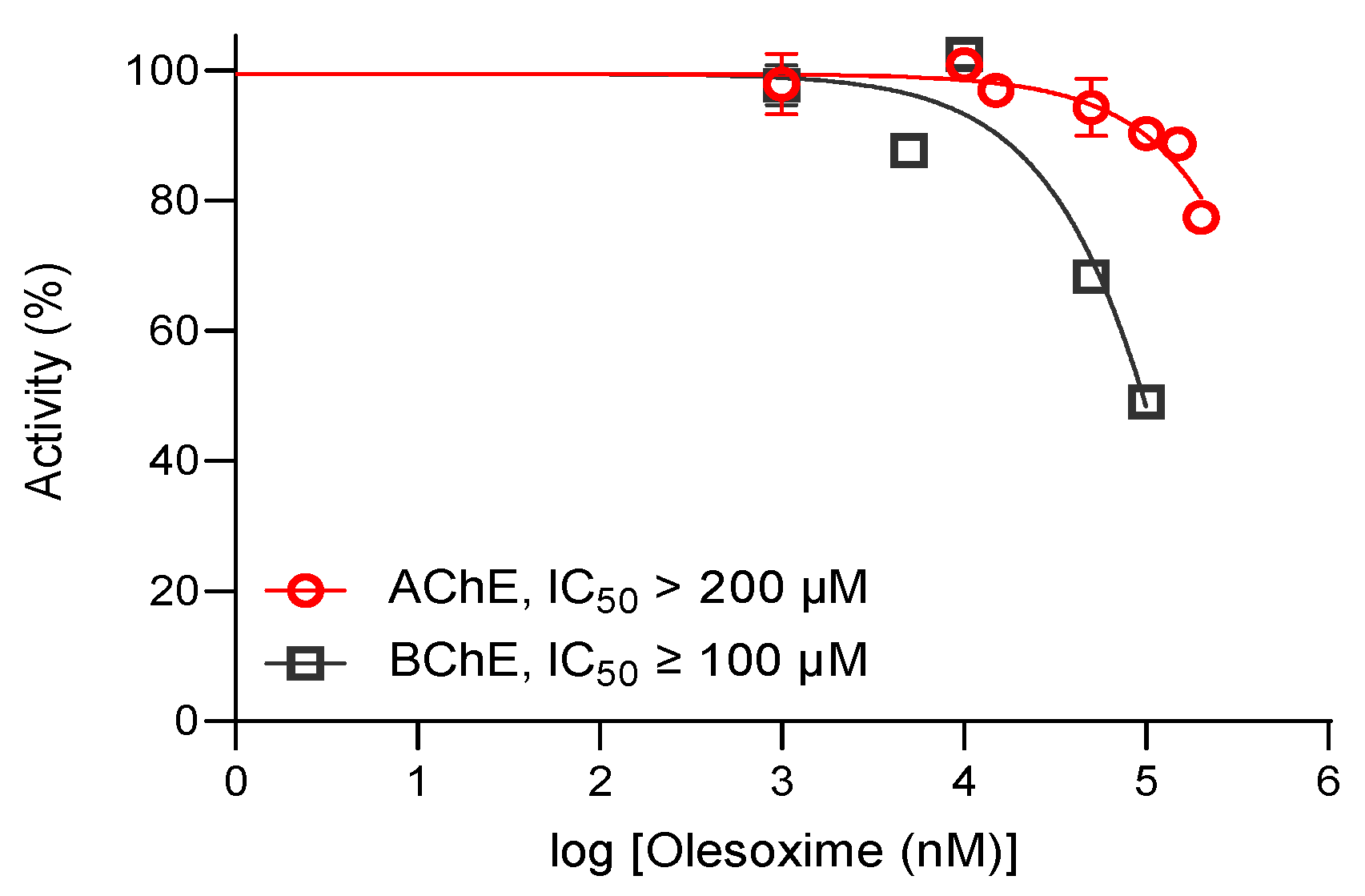
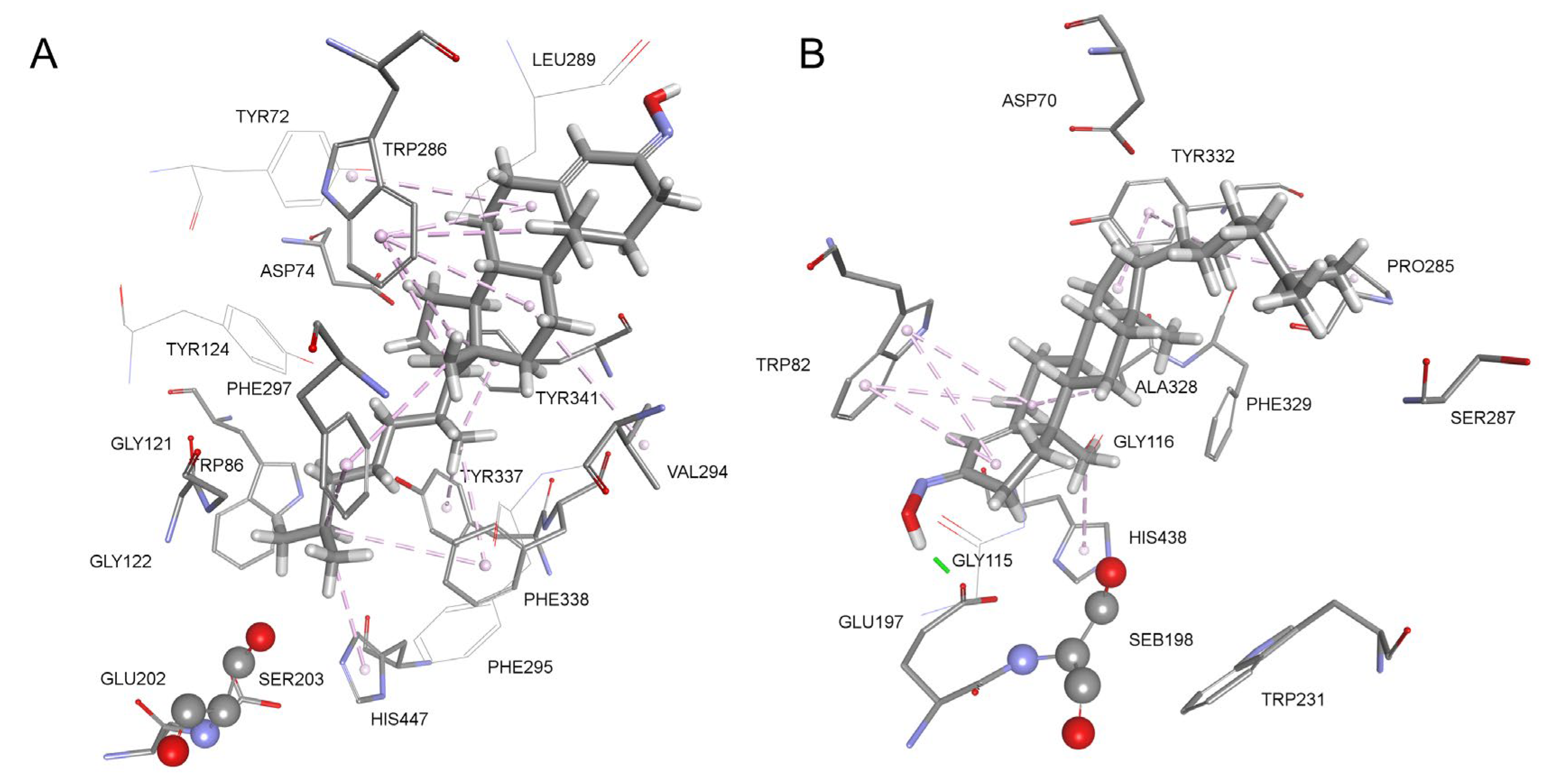

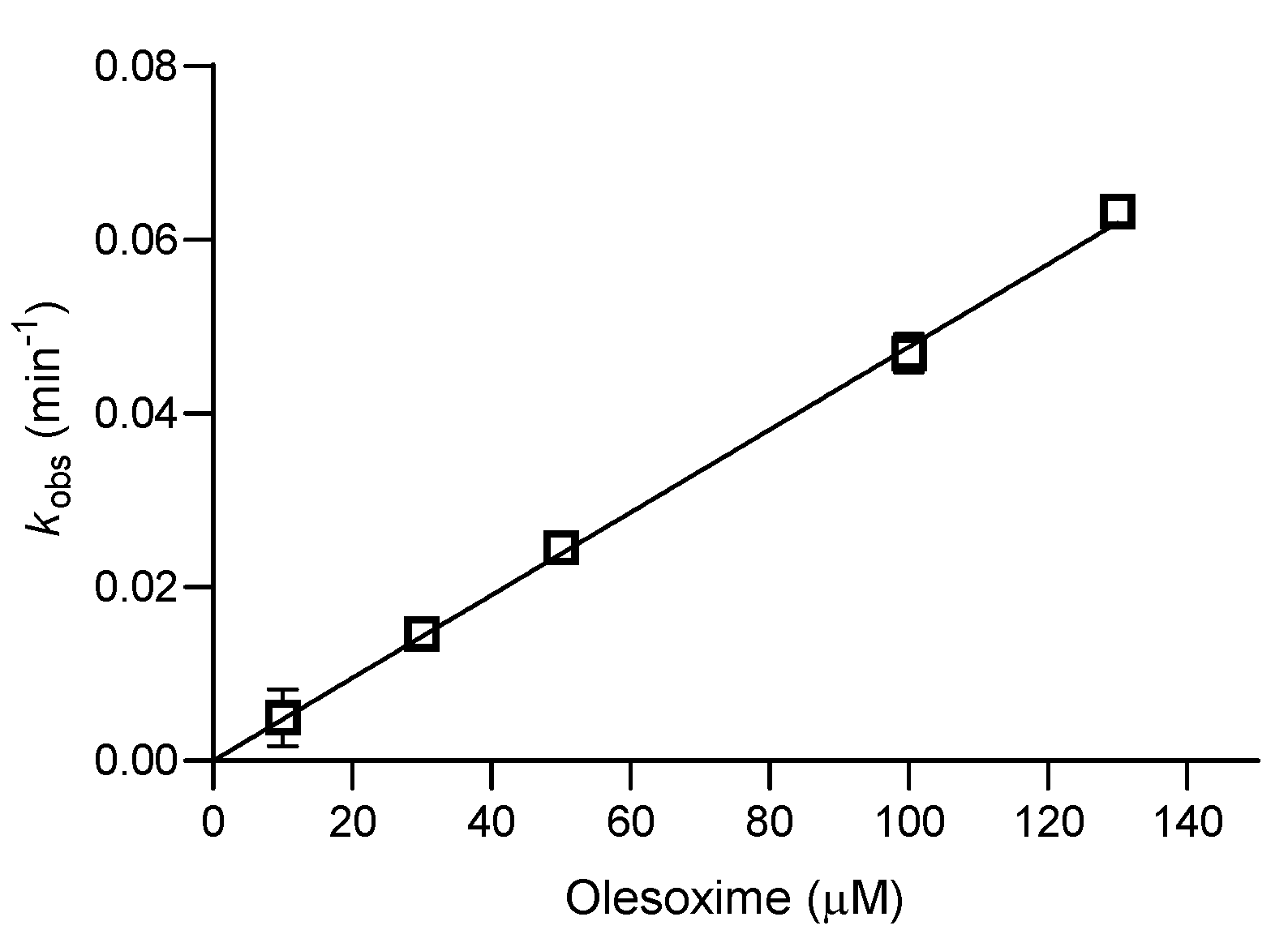

| Oxime | k2/min−1 | KOX/µM | kr/M−1 min−1 | Reactmax/% | t/h |
|---|---|---|---|---|---|
| Olesoxime * | / | / | 477 ± 12 | 70 | 3 |
| 2-PAM [35] | 0.08 ± 0.01 | 1200 ± 290 | 65 ± 10 | 80 | 1 |
| HI-6 * [21] | / | / | 780 ± 30 | 90 | 0.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kolić, D.; Šinko, G.; Jean, L.; Chioua, M.; Dias, J.; Marco-Contelles, J.; Kovarik, Z. Cholesterol Oxime Olesoxime Assessed as a Potential Ligand of Human Cholinesterases. Biomolecules 2024, 14, 588. https://doi.org/10.3390/biom14050588
Kolić D, Šinko G, Jean L, Chioua M, Dias J, Marco-Contelles J, Kovarik Z. Cholesterol Oxime Olesoxime Assessed as a Potential Ligand of Human Cholinesterases. Biomolecules. 2024; 14(5):588. https://doi.org/10.3390/biom14050588
Chicago/Turabian StyleKolić, Dora, Goran Šinko, Ludovic Jean, Mourad Chioua, José Dias, José Marco-Contelles, and Zrinka Kovarik. 2024. "Cholesterol Oxime Olesoxime Assessed as a Potential Ligand of Human Cholinesterases" Biomolecules 14, no. 5: 588. https://doi.org/10.3390/biom14050588
APA StyleKolić, D., Šinko, G., Jean, L., Chioua, M., Dias, J., Marco-Contelles, J., & Kovarik, Z. (2024). Cholesterol Oxime Olesoxime Assessed as a Potential Ligand of Human Cholinesterases. Biomolecules, 14(5), 588. https://doi.org/10.3390/biom14050588










