Estetrol Inhibits Endometriosis Development in an In Vivo Murine Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Handling
2.2. Endometriosis Surgical Induction
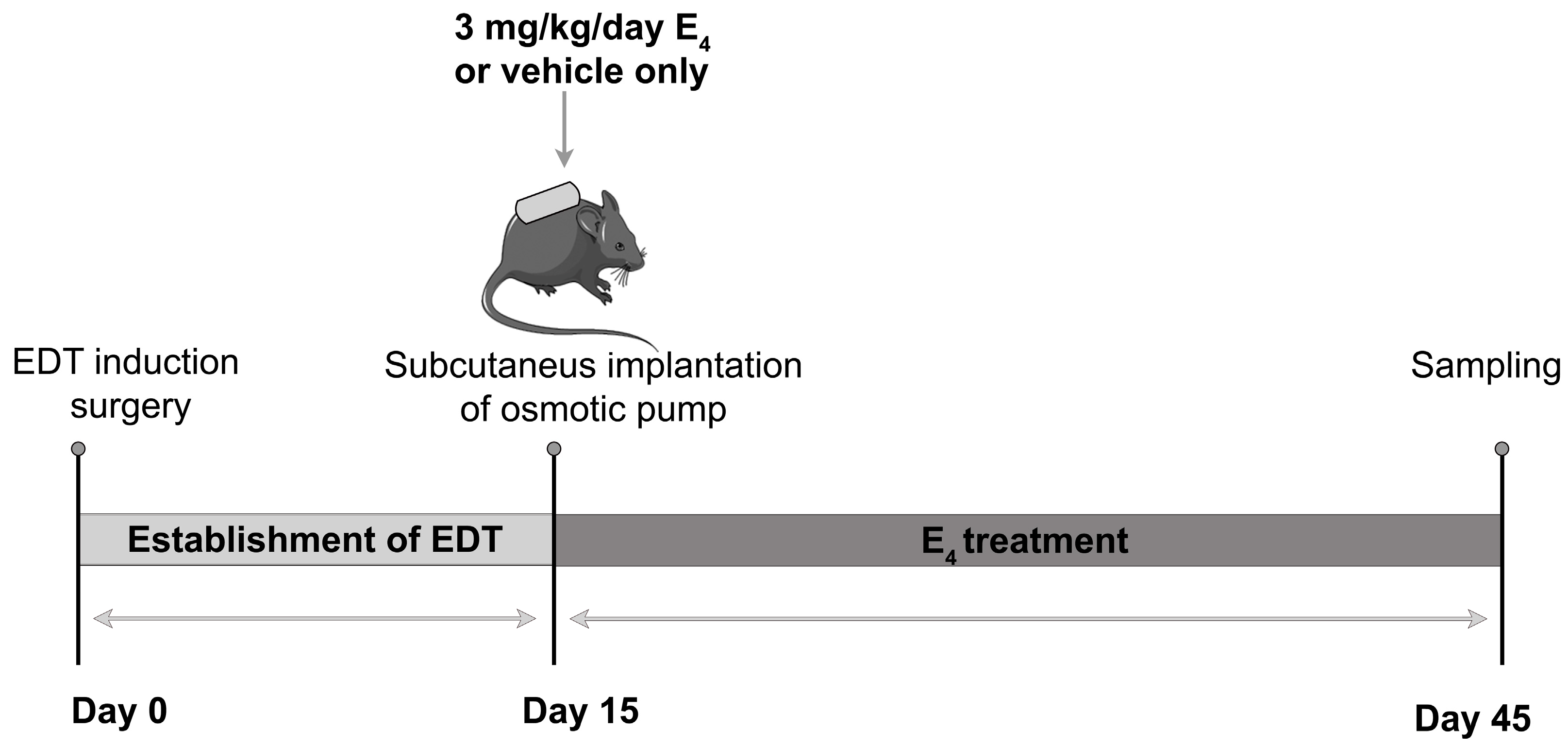
2.3. Experimental Design and Sampling of Biological Materials
2.4. Macroscopic Study of Endometriotic-like Lesions
2.5. Proliferating Cell Nuclear Antigen (PCNA) Immunohistochemistry
2.6. Terminal Deoxynucleotidyl Transferase-Mediated Nick End Labeling (TUNEL) Assay
2.7. Antioxidant Enzyme Activities
2.8. Measurement of TBARS-MDA
2.9. Enzyme-Linked Immunosorbent Assay (ELISA)
2.10. RNA Extraction and Quantitative Reverse Transcription Polymerase Chain Reaction (RT-qPCR)
2.11. Statistical Analysis
3. Results
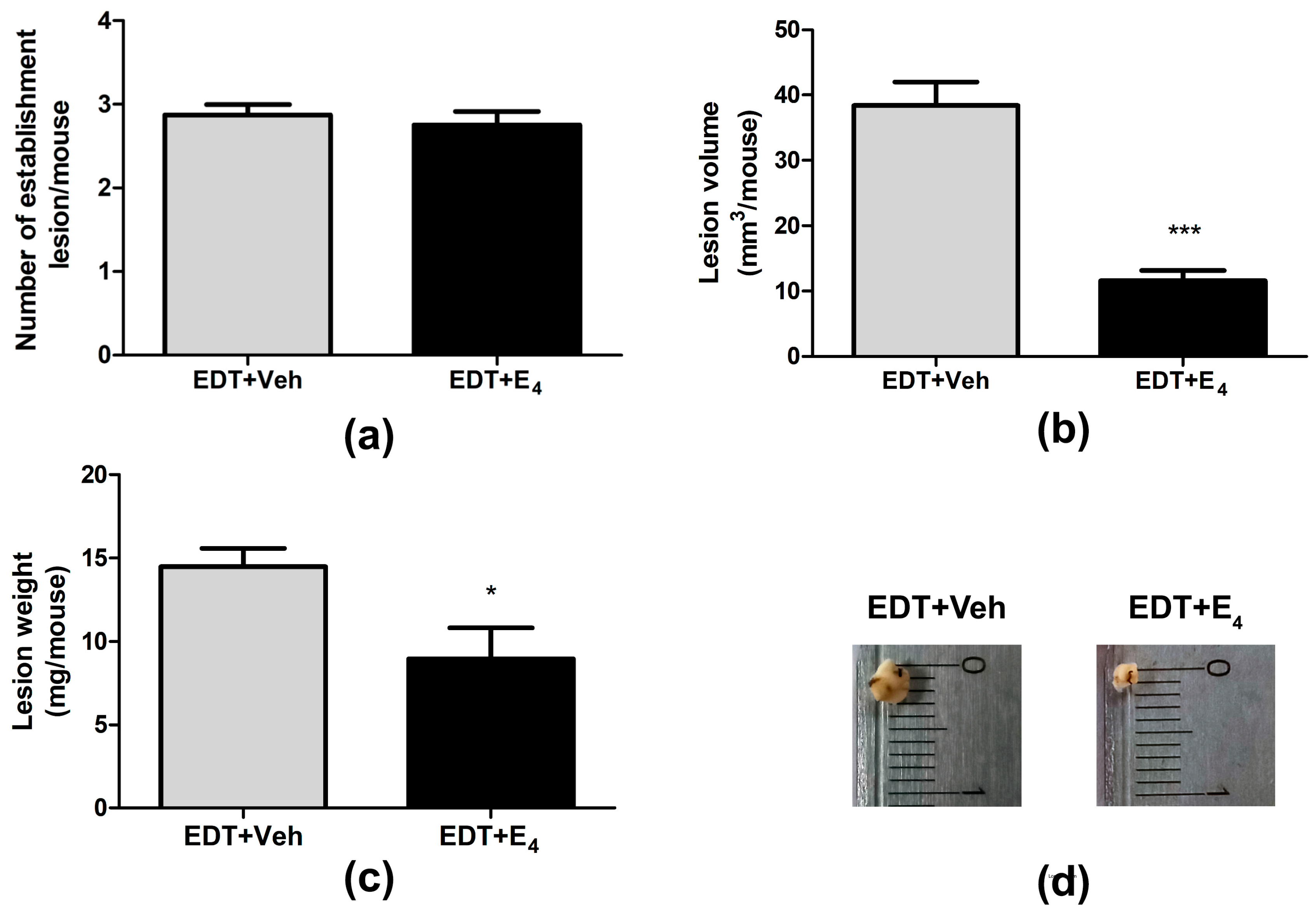
3.1. Effect of E4 on the Growth of Endometriotic-like Lesions
3.2. Effect of E4 on Cell Proliferating in Endometriotic-like Lesions
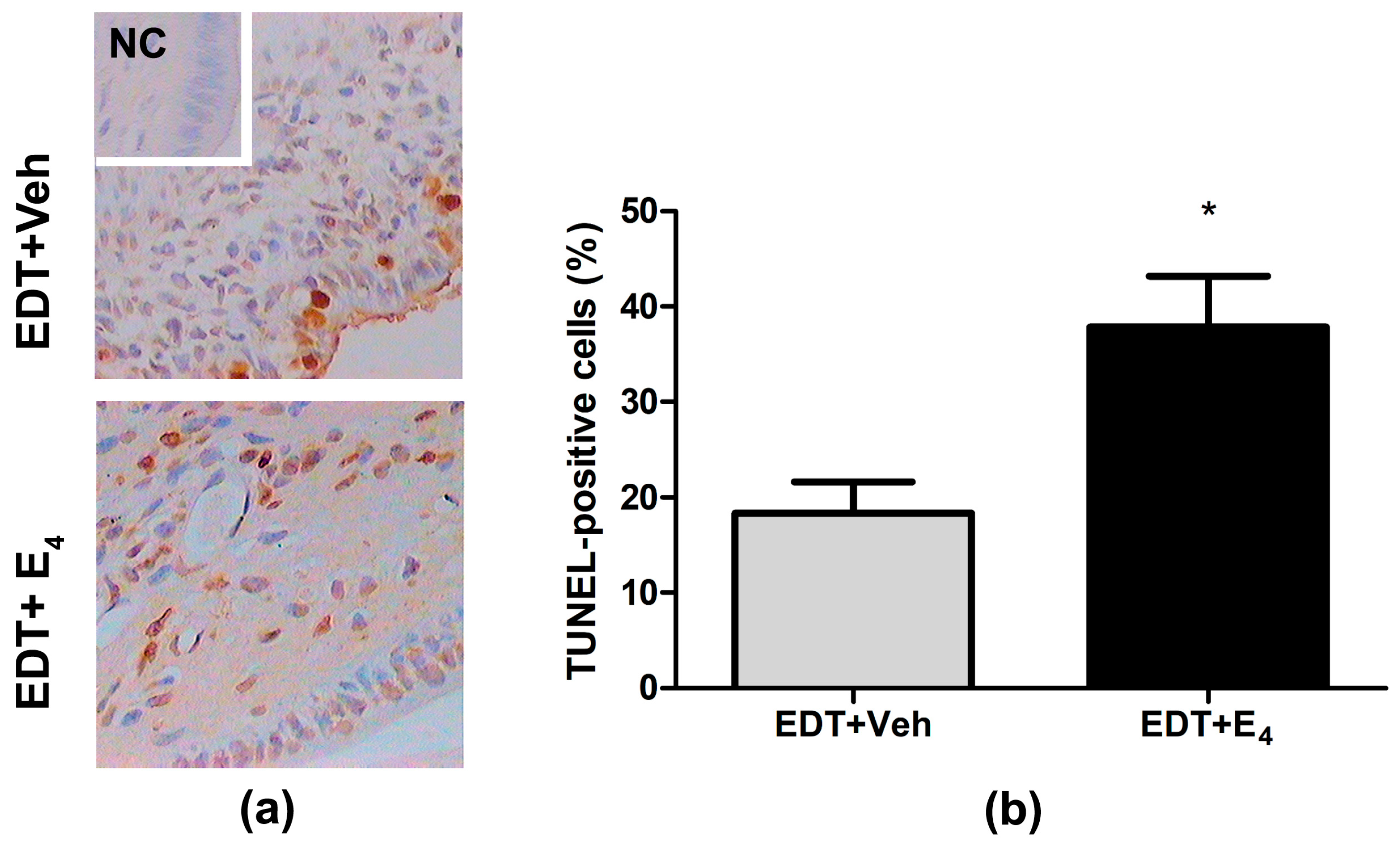
3.3. Effect of E4 on Cell Death in Endometriotic-like Lesions
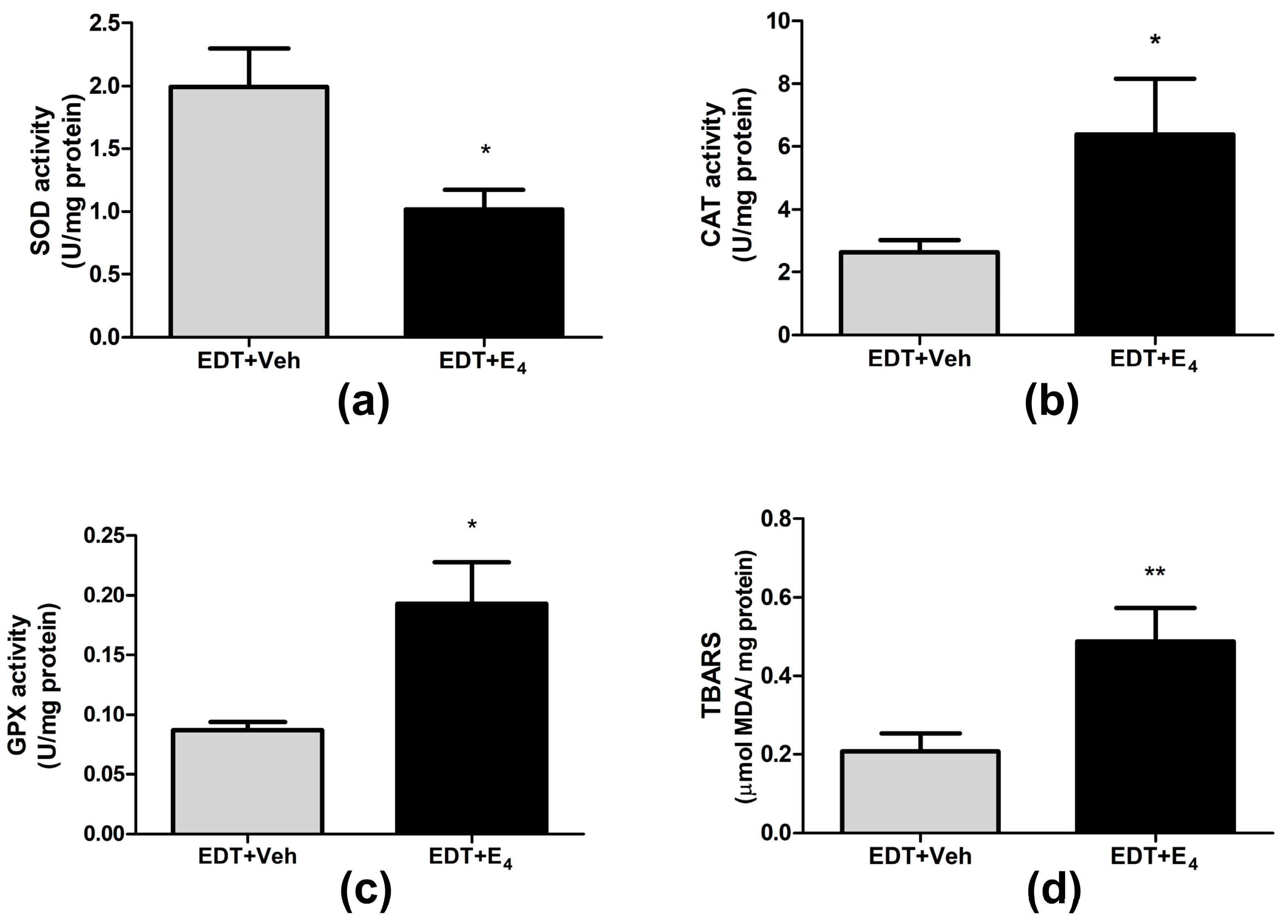
3.4. Effect of E4 on Oxidative Stress in Endometriotic-like Lesions
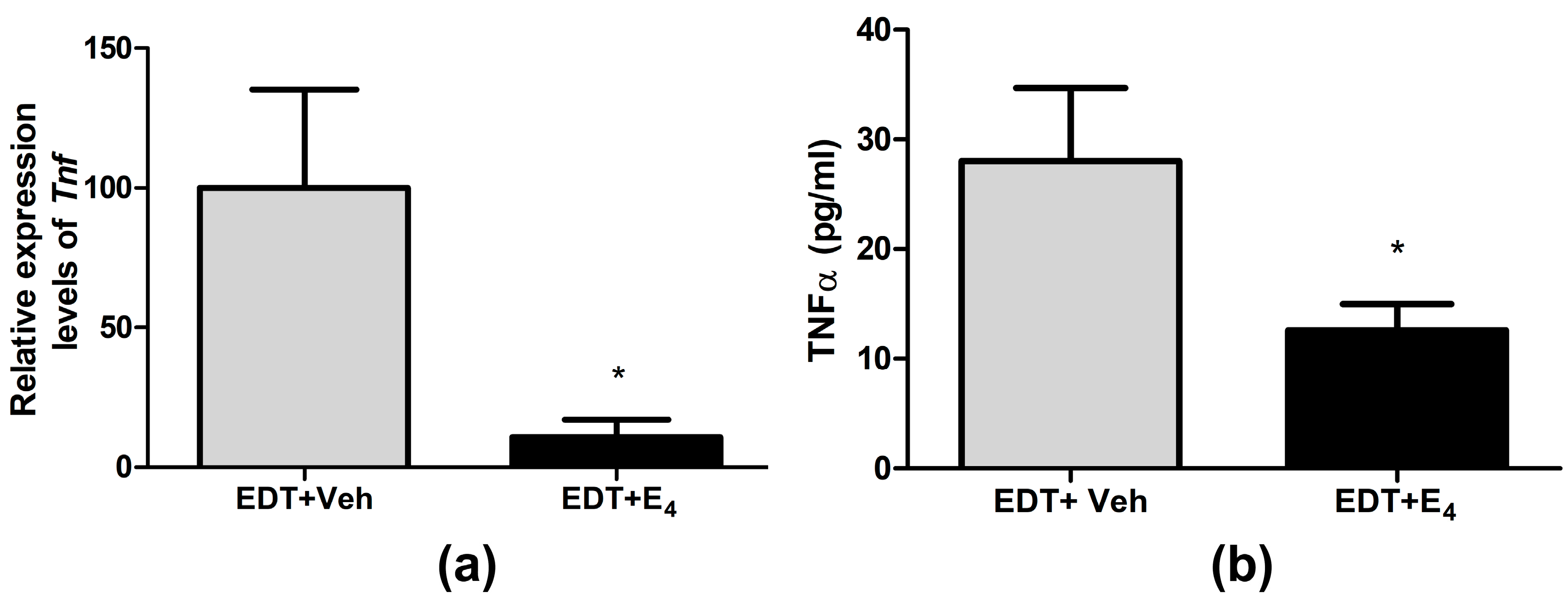
3.5. Effect of E4 on TNF-α Expression in Endometriotic-like Lesions and Peritoneal Fluid
3.6. Effect of E4 on Esr2, Esr1, and Pgr mRNA Expression in Endometriotic-like Lesions
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Smolarz, B.; Szyłło, K.; Romanowicz, H. Endometriosis: Epidemiology, Classification, Pathogenesis, Treatment and Genetics (Review of Literature). Int. J. Mol. Sci. 2021, 22, 10554. [Google Scholar] [CrossRef]
- Kobayashi, H.; Kimura, M.; Maruyama, S.; Nagayasu, M.; Imanaka, S. Revisiting Estrogen-Dependent Signaling Pathways in Endometriosis: Potential Targets for Non-Hormonal Therapeutics. Eur. J. Obstet. Gynecol. Reprod. Biol. 2021, 258, 103–110. [Google Scholar] [CrossRef]
- Lamceva, J.; Uljanovs, R.; Strumfa, I. The Main Theories on the Pathogenesis of Endometriosis. Int. J. Mol. Sci. 2023, 24, 4254. [Google Scholar] [CrossRef] [PubMed]
- Huhtinen, K.; Desai, R.; Ståhle, M.; Salminen, A.; Handelsman, D.J.; Perheentupa, A.; Poutanen, M. Endometrial and Endometriotic Concentrations of Estrone and Estradiol Are Determined by Local Metabolism Rather than Circulating Levels. J. Clin. Endocrinol. Metab. 2012, 97, 4228–4235. [Google Scholar] [CrossRef]
- Han, S.J.; Jung, S.Y.; Wu, S.-P.; Hawkins, S.M.; Park, M.J.; Kyo, S.; Qin, J.; Lydon, J.P.; Tsai, S.Y.; Tsai, M.-J.; et al. Estrogen Receptor β Modulates Apoptosis Complexes and the Inflammasome to Drive the Pathogenesis of Endometriosis. Cell 2015, 163, 960–974. [Google Scholar] [CrossRef]
- Vannuccini, S.; Clemenza, S.; Rossi, M.; Petraglia, F. Hormonal Treatments for Endometriosis: The Endocrine Background. Rev. Endocr. Metab. Disord. 2022, 23, 333–355. [Google Scholar] [CrossRef]
- Bulun, S.E.; Monsavais, D.; Pavone, M.E.; Dyson, M.; Xue, Q.; Attar, E.; Tokunaga, H.; Su, E.J. Role of Estrogen Receptor-β in Endometriosis. Semin. Reprod. Med. 2012, 30, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Grandi, G.; Barra, F.; Ferrero, S.; Sileo, F.G.; Bertucci, E.; Napolitano, A.; Facchinetti, F. Hormonal Contraception in Women with Endometriosis: A Systematic Review. Eur. J. Contracept. Reprod. Health Care 2019, 24, 61–70. [Google Scholar] [CrossRef]
- Mørch, L.S.; Skovlund, C.W.; Hannaford, P.C.; Iversen, L.; Fielding, S.; Lidegaard, Ø. Contemporary Hormonal Contraception and the Risk of Breast Cancer. N. Engl. J. Med. 2017, 377, 2228–2239. [Google Scholar] [CrossRef]
- Stanczyk, F.Z.; Archer, D.F. Biosynthesis of Estetrol in Human Pregnancy: Potential Pathways. J. Steroid Biochem. Mol. Biol. 2023, 232, 106359. [Google Scholar] [CrossRef]
- Visser, M.; Foidart, J.M.; Coelingh Bennink, H.J.T. In Vitro Effects of Estetrol on Receptor Binding, Drug Targets and Human Liver Cell Metabolism. Climacteric 2008, 11 (Suppl. 1), 64–68. [Google Scholar] [CrossRef] [PubMed]
- Křepelka, P. Estetrol and the Possibilities of Its Clinical Use. Ceska Gynekol. 2021, 86, 217–221. [Google Scholar] [CrossRef]
- Gérard, C.; Arnal, J.F.; Jost, M.; Douxfils, J.; Lenfant, F.; Fontaine, C.; Houtman, R.; Archer, D.F.; Reid, R.L.; Lobo, R.A.; et al. Profile of Estetrol, a Promising Native Estrogen for Oral Contraception and the Relief of Climacteric Symptoms of Menopause. Expert Rev. Clin. Pharmacol. 2022, 15, 121–137. [Google Scholar] [CrossRef]
- Coelingh Bennink, H.J.T.; Holinka, C.F.; Diczfalusy, E. Estetrol Review: Profile and Potential Clinical Applications. Climacteric 2008, 11, 47–58. [Google Scholar] [CrossRef]
- Fruzzetti, F.; Fidecicchi, T.; Montt Guevara, M.M.; Simoncini, T. Estetrol: A New Choice for Contraception. J. Clin. Med. 2021, 10, 5625. [Google Scholar] [CrossRef] [PubMed]
- Douxfils, J.; Morimont, L.; Gaspard, U.; Utian, W.H.; Foidart, J.M. Estetrol Is Not a SERM but a NEST and Has a Specific Safety Profile on Coagulation. Thromb. Res. 2023, 232, 148–150. [Google Scholar] [CrossRef]
- Foidart, J.M.; Gaspard, U.; Pequeux, C.; Jost, M.; Gordenne, V.; Tskitishvili, E.; Gallez, A.; Valera, M.C.; Gourdy, P.; Fontaine, C.; et al. Unique Vascular Benefits of Estetrol, a Native Fetal Estrogen with Specific Actions in Tissues (NEST); Springer: Cham, Switzerland, 2019; pp. 169–195. [Google Scholar]
- Patiño-García, D.; Palomino, J.; Pomés, C.; Celle, C.; Torres-Estay, V.; Orellana, R. Estetrol Increases Progesterone Genetic Response without Triggering Common Estrogenic Effects in Endometriotic Cell Lines and Primary Cultures. Biomedicines 2023, 11, 1169. [Google Scholar] [CrossRef]
- Vallcaneras, S.; Ghersa, F.; Bastón, J.; Delsouc, M.B.; Meresman, G.; Casais, M. TNFRp55 Deficiency Promotes the Development of Ectopic Endometriotic-like Lesions in Mice. J. Endocrinol. 2017, 234, 269–278. [Google Scholar] [CrossRef]
- Delsouc, M.B.; Ghersa, F.; Ramírez, D.; Della Vedova, M.C.; Gil, R.A.; Vallcaneras, S.S.; Casais, M. Endometriosis Progression in Tumor Necrosis Factor Receptor P55-Deficient Mice: Impact on Oxidative/Nitrosative Stress and Metallomic Profile. J. Trace Elem. Med. Biol. 2019, 52, 157–165. [Google Scholar] [CrossRef]
- Conforti, R.A.; Delsouc, M.B.; Zabala, A.S.; Vallcaneras, S.S.; Casais, M. The Copper Chelator Ammonium Tetrathiomolybdate Inhibits the Progression of Experimental Endometriosis in TNFR1-Deficient Mice. Sci. Rep. 2023, 13, 10354. [Google Scholar] [CrossRef]
- Pelch, K.E.; Schroder, A.L.; Kimball, P.A.; Sharpe-Timms, K.L.; Davis, J.W.; Nagel, S.C. Aberrant Gene Expression Profile in a Mouse Model of Endometriosis Mirrors That Observed in Women. Fertil. Steril. 2010, 93, 1615–1627.e18. [Google Scholar] [CrossRef] [PubMed]
- Burns, K.A.; Pearson, A.M.; Slack, J.L.; Por, E.D.; Scribner, A.N.; Eti, N.A.; Burney, R.O. Endometriosis in the Mouse: Challenges and Progress Toward a ‘Best Fit’ Murine Model. Front. Physiol. 2022, 12, 806574. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Li, Q.; Katzenellenbogen, B.S.; Lau, L.F.; Taylor, R.N.; Bagchi, I.C.; Bagchi, M.K. Estrogen-Induced CCN1 Is Critical for Establishment of Endometriosis-like Lesions in Mice. Mol. Endocrinol. 2014, 28, 1934–1947. [Google Scholar] [CrossRef] [PubMed]
- Mc Cormack, B.A.; Olivares, C.N.; Madanes, D.; Ricci, A.G.; Bilotas, M.A.; Barañao, R.I. Effect of Urolithins A and B on Ectopic Endometrial Growth in a Murine Model of Endometriosis. Food Funct. 2021, 12, 9894–9903. [Google Scholar] [CrossRef] [PubMed]
- Gallez, A.; Nys, G.; Wuidar, V.; Dias Da Silva, I.; Taziaux, M.; Kinet, V.; Tskitishvili, E.; Noel, A.; Foidart, J.-M.; Piel, G.; et al. Comparison of Estetrol Exposure between Women and Mice to Model Preclinical Experiments and Anticipate Human Treatment. Int. J. Mol. Sci. 2023, 24, 9718. [Google Scholar] [CrossRef] [PubMed]
- Gérard, C.; Blacher, S.; Communal, L.; Courtin, A.; Tskitishvili, E.; Mestdagt, M.; Munaut, C.; Noel, A.; Gompel, A.; Péqueux, C.; et al. Estetrol Is a Weak Estrogen Antagonizing Estradiol-Dependent Mammary Gland Proliferation. J. Endocrinol. 2015, 224, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Visser, M.; Coelingh Bennink, H.J.T. Clinical Applications for Estetrol. J. Steroid Biochem. Mol. Biol. 2009, 114, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Benoit, T.; Valera, M.-C.; Fontaine, C.; Buscato, M.; Lenfant, F.; Raymond-Letron, I.; Tremollieres, F.; Soulie, M.; Foidart, J.-M.; Game, X.; et al. Estetrol, a Fetal Selective Estrogen Receptor Modulator, Acts on the Vagina of Mice through Nuclear Estrogen Receptor α Activation. Am. J. Pathol. 2017, 187, 2499–2507. [Google Scholar] [CrossRef] [PubMed]
- Abot, A.; Fontaine, C.; Buscato, M.; Solinhac, R.; Flouriot, G.; Fabre, A.; Drougard, A.; Rajan, S.; Laine, M.; Milon, A.; et al. The Uterine and Vascular Actions of Estetrol Delineate a Distinctive Profile of Estrogen Receptor α Modulation, Uncoupling Nuclear and Membrane Activation. EMBO Mol. Med. 2014, 6, 1328–1346. [Google Scholar] [CrossRef]
- Delsouc, M.B.; Conforti, R.A.; Vitale, D.L.; Alaniz, L.; Pacheco, P.; Andujar, S.; Vallcaneras, S.S.; Casais, M. Antiproliferative and Antiangiogenic Effects of Ammonium Tetrathiomolybdate in a Model of Endometriosis. Life Sci. 2021, 287, 120099. [Google Scholar] [CrossRef]
- Strzalka, W.; Ziemienowicz, A. Proliferating Cell Nuclear Antigen (PCNA): A Key Factor in DNA Replication and Cell Cycle Regulation. Ann. Bot. 2011, 107, 1127–1140. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Zhang, J. Research Progress of PCNA in Reproductive System Diseases. Evid.-Based Complement. Altern. Med. 2021, 2021, 2391917. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Marklund, S.; Marklund, G. Involvement of the Superoxide Anion Radical in the Autoxidation of Pyrogallol and a Convenient Assay for Superoxide Dismutase. Eur. J. Biochem. 1974, 47, 469–474. [Google Scholar] [CrossRef]
- Aebi, H. [13] Catalase in Vitro. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1984; pp. 121–126. [Google Scholar]
- [12] Assays of Glutathione Flohé, L.; Günzler, W.A. Peroxidase. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1984; pp. 114–120. [Google Scholar]
- Draper, H.H.; Hadley, M. [43] Malondialdehyde Determination as Index of Lipid Peroxidation. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1990; pp. 421–431. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Delbandi, A.-A.; Mahmoudi, M.; Shervin, A.; Heidari, S.; Kolahdouz-Mohammadi, R.; Zarnani, A.-H. Evaluation of Apoptosis and Angiogenesis in Ectopic and Eutopic Stromal Cells of Patients with Endometriosis Compared to Non-Endometriotic Controls. BMC Womens Health 2020, 20, 3. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.-L.; Chang, K.-K.; Mei, J.; Zhou, W.-J.; Liu, L.-B.; Yao, L.; Meng, Y.; Wang, M.-Y.; Ha, S.-Y.; Lai, Z.-Z.; et al. Estrogen Restricts the Apoptosis of Endometrial Stromal Cells by Promoting TSLP Secretion. Mol. Med. Rep. 2018, 18, 4410–4416. [Google Scholar] [CrossRef]
- Ngô, C.; Chéreau, C.; Nicco, C.; Weill, B.; Chapron, C.; Batteux, F. Reactive Oxygen Species Controls Endometriosis Progression. Am. J. Pathol. 2009, 175, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Braun, D.P.; Ding, J.; Dmowski, W.P. Peritoneal Fluid-Mediated Enhancement of Eutopic and Ectopic Endometrial Cell Proliferation Is Dependent on Tumor Necrosis Factor-α in Women with Endometriosis. Fertil. Steril. 2002, 78, 727–732. [Google Scholar] [CrossRef]
- Holinka, C.F.; Gurpide, E. In Vivo Effects of Estetrol on the Immature Rat Uterus. Biol. Reprod. 1979, 20, 242–246. [Google Scholar] [CrossRef]
- Visser, M.; Kloosterboer, H.J.; Bennink, H.J.T.C. Estetrol Prevents and Suppresses Mammary Tumors Induced by DMBA in a Rat Model. Horm. Mol. Biol. Clin. Investig. 2012, 9, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Wakatsuki, A.; Lin, Y.; Kojima, S.; Matsushita, H.; Takeuchi, K.; Umezawa, K. Inhibitory Effects of Estetrol on the Invasion and Migration of Immortalized Human Endometrial Stromal Cells. Endocr. J. 2024, 71, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Reis, F.M.; Petraglia, F.; Taylor, R.N. Endometriosis: Hormone Regulation and Clinical Consequences of Chemotaxis and Apoptosis. Hum. Reprod. Update 2013, 19, 406–418. [Google Scholar] [CrossRef] [PubMed]
- McCubrey, J.A.; LaHair, M.M.; Franklin, R.A. Reactive Oxygen Species-Induced Activation of the MAP Kinase Signaling Pathways. Antioxid. Redox Signal 2006, 8, 1775–1789. [Google Scholar] [CrossRef] [PubMed]
- Eleutherio, E.C.A.; Silva Magalhães, R.S.; de Araújo Brasil, A.; Monteiro Neto, J.R.; de Holanda Paranhos, L. SOD1, More than Just an Antioxidant. Arch. Biochem. Biophys. 2021, 697, 108701. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Chen, Y.; Zhao, J.; Shi, J.; Wang, M.; Qiu, S.; Hu, Y.; Xu, Y.; Cui, Y.; Liu, C.; et al. The Specific Inhibition of SOD1 Selectively Promotes Apoptosis of Cancer Cells via Regulation of the ROS Signaling Network. Oxid. Med. Cell. Longev. 2019, 2019, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Khan, K.N.; Kitajima, M.; Inoue, T.; Fujishita, A.; Nakashima, M.; Masuzaki, H. 17β-Estradiol and Lipopolysaccharide Additively Promote Pelvic Inflammation and Growth of Endometriosis. Reprod. Sci. 2015, 22, 585. [Google Scholar] [CrossRef] [PubMed]
- Iwabe, T.; Harada, T.; Tsudo, T.; Nagano, Y.; Yoshida, S.; Tanikawa, M.; Terakawa, N. Tumor Necrosis Factor-Alpha Promotes Proliferation of Endometriotic Stromal Cells by Inducing Interleukin-8 Gene and Protein Expression. J. Clin. Endocrinol. Metab. 2000, 85, 824–829. [Google Scholar] [CrossRef]
- Wang, Y.; Nicholes, K.; Shih, I.M. The Origin and Pathogenesis of Endometriosis. Annu. Rev. Pathol. Mech. Dis. 2020, 15, 71–95. [Google Scholar] [CrossRef]
- Coelingh Bennink, H.J.T.; Verhoeven, C.; Zimmerman, Y.; Visser, M.; Foidart, J.-M.; Gemzell-Danielsson, K. Clinical Effects of the Fetal Estrogen Estetrol in a Multiple-Rising-Dose Study in Postmenopausal Women. Maturitas 2016, 91, 93–100. [Google Scholar] [CrossRef]
- Creinin, M.D.; Cagnacci, A.; Spaczyński, R.Z.; Stute, P.; Chabbert-Buffet, N.; Korver, T.; Simoncini, T. Experts’ View on the Role of Oestrogens in Combined Oral Contraceptives: Emphasis on Oestetrol (E4). Front. Glob. Womens Health 2024, 5, 1395863. [Google Scholar] [CrossRef]
- Hammond, G.L.; Hogeveen, K.N.; Visser, M.; Coelingh Bennink, H.J.T. Estetrol Does Not Bind Sex Hormone Binding Globulin or Increase Its Production by Human HepG2 Cells. Climacteric 2008, 11, 41–46. [Google Scholar] [CrossRef]
- Douxfils, J.; Klipping, C.; Duijkers, I.; Kinet, V.; Mawet, M.; Maillard, C.; Jost, M.; Rosing, J.; Foidart, J.M. Evaluation of the Effect of a New Oral Contraceptive Containing Estetrol and Drospirenone on Hemostasis Parameters. Contraception 2020, 102, 396–402. [Google Scholar] [CrossRef]
- Coelingh Bennink, H.J.T.T.; Heegaard, A.-M.; Visser, M.; Holinka, C.F.; Christiansen, C. Oral Bioavailability and Bone-Sparing Effects of Estetrol in an Osteoporosis Model. Climacteric 2008, 11, 2–14. [Google Scholar] [CrossRef] [PubMed]
- Pluchino, N.; Santoro, A.N.; Casarosa, E.; Giannini, A.; Genazzani, A.; Russo, M.; Russo, N.; Petignat, P.; Genazzani, A.R. Effect of Estetrol Administration on Brain and Serum Allopregnanolone in Intact and Ovariectomized Rats. J. Steroid Biochem. Mol. Biol. 2014, 143, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Gallez, A.; Dias Da Silva, I.; Wuidar, V.; Foidart, J.-M.; Péqueux, C. Estetrol and Mammary Gland: Friends or Foes? J. Mammary Gland. Biol. Neoplasia 2021, 26, 297–308. [Google Scholar] [CrossRef] [PubMed]
- Gérard, C.; Mestdagt, M.; Tskitishvili, E.; Communal, L.; Gompel, A.; Silva, E.; Arnal, J.-F.; Lenfant, F.; Noel, A.; Foidart, J.-M.; et al. Combined Estrogenic and Anti-Estrogenic Properties of Estetrol on Breast Cancer May Provide a Safe Therapeutic Window for the Treatment of Menopausal Symptoms. Oncotarget 2015, 6, 17621–17636. [Google Scholar] [CrossRef]
- Giretti, M.S.; Montt Guevara, M.M.; Cecchi, E.; Mannella, P.; Palla, G.; Spina, S.; Bernacchi, G.; Di Bello, S.; Genazzani, A.R.; Genazzani, A.D.; et al. Effects of Estetrol on Migration and Invasion in T47-D Breast Cancer Cells through the Actin Cytoskeleton. Front. Endocrinol. 2014, 5, 80. [Google Scholar] [CrossRef]
- Holinka, C.F.; Brincat, M.; Coelingh Bennink, H.J.T. Preventive Effect of Oral Estetrol in a Menopausal Hot Flush Model. Climacteric 2008, 11, 15–21. [Google Scholar] [CrossRef]
- Bulun, S.E.; Yilmaz, B.D.; Sison, C.; Miyazaki, K.; Bernardi, L.; Liu, S.; Kohlmeier, A.; Yin, P.; Milad, M.; Wei, J.J. Endometriosis. Endocr. Rev. 2019, 40, 1048–1079. [Google Scholar] [CrossRef]
- Coelingh Bennink, H.J.T.; Skouby, S.; Bouchard, P.; Holinka, C.F. Ovulation Inhibition by Estetrol in an in Vivo Model. Contraception 2008, 77, 186–190. [Google Scholar] [CrossRef] [PubMed]


| Gene | GenBank Access Number | Primer Sequences (5′–3′) | Amplicon (bp) |
|---|---|---|---|
| Esr1 | NM_001302532.1 | F: CTTGGAAGGCCGAAATGAAATG R: GGCAGGGCTATTCTTCTTAGTG | 103 |
| Esr2 | NM_207707.1 | F: CTGGGTATCATTACGGTGTCTG R: GATTCGTGGCTGGACAGATATAG | 99 |
| Pgr | NM_008829.2 | F: CCTGACACTTCCAGCTCTTT R: CGGAAACCTGGCAGAGATTTA | 100 |
| Tnf | NM_001278601.1 | F: CTACCTTGTTGCCTCCTCTTT R: GAGCAGAGGTTCAGTGATGTAG | 116 |
| Rn18s | NR_003278.3 | F: CTGAGAAACGGCTACCACATC R: GCCTCGAAAGAGTCCTGTATTG | 107 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zabala, A.S.; Conforti, R.A.; Delsouc, M.B.; Filippa, V.; Montt-Guevara, M.M.; Giannini, A.; Simoncini, T.; Vallcaneras, S.S.; Casais, M. Estetrol Inhibits Endometriosis Development in an In Vivo Murine Model. Biomolecules 2024, 14, 580. https://doi.org/10.3390/biom14050580
Zabala AS, Conforti RA, Delsouc MB, Filippa V, Montt-Guevara MM, Giannini A, Simoncini T, Vallcaneras SS, Casais M. Estetrol Inhibits Endometriosis Development in an In Vivo Murine Model. Biomolecules. 2024; 14(5):580. https://doi.org/10.3390/biom14050580
Chicago/Turabian StyleZabala, Ana Sofia, Rocío Ayelem Conforti, María Belén Delsouc, Verónica Filippa, Maria Magdalena Montt-Guevara, Andrea Giannini, Tommaso Simoncini, Sandra Silvina Vallcaneras, and Marilina Casais. 2024. "Estetrol Inhibits Endometriosis Development in an In Vivo Murine Model" Biomolecules 14, no. 5: 580. https://doi.org/10.3390/biom14050580
APA StyleZabala, A. S., Conforti, R. A., Delsouc, M. B., Filippa, V., Montt-Guevara, M. M., Giannini, A., Simoncini, T., Vallcaneras, S. S., & Casais, M. (2024). Estetrol Inhibits Endometriosis Development in an In Vivo Murine Model. Biomolecules, 14(5), 580. https://doi.org/10.3390/biom14050580







