Mitochondrial Transplantation’s Role in Rodent Skeletal Muscle Bioenergetics: Recharging the Engine of Aging
Abstract
1. Introduction
2. Materials and Methods
2.1. Overall Experimental Design
2.2. Animals and Housing
2.3. Treadmill Test
2.4. Mitochondrial Transplantation Protocol
2.5. Harvesting and Preparation of Tissues
2.6. Cytochrome c Oxidase (CcO) Activity and ATP Concentrations
2.7. Muscle Homogenization and Mitochondrial Enrichment for Blue Native PAGE (BN-PAGE)
2.8. Mitochondrial Membrane Solubilization for BN-PAGE
2.9. Mitochondrial Native Protein In-Gel Activity Assay (IGA)
2.10. Citrate Synthase Activity
2.11. Western Blot
2.12. Epigenetic Analyses
2.13. Statistical Analyses
3. Results
3.1. Anthropometrics
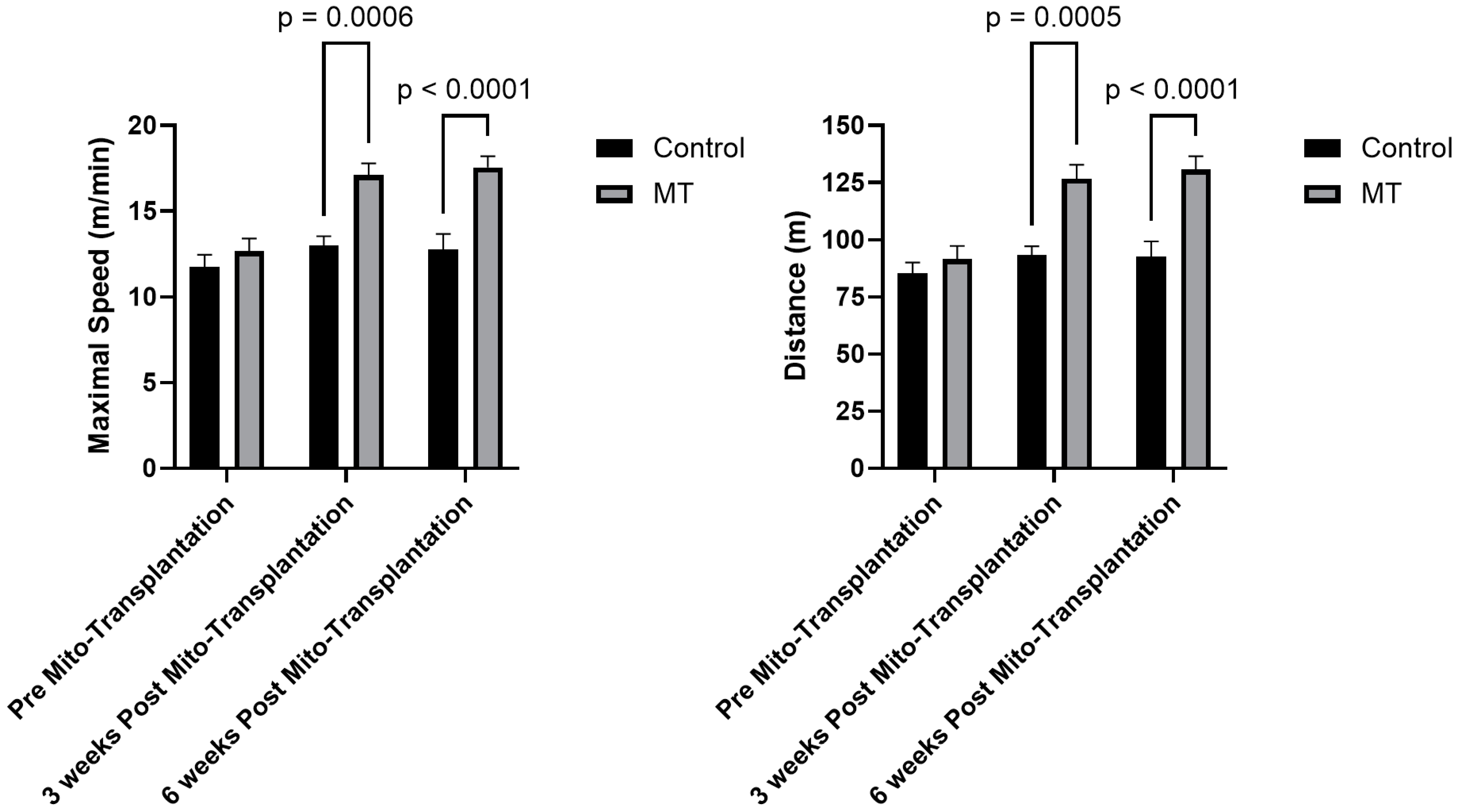
3.2. Exercise Tolerance
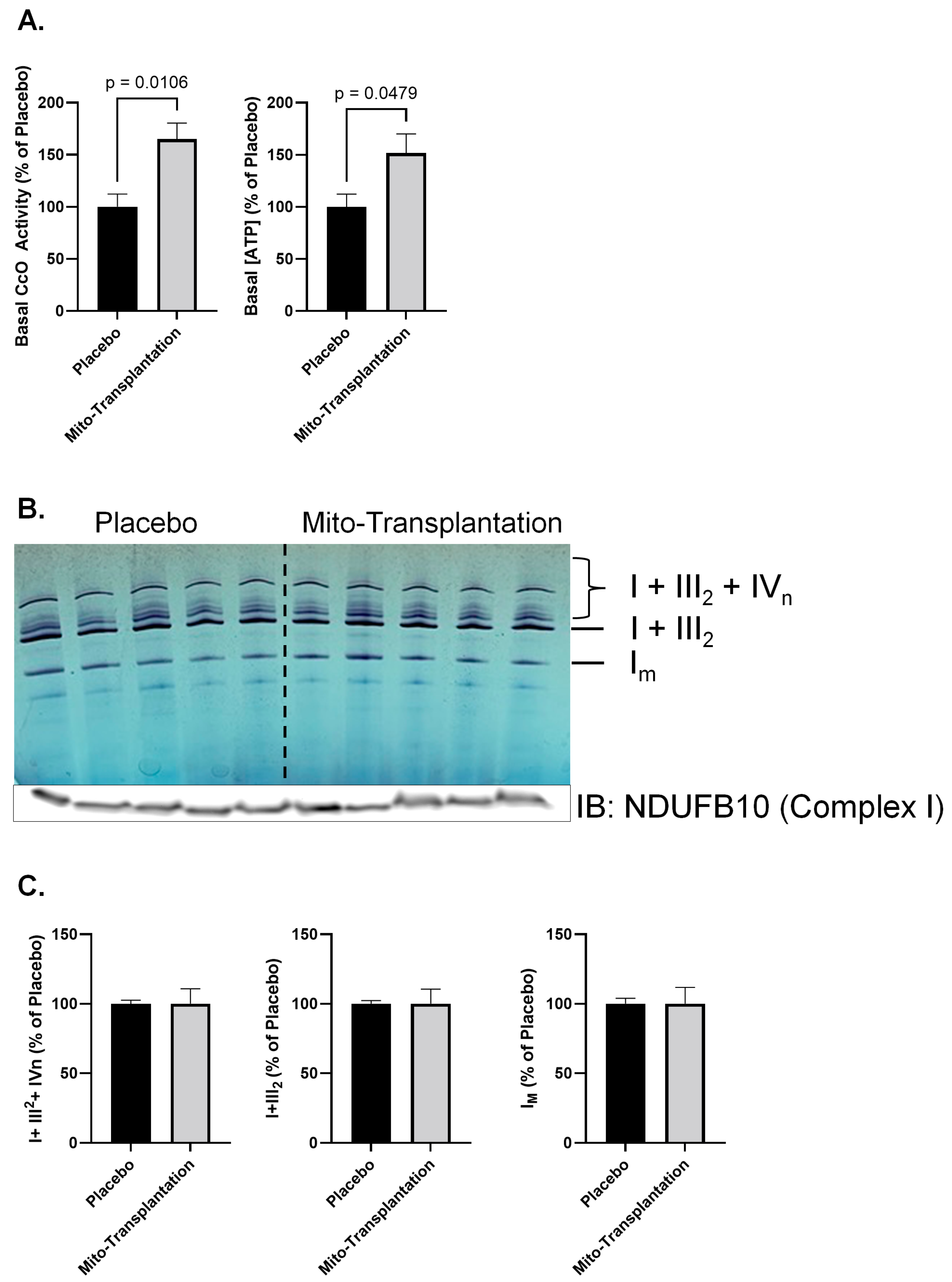
3.3. CcO and Complex I Activity, [ATP], and Supercomplex Composition
3.4. Epigenetic Response
3.5. Basal Citrate Synthase Activity in the Plantaris Muscle
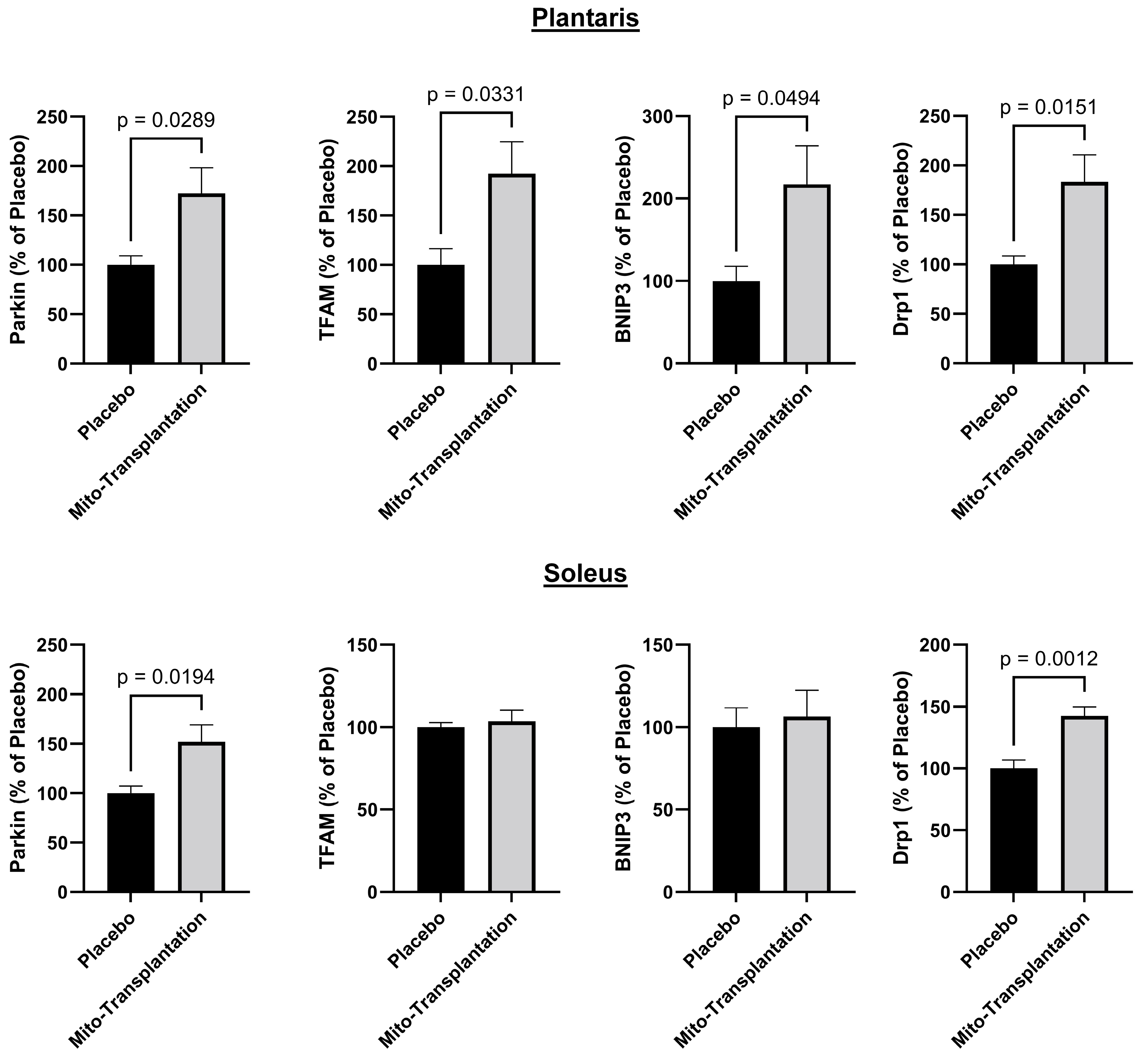
3.6. Western Blots for Mitochondrial Protein Expression
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yu, W.W.; Randhawa, A.K.; Blair, S.N.; Sui, X.; Kuk, J.L. Age- and sex- specific all-cause mortality risk greatest in metabolic syndrome combinations with elevated blood pressure from 7 U.S. cohorts. PLoS ONE 2019, 14, e0218307. [Google Scholar] [CrossRef] [PubMed]
- Lemes, I.R.; Sui, X.; Fritz, S.L.; Beattie, P.F.; Lavie, C.J.; Turi-Lynch, B.C.; Blair, S.N. Cardiorespiratory Fitness and Risk of All-Cause, Cardiovascular Disease, and Cancer Mortality in Men with Musculoskeletal Conditions. J. Phys. Act. Health 2019, 16, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Roshanravan, B.; Liu, S.Z.; Ali, A.S.; Shankland, E.G.; Goss, C.; Amory, J.K.; Robertson, H.T.; Marcinek, D.J.; Conley, K.E. In vivo mitochondrial ATP production is improved in older adult skeletal muscle after a single dose of elamipretide in a randomized trial. PLoS ONE 2021, 16, e0253849. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.Z.; Marcinek, D.J. Skeletal muscle bioenergetics in aging and heart failure. Heart Fail. Rev. 2017, 22, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Harman, D. Aging: A theory based on free radical and radiation chemistry. J. Gerontol. 1956, 11, 298–300. [Google Scholar] [CrossRef]
- Marzetti, E.; Calvani, R.; Cesari, M.; Buford, T.W.; Lorenzi, M.; Behnke, B.J.; Leeuwenburgh, C. Mitochondrial dysfunction and sarcopenia of aging: From signaling pathways to clinical trials. Int. J. Biochem. Cell Biol. 2013, 45, 2288–2301. [Google Scholar] [CrossRef]
- Lopez-Otin, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The hallmarks of aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.L.; Robinson, M.M.; Nair, K.S. Skeletal muscle aging and the mitochondrion. Trends Endocrinol. Metab. TEM 2013, 24, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Freire, M.; de Cabo, R.; Studenski, S.A.; Ferrucci, L. The Neuromuscular Junction: Aging at the Crossroad between Nerves and Muscle. Front. Aging Neurosci. 2014, 6, 208. [Google Scholar] [CrossRef]
- Kujoth, G.C.; Hiona, A.; Pugh, T.D.; Someya, S.; Panzer, K.; Wohlgemuth, S.E.; Hofer, T.; Seo, A.Y.; Sullivan, R.; Jobling, W.A.; et al. Mitochondrial DNA mutations, oxidative stress, and apoptosis in mammalian aging. Science 2005, 309, 481–484. [Google Scholar] [CrossRef]
- Kunz, W.S.; Kudin, A.; Vielhaber, S.; Elger, C.E.; Attardi, G.; Villani, G. Flux control of cytochrome c oxidase in human skeletal muscle. J. Biol. Chem. 2000, 275, 27741–27745. [Google Scholar] [CrossRef] [PubMed]
- Villani, G.; Greco, M.; Papa, S.; Attardi, G. Low reserve of cytochrome c oxidase capacity in vivo in the respiratory chain of a variety of human cell types. J. Biol. Chem. 1998, 273, 31829–31836. [Google Scholar] [CrossRef] [PubMed]
- Hajek, P.; Villani, G.; Attardi, G. Rate-limiting step preceding cytochrome c release in cells primed for Fas-mediated apoptosis revealed by analysis of cellular mosaicism of respiratory changes. J. Biol. Chem. 2001, 276, 606–615. [Google Scholar] [CrossRef] [PubMed]
- Villani, G.; Attardi, G. In vivo control of respiration by cytochrome c oxidase in wild-type and mitochondrial DNA mutation-carrying human cells. Proc. Natl. Acad. Sci. USA 1997, 94, 1166–1171. [Google Scholar] [CrossRef] [PubMed]
- Memme, J.M.; Slavin, M.; Moradi, N.; Hood, D.A. Mitochondrial Bioenergetics and Turnover during Chronic Muscle Disuse. Int. J. Mol. Sci. 2021, 22, 5179. [Google Scholar] [CrossRef]
- Hood, D.A.; Memme, J.M.; Oliveira, A.N.; Triolo, M. Maintenance of Skeletal Muscle Mitochondria in Health, Exercise, and Aging. Annu. Rev. Physiol. 2019, 81, 19–41. [Google Scholar] [CrossRef] [PubMed]
- Hüttemann, M.; Lee, I.; Malek, M.H. (-)-Epicatechin maintains endurance training adaptation in mice after 14 days of detraining. FASEB J. 2012, 26, 1413–1422. [Google Scholar] [CrossRef] [PubMed]
- Gouspillou, G.; Godin, R.; Piquereau, J.; Picard, M.; Mofarrahi, M.; Mathew, J.; Purves-Smith, F.M.; Sgarioto, N.; Hepple, R.T.; Burelle, Y.; et al. Protective role of Parkin in skeletal muscle contractile and mitochondrial function. J. Physiol. 2018, 596, 2565–2579. [Google Scholar] [CrossRef] [PubMed]
- Leduc-Gaudet, J.P.; Reynaud, O.; Hussain, S.N.; Gouspillou, G. Parkin overexpression protects from ageing-related loss of muscle mass and strength. J. Physiol. 2019, 597, 1975–1991. [Google Scholar] [CrossRef]
- Peker, N.; Sharma, M.; Kambadur, R. Parkin deficiency exacerbates fasting-induced skeletal muscle wasting in mice. NPJ Park. Dis. 2022, 8, 159. [Google Scholar] [CrossRef]
- Leduc-Gaudet, J.P.; Mayaki, D.; Reynaud, O.; Broering, F.E.; Chaffer, T.J.; Hussain, S.N.A.; Gouspillou, G. Parkin Overexpression Attenuates Sepsis-Induced Muscle Wasting. Cells 2020, 9, 1454. [Google Scholar] [CrossRef] [PubMed]
- Theilen, N.T.; Jeremic, N.; Weber, G.J.; Tyagi, S.C. TFAM overexpression diminishes skeletal muscle atrophy after hindlimb suspension in mice. Arch. Biochem. Biophys. 2019, 666, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Gao, A.; Jiang, J.; Xie, F.; Chen, L. Bnip3 in mitophagy: Novel insights and potential therapeutic target for diseases of secondary mitochondrial dysfunction. Clin. Chim. Acta 2020, 506, 72–83. [Google Scholar] [CrossRef]
- Romanello, V.; Guadagnin, E.; Gomes, L.; Roder, I.; Sandri, C.; Petersen, Y.; Milan, G.; Masiero, E.; Del Piccolo, P.; Foretz, M.; et al. Mitochondrial fission and remodelling contributes to muscle atrophy. EMBO J. 2010, 29, 1774–1785. [Google Scholar] [CrossRef] [PubMed]
- Dulac, M.; Leduc-Gaudet, J.P.; Reynaud, O.; Ayoub, M.B.; Guerin, A.; Finkelchtein, M.; Hussain, S.N.; Gouspillou, G. Drp1 knockdown induces severe muscle atrophy and remodelling, mitochondrial dysfunction, autophagy impairment and denervation. J. Physiol. 2020, 598, 3691–3710. [Google Scholar] [CrossRef] [PubMed]
- McKenna, C.F.; Salvador, A.F.; Keeble, A.R.; Khan, N.A.; De Lisio, M.; Konopka, A.R.; Paluska, S.A.; Burd, N.A. Muscle strength after resistance training correlates to mediators of muscle mass and mitochondrial respiration in middle-aged adults. J. Appl. Physiol. 2022, 133, 572–584. [Google Scholar] [CrossRef] [PubMed]
- Mesquita, P.H.C.; Lamb, D.A.; Parry, H.A.; Moore, J.H.; Smith, M.A.; Vann, C.G.; Osburn, S.C.; Fox, C.D.; Ruple, B.A.; Huggins, K.W.; et al. Acute and chronic effects of resistance training on skeletal muscle markers of mitochondrial remodeling in older adults. Physiol. Rep. 2020, 8, e14526. [Google Scholar] [CrossRef] [PubMed]
- Grevendonk, L.; Connell, N.J.; McCrum, C.; Fealy, C.E.; Bilet, L.; Bruls, Y.M.H.; Mevenkamp, J.; Schrauwen-Hinderling, V.B.; Jorgensen, J.A.; Moonen-Kornips, E.; et al. Impact of aging and exercise on skeletal muscle mitochondrial capacity, energy metabolism, and physical function. Nat. Commun. 2021, 12, 4773. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.L.; Irving, B.A.; Lanza, I.R.; Vendelbo, M.H.; Konopka, A.R.; Robinson, M.M.; Henderson, G.C.; Klaus, K.A.; Morse, D.M.; Heppelmann, C.; et al. Differential Effect of Endurance Training on Mitochondrial Protein Damage, Degradation, and Acetylation in the Context of Aging. J. Gerontol. A Biol. Sci. Med. Sci. 2015, 70, 1386–1393. [Google Scholar] [CrossRef]
- Hendrickse, P.W.; Venckunas, T.; Platkevicius, J.; Kairaitis, R.; Kamandulis, S.; Snieckus, A.; Stasiulis, A.; Vitkiene, J.; Subocius, A.; Degens, H. Endurance training-induced increase in muscle oxidative capacity without loss of muscle mass in younger and older resistance-trained men. Eur. J. Appl. Physiol. 2021, 121, 3161–3172. [Google Scholar] [CrossRef]
- Arroum, T.; Hish, G.A.; Burghardt, K.J.; Ghaloush, M.; Bazzi, B.; Mrech, A.; Morse, P.T.; Britton, S.L.; Koch, L.G.; McCully, J.D.; et al. Mitochondria Transplantation: Rescuing Innate Muscle Bioenergetic Impairment in a Model of Aging and Exercise Intolerance. J. Strength Cond Res. 2024, in press. [Google Scholar]
- Nogueira, L.; Ramirez-Sanchez, I.; Perkins, G.A.; Murphy, A.; Taub, P.R.; Ceballos, G.; Villarreal, F.J.; Hogan, M.C.; Malek, M.H. (-)-Epicatechin enhances fatigue resistance and oxidative capacity in mouse muscle. J. Physiol. 2011, 589, 4615–4631. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.; Hüttemann, M.; Kruger, A.; Bollig-Fischer, A.; Malek, M.H. (-)-Epicatechin combined with 8 weeks of treadmill exercise is associated with increased angiogenic and mitochondrial signaling in mice. Front. Pharmacol. 2015, 6, 43. [Google Scholar] [CrossRef] [PubMed]
- Hosoda, R.; Nakashima, R.; Yano, M.; Iwahara, N.; Asakura, S.; Nojima, I.; Saga, Y.; Kunimoto, R.; Horio, Y.; Kuno, A. Resveratrol, a SIRT1 activator, attenuates aging-associated alterations in skeletal muscle and heart in mice. J. Pharmacol. Sci. 2023, 152, 112–122. [Google Scholar] [CrossRef] [PubMed]
- VanderVeen, B.N.; Cardaci, T.D.; Cunningham, P.; McDonald, S.J.; Bullard, B.M.; Fan, D.; Murphy, E.A.; Velazquez, K.T. Quercetin Improved Muscle Mass and Mitochondrial Content in a Murine Model of Cancer and Chemotherapy-Induced Cachexia. Nutrients 2023, 15, 102. [Google Scholar] [CrossRef]
- Spinelli, S.; Begani, G.; Guida, L.; Magnone, M.; Galante, D.; D’Arrigo, C.; Scotti, C.; Iamele, L.; De Jonge, H.; Zocchi, E.; et al. LANCL1 binds abscisic acid and stimulates glucose transport and mitochondrial respiration in muscle cells via the AMPK/PGC-1alpha/Sirt1 pathway. Mol. Metab. 2021, 53, 101263. [Google Scholar] [CrossRef] [PubMed]
- Narkar, V.A.; Downes, M.; Yu, R.T.; Embler, E.; Wang, Y.X.; Banayo, E.; Mihaylova, M.M.; Nelson, M.C.; Zou, Y.; Juguilon, H.; et al. AMPK and PPARdelta agonists are exercise mimetics. Cell 2008, 134, 405–415. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.H.; Bylykbashi, E.; Chatila, Z.K.; Lee, S.W.; Pulli, B.; Clemenson, G.D.; Kim, E.; Rompala, A.; Oram, M.K.; Asselin, C.; et al. Combined adult neurogenesis and BDNF mimic exercise effects on cognition in an Alzheimer’s mouse model. Science 2018, 361, eaan8821. [Google Scholar] [CrossRef]
- McCully, J.D.; Cowan, D.B.; Pacak, C.A.; Toumpoulis, I.K.; Dayalan, H.; Levitsky, S. Injection of isolated mitochondria during early reperfusion for cardioprotection. Am. J. Physiol. Heart Circ. Physiol. 2009, 296, H94–H105. [Google Scholar] [CrossRef]
- Preble, J.M.; Pacak, C.A.; Kondo, H.; MacKay, A.A.; Cowan, D.B.; McCully, J.D. Rapid isolation and purification of mitochondria for transplantation by tissue dissociation and differential filtration. J. Vis. Exp. JoVE 2014, e51682. [Google Scholar] [CrossRef]
- Doulamis, I.P.; Guariento, A.; Duignan, T.; Orfany, A.; Kido, T.; Zurakowski, D.; Del Nido, P.J.; McCully, J.D. Mitochondrial transplantation for myocardial protection in diabetic hearts. Eur. J. Cardiothorac. Surg. 2020, 57, 836–845. [Google Scholar] [CrossRef] [PubMed]
- Orfany, A.; Arriola, C.G.; Doulamis, I.P.; Guariento, A.; Ramirez-Barbieri, G.; Moskowitzova, K.; Shin, B.; Blitzer, D.; Rogers, C.; Del Nido, P.J.; et al. Mitochondrial transplantation ameliorates acute limb ischemia. J. Vasc. Surg. 2020, 71, 1014–1026. [Google Scholar] [CrossRef] [PubMed]
- Guariento, A.; Doulamis, I.P.; Duignan, T.; Kido, T.; Regan, W.L.; Saeed, M.Y.; Hoganson, D.M.; Emani, S.M.; Fynn-Thompson, F.; Matte, G.S.; et al. Mitochondrial transplantation for myocardial protection in ex-situ–perfused hearts donated after circulatory death. J. Heart Lung Transplant. 2020, 39, 1279–1288. [Google Scholar] [CrossRef] [PubMed]
- Shin, B.; Cowan, D.B.; Emani, S.M.; Del Nido, P.J.; McCully, J.D. Mitochondrial Transplantation in Myocardial Ischemia and Reperfusion Injury. Adv. Exp. Med. Biol. 2017, 982, 595–619. [Google Scholar] [CrossRef] [PubMed]
- Guariento, A.; Piekarski, B.L.; Doulamis, I.P.; Blitzer, D.; Ferraro, A.M.; Harrild, D.M.; Zurakowski, D.; Del Nido, P.J.; McCully, J.D.; Emani, S.M. Autologous mitochondrial transplantation for cardiogenic shock in pediatric patients following ischemia-reperfusion injury. J. Thorac. Cardiovasc. Surg. 2021, 162, 992–1001. [Google Scholar] [CrossRef] [PubMed]
- Alway, S.E.; Paez, H.G.; Pitzer, C.R.; Ferrandi, P.J.; Khan, M.M.; Mohamed, J.S.; Carson, J.A.; Deschenes, M.R. Mitochondria transplant therapy improves regeneration and restoration of injured skeletal muscle. J. Cachexia Sarcopenia Muscle 2023, 14, 493–507. [Google Scholar] [CrossRef]
- Brossia-Root, L.J.; Alworth, L.C.; Malek, M.H. Considerations for aerobic exercise paradigms with rodent models. Lab. Anim. 2016, 45, 213–215. [Google Scholar] [CrossRef] [PubMed]
- Hüttemann, M.; Lee, I.; Perkins, G.A.; Britton, S.L.; Koch, L.G.; Malek, M.H. (-)-Epicatechin is associated with increased angiogenic and mitochondrial signalling in the hindlimb of rats selectively bred for innate low running capacity. Clin. Sci. 2013, 124, 663–674. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.; Hüttemann, M.; Malek, M.H. (-)-Epicatechin attenuates degradation in oxidative skeletal muscle associated with hindlimb suspension (given The Gary A. Dudley Memorial Paper: Scientific Manuscript Excellence Honor). J. Strength Cond Res. 2016, 30, 1–10. [Google Scholar] [CrossRef]
- Lee, I.; Salomon, A.R.; Ficarro, S.; Mathes, I.; Lottspeich, F.; Grossman, L.I.; Hüttemann, M. cAMP-dependent tyrosine phosphorylation of subunit I inhibits cytochrome c oxidase activity. J. Biol. Chem. 2005, 280, 6094–6100. [Google Scholar] [CrossRef]
- Lee, I.; Hüttemann, M.; Liu, J.; Grossman, L.I.; Malek, M.H. Deletion of heart-type cytochrome c oxidase subunit 7A1 impairs skeletal muscle angiogenesis and oxidative phosphorylation. J. Physiol. 2012, 590, 5231–5243. [Google Scholar] [CrossRef] [PubMed]
- Wittig, I.; Braun, H.P.; Schagger, H. Blue native PAGE. Nat. Protoc. 2006, 1, 418–428. [Google Scholar] [CrossRef] [PubMed]
- Jha, P.; Wang, X.; Auwerx, J. Analysis of Mitochondrial Respiratory Chain Supercomplexes Using Blue Native Polyacrylamide Gel Electrophoresis (BN-PAGE). Curr. Protoc. Mouse Biol. 2016, 6, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Srere, P.A. Citrate synthase. Methods Enzymol. 1969, 13, 3–11. [Google Scholar]
- Doulamis, I.P.; Nomoto, R.S.; Tzani, A.; Hong, X.; Duignan, T.; Celik, A.; Del Nido, P.J.; McCully, J.D. Transcriptomic and proteomic pathways of diabetic and non-diabetic mitochondrial transplantation. Sci. Rep. 2022, 12, 22101. [Google Scholar] [CrossRef] [PubMed]
- Koch, L.G.; Britton, S.L. Biology: Motion is Function. Function 2022, 3, zqac030. [Google Scholar] [CrossRef] [PubMed]
- Biesiadecki, B.J.; Brotto, M.A.; Brotto, L.S.; Koch, L.G.; Britton, S.L.; Nosek, T.M.; Jin, J.P. Rats genetically selected for low and high aerobic capacity exhibit altered soleus muscle myofilament functions. Am. J. Physiol. Cell Physiol. 2020, 318, C422–C429. [Google Scholar] [CrossRef] [PubMed]
- Koch, L.G.; Kemi, O.J.; Qi, N.; Leng, S.X.; Bijma, P.; Gilligan, L.J.; Wilkinson, J.E.; Wisloff, H.; Hoydal, M.A.; Rolim, N.; et al. Intrinsic aerobic capacity sets a divide for aging and longevity. Circ. Res. 2011, 109, 1162–1172. [Google Scholar] [CrossRef]
- Jackson, S.J.; Andrews, N.; Ball, D.; Bellantuono, I.; Gray, J.; Hachoumi, L.; Holmes, A.; Latcham, J.; Petrie, A.; Potter, P.; et al. Does age matter? The impact of rodent age on study outcomes. Lab. Anim. 2017, 51, 160–169. [Google Scholar] [CrossRef]
- Samelman, T.R.; Shiry, L.J.; Cameron, D.F. Endurance training increases the expression of mitochondrial and nuclear encoded cytochrome c oxidase subunits and heat shock proteins in rat skeletal muscle. Eur. J. Appl. Physiol. 2000, 83, 22–27. [Google Scholar] [CrossRef]
- Coggan, A.R.; Spina, R.J.; Kohrt, W.M.; Holloszy, J.O. Effect of prolonged exercise on muscle citrate concentration before and after endurance training in men. Am. J. Physiol. 1993, 264, E215–E220. [Google Scholar] [CrossRef]
- Murias, J.M.; Kowalchuk, J.M.; Ritchie, D.; Hepple, R.T.; Doherty, T.J.; Paterson, D.H. Adaptations in capillarization and citrate synthase activity in response to endurance training in older and young men. J. Gerontol. A Biol. Sci. Med. Sci. 2011, 66, 957–964. [Google Scholar] [CrossRef] [PubMed]
- King, W.T.; Axelrod, C.L.; Zunica, E.R.M.; Noland, R.C.; Davuluri, G.; Fujioka, H.; Tandler, B.; Pergola, K.; Hermann, G.E.; Rogers, R.C.; et al. Dynamin-related protein 1 regulates substrate oxidation in skeletal muscle by stabilizing cellular and mitochondrial calcium dynamics. J. Biol. Chem. 2021, 297, 101196. [Google Scholar] [CrossRef] [PubMed]
- Dulac, M.; Leduc-Gaudet, J.P.; Cefis, M.; Ayoub, M.B.; Reynaud, O.; Shams, A.; Moamer, A.; Nery Ferreira, M.F.; Hussain, S.N.; Gouspillou, G. Regulation of muscle and mitochondrial health by the mitochondrial fission protein Drp1 in aged mice. J. Physiol. 2021, 599, 4045–4063. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; Gu, Y.; Sui, X.; Shen, L.; Han, J.; Wang, H.; Xi, Q.; Zhuang, Q.; Meng, Q.; Wu, G. Phosphorylation of Dynamin-Related Protein 1 (DRP1) Regulates Mitochondrial Dynamics and Skeletal Muscle Wasting in Cancer Cachexia. Front. Cell Dev. Biol. 2021, 9, 673618. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Lee, H.Y.; Hanna, R.A.; Gustafsson, A.B. Mitochondrial autophagy by Bnip3 involves Drp1-mediated mitochondrial fission and recruitment of Parkin in cardiac myocytes. Am. J. Physiol. Heart Circ. Physiol. 2011, 301, H1924–H1931. [Google Scholar] [CrossRef]
- Wang, F.; Zhou, T.; Zhou, C.X.; Zhang, Q.B.; Wang, H.; Zhou, Y. The worsening of skeletal muscle atrophy induced by immobilization at the early stage of remobilization correlates with BNIP3-dependent mitophagy. BMC Musculoskelet. Disord. 2023, 24, 632. [Google Scholar] [CrossRef]
- Irazoki, A.; Zorzano, A.; Sebastian, D. Mitochondrial fitness sustains healthy muscle aging. Aging 2023, 15, 5956–5958. [Google Scholar] [CrossRef]
- Irazoki, A.; Martinez-Vicente, M.; Aparicio, P.; Aris, C.; Alibakhshi, E.; Rubio-Valera, M.; Castellanos, J.; Lores, L.; Palacin, M.; Guma, A.; et al. Coordination of mitochondrial and lysosomal homeostasis mitigates inflammation and muscle atrophy during aging. Aging Cell 2022, 21, e13583. [Google Scholar] [CrossRef]
- Kim, T.Y.; Wang, D.; Kim, A.K.; Lau, E.; Lin, A.J.; Liem, D.A.; Zhang, J.; Zong, N.C.; Lam, M.P.; Ping, P. Metabolic labeling reveals proteome dynamics of mouse mitochondria. Mol. Cell. Proteom. 2012, 11, 1586–1594. [Google Scholar] [CrossRef]
- Bloemberg, D.; Quadrilatero, J. Rapid determination of myosin heavy chain expression in rat, mouse, and human skeletal muscle using multicolor immunofluorescence analysis. PLoS ONE 2012, 7, e35273. [Google Scholar] [CrossRef] [PubMed]
- Wiechmann, T.; Roh, S.; Sauer, S.; Czamara, D.; Arloth, J.; Kodel, M.; Beintner, M.; Knop, L.; Menke, A.; Binder, E.B.; et al. Identification of dynamic glucocorticoid-induced methylation changes at the FKBP5 locus. Clin. Epigenet. 2019, 11, 83. [Google Scholar] [CrossRef] [PubMed]
- Baccarelli, A.; Wright, R.O.; Bollati, V.; Tarantini, L.; Litonjua, A.A.; Suh, H.H.; Zanobetti, A.; Sparrow, D.; Vokonas, P.S.; Schwartz, J. Rapid DNA methylation changes after exposure to traffic particles. Am. J. Respir. Crit. Care Med. 2009, 179, 572–578. [Google Scholar] [CrossRef] [PubMed]
- Verlinden, I.; Guiza, F.; Derese, I.; Wouters, P.J.; Joosten, K.; Verbruggen, S.C.; Van den Berghe, G.; Vanhorebeek, I. Time course of altered DNA methylation evoked by critical illness and by early administration of parenteral nutrition in the paediatric ICU. Clin. Epigenet. 2020, 12, 155. [Google Scholar] [CrossRef] [PubMed]
- Saul, D.; Kosinsky, R.L. Epigenetics of Aging and Aging-Associated Diseases. Int. J. Mol. Sci. 2021, 22, 401. [Google Scholar] [CrossRef] [PubMed]
- Bjornsson, H.T.; Sigurdsson, M.I.; Fallin, M.D.; Irizarry, R.A.; Aspelund, T.; Cui, H.; Yu, W.; Rongione, M.A.; Ekstrom, T.J.; Harris, T.B.; et al. Intra-individual change over time in DNA methylation with familial clustering. JAMA 2008, 299, 2877–2883. [Google Scholar] [CrossRef]
- Bollati, V.; Schwartz, J.; Wright, R.; Litonjua, A.; Tarantini, L.; Suh, H.; Sparrow, D.; Vokonas, P.; Baccarelli, A. Decline in genomic DNA methylation through aging in a cohort of elderly subjects. Mech. Ageing Dev. 2009, 130, 234–239. [Google Scholar] [CrossRef]


| Antibody | Company | Product ID | Lot No. | Dilution |
|---|---|---|---|---|
| Parkin | Cell Signaling Technology (Danvers, MA, USA) | 2132S | 4 | 1:1000 |
| TFAM | Abcam (Cambridge, UK) | Ab252432 | GR3455740-1 | 1:1000 |
| Anti-BNIP3 | Abcam | Ab109362 | GR3284300-7 | 1:1000 |
| Drp1 | Abcam | Ab184247 | GR3369203-23 | 1:1000 |
| α-Tubulin | Abcam | Ab7291 | 1009714-1 | 1:1000 |
| IRDye 800CW | LI-COR (Lincoln, NE, USA) | 925-32211 | D11103-01 | 1:20,000 |
| IRDye 680RD | LI-COR | 925-68070 | D10901-11 | 1:20,000 |
| Placebo | Mitochondria Transplantation | |||||
|---|---|---|---|---|---|---|
| Pre-Injection | 3 Weeks Post-Injection | 6 Weeks Post-Injection | Pre-Injection | 3 Weeks Post-Injection | 6 Weeks Post-Injection | |
| Body mass (g) | 33.7 ± 1.6 | 32.5 ± 1.2 | 33.0 ± 1.2 | 34.2 ± 1.7 | 33.5 ± 1.2 | 34.3 ± 1.2 |
| Soleus (mg) | - | - | 8.1 ± 0.8 | - | - | 7.4 ± 0.5 |
| Soleus/body mass (mg/g) | - | - | 0.25 ± 0.03 | - | - | 0.22 ± 0.02 |
| Plantaris (mg) | - | - | 13.8 ± 0.8 | - | - | 12.6 ± 0.7 |
| Plantaris/body mass (mg/g) | - | - | 0.42 ± 0.04 | - | - | 0.37 ± 0.02 |
| Quadriceps femoris (mg) | - | - | 141.4 ± 3.2 | - | - | 151.0 ± 6.8 |
| Quadriceps femoris/body mass (mg/g) | - | - | 4.32 ± 0.53 | - | - | 4.43 ± 0.68 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arroum, T.; Hish, G.A.; Burghardt, K.J.; McCully, J.D.; Hüttemann, M.; Malek, M.H. Mitochondrial Transplantation’s Role in Rodent Skeletal Muscle Bioenergetics: Recharging the Engine of Aging. Biomolecules 2024, 14, 493. https://doi.org/10.3390/biom14040493
Arroum T, Hish GA, Burghardt KJ, McCully JD, Hüttemann M, Malek MH. Mitochondrial Transplantation’s Role in Rodent Skeletal Muscle Bioenergetics: Recharging the Engine of Aging. Biomolecules. 2024; 14(4):493. https://doi.org/10.3390/biom14040493
Chicago/Turabian StyleArroum, Tasnim, Gerald A. Hish, Kyle J. Burghardt, James D. McCully, Maik Hüttemann, and Moh H. Malek. 2024. "Mitochondrial Transplantation’s Role in Rodent Skeletal Muscle Bioenergetics: Recharging the Engine of Aging" Biomolecules 14, no. 4: 493. https://doi.org/10.3390/biom14040493
APA StyleArroum, T., Hish, G. A., Burghardt, K. J., McCully, J. D., Hüttemann, M., & Malek, M. H. (2024). Mitochondrial Transplantation’s Role in Rodent Skeletal Muscle Bioenergetics: Recharging the Engine of Aging. Biomolecules, 14(4), 493. https://doi.org/10.3390/biom14040493









