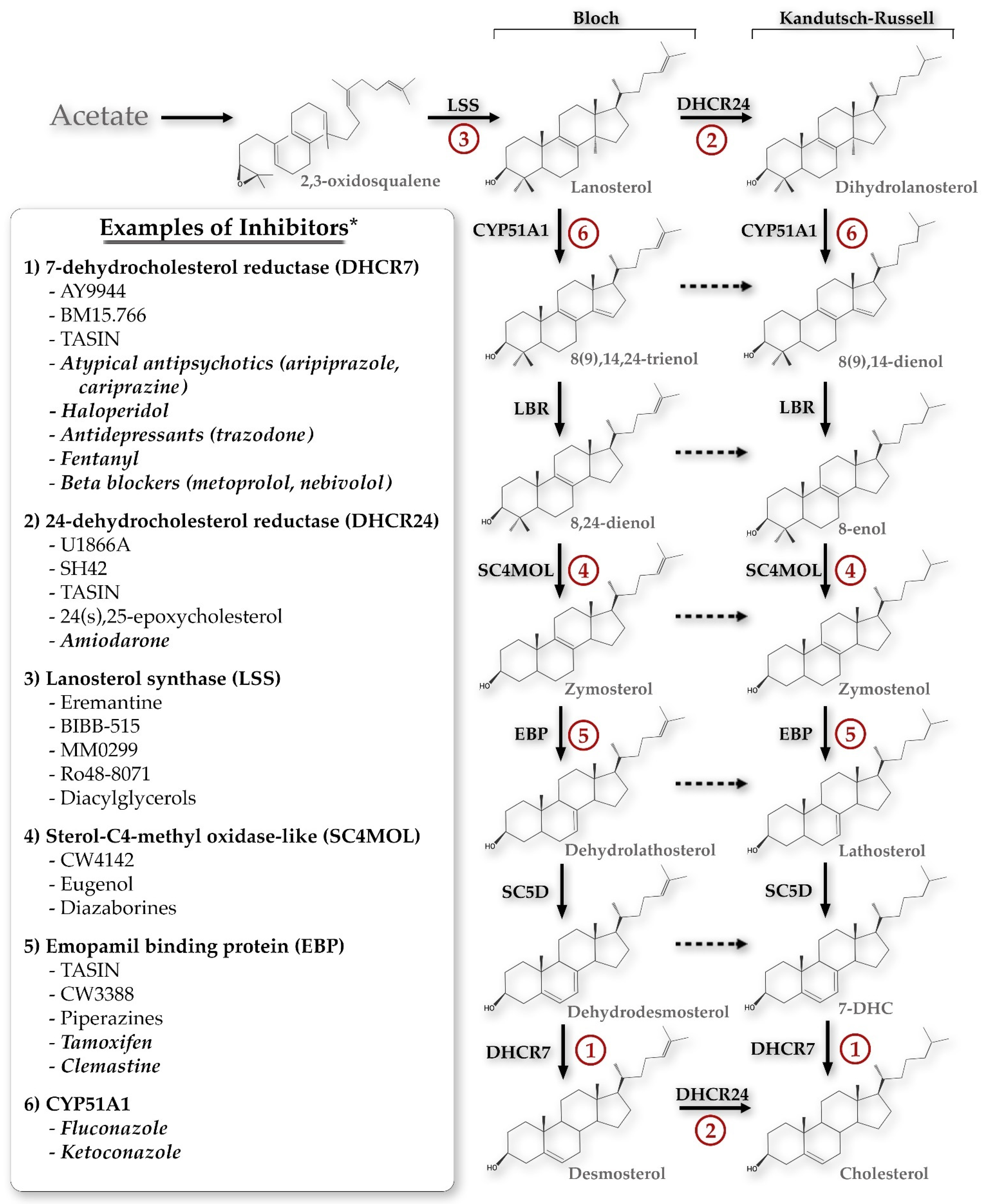
Chemical Inhibition of Sterol Biosynthesis
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Chemicals That Alter 7-Dehydrocholesterol Reductase (DHCR7) Activity
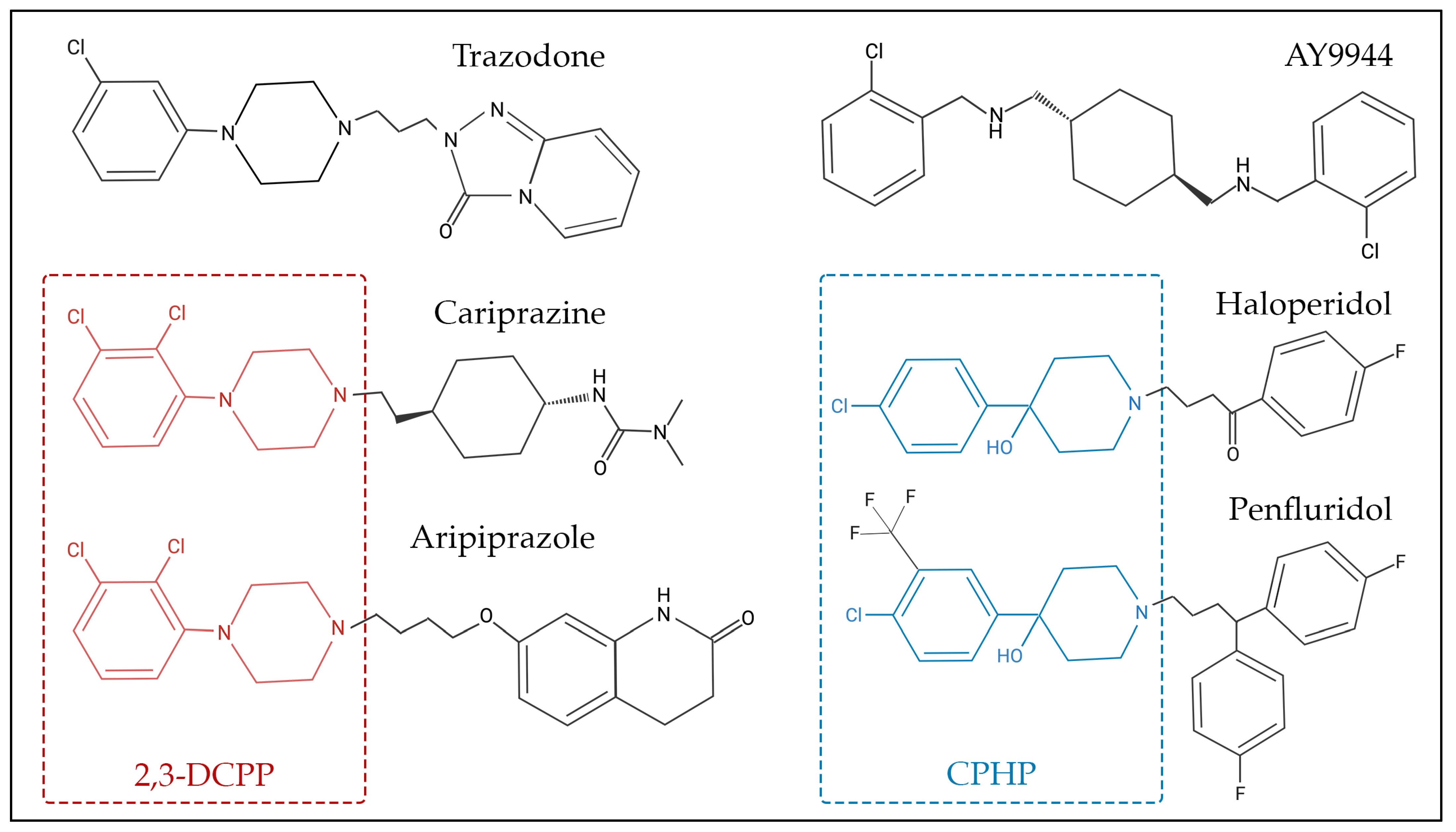
3.1.1. AY9944
3.1.2. BM15.766
3.1.3. Other DHCR7 Inhibitors
3.2. Chemicals That Alter 24-Dehydrocholesterol Reductase (DHCR24) Activity
3.2.1. U18666A
3.2.2. SH42
3.2.3. Other DHCR24 Inhibitors
3.3. Chemicals That Alter Lanosterol Synthase (LSS) Activity
3.4. Chemicals That Alter Sterol-C4-Methyl Oxidase-like (SC4MOL) Activity
3.5. Chemicals That Alter Emopamil-Binding Protein (EBP) Activity
3.6. Chemicals That Alter Cytochrome P450 Family 51 Subfamily A Member 1 (CYP51A1) Activity
3.7. Chemicals That Alter Sterol-C5-Desaturase (SC5D) Activity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Björkhem, I.; Meaney, S. Brain cholesterol: Long secret life behind a barrier. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 806–815. [Google Scholar] [CrossRef] [PubMed]
- Dietschy, J.M.; Turley, S.D. Cholesterol metabolism in the brain. Curr. Opin. Lipidol. 2001, 12, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Saher, G. Cholesterol Metabolism in Aging and Age-Related Disorders. Annu. Rev. Neurosci. 2023, 46, 59–78. [Google Scholar] [CrossRef] [PubMed]
- Gliozzi, M.; Musolino, V.; Bosco, F.; Scicchitano, M.; Scarano, F.; Nucera, S.; Zito, M.C.; Ruga, S.; Carresi, C.; Macrì, R.; et al. Cholesterol homeostasis: Researching a dialogue between the brain and peripheral tissues. Pharmacol. Res. 2021, 163, 105215. [Google Scholar] [CrossRef] [PubMed]
- Eyles, D.W.; Smith, S.; Kinobe, R.; Hewison, M.; McGrath, J.J. Distribution of the vitamin D receptor and 1 alpha-hydroxylase in human brain. J. Chem. Neuroanat. 2005, 29, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, W.J.; Wang, Y. Oxysterols as lipid mediators: Their biosynthetic genes, enzymes and metabolites. Prostaglandins Other Lipid Mediat. 2020, 147, 106381. [Google Scholar] [CrossRef]
- Wang, Y.; Sousa, K.M.; Bodin, K.; Theofilopoulos, S.; Sacchetti, P.; Hornshaw, M.; Woffendin, G.; Karu, K.; Sjövall, J.; Arenas, E.; et al. Targeted lipidomic analysis of oxysterols in the embryonic central nervous system. Mol. Biosyst. 2009, 5, 529–541. [Google Scholar] [CrossRef]
- Porter, F.D.; Herman, G.E. Malformation syndromes caused by disorders of cholesterol synthesis. J. Lipid Res. 2011, 52, 6–34. [Google Scholar] [CrossRef] [PubMed]
- Porter, F.D. Smith-Lemli-Opitz syndrome: Pathogenesis, diagnosis and management. Eur. J. Hum. Genet. 2008, 16, 535–541. [Google Scholar] [CrossRef] [PubMed]
- Porter, F.D. Cholesterol precursors and facial clefting. J. Clin. Investig. 2006, 116, 2322–2325. [Google Scholar] [CrossRef]
- Cocciadiferro, D.; Mazza, T.; Vecchio, D.; Biagini, T.; Petrizzelli, F.; Agolini, E.; Villani, A.; Minervino, D.; Martinelli, D.; Rizzo, C.; et al. Exploiting in silico structural analysis to introduce emerging genotype-phenotype correlations in DHCR24-related sterol biosynthesis disorder: A case study. Front. Genet. 2023, 14, 1307934. [Google Scholar] [CrossRef] [PubMed]
- Korade, Z.; Heffer, M.; Mirnics, K. Medication effects on developmental sterol biosynthesis. Mol. Psychiatry 2022, 27, 490–501. [Google Scholar] [CrossRef] [PubMed]
- Tonini, C.; Segatto, M.; Gagliardi, S.; Bertoli, S.; Leone, A.; Barberio, L.; Mandalà, M.; Pallottini, V. Maternal Dietary Exposure to Low-Dose Bisphenol A Affects Metabolic and Signaling Pathways in the Brain of Rat Fetuses. Nutrients 2020, 12, 1448. [Google Scholar] [CrossRef]
- Wages, P.A.; Joshi, P.; Tallman, K.A.; Kim, H.H.; Bowman, A.B.; Porter, N.A. Screening ToxCast™ for Chemicals That Affect Cholesterol Biosynthesis: Studies in Cell Culture and Human Induced Pluripotent Stem Cell-Derived Neuroprogenitors. Environ. Health Perspect. 2020, 128, 17014. [Google Scholar] [CrossRef] [PubMed]
- Wages, P.A.; Kim, H.-Y.H.; Korade, Z.; Porter, N.A. Identification and characterization of prescription drugs that change levels of 7-dehydrocholesterol and desmosterol. J. Lipid Res. 2018, 59, 1916–1926. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.Y.; Korade, Z.; Tallman, K.A.; Liu, W.; Weaver, C.D.; Mirnics, K.; Porter, N.A. Inhibitors of 7-Dehydrocholesterol Reductase: Screening of a Collection of Pharmacologically Active Compounds in Neuro2a Cells. Chem. Res. Toxicol. 2016, 29, 892–900. [Google Scholar] [CrossRef]
- Simonen, P.; Lehtonen, J.; Lampi, A.M.; Piironen, V.; Stenman, U.H.; Kupari, M.; Gylling, H. Desmosterol accumulation in users of amiodarone. J. Intern. Med. 2018, 283, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Simonen, P.; Li, S.; Chua, N.K.; Lampi, A.M.; Piironen, V.; Lommi, J.; Sinisalo, J.; Brown, A.J.; Ikonen, E.; Gylling, H. Amiodarone disrupts cholesterol biosynthesis pathway and causes accumulation of circulating desmosterol by inhibiting 24-dehydrocholesterol reductase. J. Intern. Med. (GBR) 2020, 288, 560–569. [Google Scholar] [CrossRef] [PubMed]
- Herron, J.; Reese, R.C.; Tallman, K.A.; Narayanaswamy, R.; Porter, N.A.; Xu, L. Identification of Environmental Quaternary Ammonium Compounds as Direct Inhibitors of Cholesterol Biosynthesis. Toxicol. Sci. Off. J. Soc. Toxicol. 2016, 151, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Herron, J.M.; Hines, K.M.; Tomita, H.; Seguin, R.P.; Cui, J.Y.; Xu, L. Multiomics Investigation Reveals Benzalkonium Chloride Disinfectants Alter Sterol and Lipid Homeostasis in the Mouse Neonatal Brain. Toxicol. Sci. 2019, 171, 32–45. [Google Scholar] [CrossRef] [PubMed]
- Herron, J.M.; Tomita, H.; White, C.C.; Kavanagh, T.J.; Xu, L. Benzalkonium Chloride Disinfectants Induce Apoptosis, Inhibit Proliferation, and Activate the Integrated Stress Response in a 3-D In Vitro Model of Neurodevelopment. Chem. Res. Toxicol. 2021, 34, 1265–1274. [Google Scholar] [CrossRef] [PubMed]
- Simonen, P.; Lommi, J.; Lemström, K.; Tolva, J.; Sinisalo, J.; Gylling, H. Amiodarone accumulates two cholesterol precursors in myocardium: A controlled clinical study. J. Intern. Med. 2023, 294, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Hrubec, T.C.; Seguin, R.P.; Xu, L.; Cortopassi, G.A.; Datta, S.; Hanlon, A.L.; Lozano, A.J.; McDonald, V.A.; Healy, C.A.; Anderson, T.C.; et al. Altered toxicological endpoints in humans from common quaternary ammonium compound disinfectant exposure. Toxicol. Rep. 2021, 8, 646–656. [Google Scholar] [CrossRef] [PubMed]
- Martín, M.G.; Pfrieger, F.; Dotti, C.G. Cholesterol in brain disease: Sometimes determinant and frequently implicated. EMBO Rep. 2014, 15, 1036–1052. [Google Scholar] [CrossRef] [PubMed]
- Menezes, A.R.; Lavie, C.J.; Milani, R.V.; OʼKeefe, J. The effects of statins on prevention of stroke and dementia: A review. J. Cardiopulm. Rehabil. Prev. 2012, 32, 240–249. [Google Scholar] [CrossRef]
- Siwek, M.; Woroń, J.; Gorostowicz, A.; Wordliczek, J. Adverse effects of interactions between antipsychotics and medications used in the treatment of cardiovascular disorders. Pharmacol. Rep. 2020, 72, 350–359. [Google Scholar] [CrossRef] [PubMed]
- Kidnapillai, S.; Bortolasci, C.C.; Panizzutti, B.; Spolding, B.; Connor, T.; Bonifacio, K.; Sanigorski, A.; Dean, O.M.; Crowley, T.; Jamain, S.; et al. Drugs used in the treatment of bipolar disorder and their effects on cholesterol biosynthesis—A possible therapeutic mechanism. World J. Biol. Psychiatry 2019, 20, 766–777. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Kaler, J.; Ray, S.D. The Benefits Outweigh the Risks of Treating Hypercholesterolemia: The Statin Dilemma. Cureus 2023, 15, e33648. [Google Scholar] [CrossRef]
- Dietschy, J.M.; Turley, S.D. Thematic review series: Brain Lipids. Cholesterol metabolism in the central nervous system during early development and in the mature animal. J. Lipid Res. 2004, 45, 1375–1397. [Google Scholar] [CrossRef] [PubMed]
- Tierney, E.; Bukelis, I.; Thompson, R.E.; Ahmed, K.; Aneja, A.; Kratz, L.; Kelley, R.I. Abnormalities of cholesterol metabolism in autism spectrum disorders. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2006, 141b, 666–668. [Google Scholar] [CrossRef]
- Boland, M.R.; Tatonetti, N.P. Investigation of 7-dehydrocholesterol reductase pathway to elucidate off-target prenatal effects of pharmaceuticals: A systematic review. Pharmacogenom. J. 2016, 16, 411–429. [Google Scholar] [CrossRef] [PubMed]
- Moebius, F.F.; Fitzky, B.U.; Lee, J.N.; Paik, Y.K.; Glossmann, H. Molecular cloning and expression of the human delta7-sterol reductase. Proc. Natl. Acad. Sci. USA 1998, 95, 1899–1902. [Google Scholar] [CrossRef] [PubMed]
- Fitzky, B.U.; Witsch-Baumgartner, M.; Erdel, M.; Lee, J.N.; Paik, Y.K.; Glossmann, H.; Utermann, G.; Moebius, F.F. Mutations in the Delta7-sterol reductase gene in patients with the Smith-Lemli-Opitz syndrome. Proc. Natl. Acad. Sci. USA 1998, 95, 8181–8186. [Google Scholar] [CrossRef] [PubMed]
- Wassif, C.A.; Maslen, C.; Kachilele-Linjewile, S.; Lin, D.; Linck, L.M.; Connor, W.E.; Steiner, R.D.; Porter, F.D. Mutations in the human sterol delta7-reductase gene at 11q12-13 cause Smith-Lemli-Opitz syndrome. Am. J. Hum. Genet. 1998, 63, 55–62. [Google Scholar] [CrossRef]
- Waterham, H.R.; Wijburg, F.A.; Hennekam, R.C.; Vreken, P.; Poll-The, B.T.; Dorland, L.; Duran, M.; Jira, P.E.; Smeitink, J.A.; Wevers, R.A.; et al. Smith-Lemli-Opitz syndrome is caused by mutations in the 7-dehydrocholesterol reductase gene. Am. J. Hum. Genet. 1998, 63, 329–338. [Google Scholar] [CrossRef]
- Online Mendelian Inheritance in Man (OMIM). Johns Hopkins University, Baltimore, MD. 270400: 1/12/24. Available online: https://omim.org (accessed on 20 February 2024).
- Kelley, R.I.; Hennekam, R.C. The Smith-Lemli-Opitz syndrome. J. Med. Genet. 2000, 37, 321–335. [Google Scholar] [CrossRef] [PubMed]
- Cross, J.L.; Iben, J.; Simpson, C.L.; Thurm, A.; Swedo, S.; Tierney, E.; Bailey-Wilson, J.E.; Biesecker, L.G.; Porter, F.D.; Wassif, C.A. Determination of the allelic frequency in Smith-Lemli-Opitz syndrome by analysis of massively parallel sequencing data sets. Clin. Genet. 2015, 87, 570–575. [Google Scholar] [CrossRef] [PubMed]
- Fliesler, S.J.; Peachey, N.S.; Richards, M.J.; Nagel, B.A.; Vaughan, D.K. Retinal degeneration in a rodent model of Smith-Lemli-Opitz syndrome: Electrophysiologic, biochemical, and morphologic features. Arch. Ophthalmol. 2004, 122, 1190–1200. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Liu, W.; Sheflin, L.G.; Fliesler, S.J.; Porter, N.A. Novel oxysterols observed in tissues and fluids of AY9944-treated rats: A model for Smith-Lemli-Opitz syndrome. J. Lipid Res. 2011, 52, 1810–1820. [Google Scholar] [CrossRef] [PubMed]
- Myers, B.R.; Neahring, L.; Zhang, Y.; Roberts, K.J.; Beachy, P.A. Rapid, direct activity assays for Smoothened reveal Hedgehog pathway regulation by membrane cholesterol and extracellular sodium. Proc. Natl. Acad. Sci. USA 2017, 114, e11141–e11150. [Google Scholar] [CrossRef] [PubMed]
- Roux, C.; Wolf, C.; Mulliez, N.; Gaoua, W.; Cormier, V.; Chevy, F.; Citadelle, D. Role of cholesterol in embryonic development. Am. J. Clin. Nutr. 2000, 71, 1270S–1279S. [Google Scholar] [CrossRef]
- Kolf-Clauw, M.; Chevy, F.; Wolf, C.; Siliart, B.; Citadelle, D.; Roux, C. Inhibition of 7-dehydrocholesterol reductase by the teratogen AY9944: A rat model for Smith-Lemli-Opitz syndrome. Teratology 1996, 54, 115–125. [Google Scholar] [CrossRef]
- Fliesler, S.J.; Xu, L. Oxysterols and Retinal Degeneration in a Rat Model of Smith-Lemli-Opitz Syndrome: Implications for an Improved Therapeutic Intervention. Molecules 2018, 23, 2720. [Google Scholar] [CrossRef]
- Fliesler, S.J.; Peachey, N.S.; Herron, J.; Hines, K.M.; Weinstock, N.I.; Ramachandra Rao, S.; Xu, L. Prevention of Retinal Degeneration in a Rat Model of Smith-Lemli-Opitz Syndrome. Sci. Rep. 2018, 8, 1286. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Sheflin, L.G.; Porter, N.A.; Fliesler, S.J. 7-Dehydrocholesterol-derived oxysterols and retinal degeneration in a rat model of Smith-Lemli-Opitz syndrome. Biochim. Biophys. Acta 2012, 1821, 877–883. [Google Scholar] [CrossRef] [PubMed]
- Wolf, C.; Chevy, F.; Pham, J.; Kolf-Clauw, M.; Citadelle, D.; Mulliez, N.; Roux, C. Changes in serum sterols of rats treated with 7-dehydrocholesterol-delta 7-reductase inhibitors: Comparison to levels in humans with Smith-Lemli-Opitz syndrome. J. Lipid Res. 1996, 37, 1325–1333. [Google Scholar] [CrossRef] [PubMed]
- Guirgis, F.W.; Jacob, V.; Wu, D.; Henson, M.; Daly-Crews, K.; Hopson, C.; Black, L.P.; DeVos, E.L.; Sulaiman, D.; Labilloy, G.; et al. DHCR7 Expression Predicts Poor Outcomes and Mortality from Sepsis. Res. Sq. 2023. [Google Scholar] [CrossRef] [PubMed]
- Yamada, N.; Karasawa, T.; Komada, T.; Matsumura, T.; Baatarjav, C.; Ito, J.; Nakagawa, K.; Yamamuro, D.; Ishibashi, S.; Miura, K.; et al. DHCR7 as a novel regulator of ferroptosis in hepatocytes. bioRxiv 2022. [Google Scholar] [CrossRef]
- Korade, Z.; Tallman, K.A.; Kim, H.Y.H.; Balog, M.; Genaro-Mattos, T.C.; Pattnaik, A.; Mirnics, K.; Pattnaik, A.K.; Porter, N.A. Dose-Response Effects of 7-Dehydrocholesterol Reductase Inhibitors on Sterol Profiles and Vesicular Stomatitis Virus Replication. ACS Pharm. Transl. Sci. 2022, 5, 1086–1096. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Li, W.; Zheng, X.; Qi, L.; Wang, H.; Zhang, C.; Wan, X.; Zheng, Y.; Zhong, R.; Zhou, X.; et al. Targeting 7-Dehydrocholesterol Reductase Integrates Cholesterol Metabolism and IRF3 Activation to Eliminate Infection. Immunity 2020, 52, 109–122.e106. [Google Scholar] [CrossRef] [PubMed]
- Aufenanger, J.; Pill, J.; Schmidt, F.H.; Stegmeier, K. The effects of BM 15.766, an inhibitor of 7-dehydrocholesterol delta 7-reductase, on cholesterol biosynthesis in primary rat hepatocytes. Biochem. Pharmacol. 1986, 35, 911–916. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Salen, G.; Shefer, S.; Ness, G.C.; Chen, T.S.; Zhao, Z.; Tint, G.S. Reproducing abnormal cholesterol biosynthesis as seen in the Smith-Lemli-Opitz syndrome by inhibiting the conversion of 7-dehydrocholesterol to cholesterol in rats. J. Clin. Investig. 1995, 95, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Servatius, R.J.; Shefer, S.; Tint, G.S.; O’Brien, W.T.; Batta, A.K.; Salen, G. Relationship between abnormal cholesterol synthesis and retarded learning in rats. Metabolism 1998, 47, 878–882. [Google Scholar] [CrossRef] [PubMed]
- Lindenthal, B.; Bertsch, T.; Fassbender, K.; Stroick, M.; Kühl, S.; Lütjohann, D.; Von Bergmann, K. Influence of simvastatin, pravastatin, and BM 15.766 on neutral sterols in liver and testis of guinea pigs. Metab. Clin. Exp. 2002, 51, 492–499. [Google Scholar] [CrossRef] [PubMed]
- Kolf-Clauw, M.; Chevy, F.; Siliart, B.; Wolf, C.; Mulliez, N.; Roux, C. Cholesterol biosynthesis inhibited by BM15.766 induces holoprosencephaly in the rat. Teratology 1997, 56, 188–200. [Google Scholar] [CrossRef]
- Honda, A.; Shefer, S.; Salen, G.; Xu, G.; Batta, A.K.; Tint, G.S.; Honda, M.; Chen, T.C.; Holick, M.F. Regulation of the last two enzymatic reactions in cholesterol biosynthesis in rats: Effects of BM 15.766, cholesterol, cholic acid, lovastatin, and their combinations. Hepatology 1996, 24, 435–439. [Google Scholar] [CrossRef] [PubMed]
- Tallman, K.A.; Allen, L.B.; Klingelsmith, K.B.; Anderson, A.; Genaro-Mattos, T.C.; Mirnics, K.; Porter, N.A.; Korade, Z. Prescription Medications Alter Neuronal and Glial Cholesterol Synthesis. ACS Chem. Neurosci. 2021, 12, 735–745. [Google Scholar] [CrossRef] [PubMed]
- Korade, Ž.; Liu, W.; Warren, E.B.; Armstrong, K.; Porter, N.A.; Konradi, C. Effect of psychotropic drug treatment on sterol metabolism. Schizophr. Res. 2017, 187, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Genaro-Mattos, T.C.; Tallman, K.A.; Allen, L.B.; Anderson, A.; Mirnics, K.; Korade, Z.; Porter, N.A. Dichlorophenyl piperazines, including a recently-approved atypical antipsychotic, are potent inhibitors of DHCR7, the last enzyme in cholesterol biosynthesis. Toxicol. Appl. Pharmacol. 2018, 349, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Genaro-Mattos, T.C.; Anderson, A.; Allen, L.B.; Tallman, K.A.; Porter, N.A.; Korade, Z.; Mirnics, K. Maternal cariprazine exposure inhibits embryonic and postnatal brain cholesterol biosynthesis. Mol. Psychiatry 2020, 25, 2685–2694. [Google Scholar] [CrossRef]
- Kim, J.; Neely, M.D.; Kim, H.Y.; Tallman, K.; Porter, N. 2,3-Dichlorophenylpiperazine (DCPP)-derived Antipsychotics Obstruct Cholesterol Biosynthesis in hiPSC-derived Neural Precursors and Early Postmitotic Neurons. FASEB J. 2020, 34, 1. [Google Scholar] [CrossRef]
- Speen, A.M.; Hoffman, J.R.; Kim, H.-Y.H.; Escobar, Y.N.; Nipp, G.E.; Rebuli, M.E.; Porter, N.A.; Jaspers, I. Small Molecule Antipsychotic Aripiprazole Potentiates Ozone-Induced Inflammation in Airway Epithelium. Chem. Res. Toxicol. 2019, 32, 1997–2005. [Google Scholar] [CrossRef] [PubMed]
- Korade, Z.; Allen, L.B.; Anderson, A.; Tallman, K.A.; Genaro-Mattos, T.C.; Porter, N.A.; Mirnics, K. Trazodone effects on developing brain. Transl. Psychiatry 2021, 11, 85. [Google Scholar] [CrossRef] [PubMed]
- Cenik, B.; Palka, J.M.; Thompson, B.M.; McDonald, J.G.; Tamminga, C.A.; Cenik, C.; Brown, E.S. Desmosterol and 7-dehydrocholesterol concentrations in post mortem brains of depressed people: The role of trazodone. Transl. Psychiatry 2022, 12, 139. [Google Scholar] [CrossRef] [PubMed]
- Balog, M.; Anderson, A.; Genaro-Mattos, T.C.; Korade, Z.; Mirnics, K. Individual and simultaneous treatment with antipsychotic aripiprazole and antidepressant trazodone inhibit sterol biosynthesis in the adult brain. J. Lipid Res. 2022, 63, 100249. [Google Scholar] [CrossRef] [PubMed]
- Balog, M.; Anderson, A.C.; Heffer, M.; Korade, Z.; Mirnics, K. Effects of Psychotropic Medication on Somatic Sterol Biosynthesis of Adult Mice. Biomolecules 2022, 12, 1535. [Google Scholar] [CrossRef]
- Wadman, E.; Fernandes, E.; Muss, C.; Powell-Hamilton, N.; Wojcik, M.H.; Madden, J.A.; Carreon, C.K.; Clark, R.D.; Stenftenagel, A.; Chikalard, K.; et al. A novel syndrome associated with prenatal fentanyl exposure. Genet. Med. Open 2023, 1, 100834. [Google Scholar] [CrossRef]
- Allen, L.B.; Mirnics, K. Metoprolol Inhibits Developmental Brain Sterol Biosynthesis in Mice. Biomolecules 2022, 12, 1211. [Google Scholar] [CrossRef] [PubMed]
- Theodoropoulos, P.C.; Wang, W.; Budhipramono, A.; Thompson, B.M.; Madhusudhan, N.; Mitsche, M.A.; McDonald, J.G.; De Brabander, J.K.; Nijhawan, D. A Medicinal Chemistry-Driven Approach Identified the Sterol Isomerase EBP as the Molecular Target of TASIN Colorectal Cancer Toxins. J. Am. Chem. Soc. 2020, 142, 6128–6138. [Google Scholar] [CrossRef] [PubMed]
- Ačimovič, J.; Korošec, T.; Seliškar, M.; Bjorkhem, I.; Monostory, K.; Szabo, P.; Pascussi, J.M.; Belič, A.; Urleb, U.; Kocjan, D.; et al. Inhibition of human sterol Δ7-reductase and other postlanosterol enzymes by LK-980, a novel inhibitor of cholesterol synthesis. Drug Metab. Dispos. 2011, 39, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Shin, M.H.; Lee, Y.; Kim, M.-K.; Lee, D.H.; Chung, J.H. UV increases skin-derived 1α,25-dihydroxyvitamin D 3 production, leading to MMP-1 expression by altering the balance of vitamin D and cholesterol synthesis from 7-dehydrocholesterol. J. Steroid Biochem. Mol. Biol. 2019, 195, 105449. [Google Scholar] [CrossRef] [PubMed]
- Minner, F.; Ruban, E.; Mathay, C.; Herin, M.; Poumay, Y. Involvement of DHCR7 and DHCR24 in the physiology of epidermal keratinocytes. J. Investig. Dermatol. 2009, 129, S53. [Google Scholar] [CrossRef]
- Waterham, H.R.; Koster, J.; Romeijn, G.J.; Hennekam, R.C.; Vreken, P.; Andersson, H.C.; FitzPatrick, D.R.; Kelley, R.I.; Wanders, R.J. Mutations in the 3beta-hydroxysterol Delta24-reductase gene cause desmosterolosis, an autosomal recessive disorder of cholesterol biosynthesis. Am. J. Hum. Genet. 2001, 69, 685–694. [Google Scholar] [CrossRef]
- Online Mendelian Inheritance in Man (OMIM). Johns Hopkins University, Baltimore, MD. 602398: 7/15/21. Available online: https://omim.org (accessed on 20 February 2024).
- Zolotushko, J.; Flusser, H.; Markus, B.; Shelef, I.; Langer, Y.; Heverin, M.; Björkhem, I.; Sivan, S.; Birk, O.S. The desmosterolosis phenotype: Spasticity, microcephaly and micrognathia with agenesis of corpus callosum and loss of white matter. Eur. J. Hum. Genet. 2011, 19, 942–946. [Google Scholar] [CrossRef] [PubMed]
- Schaaf, C.P.; Koster, J.; Katsonis, P.; Kratz, L.; Shchelochkov, O.A.; Scaglia, F.; Kelley, R.I.; Lichtarge, O.; Waterham, H.R.; Shinawi, M. Desmosterolosis-phenotypic and molecular characterization of a third case and review of the literature. Am. J. Med. Genet. A 2011, 155a, 1597–1604. [Google Scholar] [CrossRef] [PubMed]
- Greeve, I.; Hermans-Borgmeyer, I.; Brellinger, C.; Kasper, D.; Gomez-Isla, T.; Behl, C.; Levkau, B.; Nitsch, R.M. The human DIMINUTO/DWARF1 homolog seladin-1 confers resistance to Alzheimer’s disease-associated neurodegeneration and oxidative stress. J. Neurosci. Off. J. Soc. Neurosci. 2000, 20, 7345–7352. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Miloslavskaya, I.; Demontis, S.; Maestro, R.; Galaktionov, K. Regulation of cellular response to oncogenic and oxidative stress by Seladin-1. Nature 2004, 432, 640–645. [Google Scholar] [CrossRef] [PubMed]
- Takano, T.; Tsukiyama-Kohara, K.; Hayashi, M.; Hirata, Y.; Satoh, M.; Tokunaga, Y.; Tateno, C.; Hayashi, Y.; Hishima, T.; Funata, N.; et al. Augmentation of DHCR24 expression by hepatitis C virus infection facilitates viral replication in hepatocytes. J. Hepatol. 2011, 55, 512–521. [Google Scholar] [CrossRef] [PubMed]
- Spann, N.J.; Garmire, L.X.; McDonald, J.G.; Myers, D.S.; Milne, S.B.; Shibata, N.; Reichart, D.; Fox, J.N.; Shaked, I.; Heudobler, D.; et al. Regulated accumulation of desmosterol integrates macrophage lipid metabolism and inflammatory responses. Cell 2012, 151, 138–152. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Kambe, F.; Cao, X.; Kozaki, Y.; Kaji, T.; Ishii, T.; Seo, H. 3β-Hydroxysteroid-Δ24 Reductase Is a Hydrogen Peroxide Scavenger, Protecting Cells from Oxidative Stress-Induced Apoptosis. Endocrinology 2008, 149, 3267–3273. [Google Scholar] [CrossRef]
- Rodgers, M.A.; Villareal, V.A.; Schaefer, E.A.; Peng, L.F.; Corey, K.E.; Chung, R.T.; Yang, P.L. Lipid metabolite profiling identifies desmosterol metabolism as a new antiviral target for hepatitis C virus. J. Am. Chem. Soc. 2012, 134, 6896–6899. [Google Scholar] [CrossRef]
- Mirza, R.; Qiao, S.; Tateyama, K.; Miyamoto, T.; Xiuli, L.; Seo, H. 3β-Hydroxysterol-Delta24 reductase plays an important role in long bone growth by protecting chondrocytes from reactive oxygen species. J. Bone Miner. Metab. 2012, 30, 144–153. [Google Scholar] [CrossRef]
- Spann, N.J.; Glass, C.K. Sterols and oxysterols in immune cell function. Nat. Immunol. 2013, 14, 893–900. [Google Scholar] [CrossRef]
- Muller, C.; Hank, E.; Giera, M.; Bracher, F. Dehydrocholesterol Reductase 24 (DHCR24): Medicinal Chemistry, Pharmacology and Novel Therapeutic Options. Curr. Med. Chem. 2022, 29, 4005–4025. [Google Scholar] [CrossRef] [PubMed]
- Dai, M.; Zhu, X.L.; Liu, F.; Xu, Q.Y.; Ge, Q.L.; Jiang, S.H.; Yang, X.M.; Li, J.; Wang, Y.H.; Wu, Q.K.; et al. Cholesterol Synthetase DHCR24 Induced by Insulin Aggravates Cancer Invasion and Progesterone Resistance in Endometrial Carcinoma. Sci. Rep. 2017, 7, 41404. [Google Scholar] [CrossRef]
- Lee, G.T.; Ha, Y.S.; Jung, Y.S.; Moon, S.K.; Kang, H.W.; Lee, O.J.; Joung, J.Y.; Choi, Y.H.; Yun, S.J.; Kim, W.J.; et al. DHCR24 is an independent predictor of progression in patients with non-muscle-invasive urothelial carcinoma, and its functional role is involved in the aggressive properties of urothelial carcinoma cells. Ann. Surg. Oncol. 2014, 21 (Suppl. S4), S538–S545. [Google Scholar] [CrossRef] [PubMed]
- Quan, X.; Chen, X.; Sun, D.; Xu, B.; Zhao, L.; Shi, X.; Liu, H.; Gao, B.; Lu, X. The mechanism of the effect of U18666a on blocking the activity of 3β-hydroxysterol Δ-24-reductase (DHCR24): Molecular dynamics simulation study and free energy analysis. J. Mol. Model. 2016, 22, 46. [Google Scholar] [CrossRef]
- Liu, T.; Li, Y.; Yang, B.; Wang, H.; Lu, C.; Chang, A.K.; Huang, X.; Zhang, X.; Lu, Z.; Lu, X.; et al. Suppression of neuronal cholesterol biosynthesis impairs brain functions through insulin-like growth factor I-Akt signaling. Int. J. Biol. Sci. 2021, 17, 3702–3716. [Google Scholar] [CrossRef]
- Hernández-Jiménez, M.; Martínez-López, D.; Gabandé-Rodríguez, E.; Martín-Segura, A.; Lizasoain, I.; Ledesma, M.D.; Dotti, C.G.; Moro, M.A. Seladin-1/DHCR24 is neuroprotective by associating EAAT2 glutamate transporter to lipid rafts in experimental stroke. Stroke J. Cereb. Circ. 2016, 47, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Hagiwara, K.; Nakamura, Y.; Nishijima, M.; Yamakawa, Y. Prevention of prion propagation by dehydrocholesterol reductase inhibitors in cultured cells and a therapeutic trial in mice. Biol. Pharm. Bull. 2007, 30, 835–838. [Google Scholar] [CrossRef] [PubMed]
- Stamatakos, S.; Beretta, G.L.; Vergani, E.; Dugo, M.; Corno, C.; Corna, E.; Tinelli, S.; Frigerio, S.; Ciusani, E.; Rodolfo, M.; et al. Deregulated FASN expression in BRAF inhibitor-resistant melanoma cells unveils new targets for drug combinations. Cancers 2021, 13, 2284. [Google Scholar] [CrossRef] [PubMed]
- Tian, K.Y.; Chang, H.M.; Wang, J.; Qi, M.H.; Wang, W.L.; Qiu, Y.; Liang, K.; Chen, F.Q.; Zha, D.J.; Qiu, J.H. Inhibition of DHCR24 increases the cisplatin-induced damage to cochlear hair cells in vitro. Neurosci. Lett. 2019, 706, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Müller, C.; Hemmers, S.; Bartl, N.; Plodek, A.; Körner, A.; Mirakaj, V.; Giera, M.; Bracher, F. New chemotype of selective and potent inhibitors of human delta 24-dehydrocholesterol reductase. Eur. J. Med. Chem. 2017, 140, 305–320. [Google Scholar] [CrossRef] [PubMed]
- Körner, A.; Zhou, E.; Müller, C.; Mohammed, Y.; Herceg, S.; Bracher, F.; Rensen, P.C.N.; Wang, Y.; Mirakaj, V.; Giera, M. Inhibition of Δ24-dehydrocholesterol reductase activates pro-resolving lipid mediator biosynthesis and inflammation resolution. Proc. Natl. Acad. Sci. USA 2019, 116, 20623–20634. [Google Scholar] [CrossRef]
- Zhou, E.; Ge, X.; Nakashima, H.; Li, R.; van der Zande, H.J.P.; Liu, C.; Li, Z.; Müller, C.; Bracher, F.; Mohammed, Y.; et al. Inhibition of DHCR24 activates LXRα to ameliorate hepatic steatosis and inflammation. EMBO Mol. Med. 2023, 15, e16845. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Lu, Z.; Li, Y.; Liu, T.; Zhao, L.; Gao, T.; Lu, X.; Gao, B. Virtual Screening of Novel 24-Dehydroxysterol Reductase (DHCR24) Inhibitors and the Biological Evaluation of Irbesartan in Cholesterol-Lowering Effect. Molecules 2023, 28, 2634. [Google Scholar] [CrossRef] [PubMed]
- Allen, L.B.; Genaro-Mattos, T.C.; Anderson, A.; Porter, N.A.; Mirnics, K.; Korade, Z. Amiodarone Alters Cholesterol Biosynthesis through Tissue-Dependent Inhibition of Emopamil Binding Protein and Dehydrocholesterol Reductase 24. ACS Chem. Neurosci. 2020, 11, 1413–1423. [Google Scholar] [CrossRef] [PubMed]
- Luu, W.; Zerenturk, E.J.; Kristiana, I.; Bucknall, M.P.; Sharpe, L.J.; Brown, A.J. Signaling regulates activity of DHCR24, the final enzyme in cholesterol synthesis. J. Lipid Res. 2014, 55, 410–420. [Google Scholar] [CrossRef] [PubMed]
- Zerenturk, E.; Sharpe, L.J.; Kristiana, I.; Brown, A.J. DHCR24: Unexpected new directions for a terminal step in cholesterol synthesis. FASEB J. 2013, 27, 822.2. [Google Scholar] [CrossRef]
- Zerenturk, E.J.; Kristiana, I.; Gill, S.; Brown, A.J. The endogenous regulator 24(S),25-epoxycholesterol inhibits cholesterol synthesis at DHCR24 (Seladin-1). Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2012, 1821, 1269–1277. [Google Scholar] [CrossRef] [PubMed]
- Phull, M.S.; Jadav, S.S.; Gundla, R.; Mainkar, P.S. A perspective on medicinal chemistry approaches towards adenomatous polyposis coli and Wnt signal based colorectal cancer inhibitors. Eur. J. Med. Chem. 2021, 212, 113149. [Google Scholar] [CrossRef] [PubMed]
- Taiwo, O.A.; Dosumu, O.A.; Oni, E.O.; Akomolafe, V.O.; Elazab, S.T.; Qusti, S.; Alshammari, E.M.; Batiha, G.E.S.; Ojo, O.A. Preclinical prediction of phytochemicals identified from cannabis as novel inhibitors of TEX 11, DHCR24, and CatSper 1 in fertility drug design. Inform. Med. Unlocked 2021, 26, 100747. [Google Scholar] [CrossRef]
- Wu, J.; Guo, L.; Qiu, X.; Ren, Y.; Li, F.; Cui, W.; Song, S. Genkwadaphnin inhibits growth and invasion in hepatocellular carcinoma by blocking DHCR24-mediated cholesterol biosynthesis and lipid rafts formation. Br. J. Cancer 2020, 123, 1673–1685. [Google Scholar] [CrossRef] [PubMed]
- Nakano-Ito, K.; Fujikawa, Y.; Hihara, T.; Shinjo, H.; Kotani, S.; Suganuma, A.; Aoki, T.; Tsukidate, K. E2012-induced cataract and its predictive biomarkers. Toxicol. Sci. 2014, 137, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Giannini, S.; Benvenuti, S.; Luciani, P.; Manuelli, C.; Cellai, I.; Deledda, C.; Pezzatini, A.; Vannelli, G.B.; Maneschi, E.; Rotella, C.M.; et al. Intermittent high glucose concentrations reduce neuronal precursor survival by altering the IGF system: The involvement of the neuroprotective factor DHCR24 (Seladin-1). J. Endocrinol. 2008, 198, 523–532. [Google Scholar] [CrossRef] [PubMed]
- Baker, C.H.; Matsuda, S.P.; Liu, D.R.; Corey, E.J. Molecular cloning of the human gene encoding lanosterol synthase from a liver cDNA library. Biochem. Biophys. Res. Commun. 1995, 213, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Roessler, E.; Mittaz, L.; Du, Y.; Scott, H.S.; Chang, J.; Rossier, C.; Guipponi, M.; Matsuda, S.P.; Muenke, M.; Antonarakis, S.E. Structure of the human Lanosterol synthase gene and its analysis as a candidate for holoprosencephaly (HPE1). Hum. Genet. 1999, 105, 489–495. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Online Mendelian Inheritance in Man (OMIM). Johns Hopkins University, Baltimore, MD. 600909: 2/10/22. Available online: https://omim.org (accessed on 20 February 2024).
- Online Mendelian Inheritance in Man (OMIM). Johns Hopkins University, Baltimore, MD. 618275: 12/27/21. Available online: https://omim.org (accessed on 20 February 2024).
- Online Mendelian Inheritance in Man (OMIM). Johns Hopkins University, Baltimore, MD. 618840: 1/2/24. Available online: https://omim.org (accessed on 20 February 2024).
- Zhao, L.; Chen, X.J.; Zhu, J.; Xi, Y.B.; Yang, X.; Hu, L.D.; Ouyang, H.; Patel, S.H.; Jin, X.; Lin, D.; et al. Lanosterol reverses protein aggregation in cataracts. Nature 2015, 523, 607–611. [Google Scholar] [CrossRef] [PubMed]
- Romano, M.T.; Tafazzoli, A.; Mattern, M.; Sivalingam, S.; Wolf, S.; Rupp, A.; Thiele, H.; Altmüller, J.; Nürnberg, P.; Ellwanger, J.; et al. Bi-allelic Mutations in LSS, Encoding Lanosterol Synthase, Cause Autosomal-Recessive Hypotrichosis Simplex. Am. J. Hum. Genet. 2018, 103, 777–785. [Google Scholar] [CrossRef] [PubMed]
- Mori, M.; Li, G.; Abe, I.; Nakayama, J.; Guo, Z.; Sawashita, J.; Ugawa, T.; Nishizono, S.; Serikawa, T.; Higuchi, K.; et al. Lanosterol synthase mutations cause cholesterol deficiency-associated cataracts in the Shumiya cataract rat. J. Clin. Investig. 2006, 116, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, M.E.; Flenniken, A.M.; Ji, X.; Teboul, L.; Wong, M.D.; White, J.K.; Meehan, T.F.; Weninger, W.J.; Westerberg, H.; Adissu, H.; et al. High-throughput discovery of novel developmental phenotypes. Nature 2016, 537, 508–514. [Google Scholar] [CrossRef] [PubMed]
- Karunagaran, S.; Kavitha, R.; Vadivelu, M.; Lee, K.W.; Meganathan, C. Insight mechanism of the selective lanosterol synthase inhibitor: Molecular modeling, docking and density functional theory approaches. Curr. Comput.-Aided Drug Des. 2017, 13, 275–293. [Google Scholar] [CrossRef] [PubMed]
- Wallace, I.M.; Urbanus, M.L.; Luciani, G.M.; Burns, A.R.; Han, M.K.L.; Wang, H.; Arora, K.; Heisler, L.E.; Proctor, M.; St Onge, R.P.; et al. Compound prioritization methods increase rates of chemical probe discovery in model organisms. Chem. Biol. 2011, 18, 1273–1283. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, R.; Sakano, Y.; Shimizu, K.; Shibuya, M.; Ebizuka, Y.; Goda, Y. Constituents of Laurus nobilis L. inhibit recombinant human lanosterol synthase. J. Nat. Med. 2006, 60, 78–81. [Google Scholar] [CrossRef]
- McCrae, C.; Dzgoev, A.; Ståhlman, M.; Horndahl, J.; Svärd, R.; Große, A.; Großkopf, T.; Skujat, M.-A.; Williams, N.; Schubert, S.; et al. Lanosterol Synthase Regulates Human Rhinovirus Replication in Human Bronchial Epithelial Cells. Am. J. Respir. Cell Mol. Biol. 2018, 59, 713–722. [Google Scholar] [CrossRef]
- Bobba, R.K.; Benakanakere, I.; Bearelly, S.; Arya, M.; Sleigtholm, R.; Freter, C. Mevalonate pathway inhibitors in the treatment of B-cell chronic lymphocytic leukemia. J. Clin. Oncol. 2012, 30, 6561. [Google Scholar] [CrossRef]
- Nguyen, T.P.; Wang, W.; Sternisha, A.C.; Corley, C.D.; Wang, H.Y.L.; Wang, X.; Ortiz, F.; Lim, S.K.; Abdullah, K.G.; Parada, L.F.; et al. Selective and brain-penetrant lanosterol synthase inhibitors target glioma stem-like cells by inducing 24(S),25-epoxycholesterol production. Cell Chem. Biol. 2023, 30, 214–229.e218. [Google Scholar] [CrossRef] [PubMed]
- Castro, A.; Otero, C.; Dumrauf, B.; Rodenak-Kladniew, B.; Montero-Villegas, S.; Viña, S.; García De Bravo, M.; Crespo, R. Effect of essential oils from introduced and local plants on cholesterol metabolism in hepatic and foam cells. A search for natural antiatherogenic compounds. Biocell 2019, 43, 99. [Google Scholar]
- Sakano, Y.; Shibuya, M.; Yamaguchi, Y.; Masuma, R.; Tomoda, H.; Omura, S.; Ebizuka, Y. Epohelmins A and B, novel lanosterol synthase inhibitors from a fungal strain FKI-0929. J. Antibiot. 2004, 57, 564–568. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tanaka, R.; Sakano, Y.; Nagatsu, A.; Shibuya, M.; Ebizuka, Y.; Goda, Y. Synthesis of digalactosyl diacylglycerols and their structure-inhibitory activity on human lanosterol synthase. Bioorg. Med. Chem. Lett. 2005, 15, 159–162. [Google Scholar] [CrossRef] [PubMed]
- Online Mendelian Inheritance in Man (OMIM). Johns Hopkins University, Baltimore, MD. 616834: 1/13/22. Available online: https://omim.org (accessed on 20 February 2024).
- He, M.; Kratz, L.E.; Michel, J.J.; Vallejo, A.N.; Ferris, L.; Kelley, R.I.; Hoover, J.J.; Jukic, D.; Gibson, K.M.; Wolfe, L.A.; et al. Mutations in the human SC4MOL gene encoding a methyl sterol oxidase cause psoriasiform dermatitis, microcephaly, and developmental delay. J. Clin. Investig. 2011, 121, 976–984. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Steere, L.; Zhang, Y.-K.; McGregor, C.; Hahne, C.; Zhou, Y.; Liu, C.; Cai, Y.; Zhou, H.; Chen, X.; et al. Inhibiting C-4 Methyl Sterol Oxidase with Novel Diazaborines to Target Fungal Plant Pathogens. ACS Chem. Biol. 2022, 17, 1343–1350. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wang, Q.; Wu, X.; Jiang, M.; Jin, H.; Tao, K.; Hou, T. Unraveling the polypharmacology of a natural antifungal product, eugenol, against Rhizoctonia solani. Pest. Manag. Sci. 2021, 77, 3469–3483. [Google Scholar] [CrossRef] [PubMed]
- Sukhanova, A.; Gorin, A.; Serebriiskii, I.G.; Gabitova, L.; Zheng, H.; Restifo, D.; Egleston, B.L.; Cunningham, D.; Bagnyukova, T.; Liu, H.; et al. Targeting C4-demethylating genes in the cholesterol pathway sensitizes cancer cells to EGF receptor inhibitors via increased EGF receptor degradation. Cancer Discov. 2013, 3, 96–112. [Google Scholar] [CrossRef] [PubMed]
- Pleshinger, M.J.; Friedrich, R.M.; Hubler, Z.; Rivera-León, A.M.; Gao, F.; Yan, D.; Sax, J.L.; Srinivasan, R.; Bederman, I.; Shick, H.E.; et al. Inhibition of SC4MOL and HSD17B7 shifts cellular sterol composition and promotes oligodendrocyte formation. RSC Chem. Biol. 2022, 3, 56–68. [Google Scholar] [CrossRef] [PubMed]
- Braverman, N.; Lin, P.; Moebius, F.F.; Obie, C.; Moser, A.; Glossmann, H.; Wilcox, W.R.; Rimoin, D.L.; Smith, M.; Kratz, L.; et al. Mutations in the gene encoding 3 beta-hydroxysteroid-delta 8, delta 7-isomerase cause X-linked dominant Conradi-Hünermann syndrome. Nat. Genet. 1999, 22, 291–294. [Google Scholar] [CrossRef] [PubMed]
- Online Mendelian Inheritance in Man (OMIM). Johns Hopkins University, Baltimore, MD. 302960: 3/2/22. Available online: https://omim.org (accessed on 20 February 2024).
- Herman, G.E.; Kelley, R.I.; Pureza, V.; Smith, D.; Kopacz, K.; Pitt, J.; Sutphen, R.; Sheffield, L.J.; Metzenberg, A.B. Characterization of mutations in 22 females with X-linked dominant chondrodysplasia punctata (Happle syndrome). Genet. Med. Off. J. Am. Coll. Med. Genet. 2002, 4, 434–438. [Google Scholar] [CrossRef] [PubMed]
- Online Mendelian Inheritance in Man (OMIM). Johns Hopkins University, Baltimore, MD. 300960: 2/6/23. Available online: https://omim.org (accessed on 20 February 2024).
- Long, T.; Hassan, A.; Thompson, B.M.; McDonald, J.G.; Wang, J.; Li, X. Structural basis for human sterol isomerase in cholesterol biosynthesis and multidrug recognition. Nat. Commun. 2019, 10, 2452. [Google Scholar] [CrossRef]
- Moebius, F.F.; Reiter, R.J.; Bermoser, K.; Glossmann, H.; Cho, S.Y.; Paik, Y.K. Pharmacological analysis of sterol delta8-delta7 isomerase proteins with [3H]ifenprodil. Mol. Pharmacol. 1998, 54, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Berardi, F.; Abate, C.; Ferorelli, S.; De Robertis, A.F.; Leopoldo, M.; Colabufo, N.A.; Niso, M.; Perrone, R. Novel 4-(4-aryl)cyclohexyl-1-(2-pyridyl)piperazines as Δ8–Δ7 sterol isomerase (emopamil binding protein) selective ligands with antiproliferative activity. J. Med. Chem. 2008, 51, 7523–7531. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Theodoropoulos, P.C.; Eskiocak, U.; Wang, W.; Moon, Y.-A.; Posner, B.; Williams, N.S.; Wright, W.E.; Kim, S.B.; Nijhawan, D. Selective targeting of mutant adenomatous polyposis coli (APC) in colorectal cancer. Sci. Transl. Med. 2016, 8, 361ra140. [Google Scholar] [CrossRef]
- Allimuthu, D.; Hubler, Z.; Najm, F.J.; Tang, H.; Bederman, I.; Seibel, W.; Tesar, P.J.; Adams, D.J. Diverse Chemical Scaffolds Enhance Oligodendrocyte Formation by Inhibiting CYP51, TM7SF2, or EBP. Cell Chem. Biol. 2019, 26, 593–599.e594. [Google Scholar] [CrossRef]
- Hubler, Z.; Allimuthu, D.; Bederman, I.; Elitt, M.S.; Madhavan, M.; Allan, K.C.; Shick, H.E.; Garrison, E.; Karl, M.T.; Factor, D.C.; et al. Accumulation of 8,9-unsaturated sterols drives oligodendrocyte formation and remyelination. Nature 2018, 560, 372–376. [Google Scholar] [CrossRef]
- Sax, J.L.; Hershman, S.N.; Hubler, Z.; Allimuthu, D.; Elitt, M.S.; Bederman, I.; Adams, D.J. Enhancers of Human and Rodent Oligodendrocyte Formation Predominantly Induce Cholesterol Precursor Accumulation. ACS Chem. Biol. 2022, 17, 2188–2200. [Google Scholar] [CrossRef]
- Sheng, G.; Wang, D.; Giera, S.; Pan, Q.; Farley, J.; Radzwill, K.; Garron, C.; Byrne, A.; Merriman, G.; Samad, T.; et al. Emopamil binding protein inhibition as a remyelination therapy for multiple sclerosis. Mult. Scler. J. 2019, 25, 911. [Google Scholar] [CrossRef]
- Sax, J.L.; Hubler, Z.; Allimuthu, D.; Adams, D.J. Screening Reveals Sterol Derivatives with Pro-Differentiation, Pro-Survival, or Potent Cytotoxic Effects on Oligodendrocyte Progenitor Cells. ACS Chem. Biol. 2021, 16, 1288–1297. [Google Scholar] [CrossRef]
- Hubler, Z.; Friedrich, R.M.; Sax, J.L.; Allimuthu, D.; Gao, F.; Rivera-León, A.M.; Pleshinger, M.J.; Bederman, I.; Adams, D.J. Modulation of lanosterol synthase drives 24,25-epoxysterol synthesis and oligodendrocyte formation. Cell Chem. Biol. 2021, 28, 866–875.e865. [Google Scholar] [CrossRef]
- Lamb, D.C.; Kelly, D.E.; Kelly, S.L. Molecular diversity of sterol 14alpha-demethylase substrates in plants, fungi and humans. FEBS Lett. 1998, 425, 263–265. [Google Scholar] [CrossRef]
- Korade, Z.; Kim, H.Y.; Tallman, K.A.; Liu, W.; Koczok, K.; Balogh, I.; Xu, L.; Mirnics, K.; Porter, N.A. The Effect of Small Molecules on Sterol Homeostasis: Measuring 7-Dehydrocholesterol in Dhcr7-Deficient Neuro2a Cells and Human Fibroblasts. J. Med. Chem. 2016, 59, 1102–1115. [Google Scholar] [CrossRef]
- Hankins, E.G.; Gillespie, J.R.; Aikenhead, K.; Buckner, F.S. Upregulation of sterol C14-demethylase expression in Trypanosoma cruzi treated with sterol biosynthesis inhibitors. Mol. Biochem. Parasitol. 2005, 144, 68–75. [Google Scholar] [CrossRef]
- Parker, J.E.; Merkamm, M.; Manning, N.J.; Pompon, D.; Kelly, S.L.; Kelly, D.E. Differential azole antifungal efficacies contrasted using a Saccharomyces cerevisiae strain humanized for sterol 14 alpha-demethylase at the homologous locus. Antimicrob. Agents Chemother. 2008, 52, 3597–3603. [Google Scholar] [CrossRef]
- Lamb, D.C.; Kelly, D.E.; Waterman, M.R.; Stromstedt, M.; Rozman, D.; Kelly, S.L. Characteristics of the heterologously expressed human lanosterol 14alpha-demethylase (other names: P45014DM, CYP51, P45051) and inhibition of the purified human and Candida albicans CYP51 with azole antifungal agents. Yeast 1999, 15, 755–763. [Google Scholar] [CrossRef]
- Zhang, J.; Li, L.; Lv, Q.; Yan, L.; Wang, Y.; Jiang, Y. The Fungal CYP51s: Their Functions, Structures, Related Drug Resistance, and Inhibitors. Front. Microbiol. 2019, 10, 691. [Google Scholar] [CrossRef]
- Chen, Z.F.; Ying, G.G. Occurrence, fate and ecological risk of five typical azole fungicides as therapeutic and personal care products in the environment: A review. Environ. Int. 2015, 84, 142–153. [Google Scholar] [CrossRef]
- Mogensen, D.M.; Pihl, M.B.; Skakkebæk, N.E.; Andersen, H.R.; Juul, A.; Kyhl, H.B.; Swan, S.; Kristensen, D.M.; Andersen, M.S.; Lind, D.V.; et al. Prenatal exposure to antifungal medication may change anogenital distance in male offspring: A preliminary study. Environ. Health 2017, 16, 68. [Google Scholar] [CrossRef]
- Schoelwer, M.; Eugster, E.A. Treatment of Peripheral Precocious Puberty. Endocr. Dev. 2016, 29, 230–239. [Google Scholar] [CrossRef]
- Brunetti-Pierri, N.; Corso, G.; Rossi, M.; Ferrari, P.; Balli, F.; Rivasi, F.; Annunziata, I.; Ballabio, A.; Russo, A.D.; Andria, G.; et al. Lathosterolosis, a novel multiple-malformation/mental retardation syndrome due to deficiency of 3beta-hydroxysteroid-delta5-desaturase. Am. J. Hum. Genet. 2002, 71, 952–958. [Google Scholar] [CrossRef]
- Schade, D.S.; Shey, L.; Eaton, R.P. Cholesterol Review: A Metabolically Important Molecule. Endocr. Pract. 2020, 26, 1514–1523. [Google Scholar] [CrossRef]
- Sharpe, L.J.; Brown, A.J. Controlling cholesterol synthesis beyond 3-hydroxy-3-methylglutaryl-CoA reductase (HMGCR). J. Biol. Chem. 2013, 288, 18707–18715. [Google Scholar] [CrossRef]
- Wood, W.G.; Igbavboa, U.; Eckert, G.; Müller, W. Cholesterol-a Janus-faced molecule in the central nervous system. In Handbook of Neurochemistry and Molecular Neurobiology: Neural Membranes and Transport; Springer: Boston, MA, USA, 2007; pp. 151–170. [Google Scholar]
- Brown, M.S.; Radhakrishnan, A.; Goldstein, J.L. Retrospective on Cholesterol Homeostasis: The Central Role of Scap. Annu. Rev. Biochem. 2018, 87, 783–807. [Google Scholar] [CrossRef]
- Benachenhou, S.; Laroui, A.; Dionne, O.; Rojas, D.; Toupin, A.; Çaku, A. Cholesterol alterations in fragile X syndrome, autism spectrum disorders and other neurodevelopmental disorders. Int. Rev. Neurobiol. 2023, 173, 115–139. [Google Scholar] [CrossRef]
- Ikonen, E.; Zhou, X. Cholesterol transport between cellular membranes: A balancing act between interconnected lipid fluxes. Dev. Cell 2021, 56, 1430–1436. [Google Scholar] [CrossRef]
- Griffiths, W.J.; Wang, Y. Sterols, Oxysterols, and Accessible Cholesterol: Signalling for Homeostasis, in Immunity and during Development. Front. Physiol. 2021, 12, 723224. [Google Scholar] [CrossRef]
- Griffiths, W.J.; Wang, Y. Cholesterol metabolism: From lipidomics to immunology. J. Lipid Res. 2022, 63, 100165. [Google Scholar] [CrossRef]
- Simons, K.; Ehehalt, R. Cholesterol, lipid rafts, and disease. J. Clin. Investig. 2002, 110, 597–603. [Google Scholar] [CrossRef]
- Ačimovič, J.; Goyal, S.; Košir, R.; Goličnik, M.; Perše, M.; Belič, A.; Urlep, Ž.; Guengerich, F.P.; Rozman, D. Cytochrome P450 metabolism of the post-lanosterol intermediates explains enigmas of cholesterol synthesis. Sci. Rep. 2016, 6, 28462. [Google Scholar] [CrossRef]
- Xu, S.; Tang, C. Cholesterol and Hedgehog Signaling: Mutual Regulation and Beyond. Front. Cell Dev. Biol. 2022, 10, 774291. [Google Scholar] [CrossRef]
- Ghersi, D.; Genaro-Mattos, T.C. Identifying Molecular Fragments That Drive 7-Dehydrocholesterol Elevation. ACS Pharm. Transl. Sci. 2022, 5, 3–7. [Google Scholar] [CrossRef]
- McAinsh, J.; Cruickshank, J.M. Beta-blockers and central nervous system side effects. Pharmacol. Ther. 1990, 46, 163–197. [Google Scholar] [CrossRef]
- Shek, E.; Bardhan, S.; Cheine, M.V.; Ahonen, J.; Wahlbeck, K. Beta-blocker supplementation of standard drug treatment for schizophrenia. Schizophr. Bull. 2010, 36, 1079–1080. [Google Scholar] [CrossRef]
- Miller, C.H.; Fleischhacker, W.W. Managing antipsychotic-induced acute and chronic akathisia. Drug Saf. 2000, 22, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Stransky, A.; Tierney, E. Cognitive and behavioral aspects of Smith-Lemli-Opitz syndrome. Am. J. Med. Genet. C Semin. Med. Genet. 2012, 160c, 295–300. [Google Scholar] [CrossRef]
- Thurm, A.; Tierney, E.; Farmer, C.; Albert, P.; Joseph, L.; Swedo, S.; Bianconi, S.; Bukelis, I.; Wheeler, C.; Sarphare, G.; et al. Development, behavior, and biomarker characterization of Smith-Lemli-Opitz syndrome: An update. J. Neurodev. Disord. 2016, 8, 12. [Google Scholar] [CrossRef] [PubMed]
- Tierney, E.; Nwokoro, N.A.; Kelley, R.I. Behavioral phenotype of RSH/Smith-Lemli-Opitz syndrome. Ment. Retard. Dev. Disabil. Res. Rev. 2000, 6, 131–134. [Google Scholar] [CrossRef] [PubMed]
- Lamberson, C.R.; Muchalski, H.; McDuffee, K.B.; Tallman, K.A.; Xu, L.; Porter, N.A. Propagation rate constants for the peroxidation of sterols on the biosynthetic pathway to cholesterol. Chem. Phys. Lipids 2017, 207, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Genaro-Mattos, T.C.; Klingelsmith, K.B.; Allen, L.B.; Anderson, A.; Tallman, K.A.; Porter, N.A.; Korade, Z.; Mirnics, K. Sterol Biosynthesis Inhibition in Pregnant Women Taking Prescription Medications. ACS Pharm. Transl. Sci. 2021, 4, 848–857. [Google Scholar] [CrossRef] [PubMed]
- Chikalard, K.; Stenftenagel, A.; Kimonis, V. Smith-Lemli-Opitz Phenotype with Transiently Abnormal Sterols in the Setting of Perinatal Fentanyl Exposure, a Case Report. J. Investig. Med. 2023, 71, 327. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peeples, E.S.; Mirnics, K.; Korade, Z. Chemical Inhibition of Sterol Biosynthesis. Biomolecules 2024, 14, 410. https://doi.org/10.3390/biom14040410
Peeples ES, Mirnics K, Korade Z. Chemical Inhibition of Sterol Biosynthesis. Biomolecules. 2024; 14(4):410. https://doi.org/10.3390/biom14040410
Chicago/Turabian StylePeeples, Eric S., Karoly Mirnics, and Zeljka Korade. 2024. "Chemical Inhibition of Sterol Biosynthesis" Biomolecules 14, no. 4: 410. https://doi.org/10.3390/biom14040410
APA StylePeeples, E. S., Mirnics, K., & Korade, Z. (2024). Chemical Inhibition of Sterol Biosynthesis. Biomolecules, 14(4), 410. https://doi.org/10.3390/biom14040410






