Research Progress on Sesquiterpenoids of Curcumae Rhizoma and Their Pharmacological Effects
Abstract
1. Introduction
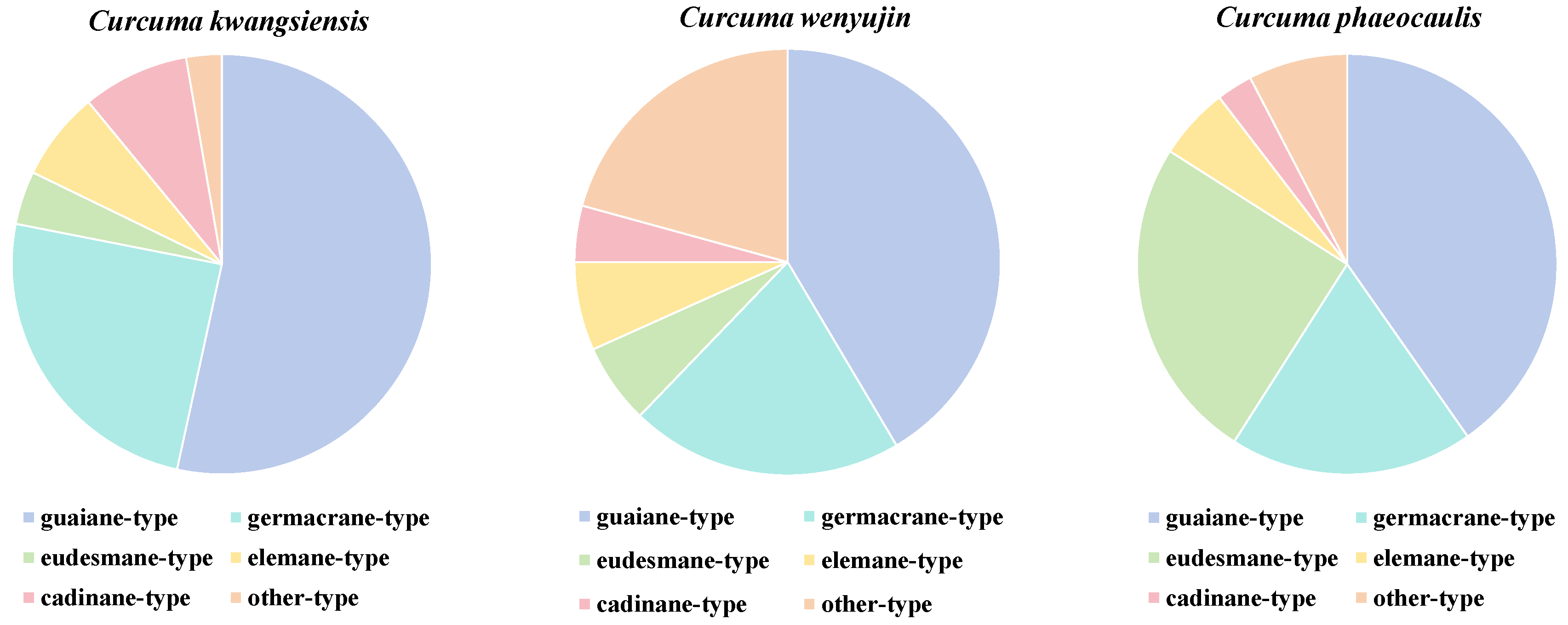
2. Medicinal Plants of Curcumae Rhizoma
3. Chemical Composition of Curcumae Rhizoma
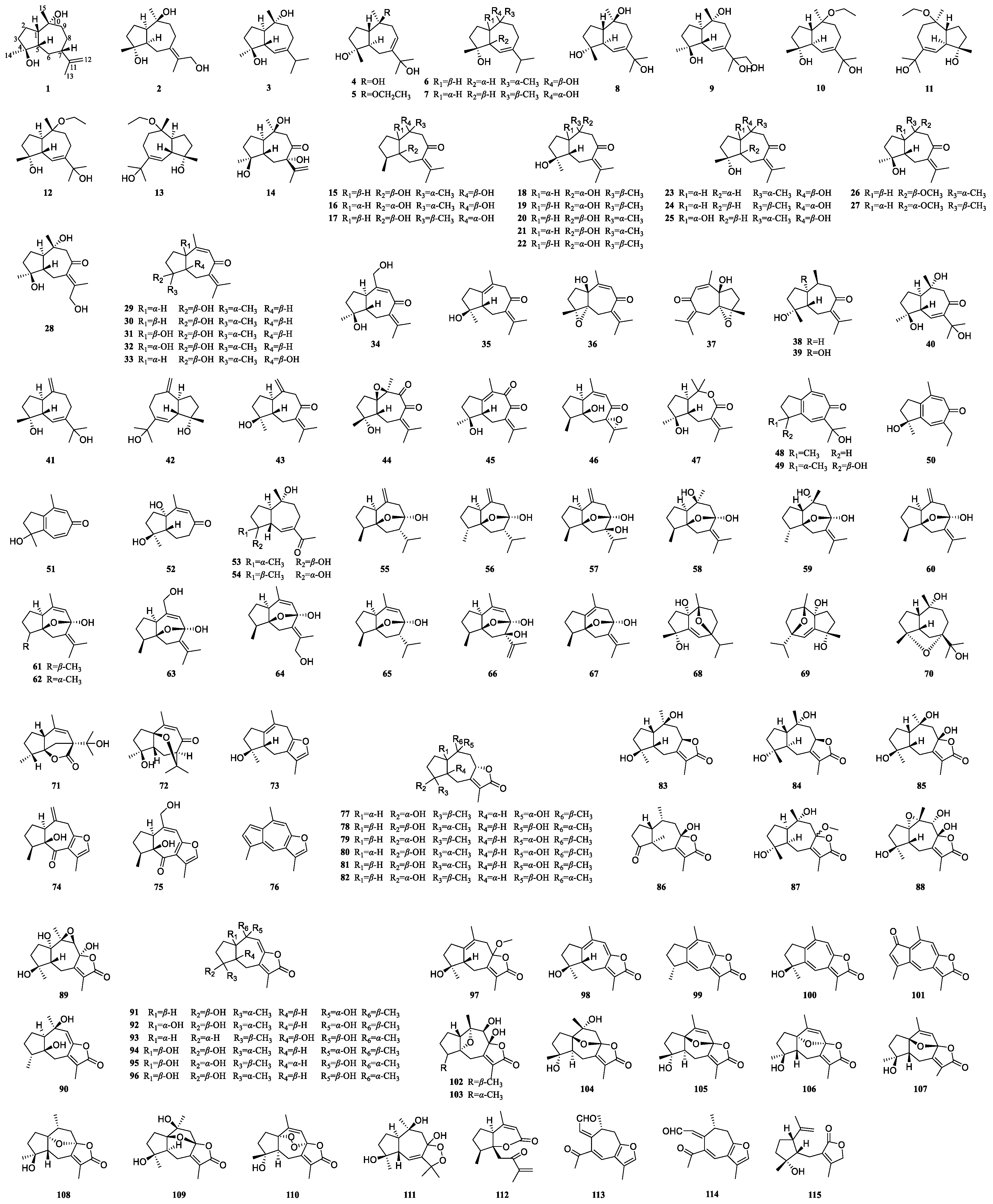
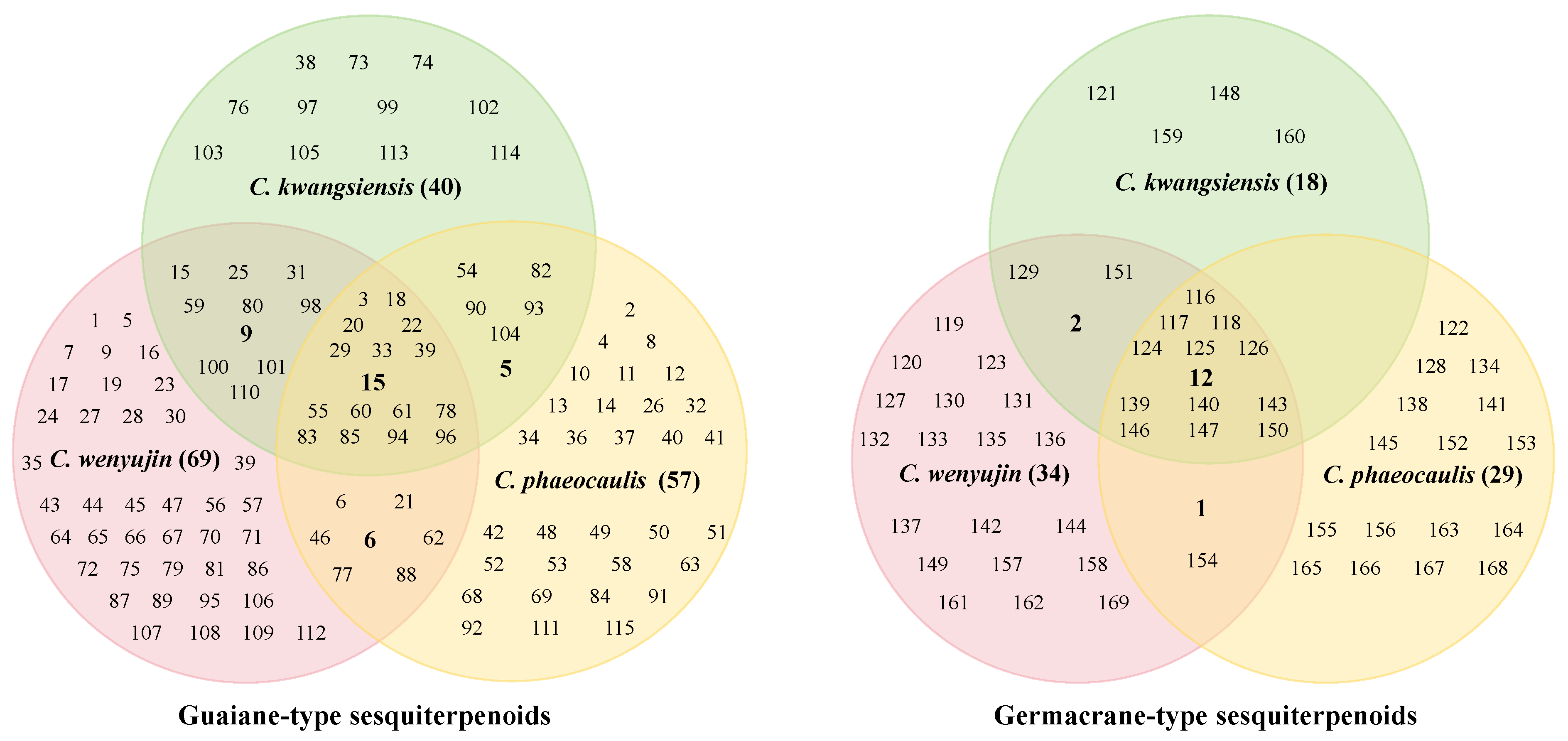
3.1. Guaiane-Type Sesquiterpenoids of Curcumae Rhizoma
| No. | Compounds | Medicinal Source | Reference |
|---|---|---|---|
| 1 | epi-Guaidiol A | C. wenyujin | [27] |
| 2 | Phaeocaulisguatriol | C. phaeocaulis | [28] |
| 3 | Alismoxide | C. wenyujin, C. phaeocaulis, C. kwangsiensis | [12,16,29,30] |
| 4 | 4α,10α,11-Trihydroxy-1βH,5βH-guai-7(8)-ene | C. phaeocaulis | [28] |
| 5 | Wenyujinol E | C. wenyujin | [27] |
| 6 | Guaianediol | C. phaeocaulis, C. wenyujin | [27,28] |
| 7 | 6-Guaiene-4α,10α-diol | C. wenyujin | [31] |
| 8 | 4α,10β,11-Trihydroxy-1,5-trans-guai-6-ene | C. phaeocaulis | [28] |
| 9 | Wenyujinol N | C. wenyujin | [32] |
| 10 | (+)-Phaeocauline A | C. phaeocaulis | [33] |
| 11 | (−)-Phaeocauline A | C. phaeocaulis | [33] |
| 12 | (+)-Phaeocauline B | C. phaeocaulis | [33] |
| 13 | (−)-Phaeocauline B | C. phaeocaulis | [33] |
| 14 | Phaeocaulisin Q | C. phaeocaulis | [34] |
| 15 | Wenyujinin A | C. wenyujin, C. kwangsiensis | [12,35] |
| 16 | Wenyujinin B | C. wenyujin | [27,35] |
| 17 | Wenyujinin Q | C. wenyujin | [36] |
| 18 | Zedoarondiol | C. wenyujin, C. phaeocaulis, C. kwangsiensis | [16,27,31,37,38] |
| 19 | Neozedoarondiol | C. wenyujin | [10] |
| 20 | Isozedoarondiol | C. wenyujin, C. phaeocaulis, C. kwangsiensis | [16,27,28,36,38] |
| 21 | Phaeocaulisin E | C. wenyujin, C. phaeocaulis | [16,31,37] |
| 22 | (1S,4S,5S,10R)-Zedoarondiol | C. phaeocaulis, C. wenyujin, C. kwangsiensis | [16,38,39] |
| 23 | (1S,4S,5S,10R)-Isozedoarondiol | C. wenyujin | [31] |
| 24 | Wenyujinin R | C. wenyujin | [36] |
| 25 | 4,10-Epizedoarondiol | C. kwangsiensis, C. wenyujin | [31,38] |
| 26 | 4-Hydroxy-10-methoxy-guai-7(11)-en-8-one | C. phaeocaulis | [28] |
| 27 | Methylzedoarondiol | C. wenyujin | [27] |
| 28 | Wenyujinol M | C. wenyujin | [32] |
| 29 | Procurcumenol | C. wenyujin, C. phaeocaulis, C. kwangsiensis | [12,27,29,31,37] |
| 30 | Epiprocurcumenol | C. wenyujin | [40] |
| 31 | Aerugidiol | C. wenyujin, C. kwangsiensis | [29,38,39] |
| 32 | 1-epi-Aerugidiol | C. phaeocaulis | [37] |
| 33 | Procurcumadiol | C. wenyujin, C. phaeocaulis, C. kwangsiensis | [16,31,37,38] |
| 34 | Phaeocaulisin F | C. phaeocaulis | [16] |
| 35 | Neoprocurcumenol | C. wenyujin | [36] |
| 36 | (+)-Phaeocauline D | C. phaeocaulis | [33] |
| 37 | (−)-Phaeocauline D | C. phaeocaulis | [33] |
| 38 | Dihydroprocurcumenol | C. kwangsiensis | [12] |
| 39 | Wenyujinol D | C. wenyujin | [27] |
| 40 | Phaeocaulisin P | C. phaeocaulis | [34] |
| 41 | (+)-Phaeocauline E | C. phaeocaulis | [33] |
| 42 | (−)-Phaeocauline E | C. phaeocaulis | [33] |
| 43 | Isoprocurcumenol | C. wenyujin | [41] |
| 44 | Wenyujinol F | C. wenyujin | [27] |
| 45 | 9-Oxo-neoprocurcumenol | C. wenyujin | [27] |
| 46 | 7α,11α-Epoxy-5β-hydroxy-9-guaiane-8-one | C. wenyujin, C. phaeocaulis | [16,31] |
| 47 | 8,9-seco-4β-Hydroxy-1α,5βH-7(11)-guaen-8,10-olide | C. wenyujin | [29] |
| 48 | Phaeocaulisin L | C. phaeocaulis | [42] |
| 49 | Phaeocaulisin D | C. phaeocaulis | [16] |
| 50 | Phaeocaulisin R | C. phaeocaulis | [37] |
| 51 | Phaeocaulisin K | C. phaeocaulis | [42] |
| 52 | Phaeocaulisin J | C. phaeocaulis | [16,28] |
| 53 | 4α,10β-Dihydroxy-1βH,5αH-guai-6(7)-en-11-one | C. phaeocaulis | [34] |
| 54 | Phaeocaulisin N | C. phaeocaulis | [34] |
| 55 | Curcumol | C. wenyujin, C. kwangsiensis, C. phaeocaulis | [26,31,36,43] |
| 56 | 4-Epicurcumol | C. wenyujin | [44] |
| 57 | 7β,8α-Dihydroxy-1α,4αH-guai-10(15)-en-5β,8β-endoxide | C. wenyujin | [29] |
| 58 | 10β-Hydroxy-9,10-dihydrocurcumenol | C. phaeocaulis | [28] |
| 59 | Wenyujinin I | C. wenyujin, C. kwangsiensis | [12,35] |
| 60 | Isocurcumenol | C. phaeocaulis, C. wenyujin, C. kwangsiensis | [16,26,45] |
| 61 | Curcumenol | C. wenyujin, C. phaeocaulis, C. kwangsiensis | [12,16,28,31,46] |
| 62 | 4-Epicurcumenol | C. wenyujin, C. phaeocaulis | [16,46] |
| 63 | 15-Hydroxycurcumenol | C. phaeocaulis | [28] |
| 64 | 12-Hydroxycurcumenol | C. wenyujin | [31] |
| 65 | Isocurcumol | C. wenyujin | [44] |
| 66 | 7β,8α-dihydroxy-1α,4αH-guai-9,11-dien-5β,8β-endoxide | C. wenyujin | [46] |
| 67 | Neocurcumenol | C. wenyujin | [46] |
| 68 | (+)-Phaeocauline C | C. phaeocaulis | [33] |
| 69 | (−)-Phaeocauline C | C. phaeocaulis | [33] |
| 70 | 4α,7α-Epoxyguaiane-10α,11-diol | C. wenyujin | [32] |
| 71 | (1R,4R,5S,7S)-Curwenyujinone | C. wenyujin | [47] |
| 72 | Wenyujinin H | C. wenyujin | [35] |
| 73 | Curcumafuranol | C. kwangsiensis | [48] |
| 74 | Zedoarol | C. kwangsiensis | [49] |
| 75 | Wenyujinin F | C. wenyujin | [35] |
| 76 | Linderazulene | C. kwangsiensis | [50] |
| 77 | (+)-Zedoalactone A | C. wenyujin, C. phaeocaulis | [28,51] |
| 78 | Zedoalactone C | C. wenyujin, C. phaeocaulis, C. kwangsiensis | [16,52,53] |
| 79 | Zedoalactone E | C. wenyujin | [39,46] |
| 80 | Zedoalactone G | C. wenyujin, C. kwangsiensis | [51,52] |
| 81 | Zedoalactone H | C. wenyujin | [46] |
| 82 | Phaeocaulisin C | C. phaeocaulis, C. kwangsiensis | [16,52] |
| 83 | Zedoalactone A | C. wenyujin, C. kwangsiensis, C. phaeocaulis | [16,28,31,52] |
| 84 | Phaeocaulisin B | C. phaeocaulis | [16] |
| 85 | Zedoarolide B | C. wenyujin, C. phaeocaulis, C. kwangsiensis | [12,16,28,31,51] |
| 86 | Wenyujinol H | C. wenyujin | [27] |
| 87 | 8-O-Methylzedoarolide B | C. wenyujin | [32] |
| 88 | Zedoarolide A | C. phaeocaulis, C. wenyujin | [16,28,32] |
| 89 | Wenyujinol G | C. wenyujin | [27] |
| 90 | Phaeocaulisin I | C. phaeocaulis, C. kwangsiensis | [12,16] |
| 91 | Phaeocaulisin G | C. phaeocaulis | [16] |
| 92 | Phaeocaulisin H | C. phaeocaulis | [16] |
| 93 | Phaeocaulisin O | C. kwangsiensis, C. phaeocaulis | [34,52] |
| 94 | Zedoalactone B | C. wenyujin, C. kwangsiensis, C. phaeocaulis | [16,27,51,52] |
| 95 | (1R,4R,5S,10S)-Zedoalactone B | C. wenyujin | [51] |
| 96 | Zedoalactone D | C. wenyujin, C. kwangsiensis, C. phaeocaulis | [16,39,52] |
| 97 | (4S)-Hydroxy-(8)-methoxy-(5S)-(H)-guaia1(10),7(11)-dien-12,8-olide | C. kwangsiensis | [12] |
| 98 | Zedoalactone F | C. wenyujin, C. kwangsiensis | [38,39] |
| 99 | Gweicurculactone | C. kwangsiensis | [54] |
| 100 | (4S)-4-Hydroxy-gweicurculactone | C. wenyujin, C. kwangsiensis | [51,54] |
| 101 | 2-Oxoguaia-1(10),3,5,7(11),8-pentaen-12,8-olide | C. wenyujin, C. kwangsiensis | [27,54] |
| 102 | 4β-Methyl-8β,9β-dihydroxy-5α,10α-epoxy-guai-12,8-olide | C. kwangsiensis | [52] |
| 103 | 4α-Methyl-8β,9β-dihydroxy-5α,10α-epoxy-guai-12,8-olide | C. kwangsiensis | [52] |
| 104 | Phaeocaulisin A | C. phaeocaulis, C. kwangsiensis | [16,54] |
| 105 | (1R,4R,5S,8S,9Z)-4-Hydroxy-1,8-epoxy-5H-guaia-7(11),9-dien-12,8-olide | C. kwangsiensis | [54] |
| 106 | Wenyujinol A | C. wenyujin | [27] |
| 107 | Wenyujinol B | C. wenyujin | [27] |
| 108 | Wenyujinol C | C. wenyujin | [27] |
| 109 | Wenyujinin G | C. wenyujin | [35] |
| 110 | 1α,8α-Epidioxy-4α-hydroxy-5αH-guai-7(11),9-dien-12,8-olide | C. wenyujin, C. kwangsiensis | [12,29] |
| 111 | Phaeocaulisin M | C. phaeocaulis | [42] |
| 112 | Curcuzedoalide | C. wenyujin | [31] |
| 113 | Kwangsiensis A | C. kwangsiensis | [55] |
| 114 | Kwangsiensis B | C. kwangsiensis | [55] |
| 115 | 12-Dehydroxy-chloraniolide | C. phaeocaulis | [56] |
3.2. Germacrane-Type Sesquiterpenoids of Curcumae Rhizoma
| No. | Compounds | Medicinal Source | Reference |
|---|---|---|---|
| 116 | Germacrone | C. wenyujin, C. kwangsiensis, C. phaeocaulis | [31,49,57] |
| 117 | Curdione | C. wenyujin, C. kwangsiensis, C. phaeocaulis | [17,26,29,31,43] |
| 118 | Neocurdione | C. wenyujin, C. kwangsiensis, C. phaeocaulis | [26,43,44,58] |
| 119 | (2R)-2β-Hydroxycurdione | C. wenyujin | [18] |
| 120 | Wenyujinone D | C. wenyujin | [18] |
| 121 | Dehydrocurdione | C. kwangsiensis | [12] |
| 122 | Heyneanone C | C. phaeocaulis | [59] |
| 123 | Heyneanone D | C. wenyujin | [18,40] |
| 124 | 13-Hydroxygermacrone | C. wenyujin, C. phaeocaulis, C. kwangsiensis | [31,56,60] |
| 125 | Germacrene D | C. wenyujin, C. phaeocaulis, C. kwangsiensis | [26] |
| 126 | (4S,5S)-Germacrone-4,5-epoxide | C. wenyujin, C. kwangsiensis, C. phaeocaulis | [49,57,58,61] |
| 127 | (+)-(4S,5S)-Germacrone-4,5-epoxide | C. wenyujin | [17,62] |
| 128 | (4S,5S)-13-Hydroxygermacrone-4,5-epoxide | C. phaeocaulis | [59] |
| 129 | Germacrone-1,10-epoxide | C. wenyujin, C. kwangsiensis | [49,58] |
| 130 | (1R,10R)-(−)-1,10-Dihydrocurdione | C. wenyujin | [63] |
| 131 | (1R,10R)-Epoxy-1,10-dihydrocurdione | C. wenyujin | [43] |
| 132 | (1S,10S),(4S,5S)-Germacrone-1(10),4(5)-diepoxide | C. wenyujin | [43,62] |
| 133 | (+)-(1S,4S,5S,10S)-Germacrone-1(10)-4-diepoxide | C. wenyujin | [17] |
| 134 | (1R,4S,5R,6R,7S,10R)-1(10),4(5)-Diepoxygermacran-11(12)-en-6-ol | C. phaeocaulis | [15] |
| 135 | Germacrone-1(10),4,7(11)-triepoxide | C. wenyujin | [62] |
| 136 | Wenyujinin J | C. wenyujin | [35] |
| 137 | Wenyujinol O | C. wenyujin | [32] |
| 138 | Phagermadiol | C. phaeocaulis | [42,59] |
| 139 | Furanodiene | C. kwangsiensis, C. wenyujin, C. phaeocaulis | [26] |
| 140 | Furanodienone | C. wenyujin, C. kwangsiensis, C. phaeocaulis | [31,49,56] |
| 141 | (1S)-1-Hydroxy-isofuranodienone | C. phaeocaulis | [37] |
| 142 | 1(10)Z,4Z-Furanodiene-6-one | C. wenyujin | [31] |
| 143 | Zederone | C. wenyujin, C. kwangsiensis, C. phaeocaulis | [31,49,56] |
| 144 | Wenyujinin K | C. wenyujin | [35] |
| 145 | (1R,4S,5R,9R,10S)-9-Hydroxy-zederone epoxide | C. phaeocaulis | [59] |
| 146 | Curdionolide B | C. wenyujin, C. phaeocaulis, C. kwangsiensis | [12,17,44,59] |
| 147 | Curdionolide A | C. wenyujin, C. phaeocaulis, C. kwangsiensis | [17,31,52,59] |
| 148 | Souliene A | C. kwangsiensis | [12] |
| 149 | Wenyujinone C | C. wenyujin | [18] |
| 150 | Aeruginolactone | C. wenyujin, C. phaeocaulis, C. kwangsiensis | [12,30,56] |
| 151 | Curcuminol G | C. wenyujin, C. kwangsiensis | [12,45] |
| 152 | (+)-Phaeocaulin C | C. phaeocaulis | [64] |
| 153 | (−)-Phaeocaulin C | C. phaeocaulis | [64] |
| 154 | (1E,4Z)-8-Hydroxy-6-oxogermacra-1(10),4,7(11)-trieno-12,8-lactone | C. wenyujin, C. phaeocaulis | [17,30,56] |
| 155 | (+)-Phaeocaulin D | C. phaeocaulis | [64] |
| 156 | (−)-Phaeocaulin D | C. phaeocaulis | [64] |
| 157 | Wenyujinone A | C. wenyujin | [18] |
| 158 | 1,8-Epoxy-7(11)-germacren-5-one-12,8-olide | C. wenyujin | [18] |
| 159 | Curkwangsien A | C. kwangsiensis | [65] |
| 160 | Curkwangsien B | C. kwangsiensis | [65] |
| 161 | Curdionolide C | C. wenyujin | [17] |
| 162 | Wenyujinone B | C. wenyujin | [18] |
| 163 | (+)-Phaeocaulin B | C. phaeocaulis | [64] |
| 164 | (−)-Phaeocaulin B | C. phaeocaulis | [64] |
| 165 | (+)-Phaeocaulin A | C. phaeocaulis | [59] |
| 166 | (−)-Phaeocaulin A | C. phaeocaulis | [59] |
| 167 | (−)-Phaeocaulin E | C. phaeocaulis | [56] |
| 168 | (+)-Phaeocaulin F | C. phaeocaulis | [56] |
| 169 | Wenjine | C. wenyujin | [62] |
3.3. Eudesmane-Type Sesquiterpenoids of Curcumae Rhizoma
3.4. Elemane-Type Sesquiterpenoids of Curcumae Rhizoma
3.5. Cadinane-Type Sesquiterpenoids of Curcumae Rhizoma
3.6. Other-Type Sesquiterpenoids of Curcumae Rhizoma
4. Biological Activity
4.1. Anti-Inflammatory Activity
| Compounds | Compound Types | Activity Types | Pharmacological Models | Effects | IC50 (μM) | Positive Control IC50 (μM) | Reference |
|---|---|---|---|---|---|---|---|
| Isozedoarondiol (20) | Guaiane-type sesquiterpenoids | Anti-inflammatory activity | LPS-induced RAW 246.7 cell inflammation model | Inhibit LPS-induced NO production | 1.4 | 12.1 (Indomethacin) 43.8 (Hydrocortisone) | [16] |
| Phaeocaulisin L (48) | 54.27 ± 4.23 | 58.66 ± 6.39 (Hydrocortisone) | [42] | ||||
| Phaeocaulisin D (49) | 5.9 | 12.1 (Indomethacin) 43.8 (Hydrocortisone) | [16] | ||||
| Phaeocaulisin N (54) | 3.58 ± 0.17 | 58.79 ± 3.32 (Hydrocortisone) | [34] | ||||
| 4-Epicurcumol (56) | 17.26 ± 1.26 | 64.34 ± 7.49 (Hydrocortisone) | [44] | ||||
| 15-Hydroxycurcumenol (63) | 6.44 ± 0.51 | 14.1 ± 0.69 (Indomethacin) | [78] | ||||
| 12-Hydroxycurcumenol (64) | 9.64 ± 0.47 | 14.1 ± 0.69 (Indomethacin) | [78] | ||||
| Isocurcumol (65) | 22.36 ± 1.32 | 64.34 ± 7.49 (Hydrocortisone) | [44] | ||||
| Zedoalactone A (83) | 1.6 | 12.1 (Indomethacin) 43.8 (Hydrocortisone) | [16] | ||||
| Phaeocaulisin B (84) | 1.9 | 12.1 (Indomethacin) 43.8 (Hydrocortisone) | [16] | ||||
| Zedoalactone B (94) | 1.3 | 12.1 (Indomethacin) 43.8 (Hydrocortisone) | [16] | ||||
| Zedoalactone D (96) | 1.6 | 12.1 (Indomethacin) 43.8 (Hydrocortisone) | [16] | ||||
| Phaeocaulisin A (104) | 8.5 | 51.4 (Hydrocortisone) | [35] | ||||
| Wenyujinin G (109) | 7.6 | 51.4 (Hydrocortisone) | [35] | ||||
| Phaeocaulisin M (111) | 6.05 ± 0.43 | 58.66 ± 6.39 (Hydrocortisone) | [42] | ||||
| Gweicurculactone (99) | Inhibit NO production and the expressions of iNOS and COX-2 mRNA | 27.3 | 5.6 ± 0.3 (CAPE) 26.3 ± 0.3 (Indomethacin) 65.0 ± 1.2 (L-NA) | [86] | |||
| Curcuzedoalide (112) | Inhibit NO production and suppress pre-inflammatory protein expressions of iNOS and COX-2 | 12.21 ± 1.67 | 4.15 ± 1.35 (Quercetin) | [87] | |||
| 4α,10α,11-Trihydroxy-1βH,5βH-guai-7(8)-ene (4) | LPS-induced THP-1 cell inflammation model | Inhibit the release of inflammatory mediator (TNF-α) | [88] | ||||
| Zedoarondiol (18) | LPS-induced RAW 264.7 cell and mouse peritoneal macrophage cell models | Inhibit iNOS, COX-2, and pro-inflammatory cytokine (TNF-α, IL-1β, and IL-6) expressions by suppressing the phosphorylations of IKK and MAPKs, and inactivating the NF-κB pathway | [89] | ||||
| LPS-induced THP-1-blue cell inflammation model | Inhibit LPS-stimulated TLR4 activation | 22.5 ± 1.0 | 2.6 ± 0.8 (Luteolin) | [37] | |||
| Procurcumenol (29) | Anti neuro-inflammatory activity | LPS-induced BV-2 cell inflammation model | Inhibit LPS-induced NO production | 20.05 | 23.53 ± 4.70 (Minocycline) | [52] | |
| Dihydroprocurcumenol (38) | Anti-inflammatory activity | LPS-induced RAW 246.7 cell inflammation model | Inhibit the secretion of inflammatory mediator (COX-2) | [12] | |||
| Anti-inflammatory and antinociceptive effects | Carrageenan-induced paw edema and acetic acid-induced writhing animal models | Inhibit the paw edema (inhibitory effects: 28.1% and 35.3% at 100 and 50 mg/kg, respectively); decrease the levels of stretching and twisting by the rates of 46.9% | [12] | ||||
| Curcumol (55) | Anti-inflammatory activity | LPS-induced RAW 246.7 cell inflammation model | Suppress iNOS mRNA expression and protein level; inhibit the transcriptional and translational levels of TNF-α, IL-1β, and IL-6; interfere with the JNK-mediated AP-1 pathway | [90] | |||
| Alleviate psoriasis-like inflammation activity | NHEK cell model | Reduce proliferation and inflammatory gene expression in stimulated keratinocytes by inhibiting JAK1/STAT3 signaling | [83] | ||||
| Ameliorate lung Inflammation activity | Asthmatic mice model established by ovalbumin induction | Inhibit the abnormal activation of the Wnt/β-catenin pathway | [82] | ||||
| Curcumenol (61) | Anti-inflammatory activity | LPS-induced RAW 246.7 cell inflammation model | Inhibit the secretion of inflammatory mediators (COX-2, IL-1β, and TNF-α) | [12] | |||
| LPS-induced macrophage inflammation model | Inhibit LPS-induced NO production | 5.42 ± 0.64 | 14.1 ± 0.69 (Indomethacin) | [78] | |||
| Anti neuro-inflammatory activity | LPS-induced BV-2 cell inflammation model | Inhibit releases of the inflammatory mediators (COX-2, IL-1β, and TNF-α) and diminish the expression of the regulatory genes by inhibiting Akt-dependent NF-κB activation and downregulating Akt and p38 MAPK signaling | [91] | ||||
| Anti-inflammatory and antinociceptive effects | Carrageenan-induced paw edema and acetic acid-induced writhing animal models | Inhibit the paw edema (inhibitory effects: 29.5% and 30% at 100 and 50 mg/kg, respectively); decrease the levels of stretching and twisting by the rate of 32.7% | [12] | ||||
| Neocurdione (118) | Germacrane-type sesquiterpenoids | Anti-inflammatory activity | LPS-induced RAW 246.7 cell inflammation model | Inhibit LPS-induced NO production | 24.18 ± 1.66 | 64.34 ± 7.49 (Hydrocortisone) | [44] |
| Curdionolide B (146) | 14.50 ± 0.87 | 64.34 ± 7.49 (Hydrocortisone) | [44] | ||||
| Germacrone (116) | Anti-inflammatory activity; alleviate bronchial asthma and rheumatoid arthritis activity, etc. | Multiple inflammation models | Regulate the expressions of related genes and proteins by PI3K III/Beclin-1/Bcl-2 and PI3K/Akt/mTOR pathways; regulate the expression of pro-inflammatory cytokines (IL-6, TNF-α, TGF-β1, and IL-10); regulate Th1/Th2 balance and NF-κB activation; upregulate TLR8 expression in THP-1 cells, etc. | [80] | |||
| Alleviate rheumatoid arthritis activity | Collagen-induced arthritis (CIA) model | Alleviate the progression of arthritis through regulating Th1/Th2 balance and inactivating the NF-κB pathway | [84] | ||||
| Dehydrocurdione (121) | Analgesic activity; antipyretic activity; anti-inflammatory activity | Acetic acid-induced writhing method; baker’s yeast-treated rat model; carrageenan-induced paw edema model | Mitigate the writhing reflex induced by acetic acid and the fever elicited by baker’s yeast; inhibit the carrageenan-induced paw edema; reduce chronic adjuvant arthritis | [79] | |||
| Souliene A (148) | Anti-inflammatory activity | LPS-induced RAW 246.7 cell inflammation model | Inhibit the secretion of inflammatory mediator (COX-2) | [12] | |||
| Anti-inflammatory and antinociceptive effects | Carrageenan-induced paw edema and acetic acid-induced writhing animal models | Inhibit the paw edema (inhibitory effects: 40.7% and 35.9% at 100 and 50 mg/kg, respectively); decrease the levels of stretching and twisting by the rate of 38.5% | [12] | ||||
| Curcuminol G (151) | Anti-inflammatory activity | LPS-induced RAW 246.7 cell inflammation model | Inhibit the secretion of inflammatory mediators (COX-2, IL-1β, and TNF-α) | [12] | |||
| Anti-inflammatory and antinociceptive effects | Carrageenan-induced paw edema and acetic acid-induced writhing animal models | Inhibit the paw edema (inhibitory effects: 31.4% and 45.4% at 100 and 50 mg/kg, respectively); decrease the levels of stretching and twisting by the rate of 26.2% | [12] | ||||
| 1α,4β-Dihydroxy-eudesm-7(11)-en-8-one (172) | Eudesmane-type sesquiterpenoids | Anti-inflammatory activity | LPS-induced RAW 246.7 cell inflammation model | Inhibit LPS-induced NO production | 5.6 | 12.1 (Indomethacin) 43.8 (Hydrocortisone) | [2] |
| 1-Hydroxyeudesma-4(14),7(11)-dien-8-one (173) | 1.2 | 12.1 (Indomethacin) 43.8 (Hydrocortisone) | [2] | ||||
| Phaeusmane A (176) | 3.2 | 12.1 (Indomethacin) 43.8 (Hydrocortisone) | [2] | ||||
| Phaeusmane B (177) | 9.6 | 12.1 (Indomethacin) 43.8 (Hydrocortisone) | [2] | ||||
| Phaeusmane D (178) | 14.4 | 12.1 (Indomethacin) 43.8 (Hydrocortisone) | [2] | ||||
| Phaeusmane C (180) | 19.6 | 12.1 (Indomethacin) 43.8 (Hydrocortisone) | [2] | ||||
| Eudesm-11-ene-4α,6α-diol (181) | 0.8 | 12.1 (Indomethacin) 43.8 (Hydrocortisone) | [2] | ||||
| 1β-Hydroxyeudesma-4,11-dien-3-one (184) | 9.3 | 12.1 (Indomethacin) 43.8 (Hydrocortisone) | [2] | ||||
| Curcolonol (186) | 16.2 | 12.1 (Indomethacin) 43.8 (Hydrocortisone) | [2] | ||||
| Chlomultin B (195) | 18.6 | 12.1 (Indomethacin) 43.8 (Hydrocortisone) | [2] | ||||
| Myrrhterpenoid N (196) | 19.3 | 12.1 (Indomethacin) 43.8 (Hydrocortisone) | [2] | ||||
| Phaeusmane F (197) | 4.8 | 12.1 (Indomethacin) 43.8 (Hydrocortisone) | [2] | ||||
| (7Z)-1β,4α-Dihydroxy-5α,8β(H)-eudesm-7(11)-en-8,12-olide (200) | 15.3 | 53.8 (Hydrocortisone) | [66] | ||||
| (7Z)-1β,4β-Dihydroxy-5α,8β(H)-eudesm-7(11)-en-8,12-olide (201) | 3.8 | 12.1 (Indomethacin) 43.8 (Hydrocortisone) | [2] | ||||
| Curcolide (202) | 0.8 | 12.1 (Indomethacin) 43.8 (Hydrocortisone) | [2] | ||||
| 1β,8β-Dihydroxy-eudesma-3,7(11)-dien-8α,12-olide (204) | 8.9 | 12.1 (Indomethacin) 43.8 (Hydrocortisone) | [2] | ||||
| Phaeusmane H (210) | 20.9 | 12.1 (Indomethacin) 43.8 (Hydrocortisone) | [2] | ||||
| Hydroxyisogermafurenolide (220) | 26.0 | 53.8 (Hydrocortisone) | [66] | ||||
| 8β(H)-Elema-1,3,7(11),8-tetraen-8,12-lactam (223) | 9.4 ± 1.6 | 42.7 ± 3.1 (Hydrocortisone) | [46] | ||||
| Curzerenone (215) | IL-6-stimulated STAT-3 expression model | Inhibit STAT-3 expression stimulated by IL-6; suppress the mRNA expression levels of the proinflammatory genes IL-1β and CRP via blockade of the IL-6-activated and ERK-MAPK signaling pathways | 4.8 | [56] | |||
| Phacadinane B (228) | Cadinane-type sesquiterpenoids | Anti-inflammatory activity | LPS-induced RAW 246.7 cell inflammation model | Inhibit LPS-induced NO production | 2.25 ± 0.71 | 43.80 ± 6.79 (Hydrocortisone) | [74] |
| Phacadinane A (229) | 3.88 ± 0.58 | 43.80 ± 6.79 (Hydrocortisone) | [74] | ||||
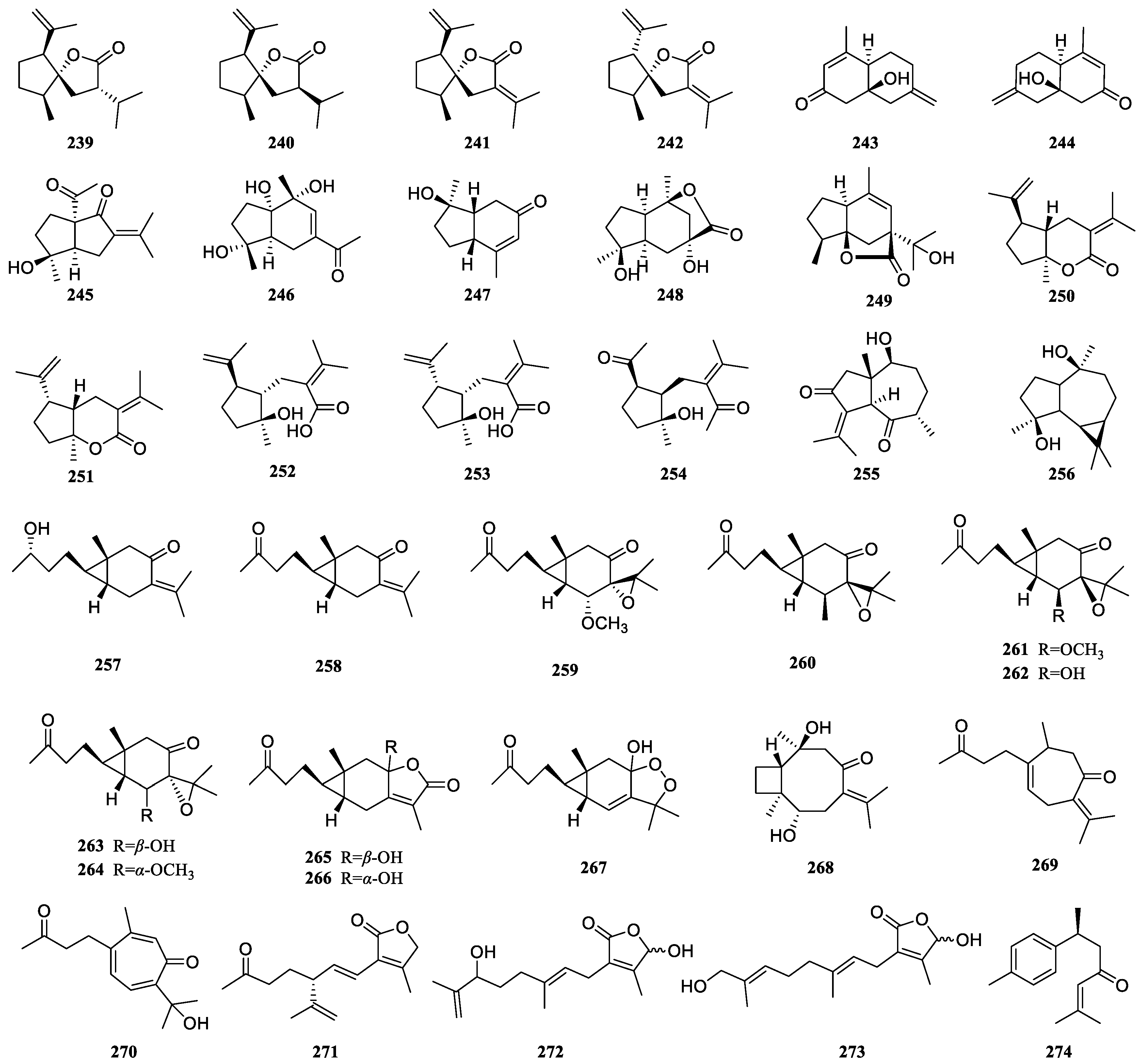
| Curcumalactone (239) | Other-type sesquiterpenoids | Anti-inflammatory activity | LPS-induced RAW 246.7 inflammation model | Inhibit LPS-induced NO production | 23.28 ± 1.47 | 64.34 ± 7.49 (Hydrocortisone) | [68] |
| 7-Epicurcumalactone (240) | 45.49 ± 2.96 | 64.34 ± 7.49 (Hydrocortisone) | [68] | ||||
| Phaeocauone (246) | 2.35 ± 0.17 | 58.79 ± 3.32 (Hydrocortisone) | [69] | ||||
| Phasalvione (255) | 7.46 ± 0.69 | 58.79 ± 3.32 (Hydrocortisone) | [69] | ||||
| 8,11-Epidioxy-8-hydroxy-4-oxo-6-carabren (267) | 25.36 ± 3.26 | 64.34 ± 7.49 (Hydrocortisone) | [44] | ||||
| Curcumolide (249) | Suppress LPS-induced NF-κB activation, including the nuclear translocation and DNA binding activity of NF-κB; decrease pro-inflammatory mediators (TNF-α, IL-6, and IL-1β); NO and ROS production | [92] |
4.2. Cancer-Related Activity
| Compounds | Compound Types | Activity Types | Pharmacological Models | Effects | IC50 | Positive Control IC50 | Reference |
|---|---|---|---|---|---|---|---|
| Zedoarondiol (18) | Guaiane-type sesquiterpenoids | Cytotoxic activity against lung carcinoma | A-549 cell model | Exhibit cytotoxic activity | 3.64 ± 0.66 μM | 0.0831 ± 0.0091 μM (Doxorubicin) | [95] |
| Cytotoxic activity against breast cancer | MCF-7 cell model | 7.34 ± 0.94 μM | 8.02 ± 1.13 μM (Doxorubicin) | [95] | |||
| Cytotoxic activity against breast cancer | MDA-MB-231 cell model | 7.51 ± 1.35 μM | 6.93 ± 1.08 μM (Doxorubicin) | [95] | |||
| Cytotoxic activity against leukemia | HL-60 cell model | 7.35 ± 0.61 μM | 0.0776 ± 0.0082 μM (Doxorubicin) | [95] | |||
| Isozedoarondiol (20) | Cytotoxic activity against lung carcinoma | A-549 cell model | 4.21 ± 0.93 μM | 0.0831 ± 0.0091 μM (Doxorubicin) | [95] | ||
| Cytotoxic activity against breast cancer | MCF-7 cell model | 9.19 ± 0.79 μM | 8.02 ± 1.13 μM (Doxorubicin) | [95] | |||
| Cytotoxic activity against breast cancer | MDA-MB-231 cell model | 9.40 ± 1.21 μM | 6.93 ± 1.08 μM (Doxorubicin) | [95] | |||
| Phaeocaulisin E (21) | Cytotoxic activity against lung carcinoma | A-549 cell model | 4.79 ± 0.81 μM | 0.0831 ± 0.0091 μM (Doxorubicin) | [95] | ||
| Cytotoxic activity against breast cancer | MCF-7 cell model | 9.85 ± 1.02 μM | 8.02 ± 1.13 μM (Doxorubicin) | [95] | |||
| Cytotoxic activity against breast cancer | MDA-MB-231 cell model | 10.15 ± 1.43 μM | 6.93 ± 1.08 μM (Doxorubicin) | [95] | |||
| Procurcumenol (29) | Cytotoxic activity against lung carcinoma | A-549 cell model | 5.82 ± 0.91 μM | 0.0831 ± 0.0091 μM (Doxorubicin) | [95] | ||
| Aerugidiol (31) | Cytotoxic activity against breast cancer | MCF-7 cell model | 7.23 ± 1.01 μM | 8.02 ± 1.13 μM (Doxorubicin) | [95] | ||
| Cytotoxic activity against breast cancer | MDA-MB-231 cell model | 7.40 ± 0.93 μM | 6.93 ± 1.08 μM (Doxorubicin) | [95] | |||
| Isoprocurcumenol (43) | Cytotoxic activity against lung carcinoma | A-549 cell model | 3.81 ± 0.65 μM | 0.0831 ± 0.0091 μM (Doxorubicin) | [95] | ||
| Cytotoxic activity against breast cancer | MCF-7 cell model | 8.13 ± 0.93 μM | 8.02 ± 1.13 μM (Doxorubicin) | [95] | |||
| Cytotoxic activity against breast cancer | MDA-MB-231 cell model | 8.34 ± 1.14 μM | 6.93 ± 1.08 μM (Doxorubicin) | [95] | |||
| Phaeocaulisguatriol (2) | Cytotoxic activity against breast cancer | MCF-7 cell model | Induce cell apoptosis by activating the expressions of TP53 and caspase 3 proteins | 40.73 ± 0.42 μM | 9.86 ± 0.13 μM (Cisplatin) | [28] | |
| Curcumol (55) | Cytotoxic activity against lung carcinoma, breast cancer, nasopharyngeal carcinoma, etc.; antitumor activity against lung cancer, nasopharyngeal carcinoma, colorectal cancer, etc. | Multi-models | Arrest the cell cycle at G0/G1 or G2/M phases; induce apoptosis in numerous cancer cells via targeting key signaling pathways, such as MAPK/ERK, PI3K/Akt, and NF-κB; regulate various signaling cascades | [98] | |||
| Cytotoxic activity against breast cancer; antitumor activity against breast cancer | MDA-MB-231 cell model; MDA-MB-231 cell xenograft model in nude mice | Trigger apoptosis of p53 mutant triple-negative human breast cancer cells via activation of p73 and PUMA | [102] | ||||
| Cytotoxic activity against hepatic cancer; antitumor activity against hepatic cancer | Hela, A549, HUVEC cell models; Hep3B cell xenograft model in murine | Inhibit the expression of PD-L1 through crosstalk between HIF-1α and p-STAT3 (T705) signaling pathways | [103] | ||||
| Cytotoxic activity against colorectal cancer; antitumor activity against colorectal cancer | LoVo and SW 480 cell models; LoVo cell xenograft model in nude mice | Inhibit growth and induce apoptosis via IGF-1R and p38 MAPK pathways | [104] | ||||
| Curcumenol (61) | Cytotoxic activity against breast cancer | MCF-7 cell model | Induce apoptosis by inhibiting the proliferation of the cancer cell | 9.3 ± 0.3 μg/mL | 0.1 ± 0.0 μg/mL (Doxorubicin) | [105] | |
| Cytotoxic activity against lung carcinoma; antitumor activity against lung carcinoma | CCD19, BEAS-2B, H1299, H460, and HEK293T cell models and mice xenograft model | Induce cell death, suppress cell proliferation, and trigger ferroptosis in lung cancer cells via the lncRNA H19/miR-19b-3p/FTH1 axis | [106] | ||||
| Curcuzedoalide (112) | Cytotoxic activity against gastric cancer | AGS cell model | Activate caspase-8, caspase-9, caspase-3, and PARP, inducing apoptosis | [107] | |||
| Germacrone (116) | Germacrane-type sesquiterpenoids | Cytotoxic activity against colorectal cancer, gastric cancer, breast cancer, cervical cancer, prostate cancer, etc. | Multi-cell models | Regulate the expressions of Akt/MDM2/p53, JAK2/STAT3, AMPK, and Akt/mTOR pathways and related proteins; inhibit the proliferation of cancer cells, promote the apoptosis of cancer cells, promote autophagy; reverse the resistance of drugs, enhance the antitumor activity of drugs, and reduce the toxicity of chemotherapeutic drugs | [80] | ||
| Cytotoxic activity against gastric cancer | BGC823 cell model | Inhibit cell proliferation through the induction of G2/M-phase cell cycle arrest and promote cell apoptosis through modulations of cell cycle-associated protein expression and mitochondria-mediated apoptosis | [108] | ||||
| Cytotoxic activity against breast cancer | MCF-7 and MDA-MB-23 cell models | Induce cell cycle arrest and apoptosis through mitochondria-mediated caspase pathway | [109] | ||||
| Cytotoxic activity against hepatic carcinoma | HepG2 and Bel7402 cell models | Regulate the expression of proteins related to G2/M cell cycle and apoptosis; p53 and oxidative damage may be involved in the inhibition of human hepatoma cells’ growth | [3] | ||||
| Cytotoxic activity against esophageal squamous cell carcinoma | Esophageal squamous cell carcinoma (ESCC) cell models | Exert an anti-esophageal effect through intrinsic apoptotic signaling pathways and by inhibiting STAT3 activity | [110] | ||||
| Curdione (117) | Cytotoxic activity against colorectal cancer; antitumor activity against colorectal cancer | CRC cell model; CRC cell xenograft model in nude mice | Induce ferroptosis in CRC by virtue of m6A methylation | [111] | |||
| Cytotoxic activity against breast cancer; antitumor activity against breast cancer | MCF-7 and MDA-MB-23 cell models; MCF-7 cell xenograft model in nude mice | Inhibit proliferation and induce apoptosis; exert a synergistically inhibitory effect with other chemotherapy drugs through MAPKs and PI3K/AKT pathways | [19] | ||||
| Cytotoxic activity against uterine leiomyosarcoma; antitumor activity against uterine leiomyosarcoma | SK-UT-1 and SK-LMS-1 cell models; SK-UT-1 cell xenograft model in nude mice | Decrease the viability and proliferation of SK-UT-1 and SK-LMS-1 cells, improve apoptosis and autophagic death, and exhibit an antitumor effect through indoleamine-2, 3-dioxygenase-1 | [112] | ||||
| Cytotoxic activity against hepatic carcinoma | HHSEC under the micro-environment of HepG2 cells | Inhibit the expressions of VEGF and VEGFR2 in HHSECs in HepG2 cell micro-environment | [113] | ||||
| Furanodiene (139) | Cytotoxic activity against breast cancer; antitumor activity against breast cancer | MCF-7 and MDA-MB-231 cell models and MCF-7 cell xenograft model in nude mice | Inhibit cell proliferation through apoptosis in a mitochondria-mediated pathway by regulating cyclin D1, CDK2, pRb, and Bcl-2 family proteins; activating caspases and PARP; and the Akt pathway is also be involved | [93] | |||
| Cytotoxic activity against breast cancer | MCF-7 cell model | Inhibit cancer cell growth via the AMPK pathway and induce cell apoptosis via metabolic regulation in chemoresistant MCF-7 breast cancer cells | [94] | ||||
| Cytotoxic activity against leukemia | HL60 cell model | Activate bid protein (a substrate of caspase-8), upregulate TNFR1, promote the formation of the TNFR1 complex and the production of TNF-α through the activation of TNFR1 signaling pathway, inducing cell apoptosis | [96] | ||||
| Cytotoxic activity against lung cancer, breast cancer, leukemia, etc.; antitumor activity against breast cancer | Multi-models | Induce apoptosis in several cancer types by modulating MAPKs/ERK, NF-κB, Akt, and other pathways | [99] | ||||
| Furanodienone (140) | Cytotoxic activity against colorectal cancer; antitumor activity against colorectal cancer | RKO and HT-29 cell models and CRC cell xenograft model in nude mice | Induce G0/G1 arrest and cause apoptosis via the ROS/MAPKs-mediated caspase-dependent pathway | [114] | |||
| Zederone (143) | Cytotoxic activity against ovarian cancer | SKOV-3 cell model | Inhibit mTOR/p70s6K signaling pathway | [115] | |||
| Curcolonol (186) | Eudesmane-type sesquiterpenoids | Cytotoxic activity against breast cancer | MDA-MB-231 cell model | Inhibit LIM kinase 1 to downregulate cofilin 1 phosphorylation | [116] | ||
| Serralactone A (205) | Cytotoxic activity against breast cancer | MDA-MB-231 and MDA-MB-468 cell models | Downregulate LIMK1 activation | [117] | |||
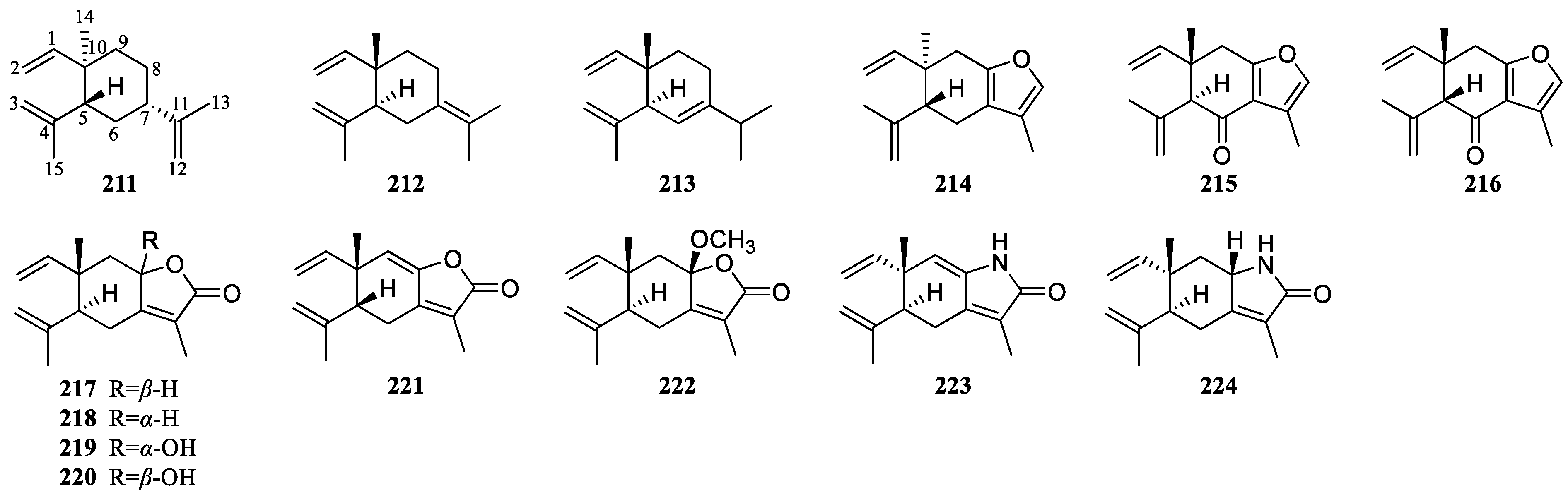
| β-Elemene (211) | Elemane-type sesquiterpenoids | Cytotoxic activity against gastric cancer, hepatocarcinoma, breast cancer, etc.; antitumor activity against hepatocarcinoma, lung cancer, etc. | Multi-models | Inhibit cell proliferation, arrest the cell cycle and induce cell apoptosis; enhance cell immune function associated with malignancy; activate cytoprotective autophagy; reverse multidrug resistance; prevent tumor angiogenesis; enhance the sensitivity of tumor cells to radiotherapy | [101] | ||
| Cytotoxic activity against lung cancer, hepatocarcinoma, breast cancer, etc.; antitumor activity against leukemia, esophageal cancer, gastric cancer, etc. | Multi-models | Inhibit and kill tumor cells through a variety of mechanisms; enhance the effect of radiotherapy or chemotherapy synergistically; regulate autoimmune activity in the treatment of tumors | [100] | ||||
| δ-Elemene (213) | Cytotoxic activity against leukemia | HL-60 cell model | Induce apoptosis by activating caspase-3 and interfering with the cell cycle at the G2/M phase | [97] | |||
| Curzerene (214) | Cytotoxic activity against lung carcinoma; antitumor activity against lung carcinoma | SPC-A1 cell model and SPC-A1 cell xenograft model in nude mice | Induce the downregulation of GSTA1 protein and mRNA expression in SPC-A1 cells | [118] | |||
| Curzerenone (215) | Cytotoxic activity against lung carcinoma | H69AR and MRC5 cell models | Mediate programmed cell death, loss of mitochondrial membrane potential, ROS; and block the ERK/MAPK and NF-κB signaling pathways | [119] | |||
| Acomadendrane-4β,10β-diol (256) | Other-type sesquiterpenoids | Cytotoxic activity against colon cancer | RKO cell model | Exhibit antimigratory activity | [65] | ||
| Curcumenone (258) | Cytotoxic activity against breast cancer | MCF-7 cell model | Exhibit cytotoxic activity | 8.3 ± 1.0 μg/mL | 0.1 ± 0.0 μg/mL (Doxorubicin) | [105] |
4.3. Effects on Cardiovascular System
| Compounds | Compound Types | Activity Types | Pharmacological Models | Effects | Value | Positive Control | Reference |
|---|---|---|---|---|---|---|---|
| (+)-Phaeocauline A (10) | Guaiane-type sesquiterpenoids | Anti-platelet effect | Abnormal platelet aggregation induced by arachidonic acid | Inhibit the platelet aggregation induced by AA | Inhibition rate: 27.78 ± 4.36% | Inhibition rate: 72.89 ± 7.65% (Aspirin) | [33] |
| (−)-Phaeocauline A (11) | Inhibition rate: 31.63 ± 7.10% | ||||||
| Procurcumenol (29) | Platelet aggregation induced by ADP | Inhibit the activity of the MAPK and PI3K/AKT pathways | Inhibitionmax: 76.3%; IC50: 0.2560 mg/mL | [8] | |||
| Isoprocurcumenol (43) | Inhibitionmax: 62.8%; IC50: 0.2680 mg/mL | ||||||
| (+)-Phaeocauline D (36) | Vasorelaxant effect | Contraction of rat aortic rings induced by KCl | Exhibit vasorelaxant effects against KCl-induced contraction | Vasorelaxation: 35.51 ± 3.65% | [33] | ||
| (−)-Phaeocauline D (37) | Maximal vasorelaxation: 38.96 ± 3.26% | ||||||
| (+)-Phaeocauline E (41) | Maximal vasorelaxation: 39.42 ± 4.63% | ||||||
| (−)-Phaeocauline E (42) | Maximal vasorelaxation: 40.93 ± 5.68% | ||||||
| (+)-Phaeocauline C (68) | Maximal vasorelaxation: 47.71 ± 4.35% | ||||||
| (−)-Phaeocauline C (69) | Maximal vasorelaxation: 45.64 ± 6.85% | ||||||
| Curcumol (55) | Protective effect against cardiac remodeling | Isoproterenol (ISO)-induced cardiac remodeling | Attenuate cardiac dysfunction, myocardial fibrosis, and hypertrophy; inhibit the inflammation and apoptosis induced by ISO and TGF-β1; inhibit the AKT/NF-κB pathway | [126] | |||
| Zedoarondiol (19) | Protective effect against ox-LDL-induced injury of endothelial cells | ox-LDL-induced endothelial cell injury | Inhibit oxidative stress and inflammation via the Nrf2/HO-1 pathway | [121] | |||
| Anti-atherosclerosis effect | Arteriosclerosis in apoE mice induced by high-fat diet; THP-1 monocyte migration and adhesion experience | Ameliorate AS plaque and inhibit monocyte migration and adhesion to endothelial cells via regulating the CXCL12/CXCR4 pathway | [122] | ||||
| Arteriosclerosis in apoE mice induced by high-fat diet | Inhibit aortic plaque, inhibit the expressions of HIF 1α and downstream protein VEGF, and alleviate oxidative stress injury | [123] | |||||
| Anti-atherosclerosis effect, intervene in-sent restenosis effect | PDGF-BB-induced VSMCs proliferation | Inhibit PDGF-BB-induced VSMCs proliferation via AMPK-mediated downregulation of the mTOR/p70S6K pathway and upregulation of the p53/p21 pathway | [124] | ||||
| Protective effect against coronary heart disease and cardiovascular events | RAW264.7 macrophage inflammation model | Regulate the expression of Sirt1 of the target gene of miRNA-34a and the downstream inflammatory pathway | [125] | ||||
| Germacrone (116) | Germacrane-type sesquiterpenoids | Protective effect against cardiac remodeling | Isoproterenol-induced mouse model; isoproterenol-induced neonatal rat cardiomyocytes | Attenuate oxidative stress, inflammation, and apoptosis in cardiac remodeling by inhibiting the PI3K/AKT pathway | [127] | ||
| Protective effect against cerebral ischemia/reperfusion injury | Cerebral ischemia–reperfusion injury model in rats | Increase the levels of Bcl-2 and inhibit the levels of caspase-3 and Bax; induce Akt activation | [128] | ||||
| Curdione (117) | Neuroprotective effects against focal cerebral ischemia reperfusion injury in rats | Cerebral ischemia–reperfusion injury model in rats | Reduce infarct size and neurological deficits, promote cognitive function recovery and recover neuronal morphologic damage; block the increase in MDA content and elevate the activities of SOD, CAT, and GSH-PX; increase the Bcl-2/Bax ratio and decrease cellular apoptosis | [1] | |||
| Anti-platelet aggregation effect | Thrombin-induced platelet aggregation | Regulate the AMP-activated protein kinase-vinculin/talin-integrin αIIbβ3 signaling pathway | [120] | ||||
| Platelet aggregation induced by ADP | Inhibit the activity of MAPK and PI3K/AKT pathways | Inhibitionmax: 85.6%; IC50: 0.1611 mg/mL | [8] | ||||
| Platelet aggregation induced by thrombin, PAF, ADP, AA, and tail thrombosis models | Increase cAMP levels, inhibit intracellular Ca2+ mobilization, and increase vasodilation | [13] | |||||
| Neocurdione (118) | Platelet aggregation induced by ADP | Inhibit the activity of the MAPK and PI3K/AKT pathways | Inhibitionmax: 77.6%; IC50: 0.2290 mg/mL | [8] | |||
| (1R,4S,5R,9R,10S)-9-Hydroxy-zederone epoxide (145) | Platelet aggregation induced by ADP and AA | Inhibit the platelet aggregation induced by ADP and AA | Inhibitionmax: 21.07 ± 8.67%; 27.73 ± 6.42% | Inhibitionmax: 44.83 ± 1.24%; 72.74 ± 7.54% (Aspirin) | [59] | ||
| β-Elemene (211) | Elemane-type sesquiterpenoids | Anti-thrombotic effect | Anticoagulant experiment and plasma recalcificatic time in wistar rabbits, acute blood-stasis rat model made by using ice-cold water, platelet aggregation induced by ADP and AA | Dissolve the thrombus and blood clots, prolong prothrombin and thrombin times, inhibit platelet aggregation | [129] | ||
| Anti-atherosclerosis effect | Arteriosclerosis in apoE mice induced by high-fat diet; HUVEC cell model | Increase the levels of plasma NO2/NO3, increase the expression of phosphorylation-eNOS; upregulate the Akt/eNOS signaling pathway and NO production in HUVECs | [130] | ||||
| Curcumadione (269) | Other-type sesquiterpenoids | Anti-platelet effect | Platelet aggregation induced by ADP | Inhibit the activity of MAPK and PI3K/AKT pathways | Inhibitionmax: 76.3%; IC50: 0.2560 mg/mL | [8] |
4.4. Hepatoprotective Activity
4.5. Anti-Diabetic Activity
4.6. Other Biological Activities
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Li, X.J.; Liang, L.; Shi, H.X.; Sun, X.P.; Wang, J.; Zhang, L.S. Neuroprotective Effects of Curdione against Focal Cerebral Ischemia Reperfusion Injury in Rats. Neuropsychiatr. Dis. Treat. 2017, 13, 1733–1740. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, J.H.; Wang, Y.; Donkor, P.O.; Li, Q.; Gao, S.Y.; Hou, Y.G.; Xu, Y.; Cui, J.N.; Ding, L.Q.; et al. Eudesmane-Type Sesquiterpenes from Curcuma phaeocaulis and Their Inhibitory Activities on Nitric Oxide Production in RAW 264.7 Cells. Eur. J. Org. Chem. 2014, 2014, 5540–5548. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, W.; Fang, B.; Ma, F.; Zheng, Q.; Deng, P.; Zhao, S.; Chen, M.; Yang, G.; He, G. Anti-Tumor Effect of Germacrone on Human Hepatoma Cell Lines through Inducing G2/M Cell Cycle Arrest and Promoting Apoptosis. Eur. J. Pharmacol. 2013, 698, 95–102. [Google Scholar] [CrossRef]
- Matsuda, H.; Morikawa, T.; Ninomiya, K.; Yoshikawa, M. Hepatoprotective Constituents from Zedoariae Rhizoma: Absolute Stereostructures of Three New Carabrane-Type Sesquiterpenes, Curcumenolactones A, B, and C. Bioorg. Med. Chem. 2001, 9, 909–916. [Google Scholar] [CrossRef]
- National PC. Pharmacopoeia of the People’s Republic of China (I); Chinese Medical Science and Technology Press: Beijing, China, 2020; pp. 286–287. [Google Scholar]
- Zhou, Y.; Xie, M.; Song, Y.; Wang, W.P.; Zhao, H.R.; Tian, Y.X.; Wang, Y.; Bai, S.J.; Zhao, Y.C.; Chen, X.Y.; et al. Two Traditional Chinese Medicines Curcumae Radix and Curcumae Rhizoma: An Ethnopharmacology, Phytochemistry, and Pharmacology Review. Evid. Based Complement. Alternat. Med. 2016, 2016, 4973128. [Google Scholar] [CrossRef]
- Zhu, X.; Quan, Y.Y.; Yin, Z.J.; Li, M.; Wang, T.; Zheng, L.Y.; Feng, S.Q.; Zhao, J.N.; Li, L. Sources, Morphology, Phytochemistry, Pharmacology of Curcumae Longae Rhizoma, Curcumae Radix, and Curcumae Rhizoma: A Review of the Literature. Front. Pharmacol. 2023, 14, 1229963. [Google Scholar] [CrossRef]
- Tong, H.J.; Yu, M.T.; Fei, C.H.; Ji, D.; Dong, J.J.; Su, L.L.; Gu, W.; Mao, C.Q.; Li, L.; Bian, Z.H.; et al. Bioactive Constituents and the Molecular Mechanism of Curcumae Rhizoma in the Treatment of Primary Dysmenorrhea Based on Network Pharmacology and Molecular Docking. Phytomedicine 2021, 86, 153558. [Google Scholar] [CrossRef]
- Gao, T.H.; Liao, W.; Lin, L.T.; Zhu, Z.P.; Lu, M.G.; Fu, C.M.; Xie, T. Curcumae Rhizoma and Its Major Constituents against Hepatobiliary Disease: Pharmacotherapeutic Properties and Potential Clinical Applications. Phytomedicine 2022, 102, 154090. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhu, Z.-P.; Chen, J.; Zheng, Y.-F.; Limsila, B.; Lu, M.-G.; Gao, T.-H.; Yang, Q.-S.; Fu, C.-M.; Liao, W. Terpenoids from Curcumae Rhizoma: Their Anticancer Effects and Clinical Uses on Combination and Versus Drug Therapies. Biomed. Pharmacother. 2021, 138, 111350. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.H.; Wu, Y.C.; Li, Y.M.; Guo, F.J. Review of the Traditional Uses, Phytochemistry, and Pharmacology of Curcuma wenyujin Y. H. Chen et C. Ling. J. Ethnopharmacol. 2021, 269, 113689. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.L.; Zhao, Y.L.; Ding, C.F.; Zhu, P.F.; Jin, Q.; Liu, Y.P.; Ding, Z.T.; Luo, X.D. Anti-Inflammatory and Antinociceptive Effects of Curcuma kwangsiensis and its Bioactive Terpenoids in vivo and in vitro. J. Ethnopharmacol. 2020, 259, 112935. [Google Scholar] [CrossRef] [PubMed]
- Xia, Q.; Wang, X.; Xu, D.J.; Chen, X.H.; Chen, F.H. Inhibition of Platelet Aggregation by Curdione from Curcuma wenyujin Essential Oil. Thromb. Res. 2012, 130, 409–414. [Google Scholar] [CrossRef]
- Chen, X.P.; Pei, L.X.; Zhong, Z.F.; Guo, J.J.; Zhang, Q.W.; Wang, Y.T. Anti-Tumor Potential of Ethanol Extract of Curcuma phaeocaulis Valeton against Breast Cancer Cells. Phytomedicine 2011, 18, 1238–1243. [Google Scholar] [CrossRef]
- Cui, T.; Ni, H.; Liu, J.; Peng, C.; Xiong, L.; Liu, F. Sesquiterpenoids from Volatile Oil of Curcuma phaeocaulis and Relaxant Effects on Uterine Smooth Muscle. Chin. Tradit. Herb. Drugs 2022, 53, 4265–4269. [Google Scholar]
- Liu, Y.; Ma, J.H.; Zhao, Q.; Liao, C.R.; Ding, L.; Chen, L.Q.; Zhao, F.; Qiu, F. Guaiane-Type Sesquiterpenes from Curcuma phaeocaulis and Their Inhibitory Effects on Nitric Oxide Production. J. Nat. Prod. 2013, 76, 1150–1156. [Google Scholar] [CrossRef]
- Lou, Y.; Zhao, F.; Wu, Z.; Peng, K.F.; Wei, X.C.; Chen, L.X.; Qiu, F. Germacrane-Type Sesquiterpenes from Curcuma wenyujin. Helv. Chim. Acta 2009, 92, 1665–1672. [Google Scholar] [CrossRef]
- Li, Y.; Wang, H.; Wang, H.; Wu, Y.C.; Li, Y.M.; Guo, F.J. Nine New Sesquiterpenes from Curcuma wenyujin Rhizomes. Fitoterapia 2022, 158, 105167. [Google Scholar] [CrossRef]
- Zhao, P.; Qiu, J.F.; Pan, C.L.; Tang, Y.Y.; Chen, M.J.; Song, H.; Yang, J.; Hao, X.J. Potential Roles and Molecular Mechanisms of Bioactive Ingredients in Curcumae Rhizoma against Breast Cancer. Phytomedicine 2023, 114, 154810. [Google Scholar] [CrossRef]
- Lu, J.-J.; Dang, Y.-Y.; Huang, M.; Xu, W.-S.; Chen, X.-P.; Wang, Y.-T. Anti-Cancer Properties of Terpenoids Isolated from Rhizoma Curcumae–A Review. J. Ethnopharmacol. 2012, 143, 406–411. [Google Scholar] [CrossRef]
- Wu, Y.-Q.; Tong, T. Curcumae Rhizoma: A Botanical Drug against Infectious Diseases. Front. Pharmacol. 2023, 13, 1015098. [Google Scholar] [CrossRef]
- Dosoky, N.; Setzer, W. Chemical Composition and Biological Activities of Essential Oils of Curcuma Species. Nutrients 2018, 10, 1196. [Google Scholar] [CrossRef]
- Yang, F.Q.; Li, S.P.; Zhao, J.; Lao, S.C.; Wang, Y.T. Optimization of GC–MS Conditions Based on Resolution and Stability of Analytes for Simultaneous Determination of Nine Sesquiterpenoids in Three Species of Curcuma rhizomes. J. Pharm. Biomed. Anal. 2007, 43, 73–82. [Google Scholar] [CrossRef]
- Xiang, Z.; Wang, X.Q.; Cai, X.J.; Zeng, S. Metabolomics Study on Quality Control and Discrimination of Three Curcuma Species Based on Gas Chromatograph–Mass Spectrometry. Phytochem. Anal. 2011, 22, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; He, T.; Wang, J.J.; Wang, L.; Ren, X.Y.; He, S.H.; Liu, X.Y.; Dong, Y.; Ma, J.M.; Song, R.L.; et al. High Performance Liquid Chromatography Fingerprint and Headspace Gas Chromatography-Mass Spectrometry Combined with Chemometrics for the Species Authentication of Curcumae rhizoma. J. Pharm. Biomed. Anal. 2021, 202, 114144. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.Q.; Li, S.P.; Chen, Y.; Lao, S.C.; Wang, Y.T.; Dong, T.T.X.; Tsim, K.W.K. Identification and Quantitation of Eleven Sesquiterpenes in Three Species of Curcuma Rhizomes by Pressurized Liquid Extraction and Gas Chromatography–Mass Spectrometry. J. Pharm. Biomed. Anal. 2005, 39, 552–558. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.H.; Liu, J.W.; Wu, Y.C.; Li, Y.M.; Guo, F.J. Guaiane-Type Sesquiterpenes from Curcuma wenyujin. Phytochemistry 2022, 198, 113164. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.J.; Yan, X.; Liu, W.R.; Tian, Y.X.; Song, R.L.; Dong, Y.; Ren, X.Y.; Zheng, Y.; Shan, D.J.; Lv, F.; et al. Sesquiterpenoids Isolated from the Rhizome of Curcuma phaeocaulis Valeton: Antitumor Activity, in Silico Molecular Docking and Molecular Dynamics Study. New J. Chem. 2023, 47, 7830–7839. [Google Scholar] [CrossRef]
- Dong, J.Y.; Ma, X.Y.; Cai, X.Q.; Yan, P.C.; Yue, L.; Lin, C.; Shao, W.W. Sesquiterpenoids from Curcuma wenyujin with Anti-Influenza Viral Activities. Phytochemistry 2013, 85, 122–128. [Google Scholar] [CrossRef]
- Lou, Y.; He, H.; Wei, X.C.; Li, X.G.; Chen, L.X.; Qiu, F. Sesquiterpenes from Curcuma wenyujin. J. Shenyang Pharm. Univ. 2010, 27, 195–199. [Google Scholar]
- Zhou, C.X.; Zhang, L.S.; Chen, F.F.; Wu, H.S.; Mo, J.X.; Gan, L.S. Terpenoids from Curcuma wenyujin Increased Glucose Consumption on HepG2 Cells. Fitoterapia 2017, 121, 141–145. [Google Scholar] [CrossRef]
- Chen, L.J.; Liu, J.W.; Wang, H.; Li, Y.H.; Li, Y.M.; Guo, F.J. Four New Sesquiterpenes from Curcuma wenyujin. Fitoterapia 2022, 163, 105344. [Google Scholar] [CrossRef]
- Liu, F.; Chen, J.F.; Qiao, M.M.; Zhao, H.Y.; Zhou, Q.M.; Guo, L.; Peng, C.; Xiong, L. Seven Pairs of New Enantiomeric Sesquiterpenoids from Curcuma phaeocaulis. Bioorg. Chem. 2020, 99, 103820. [Google Scholar] [CrossRef]
- Ma, J.H.; Zhao, F.; Wang, Y.; Liu, Y.; Gao, S.Y.; Ding, L.Q.; Chen, L.X.; Qiu, F. Four New Sesquiterpenoids as Natural Nitric Oxide (NO) Inhibitors from the Rhizomes of Curcuma phaeocaulis. Phytochem. Lett. 2015, 14, 221–225. [Google Scholar] [CrossRef]
- Yin, G.P.; Li, L.C.; Zhang, Q.Z.; An, Y.W.; Zhu, J.J.; Wang, Z.M.; Chou, G.X.; Wang, Z.T. iNOS Inhibitory Activity of Sesquiterpenoids and a Monoterpenoid from the Rhizomes of Curcuma wenyujin. J. Nat. Prod. 2014, 77, 2161–2169. [Google Scholar] [CrossRef]
- Huang, H.F.; Zheng, C.J.; Chen, G.Y.; Yin, W.Q.; Huang, X.; Mo, Z.R. Sesquiterpenoids from Curcuma wenyujin Dreg and their Biological Activities. Chin. Chem. Lett. 2016, 27, 1612–1616. [Google Scholar] [CrossRef]
- Jang, H.J.; Kim, J.H.; Oh, H.M.; Kim, M.S.; Jo, J.H.; Jung, K.; Lee, S.; Kim, Y.H.; Lee, W.S.; Lee, S.W.; et al. Sesquiterpenoids from the Rhizomes of Curcuma phaeocaulis and Their Inhibitory Effects on LPS-Induced TLR4 Activation. Chem. Pharm. Bull. 2016, 64, 1062–1066. [Google Scholar] [CrossRef][Green Version]
- Dai, W.F.; Zhang, L.L.; Liu, Y.F.; Zhang, M. A New 4,5-Secofurancadinene from the Rhizome of Curcuma kwangsiensis. Rec. Nat. Prod. 2020, 14, 297–300. [Google Scholar] [CrossRef]
- Lou, Y.; Zhao, F.; He, H.; Peng, K.F.; Zhou, X.H.; Chen, L.X.; Qiu, F. Guaiane-type Sesquiterpenes from Curcuma wenyujin and Their Inhibitory Effects on Nitric Oxide Production. J. Asian Nat. Prod. Res. 2009, 11, 737–747. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.F.; Zheng, C.J.; Mo, Z.R.; Yin, W.Q.; Chen, G.Y.; Han, C.R.; Huang, X. Antibacterial Sesquiterpenoids from the Petroleum Ether Extract of Curcuma wenyujin Dreg. Chem. Nat. Compd. 2016, 52, 527–530. [Google Scholar] [CrossRef]
- Zhan, X.R.; Zeng, Z.W.; Meng, F.L.; Wang, S.L.; Xie, T. Pharmaceutical Researches on Zedoary Turmeric Oil. J. Hangzhou Norm. Univ. Nat. Sci. Ed. 2011, 10, 454–458. [Google Scholar]
- Ma, J.H.; Wang, Y.; Liu, Y.; Gao, S.Y.; Ding, L.Q.; Zhao, F.; Chen, L.X.; Qiu, F. Four New Sesquiterpenes from the Rhizomes of Curcuma phaeocaulis and Their iNOS Inhibitory Activities. J. Asian Nat. Prod. Res. 2015, 17, 532–540. [Google Scholar] [CrossRef]
- Harimaya, K.; Gao, J.F.; Ohkura, T.; Kawamata, T.; Irraka, Y.; Guo, Y.T.; Inayama, S. A Series of Sesquiterpenes with a 7α-isopropyl Side Chain and Related Compounds Isolated from Curcuma wenyujin. Chem. Pharm. Bull. 1991, 39, 843–853. [Google Scholar] [CrossRef]
- Xia, G.Y.; Zhou, L.; Ma, J.H.; Wang, Y.; Ding, L.Q.; Zhao, F.; Chen, L.X.; Qiu, F. Sesquiterpenes from the Essential oil of Curcuma wenyujin and their Inhibitory Effects on Nitric Oxide Production. Fitoterapia 2015, 103, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.Y.; Zhang, L.S.; Zhang, J.; Zhang, Y.Y.; Pan, J.R. Chemical Constituents from Curcuma wenyujin. Chin. Tradit. Pat. Med. 2016, 38, 1534–1537. [Google Scholar]
- Qiu, G.G.; Yan, P.C.; Shao, W.W.; Zhou, J.; Lin, W.W.; Fang, L.L.; Zhao, X.W.; Dong, J.Y. Two New Sesquiterpenoids Including a Sesquiterpenoid Lactam from Curcuma wenyujin. Chem. Pharm. Bull. 2013, 61, 983–986. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.-H.; Zheng, H.-H.; Xu, Y.-T.; Zhang, P.; Chen, G.; Zhu, Y. Two New Sesquiterpenes from a Kind of TCM Pieces, Curcumae Radix. Rec. Nat. Prod. 2014, 8, 334. [Google Scholar]
- Cai, Y. A New Sesquiterpene Compound—Curcumafuranol. J. Beijing Med. Univ. 1998, 30, 49–52. [Google Scholar]
- Zhu, K. Studies on the Chemical Constituents from Curcuma kwangsiensis. Master’s Thesis, Shenyang Pharmaceutical University, Shenyang, China, 2008. [Google Scholar]
- Jiang, D.Q.; Pu, J.L.; Huang, P.; Huang, Y.M.; He, Y.Z.; He, C.H.; Zheng, Q.T. Studies on the Chemical Composition of Curcuma kwangsiensis. Chin. Pharm. J. 1989, 24, 42. [Google Scholar]
- Yin, G.P.; An, Y.W.; Hu, G.; Zhu, J.J.; Chen, L.M.; Li, L.C.; Wang, Z.M. Three New Guaiane Sesquiterpene Lactones from Rhizomes of Curcuma wenyujin. J. Asian Nat. Prod. Res. 2013, 15, 723–730. [Google Scholar] [CrossRef]
- Liao, H.B.; Feng, W.Y.; Wang, H.S.; Liang, D. Sesquiterpenoid Compounds from Curcuma kwangsiensis (Thunb.) Sweet. Chem. Biodivers. 2019, 16, e1900123. [Google Scholar] [CrossRef]
- Hu, D.; Ma, N.; Lou, Y.; Qu, G.X.; Qiu, F. Guaiane Sesquiterpenes of Curcuma wenyujin. J. Shenyang Pharm. Univ. 2008, 25, 188–190. [Google Scholar]
- Phan, M.G.; Tran, T.T.N.; Phan, T.S.; Matsunami, K.; Otsuka, H. Guaianolides from Curcuma kwangsiensis. Phytochem. Lett. 2014, 9, 137–140. [Google Scholar] [CrossRef]
- Xiang, F.F.; He, J.W.; Liu, Z.X.; Peng, Q.Z.; Wei, H. Two New Guaiane-Type Sesquiterpenes from Curcuma kwangsiensis and Their Inhibitory Activity of Nitric Oxide Production in Lipopolysaccharide-Stimulated Macrophages. Nat. Prod. Res. 2018, 32, 2670–2675. [Google Scholar] [CrossRef]
- Jang, H.J.; Lim, H.J.; Park, E.J.; Lee, S.J.; Lee, S.; Lee, S.W.; Rho, M.C. STAT3-Inhibitory Activity of Sesquiterpenoids and Diterpenoids from Curcuma phaeocaulis. Bioorg. Chem. 2019, 93, 103267. [Google Scholar] [CrossRef]
- Oh, S.; Han, A.R.; Park, H.R.; Jang, E.J.; Kim, H.K.; Jeong, M.G.; Song, H.; Park, G.H.; Seo, E.K.; Hwang, E.S. Suppression of Inflammatory Cytokine Production by ar-Turmerone Isolated from Curcuma phaeocaulis. Chem. Biodiversity 2014, 11, 1034–1041. [Google Scholar] [CrossRef]
- Liu, X.Y.; Lou, Y.; Hu, D.; Chen, L.X.; Bu, G.M.; Qiu, F. Chemical Constituents of the Essential Oil from Curcuma wenyujin Y. H. Chen et C. Ling. J. Shenyang Pharm. Univ. 2007, 24, 686. [Google Scholar]
- Li, X.C.; Chen, J.F.; Xiong, L.; Peng, C.; Guo, L.; Liu, F. Study on Germacrane-Type Sesquiterpenoids from Curcuma phaeocaulis. Chin. Tradit. Herb. Drugs 2021, 52, 28–34. [Google Scholar]
- Li, J. Studies on Chemical Substances of Curcuma kwangsiensis and Metabolites of Natural Curcuminoids in Rats. Ph.D. Thesis, Shenyang Pharmaceutical University, Shenyang, China, 2010. [Google Scholar]
- Yin, G.P.; Yang, D.; Zhu, T.; Zhang, Z.L.; Xie, W.; Hu, C.H.; Zhu, J.J.; Wang, Z.M. Wenyujindiol A, A New Sesquiterpene from the Rhizomes of Curcuma wenyujin. Tetrahedron Lett. 2020, 61, 152448. [Google Scholar] [CrossRef]
- Gao, J.F.; Xie, J.H.; Harimaya, K.; Kawamata, T.; Iitaka, Y.; Inayama, S. The Absolute Structure and Synthesis of Wenjine Isolated from Curcuma wenyujin. Chem. Pharm. Bull. 1991, 39, 854–856. [Google Scholar] [CrossRef]
- Niu, Z.G.; Chen, H.H.; Gao, C.W.; Chen, G.Y.; Li, G.N. Chemical Constituents from the Dregs of Curcuma wenyujin. Guang Dong Chem. 2014, 16, 22–23. [Google Scholar]
- Xia, G.Y.; Sun, D.J.; Ma, J.H.; Liu, Y.; Zhao, F.; Donkor, P.O.; Ding, L.Q.; Chen, L.X.; Qiu, F. (+)/(−)-Phaeocaulin A-D, Four Pairs of New Enantiomeric Germacrane-Type Sesquiterpenes from Curcuma phaeocaulis as Natural Nitric Oxide Inhibitors. Sci. Rep. 2017, 7, 43576. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.T.; Ge, D.; Qu, H.F.; Wang, G.K.; Wang, G. Chemical Constituents of Curcuma kwangsiensis and Their Antimigratory Activities in RKO Cells. Natl. Prod. Res. 2019, 33, 3493–3499. [Google Scholar] [CrossRef] [PubMed]
- Lou, Y.; Zhao, F.; He, H.; Peng, K.F.; Chen, L.X.; Qiu, F. Four New Sesquiterpenes from Curcuma wenyujin and Their Inhibitory Effects on Nitric-Oxide Production. Chem. Biodivers. 2010, 7, 1245–1253. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.S.; Zhang, J.M.; Guo, X.H.; Song, Q.L.; Zhao, W.J. A New Eudesmane Sesquiterpene Lactone from Curcuma wenyujin. Acta Pharm. Sin. B 2007, 42, 1062–1065. [Google Scholar]
- Gao, S.Y.; Xia, G.Y.; Wang, L.Q.; Zhou, L.; Zhao, F.; Huang, J.; Chen, L.X. Sesquiterpenes from Curcuma wenyujin with Their Inhibitory Activities on Nitric Oxide Production in RAW 264.7 Cells. Nat. Prod. Res. 2017, 31, 548–554. [Google Scholar] [CrossRef]
- Ma, J.H.; Zhao, F.; Wang, Y.; Liu, Y.; Gao, S.Y.; Ding, L.Q.; Chen, L.X.; Qiu, F. Natural Nitric Oxide (NO) Inhibitors from the Rhizomes of Curcuma phaeocaulis. Org. Biomol. Chem. 2015, 13, 8349–8358. [Google Scholar] [CrossRef]
- Song, G.Q.; Wu, P.; Dong, X.M.; Cheng, L.H.; Lu, H.Q.; Lin, Y.Y.; Tang, W.Y.; Xie, T.; Zhou, J.L. Elemene Induces Cell Apoptosis via Inhibiting Glutathione Synthesis in Lung Adenocarcinoma. J. Ethnopharmacol. 2023, 311, 116409. [Google Scholar] [CrossRef]
- Zhu, J.J.; Lower-Nedza, A.D.; Hong, M.; Jie, S.; Wang, Z.M.; Dong, Y.M.; Tschiggerl, C.; Bucar, F.; Brantner, A.H. Chemical Composition and Antimicrobial Activity of Three Essential Oils from Curcuma wenyujin. Nat. Prod. Commun. 2013, 8, 523–526. [Google Scholar] [CrossRef]
- Zhang, L.Y.; Yang, Z.W.; Huang, Z.B.; Zhao, M.C.; Li, P.H.; Zhou, W.; Zhang, K.; Zheng, X.; Lin, L.; Tang, J.; et al. Variation in Essential Oil and Bioactive Compounds of Curcuma kwangsiensis Collected from Natural Habitats. Chem. Biodivers. 2017, 14, e1700020. [Google Scholar] [CrossRef]
- Liang, H.; Wang, Q.; Ding, C.B.; Zhang, L.; Yang, R.W. Chemical Composition, Antioxidant and Antibacterial Activities of Essential Oil of Curcuma phaeocaulis Valeton. Bangladesh J. Bot. 2020, 49, 531–540. [Google Scholar] [CrossRef]
- Ma, J.H.; Wang, Y.; Liu, Y.; Gao, S.Y.; Ding, L.Q.; Zhao, F.; Chen, L.X.; Qiu, F. Cadinane Sesquiterpenes from Curcuma phaeocaulis with Their Inhibitory Activities on Nitric Oxide Production in RAW 264.7 Cells. Fitoterapia 2015, 103, 90–96. [Google Scholar] [CrossRef]
- Zuo, J.; Zhang, T.-H.; Peng, C.; Xu, B.-J.; Dai, O.; Lu, Y.; Zhou, Q.-M.; Xiong, L. Essential Oil from Ligusticum chuanxiong Hort. Alleviates Lipopolysaccharide-Induced Neuroinflammation: Integrating Network Pharmacology and Molecular Mechanism Evaluation. J. Ethnopharmacol. 2024, 319, 117337. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zhao, Y.; Aisa, H.A. Anti-Inflammatory Effect of Pomegranate Flower in Lipopolysaccharide (LPS)-Stimulated RAW264.7 Macrophages. Pharm. Biol. 2017, 55, 2095–2101. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Ma, L.; Cai, C.; Gong, X. Caffeine Inhibits NLRP3 Inflammasome Activation by Suppressing MAPK/NF-κB and A2aR Signaling in LPS-Induced THP-1 Macrophages. Int. J. Biol. Sci. 2019, 15, 1571–1581. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhao, Q.; Liang, Y.Y.; Ma, J.H.; Chen, L.X.; Zhang, X.; Ding, L.Q.; Zhao, F.; Qiu, F. Stereo- and Regiospecific Biotransformation of Curcumenol by Four Fungal Strains. J. Mol. Catal. B Enzym. 2015, 115, 13–19. [Google Scholar] [CrossRef]
- Yoshioka, T.; Fujii, E.; Endo, M.; Wada, K.; Tokunaga, Y.; Shiba, N.; Hohsho, H.; Shibuya, H.; Muraki, T. Antiinflammatory Potency of Dehydrocurdione, A Zedoary-Derived Sesquiterpene. Inflamm Res. 1998, 47, 476–481. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.Y.; Hao, E.W.; Cao, R.; Du, Z.C.; Liang, L.L.; Shen, Y.B.; Hou, X.T.; Deng, J.G. Research Progress on Pharmacological Action and Mechanism of Germacrone. Drugs Clin. 2022, 37, 644–652. [Google Scholar]
- Cai, Y.; Li, W.C.; Tu, H.F.; Chen, N.M.; Zhong, Z.P.; Yan, P.C.; Dong, J.Y. Curcumolide Reduces Diabetic Retinal Vascular Leukostasis and Leakage Partly via Inhibition of the p38MAPK/NF-κB Signaling. Bioorg. Med. Chem. Lett. 2017, 27, 1835–1839. [Google Scholar] [CrossRef] [PubMed]
- Jia, S.S.; Guo, P.; Lu, J.H.; Huang, X.J.; Deng, L.M.; Jin, Y.; Zhao, L.Y.; Fan, X.F. Curcumol Ameliorates Lung Inflammation and Airway Remodeling via Inhibiting the Abnormal Activation of the Wnt/β-Catenin Pathway in Chronic Asthmatic Mice. Drug Des. Devel. Ther. 2021, 15, 2641–2651. [Google Scholar] [CrossRef]
- Lv, M.F.; Shao, J.Y.; Jiang, F.; Liu, J.J. Curcumol may Alleviate Psoriasis-Like Inflammation by Inhibiting Keratinocyte ProliferAtion and Inflammatory Gene Expression via JAK1/STAT3 Signaling. Aging 2021, 13, 18392–18403. [Google Scholar] [CrossRef]
- Wang, Z.R.; Zhuo, F.; Chu, P.G.; Yang, X.L.; Zhao, G. Germacrone Alleviates Collagen-Induced Arthritis via Regulating Th1/Th2 Balance and NF-κB Activation. Biochem. Biophys. Res. Commun. 2019, 518, 560–564. [Google Scholar] [CrossRef]
- Li, Y.Q.; Li, G.Z.; Dong, Y.; Ma, X.; Dong, H.J.; Wu, Q.Q.; Zhao, W.J. Orobanone Analogues from Acid-Promoted Aromatization Rearrangement of Curcumol Inhibit Hypoxia-Inducible Factor-1 (HIF-1) in Cell-Based Reporter Assays. Bioorg. Chem. 2019, 85, 357–363. [Google Scholar] [CrossRef]
- Tungcharoen, P.; Wattanapiromsakul, C.; Tansakul, P.; Nakamura, S.; Matsuda, H.; Tewtrakul, S. Antiinflammation Constituents from Curcuma zedoaroides. Phytother. Res. 2018, 32, 2312–2320. [Google Scholar] [CrossRef]
- Lee, T.K.; Trinh, T.A.; Lee, S.R.; Kim, S.; So, H.M.; Moon, E.; Hwang, G.S.; Kang, K.S.; Kim, J.H.; Yamabe, N.; et al. Bioactivity-Based Analysis and Chemical Characterization of Anti-Inflammatory Compounds from Curcuma zedoaria Rhizomes Using LPS-Stimulated RAW264.7 Cells. Bioorg. Chem. 2019, 82, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.K.; Zhang, N.; Yao, J.N.; Yu, Y.; Wang, G.; Hung, C.C.; Cheng, Y.Y.; Morris-Natschke, S.L.; Zhou, Z.Y.; Liu, J.S.; et al. Kalshinoids A–F, Anti-Inflammatory Sesquiterpenes from Kalimeris shimadae. J. Nat. Prod. 2019, 82, 3372–3378. [Google Scholar] [CrossRef] [PubMed]
- Cho, W.; Nam, J.W.; Kang, H.J.; Windono, T.; Seo, E.K.; Lee, K.T. Zedoarondiol Isolated from the Rhizoma of Curcuma heyneana is Involved in the Inhibition of iNOS, COX-2 and Pro-Inflammatory Cytokines via the Downregulation of NF-kappa B Pathway in LPS-Stimulated Murine Macrophages. Int. Immunopharmacol. 2009, 9, 1049–1057. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zong, C.J.; Gao, Y.; Cai, R.L.; Fang, L.; Lu, J.; Liu, F.; Qi, Y. Curcumol Exhibits Anti-Inflammatory Properties by Interfering with the JNK-Mediated AP-1 Pathway in Lipopolysaccharide-Activated RAW264.7 Cells. Eur. J. Pharmacol. 2014, 723, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Lo, J.Y.; Kamarudin, M.N.A.; Hamdi, O.A.A.; Awang, K.; Kadir, H.A. Curcumenol Isolated from Curcuma zedoaria Suppresses Akt-Mediated NF-κB Activation and p38 MAPK Signaling Pathway in LPS-Stimulated BV-2 Microglial Cells. Food Funct. 2015, 6, 3550–3559. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.Y.; Shao, W.W.; Yan, P.C.; Cai, X.Q.; Fang, L.L.; Zhao, X.W.; Lin, W.W.; Cai, Y. Curcumolide, A Unique Sesquiterpenoid with Anti-Inflammatory Properties from Curcuma wenyujin. Bioorg. Med. Chem. Lett. 2015, 25, 198–202. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Z.F.; Dang, Y.Y.; Yuan, X.; Guo, W.; Li, Y.B.; Tan, W.; Cui, J.R.; Lu, J.J.; Zhang, Q.W.; Chen, X.P.; et al. Furanodiene, A Natural Product, Inhibits Breast Cancer Growth Both in vitro and in vivo. Cell. Physiol. Biochem. 2012, 30, 778–790. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Z.F.; Tan, W.; Qiang, W.W.; Scofield, V.L.; Tian, K.; Wang, C.M.; Qiang, W.A.; Wang, Y.T. Furanodiene Alters Mitochondrial Function in Doxorubicin-Resistant MCF-7 Human Breast Cancer Cells in an AMPK-Dependent Manner. Mol. Biosyst. 2016, 12, 1626–1637. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Tran, T.H.; Nguyen, T.H.; Do, T.H. Cytotoxic Sesquiterpenes and Diterpenes from the Rhizomes of Curcuma zedoaroides Chaveer. & Tanee. Biochem. Syst. Ecol. 2024, 112, 104781. [Google Scholar]
- Ma, E.; Wang, X.L.; Li, Y.C.; Sun, X.Y.; Tai, W.J.; Li, T.; Guo, T. Induction of Apoptosis by Furanodiene in HL60 Leukemia Cells through Activation of TNFR1 Signaling Pathway. Cancer Lett. 2008, 271, 158–166. [Google Scholar] [CrossRef]
- Ying, J.; Yang, W.; Xie, C.Y.; Ni, Q.C.; Pan, X.D.; Dong, J.H.; Liu, Z.M.; Wang, X.S. Induction of Caspase-3-Dependent Apoptosis in Human Leukemia HL-60 Cells by δ-Elemene. J. Pharm. Soc. Jpn. 2011, 131, 1383–1394. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wei, W.; Rasul, A.; Sadiqa, A.; Sarfraz, I.; Hussain, G.; Nageen, B.; Liu, X.; Watanabe, N.; Selamoglu, Z.; Ali, M.; et al. Curcumol: From Plant Roots to Cancer Roots. Int. J. Biol. Sci. 2019, 15, 1600–1609. [Google Scholar] [CrossRef] [PubMed]
- Batool, R.; Rasul, A.; Hussain, G.; Shah, M.A.; Nageen, B.; Sarfraz, I.; Zahoor, M.K.; Riaz, A.; Ajaz, A.; Adem, Ş. Furanodiene: A Novel, Potent, and Multitarget Cancer-Fighting Terpenoid. Curr. Pharm. Des. 2021, 27, 2628–2634. [Google Scholar] [CrossRef] [PubMed]
- Tong, H.X.; Liu, Y.H.; Jiang, L.J.; Wang, J.S. Multi-Targeting by β-Elemene and Its Anticancer Properties: A Good Choice for Oncotherapy and Radiochemotherapy Sensitization. Nutr. Cancer 2019, 72, 554–567. [Google Scholar] [CrossRef] [PubMed]
- Zhai, B.T.; Zhang, N.N.; Han, X.M.; Li, Q.J.; Zhang, M.M.; Chen, X.Y.; Li, G.H.; Zhang, R.N.; Chen, P.; Wang, W.G.; et al. Molecular Targets of β-elemene, A Herbal Extract used in Traditional Chinese Medicine, and its Potential Role in Cancer Therapy: A Review. Biomed. Pharmacother. 2019, 114, 108812. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.Z.; Li, A.; Liao, G.Z.; Yang, F.C.; Yang, J.; Chen, X.; Jiang, X.S. Curcumol Triggers Apoptosis of p53 Mutant Triple-Negative Human Breast Cancer MDA-MB 231 Cells via Activation of p73 and PUMA. Oncol. Lett. 2017, 14, 1080–1088. [Google Scholar] [CrossRef] [PubMed]
- Zuo, H.X.; Jin, Y.; Wang, Z.; Li, M.Y.; Zhang, Z.H.; Wang, J.Y.; Xing, Y.; Ri, M.H.; Jin, C.H.; Xu, G.H.; et al. Curcumol Inhibits the Expression of Programmed Cell Death-Ligand 1 through Crosstalk between Hypoxia-Inducible Factor-1α and STAT3 (T705) Signaling Pathways in Hepatic Cancer. J. Ethnopharmacol. 2020, 257, 112835. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Huang, F.X.; Bai, Z.; Chi, B.X.; Wu, J.C.; Chen, X. Curcumol Inhibits Growth and Induces Apoptosis of Colorectal Cancer LoVo Cell Line via IGF-1R and p38 MAPK Pathway. Int. J. Mol. Sci. 2015, 16, 19851–19867. [Google Scholar] [CrossRef]
- Hamdi, O.A.A.; Rahman, S.N.S.A.; Awang, K.; Wahab, N.A.; Looi, C.Y.; Thomas, N.F.; Malek, S.N.A. Cytotoxic Constituents from the Rhizomes of Curcuma zedoaria. Sci. World J. 2014, 2014, 321943. [Google Scholar]
- Zhang, R.N.; Pan, T.; Xiang, Y.; Zhang, M.M.; Xie, H.; Liang, Z.M.; Chen, B.; Xu, C.; Wang, J.; Huang, X.X.; et al. Curcumenol Triggered Ferroptosis in Lung Cancer Cells via LncRNA H19/miR-19b-3p/FTH1 Axis. Bioact. Mater. 2021, 13, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Jung, E.B.; Trinh, T.A.; Lee, T.K.; Yamabe, N.; Kang, K.S.; Song, J.H.; Choi, S.; Lee, S.; Jang, T.S.; Kim, K.H.; et al. Curcuzedoalide Contributes to the Cytotoxicity of Curcuma zedoaria Rhizomes against Human Gastric Cancer AGS Cells through Induction of Apoptosis. J. Ethnopharmacol. 2018, 213, 48–55. [Google Scholar] [CrossRef]
- Wu, L.; Wang, L.F.; Tian, X.G.; Zhang, J.Y.; Feng, H. Germacrone Exerts Anti-Cancer Effects on Gastric Cancer Through Induction of Cell Cycle Arrest and Promotion of Apoptosis. BMC Complement. Med. Ther. 2020, 20, 21. [Google Scholar] [CrossRef]
- Zhong, Z.F.; Chen, X.P.; Tan, W.; Xu, Z.T.; Zhou, K.Y.; Wu, T.; Cui, L.; Wang, Y.T. Germacrone Inhibits the Proliferation of Breast Cancer Cell Lines by Inducing Cell Cycle Arrest and Promoting Apoptosis. Eur. J. Pharmacol. 2011, 667, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Hao, J.; Guo, K.W.; Liu, W.X.; Yao, F.; Wu, Q.M.; Liu, C.; Wang, Q.; Yang, X.Z. Germacrone Inhibits Cell Proliferation and Induces Apoptosis in Human Esophageal Squamous Cell Carcinoma Cells. BioMed Res. Int. 2020, 2020, 7643248. [Google Scholar] [CrossRef]
- Wang, F.; Sun, Z.; Zhang, Q.Y.; Yang, H.; Yang, G.; Yang, Q.; Zhu, Y.M.; Wu, W.Y.; Xu, W.W.; Wu, X.Y. Curdione Induces Ferroptosis Mediated by m6A Methylation via METTL14 and YTHDF2 in Colorectal Cancer. Chin. Med. 2023, 18, 122. [Google Scholar] [CrossRef]
- Wei, C.; Li, D.H.; Liu, Y.; Wang, W.N.; Qiu, T.T. Curdione Induces Antiproliferation Effect on Human Uterine Leiomyosarcoma via Targeting IDO1. Front. Oncol. 2021, 11, 637024. [Google Scholar] [CrossRef]
- Cao, R.R.; Zhou, J.; Wang, Q.M.; Zhang, X.; Wang, R.R.; Chen, M. The Effect of the Curdione on the Proliferation of HHSEC Under the Microenvironment of HepG2 Cells via VEGF/VEGFR2 Signaling Pathway. J. Hunan Univ. Chin. Med. 2021, 41, 1835–1839. [Google Scholar]
- Jiang, Y.; Wang, X.Q.; Hu, D.D. Furanodienone Induces G0/G1 Arrest and Causes Apoptosis via the ROS/MAPKs-Mediated Caspase-Dependent Pathway in Human Colorectal Cancer Cells: A Study in vitro and in vivo. Cell Death Dis. 2017, 8, e2815. [Google Scholar] [CrossRef]
- Zhang, Z.L.; Li, L.Y.; Wang, J.M.; Liang, X.; Wang, Y.Y.; Wang, X.F.; Qiao, Y.H.; Zhao, B. A Study of Zederone for the Inhibition on Ovarian Cancer Cell Proliferation through mTOR/p70s6K Signaling Pathway. J. BUON 2020, 25, 785–791. [Google Scholar]
- Lu, H.; Chen, J.; Luo, Y.M.; Xu, H.J.; Xiong, L.; Fu, J.J. Curcolonol Suppresses the Motility of Breast Cancer Cells by Inhibiting LIM Kinase 1 to Downregulate Cofilin 1 Phosphorylation. Int. J. Oncol. 2018, 53, 2695–2704. [Google Scholar] [CrossRef]
- Fu, J.J.; Yu, J.J.; Chen, J.; Xu, H.J.; Luo, Y.M.; Lu, H. In vitro Inhibitory Properties of Sesquiterpenes from Chloranthus serratus on Cell Motility via Down-Regulation of LIMK1 Activation in Human Breast Cancer. Phytomedicine 2018, 49, 23–31. [Google Scholar] [CrossRef]
- Wang, Y.D.; Li, J.H.; Guo, J.Q.; Wang, Q.Y.; Zhu, S.G.; Gao, S.Y.; Yang, C.; Wei, M.; Pan, X.D.; Zhu, W.; et al. Cytotoxic and Antitumor Effects of Curzerene from Curcuma longa. Planta Med. 2017, 83, 23–29. [Google Scholar] [CrossRef]
- Zheng, T.T.; Xiao, H.T.; Shen, Y.H.; Zhang, X.; Jiang, K.K.; Liu, L.; Bai, X.H.; Peng, J.; Chen, Y. Anticancer Effects of Curzerenone against Drug-Resistant Human Lung Carcinoma Cells are Mediated via Programmed Cell Death, Loss of Mitochondrial Membrane Potential, ROS, and Blocking the ERK/MAPK and NF-κB Signaling Pathway. J. BUON. 2019, 24, 907–912. [Google Scholar]
- Fang, H.; Gao, B.B.; Zhao, Y.L.; Fang, X.; Bian, M.H.; Xia, Q. Curdione Inhibits Thrombin-Induced Platelet Aggregation via Regulating the AMP-Activated Protein Kinase-Vinculin/Talin-Integrin αIIbβ3 Sign Pathway. Phytomedicine 2019, 61, 152859. [Google Scholar] [CrossRef] [PubMed]
- Mao, H.M.; Tao, T.Q.; Wang, X.R.; Liu, M.; Song, D.D.; Liu, X.H.; Shi, D.Z. Zedoarondiol Attenuates Endothelial Cells Injury Induced by Oxidized Low-Density Lipoprotein via Nrf2 Activation. Cell Physiol. Biochem. 2018, 48, 1468–1479. [Google Scholar] [CrossRef] [PubMed]
- Chai, H.; Qu, H.; He, S.; Song, L.; Yang, Y.; Huang, H.B.; Shi, D.Z. Zedoarondiol Inhibits Atherosclerosis by Regulating Monocyte Migration and Adhesion via CXCL12/CXCR4 Pathway. Pharmacol. Res. 2022, 182, 106328. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.J. Research on Anti-Atherosclerosis of the Zedoarondiol an Active Ingredients of Curcuma by Mediating Hif-1α Signaling Pathway. Master’s Thesis, China Academy of Chinese Medical Sciences, Beijing, China, 2021. [Google Scholar]
- Mao, H.M.; Tao, T.Q.; Song, D.D.; Liu, M.; Wang, X.R.; Liu, X.H.; Shi, D.Z. Zedoarondiol Inhibits Platelet-Derived Growth Factor-Induced Vascular Smooth Muscle Cells Proliferation via Regulating AMP-Activated Protein Kinase Signaling Pathway. Cell. Physiol. Biochem. 2016, 40, 1506–1520. [Google Scholar] [CrossRef] [PubMed]
- Liu, J. Analysis of Risk Factors Related to Prethrombotic State and Effect of Zedoarondiol Regulation Target miRNA on Inflammatory Response. Master’s Thesis, China Academy of Chinese Medical Sciences, Beijing, China, 2018. [Google Scholar]
- Fang, Z.; Li, S.; Yushanjiang, F.; Feng, G.K.; Cui, S.Y.; Hu, S.; Jiang, X.J.; Liu, C.Y. Curcumol Alleviates Cardiac Remodeling via the AKT/NF-κB Pathway. Int. Immunopharmacol. 2023, 122, 110527. [Google Scholar] [CrossRef]
- Fang, Z.; Yushanjiang, F.; Wang, G.J.; Zheng, X.X.; Jiang, X.J. Germacrone Mitigates Cardiac Remodeling by Regulating PI3K/AKT-Mediated Oxidative Stress, Inflammation, and Apoptosis. Int. Immunopharmacol. 2023, 124, 110876. [Google Scholar] [CrossRef]
- Wu, T.H.; Yin, F.; Kong, H.M.; Peng, J. Germacrone Attenuates Cerebral Ischemia/Reperfusion Injury in Rats via Antioxidative and Antiapoptotic Mechanisms. J. Cell. Biochem. 2019, 120, 18901–18909. [Google Scholar] [CrossRef] [PubMed]
- Huo, W.M.; Duan, W.L.; Liu, J.; Shang, J. Studies on the Anticoagulant and Antithromboticm Effects of β-elemene. Asia Pac. Tradit. Med. 2013, 9, 30–33. [Google Scholar]
- Liu, M.; Chen, X.T.; Ma, J.; Hassan, W.; Wu, H.L.; Ling, J.W.; Shang, J. β-Elemene Attenuates Atherosclerosis in Apolipoprotein E-Deficient Mice via Restoring NO Levels and Alleviating Oxidative Stress. Biomed. Pharmacother. 2017, 95, 1789–1798. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, H.; Ninomiya, K.; Morikawa, T.; Yoshikawa, M. Inhibitory Effect and Action Mechanism of Sesquiterpenes from Zedoariae Rhizoma on D-galactosamine/ Lipopolysaccharide-Induced Liver Injury. Bioorg. Med. Chem. Lett. 1998, 8, 339–344. [Google Scholar] [CrossRef]
- Yang, X.; Wang, Z.M.; Kai, J.; Wang, F.X.; Jia, Y.; Wang, S.J.; Tan, S.Z.; Shen, X.K.; Chen, A.P.; Shao, J.J.; et al. Curcumol Attenuates Liver Sinusoidal Endothelial Cell Angiogenesis via Regulating Glis-PROX1-HIF-1α in Liver Fibrosis. Cell Prolif. 2020, 53, e12762. [Google Scholar] [CrossRef]
- Jia, Y.; Wang, F.X.; Guo, Q.; Li, M.M.; Wang, L.; Zhang, Z.L.; Jiang, S.Y.; Jin, H.H.; Chen, A.P.; Tan, S.Z.; et al. Curcumol Induces RIPK1/RIPK3 Complex-Dependent Necroptosis via JNK1/2-ROS Signaling in Hepatic Stellate Cells. Redox Biol. 2018, 19, 375–387. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhao, T.J.; Wang, J.-R.; Jiang, R.Z.; Huang, J.B.; Li, W.M.; Wang, J.H. Curcumol Alleviates Liver Fibrosis through Inducing Autophagy and Ferroptosis in Hepatic Stellate Cells. FASEB J. 2022, 36, e22665. [Google Scholar] [CrossRef]
- Li, Z.Y.; Wang, Z.L.; Dong, F.; Shi, W.; Dai, W.Z.; Zhao, J.; Li, Q.; Fang, Z.-E.; Ren, L.T.; Liu, T.T.; et al. Germacrone Attenuates Hepatic Stellate Cells Activation and Liver Fibrosis via Regulating Multiple Signaling Pathways. Front. Pharmacol. 2021, 12, 745561. [Google Scholar] [CrossRef]
- Ji, D.; Zhao, Q.; Qin, Y.W.; Tong, H.J.; Wang, Q.H.; Yu, M.T.; Mao, C.Q.; Lu, T.L.; Qiu, J.C.; Jiang, C.X. Germacrone Improves Liver Fibrosis by Regulating the PI3K/AKT/mTOR Signaling Pathway. Cell Biol. Int. 2021, 45, 1866–1875. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Yang, F.Q.; Li, S.P.; Gao, J.L.; Hu, G.; Lao, S.C.; Conceição, L.E.; Fung, K.P.; Wang, Y.T.; Lee, M.Y. Furanodiene Induces G2/M Cell Cycle Arrest and Apoptosis through MAPK Signaling and Mitochondria-Caspase Pathway in Human Hepatocellular Carcinoma Cells. Cancer Biol. Ther. 2007, 6, 1044–1050. [Google Scholar] [CrossRef]
- Saifudin, A.; Tanaka, K.; Kadota, S.; Tezuka, Y. Sesquiterpenes from the Rhizomes of Curcuma heyneana. J. Nat. Prod. 2013, 76, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.Y.; Wang, M.J.; Liu, H.X.; Wang, Q.; Chen, Q.C. Hypoglycemic Effect of Alismoxide in Type 2 Diabetic Mice. Chin. Pharmacol. Bull. 2019, 35, 1240–1244. [Google Scholar]
- Lin, W.W.; Tu, H.F.; Zhu, Y.; Guan, Y.J.; Liu, H.; Ling, W.; Yan, P.C.; Dong, J.Y. Curcumolide, A Unique Sesquiterpenoid from Curcuma wenyujin Displays Anti-Angiogenic Activity and Attenuates Ischemia-Induced Retinal Neovascularization. Phytomedicine 2019, 64, 152923. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Tu, H.F.; Wu, C.M.; Liu, T.; Chen, S.S.; Shen, L.L.; Xiao, Q.W.; Zhao, S.M.; Xu, S.Y.; Lin, W.W.; et al. Therapeutic Potential of Elema-1,3,7(11),8-tetraen-8,12-lactam from Curcuma wenyujin on Diabetic Retinopathy via Anti-Inflammatory and Anti-Angiogenic Pathways. J. Ethnopharmacol. 2024, 318, 116843. [Google Scholar] [CrossRef]
- Kwon, P.K.; Kim, S.W.; De, R.; Jeong, S.W.; Kim, K.T. Isoprocurcumenol Supports Keratinocyte Growth and Survival through Epidermal Growth Factor Receptor Activation. Int. J. Mol. Sci. 2021, 22, 12579. [Google Scholar] [CrossRef]
- Hamdi, O.A.A.; Ye, L.J.; Kamarudin, M.N.A.; Hazni, H.; Paydar, M.; Looi, C.Y.; Shilpi, L.A.; Kadir, H.A.; Awang, K. Neuroprotective and Antioxidant Constituents from Curcuma zedoaria Rhizomes. Rec. Nat. Prod. 2015, 9, 349–355. [Google Scholar]
- Park, J.H.; Mohamed, M.A.A.; Thi, N.N.; Seo, K.H.; Jung, Y.J.; Shrestha, S.; Lee, T.H.; Kim, J.; Baek, N.I. Guaiane Sesquiterpenes from the Rhizome of Curcuma xanthorrhiza and Their Inhibitory Effects on UVB-Induced MMP-1 Expression in Human Keratinocytes. Nat. Prod. Commun. 2017, 12, 1535–1538. [Google Scholar] [CrossRef]
- Yang, K.; Wu, B.; Wei, W.; Li, C.; Li, L.; Cong, Z.; Xiang, Q. Curdione Ameliorates Sepsis-Induced Lung Injury by Inhibiting Platelet-Mediated Neutrophil Extracellular Trap Formation. Int. Immunopharmacol. 2023, 118, 110082. [Google Scholar] [CrossRef]
- Yang, X.; Li, B.X.; Tian, H.J.; Cheng, X.F.; Zhou, T.J.; Zhao, J. Curcumenol Mitigates the Inflammation and Ameliorates the Catabolism Status of the Intervertebral Discs in vivo and in vitro via Inhibiting the TNFα/NFκB Pathway. Front. Pharmacol. 2022, 13, 905966. [Google Scholar] [CrossRef] [PubMed]
- Borah, S.; Sarkar, P.; Sharma, H.K. Zederone Improves the Fecal Microbial Profile in Dementia Induced Rat Model: A First Report. CNS Neurol. Disord. Drug Targets 2022, 21, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Kimura, Y.; Sumiyoshi, M.; Tamaki, T. Effects of the Extracts and an Active Compound Curcumenone Isolated from Curcuma zedoaria Rhizomes on Alcohol-Induced Drunkenness in Mice. Fitoterapia 2013, 84, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-H.; Mohamed, M.A.A.; Jung, Y.J.; Shrestha, S.; Lee, T.H.; Lee, C.H.; Han, D.; Kim, J.; Baek, N.I. Germacrane Sesquiterpenes Isolated from the Rhizome of Curcuma xanthorrhiza Roxb. Inhibit UVB-Induced Upregulation of MMP-1, -2, and -3 Expression in Human Keratinocytes. Arch. Pharm. Res. 2015, 38, 1752–1760. [Google Scholar] [CrossRef]
- Irie, K.; Yoshioka, T.; Nakai, A.; Ochiai, K.; Nishikori, T.; Wu, G.R.; Shibuya, H.; Muraki, T. A Ca(2+) Channel Blocker-Like Effect of Dehydrocurdione on Rodent Intestinal and Vascular Smooth Muscle. Eur. J. Pharmacol. 2000, 403, 235–242. [Google Scholar] [CrossRef]
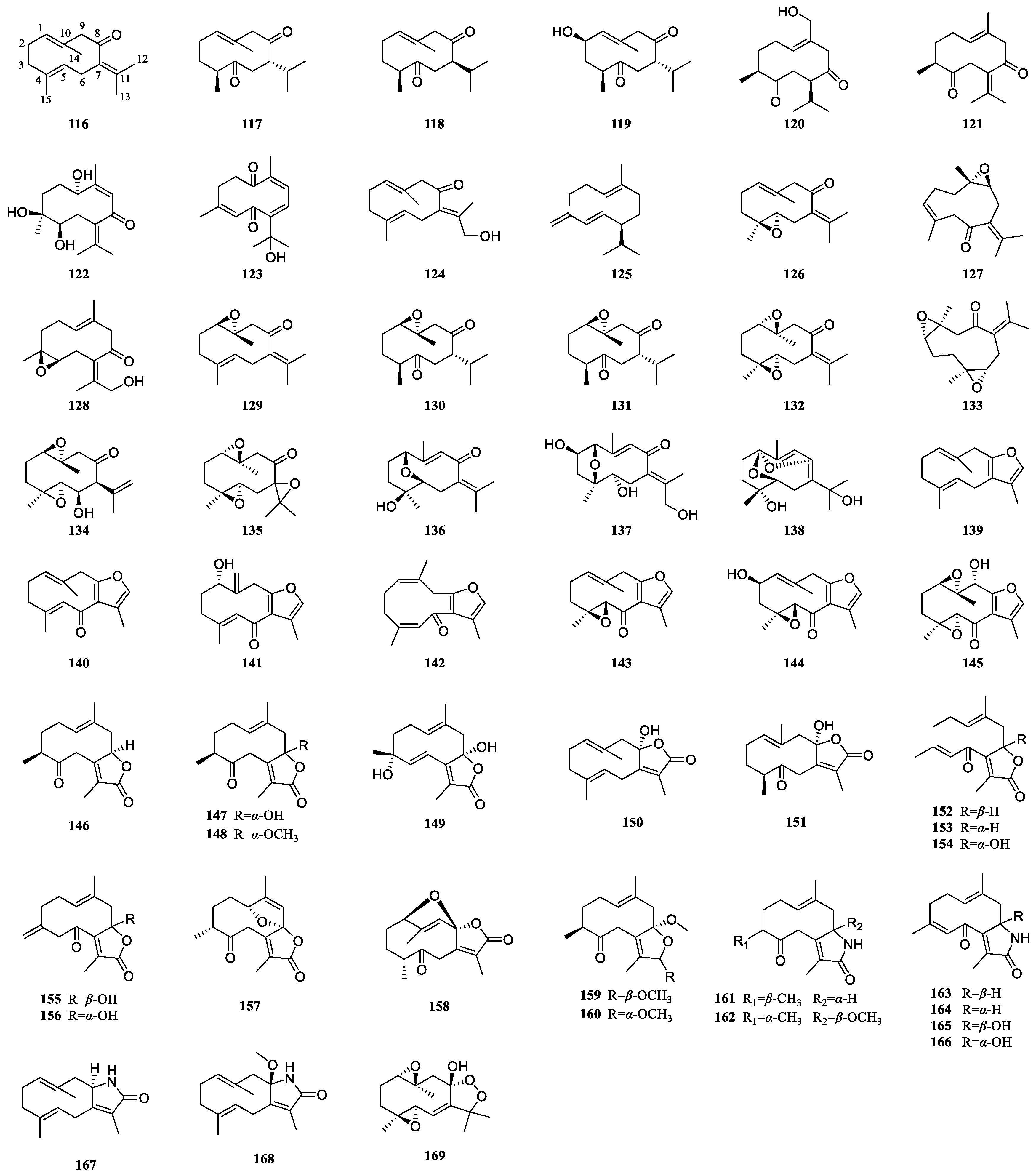


| No. | Compounds | Medicinal Source | Reference |
|---|---|---|---|
| 170 | Phaeocaulistriol A | C. phaeocaulis | [28] |
| 171 | Phaeocaulistriol B | C. phaeocaulis | [28] |
| 172 | 1α,4β-Dihydroxyeudesm-7(11)-en-8-one | C. phaeocaulis, C. kwangsiensis | [2,38] |
| 173 | 1-Hydroxyeudesma-4(14),7(11)-dien-8-one | C. phaeocaulis | [2] |
| 174 | 1-Hydroxyeudesma-3,7(11)-dien-8-one | C. phaeocaulis | [2] |
| 175 | 9-Hydroxyeudesma-3,7(11)-dien-6-one | C. phaeocaulis | [2] |
| 176 | Phaeusmane A | C. phaeocaulis | [2] |
| 177 | Phaeusmane B | C. phaeocaulis | [2] |
| 178 | Phaeusmane D | C. phaeocaulis | [2] |
| 179 | Phaeusmane E | C. phaeocaulis | [2] |
| 180 | Phaeusmane C | C. phaeocaulis | [2] |
| 181 | Eudesm-11-ene-4α,6α-diol | C. phaeocaulis, C. kwangsiensis | [2,12] |
| 182 | Capillosanane Z | C. wenyujin | [18] |
| 183 | Cyperusol C | C. wenyujin, C. phaeocaulis | [2,31] |
| 184 | 1β-Hydroxyeudesma-4,11-dien-3-one | C. phaeocaulis | [2] |
| 185 | Zedoarofuran | C. phaeocaulis | [37] |
| 186 | Curcolonol | C. wenyujin, C. phaeocaulis | [2,66] |
| 187 | 9α-Hydroxycurcolonol | C. phaeocaulis | [56] |
| 188 | (+)-Phaeocauline G | C. phaeocaulis | [33] |
| 189 | (−)-Phaeocauline G | C. phaeocaulis | [33] |
| 190 | Curcodione | C. wenyujin, C. phaeocaulis | [2,66] |
| 191 | 4α-Hydroxy-8,12-epoxyeudesma-7,11-diene-1,6-dione | C. phaeocaulis | [37] |
| 192 | Curcolone | C. phaeocaulis | [2] |
| 193 | 3α-Hydroxy-4-deoxy-5-dehydrocurcolonol | C. phaeocaulis | [56] |
| 194 | Chlorantene D | C. phaeocaulis | [28] |
| 195 | Chlomultin B | C. phaeocaulis | [2] |
| 196 | Myrrhterpenoid N | C. phaeocaulis | [2] |
| 197 | Phaeusmane F | C. phaeocaulis | [2] |
| 198 | Phaeusmane G | C. phaeocaulis | [2] |
| 199 | 1β,8β-Dihydroxyeudesma-4,7(11)-dien-8α,12-olide | C. phaeocaulis | [2] |
| 200 | (7Z)-1β,4α-Dihydroxy-5α,8β(H)-eudesm-7(11)-en-8,12-olide | C. phaeocaulis, C. wenyujin | [2,66] |
| 201 | (7Z)-1β,4β-Dihydroxy-5α,8β(H)-eudesm-7(11)-en-8,12-olide | C. phaeocaulis, C. wenyujin | [2,32] |
| 202 | Curcolide | C. wenyujin | [29,66] |
| 203 | Wenyujinlactone A | C. wenyujin | [67] |
| 204 | 1β,8β-Dihydroxyeudesma-3,7(11)-dien-8α,12-olide | C. phaeocaulis | [2] |
| 205 | Serralactone A | C. phaeocaulis | [2,56] |
| 206 | Hydroxyatractylolide | C. kwangsiensis | [60] |
| 207 | Butenolide III | C. wenyujin | [68] |
| 208 | Neolitacumone A | C. phaeocaulis, C. wenyujin | [2,37,67] |
| 209 | Phaeusmane I | C. phaeocaulis | [69] |
| 210 | Phaeusmane H | C. phaeocaulis | [2] |
| No. | Compounds | Medicinal Source | Reference |
|---|---|---|---|
| 211 | β-Elemene | C. wenyujin, C. phaeocaulis, C. kwangsiensis | [70,71,72] |
| 212 | γ-Elemene | C. wenyujin, C. phaeocaulis, C. kwangsiensis | [70,71,72] |
| 213 | δ-Elemene | C. wenyujin, C. phaeocaulis, C. kwangsiensis | [70,71,72] |
| 214 | Curzerene | C. wenyujin, C. phaeocaulis, C. kwangsiensis | [26] |
| 215 | Curzerenone | C. wenyujin, C. phaeocaulis, C. kwangsiensis | [31,38,57] |
| 216 | Epicurzerenone | C. phaeocaulis | [73] |
| 217 | Isogermafurenolide | C. wenyujin | [44,46] |
| 218 | 5-Isopropenyl-3,6-dimethyl-6-vinyl-5,6,7,7α-tetrahydro-4H-benzofuran-2-one | C. wenyujin | [58] |
| 219 | 8β-Hydroxy-isogermafureolide | C. wenyujin, C. phaeocaulis, C. kwangsiensis | [12,56,68] |
| 220 | Hydroxyisogermafurenolide | C. wenyujin, C. kwangsiensis | [44,46,52,58,66] |
| 221 | 5βH-Elema-1,3,7,8-tetraen-8,12-olide | C. wenyujin | [44] |
| 222 | 8β-Methoxy-isogermafurenolide | C. phaeocaulis | [69] |
| 223 | 8β(H)-Elema-1,3,7(11),8-tetraen-8,12-lactam | C. wenyujin, C. phaeocaulis | [36,46,69] |
| 224 | 8β(H)-Elema-1,3,7(11)-trien-8,12-lactam | C. phaeocaulis | [56] |
| No. | Compounds | Medicinal Source | Reference |
|---|---|---|---|
| 225 | Wenyujinone F | C. wenyujin | [18] |
| 226 | Wenyujinone E | C. wenyujin | [18] |
| 227 | 7-Hydroxy-5(10),6,8-cadinatriene-4-one | C. wenyujin | [29] |
| 228 | Phacadinane B | C. phaeocaulis | [37,74] |
| 229 | Phacadinane A | C. phaeocaulis | [74] |
| 230 | Curcujinone B | C. wenyujin | [31] |
| 231 | (+)-Commyrrin A | C. wenyujin, C. kwangsiensis | [18,38] |
| 232 | (−)-Commyrrin A | C. wenyujin, C. kwangsiensis | [38] |
| 233 | Pyrocurzerenone | C. kwangsiensis | [38] |
| 234 | Furanocadalene | C. kwangsiensis | [38] |
| 235 | Curcujinone A | C. wenyujin | [31] |
| 236 | Phacadinane C | C. phaeocaulis | [74] |
| 237 | Phacadinane D | C. phaeocaulis, C. kwangsiensis | [12,56,74] |
| 238 | 4,5-Seco-pyrocurzerenone | C. kwangsiensis | [38] |
| No. | Compounds | Medicinal Source | Reference |
|---|---|---|---|
| 239 | Curcumalactone | C. wenyujin | [29,31,43] |
| 240 | 7-Epicurcumalactone | C. wenyujin | [68] |
| 241 | Curcumanolide A | C. wenyujin, C. phaeocaulis | [15,31,45] |
| 242 | Curcumanolide B | C. wenyujin | [31,45] |
| 243 | (+)-Phaeocauline F | C. phaeocaulis | [33] |
| 244 | (−)-Phaeocauline F | C. phaeocaulis | [33] |
| 245 | Phaeocaudione | C. phaeocaulis | [69] |
| 246 | Phaeocauone | C. phaeocaulis, wenyujin | [32,69] |
| 247 | Wenyujinin L | C. wenyujin, C. phaeocaulis | [35,37] |
| 248 | Wenyujinol P | C. wenyujin | [32] |
| 249 | Curcumolide | C. wenyujin, C. kwangsiensis | [12,45] |
| 250 | Gajutsulactone A | C. wenyujin | [31] |
| 251 | Gajutsulactone B | C. wenyujin | [31] |
| 252 | Wenyujinin C | C. wenyujin | [31,35] |
| 253 | Wenyujinin D | C. wenyujin | [35] |
| 254 | Wenyujinin E | C. wenyujin | [35,36] |
| 255 | Phasalvione | C. phaeocaulis, C. wenyujin | [28,69] |
| 256 | Acomadendrane-4β,10β-diol | C. kwangsiensis | [65] |
| 257 | (4S)-Dihydrocurcumenone | C. wenyujin | [66] |
| 258 | Curcumenone | C. wenyujin | [44,46,66] |
| 259 | 7α,11-Epoxy-6α-hydroxy-carabrane-4,8-dione | C. wenyujin | [44,68] |
| 260 | 4,8-Dioxo-6β-methoxyl-7α,11-epoxycarabrane | C. wenyujin | [40] |
| 261 | 4,8-Dioxo-6β-methoxyl-7β,11-epoxycarabrane | C. wenyujin | [40] |
| 262 | 4,8-Dioxo-6β-hydroxyl-7β,11-epoxycarabrane | C. wenyujin | [36] |
| 263 | 4,8-Dioxo-6β-hydroxyl-7α,11-epoxycarabrane | C. wenyujin | [36,40] |
| 264 | 7α,11-Epoxy-6α-methoxy-carabrane-4,8-dione | C. wenyujin | [44] |
| 265 | Curcumenolactone A | C. phaeocaulis | [37] |
| 266 | Curcumenolactone B | C. phaeocaulis | [37] |
| 267 | 8,11-Epidioxy-8-hydroxy-4-oxo-6-carabren | C. wenyujin | [44] |
| 268 | Wenyujindiol A | C. wenyujin | [61] |
| 269 | Curcumadione | C. wenyujin | [63] |
| 270 | Curcumadionol | C. wenyujin, C. phaeocaulis | [16,66] |
| 271 | (6R)-Dehydroxysipanolinolide | C. wenyujin | [66] |
| 272 | Wenyujinone H | C. wenyujin | [18] |
| 273 | Wenyujinone I | C. wenyujin | [18] |
| 274 | αr-Turmerone | C. phaeocaulis | [57] |
| Compound | Compound Types | Activity Types | Pharmacological Models | Effects | Value | Positive Control | Reference |
|---|---|---|---|---|---|---|---|
| Zedoarondiol (18) | Guaiane-type sesquiterpenoids | Hepatoprotective effect | D-GalN/LPS-induced liver injury | Show a potent protective effect on D-GaIN/LPS-induced acute liver injury | Liver injury: 60.7 ± 10.5%, 54.7 ± 12.7% | Liver injury: 99.0 ± 0.1%, 98.3 ± 0.0% (Hydrocortisone) | [4,131] |
| Aerugidiol (31) | Liver injury: 88.0 ± 2.0%, 89.1 ± 0.7% | ||||||
| Isocurcumenol (60) | Liver injury: 77.3 ± 6.6%, 80.2 ± 5.5% | ||||||
| Curcumenol (61) | D-GalN/LPS-induced liver injury; D-GalN-induced cytotoxicity | Show a potent protective effect on D-GaIN/LPS-induced acute liver injury; inhibit D-GalN-induced cytotoxicity | Liver injury: 50.7 ± 13.8%, 53.4 ± 13.4%; hepatocytotoxicity: 25.1 ± 5.3% | ||||
| Curcumol (55) | Anti-liver fibrosis effect | LSECs accompanied by an abnormal angioarchitecture; liver fibrosis rats induced by CCl4 | Attenuate liver sinusoidal endothelial cell angiogenesis via regulating Glis-PROX1-HIF-1α in liver fibrosis | [132] | |||
| Liver fibrosis rats induced by CCl4; HSCs and LX-2 cell models | Target RIPK1/RIPK3 complex-dependent necroptosis via JNK1/2-ROS signaling for the treatment of hepatic fibrosis | [133] | |||||
| HSC cell model | Promote autophagy in HSCs, mediate the degradation of NCOA4 and FTH1 complexes, release iron ions, lead to iron overload, and induce ferroptosis | [134] | |||||
| Anti-hepatobiliary disease effect | Liver fibrosis rats induced by CCl4; HSCs, HepG2, and RBE cell models | Inhibit the activity of RhoROCK and MAPK signaling pathways, inhibit HSC migration and adhesion, and inhibit cell proliferation | [9] | ||||
| Germacrone (116) | Germacrane-type sesquiterpenoids | Anti-liver fibrosis effect | LX-2 and LO2 cell models | Reduce ROS release to avoid liver injury-induced HSC activation; inhibit the activation and survival of HSCs by regulating TGF-beta/Smad and apoptosis pathways | [135] | ||
| Liver fibrosis rats induced by CCl4; LX-2 cell model | Attenuate hepatic fibrosis via the PI3K/AKT/mTOR signaling pathway | [136] | |||||
| Anti-hepatoma effect | HepG2 and Bel7402 cell models | Regulate the expression of proteins related to the G2/M cell cycle, apoptosis and p53; oxidative damage may be involved | [3] | ||||
| Hepatoprotective effect | D-GalN/LPS-induced liver injury; D-GalN-induced cytotoxicity | Show potent protective effect on D-GaIN/LPS-induced acute liver injury; inhibit D-GalN-induced cytotoxicity | Liver injury: 82.9 ± 5.4%, 78.1 ± 6.8%; hepatocytotoxicity: 59.8 ± 6.3% | Liver injury: 99.0 ± 0.1%, 98.3 ± 0.0% (Hydrocortisone) | [4,131] | ||
| Curdione (117) | Hepatoprotective effect | D-GalN/LPS-induced liver injury; D-GalN-induced cytotoxicity | Show potent protective effect on D-GaIN/LPS-induced acute liver injury; inhibit D-GalN-induced cytotoxicity | Liver injury: 76.6 ± 4,7%, 74.6 ± 4.7%; hepatocytotoxicity: 77.1 ± 5.8% | Liver injury: 99.0 ± 0.1%, 98.3 ± 0.0% (Hydrocortisone) | [4,131] | |
| Neocurdione (118) | Liver injury: 59.3 ± 10.6%, 58.4 ± 11.1%; hepatocytotoxicity: 44.6 ± 5.3% | ||||||
| Wenyujinone D (120) | Oxidative damage induced by H2O2 in LO2 cells | Weaken the oxidative damage induced by H2O2 in LO2 cells via strengthening cell viability | Cell viability: 63.6% (H2O2: 50.7%) | [18] | |||
| Wenyujinone B (162) | Cell viability: 86.0% (H2O2: 50.7%) | ||||||
| Furanodiene (139) | Hepatoprotective effect | Oxidative damage induced by H2O2 in LO2 cells | Weaken the oxidative damage induced via strengthening cell viability | Cell viability: 85.0% (H2O2: 50.7%) | [18] | ||
| D-GalN/LPS-induced liver injury | Show potent protective effect on D-GaIN/LPS-induced acute liver injury | Liver injury: 72.9 ± 6.7%, 74.3 ± 5.7% | Liver injury: 99.0 ± 0.1%, 98.3 ± 0.0% (Hydrocortisone) | [131] | |||
| Anti-hepatoma effect | HepG2 cell model | Induce G2/M cell cycle arrest and apoptosis through MAPK signaling and mitochondria-caspase pathway in HepG2 cells | [137] | ||||
| Anti-hepatobiliary disease effect | HepG2 cell model | Induce G2/M cell cycle arrest and apoptosis | [9] | ||||
| β-Elemene (211) | Elemane-type sesquiterpenoids | Hepatoprotective effect; anti-fibrotic effect; anti-hepatoma effect | Liver fibrosis rats induced by CCl4; HSC-T6, HepG2, BNL, and H22 cell models | Inhibit the biological effect of ANG II and delayed liver fibrosis; inhibit cell migration and invasion through TGF-β1/Smad, JNK1/2-ROS, NF-κB, and other pathways | [9] | ||
| Curcumenone (258) | Other-type sesquiterpenoids | Hepatoprotective effect | D-GalN/LPS-induced liver injury | Show potent protective effect on D-GaIN/LPS-induced acute liver injury | Liver injury: 90.1 ± 0.5%, 88.0 ± 0.4% | Liver injury: 99.0 ± 0.1%, 98.3 ± 0.0% (Hydrocortisone) | [131] |
| Curcumenolactone A (265) | D-GalN-induced cytotoxicity | Inhibit D-GalN-induced cytotoxicity | Inhibition: 65.5 ± 5.7% (100 μM) | [4] | |||
| Curcumenolactone B (266) | Inhibition: 71.1 ± 4.3% (100 μM) |
| Compounds | Compound Types | Activity Types | Pharmacological Models | Effects | Value | Positive Control | Ref. |
|---|---|---|---|---|---|---|---|
| 4,10-Epizedoarondiol (25) | Guaiane-type sesquiterpenoids | Anti-diabetic effect | PTP1B inhibitory assay | Inhibit the activity of PTP1B | IC50: 35.1 μM | IC50: 5.62 μM (RK-682); 2.75 μM (Ulsolic acid) | [138] |
| Procurcumenol (29) | IC50: 45.6 μM | ||||||
| Aerugidiol (31) | IC50: 35.7 μM | ||||||
| Alismoxide (3) | Type 2 diabetes mellitus mouse model induced by combined administration of streptozotocin and nicotinamide | Accelerate 3T3-L1 pre-adipocyte differentiation and possess a hypoglycemic property | [139] | ||||
| 7α,11α-Epoxy-5β-hydroxy-9-guaiane-8-one (46) | Glucose transportation model on HepG2 cells | Increase glucose consumption in HepG2 cells | 46.1% (10 μM) | [31] | |||
| Curcumenol (61) | 47.0% (10 μM) | [31] | |||||
| Curdione (117) | Germacrane-type sesquiterpenoids | Anti-diabetic effect | Glucose transportation model on HepG2 cells | Increase glucose consumption in HepG2 cells | 74.0% (10 μM) | [31] | |
| Zederone (143) | 57.0% (10 μM) | [31] | |||||
| Heyneanone C (122) | PTP1B Inhibitory Assay | Inhibit the activity of PTP1B | IC50: 35.2 μM | IC50: 5.62 μM (RK-682); 2.75 μM (Ulsolic acid) | [138] | ||
| Germacrone (116) | Regulation of glucose–lipid metabolism | Multi-models | Regulate adipogenesis, lipolysis, and AMPKα pathway; inhibit fatty acid synthesis and uptake by suppressing the activation of the SREBP signaling pathway to alleviate hyperlipidemia and stimulate FA-β oxidation to improve lipid metabolism | [80] | |||
| 8β(H)-Elema-1,3,7(11),8-tetraen-8,12-lactam (223) | Elemane-type sesquiterpenoids | Attenuate ischemia-induced retinal neovascularization effect | Diabetic retinopathy rat models | Exert anti-inflammatory and anti-angiogenic effects through inhibiting NF-κB and VEGFR2 signaling pathways; reduce retinal microvascular leakage; induce retinal neovascularization | [141] | ||
| Gajutsulactone B (251) | Other-type sesquiterpenoids | Anti-diabetic effect | Glucose transportation model on HepG2 cells | Increase glucose consumption in HepG2 cells | 47.2% (10 μM) | [31] | |
| Wenyujinin C (252) | 49.7% (10 μM) | ||||||
| Curcumolide (249) | Attenuate diabetic retinopathy effect | STZ-induced diabetic rat model and TNF-α-stimulated HUVECs | Reduce diabetic retinal vascular leukostasis and leakage partly via the inhibition of the p38MAPK/NF-κB signaling pathway | [81] | |||
| Attenuate ischemia-induced retinal neovascularization effect | HUVEC cell model; oxygen-induced mouse retinopathy model | Exert anti-angiogenic activity and attenuate ischemia-induced retinal neovascularization via the VEGFR2 signaling pathway | [140] |
| Compounds | Compound Types | Activity Types | Pharmacological Models | Effects | Value | Positive Control | Reference |
|---|---|---|---|---|---|---|---|
| Wenyujinin Q (17) | Guaiane-type sesquiterpenoids | Antifungal activity | Broad-spectrum antifungal activities | Exhibit broad-spectrum antifungal activities | A. brassicicola: 50 μg/mL; P. parasitica var. nicotianae: 100 μg/mL; C. capsici: 50 μg/mL; B. oryzae: 50 μg/mL; D. medusaea Nitschke: 100 μg/mL; C. paradoxa Moreau: 50 μg/mL; E. turcicum: 25 μg/mL; P. theae: 25 μg/mL; A. citri: 100 μg/mL | A. brassicicola: 12.5 μg/mL; P. parasitica var. nicotianae: 50 μg/mL; C. capsici: 12.5 μg/mL; B. oryzae: 50 μg/mL; D. medusaea Nitschke: 50 μg/mL; C. paradoxa Moreau: 25 μg/mL; E. turcicum: 12.5 μg/mL; P. theae: 25 μg/mL; A. citri: 25 μg/mL (Prochloraz) | [36] |
| Phaeocaulisin E (21) | A. brassicicola: 100 μg/mL; P. parasitica var. nicotianae: 50 μg/mL; C. capsici: 50 μg/mL; B. oryzae: 100 μg/mL; D. medusaea Nitschke: 100 μg/mL; C. paradoxa Moreau: 25 μg/mL; E. turcicum: 50 μg/mL; P. theae: 25 μg/mL; A. citri: 50 μg/mL | ||||||
| Neoprocurcumenol (35) | A. brassicicola: 12.5 μg/mL; P. parasitica var. nicotianae: 50 μg/mL; C. capsici: 25 μg/mL; B. oryzae: 50 μg/mL; D. medusaea Nitschke: 100 μg/mL; C. paradoxa Moreau: 25 μg/mL; E. turcicum: 50 μg/mL; P. theae: 25 μg/mL; A. citri: 50 μg/mL | ||||||
| Procurcumadiol (33) | Antibacterial effect | Antibacterial activity against E. coli | Antibacterial activity against E. coli | E. coli: 1.25 μg/mL | E. coli: 0.3 μg/mL (Ciprofloxacin) | [40] | |
| 7β,8α-Dihydroxy-1α,4αH-guai-10(15)-en-5β,8β-endoxide (57) | Anti-viral activity | Influenza virus A | Show anti-viral activity against the influenza virus A | IC50: 9.18 ± 0.46 μM | IC50: 8.06 ± 0.64 μM (Ribavirin); 47.42 ± 1.96μM (Oseltamivir) | [29] | |
| 1α,8α-Epidioxy-4α-hydroxy-5αH-guai-7(11),9-dien-12,8-olide (110) | IC50: 6.80 ± 0.13 μM | IC50: 8.06 ± 0.64 μM (Ribavirin); 47.42 ± 1.96μM (Oseltamivir) | |||||
| 9-Oxo-neoprocurcumenol (45) | Antioxidant property | Nrf2-luciferase activity in HEK 293 cells | Exhibit antioxidant activity via the activation of the Nrf2-ARE pathway | [27] | |||
| Zedoarolide B (85) | Dual-luciferase reporter gene assay in 293 T cells | Activate the transcription of Nrf2 in 293 T cells | [32] | ||||
| Alismoxide (3) | Anti-aging effect | UVB-mediated HaCaT cell model | Inhibit the production of MMP-1 in UV-irradiated HaCaT cells | [149] | |||
| Zedoarondiol (18) | Antifungal activity | Broad-spectrum antifungal activities | Exhibit broad-spectrum antifungal activities | A. brassicicola: 100 μg/mL; P. parasitica var. nicotianae: 100 μg/mL; C. capsici: 50 μg/mL; B. oryzae: 100 μg/mL; D. medusaea Nitschke: 100 μg/mL; C. paradoxa Moreau: 25 μg/mL; E. turcicum: 50 μg/mL; P. theae: 25 μg/mL; A. citri: 50 μg/mL | A. brassicicola: 12.5 μg/mL; P. parasitica var. nicotianae: 50 μg/mL; C. capsici: 12.5 μg/mL; B. oryzae: 50 μg/mL; D. medusaea Nitschke: 50 μg/mL; C. paradoxa Moreau: 25 μg/mL; E. turcicum: 12.5 μg/mL; P. theae: 25 μg/mL; A. citri: 25 μg/mL (Prochloraz) | [36] | |
| Endothelial cell injury protective effect | ox-LDL-induced HUVEC injury | Attenuate ox-LDL-induced endothelial cell injury by inhibiting oxidative stress and inflammation via the Nrf2/HO-1 pathway | [121] | ||||
| Isozedoarondiol (20) | Antifungal activity | Broad-spectrum antifungal activities | Exhibit broad-spectrum antifungal activities | A. brassicicola: 100 μg/mL; P. parasitica var. nicotianae: 100 μg/mL; C. capsici: 100 μg/mL; B. oryzae: 50 μg/mL; D. medusaea Nitschke: 100 μg/mL; C. paradoxa Moreau: 25 μg/mL; E. turcicum: 50 μg/mL; P. theae: 50 μg/mL; A. citri: 50 μg/mL | A. brassicicola: 12.5 μg/mL; P. parasitica var. nicotianae: 50 μg/mL; C. capsici: 12.5 μg/mL; B. oryzae: 50 μg/mL; D. medusaea Nitschke: 50 μg/mL; C. paradoxa Moreau: 25 μg/mL; E. turcicum: 12.5 μg/mL; P. theae: 25 μg/mL; A. citri: 25 μg/mL (Prochloraz) | [36] | |
| Anti-aging effect | UVB-mediated HaCaT cell model | Inhibit production of MMP-1 in UV-irradiated HaCaT cells | [149] | ||||
| Procurcumenol (29) | Antioxidant activity | Nrf2-luciferase activity in HEK 293 cells | Exhibit antioxidant activity via activation of the Nrf2-ARE pathway | [27] | |||
| Antibacterial effect | Antibacterial activity against S. albus | Antibacterial activity against S. albus | S. albus: 1.25 μg/mL | S. albus: 0.6 μg/mL (Ciprofloxacin) | [40] | ||
| Neuroprotective property | H2O2-induced oxidative stress in NG108-15 cells | Show moderate protection of NG108-15 cells | Neuroprotective: cell viability: 80.00 ± 0.71% (15 μM) (H2O2: 67.63 ± 0.86) | [143] | |||
| Isoprocurcumenol (43) | Skin function maintenance activity | UVB-induced cellular damage | Activate EGFR signaling, increase the phosphorylation of ERK and AKT, upregulate the expression of genes related to cell growth and proliferation, and induce the proliferation of keratinocytes | [142] | |||
| Neuroprotective property; antioxidant activity | H2O2-induced oxidative stress in NG108-15 cells; oxygen radical antioxidant capacity assay | Show moderate protection of NG108-15 cells; antioxidant activity | Neuroprotective: cell viability: 80.96 ± 0.91% (4 μM) (H2O2: 67.63 ± 0.86) Antioxidant: TE: 26.43 ± 1.88 μM/100 μg | [143] | |||
| Curcumenol (61) | Neuroprotective property | H2O2-induced oxidative stress in NG108-15 cells | Show moderate protection of NG108-15 cells | Neuroprotective: cell viability: 103.04 ± 2.17% (4 μM) (H2O2: 67.63 ± 0.86) | [143] | ||
| Improvement of intervertebral disc catabolism status | Lumbar spine-instability mouse model | Inhibit TNFα/NF-κB signaling pathway and mitigate the expression of the MMP family (MMP-3, MMP-9, and MMP-13) | [146] | ||||
| Germacrone (116) | Germacrane-type sesquiterpenoids | Neuroprotective property; antioxidant activity | H2O2-induced oxidative stress in NG108-15 cells; oxygen radical antioxidant capacity assay | Show moderate protection of NG108-15 cells; antioxidant activity | Neuroprotective: cell viability: 89.99 ± 2.01% (15 μM) (H2O2: 67.63 ± 0.86) Antioxidant: TE: 24.86 ± 2.33 μM/100 μg | [143] | |
| Anti-aging effect | UVB-induced damage in HaCaT cells | Inhibit UVB-induced upregulation of mRNA and protein expression levels of MMP-1, MMP-2, and MMP-3 | [144] | ||||
| Curdione (117) | Effect on sepsis-induced lung injury | CLP surgery established mice sepsis model | Inhibit platelet-mediated neutrophil recruitment, infiltration, and NET formation; exert anti-inflammatory and antioxidant properties | [145] | |||
| Dehydrocurdione (121) | Ca(2+) channel blocker-like effect | Ca(2+) channel blocker-like model | Exhibit a Ca(2+) channel blocker-like effect on rodent intestinal and vascular smooth muscles | [150] | |||
| Neuroprotective property; antioxidant activity | H2O2-induced oxidative stress in NG108-15 cells; oxygen radical antioxidant capacity assay | Show obvious protection of NG108-15 cells; antioxidant activity | Neuroprotective: cell viability: 100.60 ± 1.72% (10 μM) (H2O2: 67.63 ± 0.86) Antioxidant: TE: 26.18 ± 2.59 μM/100 μg | [143] | |||
| Heyneanone D (123) | Antibacterial effect | Antibacterial activity against E. coli | Antibacterial activity against E. coli | E. coli: 1.25 μg/mL | E. coli: 0.3 μg/mL (Ciprofloxacin) | [40] | |
| 13-Hydroxygermacrone (124) | Anti-aging effect | UVB-induced damage in HaCaT cells | Inhibit UVB-induced upregulation of mRNA and protein expression levels of MMP-1, MMP-2, and MMP-3 | [144] | |||
| Zederone (143) | Alzheimer’s disease | Aluminium-induced dementia rat model | Improve fecal microbiological profiles; regulate gut bacterial ecological imbalances | [147] | |||
| Antioxidant activity | Oxygen radical antioxidant capacity assay | Antioxidant | TE: 27.78 ± 2.53 μM/100 μg | [143] | |||
| Curcolide (202) | Eudesmane-type sesquiterpenoids | Antioxidant property | Dual-luciferase reporter gene assay in 293 T cells | Activate the transcription of Nrf2 in 293 T cells | [32] | ||
| Curcumanolide A (241) | Other-type sesquiterpenoids | Relaxant effect on uterine smooth muscle | Oxytocin-induced contraction model of rat uterine smooth muscle | Show an inhibitory effect against oxytocin-induced rat uterine smooth muscle contraction | [15] | ||
| Curcumenone (258) | Protective effect on drunkenness | Alcohol-induced drunkenness model | Increase liver alcohol dehydrogenase activity and decrease the elevation of blood alcohol concentrations | [148] | |||
| Antioxidant activity | Oxygen radical antioxidant capacity assay | Antioxidant | TE: 21.16 ± 2.12 μM/100 μg | [143] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, T.; Li, B.-Y.; Liu, F.; Xiong, L. Research Progress on Sesquiterpenoids of Curcumae Rhizoma and Their Pharmacological Effects. Biomolecules 2024, 14, 387. https://doi.org/10.3390/biom14040387
Cui T, Li B-Y, Liu F, Xiong L. Research Progress on Sesquiterpenoids of Curcumae Rhizoma and Their Pharmacological Effects. Biomolecules. 2024; 14(4):387. https://doi.org/10.3390/biom14040387
Chicago/Turabian StyleCui, Ting, Bo-Yu Li, Fei Liu, and Liang Xiong. 2024. "Research Progress on Sesquiterpenoids of Curcumae Rhizoma and Their Pharmacological Effects" Biomolecules 14, no. 4: 387. https://doi.org/10.3390/biom14040387
APA StyleCui, T., Li, B.-Y., Liu, F., & Xiong, L. (2024). Research Progress on Sesquiterpenoids of Curcumae Rhizoma and Their Pharmacological Effects. Biomolecules, 14(4), 387. https://doi.org/10.3390/biom14040387






