Abstract
Polyunsaturated fatty acids (PUFAs) generate pro- and anti-inflammatory eicosanoids via three different metabolic pathways. This study profiled tear PUFAs and their metabolites and examined the relationships with dry eye (DE) and meibomian gland dysfunction (MGD) symptoms and signs. A total of 40 individuals with normal eyelids and corneal anatomies were prospectively recruited. The symptoms and signs of DE and MGD were assessed, and tear samples (from the right eye) were analyzed by mass spectrometry. Mann–Whitney U tests assessed differences between medians; Spearman tests assessed correlations between continuous variables; and linear regression models assessed the impact of potential confounders. The median age was 63 years; 95% were male; 30% were White; and 85% were non-Hispanic. The symptoms of DE/MGD were not correlated with tear PUFAs and eicosanoids. DE signs (i.e., tear break-up time (TBUT) and Schirmer’s) negatively correlated with anti-inflammatory eicosanoids (11,12-dihydroxyeicosatrienoic acid (11,12 DHET) and 14,15-dihydroxyicosatrienoic acid (14,15, DHET)). Corneal staining positively correlated with the anti-inflammatory PUFA, docosahexaenoic acid (DHA). MGD signs significantly associated with the pro-inflammatory eicosanoid 15-hydroxyeicosatetranoic acid (15-HETE) and DHA. Several relationships remained significant when potential confounders were considered. DE/MGD signs relate more to tear PUFAs and eicosanoids than symptoms. Understanding the impact of PUFA-related metabolic pathways in DE/MGD may provide targets for new therapeutic interventions.
1. Introduction
Dry eye (DE) is a prevalent, multifactorial disease that consists of a wide range of clinical manifestations that include symptoms of ocular surface pain (characterized as “dryness”, “burning”, and “discomfort”, to name a few) and visual disturbances [1]. Signs of DE can include tear instability, insufficient tear production, and/or ocular surface disruption [2]. Closely related to DE, meibomian gland dysfunction (MGD) is defined as “a chronic, diffuse abnormality of the meibomian glands, commonly characterized by terminal duct obstruction and/or qualitative/quantitative changes in the glandular secretion”, commonly caused by epithelial gland hyperkeratinization [3]. Symptoms, such as dryness and foreign body sensations, and signs, such as tear instability, can overlap between DE and MGD [3]. Together, DE/MGD symptoms and signs impact the quality of life, negatively affect mood, and limit activities of daily living [4]. Various external and internal factors can impact DE/MGD manifestations, including weather, air pollution, diet, and systemic comorbidities [5]. Inflammation has been identified as an important intermediary between such external and internal factors and DE/MGD, and thus, many studies have examined the contributions of cellular (i.e., macrophages, regulatory T cells) [6] and soluble (i.e., tumor necrosis factor-alpha (TNF-α), interleukin-1B (IL-1B), and interleukin-6 (IL-6)) [7] mediators on disease pathophysiology.
In this regard, the roles of pro-(omega 6, ω6) and anti-(omega 3, ω3) inflammatory polyunsaturated fatty acids (PUFAs) have been studied with respect to DE/MGD. PUFAs are eicosanoid precursors that are converted to bioactive products by three enzymatic metabolic pathways: the cyclooxygenase (COX), lipoxygenase (LOX), and cytochrome P450 (CYP) pathways [8]. The most abundant and precursor of ω6-derived eicosanoid is arachidonic acid (AA) [9], which is released from cell membranes to produce pro-inflammatory thromboxanes (i.e., TXB2) via the COX pathway, pro-inflammatory LOX-derived hydroxyeicosatetraenoic acids (HETEs) [8], and leukotrienes (i.e., LTB4) [10], which are involved in acute inflammation [11]. AA can also be metabolized into anti-inflammatory eicosanoids, such as epoxyeicosatrienoic acids (EETs), via the CYP pathway and further converted to more stable metabolites, dihydroxyeicosatrienoic acids (DHETs) [8]. ω3-derived anti-inflammatory PUFA eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) serve as substrates for the synthesis of a different class of bioactive lipid mediators known as specialized pro-resolving mediators (SPMs) (i.e., lipoxins, resolvins, protectins, and maresins), which are involved in the resolution of inflammation [11,12].
Previously, it was suggested that chronic inflammation in DE/MGD is driven by an imbalance between ω6 (AA) and ω3 (EPA and DHA) PUFAs, leading to the hyperproduction of pro-inflammatory lipid mediators (ω6 derivatives) and the underproduction of ω3 derivatives [13,14]. In our prior study, we examined the correlations between DE/MGD features and various eicosanoids in 41 individuals. The strongest relationships were between prostaglandin E2 (PGE2) (produced via the COX pathway) and corneal staining (ρ = 0.35) and meibomian gland (MG) plugging (ρ = 0.40), p < 0.05 for both, indicating higher PGE2 levels in individuals with worse DE signs. In a similar manner, a more inflammatory PUFA profile (higher ω6: ω3 ratio) correlated with less healthy tear parameters (tear break-up time, TBUT ρ = −0.37, Schirmer score ρ = −0.38, and corneal staining ρ = 0.31) [13]. Other studies have found that both pro- and anti-inflammatory eicosanoids are increased in DE/MGD. One Singapore-based study compared individuals with poor meibum expressibility (quality ≥ 1) from ≤2 meibomian glands (case, n = 29) to those with normal meibum expressibilty (quality = 0) from ≥3 meibomian glands (control, n = 11). Cases had higher levels of pro-inflammatory eicosanoids produced by COX and/or LOX pathways compared to the controls (5-hydroxyeicosatetraenoic acid (5-HETE): 0.69 ± 0.62 vs. 0.36 ± 0.62, p = 0.01; leukotriene B4 (LTB4): 0.14 ± 0.15 vs. 0.11 ± 0.26, p = 0.04). However, cases also had increased levels of anti-inflammatory eicosanoids derived from EPA compared to controls (18-hydroxyeicosapentaenoic acid (18-HEPE): 0.19 ± 0.24 vs. 0.06 ± 0.04, p = 0.01; 12-hydroxyeicosapentaenoic acid (12-HEPE): 0.62 ± 0.66 vs. 0.52 ± 1.06, p = 0.03; 5-hydroxyeicosapentaenoic acid (5-HEPE): 0.12 ± 0.21 vs. 0.07 ± 0.10, p = 0.05) [10]. These results suggest that pro-inflammatory responses contribute to the pathophysiology of DE/MGD, with a compensatory anti-inflammatory response that aims to restore ocular surface homeostasis.
While several studies have examined the relationships between eicosanoids produced from the LOX and COX metabolic pathways and DE/MGD, what is missing from the literature is an examination of eicosanoids produced from the AA cytochrome P450 epoxygenase pathway. Animal [15] and human studies [16] have demonstrated that cytochrome-derived eicosanoids can have potent pro- or anti-inflammatory properties, and as such, these metabolites may play a role in DE/MGD. To bridge this knowledge gap, we aimed to profile the levels of tear PUFAs, LOX, COX, and cytochrome pathway-derived eicosanoids and examine their relation to clinical symptoms and signs of DE and MGD.
2. Materials and Methods
2.1. Study Design and Population
This prospective, single-site, cross-sectional study was conducted in accordance with the tenets of the Declaration of Helsinki, complied with the requirements of the United States Health Insurance Portability and Accountability Act (HIPAA), and was approved by the Miami Veterans Affairs (VA) Institutional Review Board. Participants were recruited from 2016 to 2017 at the Miami VA Eye Clinic, and informed consent was obtained from the subjects after an explanation of the nature and possible consequences of the study. Individuals were excluded if they had concomitant ocular or systemic conditions that could confound DE, such as anatomic abnormalities of their eyelids (i.e., ectropion), conjunctiva (i.e., pterygium), and/or cornea (i.e., Salzman’s nodular degeneration and edema); history of glaucoma, refractive, or retinal surgery; cataract surgery within the last 6 months; use of contact lenses; topical medications besides artificial tears; HIV; sarcoidosis; graft-versus host disease or a collagen vascular disease.
2.2. Data Collection
Demographic information, including age, sex, race, ethnicity, smoking, medical history, oral medications, and supplements, was collected for each patient.
2.3. Ocular Symptoms
All individuals filled out validated questionnaires regarding DE symptom severity, including the five-item Dry Eye Questionnaire (DEQ-5; range: 0–22) [17] and the Ocular Surface Disease Index (OSDI; range: 0–100) [18]. Pain-specific questionnaires included a Numerical Rating Scale [19] (NRS; range: 0–10, quantifying “average intensity of eye pain during the past week”) and Neuropathic Pain Symptom Inventory-modified for the Eye [20] (NPSI-Eye) that assessed the intensity of neuropathic pain features (i.e., burning sensation, pain sensitivity to wind, light, and temperature change).
2.4. Ocular Surface Assessment
All participants underwent a comprehensive ocular surface examination of both eyes, which included the following, in the order performed:
- (1)
- Measurement of tear film osmolarity (TearLAB, San Diego, CA, USA).
- (2)
- Assessment of ocular surface inflammation via InflammaDry (Quidel, San Diego, CA, USA), identifying matrix metallopeptidase 9 (MMP9) graded as 1 = present or 0 = absent based on the appearance of pink stripe.
- (3)
- Upper or lower eyelid laxity determined by rotation (0 = 0–25%, 1 = 25–50%, and 2 = 50–100%) and the snap back test (0 = prompt snapback, 1 = slowed return, and 2 = does not return fully until blinking), respectively.
- (4)
- Anterior blepharitis graded as 0 = none, 1 = mild, 2 = moderate, and 3 = severe.
- (5)
- Telangiectasias seen on the lower eyelids as 0 = none, 1 = mild vessel engorgement, 2 = moderate vessel engorgement, and 3 = severe vessel engorgement.
- (6)
- Inferior meibomian gland plugging graded as 0 = none, 1 = less than 1/3, 2 = between 1/3 and 2/3, and 3 = greater than 2/3 lid involvement.
- (7)
- Tear stability measured by placing 5 µL fluorescein in the superior conjunctivae and assessing the tear break-up time (TBUT).
- (8)
- Fluorescein corneal staining graded to the National Eye Institute (NEI) scale with five areas assessed the inferior, nasal, superior, temporal, and central, and each scored 0–3 (maximum score: 15).
- (9)
- Conjunctivochalasis in each area of the lower eyelid (nasally, medially, and temporally) graded as 0 = none, 1 = mild, 2 = moderate, and 3 = severe.
- (10)
- Tear production graded as millimeter (mm) wetting of anesthetized Schirmer’s test placed in the inferior fornix at 5 min.
- (11)
- Inferior meibomian gland drop out graded to the Meiboscale (range: 0–4) [21].
- (12)
- Meibum quality graded as 0 = clear, 1 = cloudy, 2 = granular, 3 = toothpaste, and 4 = no meibum extracted.
2.5. Tear Collection, PUFA, and Eicosanoid Extraction and Analysis
Schirmer strips were stored in −80 °C until analysis. Eicosanoids were extracted and analyzed by UPLC ESI-MS/MS, as previously described by us and others [22,23,24,25,26,27,28,29]. Briefly, tear strips were placed in tubes with 4 mL of water and an internal standard (IS) mixture comprising 10% methanol (400 μL) and glacial acetic acid (20 μL) and an internal standard (20 μL) containing the following deuterated eicosanoids (1.5 pmol/μL, 30 pmol total) (all standards purchased from Cayman Chemicals, Ann Arbor, MI, USA): (d4) 6keto-prostaglandin F1α, (d4) prostaglandin F2α, (d4) prostaglandin E2, (d4) prostaglandin D2, (d8) 5-hydroxyeicosatetraenoic acid (5-HETE), (d8) 12-hydroxyeicosatetraenoic acid (12-HETE), (d8) 15-hydroxyeicosatetraenoic acid (15-HETE), (d6) 20-hydroxyeicosatetraenoic acid (20-HETE), (d11) 8,9 epoxyeicosa-trienoic acid, (d8) 14,15 epoxyeicosa-trienoic acid, (d8) arachidonic acid, (d5) Eicosapentaenoic acid, (d5) docosahexaenoic acid, (d4) prostaglandin A2, (d4) leukotriene B4, (d4) leukotriene C4, (d4) leukotriene D4, (d4) leukotriene E4, (d5) 5(S),6(R)-lipoxin A4, (d11) 5-iPF2α-VI, (d4) 8-iso prostaglandin F2α, (d11) (±)14,15-DHET, (d11) (±)8,9-DHET, (d11) (±)11,12-DHET, (d4) prostaglandin E1, (d4) thromboxane B2, (d6) dihomo gamma linoleic acid, (d5) resolvin D2, (d5) resolvin D1 (RvD1), (d5) Maresin2, (d7) 5-OxoETE, and (d5) resolvin D3. Samples and vial rinses (5% MeOH; 2 mL) were applied to Strata-X SPE columns (Phenomenex, Torrance, CA, USA), previously washed with methanol (2 mL) and then dH2O (2 mL). Eicosanoids eluted with isopropanol (2 mL) were dried in vacuo and reconstituted in EtOH:dH2O (50:50;100 μL) prior to an ultra-high performance liquid chromatography electrospray ionization–MS/MS (UPLC ESI-MS/MS) analysis.
Eicosanoids were separated using a Shimadzu Nexera X2 LC-30AD (Shimadzu, Kyoto, Japan) coupled with a SIL-30AC auto injector (Shimadzu, Kyoto, Japan) and a DGU-20A5R (Shimadzu, Kyoto, Japan) degassing unit in the following way: A 14 min, reversed-phase LC method utilizing an Ascentis Express C18 column (150 mm × 2.1 mm, 2.7 µm) was used to separate the eicosanoids at a 0.5 mL/min flow rate at 40 °C. The column was equilibrated with 100% Solvent A (acetonitrile/water/formic acid (20:80:0.02, v/v/v)) for 5 min and then 10 µL of sample was injected. Further, 100% Solvent A was used for the first two min of elution. Solvent B (acetonitrile/isopropanol/formic acid (20:80:0.02, v/v/v)) was increased in a linear gradient to 25% Solvent B at 3 min, to 30% at 6 min, to 55% at 6.1 min, to 70% at 10 min, and to 100% at 10.10 min. Then, 100% Solvent B was held constant until 13.0 min, where it was decreased to 0% Solvent B and 100% Solvent A from 13.0 min to 13.1 min. From 13.1 min to 14.0 min, Solvent A was held constant at 100%.
Eicosanoids were analyzed via mass spectrometric means using an AB Sciex Triple Quad 5500 Mass Spectrometer (Sciex, Toronto, Canada). Q1 and Q3 were set to detect distinctive precursor and product ion pairs. Ions were fragmented in Q2 using N2 gas for collisionally induced dissociation. The analysis used multiple-reaction monitoring in a negative-ion mode. Eicosanoids were monitored using precursor → product MRM pairs. The mass spectrometer parameters used were as follows: curtain gas: 20 psi; CAD: medium; ion spray voltage: −4500 V; temperature: 300 °C; gas 1: 40 psi; gas 2: 60 psi; declustering potential, collision energy, and cell exit potential vary per transition as reported [22,23,24,25,26,27,28,29].
2.6. Statistical Analysis
Statistical analyses were performed using SPSS Statistics Software version 25.0 (IBM Corp. Armonk, NY). Descriptive statistics were used to summarize participant demographics, comorbidities, medication use, DE/MGD symptoms, and signs. The normality of the distributions of variables of interest was assessed using the Shapiro–Wilk test. Given that measures were not normally distributed, Mann–Whitney U tests were run to assess the differences between medians, and Spearman correlation coefficients (ρ) were calculated to evaluate the relationship between demographics, DE signs/symptoms, pro, and anti-inflammatory markers. After inspecting residuals, linear regression models with the forward method were performed to predict the contribution of patient characteristics, comorbidities, tear PUFAs, and eicosanoids on DE/MGD symptoms and signs. p < 0.05 was considered statistically significant.
3. Results
3.1. Study Population
The median age of the racially diverse, predominantly male population was 63 years (interquartile range (IQR): 14) (Table 1). DE symptoms ranged from none to severe, with 90% of individuals reporting mild or greater DE symptoms as determined by a DEQ-5 ≥ 6 and 83% as determined by an OSDI ≥ 13. The majority reported some degree of ocular pain (85%, NRS ≥ 1), with 48% reporting moderate or greater pain (NRS ≥ 4). Ocular surface signs varied, with 13% displaying tear instability in the right eye (OD) (as determined by TBUT < 5 s) and 8% showing aqueous tear deficiency (as determined by Schirmer’s < 5 mm). All individuals had at least one sign of MGD, which included eyelid telangiectasias, MG plugging, or MG dropout.

Table 1.
Demographics and clinical information of the study population.
3.2. Tear PUFAs and Eicosanoids
Several tear PUFAs and eicosanoids were collected from the study population (as seen in Supplemental Table S1) with pro-inflammatory properties (i.e., arachidonic acid (AA), thromboxane B2 (TXB2), 5-hydroxyeicosatetraenoic acid (5-HETE), 12-hydroxyeicosatetraenoic acid (12-HETE), 15-hydroxyeicosatetraenoic acid (15-HETE)), and anti-inflammatory properties (i.e., docosahexaenoic acid (DHA), eicosapentaenoic acid (EPA), 11,12-dihydroxyeicosatrienoic acid (11,12 DHET, 14,15-dihydroxyicosatrienoic acid (14,15, DHET)). Ratios showing the relationship between pro- and anti-inflammatory tear PUFAs were also calculated (i.e., AA:DHA, AA:EPA, ω6: ω3). Median values were calculated for all markers, including those with undetectable levels (0.001 pmol of select lipid/mg protein), and the percentage recovered indicates the frequency of detectable quantities.
3.3. Relationships between Tear Eicosanoids and Clinical Metrics
Mann–Whitney U tests were performed to compare differences in medians between demographics, co-morbidities, tear PUFAs, and eicosanoids. Only the significant differences are summarized in Table 2. Males had higher levels of tear pro-inflammatory eicosanoids (i.e., 12 HETE and 15-HETE) compared to females. A similar pattern was noted in Hispanic individuals (compared to non-Hispanics) and in those with diabetes (compared to non-diabetics). On the other hand, smokers had increased levels of an anti-inflammatory marker (14,15 DHET) compared to non-smokers. Also, subjects who reported taking fish oil supplements had a less inflammatory profile, with higher levels of anti-inflammatory mediators and lower inflammatory ratios compared to those not on supplements (EPA: 49.83 (IQR: 66.85) vs. 7.61 (IQR: 16.3), p = 0.03; AA:EPA: 35.42 (IQR: 44.18) vs. 74.11 (IQR: 75.56), p = 0.04). Individuals who reported taking multivitamin supplements had higher levels of both pro- (i.e., 5-HETE, AA) and anti- (i.e., 14,15 DHET, 11,12 DHET, EPA, and DHA) inflammatory eicosanoids compared to those not on supplements.

Table 2.
Significant differences in medians between demographics, medical history, and eicosanoids.
3.4. Relationships between Tear PUFAs, Eicosanoids, and DE/MGD Metrics
Spearman correlations were performed to examine the relationships between DE/MGD symptoms and signs and lipid mediators. Symptoms were not related to pro- or anti-inflammatory lipid mediators (Table 3). However, several significant correlations were noted with respect to tear parameters and eicosanoids. Tear stability (TBUT) negatively correlated with anti-inflammatory mediators (DHA: ρ = −0.34, p = 0.03 and 11,12 DHET: ρ = −0.34, p = 0.03). On the other hand, tear production (Schirmer) negatively correlated with both pro- (5-HETE: ρ = −0.32, p = 0.04) and anti (14,15-DHET: ρ = −0.40, p = 0.01) inflammatory eicosanoids. Finally, corneal staining positively correlated with an anti-inflammatory eicosanoid (DHA: ρ = 0.35, p = 0.03), all of which suggest possible compensatory mechanisms to limit inflammation-associated tear instability, tear reduction, and corneal epithelial disruption.

Table 3.
Spearman correlations demonstrating relationships between DE/MGD symptoms and signs and pro- and anti-inflammatory markers.
With regards to MGD, individuals with telangiectasias had higher pro-inflammatory lipid levels compared to those without telangiectasias (15-HETE: none: 1.07 (IQR: 1.46); mild: 1.83 (IQR: 5.53); and moderate: 2.75 (IQR: 4.05), ρ = 0.32, p < 0.05).
3.5. Linear Regression Models
After inspecting residuals, forward linear regression models were built with significant DE/MGD signs (right eye only) from univariable analysis as dependent variables (Table 3) and all PUFAs and eicosanoids as independent variables. Other variables included in the models were demographics (i.e., gender and ethnicity), history of smoking, hypercholesterolemia, diabetes, sleep apnea, use of betablockers, anxiolytics, fish oil, and multivitamin supplements (Table 4). In all models, tear lipids remained when confounders were considered. Specifically, those with worse ocular surface parameters (lower TBUT and Schirmer, higher corneal staining, and more severe eyelid telangiectasias) had higher levels of eicosanoids with anti-inflammatory properties (11,12-DHET, 14,15-DHET, and DHA) (Table 4, Figure 1).

Table 4.
Linear regression models examining impact of tear PUFAs, eicosanoids, demographics, and comorbidities on DE/MGD symptoms and signs.
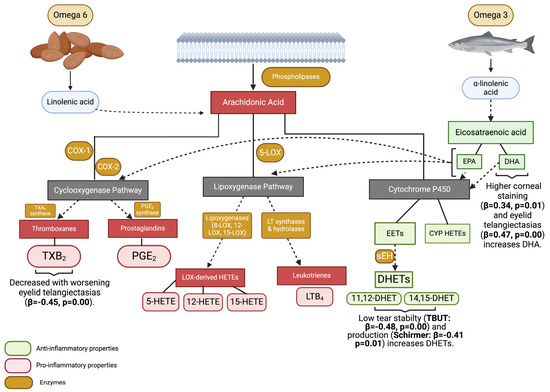
Figure 1.
Graphic representation of relationships between PUFAs, eicosanoids, and dry eye signs. COX-1: cyclooxygenase 1; COX-2: cyclooxygenase 2; TXA2: thromboxane A2; PGE2: prostagladin E2; TXB2: thromboxane B2; 5-LOX: 5-lipoxygenase; 8-LOX: 8-lipoxygenase; 12-LOX: 12-lipoxygenase; 15-LOX: 15-lipoxygenase; LT: leukotrienes; LOX: lipoxygenase; 5-HETE: 5-hydroxyeicosatetraenoic acid; 12-HETE: 12-hydroxyeicosatetraenoic acid; 15-HETE: 15-hydroxyeicosatetraenoic acid; LTB4: leukotriene B4; EPA: eicosapentaenoic acid; DHA: docosahexaenoic acid; EETs: epoxyeicosatrienoic acids; she: soluble epoxide hydrolases; DHETs: dihydroxyeicosatrienoic acids; 11,12 DHET: 11,12-dihydroxyeicosatrienoic acid; 14,15 DHET: 14,15-dihydroxyicosatrienoic acid; CYP HETEs: cytochrome P450 hydroxyicosatetraenoic acids. Overall, ocular surface parameters were more abnormal in individuals with higher anti-inflammatory properties, suggesting compensatory responses to an adverse ocular surface environment. Figure created with BioRender.com.
4. Discussion
In this study, we found some relationships between DE/MGD signs, PUFAs, and their derivatives, even when considering potential confounders. Specifically, the multivariable analysis found negative correlations between tear film parameters (stability and production) and anti-inflammatory eicosanoids (11,12-DHET and 14,15-DHET), suggesting compensatory mechanisms to a pro-inflammatory state driven by tear abnormalities. Similar findings were noted when considering corneal staining and eyelid telangiectasias, as they were related to higher levels of the anti-inflammatory eicosanoid DHA.
Our results share similarities and differences from prior studies. The similarities include detecting COX-pathway-derived pro-inflammatory eicosanoids (i.e., 5-HETE) in tears. A case (n = 40)–control (n = 30) study in subjects with a mean Schirmer value of 15.5 ± 14.0 mm, non-invasive TBUT (NIBUT) of 7.34 s, and MGD (defined as eyelid telangiectasias ≥ 1 (range: 0–3), MG plugging ≥ 1 (range: 0–3), or meibum expressibility ≥ 1 (range: 0–3)) showed that low tear production related to a pro-inflammatory state, given the negative correlations between Schirmer values and pro-inflammatory eicosanoids (5-HETE: ρ = −0.51, p = 0.017, and 9-HETE: ρ = −0.78, p < 0.001) [10]. Our univariable results, noting a negative correlation between Schirmer and 5-HETE, coincide with prior findings. Yet, studies have also detected increased anti-inflammatory metabolites derived from the CYP metabolic pathway of DHA [30], including 20-HDoHE (coefficient = −0.02, p value < 0.001) and 17-HDoHE (coefficient = −0.00, p value = 0.001), in subjects with lower tear production. The relationship between pro- (i.e., 5-HETE) and anti- (i.e., 20-HDoHE and 17-HDoHE) inflammatory markers may again represent a compensatory response of eicosanoids to counteract the pro-inflammatory state that occurs in aqueous deficiency. Our study findings were similar in that tear stability (TBUT) and tear production (Schirmer) were negatively related to the anti-inflammatory eicosanoids 11,12-DHET and 14,15-DHET, respectively.
The findings with respect to corneal staining also share similarities and differences with the literature. In some studies, corneal staining was positively related to various pro-inflammatory prostaglandins (i.e., PGE2 (ρ = 0.35, p < 0.05) [13] and PGF2α (coefficient = 0.09, p value = 0.02)) [10]. At the same time, corneal staining has also been positively correlated with various anti-inflammatory eicosanoids (18-HEPE (coefficient = 0.07, p value = 0.02), 20-HDoHE (coefficient = 0.13, p value = 0.03), and 17-HDoHE (coefficient = 0.03, p value = 0.01)) [10]. In a similar manner, we found significant relationships between more severe corneal staining and increased levels of DHA. While differences in study populations, DE/MGD definitions, and extraction methods may have contributed to the variable findings across studies, some eicosanoid signatures emerge in relation to DE/MGD signs, including prostaglandins, DHA, and HETEs.
A unique finding in our study was the detection of tear eicosanoids metabolized via the CYP pathway (i.e., 11,12 DHET, 14,15-DHET). Studies outside the eye have related DHET molecules to pro-inflammatory conditions such as coronary heart disease (CHD), with individuals with CHD having higher levels of 14,15-DHET compared to controls (2.53 ± 1.60 ng/L vs. 1.65 ± 1.54 ng/L, p = 0.036) [16]. We similarly found significant correlations between low tear stability and production and DHET molecules, indicating that higher levels of anti-inflammatory eicosanoids may be involved in disease modulation and restoration of homeostasis across disease states and organs [16].
Beyond tear and ocular surface parameters, several patient-related factors, including gender, ethnicity, the presence of comorbidities, and smoking, are related to levels of various tear eicosanoids, with ethnicity and diabetes remaining in multivariable models. Specifically, males, Hispanics, and individuals with diabetes had higher levels of pro-inflammatory eicosanoids (i.e., 12-HETE and 15-HETE) compared to their counterparts, while current or previous smokers had higher levels of an anti-inflammatory eicosanoid (i.e., 14,15-DHET) compared to non-smokers. These findings share similarities and differences with prior literature. While previous studies have not found differences in tear lipids by gender [10,13], mixed findings were noted by ethnicity, with higher [31], lower [31], and similar [32,33] levels of plasma anti-inflammatory PUFAs reported. Similar to our study, increased pro-inflammatory and angiogenic eicosanoids (i.e., LTB4 and 12-HETE) were noted in individuals with chronic diseases such as diabetes [34] and CHD [35]. Interestingly, prior studies have also noted increased plasma pro- (i.e., 5-HETE) and anti- (i.e., 11,12-DHET and 14,15-DHET) inflammatory eicosanoids in smokers, suggesting that compensatory mechanisms may occur both in blood and tears [36]. Although the aim of our study was not to describe the impact of oral supplements such as fish oil and multivitamins on tear composition, we noted that individuals who reported taking supplements had a less inflammatory tear film (lower AA: EPA, AA: DHA and ω6: ω3) compared to their counterparts. Similar findings were noted in our prior study with respect to individuals taking ω3 (DHA and EPA) supplements [13]. While the results of studies on the benefits of ω3 and ω6 supplementation in DE have been mixed [37,38,39,40,41,42], our findings suggest the need for further research on this topic.
As with all studies, our findings need to be considered in light of the limitations of this study, which included its cross-sectional nature, predominately male population, limited sample size, and sensitivity of the tear eicosanoid assay. Furthermore, the origin of lipid mediators in tears is not known, with possible sources, including the MG, lacrimal gland, and/or ocular surface epithelium. Finally, our assay did not include some pro-resolving eicosanoids, such as resolvins, maresins, and neuroprotectin D1. Despite these limitations, in this study, we found both pro- and anti-inflammatory markers in subjects with tear abnormalities and MGD, which is similar to prior studies. We also detected new findings, such as CYP-derived metabolites, which have been reported to possess anti-inflammatory properties.
5. Conclusions
Overall, our findings highlight the complexity of studying tear eicosanoids in DE/MGD, as their presence may contribute to or be a compensatory mechanism for an abnormal ocular surface environment. It is plausible that the ocular surface constantly autoregulates itself to re-establish homeostasis, and thus, longitudinal studies are needed to evaluate our findings more robustly. A better understanding of the role of eicosanoids in DE/MGD is needed, as this knowledge may improve treatment algorithms by suggesting which medications (i.e., corticosteroids that block phospholipase A2 enzyme preventing production of AA and all downstream products vs. nonsteroidal anti-inflammatory drugs (NSAIDs) that only block the COX pathway) would be optimal in an individual patient [43]. Moreover, beyond COX and LOX inhibition, selective cytochrome P-450 inhibitors may have a beneficial role, which needs to be further defined in the DE/MGD field.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/biom14030376/s1. Supplemental Table S1: Median and ranges for tear lipids collected.
Author Contributions
Conceptualization, A.G. and N.M.; methodology, A.P., D.S., C.E.C. and N.M.; validation, D.S. and C.E.C., formal analysis, S.M.-M., A.P., D.S., C.E.C. and N.M.; investigation, S.M.-M. and A.G.; resources, A.G., N.M., D.S. and C.E.C.; writing—original draft preparation, S.M.-M.; writing—review and editing, S.M.-M., A.G. and N.M.; visualization, S.M.-M. and A.G.; supervision, A.G. and N.M.; project administration, A.G. and N.M.; funding acquisition, A.G., D.S., C.E.C. and N.M. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Biomedical Laboratory R&D (BLRD) Service I01 BX004893 (Drs. Galor and Mandal). Other support: Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Clinical Sciences R&D (CSRD) I01 CX002015 (AG), VA Merit Review award, BX001792 (CEC), VA Merit Review award, BX 006063 (CEC), and a Senior Research Career Scientist Award, IK6BX004603 (CEC). Department of Defense Gulf War Illness Research Program (GWIRP) W81XWH-20-1-0579 (AG), Vision Research Program (VRP) W81XWH-20-1-0820 (AG), and Vision Research Program (VRP) grant W81XWH-20-1-0900 (NM); National Institutes of Health grants EY022071 (NM), R01 EY031316 (NM), R01 AI139072 (CEC), and P01 CA171983-Project 1 (CEC); and U24EY035102 (AG), U01EY034686 (AG), and R33EY032468 (AG). This work was also supported by a center grant to the UVA Comprehensive Cancer Center from the National Cancer Institute (2P30CA044579-26), National Eye Institute NIH Center Core Grant P30EY014801 (institutional) and Research to Prevent Blindness Unrestricted Grant (institutional). The contents of this manuscript do not represent the views of the Department of Veterans Affairs or the United States Government. The funding sources were not involved in the study design, data collection, interpretation, analysis, writing, or decision to submit this article for publication.
Institutional Review Board Statement
The study was conducted in accordance with the tenets of the Declaration of Helsinki, complied with the requirements of the United States Health Insurance Portability and Accountability Act (HIPAA), and was approved by the Miami Veterans Affairs (VA) Institutional Review Board (protocol code 3011.08 and date of approval 8 June 2020).
Informed Consent Statement
Informed consent was obtained from all subjects after explanation of the nature and possible consequences of the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Stapleton, F.; Alves, M.; Bunya, V.Y.; Jalbert, I.; Lekhanont, K.; Malet, F.; Na, K.-S.; Schaumberg, D.; Uchino, M.; Vehof, J.; et al. TFOS DEWS II Epidemiology Report. Ocul. Surf. 2017, 15, 334–365. [Google Scholar] [CrossRef]
- Wolffsohn, J.S.; Arita, R.; Chalmers, R.; Djalilian, A.; Dogru, M.; Dumbleton, K.; Gupta, P.K.; Karpecki, P.; Lazreg, S.; Pult, H.; et al. TFOS DEWS II Diagnostic Methodology report. Ocul. Surf. 2017, 15, 539–574. [Google Scholar] [CrossRef]
- Nichols, K.K.; Foulks, G.N.; Bron, A.J.; Glasgow, B.J.; Dogru, M.; Tsubota, K.; Lemp, M.A.; Sullivan, D.A. The international workshop on meibomian gland dysfunction: Executive summary. Invest Ophthalmol. Vis. Sci. 2011, 52, 1922–1929. [Google Scholar] [CrossRef]
- Pouyeh, B.; Viteri, E.; Feuer, W.; Lee, D.J.; Florez, H.; Fabian, J.A.; Perez, V.L.; Galor, A. Impact of ocular surface symptoms on quality of life in a United States veterans affairs population. Am. J. Ophthalmol. 2012, 153, 1061–1066.e3. [Google Scholar] [CrossRef]
- Pflugfelder, S.C.; de Paiva, C.S. The Pathophysiology of Dry Eye Disease: What We Know and Future Directions for Research. Ophthalmology 2017, 124, S4–S13. [Google Scholar] [CrossRef]
- Lam, H.; Bleiden, L.; de Paiva, C.S.; Farley, W.; Stern, M.E.; Pflugfelder, S.C. Tear cytokine profiles in dysfunctional tear syndrome. Am. J. Ophthalmol. 2009, 147, 198–205.e1. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Gadaria-Rathod, N.; Epstein, S.; Asbell, P. Tear cytokine profile as a noninvasive biomarker of inflammation for ocular surface diseases: Standard operating procedures. Invest. Ophthalmol. Vis. Sci. 2013, 54, 8327–8336. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Wu, L.; Chen, J.; Dong, L.; Chen, C.; Wen, Z.; Hu, J.; Fleming, I.; Wang, D.W. Metabolism pathways of arachidonic acids: Mechanisms and potential therapeutic targets. Signal Transduct. Target. Ther. 2021, 6, 94. [Google Scholar] [CrossRef] [PubMed]
- Tallima, H.; El Ridi, R. Arachidonic acid: Physiological roles and potential health benefits—A review. J. Adv. Res. 2018, 11, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Ambaw, Y.A.; Chao, C.; Ji, S.; Raida, M.; Torta, F.; Wenk, M.R.; Tong, L. Tear eicosanoids in healthy people and ocular surface disease. Sci. Rep. 2018, 8, 11296. [Google Scholar] [CrossRef] [PubMed]
- Spite, M.; Claria, J.; Serhan, C.N. Resolvins, specialized proresolving lipid mediators, and their potential roles in metabolic diseases. Cell Metab. 2014, 19, 21–36. [Google Scholar] [CrossRef]
- English, J.T.; Norris, P.C.; Hodges, R.R.; Dartt, D.A.; Serhan, C.N. Identification and Profiling of Specialized Pro-Resolving Mediators in Human Tears by Lipid Mediator Metabolomics. Prostaglandins Leukot. Essent. Fat. Acids 2017, 117, 17–27. [Google Scholar] [CrossRef]
- Walter, S.D.; Gronert, K.; McClellan, A.L.; Levitt, R.C.; Sarantopoulos, K.D.; Galor, A. omega-3 Tear Film Lipids Correlate With Clinical Measures of Dry Eye. Invest Ophthalmol. Vis. Sci. 2016, 57, 2472–2478. [Google Scholar] [CrossRef]
- Chistyakov, D.V.; Gancharova, O.S.; Baksheeva, V.E.; Tiulina, V.V.; Goriainov, S.V.; Azbukina, N.V.; Tsarkova, M.S.; Zamyatnin, A.A., Jr.; Philippov, P.P.; Sergeeva, M.G.; et al. Inflammation in Dry Eye Syndrome: Identification and Targeting of Oxylipin-Mediated Mechanisms. Biomedicines 2020, 8, 344. [Google Scholar] [CrossRef] [PubMed]
- Schwartzman, M.L.; Abraham, N.G.; Masferrer, J.; Dunn, M.W.; McGiff, J.C. Cytochrome P450 dependent metabolism of arachidonic acid in bovine corneal epithelium. Biochem. Biophys. Res. Commun. 1985, 132, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Peng, R.; Guo, Y.; Shen, L.; Zhao, S.; Xu, D. The role of 14,15-dihydroxyeicosatrienoic acid levels in inflammation and its relationship to lipoproteins. Lipids Health Dis. 2013, 12, 151. [Google Scholar] [CrossRef] [PubMed]
- Chalmers, R.L.; Begley, C.G.; Caffery, B. Validation of the 5-Item Dry Eye Questionnaire (DEQ-5): Discrimination across self-assessed severity and aqueous tear deficient dry eye diagnoses. Contact Lens Anterior Eye 2010, 33, 55–60. [Google Scholar] [CrossRef]
- Schiffman, R.M.; Christianson, M.D.; Jacobsen, G.; Hirsch, J.D.; Reis, B.L. Reliability and validity of the Ocular Surface Disease Index. Arch. Ophthalmol. 2000, 118, 615–621. [Google Scholar] [CrossRef]
- Kalangara, J.P.; Galor, A.; Levitt, R.C.; Covington, D.B.; McManus, K.T.; Sarantopoulos, C.D.; Felix, E.R. Characteristics of Ocular Pain Complaints in Patients With Idiopathic Dry Eye Symptoms. Eye Contact Lens 2017, 43, 192–198. [Google Scholar] [CrossRef]
- Farhangi, M.; Feuer, W.; Galor, A.; Bouhassira, D.; Levitt, R.C.; Sarantopoulos, C.D.; Felix, E.R. Modification of the Neuropathic Pain Symptom Inventory for use in eye pain (NPSI-Eye). Pain 2019, 160, 1541–1550. [Google Scholar] [CrossRef]
- Heiko, D.P. Improved Meiboscale for Meibography. v. 2023. Available online: https://www.heiko-pult.de/media/files/MEIBOSCALE-2016--Einseiter.pdf (accessed on 18 October 2023).
- MacKnight, H.P.; Stephenson, D.J.; Hoeferlin, L.A.; Benusa, S.D.; DeLigio, J.T.; Maus, K.D.; Ali, A.N.; Wayne, J.S.; Park, M.A.; Hinchcliffe, E.H.; et al. The interaction of ceramide 1-phosphate with group IVA cytosolic phospholipase A(2) coordinates acute wound healing and repair. Sci. Signal 2019, 12, eaav5918. [Google Scholar] [CrossRef]
- Stephenson, D.J.; MacKnight, H.P.; Hoeferlin, L.A.; Washington, S.L.; Sawyers, C.; Archer, K.J.; Strauss, J.F., III.; Walsh, S.W.; Chalfant, C.E. Bioactive lipid mediators in plasma are predictors of preeclampsia irrespective of aspirin therapy. J. Lipid Res. 2023, 64, 100377. [Google Scholar] [CrossRef]
- Maus, K.D.; Stephenson, D.J.; Ali, A.N.; MacKnight, H.P.; Huang, H.-J.; Serrats, J.; Kim, M.; Diegelmann, R.F.; Chalfant, C.E. Ceramide kinase regulates acute wound healing by suppressing 5-oxo-ETE biosynthesis and signaling via its receptor OXER1. J. Lipid Res. 2022, 63, 100187. [Google Scholar] [CrossRef]
- Maus, K.D.; Stephenson, D.J.; Macknight, H.P.; Vu, N.T.; Hoeferlin, L.A.; Kim, M.; Diegelmann, R.F.; Xie, X.; Chalfant, C.E. Skewing cPLA(2)alpha activity toward oxoeicosanoid production promotes neutrophil N2 polarization, wound healing, and the response to sepsis. Sci. Signal 2023, 16, eadd6527. [Google Scholar] [CrossRef]
- Wijesinghe, D.S.; Allegood, J.C.; Gentile, L.B.; Fox, T.E.; Kester, M.; Chalfant, C.E. Use of high performance liquid chromatography-electrospray ionization-tandem mass spectrometry for the analysis of ceramide-1-phosphate levels. J. Lipid Res. 2010, 51, 641–651. [Google Scholar] [CrossRef]
- Vu, N.T.; Kim, M.; Stephenson, D.J.; MacKnight, H.P.; Chalfant, C.E. Ceramide Kinase Inhibition Drives Ferroptosis and Sensitivity to Cisplatin in Mutant KRAS Lung Cancer by Dysregulating VDAC-Mediated Mitochondria Function. Mol. Cancer Res. 2022, 20, 1429–1442. [Google Scholar] [CrossRef]
- Wijesinghe, D.S.; Brentnall, M.; Mietla, J.A.; Hoeferlin, L.A.; Diegelmann, R.F.; Boise, L.H.; Chalfant, C.E. Ceramide kinase is required for a normal eicosanoid response and the subsequent orderly migration of fibroblasts. J. Lipid Res. 2014, 55, 1298–1309. [Google Scholar] [CrossRef] [PubMed]
- Simanshu, D.K.; Kamlekar, R.K.; Wijesinghe, D.S.; Zou, X.; Zhai, X.; Mishra, S.K.; Molotkovsky, J.G.; Malinina, L.; Hinchcliffe, E.H.; Chalfant, C.E.; et al. Non-vesicular trafficking by a ceramide-1-phosphate transfer protein regulates eicosanoids. Nature 2013, 500, 463–467. [Google Scholar] [CrossRef] [PubMed]
- Ambaw, Y.A.; Fuchs, D.; Raida, M.; Mazengia, N.T.; Torta, F.; Wheelock, C.E.; Wenk, M.R.; Tong, L. Changes of tear lipid mediators after eyelid warming or thermopulsation treatment for meibomian gland dysfunction. Prostaglandins Other Lipid Mediat. 2020, 151, 106474. [Google Scholar] [CrossRef]
- Anderson, J.S.; Nettleton, J.A.; Herrington, D.M.; Johnson, W.C.; Tsai, M.Y.; Siscovick, D. Relation of omega-3 fatty acid and dietary fish intake with brachial artery flow-mediated vasodilation in the Multi-Ethnic Study of Atherosclerosis. Am. J. Clin. Nutr. 2010, 92, 1204–1213. [Google Scholar] [CrossRef] [PubMed]
- Haffner, S.M.; Miettinen, H.; Stern, M.P.; Agil, A.; Jialal, I. Plasma oxidizability in Mexican-Americans and non-Hispanic whites. Metabolism 1996, 45, 876–881. [Google Scholar] [CrossRef]
- Steffen, B.T.; Steffen, L.M.; Tracy, R.; Siscovick, D.; Jacobs, D.; Liu, K.; He, K.; Hanson, N.Q.; Nettleton, J.A.; Tsai, M.Y. Ethnicity, plasma phospholipid fatty acid composition and inflammatory/endothelial activation biomarkers in the Multi-Ethnic Study of Atherosclerosis (MESA). Eur. J. Clin. Nutr. 2012, 66, 600–605. [Google Scholar] [CrossRef]
- Issan, Y.; Hochhauser, E.; Guo, A.; Gotlinger, K.H.; Kornowski, R.; Leshem-Lev, D.; Lev, E.; Porat, E.; Snir, E.; Thompson, C.I.; et al. Elevated level of pro-inflammatory eicosanoids and EPC dysfunction in diabetic patients with cardiac ischemia. Prostaglandins Other Lipid Mediat. 2013, 100–101, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Pascale, J.V.; Lucchesi, P.A.; Garcia, V. Unraveling the Role of 12- and 20- HETE in Cardiac Pathophysiology: G-Protein-Coupled Receptors, Pharmacological Inhibitors, and Transgenic Approaches. J. Cardiovasc. Pharmacol. 2021, 77, 707–717. [Google Scholar] [CrossRef] [PubMed]
- Titz, B.; Luettich, K.; Leroy, P.; Boue, S.; Vuillaume, G.; Vihervaara, T.; Ekroos, K.; Martin, F.; Peitsch, M.C.; Hoeng, J. Alterations in Serum Polyunsaturated Fatty Acids and Eicosanoids in Patients with Mild to Moderate Chronic Obstructive Pulmonary Disease (COPD). Int. J. Mol. Sci. 2016, 17, 1583. [Google Scholar] [CrossRef] [PubMed]
- Dry Eye Assessment and Management Study Research Group; Asbell, P.A.; Maguire, M.G.; Pistilli, M.; Ying, G.-S.; Szczotka-Flynn, L.B.; Hardten, D.R.; Lin, M.C.; Shtein, R.M. n-3 Fatty Acid Supplementation for the Treatment of Dry Eye Disease. N. Engl. J. Med. 2018, 378, 1681–1690. [Google Scholar]
- Downie, L.E.; Ng, S.M.; Lindsley, K.B.; Akpek, E.K. Omega-3 and omega-6 polyunsaturated fatty acids for dry eye disease. Cochrane Database Syst. Rev. 2019, 12, CD011016. [Google Scholar] [CrossRef] [PubMed]
- Oydanich, M.; Maguire, M.G.; Pistilli, M.; Hamrah, P.; Greiner, J.V.; Lin, M.C.; Asbell, P.A.; Dry Eye Assessment and Management Study Research Group. Effects of Omega-3 Supplementation on Exploratory Outcomes in the Dry Eye Assessment and Management Study. Ophthalmology 2020, 127, 136–138. [Google Scholar] [CrossRef]
- Ng, A.; Woods, J.; Jahn, T.; Jones, L.W.; Ritter, J.S. Effect of a Novel Omega-3 and Omega-6 Fatty Acid Supplement on Dry Eye Disease: A 3-month Randomized Controlled Trial. Optom. Vis. Sci. 2022, 99, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Giannaccare, G.; Pellegrini, M.; Sebastiani, S.; Bernabei, F.; Roda, M.; Taroni, L.; Versura, P.; Campos, E.C. Efficacy of Omega-3 Fatty Acid Supplementation for Treatment of Dry Eye Disease: A Meta-Analysis of Randomized Clinical Trials. Cornea 2019, 38, 565–573. [Google Scholar] [CrossRef]
- Christen, W.G.; Cook, N.R.; Manson, J.E.; Buring, J.E.; Lee, I.-M.; Bubes, V.; Friedenberg, G.; Dushkes, R.; Smith, D.; Schaumberg, D.A.; et al. Efficacy of Marine omega-3 Fatty Acid Supplementation vs Placebo in Reducing Incidence of Dry Eye Disease in Healthy US Adults: A Randomized Clinical Trial. JAMA Ophthalmol. 2022, 140, 707–714. [Google Scholar] [CrossRef] [PubMed]
- Hagan, S.; Martin, E.; Enriquez-de-Salamanca, A. Tear fluid biomarkers in ocular and systemic disease: Potential use for predictive, preventive and personalised medicine. EPMA J. 2016, 7, 15. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





