The Effects of the Steroids 5-Androstenediol and Dehydroepiandrosterone and Their Synthetic Derivatives on the Viability of K562, HeLa, and Wi-38 Cells and the Luminol-Stimulated Chemiluminescence of Peripheral Blood Mononuclear Cells from Healthy Volunteers
Abstract
1. Introduction
2. Materials and Methods
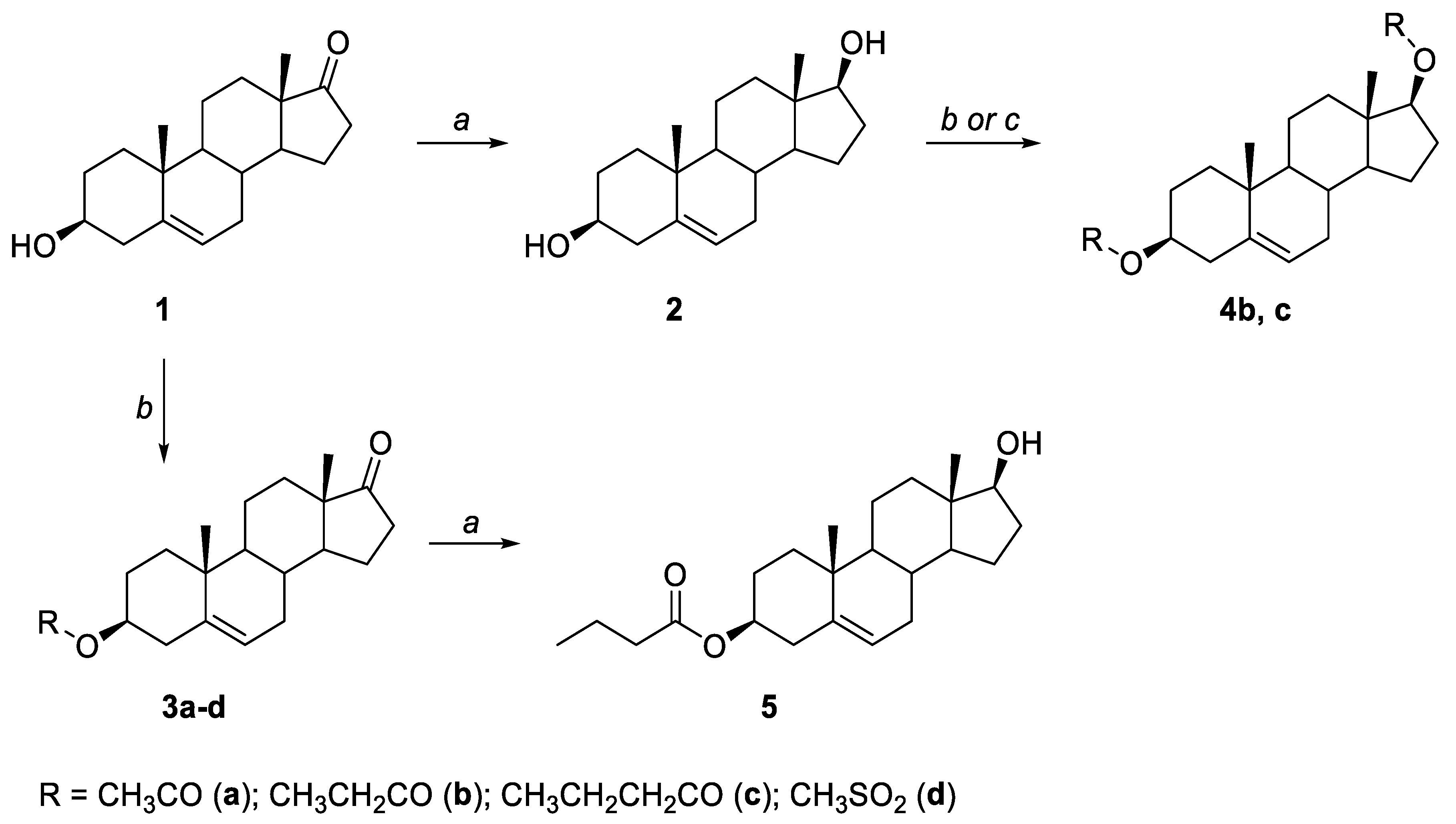
2.1. General Synthesis
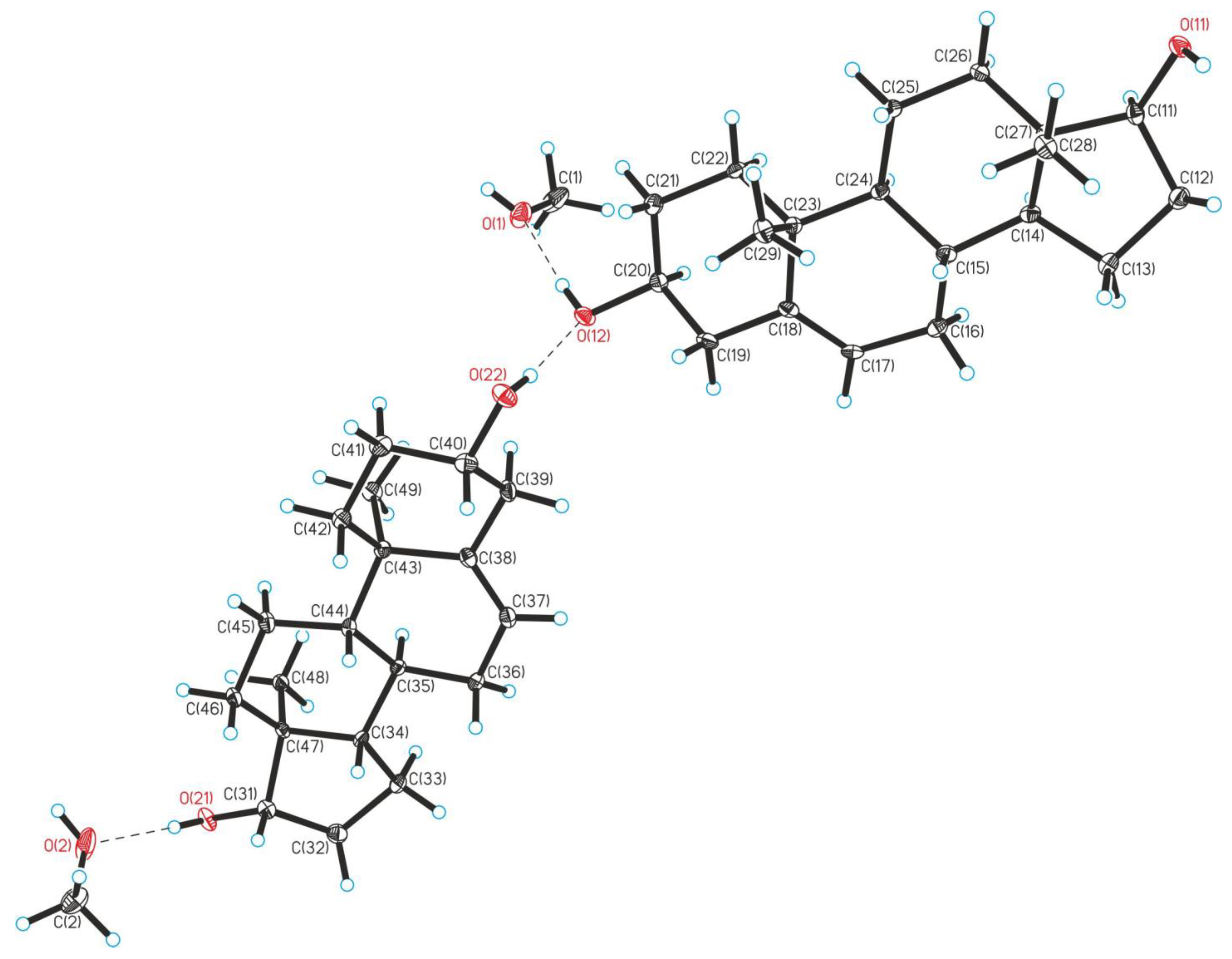
Crystallographic Details
2.2. Biological Tests
2.2.1. Cell Lines
2.2.2. MTT Assay
2.2.3. Participants
2.2.4. LSCL Measurement
2.2.5. Statistical Analysis
3. Results
3.1. Synthesis
3.2. Biological Tests
3.2.1. The Effect of the Steroid Hormones 5-AED and DHEA and Their Derivatives on the Viability of K562, HeLa, and Wi-38 Cells
3.2.2. The Effect of 5-AED and DHEA and Their Derivatives on the Luminal-Dependent Chemiluminescence of PBMCs from Healthy Volunteers
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AR | androgen receptor |
| ER | estrogen receptor |
| G-CSF | granulocyte colony-stimulating factor |
| GR | glucocorticoid receptor |
| LSCL | luminol-stimulated chemiluminescence |
| NADPH | nicotinamide adenine dinucleotide phosphate |
| NOX | NADPH oxidase |
| PBMCs | peripheral blood mononuclear cells |
| ROS | reactive oxygen species |
| SSHs | sex steroid hormones |
| THF | tetrahydrofuran |
References
- Huang, X.; Shen, Q.-K.; Zhang, H.-J.; Li, J.-L.; Tian, Y.-S.; Quan, Z.-S. Design and synthesis of novel dehydroepiandrosterone analogues as potent antiproliferative agents. Molecules 2018, 23, 2243. [Google Scholar] [CrossRef]
- Bansal, R.; Suryan, A. A Comprehensive Review on Steroidal Bioconjugates as Promising Leads in Drug Discovery. ACS Bio Med. Chem. Au 2022, 2, 340–369. [Google Scholar] [CrossRef]
- Liu, X.-K.; Ye, B.-J.; Wu, Y.; Nan, J.-X.; Lin, Z.-H.; Piao, H.-R. Synthesis and antitumor activity of dehydroepiandrosterone derivatives on Es-2, A549, and HepG2 cells in vitro. Chem. Biol. Drug Des. 2012, 79, 523–529. [Google Scholar] [CrossRef]
- Ke, S.; Shi, L.; Zhang, Z.; Yang, Z. Steroidal[17,16-d]pyrimidines derived from dehydroepiandrosterone: A convenient synthesis, antiproliferation activity, structure-activity relationships, and role of heterocyclic moiety. Sci. Rep. 2017, 7, 44439. [Google Scholar] [CrossRef]
- Savić, M.P.; Djškorić, D.Đ.; Kuzminac, I.Z.; Jakimov, D.S.; Kojić, V.V.; Rárová, L.; Strnad, M.; Djurendić, E.A. New A-homo lactam D-homo lactone androstane derivative: Synthesis and evaluation of cytotoxic and anti-inflammatory activities in vitro. Steroids 2020, 157, 108596. [Google Scholar] [CrossRef]
- Labrie, F.; Luu-The, V.; Bélanger, A.; Lin, S.X.; Simard, J.; Pelletier, G.; Labrie, C. Is dehydroepiandrosterone a hormone? J. Endocrinol. 2005, 187, 169–196. [Google Scholar] [CrossRef] [PubMed]
- William, F.; Ganong, M.D. Review of Medical Physiology, 22nd ed.; McGraw Hill: New York, NY, USA, 2005; p. 362. [Google Scholar]
- Nair, K.S.; Rizza, R.A.; O’Brien, P.; Dhatariya, K.; Short, K.R.; Nehra, A.; Vittone, J.L.; Klee, G.G.; Basu, A.; Basu, R.; et al. DHEA in elderly women and DHEA or testosterone in elderly men. N. Engl. J. Med. 2006, 355, 1647–1659. [Google Scholar] [CrossRef] [PubMed]
- Pescovitz, O.H.; Eugster, E.A. Pediatric Endocrinology: Mechanisms, Manifestations, and Management; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2004; p. 362. [Google Scholar]
- Lifshitz, F. Pediatric Endocrinology: Growth, Adrenal, Sexual, Thyroid, Calcium, and Fluid Balance Disorders; CRC Press: Boca Raton, FL, USA, 2006; p. 289. [Google Scholar]
- Salhan, S. Textbook of Gynecology; JP Medical Ltd.: London UK, 2011; p. 94. [Google Scholar]
- Webb, S.J.; Geoghegan, T.E.; Prough, R.A.; Miller, K.K.M. The biological actions of dehydroepiandrosterone involves multiple receptors. Drug Metab. Rev. 2006, 38, 89–116. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Knecht, K.; Birzin, E.; Fisher, J.; Wilkinson, H.; Mojena, M.; Moreno, C.T.; Schmidt, A.; Harada, S.; Freedman, L.P.; et al. Direct agonist/antagonist functions of dehydroepiandrosterone. Endocrinology 2005, 146, 4568–4576. [Google Scholar] [CrossRef] [PubMed]
- Weizman, A. Neuroactive Steroids in Brain Function, Behavior and Neuropsychiatric Disorders: Novel Strategies for Research and Treatment; Springer Science & Business Media: Berlin, Germany, 2008; p. 229. [Google Scholar]
- Gravanis, A.G.; Mellon, S.H. Hormones in Neurodegeneration, Neuroprotection, and Neurogenesis; John Wiley & Sons: Hoboken, NJ, USA, 2011; p. 349. [Google Scholar]
- Savic, I. Sex Difference in the Human Brain, Their Underpinnings and Implications; Elsevier: Amsterdam, The Netherlands, 2010; p. 127. [Google Scholar]
- Gordon, G.; Mackow, M.C.; Levy, H.R. On the Mechanism of Interaction of Steroids with Human Glucose 6-Phosphate Dehydrogenase. Arch. Biochem. Biophys. 1995, 318, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, A.G. Dehydroepiandrosterone, Cancer, and Aging. Aging Dis. 2022, 13, 423–432. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, A.G.; Perantoni, A. Protective effect of dehydroepiandrosterone against aflatoxin B1- and 7,12-dimethylbenz(a) anthracene-induced cytotoxicity and transformation in cultured cells. Cancer Res. 1975, 35, 2482–2487. [Google Scholar] [PubMed]
- Feo, F.; Pirisi, L.; Pascale, R.; Daino, L.; Frassetto, S.; Garcea, R.; Gaspa, L. Modulatory effect of glucose-6- phosphate dehydrogenase deficiency on benzo(a)pyrene toxicity and transforming activity for in vitro-cultured human skin fibroblasts. Cancer Res. 1984, 44, 3419–3425. [Google Scholar] [PubMed]
- Garcea, R.; Daino, L.; Frassetto, S.; Cozzolino, P.; Ruggiu, M.E.; Vannini, M.G.; Pascale, R.; Lenzerini, L.; Simile, M.M.; Puddu, M.; et al. Reversal by ribo-and deoxyribonucleosides of dehydroepiandrosteroneinduced inhibition of enzyme altered foci in the liver of rats subjected to the initiation-selection process of experimental carcinogenesis. Carcinogenesis 1988, 9, 931–938. [Google Scholar] [CrossRef] [PubMed]
- Muller, C.; Cluzeaud, F.; Pinon, G.M.; Rafestin-Oblin, M.E.; Morfin, R. Dehydroepiandrosterone and its 7-hydroxylated metabolites do not interfere with the transactivation and cellular trafficking of the glucocorticoid receptor. J. Steroid Biochem. Mol. Biol. 2004, 92, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Apostolova, G.; Schweizer, R.A.S.; Balazs, Z.; Kostadinova, R.M.; Odermatt, A. Dehydroepiandrosterone inhibits the amplification of glucocorticoid action in adipose tissue. Am. J. Physiol. Endocrinol. Metab. 2005, 288, E957–E964. [Google Scholar] [CrossRef] [PubMed]
- Nyce, J. Components of the human-specific, p53-mediated “kill switch” tumor suppression mechanism are usurped by human tumors, creating the possibility of therapeutic exploitation. Cancer Drug Resist. 2019, 2, 1207–1214. [Google Scholar] [CrossRef]
- Dondi, D.; Piccolella, M.; Biserni, A.; Della Torre, S.; Ramachandran, B.; Locatelli, A. Estrogen receptor β and the progression of prostate cancer: Role of 5α-androstane-3β,17β-diol. Endocr. Relat. Cancer 2010, 17, 731–742. [Google Scholar] [CrossRef]
- Bosland, M.C.; Prinsen, M.K. Induction of dorsolateral prostate adenocarcinomas and other accessory sex gland lesions in male Wistar rats by a single administration of n-methyl-n-nitrosourea, 7,12- dimethylbenz(a)anthracene, and 3,2′-dimethyl-4- aminobiphenyl after sequential treatment with cyproterone acetate and testosterone proprionate. Cancer Res. 1990, 50, 691–699. [Google Scholar]
- Sun, J.; Yu, L.; Qu, X.; Huang, T. The role of peroxisome proliferator-activated receptors in the tumor microenvironment, tumor cell metabolism, and anticancer therapy. Front. Pharmacol. 2023, 14, 1184794. [Google Scholar] [CrossRef]
- Loria, R.M.; Ben-Nathan, D. Protective effects of DHEA and AED against viral, bacterial and parasitic infections. Isr. J. Vet. Med. 2011, 66, 119–129. [Google Scholar]
- Whitnall, M.H.; Elliott, T.B.; Harding, R.A.; Inal, C.E.; Landauer, M.R.; Wilhelmsen, C.L.; Wilhelmsena, C.L.; McKinneya, L.; Miner, V.L.; Jackson, W.E., III.; et al. Androstenediol stimulates myelopoiesis and enhances resistance to infection in gamma-irradiated mice. Int. J. Immunopharmacol. 2000, 22, 1–14. [Google Scholar] [CrossRef]
- Stickney, D.R.; Dowding, C.; Authier, S.; Garsd, A.; Onizuka-Handa, N.; Reading, C.; Frincke, J.M. 5-Androstenediol improves survival in clinically unsupported rhesus monkeys with radiation-induced myelosuppression. Int. Immunopharmacol. 2007, 7, 500–505. [Google Scholar] [CrossRef]
- Grace, M.B.; Singh, V.K.; Rhee, J.G.; Jackson, W.E.; Kao, T.-C.; Whitnall, M.H. 5-AED enhances survival of irradiated mice in a G-CSF-dependent manner, stimulates innate immune cell function, reduces radiation-induced DNA damage and induces genes that modulate cell cycle progression and apoptosis. J. Radiat. Res. 2012, 53, 840–853. [Google Scholar] [CrossRef] [PubMed]
- Ruzicka, L.; Wettstein, A. Sexualhormone VII. Über die künstliche Herstellung des Testikelhormons Testosteron (Androsten-3-on-17-ol). Helv. Chim. Acta 1935, 18, 1264–1275. [Google Scholar] [CrossRef]
- Barton, D.H.R.; Cox, J.D. The application of the method of molecular rotation differences to steroids. Part IV. Optical anomalies. J. Chem. Soc. 1948, 783–793. [Google Scholar] [CrossRef] [PubMed]
- Ruddock, P.L.; Williams, D.J.; Reese, P.B. The scope and limitations of the reaction of δ5-steroids with mercury(II) trifluoroacetate. Steroids 1998, 63, 650–664. [Google Scholar] [CrossRef] [PubMed]
- Kovganko, N.V.; Kashkan, Z.N. Reactivity of hydroxy and keto groups on C-6 and C-17 of 3α,5α-cycloandrostanes. Chem. Nat. Comp. 2001, 37, 47–51. [Google Scholar] [CrossRef]
- Goswami, P.; Hazarika, S.; Borah, P.; Chowdhury, P. Chloro- or Bromo-trimethylsilane Induced Rapid and Quantitative Acid-Ester Conversion for Steroid Based Alcohols with Various Carboxylic Acids under Solvent Free Conditions. Ind. J. Chem.—Sect. B 2003, 42, 678–682. [Google Scholar] [CrossRef]
- Riva, S.; Klibanov, A.M. Enzymochemical regioselective oxidation of steroids without oxidoreductase. J. Am. Chem. Soc. 1988, 110, 3291–3295. [Google Scholar] [CrossRef]
- Krause, L.; Herbst-Irmer, R.; Sheldrick, G.M.; Stalke, D. Comparison of silver and molybdenum microfocus X-ray sources for single-crystal structure determination. J. Appl. Crystallogr. 2015, 48, 3–10. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Semeikin, A.V.; Fedotcheva, T.A.; Sveshnikova, E.D.; Shilov, B.V.; Smirnov, A.S.; Shimanovskii, N.L. Cytostatic activity and ligand-receptor interaction energy of the novel Russian-produced gestagen gestobutanoil and its metabolites. Pharm. Chem. J. 2021, 54, 1087–1092. [Google Scholar] [CrossRef]
- Böyum, A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of mononuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand. J. Clin. Lab. Investig. Suppl. 1968, 97, 77–89. [Google Scholar]
- Cui, H.-W.; Peng, S.; Gu, X.-Z.; Chen, H.; He, Y.; Gao, W.; Lv, F.; Wang, J.-H.; Wang, Y.; Xie, J.; et al. Synthesis and biological evaluation of D-ring fused 1,2,3-thiadiazole dehydroepiandrosterone derivatives as antitumor agents. Eur. J. Med. Chem. 2016, 111, 126–137. [Google Scholar] [CrossRef] [PubMed]
- Kudryavtsev, K.V.; Sokolov, M.N.; Varpetyan, E.E.; Kirsanova, A.A.; Fedotcheva, N.I.; Shimanovskii, N.L.; Fedotcheva, T.A. A pregnane steroid as the chiral auxiliary in 1,3-dipolar azomethine ylide’s cycloaddition: Asymmetric synthesis and anticancer activity of novel hybrid compounds. ChemistrySelect 2020, 5, 11467–11470. [Google Scholar] [CrossRef]
- Drugbank Database. Available online: https://go.drugbank.com/drugs/DB01708 (accessed on 3 January 2024).
- Cristofalo, V.J.; Rosner, B.A. Modulation of cell proliferation and senescence of WI-38 cells by hydrocortisone. Fed Proc. 1979, 38, 1851–1856. [Google Scholar]
- Mawal-Dewan, M.; Frisoni, L.; Cristofalo, V.J.; Sell, C. Extension of replicative lifespan in WI-38 human fibroblasts by dexamethasone treatment is accompanied by suppression of p21Waf1/Cip1/Sdi1 levels. Exp. Cell Res. 2003, 285, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Iemitsu, M. The Role of Dehydroepiandrosterone (DHEA) in Skeletal Muscle. Vitam. Horm. 2018, 108, 205–221. [Google Scholar] [CrossRef] [PubMed]
- Majidian, M.; Kolli, H.; Moy, R.L. Management of skin thinning and aging: Review of therapies for neocollagenesis; hormones and energy devices. Int. J. Dermatol. 2021, 60, 1481–1487. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Wright, L.S.; Marwah, P.; Lardy, H.A.; Svendsen, C.N. Mitotic and neurogenic effects of dehydroepiandrosterone (DHEA) on human neural stem cell cultures derived from the fetal cortex. Proc. Natl. Acad. Sci. USA 2004, 101, 3202–3207. [Google Scholar] [CrossRef]
- Samaras, N.; Samaras, D.; Frangos, E.; Forster, A.; Philippe, J. A review of age-related dehydroepiandrosterone decline and its association with well-known geriatric syndromes: Is treatment beneficial? Rejuvenation Res. 2013, 16, 285–294. [Google Scholar] [CrossRef]
- Buford, T.W.; Willoughby, D.S. Impact of DHEA(S) and cortisol on immune function in ag-ing: A brief review. Appl. Physiol. Nutr. Metab. 2008, 33, 429–433. [Google Scholar] [CrossRef] [PubMed]
- Fedotcheva, T.A.; Shimanovskii, N.L.; Senderovich, A.I.; Chermnykh, N.S.; Semeikin, A.V.; Rzheznikov, V.M.; Golubovskaya, L.E.; Grinenko, G.S.; Banin, V.V.; Sergeev, P.V. Comparative analysis of the effect of gestagens, antiestrogencytostatics, and androstenes on the viability of tumor and normal cells. Pharm. Chem. J. 2007, 41, 345–349. [Google Scholar] [CrossRef]
- Fedotcheva, T.A.; Fedotcheva, N.I.; Shimanovsky, N.L. Progesterone as an anti-inflammatory drug and immunomodulator: New aspects in hormonal regulation of the inflammation. Biomolecules 2022, 12, 1299. [Google Scholar] [CrossRef] [PubMed]
- Pavlik, T.I.; Shimanovsky, N.L.; Zemlyanaya, O.A.; Fedotcheva, T.A. The effect of progestins on cytokine production in the peripheral blood mononuclear cells of menopausal women and their luminol-dependent chemiluminescence. Molecules 2023, 28, 4354. [Google Scholar] [CrossRef] [PubMed]
- Yaqoob, S.; Nasim, N.; Khanam, R.; Wang, Y.; Jabeen, A.; Qureshi, U.; UlHaq, Z.; ElSeedi, H.R.; Jiang, Z.H.; Khan, F.A.; et al. Synthesis of highly potent antiInflammatory compounds (ROS inhibitors). Molecules 2021, 26, 1272. [Google Scholar] [CrossRef]
- El Newahie, A.M.S.; Nissan, Y.M.; Ismail, N.S.M.; Abou El Ella, D.A.; Khojah, S.M.; Abouzid, K.A.M. Design and synthesis of new quinoxaline derivatives as anticancer agents and apoptotic inducers. Molecules 2019, 24, 1175. [Google Scholar] [CrossRef] [PubMed]
- 5-Androstene 3B, 17B Diol for Treatment. US patent №US005641768A, 24 June 1997.
- Maggiolini, M.; Donzé, O.; Jeannin, E.; Andò, S.; Picard, D. Adrenal androgens stimulate the proliferation of breast cancer cells as direct activators of estrogen receptor alpha. Cancer Res. 1999, 59, 4864–4869. [Google Scholar] [PubMed]
- Boccuzzi, G.; Brignardello, E.; di Monaco, M.; Forte, C.; Leonardi, L.; Pizzini, A. Influence of dehydroepiandrosterone and 5-en-androstene-3 beta, 17 beta-diol on the growth of MCF-7 human breast cancer cells induced by 17 beta-estradiol. Anticancer Res. 1992, 12, 799–803. [Google Scholar]
- Toth-Fejel, S.; Cheek, J.; Calhoun, K.; Muller, P.; Pommier, R.F. Estrogen and androgen receptors as comediators of breast cancer cell proliferation: Providing a new therapeutic tool. Arch. Surg. 2004, 139, 50–54. [Google Scholar] [CrossRef]
- Ortega-Calderón, Y.N.; López-Marure, R. Dehydroepiandrosterone inhibits proliferation and suppresses migration of human cervical cancer cell lines. Anticancer Res. 2014, 34, 4039–4044. [Google Scholar]
- Girón, R.A.; Montaño, L.F.; Escobar, M.L.; López-Marure, R. Dehydroepiandrosterone inhibits the proliferation and induces the death of HPV-positive and HPV-negative cervical cancer cells through an androgen- and estrogen-receptor independent mechanism. FEBS J. 2009, 276, 5598–5609. [Google Scholar] [CrossRef] [PubMed]
- Dworkin, C.R.; Gorman, S.D.; Pashko, L.L.; Cristofalo, V.J.; Schwartz, A.G. Inhibition of growth of HeLa and WI-38 cells by dehydroepiandrosterone and its reversal by ribo- and deoxyribonucleosides. Life Sci. 1986, 38, 1451–1457. [Google Scholar] [CrossRef] [PubMed]
- Kondo, H.; Kasuga, H.; Noumura, T. Effects of various steroids on in vitro lifespan and cell growth of human fetal lung fibroblasts (WI-38). Mech. Ageing Dev. 1983, 21, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Frantz, M.C.; Prix, N.J.; Wichmann, M.W.; van den Engel, N.K.; Hernandez-Richter, T.; Faist, E.; Chaudry, I.H.; Jauch, K.W.; Angele, M.K. Dehydroepiandrosterone restores depressed peripheral blood mononuclear cell function following major abdominal surgery via the estrogen receptors. Crit. Care Med. 2005, 33, 1779–1786. [Google Scholar] [CrossRef] [PubMed]
- Ofner, P.; Vena, R.L.; Barowsky, N.J.; Singer, R.M.; Tashjian, A.H. Comparative C19-radiosteroid metabolism by MA 160 and HeLa cell lines. In Vitro 1977, 13, 378–388. [Google Scholar] [CrossRef] [PubMed]
- Vosooghi, M.; Yahyavi, H.; Divsalar, K.; Shamsa, H.; Kheirollahi, A.; Safavi, M.; Ardestani, S.K.; Sadeghi-Neshat, S.; Mohammadhosseini, N.; Edraki, N.; et al. Synthesis and in vitro cytotoxic activity evaluation of (E)-16-(substituted benzylidene) derivatives of dehydroepiandrosterone. DARU J. Pharm. Sci. 2013, 21, 34. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Iruthayanathan, M.; Homan, L.L.; Wang, Y.; Yang, L.; Wang, Y.; Dillon, J.S. Dehydroepiandrosterone stimulates endothelial proliferation and angiogenesis through extracellular signal-regulated kinase 1/2-mediated mechanisms. Endocrinology 2008, 149, 889–898. [Google Scholar] [CrossRef]
- Gaši, K.M.P.; Brenesel, M.D.D.; Djurendić, E.A.; Sakač, M.N.; Čanadi, J.J.; Daljev, J.J.; Armbruster, T.; Andrić, S.; Sladić, D.M.; Božić, T.T.; et al. Synthesis and biological evaluation of some 17-picolyl and 17-picolinylidene androst-5-ene derivatives. Steroids 2007, 72, 31–40. [Google Scholar] [CrossRef]
- Loria, R.M.; Padgett, D.A. Androstenediol regulates systemic resistance against lethal infections in mice. Arch. Virol. 1992, 127, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Stepanova, E.S.; Makarenkova, L.M.; Chistyakov, V.V.; Fedotcheva, T.A.; Parshin, V.A.; Shimanovsky, N.L. Metabolism of Gestobutanoil, a Novel Drug of Progestin Group. Sovrem. Tehnol. Med. 2019, 11, 48. [Google Scholar] [CrossRef]
- Pillerová, M.; Borbélyová, V.; Hodosy, J.; Riljak, V.; Renczés, E.; Frick, K.M.; Tóthová, Ľ. On the role of sex steroids in biological functions by classical and non-classical pathways. An update. Front. Neuroendocrinol. 2021, 62, 100926. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Kumar, B.S.; Negi, A.S. Current status on development of steroids as anticancer agents. J. Steroid Biochem. Mol. Biol. 2013, 137, 242–270. [Google Scholar] [CrossRef]
- Savić, M.P.; Ajduković, J.J.; Plavša, J.J.; Bekić, S.S.; Ćelić, A.S.; Klisurić, O.R.; Jakimov, D.S.; Petri, E.T.; Djurendić, E.A. Evaluation of A-ring fused pyridine d-modified androstane derivatives for antiproliferative and aldo–keto reductase 1C3 inhibitory activity. Medchemcomm 2018, 9, 969–981. [Google Scholar] [CrossRef]



| Compound | HeLa, 24 h | HeLa, 5 d | K562, 24 h | Wi-38, 24 h | PMBC, 24 h |
|---|---|---|---|---|---|
| DHEA (1) | 82 ± 3 | 40 ± 9 | 70 ± 21 | 144 ± 10 | 96 ± 7 |
| 5-AED (2) | 107 ± 13 | 28 ± 7 | 82 ± 5.6 | 94 ± 5.5 | 98 ± 17 |
| DHEA 3-acetate (3a) | 96 ± 17 | 27 ± 1.5 | 50 ± 20 | 143 ± 8 | 98 ± 6 |
| DHEA 3-propionate (3b) | 69 ± 2 | 32 ± 6 | 55 ± 22 | 147 ± 9 | 100 ± 5 |
| DHEA 3-butanoate (3c) | 86 ± 4 | 46 ± 7 | 56 ± 15 | 152 ± 4 | 104 ± 20 |
| DHEA 3-Methylsulfonate (3d) | 92 ± 19 | 32 ± 6 | 55 ± 31 | 122 ± 7 | 98 ± 7 |
| 5-AED 3,17-Dipropionate (4b) | 77 ± 5 | 30 ± 6 | 50 ± 7 | 129 ± 13 | 102 ± 13 |
| 5-AED 3,17-Dibutanoate (4c) | 83 ± 10 | 30 ± 5 | 55 ± 19 | 145 ± 10 | 93 ± 20 |
| 5-AED 3-Butanoate (5) | 92 ± 12 | 23 ± 2 | 96 ± 3 | 100 ± 2 | 97 ± 15 |
| Cell Line | IC50 and Other Parameters of DHEA Related to Cell Viability | Reference |
|---|---|---|
| HeLa | 50 μM | [57] |
| HeLa | 15% inhibition of growth at 100 μM, 48 h | [1] |
| T47D | 2.55 μM, 96h | [42] |
| MCF-7/WT | DHEA at 0.1 μM stimulated MCF-7 (48–144 h) by increasing the ER-alpha transcriptional activity | [58] |
| MCF-7 | DHEA at 500 nM and AED at 2 nM stimulated the growth of MCF-7 cells in a steroid-free medium but partly antagonized the stimulatory effect of 1 nM estrogen. The latter was also shown at lower DHEA concentrations (20 nM, 100 nM). | [59] |
| T47D ER-positive–AR-positive | DHEA-S induced the growth of 43.4% of cells, 120h; DHEA-S induced mitogen-activated protein kinase by 5.4-fold within a few minutes. | [60] |
| ER-negative and AR-positive HCC1937 | DHEA-S inhibited cell growth by 22%, 120 h. | [60] |
| HeLa | IC50 70 μM, 24 h | [61] |
| HeLa | IC50 70 μM, 48 h | [62] |
| HeLa | DHEA, 100 μM for 72 h totally blocked proliferation, and 10 μM blocked proliferation after 144 h; 10 μM is the IC50 for G6PD | [63] |
| Wi-38 | In the first 7 days, there was no effect; 137 µM DHEA reduced the number of cells by 65%, and 18 µM by 19%, and lower concentrations had no effect during incubation for 7–16 days. | [64] |
| Wi-38 | Only a 100 μM concentration of DHEA, and only for 3 days, had an inhibitory effect. Inhibition could be largely overcome by the addition of a combination of four deoxy- and four ribonucleosides to the culture medium. The inhibition of G6PDH by DHEA may be responsible for its anti-mitotic effect. | [63] |
| PBMCs | DHEA alone did not affect monocyte function; in combination with LPS, DHEA led to the significant activation of cultured monocytes. DHEA significantly increased the release capacities for IL-1 and TNF-α in vitro. DHEA increased the immune response. | [65] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sokolov, M.N.; Rozhkov, V.V.; Uspenskaya, M.E.; Ulchenko, D.N.; Shmygarev, V.I.; Trukhan, V.M.; Churakov, A.V.; Shimanovsky, N.L.; Fedotcheva, T.A. The Effects of the Steroids 5-Androstenediol and Dehydroepiandrosterone and Their Synthetic Derivatives on the Viability of K562, HeLa, and Wi-38 Cells and the Luminol-Stimulated Chemiluminescence of Peripheral Blood Mononuclear Cells from Healthy Volunteers. Biomolecules 2024, 14, 373. https://doi.org/10.3390/biom14030373
Sokolov MN, Rozhkov VV, Uspenskaya ME, Ulchenko DN, Shmygarev VI, Trukhan VM, Churakov AV, Shimanovsky NL, Fedotcheva TA. The Effects of the Steroids 5-Androstenediol and Dehydroepiandrosterone and Their Synthetic Derivatives on the Viability of K562, HeLa, and Wi-38 Cells and the Luminol-Stimulated Chemiluminescence of Peripheral Blood Mononuclear Cells from Healthy Volunteers. Biomolecules. 2024; 14(3):373. https://doi.org/10.3390/biom14030373
Chicago/Turabian StyleSokolov, Mikhail N., Vladimir V. Rozhkov, Maria E. Uspenskaya, Darya N. Ulchenko, Vladimir I. Shmygarev, Vladimir M. Trukhan, Andrei V. Churakov, Nikolay L. Shimanovsky, and Tatiana A. Fedotcheva. 2024. "The Effects of the Steroids 5-Androstenediol and Dehydroepiandrosterone and Their Synthetic Derivatives on the Viability of K562, HeLa, and Wi-38 Cells and the Luminol-Stimulated Chemiluminescence of Peripheral Blood Mononuclear Cells from Healthy Volunteers" Biomolecules 14, no. 3: 373. https://doi.org/10.3390/biom14030373
APA StyleSokolov, M. N., Rozhkov, V. V., Uspenskaya, M. E., Ulchenko, D. N., Shmygarev, V. I., Trukhan, V. M., Churakov, A. V., Shimanovsky, N. L., & Fedotcheva, T. A. (2024). The Effects of the Steroids 5-Androstenediol and Dehydroepiandrosterone and Their Synthetic Derivatives on the Viability of K562, HeLa, and Wi-38 Cells and the Luminol-Stimulated Chemiluminescence of Peripheral Blood Mononuclear Cells from Healthy Volunteers. Biomolecules, 14(3), 373. https://doi.org/10.3390/biom14030373









