Heterogeneity and Differentiation of the Human Arterial Tree: Focus on microRNA Expression in Vascular Disease
Abstract
1. Introduction
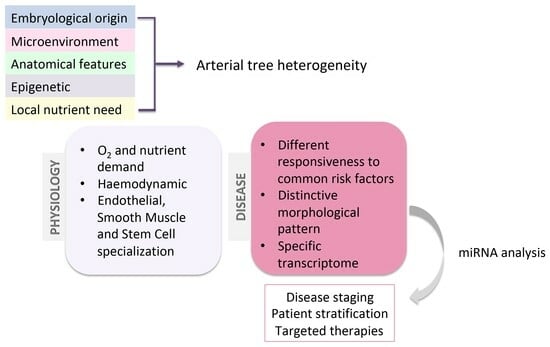
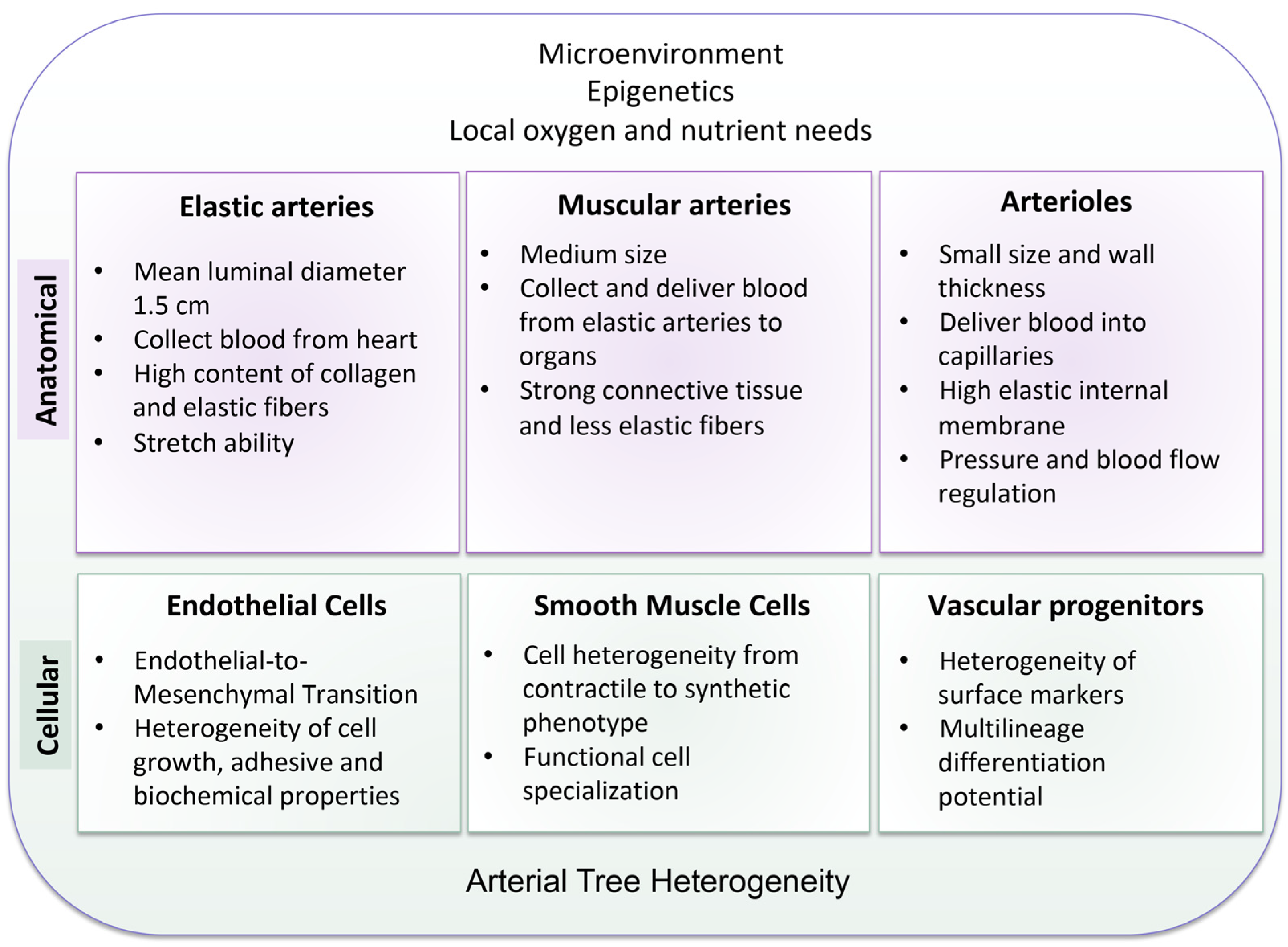
2. The Heterogeneity of the Arterial Tree: Structural and Cellular Basis
3. The Arterial Heterogeneity in Atherosclerosis
4. microRNAs (miRNAs)
4.1. miRNAs as Endogenous Regulators of Vascular Biology and Vascular Heterogeneity
4.2. miRNAs and Vascular Heterogeneity: Focus on Femoral Artery and Abdominal Aorta
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tucker, W.D.; Arora, Y.; Mahajan, K. Anatomy, Blood Vessels. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Fleischer, J.R.; Jodszuweit, C.A.; Ghadimi, M.; De Oliveira, T.; Conradi, L.-C. Vascular Heterogeneity With a Special Focus on the Hepatic Microenvironment. Front. Physiol. 2020, 11, 591901. [Google Scholar] [CrossRef] [PubMed]
- Aird, W.C. Endothelial Cell Heterogeneity and Atherosclerosis. Curr. Atheroscler. Rep. 2006, 8, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Aird, W.C. Phenotypic Heterogeneity of the Endothelium. Circ. Res. 2007, 100, 158–173. [Google Scholar] [CrossRef] [PubMed]
- Aird, W.C. Endothelial Cell Heterogeneity. Cold Spring Harb. Perspect. Med. 2012, 2, a006429. [Google Scholar] [CrossRef] [PubMed]
- Potente, M.; Mäkinen, T. Vascular Heterogeneity and Specialization in Development and Disease. Nat. Rev. Mol. Cell Biol. 2017, 18, 477–494. [Google Scholar] [CrossRef] [PubMed]
- Chi, J.-T.; Chang, H.Y.; Haraldsen, G.; Jahnsen, F.L.; Troyanskaya, O.G.; Chang, D.S.; Wang, Z.; Rockson, S.G.; van de Rijn, M.; Botstein, D.; et al. Endothelial Cell Diversity Revealed by Global Expression Profiling. Proc. Natl. Acad. Sci. USA 2003, 100, 10623–10628. [Google Scholar] [CrossRef]
- Sengoelge, G.; Luo, W.; Fine, D.; Perschl, A.M.; Fierlbeck, W.; Haririan, A.; Sorensson, J.; Rehman, T.-U.; Hauser, P.; Trevick, J.S.; et al. A SAGE-Based Comparison between Glomerular and Aortic Endothelial Cells. Am. J. Physiol.-Ren. Physiol. 2005, 288, F1290–F1300. [Google Scholar] [CrossRef][Green Version]
- Zhang, J.; Burridge, K.A.; Friedman, M.H. In Vivo Differences between Endothelial Transcriptional Profiles of Coronary and Iliac Arteries Revealed by Microarray Analysis. Am. J. Physiol. Heart Circ. Physiol. 2008, 295, H1556–H1561. [Google Scholar] [CrossRef][Green Version]
- Simmons, G.H.; Padilla, J.; Laughlin, M.H. Heterogeneity of Endothelial Cell Phenotype within and amongst Conduit Vessels of the Swine Vasculature. Exp. Physiol. 2012, 97, 1074–1082. [Google Scholar] [CrossRef]
- Thorin, E.; Shatos, M.A.; Shreeve, S.M.; Walters, C.L.; Bevan, J.A. Human Vascular Endothelium Heterogeneity. Stroke 1997, 28, 375–381. [Google Scholar] [CrossRef]
- Rensen, S.S.M.; Doevendans, P.A.F.M.; van Eys, G.J.J.M. Regulation and Characteristics of Vascular Smooth Muscle Cell Phenotypic Diversity. Neth. Heart J. 2007, 15, 100–108. [Google Scholar] [CrossRef]
- Wall, V.Z.; Bornfeldt, K.E. Arterial Smooth Muscle. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 2175–2179. [Google Scholar] [CrossRef]
- Majesky, M.W. Developmental Basis of Vascular Smooth Muscle Diversity. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 1248–1258. [Google Scholar] [CrossRef]
- Ko, Y.-S.; Yeh, H.-I.; Haw, M.; Dupont, E.; Kaba, R.; Plenz, G.; Robenek, H.; Severs, N.J. Differential Expression of Connexin43 and Desmin Defines Two Subpopulations of Medial Smooth Muscle Cells in the Human Internal Mammary Artery. Arterioscler. Thromb. Vasc. Biol. 1999, 19, 1669–1680. [Google Scholar] [CrossRef][Green Version]
- Li, S.; Fan, Y.-S.; Chow, L.H.; Van Den Diepstraten, C.; van der Veer, E.; Sims, S.M.; Pickering, J.G. Innate Diversity of Adult Human Arterial Smooth Muscle Cells. Circ. Res. 2001, 89, 517–525. [Google Scholar] [CrossRef]
- Ciavarella, C.; Valente, S.; Pasquinelli, G. The Characteristics and Survival Potential Under Sub-Lethal Stress of Mesenchymal Stromal/Stem Cells Isolated from the Human Vascular Wall. Stem Cells 2022, 40, 1071–1077. [Google Scholar] [CrossRef]
- Tang, Z.; Wang, A.; Yuan, F.; Yan, Z.; Liu, B.; Chu, J.S.; Helms, J.A.; Li, S. Differentiation of Multipotent Vascular Stem Cells Contributes to Vascular Diseases. Nat. Commun. 2012, 3, 875. [Google Scholar] [CrossRef]
- Pasquinelli, G.; Pacilli, A.; Alviano, F.; Foroni, L.; Ricci, F.; Valente, S.; Orrico, C.; Lanzoni, G.; Buzzi, M.; Luigi Tazzari, P.; et al. Multidistrict Human Mesenchymal Vascular Cells: Pluripotency and Stemness Characteristics. Cytotherapy 2010, 12, 275–287. [Google Scholar] [CrossRef]
- Pasanisi, E.; Ciavarella, C.; Valente, S.; Ricci, F.; Pasquinelli, G. Differentiation and Plasticity of Human Vascular Wall Mesenchymal Stem Cells, Dermal Fibroblasts and Myofibroblasts: A Critical Comparison Including Ultrastructural Evaluation of Osteogenic Potential. Ultrastruct. Pathol. 2019, 43, 261–272. [Google Scholar] [CrossRef]
- Sabrina, V.; Gianandrea, P. Phenotypic and Functional Mapping of Mesenchymal Stem Cells Harvested from Different Portions of the Human Arterial Tree. In Mesenchymal Stem Cells—Isolation, Characterization and Applications; IntechOpen: London, UK, 2017; ISBN 978-953-51-3616-3. [Google Scholar]
- Lahoz, C.; Mostaza, J.M. Atherosclerosis As a Systemic Disease. Rev. Esp. Cardiol. 2007, 60, 184–195. [Google Scholar] [CrossRef]
- Stary, H.C.; Chandler, A.B.; Dinsmore, R.E.; Fuster, V.; Glagov, S.; Insull, W., Jr.; Rosenfeld, M.E.; Schwartz, C.J.; Wagner, W.D.; Wissler, R.W. A Definition of Advanced Types of Atherosclerotic Lesions and a Histological Classification of Atherosclerosis. Circulation 1995, 92, 1355–1374. [Google Scholar] [CrossRef]
- Herisson, F.; Heymann, M.-F.; Chétiveaux, M.; Charrier, C.; Battaglia, S.; Pilet, P.; Rouillon, T.; Krempf, M.; Lemarchand, P.; Heymann, D.; et al. Carotid and Femoral Atherosclerotic Plaques Show Different Morphology. Atherosclerosis 2011, 216, 348–354. [Google Scholar] [CrossRef]
- Artery-Related Differences in Atherosclerosis Expression|Stroke. Available online: https://www.ahajournals.org/doi/10.1161/STROKEAHA.107.486480 (accessed on 5 January 2023).
- Sigala, F.; Oikonomou, E.; Antonopoulos, A.S.; Galyfos, G.; Tousoulis, D. Coronary versus Carotid Artery Plaques. Similarities Differ. Regarding Biomark. Morphol. Prognosis. Curr. Opin. Pharmacol. 2018, 39, 9–18. [Google Scholar] [CrossRef]
- Espitia, O.; Chatelais, M.; Steenman, M.; Charrier, C.; Maurel, B.; Georges, S.; Houlgatte, R.; Verrecchia, F.; Ory, B.; Lamoureux, F.; et al. Implication of Molecular Vascular Smooth Muscle Cell Heterogeneity among Arterial Beds in Arterial Calcification. PLoS ONE 2018, 13, e0191976. [Google Scholar] [CrossRef]
- Aavik, E.; Babu, M.; Ylä-Herttuala, S. DNA Methylation Processes in Atherosclerotic Plaque. Atherosclerosis 2019, 281, 168–179. [Google Scholar] [CrossRef]
- Lee, R.C.; Feinbaum, R.L.; Ambros, V. The C. Elegans Heterochronic Gene Lin-4 Encodes Small RNAs with Antisense Complementarity to Lin-14. Cell 1993, 75, 843–854. [Google Scholar] [CrossRef]
- Singh, M.; Stewart, R.; White, H. Importance of Frailty in Patients with Cardiovascular Disease. Eur. Heart J. 2014, 35, 1726–1731. [Google Scholar] [CrossRef]
- Dato, S.; Crocco, P.; Iannone, F.; Passarino, G.; Rose, G. Biomarkers of Frailty: miRNAs as Common Signatures of Impairment in Cognitive and Physical Domains. Biology 2022, 11, 1151. [Google Scholar] [CrossRef]
- Small, E.M.; Olson, E.N. Pervasive Roles of microRNAs in Cardiovascular Biology. Nature 2011, 469, 336–342. [Google Scholar] [CrossRef]
- Stavast, C.J.; Erkeland, S.J. The Non-Canonical Aspects of MicroRNAs: Many Roads to Gene Regulation. Cells 2019, 8, 1465. [Google Scholar] [CrossRef]
- Annese, T.; Tamma, R.; De Giorgis, M.; Ribatti, D. microRNAs Biogenesis, Functions and Role in Tumor Angiogenesis. Front. Oncol. 2020, 10, 581007. [Google Scholar] [CrossRef]
- Wang, D.; Atanasov, A.G. The microRNAs Regulating Vascular Smooth Muscle Cell Proliferation: A Minireview. Int. J. Mol. Sci. 2019, 20, 324. [Google Scholar] [CrossRef]
- Kim, D.Y.; Sung, J.H. Regulatory Role of microRNAs in the Proliferation and Differentiation of Adipose-Derived Stem Cells. Histol. Histopathol. 2016, 32, 1–10. [Google Scholar] [CrossRef]
- miR-21 and miR-146a: The microRNAs of Inflammaging and Age-Related Diseases—ScienceDirect. Available online: https://www.sciencedirect.com/science/article/pii/S1568163721001215?via%3Dihub (accessed on 6 September 2023).
- Mahesh, G.; Biswas, R. MicroRNA-155: A Master Regulator of Inflammation. J. Interferon Cytokine Res. 2019, 39, 321–330. [Google Scholar] [CrossRef]
- microRNAs at the Synapse|Nature Reviews Neuroscience. Available online: https://www.nature.com/articles/nrn2763 (accessed on 6 September 2023).
- Zheng, K.; Li, H.; Huang, H.; Qiu, M. microRNAs and Glial Cell Development. Neuroscientist 2012, 18, 114–118. [Google Scholar] [CrossRef]
- Zingale, V.D.; Gugliandolo, A.; Mazzon, E. MiR-155: An Important Regulator of Neuroinflammation. Int. J. Mol. Sci. 2021, 23, 90. [Google Scholar] [CrossRef]
- Lee, Y.S.; Dutta, A. MicroRNAs in Cancer. Annu. Rev. Pathol. Mech. Dis. 2009, 4, 199–227. [Google Scholar] [CrossRef]
- Tang, X.; Tang, G.; Özcan, S. Role of MicroRNAs in Diabetes. Biochim. Biophys. Acta 2008, 1779, 697–701. [Google Scholar] [CrossRef]
- Fish, J.E.; Santoro, M.M.; Morton, S.U.; Yu, S.; Yeh, R.-F.; Wythe, J.D.; Bruneau, B.G.; Stainier, D.Y.R.; Srivastava, D. miR-126 Regulates Angiogenic Signaling and Vascular Integrity. Dev. Cell 2008, 15, 272–284. [Google Scholar] [CrossRef]
- Zhou, Q.; Gallagher, R.; Ufret-Vincenty, R.; Li, X.; Olson, E.N.; Wang, S. Regulation of Angiogenesis and Choroidal Neovascularization by Members of microRNA-23∼27∼24 Clusters. Proc. Natl. Acad. Sci. USA 2011, 108, 8287–8292. [Google Scholar] [CrossRef]
- Urbich, C.; Kaluza, D.; Frömel, T.; Knau, A.; Bennewitz, K.; Boon, R.A.; Bonauer, A.; Doebele, C.; Boeckel, J.-N.; Hergenreider, E.; et al. MicroRNA-27a/b Controls Endothelial Cell Repulsion and Angiogenesis by Targeting Semaphorin 6A. Blood 2012, 119, 1607–1616. [Google Scholar] [CrossRef]
- Lou, Y.-L.; Guo, F.; Liu, F.; Gao, F.-L.; Zhang, P.-Q.; Niu, X.; Guo, S.-C.; Yin, J.-H.; Wang, Y.; Deng, Z.-F. miR-210 Activates Notch Signaling Pathway in Angiogenesis Induced by Cerebral Ischemia. Mol. Cell Biochem. 2012, 370, 45–51. [Google Scholar] [CrossRef]
- Xu, F.; Ahmed, A.S.I.; Kang, X.; Hu, G.; Liu, F.; Zhang, W.; Zhou, J. MicroRNA-15b/16 Attenuates Vascular Neointima Formation by Promoting the Contractile Phenotype of Vascular Smooth Muscle through Targeting YAP. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 2145–2152. [Google Scholar] [CrossRef]
- Liu, X.; Cheng, Y.; Chen, X.; Yang, J.; Xu, L.; Zhang, C. MicroRNA-31 Regulated by the Extracellular Regulated Kinase Is Involved in Vascular Smooth Muscle Cell Growth via Large Tumor Suppressor Homolog 2. J. Biol. Chem. 2011, 286, 42371–42380. [Google Scholar] [CrossRef]
- Dong, S.; Xiong, W.; Yuan, J.; Li, J.; Liu, J.; Xu, X. MiRNA-146a Regulates the Maturation and Differentiation of Vascular Smooth Muscle Cells by Targeting NF-κB Expression. Mol. Med. Rep. 2013, 8, 407–412. [Google Scholar] [CrossRef]
- Vartak, T.; Kumaresan, S.; Brennan, E. Decoding microRNA Drivers in Atherosclerosis. Biosci. Rep. 2022, 42, BSR20212355. [Google Scholar] [CrossRef]
- Parahuleva, M.S.; Lipps, C.; Parviz, B.; Hölschermann, H.; Schieffer, B.; Schulz, R.; Euler, G. MicroRNA Expression Profile of Human Advanced Coronary Atherosclerotic Plaques. Sci. Rep. 2018, 8, 7823. [Google Scholar] [CrossRef]
- Zhou, Z.; Chen, Y.; Zhang, D.; Wu, S.; Liu, T.; Cai, G.; Qin, S. MicroRNA-30-3p Suppresses Inflammatory Factor-Induced Endothelial Cell Injury by Targeting TCF21. Mediat. Inflamm. 2019, 2019, 1342190. [Google Scholar] [CrossRef]
- Vasuri, F.; Ciavarella, C.; Fittipaldi, S.; Pini, R.; Vacirca, A.; Gargiulo, M.; Faggioli, G.; Pasquinelli, G. Different Histological Types of Active Intraplaque Calcification Underlie Alternative miRNA-mRNA Axes in Carotid Atherosclerotic Disease. Virchows Arch. 2019, 476, 307–316. [Google Scholar] [CrossRef]
- Ciavarella, C.; Motta, I.; Vasuri, F.; Fittipaldi, S.; Valente, S.; Pollutri, D.; Ricci, F.; Gargiulo, M.; Pasquinelli, G. Involvement of miR-30a-5p and miR-30d in Endothelial to Mesenchymal Transition and Early Osteogenic Commitment under Inflammatory Stress in HUVEC. Biomolecules 2021, 11, 226. [Google Scholar] [CrossRef]
- Han, Y.; Zhang, J.; Huang, S.; Cheng, N.; Zhang, C.; Li, Y.; Wang, X.; Liu, J.; You, B.; Du, J. MicroRNA-223-3p Inhibits Vascular Calcification and the Osteogenic Switch of Vascular Smooth Muscle Cells. J. Biol. Chem. 2021, 296, 100483. [Google Scholar] [CrossRef]
- Li, Z.; Zhao, Y.; Suguro, S.; Suguro, R. MicroRNAs Regulate Function in Atherosclerosis and Clinical Implications. Oxid. Med. Cell Longev. 2023, 2023, 2561509. [Google Scholar] [CrossRef]
- Verma, S.; Kumar, A.; Narang, R.; Bisoi, A.K.; Mitra, D.K. Signature Transcriptome Analysis of Stage Specific Atherosclerotic Plaques of Patients. BMC Med. Genom. 2022, 15, 99. [Google Scholar] [CrossRef]
- Sun, B.; Shan, Z.; Sun, G.; Wang, X. Micro-RNA-183-5p Acts as a Potential Diagnostic Biomarker for Atherosclerosis and Regulates the Growth of Vascular Smooth Muscle Cell. J. Chin. Med. Assoc. 2021, 84, 33. [Google Scholar] [CrossRef]
- Li, Z.; Xu, C.; Sun, D. MicroRNA-488 Serves as a Diagnostic Marker for Atherosclerosis and Regulates the Biological Behavior of Vascular Smooth Muscle Cells. Bioengineered 2021, 12, 4092–4099. [Google Scholar] [CrossRef]
- Chong, H.; Wei, Z.; Na, M.; Sun, G.; Zheng, S.; Zhu, X.; Xue, Y.; Zhou, Q.; Guo, S.; Xu, J.; et al. The PGC-1α/NRF1/miR-378a Axis Protects Vascular Smooth Muscle Cells from FFA-Induced Proliferation, Migration and Inflammation in Atherosclerosis. Atherosclerosis 2020, 297, 136–145. [Google Scholar] [CrossRef]
- de Yébenes, V.G.; Briones, A.M.; Martos-Folgado, I.; Mur, S.M.; Oller, J.; Bilal, F.; González-Amor, M.; Méndez-Barbero, N.; Silla-Castro, J.C.; Were, F.; et al. Aging-Associated miR-217 Aggravates Atherosclerosis and Promotes Cardiovascular Dysfunction. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 2408. [Google Scholar] [CrossRef]
- Lagos-Quintana, M.; Rauhut, R.; Yalcin, A.; Meyer, J.; Lendeckel, W.; Tuschl, T. Identification of Tissue-Specific MicroRNAs from Mouse. Curr. Biol. 2002, 12, 735–739. [Google Scholar] [CrossRef]
- Moreau, P.R.; Tomas Bosch, V.; Bouvy-Liivrand, M.; Õunap, K.; Örd, T.; Pulkkinen, H.H.; Pölönen, P.; Heinäniemi, M.; Ylä-Herttuala, S.; Laakkonen, J.P.; et al. Profiling of Primary and Mature miRNA Expression in Atherosclerosis-Associated Cell Types. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 2149–2167. [Google Scholar] [CrossRef]
- McCall, M.N.; Kent, O.A.; Yu, J.; Fox-Talbot, K.; Zaiman, A.L.; Halushka, M.K. MicroRNA Profiling of Diverse Endothelial Cell Types. BMC Med. Genom. 2011, 4, 78. [Google Scholar] [CrossRef]
- Pereira-da-Silva, T.; Coutinho Cruz, M.; Carrusca, C.; Cruz Ferreira, R.; Napoleão, P.; Mota Carmo, M. Circulating microRNA Profiles in Different Arterial Territories of Stable Atherosclerotic Disease: A Systematic Review. Am. J. Cardiovasc. Dis. 2018, 8, 1–13. [Google Scholar]
- Teixeira, A.R.; Ferreira, V.V.; Pereira-da-Silva, T.; Ferreira, R.C. The Role of miRNAs in the Diagnosis of Stable Atherosclerosis of Different Arterial Territories: A Critical Review. Front. Cardiovasc. Med. 2022, 9, 1040971. [Google Scholar] [CrossRef]
- Collura, S.; Ciavarella, C.; Morsiani, C.; Motta, I.; Valente, S.; Gallitto, E.; Abualhin, M.; Pini, R.; Vasuri, F.; Franceschi, C.; et al. MicroRNA Profiles of Human Peripheral Arteries and Abdominal Aorta in Normal Conditions: MicroRNAs-27a-5p, -139-5p and -155-5p Emerge and in Atheroma Too. Mech. Ageing Dev. 2021, 198, 111547. [Google Scholar] [CrossRef]
- Li, X.; Ballantyne, L.L.; Yu, Y.; Funk, C.D. Perivascular Adipose Tissue–Derived Extracellular Vesicle miR-221-3p Mediates Vascular Remodeling. FASEB J. 2019, 33, 12704–12722. [Google Scholar] [CrossRef]
- Collura, S.; Morsiani, C.; Vacirca, A.; Fronterrè, S.; Ciavarella, C.; Vasuri, F.; D’Errico, A.; Franceschi, C.; Pasquinelli, G.; Gargiulo, M.; et al. The Carotid Plaque as Paradigmatic Case of Site-Specific Acceleration of Aging Process: The microRNAs and the Inflammaging Contribution. Ageing Res. Rev. 2020, 61, 101090. [Google Scholar] [CrossRef]
- Salvioli, S.; Basile, M.S.; Bencivenga, L.; Carrino, S.; Conte, M.; Damanti, S.; De Lorenzo, R.; Fiorenzato, E.; Gialluisi, A.; Ingannato, A.; et al. Biomarkers of aging in frailty and age-associated disorders: State of the art and future perspective. Ageing Res. Rev. 2023, 91, 102044. [Google Scholar] [CrossRef]

| miRNA | Roles in the Vascular System |
|---|---|
| miR-126 | Regulation of angiogenic process and maintenance of vascular integrity by activating VEGF signaling [41] |
| miR-23, miR-27 | Promotion of angiogenic process by inhibition of SPROUTY2 and SEMA6A [42,43] |
| miR-210 | Induction of NOTCH1 pathway [44] |
| miR-15b/16 | Promotion of VSMC contractile phenotype [45] |
| miR-146, miR-31 | Control of proliferative and migratory abilities in VSMCs [46,47] |
| miR-30a-5p, miR-30d | Regulation of End-MT, osteogenic differentiation, and vascular calcification by targeting SLUG [51] |
| miR-223-3p | Inhibition of vascular calcification by targeting IL-6/STAT3 signaling [56] |
| miR-155-5p, miR-27a-5p | Increased expression in atherosclerotic plaque of femoral arteries [54] |
| miR-183-5p, miR-488 | Increase of proliferation and migration in VSMCs [59,60] |
| miR-378a | Inhibition of proliferation and migration in VSMCs [61] |
| miR-217 | Promotion of endothelial dysfunction [62] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciavarella, C.; Motta, I.; Capri, M.; Gargiulo, M.; Pasquinelli, G. Heterogeneity and Differentiation of the Human Arterial Tree: Focus on microRNA Expression in Vascular Disease. Biomolecules 2024, 14, 343. https://doi.org/10.3390/biom14030343
Ciavarella C, Motta I, Capri M, Gargiulo M, Pasquinelli G. Heterogeneity and Differentiation of the Human Arterial Tree: Focus on microRNA Expression in Vascular Disease. Biomolecules. 2024; 14(3):343. https://doi.org/10.3390/biom14030343
Chicago/Turabian StyleCiavarella, Carmen, Ilenia Motta, Miriam Capri, Mauro Gargiulo, and Gianandrea Pasquinelli. 2024. "Heterogeneity and Differentiation of the Human Arterial Tree: Focus on microRNA Expression in Vascular Disease" Biomolecules 14, no. 3: 343. https://doi.org/10.3390/biom14030343
APA StyleCiavarella, C., Motta, I., Capri, M., Gargiulo, M., & Pasquinelli, G. (2024). Heterogeneity and Differentiation of the Human Arterial Tree: Focus on microRNA Expression in Vascular Disease. Biomolecules, 14(3), 343. https://doi.org/10.3390/biom14030343