Menthol Pretreatment Alleviates Campylobacter jejuni-Induced Enterocolitis in Human Gut Microbiota-Associated IL-10−/− Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Mice, Microbiota Depletion
2.2. Human Fecal Microbiota Transplantation
2.3. Prophylactic Regimen
2.4. Campylobacter jejuni Infection
2.5. Summary of Mouse Cohorts
2.6. Measurement of Gastrointestinal C. jejuni Loads
2.7. Fecal Microbiota Composition
2.8. Clinical Conditions of Mice
2.9. Sampling Procedures
2.10. Histopathology
2.11. Quantitative In Situ Immunohistochemistry
2.12. Pro-Inflammatory Mediators
2.13. Statistics
3. Results
3.1. Clinical Conditions over Time following C. jejuni Infection of hma IL-10−/− Mice with Menthol Pretreatment
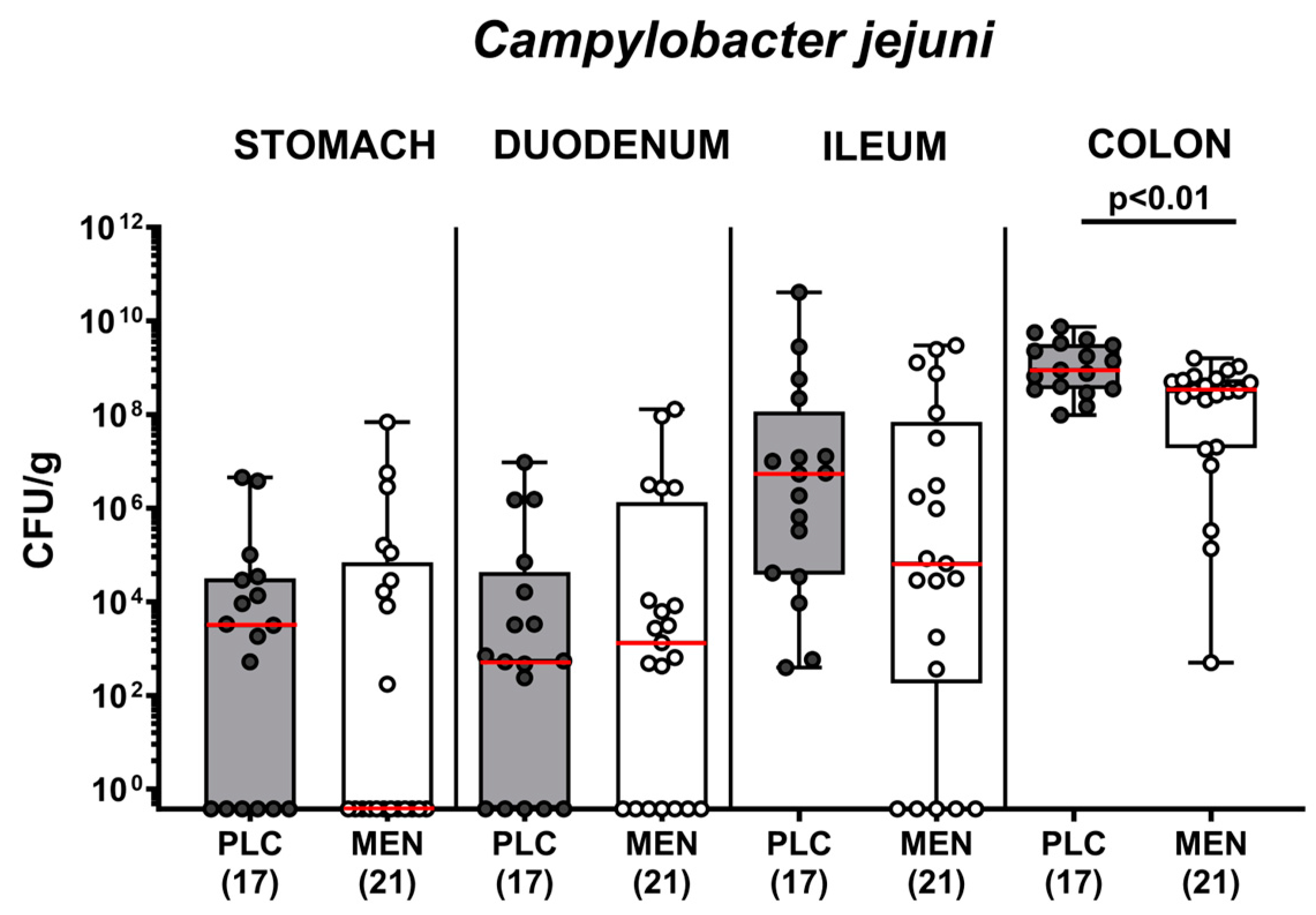
3.2. Pathogen Numbers in Gastrointestinal Luminal Samples from C. jejuni Infected hma IL-10−/− Mice with Menthol Pretreatment
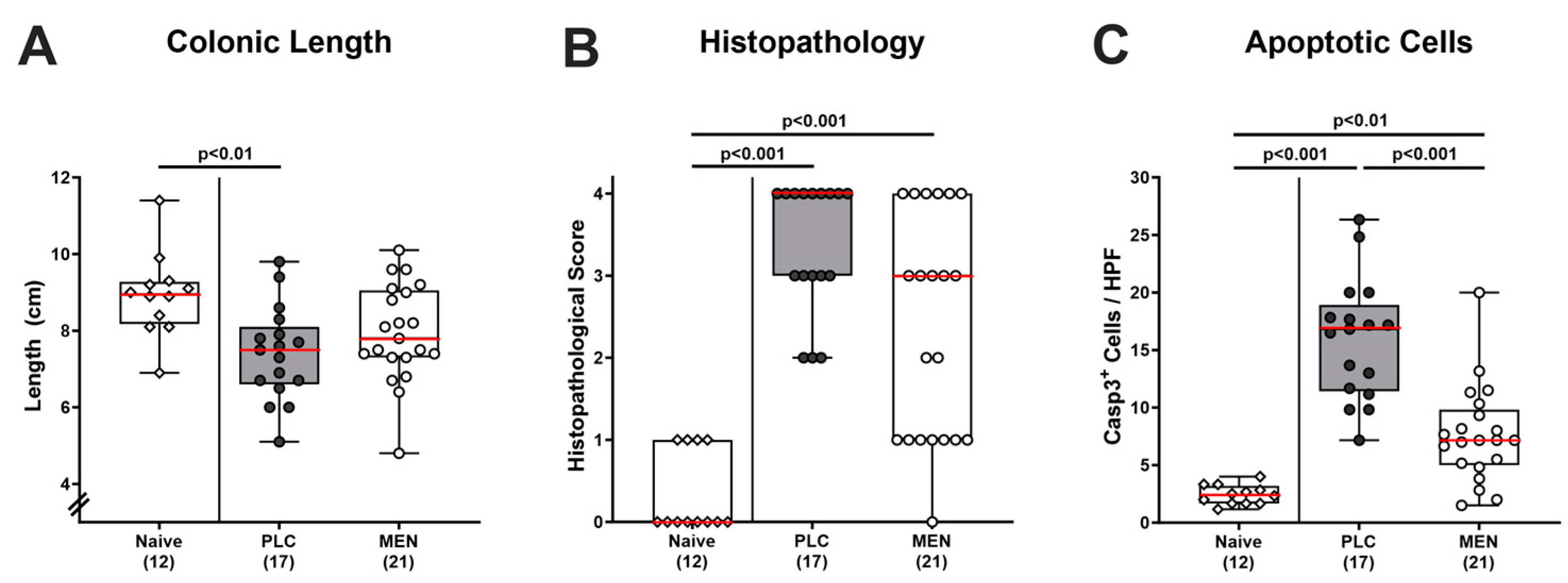
3.3. Inflammatory Changes in the Colon of C. jejuni Infected hma IL-10−/− Mice with Menthol Pretreatment
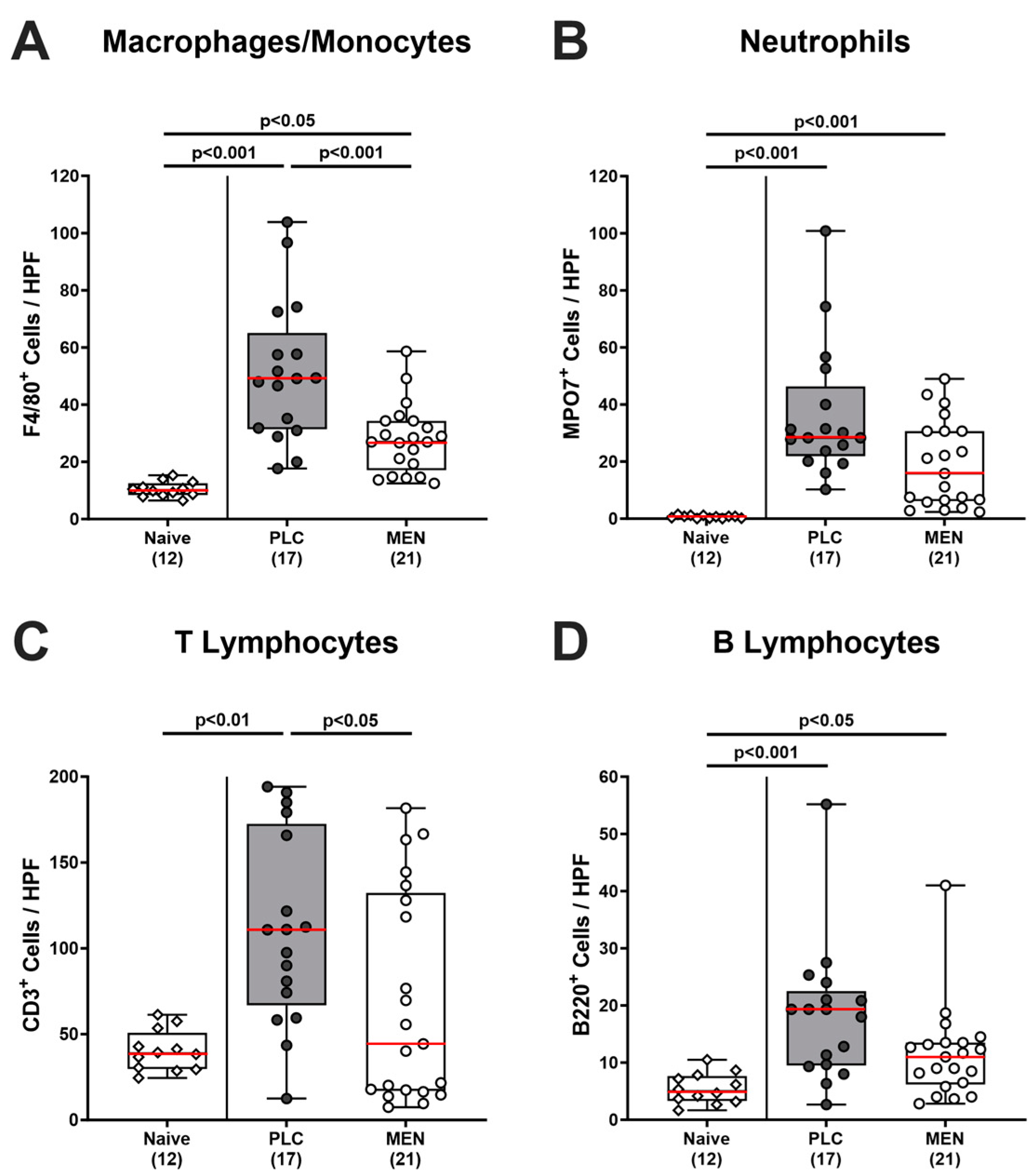
3.4. Colonic Immune Cells in C. jejuni Infected hma IL-10−/− Mice with Menthol Pretreatment
3.5. Intestinal Proinflammatory Mediator Secretion in C. jejuni Infected hma IL-10−/− Mice with Menthol Pretreatment
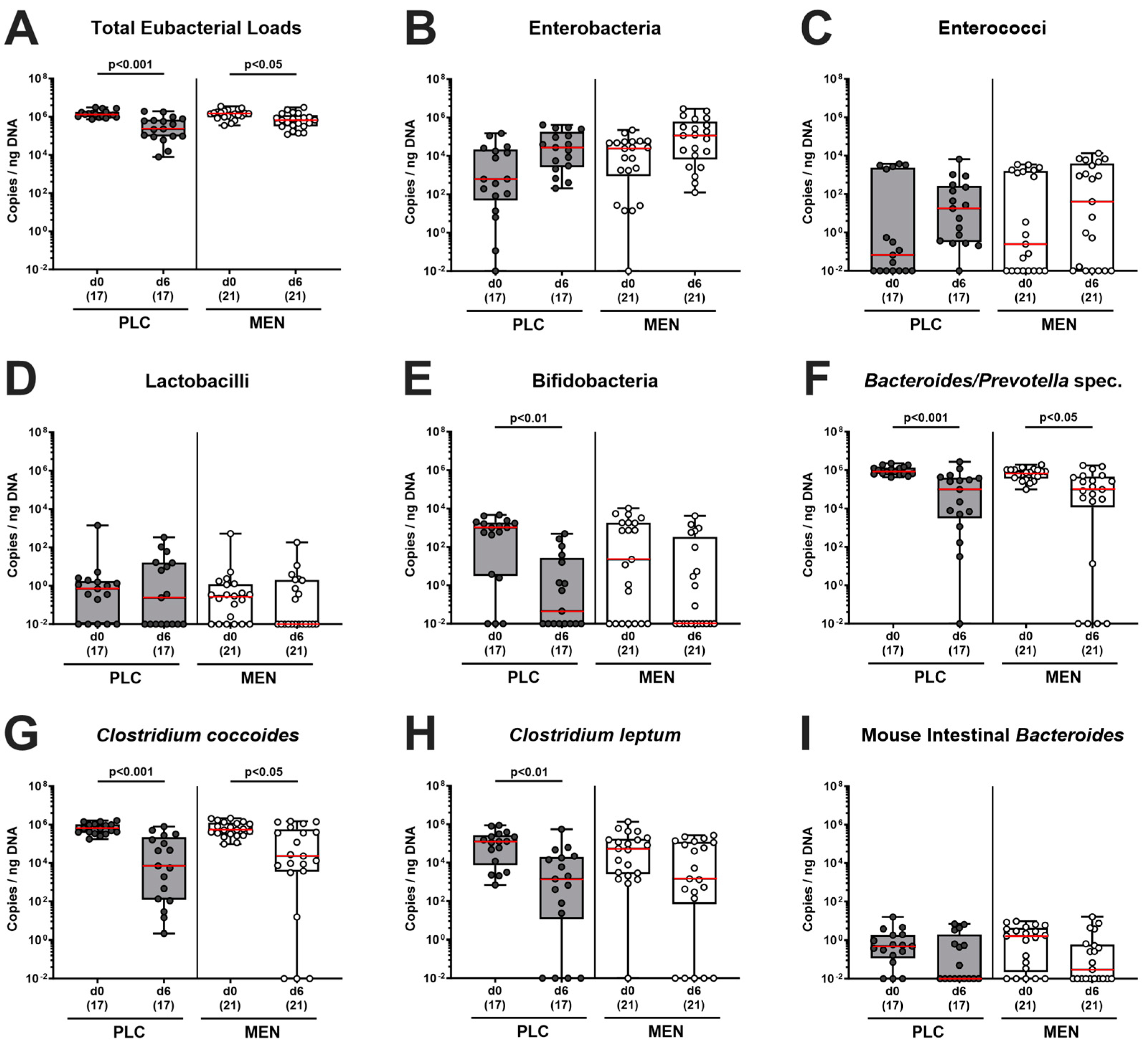
3.6. Fecal Microbiota during C. jejuni Infection of hma IL-10−/− Mice with Menthol Pretreatment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Oyarzabal, O.A.; Backert, S. Food Safety Resources. In Microbial Food Safety; Springer: Berlin/Heidelberg, Germany, 2012; pp. 235–239. [Google Scholar]
- Wilson, D.J.; Gabriel, E.; Leatherbarrow, A.J.; Cheesbrough, J.; Gee, S.; Bolton, E.; Fox, A.; Fearnhead, P.; Hart, C.A.; Diggle, P.J. Tracing the source of campylobacteriosis. PLoS Genet. 2008, 4, e1000203. [Google Scholar] [CrossRef] [PubMed]
- Pielsticker, C.; Glünder, G.; Rautenschlein, S. Colonization properties of Campylobacter jejuni in chickens. Eur. J. Microbiol. Immunol. 2012, 2, 61–65. [Google Scholar] [CrossRef]
- Fitzgerald, C. Campylobacter. Clin. Lab. Med. 2015, 35, 289–298. [Google Scholar] [CrossRef]
- Ellis-Iversen, J.; Ridley, A.; Morris, V.; Sowa, A.; Harris, J.; Atterbury, R.; Sparks, N.; Allen, V. Persistent environmental reservoirs on farms as risk factors for Campylobacter in commercial poultry. Epidemiol. Infect. 2012, 140, 916–924. [Google Scholar] [CrossRef]
- Skirrow, M. Campylobacter enteritis: A” new” disease. Br. Med. J. 1977, 2, 9–11. [Google Scholar] [CrossRef]
- Silva, J.; Leite, D.; Fernandes, M.; Mena, C.; Gibbs, P.A.; Teixeira, P. Campylobacter spp. as a foodborne pathogen: A review. Front. Microbiol. 2011, 2, 200. [Google Scholar] [CrossRef]
- Black, R.E.; Levine, M.M.; Clements, M.L.; Hughes, T.P.; Blaser, M.J. Experimental Campylobacter jejuni infection in humans. J. Infect. Dis. 1988, 157, 472–479. [Google Scholar] [CrossRef]
- Allos, B.M. Campylobacter jejuni Infections: Update on emerging issues and trends. Clin. Infect. Dis. 2001, 32, 1201–1206. [Google Scholar] [CrossRef]
- Tegtmeyer, N.; Sharafutdinov, I.; Harrer, A.; Esmaeili, D.S.; Linz, B.; Backert, S. Campylobacter Virulence Factors and Molecular Host–Pathogen Interactions. In Fighting Campylobacter Infections: Towards a One Health Approach; Springer: Cham, Switzerland, 2021; Volume 431, pp. 169–202. [Google Scholar] [CrossRef]
- Callahan, S.M.; Dolislager, C.G.; Johnson, J.G. The host cellular immune response to infection by Campylobacter spp. and its role in disease. Infect. Immun. 2021, 89, e00116–e00121. [Google Scholar] [CrossRef]
- Walker, R.I.; Caldwell, M.B.; Lee, E.C.; Guerry, P.; Trust, T.J.; Ruiz-Palacios, G.M. Pathophysiology of Campylobacter enteritis. Microbiol. Rev. 1986, 50, 81–94. [Google Scholar] [CrossRef] [PubMed]
- Wassenaar, T.M.; Blaser, M.J. Pathophysiology of Campylobacter jejuni infections of humans. Microbes Infect. 1999, 1, 1023–1033. [Google Scholar] [CrossRef]
- Young, K.T.; Davis, L.M.; Dirita, V.J. Campylobacter jejuni: Molecular biology and pathogenesis. Nat. Rev. Microbiol. 2007, 5, 665–679. [Google Scholar] [CrossRef]
- Butkevych, E.; Lobo de Sá, F.D.; Nattramilarasu, P.K.; Bücker, R. Contribution of Epithelial Apoptosis and Subepithelial Immune Responses in Campylobacter jejuni-Induced Barrier Disruption. Front. Microbiol. 2020, 11, 344. [Google Scholar] [CrossRef]
- Lobo de Sá, F.; Schulzke, J.-D.; Bücker, R. Diarrheal mechanisms and the role of intestinal barrier dysfunction in Campylobacter infections. Curr. Top. Microbiol. Immunol. 2021, 431, 203–231. [Google Scholar] [CrossRef]
- Dai, L.; Sahin, O.; Grover, M.; Zhang, Q. New and alternative strategies for the prevention, control, and treatment of antibiotic-resistant Campylobacter. Transl. Res. 2020, 223, 76–88. [Google Scholar] [CrossRef] [PubMed]
- Finsterer, J. Triggers of Guillain-Barré Syndrome: Campylobacter jejuni Predominates. Int. J. Mol. Sci. 2022, 23, 14222. [Google Scholar] [CrossRef]
- Nachamkin, I.; Allos, B.M.; Ho, T. Campylobacter species and Guillain-Barre syndrome. Clin. Microbiol. Rev. 1998, 11, 555–567. [Google Scholar] [CrossRef]
- Pope, J.E.; Krizova, A.; Garg, A.X.; Thiessen-Philbrook, H.; Ouimet, J.M. Campylobacter reactive arthritis: A systematic review. In Seminars in Arthritis and Rheumatism; Elsevier: Amsterdam, The Netherlands, 2007; pp. 48–55. [Google Scholar]
- Omarova, S.; Awad, K.; Moos, V.; Püning, C.; Gölz, G.; Schulzke, J.-D.; Bücker, R. Intestinal Barrier in Post-Campylobacter jejuni Irritable Bowel Syndrome. Biomolecules 2023, 13, 449. [Google Scholar] [CrossRef]
- Mortensen, N.P.; Kuijf, M.L.; Ang, C.W.; Schiellerup, P.; Krogfelt, K.A.; Jacobs, B.C.; van Belkum, A.; Endtz, H.P.; Bergman, M.P. Sialylation of Campylobacter jejuni lipo-oligosaccharides is associated with severe gastro-enteritis and reactive arthritis. Microbes Infect. 2009, 11, 988–994. [Google Scholar] [CrossRef]
- Mouftah, S.F.; Cobo-Díaz, J.F.; Álvarez-Ordóñez, A.; Elserafy, M.; Saif, N.A.; Sadat, A.; El-Shibiny, A.; Elhadidy, M. High-throughput sequencing reveals genetic determinants associated with antibiotic resistance in Campylobacter spp. from farm-to-fork. PLoS ONE 2021, 16, e0253797. [Google Scholar] [CrossRef]
- World Health Organisation. Campylobacter. Available online: https://www.who.int/news-room/fact-sheets/detail/campylobacter (accessed on 9 January 2024).
- European Food Safety Authority; European Centre for Disease Prevention and Control. The European Union Summary Report on Antimicrobial Resistance in zoonotic and indicator bacteria from humans, animals and food in 2019–2020. EFSA J. 2022, 20, e07209. [Google Scholar] [CrossRef]
- Kaakoush, N.O.; Castano-Rodriguez, N.; Mitchell, H.M.; Man, S.M. Global Epidemiology of Campylobacter Infection. Clin. Microbiol. Rev. 2015, 28, 687–720. [Google Scholar] [CrossRef]
- Kirk, M.D.; Pires, S.M.; Black, R.E.; Caipo, M.; Crump, J.A.; Devleesschauwer, B.; Döpfer, D.; Fazil, A.; Fischer-Walker, C.L.; Hald, T. World Health Organization estimates of the global and regional disease burden of 22 foodborne bacterial, protozoal, and viral diseases, 2010: A data synthesis. PLoS Med. 2015, 12, e1001921. [Google Scholar]
- Lackner, J.; Weiss, M.; Müller-Graf, C.; Greiner, M. The disease burden associated with Campylobacter spp. in Germany, 2014. PLoS ONE 2019, 14, e0216867. [Google Scholar] [CrossRef]
- MacDougall, J.M.; Fandrick, K.; Zhang, X.; Serafin, S.V.; Cashman, J.R. Inhibition of human liver microsomal (S)-nicotine oxidation by (−)-menthol and analogues. Chem. Res. Toxicol. 2003, 16, 988–993. [Google Scholar] [CrossRef]
- Trombetta, D.; Castelli, F.; Sarpietro, M.G.; Venuti, V.; Cristani, M.; Daniele, C.; Saija, A.; Mazzanti, G.; Bisignano, G. Mechanisms of antibacterial action of three monoterpenes. Antimicrob. Agents Chemother. 2005, 49, 2474–2478. [Google Scholar] [CrossRef]
- Sabzghabaee, A.M.; Nili, F.; Ghannadi, A.; Eizadi-Mood, N.; Anvari, M. Role of menthol in treatment of candidial napkin dermatitis. World J. Pediatr. 2011, 7, 167–170. [Google Scholar] [CrossRef]
- Rozza, A.L.; Meira de Faria, F.; Souza Brito, A.R.; Pellizzon, C.H. The gastroprotective effect of menthol: Involvement of anti-apoptotic, antioxidant and anti-inflammatory activities. PLoS ONE 2014, 9, e86686. [Google Scholar] [CrossRef]
- Matouk, A.I.; El-Daly, M.; Habib, H.A.; Senousy, S.; Naguib Abdel Hafez, S.M.; Kasem, A.W.; Almalki, W.H.; Alzahrani, A.; Alshehri, A.; Ahmed, A.-S.F. Protective effects of menthol against sepsis-induced hepatic injury: Role of mediators of hepatic inflammation, apoptosis, and regeneration. Front. Pharmacol. 2022, 13, 952337. [Google Scholar] [CrossRef]
- Goudarzi, M.A.; Radfar, M.; Goudarzi, Z. Peppermint as a promising treatment agent in inflammatory conditions: A comprehensive systematic review of literature. Phytother. Res. 2023, 38, 187–195. [Google Scholar] [CrossRef]
- Bereswill, S.; Fischer, A.; Plickert, R.; Haag, L.M.; Otto, B.; Kuhl, A.A.; Dasti, J.I.; Zautner, A.E.; Munoz, M.; Loddenkemper, C.; et al. Novel murine infection models provide deep insights into the “menage a trois” of Campylobacter jejuni, microbiota and host innate immunity. PLoS ONE 2011, 6, e20953. [Google Scholar] [CrossRef]
- Warren, H.S.; Fitting, C.; Hoff, E.; Adib-Conquy, M.; Beasley-Topliffe, L.; Tesini, B.; Liang, X.; Valentine, C.; Hellman, J.; Hayden, D. Resilience to bacterial infection: Difference between species could be due to proteins in serum. J. Infect. Dis. 2010, 201, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Lippert, E.; Karrasch, T.; Sun, X.; Allard, B.; Herfarth, H.H.; Threadgill, D.; Jobin, C. Gnotobiotic IL-10; NF-κB mice develop rapid and severe colitis following Campylobacter jejuni infection. PLoS ONE 2009, 4, e7413. [Google Scholar] [CrossRef]
- Heimesaat, M.M.; Mousavi, S.; Bandick, R.; Bereswill, S. Campylobacter jejuni infection induces acute enterocolitis in IL-10-/- mice pretreated with ampicillin plus sulbactam. Eur. J. Microbiol. Immunol. 2022, 12, 73–83. [Google Scholar] [CrossRef]
- Shayya, N.W.; Foote, M.S.; Langfeld, L.Q.; Du, K.; Bandick, R.; Mousavi, S.; Bereswill, S.; Heimesaat, M.M. Human microbiota associated IL-10−/− mice: A valuable enterocolitis model to dissect the interactions of Campylobacter jejuni with host immunity and gut microbiota. Eur. J. Microbiol. Immunol. 2023, 12, 107–122. [Google Scholar] [CrossRef]
- Weschka, D.; Mousavi, S.; Biesemeier, N.; Bereswill, S.; Heimesaat, M.M. Survey of Pathogen-Lowering and Immuno-Modulatory Effects Upon Treatment of Campylobacter coli-Infected Secondary Abiotic IL-10−/− Mice with the Probiotic Formulation Aviguard®. Microorganisms 2021, 9, 1127. [Google Scholar] [CrossRef]
- Heimesaat, M.M.; Alutis, M.; Grundmann, U.; Fischer, A.; Tegtmeyer, N.; Böhm, M.; Kühl, A.A.; Göbel, U.B.; Backert, S.; Bereswill, S. The role of serine protease HtrA in acute ulcerative enterocolitis and extra-intestinal immune responses during Campylobacter jejuni infection of gnotobiotic IL-10 deficient mice. Front. Cell. Infect. Microbiol. 2014, 4, 77. [Google Scholar] [CrossRef]
- Erben, U.; Loddenkemper, C.; Doerfel, K.; Spieckermann, S.; Haller, D.; Heimesaat, M.M.; Zeitz, M.; Siegmund, B.; Kühl, A.A. A guide to histomorphological evaluation of intestinal inflammation in mouse models. Int. J. Clin. Exp. Pathol. 2014, 7, 4557–4576. [Google Scholar]
- Heimesaat, M.M.; Giladi, E.; Kühl, A.A.; Bereswill, S.; Gozes, I. The octapetide NAP alleviates intestinal and extra-intestinal anti-inflammatory sequelae of acute experimental colitis. Peptides 2018, 101, 1–9. [Google Scholar] [CrossRef]
- Bryan, N.S.; Grisham, M.B. Methods to detect nitric oxide and its metabolites in biological samples. Free Radic. Biol. Med. 2007, 43, 645–657. [Google Scholar] [CrossRef]
- Bandick, R.; Busmann, L.V.; Mousavi, S.; Shayya, N.W.; Piwowarski, J.P.; Granica, S.; Melzig, M.F.; Bereswill, S.; Heimesaat, M.M. Therapeutic Effects of Oral Application of Menthol and Extracts from Tormentil (Potentilla erecta), Raspberry Leaves (Rubus idaeus), and Loosestrife (Lythrum salicaria) during Acute Murine Campylobacteriosis. Pharmaceutics 2023, 15, 2410. [Google Scholar] [CrossRef]
- Muruganathan, U.; Srinivasan, S.; Vinothkumar, V. Antidiabetogenic efficiency of menthol, improves glucose homeostasis and attenuates pancreatic β-cell apoptosis in streptozotocin-nicotinamide induced experimental rats through ameliorating glucose metabolic enzymes. Biomed. Pharmacother. 2017, 92, 229–239. [Google Scholar] [CrossRef]
- Santo, S.G.E.; Romualdo, G.R.; Santos, L.A.D.; Grassi, T.F.; Barbisan, L.F. Modifying effects of menthol against benzo(a)pyrene-induced forestomach carcinogenesis in female Swiss mice. Environ. Toxicol. 2021, 36, 2245–2255. [Google Scholar] [CrossRef]
- Huang, S.-S.; Su, H.-H.; Chien, S.-Y.; Chung, H.-Y.; Luo, S.-T.; Chu, Y.-T.; Wang, Y.-H.; MacDonald, I.J.; Lee, H.-H.; Chen, Y.-H. Activation of peripheral TRPM8 mitigates ischemic stroke by topically applied menthol. J. Neuroinflamm. 2022, 19, 192. [Google Scholar] [CrossRef]
- Bayat, M.; Kalantar, K.; Amirghofran, Z. Inhibition of interferon-γ production and T-bet expression by menthol treatment of human peripheral blood mononuclear cells. Immunopharmacol. Immunotoxicol. 2019, 41, 267–276. [Google Scholar] [CrossRef]
- Juergens, U.R.; Stöber, M.; Vetter, H. The anti-inflammatory activity of L-menthol compared to mint oil in human monocytes in vitro: A novel perspective for its therapeutic use in inflammatory diseases. Eur. J. Med. Res. 1998, 3, 539–545. [Google Scholar]
- Marcuzzi, A.; Tommasini, A.; Crovella, S.; Pontillo, A. Natural isoprenoids inhibit LPS-induced-production of cytokines and nitric oxide in aminobisphosphonate-treated monocytes. Int. Immunopharmacol. 2010, 10, 639–642. [Google Scholar] [CrossRef]
- Ghasemi-Pirbaluti, M.; Motaghi, E.; Bozorgi, H. The effect of menthol on acute experimental colitis in rats. Eur. J. Pharmacol. 2017, 805, 101–107. [Google Scholar] [CrossRef]
- Bastaki, S.M.; Adeghate, E.; Amir, N.; Ojha, S.; Oz, M. Menthol inhibits oxidative stress and inflammation in acetic acid-induced colitis in rat colonic mucosa. Am. J. Transl. Res. 2018, 10, 4210–4222. [Google Scholar]
- Baibars, M.; Eng, S.; Shaheen, K.; Alraiyes, A.H.; Alraies, M.C. Menthol toxicity: An unusual cause of coma. Case Rep. Med. 2012, 2012, 187039. [Google Scholar] [CrossRef]
- Kumar, A.; Baitha, U.; Aggarwal, P.; Jamshed, N. A fatal case of menthol poisoning. Int. J. Appl. Basic. Med. Res. 2016, 6, 137–139. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heimesaat, M.M.; Langfeld, L.Q.; Schabbel, N.; Shayya, N.W.; Mousavi, S.; Bereswill, S. Menthol Pretreatment Alleviates Campylobacter jejuni-Induced Enterocolitis in Human Gut Microbiota-Associated IL-10−/− Mice. Biomolecules 2024, 14, 290. https://doi.org/10.3390/biom14030290
Heimesaat MM, Langfeld LQ, Schabbel N, Shayya NW, Mousavi S, Bereswill S. Menthol Pretreatment Alleviates Campylobacter jejuni-Induced Enterocolitis in Human Gut Microbiota-Associated IL-10−/− Mice. Biomolecules. 2024; 14(3):290. https://doi.org/10.3390/biom14030290
Chicago/Turabian StyleHeimesaat, Markus M., Luis Q. Langfeld, Niklas Schabbel, Nizar W. Shayya, Soraya Mousavi, and Stefan Bereswill. 2024. "Menthol Pretreatment Alleviates Campylobacter jejuni-Induced Enterocolitis in Human Gut Microbiota-Associated IL-10−/− Mice" Biomolecules 14, no. 3: 290. https://doi.org/10.3390/biom14030290
APA StyleHeimesaat, M. M., Langfeld, L. Q., Schabbel, N., Shayya, N. W., Mousavi, S., & Bereswill, S. (2024). Menthol Pretreatment Alleviates Campylobacter jejuni-Induced Enterocolitis in Human Gut Microbiota-Associated IL-10−/− Mice. Biomolecules, 14(3), 290. https://doi.org/10.3390/biom14030290






