Circulating Biomarkers Involved in the Development of and Progression to Chronic Pancreatitis—A Literature Review
Abstract
1. Background
2. Materials and Methods
3. Results
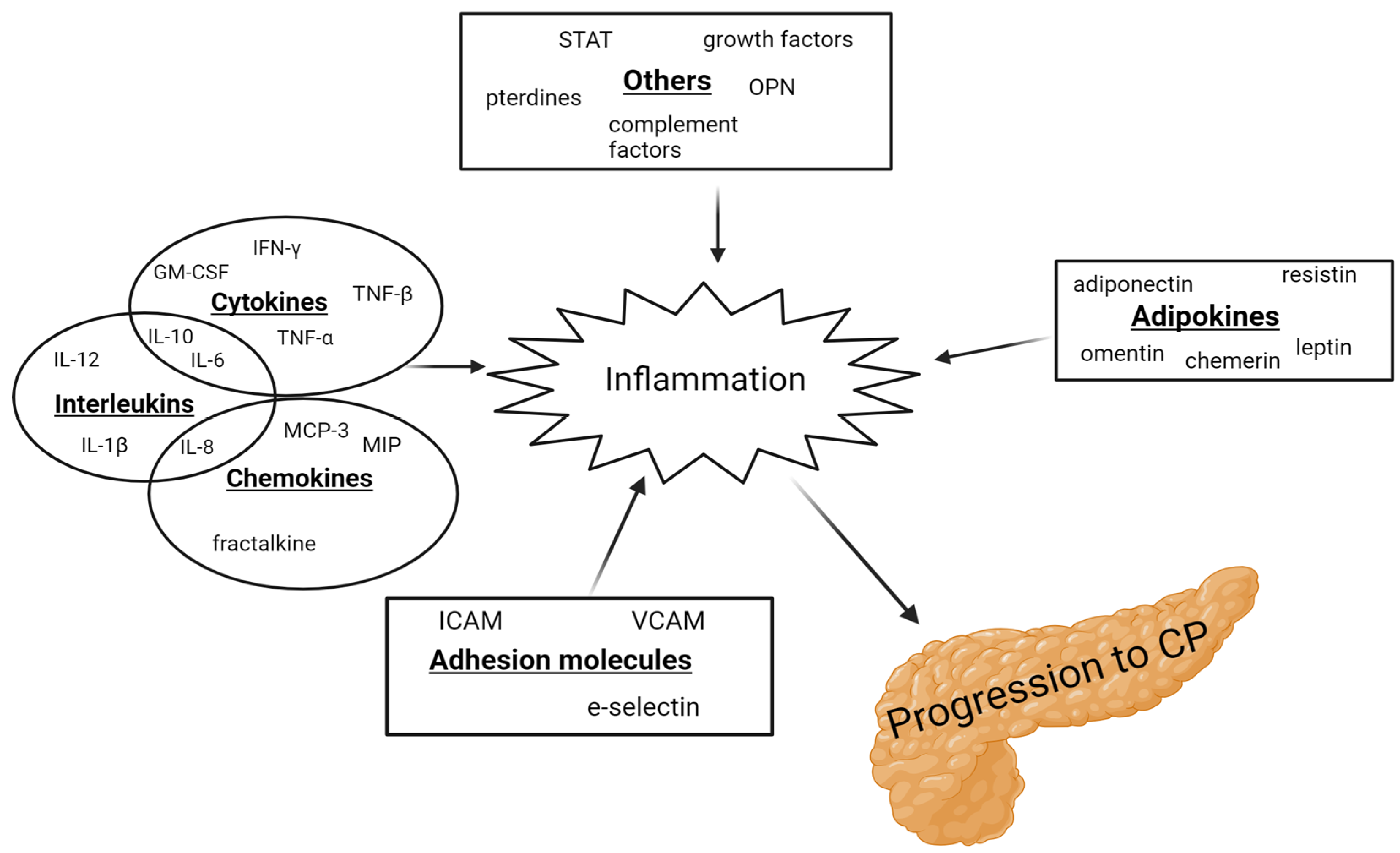
3.1. Inflammation
3.1.1. Interleukin 6
3.1.2. Tumor Necrosis Factor α
3.1.3. Leptin
| Biomarkers | Mechanism | Pancreas-Specific Effects | Blood Levels in Patients with CP | Comment |
|---|---|---|---|---|
| IL-1β [12,22,29,30,31,50] | A pro-inflammatory cytokine that activates several intracellular responses, e.g., stimulation of IL-6, IL-8, and TNF-α [51,52]. | Excessive or prolonged IL-1β activation can lead to CP [52,53]. Activates proliferation and collagen secretion in fibroblasts [54]. | = = ↓ ↓ ↑ = | Overexpression of IL-1β in murine pancreas results in CP [53]. Increases protease inhibitors having a protective effect in CP [30]. |
| IL-1α [9,29,50] | A pro-inflammatory cytokine that induces inflammation via activation of, e.g., COX2, IL-6, and TNF-α [55]. | Not typically associated with the pancreas but can indirectly be involved in pancreatic diseases. | ↓ ↓ = | |
| IL-1Ra [12,29] | An IL-1 receptor antagonist. Anti-inflammatory cytokine with protumor activity [12]. | Has a protective effect on both AP and CP [56,57]. | ↑ = | Higher levels in PDAC compared to CP [29]. |
| IL-2 [6,29,58] | A potent Th1-related cytokine that acts on NK cells and T-cells [59]. | Increases T-cells in the pancreas and induces expression of T-cell-associated proteins [60]. | ↑ ↓ ↓ | |
| IL-2R [20,61,62,63] | IL-2 receptor | ↑ = = = | ||
| IL-4 [6,9,29] | Modulates the differentiation of precursor Th cells to Th2 cells [64]; inhibition of pro-inflammatory cytokine synthesis [65]. | Secreted by PSCs, mediates macrophage activation by participating in the promotion of pancreatic fibrosis [66]. | ↓ ↑ ↓ | Potentially, levels of IL-4 in patients with CP depend on whether inflammation or fibrosis is the dominant process. |
| IL-6 [6,11,12,20,21,22,23,24,25,26,27,28,29,30,31,67] | A pro-inflammatory cytokine that causes cell proliferation, differentiation, and inflammatory responses and triggers the synthesis of acute-phase proteins [19,30]. | Promotes PSCs activation and collagen synthesis through the upregulation of TGF-β1 [68]. | ↑ ↑ ↑ = ↑ ↑ ↑ ↑ = ↑ = = = = = ↑ | Levels are closely linked to the quantity of alcohol consumed by patients with alcoholic CP [24]. Elevated in AP and reflects the severity and prognosis of the pancreatitis [37]. |
| IL-8 [9,11,12,31] | A chemoattractant that acts as a neutrophil activator and a pro-angiogenic factor [9,69]. | Circulating neutrophils from patients with CP express mRNA for IL-8 [69]. High levels of IL-8 are found in CP tissue [69,70,71]. | ↑ ↑ ↑ ↑ | Depending on the etiology, the amount of IL-8 correlates with the severity of the pancreatitis [69,70,71]. |
| IL-10 [6,23,29,31,58] | An anti-inflammatory cytokine that inhibits cytokine release from lymphocytes, e.g., IL-12, IL-6, and TNF-α [13,72]. | Has a protective effect on the pancreas during inflammation. The absence of IL-10 prevents the downregulation of inflammation [72,73]. | ↓ ↑ ↑ = = | Is seen to have a protective effect on the pancreas in mice [74]. |
| IL-12 [6,9,13,29] | Activates Th1-cells and induces the secretion of cytokines, e.g., INF-γ, IL-2, and TNF-α [6]. | The level escalates during the transition from AP to CP. Increased levels in both conditions [6]. | ↑ ↑ ↑ ↓ | Potential role in the progression of the disease [6]. |
| IL-17 [23,75] | A pro-inflammatory cytokine with a key role in the initial immune response [76]. | Triggers damage to pancreatic acinar cells by producing and releasing cytokines/chemokines recruiting immune cells [76]. | = ↑ | Valuable severity and prognostic factor in AP progression [77]. |
| GM-CSF [46,78] | A growth and differentiation factor for granulocytes and macrophages [79]. | Regulates cancer-associated inflammation in PDAC [80]. | ↓ ↑ | |
| IFN-γ [6,13,29] | A pro-inflammatory cytokine produced by activated T-cells and NK-cells, with chemotactic abilities [81]. | Stimulated by upregulated IL-18 and IL-12 in CP [6]. Elevated levels were found in CP tissue [82,83,84]. | ↑ = ↓ | Potential role in the progression of pancreatitis [6]. |
| TNF-α [8,9,11,20,22,25,29,31,39,40,41,42] | Regulates cytokines and adhesion molecules; also, a priming activator of inflammatory cells and PSCs [38]. | Induces PSC activation and collagen synthesis leading to fibrosis and inflammation in the pancreas [40]. | = ↓ ↑ = ↑ = = ↑ ↑ ↑ ↑ = | Elevated levels are also seen in patients with AP [33,36]. |
| ICAM [15,85,86,87] | An adhesion molecule that serves to mediate the adhesion of immune cells to endo-/epithelial cells [88]. | Overexpression of ICAM-1 in pancreatic endothelial cells leads to inflammatory cell infiltration in the pancreatic parenchyma [88]. | ↑ = ↑ ↑ | Elevated levels in AP correlate with higher mortality rates and necrosis development [88] |
| VEGF [14,89] | A pro-angiogenic mediator that enhances vascular permeability and stimulates immune cell migration [90]. | Not typically associated with the pancreas, it can indirectly be involved in pancreas diseases. | ↑ = | |
| Fractalkine [7,91,92] | Adhesion molecule that can be cleaved and functions as a chemoattractant [93]. | Expressed on the cell membranes of PSCs, it induces monocyte recruitment in the inflamed pancreas [7]. | ↑ ↑ ↑ | Alcohol consumption influences the levels of fractalkine. One study only found elevation in mild and severe CP [91]. |
| Chemerin [10,94,95] | An adipokine with chemoattractant properties, promotes the differentiation of adipocytes [96]. | Promotes the recruitment of macrophages to the inflamed pancreas [96]. | ↑ ↑ ↑ | No correlation between chemerin levels and alcohol intake or diabetes [94]. |
| Adiponectin [16,22,41,47,48,97] | An adipokine with anti-inflammatory properties. Reduces the levels of circulating fatty acids, activates their oxidation, and prevents lipid accumulation in cells [98,99]. | A lack of adiponectin accelerates the progression of CP in mice [100]. | = ↑ ↓ = = ↑ | Levels are inversely proportional to fat percentage. |
| Leptin [16,41,46,47,48,49] | An adipokine with pro-inflammatory and pro-fibrogenic properties [44,45]. | Inhibits SC apoptosis; therefore, lower levels are thought to induce SC apoptosis and thereby inhibit fibrosis [16]. | ↓ ↑ ↓ ↓ ↓ ↓ | Higher levels were found among patients with CP with DM [41]. |
| Resistin [16,46,101] | An adipokine that acts in a pro-inflammatory manner by upregulating IL-6 and TNF-α [102]. | Increases the concentration of TNF-α, which, in turn, activates PSCs [16]. | ↑ ↑ ↑ | Higher levels were found among patients with CP with DM [41]. |
| Osteopontin [17,103,104,105,106] | A glycophosphoprotein produced and secreted by osteoblasts, activated T cells, macrophages, and others. Functions as a chemoattractant in sites of inflammation [17,107]. | May play a part in the calcification and the formation of pancreas calculi [108]. | ↑ ↑ ↑ = = | |
| Neopterin [18,20,62] | A compound secreted by activated macrophages stimulated by INF-γ [20]. | A marker of the cellular immunity mediated by the lymphocyte–macrophage axis [20]. | = ↑ ↓ | Elevated in patients with AP and can reflect the severity and prognosis of AP [109,110]. |
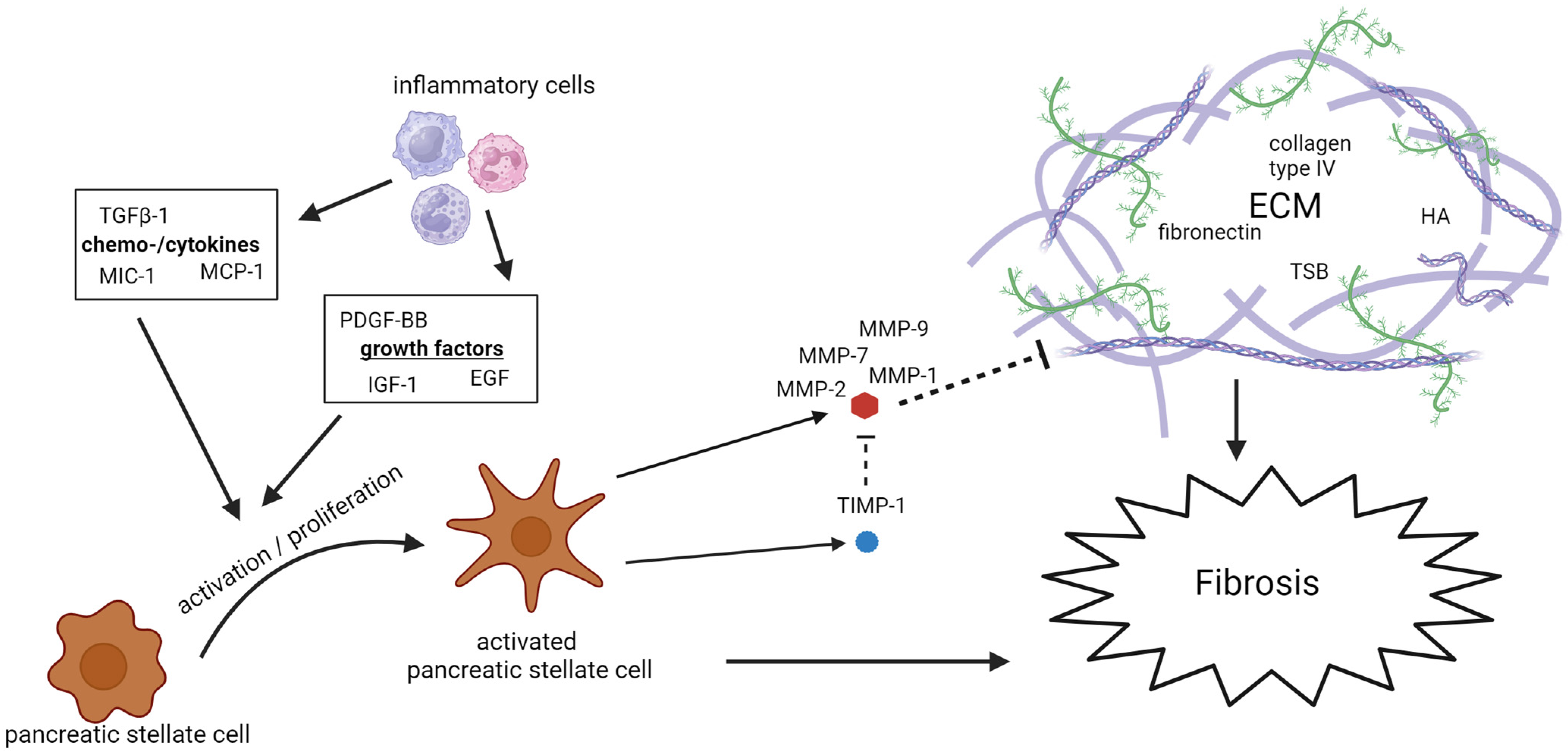
3.2. Fibrosis
3.2.1. Extracellular Matrix Remodeling
3.2.2. Activation of PSCs
| Biomarkers | Mechanism | Pancreas-Specific Effects | Blood Levels in Patients with CP | Comment |
|---|---|---|---|---|
| MMP-7 [48,87] | Enzymes secreted by activated PSCs that degrade the ECM [120]. | Degradation of basement collagen (type IV) [39,112]. | = ↑ | |
| MMP-9 [39,112,113,114] | ↑ ↑ = ↑ | One study found elevated levels in the plasma and not in the serum [114]. | ||
| TIMP-1 [15,48,85,87,105,106,113,115,116] | Enzymes secreted by activated PSCs that inhibit MMPs [120]. | Inhibits the proteolytic activity of MMPs. An imbalance between MMP And TIMPs supports the abnormal formation of the ECM [120]. | ↑ ↑ = ↑ = ↑ ↑ = | mRNA expression in the pancreas increases with disease progression [121]. |
| HA [92,101,117,118] | A protein component of the ECM [101]. | Marker of ECM proliferation. | ↑ ↑ ↑ = | |
| TGF-β [7,10,24,91,92,94,101,117,122,123] | A multipotent growth factor, with various functions, e.g., cell differentiation, proliferation, matrix production, and apoptosis. Promotes the recruitment of inflammatory cells and contributes to fibrosis [124]. | Activates PSCs leading to fibrosis formation in CP [124]. | ↑ ↑ = ↑ ↑ ↑ ↑ ↑ ↑ = | Higher in patients with pancreatic atrophy than in patients with a non-atrophic pancreas [7]. Correlates with the severity of alcoholic CP [91]. |
| PDGF [10,12,46,89,94,117,122] | A growth factor and mitogen acting on fibroblasts and promoting cell proliferation and migration [125]. | Acts as a growth factor on PSCs leading to ECM formation and, consequently, fibrosis [101]. | ↑ = ↓ = ↑ ↑ = | One paper studied PDGF-AA [122]. No correlation between PDGF-BB and alcohol intake [117]. |
| MCP-1 [7,9,12,24,25,29,48,91,92,101,111] | A chemoattractant that recruits an inflammatory infiltrate and initiates inflammation [24,126]. | Activates PSCs via TNF-β and promotes pancreatic fibrosis [101]. | = ↓ = = ↑ = = = ↑ ↑ ↑ | Negative association with alcohol [24]. Treatment with MCP-1 antagonist in rats inhibits pancreatic fibrosis [7]. |
| MIC-1 [17,106,127,128,129] | Part of the TGF-β family. An autocrine regulator of macrophage activation [130]. | The specific mechanism in the pancreas is not clear [106,129]. | ↑ ↑ ↑ ↑ ↑ = | Further elevated in patients with PDAC, making it a potential biomarker [106]. |
| M2BP [131,132] | A ligand that binds to extracellular proteins such as integrins, collagens, and fibronectin [133]. | Suggested to be associated with cell-to-cell and cell-to-ECM adhesion and plays a role in the facilitation of fibrosis [134]. | ↑ ↑ | A novel biomarker of liver fibrosis [135,136]. |
| ET-1 [22,137] | A mediator with vasoconstrictive and pro-inflammatory properties, secreted by damaged endothelial cells [137]. | Affects the activation of PSCs and stimulates the migration of PSCs [138]. | = = | Elevated levels seen in smokers [137]. |
| EGF [29,89,139] | A growth factor that stimulates the proliferation of, e.g., fibroblasts and epithelial cells [140]. | Regulates both chemoattraction and stimulation of the proliferation of PSCs [141]. | ↑ ↓ ↑ | |
| IGF-1 [24,48,50,139,142,143,144] | A growth factor that plays an important role in many bioactivities such as cell proliferation, differentiation, and survival [145]. | Stimulates migration and proliferation of PSCs [146]. | = = = = ↑ = = | One study found reduced levels of IGF-1R [144]. |
| IGFBP-2 [48,142] | Insulin growth factor-binding protein 2 | ↑ ↑ |
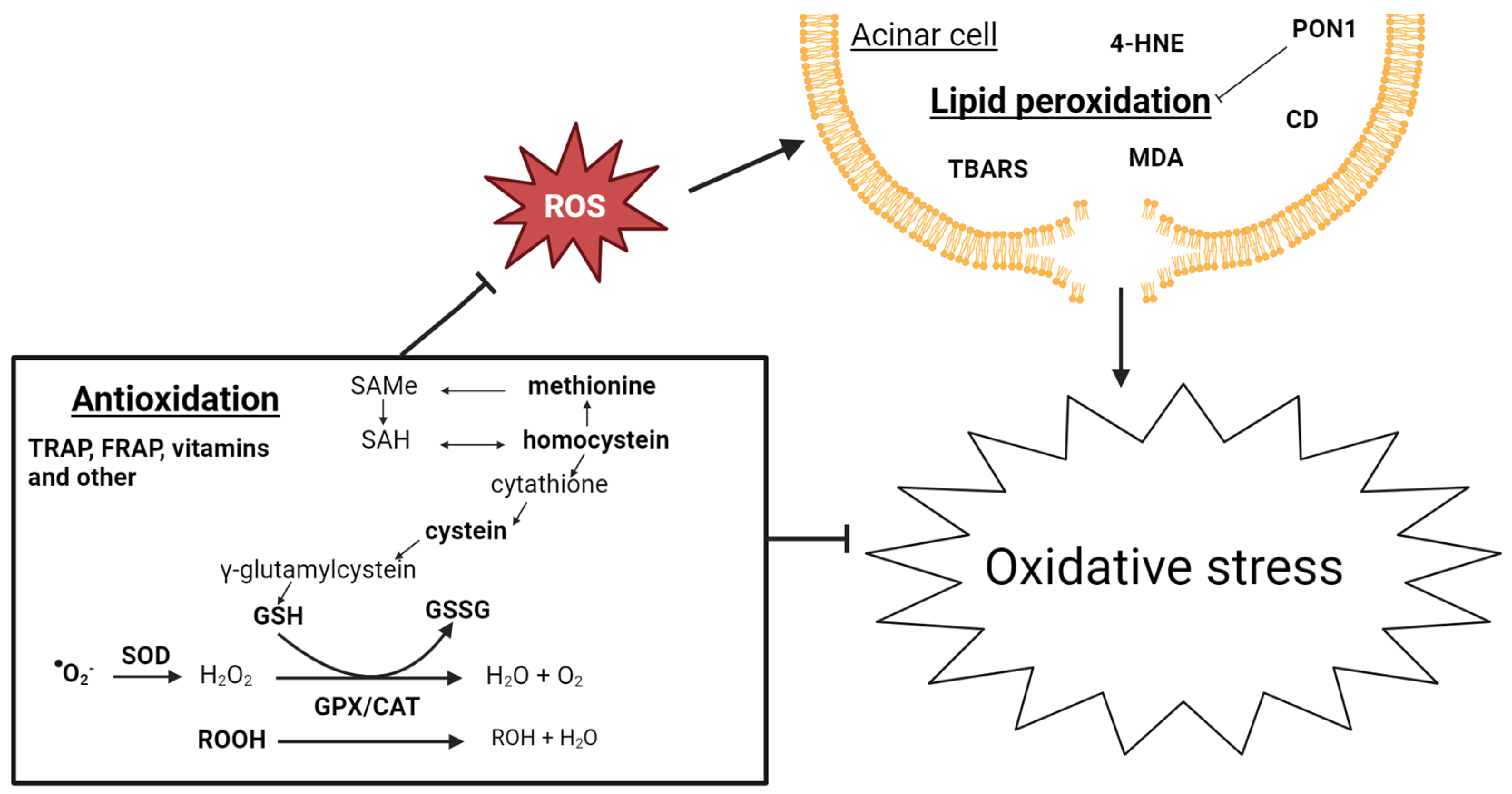
3.3. Oxidative Stress
3.3.1. Lipid Peroxidation
3.3.2. Antioxidation
| Biomarkers | Mechanism | Blood Levels in Patients with CP | Comment |
|---|---|---|---|
| TBARS [122,147,158,159,160,161,162] | A byproduct of the lipid peroxidation process [163]. | ↑ ↑ ↑ ↑ ↑ ↑ = | TBARS are higher in patients with TCP than in patients with ACP [160]. |
| 4-HNE [164,165] | A byproduct of the lipid peroxidation process [166]. | ↑ ↑ | Also elevated in RAP, especially during attacks on AP [164]. |
| MDA [149,164,165] | One of the final products of lipid peroxidation [164]. | ↑ = ↑ | Elevated levels are also found in pancreatic tissue samples [149]. |
| CD [155,157] | Primary products in lipid peroxidation in cells [157]. | = ↑ | Elevated levels are also found in pancreatic tissue samples [149]. |
| ROS [158,167] | Reactive oxygen species | = ↑ | Difficult to measure in the blood due to a short half-life. |
| [148,167] | Reactive oxygen species molecule [148]. | ↑ ↑ | Elevated in both PMA-stimulated and resting neutrophils [167]. |
| GSH [157,159,160,161,168] | The main ROS scavenger. Used by GPX to metabolize H2O2 and lipid hydroperoxides to water/alcohols [157]. | = ↓ ↓ ↓ ↓ | |
| GPX [157,159,160,162,164,167,169,170] | Catalyzes hydrogen peroxide to oxygen and water and, therefore, has an important function in the protection against oxidative stress [167]. | ↓ ↓ ↓ ↓ ↓ ↓ = ↓ | |
| CAT [150,157,167,169] | = = ↑ ↓ | ||
| SOD [157,159,160,161,164,167,169] | Catalyzes the dismutation of superoxide anions to hydrogen peroxide [157]. | = ↓ ↓ ↓ = ↑ = | One study found elevated serum SOD and lower levels of erythrocyte SOD in patients with CP [161]. |
| PON1 [156,157] | An HDL-associated enzyme. Plays a role in the hydrolyzation of active oxidized phospholipids and in the destruction of lipid hydroperoxides and H2O2 and prevents oxidation of LDL [156]. | ↓ ↓ | |
| TRAP [147,165] | Total peroxyl radical-trapping antioxidant parameter. | = = | |
| FRAP [122,158,161,164] | Ferrin-reducing ability of the plasma. A measurement of the non-enzymatic antioxidant capacity of the plasma [158]. | ↓ ↓ ↓ ↓ | Lower levels are also observed in patients with RAP [164]. |
| Vitamin A [42,161,162,169,171] | Blood antioxidant | ↓ ↓ ↓ ↓ ↓ | Dietary-dependent |
| Vitamin C [147,159,160,161,164,169] | Blood antioxidant | ↓ ↓ ↓ = = = | Dietary-dependent |
| Vitamin E [42,161,162,169,170,171] | Blood antioxidant | ↓ ↓ ↓ ↓ ↓ ↓ | Dietary-dependent |
| Zink [162,169,171] | Blood antioxidant | = = = | Elevated levels in patients with RAP [171]. |
| Copper [162,169,171] | Induces oxidative stress by increasing ROS [172]. | ↑ ↑ = | Reduced levels in patients with RAP [171]. |
| Selenium [162,169,171] | Blood antioxidant | ↓ ↓ ↓ | |
| Homocysteine [158,168] | Amino acid mediator in the synthesis of GSH. | = ↑ | |
| Cysteine [158,168] | Essential amino acid necessary for the formation of GSH. | ↓ ↓ | |
| Methionine [49,168,173] | Essential amino acid necessary for the formation of GSH. | = ↓ ↓ | One study only found elevated levels in TCP [168]. |
| β-carotene [156,169,171] | Blood antioxidant | ↓ ↓ ↓ |
| Inflammation | Fibrosis | Oxidative stress | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Group | Biomarker | Expression Changes in CP | Group | Biomarker | Expression Changes in CP | Group | Biomarker | Expression Changes in CP | Group | Biomarker | Expression Changes in CP |
| Interleukins | IL-5 [9] | ns | Adhesion molecules | CD44 [62] | ↓ | Components of the ECM | Collagen IV [87] | ↑ | Antioxidants | GR [157] | ns |
| IL-7 [9] | ns | e-selectin [22] | ns | Fibronectin [87] | ↑ | Xanthine [171] | ↓ | ||||
| IL-13 [9] | ns | VCAM [22] | ns | Laminin [117] | ↑ | Β-cyproxanthine [171] | ↓ | ||||
| IL-15 [29] | ↓ | Complement factors | C1q [9] | ↓ | MMP-1 [112] | ↑ | Lycopene [171] | ↓ | |||
| IL-16 [9] | ns | C3 [9] | ns | MMP-2 [47] | ↑ | SH groups [147] | ↓ | ||||
| IL-18 [13] | ↑ | C4 [9] | ↑ | MMP-3 [112] | ns | Lipid peroxidation | Ox-LDL/LDL [157] | ↑ | |||
| IL-23 [75] | ↑ | C4BPA [174] | ↑ | PICP [87] | ns | ROOH [170] | ↑ | ||||
| Cytokines | TNF-β [9] | ↓ | C5 [9] | ↑ | PINP [87] | ns | Lipid peroxide [170] | ↑ | |||
| GCSF [78] | ns | pro-C3 [118] | ns | THBS1 [85] | ns | Protein damage | 3-NT [157] | ↑ | |||
| MCSF [78] | ns | Pro-C5 [175] | ns | TPS [18] | ↑ | Carbonyls [158] | ↑ | ||||
| IFN-α [29] | ns | Properdin [9] | ↓ | TSP-2 [87] | ↑ | Others | Nitrites [165] | ↑ | |||
| Chemokines | CCL5 [85] | ns | Adipokines | Omentin [95] | ↑ | Others | AZGP1 [85] | ↑ | |||
| CXCL16 [176] | ns | Others | ANG-1 [89] | ↓ | CCN1 [87] | ns | |||||
| IP10 [12] | ns | HMGB1 [10] | ns | CCN2 [87] | ↑ | ||||||
| MCP-3 [9] | ns | LBP [85] | ns | PLG [87] | ns | ||||||
| MIP-1β [12] | ns | LTF [85] | ↑ | ||||||||
| MIP-3α [11] | ↑ | RORγT [75] | ↑ | ||||||||
| PPBP [85] | ns | STAT3 [75] | ↑ | ||||||||
| RBP-4 [143] | ns | YKL-40 [26] | ns | ||||||||
| Growth factors | IGF-2 [48] | ns | CD40L [29] | ↑ | |||||||
| IGFBP1,3 [48] | ↑ | ||||||||||
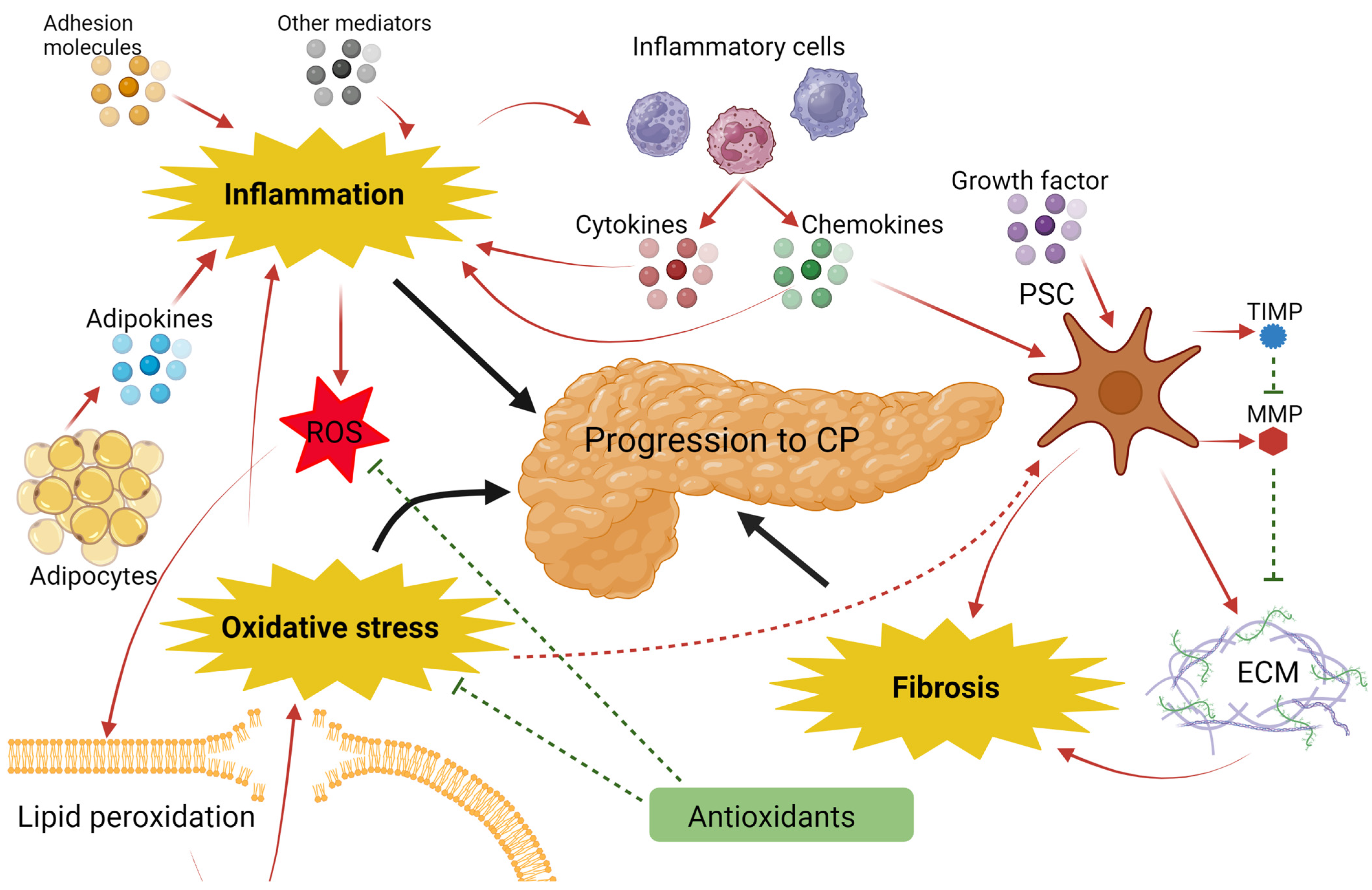
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Peery, A.F.; Crockett, S.D.; Murphy, C.C.; Lund, J.L.; Dellon, E.S.; Williams, J.L.; Jensen, E.T.; Shaheen, N.J.; Barritt, A.S.; Lieber, S.R.; et al. Burden and Cost of Gastrointestinal, Liver, and Pancreatic Diseases in the United States: Update 2018. Gastroenterology 2019, 156, 254–272.e11. [Google Scholar] [CrossRef]
- Schneider, A.; Löhr, J.M.; Singer, M.V. The M-ANNHEIM Classification of Chronic Pancreatitis: Introduction of a Unifying Classification System Based on a Review of Previous Classifications of the Disease. J. Gastroenterol. 2007, 42, 101–119. [Google Scholar] [CrossRef]
- Cai, Q.Y.; Tan, K.; Zhang, X.L.; Han, X.; Pan, J.P.; Huang, Z.Y.; Tang, C.W.; Li, J. Incidence, Prevalence, and Comorbidities of Chronic Pancreatitis: A 7-Year Population-Based Study. World J. Gastroenterol. 2023, 29, 4671–4684. [Google Scholar] [CrossRef]
- Sankaran, S.J.; Xiao, A.Y.; Wu, L.M.; Windsor, J.A.; Forsmark, C.E.; Petrov, M.S. Frequency of Progression from Acute to Chronic Pancreatitis and Risk Factors: A Meta-Analysis. Gastroenterology 2015, 149, 1490–1500.e1. [Google Scholar] [CrossRef] [PubMed]
- Hegyi, P.J.; Soós, A.; Tóth, E.; Ébert, A.; Venglovecz, V.; Márta, K.; Mátrai, P.; Mikó, A.; Bajor, J.; Sarlós, P.; et al. Evidence for Diagnosis of Early Chronic Pancreatitis after Three Episodes of Acute Pancreatitis: A Cross-Sectional Multicentre International Study with Experimental Animal Model. Sci. Rep. 2021, 11, 1367. [Google Scholar] [CrossRef] [PubMed]
- Bhatnagar, A.; Wig, J.D.; Majumdar, S. Immunological Findings in Acute and Chronic Pancreatitis. ANZ J. Surg. 2003, 73, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Ito, T. Can Measurement of Chemokines Become Useful Biological and Functional Markers of Early-Stage Chronic Pancreatitis? J. Gastroenterol. 2007, 42, 72–77. [Google Scholar] [CrossRef]
- Kıyıcı, A.; İbiş, M.; Akbulut, Ş.; Köklü, S.; Uçar, E.; Ünlü, A. Serum TNF-Alpha Levels in Acute and Chronic Pancreatitis. Eur. J. Gen. Med. 2009, 6, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Sandström, A.; Andersson, R.; Segersvärd, R.; Löhr, M.; Borrebaeck, C.A.K.; Wingren, C. Serum Proteome Profiling of Pancreatitis Using Recombinant Antibody Microarrays Reveals Disease-Associated Biomarker Signatures. Proteom. Clin. Appl. 2012, 6, 486–496. [Google Scholar] [CrossRef]
- Stojek, M.; Adrych, K.; Rojek, L.; Smoczynski, M.; Sledzinski, T.; Szrok, S.; Swierczynski, J. Decreased Serum Platelet Derived Growth Factor BB Levels in Acute and Increased in Chronic Pancreatitis. World J. Gastroenterol. 2014, 20, 13127–13132. [Google Scholar] [CrossRef]
- Miron, N.; Miron, M.-M.; Milea, V.G.I.; Cristea, V. Proinflammatory Cytokines: An Insight into Pancreatic Oncogenesis. Rom. Arch. Microbiol. Immunol. 2010, 69, 183–189. [Google Scholar]
- Shaw, V.E.; Lane, B.; Jenkinson, C.; Cox, T.; Greenhalf, W.; Halloran, C.M.; Tang, J.; Sutton, R.; Neoptolemos, J.P.; Costello, E. Serum Cytokine Biomarker Panels for Discriminating Pancreatic Cancer from Benign Pancreatic Disease. Mol. Cancer 2014, 13, 114. [Google Scholar] [CrossRef] [PubMed]
- Schneider, A.; Haas, S.L.; Hildenbrand, R.; Siegmund, S.; Reinhard, I.; Nakovics, H.; Singer, M.V.; Feick, P. Enhanced Expression of Interleukin-18 in Serum and pancreas of Patients with Chronic Pancreatitis. World J. Gastroenterol. 2006, 12, 6507. [Google Scholar] [CrossRef] [PubMed]
- Berindan-Neagoe, I.; Burz, C.; Balacescu, O.; Balacescu, L.; Seicean, A.; Cristea, V.; Irimie, A. Molecular Angiogenesis Profile as a Tool to Discriminate Chronic Pancreatitis (CP) from Pancreatic Cancer (PC). CA Cancer J. Clin. 2011, 40, 482–483. [Google Scholar] [CrossRef] [PubMed]
- Jenkinson, C.; Elliott, V.; Menon, U.; Apostolidou, S.; Fourkala, O.E.; Gentry-Maharaj, A.; Pereira, S.P.; Jacobs, I.; Cox, T.F.; Greenhalf, W.; et al. Evaluation in Pre-Diagnosis Samples Discounts ICAM-1 and TIMP-1 as Biomarkers for Earlier Diagnosis of Pancreatic Cancer. J. Proteom. 2015, 113, 400–402. [Google Scholar] [CrossRef]
- Adrych, K.; Smoczynski, M.; Sledzinski, T.; Dettlaff-Pokora, A.; Goyke, E.; Swierczynski, J. Increased Serum Resistin Concentration in Patients With Chronic Pancreatitis Possible Cause of Pancreatic Fibrosis. J. Clin. Gastroenterol. 2008, 43, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Sokoll, L.J.; Pasay, J.J.; Rubin, A.L.; Li, H.; Bach, D.M.; Chan, D.W.; Zhang, Z. Identification of Serum Biomarker Panels for the Early Detection of Pancreatic Cancer. Cancer Epidemiol. Biomark. Prev. 2019, 28, 174–182. [Google Scholar] [CrossRef]
- Talar-Wojnarowska, R.; Gasiorowska, A.; Olakowski, M.; Lekstan, A.; Lampe, P.; Malecka-Panas, E. Clinical Value of Serum Neopterin, Tissue Polypeptide-Specific Antigen and CA19-9 Levels in Differential Diagnosis between Pancreatic Cancer and Chronic Pancreatitis. Pancreatology 2011, 10, 689–694. [Google Scholar] [CrossRef]
- Manohar, M.; Verma, A.K.; Venkateshaiah, S.U.; Sanders, N.L.; Mishra, A. Pathogenic Mechanisms of Pancreatitis. World J. Gastrointest. Pharmacol. Ther. 2017, 8, 10–25. [Google Scholar] [CrossRef]
- Manes, G.; Spada, O.A.; Rabitti, P.G.; Feola, B.; Misso, S.; Minerva, A.; Uomo, G. Neopterin Serum Levels in Pancreatic Adenocarcinoma. Int. J. Pancreatol. 1999, 25, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Talar-Wojnarowska, R.; Gasiorowska, A.; Smolarz, B.; Romanowicz-Makowska, H.; Kulig, A.; Malecka-Panas, E. Clinical Significance of Interleukin-6 (Il-6) Gene Polymorphism and Il-6 Serum Level in Pancreatic Adenocarcinoma and Chronic Pancreatitis. Dig. Dis. Sci. 2009, 54, 683–689. [Google Scholar] [CrossRef] [PubMed]
- Gasiorowska, A.; Talar-Wojnarowska, R.; Kaczka, A.; Borkowska, A.; Czupryniak, L.; Małecka-Panas, E. Subclinical Inflammation and Endothelial Dysfunction in Patients with Chronic Pancreatitis and Newly Diagnosed Pancreatic Cancer. Dig. Dis. Sci. 2016, 61, 1121–1129. [Google Scholar] [CrossRef]
- Tanţău, A.; Leucuţa, D.C.; Tanţău, M.; Boţan, E.; Zaharie, R.; Mândruţiu, A.; Tomuleasa, I.C. Inflammation, Tumoral Markers and Interleukin-17, -10, and -6 Profiles in Pancreatic Adenocarcinoma and Chronic Pancreatitis. Dig. Dis. Sci. 2021, 66, 3427–3438. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, N.; Larsen, S.; Seidelin, J.B.; Nielsen, O.H. Alcohol Modulates Circulating Levels of Interleukin-6 and Monocyte Chemoattractant Protein-1 in Chronic Pancreatitis. Scand. J. Gastroenterol. 2004, 39, 277–282. [Google Scholar] [CrossRef]
- Zhang, J.; Fan, H.; Gross, M.; Liu, N.; Carlson, H.; Wood, A.; Hoffman, K.; Petrosino, J.; Pankratz, N.; Thyagarajan, B.; et al. Progressive Reduction in Circulating Levels of Carotenoids and Other Micronutrients in Patients with Chronic Pancreatitis. Pancreatology 2022, 22, 1126–1133. [Google Scholar] [CrossRef]
- Hansen, M.; Rinnov Nielsen, A.; Vilsbøll, T.; Lund, A.; Krarup, T.; Knop, F.K.; Vestergaard, H. Increased Levels of YKL-40 and Interleukin 6 in Patients With Chronic Pancreatitis and Secondary Diabetes. Pancreas 2012, 41, 1316–1318. [Google Scholar] [CrossRef] [PubMed]
- Mroczko, B.; Groblewska, M.; Gryko, M.; Kȩdra, B.; Szmitkowski, M. Diagnostic Usefulness of Serum Interleukin 6 (IL-6) and C-Reactive Protein (CRP) in the Differentiation between Pancreatic Cancer and Chronic Pancreatitis. J. Clin. Lab. Anal. 2010, 24, 256–261. [Google Scholar] [CrossRef]
- Singh, N.; Gupta, S.; Rashid, S.; Saraya, A. Association of Inflammatory Markers with the Disease & Mutation Status in Pancreatic Cancer. Indian J. Med. Res. 2022, 155, 49–55. [Google Scholar] [CrossRef]
- Chung, H.W.; Jang, S.; Lim, J.B. Clinical Implications and Diagnostic Usefulness of Correlation between Soluble Major Histocompatibility Complex Class i Chain-Related Molecule a and Protumorigenic Cytokines in Pancreatic Ductal Adenocarcinoma. Cancer 2013, 119, 233–244. [Google Scholar] [CrossRef]
- Bamba, T.; Yoshioka, U.; Hosoda, S. Serum Levels of Interleukin-Lp and Interleukin-6 in Patients with Chronic Pancreatitis. J. Gastroenterol. 1994, 29, 314–319. [Google Scholar] [CrossRef]
- Dima, S.O.; Tanase, C.; Albulescu, R.; Herlea, V.; Chivu-Economescu, M.; Purnichescu-Purtan, R.; Dumitrascu, T.; Duda, D.G.; Popescu, I. An Exploratory Study of Inflammatory Cytokines as Prognostic Biomarkers in Patients With Ductal Pancreatic Adenocarcinoma. Pancreas 2012, 41, 1001–1007. [Google Scholar] [CrossRef]
- Cho, I.R.; Do, M.Y.; Han, S.Y.; Jang, S.I.; Cho, J.H. Comparison of Interleukin-6, C-Reactive Protein, Procalcitonin, and the Computed Tomography Severity Index for Early Prediction of Severity of Acute Pancreatitis. Gut Liver 2023, 17, 629–637. [Google Scholar] [CrossRef] [PubMed]
- Heresbach, D.; Letourneur, J.P.; Bahon, I.; Pagenault, M.; Guillou, Y.M.; Dyard, F.; Fauchet, R.; Mallédant, Y.; Bretagne, J.F.; Gosselin, M. Value of Early Blood Th-1 Cytokine Determination in Predicting Severity of Acute Pancreatitis. Scand. J. Gastroenterol. 1998, 33, 554–560. [Google Scholar] [CrossRef] [PubMed]
- Berney, T.; Gasche, Y.; Robert, J.; Jenny, A.; Mensi, N.; Grau, G.; Vermeulen, B.; Morel, P. Serum Profiles of Interleukin-6, Interleukin-8, and Interleukin-10 in Patients with Severe and Mild Acute Pancreatitis. Pancreas 1999, 18, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Inagaki, T.; Hoshino, M.; Hayakawa, T.; Ohara, H.; Yamada, H.; Iida, M.; Nakazawa, T.; Ogasawara, T.; Uchida, A.; Hasegawa, C.; et al. Interleukin-6 Is a Useful Marker for Early Prediction of the Severity of Acute Pancreatitis. Pancreas 1997, 14, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Brivet, F.G.; Emilie, D.; Galanaud, P. Pro- and Anti-Inflammatory Cytokines during Acute Severe Pancreatitis: An Early and Sustained Response, Although Unpredictable of Death. Crit. Care Med. 1999, 27, 749–755. [Google Scholar] [CrossRef] [PubMed]
- Leser, H.G.; Gross, V.; Scheibenbogen, C.; Heinisch, A.; Salm, R.; Lausen, M.; Rückauer, K.; Andreesen, R.; Farthmann, E.H.; Schölmerich, J. Elevation of Serum Interleukin-6 Concentration Precedes Acute-Phase Response and Reflects Severity in Acute Pancreatitis. Gastroenterology 1991, 101, 782–785. [Google Scholar] [CrossRef] [PubMed]
- Norman, J. The Role of Cytokines in the Pathogenesis of Acute Pancreatitis. Am. J. Surg. 1998, 175, 76–83. [Google Scholar] [CrossRef]
- Manjari, K.S.; Jyothy, A.; Vidyasagar, A.; Prabhakar, B.; Nallari, P.; Venkateshwari, A. Matrix Metalloproteinase-9, Transforming Growth Factor-Β1, and Tumor Necrosis Factor-α Plasma Levels in Chronic Pancreatitis. Indian J. Gastroenterol. 2013, 32, 103–107. [Google Scholar] [CrossRef]
- Sri Manjari, K.; Jyothy, A.; Shravan Kumar, P.; Prabhakar, B.; Uma Devi, M.; Ramanna, M.; Nallari, P.; Venkateshwari, A. A Single-Nucleotide Polymorphism in Tumor Necrosis Factor-α (-308 G/A) as a Biomarker in Chronic Pancreatitis. Gene 2014, 539, 186–189. [Google Scholar] [CrossRef]
- Hontsariuk, D.O.; Ferfetska, K.V.; Khrystych, T.M.; Fediv, O.I.; Temerivska, T.G.; Jiguleva, E.O.; Honcharuk, L.M.; Olinik, O.Y. Incides of C-Reactive Protein, Tumor Necrosis Factor-α, Adiponectin, Leptin and Resistin in the Blood of Patients Suffering from Chronic Pancreatitis and Type 2 Diabetes Mellitus. J. Med. Life 2020, 13, 568–571. [Google Scholar] [CrossRef]
- Greer, J.B.; Greer, P.; Sandhu, B.S.; Alkaade, S.; Wilcox, C.M.; Anderson, M.A.; Sherman, S.; Gardner, T.B.; Lewis, M.D.; Guda, N.M.; et al. Nutrition and Inflammatory Biomarkers in Chronic Pancreatitis Patients. Nutr. Clin. Pract. 2019, 34, 387–399. [Google Scholar] [CrossRef]
- Zhao, S.; Kusminski, C.M.; Scherer, P.E. Adiponectin, Leptin and Cardiovascular Disorders. Circ. Res. 2021, 128, 136–149. [Google Scholar] [CrossRef]
- Saxena, N.K.; Titus, M.A.; Ding, X.; Floyd, J.; Srinivasan, S.; Sitaraman, S.V.; Anania, F.A. Leptin as a Novel Profibrogenic Cytokine in Hepatic Stellate Cells: Mitogenesis and Inhibition of Apoptosis Mediated by Extracellular Regulated Kinase (Erk) and Akt Phosphorylation. FASEB J. 2004, 18, 1612–1614. [Google Scholar] [CrossRef] [PubMed]
- Petrescu, A.D.; Grant, S.; Williams, E.; An, S.Y.; Seth, N.; Shell, M.; Amundsen, T.; Tan, C.; Nadeem, Y.; Tjahja, M.; et al. Leptin Enhances Hepatic Fibrosis and Inflammation in a Mouse Model of Cholestasis. Am. J. Pathol. 2022, 192, 484–502. [Google Scholar] [CrossRef] [PubMed]
- Park, W.G.; Li, L.; Appana, S.; Wei, W.; Stello, K.; Andersen, D.K.; Hughes, S.J.; Whitcomb, D.C.; Brand, R.E.; Yadav, D.; et al. Unique Circulating Immune Signatures for Recurrent Acute Pancreatitis, Chronic Pancreatitis and Pancreatic Cancer: A Pilot Study of These Conditions with and without Diabetes: Immune Profiling of Pancreatic Disorders. Pancreatology 2020, 20, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Dranka-Bojarowska, D.; Lekstan, A.; Olakowski, M.; Jablonska, B.; Lewinski, A.; Musialski, P.; Sobczyk, W.; Kapalka, A.; Lampe, P. The Assessment of Serum Concentration of Adiponectin, Leptin and Serum Carbonhydrate Antigen-19.9 in Patients with Pancreatic Cancer and Chronic Pancreatitis. J. Physiol. Pharmacol. 2015, 66, 653–663. [Google Scholar] [PubMed]
- Hrabák, P.; Šoupal, J.; Kalousová, M.; Krechler, T.; Vočka, M.; Hanuš, T.; Petruželka, L.; Svačina, Š.; Žák, A.; Zima, T. Novel Biochemical Markers for Non-Invasive Detection of Pancreatic Cancer. Neoplasma 2022, 69, 474–483. [Google Scholar] [CrossRef] [PubMed]
- Adrych, K.; Smoczynski, M.; Stojek, M.; Sledzinski, T.; Slominska, E.; Goyke, E.; Smolenski, R.T.; Swierczynski, J. Decreased Serum Essential and Aromatic Amino Acids in Patients with Chronic Pancreatitis. World J. Gastroenterol. 2010, 16, 4422–4427. [Google Scholar] [CrossRef]
- Basso, D.; Plebani, M.; Panozzo, M.; Meggiato, T.; De Paoli, M.; Del Favero, G. Insulin-like Growth Factor-I, Interleukin-1 g and in Pancreatic Cancer: Role in Tumor Invasiveness and Associated Diabetes. Int. J. Clin. Lab. Res. 1995, 25, 40–43. [Google Scholar] [CrossRef]
- Voronov, E.; Dotan, S.; Krelin, Y.; Song, X.; Elkabets, M.; Carmi, Y.; Rider, P.; Cohen, I.; Romzova, M.; Kaplanov, I.; et al. Unique versus Redundant Functions of IL-1α and IL-1β in the Tumor Microenvironment. Front. Immunol. 2013, 4, 177. [Google Scholar] [CrossRef]
- Tu, S.; Bhagat, G.; Cui, G.; Takaishi, S.; Kurt-Jones, E.A.; Rickman, B.; Betz, K.S.; Penz-Oesterreicher, M.; Bjorkdahl, O.; Fox, J.G.; et al. Overexpression of Interleukin-1β Induces Gastric Inflammation and Cancer and Mobilizes Myeloid-Derived Suppressor Cells in Mice. Cancer Cell 2008, 14, 408–419. [Google Scholar] [CrossRef] [PubMed]
- Marrache, F.; Tu, S.P.; Bhagat, G.; Pendyala, S.; Österreicher, C.H.; Gordon, S.; Ramanathan, V.; Penz-Österreicher, M.; Betz, K.S.; Song, Z.; et al. Overexpression of Interleukin-1β in the Murine Pancreas Results in Chronic Pancreatitis. Gastroenterology 2008, 135, 1277–1287. [Google Scholar] [CrossRef]
- Schmidt, J.A.; Oliver, C.N.; Lepe-Zuniga, J.L.; Green, I.; Gery, I. Silica-Stimulated Monocytes Release Fibroblast Proliferation Factors Identical to Interleukin 1. A Potential Role for Interleukin 1 in the Pathogenesis of Silicosis. J. Clin. Investig. 1984, 73, 1462–1472. [Google Scholar] [CrossRef] [PubMed]
- Malik, A.; Kanneganti, T.D. Function and Regulation of IL-1α in Inflammatory Diseases and Cancer. Immunol. Rev. 2018, 281, 124–137. [Google Scholar] [CrossRef]
- Fink, G.; Yang, J.; Carter, G.; Norman, J. Acute Pancreatitis-Induced Enzyme Release and Necrosis Are Attenuated by IL-1 Antagonism through an Indirect Mechanism. J. Surg. Res. 1997, 67, 94–97. [Google Scholar] [CrossRef]
- Shen, J.; Gao, J.; Zhang, J.; Xiang, D.; Wang, X.; Qian, L.; Shen, J.; Yang, L.; Zhu, S.; Wu, M.; et al. Recombinant Human Interleukin-1 Receptor Antagonist (RhIL-1Ra) Attenuates Caerulein-Induced Chronic Pancreatitis in Mice. Biomed. Pharmacother. 2012, 66, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.W.; Lim, J.B. Clinical Significance of Serum Levels of Immune-Associated Molecules, Uric Acid and Soluble MHC Class I Chain-Related Molecules A and B, as Diagnostic Tumor Markers for Pancreatic Ductal Adenocarcinoma. Cancer Sci. 2011, 102, 1673–1679. [Google Scholar] [CrossRef]
- Thornton, A.M.; Donovan, E.E.; Piccirillo, C.A.; Shevach, E.M. Cutting Edge: IL-2 Is Critically Required for the in Vitro Activation of CD4+CD25+ T Cell Suppressor Function. J. Immunol. 2004, 172, 6519–6523. [Google Scholar] [CrossRef]
- Grinberg-Bleyer, Y.; Baeyens, A.; You, S.; Elhage, R.; Fourcade, G.; Gregoire, S.; Cagnard, N.; Carpentier, W.; Tang, Q.; Bluestone, J.; et al. IL-2 Reverses Established Type 1 Diabetes in NOD Mice by a Local Effect on Pancreatic Regulatory T Cells. J. Exp. Med. 2010, 207, 1871–1878. [Google Scholar] [CrossRef]
- Kayhan, B.; Kayhan, M.; Akdogan, M. Can IL-2R Alpha Be a Valuable Marker along with CA 19-9 in the Diagnosis of Chronic Pancreatitis and Pancreatic Cancer? Int. J. Biol. Markers 2004, 19, 196–202. [Google Scholar] [CrossRef]
- Schlosser, W.; Gansauge, F.; Schlosser, S.; Gansauge, S.; Beger, H.G. Low Serum Levels of CD44, CD44v6, and Neopterin Indicate Immune Dysfunction in Chronic Pancreatitis. Pancreas 2001, 23, 335–340. [Google Scholar] [CrossRef]
- Gansauge, F.; Steinbach, G.; Gansauge, S.; Ko Ènig, H.-H.; Èrg, J.; Èller, M.; Ènert, A.G.; Beger, H.G. Prognostic Significance of Soluble Interleukin-2 Receptor-a in Adenocarcinoma of the Pancreas. Cancer Lett. 1998, 134, 193–199. [Google Scholar] [CrossRef]
- Bluestone, J.A.; MacKay, C.R.; O’Shea, J.J.; Stockinger, B. The Functional Plasticity of T Cell Subsets. Nat. Rev. Immunol. 2009, 9, 811–816. [Google Scholar] [CrossRef]
- Opal, S.M.; Depalo, V.A. Anti-Inflammatory Cytokines. Chest 2000, 117, 1162–1172. [Google Scholar] [CrossRef]
- Xue, J.; Sharma, V.; Hsieh, M.H.; Chawla, A.; Murali, R.; Pandol, S.J.; Habtezion, A. Alternatively Activated Macrophages Promote Pancreatic Fibrosis in Chronic Pancreatitis. Nat. Commun. 2015, 6, 7158. [Google Scholar] [CrossRef] [PubMed]
- Duggan, S.N.; Purcell, C.; Kilbane, M.; O’Keane, M.; McKenna, M.; Gaffney, P.; Ridgway, P.F.; Boran, G.; Conlon, K.C. An Association between Abnormal Bone Turnover, Systemic Inflammation, and Osteoporosis in Patients with Chronic Pancreatitis: A Case-Matched Study. Am. J. Gastroenterol. 2015, 110, 336–345. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Li, H.; Sun, L.; Brigstock, D.R.; Gao, R. NC-ND License Interleukin-6 Participates in Human Pancreatic Stellate Cell Activation and Collagen I Production via TGF-Β1/Smad Pathway. Cytokine 2021, 143, 1043–4666. [Google Scholar] [CrossRef] [PubMed]
- Saurer, L.; Reber, P.; Schaffner, T.; Buchlerbubuchler, M.W.; Buri, C.; Kappeler, A.; Walz, A.; Friess, H.; Mueller, C. Differential Expression of Chemokines in Normal Pancreas and in Chronic Pancreatitis. Gastroenterology 2000, 18, 356–367. [Google Scholar] [CrossRef] [PubMed]
- Di Sebastiano, P.; Di Mola, F.F.; Di Febbo, C.; Baccante, G.; Porreca, E.; Innocenti, P.; Friess, H.; Büchler, M.W. Expression of Interleukin 8 (IL-8) and Substance P in Human Chronic Pancreatitis. Gut 2000, 47, 423–428. [Google Scholar] [CrossRef][Green Version]
- Motoo, Y.; Xie, M.-J.; Mouri, H.; Sawabu, N. Expression of Interleukin-8 in Human Obstructive Pancreatitis. JOP 2004, 5, 138–144. [Google Scholar]
- Demols, A.; Van Laethem, J.-L.; Quertinmont, E.; Degraef, C.; Delhaye, M.; Geerts, A.; Deviere, J. Endogenous Interleukin-10 Modulates Fibrosis and Regeneration in Experimental Chronic Pancreatitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2002, 282, G1105–G1112. [Google Scholar] [CrossRef]
- Pezzilli, R.; Billi, P.; Miniero, R.; Barakat, B. Serum Interleukin-10 in Human Acute Pancreatitis. Dig. Dis. Sci. 1997, 42, 1469–1472. [Google Scholar] [CrossRef]
- Van Laethem, J.L.; Eskinazi, R.; Louis, H.; Rickaert, F.; Robberecht, P.; Devière, J. Multisystemic Production of Interleukin 10 Limits the Severity of Acute Pancreatitis in Mice. Gut 1998, 43, 408–413. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.A.; Singh, N.; Gunjan, D.; Gopi, S.; Dash, N.R.; Gupta, S.; Saraya, A. Increased Circulating Th17 Cell Populations in Patients with Pancreatic Ductal Adenocarcinoma. Immunogenetics 2023, 75, 433–443. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Chen, H.; Liu, L.; Xiao, P.; Xie, Y.; Geng, X.; Zhang, T.; Zhang, Y.; Lu, T.; Tan, H.; et al. Role of Interleukin-17 in Acute Pancreatitis. Front. Immunol. 2021, 12, 674803. [Google Scholar] [CrossRef] [PubMed]
- Jia, R.; Tang, M.; Qiu, L.; Sun, R.; Cheng, L.; Ma, X.; Yin, G.; Hu, G.; Wang, X.; Zhao, Y. Increased Interleukin-23/17 Axis and C-Reactive Protein Are Associated with Severity of Acute Pancreatitis in Patients. Pancreas 2015, 44, 321–325. [Google Scholar] [CrossRef] [PubMed]
- Mroczko, B.; Szmitkowski, M.; Wereszczyńska-Siemiatkowska, U.; Jurkowska, G. Hematopoietic Cytokines in the Sera of Patients with Pancreatic Cancer. Clin. Chem. Lab. Med. 2005, 43, 146–150. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, P.; Budnick, I.; Singh, M.; Thiruppathi, M.; Alharshawi, K.; Elshabrawy, H.; Holterman, M.J.; Prabhakar, B.S. Dual Role of GM-CSF as a Pro-Inflammatory and a Regulatory Cytokine: Implications for Immune Therapy. J. Interferon Cytokine Res. 2015, 35, 585–599. [Google Scholar] [CrossRef] [PubMed]
- Bayne, L.J.; Beatty, G.L.; Jhala, N.; Clark, C.E.; Rhim, A.D.; Stanger, B.Z.; Vonderheide, R.H. Tumor-Derived Granulocyte-Macrophage Colony Stimulating Factor Regulates Myeloid Inflammation and T Cell Immunity in Pancreatic Cancer. Cancer Cell 2012, 21, 822–835. [Google Scholar] [CrossRef] [PubMed]
- Halloran, P.F.; Autenried, P.; Ramassar, V.; Urmson, J.; Cockfield, S. Local T Cell Responses Induce Widespread MHC Expression. Evidence That IFN-Gamma Induces Its Own Expression in Remote Sites. J. Immunol. 1992, 148, 3837–3846. [Google Scholar] [CrossRef]
- Xie, M.J.; Motoo, Y.; Su, S.B.; Sawabu, N. Expression of Tumor Necrosis Factor-Alpha, Interleukin-6, and Interferon-Gamma in Spontaneous Chronic Pancreatitis in the WBN/Kob Rat. Pancreas 2001, 22, 400–408. [Google Scholar] [CrossRef] [PubMed]
- Sparmann, G.; Behrend, S.; Merkord, J.; Kleine, H.D.; Graser, E.; Ritter, T.; Liebe, S.; Emmrich, J. Cytokine MRNA Levels and Lymphocyte Infiltration in Pancreatic Tissue during Experimental Chronic Pancreatitis Induced by Dibutyltin Dichloride. Dig. Dis. Sci. 2001, 46, 1647–1656. [Google Scholar] [CrossRef] [PubMed]
- Hasel, C.; Rau, B.; Perner, S.; Sträter, J.; Möller, P. Differential and Mutually Exclusive Expression of CD95 and CD95 Ligand in Epithelia of Normal Pancreas and Chronic Pancreatitis. Lab. Investig. 2001, 81, 317–326. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pan, S.; Chen, R.; Crispin, D.A.; May, D.; Stevens, T.; McIntosh, M.W.; Bronner, M.P.; Ziogas, A.; Anton-Culver, H.; Brentnall, T.A. Protein Alterations Associated with Pancreatic Cancer and Chronic Pancreatitis Found in Human Plasma Using Global Quantitative Proteomics Profiling. J. Proteome Res. 2011, 10, 2359–2376. [Google Scholar] [CrossRef]
- Mohamed, A.; Saad, Y.; Saleh, D.; Elawady, R.; Eletreby, R.; Kharalla, A.S.; Badr, E. Can Serum ICAM 1 Distinguish Pancreatic Cancer from Chronic Pancreatitis? Asian Pac. J. Cancer Prev. 2016, 17, 4671–4675. [Google Scholar] [CrossRef]
- Resovi, A.; Bani, M.R.; Porcu, L.; Anastasia, A.; Minoli, L.; Allavena, P.; Cappello, P.; Novelli, F.; Scarpa, A.; Morandi, E.; et al. Soluble Stroma-related Biomarkers of Pancreatic Cancer. EMBO Mol. Med. 2018, 10, e8741. [Google Scholar] [CrossRef]
- Sato, T.; Shibata, W.; Maeda, S. Adhesion Molecules and Pancreatitis. J. Gastroenterol. 2019, 54, 99–107. [Google Scholar] [CrossRef]
- Rahbari, N.N.; Schmidt, T.; Falk, C.S.; Hinz, U.; Herber, M.; Bork, U.; Büchler, M.W.; Weitz, J.; Koch, M. Expression and Prognostic Value of Circulating Angiogenic Cytokines in Pancreatic Cancer. BMC Cancer 2011, 11, 286. [Google Scholar] [CrossRef]
- Shibuya, M. Vascular Endothelial Growth Factor (VEGF) and Its Receptor (VEGFR) Signaling in Angiogenesis: A Crucial Target for Anti- and Pro-Angiogenic Therapies. Genes Cancer 2011, 2, 1097–1105. [Google Scholar] [CrossRef]
- Yasuda, M.; Ito, T.; Oono, T.; Kawabe, K.; Kaku, T.; Igarashi, H.; Nakamura, T.; Takayanagi, R. Fractalkine and TGF-Β1 Levels Reflect the Severity of Chronic Pancreatitis in Humans. World J. Gastroenterol. 2008, 14, 6488–6495. [Google Scholar] [CrossRef] [PubMed]
- Kozak, A.; Talar-Wojnarowska, R.; Kaczka, A.; Borkowska, A.; Czupryniak, L.; Małecka-Panas, E.; Gąsiorowska, A. Utility of Different Serum Fibrosis Markers in Diagnosing Patients with Chronic Pancreatitis and Pancreatic Adenocarcinoma. World J. Gastrointest. Oncol. 2016, 8, 635–641. [Google Scholar] [CrossRef] [PubMed]
- Bazan, J.F.; Bacon, K.B.; Hardiman, G.; Wang, W.; Soo, K.; Rossi, D.; Greaves, D.R.; Zlotnik, A.; Schall, T.J. A New Class of Membrane-Bound Chemokine with a CX3C Motif. Nature 1997, 385, 640–644. [Google Scholar] [CrossRef] [PubMed]
- Adrych, K.; Stojek, M.; Smoczynski, M.; Sledzinski, T.; Sylwia, S.W.; Swierczynski, J. Increased Serum Chemerin Concentration in Patients with Chronic Pancreatitis. Dig. Liver Dis. 2012, 44, 393–397. [Google Scholar] [CrossRef] [PubMed]
- Kiczmer, P.; Szydło, B.; Prawdzic Seńkowska, A.; Jopek, J.; Wiewióra, M.; Piecuch, J.; Ostrowska, Z.; Świętochowska, E. Serum Omentin-1 and Chemerin Concentrations in Pancreatic Cancer and Chronic Pancreatitis. Folia Medica Cracoviensia 2018, LVIII, 77–87. [Google Scholar] [CrossRef]
- Hart, R.; Greaves, D.R. Chemerin Contributes to Inflammation by Promoting Macrophage Adhesion to VCAM-1 and Fibronectin through Clustering of VLA-4 and VLA-5. J. Immunol. 2010, 185, 3728–3739. [Google Scholar] [CrossRef]
- Chang, M.-C.; Chang, Y.-T.; Su, T.-C.; Yang, W.-S.; Chen, C.-L.; Tien, Y.-W.; Liang, P.-C.; Wei, S.-C.; Wong, J.-M. Adiponectin as a Potential Differential Marker to Distinguish Pancreatic Cancer and Chronic Pancreatitis. Pancreas 2007, 35, 16–21. [Google Scholar] [CrossRef]
- Trayhurn, P.; Wood, I.S. Adipokines: Inflammation and the Pleiotropic Role of White Adipose Tissue. Br. J. Nutr. 2004, 92, 347–355. [Google Scholar] [CrossRef]
- Fang, H.; Judd, R.L. Adiponectin Regulation and Function. Compr. Physiol. 2018, 8, 1031–1063. [Google Scholar] [CrossRef]
- Yamada, T.; Araki, H.; Watabe, K.; Kamada, Y.; Kiso, S.; Ogiyama, H.; Nishihara, T.; Kihara, S.; Funahashi, T.; Shimomura, I.; et al. Adiponectin Deficiency Enhanced the Severity of Cerulein-Induced Chronic Pancreatitis in Mice. J. Gastroenterol. 2010, 45, 742–749. [Google Scholar] [CrossRef] [PubMed]
- Kamath, M.G.; Pai, C.G.; Kamath, A.; Kurien, A. Monocyte Chemoattractant Protein-1, Transforming Growth Factor-Β1, Nerve Growth Factor, Resistin and Hyaluronic Acid as Serum Markers: Comparison between Recurrent Acute and Chronic Pancreatitis. Hepatobiliary Pancreat. Dis. Int. 2016, 15, 209–215. [Google Scholar] [CrossRef]
- Silswal, N.; Singh, A.K.; Aruna, B.; Mukhopadhyay, S.; Ghosh, S.; Ehtesham, N.Z. Human Resistin Stimulates the Pro-Inflammatory Cytokines TNF-Alpha and IL-12 in Macrophages by NF-KappaB-Dependent Pathway. Biochem. Biophys. Res. Commun. 2005, 334, 1092–1101. [Google Scholar] [CrossRef]
- Rychlíková, J.; Vecka, M.; Jáchymová, M.; Macášek, J.; Hrabák, P.; Zeman, M.; Vávrová, L.; Řoupal, J.; Krechler, T.; Ák, A. Osteopontin as a Discriminating Marker for Pancreatic Cancer and Chronic Pancreatitis. Cancer Biomark. 2016, 17, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Kolb, A.; Kleeff, J.; Guweidhi, A.; Esposito, I.; Giese, N.A.; Adwan, H.; Giese, T.; Büchler, M.W.; Berger, M.R.; Friess, H. Osteopontin Influences the Invasiveness of Pancreatic Cancer Cells and Is Increased in Neoplastic and Inflammatory Conditions. Cancer Biol. Ther. 2005, 4, 740–746. [Google Scholar] [CrossRef] [PubMed]
- Poruk, K.E.; Firpo, M.A.; Scaife, C.L.; Adler, D.G.; Emerson, L.L.; Boucher, K.M.; Mulvihill, S.J. Serum Osteopontin and Tissue Inhibitor of Metalloproteinase 1 as Diagnostic and Prognostic Biomarkers for Pancreatic Adenocarcinoma. Pancreas 2013, 42, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Koopmann, J.; Rosenzweig, C.N.W.; Zhang, Z.; Canto, M.I.; Brown, D.A.; Hunter, M.; Yeo, C.; Chan, D.W.; Breit, S.N.; Goggins, M. Serum Markers in Patients with Resectable Pancreatic Adenocarcinoma: Macrophage Inhibitory Cytokine 1 versus CA19-9. Clin. Cancer Res. 2006, 12, 442–446. [Google Scholar] [CrossRef]
- Lamort, A.-S.; Giopanou, I.; Psallidas, I.; Stathopoulos, G.T. Cells Osteopontin as a Link between Inflammation and Cancer: The Thorax in the Spotlight. Cells 2019, 8, 815. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Oka, M.; Iizuka, N.; Kawauchi, S.; Gondo, T.; Ueno, T.; Tangoku, A. Osteopontin Expression in Chronic Pancreatitis. Pancreas 2002, 25, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Z.; Gu, Y.; Huang, Y.; Zhang, L.; Zhang, X.; Xu, H.; Liu, H.; Zhong, Y. Elevated Serum Neopterin Concentration Increases Mortality Risk in Patients with Acute Pancreatitis. Pteridines 2019, 30, 16–20. [Google Scholar] [CrossRef]
- Uomo, G.; Spada, O.A.; Manes, G.; Feola, B.; Misso, S.; Cavallera, A.; Rabitti, P.G. Neopterin in Acute Pancreatitis. Scand. J. Gastroenterol. 1996, 31, 1032–1036. [Google Scholar] [CrossRef]
- Cavestro, G.M.; Zuppardo, R.A.; Bertolini, S.; Sereni, G.; Frulloni, L.; Okolicsanyi, S.; Calzolari, C.; Singh, S.K.; Sianesi, M.; Del Rio, P.; et al. Connections between Genetics and Clinical Data: Role of Mcp-1, Cftr, and Spink-1 in the Setting of Acute, Acute Recurrent, and Chronic Pancreatitis. Am. J. Gastroenterol. 2010, 105, 199–206. [Google Scholar] [CrossRef]
- Venkateshwari, A.; Sri Manjari, K.; Krishnaveni, D.; Nallari, P.; Vidyasagar, A.; Jyothy, A. Role of Plasma MMP 9 Levels in the Pathogenesis of Chronic Pancreatitis. Indian J. Clin. Biochem. 2011, 26, 136–139. [Google Scholar] [CrossRef] [PubMed]
- Mroczko, B.; Lukaszewicz-Zajac, M.; Wereszczynska-Siemiatkowska, U.; Groblewska, M.; Gryko, M.; Kedra, B.; Jurkowska, G.; Szmitkowski, M. Clinical Significance of the Measurements of Serum Matrix Metalloproteinase-9 and Its Inhibitor (Tissue Inhibitor of Metalloproteinase-1) in Patients With Pancreatic Cancer Metalloproteinase-9 as an Independent Prognostic Factor. Pancreas 2009, 38, 613–618. [Google Scholar] [CrossRef] [PubMed]
- Dranka-Bojarowska, D.; Lewinski, A.; Lekstan, A.; Gajda, M.; Ciosek, J.; Mrowiec, S. The Assessment of Serum and Diagnostic Peritoneal Lavage Concentration of Matrix Metalloproteinase-2, Matrix Metalloproteinase-9, Carbohydrate Antigen 19-9, and Carcinoembryonic Antigen in Patients with Pancreatic Cancer and Chronic Pancreatitis. J. Physiol. Pharmacol. 2020, 71, 689–704. [Google Scholar] [CrossRef]
- Grünwald, B.; Harant, V.; Schaten, S.; Frühschütz, M.; Spallek, R.; Höchst, B.; Stutzer, K.; Berchtold, S.; Erkan, M.; Prokopchuk, O.; et al. Pancreatic Premalignant Lesions Secrete Tissue Inhibitor of Metalloproteinases-1, Which Activates Hepatic Stellate Cells Via CD63 Signaling to Create a Premetastatic Niche in the Liver. Gastroenterology 2016, 151, 1011–1024.e7. [Google Scholar] [CrossRef] [PubMed]
- Prokopchuk, O.; Grünwald, B.; Nitsche, U.; Jäger, C.; Prokopchuk, O.L.; Schubert, E.C.; Friess, H.; Martignoni, M.E.; Krüger, A. Elevated Systemic Levels of the Matrix Metalloproteinase Inhibitor TIMP-1 Correlate with Clinical Markers of Cachexia in Patients with Chronic Pancreatitis and Pancreatic Cancer. BMC Cancer 2018, 18, 128. [Google Scholar] [CrossRef]
- Adrych, K.; Smoczynski, M.; Stojek, M.; Sledzinski, T.; Korczynska, J.; Goyke, E.; Swierczynski, J. Coordinated Increase in Serum Platelet-Derived Growth Factor-BB and Transforming Growth Factor-Β1 in Patients with Chronic Pancreatitis. Pancreatology 2011, 11, 434–440. [Google Scholar] [CrossRef]
- Chen, I.M.; Willumsen, N.; Dehlendorff, C.; Johansen, A.Z.; Jensen, B.V.; Hansen, C.P.; Hasselby, J.P.; Bojesen, S.E.; Pfeiffer, P.; Nielsen, S.E.; et al. Clinical Value of Serum Hyaluronan and Propeptide of Type III Collagen in Patients with Pancreatic Cancer. Int. J. Cancer 2020, 146, 2913–2922. [Google Scholar] [CrossRef]
- Suda, K. Distribution, Pathogenesis and Progression of Human Pancreatic Fibrosis. Gastroenterology 2007, 33, 67–79. [Google Scholar] [CrossRef]
- Patel, M.; Fine, D.R. Fibrogenesis in the Pancreas after Acinar Cell Injury. Scand. J. Surg. 2005, 94, 108–111. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, B.; Xu, X.-F.; Jiang, T.-T.; Zhang, X.-Q.; Shi, Y.-L.; Chen, Y.; Liu, F.; Gu, J.; Zhu, L.-J.; et al. Pathophysiology of Chronic Pancreatitis Induced by Dibutyltin Dichloride Joint Ethanol in Mice. World J. Gastroenterol. 2016, 22, 2960–2970. [Google Scholar] [CrossRef]
- Dhingra, R.; Singh, N.; Sachdev, V.; Ashish Datt Upadhyay, Þ.; Saraya, A. Effect of Antioxidant Supplementation on Surrogate Markers of Fibrosis in Chronic Pancreatitis A Randomized, Placebo-Controlled Trial. Pancreas 2013, 42, 589–595. [Google Scholar] [CrossRef] [PubMed]
- Manjari, K.S.; Nallari, P.; Vidyasagar, A.; Jyothy, A.; Venkateshwari, A. Plasma TGF-Β1, MMP-1 and MMP-3 Levels in Chronic Pancreatitis. Indian J. Clin. Biochem. 2012, 27, 152–156. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Li, Z.; Yu, X.; Werner, J.; Bazhin, A.V.; D’Haese, J.G. The Role of Interleukin-18 in Pancreatitis and Pancreatic Cancer. Cytokine Growth Factor Rev. 2019, 50, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Heldin, C.-H. Platelet-Derived Growth Factor (PDGF). In Encyclopedia of Hormones; Henry, H.L., Norman, A.W., Eds.; Academic Press: Cambridge, MA, USA, 2003; pp. 231–237. [Google Scholar]
- Singh, S.; Anshita, D.; Ravichandiran, V. MCP-1: Function, Regulation, and Involvement in Disease. Int. Immunopharmacol. 2021, 101, 107598. [Google Scholar] [CrossRef]
- Wang, X.; Li, Y.; Tian, H.; Qi, J.; Li, M.; Fu, C.; Wu, F.; Wang, Y.; Cheng, D.; Zhao, W.; et al. Macrophage Inhibitory Cytokine 1 (MIC-1/GDF15) as a Novel Diagnostic Serum Biomarker in Pancreatic Ductal Adenocarcinoma. BMC Cancer 2014, 14, 578. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.F.; Xu, L.X.; Huang, L.Y.; Guo, F.; Zhang, F.; He, X.Y.; Yuan, Y.Z.; Yao, W.Y. Combined Detection of Serum Ul16-Binding Protein 2 and Macrophage Inhibitory Cytokine-1 Improves Early Diagnosis and Prognostic Prediction of Pancreatic Cancer. Oncol. Lett. 2014, 8, 2096–2102. [Google Scholar] [CrossRef]
- Kaur, S.; Chakraborty, S.; Baine, M.J.; Mallya, K.; Smith, L.M.; Sasson, A.; Brand, R.; Guha, S.; Jain, M.; Wittel, U.; et al. Potentials of Plasma NGAL and MIC-1 as Biomarker(s) in the Diagnosis of Lethal Pancreatic Cancer. PLoS ONE 2013, 8, e55171. [Google Scholar] [CrossRef]
- Bootcov, M.R.; Bauskin, A.R.; Valenzuela, S.M.; Moore, A.G.; Bansal, M.; He, X.Y.; Zhang, H.P.; Donnellan, M.; Mahler, S.; Pryor, K.; et al. MIC-1, a Novel Macrophage Inhibitory Cytokine, Is a Divergent Member of the TGF-Superfamily. Cell Biol. South Wales 1997, 94, 11514–11519. [Google Scholar] [CrossRef]
- Fujiyama, T.; Ito, T.; Ueda, K.; Tachibana, Y.; Yasunaga, K.; Miki, M.; Takaoka, T.; Lee, L.; Kawabe, K.; Ogawa, Y. Serum Levels of Wisteria Floribunda Agglutinin-Positive Mac-2 Binding Protein Reflect the Severity of Chronic Pancreatitis. J. Dig. Dis. 2017, 18, 302–308. [Google Scholar] [CrossRef]
- Maekawa, T.; Kamada, Y.; Ebisutani, Y.; Ueda, M.; Hata, T.; Kawamoto, K.; Takamatsu, S.; Mizutani, K.; Shimomura, M.; Sobajima, T.; et al. Serum Mac-2 Binding Protein Is a Novel Biomarker for Chronic Pancreatitis. World J. Gastroenterol. 2016, 22, 4403–4410. [Google Scholar] [CrossRef]
- Sasaki, T.; Brakebusch, C.; Engel, J.; Timpl, R. Mac-2 Binding Protein Is a Cell-Adhesive Protein of the Extracellular Matrix Which Self-Assembles into Ring-like Structures and Binds Beta1 Integrins, Collagens and Fibronectin. EMBO J. 1998, 17, 1606–1613. [Google Scholar] [CrossRef]
- Inohara, H.; Akahani, S.; Koths, K.; Raz, A. Interactions between Galectin-3 and Mac-2-Binding Protein Mediate Cell-Cell Adhesion. Cancer Res. 1996, 56, 4530–4534. [Google Scholar] [PubMed]
- Fujiyoshi, M.; Kuno, A.; Gotoh, M.; Fukai, M.; Yokoo, H.; Kamachi, H.; Kamiyama, T.; Korenaga, M.; Mizokami, M.; Narimatsu, H.; et al. Clinicopathological Characteristics and Diagnostic Performance of Wisteria Floribunda Agglutinin Positive Mac-2-Binding Protein as a Preoperative Serum Marker of Liver Fibrosis in Hepatocellular Carcinoma. J. Gastroenterol. 2015, 50, 1134–1144. [Google Scholar] [CrossRef] [PubMed]
- Kamada, Y.; Fujii, H.; Fujii, H.; Sawai, Y.; Doi, Y.; Uozumi, N.; Mizutani, K.; Akita, M.; Sato, M.; Kida, S.; et al. Serum Mac-2 Binding Protein Levels as a Novel Diagnostic Biomarker for Prediction of Disease Severity and Nonalcoholic Steatohepatitis. Proteom. Clin. Appl. 2013, 7, 648–656. [Google Scholar] [CrossRef]
- Śliwińska-Mosson, M.; Milnerowicz, S.; Nabzdyk, S.; Kokot, I.; Nowak, M.; Milnerowicz, H. The Effect of Smoking on Endothelin-1 in Patients With Chronic Pancreatitis. Appl. Immunohistochem. Mol. Morphol. 2014, 23, 288–296. [Google Scholar] [CrossRef]
- Jonitz, A.; Fitzner, B.; Jaster, R. Molecular Determinants of the Profibrogenic Effects of Endothelin-1 in Pancreatic Stellate Cells. World J. Gastroenterol. 2009, 15, 4143–4149. [Google Scholar] [CrossRef]
- Meggiato, T.; Plebani, M.; Basso, D.; Panozzo, M.P.; Del Favero, G. Serum Growth Factors in Patients with Pancreatic Cancer. Tumor Biol. 1999, 20, 65–71. [Google Scholar] [CrossRef]
- Wong, R.W.C.; Guillaud, L. The Role of Epidermal Growth Factor and Its Receptors in Mammalian CNS. Cytokine Growth Factor Rev. 2004, 15, 147–156. [Google Scholar] [CrossRef]
- Blaine, S.A.; Ray, K.C.; Branch, K.M.; Robinson, P.S.; Whitehead, R.H.; Means, A.L. Epidermal Growth Factor Receptor Regulates Pancreatic Fibrosis. Am. J. Physiol. Gastrointest. Liver Physiol. 2009, 297, 434–441. [Google Scholar] [CrossRef]
- Wlodarczyk, B.; Borkowska, A.; Wlodarczyk, P.; Malecka-Panas, E.; Gasiorowska, A. Insulin-like Growth Factor 1 and Insulin-like Growth Factor Binding Protein 2 Serum Levels as Potential Biomarkers in Differential Diagnosis between Chronic Pancreatitis and Pancreatic Adenocarcinoma in Reference to Pancreatic Diabetes. Przegląd Gastroenterol. 2021, 16, 36–72. [Google Scholar] [CrossRef]
- Wlodarczyk, B.; Gasiorowska, A.; Borkowska, A.; Malecka-Panas, E. Evaluation of Insulin-like Growth Factor (IGF-1) and Retinol Binding Protein (RBP-4) Levels in Patients with Newly Diagnosed Pancreatic Adenocarcinoma (PDAC). Pancreatology 2017, 17, 623–628. [Google Scholar] [CrossRef]
- Xu, J.W.; Wang, T.X.; You, L.; Zheng, L.F.; Shu, H.; Zhang, T.P.; Zhao, Y.P. Insulin-like Growth Factor 1 Receptor (IGF-1R) as a Target of MiR-497 and Plasma IGF-1R Levels Associated with TNM Stage of Pancreatic Cancer. PLoS ONE 2014, 9, e92847. [Google Scholar] [CrossRef] [PubMed]
- Al-Samerria, S.; Radovick, S. The Role of Insulin-like Growth Factor-1 (Igf-1) in the Control of Neuroendocrine Regulation of Growth. Cells 2021, 10, 2664. [Google Scholar] [CrossRef] [PubMed]
- Rosendahl, A.H.; Gundewar, C.; Hilmersson, K.S.; Ni, L.; Saleem, M.A.; Andersson, R. Conditionally Immortalized Human Pancreatic Stellate Cell Lines Demonstrate Enhanced Proliferation and Migration in Response to IGF-I. Exp. Cell Res. 2014, 330, 300–310. [Google Scholar] [CrossRef] [PubMed]
- Sajewicz, W.; Milnerowicz, S.; Nabzdyk, S. Blood Plasma Antioxidant Defense in Patients With Pancreatitis. Pancreas 2006, 32, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, N.; Watanabe, N.; Okamoto, T.; Niitsu, Y. Specific Interaction of Pancreatic Elastase and Leucocytes to Produce Oxygen Radicals and Its Implication in Pancreatitis. Gut 1994, 35, 1659–1664. [Google Scholar] [CrossRef] [PubMed]
- Schoenberg, M.H.; Buchler, M.; Pietrzyk, C.; Uhl, W.; Birk, D.; Eisele, S.; Marzinzig, M.; Beger, H.G. Lipid Peroxidation and Glutathione Metabolism in Chronic Pancreatitis. Pancreas 1995, 10, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Fukui, M.; Kanoh, M.; Takamatsu, Y.; Arakawa, Y. Analysis of Serum Catalase Activities in Pancreatic Diseases. J. Gastroenterol. 2004, 39, 469–474. [Google Scholar] [CrossRef]
- López, M.A.; Alcaraz, C.A. Oxidative Stress and Acute Pancreatitis. World J. Gastroenterol. 2011, 103, 559–562. [Google Scholar] [CrossRef]
- Tasci, I.; Deveci, S.; Isik, A.T.; Comert, B.; Akay, C.; Mas, N.; Inal, V.; Yamanel, L.; Mas, M.R. Allopurinol in Rat Chronic Pancreatitis: Effects on Pancreatic Stellate Cell Activation. Pancreas 2007, 35, 366–371. [Google Scholar] [CrossRef]
- Kirk, G.R.; White, J.S.; McKie, L.; Stevenson, M.; Young, I.; Clements, W.D.B.; Rowlands, B.J. Combined Antioxidant Therapy Reduces Pain and Improves Quality of Life in Chronic Pancreatitis. J. Gastrointest. Surg. 2006, 10, 499–503. [Google Scholar] [CrossRef] [PubMed]
- Salim, A.S. Role of Oxygen-Derived Free Radical Scavengers in the Treatment of Recurrent Pain Produced by Chronic Pancreatitis. A New Approach. Arch. Surg. 1991, 126, 1109–1114. [Google Scholar] [CrossRef]
- Santini, S.A.; Spada, C.; Bononi, F.; Foschia, F.; Mutignani, M.; Perri, V.; Giardina, B.; Gentiloni Silveri, N.; Costamagna, G. Enhanced Lipoperoxidation Products in Pure Pancreatic Juice: Evidence for Organ-Specific Oxidative Stress in Chronic Pancreatitis. Dig. Liver Dis. 2003, 35, 888–892. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Lin, B. Decreased Serum Paraoxonase Activity in Patients With Chronic Pancreatitis. Am. J. Med. Sci. 2013, 346, 363–365. [Google Scholar] [CrossRef]
- Kodydkova, J.; Vavrova, L.; Stankova, B.; Macasek, J.; Krechler, T.; Zak, A. Antioxidant Status and Oxidative Stress Markers in Pancreatic Cancer and Chronic Pancreatitis. Pancreas 2013, 42, 614–621. [Google Scholar] [CrossRef] [PubMed]
- Verlaan, M.; Roelofs, H.M.; van Schaik, A.; Wanten, G.J.; Jansen, J.B.; Peters, W.H.; Drenth, J.P. Assessment of Oxidative Stress in Chronic Pancreatitis Patients. World J. Gastroenterol. 2006, 12, 5705. [Google Scholar] [CrossRef]
- Girish, B.N.; Rajesh, G.; Vaidyanathan, K. Deficiency of Folate and Vitamin B12 Increases Oxidative Stress in Chronic Pancreatitis Patients. Indian J. Gastroenterol. 2022, 41, 77–83. [Google Scholar] [CrossRef]
- Girish, B.; Rajesh, G.; Vaidyanathan, K.; Balakrishnan, V. Assessment of Oxidative Status in Chronic pancreatitis and Its Relation with Zinc Status. Indian J. Gastroenterol. 2011, 30, 63–65. [Google Scholar] [CrossRef]
- Bhardwaj, P.; Garg, P.K.; Maulik, S.K.; Saraya, A.; Tandon, R.K.; Acharya, S.K. A Randomized Controlled Trial of Antioxidant Supplementation for Pain Relief in Patients With Chronic Pancreatitis. Gastroenterology 2009, 136, 149–159. [Google Scholar] [CrossRef]
- Van Gossum, A.; Closset, P.; Noel, E.; Cremer, M.; Neve, J. Deficiency in Antioxidant Factors in Patients with Alcohol-Related Chronic Pancreatitis. Dig. Dis. Sci. 1996, 41, 1225–1231. [Google Scholar] [CrossRef]
- Aguilar Diaz De Leon, J.; Borges, C.R. Evaluation of Oxidative Stress in Biological Samples Using the Thiobarbituric Acid Reactive Substances Assay. J. Vis. Exp. 2020, 159, e61122. [Google Scholar] [CrossRef]
- Bopanna, S.; Nayak, B.; Prakash, S.; Shalimar; Mahapatra, S.J.; Garg, P.K. Increased Oxidative Stress and Deficient Antioxidant Levels May Be Involved in the Pathogenesis of Idiopathic Recurrent Acute Pancreatitis. Pancreatology 2017, 17, 529–533. [Google Scholar] [CrossRef] [PubMed]
- Podborska, M.; Sevcikova, A.; Trna, J.; Dite, P.; Lojek, A.; Kubala, L. Increased Markers of Oxidative Stress in Plasma of Patients with Chronic Pancreatitis. Neuroendocrinol. Lett. 2009, 30, 300709–300728. [Google Scholar]
- Trevisani, M.; Siemens, J.; Materazzi, S.; Bautista, D.M.; Nassini, R.; Campi, B.; Imamachi, N.; Andrè, E.; Patacchini, R.; Cottrell, G.S.; et al. 4-Hydroxynonenal, an Endogenous Aldehyde, Causes Pain and Neurogenic Inflammation through Activation of the Irritant Receptor TRPA1. Proc. Natl. Acad. Sci. USA 2007, 104, 13519–13524. [Google Scholar] [CrossRef] [PubMed]
- Szuster-Ciesielska, A.; Daniluk, J.; Kandefer-Szerszeń, M. Oxidative Stress in Blood of Patients with Alcohol-Related Pancreatitis. Pancreas 2001, 22, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Girish, B.N.; Vaidyanathan, K.; Nanjundarao, Þ.; Rao, A.; Rajesh, G.; Reshmi, S.; Balakrishnan, V. Chronic Pancreatitis Is Associated With Hyperhomocysteinemia and Derangements in Transsulfuration and Transmethylation Pathways. Pancreas 2009, 39, e11–e16. [Google Scholar] [CrossRef]
- Quilliot, D.; Walters, E.; Bonte, J.-P.; Fruchart, J.-C.; Duriez, P.; Ziegler, O. Diabetes Mellitus Worsens Antioxidant Status in Patients with Chronic Pancreatitis. Am. J. Clin. Nutr. 2005, 81, 1117–1125. [Google Scholar] [CrossRef][Green Version]
- Matsumoto, M.; Wakasugi, H.; Ibayashi, H. Serum Vitamine E, Lipid Peroxide and Glutathione Peroxidase in Patients with Chronic Pancreatitis. Cfinica Chimica Acta 1981, 110, 121–125. [Google Scholar] [CrossRef]
- Morris-Stiff, G.J.; Bowrey, D.J.; Oleesky, D.; Davies, M.; B Clark, G.W.; A Puntis, M.C. The Antioxidant Profiles of Patients With Recurrent Acute and Chronic Pancreatitis. Am J. Gastroenterol. 1999, 94, 2135–2140. [Google Scholar] [CrossRef]
- Liu, H.; Guo, H.; Jian, Z.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Copper Induces Oxidative Stress and Apoptosis in the Mouse Liver. Oxidative Med. Cell. Longev. 2020, 2020, 1359164. [Google Scholar] [CrossRef]
- Kawaguchi, Y.; Ogawa, M.; Ito, H.; Mine, T. Alterations in Plasma Amino Acid Levels in Alcoholic Chronic Pancreatitis in Japanese. Digestion 2012, 86, 155–160. [Google Scholar] [CrossRef]
- Sogawa, K.; Yamanaka, S.; Takano, S.; Sasaki, K.; Miyahara, Y.; Furukawa, K.; Takayashiki, T.; Kuboki, S.; Takizawa, H.; Nomura, F.; et al. Fucosylated C4b-Binding Protein α-Chain, a Novel Serum Biomarker That Predicts Lymph Node Metastasis in Pancreatic Ductal Adenocarcinoma. Oncol. Lett. 2021, 21, 127. [Google Scholar] [CrossRef]
- Nissen, N.I.; Johansen, A.Z.; Chen, I.M.; Jensen, C.; Madsen, E.A.; Hansen, C.P.; Thorlacius-Ussing, J.; Karsdal, M.; Johansen, J.S.; Diab, H.M.H.; et al. High Serum Levels of the C-Propetide of Type V Collagen (PRO-C5) Are Prognostic for Short Overall Survival in Patients with Pancreatic Ductal Adenocarcinoma. Front Mol Biosci 2023, 10, 1158058. [Google Scholar] [CrossRef]
- Slater, E.P.; Fendrich, V.; Strauch, K.; Rospleszcz, S.; Ramaswamy, A.; Matthäi, E.; Chaloupka, B.; Gress, T.M.; Langer, P.; Bartsch, D.K. LCN2 and TIMP1 as Potential Serum Markers for the Early Detection of Familial Pancreatic Cancer. Transl. Oncol. 2013, 6, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Iglesias-García, J.; Abdulkader, I.; Lariño-Noia, J.; Forteza, J.; Dominguez-Muñoz, J.E. Histological Evaluation of Chronic Pancreatitis by Endoscopic Ultrasound-Guided Fine Needle Biopsy. Gut 2006, 55, 1661–1662. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kikuta, K.; Masamune, A.; Satoh, M.; Suzuki, N.; Satoh, K.; Shimosegawa, T. Hydrogen Peroxide Activates Activator Protein-1 and Mitogen-Activated Protein Kinases in Pancreatic Stellate Cells. Mol. Cell. Biochem. 2006, 291, 11–20. [Google Scholar] [CrossRef]
- Lee, B.; Jones, E.K.; Manohar, M.; Li, L.; Yadav, D.; Conwell, D.L.; Hart, P.A.; Vege, S.S.; Fogel, E.L.; Serrano, J.; et al. Distinct Serum Immune Profiles Define the Spectrum of Acute and Chronic Pancreatitis From the Multicenter Prospective Evaluation of Chronic Pancreatitis for Epidemiologic and Translational Studies (PROCEED) Study. Gastroenterology 2023, 165, 173–186. [Google Scholar] [CrossRef]
- Novovic, S.; Borch, A.; Werge, M.; Karran, D.; Gluud, L.; Schmidt, P.N.; Hansen, E.F.; Nøjgaard, C.; Jensen, A.B.; Jensen, F.K.; et al. Characterisation of the Fibroinflammatory Process Involved in Progression from Acute to Chronic Pancreatitis: Study Protocol for a Multicentre, Prospective Cohort Study. BMJ Open 2019, 9, e028999. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poulsen, V.V.; Hadi, A.; Werge, M.P.; Karstensen, J.G.; Novovic, S. Circulating Biomarkers Involved in the Development of and Progression to Chronic Pancreatitis—A Literature Review. Biomolecules 2024, 14, 239. https://doi.org/10.3390/biom14020239
Poulsen VV, Hadi A, Werge MP, Karstensen JG, Novovic S. Circulating Biomarkers Involved in the Development of and Progression to Chronic Pancreatitis—A Literature Review. Biomolecules. 2024; 14(2):239. https://doi.org/10.3390/biom14020239
Chicago/Turabian StylePoulsen, Valborg Vang, Amer Hadi, Mikkel Parsberg Werge, John Gásdal Karstensen, and Srdan Novovic. 2024. "Circulating Biomarkers Involved in the Development of and Progression to Chronic Pancreatitis—A Literature Review" Biomolecules 14, no. 2: 239. https://doi.org/10.3390/biom14020239
APA StylePoulsen, V. V., Hadi, A., Werge, M. P., Karstensen, J. G., & Novovic, S. (2024). Circulating Biomarkers Involved in the Development of and Progression to Chronic Pancreatitis—A Literature Review. Biomolecules, 14(2), 239. https://doi.org/10.3390/biom14020239







