Therapeutic Potential of IL-1 Antagonism in Hidradenitis Suppurativa
Abstract
1. Introduction
2. The IL-1 Family
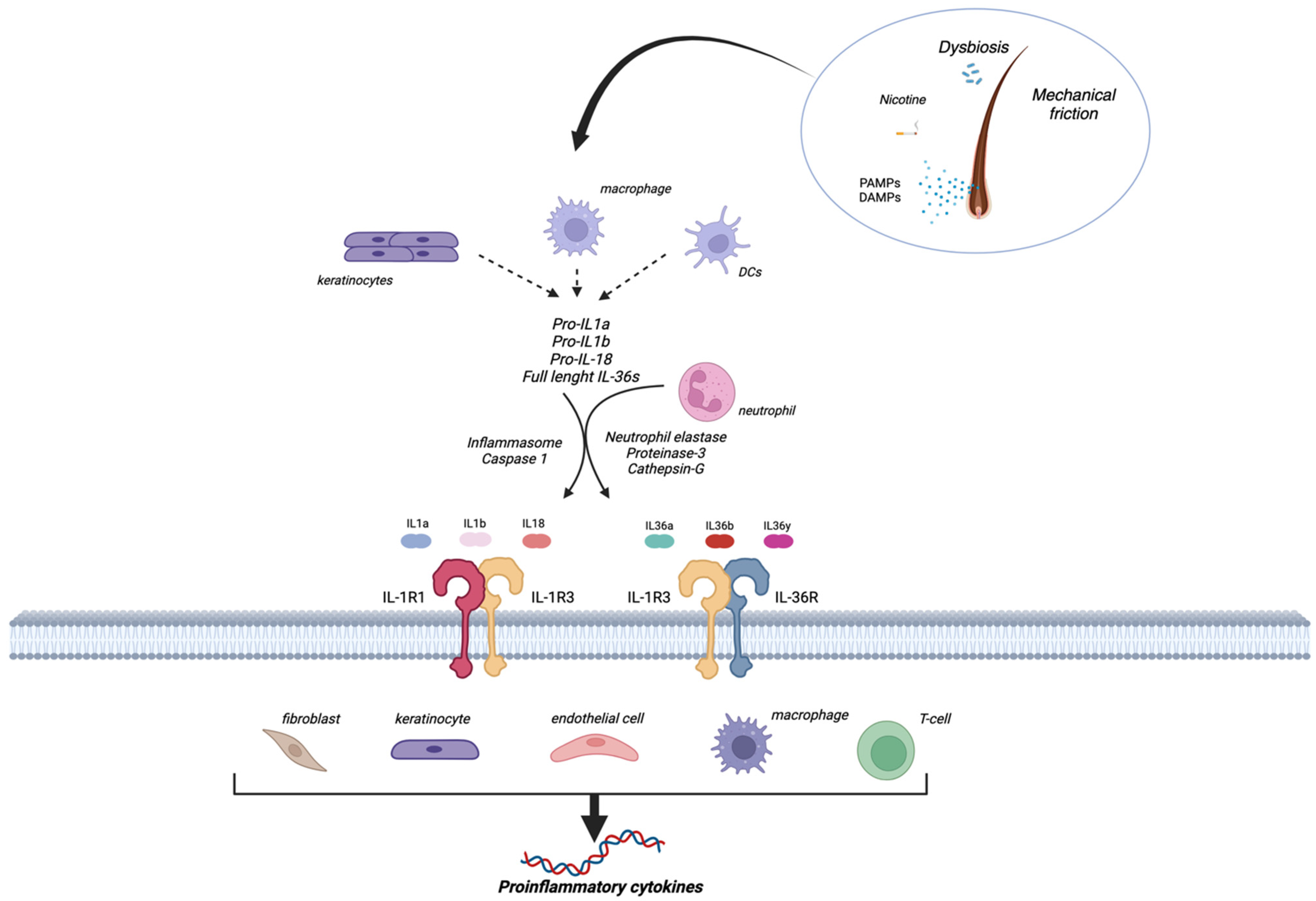
3. Hidradenitis Suppurativa Pathogenesis
4. Evidence of the Role of IL-1 in Hidradenitis Suppurativa
4.1. Preclinical Evidence
4.2. HS as an Autoinflammatory Disease
4.3. Clinical Evidence
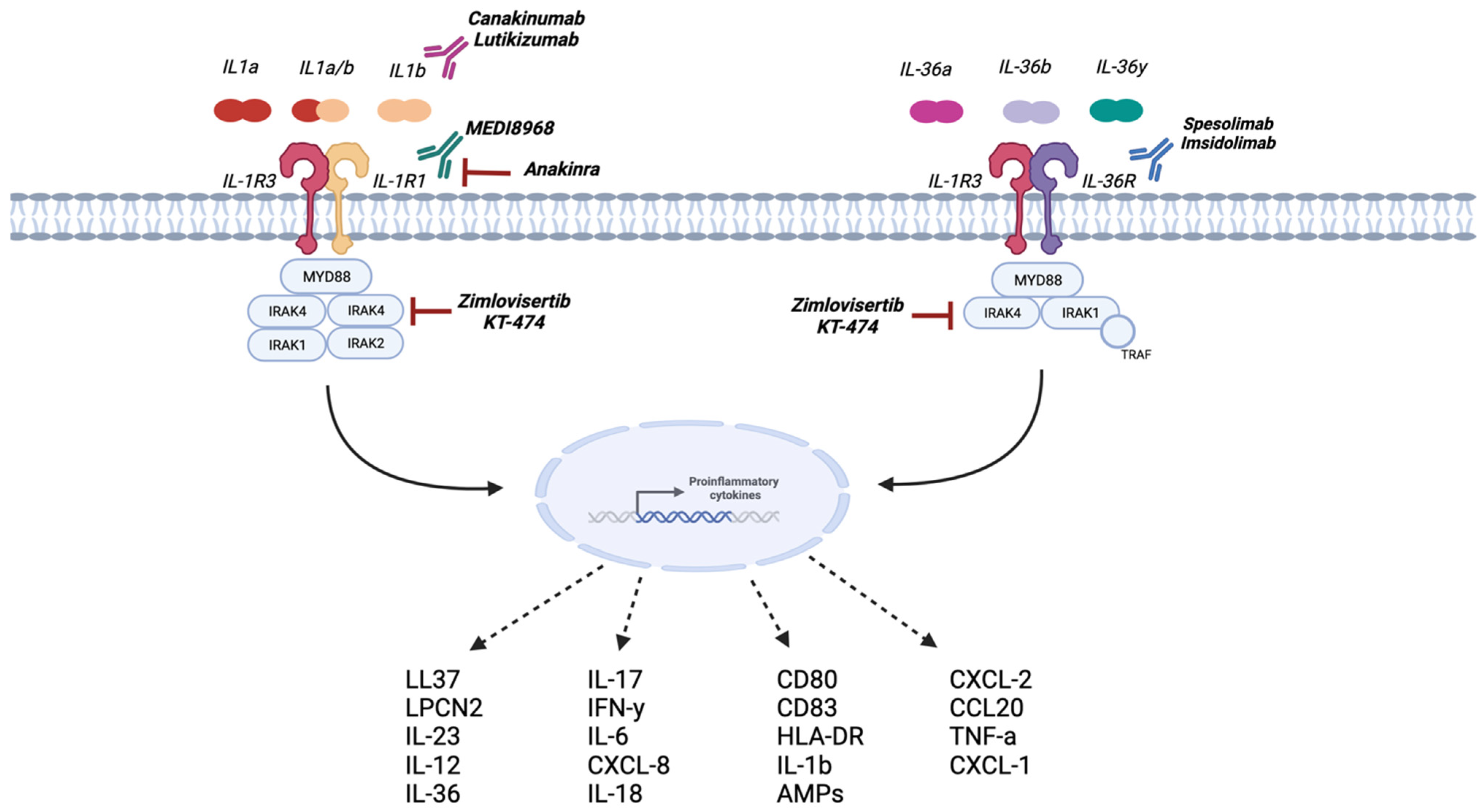
4.3.1. Anakinra
4.3.2. Bermekimab
4.3.3. MEDI8968 (AMG 108)
4.3.4. Canakinumab
4.3.5. Lutikizumab
4.3.6. Zimlovisertib and KT-474
4.3.7. MAS-825
4.3.8. Spesolimab
4.3.9. Imsidolimab
5. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Jfri, A.; Nassim, D.; O’Brien, E.; Gulliver, W.; Nikolakis, G.; Zouboulis, C.C. Prevalence of Hidradenitis Suppurativa: A Systematic Review and Meta-regression Analysis. JAMA Dermatol. 2021, 157, 924–931. [Google Scholar] [CrossRef]
- Chernyshov, P.V.; Finlay, A.Y.; Tomas-Aragones, L.; Poot, F.; Sampogna, F.; Marron, S.E.; Zemskov, S.V.; Abeni, D.; Tzellos, T.; Szepietowski, J.C.; et al. Quality of Life in Hidradenitis Suppurativa: An Update. Int. J. Environ. Res. Public. Health 2021, 18, 6131. [Google Scholar] [CrossRef]
- Sabat, R.; Jemec, G.B.E.; Matusiak, L.; Kimball, A.B.; Prens, E.; Wolk, K. Hidradenitis suppurativa. Nat. Rev. Dis. Primers 2020, 6, 18. [Google Scholar] [CrossRef]
- Cugno, M.; Borghi, A.; Marzano, A.V. PAPA, PASH and PAPASH Syndromes: Pathophysiology, Presentation and Treatment. Am. J. Clin. Dermatol. 2017, 18, 555–562. [Google Scholar] [CrossRef]
- Tzanetakou, V.; Kanni, T.; Giatrakou, S.; Katoulis, A.; Papadavid, E.; Netea, M.G.; Dinarello, C.A.; van der Meer, J.W.M.; Rigopoulos, D.; Giamarellos-Bourboulis, E.J. Safety and Efficacy of Anakinra in Severe Hidradenitis Suppurativa: A Randomized Clinical Trial. JAMA Dermatol. 2016, 152, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Gottlieb, A.; Natsis, N.E.; Kerdel, F.; Forman, S.; Gonzalez, E.; Jimenez, G.; Hernandez, L.; Kaffenberger, J.; Guido, G.; Lucas, K.; et al. A Phase II Open-Label Study of Bermekimab in Patients with Hidradenitis Suppurativa Shows Resolution of Inflammatory Lesions and Pain. J. Investig. Dermatol. 2020, 140, 1538–1545.e2. [Google Scholar] [CrossRef] [PubMed]
- Kimball, A.B.; Okun, M.M.; Williams, D.A.; Gottlieb, A.B.; Papp, K.A.; Zouboulis, C.C.; Armstrong, A.W.; Kerdel, F.; Gold, M.H.; Forman, S.B.; et al. Two Phase 3 Trials of Adalimumab for Hidradenitis Suppurativa. N. Engl. J. Med. 2016, 375, 422–434. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.novartis.com/news/media-releases/fda-approves-novartis-cosentyx-first-new-biologic-treatment-option-hidradenitis-suppurativa-patients-nearly-decade (accessed on 20 November 2023).
- Snyder, C.L.; Gibson, R.S.; Porter, M.L.; Kimball, A.B. Secukinumab in the treatment of hidradenitis suppurativa. Immunotherapy 2023, 15, 1449–1457. [Google Scholar] [CrossRef] [PubMed]
- Dinarello, C.A. Overview of the IL-1 family in innate inflammation and acquired immunity. Immunol. Rev. 2018, 281, 8–27. [Google Scholar] [CrossRef] [PubMed]
- Dinarello, C.A. Immunological and inflammatory functions of the interleukin-1 family. Annu. Rev. Immunol. 2009, 27, 519–550. [Google Scholar] [CrossRef] [PubMed]
- Fields, J.K.; Gunther, S.; Sundberg, E.J. Structural Basis of IL-1 Family Cytokine Signaling. Front. Immunol. 2019, 10, 1412. [Google Scholar] [CrossRef]
- Boraschi, D.; Italiani, P.; Weil, S.; Martin, M.U. The family of the interleukin-1 receptors. Immunol. Rev. 2018, 281, 197–232. [Google Scholar] [CrossRef]
- van de Veerdonk, F.L.; Stoeckman, A.K.; Wu, G.; Boeckermann, A.N.; Azam, T.; Netea, M.G.; Joosten, L.A.; van der Meer, J.W.; Hao, R.; Kalabokis, V.; et al. IL-38 binds to the IL-36 receptor and has biological effects on immune cells similar to IL-36 receptor antagonist. Proc. Natl. Acad. Sci. USA 2012, 109, 3001–3005. [Google Scholar] [CrossRef]
- Buhl, A.L.; Wenzel, J. Interleukin-36 in Infectious and Inflammatory Skin Diseases. Front. Immunol. 2019, 10, 1162. [Google Scholar] [CrossRef]
- Liew, F.Y.; Girard, J.P.; Turnquist, H.R. Interleukin-33 in health and disease. Nat. Rev. Immunol. 2016, 16, 676–689. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, K.; Nakanishi, K.; Tsutsui, H. Interleukin-18 in Health and Disease. Int. J. Mol. Sci. 2019, 20, 649. [Google Scholar] [CrossRef] [PubMed]
- Bufler, P.; Azam, T.; Gamboni-Robertson, F.; Reznikov, L.L.; Kumar, S.; Dinarello, C.A.; Kim, S.H. A complex of the IL-1 homologue IL-1F7b and IL-18-binding protein reduces IL-18 activity. Proc. Natl. Acad. Sci. USA 2002, 99, 13723–13728. [Google Scholar] [CrossRef]
- Pan, G.; Risser, P.; Mao, W.; Baldwin, D.T.; Zhong, A.W.; Filvaroff, E.; Yansura, D.; Lewis, L.; Eigenbrot, C.; Henzel, W.J.; et al. IL-1H, an interleukin 1-related protein that binds IL-18 receptor/IL-1Rrp. Cytokine 2001, 13, 1–7. [Google Scholar] [CrossRef]
- Wolk, K.; Join-Lambert, O.; Sabat, R. Aetiology and pathogenesis of hidradenitis suppurativa. Br. J. Dermatol. 2020, 183, 999–1010. [Google Scholar] [CrossRef]
- Hoffman, L.K.; Ghias, M.H.; Lowes, M.A. Pathophysiology of hidradenitis suppurativa. Semin. Cutan. Med. Surg. 2017, 36, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Hunger, R.E.; Surovy, A.M.; Hassan, A.S.; Braathen, L.R.; Yawalkar, N. Toll-like receptor 2 is highly expressed in lesions of acne inversa and colocalizes with C-type lectin receptor. Br. J. Dermatol. 2008, 158, 691–697. [Google Scholar] [CrossRef]
- Feldmeyer, L.; Werner, S.; French, L.E.; Beer, H.D. Interleukin-1, inflammasomes and the skin. Eur. J. Cell Biol. 2010, 89, 638–644. [Google Scholar] [CrossRef]
- Lima, A.L.; Karl, I.; Giner, T.; Poppe, H.; Schmidt, M.; Presser, D.; Goebeler, M.; Bauer, B. Keratinocytes and neutrophils are important sources of proinflammatory molecules in hidradenitis suppurativa. Br. J. Dermatol. 2016, 174, 514–521. [Google Scholar] [CrossRef]
- Giamarellos-Bourboulis, E.J. Keratinocytes as drivers of hidradenitis suppurativa inflammation: Need for priming. Br. J. Dermatol. 2023, 188, 318–319. [Google Scholar] [CrossRef] [PubMed]
- Foster, A.M.; Baliwag, J.; Chen, C.S.; Guzman, A.M.; Stoll, S.W.; Gudjonsson, J.E.; Ward, N.L.; Johnston, A. IL-36 promotes myeloid cell infiltration, activation, and inflammatory activity in skin. J. Immunol. 2014, 192, 6053–6061. [Google Scholar] [CrossRef]
- Satoh, T.K.; Mellett, M.; Contassot, E.; French, L.E. Are neutrophilic dermatoses autoinflammatory disorders? Br. J. Dermatol. 2018, 178, 603–613. [Google Scholar] [CrossRef]
- Henry, C.M.; Sullivan, G.P.; Clancy, D.M.; Afonina, I.S.; Kulms, D.; Martin, S.J. Neutrophil-Derived Proteases Escalate Inflammation through Activation of IL-36 Family Cytokines. Cell Rep. 2016, 14, 708–722. [Google Scholar] [CrossRef]
- Macleod, T.; Doble, R.; McGonagle, D.; Wasson, C.W.; Alase, A.; Stacey, M.; Wittmann, M. Neutrophil Elastase-mediated proteolysis activates the anti-inflammatory cytokine IL-36 Receptor antagonist. Sci. Rep. 2016, 6, 24880. [Google Scholar] [CrossRef] [PubMed]
- Molinelli, E.; Gioacchini, H.; Sapigni, C.; Diotallevi, F.; Brisigotti, V.; Rizzetto, G.; Offidani, A.; Simonetti, O. New Insight into the Molecular Pathomechanism and Immunomodulatory Treatments of Hidradenitis Suppurativa. Int. J. Mol. Sci. 2023, 24, 8428. [Google Scholar] [CrossRef] [PubMed]
- Witte-Handel, E.; Wolk, K.; Tsaousi, A.; Irmer, M.L.; Mossner, R.; Shomroni, O.; Lingner, T.; Witte, K.; Kunkel, D.; Salinas, G.; et al. The IL-1 Pathway Is Hyperactive in Hidradenitis Suppurativa and Contributes to Skin Infiltration and Destruction. J. Investig. Dermatol. 2019, 139, 1294–1305. [Google Scholar] [CrossRef]
- Malvaso, D.; Calabrese, L.; Chiricozzi, A.; Antonelli, F.; Coscarella, G.; Rubegni, P.; Peris, K. IL-17 Inhibition: A Valid Therapeutic Strategy in the Management of Hidradenitis Suppurativa. Pharmaceutics 2023, 15, 2450. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Debets, R.; Hegmans, J.J.; Benner, R.; Prens, E.P. IL-1 beta and IFN-gamma induce the regenerative epidermal phenotype of psoriasis in the transwell skin organ culture system. IFN-gamma up-regulates the expression of keratin 17 and keratinocyte transglutaminase via endogenous IL-1 production. J. Pathol. 1999, 187, 358–364. [Google Scholar] [CrossRef]
- van der Zee, H.H.; de Ruiter, L.; van den Broecke, D.G.; Dik, W.A.; Laman, J.D.; Prens, E.P. Elevated levels of tumour necrosis factor (TNF)-alpha, interleukin (IL)-1beta and IL-10 in hidradenitis suppurativa skin: A rationale for targeting TNF-alpha and IL-1beta. Br. J. Dermatol. 2011, 164, 1292–1298. [Google Scholar] [CrossRef] [PubMed]
- Vossen, A.; van Straalen, K.R.; Florencia, E.F.; Prens, E.P. Lesional Inflammatory Profile in Hidradenitis Suppurativa Is Not Solely Driven by IL-1. J. Investig. Dermatol. 2020, 140, 1463–1466.e2. [Google Scholar] [CrossRef]
- Kelly, G.; Hughes, R.; McGarry, T.; van den Born, M.; Adamzik, K.; Fitzgerald, R.; Lawlor, C.; Tobin, A.M.; Sweeney, C.M.; Kirby, B. Dysregulated cytokine expression in lesional and nonlesional skin in hidradenitis suppurativa. Br. J. Dermatol. 2015, 173, 1431–1439. [Google Scholar] [CrossRef]
- Calabrese, L.; Fiocco, Z.; Satoh, T.K.; Peris, K.; French, L.E. Therapeutic potential of targeting interleukin-1 family cytokines in chronic inflammatory skin diseases. Br. J. Dermatol. 2022, 186, 925–941. [Google Scholar] [CrossRef]
- Thomi, R.; Kakeda, M.; Yawalkar, N.; Schlapbach, C.; Hunger, R.E. Increased expression of the interleukin-36 cytokines in lesions of hidradenitis suppurativa. J. Eur. Acad. Dermatol. Venereol. 2017, 31, 2091–2096. [Google Scholar] [CrossRef] [PubMed]
- Hessam, S.; Sand, M.; Gambichler, T.; Skrygan, M.; Ruddel, I.; Bechara, F.G. Interleukin-36 in hidradenitis suppurativa: Evidence for a distinctive proinflammatory role and a key factor in the development of an inflammatory loop. Br. J. Dermatol. 2018, 178, 761–767. [Google Scholar] [CrossRef]
- Di Caprio, R.; Balato, A.; Caiazzo, G.; Lembo, S.; Raimondo, A.; Fabbrocini, G.; Monfrecola, G. IL-36 cytokines are increased in acne and hidradenitis suppurativa. Arch. Dermatol. Res. 2017, 309, 673–678. [Google Scholar] [CrossRef]
- Hayran, Y.; Alli, N.; Yucel, C.; Akdogan, N.; Turhan, T. Serum IL-36alpha, IL-36beta, and IL-36gamma levels in patients with hidradenitis suppurativa: Association with disease characteristics, smoking, obesity, and metabolic syndrome. Arch. Dermatol. Res. 2020, 312, 187–196. [Google Scholar] [CrossRef]
- Shanmugam, V.K.; Jones, D.; McNish, S.; Bendall, M.L.; Crandall, K.A. Transcriptome patterns in hidradenitis suppurativa: Support for the role of antimicrobial peptides and interferon pathways in disease pathogenesis. Clin. Exp. Dermatol. 2019, 44, 882–892. [Google Scholar] [CrossRef] [PubMed]
- Manthiram, K.; Zhou, Q.; Aksentijevich, I.; Kastner, D.L. The monogenic autoinflammatory diseases define new pathways in human innate immunity and inflammation. Nat. Immunol. 2017, 18, 832–842. [Google Scholar] [CrossRef] [PubMed]
- Garcovich, S.; Genovese, G.; Moltrasio, C.; Malvaso, D.; Marzano, A.V. PASH, PAPASH, PsAPASH, and PASS: The autoinflammatory syndromes of hidradenitis suppurativa. Clin. Dermatol. 2021, 39, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Nomura, T. Hidradenitis Suppurativa as a Potential Subtype of Autoinflammatory Keratinization Disease. Front. Immunol. 2020, 11, 847. [Google Scholar] [CrossRef] [PubMed]
- Marzano, A.V.; Genovese, G.; Moltrasio, C.; Tricarico, P.M.; Gratton, R.; Piaserico, S.; Garcovich, S.; Boniotto, M.; Brandao, L.; Moura, R.; et al. Whole-Exome Sequencing in 10 Unrelated Patients with Syndromic Hidradenitis Suppurativa: A Preliminary Step for a Genotype-Phenotype Correlation. Dermatology 2022, 238, 860–869. [Google Scholar] [CrossRef] [PubMed]
- Holzinger, D.; Fassl, S.K.; de Jager, W.; Lohse, P.; Rohrig, U.F.; Gattorno, M.; Omenetti, A.; Chiesa, S.; Schena, F.; Austermann, J.; et al. Single amino acid charge switch defines clinically distinct proline-serine-threonine phosphatase-interacting protein 1 (PSTPIP1)-associated inflammatory diseases. J. Allergy Clin. Immunol. 2015, 136, 1337–1345. [Google Scholar] [CrossRef]
- Broderick, L.; Hoffman, H.M. IL-1 and autoinflammatory disease: Biology, pathogenesis and therapeutic targeting. Nat. Rev. Rheumatol. 2022, 18, 448–463. [Google Scholar] [CrossRef]
- Hodak, E.; Atzmony, L.; Pavlovsky, L.; Comaneshter, D.; Cohen, A.D. Hidradenitis Suppurativa Is Associated with Familial Mediterranean Fever-A Population-Based Study. J. Investig. Dermatol. 2017, 137, 2019–2021. [Google Scholar] [CrossRef]
- Abbara, S.; Georgin-Lavialle, S.; Stankovic Stojanovic, K.; Bachmeyer, C.; Senet, P.; Buob, D.; Audia, S.; Delcey, V.; Fellahi, S.; Bastard, J.P.; et al. Association of hidradenitis suppurativa and familial Mediterranean fever: A case series of 6 patients. Jt. Bone Spine 2017, 84, 159–162. [Google Scholar] [CrossRef] [PubMed]
- Vural, S.; Gundogdu, M.; Kundakci, N.; Ruzicka, T. Familial Mediterranean fever patients with hidradenitis suppurativa. Int. J. Dermatol. 2017, 56, 660–663. [Google Scholar] [CrossRef] [PubMed]
- Guillem, P.; Mintoff, D.; Kabbani, M.; Cogan, E.; Vlaeminck-Guillem, V.; Duquesne, A.; Benhadou, F. Case Report: Comorbid Hyper-IgD Syndrome and Hidradenitis Suppurativa—A New Syndromic Form of HS? A Report of Two Cases. Front. Immunol. 2022, 13, 883811. [Google Scholar] [CrossRef]
- Calabrese, L.; Vitale, A.; Moltrasio, C.; Genovese, G.; Romagnuolo, M.; Marzano, A.V.; Maglie, R.; Rubegni, P.; Cantarini, L. Hyper-IgD syndrome and hidradenitis suppurativa: An intriguing link. J. Eur. Acad. Dermatol. Venereol. 2023. [Google Scholar] [CrossRef] [PubMed]
- Leslie, K.S.; Tripathi, S.V.; Nguyen, T.V.; Pauli, M.; Rosenblum, M.D. An open-label study of anakinra for the treatment of moderate to severe hidradenitis suppurativa. J. Am. Acad. Dermatol. 2014, 70, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Fleischmann, R.M.; Schechtman, J.; Bennett, R.; Handel, M.L.; Burmester, G.R.; Tesser, J.; Modafferi, D.; Poulakos, J.; Sun, G. Anakinra, a recombinant human interleukin-1 receptor antagonist (r-metHuIL-1ra), in patients with rheumatoid arthritis: A large, international, multicenter, placebo-controlled trial. Arthritis Rheum. 2003, 48, 927–934. [Google Scholar] [CrossRef] [PubMed]
- Andre, R.; Marescassier, H.; Gabay, C.; Pittet, B.; Laffitte, E. Long-term therapy with anakinra in hidradenitis suppurativa in three patients. Int. J. Dermatol. 2019, 58, e208–e209. [Google Scholar] [CrossRef] [PubMed]
- Kanni, T.; Argyropoulou, M.; Spyridopoulos, T.; Pistiki, A.; Stecher, M.; Dinarello, C.A.; Simard, J.; Giamarellos-Bourboulis, E.J. MABp1 Targeting IL-1alpha for Moderate to Severe Hidradenitis Suppurativa Not Eligible for Adalimumab: A Randomized Study. J. Investig. Dermatol. 2018, 138, 795–801. [Google Scholar] [CrossRef] [PubMed]
- Theut Riis, P.; Thorlacius, L.R.; Jemec, G.B. Investigational drugs in clinical trials for Hidradenitis Suppurativa. Expert. Opin. Investig. Drugs 2018, 27, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Sun, N.Z.; Ro, T.; Jolly, P.; Sayed, C.J. Non-response to Interleukin-1 Antagonist Canakinumab in Two Patients with Refractory Pyoderma Gangrenosum and Hidradenitis Suppurativa. J. Clin. Aesthet. Dermatol. 2017, 10, 36–38. [Google Scholar] [PubMed]
- Tekin, B.; Salman, A.; Ergun, T. Hidradenitis suppurativa unresponsive to canakinumab treatment: A case report. Indian J. Dermatol. Venereol. Leprol. 2017, 83, 615–617. [Google Scholar] [CrossRef]
- Houriet, C.; Seyed Jafari, S.M.; Thomi, R.; Schlapbach, C.; Borradori, L.; Yawalkar, N.; Hunger, R.E. Canakinumab for Severe Hidradenitis Suppurativa: Preliminary Experience in 2 Cases. JAMA Dermatol. 2017, 153, 1195–1197. [Google Scholar] [CrossRef]
- Akdogan, N.; Yalici-Armagan, B.; Dogan, S.; Yilmaz, R. Severe hidradenitis suppurativa (acne inversa) associated with focal segmental glomerulosclerosis and gout partially responsive to canakinumab. Dermatol. Ther. 2021, 34, edth15002. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.pfizer.com/sites/default/files/plsr-studies/C2501007%20Plain%20Language%20Study%20Results%20Summary%20%28PLSRS%29%20Phase%202-4.pdf (accessed on 1 December 2023).
- Available online: https://investors.kymeratx.com/news-releases/news-release-details/kymera-announces-positive-results-phase-1-clinical-trial/ (accessed on 1 December 2023).
- Alavi, A.; Prens, E.; Kimball, A.; Krueger, J.; Mukhopadhyay, S.; Wang, H.; Ivanhoff, N.; Hernandez, A.C.; Zouboulis, C. Spesolimab for Hidradenitis Suppurativa: A Proof-of-Concept Study. SKIN J. Cutan. Med. 2023, 7, s286. [Google Scholar] [CrossRef]
- Available online: https://ir.anaptysbio.com/news-releases/news-release-details/anaptysbio-reports-harp-phase-2-top-line-data-imsidolimab/ (accessed on 4 December 2023).
- Chopra, D.; Arens, R.A.; Amornpairoj, W.; Lowes, M.A.; Tomic-Canic, M.; Strbo, N.; Lev-Tov, H.; Pastar, I. Innate immunity and microbial dysbiosis in hidradenitis suppurativa—Vicious cycle of chronic inflammation. Front. Immunol. 2022, 13, 960488. [Google Scholar] [CrossRef] [PubMed]
- US Food and Drug Administration. Kineret [Prescribing Information]. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/103950s5136lbl.pdf (accessed on 30 September 2023).
- European Medicines Agency. Kineret [Prescribing Information]. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/kineret (accessed on 30 September 2023).
- US Food and Drug Administration. Arcalyst [Prescribing Information]. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2008/125249lbl.pdf (accessed on 30 September 2023).
- US Food and Drug Administration. Ilaris [Prescribing Information]. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/BLA125319_858687lbl.pdf (accessed on 30 September 2023).
- European Medicines Agency. Ilaris [Prescribing Information]. Available online: https://www.ema.europa.eu/en/documents/product-information/ilaris-epar-product-information_en.pdf (accessed on 30 September 2023).
- Rivera-Díaz, R.; Daudén, E.; Carrascosa, J.M.; Cueva, P.D.L.; Puig, L. Generalized Pustular Psoriasis: A Review on Clinical Characteristics, Diagnosis, and Treatment. Dermatol. Ther. 2023, 13, 673–688. [Google Scholar] [CrossRef]
| Drug Name | MoA | Study | Clinical Trial No. | Status | Primary Endpoint(s) |
|---|---|---|---|---|---|
| Anakinra | Recombinant IL-1R antagonist | Phase II, randomized, double blind, placebo controlled | NCT01558375 | Completed | Efficacy at week 24 |
| Phase II, open label, non- randomized, proof-of-concept trial | NCT01516749 | Completed | Changes in modified Sartorius score at week 8 | ||
| Bermekimab (MABp1) | Anti IL-1α mAb | Phase II, randomized, double blind, placebo controlled | NCT04019041 | Completed | Percentage of participants with HiSCR50 at week 12 |
| Phase II, randomized, double blind, placebo and active comparator controlled, dose ranging | NCT04988308 | Terminated | Part 1. Percentage of participants with HiSCR50 at week 16 Part 2. Percentage of participants with HiSCR50 at week 12 | ||
| Phase II, open label | NCT03512275 | Completed | Number of adverse events up to visit 14 (day 93) | ||
| MEDI8968 (AMG 108) | IL-1R1 inhibitor mAb | Phase II, randomized, double blind, placebo controlled | NCT01838499 | Terminated | Percentage of subjects achieving PGA score 0, 1 or 2 from baseline to week 12 |
| Lutikizumab | Anti IL-1 α/β mAb | Phase II, randomized, double blind, placebo controlled | NCT05139602 | Active, not recruiting | Percentage of participants with HiSCR50 at week 16 |
| Zimlovisertib (PF-06650833) | IRAK4 inhibitor, small molecule | Phase II, randomized, double blind, placebo controlled | NCT04092452 | Completed | Percentage of participants with HiSCR50 at week 16 |
| KT-474 (SAR444656) | IRAK4 inhibitor, small molecule | Phase I, randomized, placebo controlled, single and multiple ascending dose | NCT04772885 | Completed | Incidence and severity of TAEs. Incidence and frequency of use of concomitant medication |
| Phase II, randomized, double blind, placebo controlled | NCT06028230 | Not yet recruiting | Percent change from baseline in AN count at week 16 | ||
| MAS-825 | Anti IL-1 β/IL-18 mAb | Phase II, randomized, double blind, placebo controlled | NCT03827798 | Recruiting | Percentage of participants with HiSCR50 at week 16 |
| Imsidolimab | IL-36R antagonist | Phase II, randomized, double blind, placebo controlled | NCT04856930 | Completed | Percent change from baseline in AN count at week 16 |
| Spesolimab | IL-36R antagonist | Phase II, randomized, double blind, placebo controlled | NCT04762277 | Completed | Percent change from baseline in AN count at week 12 |
| Phase II, open-label, long-term extension study | NCT04876391 | Active, not recruiting | Occurrence of TEAEs up to the end of maintenance treatment period including residual effect period (REP) | ||
| Phase II/III, randomized, double blind, placebo controlled | NCT05819398 | Recruiting | Percent change from baseline in draining fistulas/tunnels (dT) at week 8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calabrese, L.; Malvaso, D.; Coscarella, G.; Antonelli, F.; D’Amore, A.; Gori, N.; Rubegni, P.; Peris, K.; Chiricozzi, A. Therapeutic Potential of IL-1 Antagonism in Hidradenitis Suppurativa. Biomolecules 2024, 14, 175. https://doi.org/10.3390/biom14020175
Calabrese L, Malvaso D, Coscarella G, Antonelli F, D’Amore A, Gori N, Rubegni P, Peris K, Chiricozzi A. Therapeutic Potential of IL-1 Antagonism in Hidradenitis Suppurativa. Biomolecules. 2024; 14(2):175. https://doi.org/10.3390/biom14020175
Chicago/Turabian StyleCalabrese, Laura, Dalma Malvaso, Giulia Coscarella, Flaminia Antonelli, Alessandra D’Amore, Niccolò Gori, Pietro Rubegni, Ketty Peris, and Andrea Chiricozzi. 2024. "Therapeutic Potential of IL-1 Antagonism in Hidradenitis Suppurativa" Biomolecules 14, no. 2: 175. https://doi.org/10.3390/biom14020175
APA StyleCalabrese, L., Malvaso, D., Coscarella, G., Antonelli, F., D’Amore, A., Gori, N., Rubegni, P., Peris, K., & Chiricozzi, A. (2024). Therapeutic Potential of IL-1 Antagonism in Hidradenitis Suppurativa. Biomolecules, 14(2), 175. https://doi.org/10.3390/biom14020175





