One-Pot Synthesis of β-Alanine from Fumaric Acid via an Efficient Dual-Enzyme Cascade Biotransformation
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains, Plasmids, and Chemicals
2.2. Protein Expression and Purification
2.3. Enzyme Assay
2.4. Analytical Methods
2.5. Biochemical Characterization
2.6. Kinetic Assay
2.7. Cascade Reactions
3. Results and Discussion
3.1. Design the Cascade Reaction by EcMAL and PanDs
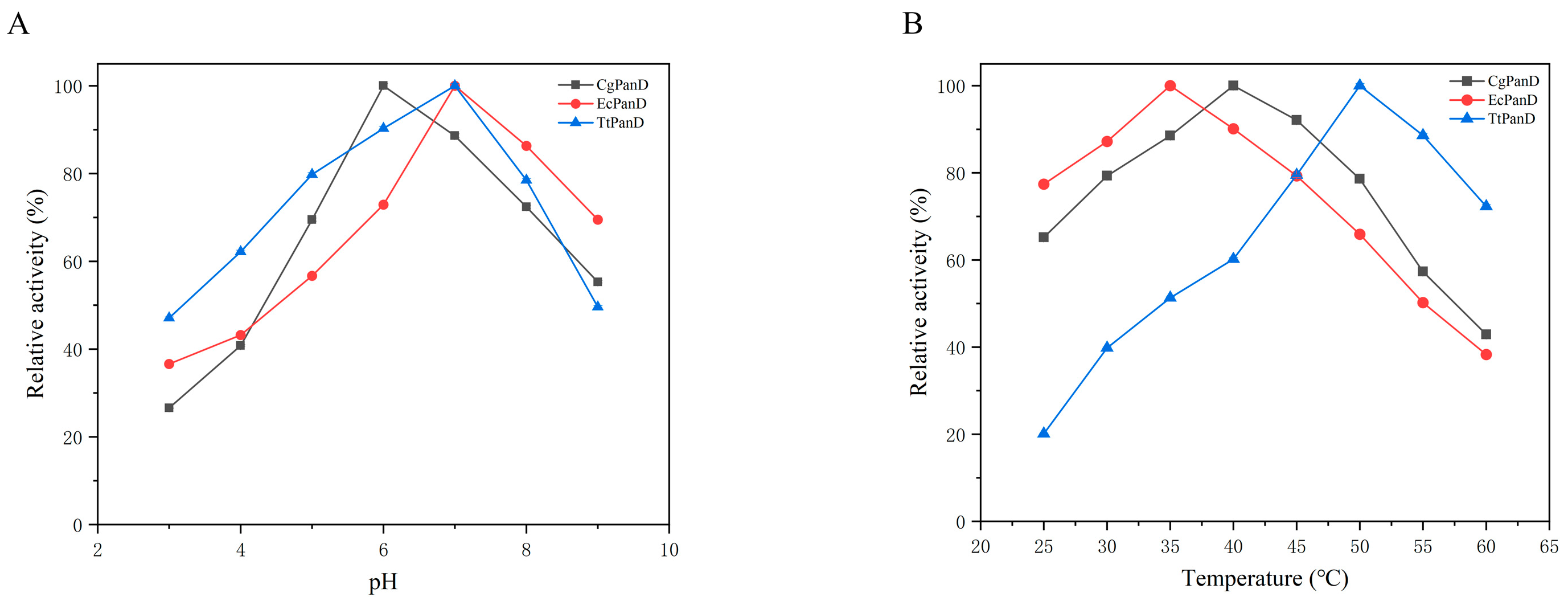
3.2. Optimization of Reaction Conditions for PanDs
3.3. Comparison of the Activity of Aspartic Acid α-Decarboxylase from Different Sources
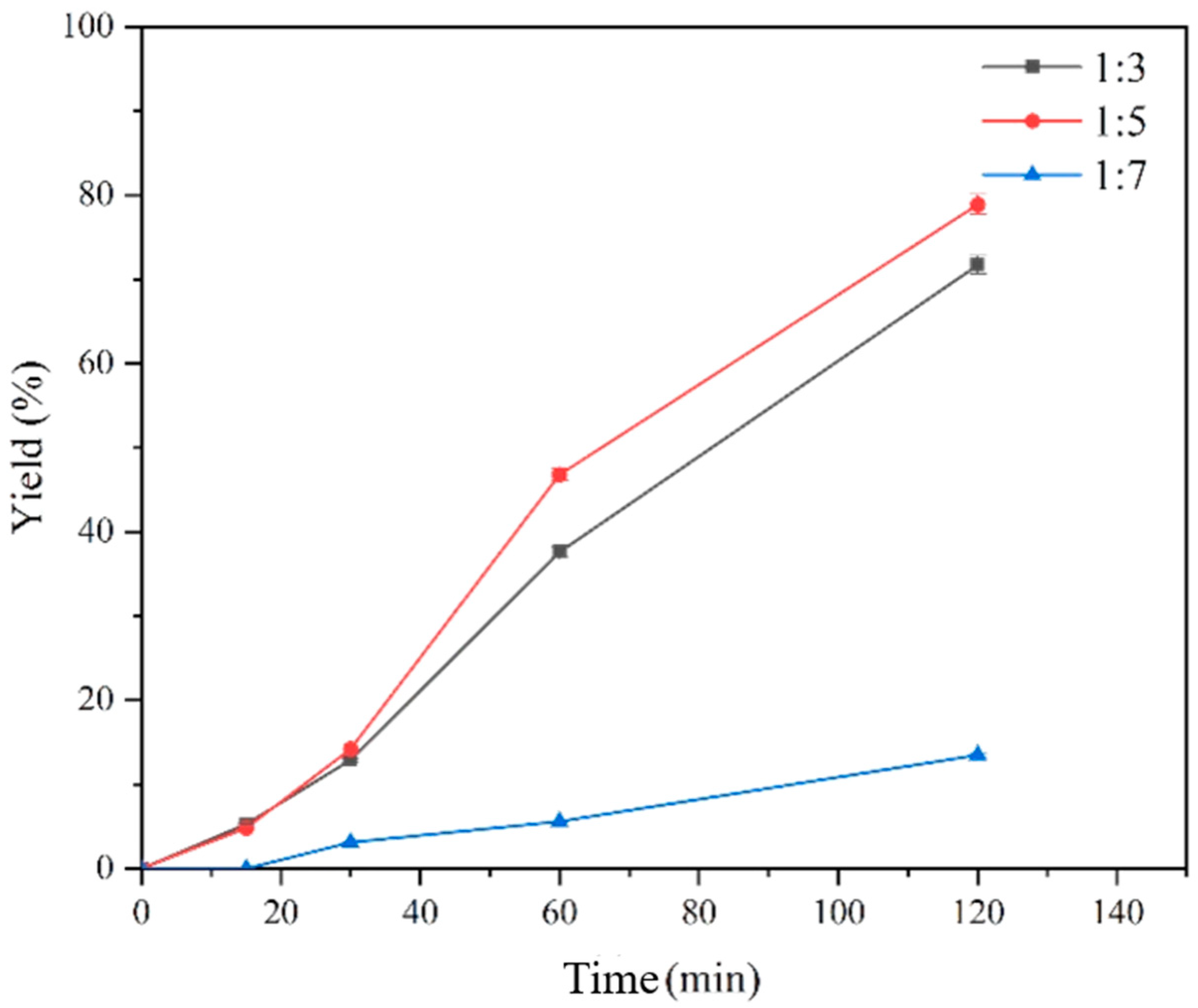
3.4. Construction and Optimization of the Free Two-Enzyme Cascade Reaction
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lu, J.; Wang, G.; Yang, C.; Peng, Z.; Yang, L.; Du, B.; Guo, C.; Sui, S.; Wang, J.; Li, J.; et al. Study on the Construction Technology of β-Alanine Synthesizing Escherichia coli Based on Cellulosome Assembly. Front. Bioeng. Biotechnol. 2023, 11, 1202483. [Google Scholar] [CrossRef]
- Shen, Y.; Zhao, L.; Li, Y.; Zhang, L.; Shi, G. Synthesis of β-Alanine from l-Aspartate Using l-Aspartate-α-Decarboxylase from Corynebacterium glutamicum. Biotechnol. Lett. 2014, 36, 1681–1686. [Google Scholar] [CrossRef] [PubMed]
- Meenukutty, M.S.; Mohan, A.P.; Vidya, V.G.; Viju Kumar, V.G. Synthesis, Characterization, DFT Analysis and Docking Studies of a Novel Schiff Base Using 5-Bromo Salicylaldehyde and β-Alanine. Heliyon 2022, 8, e09600. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Zhou, C.; Guo, X.; Du, Z.; Cheng, Y.; Wang, Z.; He, X. Enhancing Fluxes through the Mevalonate Pathway in Saccharomyces Cerevisiae by Engineering the HMGR and β-Alanine Metabolism. Microb. Biotechnol. 2022, 15, 2292–2306. [Google Scholar] [CrossRef] [PubMed]
- Poon, N.Y.; Sinskey, A.J.; Zhou, K. Engineering Escherichia coli to Assimilate β-Alanine as a Major Carbon Source. Appl. Microbiol. Biotechnol. 2023, 107, 4581–4591. [Google Scholar] [CrossRef]
- de Salazar, L.; Segarra, I.; López-Román, F.J.; Torregrosa-García, A.; Pérez-Piñero, S.; Ávila-Gandía, V. Increased Bioavailability of β-Alanine by a Novel Controlled-Release Powder Blend Compared to a Slow-Release Tablet. Pharmaceutics 2021, 13, 1517. [Google Scholar] [CrossRef]
- Xu, J.; Zhou, L.; Yin, M.; Zhou, Z. Novel Mode Engineering for β-Alanine Production in Escherichia coli with the Guide of Adaptive Laboratory Evolution. Microorganisms 2021, 9, 600. [Google Scholar] [CrossRef]
- Tan, G.; Das, M.; Keum, H.; Bellotti, P.; Daniliuc, C.; Glorius, F. Photochemical Single-Step Synthesis of β-Amino Acid Derivatives from Alkenes and (Hetero)Arenes. Nat. Chem. 2022, 14, 1174–1184. [Google Scholar] [CrossRef]
- Wang, J.; Ma, D.; Mai, D.; Li, H.; Wang, J.; Wang, X.; Chen, K.; Ouyang, P. β-Alanine Production by L-Aspartate-α-Decarboxylase from Corynebacterium glutamicum and Variants with Reduced Substrate Inhibition. Mol. Catal. 2022, 522, 112246. [Google Scholar] [CrossRef]
- Yuan, S.F.; Nair, P.H.; Borbon, D.; Coleman, S.M.; Fan, P.H.; Lin, W.L.; Alper, H.S. Metabolic Engineering of E. coli for β-Alanine Production Using a Multi-Biosensor Enabled Approach. Metab. Eng. 2022, 74, 24–35. [Google Scholar] [CrossRef]
- Ghiffary, M.R.; Prabowo, C.P.S.; Adidjaja, J.J.; Lee, S.Y.; Kim, H.U. Systems Metabolic Engineering of Corynebacterium glutamicum for the Efficient Production of β-Alanine. Metab. Eng. 2022, 74, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Zou, S.M.; Wang, J.P.; Zong, M.H.; Wang, Z.L.; Zheng, Z.J.; Li, N. One-Pot Photoenzymatic Synthesis of Maleic Acid and Its Derivatives from Bio-Based Furfural via Catalytic Cascades. Green Chem. 2023, 25, 6892–6900. [Google Scholar] [CrossRef]
- Yu, X.J.; Huang, C.Y.; Xu, X.D.; Chen, H.; Liang, M.J.; Xu, Z.X.; Xu, H.X.; Wang, Z. Protein Engineering of a Pyridoxal-50-Phosphate-Dependent l-Aspartate-α-Decarboxylase from Tribolium Castaneum for β-Alanine Production. Molecules 2020, 25, 1280. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.C.; Tian, Y.H.; Yang, J.L.; Zhu, Y.N.; Zhou, H.Y.; Zheng, Y.G.; Liu, Z.Q. Research Progress of L-Aspartate-α-Decarboxylase and Its Isoenzyme in the β-Alanine Synthesis. World J. Microbiol. Biotechnol. 2023, 39, 42. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Piao, X.; Cui, S.; Hu, M.; Tao, Y. Enhanced Production of β-Alanine through Co-Expressing Two Different Subtypes of l-Aspartate-α-Decarboxylase. J. Ind. Microbiol. Biotechnol. 2020, 47, 465–474. [Google Scholar] [CrossRef]
- Miao, L.; Li, Y.; Zhu, T. Metabolic Engineering of Methylotrophic Pichia Pastoris for the Production of β-Alanine. Bioresour. Bioprocess. 2021, 8, 89. [Google Scholar] [CrossRef]
- Wu, J.; Ma, B.-D.; Xu, Y. One-Pot Synthesis of β-Alanine from Maleic Acid via Three-Enzyme Cascade Biotransformation. Catalysts 2023, 13, 267. [Google Scholar] [CrossRef]
- Fan, A.; Li, J.; Yu, Y.; Zhang, D.; Nie, Y.; Xu, Y. Enzymatic Cascade Systems for D-Amino Acid Synthesis: Progress and Perspectives. Syst. Microbiol. Biomanuf. 2021, 1, 397–410. [Google Scholar] [CrossRef]
- Sperl, J.M.; Sieber, V. Multienzyme Cascade Reactions—Status and Recent Advances. ACS Catal. 2018, 8, 2385–2396. [Google Scholar] [CrossRef]
- Noordzij, G.J.; Wilsens, C.H.R.M. Cascade Aza-Michael Addition-Cyclizations; Toward Renewable and Multifunctional Carboxylic Acids for Melt-Polycondensation. Front. Chem. 2019, 7, 729. [Google Scholar] [CrossRef]
- Liu, R.; Wang, J.; Xiong, P.; Chen, Q.; Liu, H. De Novo Sequence Redesign of a Functional Ras-Binding Domain Globally Inverted the Surface Charge Distribution and Led to Extreme Thermostability. Biotechnol. Bioeng. 2021, 118, 2031–2042. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Xun, G.; Feng, Y. The State-of-the-Art Strategies of Protein Engineering for Enzyme Stabilization. Biotechnol. Adv. 2019, 37, 530–537. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, R.; Xu, M.; Zhang, X.; Yang, T.; Liu, F.; Yang, S.; Rao, Z. Glu56Ser Mutation Improves the Enzymatic Activity and Catalytic Stability of Bacillus subtilis L-Aspartate α-Decarboxylase for an Efficient β-Alanine Production. Process Biochem. 2018, 70, 117–123. [Google Scholar] [CrossRef]
- He, F.S.; Jin, J.H.; Yang, Z.T.; Yu, X.; Fossey, J.S.; Deng, W.P. Direct Asymmetric Synthesis of β-Bis-Aryl-α-Amino Acid Esters via Enantioselective Copper-Catalyzed Addition of p-Quinone Methides. ACS Catal. 2016, 6, 652–656. [Google Scholar] [CrossRef]
- Ni, Z.-F.; Xu, P.; Zong, M.-H.; Lou, W.-Y. Structure-Guided Protein Engineering of Ammonia Lyase for Efficient Synthesis of Sterically Bulky Unnatural Amino Acids. Bioresour. Bioprocess 2021, 8, 103. [Google Scholar] [CrossRef]
- Ni, D.; Zhang, S.; Klrtel, O.; Xu, W.; Chen, Q.; Öner, E.T.; Mu, W. Improving the Thermostability and Catalytic Activity of an Inulosucrase by Rational Engineering for the Biosynthesis of Microbial Inulin. J. Agric. Food Chem. 2021, 69, 13125–13134. [Google Scholar] [CrossRef]
- Ni, Z.F.; Zeng, Y.J.; Xu, P.; Guo, Z.W.; Ou, X.Y.; Peng, F.; Yang, J.G.; Zong, M.H.; Lou, W.Y. Characterization of a Novel Methylaspartate Ammonia Lyase from E. coli O157:H7 for Efficient Asymmetric Synthesis of Unnatural Amino Acids. ACS Sustain. Chem. Eng. 2020, 8, 329–334. [Google Scholar] [CrossRef]
- Àvila-Cabré, S.; Pérez-Trujillo, M.; Albiol, J.; Ferrer, P. Engineering the Synthetic β-Alanine Pathway in Komagataella Phaffii for Conversion of Methanol into 3-Hydroxypropionic Acid. Microb. Cell Fact. 2023, 22, 237. [Google Scholar] [CrossRef]
- Lambrughi, M.; Maršić, Ž.S.; Saez-Jimenez, V.; Mapelli, V.; Olsson, L.; Papaleo, E. Conformational Gating in Ammonia Lyases. bioRxiv 2019. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Chen, X.; Feng, J.; Wu, Q.; Zhu, D. Structure-Guided Engineering of: Meso -Diaminopimelate Dehydrogenase for Enantioselective Reductive Amination of Sterically Bulky 2-Keto Acids. Catal. Sci. Technol. 2018, 8, 4994–5002. [Google Scholar] [CrossRef]
- Yuan, S.; Chan, H.C.S.; Filipek, S.; Vogel, H. PyMOL and Inkscape Bridge the Data and the Data Visualization. Structure 2016. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Yao, P.; Li, J.; Feng, J.; Wu, Q.; Zhu, D. Improving the Catalytic Efficiency and Stereoselectivity of a Nitrilase from: Synechocystis Sp. PCC6803 by Semi-Rational Engineering En Route to Chiral γ-Amino Acids. Catal. Sci. Technol. 2019, 9, 1504–1510. [Google Scholar] [CrossRef]
- Wang, J.B.; Lonsdale, R.; Reetz, M.T. Exploring Substrate Scope and Stereoselectivity of P450 Peroxygenase OleTJE in Olefin-Forming Oxidative Decarboxylation. Chem. Commun. 2016, 52, 8131–8133. [Google Scholar] [CrossRef] [PubMed]


| Parameters | EcMAL | CgPanD | EcPanD | TtPanD |
|---|---|---|---|---|
| Km (mM) | 6.7 ± 0.1 | 4.3 ± 0.2 | 7.9 ± 0.1 | 8.5 ± 0.1 |
| Kcat (s−1) | 87 ± 3.8 | 2.8 ± 0.5 | 1.4 ± 0.2 | 2.2 ± 0.1 |
| Kcat/Km (s−1∙mM−1) | 13 | 0.65 | 0.18 | 0.26 |
| Specific enzyme activity (U mg−1 protein) | 82 ± 3.6 | 10.5 ± 2.8 | 2.3 ± 0.1 | 2.8 ± 0.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ni, Z.; Zhang, L.; Nie, A.; Wang, H.; Wu, X. One-Pot Synthesis of β-Alanine from Fumaric Acid via an Efficient Dual-Enzyme Cascade Biotransformation. Biomolecules 2024, 14, 1553. https://doi.org/10.3390/biom14121553
Ni Z, Zhang L, Nie A, Wang H, Wu X. One-Pot Synthesis of β-Alanine from Fumaric Acid via an Efficient Dual-Enzyme Cascade Biotransformation. Biomolecules. 2024; 14(12):1553. https://doi.org/10.3390/biom14121553
Chicago/Turabian StyleNi, Zifu, Linshang Zhang, Azhen Nie, Huan Wang, and Xiaoling Wu. 2024. "One-Pot Synthesis of β-Alanine from Fumaric Acid via an Efficient Dual-Enzyme Cascade Biotransformation" Biomolecules 14, no. 12: 1553. https://doi.org/10.3390/biom14121553
APA StyleNi, Z., Zhang, L., Nie, A., Wang, H., & Wu, X. (2024). One-Pot Synthesis of β-Alanine from Fumaric Acid via an Efficient Dual-Enzyme Cascade Biotransformation. Biomolecules, 14(12), 1553. https://doi.org/10.3390/biom14121553







