Acclimation of Hairless Spontaneously Hypertensive Rat to Ambient Temperature Attenuates Hypertension-Induced Pro-Arrhythmic Downregulation of Cx43 in the Left Heart Ventricle of Males
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Design
2.2. Cardiac Structure and Function Assessed by Echocardiography
2.3. Electrocardiographic Examination
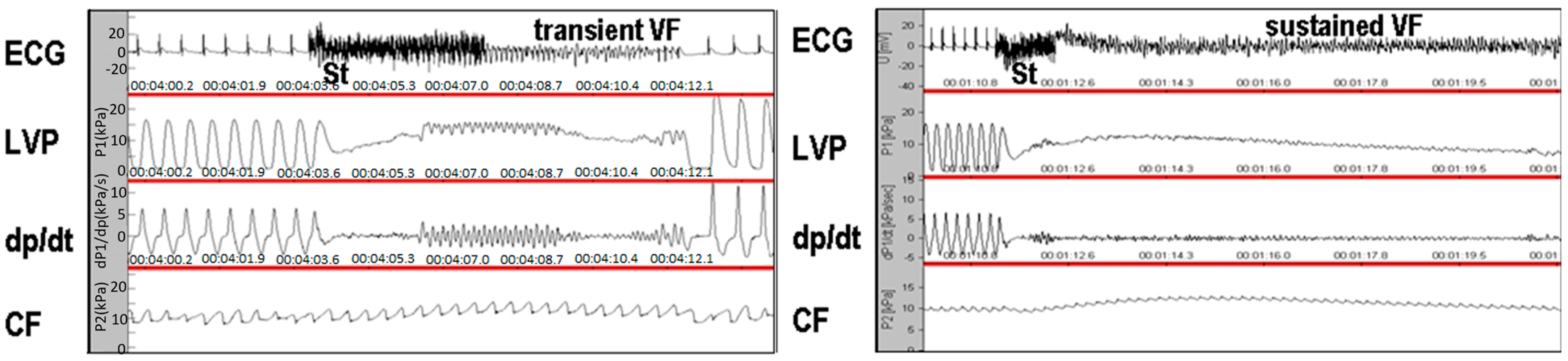
2.4. Estimation of VF Threshold Ex Vivo in Langendorff-Mode Perfused Hearts
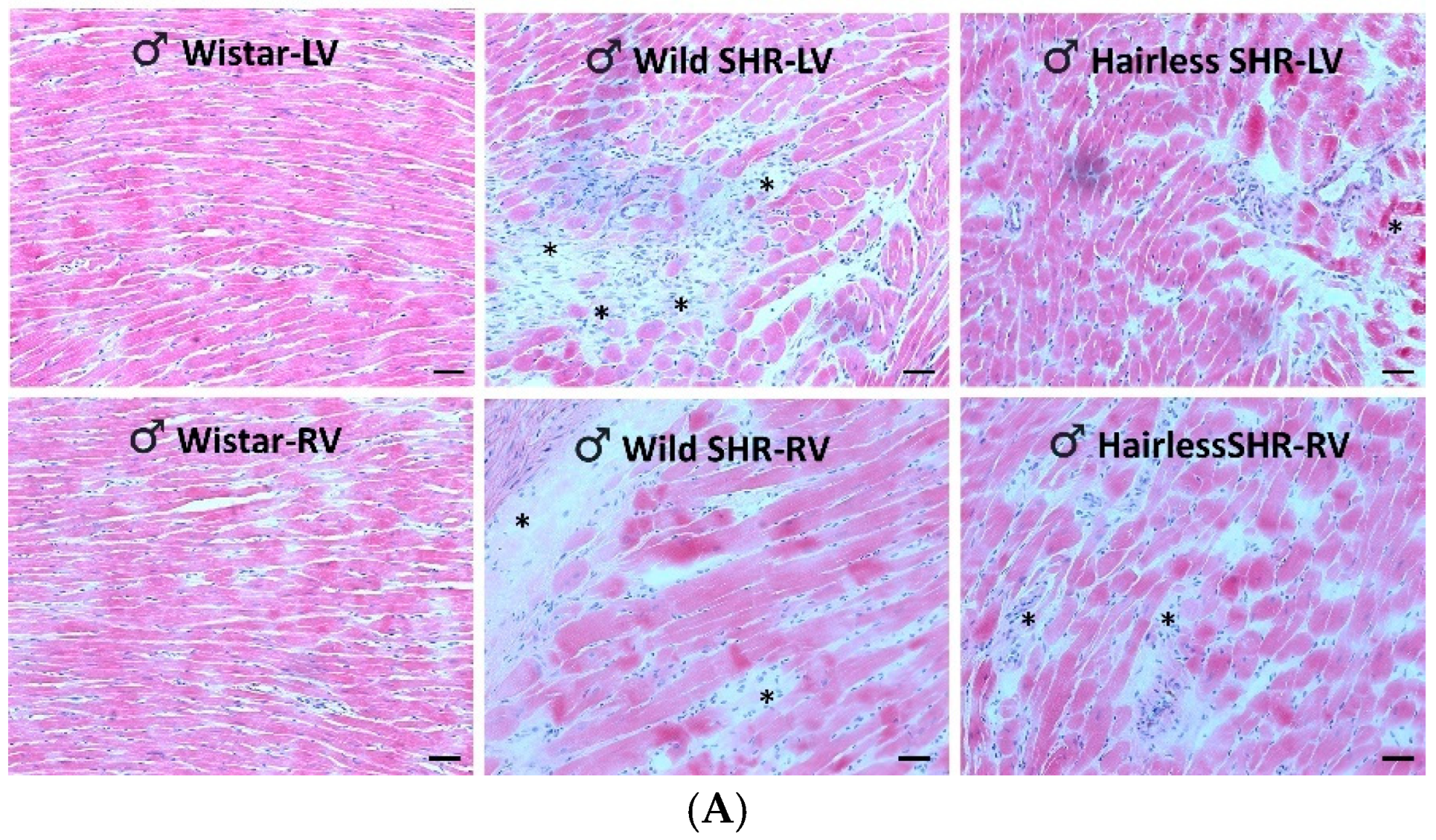
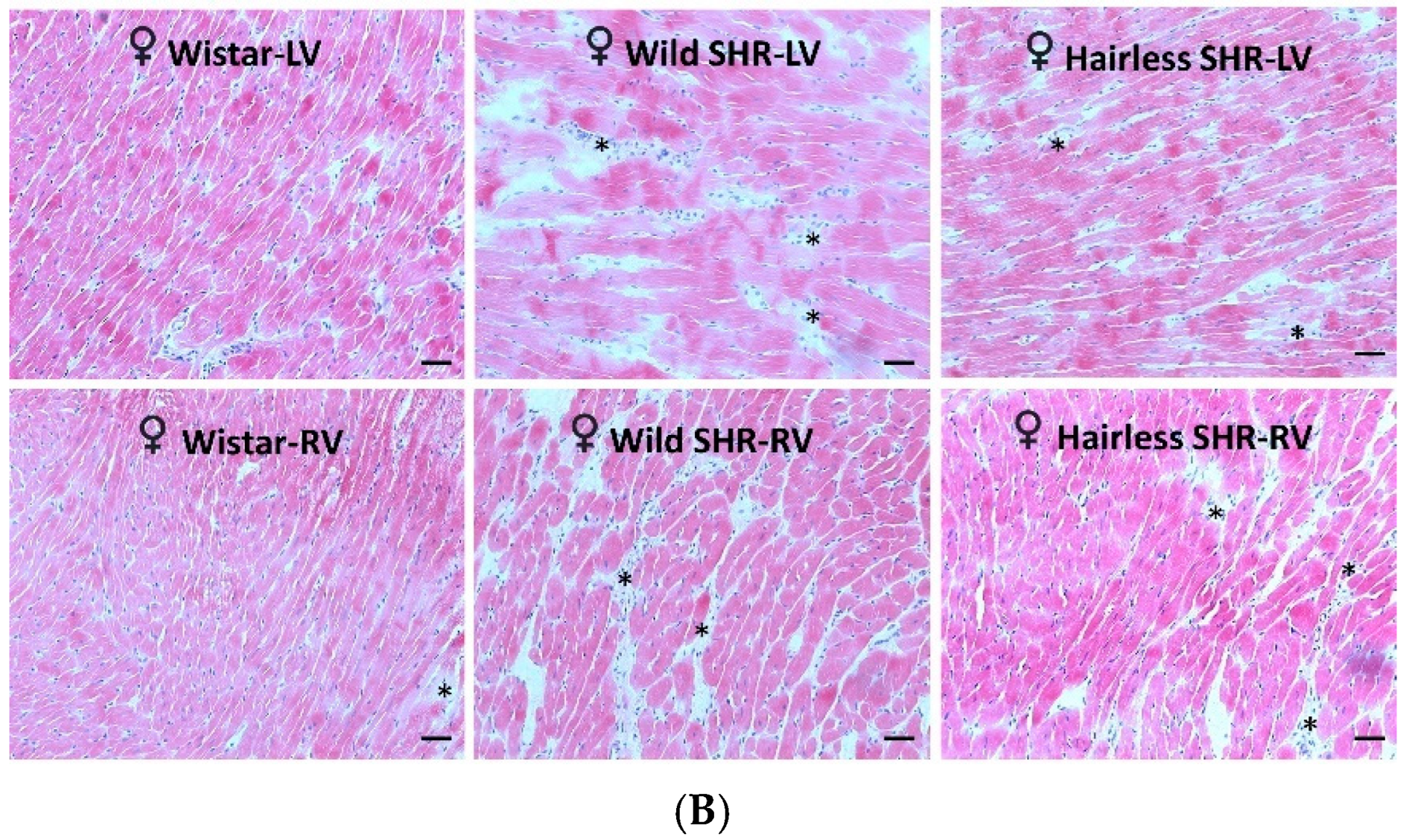
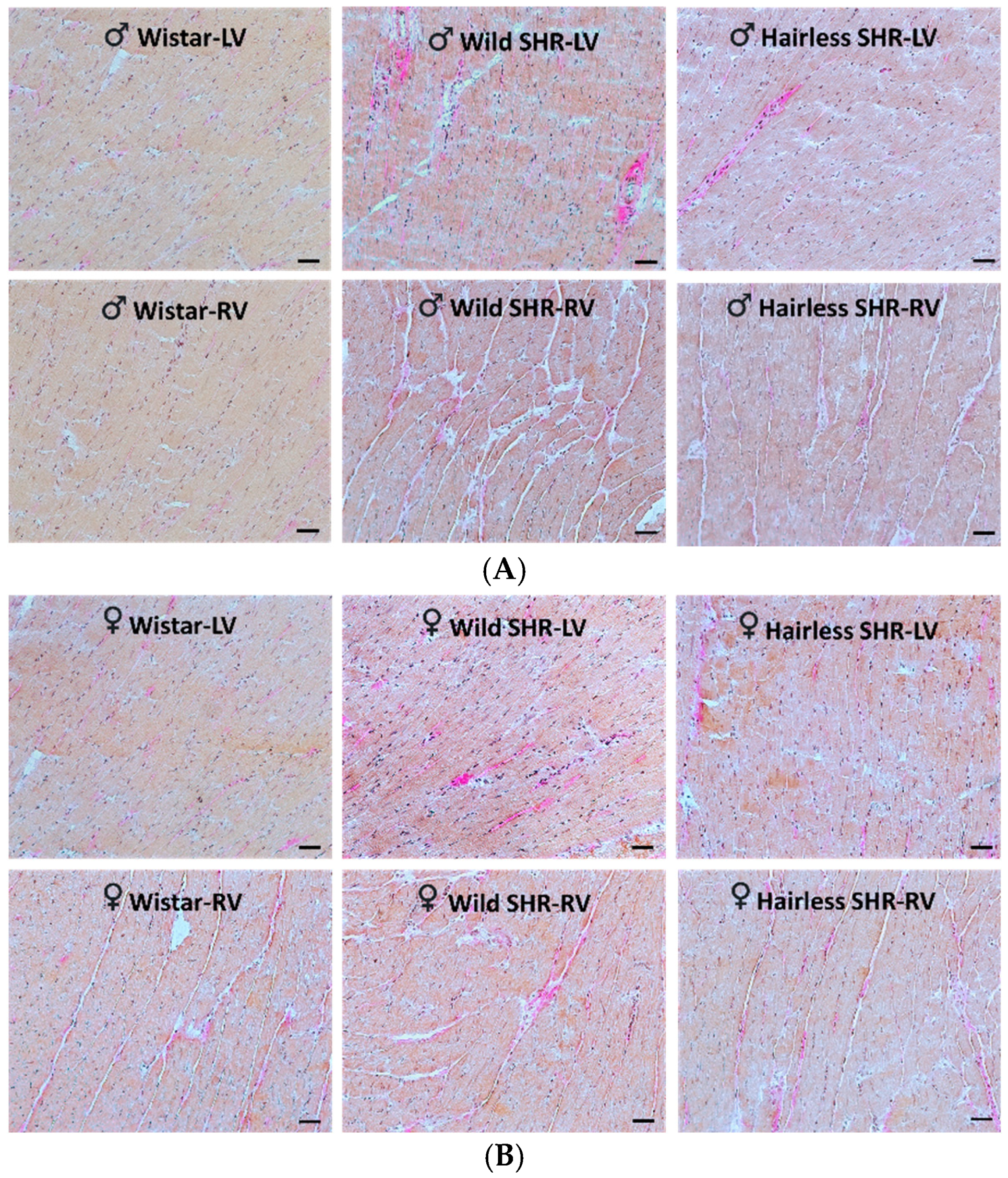
2.5. Microscopic Examination and Quantitative Image Analysis of Heart Tissue Samples
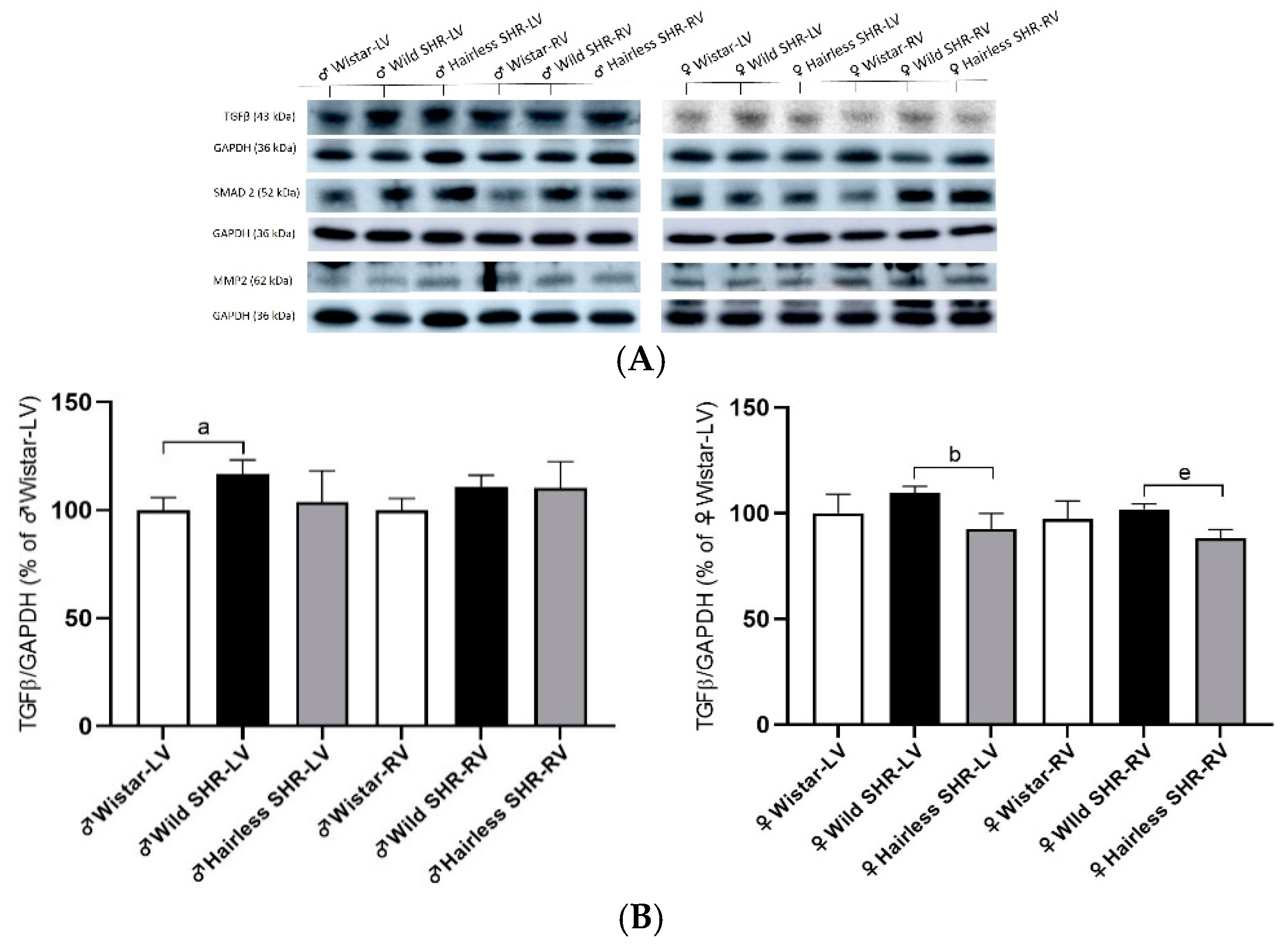
2.6. Determination of Myocardial Protein Levels of Cx43, PKCε, PKCδ, TGFb, SMAD2, and MMP-2 by Western Blotting
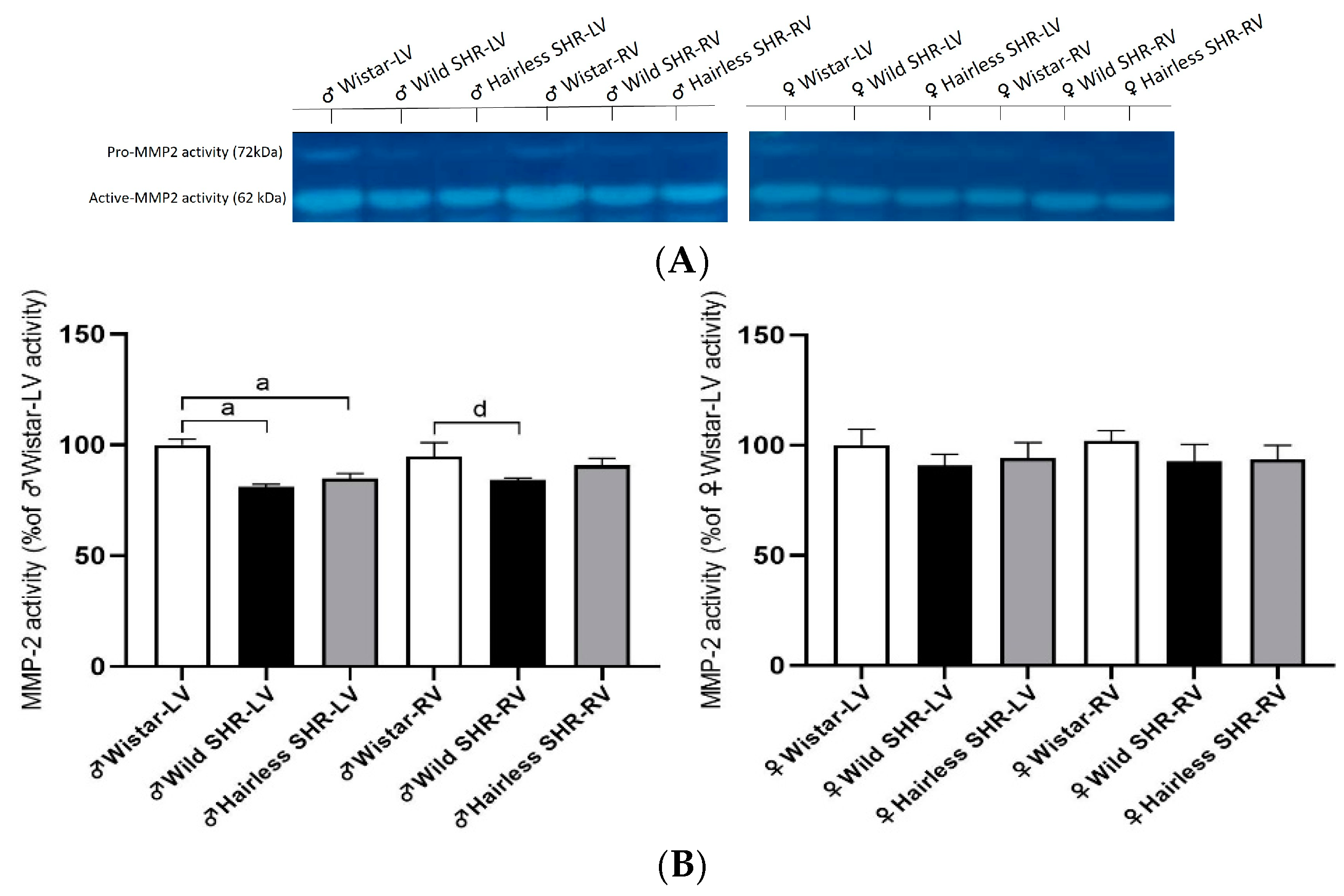
2.7. Detection of MMP-2 Activity Using Gelatine Zymography
2.8. Statistical Evaluation
3. Results
3.1. Basic Biometric Parameters
3.2. Structural and Functional Heart Parameters Assessed by Echocardiography
3.3. Basic Electrocardiographic Parameters
3.4. Incidence of Sustained Ventricular Fibrillation Assessed in Ex Vivo Perfused Rat Heart
3.5. Microscopic Examination of Heart Tissue
3.5.1. Assessment of Cardiac Tissue Structural Changes Using Hematoxylin–Eosin Staining
3.5.2. Evaluation of Collagen Deposition in Cardiac Tissue Using Van Gieson Staining
3.5.3. Detection of Alkaline Phosphatase Activity
3.5.4. Detection of Dipeptidyl Peptidase-4 Activity in Venous Capillaries
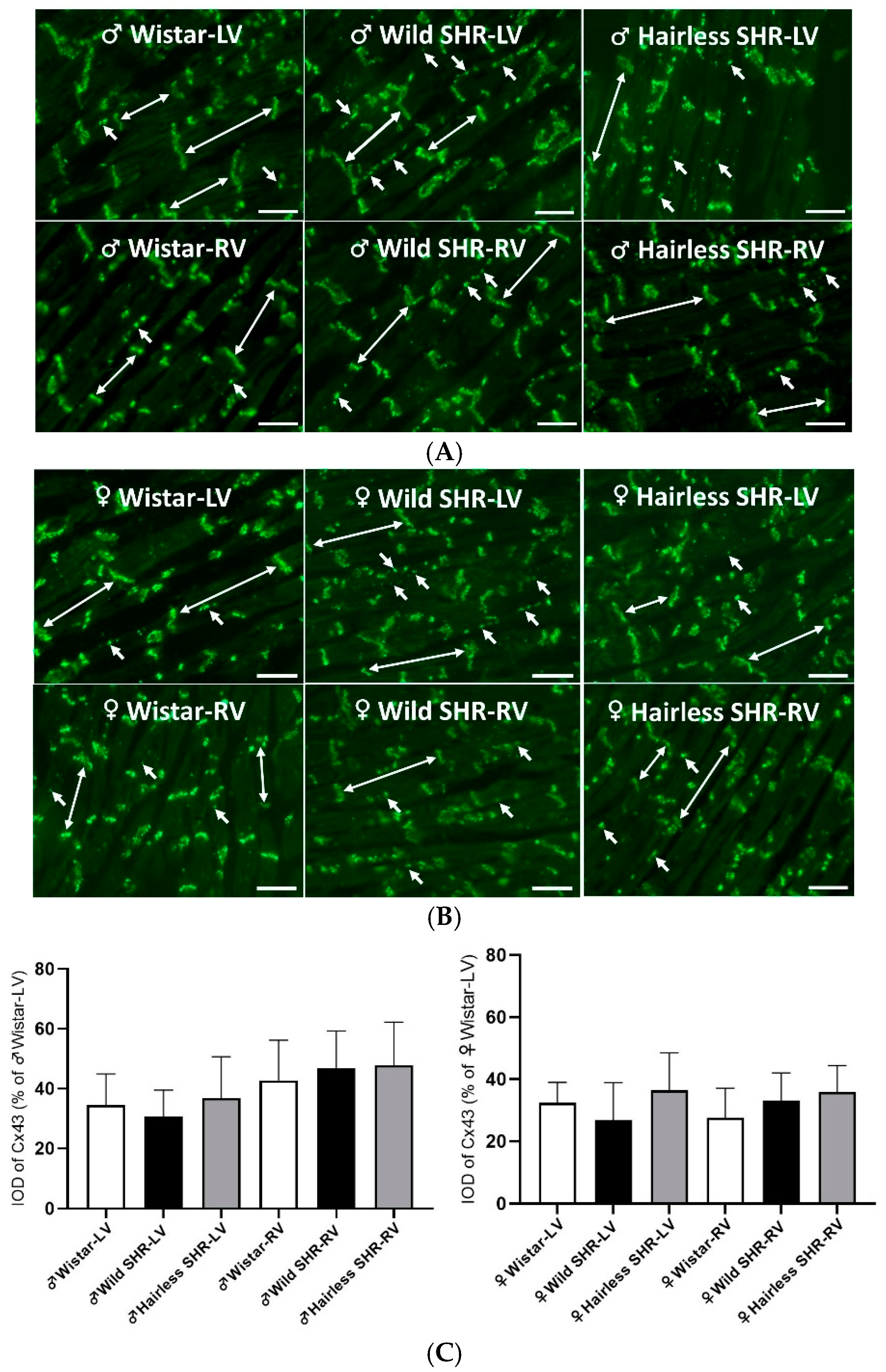
3.5.5. Immunofluorescent Detection of Myocardial Cx43
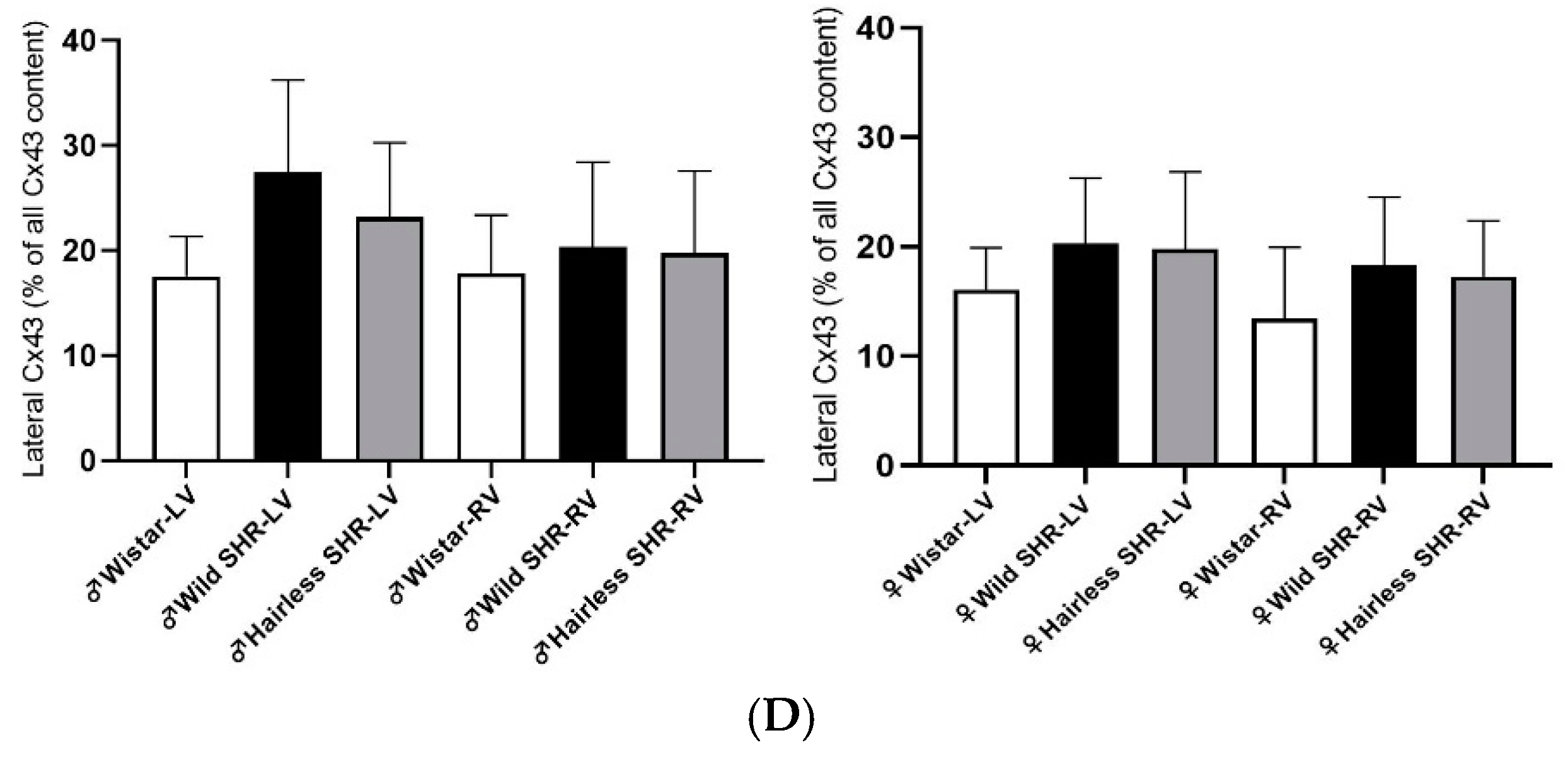
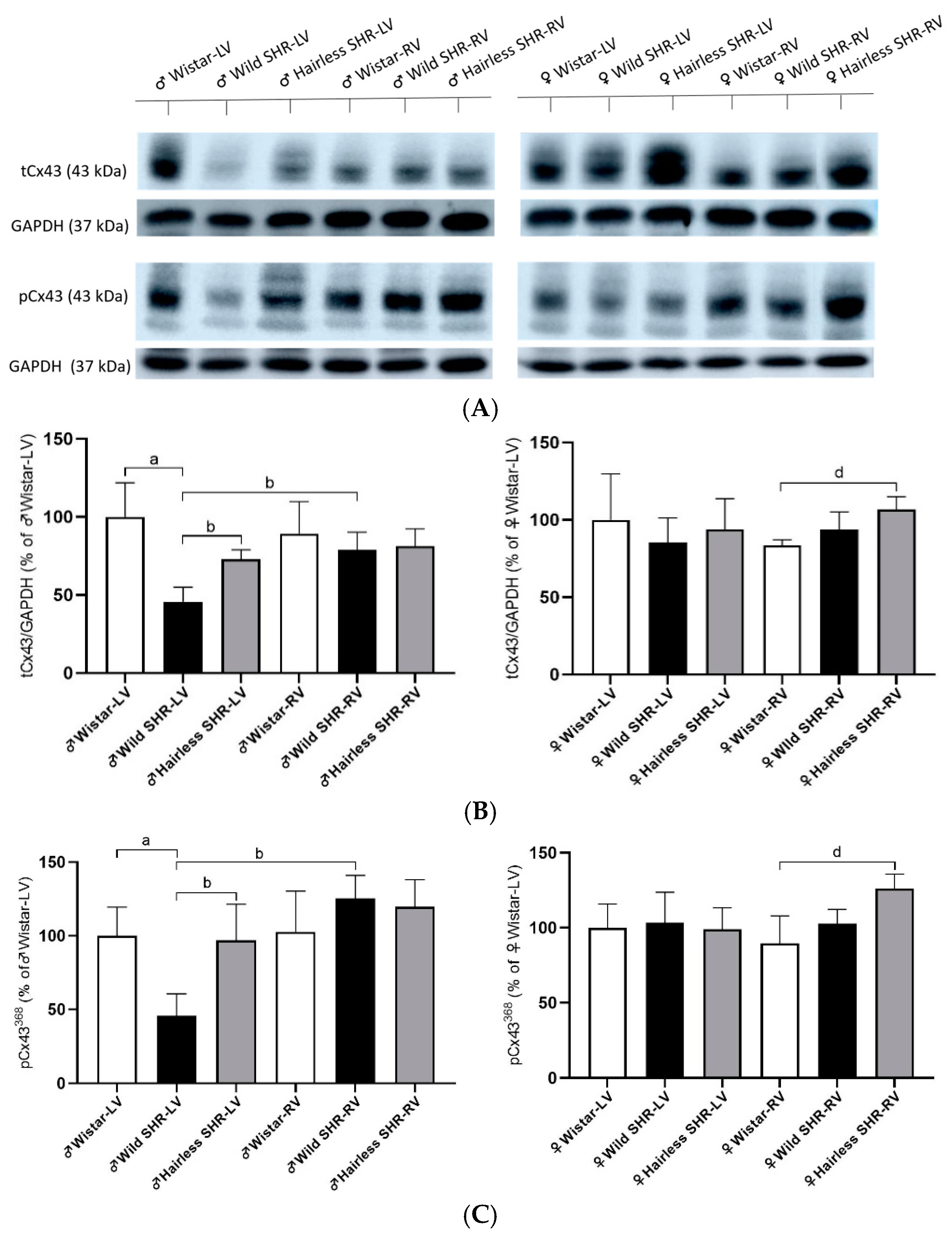
3.6. Myocardial Total tCx43 Protein Levels and Its Phosphorylated pCx43368 Variants
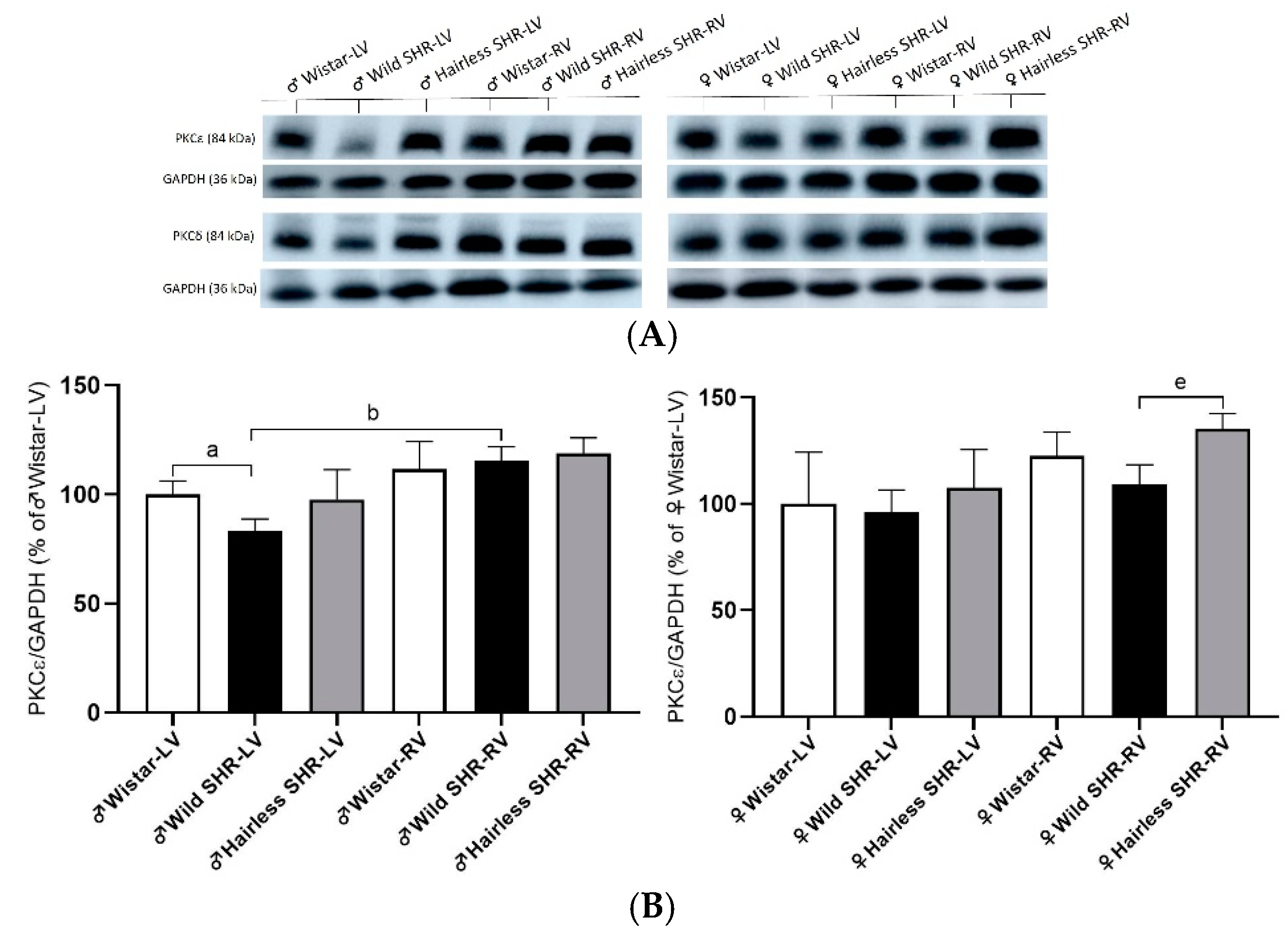
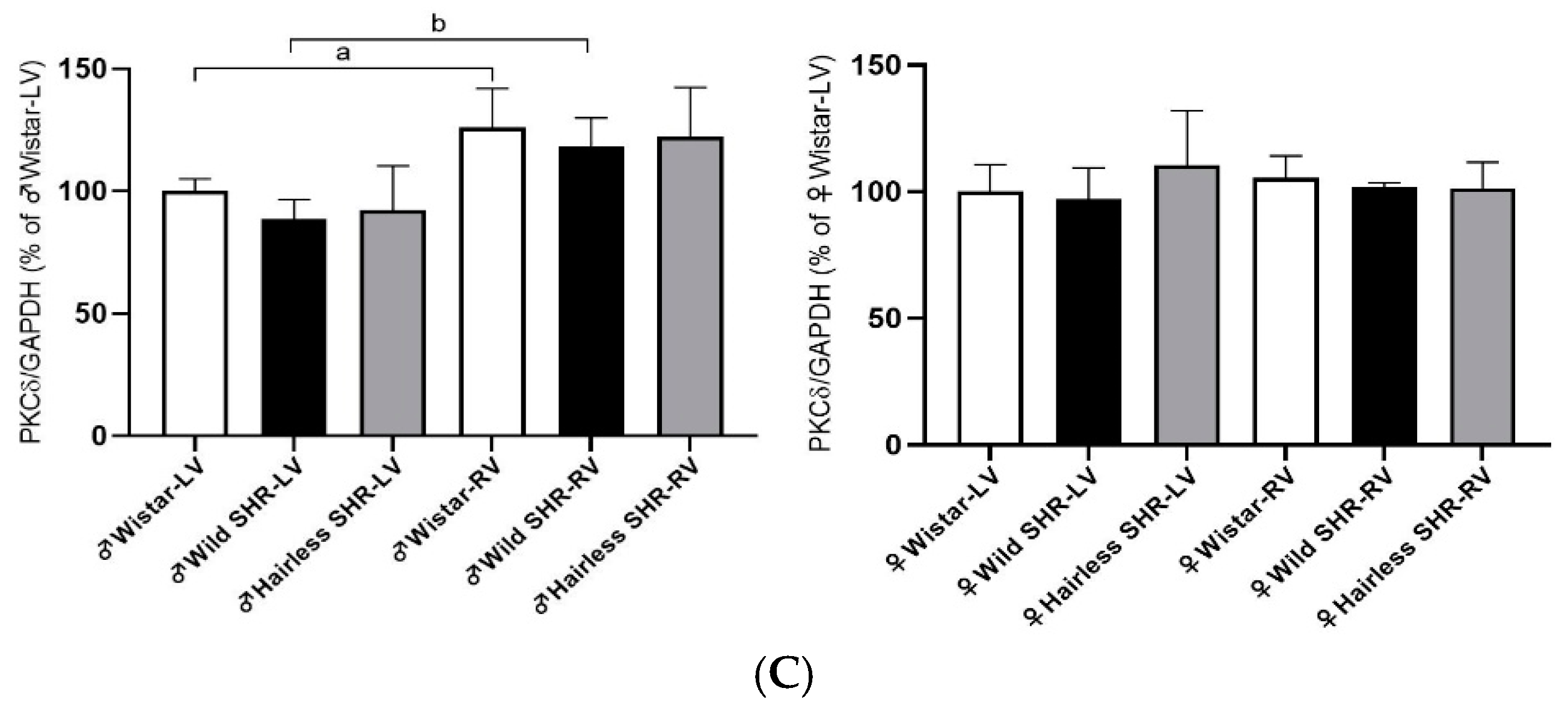
3.7. Cx43 Modulating PKCε and PKCδ
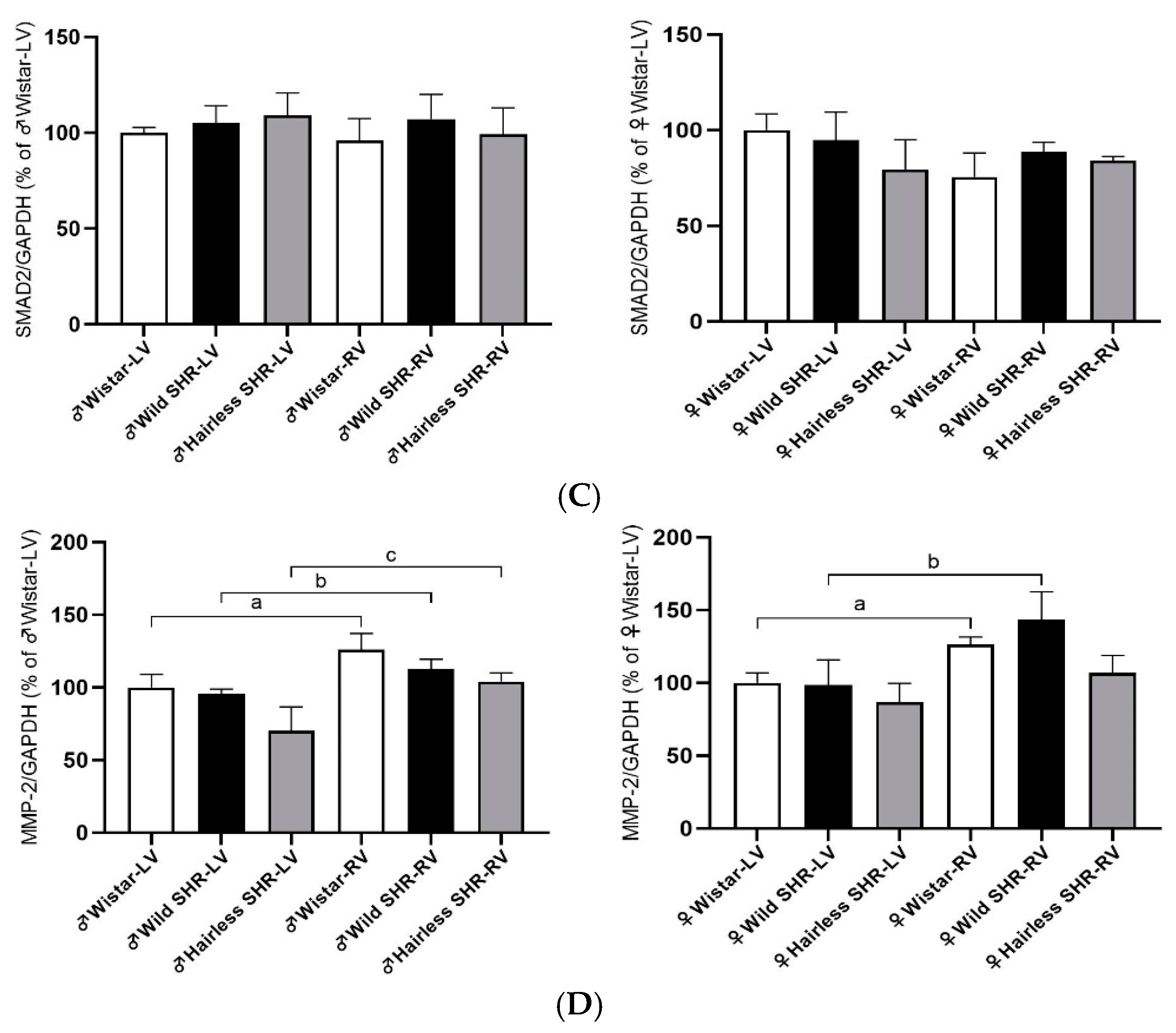
3.8. Extracellular Matrix Protein (TGFβ, SMAD2, and MMP-2) Alterations in Left and Right Heart Ventricles
3.9. Myocardial Activity of MMP-2
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kario, K.; Okura, A.; Hoshide, S.; Mogi, M. The WHO Global report 2023 on hypertension warning the emerging hypertension burden in globe and its treatment strategy. Hypertens. Res. 2024, 47, 1099–1102. [Google Scholar] [CrossRef] [PubMed]
- Unger, T.; Borghi, C.; Charchar, F.; Khan, N.A.; Poulter, N.R.; Prabhakaran, D.; Ramirez, A.; Schlaich, M.; Stergiou, G.S.; Tomaszewski, M.; et al. 2020 International Society of Hypertension Global Hypertension Practice Guidelines. Hypertension 2020, 75, 1334–1357. [Google Scholar] [CrossRef] [PubMed]
- Marazzato, J.; Blasi, F.; Golino, M.; Verdecchia, P.; Angeli, F.; De Ponti, R. Hypertension and Arrhythmias: A Clinical Overview of the Pathophysiology-Driven Management of Cardiac Arrhythmias in Hypertensive Patients. J. Cardiovasc. Dev. Dis. 2022, 9, 110. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Carrillo-Larco, R.M.; Danaei, G.; Riley, L.M.; Paciorek, C.J.; Stevens, G.A.; Gregg, E.W.; Bennett, J.E.; Solomon, B.; Singleton, R.K.; et al. Worldwide trends in hypertension prevalence and progress in treatment and control from 1990 to 2019: A pooled analysis of 1201 population-representative studies with 104 million participants. Lancet 2021, 398, 957–980. [Google Scholar] [CrossRef] [PubMed]
- Naderi, H.; Ramírez, J.; Van Duijvenboden, S.; Pujadas, E.R.; Aung, N.; Wang, L.; Anwar Ahmed Chahal, C.; Lekadir, K.; Petersen, S.E.; Munroe, P.B. Predicting left ventricular hypertrophy from the 12-lead electrocardiogram in the UK Biobank imaging study using machine learning. Eur. Heart J.-Digit. Health 2023, 4, 316–324. [Google Scholar] [CrossRef]
- Mogi, M.; Hoshide, S.; Kario, K. Optimal blood pressure and improvement of achievement rate. Hypertens. Res. 2023, 46, 2445–2446. [Google Scholar] [CrossRef]
- Dzau, V.J.; Hodgkinson, C.P. Precision Hypertension. Hypertension 2024, 81, 702–708. [Google Scholar] [CrossRef]
- Sykora, M.; Andelova, K.; Szeiffova Bacova, B.; Egan Benova, T.; Martiskova, A.; Knezl, V.; Tribulova, N. Hypertension Induces Pro-arrhythmic Cardiac Connexome Disorders: Protective Effects of Treatment. Biomolecules 2023, 13, 330. [Google Scholar] [CrossRef]
- Thomas, D.; Christ, T.; Fabritz, L.; Goette, A.; Hammwöhner, M.; Heijman, J.; Kockskämper, J.; Linz, D.; Odening, K.E.; Schweizer, P.A.; et al. German Cardiac Society Working Group on Cellular Electrophysiology state-of-the-art paper: Impact of molecular mechanisms on clinical arrhythmia management. Clin. Res. Cardiol. 2019, 108, 577–599. [Google Scholar] [CrossRef]
- Han, B.; Trew, M.L.; Zgierski-Johnston, C.M. Cardiac conduction velocity, remodeling and arrhythmogenesis. Cells 2021, 10, 2923. [Google Scholar] [CrossRef]
- Dhein, S.; Salameh, A. Remodeling of cardiac gap junctional cell–cell coupling. Cells 2021, 10, 2422. [Google Scholar] [CrossRef] [PubMed]
- Verheule, S.; Schotten, U. Electrophysiological consequences of cardiac fibrosis. Cells 2021, 10, 3220. [Google Scholar] [CrossRef] [PubMed]
- Fedorov, V.V.; Li, L.; Glukhov, A.; Shishkina, I.; Aliev, R.R.; Mikheeva, T.; Nikolski, V.P.; Rosenshtraukh, L.V.; Efimov, I.R. Hibernator Citellus undulatus maintains safe cardiac conduction and is protected against tachyarrhythmias during extreme hypothermia: Possible role of Cx43 and Cx45 up-regulation. Heart Rhythm 2005, 2, 966–975. [Google Scholar] [CrossRef] [PubMed]
- Fedorov, V.V.; Glukhov, A.V.; Sudharshan, S.; Egorov, Y.; Rosenshtraukh, L.V.; Efimov, I.R. Electrophysiological mechanisms of antiarrhythmic protection during hypothermia in winter hibernating versus nonhibernating mammals. Heart Rhythm 2008, 5, 1587–1596. [Google Scholar] [CrossRef]
- Saitongdee, P.; Milner, P.; Becker, D.L.; Knight, G.E.; Burnstock, G. Increased connexin43 gap junction protein in hamster cardiomyocytes during cold acclimatization and hibernation. Cardiovasc. Res. 2000, 47, 108–115. [Google Scholar] [CrossRef]
- Tibenska, V.; Benesova, A.; Vebr, P.; Liptakova, A.; Hejnová, L.; Elsnicová, B.; Drahota, Z.; Hornikova, D.; Galatík, F.; Kolar, D.; et al. Gradual cold acclimation induces cardioprotection without affecting β- adrenergic receptor-mediated adenylyl cyclase signaling. J. Appl. Physiol. 2020, 128, 1023–1032. [Google Scholar] [CrossRef]
- Marvanova, A.; Kasik, P.; Elsnicova, B.; Tibenska, V.; Galatik, F.; Hornikova, D.; Zvolska, V.; Vebr, P.; Vodicka, P.; Hejnova, L.; et al. Continuous short-term acclimation to moderate cold elicits cardioprotection in rats, and alters β-adrenergic signaling and immune status. Sci. Rep. 2023, 13, 18287. [Google Scholar] [CrossRef]
- Nassal, M.M.J.; Wan, X.; Dale, Z.; Deschênes, I.; Wilson, L.D.; Piktel, J.S. Mild hypothermia preserves myocardial conduction during ischemia by maintaining gap junction intracellular communication and Na+ channel function. Am. J. Physiol.-Heart Circ. Physiol. 2017, 312, H886–H895. [Google Scholar] [CrossRef]
- Trnovská, J.; Šilhavỳ, J.; Zídek, V.; Šimáková, M.; Mlejnek, P.; Landa, V.; Eigner, S.; Eigner Henke, K.; Škop, V.; Oliyarnyk, O.; et al. Gender-related effects on substrate utilization and metabolic adaptation in hairless spontaneously hypertensive rat. Physiol. Res. 2015, 64, 51–60. [Google Scholar] [CrossRef]
- Andelova, K.; Szeiffova Bacova, B.; Sykora, M.; Pavelka, S.; Rauchova, H.; Tribulova, N. Cardiac Cx43 Signaling Is Enhanced and TGF-β1/SMAD2/3 Suppressed in Response to Cold Acclimation and Modulated by Thyroid Status in Hairless SHRM. Biomedicines 2022, 10, 1707. [Google Scholar] [CrossRef]
- Galis, P.; Bartosova, L.; Farkasova, V.; Szobi, A.; Horvath, C.; Kovacova, D.; Adameova, A.; Rajtik, T. Intermittent Hypoxic Preconditioning Plays a Cardioprotective Role in Doxorubicin-Induced Cardiomyopathy. Cardiovasc. Toxicol. 2023, 23, 185–197. [Google Scholar] [CrossRef]
- Lee, W.C.; Lin, Y.W.; Shih, J.Y.; Chen, Z.C.; Wu, N.C.; Chang, W.T. Ivabradine could not decrease mitral regurgitation triggered atrial fibrosis and fibrillation compared with carvedilol. ESC Heart Fail. 2024, 11, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Benova, T.; Viczenczova, C.; Radosinska, J.; Bacova, B.; Knezl, V.; Dosenko, V.; Weismann, P.; Zeman, M.; Navarova, J.; Tribulova, N. Melatonin attenuates hypertension-related proarrhythmic myocardial maladaptation of connexin-43 and propensity of the heart to lethal arrhythmias. Can. J. Physiol. Pharmacol. 2013, 91, 633–639. [Google Scholar] [CrossRef] [PubMed]
- Koyama, T.; Xie, Z.; Gao, M.; Suzuki, J.; Batra, S. Adaptive changes in the capillary network in the left ventricle of rat heart. Jpn. J. Physiol. 1998, 48, 229–241. [Google Scholar] [CrossRef]
- Bacova, B.S.; Viczenczova, C.; Andelova, K.; Sykora, M.; Chaudagar, K.; Barancik, M.; Adamcova, M.; Knezl, V.; Benova, T.E.; Weismann, P.; et al. Antiarrhythmic effects of melatonin and omega-3 are linked with protection of myocardial cx43 topology and suppression of fibrosis in catecholamine stressed normotensive and hypertensive rats. Antioxidants 2020, 9, 546. [Google Scholar] [CrossRef]
- Bacova, B.S.; Radosinska, J.; Wallukat, G.; Barancik, M.; Wallukat, A.; Knezl, V.; Sykora, M.; Paulis, L.; Tribulova, N. Suppression of β1-adrenoceptor autoantibodies is involved in the antiarrhythmic effects of omega-3 fatty acids in male and female hypertensive rats. Int. J. Mol. Sci. 2020, 21, 526. [Google Scholar] [CrossRef] [PubMed]
- Lendeckel, U.; Arndt, M.; Wrenger, S.; Nepple, K.; Huth, C.; Ansorge, S.; Klein, H.U.; Goette, A. Expression and activity of ectopeptidases in fibrillating human atria. J. Mol. Cell. Cardiol. 2001, 33, 1273–1281. [Google Scholar] [CrossRef]
- Blenck, C.L.; Harvey, P.A.; Reckelhoff, J.F.; Leinwand, L.A. The importance of biological sex and estrogen in rodent models of cardiovascular health and disease. Circ. Res. 2016, 118, 1294–1312. [Google Scholar] [CrossRef]
- Linde, C.; Bongiorni, M.G.; Birgersdotter-Green, U.; Curtis, A.B.; Deisenhofer, I.; Furokawa, T.; Gillis, A.M.; Haugaa, K.H.; Lip, G.Y.H.; Van Gelder, I.; et al. Sex differences in cardiac arrhythmia: A consensus document of the european heart rhythm association, endorsed by the heart rhythm society and Asia pacific heart rhythm society. Europace 2018, 20, 1565–1565ao. [Google Scholar] [CrossRef]
- Chi, J.; Wu, Z.; Choi, C.H.J.; Nguyen, L.; Tegegne, S.; Ackerman, S.E.; Crane, A.; Marchildon, F.; Tessier-Lavigne, M.; Cohen, P. Three-Dimensional Adipose Tissue Imaging Reveals Regional Variation in Beige Fat Biogenesis and PRDM16-Dependent Sympathetic Neurite Density. Cell Metab. 2018, 27, 226–236.e3. [Google Scholar] [CrossRef]
- Rassler, B.; Hawlitschek, C.; Brendel, J.; Zimmer, H. How Do Young and Old Spontaneously Hypertensive Rats Respond to Antihypertensive Therapy? Comparative Studies on the Effects of Combined Captopril and Nifedipine Treatment. Biomedicines 2022, 10, 3059. [Google Scholar] [CrossRef] [PubMed]
- Klimas, J.; Stankovicova, T.; Kyselovic, J.; Bacharova, L. Prolonged QT interval is associated with blood pressure rather than left ventricular mass in spontaneously hypertensive rats. Clin. Exp. Hypertens. 2008, 30, 475–485. [Google Scholar] [CrossRef] [PubMed]
- Adabag, S.; Gravely, A.; Kattel, S.; Buelt-Gebhardt, M.; Westanmo, A. QT prolongation predicts all-cause mortality above and beyond a validated risk score. J. Electrocardiol. 2024, 83, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Kollarova, M.; Puzserova, A.; Balis, P.; Radosinska, D.; Tothova, L.; Bartekova, M.; Barancik, M.; Radosinska, J. Age-and phenotype-dependent changes in circulating MMP-2 and MMP-9 activities in normotensive and hypertensive rats. Int. J. Mol. Sci. 2020, 21, 7286. [Google Scholar] [CrossRef]
- Camici, P.G.; Tschöpe, C.; Di Carli, M.F.; Rimoldi, O.; Van Linthout, S. Coronary microvascular dysfunction in hypertrophy and heart failure. Cardiovasc. Res. 2020, 116, 806–816. [Google Scholar] [CrossRef]
- La Vecchia, G.; Fumarulo, I.; Caffè, A.; Chiatto, M.; Montone, R.A.; Aspromonte, N. Microvascular Dysfunction across the Spectrum of Heart Failure Pathology: Pathophysiology, Clinical Features and Therapeutic Implications. Int. J. Mol. Sci. 2024, 25, 7628. [Google Scholar] [CrossRef]
- Lambeir, A.M.; Durinx, C.; Scharpé, S.; De Meester, I. Dipeptidyl-peptidase IV from bench to bedside: An update on structural properties, functions, and clinical aspects of the enzyme DPP IV. Crit. Rev. Clin. Lab. Sci. 2003, 40, 209–294. [Google Scholar] [CrossRef]
- Eguchi, S.; Torimoto, K.; Adebiyi, A.; Kanthakumar, P.; Bomfim, G.F.; Wenceslau, C.F.; Dahlen, S.A.; Osei-Owusu, P. Milestone Papers on Signal Transduction Mechanisms of Hypertension and Its Complications. Hypertension 2024, 81, 977–990. [Google Scholar] [CrossRef]
- Yang, B.; Lin, H.; Xiao, J.; Lu, Y.; Luo, X.; Li, B.; Zhang, Y.; Xu, C.; Bai, Y.; Wang, H.; et al. The muscle-specific microRNA miR-1 regulates cardiac arrhythmogenic potential by targeting GJA1 and KCNJ2. Nat. Med. 2007, 13, 486–491. [Google Scholar] [CrossRef]
- Jin, Y.; Zhou, T.Y.; Cao, J.N.; Feng, Q.T.; Fu, Y.J.; Xu, X.; Yang, C.J. MicroRNA-206 Downregulates Connexin43 in Cardiomyocytes to Induce Cardiac Arrhythmias in a Transgenic Mouse Model. Heart Lung Circ. 2019, 28, 1755–1761. [Google Scholar] [CrossRef]
- Xu, R.; Li, S.; Guo, S.; Zhao, Q.; Abramson, M.J.; Li, S.; Guo, Y. Environmental temperature and human epigenetic modifications: A systematic review. Environ. Pollut. 2020, 259, 113840. [Google Scholar] [CrossRef] [PubMed]


| BP (mm Hg) | BW (g) | HW (g) | LVW (g) | RVW (g) | BAT (g) | BAT/BW | |
|---|---|---|---|---|---|---|---|
| ♂ Wistar | 128.6 ± 11.2 | 388.5 ± 40.2 | 1.41 ± 0.06 | 0.79 ± 0.09 | 0.23 ± 0.05 | 0.31 ± 0.08 | 0.76 ± 0.11 |
| ♂ Wild SHR | 162.9 ± 13.8 a | 340 ± 21.4 | 1.47 ± 0.20 | 0.91 ± 0.10 | 0.25 ± 0.08 | 0.33 ± 0.04 | 0.97 ± 0.11 |
| ♂ Hairless SHR | 165.0 ± 16.9 a | 300.1 ± 23.1 a | 1.36 ± 0.05 | 1.00 ± 0.07 | 0.25 ± 0.03 | 0.42 ± 0.06 b | 1.42 ± 0.29 ab |
| ♀ Wistar | 120.3 ± 27.7 | 286.4 ± 55.6 a | 0.88 ± 0.08 a | 0.58 ± 0.09 | 0.18 ± 0.05 | 0.24 ± 0.05 | 0.89 ± 0.27 |
| ♀ Wild SHR | 152.4 ± 11.5 d | 197.5 ± 37.7 bd | 0.86 ± 0.05 b | 0.68 ± 0.10 b | 0.16 ± 0.03 b | 0.21 ± 0.06 b | 1.04 ± 0.22 |
| ♀ Hairless SHR | 150.2 ± 10.0 d | 180.1 ± 10.3 cd | 0.98 ± 0.06 c | 0.78 ± 0.12 cd | 0.19 ± 0.04 | 0.27 ± 0.09 c | 1.46 ± 0.03 d |
| IVSd (mm) | LVPWd (mm) | LVIDd (mm) | LVIDs (mm) | RWT (mm) | |
|---|---|---|---|---|---|
| ♂ Wistar | 1.37 ± 0.16 | 1.49 ± 0.11 | 6.81 ± 0.40 | 4.28 ± 0.40 | 0.44 ± 0.04 |
| ♂ Wild SHR | 1.77 ± 0.10 a | 1.67 ± 0.09 | 7.77 ± 0.25 a | 5.17 ± 0.10 a | 0.43 ± 0.03 |
| ♂ Hairless SHR | 1.73 ± 0.15 | 1.81 ± 0.25 | 7.32 ± 0.20 | 4.21 ± 0.37 b | 0.49 ± 0.07 |
| ♀ Wistar | 1.61 ± 0.08 | 1.48 ± 0.12 | 7.12 ± 0.05 | 4.06 ± 0.21 | 0.41 ± 0.01 |
| ♀ Wild SHR | 1.86 ± 0.08 | 1.55 ± 0.22 | 6.44 ± 0.42 b | 3.81 ± 0.39 b | 0.49 ± 0.10 |
| ♀ Hairless SHR | 1.84 ± 0.30 | 1.66 ± 0.04 | 6.52 ± 0.21 | 3.59 ± 0.22 | 0.51 ± 0.01 |
| HR (beat/min) | CO (L/min) | EF (%) | FS (%) | EDV (mL) | ESV (mL) | |
|---|---|---|---|---|---|---|
| ♂ Wistar | 459 ± 6 | 0.25 ± 0.06 | 76.25 ± 3.18 | 37.60 ± 4.39 | 0.72 ± 0.11 | 0.17 ± 0.05 |
| ♂ Wild SHR | 334 ± 45 a | 0.23 ± 0.02 | 67.83 ± 2.08 a | 33.50 ± 1.80 | 1.04 ± 0.09 a | 0.33 ± 0.02 a |
| ♂ Hairless SHR | 353 ± 41 a | 0.25 ± 0.04 | 79.00 ± 4.77 b | 42.83 ± 4.19 b | 0.90 ± 0.05 | 0.19 ± 0.05 b |
| ♀ Wistar | 377 ± 12 a | 0.24 ± 0.01 | 79.50 ± 2.12 | 43.25 ± 2.47 | 0.81 ± 0.12 | 0.17 ± 0.03 |
| ♀ Wild SHR | 330 ± 21 | 0.17 ± 0.03 | 77.00 ± 2.65 b | 40.83 ± 4.36 | 0.62 ± 0.11 b | 0.14 ± 0.04 b |
| ♀ Hairless SHR | 383 ± 23 | 0.22 ± 0.04 | 82.50 ± 2.65 | 46.50 ± 2.64 | 0.64 ± 0.06 c | 0.11 ± 0.01 |
| PQ (ms) | QRS (ms) | QT (ms) | QTc | |
|---|---|---|---|---|
| ♂ Wistar | 28.65 ± 6.35 | 20.54 ± 4.35 | 56.69 ± 7.59 | 72.56 ± 9.02 |
| ♂ Wild SHR | 30.90 ± 7.64 | 20.22 ± 6.25 | 67.50 ± 8.36 | 90.35 ± 8.64 a |
| ♂ Hairless SHR | 27.00 ± 3.30 | 17.67 ± 1.89 | 57.36 ± 6.71 | 62.83 ± 10.32 b |
| ♀ Wistar | 28.86 ± 5.82 | 17.48 ± 1.81 | 54.58 ± 2.92 | 77.40 ± 17.39 |
| ♀ Wild SHR | 32.48 ± 5.58 | 16.28 ± 2.18 | 65.80 ± 6.19 | 75.26 ± 21.88 |
| ♀ Hairless SHR | 35.50 ± 12.20 | 21.50 ± 2.12 | 63.25 ± 2.47 | 82.01 ± 21.21 |
| Number of Rats | Current Magnitude (mA) | SVF | |
|---|---|---|---|
| ♂ Wistar | 3 | 50 | No |
| ♂ Wild SHR | 1 | 35 | Yes |
| 1 | 25 | Yes | |
| 1 | 30 | Yes | |
| ♂ Hairless SHR | 2 | 45 | Yes |
| 1 | 50 | No | |
| ♀ Wistar | 3 | 50 | No |
| ♀ Wild SHR | 2 | 50 | Yes |
| 1 | 50 | No | |
| ♀ Hairless SHR | 3 | 50 | No |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andelova, K.; Sykora, M.; Farkasova, V.; Stankovicova, T.; Szeiffova Bacova, B.; Knezl, V.; Benova, T.E.; Pravenec, M.; Tribulova, N. Acclimation of Hairless Spontaneously Hypertensive Rat to Ambient Temperature Attenuates Hypertension-Induced Pro-Arrhythmic Downregulation of Cx43 in the Left Heart Ventricle of Males. Biomolecules 2024, 14, 1509. https://doi.org/10.3390/biom14121509
Andelova K, Sykora M, Farkasova V, Stankovicova T, Szeiffova Bacova B, Knezl V, Benova TE, Pravenec M, Tribulova N. Acclimation of Hairless Spontaneously Hypertensive Rat to Ambient Temperature Attenuates Hypertension-Induced Pro-Arrhythmic Downregulation of Cx43 in the Left Heart Ventricle of Males. Biomolecules. 2024; 14(12):1509. https://doi.org/10.3390/biom14121509
Chicago/Turabian StyleAndelova, Katarina, Matus Sykora, Veronika Farkasova, Tatiana Stankovicova, Barbara Szeiffova Bacova, Vladimir Knezl, Tamara Egan Benova, Michal Pravenec, and Narcis Tribulova. 2024. "Acclimation of Hairless Spontaneously Hypertensive Rat to Ambient Temperature Attenuates Hypertension-Induced Pro-Arrhythmic Downregulation of Cx43 in the Left Heart Ventricle of Males" Biomolecules 14, no. 12: 1509. https://doi.org/10.3390/biom14121509
APA StyleAndelova, K., Sykora, M., Farkasova, V., Stankovicova, T., Szeiffova Bacova, B., Knezl, V., Benova, T. E., Pravenec, M., & Tribulova, N. (2024). Acclimation of Hairless Spontaneously Hypertensive Rat to Ambient Temperature Attenuates Hypertension-Induced Pro-Arrhythmic Downregulation of Cx43 in the Left Heart Ventricle of Males. Biomolecules, 14(12), 1509. https://doi.org/10.3390/biom14121509





