Common Genetic Factors May Play a Role in the Relationships Between Body Composition, Adipokines, and Low-Back-Pain-Related Disability
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population Design and Ethics
2.2. Assessment of Roland–Morris Disability Scores (RMDQ)
2.3. Demographic, Anthropometric, and Body Composition Assessment
2.4. Measurement of Adipokine Plasma Levels
2.5. Statistical Analysis
2.6. Statistical–Genetic Analysis
3. Results
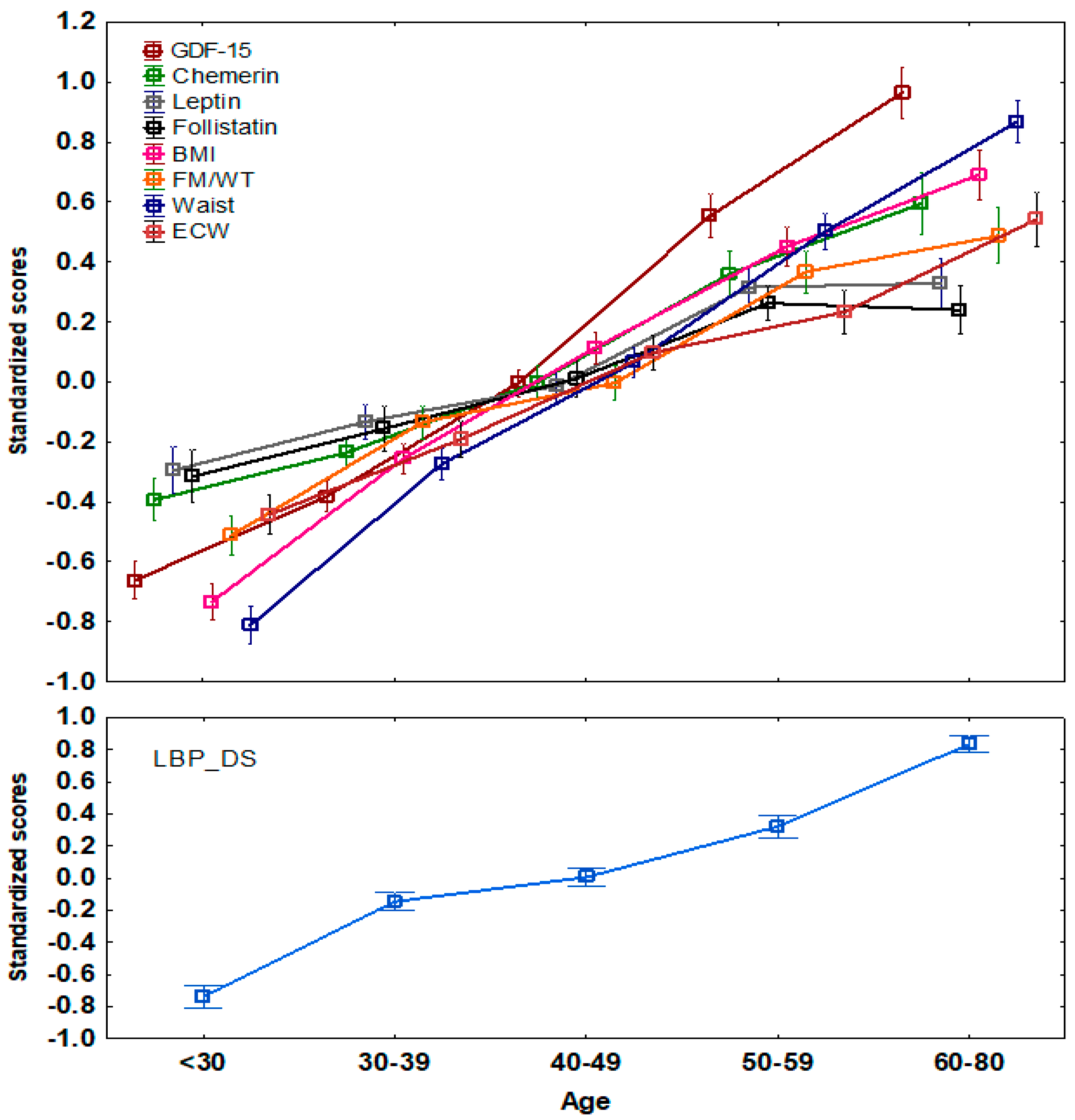
3.1. Associations of Adipokine Plasma Levels and Body Composition Parameters with LBP_DS
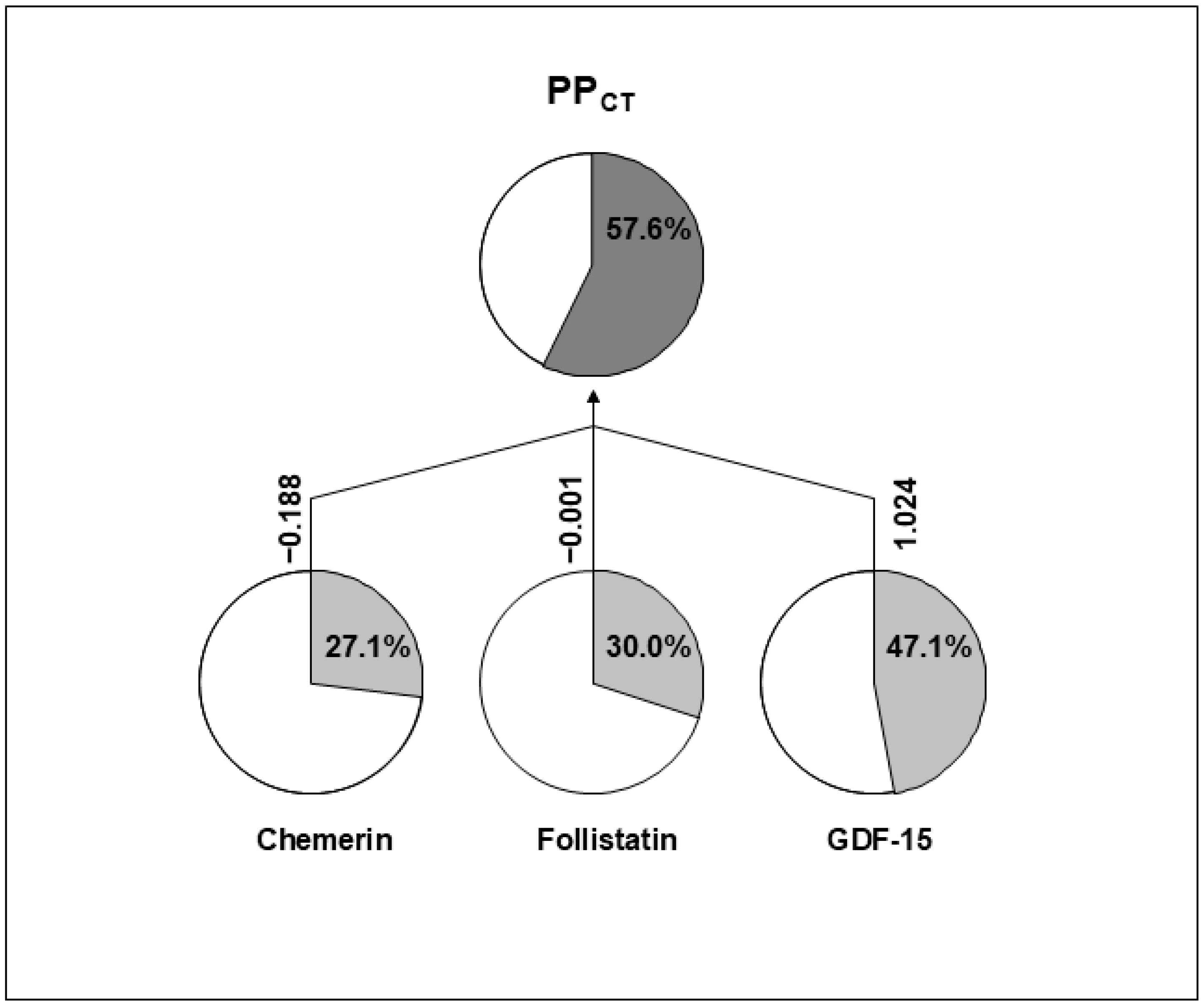
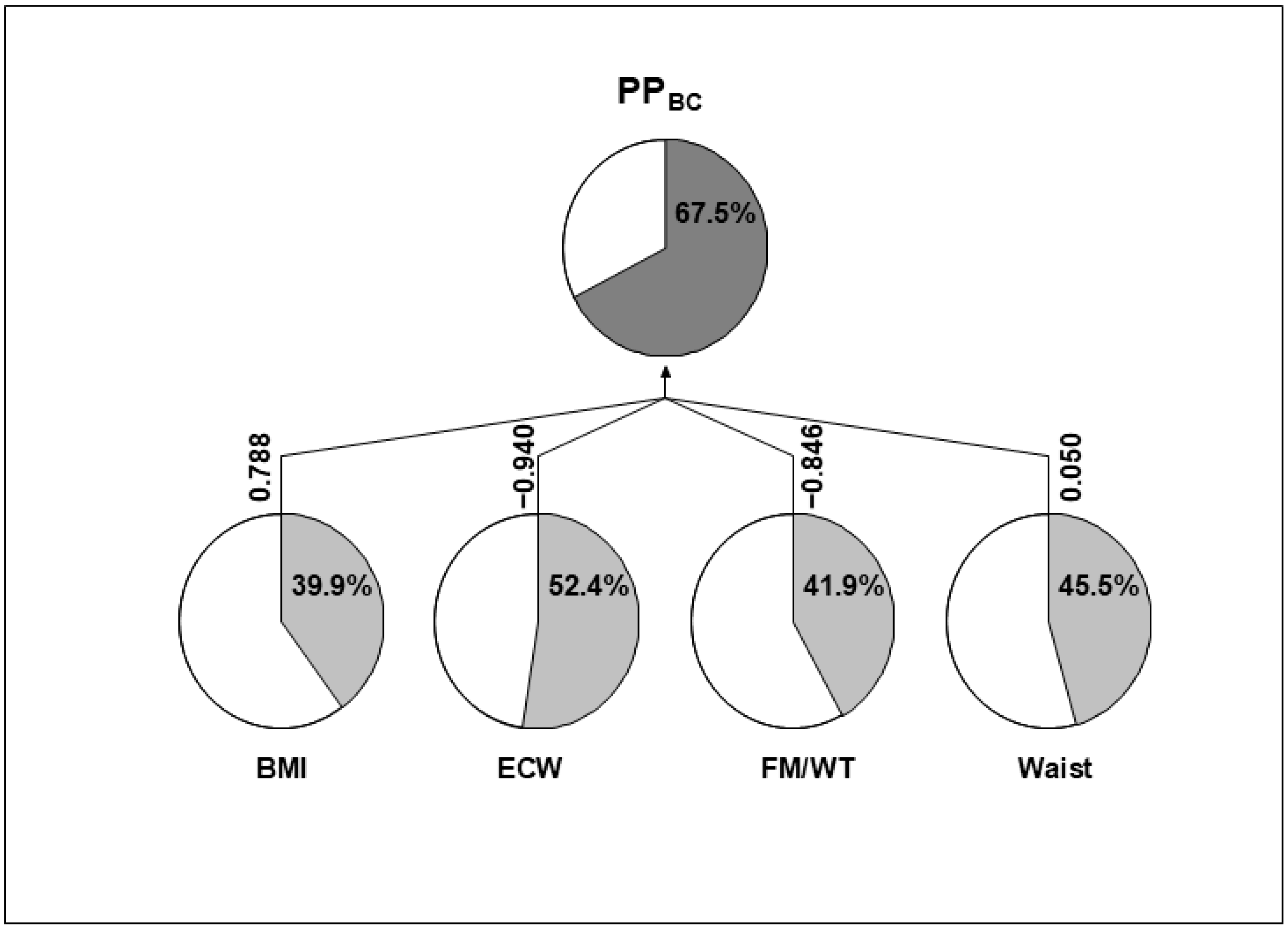
3.2. Quantitative–Genetic Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nicol, V.; Verdaguer, C.; Daste, C.; Bisseriex, H.; Lapeyre, É.; Lefèvre-Colau, M.M.; Rannou, F.; Rören, A.; Facione, J.; Nguyen, C. Chronic Low Back Pain: A Narrative Review of Recent International Guidelines for Diagnosis and Conservative Treatment. J. Clin. Med. 2023, 12, 1685. [Google Scholar] [CrossRef]
- Ferreira, M.L.; De Luca, K.; Haile, L.M.; Steinmetz, J.D.; Culbreth, G.T.; Cross, M.; Kopec, J.A.; Ferreira, P.H.; Blyth, F.M.; Buchbinder, R.; et al. Global, Regional, and National Burden of Low Back Pain, 1990–2020, Its Attributable Risk Factors, and Projections to 2050: A Systematic Analysis of the Global Burden of Disease Study 2021. Lancet Rheumatol. 2023, 5, e316–e329. [Google Scholar] [CrossRef]
- Sharma, A. The Role of Adipokines in Intervertebral Disc Degeneration. Med. Sci. 2018, 6, 34. [Google Scholar] [CrossRef]
- Ruiz-Fernández, C.; Francisco, V.; Pino, J.; Mera, A.; González-Gay, M.A.; Gómez, R.; Lago, F.; Gualillo, O. Molecular Relationships among Obesity, Inflammation and Intervertebral Disc Degeneration: Are Adipokines the Common Link? Int. J. Mol. Sci. 2019, 20, 2030. [Google Scholar] [CrossRef]
- Francisco, V.; Pino, J.; González-Gay, M.Á.; Lago, F.; Karppinen, J.; Tervonen, O.; Mobasheri, A.; Gualillo, O. A New Immunometabolic Perspective of Intervertebral Disc Degeneration. Nat. Rev. Rheumatol. 2022, 18, 47–60. [Google Scholar] [CrossRef]
- Ouchi, N.; Parker, J.L.; Lugus, J.J.; Walsh, K. Adipokines in Inflammation and Metabolic Disease. Nat. Rev. Immunol. 2011, 11, 85–97. [Google Scholar] [CrossRef]
- Nakamura, K.; Fuster, J.J.; Walsh, K. Adipokines: A Link between Obesity and Cardiovascular Disease. J. Cardiol. 2014, 63, 250–259. [Google Scholar] [CrossRef]
- Tarabeih, N.; Kalinkovich, A.; Shalata, A.; Cherny, S.S.; Livshits, G. Deciphering the Causal Relationships Between Low Back Pain Complications, Metabolic Factors, and Comorbidities. J. Pain Res. 2022, 15, 215–227. [Google Scholar] [CrossRef]
- Luo, J.; He, Z.; Li, Q.; Lv, M.; Cai, Y.; Ke, W.; Niu, X.; Zhang, Z. Adipokines in Atherosclerosis: Unraveling Complex Roles. Front. Cardiovasc. Med. 2023, 10, 1235953. [Google Scholar] [CrossRef] [PubMed]
- Low Back Pain. Available online: https://www.who.int/news-room/fact-sheets/detail/low-back-pain (accessed on 25 September 2024).
- Kumar, R.; Alostaz, M.; Bansal, A.; Varley, E.; Nemani, V.; Leveque, J.C.; Sethi, R.; Louie, P.K. Describing Functional Low Back Pain Disability in the American Population: A Crowd-Sourced Evaluation of the Oswestry Disability Index. Spine J. 2024, 24, 2165–2171. [Google Scholar] [CrossRef] [PubMed]
- Tsai, V.W.W.; Lin, S.; Brown, D.A.; Salis, A.; Breit, S.N. Anorexia-Cachexia and Obesity Treatment May Be Two Sides of the Same Coin: Role of the TGF-b Superfamily Cytokine MIC-1/GDF15. Int. J. Obes. 2016, 40, 193–197. [Google Scholar] [CrossRef]
- Breit, S.N.; Brown, D.A.; Tsai, V.W.W. The GDF15-GFRAL Pathway in Health and Metabolic Disease: Friend or Foe? Annu. Rev. Physiol. 2021, 83, 127–151. [Google Scholar] [CrossRef]
- Asrih, M.; Wei, S.; Nguyen, T.T.; Yi, H.S.; Ryu, D.; Gariani, K. Overview of Growth Differentiation Factor 15 in Metabolic Syndrome. J. Cell Mol. Med. 2023, 27, 1157–1167. [Google Scholar] [CrossRef]
- Clemente-Suárez, V.J.; Redondo-Flórez, L.; Beltrán-Velasco, A.I.; Martín-Rodríguez, A.; Martínez-Guardado, I.; Navarro-Jiménez, E.; Laborde-Cárdenas, C.C.; Tornero-Aguilera, J.F. The Role of Adipokines in Health and Disease. Biomedicines 2023, 11, 1290. [Google Scholar] [CrossRef]
- Tarabeih, N.; Shalata, A.; Trofimov, S.; Kalinkovich, A.; Livshits, G. Growth and Differentiation Factor 15 Is a Biomarker for Low Back Pain-Associated Disability. Cytokine 2019, 117, 8–14. [Google Scholar] [CrossRef]
- Dario, A.B.; Ferreira, M.L.; Refshauge, K.M.; Lima, T.S.; Ordoñana, J.R.; Ferreira, P.H. The Relationship between Obesity, Low Back Pain, and Lumbar Disc Degeneration When Genetics and the Environment Are Considered: A Systematic Review of Twin Studies. Spine J. 2015, 15, 1106–1117. [Google Scholar] [CrossRef]
- Dario, A.B.; Loureiro Ferreira, M.; Refshauge, K.; Luque-Suarez, A.; Ordoñana, J.R.; Ferreira, P.H. Obesity Does Not Increase the Risk of Chronic Low Back Pain When Genetics Are Considered. A Prospective Study of Spanish Adult Twins. Spine J. 2017, 17, 282–290. [Google Scholar] [CrossRef]
- Zhou, J.; Mi, J.; Peng, Y.; Han, H.; Liu, Z. Causal Associations of Obesity With the Intervertebral Degeneration, Low Back Pain, and Sciatica: A Two-Sample Mendelian Randomization Study. Front. Endocrinol. 2021, 12, 740200. [Google Scholar] [CrossRef]
- Ward, L.C. Bioelectrical Impedance Analysis for Body Composition Assessment: Reflections on Accuracy, Clinical Utility, and Standardisation. Eur. J. Clin. Nutr. 2019, 73, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Hartvigsen, J.; Nielsen, J.; Kyvik, K.O.H.M.; Fejer, R.; Vach, W.; Iachine, I.; Leboeuf-Yde, C. Heritability of Spinal Pain and Consequences of Spinal Pain: A Comprehensive Genetic Epidemiologic Analysis Using a Population-Based Sample of 15,328 Twins Ages 20–71 Years. Arthritis Rheum. 2009, 61, 1343–1351. [Google Scholar] [CrossRef]
- Battié, M.C.; Videman, T.; Levalahti, E.; Gill, K.; Kaprio, J. Heritability of Low Back Pain and the Role of Disc Degeneration. Pain 2007, 131, 272–280. [Google Scholar] [CrossRef]
- Livshits, G.; Popham, M.; Malkin, I.; Sambrook, P.N.; MacGregor, A.J.; Spector, T.; Williams, F.M.K. Lumbar Disc Degeneration and Genetic Factors Are the Main Risk Factors for Low Back Pain in Women: The UK Twin Spine Study. Ann. Rheum. Dis. 2011, 70, 1740–1745. [Google Scholar] [CrossRef]
- Malkin, I.; Williams, F.M.K.; LaChance, G.; Spector, T.; MacGregor, A.J.; Livshits, G. Low Back and Common Widespread Pain Share Common Genetic Determinants. Ann. Hum. Genet. 2014, 78, 357–366. [Google Scholar] [CrossRef]
- Tarabeih, N.; Kalinkovich, A.; Shalata, A.; Livshits, G. Circulating Levels of Visceral Adipose Tissue-Derived Serine Protease Inhibitor (Vaspin) Appear as a Marker of Musculoskeletal Pain Disability. Diagnostics 2020, 10, 797. [Google Scholar] [CrossRef]
- Tarabeih, N.; Shalata, A.; Higla, O.; Kalinkovich, A.; Livshits, G. The Search for Systemic Biomarkers for Monitoring Degenerative Lumbar Spinal Disorders. Osteoarthr. Cartil. Open 2022, 4, 100323. [Google Scholar] [CrossRef]
- Tarabeih, N.; Kalinkovich, A.; Ashkenazi, S.; Cherny, S.S.; Shalata, A.; Livshits, G. Relationships between Circulating Biomarkers and Body Composition Parameters in Patients with Metabolic Syndrome: A Community-Based Study. Int. J. Mol. Sci. 2024, 25, 881. [Google Scholar] [CrossRef]
- Tarabeih, N.; Kalinkovich, A.; Ashkenazi, S.; Cherny, S.S.; Shalata, A.; Livshits, G. Analysis of the Associations of Measurements of Body Composition and Inflammatory Factors with Cardiovascular Disease and Its Comorbidities in a Community-Based Study. Biomedicines 2024, 12, 1066. [Google Scholar] [CrossRef]
- Smeets, R.; Köke, A.; Lin, C.W.; Ferreira, M.; Demoulin, C. Measures of Function in Low Back Pain/Disorders: Low Back Pain Rating Scale (LBPRS), Oswestry Disability Index (ODI), Progressive Isoinertial Lifting Evaluation (PILE), Quebec Back Pain Disability Scale (QBPDS), and Roland-Morris Disability Questionnaire (RDQ). Arthritis Care Res. 2011, 63, S158–S173. [Google Scholar] [CrossRef]
- Ko, S.; Chae, S. Correlations between the SF-36, the Oswestry-Disability Index and Rolland-Morris Disability Questionnaire in Patients Undergoing Lumbar Decompression According to Types of Spine Origin Pain. Clin. Spine Surg. 2017, 30, E804–E808. [Google Scholar] [CrossRef]
- Roland, M.; Fairbank, J. The Roland-Morris Disability Questionnaire and the Oswestry Disability Questionnaire. Spine 2000, 25, 3115–3124. [Google Scholar] [CrossRef]
- Tarabeih, N.; Masharawi, Y.; Shalata, A.; Higla, O.; Kalinkovich, A.; Livshits, G. Scoliosis and Skeletal Muscle Mass Are Strongly Associated with Low Back Pain-Related Disability in Humans: An Evolutionary Anthropology Point of View. Am. J. Hum. Biol. 2022, 34, e23757. [Google Scholar] [CrossRef]
- Achamrah, N.; Colange, G.; Delay, J.; Rimbert, A.; Folope, V.; Petit, A.; Grigioni, S.; Déchelotte, P.; Coëffier, M. Comparison of Body Composition Assessment by DXA and BIA According to the Body Mass Index: A Retrospective Study on 3655 Measures. PLoS ONE 2018, 13, e0200465. [Google Scholar] [CrossRef]
- Marra, M.; Sammarco, R.; De Lorenzo, A.; Iellamo, F.; Siervo, M.; Pietrobelli, A.; Donini, L.M.; Santarpia, L.; Cataldi, M.; Pasanisi, F.; et al. Assessment of Body Composition in Health and Disease Using Bioelectrical Impedance Analysis (BIA) and Dual Energy X-ray Absorptiometry (DXA): A Critical Overview. Contrast Media Mol. Imaging 2019, 2019, 3548284. [Google Scholar] [CrossRef]
- McManus, M.L.; Churchwell, K.B.; Strange, K. Regulation of Cell Volume in Health and Disease. N. Engl. J. Med. 1995, 333, 1260–1266. [Google Scholar] [CrossRef]
- Mehdizadeh, R. Relationship between Body Water Compartments and Indexes of Adiposity in Sedentary Young Adult Girls. Braz. J. Biomotricity 2012, 6, 84–92. [Google Scholar]
- Falconer, D.S.; Douglas, S.; Mackay, T.F.C. Introduction to Quantitative Genetics; Longman: Harlow, UK, 1996; ISBN 0582243025. [Google Scholar]
- Endo, T.; Abe, T.; Akai, K.; Kijima, T.; Takeda, M.; Yamasaki, M.; Isomura, M.; Nabika, T.; Yano, S. Height Loss but Not Body Composition Is Related to Low Back Pain in Community-Dwelling Elderlies: Shimane CoHRE Study. BMC Musculoskelet. Disord. 2019, 20, 207. [Google Scholar] [CrossRef]
- Okamoto, C.S.; Dunn, A.S.; Green, B.N.; Formolo, L.R.; Chicoine, D. Correlation of Body Composition and Low Back Pain Severity in a Cross-Section of US Veterans. J. Manip. Physiol. Ther. 2017, 40, 358–364. [Google Scholar] [CrossRef]
- Shim, J.G.; Ryu, K.H.; Cho, E.A.; Ahn, J.H.; Park, J.; Lee, H.W.; Kang, S.; Han, S.Y.; Lee, S.H. Association between Body Composition and Chronic Low Back Pain in Korean Adults Aged over 50 Years: The Korea National Health and Nutrition Examination Survey (KNHANES) 2010–2011. Med. Princ. Pract. 2023, 32, 281–287. [Google Scholar] [CrossRef]
- Xu, Z.; Qi, L.; Zhang, H.; Yu, D.; Shi, Y.; Yu, Y.; Zhu, T. Smoking and BMI Mediate the Causal Effect of Education on Lower Back Pain: Observational and Mendelian Randomization Analyses. Front. Endocrinol. 2024, 15, 1288170. [Google Scholar] [CrossRef] [PubMed]
- Brener, A.; Waksman, Y.; Rosenfeld, T.; Levy, S.; Peleg, I.; Raviv, A.; Interator, H.; Lebenthal, Y. The Heritability of Body Composition. BMC Pediatr. 2021, 21, 225. [Google Scholar] [CrossRef] [PubMed]
- Hanisch, D.; Dittmar, M.; Höhler, T.; Alt, K.W. Contribution of Genetic and Environmental Factors to Variation in Body Compartments—A Twin Study in Adults. Anthropol. Anz. 2004, 62, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.M.; Urquhart, D.M.; Wang, Y.; Shaw, J.E.; Magliano, D.J.; Wluka, A.E.; Cicuttini, F.M. Fat Mass and Fat Distribution Are Associated with Low Back Pain Intensity and Disability: Results from a Cohort Study. Arthritis Res. Ther. 2017, 19, 26. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Li, Q.; Luk, A.O.Y.; Lan, H.Y.; Chan, P.K.S.; Bayés-Genís, A.; Chan, F.K.L.; Fung, E. Major Adverse Cardiovascular Events and Mortality Prediction by Circulating GDF-15 in Patients with Type 2 Diabetes: A Systematic Review and Meta-Analysis. Biomolecules 2022, 12, 934. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, A.; Kinscherf, R.; Bonaterra, G.A. Role of the Stress- and Inflammation-Induced Cytokine GDF-15 in Cardiovascular Diseases: From Basic Research to Clinical Relevance. Rev. Cardiovasc. Med. 2023, 24, 81. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Day, E.A.; Townsend, L.K.; Djordjevic, D.; Jørgensen, S.B.; Steinberg, G.R. GDF15: Emerging Biology and Therapeutic Applications for Obesity and Cardiometabolic Disease. Nat. Rev. Endocrinol. 2021, 17, 592–607. [Google Scholar] [CrossRef]
- Liu, Q.; Qin, L.; Liang, Y.; Xu, M.; Zhang, J.; Mo, X.; Tang, X.; Lu, Y.; Wang, X.; Cao, J.; et al. Correlations between Growth Differentiation Factor 15 (GDF-15) Serum Levels and Gene Polymorphism with Type 2 Diabetes Mellitus. Heliyon 2024, 10, e33044. [Google Scholar] [CrossRef]
- Xiang, Y.; Zhang, T.; Guo, J.; Peng, Y.F.; Wei, Y.S. The Association of Growth Differentiation Factor-15 Gene Polymorphisms with Growth Differentiation Factor-15 Serum Levels and Risk of Ischemic Stroke. J. Stroke Cerebrovasc. Dis. 2017, 26, 2111–2119. [Google Scholar] [CrossRef]
- Tan, L.; Lu, X.; Danser, A.H.J.; Verdonk, K. The Role of Chemerin in Metabolic and Cardiovascular Disease: A Literature Review of Its Physiology and Pathology from a Nutritional Perspective. Nutrients 2023, 15, 2878. [Google Scholar] [CrossRef]
- Macvanin, M.T.; Rizzo, M.; Radovanovic, J.; Sonmez, A.; Paneni, F.; Isenovic, E.R. Role of Chemerin in Cardiovascular Diseases. Biomedicines 2022, 10, 2970. [Google Scholar] [CrossRef]
- Zhao, K.; Ding, W.; Zhang, Y.; Ma, K.; Wang, D.; Hu, C.; Liu, J.; Zhang, X. Variants in the RARRES2 Gene Are Associated with Serum Chemerin and Increase the Risk of Diabetic Kidney Disease in Type 2 Diabetes. Int. J. Biol. Macromol. 2020, 165, 1574–1580. [Google Scholar] [CrossRef]
- Wang, D.; Wang, H.; Li, M.; Zhao, R. Chemerin Levels and Its Genetic Variants Are Associated with Gestational Diabetes Mellitus: A Hospital-Based Study in a Chinese Cohort. Gene 2022, 807, 145888. [Google Scholar] [CrossRef] [PubMed]
- Hasanvand, Z.; Sadeghi, A.; Rezvanfar, M.R.; Goodarzi, M.T.; Rahmannezhad, G.; Mashayekhi, F.J. Association between Chemerin Rs17173608 and Rs4721 Gene Polymorphisms and Gestational Diabetes Mellitus in Iranian Pregnant Women. Gene 2018, 649, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Parfenova, O.K.; Kukes, V.G.; Grishin, D.V. Follistatin-Like Proteins: Structure, Functions and Biomedical Importance. Biomedicines 2021, 9, 999. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Møller, L.L.V.; De Groote, E.; Bojsen-Møller, K.N.; Davey, J.; Henríquez-Olguin, C.; Li, Z.; Knudsen, J.R.; Jensen, T.E.; Madsbad, S.; et al. Mechanisms Involved in Follistatin-Induced Hypertrophy and Increased Insulin Action in Skeletal Muscle. bioRxiv 2019, bioRxiv 568097. [Google Scholar]
- Canalis, E.; Economides, A.N.; Gazzerro, E. Bone Morphogenetic Proteins, Their Antagonists, and the Skeleton. Endocr. Rev. 2003, 24, 218–235. [Google Scholar] [CrossRef]
- May, R.D.; Frauchiger, D.A.; Albers, C.E.; Tekari, A.; Benneker, L.M.; Klenke, F.M.; Hofstetter, W.; Gantenbein, B. Application of Cytokines of the Bone Morphogenetic Protein (BMP) Family in Spinal Fusion—Effects on the Bone, Intervertebral Disc and Mesenchymal Stromal Cells. Curr. Stem Cell Res. Ther. 2019, 14, 618–643. [Google Scholar] [CrossRef]
- Heng, A.H.S.; Say, Y.H.; Sio, Y.Y.; Ng, Y.T.; Chew, F.T. Gene Variants Associated with Acne Vulgaris Presentation and Severity: A Systematic Review and Meta-Analysis. BMC Med. Genom. 2021, 14, 103. [Google Scholar] [CrossRef]
- Hedger, M.P.; Winnall, W.R.; Phillips, D.J.; de Kretser, D.M. The Regulation and Functions of Activin and Follistatin in Inflammation and Immunity. Vitam. Horm. 2011, 85, 255–297. [Google Scholar] [CrossRef]
| Covariate | R | p | p a |
|---|---|---|---|
| Age | 0.481 | <0.00001 | - |
| BMI | 0.280 | <0.00001 | 0.04 |
| FM/WT | 0.227 | <0.00001 | 0.03 |
| SMM/WT | −0.230 | <0.00001 | NS |
| Waist | 0.360 | <0.00001 | <0.00001 |
| ECW | 0.260 | <0.00001 | 0.005 |
| Adiponectin | 0.031 | NS | NS |
| Adipsin | 0.224 | <0.0001 | 0.0002 |
| Chemerin | 0.280 | <0.00001 | 0.0002 |
| Follistatin | 0.171 | <0.00001 | 0.006 |
| GDF-15 | 0.402 | <0.00001 | <0.00001 |
| Leptin | 0.181 | <0.00001 | 0.01 |
| Variable | Additive Genetic Variance (VAD) | Additive Genetic Correlation (RAD) with LBP_DS | ||
|---|---|---|---|---|
| LBP_DS | 0.2229 ± 0.0636 | p = 0.000006 | - | |
| BMI | 0.3883 ± 0.0783 | p = 1.86 × 10−12 | 0.3239 ± 0.1346 | p = 0.01 |
| FM/WT | 0.3879 ± 0.0981 | p = 3.52 × 10−5 | 0.3924 ± 0.1295 | p = 0.002 |
| Waist | 0.4328 ± 0.0835 | p = 6.08 × 10−13 | 0.3704 ± 0.1265 | p = 0.003 |
| ECW | 0.5401 ± 0.0886 | p = 7.90 × 10−11 | 0.3817 ± 0.1050 | p = 0.0001 |
| Adipsin | 0.1199 ± 0.0772 | p > 0.05 | N/A | |
| Chemerin | 0.2615 ± 0.0814 | p = 0.0006 | 0.2876 ± 0.1421 | p = 0.03 |
| Follistatin | 0.2949 ± 0.0744 | p = 2.03 × 10−8 | 0.4055 ± 0.1566 | p = 0.01 |
| GDF-15 | 0.4289 ± 0.0899 | p = 0.0001 | 0.4296 ± 0.1049 | p = 0.00006 |
| Leptin | 0.3694 ± 0.0752 | p = 1.94 × 10−13 | 0.1581 ± 0.1462 | p > 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tarabeih, N.; Kalinkovich, A.; Ashkenazi, S.; Shalata, A.; Livshits, G. Common Genetic Factors May Play a Role in the Relationships Between Body Composition, Adipokines, and Low-Back-Pain-Related Disability. Biomolecules 2024, 14, 1426. https://doi.org/10.3390/biom14111426
Tarabeih N, Kalinkovich A, Ashkenazi S, Shalata A, Livshits G. Common Genetic Factors May Play a Role in the Relationships Between Body Composition, Adipokines, and Low-Back-Pain-Related Disability. Biomolecules. 2024; 14(11):1426. https://doi.org/10.3390/biom14111426
Chicago/Turabian StyleTarabeih, Nader, Alexander Kalinkovich, Shai Ashkenazi, Adel Shalata, and Gregory Livshits. 2024. "Common Genetic Factors May Play a Role in the Relationships Between Body Composition, Adipokines, and Low-Back-Pain-Related Disability" Biomolecules 14, no. 11: 1426. https://doi.org/10.3390/biom14111426
APA StyleTarabeih, N., Kalinkovich, A., Ashkenazi, S., Shalata, A., & Livshits, G. (2024). Common Genetic Factors May Play a Role in the Relationships Between Body Composition, Adipokines, and Low-Back-Pain-Related Disability. Biomolecules, 14(11), 1426. https://doi.org/10.3390/biom14111426








