LSD1 Demethylates and Destabilizes Autophagy Protein LC3B in Ovarian Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Line and Culture Condition
2.2. Chemical Reagents and Antibodies
2.3. Plasmid Construction and Transfection
2.4. Virus Packaging and Transduction
2.5. Creation of Dox-Inducible LSD1 Knockdown and Overexpression Cell Lines
2.6. Immunoprecipitation and Western Blotting
2.7. Purification of Recombinant Protein and GST Affinity Precipitation Assay
2.8. Immunofluorescence
2.9. Tissue Specimens and Immunohistochemistry (IHC)
2.10. Analysis of TCGA and GEO Datasets
2.11. Statistical Analysis
3. Results
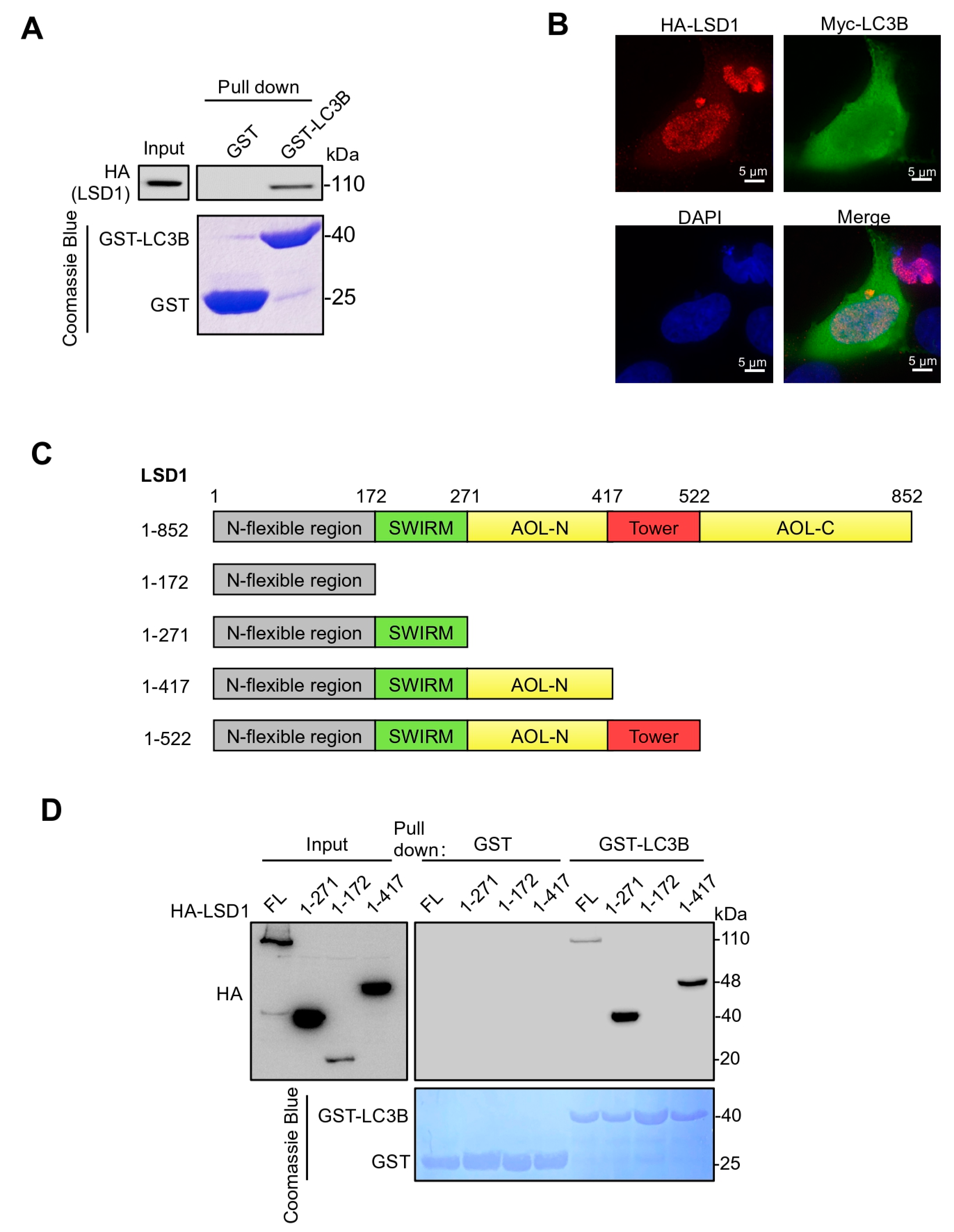
3.1. LSD1 Binds with LC3B via Its SWIRM Domain
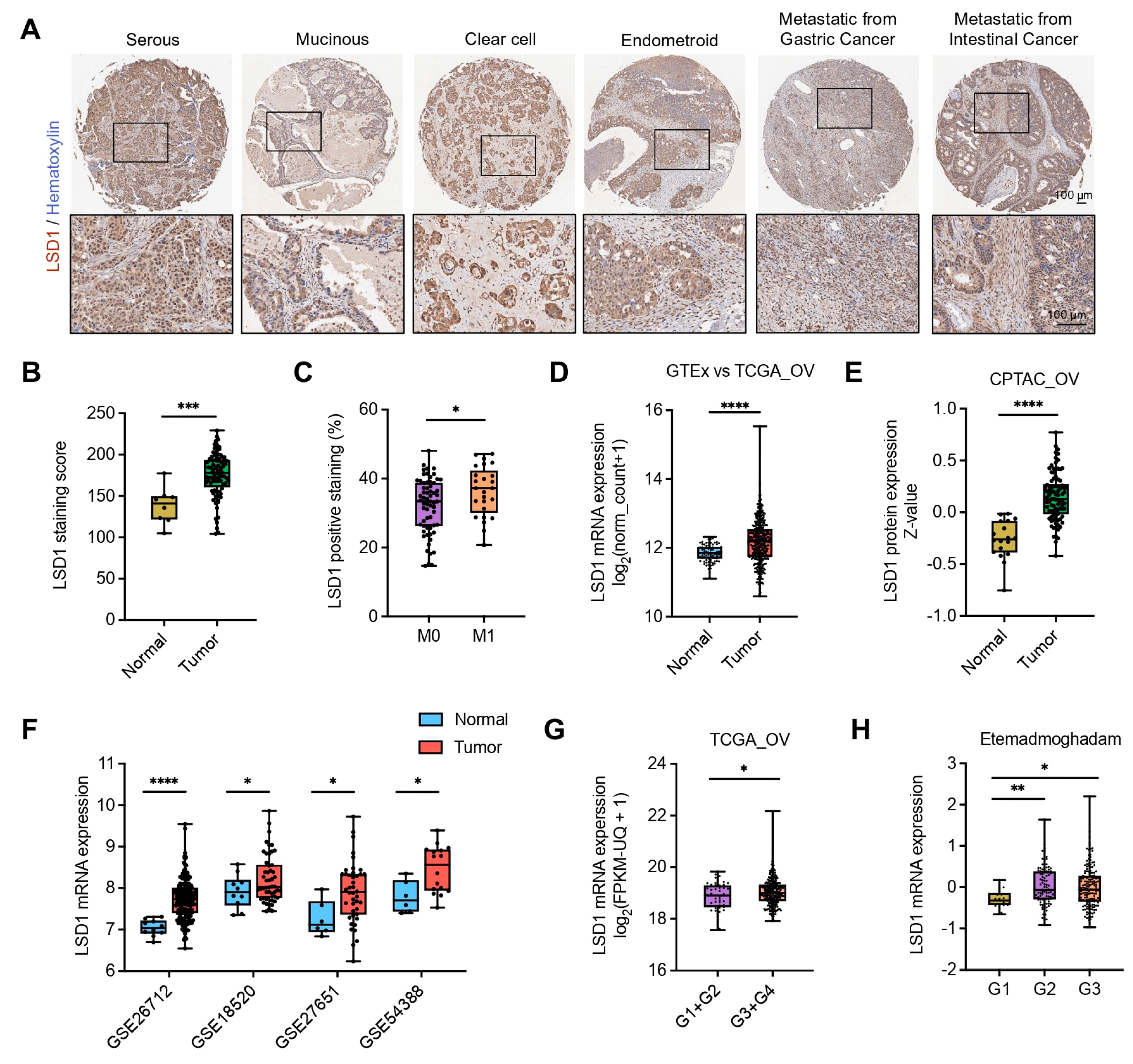
3.2. High-Level LSD1 Is Closely Associated with Aggressive Ovarian Cancer
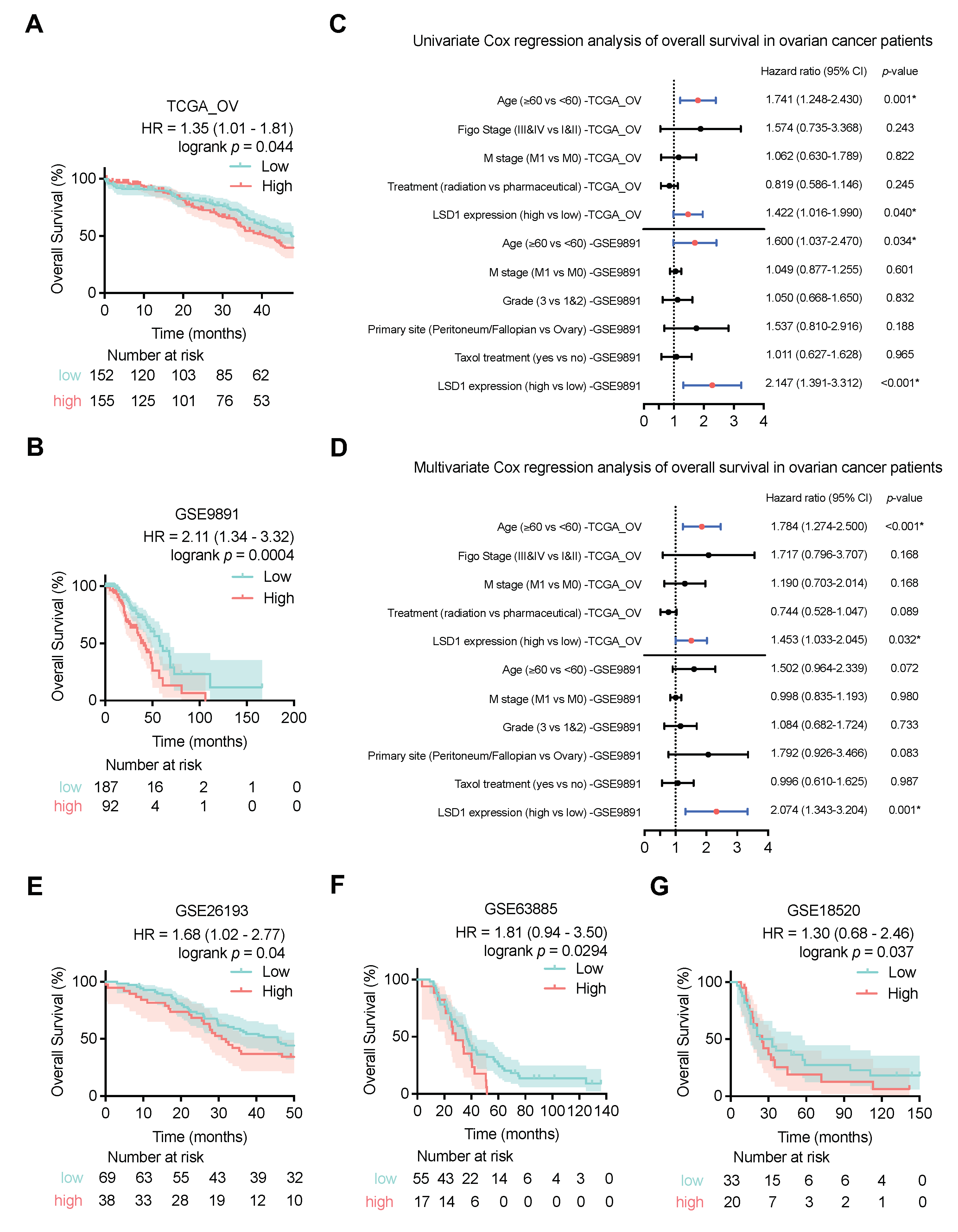
3.3. High LSD1 Expression Is Associated with Poor Outcomes of Patients with Ovarian Cancer
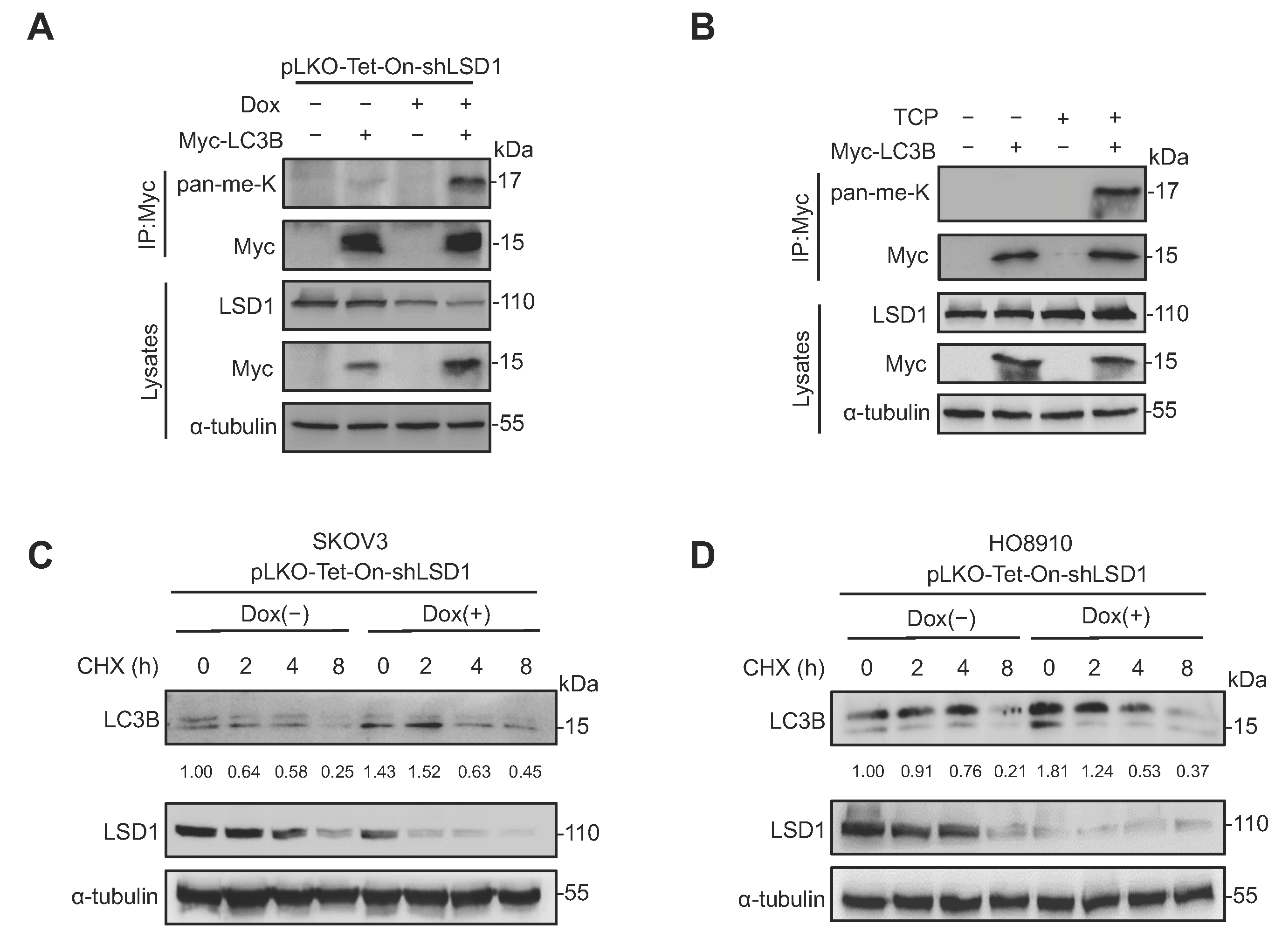
3.4. LSD1 Demethylates LC3B and Decreases LC3B Stability
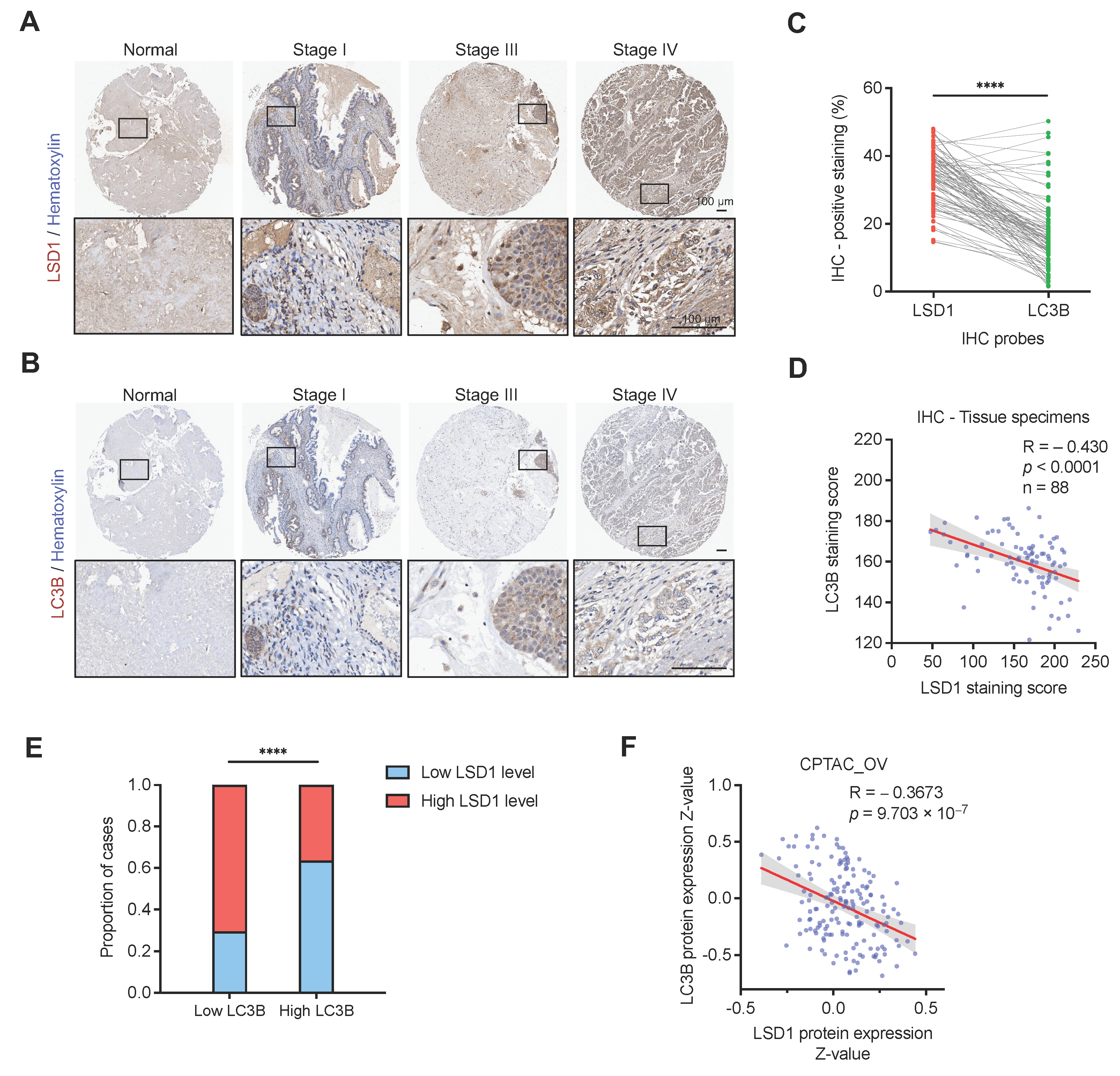
3.5. LSD1 Is Inversely Correlated with LC3B at the Protein Level in Clinical Ovarian Cancer Samples
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Klionsky, D.J. Autophagy: From Phenomenology to Molecular Understanding in Less than a Decade. Nat. Rev. Mol. Cell Biol. 2007, 8, 931–937. [Google Scholar] [CrossRef] [PubMed]
- Dikic, I.; Elazar, Z. Mechanism and Medical Implications of Mammalian Autophagy. Nat. Rev. Mol. Cell Biol. 2018, 19, 349–364. [Google Scholar] [CrossRef] [PubMed]
- Filomeni, G.; De Zio, D.; Cecconi, F. Oxidative Stress and Autophagy: The Clash between Damage and Metabolic Needs. Cell Death Differ. 2015, 22, 377–388. [Google Scholar] [CrossRef] [PubMed]
- Shang, L.; Chen, S.; Du, F.; Li, S.; Zhao, L.; Wang, X. Nutrient Starvation Elicits an Acute Autophagic Response Mediated by Ulk1 Dephosphorylation and Its Subsequent Dissociation from AMPK. Proc. Natl. Acad. Sci. USA 2011, 108, 4788–4793. [Google Scholar] [CrossRef]
- White, E.; DiPaola, R.S. The Double-Edged Sword of Autophagy Modulation in Cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2009, 15, 5308–5316. [Google Scholar] [CrossRef]
- Bhutia, S.K.; Mukhopadhyay, S.; Sinha, N.; Das, D.N.; Panda, P.K.; Patra, S.K.; Maiti, T.K.; Mandal, M.; Dent, P.; Wang, X.-Y.; et al. Autophagy: Cancer’s Friend or Foe? Adv. Cancer Res. 2013, 118, 61–95. [Google Scholar] [CrossRef] [PubMed]
- Mizushima, N. Autophagy: Process and Function. Genes Dev. 2007, 21, 2861–2873. [Google Scholar] [CrossRef]
- Weidberg, H.; Shvets, E.; Shpilka, T.; Shimron, F.; Shinder, V.; Elazar, Z. LC3 and GATE-16/GABARAP Subfamilies Are Both Essential yet Act Differently in Autophagosome Biogenesis. EMBO J. 2010, 29, 1792–1802. [Google Scholar] [CrossRef]
- Rogov, V.; Dötsch, V.; Johansen, T.; Kirkin, V. Interactions between Autophagy Receptors and Ubiquitin-like Proteins Form the Molecular Basis for Selective Autophagy. Mol. Cell 2014, 53, 167–178. [Google Scholar] [CrossRef]
- Nguyen, T.N.; Padman, B.S.; Usher, J.; Oorschot, V.; Ramm, G.; Lazarou, M. Atg8 Family LC3/GABARAP Proteins Are Crucial for Autophagosome-Lysosome Fusion but Not Autophagosome Formation during PINK1/Parkin Mitophagy and Starvation. J. Cell Biol. 2016, 215, 857–874. [Google Scholar] [CrossRef]
- Kabeya, Y. LC3, a Mammalian Homologue of Yeast Apg8p, Is Localized in Autophagosome Membranes after Processing. EMBO J. 2000, 19, 5720–5728. [Google Scholar] [CrossRef] [PubMed]
- Kabeya, Y.; Mizushima, N.; Yamamoto, A.; Oshitani-Okamoto, S.; Ohsumi, Y.; Yoshimori, T. LC3, GABARAP and GATE16 Localize to Autophagosomal Membrane Depending on Form-II Formation. J. Cell Sci. 2004, 117, 2805–2812. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Kang, R.; Sun, X.; Zhong, M.; Huang, J.; Klionsky, D.J.; Tang, D. Posttranslational Modification of Autophagy-Related Proteins in Macroautophagy. Autophagy 2015, 11, 28–45. [Google Scholar] [CrossRef] [PubMed]
- Shu, F.; Xiao, H.; Li, Q.-N.; Ren, X.-S.; Liu, Z.-G.; Hu, B.-W.; Wang, H.-S.; Wang, H.; Jiang, G.-M. Epigenetic and Post-Translational Modifications in Autophagy: Biological Functions and Therapeutic Targets. Signal Transduct. Target. Ther. 2023, 8, 32. [Google Scholar] [CrossRef] [PubMed]
- Wani, W.Y.; Boyer-Guittaut, M.; Dodson, M.; Chatham, J.; Darley-Usmar, V.; Zhang, J. Regulation of Autophagy by Protein Post-Translational Modification. Lab. Investig. J. Tech. Methods Pathol. 2015, 95, 14–25. [Google Scholar] [CrossRef]
- Shi, Y.; Whetstine, J.R. Dynamic Regulation of Histone Lysine Methylation by Demethylases. Mol. Cell 2007, 25, 1–14. [Google Scholar] [CrossRef]
- Li, R.; Wei, X.; Jiang, D.-S. Protein Methylation Functions as the Posttranslational Modification Switch to Regulate Autophagy. Cell. Mol. Life Sci. CMLS 2019, 76, 3711–3722. [Google Scholar] [CrossRef]
- Jeon, M.; Park, J.; Yang, E.; Baek, H.-J.; Kim, H. Regulation of Autophagy by Protein Methylation and Acetylation in Cancer. J. Cell. Physiol. 2022, 237, 13–28. [Google Scholar] [CrossRef]
- Song, H.; Feng, X.; Zhang, M.; Jin, X.; Xu, X.; Wang, L.; Ding, X.; Luo, Y.; Lin, F.; Wu, Q.; et al. Crosstalk between Lysine Methylation and Phosphorylation of ATG16L1 Dictates the Apoptosis of Hypoxia/Reoxygenation-Induced Cardiomyocytes. Autophagy 2018, 14, 825–844. [Google Scholar] [CrossRef]
- Dai, Y.; Luo, W.; Li, W.; Chen, Z.; Wang, X.; Chang, J. FIP200 Methylation by SETD2 Prevents Trim21-Induced Degradation and Preserves Autophagy Initiation. Cells 2022, 11, 3333. [Google Scholar] [CrossRef]
- Huang, R.; Xu, Y.; Wan, W.; Shou, X.; Qian, J.; You, Z.; Liu, B.; Chang, C.; Zhou, T.; Lippincott-Schwartz, J.; et al. Deacetylation of Nuclear LC3 Drives Autophagy Initiation under Starvation. Mol. Cell 2015, 57, 456–466. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Lu, X.-X.; Wang, J.-R.; Yang, T.-Y.; Li, X.-M.; He, X.-S.; Li, Y.; Ye, W.-L.; Wu, Y.; Gan, W.-J.; et al. TRAF6 Inhibits Colorectal Cancer Metastasis through Regulating Selective Autophagic CTNNB1/β-Catenin Degradation and Is Targeted for GSK3B/GSK3β-Mediated Phosphorylation and Degradation. Autophagy 2019, 15, 1506–1522. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Lan, F.; Matson, C.; Mulligan, P.; Whetstine, J.R.; Cole, P.A.; Casero, R.A.; Shi, Y. Histone Demethylation Mediated by the Nuclear Amine Oxidase Homolog LSD1. Cell 2004, 119, 941–953. [Google Scholar] [CrossRef]
- Shi, Y.-J.; Matson, C.; Lan, F.; Iwase, S.; Baba, T.; Shi, Y. Regulation of LSD1 Histone Demethylase Activity by Its Associated Factors. Mol. Cell 2005, 19, 857–864. [Google Scholar] [CrossRef]
- Metzger, E.; Wissmann, M.; Yin, N.; Müller, J.M.; Schneider, R.; Peters, A.H.F.M.; Günther, T.; Buettner, R.; Schüle, R. LSD1 Demethylates Repressive Histone Marks to Promote Androgen-Receptor-Dependent Transcription. Nature 2005, 437, 436–439. [Google Scholar] [CrossRef]
- Majello, B.; Gorini, F.; Saccà, C.D.; Amente, S. Expanding the Role of the Histone Lysine-Specific Demethylase LSD1 in Cancer. Cancers 2019, 11, 324. [Google Scholar] [CrossRef] [PubMed]
- Perillo, B.; Tramontano, A.; Pezone, A.; Migliaccio, A. LSD1: More than Demethylation of Histone Lysine Residues. Exp. Mol. Med. 2020, 52, 1936–1947. [Google Scholar] [CrossRef]
- Huang, J.; Sengupta, R.; Espejo, A.B.; Lee, M.G.; Dorsey, J.A.; Richter, M.; Opravil, S.; Shiekhattar, R.; Bedford, M.T.; Jenuwein, T.; et al. P53 Is Regulated by the Lysine Demethylase LSD1. Nature 2007, 449, 105–108. [Google Scholar] [CrossRef]
- Lee, J.-Y.; Park, J.-H.; Choi, H.-J.; Won, H.-Y.; Joo, H.-S.; Shin, D.-H.; Park, M.K.; Han, B.; Kim, K.P.; Lee, T.J.; et al. LSD1 Demethylates HIF1α to Inhibit Hydroxylation and Ubiquitin-Mediated Degradation in Tumor Angiogenesis. Oncogene 2017, 36, 5512–5521. [Google Scholar] [CrossRef]
- Maiques-Diaz, A.; Somervaille, T.C. LSD1: Biologic Roles and Therapeutic Targeting. Epigenomics 2016, 8, 1103–1116. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Kim, K.I.; Baek, S.H. Roles of Lysine-Specific Demethylase 1 (LSD1) in Homeostasis and Diseases. J. Biomed. Sci. 2021, 28, 41. [Google Scholar] [CrossRef] [PubMed]
- Shao, G.; Wang, J.; Li, Y.; Liu, X.; Xie, X.; Wan, X.; Yan, M.; Jin, J.; Lin, Q.; Zhu, H.; et al. Lysine-Specific Demethylase 1 Mediates Epidermal Growth Factor Signaling to Promote Cell Migration in Ovarian Cancer Cells. Sci. Rep. 2015, 5, 15344. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Han, T.; Wang, R.; Wei, J.; Peng, K.; Lin, Q.; Shao, G. LSD1 Negatively Regulates Autophagy through the mTOR Signaling Pathway in Ovarian Cancer Cells. Oncol. Rep. 2018, 40, 425–433. [Google Scholar] [CrossRef] [PubMed]
- Shao, G.; Wang, R.; Sun, A.; Wei, J.; Peng, K.; Dai, Q.; Yang, W.; Lin, Q. The E3 Ubiquitin Ligase NEDD4 Mediates Cell Migration Signaling of EGFR in Lung Cancer Cells. Mol. Cancer 2018, 17, 24. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wan, X.; Wei, Y.; Liu, X.; Lai, W.; Zhang, L.; Jin, J.; Wu, C.; Shao, Q.; Shao, G.; et al. LSD1-Mediated Epigenetic Modification Contributes to Ovarian Cancer Cell Migration and Invasion. Oncol. Rep. 2016, 35, 3586–3592. [Google Scholar] [CrossRef]
- Shao, G.; Wan, X.; Lai, W.; Wu, C.; Jin, J.; Liu, X.; Wei, Y.; Lin, Q.; Zhang, L.; Shao, Q. Inhibition of Lysine-Specific Demethylase 1 Prevents Proliferation and Mediates Cisplatin Sensitivity in Ovarian Cancer Cells. Oncol. Lett. 2018, 15, 9025–9032. [Google Scholar] [CrossRef] [PubMed]
- Etemadmoghadam, D.; deFazio, A.; Beroukhim, R.; Mermel, C.; George, J.; Getz, G.; Tothill, R.; Okamoto, A.; Raeder, M.B.; Harnett, P.; et al. Integrated Genome-Wide DNA Copy Number and Expression Analysis Identifies Distinct Mechanisms of Primary Chemoresistance in Ovarian Carcinomas. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2009, 15, 1417–1427. [Google Scholar] [CrossRef]
- Shen, N.; Wang, L.; Wu, J.; Chen, X.; Hu, F.; Su, Y. Meta-analysis of the Autophagy-associated Protein LC3 as a Prognostic Marker in Colorectal Cancer. Exp. Ther. Med. 2023, 26, 492. [Google Scholar] [CrossRef]
- Sakurai, T.; Okumura, H.; Matsumoto, M.; Uchikado, Y.; Setoyama, T.; Omoto, I.; Owaki, T.; Maemura, K.; Ishigami, S.; Natsugoe, S. The Expression of LC-3 Is Related to Tumor Suppression through Angiogenesis in Esophageal Cancer. Med. Oncol. 2013, 30, 701. [Google Scholar] [CrossRef]
- Miyamoto, M.; Takano, M.; Aoyama, T.; Soyama, H.; Yoshikawa, T.; Tsuda, H.; Furuya, K. Inhibition of Autophagy Protein LC3A as a Therapeutic Target in Ovarian Clear Cell Carcinomas. J. Gynecol. Oncol. 2017, 28, e33. [Google Scholar] [CrossRef]
- Spowart, J.E.; Townsend, K.N.; Huwait, H.; Eshragh, S.; West, N.R.; Ries, J.N.; Kalloger, S.; Anglesio, M.; Gorski, S.M.; Watson, P.H.; et al. The Autophagy Protein LC3A Correlates with Hypoxia and Is a Prognostic Marker of Patient Survival in Clear Cell Ovarian Cancer. J. Pathol. 2012, 228, 437–447. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Sui, Y.; Sun, Y.; Chen, X.; Chen, Z.; Chen, L.; Li, Y.; Wang, L. Expression of autophagy-related genes Beclin1 and LC3 in ovarian serous carcinoma and their clinical significance. Chin. J. Clin. Exp. Pathol. 2017, 33, 1219–1224. [Google Scholar] [CrossRef]
- Minamoto, T.; Nakayama, K.; Nakamura, K.; Katagiri, H.; Sultana, R.; Ishibashi, T.; Ishikawa, M.; Yamashita, H.; Sanuki, K.; Iida, K.; et al. Loss of Beclin 1 Expression in Ovarian Cancer: A Potential Biomarker for Predicting Unfavorable Outcomes. Oncol. Lett. 2018, 15, 1170–1176. [Google Scholar] [CrossRef] [PubMed]
- Ruggero, K.; Farran-Matas, S.; Martinez-Tebar, A.; Aytes, A. Epigenetic Regulation in Prostate Cancer Progression. Curr. Mol. Biol. Rep. 2018, 4, 101–115. [Google Scholar] [CrossRef] [PubMed]
- Kahl, P.; Gullotti, L.; Heukamp, L.C.; Wolf, S.; Friedrichs, N.; Vorreuther, R.; Solleder, G.; Bastian, P.J.; Ellinger, J.; Metzger, E.; et al. Androgen Receptor Coactivators Lysine-Specific Histone Demethylase 1 and Four and a Half LIM Domain Protein 2 Predict Risk of Prostate Cancer Recurrence. Cancer Res. 2006, 66, 11341–11347. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Xu, G.; Liu, J.; Zhang, N.; Li, L.; Ji, J.; Zhang, J.; Zhang, L.; Wang, G.; Wang, X.; et al. Phosphorylation of LSD1 at Ser112 Is Crucial for Its Function in Induction of EMT and Metastasis in Breast Cancer. Breast Cancer Res. Treat. 2016, 159, 443–456. [Google Scholar] [CrossRef] [PubMed]
- Boulding, T.; McCuaig, R.D.; Tan, A.; Hardy, K.; Wu, F.; Dunn, J.; Kalimutho, M.; Sutton, C.R.; Forwood, J.K.; Bert, A.G.; et al. LSD1 Activation Promotes Inducible EMT Programs and Modulates the Tumour Microenvironment in Breast Cancer. Sci. Rep. 2018, 8, 73. [Google Scholar] [CrossRef]
- Venkata, P.P.; Jayamohan, S.; He, Y.; Alejo, S.; Johnson, J.D.; Palacios, B.E.; Pratap, U.P.; Chen, Y.; Liu, Z.; Zou, Y.; et al. Pharmacological Inhibition of KDM1A/LSD1 Enhances Estrogen Receptor Beta-Mediated Tumor Suppression in Ovarian Cancer. Cancer Lett. 2023, 575, 216383. [Google Scholar] [CrossRef]
- Fang, Y.; Liao, G.; Yu, B. LSD1/KDM1A Inhibitors in Clinical Trials: Advances and Prospects. J. Hematol. Oncol. 2019, 12, 129. [Google Scholar] [CrossRef]
- Noce, B.; Di Bello, E.; Fioravanti, R.; Mai, A. LSD1 Inhibitors for Cancer Treatment: Focus on Multi-Target Agents and Compounds in Clinical Trials. Front. Pharmacol. 2023, 14, 1120911. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, M.; Feng, J.; Zhao, K.; Huang, T.; Zhang, B.; Yang, Y.; Sun, A.; Lin, Q.; Shao, G. LSD1 Demethylates and Destabilizes Autophagy Protein LC3B in Ovarian Cancer. Biomolecules 2024, 14, 1377. https://doi.org/10.3390/biom14111377
Li M, Feng J, Zhao K, Huang T, Zhang B, Yang Y, Sun A, Lin Q, Shao G. LSD1 Demethylates and Destabilizes Autophagy Protein LC3B in Ovarian Cancer. Biomolecules. 2024; 14(11):1377. https://doi.org/10.3390/biom14111377
Chicago/Turabian StyleLi, Mingyang, Jie Feng, Kangrong Zhao, Ting Huang, Bowen Zhang, Yifan Yang, Aiqin Sun, Qiong Lin, and Genbao Shao. 2024. "LSD1 Demethylates and Destabilizes Autophagy Protein LC3B in Ovarian Cancer" Biomolecules 14, no. 11: 1377. https://doi.org/10.3390/biom14111377
APA StyleLi, M., Feng, J., Zhao, K., Huang, T., Zhang, B., Yang, Y., Sun, A., Lin, Q., & Shao, G. (2024). LSD1 Demethylates and Destabilizes Autophagy Protein LC3B in Ovarian Cancer. Biomolecules, 14(11), 1377. https://doi.org/10.3390/biom14111377






