Serum from Hypertensive Patients Induces Cancer-Supporting Phenotype of Vascular Endothelium In Vitro
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Consumables
2.2. Hypertensive Patients and Healthy Volunteers
2.3. Cell Cultures
2.4. Experimental Protocol
2.5. Cancer Cell Progression
2.6. Epithelial–Mesenchymal Transition (EMT)
2.7. Intracellular Junctions
2.8. Endothelial Cell Secretome
2.9. Cancer Cell Transcriptome
2.10. Statistics
3. Results
3.1. Serum from HT Patients Promotes Endothelial-Cell-Dependent Cancer Cell Progression In Vitro
3.2. Serum from HT Patients Affects EMT Phenotype and Expression of Junctional Proteins
3.3. Serum from HT Patients Stimulates the Production of Pro-Cancerogenic Cytokines by Endothelial Cells
3.4. Serum from HT Patients Modulates Endothelial-Cell-Dependent Expression of Growth-Promoting TRANSCRIPTS in Cancer Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mills, K.T.; Bundy, J.D.; Kelly, T.N.; Reed, J.E.; Kearney, P.M.; Reynolds, K.; Chen, J.; He, J. Global Disparities of Hypertension Prevalence and Control: A Systematic Analysis of Population-Based Studies from 90 Countries. Circulation 2016, 134, 441–450. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Koene, R.J.; Prizment, A.E.; Blaes, A.; Konety, S.H. Shared Risk Factors in Cardiovascular Disease and Cancer. Circulation 2016, 133, 1104–1114. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, G.T.; Oeffinger, K.C.; Chen, Y.; Kawashima, T.; Yasui, Y.; Leisenring, W.; Stovall, M.; Chow, E.J.; Sklar, C.A.; Mulrooney, D.A.; et al. Modifiable risk factors and major cardiac events among adult survivors of childhood cancer. J. Clin. Oncol. 2013, 31, 3673–3680. [Google Scholar] [CrossRef] [PubMed]
- Uruski, P.; Matuszewska, J.; Lesniewska, A.; Rychlewski, D.; Niklas, A.; Mikula-Pietrasik, J.; Tykarski, A.; Ksiazek, K. An integrative review of nonobvious puzzles of cellular and molecular cardiooncology. Cell. Mol. Biol. Lett. 2023, 28, 44. [Google Scholar] [CrossRef]
- Seretis, A.; Cividini, S.; Markozannes, G.; Tseretopoulou, X.; Lopez, D.S.; Ntzani, E.E.; Tsilidis, K.K. Association between blood pressure and risk of cancer development: A systematic review and meta-analysis of observational studies. Sci. Rep. 2019, 9, 8565. [Google Scholar] [CrossRef]
- Connaughton, M.; Dabagh, M. Association of Hypertension and Organ-Specific Cancer: A Meta-Analysis. Healthcare 2022, 10, 1074. [Google Scholar] [CrossRef]
- Gkretsi, V.; Stylianopoulos, T. Cell Adhesion and Matrix Stiffness: Coordinating Cancer Cell Invasion and Metastasis. Front. Oncol. 2018, 8, 145. [Google Scholar] [CrossRef]
- Intengan, H.D.; Schiffrin, E.L. Vascular remodeling in hypertension: Roles of apoptosis, inflammation, and fibrosis. Hypertension 2001, 38, 581–587. [Google Scholar] [CrossRef]
- Yang, P.; Elhalawani, H.; Shi, Y.; Tang, Y.; Han, Y.; Zhao, Y.; Lou, F.; Jin, H. A large-scale retrospective study of the overall survival outcome in nasopharyngeal carcinoma with hypertension in Chinese population. Oncotarget 2017, 8, 75577–75586. [Google Scholar] [CrossRef]
- Griendling, K.K.; Camargo, L.L.; Rios, F.J.; Alves-Lopes, R.; Montezano, A.C.; Touyz, R.M. Oxidative Stress and Hypertension. Circ. Res. 2021, 128, 993–1020. [Google Scholar] [CrossRef] [PubMed]
- Jelic, M.D.; Mandic, A.D.; Maricic, S.M.; Srdjenovic, B.U. Oxidative stress and its role in cancer. J. Cancer Res. Ther. 2021, 17, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Deckers, I.A.; van den Brandt, P.A.; van Engeland, M.; van Schooten, F.J.; Godschalk, R.W.; Keszei, A.P.; Schouten, L.J. Polymorphisms in genes of the renin-angiotensin-aldosterone system and renal cell cancer risk: Interplay with hypertension and intakes of sodium, potassium and fluid. Int. J. Cancer 2015, 136, 1104–1116. [Google Scholar] [CrossRef]
- Tanase, D.M.; Gosav, E.M.; Radu, S.; Ouatu, A.; Rezus, C.; Ciocoiu, M.; Costea, C.F.; Floria, M. Arterial Hypertension and Interleukins: Potential Therapeutic Target or Future Diagnostic Marker? Int. J. Hypertens. 2019, 2019, 3159283. [Google Scholar] [CrossRef]
- Gallo, G.; Volpe, M.; Savoia, C. Endothelial Dysfunction in Hypertension: Current Concepts and Clinical Implications. Front. Med. 2021, 8, 798958. [Google Scholar] [CrossRef]
- Lopes-Bastos, B.M.; Jiang, W.G.; Cai, J. Tumour-Endothelial Cell Communications: Important and Indispensable Mediators of Tumour Angiogenesis. Anticancer Res. 2016, 36, 1119–1126. [Google Scholar]
- Shenoy, A.K.; Lu, J. Cancer cells remodel themselves and vasculature to overcome the endothelial barrier. Cancer Lett. 2016, 380, 534–544. [Google Scholar] [CrossRef]
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti Rosei, E.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; de Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension. J. Hypertens. 2018, 36, 1953–2041. [Google Scholar]
- Mikula-Pietrasik, J.; Uruski, P.; Aniukiewicz, K.; Sosinska, P.; Krasinski, Z.; Tykarski, A.; Ksiazek, K. Serum from Varicose Patients Induces Senescence-Related Dysfunction of Vascular Endothelium Generating Local and Systemic Proinflammatory Conditions. Oxidative Med. Cell. Longev. 2016, 2016, 2069290. [Google Scholar] [CrossRef]
- Uruski, P.; Aniukiewicz, K.; Mikula-Pietrasik, J.; Sosinska, P.; Tykarski, A.; Ksiazek, K.; Krasinski, Z. Endovenous Laser Ablation of Varicose Veins Preserves Biological Properties of Vascular Endothelium and Modulates Proinflammatory Agent Profile More Favorably Than Classic Vein Stripping. Biomed. Res. Int. 2017, 2017, 6167480. [Google Scholar] [CrossRef]
- Pakula, M.; Witucka, A.; Uruski, P.; Radziemski, A.; Moszynski, R.; Szpurek, D.; Maksin, K.; Wozniak, A.; Sajdak, S.; Tykarski, A.; et al. Senescence-related deterioration of intercellular junctions in the peritoneal mesothelium promotes the transmesothelial invasion of ovarian cancer cells. Sci. Rep. 2019, 9, 7587. [Google Scholar] [CrossRef] [PubMed]
- Ksiazek, K.; Mikula-Pietrasik, J.; Korybalska, K.; Dworacki, G.; Jorres, A.; Witowski, J. Senescent peritoneal mesothelial cells promote ovarian cancer cell adhesion: The role of oxidative stress-induced fibronectin. Am. J. Pathol. 2009, 174, 1230–1240. [Google Scholar] [CrossRef] [PubMed]
- Pakula, M.; Uruski, P.; Niklas, A.; Wozniak, A.; Szpurek, D.; Tykarski, A.; Mikula-Pietrasik, J.; Ksiazek, K. A Unique Pattern of Mesothelial-Mesenchymal Transition Induced in the Normal Peritoneal Mesothelium by High-Grade Serous Ovarian Cancer. Cancers 2019, 11, 662. [Google Scholar] [CrossRef]
- Mikula-Pietrasik, J.; Uruski, P.; Szubert, S.; Szpurek, D.; Sajdak, S.; Tykarski, A.; Ksiazek, K. Malignant ascites determine the transmesothelial invasion of ovarian cancer cells. Int. J. Biochem. Cell Biol. 2017, 92, 6–13. [Google Scholar] [CrossRef]
- Liu, W.; Wang, L.; Zhang, J.; Qiao, L.; Liu, Y.; Yang, X.; Zhang, J.; Zheng, W.; Ma, Z. Purification of recombinant human chemokine CCL2 in E. coli and its function in ovarian cancer. 3 Biotech 2021, 11, 8. [Google Scholar] [CrossRef]
- An, J.; Xue, Y.; Long, M.; Zhang, G.; Zhang, J.; Su, H. Targeting CCR2 with its antagonist suppresses viability, motility and invasion by downregulating MMP-9 expression in non-small cell lung cancer cells. Oncotarget 2017, 8, 39230–39240. [Google Scholar] [CrossRef]
- Zhuo, C.; Ruan, Q.; Zhao, X.; Shen, Y.; Lin, R. CXCL1 promotes colon cancer progression through activation of NF-kappaB/P300 signaling pathway. Biol. Direct 2022, 17, 34. [Google Scholar] [CrossRef]
- Xu, H.; Zeng, Y.; Liu, L.; Gao, Q.; Jin, S.; Lan, Q.; Lai, W.; Luo, X.; Wu, H.; Huang, Y.; et al. PRL-3 improves colorectal cancer cell proliferation and invasion through IL-8 mediated glycolysis metabolism. Int. J. Oncol. 2017, 51, 1271–1279. [Google Scholar] [CrossRef]
- Deying, W.; Feng, G.; Shumei, L.; Hui, Z.; Ming, L.; Hongqing, W. CAF-derived HGF promotes cell proliferation and drug resistance by up-regulating the c-Met/PI3K/Akt and GRP78 signalling in ovarian cancer cells. Biosci. Rep. 2017, 37, BSR20160470. [Google Scholar] [CrossRef]
- Zhang, Y.; He, H.; He, L.; Shi, B. IL-6 Accelerates the Proliferation and Metastasis of Pancreatic Cancer Cells via the miR-455-5p/IGF-1R Axis. Cancer Biother. Radiopharm. 2024, 39, 255–263. [Google Scholar] [CrossRef]
- Zhou, Y.; Yu, Q.; Chu, Y.; Zhu, X.; Deng, J.; Liu, Q.; Wang, Q. Downregulation of fibroblast growth factor 5 inhibits cell growth and invasion of human nonsmall-cell lung cancer cells. J. Cell. Biochem. 2019, 120, 8238–8246. [Google Scholar] [CrossRef] [PubMed]
- Boilly, B.; Vercoutter-Edouart, A.S.; Hondermarck, H.; Nurcombe, V.; Le Bourhis, X. FGF signals for cell proliferation and migration through different pathways. Cytokine Growth Factor Rev. 2000, 11, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Ardizzone, A.; Bova, V.; Casili, G.; Repici, A.; Lanza, M.; Giuffrida, R.; Colarossi, C.; Mare, M.; Cuzzocrea, S.; Esposito, E.; et al. Role of Basic Fibroblast Growth Factor in Cancer: Biological Activity, Targeted Therapies, and Prognostic Value. Cells 2023, 12, 1002. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Brekken, R.A.; Hyder, S.M. Vascular endothelial growth factor induces proliferation of breast cancer cells and inhibits the anti-proliferative activity of anti-hormones. Endocr. Relat. Cancer 2006, 13, 905–919. [Google Scholar] [CrossRef]
- Mikula-Pietrasik, J.; Uruski, P.; Sosinska, P.; Maksin, K.; Piotrowska-Kempisty, H.; Kucinska, M.; Murias, M.; Szubert, S.; Wozniak, A.; Szpurek, D.; et al. Senescent peritoneal mesothelium creates a niche for ovarian cancer metastases. Cell Death Dis. 2016, 7, e2565. [Google Scholar] [CrossRef]
- Youngs, S.J.; Ali, S.A.; Taub, D.D.; Rees, R.C. Chemokines induce migrational responses in human breast carcinoma cell lines. Int. J. Cancer 1997, 71, 257–266. [Google Scholar] [CrossRef]
- Ning, Y.; Manegold, P.C.; Hong, Y.K.; Zhang, W.; Pohl, A.; Lurje, G.; Winder, T.; Yang, D.; LaBonte, M.J.; Wilson, P.M.; et al. Interleukin-8 is associated with proliferation, migration, angiogenesis and chemosensitivity in vitro and in vivo in colon cancer cell line models. Int. J. Cancer 2011, 128, 2038–2049. [Google Scholar] [CrossRef]
- Thongchot, S.; Jamjuntra, P.; Therasakvichya, S.; Warnnissorn, M.; Ferraresi, A.; Thuwajit, P.; Isidoro, C.; Thuwajit, C. Interleukin-8 released by cancer-associated fibroblasts attenuates the autophagy and promotes the migration of ovarian cancer cells. Int. J. Oncol. 2021, 58, 14. [Google Scholar] [CrossRef]
- Ueoka, Y.; Kato, K.; Kuriaki, Y.; Horiuchi, S.; Terao, Y.; Nishida, J.; Ueno, H.; Wake, N. Hepatocyte growth factor modulates motility and invasiveness of ovarian carcinomas via Ras-mediated pathway. Br. J. Cancer 2000, 82, 891–899. [Google Scholar] [CrossRef]
- Razidlo, G.L.; Burton, K.M.; McNiven, M.A. Interleukin-6 promotes pancreatic cancer cell migration by rapidly activating the small GTPase CDC42. J. Biol. Chem. 2018, 293, 11143–11153. [Google Scholar] [CrossRef]
- Jayson, G.C.; Evans, G.S.; Pemberton, P.W.; Lobley, R.W.; Allen, T. Basic fibroblast growth factor increases the multiplication and migration of a serum-free derivative of CACO-2 but does not affect differentiation. Cancer Res. 1994, 54, 5718–5723. [Google Scholar] [PubMed]
- Imamichi, Y.; Waidmann, O.; Hein, R.; Eleftheriou, P.; Giehl, K.; Menke, A. TGF beta-induced focal complex formation in epithelial cells is mediated by activated ERK and JNK MAP kinases and is independent of Smad4. Biol. Chem. 2005, 386, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Friedl, P.; Wolf, K. Tumour-cell invasion and migration: Diversity and escape mechanisms. Nat. Rev. Cancer 2003, 3, 362–374. [Google Scholar] [CrossRef] [PubMed]
- Son, H.; Moon, A. Epithelial-mesenchymal Transition and Cell Invasion. Toxicol. Res. 2010, 26, 245–252. [Google Scholar] [CrossRef]
- Xu, J.; Lamouille, S.; Derynck, R. TGF-beta-induced epithelial to mesenchymal transition. Cell Res. 2009, 19, 156–172. [Google Scholar] [CrossRef]
- Wang, N.; Liu, W.; Zheng, Y.; Wang, S.; Yang, B.; Li, M.; Song, J.; Zhang, F.; Zhang, X.; Wang, Q.; et al. CXCL1 derived from tumor-associated macrophages promotes breast cancer metastasis via activating NF-kappaB/SOX4 signaling. Cell Death Dis. 2018, 9, 880. [Google Scholar] [CrossRef]
- Xu, J.; Liu, S.; Yang, X.; Cao, S.; Zhou, Y. Paracrine HGF promotes EMT and mediates the effects of PSC on chemoresistance by activating c-Met/PI3K/Akt signaling in pancreatic cancer in vitro. Life Sci. 2020, 263, 118523. [Google Scholar] [CrossRef]
- Dongre, A.; Weinberg, R.A. New insights into the mechanisms of epithelial-mesenchymal transition and implications for cancer. Nat. Rev. Mol. Cell Biol. 2019, 20, 69–84. [Google Scholar] [CrossRef]
- Nieto, M.A.; Huang, R.Y.; Jackson, R.A.; Thiery, J.P. Emt: 2016. Cell 2016, 166, 21–45. [Google Scholar] [CrossRef]
- Rubtsova, S.N.; Zhitnyak, I.Y.; Gloushankova, N.A. A Novel Role of E-Cadherin-Based Adherens Junctions in Neoplastic Cell Dissemination. PLoS ONE 2015, 10, e0133578. [Google Scholar] [CrossRef]
- Rubtsova, S.N.; Zhitnyak, I.Y.; Gloushankova, N.A. Dual role of E-cadherin in cancer cells. Tissue Barriers 2022, 10, 2005420. [Google Scholar] [CrossRef] [PubMed]
- Weis, S.; Cui, J.; Barnes, L.; Cheresh, D. Endothelial barrier disruption by VEGF-mediated Src activity potentiates tumor cell extravasation and metastasis. J. Cell Biol. 2004, 167, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Humphries, B.A.; Buschhaus, J.M.; Chen, Y.C.; Haley, H.R.; Qyli, T.; Chiang, B.; Shen, N.; Rajendran, S.; Cutter, A.; Cheng, Y.H.; et al. Plasminogen Activator Inhibitor 1 (PAI1) Promotes Actin Cytoskeleton Reorganization and Glycolytic Metabolism in Triple-Negative Breast Cancer. Mol. Cancer Res. 2019, 17, 1142–1154. [Google Scholar] [CrossRef]
- Diaz, V.M.; Planaguma, J.; Thomson, T.M.; Reventos, J.; Paciucci, R. Tissue plasminogen activator is required for the growth, invasion, and angiogenesis of pancreatic tumor cells. Gastroenterology 2002, 122, 806–819. [Google Scholar] [CrossRef]
- Matsuo, Y.; Ochi, N.; Sawai, H.; Yasuda, A.; Takahashi, H.; Funahashi, H.; Takeyama, H.; Tong, Z.; Guha, S. CXCL8/IL-8 and CXCL12/SDF-1alpha co-operatively promote invasiveness and angiogenesis in pancreatic cancer. Int. J. Cancer 2009, 124, 853–861. [Google Scholar] [CrossRef]
- Sipahi, I.; Debanne, S.M.; Rowland, D.Y.; Simon, D.I.; Fang, J.C. Angiotensin-receptor blockade and risk of cancer: Meta-analysis of randomised controlled trials. Lancet Oncol. 2010, 11, 627–636. [Google Scholar] [CrossRef]
- Cho, I.J.; Shin, J.H.; Jung, M.H.; Kang, C.Y.; Hwang, J.; Kwon, C.H.; Kim, W.; Kim, D.H.; Lee, C.J.; Kang, S.H.; et al. Antihypertensive Drugs and the Risk of Cancer: A Nationwide Cohort Study. J. Clin. Med. 2021, 10, 771. [Google Scholar] [CrossRef]
- Monami, M.; Filippi, L.; Ungar, A.; Sgrilli, F.; Antenore, A.; Dicembrini, I.; Bagnoli, P.; Marchionni, N.; Rotella, C.M.; Mannucci, E. Further data on beta-blockers and cancer risk: Observational study and meta-analysis of randomized clinical trials. Curr. Med. Res. Opin. 2013, 29, 369–378. [Google Scholar] [CrossRef]
- Coleman, C.I.; Baker, W.L.; Kluger, J.; White, C.M. Antihypertensive medication and their impact on cancer incidence: A mixed treatment comparison meta-analysis of randomized controlled trials. J. Hypertens. 2008, 26, 622–629. [Google Scholar] [CrossRef]
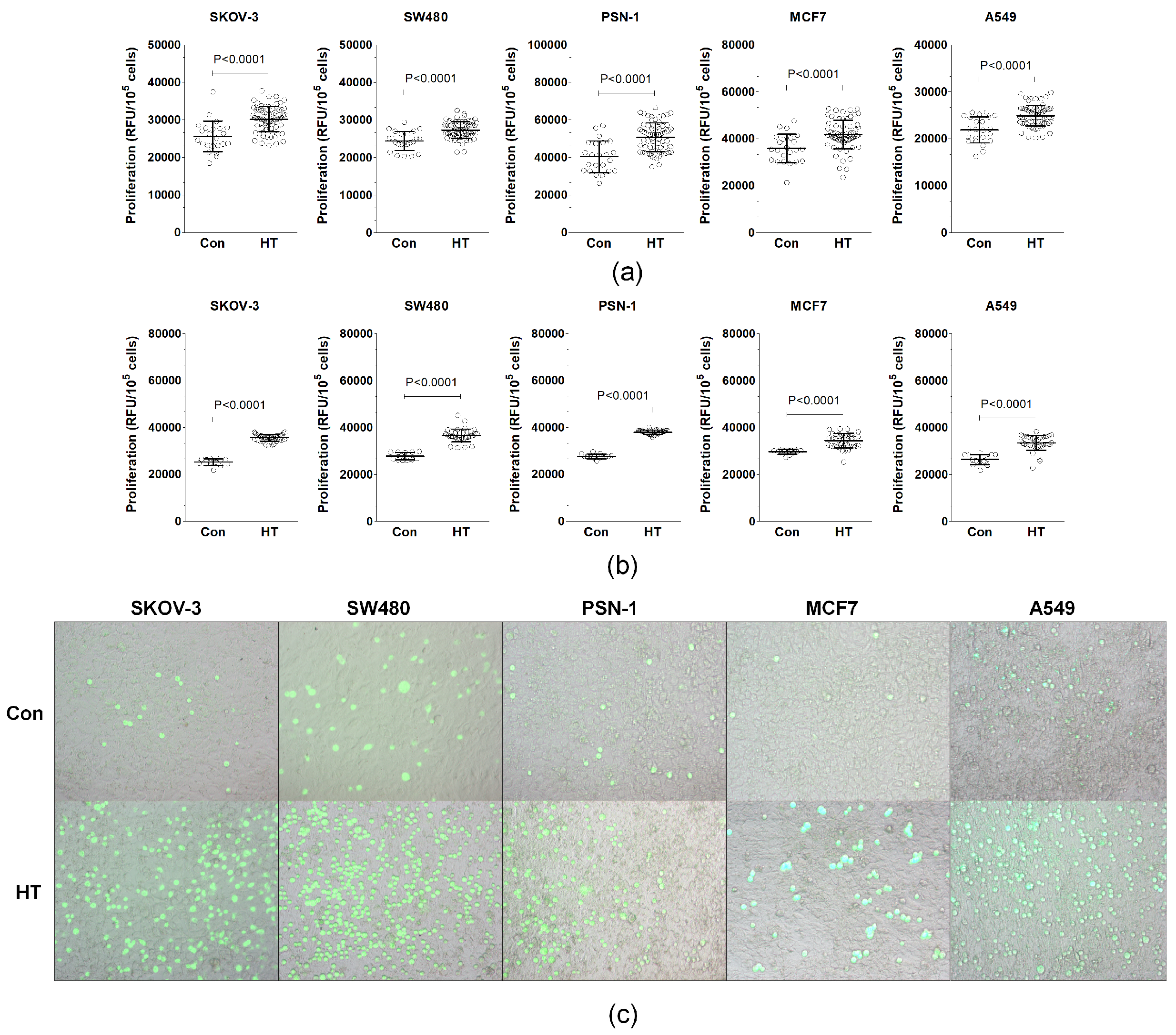
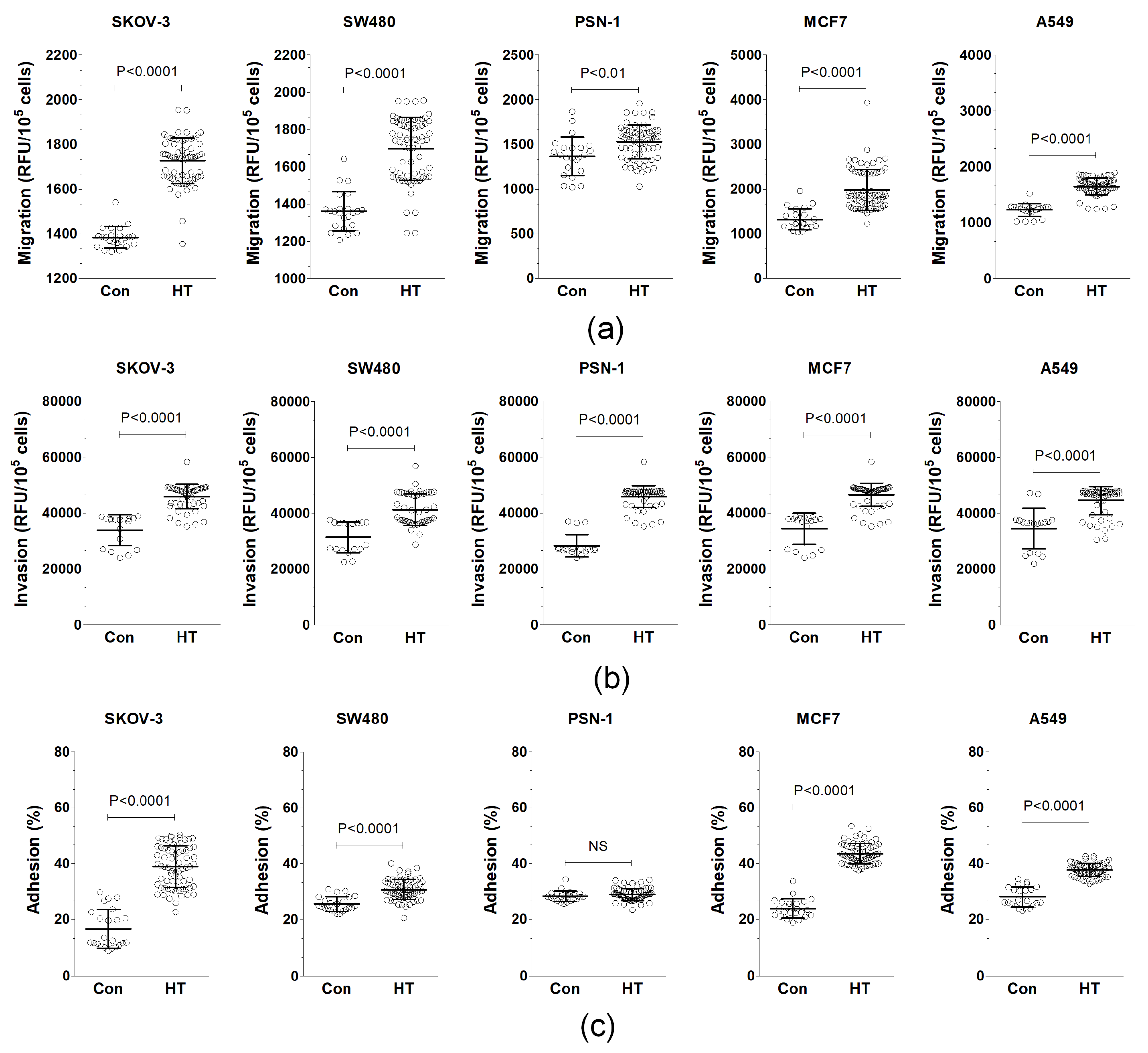
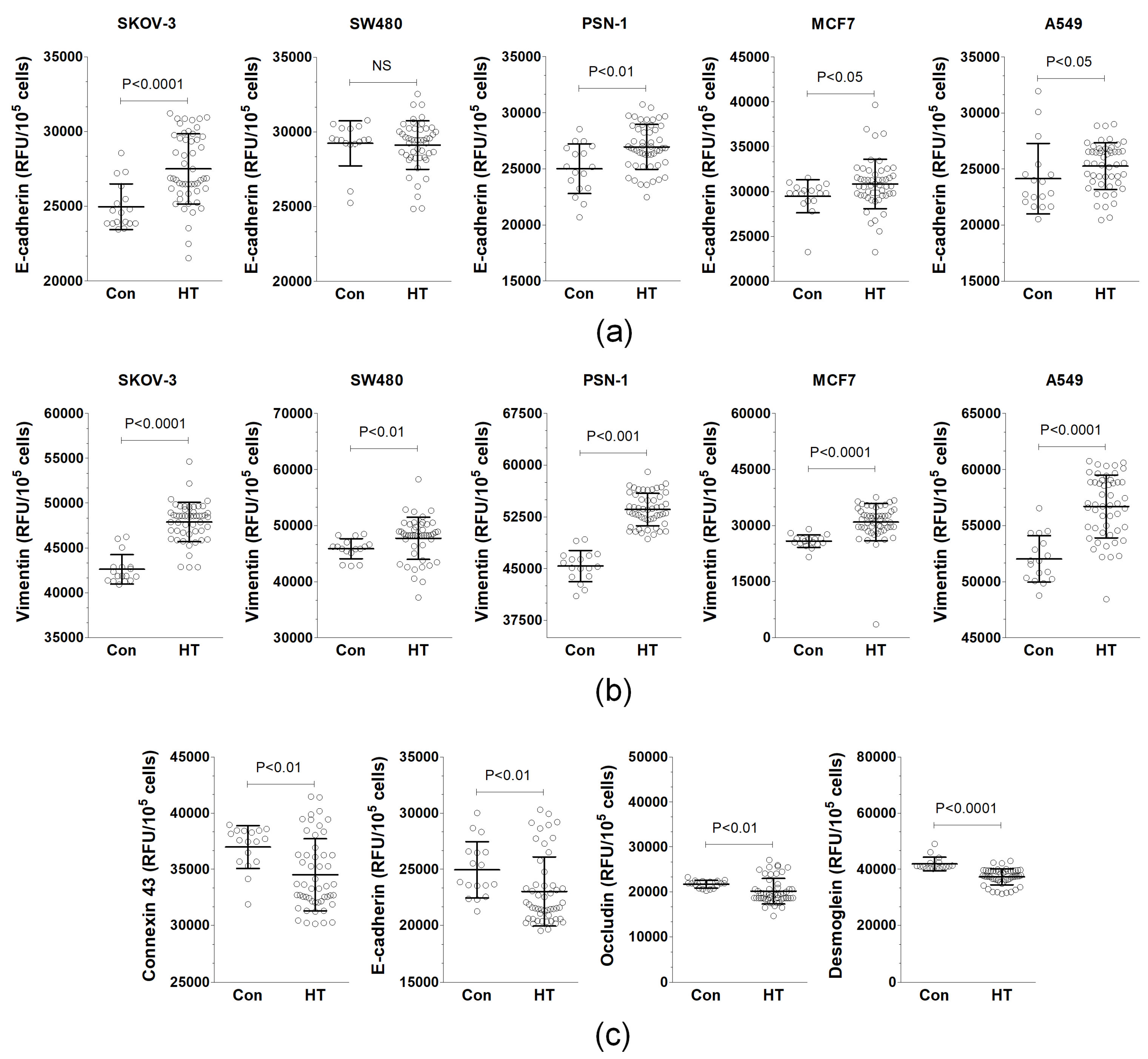

| Parameter | Control Donors | HT Patients |
|---|---|---|
| Number of patients (N), | 23 | 69 |
| Sex (Male/Female) | 14/9 | 50/19 |
| Age (years) | 42.5 ± 12.6 | 42.4 ± 11.6 |
| Office SBP (mmHg) | 116.4 ± 27.1 | 145.5 ± 14.5 |
| Office DBP (mmHg) | 69.6 ± 16.6 | 87.7 ± 9.8 |
| Gene | Forward | Reverse |
|---|---|---|
| CCL2 | AGAATCACCAGCAGCAAGTGTCC | TCCTGAACCCACTTCTGCTTGG |
| CXCL1 | AGCTTGCCTCAATCCTGCATCC | TCCTTCAGGAACAGCCACCAGT |
| CXCL8 | GAGAGTGATTGAGAGTGGACCAC | CACAACCCTCTGCACCCAGTTT |
| IL-6 | AGACAGCCACTCACCTCTTCAG | TTCTGCCAGTGCCTCTTTGCTG |
| tPA | TGGTGCTACGTCTTTAAGGCGG | GCTGACCCATTCCCAAAGTAGC |
| PAI-1 | CTCATCAGCCACTGGAAAGGCA | GACTCGTGAAGTCAGCCTGAAAC |
| FGF5 | GGAATACGAGGAGTTTTCAGCAAC | CTCCCTGAACTTGCAGTCATCTG |
| TGF-β1 | TACCTGAACCCGTGTTGCTCTC | GTTGCTGAGGTATCGCCAGGAA |
| VEGF | TTGCCTTGCTGCTCTACCTCCA | GATGGCAGTAGCTGCGCTGATA |
| GAPDH | GTCTCCTCTGACTTCAACAGCG | ACCACCCTGTTGCTGTAGCCAA |
| Gene | SKOV-3 | SW480 | PSN-1 | |||
|---|---|---|---|---|---|---|
| Con | HT | Con | HT | Con | HT | |
| CCL2 | n.d. | n.d. | 1.1 ± 0.4 | 1.5 ± 0.7 | 1.0 ± 0.1 | 1.7 ± 0.8 ** |
| CXCL1 | 1.0 ± 0.2 | 3.4 ± 1.7 *** | 1.1 ± 0.4 | 2.9 ± 3.7 | 1.0 ± 0.2 | 0.4 ± 0.2 ** |
| CXCL8 | 1.0 ± 0.2 | 4.3 ± 1.8 *** | n.d. | n.d. | 1.0 ± 0.1 | 1.5 ± 1.3 *** |
| FGF5 | 1.0 ± 0.2 | 3.2 ± 1.7 ** | 1.0 ± 0.1 | 1.3 ± 0.6 | 1.0 ± 0.1 | 0.9 ± 0.2 |
| IL-6 | 1.0 ± 0.1 | 3.8 ± 1.7 *** | 1.0 ± 0.3 | 5.2 ± 7.4 | 1.0 ± 0.2 | 0.8 ± 0.4 ** |
| PAI-1 | n.d. | n.d. | 1.0 ± 0.2 | 12.7 ± 19.3 | 1.0 ± 0.2 | 0.6 ± 0.1 ** |
| TGFβ1 | 1.0 ± 0.1 | 1.1 ± 0.3 | 1.1 ± 0.1 | 1.3 ± 0.2 *** | 1.0 ± 0.1 | 1.3 ± 0.3 *** |
| tPA | 1.0 ± 0.3 | 16.9 ± 10.4 *** | 1.0 ± 0.2 | 6.5 ± 4.6 *** | 1.0 ± 0.1 | 0.5 ± 0.2 *** |
| VEGF | 1.0 ± 0.1 | 2.5 ± 1.0 *** | 0.9 ± 0.3 | 3.0 ± 1.0 *** | 1.0 ± 0.2 | 1.7 ± 0.4 *** |
| Gene | MCF7 | A549 | ||||
| Con | HT | Con | HT | |||
| CCL2 | 1.0 ± 0.3 | 2.8 ± 1.6 ** | 1.0 ± 0.2 | 7.0 ± 2.1 *** | ||
| CXCL1 | 1.0 ± 0.3 | 11.5 ± 3.8 *** | 1.0 ± 0.1 | 4.4 ± 2.5 *** | ||
| CXCL8 | 1.0 ± 0.1 | 10.4 ± 2.5 *** | 1.0 ± 0.1 | 8.8 ± 3.4 *** | ||
| FGF5 | n.d. | n.d. | n.d. | n.d. | ||
| IL-6 | n.d. | n.d. | 1.0 ± 0.1 | 50.6 ± 32.2 ** | ||
| PAI-1 | n.d. | n.d. | 1.0 ± 0.1 | 13.2 ± 2.9 *** | ||
| TGFβ1 | 1.0 ± 0.1 | 3.6 ± 0.9 *** | 1.0 ± 0.1 | 1.3 ± 0.3 ** | ||
| tPA | 1.0 ± 0.2 | 6.2 ± 2.0 *** | 1.0 ± 0.1 | 2.0 ± 0.4 *** | ||
| VEGF | 1.0 ± 0.1 | 1.7 ± 0.4 *** | 1.0 ± 0.2 | 7.6 ± 2.2 *** | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uruski, P.; Mikuła-Pietrasik, J.; Tykarski, A.; Książek, K. Serum from Hypertensive Patients Induces Cancer-Supporting Phenotype of Vascular Endothelium In Vitro. Biomolecules 2024, 14, 1374. https://doi.org/10.3390/biom14111374
Uruski P, Mikuła-Pietrasik J, Tykarski A, Książek K. Serum from Hypertensive Patients Induces Cancer-Supporting Phenotype of Vascular Endothelium In Vitro. Biomolecules. 2024; 14(11):1374. https://doi.org/10.3390/biom14111374
Chicago/Turabian StyleUruski, Paweł, Justyna Mikuła-Pietrasik, Andrzej Tykarski, and Krzysztof Książek. 2024. "Serum from Hypertensive Patients Induces Cancer-Supporting Phenotype of Vascular Endothelium In Vitro" Biomolecules 14, no. 11: 1374. https://doi.org/10.3390/biom14111374
APA StyleUruski, P., Mikuła-Pietrasik, J., Tykarski, A., & Książek, K. (2024). Serum from Hypertensive Patients Induces Cancer-Supporting Phenotype of Vascular Endothelium In Vitro. Biomolecules, 14(11), 1374. https://doi.org/10.3390/biom14111374







