Chemical Profiling of Polar Lipids and the Polyphenolic Fraction of Commercial Italian Phaseolus Seeds by UHPLC-HRMS and Biological Evaluation
Abstract
1. Introduction
2. Materials and Methods
2.1. Standards and Reagents
2.2. Plant Material and Extraction Procedure
2.3. UHPLC-Q Exactive Orbitrap-HRMS System
2.4. Data Processing and Multivariate Analysis
2.5. Identification of Bioactive Compounds by LC-ESI-Q-Exactive MSn Experiments
2.6. Mineral Analysis
2.7. Cell-Free Activity Assay on COX-2 Enzyme
2.8. Anti-Inflammatory Activity of PVCO on Murine Macrophage Cell Line
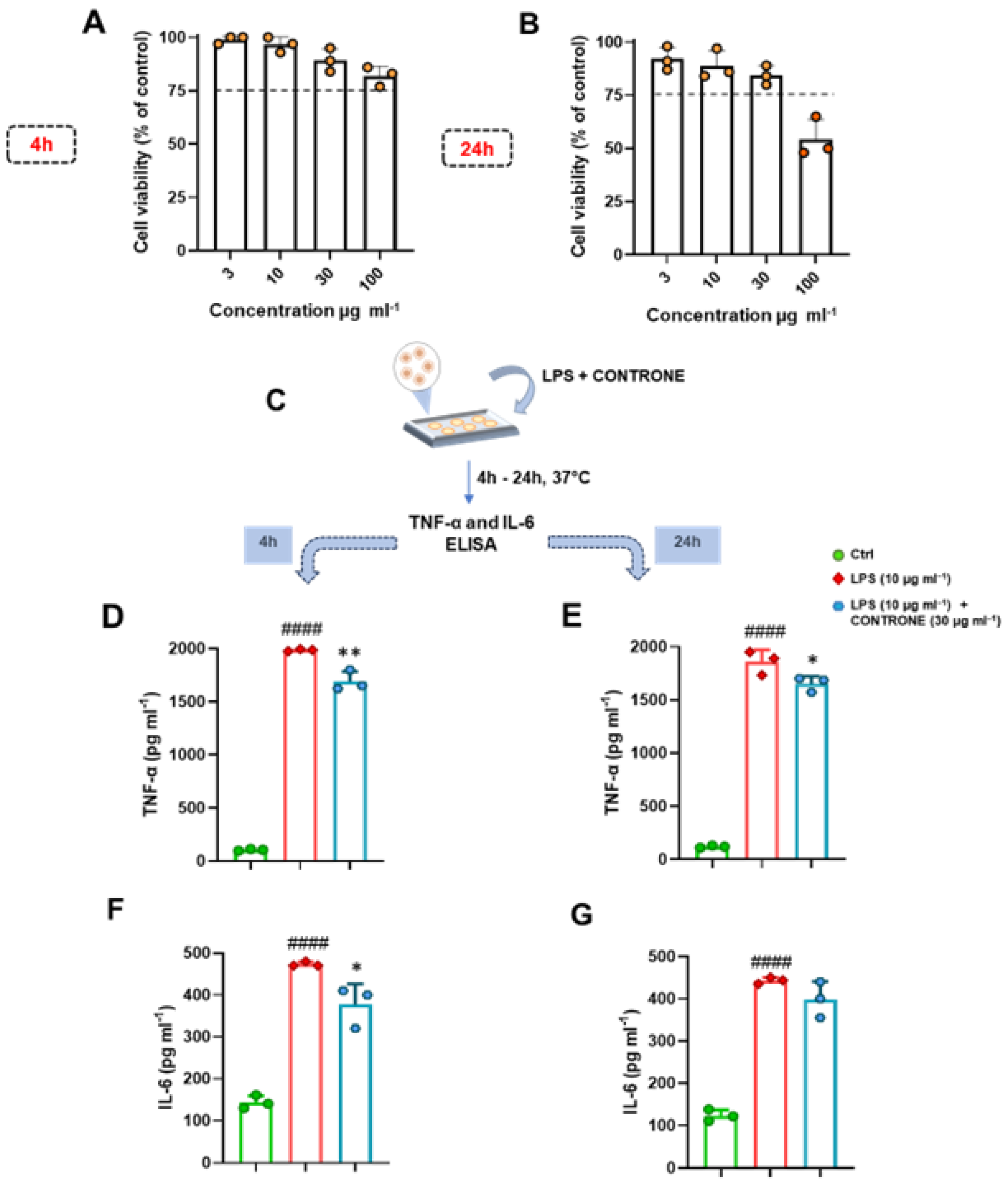
2.8.1. Cell Culture and Viability
2.8.2. Anti-Inflammatory Activity on Murine Macrophages Cell Line
2.8.3. ELISA Assay
3. Results and Discussion
3.1. Identification of Polar Lipids in Five Italian Cultivars of P. vulgaris Through LC-ESI-FT-MS Analysis
3.1.1. Polyunsaturated Fatty Acids (PUFAs) and Oxylipins Identified in P. vulgaris
| N° | Compound | Rt (min) | Molecular Formula | [M − H]− | ppm | MS/MS |
|---|---|---|---|---|---|---|
| 1 | 9,10,13-TriHODE | 13.05 | C18H32O5 | 327.2163 | 0.02 | 211.13/291.20/309.21/269.17/183.14/141.18 |
| 2 | 9,12,13-TriHOME (10) | 13.72 | C18H34O5 | 329.2320 | −0.12 | 229.14/311.22/293.21/211.13/171.10 |
| 3 | 10,16-Dihydroxyhexadecanoic acid | 14.75 | C16H32O4 | 287.2217 | 0.29 | 269.21/141.18/189.98/109.03 |
| 4 | 11-methyl dodecadienoic acid | 17.05 | C13H22O2 | 209.1538 | −0.97 | 165.16515 |
| 5 | L-PI (18:3) | 17.51 | C27H47O12P | 593.2719 | −0.38 | 315.04/241.01/277.21 |
| 6 | 9,10-DiHODE | 18.37 | C18H32O4 | 311.2222 | 1.65 | 201.11/275.20/293.21/171.10 |
| 7 | L-PE (18:3) | 18.50 | C23H42NO7P | 474.2620 | 0.36 | 277.21/214.05/196.04 |
| 8 | 15,16-DiHODE | 18.52 | C18H32O4 | 311.2221 | 0.55 | 223.17/235.17/275.20/293.21 |
| 9 | PE(18:1(9Z)/0:0) | 19.12 | C23H46NO7P | 478.2928 | 0.01 | 281.24/214.05/196.04 |
| 10 | 9,11-Linoleic acid | 19.78 | C18H32O2 | 279.2323 | 1.69 | 183.70/112.9 |
| 11 | L-PE (18:2) | 19.78 | C23H44NO7P | 476.2776 | 0.36 | 279.23/214.05/196.04 |
| 12 | OKHdiA-PE | 20.15 | C30H52NO11P | 632.3192 | −0.29 | 279.23/255.23/112.98/ |
| 13 | PE(16:0/0:0) | 20.19 | C21H44NO7P | 452.2769 | −0.47 | 255.23/214.05/196.04 |
| 14 | PI (16:0_18:3) | 20.40 | C43H77O13P | 831.5008 | −1.14 | 770.57/553.28/391.22/277.22/255.23/241.01/297.04/ |
| 15 | L-PE (16:0) | 20.63 | C21H44NO7P | 452.2776 | 0.31 | 255.23/214.05/196.04 |
| 16 | Palmitic acid | 20.63 | C16H32O2 | 255.2322 | 1.61 | 214.07/187.06/145.02/112.98 |
| 17 | PE(18:0/0:0) | 20.70 | C23H48NO7P | 480.3084 | 0.13 | 255.23/224.07/214.05/196.04/153.00 |
| 18 | PE(16:0/18:3(9Z,12Z,15Z)) | 20.99 | C39H72NO8P | 712.4914 | 0.39 | 277.22/255.23/452.28/214.05/196.04 |
| 19 | PHHdiA-PE | 21.13 | C28H52NO11P | 608.3195 | 0.15 | 255.23/313.61/401.61/112.98 |
| 20 | (6Z)-Octadecenoicacid | 21.47 | C18H34O2 | 281.2479 | 1.61 | 106.04/171.07/212.09 |
| 21 | L-PE (18:1) | 21.47 | C23H46NO7P | 478.2930 | 0.51 | 281.24/214.05/196.04 |
| 22 | (9Z)-(13S)-12_13-Epoxyoctadeca-9,11-dienoic acid | 22.14 | C18H30O3 | 293.2116 | −1.25 | 236.10551; 221.15480 |
| 23 | PE (16:0, 18:2) | 22.84 | C39H74NO8P | 714.5062 | −0.75 | 452.27/279.23/ 255.23/214.05/196.04 |
| N° | Compound | Rt (min) | Molecular Formula | [M + H]+ | ppm | MS/MS |
|---|---|---|---|---|---|---|
| 24 | L-PC (18:3) | 22.62 | C26H48O7NP | 518.3230 | −2.15 | 184.07/469.80/335.25/268.89 |
| 25 | L-PC (18:3) | 22.68 | C26H48O7NP | 518.3230 | −2.15 | 184.07/104.11 |
| 26 | L-PC (18:2) | 23.64 | C26H50O7NP | 520.3368 | −1.72 | 184.07 |
| 27 | L-PC (18:2) | 23.98 | C26H50O7NP | 520.3368 | −1.72 | 184.07 |
| 28 | L-PC(16:0) | 24.49 | C24H50O7NP | 496.3389 | 1.56 | 184.07/104.11/313.27 |
| 29 | L-PC(16:0) | 24.88 | C24H50O7NP | 496.3389 | 1.56 | 184.07/104.11/313.27 |
| 30 | L-PC(18:1) | 25.32 | C26H52O7NP | 522.3550 | 1.85 | 184.07 |
| 31 | L-PC(18:1) | 25.68 | C26H52O7NP | 522.3550 | 1.85 | 184.07/104.11 |
| 32 | NA-GPE | 22.95 | C23H44O7NP | 478.2925 | −0.57 | 337.27/306.28 |
| 33 | NA-GPE | 23.30 | C23H44O7NP | 478.2925 | −0.57 | 337.27/306.28/155.01 |
3.1.2. Polar Glycerolipids
3.1.3. Other Fatty Acids
3.2. Multivariate Analysis of Lipophilic Extracts
3.3. Elemental Profiles of Seeds
3.4. Unveiling the Metabolites in the Hydroalcoholic Extracts of P. vulgaris Varieties Through LC-ESI-HRMS Analysis
3.5. Biochemical Evaluation of the COX-2 Enzyme
3.6. Unveiling the Safety Profile and Anti-Inflammatory Activity of PVCO on the Murine Macrophage Cell Line J774A.1
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Llorach, R.; Favari, C.; Alonso, D.; Garcia-Aloy, M.; Andres-Lacueva, C.; Urpi-Sarda, M. Comparative metabolite fingerprinting of legumes using LC-MS-based untargeted metabolomics. Food Res. Int. 2019, 126, 108666. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.X.; Tang, Y.; Marcone, M.F.; Pauls, P.K.; Zhang, B.; Liu, R.; Tsao, R. Characterization of free, conjugated and bound phenolics and lipophilic antioxidants in regular- and non-darkening cranberry beans (Phaseolus vulgaris L.). Food Chem. 2015, 185, 298–308. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Guerrero, C.J.; Villa-Ruano, N.; Zepeda-Vallejo, L.G.; Hernández-Fuentes, A.D.; Ramirez-Estrada, K.; Zamudio-Lucero, S.; Hidalgo-Martínez, D.; Becerra-Martínez, E. Bean cultivars (Phaseolus vulgaris L.) under the spotlight of NMR metabolomics. Food Res. Int. 2021, 150, 110805. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, D.J.A.; Kendall, C.W.C.; Augustin, L.S.A.; Mitchell, S.; Sahye-Pudaruth, S.; Mejia, S.B.; Chiavaroli, L.; Mirrahimi, A.; Ireland, C.; Bashyam, B.; et al. Effect of Legumes as Part of a Low Glycemic Index Diet on Glycemic Control and Cardiovascular Risk Factors in Type 2 Diabetes Mellitus. Arch. Intern. Med. 2012, 172, 1653–1660. [Google Scholar] [CrossRef]
- Flight, I.; Clifton, P. Cereal grains and legumes in the prevention of coronary heart disease and stroke: A review of the literature. Eur. J. Clin. Nutr. 2006, 60, 1145–1159. [Google Scholar] [CrossRef]
- Messina, V. Nutritional and health benefits of dried beans. Am. J. Clin. Nutr. 2014, 100 (Suppl. 1), 437S–442S. [Google Scholar] [CrossRef]
- Mollard, R.C.; Luhovyy, B.L.; Panahi, S.; Nunez, M.; Hanley, A.; Anderson, G.H. Regular consumption of pulses for 8 weeks reduces metabolic syndrome risk factors in overweight and obese adults. Br. J. Nutr. 2012, 108 (Suppl. 1), S111–S122. [Google Scholar] [CrossRef]
- Fantasma, F.; Samukha, V.; Saviano, G.; Chini, M.G.; Iorizzi, M.; Caprari, C. Nutraceutical Aspects of Selected Wild Edible Plants of the Italian Central Apennines. Nutraceuticals 2024, 4, 190–231. [Google Scholar] [CrossRef]
- Margier, M.; George, S.; Hafnaoui, N.; Remond, D.; Nowicki, M.; Du Chaffaut, L.; Amiot, M.J.; Reboul, E. Nutritional Composition and Bioactive Content of Legumes: Characterization of Pulses Frequently Consumed in France and Effect of the Cooking Method. Nutrients 2018, 10, 1668. [Google Scholar] [CrossRef]
- Ha, T.J.; Lee, B.W.; Park, K.H.; Jeong, S.H.; Kim, H.T.; Ko, J.M.; Baek, I.Y.; Lee, J.H. Rapid characterisation and comparison of saponin profiles in the seeds of Korean Leguminous species using ultra performance liquid chromatography with photodiode array detector and electrospray ionisation/mass spectrometry (UPLC-PDA-ESI/MS) analysis. Food Chem. 2014, 146, 270–277. [Google Scholar] [CrossRef]
- Gan, R.Y.; Deng, Z.Q.; Yan, A.X.; Shah, N.P.; Lui, W.Y.; Chan, C.L.; Corke, H. Pigmented edible bean coats as natural sources of polyphenols with antioxidant and antibacterial effects. LWT-Food Sci. Technol. 2016, 73, 168–177. [Google Scholar] [CrossRef]
- Aparicio-Fernandez, X.; Garcia-Gasca, T.; Yousef, G.G.; Lila, M.A.; Gonzalez de Mejia, E.; Loarca-Pina, G. Chemopreventive activity of polyphenolics from black Jamapa bean (Phaseolus vulgaris L.) on HeLa and HaCaT cells. J. Agric. Food Chem. 2006, 54, 2116–2122. [Google Scholar] [CrossRef] [PubMed]
- Ranilla, L.G.; Genovese, M.I.; Lajolo, F.M. Polyphenols and antioxidant capacity of seed coat and cotyledon from Brazilian and Peruvian bean cultivars (Phaseolus vulgaris L.). J. Agric. Food Chem. 2007, 55, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Cardador-Martinez, A.; Loarca-Pina, G.; Oomah, B.D. Antioxidant activity in common beans (Phaseolus vulgaris L.). J. Agric. Food Chem. 2002, 50, 6975–6980. [Google Scholar] [CrossRef]
- Chavez-Mendoza, C.; Sanchez, E. Bioactive Compounds from Mexican Varieties of the Common Bean (Phaseolus vulgaris): Implications for Health. Molecules 2017, 22, 1360. [Google Scholar] [CrossRef]
- Telles, A.C.; Kupski, L.; Furlong, E.B. Phenolic compound in beans as protection against mycotoxins. Food Chem. 2017, 214, 293–299. [Google Scholar] [CrossRef]
- Dai, Z.; Lyu, W.; Xiang, X.; Tang, Y.; Hu, B.; Ou, S.; Zeng, X. Immunomodulatory Effects of Enzymatic-Synthesized alpha-Galactooligosaccharides and Evaluation of the Structure-Activity Relationship. J. Agric. Food Chem. 2018, 66, 9070–9079. [Google Scholar] [CrossRef]
- Elango, D.; Rajendran, K.; van der Laan, L.; Sebastiar, S.; Raigne, J.; Thaiparambil, N.A.; El Haddad, N.; Raja, B.; Wang, W.Y.; Ferela, A.; et al. Raffinose Family Oligosaccharides: Friend or Foe for Human and Plant Health? Front. Plant Sci. 2022, 13, 829118. [Google Scholar] [CrossRef]
- Rodhouse, J.C.; Haugh, C.A.; Roberts, D.; Gilbert, R.J. Red Kidney Bean Poisoning in the Uk—An Analysis of 50 Suspected Incidents between 1976 and 1989. Epidemiol. Infect. 1990, 105, 485–491. [Google Scholar] [CrossRef]
- Borowska, J.; Giczewska, A.; Zadernowski, R. Nutritional value of broad bean seeds. Part 2: Selected biologically active components. Nahrung 2003, 47, 98–101. [Google Scholar] [CrossRef]
- He, S.; Simpson, B.K.; Sun, H.; Ngadi, M.O.; Ma, Y.; Huang, T. Phaseolus vulgaris lectins: A systematic review of characteristics and health implications. Crit. Rev. Food Sci. Nutr. 2018, 58, 70–83. [Google Scholar] [CrossRef] [PubMed]
- Mudryj, A.N.; Yu, N.; Aukema, H.M. Nutritional and health benefits of pulses. Appl. Physiol. Nutr. Metab. 2014, 39, 1197–1204. [Google Scholar] [CrossRef] [PubMed]
- Alcazar-Valle, M.; Lugo-Cervantes, E.; Mojica, L.; Morales-Hernandez, N.; Reyes-Ramirez, H.; Enriquez-Vara, J.N.; Garcia-Morales, S. Bioactive Compounds, Antioxidant Activity, and Antinutritional Content of Legumes: A Comparison between Four Phaseolus Species. Molecules 2020, 25, 3528. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, R.; Mateus, N.; Pianet, I.; Laguerre, M.; de Freitas, V. Mechanisms of tannin-induced trypsin inhibition: A molecular approach. Langmuir 2011, 27, 13122–13129. [Google Scholar] [CrossRef]
- Kato, C.G.; Goncalves, G.A.; Peralta, R.A.; Seixas, F.A.V.; de Sa-Nakanishi, A.B.; Bracht, L.; Comar, J.F.; Bracht, A.; Peralta, R.M. Inhibition of alpha-Amylases by Condensed and Hydrolysable Tannins: Focus on Kinetics and Hypoglycemic Actions. Enzym. Res. 2017, 2017, 5724902. [Google Scholar] [CrossRef]
- Samukha, V.; Fantasma, F.; D’Urso, G.; Caprari, C.; De Felice, V.; Saviano, G.; Lauro, G.; Casapullo, A.; Chini, M.G.; Bifulco, G.; et al. NMR Metabolomics and Chemometrics of Commercial Varieties of Phaseolus vulgaris L. Seeds from Italy and In Vitro Antioxidant and Antifungal Activity. Plants 2024, 13, 227. [Google Scholar] [CrossRef]
- Yoshida, H.; Tomiyama, Y.; Mizushina, Y. Characterization in the fatty acid distributions of triacylglycerols and phospholipids in kidney beans (Phaseolus vulgaris L.). J. Food Lipids 2005, 12, 169–180. [Google Scholar] [CrossRef]
- Kullenberg, D.; Taylor, L.A.; Schneider, M.; Massing, U. Health effects of dietary phospholipids. Lipids Health Dis. 2012, 11, 3. [Google Scholar] [CrossRef]
- Schverer, M.; O’Mahony, S.M.; O’Riordan, K.J.; Donoso, F.; Roy, B.L.; Stanton, C.; Dinan, T.G.; Schellekens, H.; Cryan, J.F. Dietary phospholipids: Role in cognitive processes across the lifespan. Neurosci. Biobehav. Rev. 2020, 111, 183–193. [Google Scholar] [CrossRef]
- Lordan, R.; Tsoupras, A.; Zabetakis, I. Phospholipids of Animal and Marine Origin: Structure, Function, and Anti-Inflammatory Properties. Molecules 2017, 22, 1964. [Google Scholar] [CrossRef]
- Ajila, C.M.; Brar, S.K.; Verma, M.; Tyagi, R.D.; Godbout, S.; Valero, J.R. Extraction and analysis of polyphenols: Recent trends. Crit. Rev. Biotechnol. 2011, 31, 227–249. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Alonso, C.; Taroncher, M.; Castaldo, L.; Izzo, L.; Rodriguez-Carrasco, Y.; Ritieni, A.; Ruiz, M.J. Effect of Phenolic Extract from Red Beans (Phaseolus vulgaris L.) on T-2 Toxin-Induced Cytotoxicity in HepG2 Cells. Foods 2022, 11, 1033. [Google Scholar] [CrossRef] [PubMed]
- Raks, V.; Al-Suod, H.; Buszewski, B. Isolation, Separation, and Preconcentration of Biologically Active Compounds from Plant Matrices by Extraction Techniques. Chromatographia 2018, 81, 189–202. [Google Scholar] [CrossRef] [PubMed]
- Di Masi, S.; De Benedetto, G.E.; Malitesta, C.; Saponari, M.; Citti, C.; Cannazza, G.; Ciccarella, G. HPLC-MS/MS method applied to an untargeted metabolomics approach for the diagnosis of “olive quick decline syndrome”. Anal. Bioanal. Chem. 2022, 414, 465–473. [Google Scholar] [CrossRef]
- Salem, M.A.; de Souza, L.P.; Serag, A.; Fernie, A.R.; Farag, M.A.; Ezzat, S.M.; Alseekh, S. Metabolomics in the Context of Plant Natural Products Research: From Sample Preparation to Metabolite Analysis. Metabolites 2020, 10, 37. [Google Scholar] [CrossRef]
- Want, E.; Masson, P. Processing and analysis of GC/LC-MS-based metabolomics data. Methods Mol. Biol. 2011, 708, 277–298. [Google Scholar] [CrossRef]
- De Bernardo, G.; D’Urso, G.; Spadarella, S.; Giordano, M.; Leone, G.; Casapullo, A. Analysis of the Fecal Metabolomic Profile in Breast vs. Different Formula Milk Feeding in Late Preterm Infants. Metabolites 2024, 14, 72. [Google Scholar] [CrossRef]
- Seco-Gesto, E.M.; Moreda-Pineiro, A.; Bermejo-Barrera, A.; Bermejo-Barrera, P. Multi-element determination in raft mussels by fast microwave-assisted acid leaching and inductively coupled plasma-optical emission spectrometry. Talanta 2007, 72, 1178–1185. [Google Scholar] [CrossRef]
- Bellavita, R.; Raucci, F.; Merlino, F.; Piccolo, M.; Ferraro, M.G.; Irace, C.; Santamaria, R.; Iqbal, A.J.; Novellino, E.; Grieco, P.; et al. Temporin L-derived peptide as a regulator of the acute inflammatory response in zymosan-induced peritonitis. Biomed. Pharmacother. 2020, 123, 109788. [Google Scholar] [CrossRef]
- Saviano, A.; Raucci, F.; Casillo, G.M.; Mansour, A.A.; Piccolo, V.; Montesano, C.; Smimmo, M.; Vellecco, V.; Capasso, G.; Boscaino, A.; et al. Anti-inflammatory and immunomodulatory activity of Mangifera indica L. reveals the modulation of COX-2/mPGES-1 axis and Th17/Treg ratio. Pharmacol. Res. 2022, 182, 106283. [Google Scholar] [CrossRef]
- Bellavita, R.; Buommino, E.; Casciaro, B.; Merlino, F.; Cappiello, F.; Marigliano, N.; Saviano, A.; Maione, F.; Santangelo, R.; Mangoni, M.L.; et al. Synthetic Amphipathic beta-Sheet Temporin-Derived Peptide with Dual Antibacterial and Anti-Inflammatory Activities. Antibiotics 2022, 11, 1285. [Google Scholar] [CrossRef]
- Saviano, A.; Schettino, A.; Iaccarino, N.; Mansour, A.A.; Begum, J.; Marigliano, N.; Raucci, F.; Romano, F.; Riccardi, G.; Mitidieri, E.; et al. A reverse translational approach reveals the protective roles of Mangifera indica in inflammatory bowel disease. J. Autoimmun. 2024, 144, 103181. [Google Scholar] [CrossRef] [PubMed]
- David, I.; Orboi, M.D.; Simandi, M.D.; Chirila, C.A.; Megyesi, C.I.; Radulescu, L.; Draghia, L.P.; Lukinich-Gruia, A.T.; Muntean, C.; Hadaruga, D.I.; et al. Fatty acid profile of Romanian’s common bean (Phaseolus vulgaris L.) lipid fractions and their complexation ability by beta-cyclodextrin. PLoS ONE 2019, 14, e0225474. [Google Scholar] [CrossRef] [PubMed]
- Celmeli, T.; Sari, H.; Canci, H.; Sari, D.; Adak, A.; Eker, T.; Toker, C. The Nutritional Content of Common Bean (Phaseolus vulgaris L.) Landraces in Comparison to Modern Varieties. Agronomy 2018, 8, 166. [Google Scholar] [CrossRef]
- Misheva, M.; Johnson, J.; McCullagh, J. Role of Oxylipins in the Inflammatory-Related Diseases NAFLD, Obesity, and Type 2 Diabetes. Metabolites 2022, 12, 1238. [Google Scholar] [CrossRef] [PubMed]
- Napolitano, A.; Cerulli, A.; Pizza, C.; Piacente, S. Multi-class polar lipid profiling in fresh and roasted hazelnut (Corylus avellana cultivar “Tonda di Giffoni”) by LC-ESI/LTQOrbitrap/MS/MS(n). Food Chem. 2018, 269, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Black, B.A.; Sun, C.X.; Zhao, Y.Y.; Gänzle, M.G.; Curtis, J.M. Antifungal Lipids Produced by Lactobacilli and Their Structural Identification by Normal Phase LC/Atmospheric Pressure Photoionization-MS/MS. J. Agric. Food Chem. 2013, 61, 5338–5346. [Google Scholar] [CrossRef]
- Wheelan, P.; Zirrolli, J.A.; Murphy, R.C. Low-energy fast atom bombardment tandem mass spectrometry of monohydroxy substituted unsaturated fatty acids. Biol. Mass Spectrom. 1993, 22, 465–473. [Google Scholar] [CrossRef]
- Gobel, C.; Feussner, I.; Hamberg, M.; Rosahl, S. Oxylipin profiling in pathogen-infected potato leaves. Biochim. Biophys. Acta 2002, 1584, 55–64. [Google Scholar] [CrossRef]
- Ustunes, L.; Claeys, M.; Laekeman, G.; Herman, A.G.; Vlietinck, A.J.; Ozer, A. Isolation and identification of two isomeric trihydroxy octadecenoic acids with prostaglandin E-like activity from onion bulbs (Allium cepa). Prostaglandins 1985, 29, 847–865. [Google Scholar] [CrossRef]
- Moe, M.K.; Jensen, E. Structure elucidation of unsaturated fatty acids after vicinal hydroxylation of the double bonds by negative electrospray ionisation low-energy tandem mass spectrometry. Eur. J. Mass Spectrom. 2004, 10, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Di Paolo, G.; De Camilli, P. Phosphoinositides in cell regulation and membrane dynamics. Nature 2006, 443, 651–657. [Google Scholar] [CrossRef] [PubMed]
- Hsu, F.F.; Kuhlmann, F.M.; Turk, J.; Beverley, S.M. Multiple-stage linear ion-trap with high resolution mass spectrometry towards complete structural characterization of phosphatidylethanolamines containing cyclopropane fatty acyl chain in Leishmania infantum. J. Mass Spectrom. 2014, 49, 201–209. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Grembecka, M.; Szefer, P. Elemental Profiles of Legumes and Seeds in View of Chemometric Approach. Appl. Sci. 2022, 12, 1577. [Google Scholar] [CrossRef]
- Weyh, C.; Kruger, K.; Peeling, P.; Castell, L. The Role of Minerals in the Optimal Functioning of the Immune System. Nutrients 2022, 14, 644. [Google Scholar] [CrossRef]
- Bosmali, I.; Giannenas, I.; Christophoridou, S.; Ganos, C.G.; Papadopoulos, A.; Papathanasiou, F.; Kolonas, A.; Gortzi, O. Microclimate and Genotype Impact on Nutritional and Antinutritional Quality of Locally Adapted Landraces of Common Bean (Phaseolus vulgaris L.). Foods 2023, 12, 1119. [Google Scholar] [CrossRef]
- Babaahmadifooladi, M.; Jacxsens, L. Chronic dietary exposure to nickel from selected foods consumed in Belgium. Food Addit. Contam. Part A 2021, 38, 95–112. [Google Scholar] [CrossRef]
- Nicolas-Garcia, M.; Perucini-Avendano, M.; Jimenez-Martinez, C.; Perea-Flores, M.J.; Gomez-Patino, M.B.; Arrieta-Baez, D.; Davila-Ortiz, G. Bean phenolic compound changes during processing: Chemical interactions and identification. J. Food Sci. 2021, 86, 643–655. [Google Scholar] [CrossRef]
- Beninger, C.W.; Hosfield, G.L. Flavonol glycosides from Montcalm dark red kidney bean: Implications for the genetics of seed coat color in Phaseolus vulgaris L. J. Agric. Food Chem. 1999, 47, 4079–4082. [Google Scholar] [CrossRef]
- Ramirez-Jimenez, A.K.; Reynoso-Camacho, R.; Mendoza-Diaz, S.; Loarca-Pina, G. Functional and technological potential of dehydrated Phaseolus vulgaris L. flours. Food Chem. 2014, 161, 254–260. [Google Scholar] [CrossRef]
- Morita, I. Distinct functions of COX-1 and COX-2. Prostaglandins Other Lipid Mediat. 2002, 68–69, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Cambiaggi, L.; Chakravarty, A.; Noureddine, N.; Hersberger, M. The Role of alpha-Linolenic Acid and Its Oxylipins in Human Cardiovascular Diseases. Int. J. Mol. Sci. 2023, 24, 6110. [Google Scholar] [CrossRef] [PubMed]
- Rossol, M.; Heine, H.; Meusch, U.; Quandt, D.; Klein, C.; Sweet, M.J.; Hauschildt, S. LPS-induced cytokine production in human monocytes and macrophages. Crit. Rev. Immunol. 2011, 31, 379–446. [Google Scholar] [CrossRef] [PubMed]

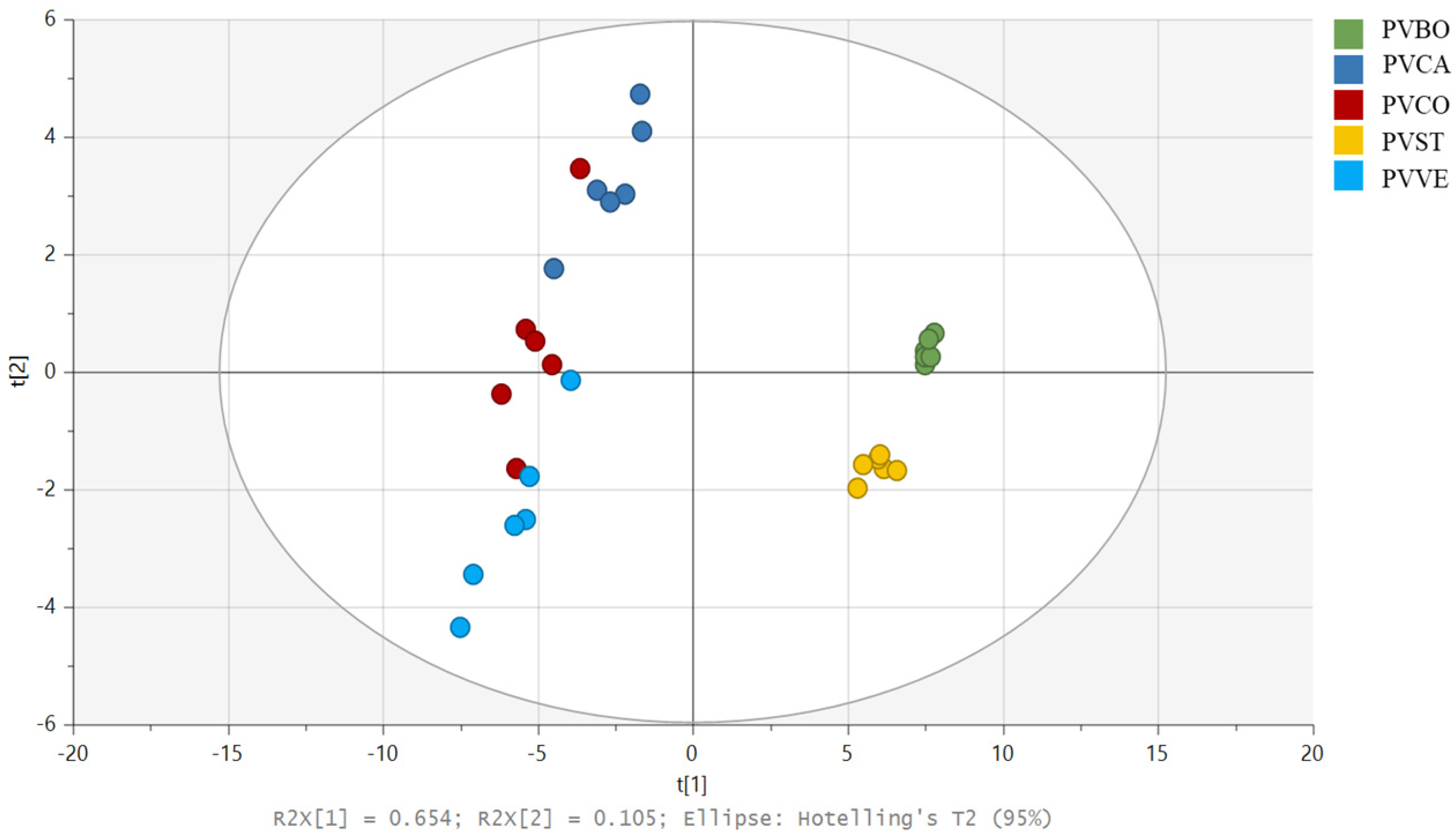

| Elements | Bean Varieties | ||||
|---|---|---|---|---|---|
| PVBO | PVCA | PVCO | PVST | PVVE | |
| Ca (λ 393.366 nm) | 1013.97 ± 0.36 | 1114.75 ± 0.09 | 1441.97 ± 0.58 | 1083.33 ± 0.16 | 1127.33 ± 0.04 |
| Cu (λ 327.395 nm) | 7.88 ± 0.01 | 9.77 ± 0.01 | 8.22 ± 0.01 | 7.06 ± 0.01 | 6.53 ± 0.01 |
| Fe (λ 259.940 nm) | 64.39 ± 0.01 | 55.66 ± 0.01 | 46.42 ± 0.01 | 59.79 ± 0.01 | 59.24 ± 0.01 |
| K (λ 766.491 nm) | 13,504.24 ± 0.16 | 15,143.07 ± 0.19 | 13,886.36 ± 0.49 | 15,073.92 ± 0.82 | 15,760.73 ± 2.50 |
| Mg (λ 280.270 nm) | 1548.22 ± 0.37 | 1679.20 ± 0.05 | 1600.58 ± 0.12 | 1341.81 ± 0.31 | 1455.22 ± 0.15 |
| Mn (λ 257.610 nm) | 15.29 ± 0.01 | 20.02 ± 0.01 | 10.64 ± 0.01 | 11.77 ± 0.01 | 15.39 ± 0.01 |
| Ni (λ 216.555 nm) | 19.71 ± 0.01 | 1.46 ± 0.01 | 0.48 ± 0.01 | 0.47 ± 0.01 | 0.93 ± 0.01 |
| P (λ 213.618 nm) | 4724.68 ± 0.69 | 5095.70 ± 0.74 | 3581.24 ± 0.60 | 4450.56 ± 0.46 | 5031.72 ± 0.32 |
| S (λ 181.972 nm) | 2090.96 ± 0.18 | 1805.66 ± 0.33 | 1893.62 ± 0.11 | 1931.73 ± 0.45 | 2247.67 ± 0.20 |
| N° | Compound | Rt (min) | Molecular Formula | [M − H]− | ppm | MS/MS | Detection |
| 34 | (epi)catechin-hexoside | 7.77; 8.26 | C21H24O11 | 451.1249 | 3.28 | 289.07 245.08 125.02 | PVVE PVST PVBO |
| 35 | L-glutamyl-L-leucine | 8.70 | C11H20O5N2 | 259.1302 | 5.5 | 128.03 130.09 | All |
| 36 | feruloylglucaric acid derivative | 8.87 | C16H18O11 | 385.0783 | 4.60 | 85.03 191.02 209.03 | All |
| 37 | catechin/(epi-) | 10.05; 10.24 | C15H14O6 | 289.0723 | 2.05 | 245.08 205.05 203.07 179.03 125.02 109.03 | PVVE PVST PVBO |
| 38 | tuberonic acid hexoside isomer | 10.59 | C18H28O9 | 387.1662 | 3.36 | 207.10 163.11 | All |
| 39 | eriodictyol-hexoside | 11.16 | C21H22O11 | 449.1097 | 4.23 | 259.06 287.06 269.05 179.00 125.02 | PVVE PVST PVBO |
| 40 | quercetin xylopyranosyl-rutinoside | 11.32 | C32H38O20 | 741.1908 | 4.84 | 301.03 179.00 | PVVE |
| 41 | quercetin sambubioside | 11.84 | C26H28O16 | 595.1306 | 2.21 | 301.03 | PVVE |
| 42 | rutin | 12.13 | C27H30O16 | 609.1478 | 4.62 | 301.03 | PVVE |
| 43 | quercetin hexoside | 12.56–12.59–12.62 | C21H20O12 | 463.0888 | 3.81 | 301.03 | All |
| 44 | p-coumaric acid | 12.83 | C9H8O3 | 163.0395 | 3.37 | 119.05 | All |
| 45 | quercetin malonylhexoside | 12.96 | C24H22O15 | 549.0899 | 4.50 | 301.03 | All |
| 46 | quercetin acetylhexoside | 13.10 | C23H22O13 | 505.0999 | 4.52 | 301.03 | All |
| 47 | sinapic acid | 13.18 | C11H12O5 | 223.0613 | 0.68 | 208.04 193.01 179.07 169.05 164.05 152.01 149.02 | All |
| 48 | ferulic acid | 13.33 | C10H10O4 | 193.0503 | 4.37 | 178.03 134.04 149.06 | All |
| 49 | kaempferol-hexoside | 13.40 | C21H20O11 | 447.0943 | 4.85 | 285.04 | PVVE PVST PVBO |
| 50 | taxifolin | 13.47 | C15H12O7 | 303.0517 | 2.41 | 285.04 125.02 | PVVE PVST PVBO PVCO |
| 51 | homovanillic acid | 13.70 | C9H10O4 | 181.0500 | 3.00 | 166.03 | All |
| 52 | hesperetin | 14.65 | C16H14O6 | 301.0724 | 2.35 | 257.05 | PVVE |
| 53 | quercetin | 16.53 | C15H10O7 | 301.0361 | 2.61 | 151.00 179.00 | PVVE |
| 54 | α-hydroxyacetovanillone | 17.40 | C9H10O4 | 181.0500 | 3.00 | 166.03 | All |
| 55 | soyasaponin V | 18.72 | C48H78O19 | 957.5073 | 2.12 | 457.37 221.07 | All |
| 56 | soyasaponin I | 19.07 | C48H78O18 | 941.5122 | 1.94 | 457.37 205.07 | All |
| 57 | sandosaponin (A/B) | 19.90 | C48H76O19 | 955.4939 | 4.38 | 455.35 221.07 | All |
| 58 | dehydrosoyasaponin I | 20.21 | C48H76O18 | 939.49908 | 4.56 | 205.07 | All |
| Positive ion mode | |||||||
| Compound | Rt (min) | Molecular Formula | [M + H]+ | ppm | MS/MS | Detection | |
| 59 | delphinidin-hexoside | 11.81 | C21H21O12 | 465.1012 | −3.33 | 303.05 | PVVE |
| 60 | cyanidin-hexoside | 12.47 | C21H21O11 | 449.1069 | −2.08 | 287.05 | PVVE |
| Compound (40 µg/mL) | Inhibition Percentage ± SD | IC50 ± SD (µg/mL) |
|---|---|---|
| PVCO | 47.5 ± 7.3 | 31.15 ± 2.16 |
| PVBO | 44.3 ± 2.6 | 42.29 ± 3.11 |
| PVST | 43.7 ± 5.4 | 43.92 ± 1.86 |
| PVCA | 38.1 ± 7.5 | \ |
| PVVE | 19.6 ± 3.9 | \ |
| Celecoxib (known inhibitor) | 85.2 ± 2.3 | \ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samukha, V.; Fantasma, F.; D’Urso, G.; Colarusso, E.; Schettino, A.; Marigliano, N.; Chini, M.G.; Saviano, G.; De Felice, V.; Lauro, G.; et al. Chemical Profiling of Polar Lipids and the Polyphenolic Fraction of Commercial Italian Phaseolus Seeds by UHPLC-HRMS and Biological Evaluation. Biomolecules 2024, 14, 1336. https://doi.org/10.3390/biom14101336
Samukha V, Fantasma F, D’Urso G, Colarusso E, Schettino A, Marigliano N, Chini MG, Saviano G, De Felice V, Lauro G, et al. Chemical Profiling of Polar Lipids and the Polyphenolic Fraction of Commercial Italian Phaseolus Seeds by UHPLC-HRMS and Biological Evaluation. Biomolecules. 2024; 14(10):1336. https://doi.org/10.3390/biom14101336
Chicago/Turabian StyleSamukha, Vadym, Francesca Fantasma, Gilda D’Urso, Ester Colarusso, Anna Schettino, Noemi Marigliano, Maria Giovanna Chini, Gabriella Saviano, Vincenzo De Felice, Gianluigi Lauro, and et al. 2024. "Chemical Profiling of Polar Lipids and the Polyphenolic Fraction of Commercial Italian Phaseolus Seeds by UHPLC-HRMS and Biological Evaluation" Biomolecules 14, no. 10: 1336. https://doi.org/10.3390/biom14101336
APA StyleSamukha, V., Fantasma, F., D’Urso, G., Colarusso, E., Schettino, A., Marigliano, N., Chini, M. G., Saviano, G., De Felice, V., Lauro, G., Maione, F., Bifulco, G., Casapullo, A., & Iorizzi, M. (2024). Chemical Profiling of Polar Lipids and the Polyphenolic Fraction of Commercial Italian Phaseolus Seeds by UHPLC-HRMS and Biological Evaluation. Biomolecules, 14(10), 1336. https://doi.org/10.3390/biom14101336











