Abstract
Inositol 1,4,5-trisphosphate receptors (IP3Rs) play a crucial role in maintaining intracellular/cytosolic calcium ion (Ca2+i) homeostasis. The release of Ca2+ from IP3Rs serves as a second messenger and a modulatory factor influencing various intracellular and interorganelle communications during both physiological and pathological processes. Accumulating evidence from in vitro, in vivo, and clinical studies supports the notion that the overactivation of IP3Rs is linked to the pathogenesis of various cardiac conditions. The overactivation of IP3Rs results in the dysregulation of Ca2+ concentration ([Ca2+]) within cytosolic, mitochondrial, and nucleoplasmic cellular compartments. In cardiovascular pathologies, two isoforms of IP3Rs, i.e., IP3R1 and IP3R2, have been identified. Notably, IP3R1 plays a pivotal role in cardiac ischemia and diabetes-induced arrhythmias, while IP3R2 is implicated in sepsis-induced cardiomyopathy and cardiac hypertrophy. Furthermore, IP3Rs have been reported to be involved in various programmed cell death (PCD) pathways, such as apoptosis, pyroptosis, and ferroptosis underscoring their multifaceted roles in cardiac pathophysiology. Based on these findings, it is evident that exploring potential therapeutic avenues becomes crucial. Both genetic ablation and pharmacological intervention using IP3R antagonists have emerged as promising strategies against IP3R-related pathologies suggesting their potential therapeutic potency. This review summarizes the roles of IP3Rs in cardiac physiology and pathology and establishes a foundational understanding with a particular focus on their involvement in the various PCD pathways within the context of cardiovascular diseases.
1. Introduction
Inositol 1,4,5-trisphosphate receptors (IP3Rs) are specialized membrane glycoprotein cationic channels responsible for releasing calcium ions (Ca2+) from the sarco/endoplasmic reticulum (SR/ER) which is known to be the predominant site of IP3Rs localization and serves as the principal site for intracellular Ca2+ (Ca2+i) storage [1,2,3]. The endogenous ligand of IP3Rs is inositol 1,4,5-trisphosphate (IP3) [1,2]. Upon the interaction between IP3 and the inositol binding core of IP3Rs, the activation and opening of large-conductance cation channels is initiated [2]. The opening of IP3Rs facilitates the release of Ca2+ from the SR/ER leading to an elevation in Ca2+i concentration ([Ca2+]i) [2]. There are three isoforms of IP3Rs expressed in mammalian cells, namely IP3R1, IP3R2, and IP3R3 [4]. The structural analysis of each IP3Rs subtype shows ~70% amino acid sequence homology, despite the fact that all these isoforms originate from three distinct genes [1,5]. All IP3R subtypes are essential for regulating Ca2+i homeostasis. The binding of IP3 to IP3Rs plays a crucial role in intracellular communication and in initiating both primary and modulatory signaling [6]. For the primary role, the Ca2+ released from IP3Rs is used ubiquitously as a secondary messenger to control a variety of intracellular to interorgan physiology and communications including secretion, contraction, protein synthesis, cell division and differentiation, and fertilization [6,7]. The modulatory function of IP3R-mediated Ca2+ release has been observed in various excitable cell types such as endocrine cells, neurons, and cardiac cells [6]. In these cells, IP3Rs modulate the [Ca2+]i signal generated by major voltage-operated channels [6].
In addition to their presence in the SR/ER, IP3Rs are also distributed in other organelles such as the plasma membrane, Golgi apparatus, mitochondria, and perinuclear/nuclear membranes [8,9,10]. This distribution is observed in various cell types, including neurons and cardiac cells [8,9,10,11]. In the past several decades, a growing body of scientific investigation has demonstrated that IP3R signaling participates in different cardiovascular abnormalities [10,12,13,14,15]. More interestingly, a few studies have shown a tight association between IP3R signaling and different types of programmed cell death (PCD) [13,14,15,16]. This review article highlights the potential role of IP3Rs in various types of PCD, particularly focusing on cardiovascular diseases. In addition, the potential effects of IP3R agonists and antagonists are also explored in different disease settings.
2. Physiology and Pathology of Calcium Handling in the Heart
It is well established that Ca2+i plays a critical role in cardiomyocyte excitation–contraction (E-C) coupling [17,18]. Under physiological conditions, proper cardiac contractility is regulated by changes in [Ca2+]i during both systole and diastole (Figure 1A). Cardiac contraction during systole is initiated by membrane depolarization leading to the activation of L-type voltage-gated calcium channels (LTCC) located on transverse tubules (T-tubule) [17,19,20]. A cluster of CaV1.2, a predominant cardiac subtype of LTCC, on T-tubules allows trans-sarcolemma Ca2+ influx to a cardiac dyad via its concentration gradient and into the cytosol [17,19,20]. Subsequently this change in [Ca2+i] triggers Ca2+-induced Ca2+ release (CICR) through the opening of Ca2+-dependent ryanodine receptor type-2 (RyR2) on the SR membrane [17,19,20]. As a result, CICR results in an increase in [Ca2+]i, which interacts with a diverse group of target proteins thereby controlling the biochemical and electromechanical function of the cardiac cell. Ca2+i released from the SR binds to cardiac troponin C (cTnC) at the level of the thin myofilaments and initiates cardiac contraction [17]. Therefore, trans-sarcolemma Ca2+ influx does not directly cause the contraction of the myofilaments; instead, the myofilaments are activated by the elevation of [Ca2+i] resulting from Ca2+i released from the SR [18].
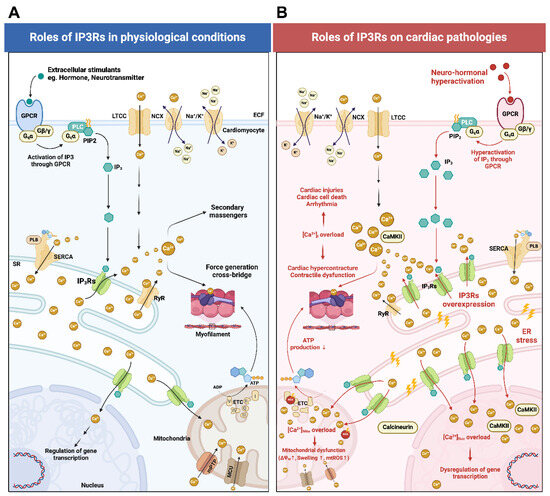
Figure 1.
A schematic diagram illustrating the regulatory mechanisms of IP3Rs in cardiomyocytes under both physiological and pathological conditions. (A) During physiological conditions, external stimuli such as adenosine, angiotensin II, bradykinin, endothelin, vasopressin, dopamine, epinephrine, norepinephrine, etc., bind and activate GPCRs on the plasma membrane. Subsequently, the Gqα-subunit dissociates from the trimeric GPCR structure, activating PLC to produce IP3 from PIP2. IP3 then binds to the IP3 binding core domain of IP3Rs leading to the release of Ca2+ from the SR. However, the amount of Ca2+ flux via IP3Rs is relatively lower compared to the Ca2+ transients generated by CICR and other major voltage-operated channels and ligand-gated channels. IP3R-mediated Ca2+ release causes Ca2+ translocation from the SR to the cytosol, mitochondria, and nucleus. In the cytosol, Ca2+ serves as a secondary messenger playing modulatory roles. Ca2+i binds to cTnC and initiates cardiac contraction. In mitochondria, Ca2+Mito influences mitochondrial energy production and metabolic activity. Mitochondria also act as secondary storage sites and modulate [Ca2+]i levels. In the nucleus, Ca2+Nuc regulates gene expression and transcription. (B) During cardiac pathologies (e.g., ischemia, I/R injuries, arrhythmias, sepsis-induced cardiomyopathy, cardiac hypertrophy, and heart failure), neurohormonal hyperactivation leads to exacerbated IP3 production from GPCR. Additionally, these pathological conditions cause IP3Rs overexpression leading to Ca2+i, Ca2+Mito, and Ca2+Nuc overload. Increased Ca2+i binds with calmodulin thus leading to CaMKII overactivation. Ca2+i overload causes cardiac hypercontracture, contractile dysfunction, cardiac cell injuries, cardiac cell death, and arrhythmias. Ca2+Mito overload causes mitochondrial dysfunction and impaired mitochondrial ATP production. Ca2+Nuc overload enhances the function of transcription factors that control the expression of pro-hypertrophic and pro-arrhythmic genes. It should be noted that the remodeling of IP3Rs and Ca2+ signaling contribute to different cardiovascular diseases and pathophysiological conditions. Endothelin-induced excessive IP3R1 expression and overactivation lead to Ca2+i and Ca2+Nuc overload, subsequently contributing to the development of atrial arrhythmias [6,10,21]. In sepsis-induced cardiomyopathy, the LPS-induced overexpression of IP3R2 leads to Ca2+i overload triggering myocardial cell death through pyroptosis and apoptosis [14]. In ischemic heart disease, the upregulation of IP3R1 in ventricular cardiomyocytes results in Ca2+i and Ca2+Mito overload leading to myocardial cell death through pyroptosis [6,13]. In the failing heart, persistent increased cardiac workload leads to chronic neurohormonal hyperactivation thereby triggering IP3R2/calcineurin/CaMKII-mediated NFAT nuclear translocation, which contributes to the transcription of hypertrophic genes [6,22,23]. This figure was created using BioRender. Abbreviations: ADP, adenosine diphosphate; ATP, adenosine triphosphate; CaMKII, Ca2+/calmodulin-dependent protein kinase II; CICR, calcium-induced calcium release; cTnC, cardiac troponin C; GPCR, G protein-coupled receptors; IP3, inositol 1,4,5-trisphosphate; IP3Rs, inositol 1,4,5-trisphosphate receptors; LTCC, L-type calcium channel; PIP2, phosphatidylinositol 4,5-bisphosphate (PIP2); PLB, phospholamban; PLC, phospholipase C; MCU, mitochondrial calcium uniporter; mPTP, mitochondrial permeability transition pore; mtROS, mitochondrial ROS production; NCX, sodium–calcium exchanger; RyR, ryanodine receptors; SERCA, sarcoplasmic/endoplasmic reticulum Ca2+-ATPase; SR, sarcoplasmic reticulum; [Ca2+], calcium ion concentration; [Ca2+]i, calcium ion concentration in the cytosol; [Ca2+]Mito, calcium ion concentration in mitochondria; [Ca2+]Nuc calcium ion concentration in the nucleus; ΔΨm mitochondrial membrane potential.
Following membrane depolarization, the LTCC reverts to a slow inactivation state due to its voltage-dependent inactivation (VDI) characteristics [17,20]. Conversely, the resultant Ca2+i transient induces the LTCC to enter its fast inactivation state through the Ca2+-dependent inactivation (CDI) process [17,20]. This process stops further Ca2+ influx from the T-tubules. Under physiological conditions during each cardiac cycle, an equal quantity of Ca2+ should be pumped out of the cell as the amount entering [17].
There are two primary mechanisms responsible for lowering Ca2+i following E-C coupling. First, Ca2+i is efficiently re-sequestered back into the SR via the sarco/endoplasmic reticulum calcium ATPase (SERCA) transporter in an ATP-dependent manner [24]. Indeed, the SR Ca2+ re-sequestration by SERCA during diastole is negatively regulated by phospholamban (PLN), which inhibits SERCA during its dephosphorylated state [18,25]. Secondly, the raised Ca2+i is pumped out via plasma membrane Ca2+-ATPase (PMCA) or exchanged with Na+ through the forward mode of the sodium–calcium exchanger (NCX), which serves as the predominant regulation unit for Ca2+ efflux in ventricular cardiomyocytes [26,27]. During diastole, about 30% of the Ca2+i released from CICR is discharged from the cell via NCX-forward mode and PMCA whereas approximately 70% is resequestered back into the SR by SERCA [28,29].
Interestingly, Ca2+i dysregulation is considered one of the pathologic hallmarks across multiple phenotypes of heart failure. In failing myocardium, there are three major components of Ca2+i dysregulation: (i) excessive Ca2+ entry, (ii) compromised SR Ca2+ resequestration, and (iii) exacerbated SR Ca2+ leakage [30,31]. These Ca2+i dysregulations cumulatively contribute to the impairment of contractility and relaxation of the heart [31,32]. The excessive Ca2+ entry is caused predominantly by the hyperphosphorylation of LTCC and the overactivation of the NCX operating in reverse mode (i.e., bringing Ca2+i into the cytosol) [29]. The foundation work from Schröder et al. (1998) reported that the channel availability and the open probability of LTCC were importantly higher in cardiomyocytes isolated from failing human hearts compared to samples from non-failing human hearts [33]. They also reported the augmented steady-state level of LTCC phosphorylation in failing human myocardium [33] which allowed excessive Ca2+ entry [29]. The recent work of Sanchez-Alonso and colleagues further clarified that the increase in the open probability of LTCC is regulated by the loss of coupling regulation of β-2 adrenergic receptors during heart failure [34]. In addition, the upregulation of NCX in the sarcolemma combined with the NCX operating in the reverse mode during heart failure magnifies the impact of an elevation in intracellular sodium concentration ([Na+]i) [35]. The elevation of [Na+]i might be partially caused by an elevated late Na+ current, diastolic Na+ influx, and/or a reduced sodium–potassium pump function, which activates the reverse mode of the NCX in human cardiomyocytes during heart failure [36,37]. The resulting NCX-reverse mode overactivation facilitates Ca2+ influx and impairs Ca2+ discharge during diastole, culminating in Ca2+i overload and subsequent diastolic dysfunction [31,38].
In addition, the store-operated calcium entry (SOCE) mechanism has also been reported to enhance Ca2+ influx during human heart failure [39,40,41]. Previous studies have reported a reduction in SERCA expression [42] alongside a decrease in PLN phosphorylation [43] resulting in compromised SR Ca2+ resequestration by SERCA in human failing myocardium. Unlike SERCA, the expression of RyR during heart failure is not changed [44]. Rather, increased β-adrenergic signaling increases adenylyl cyclase (AC) cyclic adenosine monophosphate (cAMP) production and cAMP-dependent protein kinase (PKA) phosphorylation facilitates RyR hyperphosphorylation at Ser 2809 [45,46,47]. The resultant RyR hyperphosphorylation leads to channel instability, dysfunction, and Ca2+ leakage ultimately resulting in diastolic SR Ca2+ leakage during heart failure [45,46,47]. The pathological SR Ca2+ leakage not only impacts RyR hyperphosphorylation, but also triggers Ca2+/calmodulin-stimulated protein kinase II (CaMKII) which subsequently phosphorylates LTCC exacerbating excessive Ca2+ entry [48]. Taken together, the optimal and tightly controlled regulation of [Ca2+]i is essential for the effective electromechanical coupling of the heart during both systole and diastole. The dysregulation of Ca2+i and/or the alteration in Ca2+ modulation proteins in the heart can inevitably compromise both contraction and relaxation ultimately leading to heart failure.
3. Physiology of IP3R-Mediated Ca2+ Signaling
In the heart, Ca2+i is known as a crucial regulator of intracellular signaling, arrhythmogenesis, gene transcription, and controlling cardiac contractility [12,49]. It has been recognized that the SR is also the primary site for harboring IP3Rs in addition to RyRs, both of which regulate the release of Ca2+ from the SR (Figure 1A). Both types of channels share many similar features and functions. Although the Ca2+ conductance of IP3Rs is restricted to IP3 engagement, both RyRs and IP3Rs display a biphasic response to changes in [Ca2+] in the lumen of the SR [50]. At lower concentrations, Ca2+ facilitates channel conduction, whereas at higher concentrations it induces channel inactivation [50]. In general, it has been demonstrated that IP3 and IP3Rs serve as precise modulators that finely tune the Ca2+i signals produced by major voltage-operated channels [6]. The process in which IP3 interacts with IP3Rs leading to the release of Ca2+ is termed IP3-induced Ca2+ release or IICR [51]. Although the amount of Ca2+ flux via IP3Rs is relatively lower in comparison to the Ca2+ transients generated by major voltage-operated channels or ligand-gated channels [12] and SR Ca2+ release, the IP3Rs are large-conductance cationic channels that are found localized in the ER/SR, plasma membrane, Golgi apparatus, mitochondria, and perinuclear/nuclear membrane of cardiomyocytes [2,6,10]. The subcellular distribution, spatial relationships, and functional roles of IP3Rs responsible for the release of Ca2+ from the ER/SR lumen into the cytosol and associated organelles have been studied [6,52]. The primary function of IP3Rs in cardiac cells is to amplify the frequency and amplitude of Ca2+ sparks and to sensitize Ca2+ oscillations triggered by major voltage-operated channels, thereby facilitating myocardial contraction [6]. In cardiac cells including atrial and ventricular cardiomyocytes as well as in Purkinje cells, IP3Rs are located at the SR adjacent to the T-tubule, Z-lines, mitochondria-associated SR membranes, and perinuclear region [2,8,53,54,55,56,57]. Additionally, it has been demonstrated that the presence of IP3Rs is not only necessary for ER–mitochondrial Ca2+ propagation, but also crucial for maintaining physical ER–mitochondrial contact [2]. The formation of IP3Rs into a channel complex with mitochondrial-associated proteins (i.e., GRP75, VDAC, and MCU) at the mitochondria-associated ER membranes is responsible for mitochondrial Ca2+ uptake and ER–mitochondrial oxidative stress in both cardiac and non-cardiac cells [58,59,60]. In atrial cardiomyocytes, the activation of IP3Rs on the nuclear envelope is responsible for the modification of arrhythmic gene transcription through calcineurin and CaMKII which promotes class-II histone deacetylase contributing to cardiac remodeling and arrhythmias [10,54]. In ventricular cardiomyocytes, the activation of nucleoplasmic IP3Rs triggers calcineurin/CaMKII signaling leading to the nuclear translocation of the nuclear factor of activated T cells (NFAT), which contributes to hypertrophic remodeling [8].
The expression of all three IP3Rs isoforms have been reported in cardiomyocytes [61,62]. However, previous studies have reported that among the three IP3Rs subtypes, IP3R2 appears to be the predominant isoform expressed in adult cardiomyocytes being approximately six times more prevalent in the atria compared to the ventricles [14,61,62,63]. In the fetal heart, IP3R1 expression is detected predominantly, albeit at substantially lower levels compared to the predominant expression of IP3R2 in adult atria [22]. IP3R1 has also been found in Purkinje myocyte [8,53]. Meanwhile, IP3R3 is nearly absent in adult myocardium [22]; rather, it plays a crucial role in cardiomyocyte differentiation during embryogenesis [64,65]. The structure of all IP3R isoforms comprises a tetrameric complex consisting of five domains: the IP3 binding core domain, the central regulatory domain, the suppressor domain, the transmembrane domain, and the C-terminus domain [66]. IP3Rs are activated through the selective binding of IP3 to an allosteric site at the IP3 binding core (IBC) domain [66]. This interaction triggers the channel opening of IP3Rs by facilitating the binding of Ca2+ to a stimulatory Ca2+-binding site ultimately resulting in Ca2+ efflux from storage sites [67]. The release of Ca2+ from IP3Rs activates Ca2+-sensitive adenylyl cyclase (i.e., AC1 and AC8), consequently enhancing the cAMP/PKA pathway, thus strengthening CICR mediated by the RyR [22]. In addition, Ca2+ released from IP3Rs also facilitates the distribution of Ca2+ into mitochondria and nuclei, thereby influencing and regulating mitochondrial bioenergetics and nuclear gene transcription [2,10,22]. However, in the failing heart, the heightened Ca2+i released from RyR caused by IP3Rs also exacerbates SR Ca2+ leakage while impairing mitochondria Ca2+ ([Ca2+]Mito) sequestration [22,68]. If the resultant SR Ca2+ leak reaches sufficient magnitude, it can enhance the activity of sarcolemmal NCX inducing membrane depolarization that can result in delayed afterdepolarization (DAD), and potentially amplify the generation of triggered action potentials (or triggered activities) [22,68]. Also, it has been reported that Ca2+i transients in tissues from patients with heart failure are markedly prolonged, which is related to depleted SR Ca2+ re-sequestration during diastole [18,30,69]. These cumulative effects amplify disruptions in myocardial Ca2+ homeostasis potentially contributing to compromised cardiac function. Recently reported to be associated with the pathogenesis of several cardiac and non-cardiac diseases, IP3R-mediated Ca2+ flux has been shown to contribute to an augmentation of intracellular and intra-organelle [Ca2+]. In the subsequent section, we review and discuss the remodeling of IP3Rs and their intricate roles in cardiac pathologies. A schematic diagram summarizing the regulatory mechanism of IP3Rs in cardiovascular diseases is illustrated in Figure 1B.
4. Remodeling of IP3Rs and Roles in Cardiac Pathologies
IP3Rs are more highly expressed in the heart during embryogenesis compared to during the adult stage [70]. Although IP3R2 has been reported to be the predominant isoform in adult cardiomyocytes, the genetic ablation of IP3R2 (i.e., homozygous IP3R2−/− mice) did not alter embryonic development, animal fertility, or overall systemic physiological functions [21]. In contrast, the selective overexpression of cardiac IP3R2 was observed to be associated with cardiac hypertrophy and the occurrence of increased arrhythmias [23,71]. Meanwhile, homozygous IP3R1−/− mice were found to have embryonic fatality and post-developmental abnormalities [21]. These findings suggest that in physiological versus pathological conditions, IP3Rs may play distinct roles indicating a nuanced interplay between their functions in different contexts. In line with this, several studies have reported that the elevation of IP3 and IP3Rs in adult cardiomyocytes is associated with the development of various cardiac abnormalities including ischemia, ischemic/reperfusion (I/R) injury, arrhythmias, sepsis-induced cardiomyopathy, cardiac hypertrophy, and heart failure. Gomez and colleagues reported that IP3R1 which is phosphorylated at Ser1756 (p-Ser1756 IP3R1) and glycogen synthase kinase-3β (GSK3β) were both increased in cultured cardiomyoblasts (H9c2 cells) under hypoxic–reoxygenation (H/R) insults resulting in mitochondrial Ca2+ (Ca2+Mito) overload and cell death [72]. The elevation of IP3R1 and p-Ser1756 IP3R1 protein expression were also observed in cardiac tissue after I/R in mice [72]. In this study, they reported that GSK3β specifically interacted with IP3Rs as a channel complex at the mitochondria-associated ER membranes [72]. Treatment with the GSK3β inhibitor SB216763 mitigated the increase in IP3R1 and p-Ser1756 IP3R1 protein expression and ER-mediated Ca2+i and Ca2+Mito overload in the I/R mouse hearts [72]. These results suggest that cardiac I/R mediates the overexpression of IP3R1, p-Ser1756 IP3R1, and GSK3β. These proteins have been shown to have an interaction as a channel complex responsible for Ca2+ flux from the ER to mitochondria resulting in Ca2+Mito overload and cardiac cell death. In line with these finding, Mo et al. observed a consistent upregulation of IP3R1 in cultured primary rats cardiomyocytes and H9c2 cells subjected to hypoxia/reperfusion (H/R) conditions [13]. Additionally, this upregulation was also noted in cardiac tissue in rats experiencing I/R [13]. In addition, the downregulation of endoplasmic reticulum resident protein 44 (ERP44) which is known to participate in Ca2+ homeostasis and to modulate IP3R1 was also observed [13]. In contrast, genetic modifications such as IP3R1 ablation and ERP44 overexpression were found to limit Ca2+i and Ca2+Mito overload and to reduce infarct size and cardiac injury, as well as to reduce cardiac cell death, among rats subjected to I/R insult and in cells exposed to H/R [13]. Taken together, upon H/R and I/R, there was an upregulation of IP3R1, p-Ser1756 IP3R1, and GSK3β and a downregulation of ERP44 leading to Ca2+i and Ca2+Mito overload and cardiac cell death. Both the pharmacological modulation and the genetic modification of IP3R1 and its related proteins have shown promising therapeutic potential by mitigating IP3R1-mediated Ca2+i and Ca2+Mito overload and improving cardiac cell survival in I/R-related pathological conditions. Additionally, a study from Santullia and colleagues demonstrated that IP3R2 levels were upregulated at both the mRNA and protein levels and were accompanied by changes in Ca2+ handling in the cytoplasm and mitochondria [73]. There was also increased mitochondrial oxidative stress after non-reperfused myocardial infarction [73]. However, the genetic ablation of IP3R2 did not impact Ca2+ spark frequency, SR Ca2+ load, Ca2+Mito overload, or mitochondrial oxidative stress caused by non-reperfused myocardial infarction [73]. Collectively, these data suggest that IP3R2 may not contribute to mitochondrial dysfunction in the context of non-reperfused myocardial infarction [74]. Nevertheless, it is important to note that the expression of other IP3R isoforms (i.e., IP3R1 and IP3R3) were not assessed in this study [73].
In addition to cardiac ischemia, previous studies have also addressed the essential roles of IP3Rs in relation to arrhythmogenesis when exposed to high glucose concentrations and during diabetes-related conditions. Yuan and colleagues demonstrated a significant increase in IP3R1 and its corresponding protein glucose-regulated protein 75 (GRP75) expression in isolated primary rat atrial cardiomyocytes cultured under high glucose conditions [15]. They observed an increase in both [Ca2+]i and [Ca2+]Mito compared to the control (normal glucose) group [15]. Their recent work in 2022 also assessed the marked increase in IP3R1 both in the cultured HL-1 cell line (mouse atrial cardiomyocytes) under high glucose conditions and in rats with type II diabetes [60]. More interestingly, the in situ proximity ligation results revealed that IP3R1 and GRP75 have interactions with voltage-dependent anion channels (VDAC) as an IP3R1-GRP75-VDAC complex [60]. The increase in IP3R1 expression and its complex formation was responsible for ER stress, which elevated [Ca2+]i and [Ca2+]Mito in the diabetic rats [60]. Consequently, the IP3R1-mediated Ca2+i and Ca2+Mito overload were found to be associated with mitochondrial dysfunction, adverse atrial remodeling, and increased atrial fibrillation (AF) inducibility in diabetic rats [60]. Furthermore, the genetic ablation of the protein GRP75 was found to effectively attenuate atrial remodeling and arrhythmias in type II diabetic rats [60]. Collectively, high glucose and diabetic conditions significantly augmented the expression of IP3R1 and IP3R1-related protein GRP75 leading to ER–mitochondrial channel complex formation. This ultimately caused Ca2+i and Ca2+Mito overload, mitochondrial dysfunction, atrial remodeling, and AFs. Genetic modification of the IP3R1-related protein GRP75 showed promising beneficial effects against diabetes-induced AFs.
One of the underlying mechanisms for AF has been attributed to abnormalities in atrial cardiomyocyte Ca2+i, such as spontaneous SR Ca2+ release, which augments [Ca2+]i [75]. Li and colleagues reported that the global ablation of IP3R2 abolished the increase in diastolic [Ca2+]i and Ca2+i transient amplitude in atrial cardiomyocytes to a similar extent as the IP3R antagonist 2-aminoethoxy-diphenyl borate (2-APB) in endothelin-1-stimulated mice [21]. In a previous study, Qi et al. elucidated the underlying mechanisms by which AF not only altered Ca2+i handling, but also affected nuclear Ca2+ concentration ([Ca2+]Nuc) through the involvement of IP3R1 [10]. In this study, they isolated atrial cardiomyocytes from the atria of tachycardia-paced dogs. The results showed that these AF dogs had upregulation of IP3R1 in both the nucleoplasmic and cytoplasmic fraction, while an increase in IP3R2 was observed only in the nucleoplasmic fraction [10]. In this study, diastolic [Ca2+] was determined during the depolarization phase under 1Hz stimulation followed by a 10 s pause to assess [Ca2+] during the resting stage. The isolated dog atrial cardiomyocytes with AF were found to have significantly higher [Ca2+]i and [Ca2+]Nuc levels during both the diastolic and the resting phase when compared to the control group [10]. The administration of IP3, the endogenous ligand of IP3Rs, further increased [Ca2+]i and [Ca2+]Nuc in isolated atrial cardiomyocytes from tachycardia-paced dogs [10]. Indeed, only IP3R1 knockdown could mitigate diastolic [Ca2+]Nuc alterations. This cardioprotective effect was not observed when IP3R2 was knocked down [10]. Furthermore, treatment with the IP3R antagonist 2-APB prevented IP3-mediated increase in Ca2+i and Ca2+Nuc overload [10]. These findings suggest that AF-associated [Ca2+]Nuc alterations are likely via IP3R1, but not IP3R2. IP3R1 plays a predominant role in regulating the [Ca2+]Nuc in atrial cardiomyocytes isolated from AF dogs and in vitro tachy-paced atrial cardiomyocytes.
Very recently, Wu and colleagues studied sepsis-induced cardiomyopathy by injecting lipopolysaccharide (LPS) into rats. They found that LPS administration aggravated ER stress and increased the expression of IP3R2 resulting in Ca2+i overload and cardiac cell death [14]. In this study, treatment with ER stress antagonist 4-phenylbutyric acid (4-PBA), genetic ablation of IP3R2, or pharmacological inhibition of IP3R2 using Xestospongin C reversed LPS-induced cardiomyocyte Ca2+i overload [14]. Hence, this study highlights the role of IP3R2 in being responsible for LPS-induced cardiomyocyte Ca2+i overload.
Moreover, there is also evidence implicating IP3R2 in cardiac hypertrophy. The previous work by Roderick et al. observed that IP3R2 was upregulated in spontaneously hypertensive rats (SHRs), aortic banded mice, and in heart failure patients with cardiac hypertrophy and secondary ischemic dilated cardiomyopathy [12]. Their SHR strain, known for spontaneous cardiomyocyte hypertrophy, showed higher systolic Ca2+i transient amplitudes. There was a tendency for the left ventricular fraction shortening (%LVFS) to be lower compared to wildtype mice under basal conditions, although this difference was not statistically significant [12]. Stimulation with IP3 promoted greater Ca2+i transient amplitudes and increased LVFS among the SHR strain more than in the wildtype strain. These effects were abolished by the IP3R antagonist 2-APB [12]. In addition to elucidating the roles of IP3R2 in cardiac hypertrophy, their findings also underscored the effectiveness of an IP3R antagonist in mitigating alterations in Ca2+i transients induced by IP3 stimulation. Their later work in 2012 also reported a significant increase in IP3R2 protein in the ventricular tissue of aortic banded mice [76]. These mice exhibited higher ventricular weight, elevated atrial natriuretic peptide levels, and lower LVFS indicating pressure overload-induced cardiac hypertrophy and heart failure [76]. The expression levels of miRNA-133a, known for its anti-hypertrophic properties, were also decreased in the aortic banded mice [76]. The researchers also performed miRNA-133a double knockout in mice and found that IP3R2 protein levels were increased, while the protein expression of IP3R1 and IP3R3 was not affected [76]. Conversely, the overexpression of miR-133a mitigated IP3R2 protein expression [76]. Taken together, miRNA-133a negatively regulated IP3R2 expression, Ca2+ signals, and cardiac hypertrophy. Reports from in vitro, in vivo, and clinical studies support the concept that the overactivation of IP3Rs is associated with the pathogenesis of various cardiac pathologies through the dysregulation of Ca2+ handling in cytosolic, mitochondrial, and nucleoplasmic compartments. According to previous reports mentioned above, it is evident that IP3R1 and IP3R2 exhibit distinct involvement in different cardiovascular abnormalities. IP3R1 emerges as playing a pivotal role in the development of cardiac ischemic injury and arrhythmias, while IP3R2 is implicated in sepsis-induced cardiomyopathy and cardiac hypertrophy. Both genetic modification and pharmacological intervention targeted on IP3Rs and their associated proteins exhibit promising therapeutic efficacy in alleviating pathologies linked to IP3Rs.
5. The Roles of IP3Rs and Ca2+ Signaling in Different Types of Programmed Cell Death
5.1. IP3Rs, Ca2+ Signaling, and Apoptosis (Figure 2A)
PCD in the heart is a fundamental process that occurs in both physiological and pathological conditions. This process is intricately ‘programmed’ by various predominant signaling pathways including apoptosis, pyroptosis, necroptosis, and ferroptosis [77,78]. Apoptosis is a form of programmed cell death (PCD) that plays a crucial role in various cardiovascular abnormalities including atherosclerosis, diabetic cardiomyopathy, cardiac arrhythmias, ischemic heart diseases, and heart failure [78,79]. The major mechanisms of apoptosis consist of extrinsic (death receptor dependent) and intrinsic (mitochondrial dependent) pathways [80]. The extrinsic apoptosis pathway is activated by ligand binding and the activation of transmembrane receptors such as tumor necrosis factor receptor 1 (TNFR1), toll-like receptors (TLRs), and FAS receptors (FASR) [81]. The interaction of these transmembrane receptors with their corresponding ligands (e.g., TNF-α for TNFR1, LPS for TLR4, and FAS ligand for FASR) activate downstream signaling molecules. This activation eventually leads to proteolytic caspase-mediated processes including cell shrinkage, chromosomal condensation, DNA fragmentation, and ultimately cell death [77,78,79,80,81]. In contrast to extrinsic apoptosis, internal stressors such as oxidative stress, osmotic stress, proteotoxic stress, nutritional stress, or DNA damage can trigger mitochondrial stress and injury [77,78,79,80,81]. This convergence leads to the opening of mitochondrial permeability transition pores (mPTP) followed by the release of cytochrome c from the mitochondrial intermembrane space into the cytoplasm [77,78,79,80,81]. The presence of intracytoplasmic cytochrome c facilitates the assembly of the apoptosome, which in turn activates downstream caspases, ultimately initiating apoptotic execution [77,78,79,80,81]. Substantial evidence has demonstrated that apoptosis contributes to the progression of heart failure by causing the ongoing loss of functional myocardium leading to adverse clinical outcomes [82,83]. It has been reported that evidence of apoptosis phenotypes (i.e., DNA fragmentation and caspase 3) can be identified in the myocardial tissue obtained from patients suffering from conditions such as myocarditis, dilated cardiomyopathy, diabetic cardiomyopathy, myocardial infraction, and end-stage congestive heart failure [79,84,85].
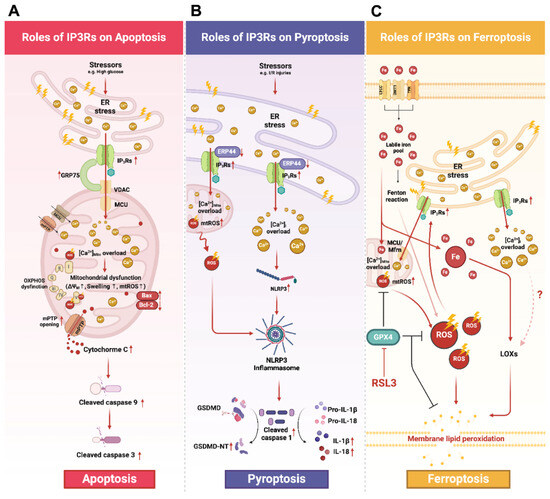
Figure 2.
A schematic diagram illustrating the roles of IP3Rs in apoptosis, pyroptosis, and ferroptosis. (A) Apoptosis: Several stress conditions (e.g., high glucose condition) mediate ER stress resulting in the upregulation of IP3Rs and GRP75 expression. Formation of the IP3Rs-GRP75-VDAC complex facilitates ER-mediated Ca2+Mito overload. Excessive [Ca2+]Mito causes mitochondrial dysfunction and facilitates mPTP opening, leading to Cytochrome C being released. The access of Cytochrome C to the cytosol initiates mitochondrial-mediated apoptosis by activating caspase cascades, including the cleavage of caspase 9 and caspase 3. (B) Pyroptosis: Stressors such as I/R or H/R induce the overexpression of IP3Rs and the downregulation of ERP44, thereby mediating Ca2+Mito and Ca2+i overload. Ca2+Mito overload impairs mitochondrial functions, disrupts oxidative phosphorylation, and increases mtROS production. The resultant overproduction of mtROS and Ca2+i overload lead to NLRP3 activation and inflammasome assembly. Once activated, the NLRP3 inflammasome cleaves pro-caspase 1 to its activated form. Then, cleaved caspase 1 further cleaves and activates GSDMD into its N-terminal fragment (GSDMD-NT). As a result, GSDMD-NT translocates and oligomerizes at the inner leaflet of the plasma membrane resulting in the formation of transmembrane pores. (C) Ferroptosis: It is proposed that ER stress could mediate ferroptosis through the overactivation of IP3Rs, thereby, leading to Ca2+Mito overload and mtROS overproduction. The administration of ferroptosis activator RSL3 inhibited the antioxidant property of GPX4 exacerbating mitochondrial and intracellular ROS levels. The subsequent augmentation of ROS leads to membrane lipid peroxidation and ultimately causes membrane rupture. In addition, Ca2+i overload caused by IP3Rs also enhances the catalytic activity of LOX, further promoting lipid membrane peroxidation. However, the underlying mechanism of whether and how Ca2+i impacts LOX remains unclear. This figure was created using BioRender. Abbreviations: BAX, Bcl-2-associated protein X; Bcl-2, B-cell lymphoma 2; GSDMD, gasdermin D; GSDMD-NT, gasdermin D N-terminus; H/R, hypoxia-reoxygenation; IP3Rs, inositol 1,4,5-trisphosphate receptors; I/R, ischemia–reperfusion; LOX, lipoxygenases; MCU, mitochondrial calcium uniporter; MFRN, mitoferrin 2; mPTP, mitochondrial permeability transition pore; mtROS, mitochondrial ROS; NLRP3, nod-like receptor protein-3; RSL3, RAS-selective lethal 3; [Ca2+], calcium ion concentration; VDAC, voltage-dependent anion channel; [Ca2+]i, calcium ion concentration in the cytosol; [Ca2+]Mito, calcium ion concentration in mitochondria; [Ca2+]Nuc calcium ion concentration in the nucleus; ΔΨm, mitochondrial membrane potential.
Recently, Ca2+ signaling and its related proteins have been extensively demonstrated to play critical roles in the development of different types of PCD pathways [80,86,87]. However, there have been limited investigations into the roles of IP3Rs in various types of PCD and even fewer studies have addressed their role in the context of cardiovascular abnormalities. As previously mentioned, high glucose conditions were found to induce an upregulation of IP3R1 and its associated protein GRP75 accompanied by Ca2+Mito overload and subsequent cardiac mitochondrial dysfunction in cultured HL-1 cells [15,60]. It is well noted that Ca2+Mito overload can ultimately induce mitochondrial dysfunction [88,89]. This process, in turn, triggers the opening of mPTP ultimately leading to mitochondrial-dependent apoptosis [88,89]. Consistently, there is also a concurrent increase in the ratio of Bcl-2-associated protein X (BAX) to B-cell lymphoma 2 (Bcl-2) and annexin V-positive cells indicating the activation of apoptosis in HL-1 cells under high glucose conditions [15,60]. Notably, the genetic ablation of GRP75 has been shown to mitigate the upregulation of pro-apoptotic proteins including cleaved caspase 9/pro-caspase 9, cleaved caspase 3/pro-caspase 3, and the BAX/Bcl-2 ratio in atrial cardiomyocytes under ER stress conditions [60]. Moreover, GRP75 silencing has also been shown to reduce the distance between the ER and the mitochondrial outer membrane, to improve [Ca2+]Mito levels, and to alleviate cardiac mitochondrial dysfunction [60]. Taken together, these finding indicate that under high glucose conditions, the disruption of ER–mitochondria Ca2+ handling occurs via the interaction of the IP3R1-GRP75-VDAC complex. This disruption mediates ER stress, Ca2+Mito overload, mitochondrial dysfunction, and subsequent apoptosis. Therefore, genetic ablation of the IP3R1-related protein GRP75 effectively attenuates high-glucose-induced apoptosis in atrial cardiomyocytes by mitigating ER–mitochondria interactions.
5.2. IP3Rs, Ca2+ Signaling, and Pyroptosis (Figure 2B)
Pyroptosis is a type of PCD that primarily arises from the caspase-1-dependent generation of transmembrane pores leading to the release of pro-inflammatory cytokines and cell lysis [90,91]. An increasing body of evidence indicates that pyroptosis plays a significant role in various cardiovascular abnormalities such as cardiac I/R injury, myocardial infarction, and heart failure [92,93,94,95]. In general, pyroptosis is initiated by the interaction between pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs) with pattern recognition receptors (PRRs), which leads to the activation of inflammasomes [80,96]. The activation and assembly of inflammasomes lead to the activation of proteolytic caspase 1, which then cleaves gasdermin D (GSDMD) into the gasdermin D C-terminus (GSDMD-CT) and N-terminus (GSDMD-NT) [80,96]. As a result, GSDMD-NT translocates and oligomerizes at the inner leaflet of the plasma membrane leading to the formation of transmembrane pores and cell lysis [80,96,97]. Apart from an external stressor, several internal stimuli have been identified as contributors to inflammasome-mediated pyroptosis. It has been reported that the assembly of inflammasomes and the occurrence of pyroptosis are facilitated by an increase in mitochondrial reactive oxygen species (mtROS) and the release of mitochondrial DNA (mtDNA) from impaired and dysfunctional mitochondria [94,95].
In addition, a study from Mo et al. also reported the involvement of IP3R1 in Ca2+ transport and pyroptosis in a cardiac I/R model [13]. In this study, they reported the upregulation of IP3R1 and downregulation of IP3R1 modulator ERP44 in cardiac tissue from rats exposed to I/R [13]. They also noted a resultant Ca2+i and Ca2+Mito overload [13]. The upregulation of pyroptosis markers, including caspase 1, nod-like receptor protein-3 (NLRP3), apoptosis-associated speck-like protein containing a CARD, caspase recruitment domain (ASC), GSDMD-NT, interleukin 1β (IL-1β), and interleukin 18 (IL-18) were also observed in both I/R rats and H/R cells [13]. Notably, IP3R1 silencing and ERP44 overexpression effectively alleviated IP3R1-mediated Ca2+i and Ca2+Mito overload leading to a reduction in pyroptosis-pertinent proteins in rats subjected to ischemia/reperfusion (I/R) [13]. Taken together, the perturbation of Ca2+i is commonly recognized as one of the prerequisites for NLRP3 activation and subsequent pyroptosis [98,99,100]. These results indicated that cardiac I/R gave rise to IP3R1 and ERP44 overexpression, thus leading to Ca2+i and Ca2+Mito overload and subsequently myocardial pyroptosis. Notably, genetic manipulation involving IP3R1 silencing and ERP44 overexpression have been shown to potentially mitigate IP3R1-mediated pyroptosis under I/R conditions.
Furthermore, Wu et al. highlighted that LPS administration caused IP3R2-mediated Ca2+i overload, triggering NLRP3-driven pyroptosis, and cardiac dysfunction in Sprague Dawley rats administered LPS [14]. In this study, the intraperitoneal administration of LPS augmented IP3R2 expression, which activated the formation of NLRP3 inflammasomes and elevated pyroptosis markers (i.e., IL-1β, IL-18, cleaved caspase 1, and GSDMD-NT) in the left ventricle leading to contractile dysfunction [14]. In vitro experiments also confirmed that LPS treatment induced IP3R2 overexpression and Ca2+i overload in isolated neonatal rat atrial cardiomyocytes (NRCMs) [14]. Silencing IP3R2 effectively reversed LPS-induced Ca2+i overload in NRCM cardiomyocytes [14]. Additionally, either the genetic ablation of IP3R2 or the pharmacological inhibition of IP3R2 significantly reduced pyroptosis activation in NRCMs under LPS stimulation [14]. In addition to IP3R2 overactivation, the upregulation of ER stress markers was also observed, including the activation of transcription factor 4 (ATF4) and CCAAT-enhancer-binding protein homologous protein (CHOP) in both myocardial tissues and NRCM cells following LPS stimulation [14]. These insults could be mitigated by the ER stress antagonist 4-BPA [14]. Collectively, LPS-induced IP3R2 expression led to Ca2+i overload, ER stress, and myocardial pyroptosis while treatment targeting IP3R2 and ER stress provided cardioprotective benefits against LPS-induced cardiomyocyte pyroptosis.
5.3. IP3Rs, Ca2+ Signaling, and Ferroptosis (Figure 2C)
Ferroptosis is defined as being iron-dependent and lipid peroxidation-driven PCD [87,101]. The disturbance of intracellular iron and the perturbation of ROS are considered crucial factors in the initiation of ferroptosis [102]. Unlike other types of PCDs, ferroptosis does not require the involvement of traditional death executioner proteins to induce its lethal consequences [103]. Instead, ferroptosis is driven by an imbalance between the accumulation of lipid peroxidation, specifically phospholipid hydroperoxides (PLOOHs) [104], and the insufficiency of endogenous antioxidation systems such as glutathione peroxidase 4 (GPX4), coenzyme Q10 (CoQ10), and tetrahydrobiopterin (BH4) [105,106,107,108]. This imbalance leads to plasma membrane damage and rupture [101,102,103]. Ferroptosis is executed by the accumulation of peroxidized lipids, which leads to the permeabilization of the plasma membrane [107]. In contrast to other types of cell death, ferroptosis has been shown to propagate to neighboring cells [109]. The intercellular propagation of ferroptosis has been proposed to occur through the release of oxidized lipids via exocytic vesicles [107,109]. It has been recognized that ferroptosis plays a significant role in the pathogenesis and progression of numerous cardiovascular diseases including ischemia, cardiac I/R injuries, arrhythmias, sepsis-induced cardiomyopathy, chemotherapy-related cardiotoxicity, iron overload cardiomyopathy, diabetic cardiomyopathy, cardiac hypertrophy, and heart failure [110,111,112]. Among these diseases, mitochondria-dependent ferroptosis has garnered increasing attention due to its pivotal role in subcellular metabolic, redox, and ion homeostasis [113,114]. Alterations in mitochondrial morphology and function commonly predispose cardiomyocytes to ferroptosis [113,114]. Mitochondrial fragmentation, Ca2+Mito overload, mtROS overproduction, excessive mitochondrial lipid peroxidation, and disruption of the mitochondrial membrane potential (ΔΨm) have been associated with cardiac ferroptosis and impaired contraction [107,109,110,111,112,113].
Specifically, previous studies have suggested that the sustained elevation of [Ca2+]i is linked to the incidence of ferroptosis [115,116,117,118]. Mendoza et al. reported that subtoxic levels of ROS and Ca2+ synergistically sensitize Ca2+-dependent mPTP opening, resulting in mitochondrial swelling and dysfunction [119]. The dual inhibition of mPTP using cyclosporine A and lipid peroxidation using the selective ferroptosis inhibitor ferrostatin-1 and MitoQ provided protection against I/R injury-mediated mPTP-dependent cell death [119] suggesting the involvement of ferroptosis. Likewise, ferroptosis can also be initiated by intracellular stressors that induce ER stress and Ca2+Mito overload mediated by IP3Rs [120]. Mitochondria–ER contact sites (MERCS) are specialized subdomains of the ER/SR that physically reside in close apposition to mitochondria [121]. This specific surface enables the local conveyance of Ca2+ between IP3Rs on the SR/ER membrane and MCU on the mitochondrial membrane, thereby facilitating the Ca2+Mito sequestration [2,114]. Mechanistically, the activation of IP3Rs causes a significant rise in [Ca2+]Mito. However, the overwhelming activation of IP3Rs can promote electron transport chain (ETC) dysfunction and enhance mtROS production [120]. Consistent with this thought, Pedrera et al. demonstrated a sustained increase in [Ca2+]i in NIH-3T3 cells (an embryonic mouse fibroblast cell line) upon treatment with ferroptosis inducers Erastin-1 and RAS-selective lethal 3 (RSL3) [118]. In addition, the occurrence of Ca2+i overload and ferroptosis-related phenotypes can be effectively inhibited by the ferroptosis inhibitor Ferrostatin-1 [118]. Other studies have also reported that the rise of [Ca2+]i and [Ca2+]Mito occurs before the complete lysis of the plasma membrane during ferroptosis [116,122]. Furthermore, IP3R-mediated Ca2+ release has also been reported to be associated with ferroptosis. In a very recent study by Campos and colleagues, ferroptosis was induced in SH-SY5Y neuroblastoma cells using RSL3, known as an inhibitor of the anti-ferroptosis protein GPX4 [16]. Under RSL3 treatment, they found an increase in ferroptosis, elevated lipid peroxidation products, and a time-dependent overload of Ca2+i [16]. Treatment with IP3R antagonists either Xestospongin B or carbachol improved cell viability, while carbachol treatment reduced the expression levels of IP3R1 under RSL3-induced ferroptosis conditions [16]. Additionally, the knockdown of IP3R1 alleviated Ca2+i overload and reduced the occurrence of the ferroptosis phenotype in RSL3-treated SH-SY5Y cells [16]. Additionally, Zhou et al. also reported that the administration of the IP3R antagonist 2-APB strongly suppressed RSL3-induced ferroptosis in primary rat articular chondrocytes and C28/I2 cells (human chondrocyte cells) [123]. In line with these findings, the anti-ferroptosis effect of IP3R antagonists was also observed by Hirata et al. who reported that 2-APB inhibited RSL3-induced ferroptosis in RAW 264.7 cells and HeLa cells [124]. Taken together, the sustained elevation of [Ca2+]i reported in association with IP3Rs activation can promote ferroptosis in several types of non-cardiac cells. Furthermore, IP3R antagonists such as Xestospongin B, carbachol, and 2-APB have been shown to improve cell viability, reduce IP3R1 expression, and alleviate Ca2+i overload. This indicates the potential role of IP3Rs in regulating ferroptosis across various cell types. Further studies are indeed warranted to explore the role of IP3Rs and Ca2+ in ferroptosis specifically within cardiomyocytes. This research could provide valuable insights into the mechanisms underlying ferroptosis in cardiovascular diseases. A summary of the contribution of IP3Rs in different types of programmed cell death is shown in Table 1.

Table 1.
A summary of the contribution of IP3Rs in different types of programmed cell death (PCDs).
6. Conclusions
The intricate involvement of IP3Rs in diverse cardiac pathologies and PCD pathways has been progressively explored. The elevation of IP3Rs along with the dysregulation of related proteins have been reported in several cardiac pathological conditions including ischemia, I/R injuries, arrhythmias, sepsis-induced cardiomyopathy, cardiac hypertrophy, and heart failure. IP3R1 plays a pivotal role in cardiac ischemia and diabetes-induced arrhythmias. On the other hand, IP3R2 demonstrates significance in sepsis-induced cardiomyopathy and cardiac hypertrophy. Findings from previous studies also suggest that, upon exposure to various stressors or pathological conditions, there is an initial upregulation of IP3Rs which exacerbates Ca2+ overload and subsequently activates PCDs. The modulation of IP3Rs and their associated proteins, either through genetic interventions or pharmacological approaches, holds therapeutic promise for mitigating Ca2+ handling abnormalities and improving cardiac cell survival. Furthermore, IP3Rs have been implicated in diverse PCD pathways, including apoptosis, pyroptosis, and ferroptosis emphasizing their multifaceted roles in cardiac pathophysiology. However, there is still a lack of studies investigating the distinct role of IP3Rs in other types of PCDs, such as necroptosis and autophagy-dependent cell death. In addition, the potential effects of genetic ablation and pharmacological intervention using IP3R antagonists pose promising therapeutic potency against IP3R-related pathologies. Understanding the roles of IP3Rs provides a foundation for addressing IP3R-related pathologies particularly in relation to the initiation of PCD in cardiovascular diseases. Further studies are warranted to gain a comprehensive understanding of the involvement of IP3Rs in cardiac pathologies and to unveil their potential implications for future therapeutic interventions.
Author Contributions
L.-H.X. conceptualized the manuscript. C.P. and L.-H.X. collected the literature and prepared the initial draft. N.F., S.H.P., J.K.G., S.C.C., N.C. and L.-H.X. oversaw and critically revised the article. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Institutes of Health (R01s HL157116 to L.-H.X. and J.K.G. and HL133294 to L.-H.X.), the American Heart Association (19TPA34900003 to L.H.X. and J.K.G.), the NSTDA Research Chair grant from the National Science and Technology Development Agency Thailand (N42A670594 to N.C.), the Distinguished Research Professor Grant from the National Research Council of Thailand (No. N42A660301 to S.C.C.), the Chiang Mai University Center of Excellence Award (to N.C.), and the NRCT-Royal Golden Jubilee Program (No. N41A640203 to C.P. and N.C.).
Conflicts of Interest
The authors declared they have no conflicts of interests.
List of Abbreviation
AF, atrial fibrillation; ASC, apoptosis-associated speck-like protein containing a CARD; Bax, BCL2 Associated X; Bcl-2, B-cell leukemia/lymphoma 2; CaMKII, Ca2+/calmodulin-dependent protein kinase II; CICR, Ca2+-induced Ca2+ release; CMs, cardiomyocytes; cTnC, cardiac troponin C; ECC, excitation–contraction coupling; ER, endoplasmic reticulum; ERP44, endoplasmic reticulum resident protein 44; GPX4, glutathione peroxidase 4; GRP75, glucose-regulated protein 75; GSDMD-NT, gasdermin-D N-terminus; HG, high glucose; H/R, hypoxia-reoxygenation; IL-1β, interleukin 1β; IL-6, interleukin 6; IP3, inositol 1,4,5-trisphosphate; IP3R1, inositol 1,4,5-trisphosphate receptor type 1; IP3R2, inositol 1,4,5-trisphosphate receptor type 2; IP3R3, inositol 1,4,5-trisphosphate receptor type 3; I/R, ischemia–reperfusion; LPS, lipopolysaccharide; LTCC, L-type voltage-gated calcium channels; MCU, mitochondrial uniporter; Mfrn, mitoferrin 2; mPTP, mitochondrial permeability transition pore; NCX, sodium–calcium exchanger; NRAMs, neonatal rat atrial myocytes; NRCMs, neonatal rat cardiomyocytes; N/A, not applicable; PCD, programmed cell death; PLN, phospholamban; RyR2, ryanodine receptor type-2; SERCA, sarco/endoplasmic reticulum calcium ATPase; SR, sarcoplasmic reticulum; STZ, streptozotocin; TLRs, toll-like receptors; TM, tunicamycin; TNFR1, tumor necrosis factor receptor 1; TNF-α, tumor necrosis factor-α; TUNEL, terminal deoxynucleotidyl transferase dUTP nick end labeling; T2DM, type 2 diabetes mellitus; VDAC, voltage-dependent anion channel; [Ca2+], Ca2+ concentration; [Ca2+]i, intercellular/cytoplasmic Ca2+ (Ca2+i) concentration; [Ca2+]Mito, intramitochondrial Ca2+ concentration; mitochondrial membrane potential (ΔΨm).
References
- Gambardella, J.; Morelli, M.B.; Wang, X.; Castellanos, V.; Mone, P.; Santulli, G. The discovery and development of IP3 receptor modulators: An update. Expert Opin. Drug Discov. 2021, 16, 709–718. [Google Scholar] [CrossRef] [PubMed]
- Bartok, A.; Weaver, D.; Golenar, T.; Nichtova, Z.; Katona, M.; Bansaghi, S.; Alzayady, K.J.; Thomas, V.K.; Ando, H.; Mikoshiba, K.; et al. IP(3) receptor isoforms differently regulate ER-mitochondrial contacts and local calcium transfer. Nat. Commun. 2019, 10, 3726. [Google Scholar] [CrossRef] [PubMed]
- Atakpa-Adaji, P.; Thillaiappan, N.B.; Taylor, C.W. IP3 receptors and their intimate liaisons. Curr. Opin. Physiol. 2020, 17, 9–16. [Google Scholar] [CrossRef]
- Ivanova, H.; Vervliet, T.; Missiaen, L.; Parys, J.B.; De Smedt, H.; Bultynck, G. Inositol 1,4,5-trisphosphate receptor-isoform diversity in cell death and survival. Biochim. Biophys Acta 2014, 1843, 2164–2183. [Google Scholar] [CrossRef]
- Baker, M.R.; Fan, G.; Serysheva, I.I. Structure of IP(3)R channel: High-resolution insights from cryo-EM. Curr. Opin. Struct. Biol. 2017, 46, 38–47. [Google Scholar] [CrossRef]
- Berridge, M.J. The Inositol Trisphosphate/Calcium Signaling Pathway in Health and Disease. Physiol. Rev. 2016, 96, 1261–1296. [Google Scholar] [CrossRef]
- Berridge, M.J. Inositol trisphosphate and calcium signalling. Nature 1993, 361, 315–325. [Google Scholar] [CrossRef]
- Garcia, M.I.; Boehning, D. Cardiac inositol 1,4,5-trisphosphate receptors. Biochim. Biophys Acta Mol. Cell Res. 2017, 1864, 907–914. [Google Scholar] [CrossRef]
- Gerasimenko, O.V.; Gerasimenko, J.V.; Belan, P.V.; Petersen, O.H. Inositol trisphosphate and cyclic ADP-ribose-mediated release of Ca2+ from single isolated pancreatic zymogen granules. Cell 1996, 84, 473–480. [Google Scholar] [CrossRef]
- Qi, X.Y.; Vahdati Hassani, F.; Hoffmann, D.; Xiao, J.; Xiong, F.; Villeneuve, L.R.; Ljubojevic-Holzer, S.; Kamler, M.; Abu-Taha, I.; Heijman, J.; et al. Inositol Trisphosphate Receptors and Nuclear Calcium in Atrial Fibrillation. Circ. Res. 2021, 128, 619–635. [Google Scholar] [CrossRef]
- Cardenas, C.; Liberona, J.L.; Molgo, J.; Colasante, C.; Mignery, G.A.; Jaimovich, E. Nuclear inositol 1,4,5-trisphosphate receptors regulate local Ca2+ transients and modulate cAMP response element binding protein phosphorylation. J. Cell Sci. 2005, 118, 3131–3140. [Google Scholar] [CrossRef] [PubMed]
- Harzheim, D.; Movassagh, M.; Foo, R.S.; Ritter, O.; Tashfeen, A.; Conway, S.J.; Bootman, M.D.; Roderick, H.L. Increased InsP3Rs in the junctional sarcoplasmic reticulum augment Ca2+ transients and arrhythmias associated with cardiac hypertrophy. Proc. Natl. Acad. Sci. USA 2009, 106, 11406–11411. [Google Scholar] [CrossRef]
- Mo, G.; Liu, X.; Zhong, Y.; Mo, J.; Li, Z.; Li, D.; Zhang, L.; Liu, Y. IP3R1 regulates Ca2+ transport and pyroptosis through the NLRP3/Caspase-1 pathway in myocardial ischemia/reperfusion injury. Cell Death Discov. 2021, 7, 31. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.R.; Yang, H.; Zhang, H.D.; Cai, Y.J.; Zheng, Y.X.; Fang, H.; Wang, Z.F.; Kuang, S.J.; Rao, F.; Huang, H.L.; et al. IP3R2-mediated Ca2+ release promotes LPS-induced cardiomyocyte pyroptosis via the activation of NLRP3/Caspase-1/GSDMD pathway. Cell Death Discov. 2024, 10, 91. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.; Gong, M.; Zhang, Z.; Meng, L.; Tse, G.; Zhao, Y.; Bao, Q.; Zhang, Y.; Yuan, M.; Liu, X.; et al. Hyperglycemia Induces Endoplasmic Reticulum Stress in Atrial Cardiomyocytes, and Mitofusin-2 Downregulation Prevents Mitochondrial Dysfunction and Subsequent Cell Death. Oxidative Med. Cell Longev. 2020, 2020, 6569728. [Google Scholar] [CrossRef]
- Campos, J.; Gleitze, S.; Hidalgo, C.; Núñez, M.T. IP3R-Mediated Calcium Release Promotes Ferroptotic Death in SH-SY5Y Neuroblastoma Cells. Antioxidants 2024, 13, 196. [Google Scholar] [CrossRef]
- Eisner, D.A.; Caldwell, J.L.; Kistamas, K.; Trafford, A.W. Calcium and Excitation-Contraction Coupling in the Heart. Circ. Res. 2017, 121, 181–195. [Google Scholar] [CrossRef]
- Siri-Angkul, N.; Dadfar, B.; Jaleel, R.; Naushad, J.; Parambathazhath, J.; Doye, A.A.; Xie, L.H.; Gwathmey, J.K. Calcium and Heart Failure: How Did We Get Here and Where Are We Going? Int. J. Mol. Sci. 2021, 22, 7392. [Google Scholar] [CrossRef] [PubMed]
- Eisner, D. Calcium in the heart: From physiology to disease. Exp. Physiol. 2014, 99, 1273–1282. [Google Scholar] [CrossRef]
- Lin, Y.C.; Huang, J.; Zhang, Q.; Hollander, J.M.; Frisbee, J.C.; Martin, K.H.; Nestor, C.; Goodman, R.; Yu, H.G. Inactivation of L-type calcium channel modulated by HCN2 channel. Am. J. Physiol. Cell Physiol. 2010, 298, C1029–C1037. [Google Scholar] [CrossRef]
- Li, X.; Zima, A.V.; Sheikh, F.; Blatter, L.A.; Chen, J. Endothelin-1-induced arrhythmogenic Ca2+ signaling is abolished in atrial myocytes of inositol-1,4,5-trisphosphate(IP3)-receptor type 2-deficient mice. Circ. Res. 2005, 96, 1274–1281. [Google Scholar] [CrossRef] [PubMed]
- Demydenko, K.; Ekhteraei-Tousi, S.; Roderick, H.L. Inositol 1,4,5-trisphosphate receptors in cardiomyocyte physiology and disease. Philos. Trans. R. Soc. B 2022, 377, 20210319. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, H.; Bodi, I.; Maillet, M.; DeSantiago, J.; Domeier, T.L.; Mikoshiba, K.; Lorenz, J.N.; Blatter, L.A.; Bers, D.M.; Molkentin, J.D. The IP3 receptor regulates cardiac hypertrophy in response to select stimuli. Circ. Res. 2010, 107, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Viskupicova, J.; Rezbarikova, P. Natural Polyphenols as SERCA Activators: Role in the Endoplasmic Reticulum Stress-Related Diseases. Molecules 2022, 27, 5095. [Google Scholar] [CrossRef]
- Frank, K.; Kranias, E.G. Phospholamban and cardiac contractility. Ann. Med. 2000, 32, 572–578. [Google Scholar] [CrossRef]
- Ottolia, M.; Torres, N.; Bridge, J.H.; Philipson, K.D.; Goldhaber, J.I. Na/Ca exchange and contraction of the heart. J. Mol. Cell Cardiol. 2013, 61, 28–33. [Google Scholar] [CrossRef]
- Pott, C.; Philipson, K.D.; Goldhaber, J.I. Excitation-contraction coupling in Na+-Ca2+ exchanger knockout mice: Reduced transsarcolemmal Ca2+ flux. Circ. Res. 2005, 97, 1288–1295. [Google Scholar] [CrossRef]
- Bers, D.M. Calcium cycling and signaling in cardiac myocytes. Annu. Rev. Physiol. 2008, 70, 23–49. [Google Scholar] [CrossRef]
- Gorski, P.A.; Ceholski, D.K.; Hajjar, R.J. Altered myocardial calcium cycling and energetics in heart failure--a rational approach for disease treatment. Cell Metab. 2015, 21, 183–194. [Google Scholar] [CrossRef]
- Del Monte, F.; Johnson, C.M.; Stepanek, A.C.; Doye, A.A.; Gwathmey, J.K. Defects in calcium control. J. Card. Fail 2002, 8, S421–S431. [Google Scholar] [CrossRef]
- Luo, M.; Anderson, M.E. Mechanisms of altered Ca2+ handling in heart failure. Circ. Res. 2013, 113, 690–708. [Google Scholar] [CrossRef] [PubMed]
- Piacentino, V., III; Weber, C.R.; Chen, X.; Weisser-Thomas, J.; Margulies, K.B.; Bers, D.M.; Houser, S.R. Cellular basis of abnormal calcium transients of failing human ventricular myocytes. Circ. Res. 2003, 92, 651–658. [Google Scholar] [CrossRef]
- Schroder, F.; Handrock, R.; Beuckelmann, D.J.; Hirt, S.; Hullin, R.; Priebe, L.; Schwinger, R.H.; Weil, J.; Herzig, S. Increased availability and open probability of single L-type calcium channels from failing compared with nonfailing human ventricle. Circulation 1998, 98, 969–976. [Google Scholar] [CrossRef]
- Sanchez-Alonso, J.L.; Fedele, L.; Copier, J.S.; Lucarelli, C.; Mansfield, C.; Judina, A.; Houser, S.R.; Brand, T.; Gorelik, J. Functional LTCC-beta(2)AR Complex Needs Caveolin-3 and Is Disrupted in Heart Failure. Circ. Res. 2023, 133, 120–137. [Google Scholar] [CrossRef]
- Despa, S.; Islam, M.A.; Weber, C.R.; Pogwizd, S.M.; Bers, D.M. Intracellular Na+ concentration is elevated in heart failure but Na/K pump function is unchanged. Circulation 2002, 105, 2543–2548. [Google Scholar] [CrossRef]
- Flesch, M.; Schwinger, R.H.; Schiffer, F.; Frank, K.; Sudkamp, M.; Kuhn-Regnier, F.; Arnold, G.; Bohm, M. Evidence for functional relevance of an enhanced expression of the Na+-Ca2+ exchanger in failing human myocardium. Circulation 1996, 94, 992–1002. [Google Scholar] [CrossRef] [PubMed]
- Studer, R.; Reinecke, H.; Bilger, J.; Eschenhagen, T.; Bohm, M.; Hasenfuss, G.; Just, H.; Holtz, J.; Drexler, H. Gene expression of the cardiac Na+-Ca2+ exchanger in end-stage human heart failure. Circ. Res. 1994, 75, 443–453. [Google Scholar] [CrossRef] [PubMed]
- Gwathmey, J.K.; Slawsky, M.T.; Briggs, G.M.; Morgan, J.P. Role of intracellular sodium in the regulation of intracellular calcium and contractility. Effects of DPI 201-106 on excitation-contraction coupling in human ventricular myocardium. J. Clin. Investig. 1988, 82, 1592–1605. [Google Scholar] [CrossRef]
- Bonilla, I.M.; Belevych, A.E.; Baine, S.; Stepanov, A.; Mezache, L.; Bodnar, T.; Liu, B.; Volpe, P.; Priori, S.; Weisleder, N.; et al. Enhancement of Cardiac Store Operated Calcium Entry (SOCE) within Novel Intercalated Disk Microdomains in Arrhythmic Disease. Sci. Rep. 2019, 9, 10179. [Google Scholar] [CrossRef]
- Rosenberg, P.; Katz, D.; Bryson, V. SOCE and STIM1 signaling in the heart: Timing and location matter. Cell Calcium 2019, 77, 20–28. [Google Scholar] [CrossRef]
- Wen, H.; Gwathmey, J.K.; Xie, L.H. Role of Transient Receptor Potential Canonical Channels in Heart Physiology and Pathophysiology. Front. Cardiovasc. Med. 2020, 7, 24. [Google Scholar] [CrossRef] [PubMed]
- Hasenfuss, G.; Reinecke, H.; Studer, R.; Meyer, M.; Pieske, B.; Holtz, J.; Holubarsch, C.; Posival, H.; Just, H.; Drexler, H. Relation between myocardial function and expression of sarcoplasmic reticulum Ca2+-ATPase in failing and nonfailing human myocardium. Circ. Res. 1994, 75, 434–442. [Google Scholar] [CrossRef]
- Schwinger, R.H.; Munch, G.; Bolck, B.; Karczewski, P.; Krause, E.G.; Erdmann, E. Reduced Ca2+-sensitivity of SERCA 2a in failing human myocardium due to reduced serin-16 phospholamban phosphorylation. J. Mol. Cell Cardiol. 1999, 31, 479–491. [Google Scholar] [CrossRef] [PubMed]
- Sainte Beuve, C.; Allen, P.D.; Dambrin, G.; Rannou, F.; Marty, I.; Trouve, P.; Bors, V.; Pavie, A.; Gandgjbakch, I.; Charlemagne, D. Cardiac calcium release channel (ryanodine receptor) in control and cardiomyopathic human hearts: mRNA and protein contents are differentially regulated. J. Mol. Cell Cardiol. 1997, 29, 1237–1246. [Google Scholar] [CrossRef]
- Zhang, H.; Makarewich, C.A.; Kubo, H.; Wang, W.; Duran, J.M.; Li, Y.; Berretta, R.M.; Koch, W.J.; Chen, X.; Gao, E.; et al. Hyperphosphorylation of the cardiac ryanodine receptor at serine 2808 is not involved in cardiac dysfunction after myocardial infarction. Circ. Res. 2012, 110, 831–840. [Google Scholar] [CrossRef] [PubMed]
- Eschenhagen, T. Is ryanodine receptor phosphorylation key to the fight or flight response and heart failure? J. Clin. Investig. 2010, 120, 4197–4203. [Google Scholar] [CrossRef]
- Dobrev, D.; Wehrens, X.H. Role of RyR2 phosphorylation in heart failure and arrhythmias: Controversies around ryanodine receptor phosphorylation in cardiac disease. Circ. Res. 2014, 114, 1311–1319, discussion 1319. [Google Scholar] [CrossRef]
- Dridi, H.; Kushnir, A.; Zalk, R.; Yuan, Q.; Melville, Z.; Marks, A.R. Intracellular calcium leak in heart failure and atrial fibrillation: A unifying mechanism and therapeutic target. Nat. Rev. Cardiol. 2020, 17, 732–747. [Google Scholar] [CrossRef]
- Wu, X.; Bers, D.M. Sarcoplasmic reticulum and nuclear envelope are one highly interconnected Ca2+ store throughout cardiac myocyte. Circ. Res. 2006, 99, 283–291. [Google Scholar] [CrossRef]
- Woll, K.A.; Van Petegem, F. Calcium-release channels: Structure and function of IP(3) receptors and ryanodine receptors. Physiol. Rev. 2022, 102, 209–268. [Google Scholar] [CrossRef]
- Chan, C.; Ooashi, N.; Akiyama, H.; Fukuda, T.; Inoue, M.; Matsu-Ura, T.; Shimogori, T.; Mikoshiba, K.; Kamiguchi, H. Inositol 1,4,5-Trisphosphate Receptor Type 3 Regulates Neuronal Growth Cone Sensitivity to Guidance Signals. Science 2020, 23, 100963. [Google Scholar] [CrossRef] [PubMed]
- Smith, H.A.; Thillaiappan, N.B.; Rossi, A.M. IP(3) receptors: An “elementary” journey from structure to signals. Cell Calcium 2023, 113, 102761. [Google Scholar] [CrossRef] [PubMed]
- Gorza, L.; Schiaffino, S.; Volpe, P. Inositol 1,4,5-trisphosphate receptor in heart: Evidence for its concentration in Purkinje myocytes of the conduction system. J. Cell Biol. 1993, 121, 345–353. [Google Scholar] [CrossRef]
- Hund, T.J.; Mohler, P.J. Role of CaMKII in cardiac arrhythmias. Trends Cardiovasc. Med. 2015, 25, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Mackenzie, L.; Bootman, M.D.; Laine, M.; Berridge, M.J.; Thuring, J.; Holmes, A.; Li, W.H.; Lipp, P. The role of inositol 1,4,5-trisphosphate receptors in Ca2+ signalling and the generation of arrhythmias in rat atrial myocytes. J. Physiol. 2002, 541, 395–409. [Google Scholar] [CrossRef]
- Mohler, P.J.; Davis, J.Q.; Bennett, V. Ankyrin-B coordinates the Na/K ATPase, Na/Ca exchanger, and InsP3 receptor in a cardiac T-tubule/SR microdomain. PLoS Biol. 2005, 3, e423. [Google Scholar] [CrossRef]
- Sankar, N.; deTombe, P.P.; Mignery, G.A. Calcineurin-NFATc regulates type 2 inositol 1,4,5-trisphosphate receptor (InsP3R2) expression during cardiac remodeling. J. Biol. Chem. 2014, 289, 6188–6198. [Google Scholar] [CrossRef]
- Atakpa-Adaji, P.; Ivanova, A. IP(3)R at ER-Mitochondrial Contact Sites: Beyond the IP(3)R-GRP75-VDAC1 Ca2+ Funnel. Contact 2023, 6, 25152564231181020. [Google Scholar] [CrossRef]
- Xu, H.; Guan, N.; Ren, Y.L.; Wei, Q.J.; Tao, Y.H.; Yang, G.S.; Liu, X.Y.; Bu, D.F.; Zhang, Y.; Zhu, S.N. IP(3)R-Grp75-VDAC1-MCU calcium regulation axis antagonists protect podocytes from apoptosis and decrease proteinuria in an Adriamycin nephropathy rat model. BMC Nephrol. 2018, 19, 140. [Google Scholar] [CrossRef]
- Yuan, M.; Gong, M.; He, J.; Xie, B.; Zhang, Z.; Meng, L.; Tse, G.; Zhao, Y.; Bao, Q.; Zhang, Y.; et al. IP3R1/GRP75/VDAC1 complex mediates endoplasmic reticulum stress-mitochondrial oxidative stress in diabetic atrial remodeling. Redox Biol. 2022, 52, 102289. [Google Scholar] [CrossRef]
- Lipp, P.; Laine, M.; Tovey, S.C.; Burrell, K.M.; Berridge, M.J.; Li, W.; Bootman, M.D. Functional InsP3 receptors that may modulate excitation-contraction coupling in the heart. Curr. Biol. 2000, 10, 939–942. [Google Scholar] [CrossRef] [PubMed]
- Garcia, M.I.; Karlstaedt, A.; Chen, J.J.; Amione-Guerra, J.; Youker, K.A.; Taegtmeyer, H.; Boehning, D. Functionally redundant control of cardiac hypertrophic signaling by inositol 1,4,5-trisphosphate receptors. J. Mol. Cell Cardiol. 2017, 112, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Perez, P.J.; Ramos-Franco, J.; Fill, M.; Mignery, G.A. Identification and functional reconstitution of the type 2 inositol 1,4,5-trisphosphate receptor from ventricular cardiac myocytes. J. Biol. Chem. 1997, 272, 23961–23969. [Google Scholar] [CrossRef]
- Nakazawa, M.; Uchida, K.; Aramaki, M.; Kodo, K.; Yamagishi, C.; Takahashi, T.; Mikoshiba, K.; Yamagishi, H. Inositol 1,4,5-trisphosphate receptors are essential for the development of the second heart field. J. Mol. Cell Cardiol. 2011, 51, 58–66. [Google Scholar] [CrossRef]
- Wei, W.; Huang, W.; Yue, J. Requirement of IP3 receptor 3 (IP3R3) in nitric oxide induced cardiomyocyte differentiation of mouse embryonic stem cells. Exp. Cell Res. 2016, 346, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Taylor, C.W.; Tovey, S.C. IP(3) receptors: Toward understanding their activation. Cold Spring Harb. Perspect. Biol. 2010, 2, a004010. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, J.; Tong, X. Cross-Talk between Mechanosensitive Ion Channels and Calcium Regulatory Proteins in Cardiovascular Health and Disease. Int. J. Mol. Sci. 2021, 22, 8782. [Google Scholar] [CrossRef]
- Hohendanner, F.; Walther, S.; Maxwell, J.T.; Kettlewell, S.; Awad, S.; Smith, G.L.; Lonchyna, V.A.; Blatter, L.A. Inositol-1,4,5-trisphosphate induced Ca2+ release and excitation-contraction coupling in atrial myocytes from normal and failing hearts. J. Physiol. 2015, 593, 1459–1477. [Google Scholar] [CrossRef]
- Gwathmey, J.K.; Copelas, L.; MacKinnon, R.; Schoen, F.J.; Feldman, M.D.; Grossman, W.; Morgan, J.P. Abnormal intracellular calcium handling in myocardium from patients with end-stage heart failure. Circ. Res. 1987, 61, 70–76. [Google Scholar] [CrossRef]
- Rosemblit, N.; Moschella, M.C.; Ondriasova, E.; Gutstein, D.E.; Ondrias, K.; Marks, A.R. Intracellular calcium release channel expression during embryogenesis. Dev. Biol. 1999, 206, 163–177. [Google Scholar] [CrossRef][Green Version]
- Blanch, I.S.J.; Egger, M. Obstruction of ventricular Ca2+-dependent arrhythmogenicity by inositol 1,4,5-trisphosphate-triggered sarcoplasmic reticulum Ca2+ release. J. Physiol. 2018, 596, 4323–4340. [Google Scholar] [CrossRef] [PubMed]
- Gomez, L.; Thiebaut, P.A.; Paillard, M.; Ducreux, S.; Abrial, M.; Crola Da Silva, C.; Durand, A.; Alam, M.R.; Van Coppenolle, F.; Sheu, S.S.; et al. The SR/ER-mitochondria calcium crosstalk is regulated by GSK3beta during reperfusion injury. Cell Death Differ. 2016, 23, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Santulli, G.; Xie, W.; Reiken, S.R.; Marks, A.R. Mitochondrial calcium overload is a key determinant in heart failure. Proc. Natl. Acad. Sci. USA 2015, 112, 11389–11394. [Google Scholar] [CrossRef]
- Seidlmayer, L.K.; Kuhn, J.; Berbner, A.; Arias-Loza, P.A.; Williams, T.; Kaspar, M.; Czolbe, M.; Kwong, J.Q.; Molkentin, J.D.; Heinze, K.G.; et al. Inositol 1,4,5-trisphosphate-mediated sarcoplasmic reticulum-mitochondrial crosstalk influences adenosine triphosphate production via mitochondrial Ca2+ uptake through the mitochondrial ryanodine receptor in cardiac myocytes. Cardiovasc. Res. 2016, 112, 491–501. [Google Scholar] [CrossRef]
- Hove-Madsen, L.; Llach, A.; Bayes-Genis, A.; Roura, S.; Rodriguez Font, E.; Aris, A.; Cinca, J. Atrial fibrillation is associated with increased spontaneous calcium release from the sarcoplasmic reticulum in human atrial myocytes. Circulation 2004, 110, 1358–1363. [Google Scholar] [CrossRef]
- Drawnel, F.M.; Wachten, D.; Molkentin, J.D.; Maillet, M.; Aronsen, J.M.; Swift, F.; Sjaastad, I.; Liu, N.; Catalucci, D.; Mikoshiba, K.; et al. Mutual antagonism between IP(3)RII and miRNA-133a regulates calcium signals and cardiac hypertrophy. J. Cell Biol. 2012, 199, 783–798. [Google Scholar] [CrossRef] [PubMed]
- Whelan, R.S.; Kaplinskiy, V.; Kitsis, R.N. Cell death in the pathogenesis of heart disease: Mechanisms and significance. Annu. Rev. Physiol. 2010, 72, 19–44. [Google Scholar] [CrossRef]
- Konstantinidis, K.; Whelan, R.S.; Kitsis, R.N. Mechanisms of cell death in heart disease. Arter. Thromb. Vasc. Biol. 2012, 32, 1552–1562. [Google Scholar] [CrossRef]
- Bennett, M.R. Apoptosis in the cardiovascular system. Heart 2002, 87, 480–487. [Google Scholar] [CrossRef]
- Del Re, D.P.; Amgalan, D.; Linkermann, A.; Liu, Q.; Kitsis, R.N. Fundamental Mechanisms of Regulated Cell Death and Implications for Heart Disease. Physiol. Rev. 2019, 99, 1765–1817. [Google Scholar] [CrossRef]
- Chiong, M.; Wang, Z.V.; Pedrozo, Z.; Cao, D.J.; Troncoso, R.; Ibacache, M.; Criollo, A.; Nemchenko, A.; Hill, J.A.; Lavandero, S. Cardiomyocyte death: Mechanisms and translational implications. Cell Death Dis. 2011, 2, e244. [Google Scholar] [CrossRef]
- Williams, R.S. Apoptosis and heart failure. N. Engl. J. Med. 1999, 341, 759–760. [Google Scholar] [CrossRef]
- Kang, P.M.; Izumo, S. Apoptosis and heart failure: A critical review of the literature. Circ. Res. 2000, 86, 1107–1113. [Google Scholar] [CrossRef] [PubMed]
- Condorelli, G.; Roncarati, R.; Ross, J., Jr.; Pisani, A.; Stassi, G.; Todaro, M.; Trocha, S.; Drusco, A.; Gu, Y.; Russo, M.A.; et al. Heart-targeted overexpression of caspase3 in mice increases infarct size and depresses cardiac function. Proc. Natl. Acad. Sci. USA 2001, 98, 9977–9982. [Google Scholar] [CrossRef]
- Lee, Y.; Gustafsson, A.B. Role of apoptosis in cardiovascular disease. Apoptosis 2009, 14, 536–548. [Google Scholar] [CrossRef] [PubMed]
- Danese, A.; Leo, S.; Rimessi, A.; Wieckowski, M.R.; Fiorica, F.; Giorgi, C.; Pinton, P. Cell death as a result of calcium signaling modulation: A cancer-centric prospective. Biochim. Biophys. Acta Mol. Cell Res. 2021, 1868, 119061. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, L.; Vitale, I.; Aaronson, S.A.; Abrams, J.M.; Adam, D.; Agostinis, P.; Alnemri, E.S.; Altucci, L.; Amelio, I.; Andrews, D.W.; et al. Molecular mechanisms of cell death: Recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018, 25, 486–541. [Google Scholar] [CrossRef]
- Giorgi, C.; Baldassari, F.; Bononi, A.; Bonora, M.; De Marchi, E.; Marchi, S.; Missiroli, S.; Patergnani, S.; Rimessi, A.; Suski, J.M.; et al. Mitochondrial Ca2+ and apoptosis. Cell Calcium 2012, 52, 36–43. [Google Scholar] [CrossRef]
- Matuz-Mares, D.; Gonzalez-Andrade, M.; Araiza-Villanueva, M.G.; Vilchis-Landeros, M.M.; Vazquez-Meza, H. Mitochondrial Calcium: Effects of Its Imbalance in Disease. Antioxidants 2022, 11, 801. [Google Scholar] [CrossRef]
- Cookson, B.T.; Brennan, M.A. Pro-inflammatory programmed cell death. Trends Microbiol. 2001, 9, 113–114. [Google Scholar] [CrossRef]
- Bergsbaken, T.; Fink, S.L.; Cookson, B.T. Pyroptosis: Host cell death and inflammation. Nat. Rev. Microbiol. 2009, 7, 99–109. [Google Scholar] [CrossRef]
- Liu, Y.; Li, X.; Sun, T.; Li, T.; Li, Q. Pyroptosis in myocardial ischemia/reperfusion and its therapeutic implications. Eur. J. Pharmacol. 2024, 971, 176464. [Google Scholar] [CrossRef] [PubMed]
- Piamsiri, C.; Maneechote, C.; Jinawong, K.; Arunsak, B.; Chunchai, T.; Nawara, W.; Chattipakorn, S.C.; Chattipakorn, N. GSDMD-mediated pyroptosis dominantly promotes left ventricular remodeling and dysfunction in post-myocardial infarction: A comparison across modes of programmed cell death and mitochondrial involvement. J. Transl. Med. 2023, 21, 16. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, X.; Liu, S.; Brickell, A.N.; Zhang, J.; Wu, Z.; Zhou, S.; Ding, Z. PCSK9 regulates pyroptosis via mtDNA damage in chronic myocardial ischemia. Basic Res. Cardiol. 2020, 115, 66. [Google Scholar] [CrossRef] [PubMed]
- Yanpiset, P.; Maneechote, C.; Sriwichaiin, S.; Siri-Angkul, N.; Chattipakorn, S.C.; Chattipakorn, N. Gasdermin D-mediated pyroptosis in myocardial ischemia and reperfusion injury: Cumulative evidence for future cardioprotective strategies. Acta Pharm. Sin. B 2023, 13, 29–53. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.; Zhang, X.; Liu, N.; Tang, L.; Peng, C.; Chen, X. Pyroptosis: Mechanisms and diseases. Signal Transduct. Target. Ther. 2021, 6, 128. [Google Scholar] [CrossRef]
- Burdette, B.E.; Esparza, A.N.; Zhu, H.; Wang, S. Gasdermin D in pyroptosis. Acta Pharm. Sin. B 2021, 11, 2768–2782. [Google Scholar] [CrossRef]
- Rossol, M.; Pierer, M.; Raulien, N.; Quandt, D.; Meusch, U.; Rothe, K.; Schubert, K.; Schoneberg, T.; Schaefer, M.; Krugel, U.; et al. Extracellular Ca2+ is a danger signal activating the NLRP3 inflammasome through G protein-coupled calcium sensing receptors. Nat. Commun. 2012, 3, 1329. [Google Scholar] [CrossRef]
- He, Y.; Hara, H.; Nunez, G. Mechanism and Regulation of NLRP3 Inflammasome Activation. Trends Biochem. Sci. 2016, 41, 1012–1021. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, H.; Kouadir, M.; Song, H.; Shi, F. Recent advances in the mechanisms of NLRP3 inflammasome activation and its inhibitors. Cell Death Dis. 2019, 10, 128. [Google Scholar] [CrossRef]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.H.; Fefelova, N.; Pamarthi, S.H.; Gwathmey, J.K. Molecular Mechanisms of Ferroptosis and Relevance to Cardiovascular Disease. Cells 2022, 11, 2726. [Google Scholar] [CrossRef]
- Dang, Q.; Sun, Z.; Wang, Y.; Wang, L.; Liu, Z.; Han, X. Ferroptosis: A double-edged sword mediating immune tolerance of cancer. Cell Death Dis. 2022, 13, 925. [Google Scholar] [CrossRef]
- Jiang, X.; Stockwell, B.R.; Conrad, M. Ferroptosis: Mechanisms, biology and role in disease. Nat. Rev. Mol. Cell Biol. 2021, 22, 266–282. [Google Scholar] [CrossRef] [PubMed]
- Berndt, C.; Alborzinia, H.; Amen, V.S.; Ayton, S.; Barayeu, U.; Bartelt, A.; Bayir, H.; Bebber, C.M.; Birsoy, K.; Bottcher, J.P.; et al. Ferroptosis in health and disease. Redox Biol. 2024, 75, 103211. [Google Scholar] [CrossRef]
- Dixon, S.J.; Olzmann, J.A. The cell biology of ferroptosis. Nat. Rev. Mol. Cell Biol. 2024, 25, 424–442. [Google Scholar] [CrossRef]
- Stockwell, B.R. Ferroptosis turns 10: Emerging mechanisms, physiological functions, and therapeutic applications. Cell 2022, 185, 2401–2421. [Google Scholar] [CrossRef] [PubMed]
- Stockwell, B.R.; Friedmann Angeli, J.P.; Bayir, H.; Bush, A.I.; Conrad, M.; Dixon, S.J.; Fulda, S.; Gascon, S.; Hatzios, S.K.; Kagan, V.E.; et al. Ferroptosis: A Regulated Cell Death Nexus Linking Metabolism, Redox Biology, and Disease. Cell 2017, 171, 273–285. [Google Scholar] [CrossRef] [PubMed]
- Riegman, M.; Sagie, L.; Galed, C.; Levin, T.; Steinberg, N.; Dixon, S.J.; Wiesner, U.; Bradbury, M.S.; Niethammer, P.; Zaritsky, A.; et al. Ferroptosis occurs through an osmotic mechanism and propagates independently of cell rupture. Nat. Cell Biol. 2020, 22, 1042–1048. [Google Scholar] [CrossRef]
- Fefelova, N.; Wongjaikam, S.; Pamarthi, S.H.; Siri-Angkul, N.; Comollo, T.; Kumari, A.; Garg, V.; Ivessa, A.; Chattipakorn, S.C.; Chattipakorn, N.; et al. Deficiency of mitochondrial calcium uniporter abrogates iron overload-induced cardiac dysfunction by reducing ferroptosis. Basic Res. Cardiol. 2023, 118, 21. [Google Scholar] [CrossRef]
- Tang, D.; Chen, X.; Kang, R.; Kroemer, G. Ferroptosis: Molecular mechanisms and health implications. Cell Res. 2021, 31, 107–125. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Ardehali, H.; Min, J.; Wang, F. The molecular and metabolic landscape of iron and ferroptosis in cardiovascular disease. Nat. Rev. Cardiol. 2023, 20, 7–23. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Wang, H.; Han, D.; Xie, E.; Yang, X.; Wei, J.; Gu, S.; Gao, F.; Zhu, N.; Yin, X.; et al. Ferroptosis as a target for protection against cardiomyopathy. Proc. Natl. Acad. Sci. USA 2019, 116, 2672–2680. [Google Scholar] [CrossRef] [PubMed]
- Ahola, S.; Langer, T. Ferroptosis in mitochondrial cardiomyopathy. Trends Cell Biol. 2024, 34, 150–160. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Wu, Q.; Feng, J.; Yan, L.; Sun, Y.; Liu, S.; Xiang, Y.; Zhang, M.; Pan, T.; Chen, X.; et al. Erianin, a novel dibenzyl compound in Dendrobium extract, inhibits lung cancer cell growth and migration via calcium/calmodulin-dependent ferroptosis. Signal Transduct. Target. Ther. 2020, 5, 51. [Google Scholar] [CrossRef]
- Dhaouadi, N.; Vitto, V.A.M.; Pinton, P.; Galluzzi, L.; Marchi, S. Ca2+ signaling and cell death. Cell Calcium 2023, 113, 102759. [Google Scholar] [CrossRef]
- Maher, P.; van Leyen, K.; Dey, P.N.; Honrath, B.; Dolga, A.; Methner, A. The role of Ca2+ in cell death caused by oxidative glutamate toxicity and ferroptosis. Cell Calcium 2018, 70, 47–55. [Google Scholar] [CrossRef]
- Pedrera, L.; Espiritu, R.A.; Ros, U.; Weber, J.; Schmitt, A.; Stroh, J.; Hailfinger, S.; von Karstedt, S.; Garcia-Saez, A.J. Ferroptotic pores induce Ca2+ fluxes and ESCRT-III activation to modulate cell death kinetics. Cell Death Differ. 2021, 28, 1644–1657. [Google Scholar] [CrossRef]
- Mendoza, A.; Patel, P.; Robichaux, D.; Ramirez, D.; Karch, J. Inhibition of the mPTP and Lipid Peroxidation Is Additively Protective Against I/R Injury. Circ. Res. 2024, 134, 1292–1305. [Google Scholar] [CrossRef]
- Martens, M.D.; Karch, J.; Gordon, J.W. The molecular mosaic of regulated cell death in the cardiovascular system. Biochim. Biophys. Acta Mol. Basis Dis. 2022, 1868, 166297. [Google Scholar] [CrossRef]
- Rieusset, J. The role of endoplasmic reticulum-mitochondria contact sites in the control of glucose homeostasis: An update. Cell Death Dis. 2018, 9, 388. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Ogawa, M.; Kojima, K.; Takayanagi, S.; Ishihara, S.; Hattori, K.; Naguro, I.; Ichijo, H. The mitochondrial Ca2+ uptake regulator, MICU1, is involved in cold stress-induced ferroptosis. EMBO Rep. 2021, 22, e51532. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Chen, Y.; Li, S.; Wei, X.; Hu, W.; Tang, S.; Ding, J.; Fu, W.; Zhang, H.; Chen, F.; et al. TRPM7 channel inhibition attenuates rheumatoid arthritis articular chondrocyte ferroptosis by suppression of the PKCalpha-NOX4 axis. Redox Biol. 2022, 55, 102411. [Google Scholar] [CrossRef] [PubMed]
- Hirata, Y.; Cai, R.; Volchuk, A.; Steinberg, B.E.; Saito, Y.; Matsuzawa, A.; Grinstein, S.; Freeman, S.A. Lipid peroxidation increases membrane tension, Piezo1 gating, and cation permeability to execute ferroptosis. Curr. Biol. 2023, 33, 1282–1294.e5. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).