Abstract
Cannabis sativa is known for producing over 120 distinct phytocannabinoids, with Δ9-tetrahydrocannabinol (Δ9-THC) and cannabidiol (CBD) being the most prominent, primarily in their acidic forms. Beyond Δ9-THC and CBD, a wide array of lesser-known phytocannabinoids, along with terpenes, flavonoids, and alkaloids, demonstrate diverse pharmacological activities, interacting with the endocannabinoid system (eCB) and other biological pathways. These compounds, characterized by phenolic structures and hydroxyl groups, possess lipophilic properties, allowing them to cross the blood–brain barrier (BBB) effectively. Notably, their antioxidant, anti-inflammatory, and neuro-modulatory effects position them as promising agents in treating neurodegenerative disorders. While research has extensively examined the neuropsychiatric and neuroprotective effects of Δ9-THC, other minor phytocannabinoids remain underexplored. Due to the well-established neuroprotective potential of CBD, there is growing interest in the therapeutic benefits of non-psychotropic minor phytocannabinoids (NMPs) in brain disorders. This review highlights the emerging research on these lesser-known compounds and their neuroprotective potential. It offers insights into their therapeutic applications across various major neurological conditions.
1. Introduction
Neurodegenerative disorders (NDs) pose a significant global challenge, contributing extensively to disability and mortality [1,2,3]. The World Health Organization (WHO) projects that by 2040, NDs will be the second-leading cause of death worldwide [4]. These disorders involve the steady loss of neurons, resulting in impaired brain function, the breakdown of neuronal networks, and ultimately leading to disability. Despite substantial research efforts, current treatments primarily focus on managing symptoms, as neuronal degeneration often progresses silently for years before clinical signs such as cognitive, motor, or other neurological symptoms manifest. Cell death, synaptic dysfunction, and behavioral abnormalities underlie most neurodegenerative diseases, such as Alzheimer’s disease (AD), Parkinson’s disease (PD), and Huntington’s disease (HD). In AD, amyloid plaques and tau protein aggregates drive neurodegeneration, while synucleinopathies in PD are marked by Lewy body accumulations [5]. HD, in contrast, is caused by a genetic mutation in the huntingtin gene (Htt) involving expanded CAG repeats [6,7,8]. Other conditions like epilepsy and stroke, though distinct in pathology [9], share overlapping features with NDs, including neuronal damage and behavioral symptoms. Environmental factors, such as substance abuse, can exacerbate these conditions, which often lead to chronic cognitive, emotional, and behavioral impairments. Current therapies for NDs primarily focus on symptom management and slowing disease progression. Medications like cholinesterase inhibitors and NMDA receptor antagonists offer limited cognitive improvement in AD [10,11]. At the same time, levodopa and dopamine agonists alleviate PD motor symptoms but fail to halt disease progression [12]. In HD, tetrabenazine reduces chorea but introduces neuropsychiatric side effects [13,14,15,16]. Although advances in gene therapy, stem cell research, and precision medicine offer hope for future treatments, the urgent need remains for novel therapies that manage symptoms and address underlying disease mechanisms with fewer side effects.
The discovery of cannabinoid receptors sparked interest in the endocannabinoid system (eCB), with endogenous ligands like anandamide (AEA) and 2-arachidonoyl-glycerol (2-AG) [17,18] playing critical roles in CNS functions, including pain regulation, neurogenesis, and immune modulation. Though CB1 receptors are abundant in the brain and mediate the psychoactive effects of THC (for references, see [19]), CB2 receptors, primarily found in immune cells, exhibit minimal psychoactivity [20,21,22]. Both receptor types are now recognized for their broader involvement in neuroprotective and anti-inflammatory processes. In recent years, we and others have reviewed a detailed discussion on the function of the eCB system in health and disease [23,24,25,26,27]. Over 120 phytocannabinoids have been identified in Cannabis sativa [28], with Δ9-tetrahydrocannabinol (Δ9-THC) and cannabidiol (CBD) being the most well-known. While Δ9-THC is primarily associated with psychotropic effects, both Δ9-THC and Δ8-THC [29] have demonstrated therapeutic benefits, including appetite stimulation, analgesia, and antiemetic properties [30,31,32,33]. Non-psychotropic cannabinoids like CBD have gained attention for their anti-inflammatory, anxiolytic, and neuroprotective effects [34]. These compounds are now understood to target multiple receptors and biological pathways beyond the classical CB1 and CB2 receptors. Their phenolic structures endow them with potent antioxidant properties, while their lipophilicity facilitates blood–brain barrier (BBB) penetration [35,36,37], making them attractive candidates for treating neurodegenerative diseases.
This review aims to explore the neuroprotective potential of non-psychotropic minor phytocannabinoids (NMPs)—such as CBD, Δ9-tetrahydrocannabivarin (THCV), cannabidivarin (CBDV), and cannabigerol (CBG)—in neurodegenerative conditions. These phytocannabinoids are selected based on their abundance, ease of synthesis, and demonstrated therapeutic potential in other areas, such as cancer and inflammatory disorders. Their structural similarity to CBD, a well-studied neuroprotective agent, suggests they may also hold significant promise for future clinical applications.
2. Role of NMPs in Epilepsy
2.1. Cannabidiol
CBD functions as a negative allosteric modulator on CB1 and CB2 receptors (Figure 1). It also exhibits affinity for various other receptors, such as the transient receptor potential vanilloid Type 1 channel (TRPV1), peroxisome proliferator-activated receptor γ (PPARγ), G protein-coupled receptor 55 (GPR55), 5-hydroxytryptamine family 1A (5-HT1A) receptor, γ-aminobutyric acid type A (GABAA) receptor, and transient receptor potential cation channel subfamily M member 8 (TRPM8) (for references, see [19]). However, challenges remain in establishing the neuroprotective function of CBD through any one of these targets. Studies have indicated that eCBs regulate seizure mechanisms in developing animals through CB1 receptor signaling [38]. In pre-clinical seizure models, an anticonvulsant effect was observed due to a pharmacological increase in AEA and 2-AG following neuronal hyperexcitability, which reduces glutamate excitotoxicity during seizures [38,39,40]. Many axon terminals in the CNS express CB1 receptors, which inhibit the release of both excitatory and inhibitory neurotransmitters [41,42].
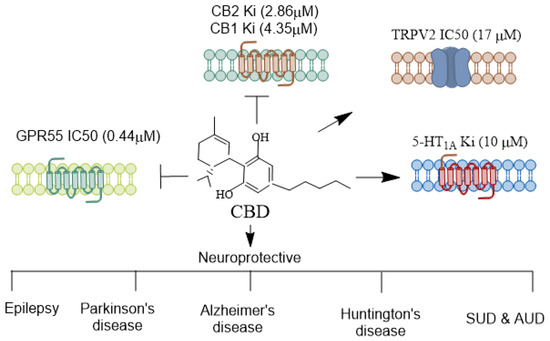
Figure 1.
Receptors mediating CBD’s agonistic and antagonistic actions. CBD interacts with various receptors, exhibiting a diverse pharmacological profile. Unlike THC, CBD does not exhibit a high affinity at the CB1 and CB2 receptors but can function as a negative allosteric modulator of CB1 [43], indirectly influencing endocannabinoid signaling. Additionally, CBD acts as an agonist at the 5-HT1A serotonin receptor [44], contributing to its anxiolytic and antidepressant effects. It also modulates TRPV1/2 [45], which are involved in inflammation and pain perception. CBD is an antagonist of GPR55, a receptor implicated in anxiety-related behaviors [46], learning and memory [47], and pain perception [48]. CBD’s receptor specificity contributes to its broad therapeutic potential across various neurological disorders. CBD, Cannabidiol; THC, Δ9-tetrahydrocannabinol; TRPV, Transient receptor potential vanilloid. →, agonist;  , antagonist.
, antagonist.
 , antagonist.
, antagonist.
Additionally, the anticonvulsant action of CBD involves several mechanisms beyond neuronal death prevention, including reinstating functions of hippocampal interneuron in a temporal lobe epilepsy model [49,50,51]. Furthermore, CBD enhances key molecular components of downstream ERK1/2 [52], PI3K/AKT [53], and pCREB pathways [54] typically linked to endocannabinoid-driven facilitation of adult neurogenesis. Subchronic CBD treatments have been shown to upregulate critical effectors involved in protein synthesis and neuronal survival in the hippocampus, including brain-derived neurotrophic factor (BDNF), MAP-2, calbindin, synapsin 1, and the activation of PPARγ. Further, CBD activates different survival and synaptic remodeling-signaling cascades such as ERK1/2-CREB [55], GSK3β, and PSD95 [56], or PI3K/mTOR/p70S6K [52,57,58,59] (for THE general signaling mechanism see Figure 2).
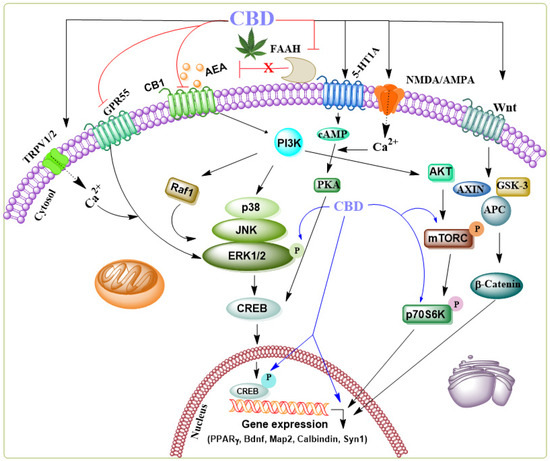
Figure 2.
Schematic representation of the signaling pathways modulated by CBD through various receptors in the brain. CBD acts as an agonist, activating several receptors such as 5-HT1A, NMDA/AMPA, Wnt-Frizzled (Fz) family receptors, and TRPV1/2—additionally, CBD functions as a negative allosteric modulator of CB1 and an inhibitor of FAAH, an enzyme involved in the degradation of anandamide (AEA) and an antagonist of GPR55. While the precise mechanisms of action in specific neurodegenerative diseases remain unclear, the collective synergistic effects of CBD have been shown to contribute to its neuroprotective role in several neurological disorder models. CBD’s influence on the ERK1/2 [52], PI3K/AKT [53], and pCREB [60] pathways, which are critical for regulating genes required for cell death, survival, neurogenesis, and synaptic plasticity, including BDNF, MAP-2, synapsin 1, calbindin, and PPARγ, suggests its potential to elicit neuroprotective effects.
CBD decreases epileptiform local field potential burst amplitude in the CA1 and dentate gyrus regions in a CB1 receptor-independent manner [61]. This observation is significant because, in addition to CB1 and CB2 receptors, CBD also binds to 5-HT1A receptors in the human brain [62]. These Gi/o protein-coupled serotonergic receptors, when activated, induce inhibitory effects. In temporal lobe epilepsy, 5-HT1A receptor signaling appears crucial in activating seizure-induced cell proliferation and survival in the dentate gyrus [63]. Experimental and clinical findings suggest that serotonergic signaling impacts seizure susceptibility and epilepsy-associated psychiatric comorbidities [64]. A reduction in serotonergic receptor activity in the dentate gyrus may lead to an excitatory/inhibitory imbalance in the trisynaptic circuit, triggering the neuropathology of epilepsy. Another study observed a significant loss of 5-HT1A binding in the hippocampus, temporal cortex, amygdaloid complex, and frontal lobe in patients with temporal lobe epilepsy [65]. Additionally, the downregulation of postsynaptic 5-HT1A receptors exacerbated the severity of epilepsy-associated depression [66]. Decreased serotonin signaling in brain regions related to epilepsy and emotions, such as the hippocampus, amygdaloid complex, and medial prefrontal lobe structures, can promote epilepsy and mental disorders, making CBD a potential therapeutic agent. CBD has also attenuated seizures in genetic models of brainstem and limbic seizures [67]. Thus, several well-designed trials have demonstrated the effectiveness of CBD in controlling epileptic seizures, with minimal significant adverse effects, although agitation, irritability, mood changes, and aggressive behavior have been noted [68]. Based on preclinical and clinical trials, the use of CBD (branded as Epidiolex) for Dravet syndrome, Lennox–Gastaut syndrome, or tuberous sclerosis complex was authorized by the FDA in 2018 and by the European Medicines Agency in 2019.
2.2. Δ9-Tetrahydrocannabivarin
THCV (Figure 3) is a relatively abundant, non-psychoactive phytocannabinoid found in the cannabis sativa [69] plant. It primarily functions as a CB1 receptor antagonist, displacing radiolabeled CB1 agonists in mouse whole-brain membranes [70].
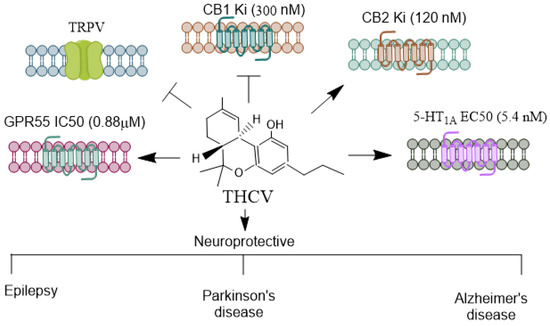
Figure 3.
Receptors mediating THCV’s agonistic and antagonistic actions. THCV is a minor cannabinoid with a distinctive pharmacological profile, exhibiting both agonistic and antagonistic effects on cannabinoid and non-cannabinoid receptors. THCV has a unique interaction with CB1 receptors [21,71]. At low doses, it acts as a CB1 antagonist, which can counteract the psychoactive effects of THC. However, at higher doses, THCV can act as a CB1 agonist, producing effects similar to THC, such as appetite suppression and potential psychoactivity. THCV is generally regarded as a partial agonist of CB2, which may contribute to its anti-inflammatory and immunomodulatory properties [21,71]. THCV also modulates transient receptor potential (TRP) channels, particularly TRPV1 [72]. These channels play roles in pain perception, thermoregulation, and inflammation, suggesting THCV’s potential in managing pain and inflammatory conditions. Similar to CBD, THCV has been shown to interact with the 5-HT1A receptor [73], which is involved in spatial learning and memory, anxiety regulation, and mood modulation [74,75]. This receptor interaction may underlie some of THCV’s potential anxiolytic and antipsychotic effects. THCV activates GPR55 [76] anxiety-related behaviors [46], learning, and memory [47]. THCV’s receptor specificity and its ability to shift between agonist and antagonist roles provide it diverse therapeutic potential, particularly in areas like metabolic disorders and neuroprotection. THCV, Δ9-Tetrahydrocannabivarin; CBD, Cannabidiol; THC, Δ9-tetrahydrocannabinol; TRPV, Transient receptor potential vanilloid. →, agonist;  , antagonist.
, antagonist.
 , antagonist.
, antagonist.
THCV also antagonizes CB1-specific GTPγS binding in various mouse brain regions, including the whole brain, cerebellum, and piriform cortex [70,77]. Moreover, it inhibits Δ9-THC-induced anti-nociception and hypothermia in mice [78]. There is evidence from a study in rats that THCV is anti-psychotic in a phencyclidine model of psychosis to a similar degree as clozapine [73]. In tissues enriched with CB2 receptors, THCV acts as a partial antagonist in Gi protein-coupled activities, as evidenced by its effect on forskolin-induced cytosolic cAMP levels [79]. Additionally, THCV regulates calcium transport in epithelial cells by inhibiting TRPV channels [80].
THCV has also demonstrated protective effects in healthy volunteers who exhibited enhanced neural responses to various stimuli [64]. It was shown to be safe, which holds likely for treating central nervous system (CNS) disorders. This makes THCV one of the most promising phytocannabinoids poised for drug approval by regulatory bodies. Indeed, THCV acts as a CB1 receptor antagonist without exerting agonist effects on [³⁵S] GTPγS binding, significantly reducing seizure incidence in adult rat piriform cortex slices [81]. In another study, THCV reduced Purkinje cell firing by enhancing inhibitory neurotransmission at interneuron–Purkinje cell synapses in mouse cerebellar brain slices. This was achieved by decreasing CB1 receptor-mediated, endocannabinoid-induced inhibition of GABA release [82]. The proposed mechanism for THCV’s anticonvulsant action is the increased GABA release through the blockade of CB1 receptors for endocannabinoids [21]. THCV has also shown efficacy in animal models of epilepsy and in NMR neuroimaging studies involving patients with epilepsy [83,84]. However, further studies with varying doses are needed to evaluate its efficacy in epilepsy treatment.
2.3. Cannabidivarin
CBDV (Figure 4) is found in unhybridized pure sativa and indica cannabis plants, known as landrace strains. These strains contain high amounts of CBD and low amounts of Δ9-THC. CBDV exhibits low binding affinity for the CB1 and CB2 receptors [85,86] and, therefore, appears to lack the psychotropic effects seen with THC. Consistent with this function, high concentrations of CBDV are required to stimulate CB1 receptor-coupled activation of [35S] GTPγS binding, inhibit cAMP synthesis, and recruit β-arrestin 2 (βarr2) [85,87,88]. Primarily, CBDV is a more potent and efficacious agonist at CB2 receptors [87,88]. It also activates TRPV1-4 channels [89,90] and stimulates ERK1/2 phosphorylation, inhibiting LPS-mediated signaling [76] through the GPR55 receptor. Additionally, CBDV acts as an inverse agonist at the Gs-coupled GPR6 receptor, leading to the stimulation of adenylyl cyclase and the recruitment of β-arrestin 2 [91]. CBDV has poor oral bioavailability. However, its liposoluble properties enable it to cross the blood–brain barrier [78] efficiently.
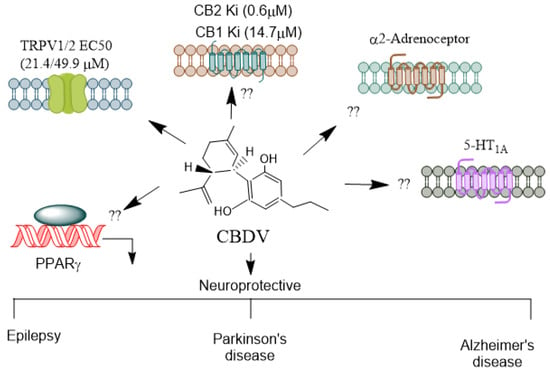
Figure 4.
Receptors mediating CBDV’s agonistic and antagonistic actions. CBDV, a non-psychoactive cannabinoid, exhibits both agonistic and antagonistic activity on several receptors, contributing to its potential therapeutic benefits in conditions like epilepsy, developmental disorders, and inflammation. Unlike THC, CBDV has a low affinity for CB1 and CB2 receptors [86]. It is thought to act more as a modulator of the endocannabinoid system rather than directly engaging as an agonist or antagonist at these receptors. This indirect modulation could influence the signaling pathways mediated by endocannabinoids, contributing to its anticonvulsant properties [92]. CBDV significantly affects TRPV1 and TRPV2, where it acts as an agonist [72]. These channels regulate pain, inflammation, and sensory perception, making CBDV potentially useful in managing neuropathic pain and inflammatory conditions. It is unknown whether CBDV activates other receptors such as α2-adrenoreceptors, PPARs, and 5-HT1A. CBDV’s receptor specificity highlights its therapeutic potential, particularly in epilepsy, neuroinflammation, and pain management, making it a promising candidate for further medical research. CBD, Cannabidiol; CBDV, Cannabidivarin; THC, Δ9-tetrahydrocannabinol; TRPV, Transient receptor potential vanilloid; PPARs, Peroxisome proliferator-activated receptor. →, agonis.
CBDV exhibited an antiseizure effect in animal models and a favorable safety profile in human studies [93,94]. In MECP2-308 mouse models, CBDV improved sociability, brain weight, and overall health and partially improved motor function [95,96]. In rat studies, CBDV significantly reduced pentylenetetrazol (PTZ) seizure severity and mortality [93,97]. However, CBDV did not affect the severity of pilocarpine convulsions. Further, CBDV significantly suppressed PTZ seizures, decreased seizure severity, and rescued PTZ-induced gene expression [98]. In another study, long-term CBDV administration in RTT mice significantly improved brain weight without affecting the neurotrophin levels [95]. None of these in vivo studies used antagonist experiments further to elucidate the mechanism of CBDV’s anticonvulsant effects. In an adult focal epilepsy cohort, CBDV exhibited safety in humans and significantly reduced seizure frequency [99]. In a pediatric RTT cohort, CBDV significantly improved seizure control in children with MECP2-related RTT [100]. Future studies are warranted to establish the specific mechanism CBDV offers for neuroprotective effects.
2.4. Cannabigerol
CBG (Figure 5) activates a2-adrenoceptors [101], PPARγ [102], which binds to cannabinoid CB1 and CB2 receptors [101] and blocks CB1 and 5-HT1A receptors [103], and it has been shown to inhibit sodium channel currents in vitro. However, it was ineffective as an anticonvulsant in a PTZ-induced acute seizure model [104]. Furthermore, CBG did not prevent hyperthermia-induced seizures in a Scn1a+/− mouse model of Dravet syndrome, which involves mutations affecting NaV channels [105]. In stably transfected HEK cells and primary dorsal root ganglion (DRG) neurons expressing NaV channels, CBG acted as a low-affinity inhibitor of sodium channels [106]. Another study found that CBG produced modest inhibitory effects on peak currents elicited by this subset of sodium channels in human voltage-gated sodium channel subtypes expressed in mammalian cells [107]. These findings suggest that CBG may induce neuronal hyperexcitability. However, more in vivo studies are warranted to explore its neuroprotective mechanisms in controlling epileptic seizures. The neuroprotective functions of NMPs in epilepsy models are summarized in Table 1.
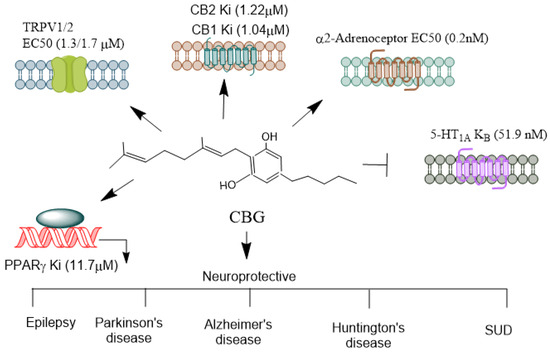
Figure 5.
Receptors mediating CBG’s agonistic and antagonistic actions. CBG, often referred to as the “mother cannabinoid” due to its role as a precursor to other cannabinoids like THC and CBD, exhibits a diverse pharmacological profile, interacting with various receptors and modulating multiple physiological pathways. CBG is considered a partial agonist at both CB1 and CB2 receptors [101]. However, its affinity for these receptors is relatively low compared to THC. Its interaction with CB1 is thought to be non-psychoactive and may even counteract some of the psychoactive effects of THC, which acts as a full agonist. CBG’s CB2 agonism has been linked to immunomodulatory and anti-inflammatory effects, which may aid in its therapeutic potential in conditions like neuroinflammation. CBG acts as an agonist at particularly TRPV1/2 [90], which is involved in pain, inflammation, and thermoregulation. CBG can activate alpha (2)-adrenoceptors, and block 5-HT1A receptors [103]. CBG activates PPARγ [102], which are involved in lipid metabolism, inflammation, and cell differentiation. CBG’s receptor interactions highlight its diverse pharmacological profile, making it a likely candidate for therapeutic applications in pain management, neuroprotection, mood disorders, and inflammation. Its ability to act as both an agonist and antagonist across different receptor systems contributes to its potential to treat a wide range of medical conditions. CBG, Cannabigerol; THC, Δ9-tetrahydrocannabinol; TRPV, Transient receptor potential vanilloid; PPARγ, Peroxisome proliferator-activated receptor-γ. →, agonist;  , antagonist.
, antagonist.
 , antagonist.
, antagonist.

Table 1.
Neuroprotective functions of NMPs in epilepsy models.
3. Role of NMPs in Parkinson’s Disease
Parkinson’s disease (PD) is the second-most-dominant neurodegenerative condition, affecting approximately 5% of individuals over the age of 85. Recently, the global burden of PD has doubled, making it one of the fastest-growing neurodegenerative diseases. Both genetic and environmental factors contribute to the risk of developing PD [110]. The PD is characterized by motor symptoms such as tremors, rigidity, bradykinesia, and a range of non-motor symptoms [111]. Motor symptoms are strongly associated with the damage and dysfunction of dopaminergic neurons in the nigrostriatal pathway, particularly during the intermediate stages of the disease [112]. This damage is often linked to the presence of α-synuclein, a protein involved in synaptic vesicle release, which can misfold and form aggregates known as Lewy bodies [113]. Developing α-synuclein aggregates can take more than 20 years, indicating that other neurotransmission systems may contribute to early non-motor symptoms, such as mild olfactory and cognitive impairments and depression [111,114]. eCBs, acting via CB1 and CB2 receptors, regulate synaptic and motor functions and can impact striatal rearrangement following dopamine depletion [115,116,117]. In PD subjects, cerebrospinal fluid exhibits heightened levels of AEA [117], and a reduction in CB1 receptors is observed in the substantia nigra [118]. Conversely, increased CB1 receptor expression is found in the nigrostriatal, mesolimbic, and mesocortical dopaminergic projection areas [118]. CB1 receptors also appear to be involved in the action of 3,4-dihydroxy-L-phenylalanine (L-DOPA); CB1 agonists have been shown to prevent the motor fluctuations commonly observed during L-DOPA therapy [119]. Additionally, increased CB2 receptor expression has been found in glial cells in postmortem tissues of PD subjects [120], suggesting a role in the inflammatory aspects of the disease. Due to the role of the eCB system in PD, pharmacological manipulation of the eCB system may represent a potential therapeutic approach for managing the disease.
3.1. Cannabidiol
A recent meta-analysis suggested that treatment with CBD significantly improves PD symptoms [121,122]. Nabilone has also been shown to be effective in alleviating anxious moods and night-time sleep problems [123], which are non-motor symptoms of PD. Additionally, CBD exhibits neuroprotective effects on the nigrostriatal pathway [124,125], and in vitro studies indicate its ability to activate tropomyosin receptor kinase A [126]. The anti-apoptotic effects of CBD are mediated by the ERK and Akt/mTOR pathways, with ERK activation modulated by TRPV1 and CB2 receptors [53]. Further studies have demonstrated that CBD exerts anti-apoptotic effects on dopaminergic neurons and reduces neuroinflammation by inhibiting the expression of NLRP3/caspase-1/IL-1β, Bax, and caspase-3 while upregulating Bcl-2 [127]. Therefore, the neuroprotective effects of CBD in PD appear to be mediated by CB2, but not CB1, receptors [53,124]. CBD prevented memory impairments and decreased despair-like behavior that was induced by bilateral intraoral 6-OHDA lesions [128]. In addition, CBD prevented dopaminergic neuronal loss in the striatum, ventral tegmental area, substantia nigra pars compacta (SNpc) and a reduced hippocampal neurogenesis, reduced the mortality rate, and decreased neuroinflammation in 6-OHDA-lesioned rats [128]. Repeated treatment with CBD favored the neuronal maturation of newborn neurons in the hippocampus in Parkinsonian rats [128]. Although these studies provide evidence of the therapeutic potential of CBD, further research is warranted before considering CBD for the treatment of PD.
3.2. Δ9-Tetrahydrocannabivarin
THCV attenuated the motor impairments induced by 6-hydroxydopamine, likely through changes in glutamatergic transmission [109]. Also, chronic administration of THCV rescued the loss of tyrosine hydroxylase-positive neurons caused by 6-hydroxydopamine in the substantia nigra as antioxidant effects [109]. Additionally, THCV was protective against 6-hydroxydopamine (6-OHDA) or LPS-induced motor impairments similar to rimonabent in rats [109]. Chronic THCV impaired microglial activation and preserved nigrostriatal dopaminergic neurons after 6-OHDA administration and in the LPS model of PD. Additionally, THCV preserved TH-positive neurons, possibly through CB2 receptors [109]. Also, THCV antagonized the effects of the CB1 receptor agonist, CP55,940, indicating its effects as an antagonist of the CB1 receptor. In Pitx3ak mutant mice, THCV rescued abnormal involuntary movements and reduced the FosB protein and the histone pAcH3, which had previously been found to be enhanced in the basal ganglia in L-DOPA-induced dyskinesia [129]. While future investigations are warranted to examine the clinical significance of THCV in humans, the findings establish THCV as a promising agent for developing a nonpsychotrophic cannabinoid-based therapy for patients with PD.

Table 2.
Neuroprotective functions of NMPs in PD models.
Table 2.
Neuroprotective functions of NMPs in PD models.
| Model | NMPs | Effect | Reference |
|---|---|---|---|
| PD Patients | CBD | PD symptoms ↓ | [121] |
| PD Patients | CBD | Anxiety, tremor amplitude ↓ | [122] |
| Unilateral lesions rat model | CBD | Hydroxydopamine-induced DA depletion | [124] |
| Sprague–Dawley rats | CBD | Neuroprotection ↑ | [125] |
| PC12 cells | CBD | Cell viability, differentiation, axonal (GAP-43), synaptophysin, and Synapsin I ↑ | [126] |
| SH-SY5Y cells | CBD | Cell viability ↑ Apoptosis, bax, and caspase 3. Moreover, nuclear PARP-1 ↓ | [53] |
| Mice | CBD | Cognitive dysfunction, TNF-α, IL-1β, IL-6, bax, caspase-3, and NLRP3/caspase-1/IL-1β inflammasome pathway ↓ Locomotion, 5-HT, DA, IL-10, TH, and Bcl-2 ↑ | [127] |
| Rats | CBD | SNpc, mortality rate, hippocampal neurogenesis, despair- behavior, memory impairments, and neuroinflammation ↓ Neuronal maturation ↑ | [128] |
| Mice | CBG | Motor tests, LAMP-1, TNF-α, IL-1β, nitric oxide synthase, and COX2 ↓ | [130] |
| SH-SY5Y cells Mice (unilaterally lesioned) | CBG | Cytoprotection, GFAP, and CD68 ↓ Motor activity ↑ | [131] |
| SH-SY5Y cells Mice (unilaterally lesioned | CBG | TH-positive neurons, motor activity ↑ | [132] |
| Rat | Δ9-THCV | Motor activity, TH-positive neurons ↑ | [109] |
| Pitx3ak mutant mice | AIMs, horizontal and vertical activities, FosB, pAcH3, and dyskinesia ↓ | [129] | |
| C. elegans | CBDV | α-syn, DAergic neurons ↓ | [133] |
CBD, Cannabidiol; CBDV, Cannabidivarin; DA, Dopamine; CBG, Cannabigerol; TH, Tyrosine hydroxylase; GFAP, Glial fibrillary acidic protein; Δ9-THCV, Δ9-tetrahydrocannabivarin; SH-SY5Y is a thrice-subcloned cell line derived from the SK-N-SH neuroblastoma cell line; C. elegans, Caenorhabditis elegans; FosB, FosB Proto-Oncogene, AP-1 Transcription Factor Subunit; pAcH3 phosphoacetyl H3; CD68, Cluster of Differentiation 68; LAMP-1, Lysosome-associated membrane glycoprotein 1; TNF-α, Tumor necrosis factor alpha; IL-1β, Interleukin 1 Beta; IL-10; Interleukin 10; IL-6, Interleukin 6; COX2, Cyclooxygenase 2; Bax, Bcl-2-associated X protein; NLRP3, Nod-like receptor protein 3; 5-HT, 5-hydroxytryptamine; Bcl-2, B-cell lymphoma-2, PARP-1, Poly (ADP-ribose) polymerase 1; SNpc, Substantia Nigra Pars Compacta; GAP-43, Growth-associated protein 43. Pitx3ak mice, Microphthalmia and aphakia mice; PC12 cells, Rat adrenal tumor cells; ↓, reduced; ↑, enhanced.
3.3. Cannabidivarin
CBDV has been shown to block α-synuclein (α-syn) aggregation via DAF-16, a key transcription factor involved in regulating oxidative stress, in a transgenic Caenorhabditis elegans (C. elegans) model [133]. Additionally, CBDV protects dopaminergic neurons from injury and degeneration induced by 6-OHDA [133]. Further exploring the molecular targets through which CBDV ameliorates α-synuclein aggregation in vivo would be valuable for understanding its therapeutic potential in PD. CBDV blocked α-synuclein (α-syn) aggregation via DAF-16, a key transcription factor for regulating oxidative stress in the transgenetic C. elegans model [133]. CBDV prevented dopaminergic neurons from 6-OHDA-induced injury and degeneration [133]. The molecular target (s) for CBDV ameliorating α-synuclein aggregation in vivo would be deserving of exploration in the future to understand its therapeutic potential of PD.
3.4. Cannabigerol
CBG has recently been investigated for its neuroprotective properties in inflammatory models of PD in mice. In one study, CBG improved motor function and rescued the loss of tyrosine hydroxylase-containing nigrostriatal neurons in mice that were unilaterally lesioned with lipopolysaccharide (LPS) [130]. Additionally, CBG reduced the elevated levels of LAMP-1 immunolabeling (a marker for autophagy impairment) and proinflammatory mediators such as tumor necrosis factor-α, interleukin-1β, inducible nitric oxide synthase and cyclooxygenase-2, and glial reactivity caused by the LPS lesion [130]. In another study using a neurotoxin model of PD, CBG exhibited cytoprotective effects in cultured SH-SY5Y cells treated with 6-hydroxydopamine (6-OHDA) [131]. Additionally, in mice unilaterally lesioned with 6-OHDA, CBG rescued tyrosine hydroxylase (TH)-positive nigral neurons from 6-OHDA-induced damage. It also completely prevented astroglial (GFAP) and microglial (CD68) reactivity in the substantia nigra of lesioned mice, leading to recovery from the motor deficiencies caused by 6-OHDA [130,132]. Furthermore, CBG treatment restored dopamine levels and its metabolite DOPAC in the striatum of 6-OHDA-lesioned mice [131]. These neuroprotective effects were attributed to CBG’s activity at the peroxisome proliferator-activated receptor-γ (PPAR-γ) rather than the CB2 [132]. The neuroprotective functions of NMPs in PD models are summarized in Table 2.
4. Role of NMPs in Alzheimer’s Disease
Alzheimer’s disease (AD) is a chronic neurodegenerative condition that affects the central nervous system, leading to a decline in cognitive functions. Similar to other neurodegenerative diseases, AD involves multiple pathologies influenced by a combination of genetic and environmental factors. Several potential causes are associated with the onset of AD, including the deposition of beta-amyloid (Aβ) aggregates forming senile plaques, hyperphosphorylation of Tau protein resulting in neurofibrillary tangles (NFTs), oxidative stress, neuroinflammation due to microglial activation, metabolic dysfunction, and obesity (for references, see [134]).
Despite numerous pharmacological strategies designed to slow down AD symptoms, these approaches have largely been ineffective. In AD patients, senile plaques have been shown to express CB1 and CB2 receptors along with microglial activation markers [135]. However, the number of CB1-positive neurons abundant in healthy individuals is significantly reduced in areas with activated microglia. Additionally, G protein coupling and CB1 receptor protein expression are markedly diminished in AD brains [135]. Furthermore, enhanced CB2 levels are found in AD patients and correlated with brain Aβ42 levels and senile plaque score [136]. The marked eCBs changes, as found in AD patients, were also observed in several AD mouse models [137,138,139]. These findings indicate that cannabinoid receptors play a vital role in the development of AD pathology and suggest that cannabinoids lacking psychiatric effects may have the potential to prevent the neurodegenerative process in AD.
4.1. Cannabidiol
In pre-clinical studies, CBD exhibited neuroprotection against many aspects of AD pathology. For example, the application of CBD prevented Aβ-peptide toxicity in PC12 cells by inhibiting oxidative stress, caspase-3 activation [140], and Tau hyperphosphorylation [141]. Additionally, CBD downregulated p-GSK-3β, an inhibitor of the Wnt pathway, causing enhanced Wnt/β-catenin signaling and stimulation of PPARγ and amyloid precursor protein (APP) ubiquitination. In the brain, Wnt/β-catenin signaling is crucial for neuronal survival and neurogenesis. It regulates synaptic plasticity and blood–brain barrier integrity and function [142]. Furthermore, CBD attenuated oxidative stress and rescued mitochondrial function [141]. In mesenchymal stem cells, CBD exposure reduced genes coding for the kinases responsible for Tau phosphorylation and the secretase enzymes involved in Aβ production [143]. Studies have shown that CBD rescues the formation of NFTs and prevents neuronal apoptosis via functioning as an endogenous cannabinoid receptor agonist [144].
In adult mice, intraventricular (i.v.) injection of fibrillar Aβ caused IL-6 gene expression and spatial memory deficits, which were rescued by CBD injection for 2 weeks [145]. In APP × PS1 mice, CBD administration rescued cognitive deficits measured by object recognition and social recognition memory without affecting anxiety behavior [146]. In a similar study, CBD was provided as pellets to APP × PS1 mice at the age of 2.5 months for 8 months and could prevent the development of social recognition memory deficits without affecting Aβ load, oxidative damage, or anxiety behavior [147]. In 5xFAD mice, chronic, low-dose CBD administration significantly improved memory and enhanced an insoluble form of Aβ levels in the cortical regions [148]. Oral administration of CBD for an extended period reversed pathophysiology and rescued social recognition memory deficits in the APP × PS1 mouse model for AD [149]. CBD administration in the senescence-accelerated mouse prone 8 (SAMP8) model improved cognitive function, increased hippocampal-activated microglia shift from M1 to M2, and restored gut microbial functions [150]. Additionally, CBD stimulates autophagy signal transduction through crosstalk between ERK1/2 and AKT kinases [52], which are key cell proliferation and survival regulators and have been implicated in AD pathology. In a randomized, double-blinded, placebo-controlled trial, CBD improved behavioral symptoms in AD [151]. Future studies are warranted to address research issues related to CBD’s safety, efficacy, and variability. In this direction, nanoparticle-coated CBD exhibited better beneficial effects on rescuing learning and memory, increasing hippocampal CB1 and CB2 receptors while reducing amyloid plaques in an AD rat model [152]. Additionally, administering CBD along with THC, a combination known to provide more significant therapeutic benefits than phytocannabinoids alone [153] was shown to improve behavior problems and rigidity in severely demented patients [154].

Table 3.
Neuroprotective role of NMPs in AD models.
Table 3.
Neuroprotective role of NMPs in AD models.
| Model | NMPs | Effect | Reference |
|---|---|---|---|
| PC12 cells | CBD | Cell Survival ↑ ROS, lipid peroxidation, and caspase 3 ↓ | [140] |
| PC12 cells | CBD | Wnt/β-catenin ↑ Tau hyperphosphorylation and p-GSK-3β ↓ | [141] |
| MSC cells | CBD | GSK3β, CDK5, DYRK1A, CAMK2A, MAPK1, MAPK12, MAPK14, and BACE1 ↓, | [143] |
| N13 microglial cells Rat primary microglia Mice | CBD | Intracellular calcium ↓ Nitrite generation and IL-6 gene expression ↓ Spatial memory and microglial migration ↑ | [145] |
| APPxPS1 mice | CBD | Social recognition and novel object recognition ↑ | [146] |
| AβPPSwe/PS1ΔE9 | CBD | Social recognition ↑ | [147] |
| 5x FAD mice | CBD and THC | Spatial memory and beta amyloid ↑ | [148] |
| SAMP8 mice | CBD | Bacteriodetes and hippocampal-activated microglia shift from M1 to M2 ↑ and LPS ↓ | [150] |
| Male wistar rats | CBD coated by nano-chitosan | Learning and memory and CB1 and CB2 protein expression ↑ Amyloid plaques ↑ | [152] |
| Female patients | CBD | Direct contact and behavior ↑ | [154] |
| MC65 cell | CBG | Aβ aggregation ↓ | [155] |
| PC12 cells | CBG | Aβ aggregation and Aβ1-42 neurotoxicity ↓ Neuroprotection | [156] |
| Male human subjects | ∆9-THCV | Memory impairment ↓ | [157] |
| In vitro assay | CBD, CBDV, CBG | AChE and BChE ↓ | [158] |
CBD, Cannabidiol; CBDV, Cannabidivarin; THC, Tetrahydrocannabinol; CBG, Cannabigerol; ∆9-THCV, ∆9-tetrahydrocannabivarin; ROS, Reactive oxygen species, MSCs, Mesenchymal stem cells; LPS, Lipopolysaccharide; AChE, Acetylcholinesterase; BChE, Butyrylcholinesteras; BDNF, Brain-derived neurotrophic factor; PGC1α, Peroxisome proliferator-activated receptor gamma coactivator 1-alpha; CDK5, Cyclin-dependent kinases 5 gene; DYRK1A, Dual-specificity tyrosine phosphorylation-regulated linase 1A; CAMK2A, Calcium-/calmpdulin-dependent protein kinase II α; MAPK1, Mitogen-activated protein kinase 1; MAPK12, Mitogen-activated protein kinase 12; MAPK14, Mitogen-activated protein kinase 14; BACE, β-Secretase 1; PC12 cells, Rat adrenal tumor cells; MSC, Multipotent stromal cells; N13 cells and primary mice microglial cells; APP, Amyloid precursor protein; PS1, Presenilin 1; FAD, 5 familial AD; SAMP8, Senescence accelerated mouse prone 8; MC65, Human CNS nerve cell line; HT22, Mouse hippocampal Neuron cell; ↓, reduced; ↑, enhanced.
4.2. Δ9-Tetrahydrocannabivarin
While THCV has not yet been tested in AD models, it has demonstrated a strong safety profile in healthy adults. In a placebo-controlled, double-blind, crossover pilot study, THCV was shown to reverse THC-induced memory impairment [157]. Future studies using THCV in AD animal models could provide potential neuroprotective mechanisms and therapeutic benefits.
4.3. Cannabidivarin
CBDV has demonstrated efficacy in an in vitro AD model by blocking oxytosis and energy loss in HT22 cells and reducing Aβ toxicity and trophic withdrawal. However, these experiments did not investigate the specific mechanisms underlying these effects [155]. Additionally, CBDV was found to inhibit the activities of acetylcholinesterase (AChE) and butyrylcholinesterase (BChE) [158]. Abnormal hyperactivity of AChE and BChE contributes to cholinergic deficiency, associated with several neurological disorders, including the memory impairments and cognitive decline observed in AD [159]. Therefore, further research to elucidate the molecular mechanisms of CBDV in AD models could provide valuable insights into its therapeutic potential for this condition.
4.4. Cannabigerol
CBG has been shown to reduce the accumulation of Aβ deposits in vitro. Specifically, CBG cleared the preformed and aggregated Aβ from neurons and stimulated Aβ degradation in the MC65 cell line [155]. This widely recognized human neuron-like cell line lacks CB1 or CB2 receptors extensively used for proteotoxicity studies [155]. Additionally, CBG inhibited Aβ1-42 -induced neurotoxicity and morphological changes such as reduced neuritic projections and rounded cell morphology in PC12 cells [156]. Future studies with CBG in AD animal models could help elucidate this phytocannabinoid’s neuroprotective mechanism. The neuroprotective functions of NMPs in AD models are summarized in Table 3.
5. NMPs’ Neuroprotective Role in Huntington’s Disease
Huntington’s disease (HD) is a progressive neurodegenerative condition characterized by abnormal motor and cognitive functions. HD is triggered by mutations in the huntingtin gene (Htt) on chromosome 4, involving multiple cytosine-adenine-guanine (CAG) repeats at the exon 1 of the Htt gene [6,7,8]. Current pharmacological treatment for HD primarily utilizes atypical anti-psychotic drugs to manage hypermotor activity symptoms. However, the exact mechanisms underlying HD pathogenesis remain largely unclear, and there is currently no specific drug available to address the cognitive impairments associated with the disease [160]. Early in HD progression, research has revealed reduced CB1 mRNA and protein levels in medium spiny projection neurons of the caudate and putamen [161,162,163]. Moreover, CB1 transcription is suppressed by mutant huntingtin protein (mHtt) [164,165]. These changes in CB1 functionality significantly contribute to the cognitive, behavioral, and motor deficits observed in animal models of HD [166,167]. Consequently, there is increasing interest in pharmacological strategies to enhance CB1 signaling as a potential therapeutic approach for treating and managing HD.
5.1. Cannabidiol
Recent studies have evaluated the neuroprotective role of CBD in vitro using models of medium spiny projection neurons expressing mutant huntingtin protein (STHdhQ111/Q111) compared to wild type (STHdhQ7/Q7). In these models, CBD enhanced CB1 expression and GABA release and promoted CB1-independent but 5HT1A-dependent phosphorylation of CREB (p-CREB). Additionally, CBD administration in rats with 3-nitropropionic acid (3NP)-induced HD reduced CB1 receptor expression and insulin-like growth factor 1 (IGF-1) while enhancing calpain expression in striatal neurons [60]. This treatment also restored levels of GABA, substance P, and neuron-specific enolase, which are involved in generating proinflammatory markers that contribute to neuronal atrophy. Furthermore, CBD reinstated antioxidant enzyme SOD-1 and proenkephalin levels in the striatum and substantia nigra, which play crucial roles in HD pathogenesis [60]. However, despite these insights into CBD’s protective function in HD, its therapeutic utility remains controversial. While some studies indicate no improvement in animal models and human trials [168,169], others have reported beneficial effects in animal models [60,170].

Table 4.
Neuroprotective functions of NMPs in different HD models.
Table 4.
Neuroprotective functions of NMPs in different HD models.
| Model | NMPs | Effect | Reference |
|---|---|---|---|
| STHdh(7/7) cells | CBD | ATP production, BDNF-2, and PGC1α CB1 mRNA levels ↑ | [164] |
| Rats | CBD | mRNA SP, mRNA NSE, and mRNA SOD-2 ↑ | [60] |
| Rats | CBD | 3NP-induced GABA, Nissl-stained neurons, CB1 and IGF-1 expression, and SOD-1 expression ↓ Calpain expression ↑ | [170] |
| RBL-2H3 cells | CBG | Human TRPV1, and rat TRPV2 ↓ | [89] |
| HT29 cells | CBG | COX-2 enzyme, and prostaglandins ↓ | [171] |
| Mice | CBG | Reactive microgliosis, expression of COX-2, iNOS, TNF-α, Cd44, and Sgk1 ↓ PPARγ, catalase, and SOD and GSH ↑ | [172] |
| NSC-34 | CBG | HAP1, SLC32A1, ADCY5, AKT, ATF4, DLGAP1,DRD4, GNB4, PRKCA ↑ ADCY9, CAMK2B, CLOCK, CREB1, DRD2, GNAL, PLD1, PPP3R1, PRKCB, SHANK1, SLC1A2, SLC18A1, and SLC38A1 ↓ | [173] |
CBD, Cannabidiol; CBG, Cannabigerol; SOD-1, Superoxide dismutase-1; SOD-2, Superoxide dismutase-2; SP, Substance P; NSE, Neuron-specific enolase; TRPV1, Transient receptor potential vanilloid 1; TRPV2, Transient receptor potential vanilloid 2; COX-2, Cyclooxygenase enzyme 2; iNOS, Inducible nitric oxide synthase; TNF-α, Tumor necrosis factor alpha; IL-6, Interleukin-6; PPARγ, peroxisome proliferator-activated receptor-γ; cd44, Non-kinase transmembrane glycoprotein gene; SGK1 Serum/glucocorticoid-regulated kinase 1 gene; GSH, Glutathione; SLC32A1, Solute carrier family 32 member 1 gene; ADCY5, Adenylate cyclase 5 gene, AKT, Serine/threonine kinase 1 gene; ATF4, Activating transcription factor4 gene; DLGAP1, DLG-associated protein 1 gene; DRD4, Dopamine receptor D4 gene; G protein, Guanine nucleotide-binding protein gene; GNAI2, Alpha inhibiting 2 gene; GNB4, Beta 4 gene; HAP1, Huntingtin-associated protein 1 gene; PRKCA, Protein kinase C, alpha gene; ADCY9, Adenylate cyclase 9 gene; CAMK2B, Calcium-/calmodulin-dependent protein kinase II, beta; CLOCK, Circadian locomotor output cycles kaput; CREB1, AMP responsive element-binding protein 1; DRD2, Dopamine receptor D2; GNAL, Guanine nucleotide-binding protein, alpha stimulating, olfactory type; PLD1, Phospholipase; D1PPP3R1, Protein phosphatase 3, regulatory subunit B, alpha isoform; PRKCB, Protein kinase C, beta; SHANK1, SH3 and multiple ankyrin repeat domains 1; SLC1A2, Solute carrier family 1 (glial high affinity glutamate transporter), member 2; SLC18A1, solute carrier family 18 (vesicular monoamine), member 1; SLC38A1, solute carrier family 38, member 1; STHdh(7/7) cells, Cell line derived from the striatum of a mouse; RBL-2H3 cells, Basophilic leukemia cell line; HT29, Colon Cancer Line; NSC-34, Motor neuron-like cells; ↓, reduced; ↑, enhanced.
5.2. Δ9-Tetrahydrocannabivarin
Therapeutic studies of cannabinoid-based agents in HD animal models suggest that CB1 and endo vanilloid receptor agonists [174,175] and AEA reuptake inhibitors [176] can prevent hyperkinesia in the early phases of HD. This is likely due to the gradual loss of CB1 receptors [177] in the advanced stages of the disease. Although further studies are warranted, the potential use of THCV in HD is limited since it functions as an antagonist of CB1 and CB2 receptors. An improved understanding of the eCB system in HD may help identify specific NMPs or combinations that provide therapeutic or neuroprotective benefits in patients with HD.
5.3. Cannabidivarin
While phytocannabinoids show promise as potential treatments for motor-related diseases, no studies have evaluated the neuroprotective effects of CBDV in models of HD. Future research is needed to explore neuroprotective mechanisms within the relevant brain regions in animal models treated with CBDV.
5.4. Cannabigerol
CBG is a biologically active constituent of the marijuana plant, present in much smaller quantities compared to other cannabinoids. It acts as a precursor to various phytocannabinoids. CBG is a non-psychoactive compound that exhibits a wide range of biological activities [178] and is potentially a therapeutic compound for treating diseases requiring multidirectional pharmacotherapy. CBG interacts with CB1, CB2, TRPV1, and PPAR receptors [88,179,180]. Additionally, CBG suppresses the activity of FAAH, an enzyme that metabolizes anandamide (AEA), affecting its levels and biological effects. However, compared to CBD, CBG is less effective as an FAAH inhibitor [89,181]. Furthermore, CBG reduces the activity of DAGL, the enzyme responsible for the biosynthesis of 2-AG, and inhibits the activities of COX-1 and COX-2, which metabolize polyunsaturated fatty acids (PUFAs), mainly arachidonic acid, into lipid mediators [171,182,183]. CBG also acts as a neutral 5-HT1A receptor antagonist, a CB1 receptor antagonist, and a potent α2-adrenoceptor agonist [103].
CBG exhibits similar pharmacokinetic (PK) profiles in rats and mice, though it shows slower brain penetration in mice. Both species have higher concentrations of CBG following intraperitoneal (i.p.) injection compared to oral administration. However, in rats, this does not correspond to higher concentrations in brain tissue [184]. CBG has demonstrated neuroprotective effects in experimental HD models through both cannabinoid receptor-dependent and independent mechanisms. For instance, in an in vivo model of HD using 3-NP, CBG significantly reduced the expression of upregulated inflammatory markers such as COX-2, iNOS, IL-6, and TNF-α [172] and prevented the 3-NP-induced neuronal loss. Additionally, CBG reversed the activities of antioxidant enzymes, including catalase, superoxide dismutase (SOD), and glutathione (GSH), compared to control groups. CBG also rescued the expression of downregulated genes directly related to HD, such as sgk1, Cd44, and huntingtin-associated protein-1, while reducing mutant huntingtin (mHTT) protein aggregation and improving motor function [172]. Moreover, CBG was found to activate PPARγ dose-dependently in cultured striatal cells containing both wild-type and mutant huntingtin [172]. A recent transcriptomic study in motor neuron-like cells (NSC-34) revealed that CBG reduced the expression of genes involved in glutamate release, enhanced the expression of genes related to GABA release, and upregulated the dopamine D4 receptor and its downstream effectors [173], suggesting CBG’s influence on neurotransmission pathways. Future studies are needed to establish the link between these transcriptomic changes and behavioral and neuronal signaling to understand the role of CBG in neuroprotection better. The neuroprotective functions of NMPs in HD models are summarized in Table 4.
6. Neuroprotective Role of NMPs in Substance and Alcohol Use Disorders
Cannabinoid receptors are densely enriched in brain areas related to reward function and developing and maintaining addictive behaviors [185]. The eCB system has been implicated in the pathophysiology of addiction by modulating pathways that affect drug- and alcohol-seeking behaviors, cravings, withdrawal [24,186,187], and memory and emotional processes [188]. Alcohol [189,190] and drug abuse [191,192] act as one of the environmental factors that promote many of the neurodegenerative disorders. Therefore, studies have explored whether phytocannabinoids provide neuroprotection against substance and AUD-related disorders. In the following sections, we discuss the current evidence on the neuroprotective functions of phytocannabinoids on substance- and alcohol-use disorders.
6.1. Substance-Use Disorders (SUD)
In a randomized, double-blind, placebo-controlled study, CBD significantly reduced cigarette smoking, although it did not affect cravings [193]. Similarly, another study found that CBD decreased the salience and pleasantness of cigarette cues without impacting cravings, withdrawal symptoms [194], or impulsivity [195]. In an open-label crossover study involving daily users of nicotine-containing e-cigarettes, CBD was shown to reduce both the severity of nicotine withdrawal symptoms and state anxiety during e-cigarette abstinence [196]. Additionally, CBD has been found to mitigate nicotine withdrawal and hyperalgesia-inducing effects in both mice [197] and rats [198]. While the potential of other phytocannabinoids to protect against nicotine-use disorder remains unknown, future research is warranted to explore this possibility.
There has been substantial scientific discussion regarding the potential of CBD as a treatment for cannabinoid-use disorder (CUD) [199,200]. In animal models, CBD has been shown to reduce spontaneous withdrawal symptoms in THC-dependent rodents [201,202,203]. In a case study, CBD alleviated cannabis-withdrawal symptoms and improved anxiety and sleep function [203,204,205]. Additionally, CBD significantly reduced cannabis use in participants of a non-randomized open-label study [206]. Moreover, CBD mitigated adverse cognitive and mental health effects [201,202] in long-term THC users, including reduced cognitive deficits, psychotic-like and depressive symptoms, and increased hippocampal volumes [207] and resting-state functional connectivity [208]. These studies suggest that the antipsychotic and anxiolytic properties of CBD may improve psychological and cognitive functions related to long-term cannabis use—the use of other NMPs to combat the effects of THC warrants further study.
CBD has been studied in opioid-use disorders and has been shown to mitigate morphine reward and reduce the reinstatement of heroin-seeking behavior in rats [209,210], as well as alleviate opioid withdrawal symptoms in rodents [211,212]. It also decreased naloxone-precipitated jumping behavior in mice [213]. CBD prevented the reinstatement of oxycodone-induced conditioned place preference (CPP) and rescued recognition memory deficits in adolescent male mice. These mice exhibited increased CB1 receptors and a reduced mu-opioid receptor (MOR) expression in the prefrontal cortex (PFC) [214]. CBG was found to reverse oxaliplatin-associated mechanical sensitivity [215] and attenuate the acute antinociceptive effects of morphine through interactions with α2-adrenergic, CB1, or CB2 receptors [213]. These findings suggest that continued research on CBD, CBG, and other NMPs could enhance our understanding of their potential in treating pain and substance use disorders. The neuroprotective functions of NMPs in SUD models are summarized in Table 5.

Table 5.
Neuroprotective functions of NMPs in different SUD models.
6.2. Alcohol-Use Disorders (AUD)
CBD has shown promise in preventing alcohol abuse and mitigating alcohol-related effects, owing to its anxiolytic properties [216,217]. Preclinical studies strongly suggest that the eCB system plays a significant role in the motivational properties of alcohol and its effects [24,218,219,220]. Specifically, antagonism of the CB1 receptor has been shown to suppress rodent alcohol consumption [24,218,219,220,221]. As CBD may act as a negative allosteric modulator of the CB1 receptor, this supports the notion that CBD could be a promising treatment for AUD.
CBD has demonstrated protective effects against alcohol-induced neurodegeneration. When administered alongside binge ethanol exposure, CBD rescued neurodegeneration in the hippocampus and entorhinal cortex [222]. Transdermal administration of CBD also significantly reduced neurodegeneration in the entorhinal cortex [223]. Furthermore, CBD has been shown to prevent alcohol-induced cell death and improve neuronal viability in rat hippocampal cultures [224]. In alcohol-dependence models, CBD inhibited impulsive choice behavior [225,226], a factor linked to both alcohol use and the risk of relapse [227,228]. CBD has also been found to reduce alcohol consumption [203], motivation, and relapse in a two-bottle choice paradigm [229]. In these studies, CBD reduced ethanol-induced hypothermia and handling-induced convulsions without affecting blood ethanol concentration [229]. Moreover, CBD does not alter breath alcohol levels in humans [230]. CBD also reversed alcohol-induced changes in gene expression, such as tyrosine hydroxylase in the ventral tegmental area, Oprm1, CNR1, and GPR55 in the nucleus accumbens (NAcc) while significantly enhancing CB2 receptor expression in the Nacc [229].
In selectively bred Sardinian alcohol-preferring (sP) rats, CBD significantly reduced lever responses for alcohol and the amount of self-administered alcohol [231]. Although the exact neuroprotective mechanisms of CBD require further investigation, these findings strongly suggest that CBD may be beneficial for treating AUDs. In another study, CBD significantly reduced alcohol-withdrawal symptoms such as anxiety behavior and altered gene expression in related brain regions [232]. In the alcohol binge-like drinking model, CBD inhibited alcohol consumption and preference, normalized abnormal corticolimbic calcitonin gene-related peptide (CGRP) expression, and reduced reward and aversion-related hyper-responsivity and glucocorticoid levels in rats [233]. CBD also significantly mitigated the blunted stress response and corticosterone levels caused by binge-like alcohol exposure, restored dopamine transmission, and facilitated excitatory postsynaptic strength and remodeling in rats [234]. Additionally, CBD inhibited the development of tolerance to alcohol’s hypothermic and sedative effects and restored reduced CB2R gene transcription in the striatum caused by ethanol [235]. In another alcohol-withdrawal study, CBD reduced anxiety-like behaviors in mice [236]. However, some studies show limitations: CBD failed to attenuate alcohol-induced locomotor sensitization [237], and oral CBD did not prevent alcohol seeking, self-administration, or intake in baboons [238]. Although the precise mechanisms remain unknown, these studies indicate that CBD and potentially other phytocannabinoids could offer neuroprotection against alcohol toxicity and AUD.
Fetal Alcohol Spectrum Disorder (FASD) embodies a range of neurobehavioral impairments caused by prenatal and postnatal exposure to ethanol. Recent studies have shown that CBD treatment during the peri-adolescence period can mitigate the increased levels of TNFα and IL-6 in the hippocampus, as well as the cognitive deficits associated with prenatal and postnatal alcohol exposure [239]. Data from four case studies suggest that CBD has neuroprotective effects, reducing FASD symptoms such as restlessness, aggression, agitation, impulsivity, and high scores on the Nisonger Disruptive Behavior Scale [240]. Additionally, CBD administration has been shown to reverse alcohol-induced gene-expression defects, anxiety, depressive-like behaviors, and cognitive impairments in animal models exposed to alcohol both prenatally and postnatally [241]. Although the precise mechanisms are not yet fully understood, early research suggests that CBD, or possibly other phytocannabinoids, could provide neuroprotective effects against the developmental impact of alcohol exposure. The neuroprotective functions of NMPs in AUD models are summarized in Table 6.

Table 6.
Neuroprotective functions of NMPs in different AUD models.
7. Conclusions
In summary, the therapeutic potential of cannabis sativa extends well beyond the widely studied CBD, encompassing a diverse range of lesser-known phytocannabinoids that show promise in addressing various neurological disorders. The neuroprotective functions of these NMPs, particularly their antioxidant, anti-inflammatory, and immune-modulating properties, offer new avenues for research and treatment. While the pharmacological mechanisms of many NMPs remain underexplored, emerging studies suggest their potential to develop novel therapies for brain disorders. As research continues to unfold, these findings could pave the way for innovative cannabinoid plant-based treatments that go beyond the scope of traditional approaches, offering new hope in neuroprotection and disease management.
Author Contributions
B.S.B. and S.S. participated in the collection of literature, writing, and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported in part by NIH/NIAAA grant AA029686 (BSB).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Basavarajappa, B.S.; Shivakumar, M.; Joshi, V.; Subbanna, S. Endocannabinoid system in neurodegenerative disorders. J. Neurochem. 2017, 142, 624–648. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, R.; Kapogiannis, D.; Huey, E.D.; Momeni, P. FTD and ALS: A tale of two diseases. Curr. Alzheimer Res. 2011, 8, 273–294. [Google Scholar] [CrossRef] [PubMed]
- Gibson, S.B.; Figueroa, K.P.; Bromberg, M.B.; Pulst, S.M.; Cannon-Albright, L. Familial clustering of ALS in a population-based resource. Neurology 2014, 82, 17–22. [Google Scholar] [CrossRef]
- Gammon, K. Neurodegenerative disease: Brain windfall. Nature 2014, 515, 299–300. [Google Scholar] [CrossRef]
- Kovacs, G.G. Concepts and classification of neurodegenerative diseases. In Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2017; Volume 145, pp. 301–307. [Google Scholar] [CrossRef]
- Gonzalez-Alegre, P. Recent advances in molecular therapies for neurological disease: Triplet repeat disorders. Hum. Mol. Genet. 2019, 28, R80–R87. [Google Scholar] [CrossRef] [PubMed]
- Nopoulos, P.C. Huntington disease: A single-gene degenerative disorder of the striatum. Dialogues Clin. Neurosci. 2016, 18, 91–98. [Google Scholar] [CrossRef]
- Paulson, H. Repeat expansion diseases. In Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2018; Volume 147, pp. 105–123. [Google Scholar] [CrossRef]
- Taylor, J.J.; Williams, N.R.; George, M.S. Beyond neural cubism: Promoting a multidimensional view of brain disorders by enhancing the integration of neurology and psychiatry in education. Acad. Med. 2015, 90, 581–586. [Google Scholar] [CrossRef]
- Hampel, H.; Vergallo, A.; Caraci, F.; Cuello, A.C.; Lemercier, P.; Vellas, B.; Giudici, K.V.; Baldacci, F.; Hanisch, B.; Haberkamp, M.; et al. Future avenues for Alzheimer’s disease detection and therapy: Liquid biopsy, intracellular signaling modulation, systems pharmacology drug discovery. Neuropharmacology 2021, 185, 108081. [Google Scholar] [CrossRef]
- Trinh, N.H.; Hoblyn, J.; Mohanty, S.; Yaffe, K. Efficacy of cholinesterase inhibitors in the treatment of neuropsychiatric symptoms and functional impairment in Alzheimer disease: A meta-analysis. JAMA 2003, 289, 210–216. [Google Scholar] [CrossRef]
- Bhushan, M.; Akash, S.; Pettarusp, W. Adverse effects of medications used to treat motor symptoms of Parkinson’s disease: A narrative review. Ann. Mov. Disord. 2023, 6, 45–57. [Google Scholar] [CrossRef]
- Hayden, M.R.; Leavitt, B.R.; Yasothan, U.; Kirkpatrick, P. Tetrabenazine. Nat. Rev. Drug. Discov. 2009, 8, 17–18. [Google Scholar] [CrossRef] [PubMed]
- Kaur, N.; Kumar, P.; Jamwal, S.; Deshmukh, R.; Gauttam, V. Tetrabenazine: Spotlight on Drug Review. Ann. Neurosci. 2016, 23, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Sampaio, C. Huntington disease—Update on ongoing therapeutic developments and a look toward the future. Parkinsonism Relat. Disord. 2024, 122, 106049. [Google Scholar] [CrossRef] [PubMed]
- Wyant, K.J.; Ridder, A.J.; Dayalu, P. Huntington’s Disease-Update on Treatments. Curr. Neurol. Neurosci. Rep. 2017, 17, 33. [Google Scholar] [CrossRef]
- Mechoulam, R.; Fride, E.; Di Marzo, V. Endocannabinoids. Eur. J. Pharmacol. 1998, 359, 1–18. [Google Scholar] [CrossRef]
- Di Marzo, V.; Melck, D.; Bisogno, T.; De Petrocellis, L. Endocannabinoids: Endogenous cannabinoid receptor ligands with neuromodulatory action. Trends Neurosci. 1998, 21, 521–528. [Google Scholar] [CrossRef]
- Peng, J.; Fan, M.; An, C.; Ni, F.; Huang, W.; Luo, J. A narrative review of molecular mechanism and therapeutic effect of cannabidiol (CBD). Basic Clin. Pharmacol. Toxicol. 2022, 130, 439–456. [Google Scholar] [CrossRef]
- Guindon, J.; Hohmann, A.G. Cannabinoid CB2 receptors: A therapeutic target for the treatment of inflammatory and neuropathic pain. Br. J. Pharmacol. 2008, 153, 319–334. [Google Scholar] [CrossRef] [PubMed]
- Pertwee, R.G. The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: Delta9-tetrahydrocannabinol, cannabidiol and delta9-tetrahydrocannabivarin. Br. J. Pharmacol. 2008, 153, 199–215. [Google Scholar] [CrossRef]
- Wilson, R.I.; Nicoll, R.A. Endocannabinoid Signaling in the Brain. Science 2002, 296, 678–682. [Google Scholar] [CrossRef]
- Aoki, J.; Isokawa, M. Understanding Cellular, Molecular, and Functional Specificity, Heterogeneity, and Diversity of the Endocannabinoid System. Cells 2024, 13, 1049. [Google Scholar] [CrossRef] [PubMed]
- Basavarajappa, B.S. Endocannabinoid System and Alcohol Abuse Disorders. In Recent Advances in Cannabinoid Physiology and Pathology; Bukiya, A.N., Ed.; Nature Springer: Cham, Switzerland, 2019; pp. 89–127. [Google Scholar]
- Basavarajappa, B.S.; Subbanna, S. Molecular Insights into Epigenetics and Cannabinoid Receptors. Biomolecules 2022, 12, 1560. [Google Scholar] [CrossRef] [PubMed]
- Dallabrida, K.G.; de Oliveira Bender, J.M.; Chade, E.S.; Rodrigues, N.; Sampaio, T.B. Endocannabinoid System Changes throughout Life: Implications and Therapeutic Potential for Autism, ADHD, and Alzheimer’s Disease. Brain Sci. 2024, 14, 592. [Google Scholar] [CrossRef] [PubMed]
- Wright, N.J.D. A review of the direct targets of the cannabinoids cannabidiol, Delta9-tetrahydrocannabinol, N-arachidonoylethanolamine and 2-arachidonoylglycerol. AIMS Neurosci. 2024, 11, 144–165. [Google Scholar] [CrossRef]
- Morales, P.; Hurst, D.P.; Reggio, P.H. Molecular Targets of the Phytocannabinoids: A Complex Picture. Prog. Chem. Org. Nat. Prod. 2017, 103, 103–131. [Google Scholar] [CrossRef] [PubMed]
- Mechoulam, R.; Gaoni, Y. The absolute configuration of delta-1-tetrahydrocannabinol, the major active constituent of hashish. Tetrahedron Lett. 1967, 12, 1109–1111. [Google Scholar] [CrossRef]
- Boychuk, D.G.; Goddard, G.; Mauro, G.; Orellana, M.F. The effectiveness of cannabinoids in the management of chronic nonmalignant neuropathic pain: A systematic review. J. Oral Facial Pain Headache 2015, 29, 7–14. [Google Scholar] [CrossRef]
- Novack, G.D. Cannabinoids for treatment of glaucoma. Curr. Opin. Ophthalmol. 2016, 27, 146–150. [Google Scholar] [CrossRef]
- Phillips, R.S.; Friend, A.J.; Gibson, F.; Houghton, E.; Gopaul, S.; Craig, J.V.; Pizer, B. Antiemetic medication for prevention and treatment of chemotherapy-induced nausea and vomiting in childhood. Cochrane Database Syst. Rev. 2016, 2, CD007786. [Google Scholar] [CrossRef]
- Pomorska, D.K.; do-Rego, J.C.; do-Rego, J.L.; Zubrzycka, M.; Janecka, A. Opioid and Cannabinoid System in Food Intake. Curr. Pharm. Des. 2016, 22, 1361–1370. [Google Scholar] [CrossRef]
- Mechoulam, R.; Peters, M.; Murillo-Rodriguez, E.; Hanus, L.O. Cannabidiol—Recent advances. Chem. Biodivers. 2007, 4, 1678–1692. [Google Scholar] [CrossRef] [PubMed]
- Borges, R.S.; Batista, J., Jr.; Viana, R.B.; Baetas, A.C.; Orestes, E.; Andrade, M.A.; Honorio, K.M.; da Silva, A.B. Understanding the molecular aspects of tetrahydrocannabinol and cannabidiol as antioxidants. Molecules 2013, 18, 12663–12674. [Google Scholar] [CrossRef] [PubMed]
- Hampson, A.J.; Grimaldi, M.; Axelrod, J.; Wink, D. Cannabidiol and (-)Delta9-tetrahydrocannabinol are neuroprotective antioxidants. Proc. Natl. Acad. Sci. USA 1998, 95, 8268–8273. [Google Scholar] [CrossRef] [PubMed]
- Yamaori, S.; Ebisawa, J.; Okushima, Y.; Yamamoto, I.; Watanabe, K. Potent inhibition of human cytochrome P450 3A isoforms by cannabidiol: Role of phenolic hydroxyl groups in the resorcinol moiety. Life Sci. 2011, 88, 730–736. [Google Scholar] [CrossRef] [PubMed]
- Huizenga, M.N.; Wicker, E.; Beck, V.C.; Forcelli, P.A. Anticonvulsant effect of cannabinoid receptor agonists in models of seizures in developing rats. Epilepsia 2017, 58, 1593–1602. [Google Scholar] [CrossRef]
- Cristino, L.; Bisogno, T.; Di Marzo, V. Cannabinoids and the expanded endocannabinoid system in neurological disorders. Nat. Rev. Neurol. 2020, 16, 9–29. [Google Scholar] [CrossRef]
- Wallace, M.J.; Blair, R.E.; Falenski, K.W.; Martin, B.R.; DeLorenzo, R.J. The endogenous cannabinoid system regulates seizure frequency and duration in a model of temporal lobe epilepsy. J. Pharmacol. Exp. Ther. 2003, 307, 129–137. [Google Scholar] [CrossRef]
- Alger, B.E. Retrograde signaling in the regulation of synaptic transmission: Focus on endocannabinoids. Prog. Neurobiology. 2002, 68, 247–286. [Google Scholar] [CrossRef] [PubMed]
- Schlicker, E.; Kathmann, M. Modulation of transmitter release via presynaptic cannabinoid receptors. Trends Pharmacol. Sci. 2001, 22, 565–572. [Google Scholar] [CrossRef]
- Laprairie, R.B.; Bagher, A.M.; Kelly, M.E.; Denovan-Wright, E.M. Cannabidiol is a negative allosteric modulator of the cannabinoid CB1 receptor. Br. J. Pharmacol. 2015, 172, 4790–4805. [Google Scholar] [CrossRef]
- Russo, E.B.; Burnett, A.; Hall, B.; Parker, K.K. Agonistic properties of cannabidiol at 5-HT1a receptors. Neurochem. Res. 2005, 30, 1037–1043. [Google Scholar] [CrossRef] [PubMed]
- Anand, U.; Jones, B.; Korchev, Y.; Bloom, S.R.; Pacchetti, B.; Anand, P.; Sodergren, M.H. CBD Effects on TRPV1 Signaling Pathways in Cultured DRG Neurons. J. Pain Res. 2020, 13, 2269–2278. [Google Scholar] [CrossRef]
- Shi, Q.X.; Yang, L.K.; Shi, W.L.; Wang, L.; Zhou, S.M.; Guan, S.Y.; Zhao, M.G.; Yang, Q. The novel cannabinoid receptor GPR55 mediates anxiolytic-like effects in the medial orbital cortex of mice with acute stress. Mol. Brain 2017, 10, 38. [Google Scholar] [CrossRef]
- Marichal-Cancino, B.A.; Fajardo-Valdez, A.; Ruiz-Contreras, A.E.; Mendez-Diaz, M.; Prospero-Garcia, O. Advances in the Physiology of GPR55 in the Central Nervous System. Curr. Neuropharmacol. 2017, 15, 771–778. [Google Scholar] [CrossRef] [PubMed]
- Armin, S.; Muenster, S.; Abood, M.; Benamar, K. GPR55 in the brain and chronic neuropathic pain. Behav. Brain Res. 2021, 406, 113248. [Google Scholar] [CrossRef]
- Do Val-da Silva, R.A.; Peixoto-Santos, J.E.; Kandratavicius, L.; De Ross, J.B.; Esteves, I.; De Martinis, B.S.; Alves, M.N.; Scandiuzzi, R.C.; Hallak, J.E.; Zuardi, A.W.; et al. Protective Effects of Cannabidiol against Seizures and Neuronal Death in a Rat Model of Mesial Temporal Lobe Epilepsy. Front. Pharmacol. 2017, 8, 131. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.A.; Shekh-Ahmad, T.; Khalil, A.; Walker, M.C.; Ali, A.B. Cannabidiol exerts antiepileptic effects by restoring hippocampal interneuron functions in a temporal lobe epilepsy model. Br. J. Pharmacol. 2018, 175, 2097–2115. [Google Scholar] [CrossRef]
- Legare, C.A.; Raup-Konsavage, W.M.; Vrana, K.E. Therapeutic Potential of Cannabis, Cannabidiol, and Cannabinoid-Based Pharmaceuticals. Pharmacology 2022, 107, 131–149. [Google Scholar] [CrossRef]
- Vrechi, T.A.M.; Leao, A.; Morais, I.B.M.; Abilio, V.C.; Zuardi, A.W.; Hallak, J.E.C.; Crippa, J.A.; Bincoletto, C.; Ureshino, R.P.; Smaili, S.S.; et al. Cannabidiol induces autophagy via ERK1/2 activation in neural cells. Sci. Rep. 2021, 11, 5434. [Google Scholar] [CrossRef]
- Gugliandolo, A.; Pollastro, F.; Bramanti, P.; Mazzon, E. Cannabidiol exerts protective effects in an in vitro model of Parkinson’s disease activating AKT/mTOR pathway. Fitoterapia 2020, 143, 104553. [Google Scholar] [CrossRef]
- Lujan, M.A.; Valverde, O. The Pro-neurogenic Effects of Cannabidiol and Its Potential Therapeutic Implications in Psychiatric Disorders. Front. Behav. Neurosci. 2020, 14, 109. [Google Scholar] [CrossRef] [PubMed]
- Lujan, M.A.; Castro-Zavala, A.; Alegre-Zurano, L.; Valverde, O. Repeated Cannabidiol treatment reduces cocaine intake and modulates neural proliferation and CB1R expression in the mouse hippocampus. Neuropharmacology 2018, 143, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Campos, A.C.; Ortega, Z.; Palazuelos, J.; Fogaca, M.V.; Aguiar, D.C.; Diaz-Alonso, J.; Ortega-Gutierrez, S.; Vazquez-Villa, H.; Moreira, F.A.; Guzman, M.; et al. The anxiolytic effect of cannabidiol on chronically stressed mice depends on hippocampal neurogenesis: Involvement of the endocannabinoid system. Int. J. Neuropsychopharmacol. 2013, 16, 1407–1419. [Google Scholar] [CrossRef]
- Lanza Cariccio, V.; Scionti, D.; Raffa, A.; Iori, R.; Pollastro, F.; Diomede, F.; Bramanti, P.; Trubiani, O.; Mazzon, E. Treatment of Periodontal Ligament Stem Cells with MOR and CBD Promotes Cell Survival and Neuronal Differentiation via the PI3K/Akt/mTOR Pathway. Int. J. Mol. Sci. 2018, 19, 2341. [Google Scholar] [CrossRef] [PubMed]
- Giacoppo, S.; Pollastro, F.; Grassi, G.; Bramanti, P.; Mazzon, E. Target regulation of PI3K/Akt/mTOR pathway by cannabidiol in treatment of experimental multiple sclerosis. Fitoterapia 2017, 116, 77–84. [Google Scholar] [CrossRef]
- Renard, J.; Loureiro, M.; Rosen, L.G.; Zunder, J.; de Oliveira, C.; Schmid, S.; Rushlow, W.J.; Laviolette, S.R. Cannabidiol Counteracts Amphetamine-Induced Neuronal and Behavioral Sensitization of the Mesolimbic Dopamine Pathway through a Novel mTOR/p70S6 Kinase Signaling Pathway. J. Neurosci. 2016, 36, 5160–5169. [Google Scholar] [CrossRef]
- Sagredo, O.; Ramos, J.A.; Decio, A.; Mechoulam, R.; Fernandez-Ruiz, J. Cannabidiol reduced the striatal atrophy caused 3-nitropropionic acid in vivo by mechanisms independent of the activation of cannabinoid, vanilloid TRPV1 and adenosine A2A receptors. Eur. J. Neurosci. 2007, 26, 843–851. [Google Scholar] [CrossRef] [PubMed]
- Jones, N.A.; Hill, A.J.; Smith, I.; Bevan, S.A.; Williams, C.M.; Whalley, B.J.; Stephens, G.J. Cannabidiol displays antiepileptiform and antiseizure properties in vitro and in vivo. J. Pharmacol. Exp. Ther. 2010, 332, 569–577. [Google Scholar] [CrossRef]
- Martinez-Aguirre, C.; Carmona-Cruz, F.; Velasco, A.L.; Velasco, F.; Aguado-Carrillo, G.; Cuellar-Herrera, M.; Rocha, L. Cannabidiol Acts at 5-HT(1A) Receptors in the Human Brain: Relevance for Treating Temporal Lobe Epilepsy. Front. Behav. Neurosci. 2020, 14, 611278. [Google Scholar] [CrossRef]
- Radley, J.J.; Jacobs, B.L. Pilocarpine-induced status epilepticus increases cell proliferation in the dentate gyrus of adult rats via a 5-HT1A receptor-dependent mechanism. Brain Res. 2003, 966, 1–12. [Google Scholar] [CrossRef]
- Schonhoff, K.; von Ruden, E.L.; Koska, I.; Seiffert, I.; Potschka, H. Hippocampal and Septal 5-HT(1A) Receptor Expression in Two Rat Models of Temporal Lobe Epilepsy. Neuroscience 2021, 465, 219–230. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Xu, Z.; Luo, H.; He, Q.; Diao, L.; Gui, X.; Wei, L. The association between 5-HT1A binding and temporal lobe epilepsy: A meta-analysis of molecular imaging studies. Epilepsy Behav. 2023, 145, 109354. [Google Scholar] [CrossRef] [PubMed]
- Pineda, E.A.; Hensler, J.G.; Sankar, R.; Shin, D.; Burke, T.F.; Mazarati, A.M. Plasticity of presynaptic and postsynaptic serotonin 1A receptors in an animal model of epilepsy-associated depression. Neuropsychopharmacology 2011, 36, 1305–1316. [Google Scholar] [CrossRef] [PubMed]
- Lazarini-Lopes, W.; Campos-Rodriguez, C.; Garcia-Cairasco, N.; N’Gouemo, P.; Forcelli, P.A. Cannabidiol attenuates generalized tonic-clonic and suppresses limbic seizures in the genetically epilepsy-prone rats (GEPR-3) strain. Pharmacol. Rep. 2023, 75, 166–176. [Google Scholar] [CrossRef]
- Barnes, J.P.; Dial, H.; Owens, W.; DeClercq, J.; Choi, L.; Shah, N.B.; Zuckerman, A.D.; Johnson, K. Adherence and discontinuation of prescription cannabidiol for the management of seizure disorders at an integrated care center. Epilepsy Res. 2024, 200, 107300. [Google Scholar] [CrossRef] [PubMed]
- Gill, E.W.; Paton, W.D.; Pertwee, R.G. Preliminary experiments on the chemistry and pharmacology of cannabis. Nature 1970, 228, 134–136. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.; Stevenson, L.A.; Wease, K.N.; Price, M.R.; Baillie, G.; Ross, R.A.; Pertwee, R.G. Evidence that the plant cannabinoid Delta9-tetrahydrocannabivarin is a cannabinoid CB1 and CB2 receptor antagonist. Br. J. Pharmacol. 2005, 146, 917–926. [Google Scholar] [CrossRef]
- Franco, R.; Rivas-Santisteban, R.; Reyes-Resina, I.; Casanovas, M.; Perez-Olives, C.; Ferreiro-Vera, C.; Navarro, G.; Sanchez de Medina, V.; Nadal, X. Pharmacological potential of varinic-, minor-, and acidic phytocannabinoids. Pharmacol. Res. 2020, 158, 104801. [Google Scholar] [CrossRef]
- Muller, C.; Morales, P.; Reggio, P.H. Cannabinoid Ligands Targeting TRP Channels. Front. Mol. Neurosci. 2018, 11, 487. [Google Scholar] [CrossRef]
- Cascio, M.G.; Zamberletti, E.; Marini, P.; Parolaro, D.; Pertwee, R.G. The phytocannabinoid, Delta(9)-tetrahydrocannabivarin, can act through 5-HT(1)A receptors to produce antipsychotic effects. Br. J. Pharmacol. 2015, 172, 1305–1318. [Google Scholar] [CrossRef]
- Garcia-Garcia, A.L.; Newman-Tancredi, A.; Leonardo, E.D. 5-HT(1A) [corrected] receptors in mood and anxiety: Recent insights into autoreceptor versus heteroreceptor function. Psychopharmacology 2014, 231, 623–636. [Google Scholar] [CrossRef] [PubMed]
- Glikmann-Johnston, Y.; Saling, M.M.; Reutens, D.C.; Stout, J.C. Hippocampal 5-HT1A Receptor and Spatial Learning and Memory. Front. Pharmacol. 2015, 6, 289. [Google Scholar] [CrossRef] [PubMed]
- Anavi-Goffer, S.; Baillie, G.; Irving, A.J.; Gertsch, J.; Greig, I.R.; Pertwee, R.G.; Ross, R.A. Modulation of L-alpha-lysophosphatidylinositol/GPR55 mitogen-activated protein kinase (MAPK) signaling by cannabinoids. J. Biol. Chem. 2012, 287, 91–104. [Google Scholar] [CrossRef]
- Dennis, I.; Whalley, B.J.; Stephens, G.J. Effects of Delta9-tetrahydrocannabivarin on [35S]GTPgammaS binding in mouse brain cerebellum and piriform cortex membranes. Br. J. Pharmacol. 2008, 154, 1349–1358. [Google Scholar] [CrossRef] [PubMed]
- Pertwee, R.G.; Thomas, A.; Stevenson, L.A.; Ross, R.A.; Varvel, S.A.; Lichtman, A.H.; Martin, B.R.; Razdan, R.K. The psychoactive plant cannabinoid, Delta9-tetrahydrocannabinol, is antagonized by Delta8- and Delta9-tetrahydrocannabivarin in mice in vivo. Br. J. Pharmacol. 2007, 150, 586–594. [Google Scholar] [CrossRef]
- Bolognini, D.; Costa, B.; Maione, S.; Comelli, F.; Marini, P.; Di Marzo, V.; Parolaro, D.; Ross, R.A.; Gauson, L.A.; Cascio, M.G.; et al. The plant cannabinoid Delta9-tetrahydrocannabivarin can decrease signs of inflammation and inflammatory pain in mice. Br. J. Pharmacol. 2010, 160, 677–687. [Google Scholar] [CrossRef] [PubMed]
- Janssens, A.; Silvestri, C.; Martella, A.; Vanoevelen, J.M.; Di Marzo, V.; Voets, T. Delta(9)-tetrahydrocannabivarin impairs epithelial calcium transport through inhibition of TRPV5 and TRPV6. Pharmacol. Res. 2018, 136, 83–89. [Google Scholar] [CrossRef]
- Hill, A.J.; Weston, S.E.; Jones, N.A.; Smith, I.; Bevan, S.A.; Williamson, E.M.; Stephens, G.J.; Williams, C.M.; Whalley, B.J. Delta(9)-Tetrahydrocannabivarin suppresses in vitro epileptiform and in vivo seizure activity in adult rats. Epilepsia 2010, 51, 1522–1532. [Google Scholar] [CrossRef]
- Ma, Y.L.; Weston, S.E.; Whalley, B.J.; Stephens, G.J. The phytocannabinoid Delta(9)-tetrahydrocannabivarin modulates inhibitory neurotransmission in the cerebellum. Br. J. Pharmacol. 2008, 154, 204–215. [Google Scholar] [CrossRef]
- Allendorfer, J.B.; Szaflarski, J.P. Neuroimaging studies towards understanding the central effects of pharmacological cannabis products on patients with epilepsy. Epilepsy Behav. 2017, 70 Pt B, 349–354. [Google Scholar] [CrossRef]
- Gaston, T.E.; Friedman, D. Pharmacology of cannabinoids in the treatment of epilepsy. Epilepsy Behav. 2017, 70 Pt B, 313–318. [Google Scholar] [CrossRef]
- Husni, A.S.; McCurdy, C.R.; Radwan, M.M.; Ahmed, S.A.; Slade, D.; Ross, S.A.; ElSohly, M.A.; Cutler, S.J. Evaluation of Phytocannabinoids from High Potency Cannabis sativa using In Vitro Bioassays to Determine Structure-Activity Relationships for Cannabinoid Receptor 1 and Cannabinoid Receptor 2. Med. Chem. Res. 2014, 23, 4295–4300. [Google Scholar] [CrossRef] [PubMed]
- Rosenthaler, S.; Pohn, B.; Kolmanz, C.; Huu, C.N.; Krewenka, C.; Huber, A.; Kranner, B.; Rausch, W.D.; Moldzio, R. Differences in receptor binding affinity of several phytocannabinoids do not explain their effects on neural cell cultures. Neurotoxicol. Teratol. 2014, 46, 49–56. [Google Scholar] [CrossRef]
- Navarro, G.; Varani, K.; Lillo, A.; Vincenzi, F.; Rivas-Santisteban, R.; Raich, I.; Reyes-Resina, I.; Ferreiro-Vera, C.; Borea, P.A.; Sanchez de Medina, V.; et al. Pharmacological data of cannabidiol- and cannabigerol-type phytocannabinoids acting on cannabinoid CB(1), CB(2) and CB(1)/CB(2) heteromer receptors. Pharmacol. Res. 2020, 159, 104940. [Google Scholar] [CrossRef] [PubMed]
- Zagzoog, A.; Mohamed, K.A.; Kim, H.J.J.; Kim, E.D.; Frank, C.S.; Black, T.; Jadhav, P.D.; Holbrook, L.A.; Laprairie, R.B. In vitro and in vivo pharmacological activity of minor cannabinoids isolated from Cannabis sativa. Sci. Rep. 2020, 10, 20405. [Google Scholar] [CrossRef] [PubMed]
- De Petrocellis, L.; Ligresti, A.; Moriello, A.S.; Allara, M.; Bisogno, T.; Petrosino, S.; Stott, C.G.; Di Marzo, V. Effects of cannabinoids and cannabinoid-enriched Cannabis extracts on TRP channels and endocannabinoid metabolic enzymes. Br. J. Pharmacol. 2011, 163, 1479–1494. [Google Scholar] [CrossRef] [PubMed]
- De Petrocellis, L.; Vellani, V.; Schiano-Moriello, A.; Marini, P.; Magherini, P.C.; Orlando, P.; Di Marzo, V. Plant-derived cannabinoids modulate the activity of transient receptor potential channels of ankyrin type-1 and melastatin type-8. J. Pharmacol. Exp. Ther. 2008, 325, 1007–1015. [Google Scholar] [CrossRef]
- Laun, A.S.; Shrader, S.H.; Song, Z.H. Novel inverse agonists for the orphan G protein-coupled receptor 6. Heliyon 2018, 4, e00933. [Google Scholar] [CrossRef]
- Devinsky, O.; Cilio, M.R.; Cross, H.; Fernandez-Ruiz, J.; French, J.; Hill, C.; Katz, R.; Di Marzo, V.; Jutras-Aswad, D.; Notcutt, W.G.; et al. Cannabidiol: Pharmacology and potential therapeutic role in epilepsy and other neuropsychiatric disorders. Epilepsia 2014, 55, 791–802. [Google Scholar] [CrossRef]
- Hill, A.J.; Mercier, M.S.; Hill, T.D.; Glyn, S.E.; Jones, N.A.; Yamasaki, Y.; Futamura, T.; Duncan, M.; Stott, C.G.; Stephens, G.J.; et al. Cannabidivarin is anticonvulsant in mouse and rat. Br. J. Pharmacol. 2012, 167, 1629–1642. [Google Scholar] [CrossRef]
- Huizenga, M.N.; Sepulveda-Rodriguez, A.; Forcelli, P.A. Preclinical safety and efficacy of cannabidivarin for early life seizures. Neuropharmacology 2019, 148, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Vigli, D.; Cosentino, L.; Raggi, C.; Laviola, G.; Woolley-Roberts, M.; De Filippis, B. Chronic treatment with the phytocannabinoid Cannabidivarin (CBDV) rescues behavioural alterations and brain atrophy in a mouse model of Rett syndrome. Neuropharmacology 2018, 140, 121–129. [Google Scholar] [CrossRef]
- Zamberletti, E.; Gabaglio, M.; Piscitelli, F.; Brodie, J.S.; Woolley-Roberts, M.; Barbiero, I.; Tramarin, M.; Binelli, G.; Landsberger, N.; Kilstrup-Nielsen, C.; et al. Cannabidivarin completely rescues cognitive deficits and delays neurological and motor defects in male Mecp2 mutant mice. J. Psychopharmacol. 2019, 33, 894–907. [Google Scholar] [CrossRef] [PubMed]
- Hill, T.D.; Cascio, M.G.; Romano, B.; Duncan, M.; Pertwee, R.G.; Williams, C.M.; Whalley, B.J.; Hill, A.J. Cannabidivarin-rich cannabis extracts are anticonvulsant in mouse and rat via a CB1 receptor-independent mechanism. Br. J. Pharmacol. 2013, 170, 679–692. [Google Scholar] [CrossRef]
- Amada, N.; Yamasaki, Y.; Williams, C.M.; Whalley, B.J. Cannabidivarin (CBDV) suppresses pentylenetetrazole (PTZ)-induced increases in epilepsy-related gene expression. PeerJ 2013, 1, e214. [Google Scholar] [CrossRef] [PubMed]
- Brodie, M.J.; Czapinski, P.; Pazdera, L.; Sander, J.W.; Toledo, M.; Napoles, M.; Sahebkar, F.; Schreiber, A.; Group, G.S. A Phase 2 Randomized Controlled Trial of the Efficacy and Safety of Cannabidivarin as Add-on Therapy in Participants with Inadequately Controlled Focal Seizures. Cannabis Cannabinoid Res. 2021, 6, 528–536. [Google Scholar] [CrossRef] [PubMed]
- Hurley, E.N.; Ellaway, C.J.; Johnson, A.M.; Truong, L.; Gordon, R.; Galettis, P.; Martin, J.H.; Lawson, J.A. Efficacy and safety of cannabidivarin treatment of epilepsy in girls with Rett syndrome: A phase 1 clinical trial. Epilepsia 2022, 63, 1736–1747. [Google Scholar] [CrossRef]
- Navarro, G.; Varani, K.; Reyes-Resina, I.; Sanchez de Medina, V.; Rivas-Santisteban, R.; Sanchez-Carnerero Callado, C.; Vincenzi, F.; Casano, S.; Ferreiro-Vera, C.; Canela, E.I.; et al. Cannabigerol Action at Cannabinoid CB(1) and CB(2) Receptors and at CB(1)-CB(2) Heteroreceptor Complexes. Front. Pharmacol. 2018, 9, 632. [Google Scholar] [CrossRef]
- Nadal, X.; Del Rio, C.; Casano, S.; Palomares, B.; Ferreiro-Vera, C.; Navarrete, C.; Sanchez-Carnerero, C.; Cantarero, I.; Bellido, M.L.; Meyer, S.; et al. Tetrahydrocannabinolic acid is a potent PPARgamma agonist with neuroprotective activity. Br. J. Pharmacol. 2017, 174, 4263–4276. [Google Scholar] [CrossRef]
- Cascio, M.G.; Gauson, L.A.; Stevenson, L.A.; Ross, R.A.; Pertwee, R.G. Evidence that the plant cannabinoid cannabigerol is a highly potent alpha2-adrenoceptor agonist and moderately potent 5HT1A receptor antagonist. Br. J. Pharmacol. 2010, 159, 129–141. [Google Scholar] [CrossRef]
- Hill, A.J.; Jones, N.A.; Smith, I.; Hill, C.L.; Williams, C.M.; Stephens, G.J.; Whalley, B.J. Voltage-gated sodium (NaV) channel blockade by plant cannabinoids does not confer anticonvulsant effects per se. Neurosci. Lett. 2014, 566, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Anderson, L.L.; Heblinski, M.; Absalom, N.L.; Hawkins, N.A.; Bowen, M.T.; Benson, M.J.; Zhang, F.; Bahceci, D.; Doohan, P.T.; Chebib, M.; et al. Cannabigerolic acid, a major biosynthetic precursor molecule in cannabis, exhibits divergent effects on seizures in mouse models of epilepsy. Br. J. Pharmacol. 2021, 178, 4826–4841. [Google Scholar] [CrossRef]
- Ghovanloo, M.R.; Estacion, M.; Higerd-Rusli, G.P.; Zhao, P.; Dib-Hajj, S.; Waxman, S.G. Inhibition of sodium conductance by cannabigerol contributes to a reduction of dorsal root ganglion neuron excitability. Br. J. Pharmacol. 2022, 179, 4010–4030. [Google Scholar] [CrossRef] [PubMed]
- Milligan, C.J.; Anderson, L.L.; McGregor, I.S.; Arnold, J.C.; Petrou, S. Beyond CBD: Inhibitory effects of lesser studied phytocannabinoids on human voltage-gated sodium channels. Front. Physiol. 2023, 14, 1081186. [Google Scholar] [CrossRef] [PubMed]
- Iannotti, F.A.; Hill, C.L.; Leo, A.; Alhusaini, A.; Soubrane, C.; Mazzarella, E.; Russo, E.; Whalley, B.J.; Di Marzo, V.; Stephens, G.J. Nonpsychotropic plant cannabinoids, cannabidivarin (CBDV) and cannabidiol (CBD), activate and desensitize transient receptor potential vanilloid 1 (TRPV1) channels in vitro: Potential for the treatment of neuronal hyperexcitability. ACS Chem. Neurosci. 2014, 5, 1131–1141. [Google Scholar] [CrossRef]
- Garcia, C.; Palomo-Garo, C.; Garcia-Arencibia, M.; Ramos, J.; Pertwee, R.; Fernandez-Ruiz, J. Symptom-relieving and neuroprotective effects of the phytocannabinoid Delta(9)-THCV in animal models of Parkinson’s disease. Br. J. Pharmacol. 2011, 163, 1495–1506. [Google Scholar] [CrossRef]
- Nalls, M.A.; Blauwendraat, C.; Vallerga, C.L.; Heilbron, K.; Bandres-Ciga, S.; Chang, D.; Tan, M.; Kia, D.A.; Noyce, A.J.; Xue, A.; et al. Identification of novel risk loci, causal insights, and heritable risk for Parkinson’s disease: A meta-analysis of genome-wide association studies. Lancet Neurol. 2019, 18, 1091–1102. [Google Scholar] [CrossRef]
- Mack, J.M.; Schamne, M.G.; Sampaio, T.B.; Pertile, R.A.; Fernandes, P.A.; Markus, R.P.; Prediger, R.D. Melatoninergic System in Parkinson’s Disease: From Neuroprotection to the Management of Motor and Nonmotor Symptoms. Oxid. Med. Cell. Longev. 2016, 2016, 3472032. [Google Scholar] [CrossRef]
- Braak, H.; Del Tredici, K. Neuropathological Staging of Brain Pathology in Sporadic Parkinson’s disease: Separating the Wheat from the Chaff. J. Parkinsons Dis. 2017, 7, S71–S85. [Google Scholar] [CrossRef]
- Yasuda, T.; Nakata, Y.; Mochizuki, H. alpha-Synuclein and neuronal cell death. Mol. Neurobiol. 2013, 47, 466–483. [Google Scholar] [CrossRef]
- Braak, H.; Ghebremedhin, E.; Rub, U.; Bratzke, H.; Del Tredici, K. Stages in the development of Parkinson’s disease-related pathology. Cell Tissue Res. 2004, 318, 121–134. [Google Scholar] [CrossRef] [PubMed]
- Garcia, C.; Palomo-Garo, C.; Gomez-Galvez, Y.; Fernandez-Ruiz, J. Cannabinoid-dopamine interactions in the physiology and physiopathology of the basal ganglia. Br. J. Pharmacol. 2016, 173, 2069–2079. [Google Scholar] [CrossRef] [PubMed]
- Oz, M.; Jaligam, V.; Galadari, S.; Petroianu, G.; Shuba, Y.M.; Shippenberg, T.S. The endogenous cannabinoid, anandamide, inhibits dopamine transporter function by a receptor-independent mechanism. J. Neurochem. 2010, 112, 1454–1464. [Google Scholar] [CrossRef] [PubMed]
- Pisani, A.; Fezza, F.; Galati, S.; Battista, N.; Napolitano, S.; Finazzi-Agro, A.; Bernardi, G.; Brusa, L.; Pierantozzi, M.; Stanzione, P.; et al. High endogenous cannabinoid levels in the cerebrospinal fluid of untreated Parkinson’s disease patients. Ann. Neurol. 2005, 57, 777–779. [Google Scholar] [CrossRef]
- Van Laere, K.; Casteels, C.; Lunskens, S.; Goffin, K.; Grachev, I.D.; Bormans, G.; Vandenberghe, W. Regional changes in type 1 cannabinoid receptor availability in Parkinson’s disease in vivo. Neurobiol. Aging 2012, 33, 620.e1–620.e8. [Google Scholar] [CrossRef]
- Song, L.; Yang, X.; Ma, Y.; Wu, N.; Liu, Z. The CB1 cannabinoid receptor agonist reduces L-DOPA-induced motor fluctuation and ERK1/2 phosphorylation in 6-OHDA-lesioned rats. Drug Des. Devel. Ther. 2014, 8, 2173–2179. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gomez-Galvez, Y.; Palomo-Garo, C.; Fernandez-Ruiz, J.; Garcia, C. Potential of the cannabinoid CB(2) receptor as a pharmacological target against inflammation in Parkinson’s disease. Prog. Neuropsychopharmacol. Biol. Psychiatry 2016, 64, 200–208. [Google Scholar] [CrossRef]
- Chagas, M.H.; Zuardi, A.W.; Tumas, V.; Pena-Pereira, M.A.; Sobreira, E.T.; Bergamaschi, M.M.; dos Santos, A.C.; Teixeira, A.L.; Hallak, J.E.; Crippa, J.A. Effects of cannabidiol in the treatment of patients with Parkinson’s disease: An exploratory double-blind trial. J. Psychopharmacol. 2014, 28, 1088–1098. [Google Scholar] [CrossRef]
- de Faria, S.M.; de Morais Fabricio, D.; Tumas, V.; Castro, P.C.; Ponti, M.A.; Hallak, J.E.; Zuardi, A.W.; Crippa, J.A.S.; Chagas, M.H.N. Effects of acute cannabidiol administration on anxiety and tremors induced by a Simulated Public Speaking Test in patients with Parkinson’s disease. J. Psychopharmacol. 2020, 34, 189–196. [Google Scholar] [CrossRef]
- Peball, M.; Krismer, F.; Knaus, H.G.; Djamshidian, A.; Werkmann, M.; Carbone, F.; Ellmerer, P.; Heim, B.; Marini, K.; Valent, D.; et al. Non-Motor Symptoms in Parkinson’s Disease are Reduced by Nabilone. Ann. Neurol. 2020, 88, 712–722. [Google Scholar] [CrossRef]
- Garcia-Arencibia, M.; Gonzalez, S.; de Lago, E.; Ramos, J.A.; Mechoulam, R.; Fernandez-Ruiz, J. Evaluation of the neuroprotective effect of cannabinoids in a rat model of Parkinson’s disease: Importance of antioxidant and cannabinoid receptor-independent properties. Brain Res. 2007, 1134, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Lastres-Becker, I.; Molina-Holgado, F.; Ramos, J.A.; Mechoulam, R.; Fernandez-Ruiz, J. Cannabinoids provide neuroprotection against 6-hydroxydopamine toxicity in vivo and in vitro: Relevance to Parkinson’s disease. Neurobiol. Dis. 2005, 19, 96–107. [Google Scholar] [CrossRef] [PubMed]
- Santos, N.A.; Martins, N.M.; Sisti, F.M.; Fernandes, L.S.; Ferreira, R.S.; Queiroz, R.H.; Santos, A.C. The neuroprotection of cannabidiol against MPP(+)-induced toxicity in PC12 cells involves trkA receptors, upregulation of axonal and synaptic proteins, neuritogenesis, and might be relevant to Parkinson’s disease. Toxicol. Vitr. 2015, 30 Pt B, 231–240. [Google Scholar] [CrossRef]
- Wang, L.; Wu, X.; Yang, G.; Hu, N.; Zhao, Z.; Zhao, L.; Li, S. Cannabidiol Alleviates the Damage to Dopaminergic Neurons in 1-Methyl-4-Phenyl-1,2,3,6-Tetrahydropyridine-Induced Parkinson’s Disease Mice Via Regulating Neuronal Apoptosis and Neuroinflammation. Neuroscience 2022, 498, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Mattos, B.A.; Bonato, J.M.; Splendor, M.C.; Del Bel, E.; Milani, H.; Oliveira, R.M.W. Cannabidiol improves nonmotor symptoms, attenuates neuroinflammation and favors hippocampal newborn neuronal maturation in a rat model of Parkinsonism. Acta Neuropsychiatr. 2024, 1–13. [Google Scholar] [CrossRef]
- Espadas, I.; Keifman, E.; Palomo-Garo, C.; Burgaz, S.; Garcia, C.; Fernandez-Ruiz, J.; Moratalla, R. Beneficial effects of the phytocannabinoid Delta(9)-THCV in L-DOPA-induced dyskinesia in Parkinson’s disease. Neurobiol. Dis. 2020, 141, 104892. [Google Scholar] [CrossRef]
- Burgaz, S.; Garcia, C.; Gomez-Canas, M.; Munoz, E.; Fernandez-Ruiz, J. Development of An Oral Treatment with the PPAR-gamma-Acting Cannabinoid VCE-003.2 Against the Inflammation-Driven Neuronal Deterioration in Experimental Parkinson’s Disease. Molecules 2019, 24, 2702. [Google Scholar] [CrossRef]
- Burgaz, S.; Garcia, C.; Gomez-Canas, M.; Navarrete, C.; Garcia-Martin, A.; Rolland, A.; Del Rio, C.; Casarejos, M.J.; Munoz, E.; Gonzalo-Consuegra, C.; et al. Neuroprotection with the cannabigerol quinone derivative VCE-003.2 and its analogs CBGA-Q and CBGA-Q-Salt in Parkinson’s disease using 6-hydroxydopamine-lesioned mice. Mol. Cell. Neurosci. 2021, 110, 103583. [Google Scholar] [CrossRef]
- Burgaz, S.; Garcia, C.; Gomez-Canas, M.; Rolland, A.; Munoz, E.; Fernandez-Ruiz, J. Neuroprotection with the Cannabidiol Quinone Derivative VCE-004.8 (EHP-101) against 6-Hydroxydopamine in Cell and Murine Models of Parkinson’s Disease. Molecules 2021, 26, 3245. [Google Scholar] [CrossRef]
- Wang, F.; Jin, T.; Li, H.; Long, H.; Liu, Y.; Jin, S.; Lu, Y.; Peng, Y.; Liu, C.; Zhao, L.; et al. Cannabidivarin alleviates alpha-synuclein aggregation via DAF-16 in Caenorhabditis elegans. FASEB J. 2023, 37, e22735. [Google Scholar] [CrossRef]
- Harrell, E.R.; King, J.W.; Stoeckel, L.E.; Trevino, M. National Institute on Aging’s 50th anniversary: Advancing cognitive aging research and the cognitive health of older adults. J. Gerontol. B Psychol. Sci. Soc. Sci. 2024, 79, gbae120. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, B.G.; Blazquez, C.; Gomez del Pulgar, T.; Guzman, M.; de Ceballos, M.L. Prevention of Alzheimer’s disease pathology by cannabinoids: Neuroprotection mediated by blockade of microglial activation. J. Neurosci. 2005, 25, 1904–1913. [Google Scholar] [CrossRef] [PubMed]
- Solas, M.; Francis, P.T.; Franco, R.; Ramirez, M.J. CB2 receptor and amyloid pathology in frontal cortex of Alzheimer’s disease patients. Neurobiol. Aging 2013, 34, 805–808. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Huang, Y.; Yu, L.; Ji, X.; Wu, J. Impact of the Cannabinoid System in Alzheimer’s Disease. Curr. Neuropharmacol. 2023, 21, 715–726. [Google Scholar] [CrossRef]
- Khavandi, M.; Rao, P.P.N.; Beazely, M.A. Differential Effects of Endocannabinoids on Amyloid-Beta Aggregation and Toxicity. Int. J. Mol. Sci. 2023, 24, 911. [Google Scholar] [CrossRef] [PubMed]
- Rapaka, D.; Adiukwu, P.C.; Challa, S.R.; Bitra, V.R. Interplay Between Astroglial Endocannabinoid System and the Cognitive Dysfunction in Alzheimer’s Disease. Physiol. Res. 2023, 72, 575–586. [Google Scholar] [CrossRef]
- Iuvone, T.; Esposito, G.; Esposito, R.; Santamaria, R.; Di Rosa, M.; Izzo, A.A. Neuroprotective effect of cannabidiol, a non-psychoactive component from Cannabis sativa, on beta-amyloid-induced toxicity in PC12 cells. J. Neurochem. 2004, 89, 134–141. [Google Scholar] [CrossRef]
- Vallee, A.; Lecarpentier, Y.; Guillevin, R.; Vallee, J.N. Effects of cannabidiol interactions with Wnt/beta-catenin pathway and PPARgamma on oxidative stress and neuroinflammation in Alzheimer’s disease. Acta Biochim. Biophys. Sin. 2017, 49, 853–866. [Google Scholar] [CrossRef]
- Zhang, N.; Parr, C.J.C.; Birch, A.M.; Goldfinger, M.H.; Sastre, M. The amyloid precursor protein binds to beta-catenin and modulates its cellular distribution. Neurosci. Lett. 2018, 685, 190–195. [Google Scholar] [CrossRef]
- Libro, R.; Diomede, F.; Scionti, D.; Piattelli, A.; Grassi, G.; Pollastro, F.; Bramanti, P.; Mazzon, E.; Trubiani, O. Cannabidiol Modulates the Expression of Alzheimer’s Disease-Related Genes in Mesenchymal Stem Cells. Int. J. Mol. Sci. 2016, 18, 26. [Google Scholar] [CrossRef]
- Li, H.; Liu, Y.; Tian, D.; Tian, L.; Ju, X.; Qi, L.; Wang, Y.; Liang, C. Overview of cannabidiol (CBD) and its analogues: Structures, biological activities, and neuroprotective mechanisms in epilepsy and Alzheimer’s disease. Eur. J. Med. Chem. 2020, 192, 112163. [Google Scholar] [CrossRef] [PubMed]
- Martin-Moreno, A.M.; Reigada, D.; Ramirez, B.G.; Mechoulam, R.; Innamorato, N.; Cuadrado, A.; de Ceballos, M.L. Cannabidiol and other cannabinoids reduce microglial activation in vitro and in vivo: Relevance to Alzheimer’s disease. Mol. Pharmacol. 2011, 79, 964–973. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.; Low, J.K.; Logge, W.; Garner, B.; Karl, T. Chronic cannabidiol treatment improves social and object recognition in double transgenic APPswe/PS1∆E9 mice. Psychopharmacology 2014, 231, 3009–3017. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.; Spiro, A.S.; Jenner, A.M.; Garner, B.; Karl, T. Long-term cannabidiol treatment prevents the development of social recognition memory deficits in Alzheimer’s disease transgenic mice. J. Alzheimer’s Dis. 2014, 42, 1383–1396. [Google Scholar] [CrossRef] [PubMed]
- Arnanz, M.A.; Ruiz de Martin Esteban, S.; Martinez Relimpio, A.M.; Rimmerman, N.; Tweezer Zaks, N.; Grande, M.T.; Romero, J. Effects of Chronic, Low-Dose Cannabinoids, Cannabidiol, Delta-9-Tetrahydrocannabinol and a Combination of Both, on Amyloid Pathology in the 5xFAD Mouse Model of Alzheimer’s Disease. Cannabis Cannabinoid Res. 2023, 9, 1312–1325. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Neary, J.P. Neuroprotection Following Concussion: The Potential Role for Cannabidiol. Can. J. Neurol. Sci. 2020, 47, 289–300. [Google Scholar] [CrossRef]
- Ma, B.Q.; Jia, J.X.; Wang, H.; Li, S.J.; Yang, Z.J.; Wang, X.X.; Yan, X.S. Cannabidiol improves the cognitive function of SAMP8 AD model mice involving the microbiota-gut-brain axis. J. Toxicol. Environ. Health A 2024, 87, 471–479. [Google Scholar] [CrossRef]
- Velayudhan, L.; Dugonjic, M.; Pisani, S.; Harborow, L.; Aarsland, D.; Bassett, P.; Bhattacharyya, S. Cannabidiol for behavior symptoms in Alzheimer’s disease (CANBiS-AD): A randomized, double-blind, placebo-controlled trial. Int. Psychogeriatr. 2024. Online ahead of print. [Google Scholar] [CrossRef]
- Amini, M.; Abdolmaleki, Z. The Effect of Cannabidiol Coated by Nano-Chitosan on Learning and Memory, Hippocampal CB1 and CB2 Levels, and Amyloid Plaques in an Alzheimer’s Disease Rat Model. Neuropsychobiology 2022, 81, 171–183. [Google Scholar] [CrossRef]
- Novotna, A.; Mares, J.; Ratcliffe, S.; Novakova, I.; Vachova, M.; Zapletalova, O.; Gasperini, C.; Pozzilli, C.; Cefaro, L.; Comi, G.; et al. A randomized, double-blind, placebo-controlled, parallel-group, enriched-design study of nabiximols* (Sativex(®)), as add-on therapy, in subjects with refractory spasticity caused by multiple sclerosis. Eur. J. Neurol. 2011, 18, 1122–1131. [Google Scholar] [CrossRef]
- Broers, B.; Pata, Z.; Mina, A.; Wampfler, J.; de Saussure, C.; Pautex, S. Prescription of a THC/CBD-Based Medication to Patients with Dementia: A Pilot Study in Geneva. Med. Cannabis Cannabinoids 2019, 2, 56–59. [Google Scholar] [CrossRef] [PubMed]
- Schubert, D.; Kepchia, D.; Liang, Z.; Dargusch, R.; Goldberg, J.; Maher, P. Efficacy of Cannabinoids in a Pre-Clinical Drug-Screening Platform for Alzheimer’s Disease. Mol. Neurobiol. 2019, 56, 7719–7730. [Google Scholar] [CrossRef] [PubMed]
- Marsh, D.T.; Sugiyama, A.; Imai, Y.; Kato, R.; Smid, S.D. The structurally diverse phytocannabinoids cannabichromene, cannabigerol and cannabinol significantly inhibit amyloid beta-evoked neurotoxicity and changes in cell morphology in PC12 cells. Basic Clin. Pharmacol. Toxicol. 2024, 134, 293–309. [Google Scholar] [CrossRef] [PubMed]
- Englund, A.; Atakan, Z.; Kralj, A.; Tunstall, N.; Murray, R.; Morrison, P. The effect of five day dosing with THCV on THC-induced cognitive, psychological and physiological effects in healthy male human volunteers: A placebo-controlled, double-blind, crossover pilot trial. J. Psychopharmacol. 2016, 30, 140–151. [Google Scholar] [CrossRef]
- Puopolo, T.; Liu, C.; Ma, H.; Seeram, N.P. Inhibitory Effects of Cannabinoids on Acetylcholinesterase and Butyrylcholinesterase Enzyme Activities. Med. Cannabis Cannabinoids 2022, 5, 85–94. [Google Scholar] [CrossRef]
- Melnikova, I. Therapies for Alzheimer’s disease. Nat. Rev. Drug Discov. 2007, 6, 341–342. [Google Scholar] [CrossRef]
- McColgan, P.; Tabrizi, S.J. Huntington’s disease: A clinical review. Eur. J. Neurol. 2018, 25, 24–34. [Google Scholar] [CrossRef]
- Denovan-Wright, E.M.; Robertson, H.A. Cannabinoid receptor messenger RNA levels decrease in a subset of neurons of the lateral striatum, cortex and hippocampus of transgenic Huntington’s disease mice. Neuroscience 2000, 98, 705–713. [Google Scholar] [CrossRef] [PubMed]
- Glass, M.; Dragunow, M.; Faull, R.L. The pattern of neurodegeneration in Huntington’s disease: A comparative study of cannabinoid, dopamine, adenosine and GABA(A) receptor alterations in the human basal ganglia in Huntington’s disease. Neuroscience. 2000, 97, 505–519. [Google Scholar] [CrossRef]
- Van Laere, K.; Casteels, C.; Dhollander, I.; Goffin, K.; Grachev, I.; Bormans, G.; Vandenberghe, W. Widespread decrease of type 1 cannabinoid receptor availability in Huntington disease in vivo. J. Nucl. Med. 2010, 51, 1413–1417. [Google Scholar] [CrossRef]
- Laprairie, R.B.; Kelly, M.E.; Denovan-Wright, E.M. Cannabinoids increase type 1 cannabinoid receptor expression in a cell culture model of striatal neurons: Implications for Huntington’s disease. Neuropharmacology 2013, 72, 47–57. [Google Scholar] [CrossRef] [PubMed]
- McCaw, E.A.; Hu, H.; Gomez, G.T.; Hebb, A.L.; Kelly, M.E.; Denovan-Wright, E.M. Structure, expression and regulation of the cannabinoid receptor gene (CB1) in Huntington’s disease transgenic mice. Eur. J. Biochem. 2004, 271, 4909–4920. [Google Scholar] [CrossRef] [PubMed]
- Blazquez, C.; Chiarlone, A.; Sagredo, O.; Aguado, T.; Pazos, M.R.; Resel, E.; Palazuelos, J.; Julien, B.; Salazar, M.; Borner, C.; et al. Loss of striatal type 1 cannabinoid receptors is a key pathogenic factor in Huntington’s disease. Brain 2011, 134 Pt 1, 119–136. [Google Scholar] [CrossRef] [PubMed]
- Chiarlone, A.; Bellocchio, L.; Blazquez, C.; Resel, E.; Soria-Gomez, E.; Cannich, A.; Ferrero, J.J.; Sagredo, O.; Benito, C.; Romero, J.; et al. A restricted population of CB1 cannabinoid receptors with neuroprotective activity. Proc. Natl. Acad. Sci. USA 2014, 111, 8257–8262. [Google Scholar] [CrossRef]
- Consroe, P.; Laguna, J.; Allender, J.; Snider, S.; Stern, L.; Sandyk, R.; Kennedy, K.; Schram, K. Controlled clinical trial of cannabidiol in Huntington’s disease. Pharmacol. Biochem. Behav. 1991, 40, 701–708. [Google Scholar] [CrossRef]
- Valdeolivas, S.; Satta, V.; Pertwee, R.G.; Fernandez-Ruiz, J.; Sagredo, O. Sativex-like combination of phytocannabinoids is neuroprotective in malonate-lesioned rats, an inflammatory model of Huntington’s disease: Role of CB1 and CB2 receptors. ACS Chem. Neurosci. 2012, 3, 400–406. [Google Scholar] [CrossRef]
- Sagredo, O.; Pazos, M.R.; Satta, V.; Ramos, J.A.; Pertwee, R.G.; Fernandez-Ruiz, J. Neuroprotective effects of phytocannabinoid-based medicines in experimental models of Huntington’s disease. J. Neurosci. Res. 2011, 89, 1509–1518. [Google Scholar] [CrossRef]
- Ruhaak, L.R.; Felth, J.; Karlsson, P.C.; Rafter, J.J.; Verpoorte, R.; Bohlin, L. Evaluation of the cyclooxygenase inhibiting effects of six major cannabinoids isolated from Cannabis sativa. Biol. Pharm. Bull. 2011, 34, 774–778. [Google Scholar] [CrossRef]
- Valdeolivas, S.; Navarrete, C.; Cantarero, I.; Bellido, M.L.; Munoz, E.; Sagredo, O. Neuroprotective properties of cannabigerol in Huntington’s disease: Studies in R6/2 mice and 3-nitropropionate-lesioned mice. Neurotherapeutics 2015, 12, 185–199. [Google Scholar] [CrossRef]
- Gugliandolo, A.; Silvestro, S.; Chiricosta, L.; Pollastro, F.; Bramanti, P.; Mazzon, E. The Transcriptomic Analysis of NSC-34 Motor Neuron-Like Cells Reveals That Cannabigerol Influences Synaptic Pathways: A Comparative Study with Cannabidiol. Life 2020, 10, 227. [Google Scholar] [CrossRef]
- de Lago, E.; Urbani, P.; Ramos, J.A.; Di Marzo, V.; Fernandez-Ruiz, J. Arvanil, a hybrid endocannabinoid and vanilloid compound, behaves as an antihyperkinetic agent in a rat model of Huntington’s disease. Brain Res. 2005, 1050, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Lastres-Becker, I.; Bizat, N.; Boyer, F.; Hantraye, P.; Brouillet, E.; Fernandez-Ruiz, J. Effects of cannabinoids in the rat model of Huntington’s disease generated by an intrastriatal injection of malonate. Neuroreport 2003, 14, 813–816. [Google Scholar] [CrossRef] [PubMed]
- de Lago, E.; Fernandez-Ruiz, J.; Ortega-Gutierrez, S.; Cabranes, A.; Pryce, G.; Baker, D.; Lopez-Rodriguez, M.; Ramos, J.A. UCM707, an inhibitor of the anandamide uptake, behaves as a symptom control agent in models of Huntington’s disease and multiple sclerosis, but fails to delay/arrest the progression of different motor-related disorders. Eur. Neuropsychopharmacol. 2006, 16, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Lastres-Becker, I.; Fezza, F.; Cebeira, M.; Bisogno, T.; Ramos, J.A.; Milone, A.; Fernandez-Ruiz, J.; Di Marzo, V. Changes in endocannabinoid transmission in the basal ganglia in a rat model of Huntington’s disease. Neuroreport 2001, 12, 2125–2129. [Google Scholar] [CrossRef] [PubMed]
- Borrelli, F.; Fasolino, I.; Romano, B.; Capasso, R.; Maiello, F.; Coppola, D.; Orlando, P.; Battista, G.; Pagano, E.; Di Marzo, V.; et al. Beneficial effect of the non-psychotropic plant cannabinoid cannabigerol on experimental inflammatory bowel disease. Biochem. Pharmacol. 2013, 85, 1306–1316. [Google Scholar] [CrossRef]
- Nachnani, R.; Raup-Konsavage, W.M.; Vrana, K.E. The Pharmacological Case for Cannabigerol. J. Pharmacol. Exp. Ther. 2021, 376, 204–212. [Google Scholar] [CrossRef]
- Starkus, J.; Jansen, C.; Shimoda, L.M.N.; Stokes, A.J.; Small-Howard, A.L.; Turner, H. Diverse TRPV1 responses to cannabinoids. Channels 2019, 13, 172–191. [Google Scholar] [CrossRef]
- de Almeida, D.L.; Devi, L.A. Diversity of molecular targets and signaling pathways for CBD. Pharmacol. Res. Perspect. 2020, 8, e00682. [Google Scholar] [CrossRef]
- Di Marzo, V.; Piscitelli, F. The Endocannabinoid System and its Modulation by Phytocannabinoids. Neurotherapeutics 2015, 12, 692–698. [Google Scholar] [CrossRef]
- Ramer, R.; Heinemann, K.; Merkord, J.; Rohde, H.; Salamon, A.; Linnebacher, M.; Hinz, B. COX-2 and PPAR-gamma confer cannabidiol-induced apoptosis of human lung cancer cells. Mol. Cancer Ther. 2013, 12, 69–82. [Google Scholar] [CrossRef]
- Deiana, S.; Watanabe, A.; Yamasaki, Y.; Amada, N.; Arthur, M.; Fleming, S.; Woodcock, H.; Dorward, P.; Pigliacampo, B.; Close, S.; et al. Plasma and brain pharmacokinetic profile of cannabidiol (CBD), cannabidivarine (CBDV), Delta(9)-tetrahydrocannabivarin (THCV) and cannabigerol (CBG) in rats and mice following oral and intraperitoneal administration and CBD action on obsessive-compulsive behaviour. Psychopharmacology 2012, 219, 859–873. [Google Scholar] [CrossRef] [PubMed]
- Glass, M.; Dragunow, M.; Faull, R.L. Cannabinoid receptors in the human brain: A detailed anatomical and quantitative autoradiographic study in the fetal, neonatal and adult human brain. Neuroscience 1997, 77, 299–318. [Google Scholar] [CrossRef] [PubMed]
- Basavarajappa, B.S.; Joshi, V.; Shivakumar, M.; Subbanna, S. Distinct Functions of Endogenous Cannabinoid System in Alcohol Abuse Disorders. Br. J. Pharmacol. 2019, 176, 3085–3109. [Google Scholar] [CrossRef] [PubMed]
- Parsons, L.H.; Hurd, Y.L. Endocannabinoid signalling in reward and addiction. Nat. Rev. Neurosci. 2015, 16, 579–594. [Google Scholar] [CrossRef]
- Stern, C.A.J.; de Carvalho, C.R.; Bertoglio, L.J.; Takahashi, R.N. Effects of Cannabinoid Drugs on Aversive or Rewarding Drug-Associated Memory Extinction and Reconsolidation. Neuroscience 2018, 370, 62–80. [Google Scholar] [CrossRef]
- Sabia, S.; Fayosse, A.; Dumurgier, J.; Dugravot, A.; Akbaraly, T.; Britton, A.; Kivimaki, M.; Singh-Manoux, A. Alcohol consumption and risk of dementia: 23 year follow-up of Whitehall II cohort study. BMJ 2018, 362, k2927. [Google Scholar] [CrossRef]
- Stampfer, M.J.; Kang, J.H.; Chen, J.; Cherry, R.; Grodstein, F. Effects of moderate alcohol consumption on cognitive function in women. N. Engl. J. Med. 2005, 352, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.J.; Fang, Z.Y.; Yang, Y.; Deng, H.M.; Wang, J.Z. Alzheimer-like phosphorylation of tau and neurofilament induced by cocaine in vivo. Acta Pharmacol. Sin. 2003, 24, 512–518. [Google Scholar]
- Shukla, M.; Vincent, B. The multi-faceted impact of methamphetamine on Alzheimer’s disease: From a triggering role to a possible therapeutic use. Ageing Res. Rev. 2020, 60, 101062. [Google Scholar] [CrossRef]
- Morgan, C.J.; Das, R.K.; Joye, A.; Curran, H.V.; Kamboj, S.K. Cannabidiol reduces cigarette consumption in tobacco smokers: Preliminary findings. Addict. Behav. 2013, 38, 2433–2436. [Google Scholar] [CrossRef]
- Hindocha, C.; Freeman, T.P.; Grabski, M.; Stroud, J.B.; Crudgington, H.; Davies, A.C.; Das, R.K.; Lawn, W.; Morgan, C.J.A.; Curran, H.V. Cannabidiol reverses attentional bias to cigarette cues in a human experimental model of tobacco withdrawal. Addiction 2018, 113, 1696–1705. [Google Scholar] [CrossRef] [PubMed]
- Hindocha, C.; Freeman, T.P.; Grabski, M.; Crudgington, H.; Davies, A.C.; Stroud, J.B.; Das, R.K.; Lawn, W.; Morgan, C.J.A.; Curran, H.V. The effects of cannabidiol on impulsivity and memory during abstinence in cigarette dependent smokers. Sci. Rep. 2018, 8, 7568. [Google Scholar] [CrossRef] [PubMed]
- Gournay, L.R.; Petry, J.; Bilsky, S.; Hill, M.A.; Feldner, M.; Peters, E.; Bonn-Miller, M.; Leen-Feldner, E. Cannabidiol Reduces Nicotine Withdrawal Severity and State Anxiety During an Acute E-cigarette Abstinence Period: A Novel, Open-Label Study. Cannabis Cannabinoid Res. 2023, 9, 996–1005. [Google Scholar] [CrossRef] [PubMed]
- Cheeks, S.N.; Buzzi, B.; Valdez, A.; Mogul, A.S.; Damaj, M.I.; Fowler, C.D. Cannabidiol as a potential cessation therapeutic: Effects on intravenous nicotine self-administration and withdrawal symptoms in mice. Neuropharmacology 2024, 246, 109833. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.C.; Tieu, L.; Suhandynata, R.T.; Boomhower, B.; Hoffman, M.; Sepulveda, Y.; Carrette, L.L.G.; Momper, J.D.; Fitzgerald, R.L.; Hanham, K.; et al. Cannabidiol reduces withdrawal symptoms in nicotine-dependent rats. Psychopharmacology 2021, 238, 2201–2211. [Google Scholar] [CrossRef]
- Black, N.; Stockings, E.; Campbell, G.; Tran, L.T.; Zagic, D.; Hall, W.D.; Farrell, M.; Degenhardt, L. Cannabinoids for the treatment of mental disorders and symptoms of mental disorders: A systematic review and meta-analysis. Lancet Psychiatry 2019, 6, 995–1010. [Google Scholar] [CrossRef]
- Hindocha, C.; Freeman, T.P.; Schafer, G.; Gardner, C.; Bloomfield, M.A.P.; Bramon, E.; Morgan, C.J.A.; Curran, H.V. Acute effects of cannabinoids on addiction endophenotypes are moderated by genes encoding the CB1 receptor and FAAH enzyme. Addict. Biol. 2020, 25, e12762. [Google Scholar] [CrossRef]
- Broyd, S.J.; van Hell, H.H.; Beale, C.; Yucel, M.; Solowij, N. Acute and Chronic Effects of Cannabinoids on Human Cognition-A Systematic Review. Biol. Psychiatry 2016, 79, 557–567. [Google Scholar] [CrossRef]
- Freeman, A.M.; Petrilli, K.; Lees, R.; Hindocha, C.; Mokrysz, C.; Curran, H.V.; Saunders, R.; Freeman, T.P. How does cannabidiol (CBD) influence the acute effects of delta-9-tetrahydrocannabinol (THC) in humans? A systematic review. Neurosci. Biobehav. Rev. 2019, 107, 696–712. [Google Scholar] [CrossRef]
- Pokorski, I.; Clement, N.; Phung, N.; Weltman, M.; Fu, S.; Copelad, J. Cannabidiol in the management of inpatient cannabis withdrawal: Clinical case series. Future Neurol. 2017, 12, 133–140. [Google Scholar] [CrossRef]
- Crippa, J.A.; Hallak, J.E.; Machado-de-Sousa, J.P.; Queiroz, R.H.; Bergamaschi, M.; Chagas, M.H.; Zuardi, A.W. Cannabidiol for the treatment of cannabis withdrawal syndrome: A case report. J. Clin. Pharm. Ther. 2013, 38, 162–164. [Google Scholar] [CrossRef] [PubMed]
- Shannon, S.; Opila-Lehman, J. Cannabidiol Oil for Decreasing Addictive Use of Marijuana: A Case Report. Integr. Med. 2015, 14, 31–35. [Google Scholar]
- Cleirec, G.; Desmier, E.; Lacatus, C.; Lesgourgues, S.; Braun, A.; Peloso, C.; Obadia, C. Efficiency of Inhaled Cannabidiol in Cannabis Use Disorder: The Pilot Study Cannavap. Front. Psychiatry 2022, 13, 899221. [Google Scholar] [CrossRef] [PubMed]
- Solowij, N.; Broyd, S.J.; Beale, C.; Prick, J.A.; Greenwood, L.M.; van Hell, H.; Suo, C.; Galettis, P.; Pai, N.; Fu, S.; et al. Therapeutic Effects of Prolonged Cannabidiol Treatment on Psychological Symptoms and Cognitive Function in Regular Cannabis Users: A Pragmatic Open-Label Clinical Trial. Cannabis Cannabinoid Res. 2018, 3, 21–34. [Google Scholar] [CrossRef] [PubMed]
- Lorenzetti, V.; McTavish, E.; Broyd, S.; van Hell, H.; Thomson, D.; Ganella, E.; Kottaram, A.R.; Beale, C.; Martin, J.; Galettis, P.; et al. Daily Cannabidiol Administration for 10 Weeks Modulates Hippocampal and Amygdalar Resting-State Functional Connectivity in Cannabis Users: A Functional Magnetic Resonance Imaging Open-Label Clinical Trial. Cannabis Cannabinoid Res. 2023, 9, e1108–e1121. [Google Scholar] [CrossRef] [PubMed]
- de Carvalho, C.R.; Takahashi, R.N. Cannabidiol disrupts the reconsolidation of contextual drug-associated memories in Wistar rats. Addict. Biol. 2017, 22, 742–751. [Google Scholar] [CrossRef]
- Ren, Y.; Whittard, J.; Higuera-Matas, A.; Morris, C.V.; Hurd, Y.L. Cannabidiol, a nonpsychotropic component of cannabis, inhibits cue-induced heroin seeking and normalizes discrete mesolimbic neuronal disturbances. J. Neurosci. 2009, 29, 14764–14769. [Google Scholar] [CrossRef]
- Navarrete, F.; Gasparyan, A.; Manzanares, J. CBD-mediated regulation of heroin withdrawal-induced behavioural and molecular changes in mice. Addict. Biol. 2022, 27, e13150. [Google Scholar] [CrossRef]
- Scicluna, R.L.; Wilson, B.B.; Thelaus, S.H.; Arnold, J.C.; McGregor, I.S.; Bowen, M.T. Cannabidiol Reduced the Severity of Gastrointestinal Symptoms of Opioid Withdrawal in Male and Female Mice. Cannabis Cannabinoid Res. 2024, 9, 547–560. [Google Scholar] [CrossRef]
- Hayduk, S.A.; Hughes, A.C.; Winter, R.L.; Milton, M.D.; Ward, S.J. Single and Combined Effects of Cannabigerol (CBG) and Cannabidiol (CBD) in Mouse Models of Oxaliplatin-Associated Mechanical Sensitivity, Opioid Antinociception, and Naloxone-Precipitated Opioid Withdrawal. Biomedicines 2024, 12, 1145. [Google Scholar] [CrossRef]
- Socha, J.; Grochecki, P.; Marszalek-Grabska, M.; Skrok, A.; Smaga, I.; Slowik, T.; Prazmo, W.; Kotlinski, R.; Filip, M.; Kotlinska, J.H. Cannabidiol Protects against the Reinstatement of Oxycodone-Induced Conditioned Place Preference in Adolescent Male but Not Female Rats: The Role of MOR and CB1R. Int. J. Mol. Sci. 2024, 25, 6651. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Ward, S.J. Paclitaxel-Associated Mechanical Sensitivity and Neuroinflammation Are Sex-, Time-, and Site-Specific and Prevented through Cannabigerol Administration in C57Bl/6 Mice. Int. J. Mol. Sci. 2024, 25, 4277. [Google Scholar] [CrossRef]
- Guimaraes, F.S.; Chiaretti, T.M.; Graeff, F.G.; Zuardi, A.W. Antianxiety effect of cannabidiol in the elevated plus-maze. Psychopharmacology 1990, 100, 558–559. [Google Scholar] [CrossRef] [PubMed]
- Almeida, V.; Levin, R.; Peres, F.F.; Niigaki, S.T.; Calzavara, M.B.; Zuardi, A.W.; Hallak, J.E.; Crippa, J.A.; Abilio, V.C. Cannabidiol exhibits anxiolytic but not antipsychotic property evaluated in the social interaction test. Prog. Neuropsychopharmacol. Biol. Psychiatry 2013, 41, 30–35. [Google Scholar] [CrossRef]
- Arnone, M.; Maruani, J.; Chaperon, F.; Thiebot, M.; Poncelet, M.; Soubrie, P.; Le Fur, G. Selective inhibition of sucrose and ethanol intake by SR 141716, an antagonist of central cannabinoid (CB1) receptors. Psychopharmacology. 1997, 132, 104–106. [Google Scholar] [CrossRef] [PubMed]
- Basavarajappa, B.S. Endocannabinoid system in the development of tolerance to alcohol. Klin. Forsch. J. Clin. Res. 2005, 11, 16–19. [Google Scholar] [CrossRef][Green Version]
- Colombo, G.; Agabio, R.; Fa, M.; Guano, L.; Lobina, C.; Loche, A.; Reali, R.; Gessa, G. Reduction of voluntary ethanol intake in ethanol-preferring sP rats by the cannabinoid antagonist SR-141716. Alcohol Alcohol. 1998, 33, 126–130. [Google Scholar] [CrossRef]
- Hungund, B.L.; Szakall, I.; Adam, A.; Basavarajappa, B.S.; Vadasz, C. Cannabinoid CB1 receptor knockout mice exhibit markedly reduced voluntary alcohol consumption and lack alcohol-induced dopamine release in the nucleus accumbens. J. Neurochem. 2003, 84, 698–704. [Google Scholar] [CrossRef]
- Hamelink, C.; Hampson, A.; Wink, D.A.; Eiden, L.E.; Eskay, R.L. Comparison of cannabidiol, antioxidants, and diuretics in reversing binge ethanol-induced neurotoxicity. J. Pharmacol. Exp. Ther. 2005, 314, 780–788. [Google Scholar] [CrossRef]
- Liput, D.J.; Hammell, D.C.; Stinchcomb, A.L.; Nixon, K. Transdermal delivery of cannabidiol attenuates binge alcohol-induced neurodegeneration in a rodent model of an alcohol use disorder. Pharmacol. Biochem. Behav. 2013, 111, 120–127. [Google Scholar] [CrossRef]
- Brenneman, D.E.; Petkanas, D.; Kinney, W.A. Pharmacological Comparisons Between Cannabidiol and KLS-13019. J. Mol. Neurosci. 2018, 66, 121–134. [Google Scholar] [CrossRef] [PubMed]
- Oberlin, B.G.; Grahame, N.J. High-alcohol preferring mice are more impulsive than low-alcohol preferring mice as measured in the delay discounting task. Alcohol. Clin. Exp. Res. 2009, 33, 1294–1303. [Google Scholar] [CrossRef]
- Wilhelm, C.J.; Mitchell, S.H. Strain differences in delay discounting using inbred rats. Genes Brain Behav. 2009, 8, 426–434. [Google Scholar] [CrossRef] [PubMed]
- Amlung, M.; Vedelago, L.; Acker, J.; Balodis, I.; MacKillop, J. Steep delay discounting and addictive behavior: A meta-analysis of continuous associations. Addiction 2017, 112, 51–62. [Google Scholar] [CrossRef]
- MacKillop, J.; Amlung, M.T.; Few, L.R.; Ray, L.A.; Sweet, L.H.; Munafo, M.R. Delayed reward discounting and addictive behavior: A meta-analysis. Psychopharmacology 2011, 216, 305–321. [Google Scholar] [CrossRef]
- Viudez-Martinez, A.; Garcia-Gutierrez, M.S.; Navarron, C.M.; Morales-Calero, M.I.; Navarrete, F.; Torres-Suarez, A.I.; Manzanares, J. Cannabidiol reduces ethanol consumption, motivation and relapse in mice. Addict. Biol. 2018, 23, 154–164. [Google Scholar] [CrossRef]
- Karoly, H.C.; Drennan, M.L.; Prince, M.A.; Zulic, L.; Dooley, G. Consuming oral cannabidiol prior to a standard alcohol dose has minimal effect on breath alcohol level and subjective effects of alcohol. Psychopharmacology 2023, 240, 1119–1129. [Google Scholar] [CrossRef]
- Maccioni, P.; Bratzu, J.; Carai, M.A.M.; Colombo, G.; Gessa, G.L. Reducing Effect of Cannabidiol on Alcohol Self-Administration in Sardinian Alcohol-Preferring Rats. Cannabis Cannabinoid Res. 2022, 7, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Gasparyan, A.; Navarrete, F.; Navarro, D.; Manzanares, J. Cannabidiol regulates behavioral and brain alterations induced by spontaneous alcohol withdrawal. Neuropharmacology 2023, 233, 109549. [Google Scholar] [CrossRef]
- Tringali, G.; Lavanco, G.; Castelli, V.; Pizzolanti, G.; Kuchar, M.; Curro, D.; Cannizzaro, C.; Brancato, A. Cannabidiol tempers alcohol intake and neuroendocrine and behavioural correlates in alcohol binge drinking adolescent rats. Focus on calcitonin gene-related peptide’s brain levels. Phytother. Res. 2023, 37, 4870–4884. [Google Scholar] [CrossRef]
- Brancato, A.; Castelli, V.; Lavanco, G.; D’Amico, C.; Feo, S.; Pizzolanti, G.; Kuchar, M.; Cannizzaro, C. Social stress under binge-like alcohol withdrawal in adolescence: Evidence of cannabidiol effect on maladaptive plasticity in rats. Psychol. Med. 2023, 53, 5538–5550. [Google Scholar] [CrossRef] [PubMed]
- Szulc, M.; Kujawski, R.; Pacholak, A.; Poprawska, M.; Czora-Poczwardowska, K.; Geppert, B.; Mikolajczak, P.L. Cannabidiol as a Modulator of the Development of Alcohol Tolerance in Rats. Nutrients 2023, 15, 1702. [Google Scholar] [CrossRef] [PubMed]
- Melkumyan, M.; Annaswamy, V.M.; Evans, A.M.; Showemimo, O.F.; McCullers, Z.E.; Sun, D.; Murphy, T.E.; Vrana, K.E.; Arnold, A.C.; Raup-Konsavage, W.M.; et al. Effects of cannabidiol, with and without ∆9-tetrahydrocannabinol, on anxiety-like behavior following alcohol withdrawal in mice. Front. Neurosci. 2024, 18, 1375440. [Google Scholar] [CrossRef] [PubMed]
- Filev, R.; Engelke, D.S.; Da Silveira, D.X.; Mello, L.E.; Santos-Junior, J.G. THC inhibits the expression of ethanol-induced locomotor sensitization in mice. Alcohol 2017, 65, 31–35. [Google Scholar] [CrossRef]
- Moore, C.F.; Zamarripa, C.A.; Weerts, E.M. Oral Cannabidiol does not alter Alcohol Seeking and Self-Administration in Baboons. Drug Alcohol Depend. 2023, 245, 109829. [Google Scholar] [CrossRef]
- Garcia-Baos, A.; Puig-Reyne, X.; Garcia-Algar, O.; Valverde, O. Cannabidiol attenuates cognitive deficits and neuroinflammation induced by early alcohol exposure in a mice model. Biomed. Pharmacother. 2021, 141, 111813. [Google Scholar] [CrossRef]
- Koren, G.; Cohen, R.; Sachs, O. Use of Cannabis in Fetal Alcohol Spectrum Disorder. Cannabis Cannabinoid Res. 2021, 6, 74–76. [Google Scholar] [CrossRef]
- Gasparyan, A.; Navarro, D.; Navarrete, F.; Austrich-Olivares, A.; Scoma, E.R.; Hambardikar, V.D.; Acosta, G.B.; Solesio, M.E.; Manzanares, J. Cannabidiol repairs behavioral and brain disturbances in a model of fetal alcohol spectrum disorder. Pharmacol. Res. 2023, 188, 106655. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).