Chemical and Biological Characterization of Metabolites from Silene viridiflora Using Mass Spectrometric and Cell-Based Assays
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Plant Material
2.3. Preparation of Extract for Bioassays
2.4. Preparation of Extract for UHPLC-MS Measurements
2.5. UHPLC-QTOF-MS/MS Analysis
2.6. Cell Cultures
2.7. Sample Preparation for Bioassays and Treatment of Cells
2.8. MTT Assay
2.9. TOPFlash Assay
2.10. Statistical Analysis
3. Results and Discussion
3.1. UHPLC-HR-MS Profiling of Metabolites in a S. viridiflora Extract
3.1.1. Flavonoids
| N | Rt (min) | Tentatively Identified Metabolites | Average m/z | Reference m/z | Error (ppm) | Adduct Type | MS/MS Fragments | Formula | Resource | Reference | Class |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 0.77 | Histidine | 156.0769 | 156.0768 | 0.8 | [M + H]+ | C6H9N3O2 | S. colorata, S. dioica | [23,24] | Amino acids | |
| 2 | 0.78 | Arginine | 175.1189 | 175.1195 | −3.4 | [M + H]+ | C6H14N4O2 | S. colorata, S. dioica | [23,24] | Amino acids | |
| 3 | 0.81 | Glutamine | 145.0622 | 145.0619 | 2.1 | [M − H]− | C5H10N2O3 | S. colorata | [23] | Amino acids | |
| 147.0765 | 147.0764 | 0.4 | [M + H]+ | ||||||||
| 4 | 0.83 | Glutamic acid | 146.0452 | 146.0459 | −4.7 | [M − H]− | C5H9NO4 | S. alba, S. colorata, S. dioica | [23,24] | Amino acids | |
| 5 | 0.84 | Glucaric acid | 209.0312 | 209.0303 | 4.4 | [M − H]− | C6H10O8 | Sugar compounds | |||
| 6 | 0.85 | Pinitol | 195.0858 | 195.0863 | −2.3 | [M + H]+ | C7H14O6 | S. brahuica, S. ruscifolia | [25,26] | Polyols | |
| 7 * | 0.85 | Sucrose | 341.1069 | 341.1084 | −4.3 | [M − H]− | 387.1133 [M + HCOO]− | C12H22O11 | S. vulgaris, S. nutans, S. noctiflora, S. ruscifolia | [26,27] | Sugar compounds |
| 343.1213 | 343.1235 | −6.5 | [M + H]+ | 365.1044 [M + Na]+ | |||||||
| 8 | 0.88 | Trehalose | 387.1130 | 387.1144 | −3.6 | [M + HCOO]− | 729.2293 [2M + HCOO]− | C12H22O11 | S. ruscifolia | [26] | Sugar compounds |
| 360.1493 | 360.1500 | −2.0 | [M + NH4]+ | ||||||||
| 9 | 0.86 | Proline | 114.0549 | 114.0549 | 0.2 | [M − H]− | C5H9NO2 | S. colorata | [23] | Amino acids | |
| 116.0709 | 116.0706 | 2.8 | [M + H]+ | ||||||||
| 10 | 0.86 | Norvaline | 118.0859 | 118.0863 | −3.6 | [M + H]+ | C5H11NO2 | Amino acids | |||
| 11 | 0.87 | Threonine | 120.0663 | 120.0661 | 1.7 | [M + H]+ | C4H9NO3 | S. colorata, S. dioica | [23,24] | Amino acids | |
| 12 * | 0.89 | Quinic acid | 191.0567 | 191.05611 | 3.1 | [M − H]− | C7H12O6 | S. alba, S. conoidea, S. compacta, S. dichotoma, S. italica, S. supine, S. vulgaris | [20,22] | Polyols | |
| 193.07133 | 193.0707 | 3.3 | [M + H]+ | ||||||||
| 13 | 0.89 | Stachydrine | 144.1017 | 144.1019 | −1.2 | [M + H]+ | C7H13NO2 | Amino acids | |||
| 14 | 0.91 | Diglycolic acid | 133.0144 | 133.0142 | 1.0 | [M − H]− | C4H6O5 | Organic acid | |||
| 15 | 0.93 | Tyrosine | 182.0803 | 182.0812 | −4.8 | [M + NH4]+ | C9H11NO3 | S. colorata | [23] | Amino acids | |
| 16 | 0.94 | Fumaric acid | 115.0036 | 115.0037 | −0.5 | [M − H]− | C4H4O4 | Organic acid | |||
| 17 | 1.3 | Citric acid | 191.0193 | 191.0197 | −2.1 | [M − H]− | C6H8O7 | S. vulgaris | [28] | Organic acid | |
| 18 | 6.34 | Tryptophan | 203.0815 | 203.0826 | −5.4 | [M − H]− | C11H12N2O2 | Amino acids | |||
| 205.0978 | 205.0972 | 3.1 | [M + H]+ | ||||||||
| 19 | 6.47 * | Ferulic acid | 193.0497 | 193.0506 | −4.6 | [M − H]− | C10H10O4 | S. pratensis, S. schimperiana | [21,29] | Phenolics | |
| 20 | 6.51 | Salidroside | 299.1121 | 299.1136 | −5.1 | [M − H]− | 599.2322 [2M − H]−, 345.1189 [M + HCOO]− | C14H20O7 | Phenolics | ||
| 21 | 6.53 | p-Coumaric acid | 163.0395 | 163.0401 | −3.4 | [M − H]− | C9H8O3 | S. alba, S. conoidea, S. compacta, S. dichotoma, S. italica, S. supine, S. vulgaris | [20,22] | Phenolics | |
| 165.0532 | 165.0530 | 1.2 | [M + H]+ | 147.0440 [M + H − H2O]+ | |||||||
| 22 | 6.69 | Chlorogenic acid | 353.0874 | 353.0878 | −1.3 | [M − H]− | C16H18O9 | S. alba, S. dichotoma, S. italica, S. supine, S. vulgaris, S. albae, S. pendulae, S. compacta | [20,22,30] | Phenolics | |
| 355.1004 | 355.1000 | 1.0 | [M + H]+ | 372.1285 [M + NH4]+, 377.0836 [M + Na]+ | |||||||
| 23 | 6.71 | Isovitexin 7,2″-di-O-glucoside | 757.2169 | 757.2186 | −2.3 | [M + H]+ | 779.1987 [M + Na]+ | C33H40O20 | Flavonoid glycoside | ||
| 755.2010 | 755.2040 | −4.0 | [M − H]− | ||||||||
| 24 | 6.88 | Isosaponarin (isovitexin 4′-O-β-D-glucopyranoside) | 593.1499 | 593.1512 | −2.1 | [M − H]− | C27H30O15 | S. armeria, S. bupleuroides, S. chlorifolia, S. compacta, S. cretacea, S. cubanensis, S. polaris | [2,31] | Flavonoid glycoside | |
| 25 | 6.89 | Luteolin 6-C-β-D-glucoside-8-C-α-L-arabinoside (carlinoside) | 579.1355 | 579.1350 | 0.9 | [M − H]− | 563.1368, 557.2928, 511.2869, 447.1487, 401.1426, 387.1638, 355.0651 | C26H28O15 | S. repens, S. sibirica | [32,33] | Flavonoid glycoside |
| 26 | 6.92 | Cosmosiin (apigenin 7-O-glucoside) | 433.1128 | 433.1129 | −0.4 | [M + H]+ | 455.0948 [M + Na]+ | C21H20O10 | S. succulenta | [34] | Flavonoid glycoside |
| 431.0981 | 431.0984 | −0.7 | [M − H]− | ||||||||
| 27 | 7.06 | Vanillic acid | 167.0345 | 167.0350 | −3.0 | [M − H]− | C8H8O4 | S. schimperiana | [21] | Phenolics | |
| 28 | 7.12 | Caffeic acid | 181.0505 | 181.0501 | 2.2 | [M + H]+ | 163.0385 | C9H8O4 | S. dichotoma, S. italica, S. schimperiana, S. albae, S. pendulae | [21,22,30] | Phenolics |
| 29 | 7.14 | Schaftoside (apigenin 6-C-β-D -glucoside-8-C-α-L-arabinoside) | 563.1406 | 563.1401 | 0.9 | [M − H]− | 511.2904, 473.1054, 443.0950, 431.1878, 383.0759 | C26H28O14 | S. aprica, S. repens, S. schafta, S. nemoralis, S. caramanica, S. sendtneri, S. frivaldszkyana, S. paradoxa, S. chalcedonica | [17,32] | Flavonoid glycoside |
| 565.1538 | 565.1552 | −2.6 | [M + H]+ | 587.1359 [M + Na]+, 547.1468 [M + H − H2O]+ | |||||||
| 30 | 7.21 | Isovitexin 7-O-glucoside-2′’-O-rhamnoside | 739.2094 | 739.2091 | 0.4 | [M − H]− | C33H40O19 | S. pratensis | [35] | Flavonoid glycoside | |
| 741.2219 | 741.2236 | −2.4 | [M + H]+ | ||||||||
| 31 * | 7.23 | 5,20,26-Trihydroxyecdysone (26-hydroxypolypodine B) | 511.2913 | 511.2907 | 1.2 | [M − H]− | 557.2967 [M + HCOO]−, 447.0890 | C27H44O9 | S. viridiflora | [16] | Ecdysteroids |
| 32 | 7.30 | 2-Deoxy-5,20,26-trihydroxyecdysone | 519.2931 | 519.2928 | 0.7 | [M + Na]+ | 479.30023, 461.29126, 443.27814 | C27H44O8 | S. viridiflora | [15] | Ecdysteroids |
| 33 | 7.34 | 20-Hydroxyecdysone galactoside | 641.3543 | 641.3537 | −0.9 | [M − H]− | 687.3597 [M + HCOO]−, 613.2096, 563.1367, 461.1630 | C33H54O12 | S. brachuica, S. viridiflora | [7] | Ecdysteroids |
| 34 | 7.40 | Vicenin 2 (apigenin-6,8-di-C-glucopyranoside) | 594.1589 | 594.1585 | 0.7 | [M − H]− | C27H30O15 | S. boissieri, S. caramanica, S. chlorantha, S. colpophylla, S. commutata, S. cyri, S. foliosa, S. frivaldszkyana, S. graminifolia, S. jenissensis, S. italic, S. linicola, S. macrostyla, S. nemoralis, S. nutans, S. paradoxa, S. saxatilis, S. sendtneri, S. roemeri, S. wolgensis | [2,36] | Flavonoid glycoside | |
| 595.1638 | 595.1658 | −3.3 | [M + H]+ | ||||||||
| 35 | 7.43 | Orientin (luteolin-8-C-β-D-glucoside) | 447.0919 | 447.0933 | −3.2 | [M − H]− | C21H20O11 | S. armeria, S. boissieri, S. bupleuroides, S. chlorantha, S. chlorifolia, S. commutata, S. compacta, S. cretacea, S. cubanensis, S. cyri, S. foliosa, S. graminifolia, S. jenissensis, S. italica, S. linicola, S. macrostyla, S. nutans, S. polaris, S. saxatilis, S. vulgaris, S. wolgensis | [2] | Flavonoid glycoside | |
| 449.1081 | 449.1079 | 0.5 | [M + H]+ | ||||||||
| 36 | 7.45 | 20,26-Dihydroxyecdysone | 495.2963 | 495.2958 | 1.0 | [M − H]− | 541.3018 [M + HCOO]−, 439.1798, 393.1740 | C27H44O8 | S. repens, S. viridiflora | [17,37] | Ecdysteroids |
| 37 | 7.49 | Isoorientin (luteolin-6-C-β-D glucoside) | 447.0925 | 447.0933 | −1.8 | [M − H]− | C21H20O11 | S. aprica, S. armeria, S. boissieri, S. bupleuroides, S. chlorantha, S. chlorifolia, S. commutata, S. compacta, S. cretacea, S. cubanensis, S. cyri, S. italic, S. littorea, S. foliosa, S. graminifolia, S. jenissensis, S. italica, S. macrostyla, S. nutans, S. polaris, S. saxatilis, S. viscariopsis, S. vulgaris, S. wolgensis | [2] | Flavonoid glycoside | |
| 449.1101 | 449.1078 | 5.2 | [M + H]+ | 471.0893 [M + Na]+ | |||||||
| 38 * | 7.51 | Saponarin (isovitexin 7-O-β-D-glucoside) | 593.1498 | 593.1512 | −2.3 | [M − H]− | C27H30O15 | S. colorata, S. repens, S. nutans | [1,38] | Flavonoid glycoside | |
| 577.1530 | 577.1552 | −3.9 | [M + H]+ | 617.15063 [M + Na]+ | |||||||
| 39 | 7.52 | Vitexin-2″-O-rhamnoside (apigenin 8-C-β-D-glucoside-2″-O-rhamnoside) | 577.1563 | 577.1557 | 1.0 | [M − H]− | 503.1104, 471.0893, 413.0855 | C27H30O14 | S. nutans | [38] | Flavonoid glycoside |
| 579.1704 | 579.1708 | −0.7 | [M + H]+ | 601.15365 [M + Na]+ | |||||||
| 40 | 7.57 | Integristerone A | 541.3018 | 541.3013 | 1.0 | [M + HCOO]− | 447.0884 | C27H44O8 | S. viridiflora | [16] | Ecdysteroids |
| 519.2947 | 519.2928 | 3.7 | [M + Na]+ | 479.30194, 461.29297, 443.27985 | |||||||
| 41 * | 7.58 | Rutin (quercetin-3-O-rutinoside) | 609.1440 | 609.1461 | −3.5 | [M − H]− | C27H30O16 | S. alba, S. conoidea, S. compacta, S. dichotoma, S. italica, S. supine, S. vulgaris, S. schimperiana | [20,21,22] | Flavonoid glycoside | |
| 611.1611 | 611.1607 | 0.7 | [M + H]+ | ||||||||
| 42 | 7.73 | Isovitexin (apigenin 6-C-β-D-glucopyranoside) | 431.0984 | 431.0984 | 0.1 | [M − H]− | C21H20O10 | S. alba, S. aprica, S. armeria, S. boissieri, S. brachuica, S. bupleuroides, S. chlorantha, S. chlorifolia, S. commutata, S. compacta, S. cretacea, S. cubanensis, S. cyri, S. diclinis, S. dioica, S. foliosa, S. graminifolia, S. jenissensis, S. italica, S. macrostyla, S. multifida, S. nutans, S. polaris, S. repens, S. supina, S. turgida, S. wolgensis | [2,18] | Flavonoid glycoside | |
| 433.1144 | 433.1129 | 3.3 | [M + H]+ | 455.0926 [M + Na]+ | |||||||
| 43 | 7.74 | Quercetin-3-O-(6′’-O-malonyl)-β-glucoside | 551.1030 | 551.1037 | −1.3 | [M + H]+ | 515.3002, 392.2085, 279.1587 | C24H22O15 | Flavonoid glycoside | ||
| 44 | 7.79 | 26-Hydroxyecdysone | 479.3014 | 479.3009 | 1.1 | [M − H]− | 1005.6133, 525.3069 [M + HCOO]− | C27H44O7 | S. repens | [37] | Ecdysteroids |
| 45 | 7.80 | Diosmin (luteolin 4′-methyl ether-7-O-rutinoside) | 607.1992 | 607.2000 | −1.3 | [M − H]− | C28H32O15 | S. succulent, S. schimperiana | [21,34] | Flavonoid glycoside | |
| 609.1842 | 609.1814 | 4.6 | [M + H]+ | 631.1658 [M + Na]+ | |||||||
| 46 | 7.82 | Hyperoside (quercetin-3-O-β-D-galactoside) | 463.0867 | 463.0873 | −1.3 | [M − H]− | C21H20O12 | S. albae, S. pendulae, S. compacta | [20,30] | Flavonoid glycoside | |
| 47 | 7.83 | Narcissin (3-methylquercetin-3-O-rutinoside) | 623.1619 | 623.1618 | 0.2 | [M − H]− | C28H32O16 | S. ruscifolia | [26] | Flavonoid glycoside | |
| 48 | 7.83 | Dihydroferulic acid | 195.0654 | 195.0663 | −4.7 | [M − H]− | C10H12O4 | Gypsophila paniculata | [39] | Phenolics | |
| 49 | 7.84 | 2-Deoxy-5,20,26-trihydroxyecdysone | 495.2963 | 495.2958 | 1.0 | [M − H]− | 1037.6054, [2M + HCOO]−, 541.3018 [M + HCOO]− | C27H44O8 | S. viridiflora | [15] | Ecdysteroids |
| 519.2918 | 519.2934 | −3.1 | [M + Na]+ | 479.2991, 461.2902, 443.2801 | |||||||
| 50 * | 7.91 | 20-Hydroxyecdysone | 479.3014 | 479.3009 | 1.1 | [M − H]− | 525.3067 [M + HCOO]− | C27H44O7 | S. viridiflora | [7] | Ecdysteroids |
| 481.3160 | 481.3160 | −0.1 | [M + H]+ | 503.2966 [M + Na]+, 463.3051 [M + H − H2O]+, 445.2938 [M + H − 2H2O]+, 427.2853 [M + H − 3H2O]+ | |||||||
| 51 | 7.93 | 2-Deoxyintegristerone A | 479.3014 | 479.3009 | 1.1 | [M − H]− | 525.3069 [M + HCOO]−, 441.1962, 369.0882 | C27H44O7 | S. otitis, S. italica ssp. nemoralis, S. viridiflora | [16] | Ecdysteroids |
| 498.3402 | 498.3425 | −4.6 | [M + NH4]+ | ||||||||
| 52 | 8.09 | Vaccaroside B | 1296.6219 | 1296.6225 | −0.4 | [M + NH4]+ | 1006.5222, 798.4625 | C60H94O29 | Vaccaria segetalis | [40] | Triterpenoids |
| 53 | 8.12 | Hesperidin | 611.1980 | 611.1976 | 0.7 | [M + H]+ | 628.2221 [M + NH4]+ | C28H34O15 | S. alba, S. conoidea, S. compacta, S. dichotoma, S. italica, S. supine, S. vulgaris, S. schimperiana | [20,21,22,23,24,25,26,27,28,29,30] | Flavonoid glycoside |
| 54 * | 8.34 | Quercitrin (quercetin 3-O-α-L-rhamnoside) | 449.1079 | 449.1078 | 0.2 | [M + H]+ | C21H20O11 | S. albae, S. pendulae | [30] | Flavonoid glycoside | |
| 55 | 8.52 | Armeroside E | 1131.5229 | 1131.5223 | 0.5 | [M − H]− | 677.3533, 565.2568, 367.1237 | C54H84O25 | S. armeria | [41] | Triterpenoids |
| 56 | 8.63 | Silenegallisaponin J | 1117.5436 | 1117.5431 | 0.5 | [M − H]− | 707.2547, 649.3389, 581.2617, 509.3094 | C54H86O24 | S. gallica | [42] | Triterpenoids |
| 57 | 8.68 | Sinocrassuloside II | 1131.5229 | 1131.5223 | 0.5 | [M − H]− | 978.4760, 825.4244, 588.2588 | C54H84O25 | S. viscidula | [43] | Triterpenoids |
| 58 * | 8.70 | 2-Deoxy-20-hydroxyecdysone | 509.3120 | 509.3114 | 1.1 | [M + HCOO]− | 463.3023 [M − H]−, 973.6228 [2M + HCOO]− | C27H44O6 | S. viridiflora | [16] | Ecdysteroids |
| 465.3211 | 465.3216 | −1.1 | [M + H]+ | 487.3024 [M + Na]+, 447.3113 [M + H − H2O]+, 429.2997 [M + H − 2H2O]+, 411.2900 [M + H − 3H2O]+, 393.2787 [M + H − 4H2O]+, 355.2270, 331.2272, 287.2005 | |||||||
| 59 | 8.71 | Armeroside D | 1149.5335 | 1149.53291 | 0.5 | [M − H]− | C54H86O26 | S. armeria | [41] | Triterpenoids | |
| 60 | 8.73 | Makisterone C | 553.3382 | 553.3377 | 1.0 | [M + HCOO]− | C29H48O7 | Ecdysteroids | |||
| 507.3327 | 507.3322 | 1.0 | [M − H]− | ||||||||
| 61 | 8.89 | Armeroside G | 1015.4755 | 1015.4750 | 0.5 | [M − H]− | C49H76O22 | S. armeria | [41] | Triterpenoids | |
| 62 | 8.97 | Armeroside F | 987.4806 | 987.4801 | 0.5 | [M − H]− | C48H76O21 | S. armeria, S. viscidula | [41,43] | Triterpenoids | |
| 63 | 9.40 | 3β,22α-Dihydroxyolean-12-en-23-al-28-oic acid 3-O-α-L-arabinopyranosyl-(1→3)-β-D-glucuronopyranoside | 793.4016 | 793.4011 | 0.7 | [M − H]− | C41H62O15 | S. odontopetala | [44] | Triterpenoids | |
| 64 | 9.46 | 2-Deoxy-20-hydroxyecdysone-acetate | 505.3171 | 505.31653 | −4.1 | [M − H]− | C29H46O7 | S. praemixta, S. wallichiana | [45,46] | Ecdysteroids | |
| 65 * | 10.12 | Silviridoside | 1115.5304 | 1115.5274 | 2.7 | [M − H]− | 774.3528, 580.2618, 557.2574 | C54H84O24 | S. viridiflora | [3] | Triterpenoids |
| 1117.5425 | 1117.5431 | −0.5 | [M + H]+ | 1134.5691, 253.1077 | |||||||
| 66 | 10.45 | 3-O-β-D-Glycuronopyranosyl-quillaic acid 28-O-hexapyranosyl-pentapyranosyl-xylopyranosyl ester | 1101.5123 | 1101.5118 | 0.5 | [M − H]− | 749.3395, 573.2545, 550.2504, 311.1664 | C53H82O24 | Triterpenoids | ||
| 1120.5534 | 1120.5540 | −0.5 | [M + NH4]+ | 971.4906, 680.4043, 571.2443 | |||||||
| 67 | 10.55 | 3-O-[β-D-Glucopyranosyl-(1→2)-(β-D-xylopyranosyl-(1→3))-β-D-glucuronopyranosyl]-28-O-β-D-glucopyranosyl-3β-hydroxyolean-12-en-28-oic acid | 1087.5331 | 1087.5325 | 0.5 | [M − H]− | 601.2628, 566.2651, 543.2607 | C53H84O23 | Beta vulgaris | [47,48] | Triterpenoids |
| 1106.5742 | 1106.5747 | −0.5 | [M + NH4]+ | 958.5082, 810.4453, 610.1826 | |||||||
| 68 | 10.96 | Acetyl-23-hydroxyolean-12-en-28-oic acid-dipentosyl-hexosyl-deoxy-pentoside | 1085.5538 | 1085.5533 | 0.5 | [M − H]− | 965.4340, 666.2781, 565.2729, 482.2128 | C54H86O22 | Triterpenoids | ||
| 1104.5949 | 1104.5955 | −0.5 | [M + NH4]+ | 678.3821, 536.1646 | |||||||
| 69 | 11.75 | Quillaic acid-3-O-β-D-glucuronopyranoside | 661.3593 | 661.3588 | 0.8 | [M − H]− | 535.3222, 279.1495, 185.0288 | C36H54O11 | S. vulgaris, Psammosilene tunicoides | [49,50] | Triterpenoids |
| 680.4004 | 680.4010 | −0.9 | [M + NH4]+ | 536.1642 | |||||||
| 70 | 13.30 | 3β,22α-Dihydroxyolean-12-en-23-al-28-oic acid 3-O-β-D-glucuronopyranoside | 707.3648 | 707.36427 | −0.7 | [M + HCOO]− | 661.3556, 330.1734, 311.1661 | C36H54O11 | S. odontopetala | [44] | Triterpenoids |
| 71 | 18.23 | Oleanolic acid | 455.3509 | 455.3525 | −3.5 | [M − H]− | C30H48O3 | S. succulenta | [51] | Triterpenoids |
3.1.2. Triterpenes
3.1.3. Ecdysteroids
3.2. Anticancer Activities
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jakimiuk, K.; Wink, M.; Tomczyk, M. Flavonoids of the Caryophyllaceae. Phytochem. Rev. 2022, 21, 179–218. [Google Scholar] [CrossRef]
- Mamadalieva, N.Z.; Lafont, R.; Wink, M. Diversity of secondary metabolites in the genus Silene L. (Caryophyllaceae)—Structures, Distribution, and Biological properties. Diversity 2014, 6, 415–499. [Google Scholar] [CrossRef]
- Makhmudova, M.M.; Bacher, M.; Zengin, G.; Rosenau, T.; Youssef, F.S.; Almasri, D.M.; Elhady, S.S.; Mamadalieva, N.Z. Silviridoside: A New Triterpene Glycoside from Silene viridiflora with Promising Antioxidant and Enzyme Inhibitory Potential. Molecules 2022, 27, 8781. [Google Scholar] [CrossRef] [PubMed]
- Dzakhangirova, M.; Syrov, V. Experimental evaluation of effect of stimulation of sum of ecdysteroids from Silene brachuica and S. viridiflora for erythropoiesis in laboratory animals. Pathology 2005, 2, 7–9. [Google Scholar]
- Shakhmurova, G.A.; Mamadalieva, N.Z.; Zhanibekov, A.A.; Khushbaktova, Z.A.; Syrov, V.N. Effect of total ecdysteroid preparation from Silene viridiflora on the immune state of experimental animals under normal and secondary immunodeficiency conditions. Pharm. Chem. J. 2012, 46, 222–224. [Google Scholar] [CrossRef]
- Mamadalieva, N.Z.; Böhmdorfer, S.; Zengin, G.; Bacher, M.; Potthast, A.; Akramov, D.K.; Janibekov, A.; Rosenau, T. Phytochemical and biological activities of Silene viridiflora extractives. Development and validation of a HPTLC method for quantification of 20-hydroxyecdysone. Ind. Crop. Prod. 2019, 129, 542–548. [Google Scholar] [CrossRef]
- Mamadalieva, N.Z.; Zibareva, L.N.; Saatov, Z.; Lafont, R. Phytoecdysteroids of Silene viridiflora. Chem. Nat. Compd. 2003, 39, 199–203. [Google Scholar] [CrossRef]
- Mamadalieva, N.Z.; Janibekov, A.A.; Lafont, R.; Girault, J.-P. Two minor phytoecdysteroids of the plant Silene viridiflora. Nat. Prod. Commun. 2010, 5, 1579–1582. Available online: https://journals.sagepub.com/doi/pdf/10.1177/1934578X1000501013 (accessed on 7 August 2024). [CrossRef]
- Blagodatski, A.; Yatsunskaya, M.; Mikhailova, V.; Tiasto, V.; Kagansky, A.; Katanaev, V.L. Medicinal mushrooms as an attractive new source of natural compounds for future cancer therapy. Oncotarget 2018, 9, 29259–29274. [Google Scholar] [CrossRef]
- Katanaev, V.L.; Di Falco, S.; Khotimchenko, Y. The Anticancer Drug Discovery Potential of Marine Invertebrates from Russian Pacific. Mar. Drugs 2019, 17, 474. [Google Scholar] [CrossRef]
- Katanaev, V.L.; Blagodatski, A.; Xu, J.; Khotimchenko, Y.; Koval, A. Mining Natural Compounds to Target WNT Signaling: Land and Sea Tales. In Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2021; Volume 269, pp. 215–248. [Google Scholar] [CrossRef]
- Boudou, C.; Mattio, L.; Koval, A.; Soulard, V.; Katanaev, V.L. Wnt-pathway inhibitors with selective activity against triple-negative breast cancer: From thienopyrimidine to quinazoline inhibitors. Front. Pharmacol. 2022, 13, 1045102, Erratum in Front. Pharmacol. 2023, 14, 1173490. [Google Scholar] [CrossRef] [PubMed]
- Koval, A.; Bassanini, I.; Xu, J.; Tonelli, M.; Boido, V.; Sparatore, F.; Amant, F.; Annibali, D.; Leucci, E.; Sparatore, A.; et al. Optimization of the clofazimine structure leads to a highly water-soluble C3-aminopyridinyl riminophenazine endowed with improved anti-Wnt and anti-cancer activity in vitro and in vivo. Eur. J. Med. Chem. 2021, 222, 113562. [Google Scholar] [CrossRef] [PubMed]
- Mamadalieva, N.Z.; Egamberdieva, D.; Zhanibekov, A.A.; Triggiani, D.; Tiezzi, A. Chemical components of Silene viridiflora and their biological properties. Chem. Nat. Compd. 2009, 45, 589–591. [Google Scholar] [CrossRef]
- Tóth, N.; Simon, A.; Tóth, G.; Kele, Z.; Hunyadi, A.; Báthori, M. 26-Hydroxylated ecdysteroids from Silene viridiflora. J. Nat. Prod. 2008, 71, 1461–1463. [Google Scholar] [CrossRef]
- Simon, A.; Tóth, N.; Tóth, G.; Kele, Z.; Groska, J.; Báthori, M. Ecdysteroids from Silene viridiflora. Helv. Chim. Acta 2009, 92, 753–761. [Google Scholar] [CrossRef]
- Zibareva, L.N.; Filonenko, E.S.; Chernyak, E.I.; Morozov, S.V.; Kotelnikov, O.A. Flavonoids of some Plant Species of the Genus Silene. Russ. J. Bioorg. Chem. 2023, 49, 1714–1722. [Google Scholar] [CrossRef]
- Olennikov, D.N.; Chirikova, N.K. C-Glycosyl Flavones from Two Eastern Siberian Species of Silene. Chem. Nat. Compd. 2019, 55, 642–647. [Google Scholar] [CrossRef]
- Liu, R.; Meng, C.; Zhang, Z.; Ma, H.; Lv, T.; Xie, S.; Liu, Y.; Wang, C. Comparative metabolism of schaftoside in healthy and calcium oxalate kidney stone rats by UHPLC-Q-TOF-MS/MS method. Anal. Biochem. 2020, 597, 113673. [Google Scholar] [CrossRef]
- Boğa, M. Chemical constituents, cytotoxic, antioxidant and cholinesterases inhibitory activities of Silene compacta (Fischer) extracts. Marmara Pharm. J. 2017, 21, 445–454. [Google Scholar] [CrossRef]
- Hussein, I.A.; Srivedavyasasri, R.; El-Hela, A.A.; Mohammad, A.-E.I.; Ross, S.A. Chemical constituents from Silene schimperiana Boiss. belonging to caryophyllaceae and their chemotaxonomic significance. Biochem. Syst. Ecol. 2020, 92, 104113. [Google Scholar] [CrossRef]
- Zengin, G.; Mahomoodally, M.F.; Aktumsek, A.; Ceylan, R.; Uysal, S.; Mocan, A.; Abdullah Yilmaz, M.; Picot-Allain, C.M.N.; Ćirić, A.; Glamočlija, J.; et al. Functional constituents of six wild edible Silene species: A focus on their phytochemical profiles and bioactive properties. Food Biosci. 2018, 23, 75–82. [Google Scholar] [CrossRef]
- Terrab, A.; Garcia-Castano, J.L.; Romero, J.M.; Berjano, R.; De Vega, C.; Talavera, S. Analysis of amino acids in nectar from Silene colorata Poiret (Caryophyllaceae). Bot. J. Linn. Soc. 2007, 155, 49–56. [Google Scholar] [CrossRef]
- Baker, I.; Baker, H.G. Analysis of amino acids in flower nectars of hybrids and their parents, with phylogenetic implications. New Phytol. 1976, 76, 87–98. [Google Scholar] [CrossRef]
- Al’magambetov, A.M.; Temirgaziev, B.S.; Zavarzin, I.V.; Kachala, V.V.; Kudabaeva, P.K.; Tuleuov, B.I.; Adekenov, S.M. New prospective herbal source of D-pinitol possessing anti-diabetic and hypoglycemic properties. Chem. Plant Raw Mater. 2016, 3, 79–84. [Google Scholar] [CrossRef]
- Tok, K.C.; Hurkul, M.M.; Bozkurt, N.N.; Aysal, A.I.; Yayla, Ş. Investigation of phytochemicals in methanolic herba extract of Silene ruscifolia by LC-QTOF/MS and GC/MS. J. Fac. Pharm. Ankara Ankara Ecz. Fak. Derg. 2022, 46, 827–838. [Google Scholar] [CrossRef]
- Witt, T.; Jürgens, A.; Geyer, R.; Gottsberger, G. Nectar dynamics and sugar composition in flowers of Silene and Saponaria species (Caryophyllaceae). Plant Biol. 1999, 1, 334–345. [Google Scholar] [CrossRef]
- García-Gonzalo, P.; del Real, A.E.P.; Lobo, M.C.; Pérez-Sanz, A. Different genotypes of Silene vulgaris (Moench) Garcke grown on chromium-contaminated soils influence root organic acid composition and rhizosphere bacterial communities. Environ. Sci. Pollut. Res. 2017, 24, 25713–25724. [Google Scholar] [CrossRef]
- Van Brederode, J.; Kamps-Heinsbroek, R.; Mastenbroek, O. Biochemical and ontogenetic evidence that the ferulic acid and isoscoparin formation in Silene are catalyzed by different enzymes. Z. Pflanzenphysiol. 1982, 106, 43–53. [Google Scholar] [CrossRef]
- Tircomnicu, V.; Gird, C.E.; Radu, S. Researches on the preparation and characterization of some tinctures from Silene albae herba and Silene pendulae herba. Curr. Health Sci. J. 2012, 38, 80–83. [Google Scholar] [PubMed]
- Darmograi, V. Flavonoids of plants of the genera Silene and Otites adans, family Caryophyllaceae. Chem. Nat. Compd. 1977, 13, 102–103. [Google Scholar] [CrossRef]
- Olennikov, D.N. Silenerepin—A new C-glycosylflavone from Silene repens. Chem. Nat. Compd. 2020, 56, 423–426. [Google Scholar] [CrossRef]
- Olennikov, D.N.; Kashchenko, N.I. Ecdysteroids and glycosylflavones of Silene sibirica (Caryophyllaceae). Chem. Plant Raw Mater. 2020, 4, 109–119. [Google Scholar] [CrossRef]
- Mahmoud, S.; Hassan, A.; Abu El Wafa, S.; Mohamed, A. UPLC-MS/MS profiling and antitumor activity of Silene succulenta Forssk. growing in Egypt. Azhar Int. J. Pharm. Med. Sci. 2021, 1, 58–62. [Google Scholar] [CrossRef]
- Mastenbroek, O.; Van Brederode, J. The possible evolution of Silene pratensis as deduced from present day variation patterns. Biochem. Syst. Ecol. 1986, 14, 165–181. [Google Scholar] [CrossRef]
- Zibareva, L.N.; Filonenko, E.S.; Chernyak, E.I.; Morozov, S.V.; Kotelnikov, O.A. Flavonoids of some plant species of the genera Silene. Chem. Plant Raw Mater. 2022, 3, 109–118. [Google Scholar] [CrossRef]
- Olennikov, D.N. Ecdysteroids of Silene repens from Eastern Siberia. Chem. Nat. Compd. 2019, 55, 770–772. [Google Scholar] [CrossRef]
- Olennikov, D.N. Ecdysteroids, Flavonoids, and Phenylpropanoids from Silene nutans. Chem. Nat. Compd. 2019, 55, 127–130. [Google Scholar] [CrossRef]
- Chou, S.-C.; Everngam, M.C.; Beck, J.J. Allelochemical phenolic acids from Gypsophila paniculata. J. Undergrad. Chem. Res. 2008, 7, 40–42. [Google Scholar]
- Koike, K.; Jia, Z.; Nikaido, T. Triterpenoid saponins from Vaccaria segetalis. Phytochemistry 1998, 47, 1343–1349. [Google Scholar] [CrossRef]
- Takahashi, N.; Li, W.; Koike, K. Oleanane-type triterpenoid saponins from Silene armeria. Phytochemistry 2016, 129, 77–85. [Google Scholar] [CrossRef]
- Bechkri, S.; Alabdul Magid, A.; Sayagh, C.; Berrehal, D.; Harakat, D.; Voutquenne-Nazabadioko, L.; Kabouche, Z.; Kabouche, A. Triterpene saponins from Silene gallica collected in North-Eastern Algeria. Phytochemistry 2020, 172, 112274. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Fang, J.; Zhu, Z.; Wu, J.; Li, Y. A new triterpenoid saponin from the roots of Silene viscidula. Nat. Prod. Res. 2012, 26, 2002–2007. [Google Scholar] [CrossRef] [PubMed]
- Ün, R.N.; Masullo, M.; Karayildirim, T.; Nalbantsoy, A.; Alankus, O.; Piacente, S. Triterpene glycosides from Silene odontopetala. Phytochemistry 2020, 176, 112404. [Google Scholar] [CrossRef] [PubMed]
- Mamadalieva, N.Z.; Saatov, Z.; Kachala, V.V.; Shashkov, A.S. 2-Deoxyecdysterone-25-Acetate from Silene wallichiana. Chem. Nat. Compd. 2002, 38, 179–181. [Google Scholar] [CrossRef]
- Saatov, Z.; Gorovits, M.B.; Abdullaev, N.D.; Usmanov, B.Z.; Abubakirov, N.K. Phytoecdysteroids of plants of the genus Silene. VIII. 2-Deoxyecdysterone 3-acetate from Silene praemixta. Khimiya Prir. Soedin. 1985, 21, 56–58. [Google Scholar]
- Edelmann, M.; Dawid, C.; Hochreiter, K.; Ralla, T.; Stark, T.D.; Salminen, H.; Weiss, J.; Hofmann, T. Molecularization of foam-active saponins from sugar beet side streams (Beta vulgaris ssp. vulgaris var. altissima). J. Agric. Food Chem. 2020, 68, 10962–10974. [Google Scholar] [CrossRef]
- Edelmann, M.; Dawid, C.; Ralla, T.; Stark, T.D.; Salminen, H.; Weiss, J.; Hofmann, T. Fast and sensitive LC–MS/MS method for the quantitation of saponins in various sugar beet materials. J. Agric. Food Chem. 2020, 68, 15027–15035. [Google Scholar] [CrossRef]
- Bouguet-Bonnet, S.; Rochd, M.; Mutzenhardt, P.; Henry, M. Total assignment of 1H and 13C NMR spectra of three triterpene saponins from roots of Silene vulgaris (Moench) Garcke. Magn. Reson. Chem. 2002, 40, 618–621. [Google Scholar] [CrossRef]
- Gevrenova, R.; Bardarov, K.; Bouguet-Bonnet, S.; Voynikov, Y.; Balabanova, V.; Zheleva-Dimitrova, D.; Henry, M. A new liquid chromatography-high resolution Orbitrap mass spectrometry-based strategy to characterize Glucuronide Oleanane-type Triterpenoid Carboxylic Acid 3,28-O-Bidesmosides (GOTCAB) saponins. A case study of Gypsophila glomerata Pall ex M. B. (Caryophyllaceae). J. Pharm. Biomed. Anal. 2018, 159, 567–581. [Google Scholar] [CrossRef]
- Karawya, M.; Elgamal, M.; Shalaby, N.; Soliman, H. Saponins of Silene succulenta forssk, growing locally. Egypt. J. Pharm. Sci. 1991, 32, 879–887. [Google Scholar]
- Bechkri, S.; Alabdul Magid, A.; Voutquenne-Nazabadioko, L.; Kabouche, A.; Sayagh, C.; Harakat, D.; Kabouche, Z. Triterpenoid saponins from Silene coeli-rosa. Phytochem. Lett. 2023, 54, 50–56. [Google Scholar] [CrossRef]
- Cheikh-Ali, S.; Farman, M.; Lacaille-Dubois, M.A.; Semmar, N. Structural organization of saponins in Caryophyllaceae. Phytochem. Rev. 2019, 18, 405–441. [Google Scholar] [CrossRef]
- Li, Y.H.; Bai, X.S.; Yang, X.X.; Li, Y.X.; Li, H.R.; Wang, Z.L.; Wang, W.; Tian, K.; Huang, X.Z. Triterpenoid saponins from Psammosilene tunicoides and their antinociceptive activities. Phytochemistry 2023, 214, 113795. [Google Scholar] [CrossRef] [PubMed]
- Moniuszko-Szajwaj, B.; Pecio, L.; Kowalczyk, M.; Simonet, A.M.; Macias, F.A.; Szumacher-Strabel, M.; Cieslak, A.; Oleszek, W.; Stochmal, A. New triterpenoid saponins from the roots of Saponaria officinalis. Nat. Prod. Commun. 2013, 8, 1687–1690. [Google Scholar] [CrossRef] [PubMed]
- Stevens, J.F.; Reed, R.L.; Morré, J.T. Characterization of Phytoecdysteroid Glycosides in Meadowfoam (Limnanthes alba) Seed Meal by Positive and Negative Ion LC-MS/MS. J. Agric. Food Chem. 2008, 56, 3945–3952. [Google Scholar] [CrossRef]
- Badawy, S.A.; Hassan, A.R.; Elkousy, R.H.; Abu El Wafa, S.A.; Mohammad, A.S.I. New cyclic glycolipids from Silene succulenta promote in vitro MCF-7 breast carcinoma cell apoptosis by cell cycle arrest and in silico mitotic Mps1/TTK inhibition. RSC Adv. 2023, 13, 18627–18638. [Google Scholar] [CrossRef]
- Martins, A.; Tóth, N.; Ványolós, A.; Béni, Z.; Zupkó, I.; Molnár, J. Significant activity of ecdysteroids on the resistance to doxorubicin in mammalian cancer cells expressing the human ABCB1 transporter. J. Med. Chem. 2012, 55, 5034–5043. [Google Scholar] [CrossRef]
- Martins, A.; Sipos, P.; Dér, K.; Csábi, J.; Miklos, W.; Berger, W.; Zalatnai, A.; Amaral, L.; Molnár, J.; Szabó-Révész, P.; et al. Ecdysteroids sensitize MDR and non-MDR cancer cell lines to doxorubicin, paclitaxel, and vincristine but tend to protect them from cisplatin. BioMed Res. Int. 2015, 2015, 895360. [Google Scholar] [CrossRef]
- Romaniuk-Drapała, A.; Lisiak, N.; Totoń, E.; Matysiak, A.; Nawrot, J.; Nowak, G.; Kaczmarek, M.; Rybczyńska, M.; Rubiś, B. Proapoptotic and proautophagic activity of 20-hydroxyecdysone in breast cancer cells in vitro. Chem. Biol. Interact. 2021, 342, 109479. [Google Scholar] [CrossRef]
- Shuvalov, O.; Fedorova, O.; Tananykina, E.; Gnennaya, Y.; Daks, A.; Petukhov, A.; Barlev, N.A. An Arthropod Hormone, Ecdysterone, Inhibits the Growth of Breast Cancer Cells via Different Mechanisms. Front. Pharmacol. 2020, 11, 561537. [Google Scholar] [CrossRef]
- Zibareva, L.; Eremina, V.; Ivanova, N.; Lazkov, G. Distribution of phytoecdysteriods in the tribe Sileneae Dumort. Fam. Caryophyllaaceae. Rastit. Resur. 2003, 39, 45–53. [Google Scholar]
- Martins, A.; Csábi, J.; Kitka, D.; Balázs, A.; Amaral, L.; Molnár, J.; Simon, A.; Tóth, G.; Hunyadi, A. Synthesis and Structure-Activity Relationship Study of Novel Ecdysteroid Dioxolanes as MDR Modulators in Cancer. Molecules 2013, 18, 15255–15275. [Google Scholar] [CrossRef] [PubMed]
- Katanaev, V.L.; Baldin, A.; Denisenko, T.V.; Silachev, D.N.; Ivanova, A.E.; Sukhikh, G.T.; Jia, L.; Ashrafyan, L.A. Cells of the tumor microenvironment speak the Wnt language. Trends Mol. Med. 2023, 29, 468–480. [Google Scholar] [CrossRef] [PubMed]
- Koval, A.; Katanaev, V.L. Dramatic dysbalancing of the Wnt pathway in breast cancers. Sci. Rep. 2018, 8, 7329. [Google Scholar] [CrossRef]
- Pellissier, L.; Koval, A.; Marcourt, L.; Ferreira Queiroz, E.; Lecoultre, N.; Leoni, S.; Quiros-Guerrero, L.M.; Barthélémy, M.; Duivelshof, B.L.; Guillarme, D.; et al. Isolation and Identification of Isocoumarin Derivatives With Specific Inhibitory Activity Against Wnt Pathway and Metabolome Characterization of Lasiodiplodia venezuelensis. Front. Chem. 2021, 9, 664489. [Google Scholar] [CrossRef]
- Koval, A.; Ahmed, K.; Katanaev, V.L. Inhibition of Wnt signalling and breast tumour growth by the multi-purpose drug suramin through suppression of heterotrimeric G proteins and Wnt endocytosis. Biochem. J. 2016, 473, 371–381. [Google Scholar] [CrossRef]
- Koval, A.; Pieme, C.A.; Queiroz, E.F.; Ragusa, S.; Ahmed, K.; Blagodatski, A.; Wolfender, J.L.; Petrova, T.V.; Katanaev, V.L. Tannins from Syzygium guineense suppress Wnt signaling and proliferation of Wnt-dependent tumors through a direct effect on secreted Wnts. Cancer Lett. 2018, 435, 110–120. [Google Scholar] [CrossRef]
- Quiros-Guerrero, L.M.; Marcourt, L.; Chaiwangrach, N.; Koval, A.; Ferreira Queiroz, E.; David, B.; Grondin, A.; Katanaev, V.L.; Wolfender, J.L. Integration of Wnt-inhibitory activity and structural novelty scoring results to uncover novel bioactive natural products: New Bicyclo [3.3.1] non-3-ene-2,9-diones from the leaves of Hymenocardia punctata. Front. Chem. 2024, 12, 1371982. [Google Scholar] [CrossRef]
- Liu, D.; Chen, L.; Zhao, H.; Vaziri, N.D.; Ma, S.C.; Zhao, Y.Y. Small molecules from natural products targeting the Wnt/β-catenin pathway as a therapeutic strategy. Biomed. Pharmacother. 2019, 117, 108990. [Google Scholar] [CrossRef]
- Yu, W.K.; Xu, Z.Y.; Yuan, L.; Mo, S.; Xu, B.; Cheng, X.D.; Qin, J.J. Targeting β-Catenin Signaling by Natural Products for Cancer Prevention and Therapy. Front. Pharmacol. 2020, 11, 984. [Google Scholar] [CrossRef]
- Shaw, H.V.; Koval, A.; Katanaev, V.L. A high-throughput assay pipeline for specific targeting of frizzled GPCRs in cancer. Methods Cell Biol. 2019, 149, 57–75. [Google Scholar] [CrossRef] [PubMed]

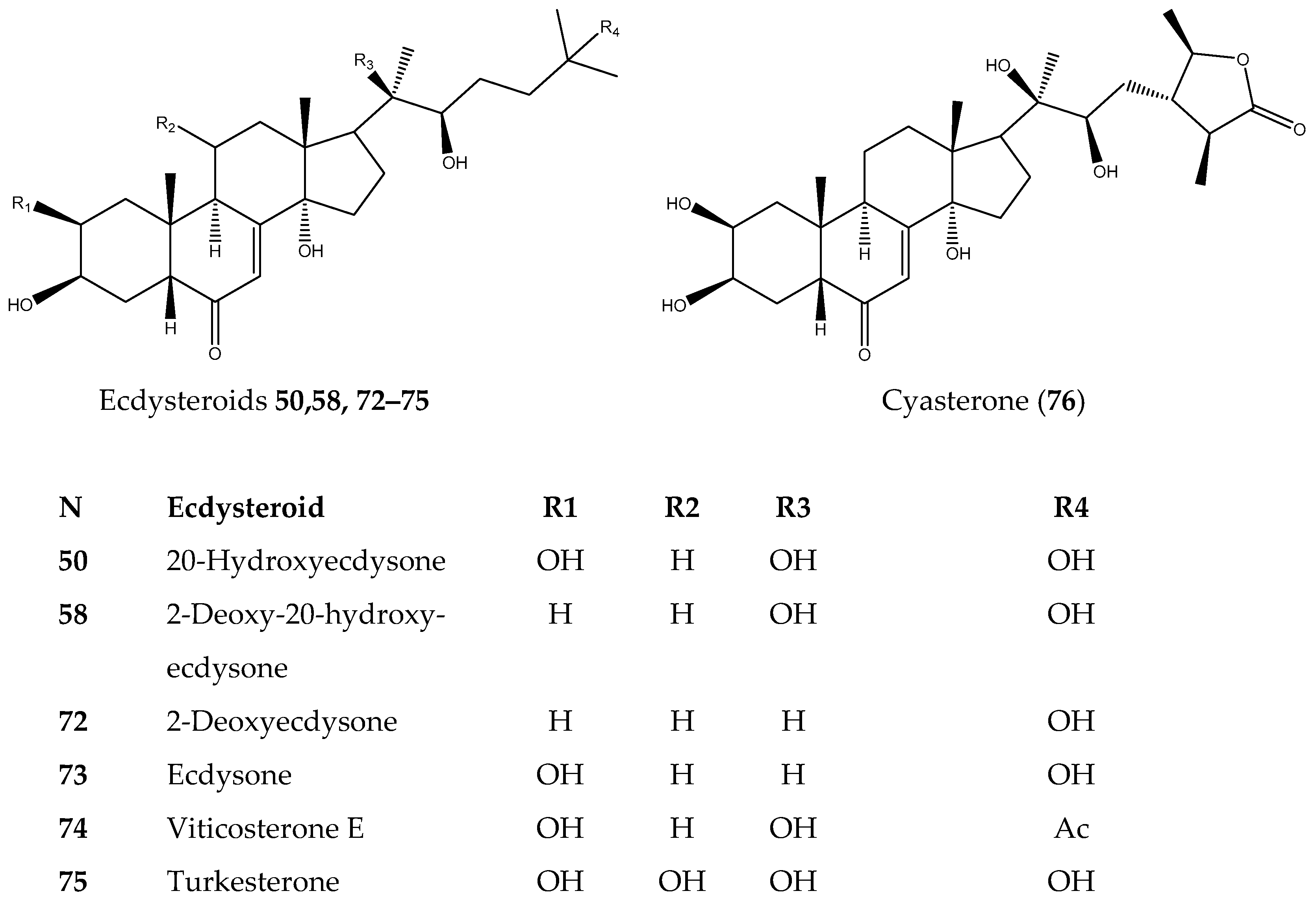
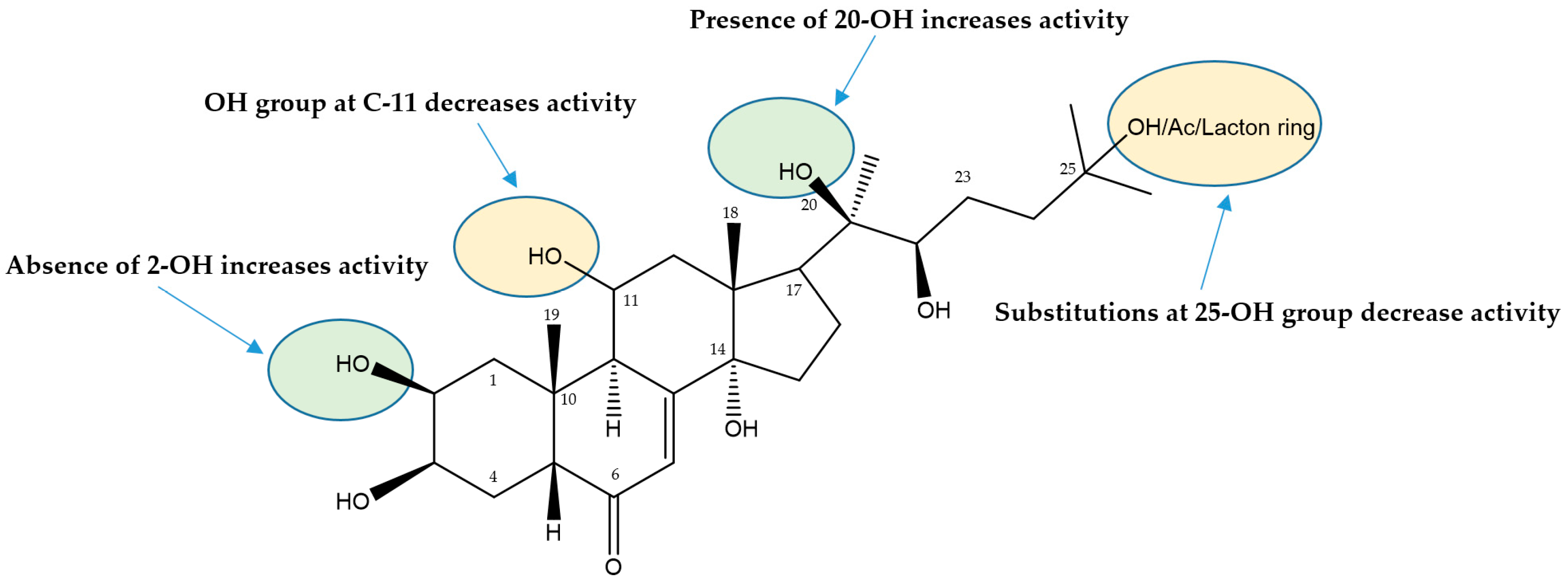
| N | Sample | BT-20 | MDA-MB-231 | HCC1395 | HEK293 |
|---|---|---|---|---|---|
| IC50, µM | |||||
| 12 | Quinic acid | >20 | >20 | >20 | >20 |
| 19 | Ferulic acid | >20 | >20 | >20 | >20 |
| 21 | p-Coumaric acid | >20 | >20 | >20 | >20 |
| 29 | Schaftoside | >20 | >20 | >20 | >20 |
| 38 | Isovitexin-7-O-glucoside | >20 | >20 | >20 | >20 |
| 41 | Rutin | >20 | >20 | >20 | >20 |
| 50 | 20-Hydroxyecdysone | >20 | >20 | >20 | >20 |
| 58 | 2-Deoxy-20-hydroxyecdysone | 0.12 ± 0.006 | 0.53 ± 0.14 | 0.21 ± 0.01 | 0.27 ± 0.01 |
| 71 | Oleanolic acid | >20 | >20 | >20 | >20 |
| 72 | 2-Deoxyecdysone | >20 | >20 | >20 | >20 |
| 73 | Ecdysone | >20 | >20 | >20 | >20 |
| 74 | Viticosterone E | >20 | >20 | >20 | >20 |
| 75 | Turkesterone | >20 | >20 | >20 | >20 |
| 76 | Cyasterone | >20 | >20 | >20 | >20 |
| MeOH extract (μg/mL) | 10.1 ± 1.3 | 10.4 ± 2.1 | 9.27 ± 1.8 | >20 | |
| Docetaxel (nM) | 4.4 ± 0.0008 | 17.7 ± 0.0029 | 6.5 ± 0.0007 | 4.47 ± 0.0013 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mamadalieva, N.Z.; Koval, A.; Dusmuratov, M.M.; Hussain, H.; Katanaev, V.L. Chemical and Biological Characterization of Metabolites from Silene viridiflora Using Mass Spectrometric and Cell-Based Assays. Biomolecules 2024, 14, 1285. https://doi.org/10.3390/biom14101285
Mamadalieva NZ, Koval A, Dusmuratov MM, Hussain H, Katanaev VL. Chemical and Biological Characterization of Metabolites from Silene viridiflora Using Mass Spectrometric and Cell-Based Assays. Biomolecules. 2024; 14(10):1285. https://doi.org/10.3390/biom14101285
Chicago/Turabian StyleMamadalieva, Nilufar Z., Alexey Koval, Maksud M. Dusmuratov, Hidayat Hussain, and Vladimir L. Katanaev. 2024. "Chemical and Biological Characterization of Metabolites from Silene viridiflora Using Mass Spectrometric and Cell-Based Assays" Biomolecules 14, no. 10: 1285. https://doi.org/10.3390/biom14101285
APA StyleMamadalieva, N. Z., Koval, A., Dusmuratov, M. M., Hussain, H., & Katanaev, V. L. (2024). Chemical and Biological Characterization of Metabolites from Silene viridiflora Using Mass Spectrometric and Cell-Based Assays. Biomolecules, 14(10), 1285. https://doi.org/10.3390/biom14101285







