Serum Otoconin-90 and Otolin-1 Concentrations in Benign Paroxysmal Positional Vertigo
Abstract
1. Introduction
2. Material and Method
2.1. Ethical Approval
2.2. Research Design
2.3. Inclusion Criteria
2.4. Exclusion Criteria
2.5. Sample Collection and Measurements
2.6. Measurement of Serum Otoconin 90 (OC90) Concentrations
2.7. Measurement of Serum Otolin-1 Concentrations
2.8. Statistical Analysis
3. Results
4. Discussion
Limitations of Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Strupp, M.; Mandalà, M.; López-Escámez, J.A. Peripheral vestibular disorders. Curr. Opin. Neurol. 2019, 32, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.-H.; Xirasagar, S.; Cheng, Y.-F.; Chen, C.-S.; Lin, H.-C. Increased prevalence of peripheral vestibular disorder among patients with Fabry disease. Orphanet J. Rare Dis. 2024, 19, 97. [Google Scholar] [CrossRef] [PubMed]
- Welgampola, M.S.; Akdal, G.; Halmagyi, G.M. Neuro-otology-some recent clinical advances. J. Neurol. 2017, 264, 188–203, Correction in J. Neurol. 2017, 264, 204. [Google Scholar] [CrossRef] [PubMed]
- Maudoux, A.; Vitry, S.; El-Amraoui, A. Vestibular Deficits in Deafness: Clinical Presentation, Animal Modeling, and Treatment Solutions. Front. Neurol. 2022, 13, 816534. [Google Scholar] [CrossRef]
- Gomaa, N.A.; Jimoh, Z.; Campbell, S.; Zenke, J.K.; Szczepek, A.J. Biomarkers for Inner Ear Disorders: Scoping Review on the Role of Biomarkers in Hearing and Balance Disorders. Diagnostics 2020, 11, 42. [Google Scholar] [CrossRef]
- Deans, M.R.; Peterson, J.M.; Wong, G.W. Mammalian Otolin: A multimeric glycoprotein specific to the inner ear that interacts with otoconial matrix protein Otoconin-90 and Cerebellin-1. PLoS ONE 2010, 5, e12765. [Google Scholar] [CrossRef]
- Yang, H.; Zhao, X.; Xu, Y.; Wang, L.; He, Q.; Lundberg, Y.W. Matrix recruitment and calcium sequestration for spatial specific otoconia development. PLoS ONE 2011, 6, e20498. [Google Scholar] [CrossRef]
- Andrade, L.R.; Lins, U.; Farina, M.; Kachar, B.; Thalmann, R. Immunogold TEM of otoconin 90 and otolin—Relevance to mineralization of otoconia, and pathogenesis of benign positional vertigo. Hear. Res. 2012, 292, 14–25. [Google Scholar] [CrossRef]
- Fan, Z.; Hu, Z.; Han, W.; Lu, X.; Liu, X.; Zhou, M.; Yan, W.; Wu, Y. High Serum Levels of Otolin-1 in Patients with Benign Paroxysmal Positional Vertigo Predict Recurrence. Front. Neurol. 2022, 13, 841677. [Google Scholar] [CrossRef]
- Zhao, X.; Yang, H.; Yamoah, E.N.; Lundberg, Y.W. Gene targeting reveals the role of Oc90 as the essential organizer of the otoconial organic matrix. Dev. Biol. 2007, 304, 508–524. [Google Scholar] [CrossRef]
- Huang, S.; Qian, S. Advances in otolith-related protein research. Front. Neurosci. 2022, 16, 956200. [Google Scholar] [CrossRef] [PubMed]
- Epley, J.M. Human experience with canalith repositioning maneuvers. Ann. N. Y. Acad. Sci. 2001, 942, 179–191. [Google Scholar] [CrossRef]
- Nuti, D.; Zee, D.S.; Mandalà, M. Benign Paroxysmal Positional Vertigo: What We Do and Do Not Know. Semin. Neurol. 2020, 40, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Guerra, J.; Devesa, J. Causes and treatment of idiopathic benign paroxysmal positional vertigo based on endocrinological and other metabolic factors. J. Otol. 2020, 15, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, Y.W.; Xu, Y.; Thiessen, K.D.; Kramer, K.L. Mechanisms of otoconia and otolith development. Dev. Dyn. 2015, 244, 239–253. [Google Scholar] [CrossRef]
- Bi, J.; Liu, B.; Zhang, Y.; Zhou, Q. Study on the Bone Metabolism Indices and Otoconin-90 in Benign Paroxysmal Positional Vertigo. Otol. Neurotol. 2021, 42, e744–e749. [Google Scholar] [CrossRef]
- Zhang, S.; Xing, J.; Gong, Y.; Li, P.; Wang, B.; Xu, L. Downregulation of VDR in benign paroxysmal positional vertigo patients inhibits otolith-associated protein expression levels. Mol. Med. Rep. 2021, 24, 591. [Google Scholar] [CrossRef]
- Parham, K.; Sacks, D.; Bixby, C.; Fall, P. Inner ear protein as a biomarker in circulation? Otolaryngol. Neck Surg. 2014, 151, 1038–1040. [Google Scholar] [CrossRef]
- Tabtabai, R.; Haynes, L.; Kuchel, G.A.; Parham, K. Age-Related Increase in Blood Levels of Otolin-1 in Humans. Otol. Neurotol. 2017, 38, 865–869. [Google Scholar] [CrossRef]
- Feng, M.Y.; Zhuang, J.H.; Gu, H.H.; Tian, Q.; Zhang, Z.H. Changes of serum E2 and Otolin-1 levels in postmenopausal women with BPPV. J. Clin. Otorhinolaryngol. Head Neck Surg. 2019, 33, 1138–1147. [Google Scholar]
- Sacks, D.; Parham, K. Preliminary Report on the Investigation of the Association Between BPPV and Osteoporosis Using Biomarkers. Otol. Neurotol. 2015, 36, 1532–1536. [Google Scholar] [CrossRef] [PubMed]
- Parham, K.; Kuchel, G.A. A Geriatric Perspective on Benign Paroxysmal Positional Vertigo. J. Am. Geriatr. Soc. 2016, 64, 378–385. [Google Scholar] [CrossRef] [PubMed]
- Qian, S.; Zhang, X.; Wang, Y. Serum Estradiol Correlates with Benign Paroxysmal Positional Vertigo in Postmenopausal Women. Endocr. Pract. 2022, 28, 673–677. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Han, W.; Yan, W.; Lu, X.; Zhou, M.; Li, L.; Guan, Q.; Fan, Z. Increased Otolin-1 in Serum as a Potential Biomarker for Idiopathic Benign Paroxysmal Positional Vertigo Episodes. Front. Neurol. 2020, 11, 367. [Google Scholar] [CrossRef]
- McKenna, K.; Rahman, K.; Parham, K. Otoconia degeneration as a consequence of primary hyperparathyroidism. Med. Hypotheses 2020, 144, 109982. [Google Scholar] [CrossRef]
- Irugu, D.V.K.; Singh, A.; Yadav, H.; Verma, H.; Kumar, R.; A Abraham, R.; Ramakrishnan, L. Serum otolin-1 as a biomarker for benign paroxysmal positional vertigo: A case-control study. J. Laryngol. Otol. 2021, 135, 589–592. [Google Scholar] [CrossRef]
- Liu, X.; Han, K.; Zhou, M.; Wu, Y. Association between otolin-1 and benign paroxysmal positional vertigo: A meta-analysis. Front. Neurol. 2022, 13, 950023. [Google Scholar] [CrossRef]
- Singh, A.; Yadav, H.; Verma, H.; Sikka, K.; Abraham, R.A.; Irugu, D.V.K. Normal Serum Levels of Otolin-1 in Patients with Meniere Disease in Remission. Int. Arch. Otorhinolaryngol. 2023, 27, e440–e444. [Google Scholar] [CrossRef]
- Hoenderop, J.G.; Nilius, B.; Bindels, R.J. Calcium absorption across epithelia. Physiol. Rev. 2005, 85, 373–422. [Google Scholar] [CrossRef]
- Yamauchi, D.; Raveendran, N.N.; Pondugula, S.R.; Kampalli, S.B.; Sanneman, J.D.; Harbidge, D.G.; Marcus, D.C. Vitamin D upregulates expression of ECaC1 mRNA in semicircular canal. Biochem. Biophys. Res. Commun. 2005, 331, 1353–1357. [Google Scholar] [CrossRef]
- Jeong, S.-H.; Kim, J.-S.; Shin, J.W.; Kim, S.; Lee, H.; Lee, A.Y.; Kim, J.-M.; Jo, H.; Song, J.; Ghim, Y. Decreased serum vitamin D in idiopathic benign paroxysmal positional vertigo. J. Neurol. 2013, 260, 832–838. [Google Scholar] [CrossRef] [PubMed]
- Büki, B.; Ecker, M.; Jünger, H.; Lundberg, Y.W. Vitamin D deficiency and benign paroxysmal positioning vertigo. Med. Hypotheses 2013, 80, 201–204. [Google Scholar] [CrossRef] [PubMed]
- Talaat, H.S.; Abuhadied, G.; Talaat, A.S.; Abdelaal, M.S.S. Low bone mineral density and vitamin D deficiency in patients with benign positional paroxysmal vertigo. Eur. Arch. Oto-Rhino-Laryngol. 2015, 272, 2249–2253. [Google Scholar] [CrossRef] [PubMed]
- Sheikhzadeh, M.; Lotfi, Y.; Mousavi, A.; Heidari, B.; Bakhshi, E. The effect of serum vitamin D normalization in preventing recurrences of benign paroxysmal positional vertigo: A case-control study. Casp. J. Intern. Med. 2016, 7, 173–177. [Google Scholar]
- Rhim, G.I. Serum vitamin D and recurrent benign paroxysmal positional vertigo. Laryngoscope Investig. Otolaryngol. 2016, 1, 150–153. [Google Scholar] [CrossRef]
- Karatas, A.; Yuceant, G.A.; Yuce, T.; Haci, C.; Cebi, I.T.; Salviz, M. Association of Benign Paroxysmal Positional Vertigo with Osteoporosis and Vitamin D Deficiency: A Case Controlled Study. J. Int. Adv. Otol. 2017, 13, 259–265. [Google Scholar] [CrossRef]
- Ren, Y.-Y.; Wang, Y.-J.; Li, J.-L.; Liu, M.; Xia, F. Low vitamin D and uric acid status in patients with benign paroxysmal positional vertigo. Sci. Prog. 2023, 106, 368504231205397. [Google Scholar] [CrossRef]
- Rhim, G.; Kim, M.-J. Vitamin D Supplementation and Recurrence of Benign Paroxysmal Positional Vertigo. Nutrients 2024, 16, 689. [Google Scholar] [CrossRef]
- Jeong, S.H.; Kim, J.S.; Kim, H.J.; Choi, J.Y.; Koo, J.W.; Choi, K.D.; Park, J.Y.; Lee, S.H.; Choi, S.Y.; Oh, S.Y.; et al. Prevention of benign paroxysmal positional vertigo with vitamin D supplementation: A randomized trial. Neurology 2020, 95, e1117–e1125. [Google Scholar] [CrossRef]
- Abdelmaksoud, A.A.; Fahim, D.F.M.; Bazeed, S.E.S.; Alemam, M.F.; Aref, Z.F. Relation between vitamin D deficiency and benign paroxysmal positional vertigo. Sci. Rep. 2021, 11, 16855. [Google Scholar] [CrossRef]
- Sharma, K.; Ojha, T.; Dabaria, R.; Chhabra, B.; Trivedi, B.B.; Bansal, M. Relation Between Posterior Canal Benign Paroxysmal Positional Vertigo and Vitamin D Deficiency. Indian J. Otolaryngol. Head Neck Surg. 2022, 74 (Suppl. S3), 4405–4408. [Google Scholar] [CrossRef]
| Case | Control | ||||
|---|---|---|---|---|---|
| n | % | n | % | p-Value | |
| Gender | |||||
| Male | 24 | 45.83% | 13 | 34.62% | 0.350 * |
| Female | 26 | 54.17% | 17 | 65.38% | |
| Age (mean ± std) | 42.46 ± 11.4 | 38.69 ± 11.2 | 0.176 † | ||
| Tinnitus | 14 | 29.17% | |||
| Right | 5 | 35.71% | - | - | - |
| Left | 9 | 64.29% | - | - | |
| Duration | |||||
| <1 week | 14 | 29.17% | - | - | - |
| 1–3 week | 26 | 54.17% | - | - | |
| ≥4 week | 8 | 16.67% | - | - | |
| Cause | |||||
| Idiopathic | 26 | 52% | - | - | - |
| After the URI | 20 | 40% | - | - | |
| Traumatic | 2 | 4% | - | - | |
| Pressure difference | 2 | 4% | - | - | |
| Neurological examination | |||||
| Normal | 30 | 60.00% | - | - | - |
| Nystagmus | 20 | 40.00% | - | - | |
| Case | Control | ||
|---|---|---|---|
| Mean ± Std or Median (25 p–75 p) | Mean ± Std or Median (25 p–75 p) | p-Value | |
| Otoconin-90 (ng/L) | 655.13 (553.47–1505.27) | 712.75 (623.59–832.12) | 0.892 ¥ |
| Calcium (mg/dL) | 8.93 ± 0.57 | 9.18 ± 0.55 | 0.091 † |
| Parathormone (pg/mL) | 48.25 ± 19.57 | 38.71 ± 12.32 | 0.051 † |
| Vitamin D (ng/mL) | 14.3 (8.45–17.5) | 26.25 (21–32) | <0.001 ¥ |
| Immature granulocyte (IG) (%) | 0.2 (0.2–0.3) | 0.2 (0.2–0.3) | 0.918 ¥ |
| White blood cell (103/µL) | 7.1 (5.8–9.1) | 6.42 (5.9–8.17) | 0.389 ¥ |
| Red blood cell (106/µL) | 4.7 (4.5–4.9) | 4.6 (4.25–5) | 0.258 ¥ |
| Hemoglobin (g/dL) | 13.38 ± 1.43 | 13.6 ± 1.49 | 0.539 † |
| Hematocrit (%) | 41.5 ± 2.34 | 40.55 ± 3.9 | 0.264 † |
| Platelet (106/µL) | 200.5 (180–223.5) | 226 (210–259) | 0.004 ¥ |
| Lymphocytes (103/µL) | 2.65 (2.25–3.1) | 2.44 (2.04–2.6) | 0.107 ¥ |
| Lymphocytes (%) | 29.7 (27.5–34.5) | 32 (28–33.6) | 0.662 ¥ |
| Neutrophil (103/µL) | 3.95 (3–5.45) | 2.98 (2.57–3.31) | <0.001 ¥ |
| Neutrophil (%) | 54.01 ± 7.8 | 29.8 ± 3.91 | <0.001 † |
| Monocyte (103/µL) | 0.6 (0.5–0.72) | 0.5 (0.42–0.6) | 0.013 ¥ |
| Monocyte (%) | 7.2 (6.4–8.9) | 7.6 (6.8–8.4) | 0.747 ¥ |
| Eosinophil (103/µL) | 0.21 (0.11–0.32) | 0.15 (0.1–0.2) | 0.094 ¥ |
| Eosinophil (%) | 1.8 (1.2–3.1) | 2 (1.5–2.9) | 0.765 ¥ |
| CRP (mg/L) | 3.3 (2–5.35) | 1.18 (0.68–1.7) | <0.001 ¥ |
| Neutrophil lymphocyte ratio (NLR) | 1.66 ± 0.52 | 1.29 ± 0.37 | 0.001 † |
| Platelet lymphocyte ratio (PLR) | 78.04 (62–94.74) | 98 (85.6–117.96) | 0.003 ¥ |
| Systemic immune inflammation index (SII) | 335.6 (227.89–425.03) | 314.22 (227.5–365.69) | 0.389 ¥ |
| Case | Control | ||
|---|---|---|---|
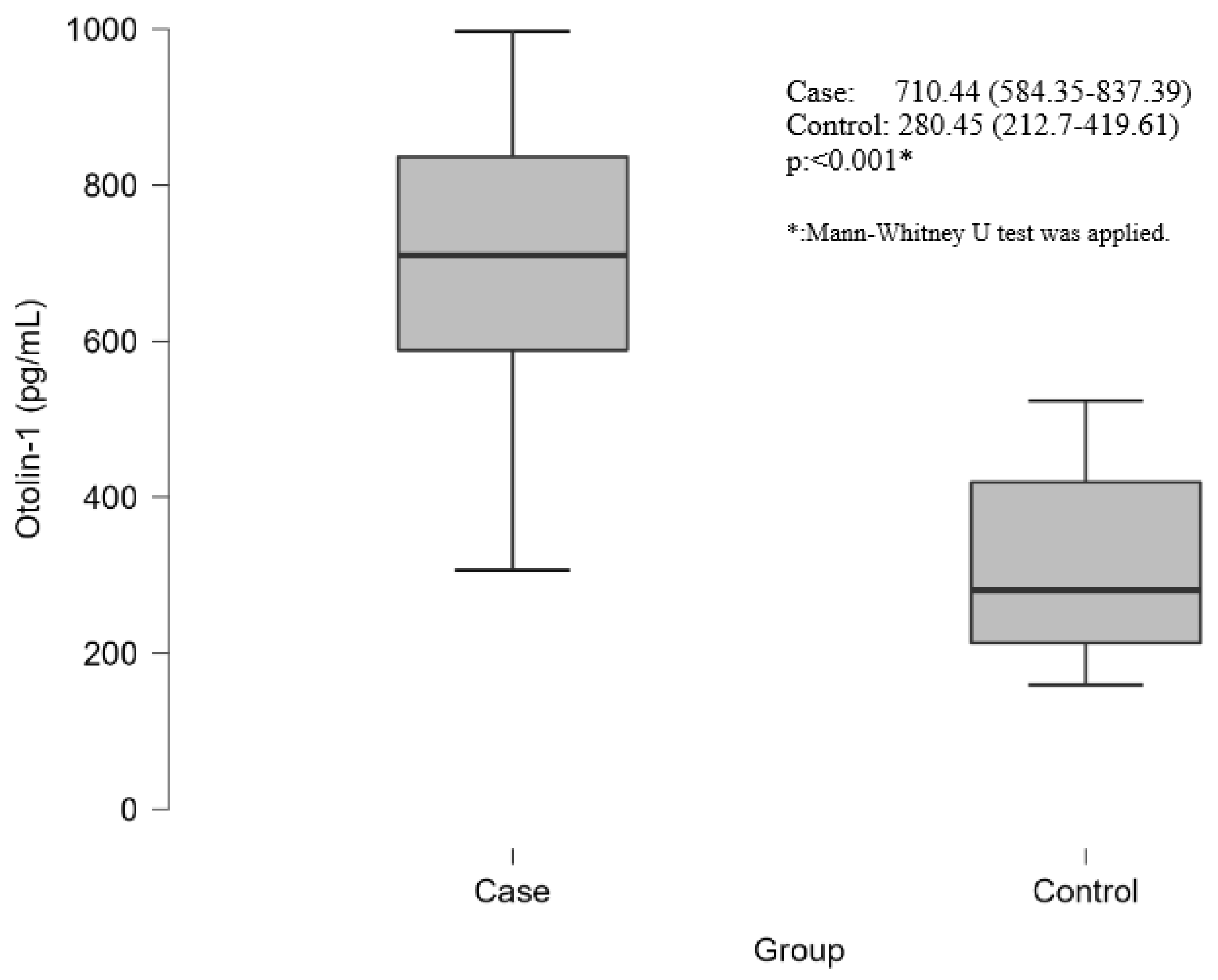
| Otolin-1 | mean ± std | 685.85 ± 202.72 | 307.88 ± 110.43 |
| Median (25 p–75 p) | 710.44 (584.35–837.39) | 280.45 (212.7–419.61) | |
| Median (Min–Max) | 710.44 (307.17–997.37) | 280.45 (159.31–523.51) | |
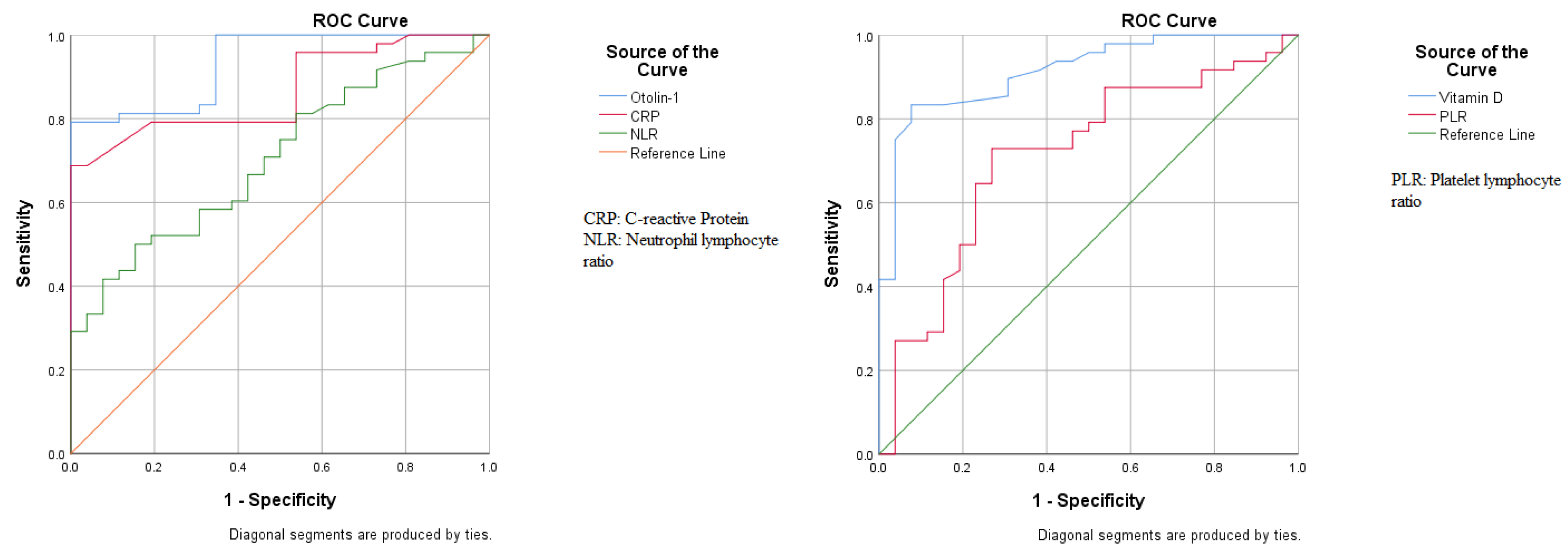
| Variables | AUC | 95 CI of AUC | p-Value | Cutoff | Sensitivity | Specificity |
|---|---|---|---|---|---|---|
| Otolin-1 | 0.933 | 0.881–0.986 | <0.001 | 525 ¶ | 79.17% | 100% |
| CRP | 0.867 | 0.787–0.947 | <0.001 | 2 ¶ | 79.17% | 80.77% |
| 2.2 ¶ | 68.75% | 96.15% | ||||
| NLR | 0.705 | 0.587–0.823 | 0.004 | 1.65 ¶ | 50.00% | 84.62% |
| PLR | 0.713 | 0.588–0.838 | 0.003 | 87.5 * | 72.92% | 73.08% |
| Vitamin D | 0.913 | 0.848–0.979 | <0.001 | 20 * | 83.33% | 88.46% |
| All Group | |||
|---|---|---|---|
| Otoconin-90 (ng/L) | Vitamin D | ||
| Otolin-1 (pg/mL) | r | 0.393 | −0.682 |
| p | 0.001 | <0.001 | |
| Otoconin-90 (ng/L) | r | −0.404 | |
| p | <0.001 | ||
| Patients | |||
| Otoconin-90 (ng/L) | Vitamin D | ||
| Otolin-1 (pg/mL) | r | 0.693 | −0.586 |
| p | <0.001 | <0.001 | |
| Otoconin-90 (ng/L) | r | −0.672 | |
| p | <0.001 | ||
| Control | |||
| Otoconin-90 (ng/L) | Vitamin D | ||
| Otolin-1 (pg/mL) | r | 0.039 | 0.181 |
| p | 0.851 | 0.376 | |
| Otoconin-90 (ng/L) | r | −0.151 | |
| p | 0.460 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aygun, D.; Dumur, S.; Elgormus, M.N.; Alpaslan, M.S.; Uzun, H. Serum Otoconin-90 and Otolin-1 Concentrations in Benign Paroxysmal Positional Vertigo. Biomolecules 2024, 14, 1279. https://doi.org/10.3390/biom14101279
Aygun D, Dumur S, Elgormus MN, Alpaslan MS, Uzun H. Serum Otoconin-90 and Otolin-1 Concentrations in Benign Paroxysmal Positional Vertigo. Biomolecules. 2024; 14(10):1279. https://doi.org/10.3390/biom14101279
Chicago/Turabian StyleAygun, Demet, Seyma Dumur, Mehmet Nuri Elgormus, Mehmet Serkan Alpaslan, and Hafize Uzun. 2024. "Serum Otoconin-90 and Otolin-1 Concentrations in Benign Paroxysmal Positional Vertigo" Biomolecules 14, no. 10: 1279. https://doi.org/10.3390/biom14101279
APA StyleAygun, D., Dumur, S., Elgormus, M. N., Alpaslan, M. S., & Uzun, H. (2024). Serum Otoconin-90 and Otolin-1 Concentrations in Benign Paroxysmal Positional Vertigo. Biomolecules, 14(10), 1279. https://doi.org/10.3390/biom14101279






