Exercise, Neuroprotective Exerkines, and Parkinson’s Disease: A Narrative Review
Abstract
1. Introduction
1.1. Neurodegenerative Diseases and the Positive Impact of Exercise
1.2. Parkinson’s Disease (PD)
1.3. Exercise and Exerkines
1.4. Parkinson’s Disease, Exercise, and Exerkines
2. Exercise as Therapy for Parkinson’s Disease
“Movement is a medicine for creating change in a person’s physical, emotional, and mental states.” Carol Welch-Baril, Neuromuscular Therapist
2.1. Defining Physical Activity and Exercise
2.1.1. Exercise Intensity Defined
2.1.2. Calculating Maximal Heart Rate during Exercise
2.2. Aerobic Exercise
2.2.1. Motor Symptoms
2.2.2. Brain Structure and Cognitive Symptoms
2.3. Resistance Training
2.3.1. Motor Symptoms, Disease Severity, and Motor Function
2.3.2. Non-Motor Symptoms and Quality of Life
2.4. Neuromotor Exercises to Improve Gait, Posture, Balance, and Reduce Risk of Falls
2.5. Stretching and Flexibility Exercises to Reduce Muscle Rigidity
2.6. Review of Systematic and Meta-Analysis Studies of Exercise for Treating Parkinson’s Disease
2.7. Exercise Suggestions
2.8. Strategies for Overcoming Barriers to Exercise for People with Parkinson’s Disease
2.8.1. Reworking Aerobic Exercise
2.8.2. Revisiting Resistance Training
2.8.3. Adaption of Neuromotor Exercises: Agility and Balance
2.8.4. Refining Flexibility
2.9. Examples of a Structured Exercise Strategy for Parkinson’s Disease
2.10. The Effect of Exercise on the Central Nervous System (CNS) and Motor Unit in Parkinson’s Disease
2.10.1. Exercise Enhances the Function of the CNS
2.10.2. Exercise Improves Motor Function
2.10.3. Long-Term Effect of Exercise on Parkinson’s Disease
2.11. Summary of Exercise and Introduction to Exerkines
3. Exercise-Induced Neuroprotective Exerkines
“Natural forces within us are the true healers of disease.” Hippocrates (460–375 BCE) [2]
3.1. The Association between Cardiorespiratory Fitness and the Risk of Parkinson’s Disease
3.2. Neuroprotective Exerkines
3.2.1. Adiponectin
3.2.2. Apelin
3.2.3. Beta-Aminoisobutyric Acid (BAIBA)
3.2.4. Beta-Hydroxybutyrate (BHB)
3.2.5. Brain-Derived Neurotrophic Factor (BDNF)
3.2.6. Cathepsin B (CTSB)
3.2.7. Fetuin-A
3.2.8. Fibroblast Growth Factor 21 (FGF21)
3.2.9. Glial Cell Line-Derived Neurotrophic Factor (GDNF)
3.2.10. Glycosylphosphatidylinositol-Specific Phospholipase D1 (GPLD1)
3.2.11. Insulin-like Growth Factor-1 (IGF-1)
3.2.12. Interleukin-6 (IL-6)
3.2.13. Irisin (FNDC5)
3.2.14. Lactate
3.2.15. Lac-Phe
3.2.16. miR451a and miR-150-5p in Small Extracellular Vesicles (sEVs)
3.2.17. Monoamine Neurotransmitters (Dopamine (DA), Norepinephrine (NE), and Serotonin (5-HT))
3.2.18. Peroxisome Proliferator-Activated Receptor-Gamma Coactivator (PGC)-1alpha (PGC-1α)/Kynurenine (Kyn) Interaction
3.2.19. Vascular Endothelial Growth Factor (VEGF)
3.3. Exerkines and the Ability to Cross the Blood–Brain Barrier
3.4. Summary of Exerkines
4. Factors That Modulate the Production of Exerkines
“Look to the nervous system as the key to maximum health.” Galen (130 AD–210 AD) [2]
4.1. Aerobic Exercise (Endurance-Based) versus Resistance Training (Strength-Based)
4.2. Age
4.3. Muscle Wasting
4.4. Sedentary Lifestyle
5. The Neuroprotective Theory to Slow the Progression of Parkinson’s Disease
“Your age is the sum total of your physical condition, the condition of your mind, and how you feel.” Jack LaLanne (1914–2011; LaLanne hosted the first and longest-running syndicated fitness television program in the U.S., from 1951 to 1985)
5.1. Assessing the Neuroprotective Theory
5.2. Initiation Pathways for Aerobic Exercise and Resistance Training
5.3. Neuroprotective Action from Myokines in Model Systems of Parkinson’s Disease
5.4. Neuroprotective Components Provided by Exerkines
6. Strengths, Limitations, and Challenges
“We must turn to nature itself, to the observations of the body in health and in disease to learn the truth.” Hippocrates (460–375 BCE) [2]
7. Conclusions
“The use of exercise has had an important share in the treatment of disease since Hippocrates used it at the sanitarium at Cos, and Galen advocated it in words that are as true now as they were eighteen hundred years ago…” R. Tait McKenzie, M.D. (1909) in “Exercise in Education and Medicine” [468].
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s disease |
| ALS | amyotrophic lateral sclerosis |
| AMPK | AMP-activated protein kinase |
| BAIBA | beta-aminoisobutyric acid |
| BBB | blood–brain barrier |
| BDNF | brain-derived neurotrophic factor |
| BHB | beta-hydroxybutyrate |
| Bpm | beats per minute |
| CNS | central nervous system |
| CTSB | cathepsin B |
| DA | dopamine |
| ERK 1/2 | extracellular signal-regulated kinase 1/2 |
| FGF21 | fibroblast growth factor 21 |
| FNDC5 | fibronectin type III domain-containing Protein 5 |
| GDNF | glial cell line-derived neurotropic factor |
| GPLD1 | glycosylphosphatidylinositol-specific phospholipase D1 |
| GPCR | G protein-coupled receptor |
| % HRmax | % maximal heart rate |
| HCAR2 | hydroxycarboxylic acid receptor 2 |
| H&Y | Hoehn and Yahr scale of PD stage |
| 6-OHDA | 6-hydroxydopamine |
| IGF-1 | insulin-like growth factor 1 |
| IL-1, -6, -10 | interleukin-1, -6, -10 |
| Kyn | Kynurenine |
| Lac-Phe | Lactoylphenylalanine |
| miR | microRNA |
| MPTP, MPP+ | 1-methyl-4-phenylpyridinium (Inactive/active forms) |
| MCI | mild cognitive impairment |
| p38 MAPK | p38 mitogen-activated protein kinase |
| ND | neurodegenerative |
| NE | norepinephrine |
| NF-κB | nuclear factor kappa-light-chain-enhancer of activated B cells |
| OT | occupational therapist |
| PD | Parkinson’s disease |
| PwP | people (person)-with-Parkinson’s |
| PGC-1α | peroxisome proliferator-activated receptor gamma coactivator 1-alpha |
| PPARα/δ/γ | peroxisome proliferator-activated receptor alpha/delta/gamma |
| PI3K/AKT/mTOR | phosphatidylinositol 3-kinase/protein kinase B/mammalian target of rapamycin |
| PT | physical therapist |
| PTSD | Post traumatic stress disorder |
| Irisin | proteolyzed fragment of FNDC5 |
| PAI-1 | plasminogen activator inhibitor-1 |
| 5-HT | serotonin |
| SIRT1 | sirtuin 1 |
| SLP | speech–language pathologist |
| sEV | small extracellular vesicles |
| TBI | Traumatic brain injury |
| TrkB | tropomyosin receptor kinase B |
| TNF-α | Tumor necrosis factor-alpha |
| UPDRS | Unified Parkinson’s Disease Rating Scale |
| VEGF(R) | vascular endothelial growth factor (receptor) |
| WAT | white-adipose tissue |
References
- Tipton, C.M. Susruta of India, an unrecognized contributor to the history of exercise physiology. J. Appl. Physiol. 2008, 104, 1553–1556. [Google Scholar] [CrossRef] [PubMed]
- Tipton, C.M. The history of “Exercise Is Medicine” in ancient civilizations. Adv. Physiol. Educ. 2014, 38, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Skovronsky, D.M.; Lee, V.M.-Y.; Trojanowski, J.Q. Neurodegenerative diseases: New concepts of pathogenesis and their therapeutic implications. Annu. Rev. Pathol. Mech. Dis. 2006, 1, 151–170. [Google Scholar] [CrossRef] [PubMed]
- Dugger, B.N.; Dickson, D.W. Pathology of neurodegenerative diseases. Cold Spring Harb. Perspect. Biol. 2017, 9, a028035. [Google Scholar] [CrossRef] [PubMed]
- Hansson, O. Biomarkers for neurodegenerative diseases. Nat. Med. 2021, 27, 954–963. [Google Scholar] [CrossRef]
- Barnham, K.J.; Masters, C.L.; Bush, A.I. Neurodegenerative diseases and oxidative stress. Nat. Rev. Drug Discov. 2004, 3, 205–214. [Google Scholar] [CrossRef]
- Amor, S.; Puentes, F.; Baker, D.; Van Der Valk, P. Inflammation in neurodegenerative diseases. Immunology 2010, 129, 154–169. [Google Scholar] [CrossRef]
- Brettschneider, J.; Tredici, K.D.; Lee, V.M.-Y.; Trojanowski, J.Q. Spreading of pathology in neurodegenerative diseases: A focus on human studies. Nat. Rev. Neurosci. 2015, 16, 109–120. [Google Scholar] [CrossRef]
- Spires-Jones, T.L.; Attems, J.; Thal, D.R. Interactions of pathological proteins in neurodegenerative diseases. Acta Neuropathol. 2017, 134, 187–205. [Google Scholar] [CrossRef]
- Spillantini, M.G.; Goedert, M. Tau protein pathology in neurodegenerative diseases. Trends Neurosci. 1998, 21, 428–433. [Google Scholar] [CrossRef]
- Ross, C.A.; Poirier, M.A. What is the role of protein aggregation in neurodegeneration? Nat. Rev. Mol. Cell Biol. 2005, 6, 891–898. [Google Scholar] [CrossRef] [PubMed]
- Ross, C.A.; Poirier, M.A. Protein aggregation and neurodegenerative disease. Nat. Med. 2004, 10, S10–S17. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yan, T.; Chu, J.M.-T.; Chen, Y.; Dunnett, S.; Ho, Y.-S.; Wong, G.T.-C.; Chang, R.C.-C. The beneficial effects of physical exercise in the brain and related pathophysiological mechanisms in neurodegenerative diseases. Lab. Investig. 2019, 99, 943–957. [Google Scholar] [CrossRef] [PubMed]
- Marques-Aleixo, I.; Beleza, J.; Sampaio, A.; Stevanović, J.; Coxito, P.; Gonçalves, I.; Ascensão, A.; Magalhães, J. Preventive and therapeutic potential of physical exercise in neurodegenerative diseases. Antioxid. Redox Signal. 2021, 34, 674–693. [Google Scholar] [CrossRef] [PubMed]
- Quan, H.; Koltai, E.; Suzuki, K.; Aguiar, A.S., Jr.; Pinho, R.; Boldogh, I.; Berkes, I.; Radak, Z. Exercise, redox system and neurodegenerative diseases. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2020, 1866, 165778. [Google Scholar] [CrossRef]
- Memon, A.A.; Coleman, J.J.; Amara, A.W. Effects of exercise on sleep in neurodegenerative disease. Neurobiol. Dis. 2020, 140, 104859. [Google Scholar] [CrossRef]
- Farì, G.; Lunetti, P.; Pignatelli, G.; Raele, M.V.; Cera, A.; Mintrone, G.; Ranieri, M.; Megna, M.; Capobianco, L. The effect of physical exercise on cognitive impairment in neurodegenerative disease: From pathophysiology to clinical and rehabilitative aspects. Int. J. Mol. Sci. 2021, 22, 11632. [Google Scholar] [CrossRef]
- Smith, M.; Barker, R.; Williams, G.; Carr, J.; Gunnarsson, R. The effect of exercise on high-level mobility in individuals with neurodegenerative disease: A systematic literature review. Physiotherapy 2020, 106, 174–193. [Google Scholar] [CrossRef]
- Lee, B.; Shin, M.; Park, Y.; Won, S.-Y.; Cho, K.S. Physical exercise-induced myokines in neurodegenerative diseases. Int. J. Mol. Sci. 2021, 22, 5795. [Google Scholar] [CrossRef]
- Valenzuela, P.L.; Castillo-Garcia, A.; Morales, J.S.; de la Villa, P.; Hampel, H.; Emanuele, E.; Lista, S.; Lucia, A. Exercise benefits on Alzheimer’s disease: State-of-the-science. Ageing Res. Rev. 2020, 62, 101108. [Google Scholar] [CrossRef]
- Gupta, R.; Khan, R.; Cortes, C.J. Forgot to Exercise? Exercise Derived Circulating Myokines in Alzheimer’s Disease: A Perspective. Front. Neurol. 2021, 12, 649452. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zhang, H.; Liang, J.; Huang, J.; Chen, N. Exercise suppresses neuroinflammation for alleviating Alzheimer’s disease. J. Neuroinflammation 2023, 20, 76. [Google Scholar] [CrossRef] [PubMed]
- Rody, T.; De Amorim, J.A.; De Felice, F.G. The emerging neuroprotective roles of exerkines in Alzheimer’s disease. Front. Aging Neurosci. 2022, 14, 965190. [Google Scholar] [CrossRef] [PubMed]
- Mattson, M.P. Energy intake and exercise as determinants of brain health and vulnerability to injury and disease. Cell Metab. 2012, 16, 706–722. [Google Scholar] [CrossRef]
- Cotman, C.W.; Berchtold, N.C. Exercise: A behavioral intervention to enhance brain health and plasticity. Trends Neurosci. 2002, 25, 295–301. [Google Scholar] [CrossRef]
- Safdar, A.; Saleem, A.; Tarnopolsky, M.A. The potential of endurance exercise-derived exosomes to treat metabolic diseases. Nat. Rev. Endocrinol. 2016, 12, 504–517. [Google Scholar] [CrossRef] [PubMed]
- Loprinzi, P.D.; Moore, D.; Loenneke, J.P. Does Aerobic and Resistance Exercise Influence Episodic Memory through Unique Mechanisms? Brain Sci. 2020, 10, 913. [Google Scholar] [CrossRef] [PubMed]
- Booth, F.W.; Roberts, C.K.; Thyfault, J.P.; Ruegsegger, G.N.; Toedebusch, R.G. Role of inactivity in chronic diseases: Evolutionary insight and pathophysiological mechanisms. Physiol. Rev. 2017, 97, 1351–1402. [Google Scholar] [CrossRef] [PubMed]
- Booth, F.W.; Roberts, C.K.; Laye, M.J. Lack of exercise is a major cause of chronic diseases. Compr. Physiol. 2012, 2, 1143. [Google Scholar]
- Xu, D.-C.; Chen, Y.; Xu, Y.; ShenTu, C.-Y.; Peng, L.-H. Signaling pathways in Parkinson’s disease: Molecular mechanisms and therapeutic interventions. Signal Transduct. Target. Ther. 2023, 8, 73. [Google Scholar]
- Kalia, L.; Lang, A. Parkinson’s disease. Lancet 2015, 386, 896–912. [Google Scholar] [CrossRef]
- Ahlskog, J.E. The New Parkinson’s Disease Treatment Book: Partnering with Your Doctor to Get the Most from Your Medications; Oxford University Press: New York, NY, USA, 2015. [Google Scholar]
- Poewe, W.; Seppi, K.; Tanner, C.M.; Halliday, G.M.; Brundin, P.; Volkmann, J.; Schrag, A.-E.; Lang, A.E. Parkinson disease. Nat. Rev. Dis. Primers 2017, 3, 17013. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, M.J.; Okun, M.S. Diagnosis and treatment of Parkinson disease: A review. JAMA 2020, 323, 548–560. [Google Scholar] [CrossRef]
- Jankovic, J. Motor fluctuations and dyskinesias in Parkinson’s disease: Clinical manifestations. Mov. Disord. 2005, 20, S11–S16. [Google Scholar] [CrossRef]
- Santens, P.; Boon, P.; Van Roost, D.; Caemaert, J. The pathophysiology of motor symptoms in Parkinson’s disease. Acta Neurol. Belg. 2003, 103, 129–134. [Google Scholar]
- Ferrazzoli, D.; Ortelli, P.; Cucca, A.; Bakdounes, L.; Canesi, M.; Volpe, D. Motor-cognitive approach and aerobic training: A synergism for rehabilitative intervention in Parkinson’s disease. Neurodegener. Dis. Manag. 2020, 10, 41–55. [Google Scholar] [CrossRef]
- Chaudhuri, K.R.; Schapira, A.H. Non-motor symptoms of Parkinson’s disease: Dopaminergic pathophysiology and treatment. Lancet Neurol. 2009, 8, 464–474. [Google Scholar] [CrossRef]
- Berganzo, K.; Tijero, B.; Gonzalez-Eizaguirre, A.; Somme, J.; Lezcano, E.; Gabilondo, I.; Fernandez, M.; Zarranz, J.; Gómez-Esteban, J. Motor and non-motor symptoms of Parkinson’s disease and their impact on quality of life and on different clinical subgroups. Neurología 2016, 31, 585–591. (In English) [Google Scholar] [CrossRef]
- Vuletić, V. Non-motor Symptoms in Parkinson’s Disease. In Mind and Brain; Springer Nature: Cham, Switzerland, 2020; pp. 109–118. [Google Scholar] [CrossRef]
- Schapira, A.H.; Chaudhuri, K.R.; Jenner, P. Non-motor features of Parkinson disease. Nat. Rev. Neurosci. 2017, 18, 435. [Google Scholar] [CrossRef]
- Pfeiffer, R.F. Non-motor symptoms in Parkinson’s disease. Park. Relat. Disord. 2016, 22, S119–S122. [Google Scholar] [CrossRef]
- Church, F.C. Treatment Options for Motor and Non-Motor Symptoms of Parkinson’s Disease. Biomolecules 2021, 11, 612. [Google Scholar] [CrossRef] [PubMed]
- Connolly, B.S.; Lang, A.E. Pharmacological treatment of Parkinson disease: A review. JAMA 2014, 311, 1670–1683. [Google Scholar] [CrossRef] [PubMed]
- van der Kolk, N.M.; de Vries, N.M.; Kessels, R.P.; Joosten, H.; Zwinderman, A.H.; Post, B.; Bloem, B.R. Effectiveness of home-based and remotely supervised aerobic exercise in Parkinson’s disease: A double-blind, randomised controlled trial. Lancet Neurol. 2019, 18, 998–1008. [Google Scholar] [CrossRef] [PubMed]
- Johansson, M.E.; Cameron, I.G.; Van der Kolk, N.M.; de Vries, N.M.; Klimars, E.; Toni, I.; Bloem, B.R.; Helmich, R.C. Aerobic Exercise Alters Brain Function and Structure in Parkinson’s Disease: A Randomized Controlled Trial. Ann. Neurol. 2022, 91, 203–216. [Google Scholar] [CrossRef] [PubMed]
- Vogel, T.; Brechat, P.H.; Leprêtre, P.M.; Kaltenbach, G.; Berthel, M.; Lonsdorfer, J. Health benefits of physical activity in older patients: A review. Int. J. Clin. Pract. 2009, 63, 303–320. [Google Scholar] [CrossRef]
- Chow, L.S.; Gerszten, R.E.; Taylor, J.M.; Pedersen, B.K.; Van Praag, H.; Trappe, S.; Febbraio, M.A.; Galis, Z.S.; Gao, Y.; Haus, J.M. Exerkines in health, resilience and disease. Nat. Rev. Endocrinol. 2022, 18, 273–289. [Google Scholar] [CrossRef]
- Giudice, J.; Taylor, J.M. Muscle as a paracrine and endocrine organ. Curr. Opin. Pharmacol. 2017, 34, 49–55. [Google Scholar] [CrossRef]
- Türkel, İ.; Özerkliğ, B.; Atakan, M.M.; Aktitiz, S.; Koşar, Ş.N.; Yazgan, B. Exercise and metabolic health: The emerging roles of novel exerkines. Curr. Protein Pept. Sci. 2022, 23, 437–455. [Google Scholar]
- Robbins, J.M.; Gerszten, R.E. Exercise, exerkines, and cardiometabolic health: From individual players to a team sport. J. Clin. Investig. 2023, 133, e168121. [Google Scholar] [CrossRef]
- Brooks, G.A.; Osmond, A.D.; Arevalo, J.A.; Duong, J.J.; Curl, C.C.; Moreno-Santillan, D.D.; Leija, R.G. Lactate as a myokine and exerkine: Drivers and signals of physiology and metabolism. J. Appl. Physiol. 2023, 134, 529–548. [Google Scholar] [CrossRef]
- Westphal, A. New insights into the molecular basis of how physical activity contributes to human health. Acta Physiol. 2023, 239, e14047. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, B.K. The physiology of optimizing health with a focus on exercise as medicine. Annu. Rev. Physiol. 2019, 81, 607–627. [Google Scholar] [CrossRef] [PubMed]
- Brooks, G.A.; Osmond, A.D.; Arevalo, J.A.; Curl, C.C.; Duong, J.J.; Horning, M.A.; Moreno Santillan, D.D.; Leija, R.G. Lactate as a major myokine and exerkine. Nat. Rev. Endocrinol. 2022, 18, 712. [Google Scholar] [CrossRef] [PubMed]
- Górecka, M.; Krzemiński, K.; Mikulski, T.; Ziemba, A.W. ANGPTL4, IL-6 and TNF-α as regulators of lipid metabolism during a marathon run. Sci. Rep. 2022, 12, 19940. [Google Scholar] [CrossRef] [PubMed]
- Silva, G.J.; Bye, A.; El Azzouzi, H.; Wisløff, U. MicroRNAs as important regulators of exercise adaptation. Prog. Cardiovasc. Dis. 2017, 60, 130–151. [Google Scholar] [CrossRef] [PubMed]
- Isaac, A.R.; Lima-Filho, R.A.; Lourenco, M.V. How does the skeletal muscle communicate with the brain in health and disease? Neuropharmacology 2021, 197, 108744. [Google Scholar] [CrossRef]
- Townsend, L.K.; MacPherson, R.E.; Wright, D.C. New horizon: Exercise and a focus on tissue-brain crosstalk. J. Clin. Endocrinol. Metab. 2021, 106, 2147–2163. [Google Scholar] [CrossRef]
- Benarroch, E. What Muscle Signals Mediate the Beneficial Effects of Exercise on Cognition? Neurology 2022, 99, 298–304. [Google Scholar] [CrossRef]
- NHIS. Adult Physical Activity—Glossary. Available online: https://www.cdc.gov/nchs/nhis/physical_activity/pa_glossary.htm (accessed on 17 April 2024).
- Tolwani, R.J.; Jakowec, M.W.; Petzinger, G.M.; Green, S.; Waggie, K. Experimental models of Parkinson’s disease: Insights from many models. Comp. Med. 1999, 49, 363–371. [Google Scholar]
- Lau, Y.S.; Patki, G.; Das-Panja, K.; Le, W.D.; Ahmad, S.O. Neuroprotective effects and mechanisms of exercise in a chronic mouse model of Parkinson’s disease with moderate neurodegeneration. Eur. J. Neurosci. 2011, 33, 1264–1274. [Google Scholar] [CrossRef]
- Goes, A.; Souza, L.; Del Fabbro, L.; De Gomes, M.; Boeira, S.; Jesse, C. Neuroprotective effects of swimming training in a mouse model of Parkinson’s disease induced by 6-hydroxydopamine. Neuroscience 2014, 256, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Petzinger, G.M.; Walsh, J.P.; Akopian, G.; Hogg, E.; Abernathy, A.; Arevalo, P.; Turnquist, P.; Vučković, M.; Fisher, B.E.; Togasaki, D.M. Effects of treadmill exercise on dopaminergic transmission in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-lesioned mouse model of basal ganglia injury. J. Neurosci. 2007, 27, 5291–5300. [Google Scholar] [CrossRef] [PubMed]
- Palasz, E.; Niewiadomski, W.; Gasiorowska, A.; Wysocka, A.; Stepniewska, A.; Niewiadomska, G. Exercise-induced neuroprotection and recovery of motor function in animal models of Parkinson’s disease. Front. Neurol. 2019, 10, 1143. [Google Scholar] [CrossRef] [PubMed]
- Schenkman, M.; Moore, C.G.; Kohrt, W.M.; Hall, D.A.; Delitto, A.; Comella, C.L.; Josbeno, D.A.; Christiansen, C.L.; Berman, B.D.; Kluger, B.M. Effect of high-intensity treadmill exercise on motor symptoms in patients with de novo Parkinson disease: A phase 2 randomized clinical trial. JAMA Neurol. 2018, 75, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Tollár, J.; Nagy, F.; Hortobágyi, T. Vastly different exercise programs similarly improve parkinsonian symptoms: A randomized clinical trial. Gerontology 2019, 65, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Mak, M.K.; Wong-Yu, I.S. Six-month community-based brisk walking and balance exercise alleviates motor symptoms and promotes functions in people with Parkinson’s disease: A randomized controlled trial. J. Park. Dis. 2021, 11, 1431–1441. [Google Scholar] [CrossRef]
- How Much Should the Average Adult Exercise Every Day? Available online: https://www.mayoclinic.org/healthy-lifestyle/fitness/expert-answers/exercise/faq-20057916 (accessed on 1 August 2024).
- Exercise Intensity: How to Measure, It. Available online: https://www.mayoclinic.org/healthy-lifestyle/fitness/in-depth/exercise-intensity/art-20046887 (accessed on 1 August 2024).
- How Much Physical Activity Do You Need? Available online: https://www.heart.org/en/healthy-living/fitness/fitness-basics/aha-recs-for-physical-activity-infographic (accessed on 1 August 2024).
- Zhen, K.; Zhang, S.; Tao, X.; Li, G.; Lv, Y.; Yu, L. A systematic review and meta-analysis on effects of aerobic exercise in people with Parkinson’s disease. npj Park. Dis. 2022, 8, 146. [Google Scholar] [CrossRef]
- Schootemeijer, S.; Darweesh, S.K.L.; de Vries, N.M. Clinical Trial Highlights—Aerobic Exercise for Parkinson’s Disease. J. Park. Dis. 2022, 12, 2297–2306. [Google Scholar] [CrossRef]
- Alberts, J.L.; Rosenfeldt, A.B. The universal prescription for Parkinson’s disease: Exercise. J. Park. Dis. 2020, 10, S21–S27. [Google Scholar] [CrossRef]
- Osborne, J.A.; Botkin, R.; Colon-Semenza, C.; DeAngelis, T.R.; Gallardo, O.G.; Kosakowski, H.; Martello, J.; Pradhan, S.; Rafferty, M.; Readinger, J.L. Physical therapist management of Parkinson disease: A clinical practice guideline from the American Physical Therapy Association. Phys. Ther. 2022, 102, pzab302. [Google Scholar] [CrossRef]
- Carroll, L.M.; Volpe, D.; Morris, M.E.; Saunders, J.; Clifford, A.M. Aquatic exercise therapy for people with Parkinson disease: A randomized controlled trial. Arch. Phys. Med. Rehabil. 2017, 98, 631–638. [Google Scholar] [CrossRef] [PubMed]
- Landers, M.R.; Navalta, J.W.; Murtishaw, A.S.; Kinney, J.W.; Richardson, S.P. A high-intensity exercise boot camp for persons with Parkinson disease: A phase II, pragmatic, randomized clinical trial of feasibility, safety, signal of efficacy, and disease mechanisms. J. Neurol. Phys. Ther. 2019, 43, 12–25. [Google Scholar] [CrossRef] [PubMed]
- Penko, A.L.; Zimmerman, N.M.; Crawford, M.; Linder, S.M.; Alberts, J.L. Effect of aerobic exercise on cardiopulmonary responses and predictors of change in individuals with Parkinson’s disease. Arch. Phys. Med. Rehabil. 2021, 102, 925–931. [Google Scholar] [CrossRef] [PubMed]
- Cugusi, L.; Manca, A.; Bergamin, M.; Di Blasio, A.; Monticone, M.; Deriu, F.; Mercuro, G. Aquatic exercise improves motor impairments in people with Parkinson’s disease, with similar or greater benefits than land-based exercise: A systematic review. J. Physiother. 2019, 65, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Cugusi, L.; Solla, P.; Serpe, R.; Carzedda, T.; Piras, L.; Oggianu, M.; Gabba, S.; Di Blasio, A.; Bergamin, M.; Cannas, A. Effects of a Nordic Walking program on motor and non-motor symptoms, functional performance and body composition in patients with Parkinson’s disease. NeuroRehabilitation 2015, 37, 245–254. [Google Scholar] [CrossRef]
- de Almeida, F.O.; Santana, V.; Corcos, D.M.; Ugrinowitsch, C.; Silva-Batista, C. Effects of endurance training on motor signs of Parkinson’s disease: A systematic review and meta-analysis. Sports Med. 2022, 52, 1789–1815. [Google Scholar] [CrossRef]
- Erickson, K.I.; Voss, M.W.; Prakash, R.S.; Basak, C.; Szabo, A.; Chaddock, L.; Kim, J.S.; Heo, S.; Alves, H.; White, S.M. Exercise training increases size of hippocampus and improves memory. Proc. Natl. Acad. Sci. USA 2011, 108, 3017–3022. [Google Scholar] [CrossRef]
- Blumenthal, J.A.; Babyak, M.A.; Moore, K.A.; Craighead, W.E.; Herman, S.; Khatri, P.; Waugh, R.; Napolitano, M.A.; Forman, L.M.; Appelbaum, M. Effects of exercise training on older patients with major depression. Arch. Intern. Med. 1999, 159, 2349–2356. [Google Scholar] [CrossRef]
- Altmann, L.J.; Stegemöller, E.; Hazamy, A.A.; Wilson, J.P.; Bowers, D.; Okun, M.S.; Hass, C.J. Aerobic exercise improves mood, cognition, and language function in Parkinson’s disease: Results of a controlled study. J. Int. Neuropsychol. Soc. 2016, 22, 878–889. [Google Scholar] [CrossRef]
- David, F.J.; Robichaud, J.A.; Leurgans, S.E.; Poon, C.; Kohrt, W.M.; Goldman, J.G.; Comella, C.L.; Vaillancourt, D.E.; Corcos, D.M. Exercise improves cognition in Parkinson’s disease: The PRET-PD randomized, clinical trial. Mov. Disord. 2015, 30, 1657–1663. [Google Scholar] [CrossRef]
- Duchesne, C.; Lungu, O.; Nadeau, A.; Robillard, M.; Boré, A.; Bobeuf, F.; Lafontaine, A.; Gheysen, F.; Bherer, L.; Doyon, J. Enhancing both motor and cognitive functioning in Parkinson’s disease: Aerobic exercise as a rehabilitative intervention. Brain Cogn. 2015, 99, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; de Quadros, A.C., Jr.; Santos, R.F.; Stella, F.; Gobbi, L.T.B.; Gobbi, S. Benefits of physical exercise on executive functions in older people with Parkinson’s disease. Brain Cogn. 2009, 69, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Sacheli, M.A.; Neva, J.L.; Lakhani, B.; Murray, D.K.; Vafai, N.; Shahinfard, E.; English, C.; McCormick, S.; Dinelle, K.; Neilson, N. Exercise increases caudate dopamine release and ventral striatal activation in Parkinson’s disease. Mov. Disord. 2019, 34, 1891–1900. [Google Scholar] [CrossRef]
- Niemann, C.; Godde, B.; Staudinger, U.M.; Voelcker-Rehage, C. Exercise-induced changes in basal ganglia volume and cognition in older adults. Neuroscience 2014, 281, 147–163. [Google Scholar] [CrossRef] [PubMed]
- Marusiak, J.; Fisher, B.E.; Jaskólska, A.; Słotwiński, K.; Budrewicz, S.; Koszewicz, M.; Kisiel-Sajewicz, K.; Kamiński, B.; Jaskólski, A. Eight weeks of aerobic interval training improves psychomotor function in patients with Parkinson’s disease—Randomized controlled trial. Int. J. Environ. Res. Public Health 2019, 16, 880. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela, C.S.M.; Moscardó, L.D.; López-Pascual, J.; Serra-Añó, P.; Tomás, J.M. Effects of dual-task group training on gait, cognitive executive function, and quality of life in people with Parkinson disease: Results of randomized controlled DUALGAIT trial. Arch. Phys. Med. Rehabil. 2020, 101, 1849–1856.e1. [Google Scholar] [CrossRef]
- de Laat, B.; Hoye, J.; Stanley, G.; Hespeler, M.; Ligi, J.; Mohan, V.; Wooten, D.W.; Zhang, X.; Nguyen, T.D.; Key, J. Intense exercise increases dopamine transporter and neuromelanin concentrations in the substantia nigra in Parkinson’s disease. npj Park. Dis. 2024, 10, 34. [Google Scholar] [CrossRef]
- Chung, C.L.H.; Thilarajah, S.; Tan, D. Effectiveness of resistance training on muscle strength and physical function in people with Parkinson’s disease: A systematic review and meta-analysis. Clin. Rehabil. 2016, 30, 11–23. [Google Scholar] [CrossRef]
- Hirsch, M.A.; Toole, T.; Maitland, C.G.; Rider, R.A. The effects of balance training and high-intensity resistance training on persons with idiopathic Parkinson’s disease. Arch. Phys. Med. Rehabil. 2003, 84, 1109–1117. [Google Scholar] [CrossRef]
- Stone, M.H.; Stone, M.; Sands, W.A. Principles and Practice of Resistance Training; Human Kinetics: Houston, TX, USA, 2007. [Google Scholar]
- Hunter, G.R.; McCarthy, J.P.; Bamman, M.M. Effects of resistance training on older adults. Sports Med. 2004, 34, 329–348. [Google Scholar] [CrossRef]
- Corcos, D.M.; Lamotte, G.; Luthra, N.S.; McKee, K.E. Advice to People with Parkinson’s in My Clinic: Exercise. J. Park. Dis. 2024, 14, 609–617. [Google Scholar] [CrossRef] [PubMed]
- de Lima, T.A.; Ferreira-Moraes, R.; Alves, W.M.G.d.C.; Alves, T.G.G.; Pimentel, C.P.; Sousa, E.C.; Abrahin, O.; Cortinhas-Alves, E.A. Resistance training reduces depressive symptoms in elderly people with Parkinson disease: A controlled randomized study. Scand. J. Med. Sci. Sports 2019, 29, 1957–1967. [Google Scholar] [CrossRef] [PubMed]
- Leal, L.C.; Abrahin, O.; Rodrigues, R.P.; da Silva, M.C.; Araújo, A.P.; de Sousa, E.C.; Pimentel, C.P.; Cortinhas-Alves, E.A. Low-volume resistance training improves the functional capacity of older individuals with Parkinson’s disease. Geriatr. Gerontol. Int. 2019, 19, 635–640. [Google Scholar] [CrossRef] [PubMed]
- Silva-Batista, C.; Corcos, D.M.; Kanegusuku, H.; Piemonte, M.E.P.; Gobbi, L.T.B.; de Lima-Pardini, A.C.; de Mello, M.T.; Forjaz, C.L.; Ugrinowitsch, C. Balance and fear of falling in subjects with Parkinson’s disease is improved after exercises with motor complexity. Gait Posture 2018, 61, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Cherup, N.P.; Buskard, A.N.; Strand, K.L.; Roberson, K.B.; Michiels, E.R.; Kuhn, J.E.; Lopez, F.A.; Signorile, J.F. Power vs strength training to improve muscular strength, power, balance and functional movement in individuals diagnosed with Parkinson’s disease. Exp. Gerontol. 2019, 128, 110740. [Google Scholar] [CrossRef] [PubMed]
- Gamborg, M.; Hvid, L.G.; Dalgas, U.; Langeskov-Christensen, M. Parkinson’s disease and intensive exercise therapy—An updated systematic review and meta-analysis. Acta Neurol. Scand. 2022, 145, 504–528. [Google Scholar] [CrossRef]
- Silva-Batista, C.; Corcos, D.M.; Barroso, R.; David, F.J.; Kanegusuku, H.; Forjaz, C.; MT, D.E.M.; Roschel, H.; Tricoli, V.; Ugrinowitsch, C. Instability Resistance Training Improves Neuromuscular Outcome in Parkinson’s Disease. Med. Sci. Sports Exerc. 2017, 49, 652–660. [Google Scholar] [CrossRef] [PubMed]
- Canning, C.G.; Sherrington, C.; Lord, S.R.; Close, J.C.; Heritier, S.; Heller, G.Z.; Howard, K.; Allen, N.E.; Latt, M.D.; Murray, S.M.; et al. Exercise for falls prevention in Parkinson disease: A randomized controlled trial. Neurology 2015, 84, 304–312. [Google Scholar] [CrossRef]
- Bloem, B.R.; Marinus, J.; Almeida, Q.; Dibble, L.; Nieuwboer, A.; Post, B.; Ruzicka, E.; Goetz, C.; Stebbins, G.; Martinez-Martin, P.; et al. Measurement instruments to assess posture, gait, and balance in Parkinson’s disease: Critique and recommendations. Mov. Disord. 2016, 31, 1342–1355. [Google Scholar] [CrossRef]
- Shulman, L.M.; Katzel, L.I.; Ivey, F.M.; Sorkin, J.D.; Favors, K.; Anderson, K.E.; Smith, B.A.; Reich, S.G.; Weiner, W.J.; Macko, R.F. Randomized clinical trial of 3 types of physical exercise for patients with Parkinson disease. JAMA Neurol. 2013, 70, 183–190. [Google Scholar] [CrossRef]
- Vieira-Yano, B.; Martini, D.N.; Horak, F.B.; de Lima-Pardini, A.; Almeida, F.; Santana, V.P.; Lima, D.; Batista, A.X.; Marquesini, R.; Lira, J.; et al. The Adapted Resistance Training with Instability Randomized Controlled Trial for Gait Automaticity. Mov. Disord. 2021, 36, 152–163. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, K.R.; Healy, D.G.; Schapira, A.H. Non-motor symptoms of Parkinson’s disease: Diagnosis and management. Lancet Neurol. 2006, 5, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Santos, L.; Fernandez-Rio, J.; Winge, K.; Barragán-Pérez, B.; González-Gómez, L.; Rodríguez-Pérez, V.; González-Díez, V.; Lucía, A.; Iglesias-Soler, E.; Dopico-Calvo, X.; et al. Effects of progressive resistance exercise in akinetic-rigid Parkinson’s disease patients: A randomized controlled trial. Eur. J. Phys. Rehabil. Med. 2017, 53, 651–663. [Google Scholar] [CrossRef]
- Alves, W.M.; Alves, T.G.; Ferreira, R.M.; Lima, T.A.; Pimentel, C.P.; Sousa, E.C.; Abrahin, O.; Alves, E.A. Strength training improves the respiratory muscle strength and quality of life of elderly with Parkinson disease. J. Sports Med. Phys. Fit. 2019, 59, 1756–1762. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, R.M.; Alves, W.; de Lima, T.A.; Alves, T.G.G.; Alves Filho, P.A.M.; Pimentel, C.P.; Sousa, E.C.; Cortinhas-Alves, E.A. The effect of resistance training on the anxiety symptoms and quality of life in elderly people with Parkinson’s disease: A randomized controlled trial. Arq. Neuropsiquiatr. 2018, 76, 499–506. [Google Scholar] [CrossRef]
- Park, J.-H.; Kang, Y.-J.; Horak, F.B. What is wrong with balance in Parkinson’s disease? J. Mov. Disord. 2015, 8, 109. [Google Scholar] [CrossRef]
- Grabli, D.; Karachi, C.; Welter, M.-L.; Lau, B.; Hirsch, E.C.; Vidailhet, M.; François, C. Normal and pathological gait: What we learn from Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 2012, 83, 979–985. [Google Scholar] [CrossRef]
- Morris, M.E.; Iansek, R. Characteristics of motor disturbance in Parkinson’s disease and strategies for movement rehabilitation. Hum. Mov. Sci. 1996, 15, 649–669. [Google Scholar] [CrossRef]
- Angel, R.W.; Alston, W.; Higgins, J.R. Control of movement in Parkinson’s disease. Brain 1970, 93, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Farley, B.G.; Fox, C.M.; Ramig, L.O.; McFarland, D.H. Intensive amplitude-specific therapeutic approaches for Parkinson’s disease: Toward a neuroplasticity-principled rehabilitation model. Top. Geriatr. Rehabil. 2008, 24, 99–114. [Google Scholar] [CrossRef]
- Hall, M.-F.E.; Church, F.C. Integrative medicine and health therapy for Parkinson disease. Top. Geriatr. Rehabil. 2020, 36, 176–186. [Google Scholar] [CrossRef]
- Larson, D.; Yeh, C.; Rafferty, M.; Bega, D. High satisfaction and improved quality of life with Rock Steady Boxing in Parkinson’s disease: Results of a large-scale survey. Disabil. Rehabil. 2022, 44, 6034–6041. [Google Scholar] [CrossRef] [PubMed]
- van Eijkeren, F.J.; Reijmers, R.S.; Kleinveld, M.J.; Minten, A.; Bruggen, J.P.t.; Bloem, B.R. Nordic walking improves mobility in Parkinson’s disease. Mov. Disord. Off. J. Mov. Disord. Soc. 2008, 23, 2239–2243. [Google Scholar] [CrossRef] [PubMed]
- Westheimer, O. Why dance for Parkinson’s disease. Top. Geriatr. Rehabil. 2008, 24, 127–140. [Google Scholar] [CrossRef]
- Gao, Q.; Leung, A.; Yang, Y.; Wei, Q.; Guan, M.; Jia, C.; He, C. Effects of Tai Chi on balance and fall prevention in Parkinson’s disease: A randomized controlled trial. Clin. Rehabil. 2014, 28, 748–753. [Google Scholar] [CrossRef] [PubMed]
- Cash, M.F.; Ulanowski, E.; Danzl, M. Development of a community-based golf and exercise program for people with Parkinson’s disease. Complement. Ther. Clin. Pract. 2018, 33, 149–155. [Google Scholar] [CrossRef]
- Bliss, R.R.; Church, F.C. Golf as a Physical Activity to Potentially Reduce the Risk of Falls in Older Adults with Parkinson’s Disease. Sports 2021, 9, 72. [Google Scholar] [CrossRef]
- Van Puymbroeck, M.; Walter, A.; Hawkins, B.L.; Sharp, J.L.; Woschkolup, K.; Urrea-Mendoza, E.; Revilla, F.; Adams, E.V.; Schmid, A.A. Functional improvements in Parkinson’s disease following a randomized trial of yoga. Evid.-Based Complement. Altern. Med. 2018, 2018, 8516351. [Google Scholar] [CrossRef]
- Bloem, B.R.; Hausdorff, J.M.; Visser, J.E.; Giladi, N. Falls and freezing of gait in Parkinson’s disease: A review of two interconnected, episodic phenomena. Mov. Disord. Off. J. Mov. Disord. Soc. 2004, 19, 871–884. [Google Scholar] [CrossRef]
- Tolosa, E.; Compta, Y. Dystonia in Parkinson’s disease. J. Neurol. 2006, 253, vii7–vii13. [Google Scholar] [CrossRef]
- Robbins, J.A.; Logemann, J.A.; Kirshner, H.S. Swallowing and speech production in Parkinson’s disease. Ann. Neurol. 1986, 19, 283–287. [Google Scholar] [CrossRef] [PubMed]
- Fox, C.M.; Ramig, L.O.; Ciucci, M.R.; Sapir, S.; McFarland, D.H.; Farley, B.G. The science and practice of LSVT/LOUD: Neural plasticity-principled approach to treating individuals with Parkinson disease and other neurological disorders. Semin. Speech Lang. 2006, 27, 283–299. [Google Scholar] [CrossRef] [PubMed]
- Sapir, S.; Ramig, L.O.; Hoyt, P.; Countryman, S.; O’Brien, C.; Hoehn, M. Speech loudness and quality 12 months after intensive voice treatment (LSVT®) for Parkinson’s disease: A comparison with an alternative speech treatment. Folia Phoniatr. Logop. 2002, 54, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Fox, C.; Ebersbach, G.; Ramig, L.; Sapir, S. LSVT LOUD and LSVT BIG: Behavioral treatment programs for speech and body movement in Parkinson disease. Park. Dis. 2012, 2012, 391946. [Google Scholar] [CrossRef] [PubMed]
- Kraus, A.; Joyner, N.; Simpson, H.; Farley, B. The Application of PD Specific Functional Training in Group Rehabilitative Sessions-Physical Performance Outcomes. 2024. Available online: https://files.constantcontact.com/fb612c26101/0ee251fe-1346-4e34-8e13-eff9a368c859.pdf (accessed on 7 September 2024).
- Combs, S.A.; Diehl, M.D.; Staples, W.H.; Conn, L.; Davis, K.; Lewis, N.; Schaneman, K. Boxing training for patients with Parkinson disease: A case series. Phys. Ther. 2011, 91, 132–142. [Google Scholar] [CrossRef] [PubMed]
- Bek, J.; Arakaki, A.I.; Lawrence, A.; Sullivan, M.; Ganapathy, G.; Poliakoff, E. Dance and Parkinson’s: A review and exploration of the role of cognitive representations of action. Neurosci. Biobehav. Rev. 2020, 109, 16–28. [Google Scholar] [CrossRef]
- Kalyani, H.; Sullivan, K.; Moyle, G.; Brauer, S.; Jeffrey, E.R.; Kerr, G. Impacts of dance on cognition, psychological symptoms and quality of life in Parkinson’s disease. NeuroRehabilitation 2019, 45, 273–283. [Google Scholar] [CrossRef]
- de Natale, E.R.; Paulus, K.S.; Aiello, E.; Sanna, B.; Manca, A.; Sotgiu, G.; Leali, P.T.; Deriu, F. Dance therapy improves motor and cognitive functions in patients with Parkinson’s disease. NeuroRehabilitation 2017, 40, 141–144. [Google Scholar] [CrossRef]
- Bearss, K.A.; DeSouza, J.F. Parkinson’s disease motor symptom progression slowed with multisensory dance learning over 3-years: A preliminary longitudinal investigation. Brain Sci. 2021, 11, 895. [Google Scholar] [CrossRef]
- Hashimoto, H.; Takabatake, S.; Miyaguchi, H.; Nakanishi, H.; Naitou, Y. Effects of dance on motor functions, cognitive functions, and mental symptoms of Parkinson’s disease: A quasi-randomized pilot trial. Complement. Ther. Med. 2015, 23, 210–219. [Google Scholar] [CrossRef]
- Heiberger, L.; Maurer, C.; Amtage, F.; Mendez-Balbuena, I.; Schulte-Mönting, J.; Hepp-Reymond, M.-C.; Kristeva, R. Impact of a weekly dance class on the functional mobility and on the quality of life of individuals with Parkinson’s disease. Front. Aging Neurosci. 2011, 3, 14. [Google Scholar] [CrossRef] [PubMed]
- Hackney, M.E.; Kantorovich, S.; Levin, R.; Earhart, G.M. Effects of tango on functional mobility in Parkinson’s disease: A preliminary study. J. Neurol. Phys. Ther. 2007, 31, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Berardelli, A.; Sabra, A.; Hallett, M. Physiological mechanisms of rigidity in Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 1983, 46, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Andrews, C.J.; Burke, D.; Lance, J.W. The response to muscle stretch and shortening in Parkinsonian rigidity. Brain 1972, 95, 795–812. [Google Scholar] [CrossRef] [PubMed]
- Ferreira-Sánchez, M.d.R.; Moreno-Verdú, M.; Cano-de-La-Cuerda, R. Quantitative measurement of rigidity in Parkinson’s disease: A systematic review. Sensors 2020, 20, 880. [Google Scholar] [CrossRef]
- Benatru, I.; Vaugoyeau, M.; Azulay, J.-P. Postural disorders in Parkinson’s disease. Neurophysiol. Clin./Clin. Neurophysiol. 2008, 38, 459–465. [Google Scholar] [CrossRef]
- Mak, M.K.; Wong-Yu, I.S. Exercise for Parkinson’s disease. Int. Rev. Neurobiol. 2019, 147, 1–44. [Google Scholar]
- Palmer, S.S.; Mortimer, J.A.; Webster, D.D.; Bistevins, R.; Dickinson, G.L. Exercise therapy for Parkinson’s disease. Arch. Phys. Med. Rehabil. 1986, 67, 741–745. [Google Scholar] [CrossRef]
- Emig, M.; George, T.; Zhang, J.K.; Soudagar-Turkey, M. The role of exercise in Parkinson’s disease. J. Geriatr. Psychiatry Neurol. 2021, 34, 321–330. [Google Scholar] [CrossRef]
- Gobbi, L.T.; Oliveira-Ferreira, M.D.; Caetano, M.J.D.; Lirani-Silva, E.; Barbieri, F.A.; Stella, F.; Gobbi, S. Exercise programs improve mobility and balance in people with Parkinson’s disease. Park. Relat. Disord. 2009, 15, S49–S52. [Google Scholar] [CrossRef]
- Flach, A.; Jaegers, L.; Krieger, M.; Bixler, E.; Kelly, P.; Weiss, E.P.; Ahmad, S.O. Endurance exercise improves function in individuals with Parkinson’s disease: A meta-analysis. Neurosci. Lett. 2017, 659, 115–119. [Google Scholar] [CrossRef] [PubMed]
- Steiger, L.; Homann, C.N. Exercise therapy in Parkinson’s disease–An overview of current interventional studies. Physiother. Res. Rep. 2019, 1, 1–10. [Google Scholar] [CrossRef]
- Choi, H.; Cho, K.-H.; Jin, C.; Lee, J.; Kim, T.-H.; Jung, W.-S.; Moon, S.-K.; Ko, C.-N.; Cho, S.-Y.; Jeon, C.-Y. Exercise Therapies for Parkinson’s Disease: A Systematic Review and Meta-Analysis. Park. Dis. 2020, 2020, 2565320. [Google Scholar] [CrossRef] [PubMed]
- PRISMA. Preferred Reporting Items for Systematic Reviews and Meta Analyses (PRISMA). Available online: http://www.prismastatement.org (accessed on 1 August 2024).
- Higgins, J. Cochrane Handbook for Systematic Reviews of Interventions; Cochrane Collaboration and John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2008. [Google Scholar]
- Schootemeijer, S.; van der Kolk, N.M.; Bloem, B.R.; de Vries, N.M. Current perspectives on aerobic exercise in people with Parkinson’s disease. Neurotherapeutics 2020, 17, 1418–1433. [Google Scholar] [CrossRef] [PubMed]
- Martignon, C.; Pedrinolla, A.; Ruzzante, F.; Giuriato, G.; Laginestra, F.G.; Bouca-Machado, R.; Ferreira, J.J.; Tinazzi, M.; Schena, F.; Venturelli, M. Guidelines on exercise testing and prescription for patients at different stages of Parkinson’s disease. Aging Clin. Exp. Res. 2021, 33, 221–246. [Google Scholar] [CrossRef]
- Hao, Z.; Zhang, X.; Chen, P. Effects of ten different exercise interventions on motor function in Parkinson’s disease patients—A Network meta-analysis of randomized controlled trials. Brain Sci. 2022, 12, 698. [Google Scholar] [CrossRef]
- Li, J.A.; Loevaas, M.B.; Guan, C.; Goh, L.; Allen, N.E.; Mak, M.K.; Lv, J.; Paul, S.S. Does exercise attenuate disease progression in people with Parkinson’s disease? A systematic review with meta-analyses. Neurorehabilit. Neural Repair 2023, 37, 328–352. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Ann. Intern. Med. 2009, 151, 264–269. [Google Scholar] [CrossRef]
- Ernst, M.; Folkerts, A.-K.; Gollan, R.; Lieker, E.; Caro-Valenzuela, J.; Adams, A.; Cryns, N.; Monsef, I.; Dresen, A.; Roheger, M. Physical exercise for people with Parkinson’s disease: A systematic review and network meta-analysis. Cochrane Database Syst. Rev. 2024, 1, CD013856. [Google Scholar]
- Langeskov-Christensen, M.; Franzén, E.; Hvid, L.G.; Dalgas, U. Exercise as medicine in Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 2024. ahead of print. [Google Scholar] [CrossRef]
- Padilha, C.; Souza, R.; Grossl, F.S.; Gauer, A.P.M.; de Sá, C.A.; Rodrigues-Junior, S.A. Physical exercise and its effects on people with Parkinson’s disease: Umbrella review. PLoS ONE 2023, 18, e0293826. [Google Scholar] [CrossRef] [PubMed]
- Moratelli, J.A.; Lima, A.G.; Alexandre, K.H.; Fausto, D.Y.; Haas, A.N.; de Azevedo Guimarães, A.C. Evidence of physical activity interventions on non-motor symptoms of people with Parkinson’s disease: An umbrella review. Sport Sci. Health 2024, 20, 321–336. [Google Scholar] [CrossRef]
- Yu, J.; Wu, J.; Lu, J.; Wei, X.; Zheng, K.; Liu, B.; Xiao, W.; Shi, Q.; Xiong, L.; Ren, Z. Efficacy of virtual reality training on motor performance, activity of daily living, and quality of life in patients with Parkinson’s disease: An umbrella review comprising meta-analyses of randomized controlled trials. J. Neuro Eng. Rehabil. 2023, 20, 133. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues-Juniora, S.A.; Padilhaa, C.; Souzaa, R.; de Sáa, C.A. Physical exercise interventions for people with Parkinson’s disease: A bibliometric review of systematic reviews. Geriatr. Gerontol. Aging 2023, 18, e0230035. [Google Scholar] [CrossRef]
- Chekroud, S.R.; Gueorguieva, R.; Zheutlin, A.B.; Paulus, M.; Krumholz, H.M.; Krystal, J.H.; Chekroud, A.M. Association between physical exercise and mental health in 1·2 million individuals in the USA between 2011 and 2015: A cross-sectional study. Lancet Psychiatry 2018, 5, 739–746. [Google Scholar] [CrossRef]
- Cordani, C.; Mosconi, B. What type of physical exercise works best to improve movement and quality of life for people with Parkinson’s disease?-A Cochrane Review summary with commentary. NeuroRehabilitation 2024, 54, 699–702. [Google Scholar] [CrossRef]
- Chodzko-Zajko, W.; Proctor, D.; Fiatarone Singh, M.; Minson, C.; Nigg, C.; Salem, G.; Skinner, J. Exercise and physical activity for older adults. American College of Sports Medicine position stand. Med. Sci. Sports Exerc. 2009, 41, 1510–1530. [Google Scholar] [CrossRef]
- Piercy, K.L.; Troiano, R.P.; Ballard, R.M.; Carlson, S.A.; Fulton, J.E.; Galuska, D.A.; George, S.M.; Olson, R.D. The physical activity guidelines for Americans. JAMA 2018, 320, 2020–2028. [Google Scholar] [CrossRef]
- Goldman, J.G.; Volpe, D.; Ellis, T.D.; Hirsch, M.A.; Johnson, J.; Wood, J.; Aragon, A.; Biundo, R.; Di Rocco, A.; Kasman, G.S. Delivering multidisciplinary rehabilitation Care in Parkinson’s disease: An international consensus statement. J. Park. Dis. 2024, 14, 135–166. [Google Scholar] [CrossRef]
- Domingos, J.; Keus, S.H.J.; Dean, J.; de Vries, N.M.; Ferreira, J.J.; Bloem, B.R. The European Physiotherapy Guideline for Parkinson’s Disease: Implications for Neurologists. J. Park. Dis. 2018, 8, 499–502. [Google Scholar] [CrossRef]
- Rafferty, M.R.; Schmidt, P.N.; Luo, S.T.; Li, K.; Marras, C.; Davis, T.L.; Guttman, M.; Cubillos, F.; Simuni, T.; all NPF-QII Investigators. Regular exercise, quality of life, and mobility in Parkinson’s disease: A longitudinal analysis of national Parkinson foundation quality improvement initiative data. J. Park. Dis. 2017, 7, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Speelman, A.D.; Van De Warrenburg, B.P.; Van Nimwegen, M.; Petzinger, G.M.; Munneke, M.; Bloem, B.R. How might physical activity benefit patients with Parkinson disease? Nat. Rev. Neurol. 2011, 7, 528–534. [Google Scholar] [CrossRef] [PubMed]
- van Nimwegen, M.; Speelman, A.D.; Hofman-van Rossum, E.J.; Overeem, S.; Deeg, D.J.; Borm, G.F.; van der Horst, M.H.; Bloem, B.R.; Munneke, M. Physical inactivity in Parkinson’s disease. J. Neurol. 2011, 258, 2214–2221. [Google Scholar] [CrossRef] [PubMed]
- Ghadieh, A.S.; Saab, B. Evidence for exercise training in the management of hypertension in adults. Can. Fam. Physician 2015, 61, 233–239. [Google Scholar] [PubMed]
- Speelman, A.D.; Groothuis, J.T.; van Nimwegen, M.; van der Scheer, E.S.; Borm, G.F.; Bloem, B.R.; Hopman, M.T.; Munneke, M. Cardiovascular responses during a submaximal exercise test in patients with Parkinson’s disease. J. Park. Dis. 2012, 2, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Borg, G.A. Psychophysical bases of perceived exertion. Med. Sci. Sports Exerc. 1982, 14, 377–381. [Google Scholar] [CrossRef]
- Chen, M.J.; Fan, X.; Moe, S.T. Criterion-related validity of the Borg ratings of perceived exertion scale in healthy individuals: A meta-analysis. J. Sports Sci. 2002, 20, 873–899. [Google Scholar] [CrossRef]
- Cook, D.; McRae, C.; Kumar, R. Impact of self-efficacy education on physical and psychosocial functioning in newly-diagnosed Parkinson patients. Mov. Disord. 2016, 31, S170. [Google Scholar]
- Li, G.; Huang, P.; Cui, S.-S.; Tan, Y.-Y.; He, Y.-C.; Shen, X.; Jiang, Q.-Y.; Huang, P.; He, G.-Y.; Li, B.-Y. Mechanisms of motor symptom improvement by long-term Tai Chi training in Parkinson’s disease patients. Transl. Neurodegener. 2022, 11, 6. [Google Scholar] [CrossRef]
- Foundation, P.s. Exercise Convening Summary Report. Available online: https://cdn.flipsnack.com/widget/v2/widget.html?hash=vp3ycpz2gx (accessed on 22 July 2024).
- Morgan, J.A.; Corrigan, F.; Baune, B.T. Effects of physical exercise on central nervous system functions: A review of brain region specific adaptations. J. Mol. Psychiatry 2015, 3, 3. [Google Scholar] [CrossRef]
- Perrey, S. Promoting motor function by exercising the brain. Brain Sci. 2013, 3, 101–122. [Google Scholar] [CrossRef] [PubMed]
- Lavin, K.M.; Ge, Y.; Sealfon, S.C.; Nair, V.D.; Wilk, K.; McAdam, J.S.; Windham, S.T.; Kumar, P.L.; McDonald, M.-L.N.; Bamman, M.M. Rehabilitative impact of exercise training on human skeletal muscle transcriptional programs in Parkinson’s disease. Front. Physiol. 2020, 11, 653. [Google Scholar] [CrossRef] [PubMed]
- Kelly, N.A.; Hammond, K.G.; Bickel, C.S.; Windham, S.T.; Tuggle, S.C.; Bamman, M.M. Effects of aging and Parkinson’s disease on motor unit remodeling: Influence of resistance exercise training. J. Appl. Physiol. 2018, 124, 888–898. [Google Scholar] [CrossRef] [PubMed]
- Reiner, M.; Niermann, C.; Jekauc, D.; Woll, A. Long-term health benefits of physical activity—A systematic review of longitudinal studies. BMC Public Health 2013, 13, 813. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Guo, J.; Sun, W.; Mei, J.; Wang, Y.; Zhang, L.; Zhang, J.; Gao, J.; Su, K.; Lv, Z. Effects of exercise on Parkinson’s disease: A meta-analysis of brain imaging studies. Front. Hum. Neurosci. 2022, 16, 796712. [Google Scholar] [CrossRef]
- Tsukita, K.; Sakamaki-Tsukita, H.; Takahashi, R. Long-term effect of regular physical activity and exercise habits in patients with early Parkinson disease. Neurology 2022, 98, e859–e871. [Google Scholar] [CrossRef]
- Müller, J.; Myers, J. Association between physical fitness, cardiovascular risk factors, and Parkinson’s disease. Eur. J. Prev. Cardiol. 2018, 25, 1409–1415. [Google Scholar] [CrossRef]
- Thacker, E.L.; Chen, H.; Patel, A.V.; McCullough, M.L.; Calle, E.E.; Thun, M.J.; Schwarzschild, M.A.; Ascherio, A. Recreational physical activity and risk of Parkinson’s disease. Mov. Disord. 2008, 23, 69–74. [Google Scholar] [CrossRef]
- Yang, F.; Trolle Lagerros, Y.; Bellocco, R.; Adami, H.-O.; Fang, F.; Pedersen, N.L.; Wirdefeldt, K. Physical activity and risk of Parkinson’s disease in the Swedish National March Cohort. Brain 2015, 138, 269–275. [Google Scholar] [CrossRef]
- Sääksjärvi, K.; Knekt, P.; Männistö, S.; Lyytinen, J.; Jääskeläinen, T.; Kanerva, N.; Heliövaara, M. Reduced risk of Parkinson’s disease associated with lower body mass index and heavy leisure-time physical activity. Eur. J. Epidemiol. 2014, 29, 285–292. [Google Scholar] [CrossRef]
- Logroscino, G.; Sesso, H.; Paffenbarger, R.; Lee, I.-M. Physical activity and risk of Parkinson’s disease: A prospective cohort study. J. Neurol. Neurosurg. Psychiatry 2006, 1318–1322. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Armengol, J.; Fajas, L.; Lopez-Mejia, I.C. Inter-organ communication: A gatekeeper for metabolic health. EMBO Rep. 2019, 20, e47903. [Google Scholar] [CrossRef] [PubMed]
- Zhou, N.; Gong, L.; Zhang, E.; Wang, X. Exploring exercise-driven exerkines: Unraveling the regulation of metabolism and inflammation. PeerJ 2024, 12, e17267. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, T.; Kamon, J.; Minokoshi, Y.a.; Ito, Y.; Waki, H.; Uchida, S.; Yamashita, S.; Noda, M.; Kita, S.; Ueki, K. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat. Med. 2002, 8, 1288–1295. [Google Scholar] [CrossRef] [PubMed]
- Bouassida, A.; Chamari, K.; Zaouali, M.; Feki, Y.; Zbidi, A.; Tabka, Z. Review on leptin and adiponectin responses and adaptations to acute and chronic exercise. Br. J. Sports Med. 2010, 44, 620–630. [Google Scholar] [CrossRef]
- Fang, H.; Judd, R.L. Adiponectin regulation and function. Compr. Physiol. 2011, 8, 1031–1063. [Google Scholar]
- Polito, R.; Di Meo, I.; Barbieri, M.; Daniele, A.; Paolisso, G.; Rizzo, M.R. Adiponectin role in neurodegenerative diseases: Focus on nutrition review. Int. J. Mol. Sci. 2020, 21, 9255. [Google Scholar] [CrossRef]
- Zhou, J.-X.; Shuai, N.-N.; Wang, B.; Jin, X.; Kuang, X.; Tian, S.-W. Neuroprotective gain of Apelin/APJ system. Neuropeptides 2021, 87, 102131. [Google Scholar] [CrossRef]
- Kammoun, H.L.; Febbraio, M.A. Come on BAIBA light my fire. Cell Metab. 2014, 19, 1–2. [Google Scholar] [CrossRef]
- Tanianskii, D.A.; Jarzebska, N.; Birkenfeld, A.L.; O’Sullivan, J.F.; Rodionov, R.N. Beta-Aminoisobutyric Acid as a Novel Regulator of Carbohydrate and Lipid Metabolism. Nutrients 2019, 11, 524. [Google Scholar] [CrossRef]
- Yi, X.; Yang, Y.; Li, T.; Li, M.; Yao, T.; Hu, G.; Wan, G.; Chang, B. Signaling metabolite beta-aminoisobutyric acid as a metabolic regulator, biomarker, and potential exercise pill. Front. Endocrinol. 2023, 14, 1192458. [Google Scholar] [CrossRef] [PubMed]
- Thickbroom, G.W. The therapeutic potential of ketone bodies in Parkinson’s disease. Expert Rev. Neurother. 2021, 21, 255–257. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.; Muhammad, S.; Khan, M.A.; Chen, H.; Ridder, D.A.; Müller-Fielitz, H.; Pokorná, B.; Vollbrandt, T.; Stölting, I.; Nadrowitz, R. The β-hydroxybutyrate receptor HCA2 activates a neuroprotective subset of macrophages. Nat. Commun. 2014, 5, 3944. [Google Scholar] [CrossRef] [PubMed]
- Newman, J.C.; Verdin, E. beta-Hydroxybutyrate: A Signaling Metabolite. Annu. Rev. Nutr. 2017, 37, 51–76. [Google Scholar] [CrossRef] [PubMed]
- Neeper, S.A.; Góauctemez-Pinilla, F.; Choi, J.; Cotman, C. Exercise and brain neurotrophins. Nature 1995, 373, 109. [Google Scholar] [CrossRef] [PubMed]
- Matthews, V.B.; Åström, M.-B.; Chan, M.; Bruce, C.R.; Krabbe, K.; Prelovsek, O.; Åkerström, T.; Yfanti, C.; Broholm, C.; Mortensen, O.H. Brain-derived neurotrophic factor is produced by skeletal muscle cells in response to contraction and enhances fat oxidation via activation of AMP-activated protein kinase. Diabetologia 2009, 52, 1409–1418. [Google Scholar] [CrossRef] [PubMed]
- Koliatsos, V.E.; Clatterbuck, R.E.; Winslow, J.W.; Cayouette, M.H.; Prices, D.L. Evidence that brain-derived neurotrophic factor is a trophic factor for motor neurons in vivo. Neuron 1993, 10, 359–367. [Google Scholar] [CrossRef]
- Nicolini, C.; Michalski, B.; Toepp, S.L.; Turco, C.V.; D’Hoine, T.; Harasym, D.; Gibala, M.J.; Fahnestock, M.; Nelson, A.J. A single bout of high-intensity interval exercise increases corticospinal excitability, brain-derived neurotrophic factor, and uncarboxylated osteolcalcin in sedentary, healthy males. Neuroscience 2020, 437, 242–255. [Google Scholar] [CrossRef]
- Moon, H.Y.; Becke, A.; Berron, D.; Becker, B.; Sah, N.; Benoni, G.; Janke, E.; Lubejko, S.T.; Greig, N.H.; Mattison, J.A. Running-induced systemic cathepsin B secretion is associated with memory function. Cell Metab. 2016, 24, 332–340. [Google Scholar] [CrossRef]
- Hummel, R.P.; James, J.H.; Warner, B.W.; Hasselgren, P.-O.; Fischer, J.E. Evidence that cathepsin B contributes to skeletal muscle protein breakdown during sepsis. Arch. Surg. 1988, 123, 221–224. [Google Scholar] [CrossRef]
- Watt, M.J.; Miotto, P.M.; De Nardo, W.; Montgomery, M.K. The liver as an endocrine organ—Linking NAFLD and insulin resistance. Endocr. Rev. 2019, 40, 1367–1393. [Google Scholar] [CrossRef] [PubMed]
- Sargeant, J.A.; Aithal, G.P.; Takamura, T.; Misu, H.; Takayama, H.; Douglas, J.A.; Turner, M.C.; Stensel, D.J.; Nimmo, M.A.; Webb, D.R. The influence of adiposity and acute exercise on circulating hepatokines in normal-weight and overweight/obese men. Appl. Physiol. Nutr. Metab. 2018, 43, 482–490. [Google Scholar] [CrossRef] [PubMed]
- Keihanian, A.; Arazi, H.; Kargarfard, M. Effects of aerobic versus resistance training on serum fetuin-A, fetuin-B, and fibroblast growth factor-21 levels in male diabetic patients. Physiol. Int. 2019, 106, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Kim, S.H.; Min, Y.-K.; Yang, H.-M.; Lee, J.-B.; Lee, M.-S. Acute exercise induces FGF21 expression in mice and in healthy humans. PLoS ONE 2013, 8, e63517. [Google Scholar] [CrossRef] [PubMed]
- Izumiya, Y.; Bina, H.A.; Ouchi, N.; Akasaki, Y.; Kharitonenkov, A.; Walsh, K. FGF21 is an Akt-regulated myokine. FEBS Lett. 2008, 582, 3805–3810. [Google Scholar] [CrossRef] [PubMed]
- Hondares, E.; Iglesias, R.; Giralt, A.; Gonzalez, F.J.; Giralt, M.; Mampel, T.; Villarroya, F. Thermogenic activation induces FGF21 expression and release in brown adipose tissue. J. Biol. Chem. 2011, 286, 12983–12990. [Google Scholar] [CrossRef] [PubMed]
- McCullough, M.J.; Gyorkos, A.M.; Spitsbergen, J.M. Short-term exercise increases GDNF protein levels in the spinal cord of young and old rats. Neuroscience 2013, 240, 258–268. [Google Scholar] [CrossRef] [PubMed]
- Gyorkos, A.M.; McCullough, M.J.; Spitsbergen, J.M. Glial cell line-derived neurotrophic factor (GDNF) expression and NMJ plasticity in skeletal muscle following endurance exercise. Neuroscience 2014, 257, 111–118. [Google Scholar] [CrossRef]
- Treanor, J.J.; Goodman, L.; de Sauvage, F.; Stone, D.M.; Poulsen, K.T.; Beck, C.D.; Gray, C.; Armanini, M.P.; Pollock, R.A.; Hefti, F.; et al. Characterization of a multicomponent receptor for GDNF. Nature 1996, 382, 80–83. [Google Scholar] [CrossRef]
- Horowitz, A.M.; Fan, X.; Bieri, G.; Smith, L.K.; Sanchez-Diaz, C.I.; Schroer, A.B.; Gontier, G.; Casaletto, K.B.; Kramer, J.H.; Williams, K.E. Blood factors transfer beneficial effects of exercise on neurogenesis and cognition to the aged brain. Science 2020, 369, 167–173. [Google Scholar] [CrossRef]
- Abdolmaleki, F.; Heidarianpour, A. Endurance exercise training restores diabetes-induced alteration in circulating Glycosylphosphatidylinositol-specific phospholipase D levels in rats. Diabetol. Metab. Syndr. 2020, 12, 43. [Google Scholar] [CrossRef] [PubMed]
- Voss, M.W.; Nagamatsu, L.S.; Liu-Ambrose, T.; Kramer, A.F. Exercise, brain, and cognition across the life span. J. Appl. Physiol. 2011, 111, 1505–1513. [Google Scholar] [CrossRef] [PubMed]
- Stein, A.M.; Silva, T.M.V.; Coelho, F.G.D.M.; Arantes, F.J.; Costa, J.L.R.; Teodoro, E.; Santos-Galduróz, R.F. Physical exercise, IGF-1 and cognition A systematic review of experimental studies in the elderly. Dement. Neuropsychol. 2018, 12, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Cotman, C.W.; Berchtold, N.C.; Christie, L.A. Exercise builds brain health: Key roles of growth factor cascades and inflammation. Trends Neurosci. 2007, 30, 464–472. [Google Scholar] [CrossRef] [PubMed]
- Steensberg, A.; van Hall, G.; Osada, T.; Sacchetti, M.; Saltin, B.; Klarlund Pedersen, B. Production of interleukin-6 in contracting human skeletal muscles can account for the exercise-induced increase in plasma interleukin-6. J. Physiol. 2000, 529 Pt 1, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Spooren, A.; Kolmus, K.; Laureys, G.; Clinckers, R.; De Keyser, J.; Haegeman, G.; Gerlo, S. Interleukin-6, a mental cytokine. Brain Res. Rev. 2011, 67, 157–183. [Google Scholar] [CrossRef]
- Juttler, E.; Tarabin, V.; Schwaninger, M. Interleukin-6 (IL-6): A possible neuromodulator induced by neuronal activity. Neuroscientist 2002, 8, 268–275. [Google Scholar] [CrossRef]
- Bostrom, P.; Wu, J.; Jedrychowski, M.P.; Korde, A.; Ye, L.; Lo, J.C.; Rasbach, K.A.; Bostrom, E.A.; Choi, J.H.; Long, J.Z.; et al. A PGC1-alpha-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 2012, 481, 463–468. [Google Scholar] [CrossRef]
- Hashemi, M.S.; Ghaedi, K.; Salamian, A.; Karbalaie, K.; Emadi-Baygi, M.; Tanhaei, S.; Nasr-Esfahani, M.H.; Baharvand, H. Fndc5 knockdown significantly decreased neural differentiation rate of mouse embryonic stem cells. Neuroscience 2013, 231, 296–304. [Google Scholar] [CrossRef]
- Wrann, C.D.; White, J.P.; Salogiannnis, J.; Laznik-Bogoslavski, D.; Wu, J.; Ma, D.; Lin, J.D.; Greenberg, M.E.; Spiegelman, B.M. Exercise induces hippocampal BDNF through a PGC-1alpha/FNDC5 pathway. Cell Metab. 2013, 18, 649–659. [Google Scholar] [CrossRef]
- Nalbandian, M.; Takeda, M. Lactate as a Signaling Molecule That Regulates Exercise-Induced Adaptations. Biology 2016, 5, 38. [Google Scholar] [CrossRef] [PubMed]
- Willkomm, L.; Schubert, S.; Jung, R.; Elsen, M.; Borde, J.; Gehlert, S.; Suhr, F.; Bloch, W. Lactate regulates myogenesis in C2C12 myoblasts in vitro. Stem Cell Res. 2014, 12, 742–753. [Google Scholar] [CrossRef] [PubMed]
- Takimoto, M.; Hamada, T. Acute exercise increases brain region-specific expression of MCT1, MCT2, MCT4, GLUT1, and COX IV proteins. J. Appl. Physiol. (1985) 2014, 116, 1238–1250. [Google Scholar] [CrossRef] [PubMed]
- Li, V.L.; He, Y.; Contrepois, K.; Liu, H.; Kim, J.T.; Wiggenhorn, A.L.; Tanzo, J.T.; Tung, A.S.-H.; Lyu, X.; Zushin, P.-J.H. An exercise-inducible metabolite that suppresses feeding and obesity. Nature 2022, 606, 785–790. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.; Li, V.L.; Long, J.Z. Lac-Phe (N-lactoyl-phenylalanine). Trends Endocrinol. Metab. 2024, 35, 758–759. [Google Scholar] [CrossRef]
- Xiao, S.; Li, V.L.; Lyu, X.; Chen, X.; Wei, W.; Abbasi, F.; Knowles, J.W.; Tung, A.S.-H.; Deng, S.; Tiwari, G. Lac-Phe mediates the effects of metformin on food intake and body weight. Nat. Metab. 2024, 6, 659–669. [Google Scholar] [CrossRef]
- Fuller, O.K.; Whitham, M.; Mathivanan, S.; Febbraio, M.A. The Protective Effect of Exercise in Neurodegenerative Diseases: The Potential Role of Extracellular Vesicles. Cells 2020, 9, 2182. [Google Scholar] [CrossRef]
- Fischetti, F.; Poli, L.; De Tommaso, M.; Paolicelli, D.; Greco, G.; Cataldi, S. The role of exercise parameters on small extracellular vesicles and microRNAs cargo in preventing neurodegenerative diseases. Front. Physiol. 2023, 14, 1241010. [Google Scholar] [CrossRef]
- Khoury, R.; Nagy, C. Running from stress: A perspective on the potential benefits of exercise-induced small extracellular vesicles for individuals with major depressive disorder. Front. Mol. Biosci. 2023, 10, 1154872. [Google Scholar] [CrossRef]
- Lin, T.W.; Kuo, Y.M. Exercise benefits brain function: The monoamine connection. Brain Sci. 2013, 3, 39–53. [Google Scholar] [CrossRef]
- van Praag, H. Exercise and the brain: Something to chew on. Trends Neurosci 2009, 32, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Chaouloff, F. Physical exercise and brain monoamines: A review. Acta Physiol. Scand. 1989, 137, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Panes, J.D.; Wendt, A.; Ramirez-Molina, O.; Castro, P.A.; Fuentealba, J. Deciphering the role of PGC-1alpha in neurological disorders: From mitochondrial dysfunction to synaptic failure. Neural Regen. Res. 2022, 17, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Baar, K.; Wende, A.R.; Jones, T.E.; Marison, M.; Nolte, L.A.; Chen, M.; Kelly, D.P.; Holloszy, J.O. Adaptations of skeletal muscle to exercise: Rapid increase in the transcriptional coactivator PGC-1. FASEB J. 2002, 16, 1879–1886. [Google Scholar] [CrossRef] [PubMed]
- Wright, D.C.; Han, D.H.; Garcia-Roves, P.M.; Geiger, P.C.; Jones, T.E.; Holloszy, J.O. Exercise-induced mitochondrial biogenesis begins before the increase in muscle PGC-1alpha expression. J. Biol. Chem. 2007, 282, 194–199. [Google Scholar] [CrossRef]
- Fabel, K.; Fabel, K.; Tam, B.; Kaufer, D.; Baiker, A.; Simmons, N.; Kuo, C.J.; Palmer, T.D. VEGF is necessary for exercise-induced adult hippocampal neurogenesis. Eur. J. Neurosci. 2003, 18, 2803–2812. [Google Scholar] [CrossRef]
- Richardson, R.S.; Wagner, H.; Mudaliar, S.R.; Saucedo, E.; Henry, R.; Wagner, P.D. Exercise adaptation attenuates VEGF gene expression in human skeletal muscle. Am. J. Physiol. Heart Circ. Physiol. 2000, 279, H772–H778. [Google Scholar] [CrossRef]
- Rosenstein, J.M.; Krum, J.M.; Ruhrberg, C. VEGF in the nervous system. Organogenesis 2010, 6, 107–114. [Google Scholar] [CrossRef]
- Ohashi, K.; Parker, J.L.; Ouchi, N.; Higuchi, A.; Vita, J.A.; Gokce, N.; Pedersen, A.A.; Kalthoff, C.; Tullin, S.; Sams, A. Adiponectin promotes macrophage polarization toward an anti-inflammatory phenotype. J. Biol. Chem. 2010, 285, 6153–6160. [Google Scholar] [CrossRef]
- Lovren, F.; Pan, Y.; Quan, A.; Szmitko, P.E.; Singh, K.K.; Shukla, P.C.; Gupta, M.; Chan, L.; Al-Omran, M.; Teoh, H. Adiponectin primes human monocytes into alternative anti-inflammatory M2 macrophages. Am. J. Physiol.-Heart Circ. Physiol. 2010, 299, H656–H663. [Google Scholar] [CrossRef]
- Kataoka, H.; Sugie, K. Serum adiponectin levels between patients with Parkinson’s disease and those with PSP. Neurol. Sci. 2020, 41, 1125–1131. [Google Scholar] [CrossRef] [PubMed]
- Besse-Patin, A.; Montastier, E.; Vinel, C.; Castan-Laurell, I.; Louche, K.; Dray, C.; Daviaud, D.; Mir, L.; Marques, M.; Thalamas, C. Effect of endurance training on skeletal muscle myokine expression in obese men: Identification of apelin as a novel myokine. Int. J. Obes. 2014, 38, 707–713. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhang, X.; Cui, H.; Zhang, C.; Zhu, C.; Li, L. Apelin-13 protects the brain against ischemia/reperfusion injury through activating PI3K/Akt and ERK1/2 signaling pathways. Neurosci. Lett. 2014, 568, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Jung, T.W.; Hwang, H.J.; Hong, H.C.; Yoo, H.J.; Baik, S.H.; Choi, K.M. BAIBA attenuates insulin resistance and inflammation induced by palmitate or a high fat diet via an AMPK-PPARdelta-dependent pathway in mice. Diabetologia 2015, 58, 2096–2105. [Google Scholar] [CrossRef] [PubMed]
- Roberts, L.D.; Bostrom, P.; O’Sullivan, J.F.; Schinzel, R.T.; Lewis, G.D.; Dejam, A.; Lee, Y.K.; Palma, M.J.; Calhoun, S.; Georgiadi, A.; et al. beta-Aminoisobutyric acid induces browning of white fat and hepatic beta-oxidation and is inversely correlated with cardiometabolic risk factors. Cell Metab. 2014, 19, 96–108. [Google Scholar] [CrossRef] [PubMed]
- Kitase, Y.; Vallejo, J.A.; Gutheil, W.; Vemula, H.; Jahn, K.; Yi, J.; Zhou, J.; Brotto, M.; Bonewald, L.F. beta-aminoisobutyric Acid, l-BAIBA, Is a Muscle-Derived Osteocyte Survival Factor. Cell Rep. 2018, 22, 1531–1544. [Google Scholar] [CrossRef]
- Jung, T.W.; Park, H.S.; Choi, G.H.; Kim, D.; Lee, T. beta-aminoisobutyric acid attenuates LPS-induced inflammation and insulin resistance in adipocytes through AMPK-mediated pathway. J. Biomed. Sci. 2018, 25, 27. [Google Scholar] [CrossRef]
- Minato, T.; Nakamura, N.; Saiki, T.; Miyabe, M.; Ito, M.; Matsubara, T.; Naruse, K. beta-Aminoisobutyric acid, L-BAIBA, protects PC12 cells from hydrogen peroxide-induced oxidative stress and apoptosis via activation of the AMPK and PI3K/Akt pathway. IBRO Neurosci. Rep. 2022, 12, 65–72. [Google Scholar] [CrossRef]
- Stautemas, J.; Van Kuilenburg, A.B.P.; Stroomer, L.; Vaz, F.; Blancquaert, L.; Lefevere, F.B.D.; Everaert, I.; Derave, W. Acute Aerobic Exercise Leads to Increased Plasma Levels of R- and S-beta-Aminoisobutyric Acid in Humans. Front. Physiol. 2019, 10, 1240. [Google Scholar] [CrossRef]
- Priyadarshini, M.; Layden, B.T. FFAR3 modulates insulin secretion and global gene expression in mouse islets. Islets 2015, 7, e1045182. [Google Scholar] [CrossRef]
- Neudorf, H.; Little, J.P. Impact of fasting & ketogenic interventions on the NLRP3 inflammasome: A narrative review. Biomed. J. 2023, 47, 100677. [Google Scholar]
- Jensen, N.J.; Wodschow, H.Z.; Nilsson, M.; Rungby, J. Effects of ketone bodies on brain metabolism and function in neurodegenerative diseases. Int. J. Mol. Sci. 2020, 21, 8767. [Google Scholar] [CrossRef] [PubMed]
- Nagappan, G.; Lu, B. Activity-dependent modulation of the BDNF receptor TrkB: Mechanisms and implications. Trends Neurosci. 2005, 28, 464–471. [Google Scholar] [CrossRef] [PubMed]
- Numakawa, T.; Odaka, H.; Adachi, N. Actions of brain-derived neurotrophin factor in the neurogenesis and neuronal function, and its involvement in the pathophysiology of brain diseases. Int. J. Mol. Sci. 2018, 19, 3650. [Google Scholar] [CrossRef] [PubMed]
- Colucci-D’Amato, L.; Speranza, L.; Volpicelli, F. Neurotrophic factor BDNF, physiological functions and therapeutic potential in depression, neurodegeneration and brain cancer. Int. J. Mol. Sci. 2020, 21, 7777. [Google Scholar] [CrossRef]
- Zuccato, C.; Cattaneo, E. Brain-derived neurotrophic factor in neurodegenerative diseases. Nat. Rev. Neurol. 2009, 5, 311–322. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, H.; Zhang, B.-S.; Soares, J.C.; Zhang, X.Y. Low BDNF is associated with cognitive impairments in patients with Parkinson’s disease. Park. Relat. Disord. 2016, 29, 66–71. [Google Scholar] [CrossRef]
- Paterno, A.; Polsinelli, G.; Federico, B. Changes of brain-derived neurotrophic factor (BDNF) levels after different exercise protocols: A systematic review of clinical studies in Parkinson’s disease. Front. Physiol. 2024, 15, 1352305. [Google Scholar] [CrossRef]
- Zhao, P.; Wei, Y.; Sun, G.; Xu, L.; Wang, T.; Tian, Y.; Chao, H.; Tu, Y.; Ji, J. Fetuin-A alleviates neuroinflammation against traumatic brain injury-induced microglial necroptosis by regulating Nrf-2/HO-1 pathway. J. Neuroinflammation 2022, 19, 269. [Google Scholar] [CrossRef]
- Yoon, S.; Boonpraman, N.; Kim, C.Y.; Moon, J.-S.; Yi, S.S. Reduction of fetuin-A levels contributes to impairment of Purkinje cells in cerebella of patients with Parkinson’s disease. BMB Rep. 2023, 56, 308. [Google Scholar] [CrossRef]
- Chung, H.S.; Choi, K.M. Organokines in disease. Adv. Clin. Chem. 2020, 94, 261–321. [Google Scholar] [PubMed]
- Hill, C.M.; Laeger, T.; Dehner, M.; Albarado, D.C.; Clarke, B.; Wanders, D.; Burke, S.J.; Collier, J.J.; Qualls-Creekmore, E.; Solon-Biet, S.M.; et al. FGF21 Signals Protein Status to the Brain and Adaptively Regulates Food Choice and Metabolism. Cell Rep. 2019, 27, 2934–2947.e3. [Google Scholar] [CrossRef] [PubMed]
- Liang, Q.; Zhong, L.; Zhang, J.; Wang, Y.; Bornstein, S.R.; Triggle, C.R.; Ding, H.; Lam, K.S.; Xu, A. FGF21 maintains glucose homeostasis by mediating the cross talk between liver and brain during prolonged fasting. Diabetes 2014, 63, 4064–4075. [Google Scholar] [CrossRef] [PubMed]
- Kazmerova, Z.; Zilka, N.; Cente, M.; Neradil, P.; Zilkova, M.; Novak, M. Can we teach old dogs new tricks? Neuroprotective cell therapy in Alzheimer’s and Parkinson’s disease. J. Alzheimer’s Dis. 2013, 37, 251–272. [Google Scholar] [CrossRef]
- Distefano, G.; Goodpaster, B.H. Effects of exercise and aging on skeletal muscle. Cold Spring Harb. Perspect. Med. 2018, 8, a029785. [Google Scholar] [CrossRef]
- Brown, B.; Peiffer, J.; Martins, R. Multiple effects of physical activity on molecular and cognitive signs of brain aging: Can exercise slow neurodegeneration and delay Alzheimer’s disease? Mol. Psychiatry 2013, 18, 864–874. [Google Scholar] [CrossRef]
- Horowitz, A.M.; Villeda, S.A. Therapeutic potential of systemic brain rejuvenation strategies for neurodegenerative disease. F1000Research 2017, 6, 1291. [Google Scholar] [CrossRef]
- Rau, J.; Beaulieu, L.; Huntington, J.; Church, F. Serpins in thrombosis, hemostasis and fibrinolysis. J. Thromb. Haemost. 2007, 5, 102–115. [Google Scholar] [CrossRef]
- Gramling, M.W.; Church, F.C. Plasminogen activator inhibitor-1 is an aggregate response factor with pleiotropic effects on cell signaling in vascular disease and the tumor microenvironment. Thromb. Res. 2010, 125, 377–381. [Google Scholar] [CrossRef]
- Reuland, C.J.; Church, F.C. Synergy between plasminogen activator inhibitor-1, α-synuclein, and neuroinflammation in Parkinson’s disease. Med. Hypotheses 2020, 138, 109602. [Google Scholar] [CrossRef]
- Trejo, J.L.; Llorens-Martín, M.V.; Torres-Alemán, I. The effects of exercise on spatial learning and anxiety-like behavior are mediated by an IGF-I-dependent mechanism related to hippocampal neurogenesis. Mol. Cell. Neurosci. 2008, 37, 402–411. [Google Scholar] [CrossRef] [PubMed]
- McCall, G.E.; Allen, D.L.; Haddad, F.; Baldwin, K.M. Transcriptional regulation of IGF-I expression in skeletal muscle. Am. J. Physiol.-Cell Physiol. 2003, 285, C831–C839. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.L.; Wang, C.-H.; Pan, C.Y.; Chen, F.C. The effects of long-term resistance exercise on the relationship between neurocognitive performance and GH, IGF-1, and homocysteine levels in the elderly. Front. Behav. Neurosci. 2015, 9, 23. [Google Scholar] [CrossRef] [PubMed]
- Voss, M.W.; Erickson, K.I.; Prakash, R.S.; Chaddock, L.; Kim, J.S.; Alves, H.; Szabo, A.; Phillips, S.M.; Wójcicki, T.R.; Mailey, E.L.; et al. Neurobiological markers of exercise-related brain plasticity in older adults. Brain Behav. Immun. 2013, 28, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Kappeler, L.; Filho, C.D.M.; Dupont, J.; Leneuve, P.; Cervera, P.; Périn, L.; Loudes, C.; Blaise, A.; Klein, R.; Epelbaum, J.; et al. Brain IGF-1 receptors control mammalian growth and lifespan through a neuroendocrine mechanism. PLoS Biol. 2008, 6, e254. [Google Scholar] [CrossRef]
- Ayadi, A.E.; Zigmond, M.J.; Smith, A.D. IGF-1 protects dopamine neurons against oxidative stress: Association with changes in phosphokinases. Exp. Brain Res. 2016, 234, 1863–1873. [Google Scholar] [CrossRef]
- Bassil, F.; Delamarre, A.; Canron, M.H.; Dutheil, N.; Vital, A.; Négrier-Leibreich, M.L.; Bezard, E.; Fernagut, P.O.; Meissner, W.G. Impaired brain insulin signalling in Parkinson’s disease. Neuropathol. Appl. Neurobiol. 2022, 48, e12760. [Google Scholar] [CrossRef]
- Meex, R.C.; Watt, M.J. Hepatokines: Linking nonalcoholic fatty liver disease and insulin resistance. Nat. Rev. Endocrinol. 2017, 13, 509–520. [Google Scholar] [CrossRef]
- Mihara, M.; Hashizume, M.; Yoshida, H.; Suzuki, M.; Shiina, M. IL-6/IL-6 receptor system and its role in physiological and pathological conditions. Clin. Sci. 2012, 122, 143–159. [Google Scholar] [CrossRef]
- Sawada, M.; Imamura, K.; Nagatsu, T. Role of cytokines in inflammatory process in Parkinson’s disease. J. Neural. Transm. Suppl. 2006, 70, 373–381. [Google Scholar] [CrossRef]
- Halling, J.F.; Pilegaard, H. PGC-1alpha-mediated regulation of mitochondrial function and physiological implications. Appl. Physiol. Nutr. Metab. 2020, 45, 927–936. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xu, S.; Hu, Y.; Liu, Q.; Liu, C.; Chai, H.; Luo, Y.; Jin, L.; Li, S. Irisin exhibits neuroprotection by preventing mitochondrial damage in Parkinson’s disease. npj Park. Dis. 2023, 9, 13. [Google Scholar] [CrossRef] [PubMed]
- Cai, M.; Wang, H.; Song, H.; Yang, R.; Wang, L.; Xue, X.; Sun, W.; Hu, J. Lactate Is Answerable for Brain Function and Treating Brain Diseases: Energy Substrates and Signal Molecule. Front. Nutr. 2022, 9, 800901. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Wu, J.; Zhu, J.; Kuei, C.; Yu, J.; Shelton, J.; Sutton, S.W.; Li, X.; Yun, S.J.; Mirzadegan, T.; et al. Lactate inhibits lipolysis in fat cells through activation of an orphan G-protein-coupled receptor, GPR81. J. Biol. Chem. 2009, 284, 2811–2822. [Google Scholar] [CrossRef] [PubMed]
- Bergersen, L.H.; Gjedde, A. Is lactate a volume transmitter of metabolic states of the brain? Front. Neuroenergetics 2012, 4, 5. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, R.F.; Woodhead, J.S.; Zeng, N.; Blenkiron, C.; Merry, T.L.; Cameron-Smith, D.; Mitchell, C.J. Circulatory exosomal miRNA following intense exercise is unrelated to muscle and plasma miRNA abundances. Am. J. Physiol.-Endocrinol. Metab. 2018, 315, E723–E733. [Google Scholar] [CrossRef]
- Kuang, W.-H.; Dong, Z.-Q.; Tian, L.-T.; Li, J. MicroRNA-451a, microRNA-34a-5p, and microRNA-221-3p as predictors of response to antidepressant treatment. Braz. J. Med. Biol. Res. 2018, 51, e7212. [Google Scholar] [CrossRef]
- Zhang, W.-J.; Cao, W.-Y.; Huang, Y.-Q.; Cui, Y.-H.; Tu, B.-X.; Wang, L.-F.; Zou, G.-J.; Liu, Y.; Hu, Z.-L.; Hu, R. The role of miR-150 in stress-induced anxiety-like behavior in mice. Neurotox. Res. 2019, 35, 160–172. [Google Scholar] [CrossRef]
- Carek, P.J.; Laibstain, S.E.; Carek, S.M. Exercise for the treatment of depression and anxiety. Int. J. Psychiatry Med. 2011, 41, 15–28. [Google Scholar] [CrossRef]
- Hou, L.; Chen, W.; Liu, X.; Qiao, D.; Zhou, F.-M. Exercise-induced neuroprotection of the nigrostriatal dopamine system in Parkinson’s disease. Front. Aging Neurosci. 2017, 9, 358. [Google Scholar] [CrossRef]
- da Costa Daniele, T.M.; de Bruin, P.F.C.; de Matos, R.S.; de Bruin, G.S.; Junior, C.M.C.; de Bruin, V.M.S. Exercise effects on brain and behavior in healthy mice, Alzheimer’s disease and Parkinson’s disease model—A systematic review and meta-analysis. Behav. Brain Res. 2020, 383, 112488. [Google Scholar] [CrossRef] [PubMed]
- Pietrelli, A.; Matković, L.; Vacotto, M.; Lopez-Costa, J.; Basso, N.; Brusco, A. Aerobic exercise upregulates the BDNF-Serotonin systems and improves the cognitive function in rats. Neurobiol. Learn. Mem. 2018, 155, 528–542. [Google Scholar] [CrossRef] [PubMed]
- Agudelo, L.Z.; Femenía, T.; Orhan, F.; Porsmyr-Palmertz, M.; Goiny, M.; Martinez-Redondo, V.; Correia, J.C.; Izadi, M.; Bhat, M.; Schuppe-Koistinen, I. Skeletal muscle PGC-1α1 modulates kynurenine metabolism and mediates resilience to stress-induced depression. Cell 2014, 159, 33–45. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.; Cai, T.; Tagle, D.A.; Li, J. Structure, expression, and function of kynurenine aminotransferases in human and rodent brains. Cell. Mol. Life Sci. 2010, 67, 353–368. [Google Scholar] [CrossRef] [PubMed]
- Myint, A.-M.; Kim, Y.K.; Verkerk, R.; Scharpé, S.; Steinbusch, H.; Leonard, B. Kynurenine pathway in major depression: Evidence of impaired neuroprotection. J. Affect. Disord. 2007, 98, 143–151. [Google Scholar] [CrossRef]
- Wang, X.; Bove, A.M.; Simone, G.; Ma, B. Molecular bases of VEGFR-2-mediated physiological function and pathological role. Front. Cell Dev. Biol. 2020, 8, 599281. [Google Scholar] [CrossRef]
- Millauer, B.; Wizigmann-Voos, S.; Schnürch, H.; Martinez, R.; Møller, N.P.H.; Risau, W.; Ullrich, A. High affinity VEGF binding and developmental expression suggest Flk-1 as a major regulator of vasculogenesis and angiogenesis. Cell 1993, 72, 835–846. [Google Scholar] [CrossRef]
- Kanno, S.; Oda, N.; Abe, M.; Terai, Y.; Ito, M.; Shitara, K.; Tabayashi, K.; Shibuya, M.; Sato, Y. Roles of two VEGF receptors, Flt-1 and KDR, in the signal transduction of VEGF effects in human vascular endothelial cells. Oncogene 2000, 19, 2138–2146. [Google Scholar] [CrossRef]
- Ceci, C.; Lacal, P.M.; Barbaccia, M.L.; Mercuri, N.B.; Graziani, G.; Ledonne, A. The VEGFs/VEGFRs system in Alzheimer’s and Parkinson’s diseases: Pathophysiological roles and therapeutic implications. Pharmacol. Res. 2024, 201, 107101. [Google Scholar] [CrossRef]
- Higuchi, K.; Masaki, T.; Gotoh, K.; Chiba, S.; Katsuragi, I.; Tanaka, K.; Kakuma, T.; Yoshimatsu, H. Apelin, an APJ Receptor Ligand, Regulates Body Adiposity and Favors the Messenger Ribonucleic Acid Expression of Uncoupling Proteins in Mice. Endocrinology 2007, 148, 2690–2697. [Google Scholar] [CrossRef]
- Wang, B.L.; Wu, J.F.; Xiao, D.; Wu, B.; Wei, D.X. 3-hydroxybutyrate in the brain: Biosynthesis, function, and disease therapy. Brain-X 2023, 1, e6. [Google Scholar] [CrossRef]
- Pan, W.; Banks, W.A.; Fasold, M.B.; Bluth, J.; Kastin, A.J. Transport of brain-derived neurotrophic factor across the blood–brain barrier. Neuropharmacology 1998, 37, 1553–1561. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, W.; Zhu, S.; Li, J.; D’amore, J.; Ward, M.F.; Yang, H.; Wu, R.; Jahnen-Dechent, W.; Tracey, K.J. Peripheral administration of fetuin-A attenuates early cerebral ischemic injury in rats. J. Cereb. Blood Flow Metab. 2010, 30, 493–504. [Google Scholar] [CrossRef] [PubMed]
- Hsuchou, H.; Pan, W.; Kastin, A.J. The fasting polypeptide FGF21 can enter brain from blood. Peptides 2007, 28, 2382–2386. [Google Scholar] [CrossRef] [PubMed]
- Pan, W.; Kastin, A.J. Interactions of IGF-1 with the blood-brain barrier in vivo and in situ. Neuroendocrinology 2000, 72, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Banks, W.A.; Kastin, A.J.; Broadwell, R.D. Passage of cytokines across the blood-brain barrier. Neuroimmunomodulation 1995, 2, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; Yang, L.; Wang, X.; Wang, M.; Li, X.; Feng, B.; Wu, Y.; Liu, S.; Zhang, K. Mechanism of CNS regulation by irisin, a multifunctional protein. Brain Res. Bull. 2022, 188, 11–20. [Google Scholar] [CrossRef]
- Van Hall, G.; Stømstad, M.; Rasmussen, P.; Jans, Ø.; Zaar, M.; Gam, C.; Quistorff, B.; Secher, N.H.; Nielsen, H.B. Blood lactate is an important energy source for the human brain. J. Cereb. Blood Flow Metab. 2009, 29, 1121–1129. [Google Scholar] [CrossRef]
- Koopaei, N.N.; Chowdhury, E.A.; Jiang, J.; Noorani, B.; da Silva, L.; Bulut, G.; Hakimjavadi, H.; Chamala, S.; Bickel, U.; Schmittgen, T.D. Enrichment of the erythrocyte miR-451a in brain extracellular vesicles following impairment of the blood-brain barrier. Neurosci. Lett. 2021, 751, 135829. [Google Scholar] [CrossRef]
- Rani, A.; O’Shea, A.; Ianov, L.; Cohen, R.A.; Woods, A.J.; Foster, T.C. miRNA in circulating microvesicles as biomarkers for age-related cognitive decline. Front. Aging Neurosci. 2017, 9, 323. [Google Scholar] [CrossRef]
- Zhang, Z.G.; Zhang, L.; Jiang, Q.; Zhang, R.; Davies, K.; Powers, C.; Van Bruggen, N.; Chopp, M. VEGF enhances angiogenesis and promotes blood-brain barrier leakage in the ischemic brain. J. Clin. Investig. 2000, 106, 829–838. [Google Scholar] [CrossRef] [PubMed]
- Spranger, J.; Verma, S.; Göhring, I.; Bobbert, T.; Seifert, J.; Sindler, A.L.; Pfeiffer, A.; Hileman, S.M.; Tschöp, M.; Banks, W.A. Adiponectin does not cross the blood-brain barrier but modifies cytokine expression of brain endothelial cells. Diabetes 2006, 55, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Yau, S.Y.; Li, A.; Hoo, R.L.; Ching, Y.P.; Christie, B.R.; Lee, T.M.; Xu, A.; So, K.-F. Physical exercise-induced hippocampal neurogenesis and antidepressant effects are mediated by the adipocyte hormone adiponectin. Proc. Natl. Acad. Sci. USA 2014, 111, 15810–15815. [Google Scholar] [CrossRef] [PubMed]
- Bloemer, J.; Pinky, P.D.; Govindarajulu, M.; Hong, H.; Judd, R.; Amin, R.H.; Moore, T.; Dhanasekaran, M.; Reed, M.N.; Suppiramaniam, V. Role of Adiponectin in Central Nervous System Disorders. Neural Plast. 2018, 2018, 4593530. [Google Scholar] [CrossRef] [PubMed]
- Boado, R.J.; Zhang, Y.; Zhang, Y.; Wang, Y.; Pardridge, W.M. GDNF fusion protein for targeted-drug delivery across the human blood–brain barrier. Biotechnol. Bioeng. 2008, 100, 387–396. [Google Scholar] [CrossRef]
- Bertler, A.; Falck, B.; Owman, C.; Rosengrenn, E.B. The localization of monoaminergic blood-brain barrier mechanisms. Pharmacol. Rev. 1966, 18, 369–385. [Google Scholar]
- Hardebo, J.E.; Owman, C. Barrier mechanisms for neurotransmitter monoamines and their precursors at the blood-brain interface. Ann. Neurol. 1980, 8, 1–31. [Google Scholar] [CrossRef]
- Lindvall, M.; Hardebo, J.E.; Owman, C. Barrier mechanisms for neurotransmitter monoamines in the choroid plexus. Acta Physiol. Scand. 1980, 108, 215–221. [Google Scholar] [CrossRef]
- Egan, B.; Zierath, J.R. Exercise metabolism and the molecular regulation of skeletal muscle adaptation. Cell Metab. 2013, 17, 162–184. [Google Scholar] [CrossRef]
- Mann, S.; Beedie, C.; Jimenez, A. Differential effects of aerobic exercise, resistance training and combined exercise modalities on cholesterol and the lipid profile: Review, synthesis and recommendations. Sports Med. 2014, 44, 211–221. [Google Scholar] [CrossRef]
- Herrero, F.; San Juan, A.; Fleck, S.; Balmer, J.; Perez, M.; Canete, S.; Earnest, C.P.; Foster, C.; Lucia, A. Combined aerobic and resistance training in breast cancer survivors: A randomized, controlled pilot trial. Int. J. Sports Med. 2006, 27, 573–580. [Google Scholar] [CrossRef] [PubMed]
- Contrepois, K.; Wu, S.; Moneghetti, K.J.; Hornburg, D.; Ahadi, S.; Tsai, M.S.; Metwally, A.A.; Wei, E.; Lee-McMullen, B.; Quijada, J.V.; et al. Molecular choreography of acute exercise. Cell 2020, 181, 1112–1130. [Google Scholar] [CrossRef] [PubMed]
- Morville, T.; Sahl, R.E.; Moritz, T.; Helge, J.W.; Clemmensen, C. Plasma metabolome profiling of resistance exercise and endurance exercise in humans. Cell Rep. 2020, 33, 108554. [Google Scholar] [CrossRef] [PubMed]
- Huh, J.Y. The role of exercise-induced myokines in regulating metabolism. Arch. Pharmacal Res. 2018, 41, 14–29. [Google Scholar] [CrossRef] [PubMed]
- Zunner, B.E.; Wachsmuth, N.B.; Eckstein, M.L.; Scherl, L.; Schierbauer, J.R.; Haupt, S.; Stumpf, C.; Reusch, L.; Moser, O. Myokines and resistance training: A narrative review. Int. J. Mol. Sci. 2022, 23, 3501. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Liu, W.; Feng, F.; Chen, L. Apelin/APJ system: A novel therapeutic target for locomotor system diseases. Eur. J. Pharmacol. 2021, 906, 174286. [Google Scholar] [CrossRef]
- Arefi Shirvan, R.; Ghorbanian, B.; Ghorbanzadeh, B. The effect of 12 weeks of aerobic exercise on the serum levels of angiopoietin-like protein 4 (ANGPTL4) and beta-aminoisobutyric acid (BAIBA) in men with metabolic syndrome. J. Sport Biosci. 2022, 14, 19–32. [Google Scholar]
- Griffin, É.W.; Mullally, S.; Foley, C.; Warmington, S.A.; O’Mara, S.M.; Kelly, Á.M. Aerobic exercise improves hippocampal function and increases BDNF in the serum of young adult males. Physiol. Behav. 2011, 104, 934–941. [Google Scholar] [CrossRef]
- Ribeiro, D.; Petrigna, L.; Pereira, F.C.; Muscella, A.; Bianco, A.; Tavares, P. The impact of physical exercise on the circulating levels of BDNF and NT 4/5: A review. Int. J. Mol. Sci. 2021, 22, 8814. [Google Scholar] [CrossRef]
- Mazo, C.E.; Miranda, E.R.; Shadiow, J.; Vesia, M.; Haus, J.M. High intensity acute aerobic exercise elicits alterations in circulating and skeletal muscle tissue expression of neuroprotective exerkines. Brain Plast. 2022, 8, 5–18. [Google Scholar] [CrossRef]
- Tanimura, Y.; Aoi, W.; Takanami, Y.; Kawai, Y.; Mizushima, K.; Naito, Y.; Yoshikawa, T. Acute exercise increases fibroblast growth factor 21 in metabolic organs and circulation. Physiol. Rep. 2016, 4, e12828. [Google Scholar] [CrossRef] [PubMed]
- Catoire, M.; Mensink, M.; Kalkhoven, E.; Schrauwen, P.; Kersten, S. Identification of human exercise-induced myokines using secretome analysis. Physiol. Genom. 2014, 46, 256–267. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Wang, Q.; Jiang, N. The role of IGF-1 in exercise to improve obesity-related cognitive dysfunction. Front. Neurosci. 2023, 17, 1229165. [Google Scholar] [CrossRef] [PubMed]
- Ostrowski, K.; Schjerling, P.; Pedersen, B.K. Physical activity and plasma interleukin-6 in humans–effect of intensity of exercise. Eur. J. Appl. Physiol. 2000, 83, 512–515. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, J.B.; Phillips, M.D.; Yellott, R.C.; Currie, L.M. Resistance and aerobic exercise: The influence of mode on the relationship between IL-6 and glucose tolerance in young men who are obese. J. Strength Cond. Res. 2011, 25, 1529–1537. [Google Scholar] [CrossRef]
- Shin, K.O.; Bae, J.Y.; Woo, J.; Jang, K.S.; Kim, K.S.; Park, J.S.; Kim, I.K.; Kang, S. The effect of exercise on expression of myokine and angiogenesis mRNA in skeletal muscle of high fat diet induced obese rat. J. Exerc. Nutr. Biochem. 2015, 19, 91. [Google Scholar] [CrossRef]
- Spriet, L.L.; Howlett, R.A.; Heigenhauser, G.J. An enzymatic approach to lactate production in human skeletal muscle during exercise. Med. Sci. Sports Exerc. 2000, 32, 756–763. [Google Scholar] [CrossRef]
- Broholm, C.; Mortensen, O.H.; Nielsen, S.; Akerstrom, T.; Zankari, A.; Dahl, B.; Pedersen, B.K. Exercise induces expression of leukaemia inhibitory factor in human skeletal muscle. J. Physiol. 2008, 586, 2195–2201. [Google Scholar] [CrossRef]
- Subbotina, E.; Sierra, A.; Zhu, Z.; Gao, Z.; Koganti, S.R.K.; Reyes, S.; Stepniak, E.; Walsh, S.A.; Acevedo, M.R.; Perez-Terzic, C.M. Musclin is an activity-stimulated myokine that enhances physical endurance. Proc. Natl. Acad. Sci. USA 2015, 112, 16042–16047. [Google Scholar] [CrossRef]
- Seldin, M.M.; Peterson, J.M.; Byerly, M.S.; Wei, Z.; Wong, G.W. Myonectin (CTRP15), a novel myokine that links skeletal muscle to systemic lipid homeostasis. J. Biol. Chem. 2012, 287, 11968–11980. [Google Scholar] [CrossRef]
- Hittel, D.S.; Axelson, M.; Sarna, N.; Shearer, J.; Huffman, K.M.; Kraus, W.E. Myostatin decreases with aerobic exercise and associates with insulin resistance. Med. Sci. Sports Exerc. 2010, 42, 2023. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.; Chung, E.; Diffee, G.; Ji, L.L. Exercise training attenuates aging-associated mitochondrial dysfunction in rat skeletal muscle: Role of PGC-1α. Exp. Gerontol. 2013, 48, 1343–1350. [Google Scholar] [CrossRef] [PubMed]
- Wang, G. Aerobic exercise ameliorates myocardial ischemia/reperfusion injury and thrombosis of diabetic rats via activation of AMPK/Sirt1/PGC-1α pathway. Gen. Physiol. Biophys. 2022, 41, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Puchert, M.; Adams, V.; Linke, A.; Engele, J. Evidence for the involvement of the CXCL12 system in the adaptation of skeletal muscles to physical exercise. Cell. Signal. 2016, 28, 1205–1215. [Google Scholar] [CrossRef] [PubMed]
- Aoi, W.; Naito, Y.; Takagi, T.; Tanimura, Y.; Takanami, Y.; Kawai, Y.; Sakuma, K.; Hang, L.P.; Mizushima, K.; Hirai, Y. A novel myokine, secreted protein acidic and rich in cysteine (SPARC), suppresses colon tumorigenesis via regular exercise. Gut 2013, 62, 882–889. [Google Scholar] [CrossRef]
- Arany, Z.; Foo, S.-Y.; Ma, Y.; Ruas, J.L.; Bommi-Reddy, A.; Girnun, G.; Cooper, M.; Laznik, D.; Chinsomboon, J.; Rangwala, S.M. HIF-independent regulation of VEGF and angiogenesis by the transcriptional coactivator PGC-1α. Nature 2008, 451, 1008–1012. [Google Scholar] [CrossRef]
- Laker, R.; Garde, C.; Camera, D.; Smiles, W.; Zierath, J.; Hawley, J.; Barrès, R. Transcriptomic and epigenetic responses to short-term nutrient-exercise stress in humans. Sci. Rep. 2017, 7, 15134. [Google Scholar] [CrossRef]
- Church, D.D.; Hoffman, J.R.; Mangine, G.T.; Jajtner, A.R.; Townsend, J.R.; Beyer, K.S.; Wang, R.; La Monica, M.B.; Fukuda, D.H.; Stout, J.R. Comparison of high-intensity vs. high-volume resistance training on the BDNF response to exercise. J. Appl. Physiol. 2016, 121, 123–128. [Google Scholar] [CrossRef]
- Kim, J.S.; Lee, Y.H.; Yi, H.K. Gradual downhill running improves age-related skeletal muscle and bone weakness: Implication of autophagy and bone morphogenetic proteins. Exp. Physiol. 2016, 101, 1528–1540. [Google Scholar] [CrossRef]
- Kim, J.; McKenna, C.F.; Askow, A.T.; Salvador, A.F.; Scaroni, S.E.; Cerna, J.; Cannavale, C.N.; Paluska, S.A.; De Lisio, M.; Petruzzello, S.J. Higher Protein Intake does not Modulate Resistance Training–Induced Changes in Myokines and Cognitive Function in Middle-Aged Adults. J. Cogn. Enhanc. 2024, 8, 76–94. [Google Scholar] [CrossRef]
- Kanzleiter, T.; Rath, M.; Görgens, S.W.; Jensen, J.; Tangen, D.S.; Kolnes, A.J.; Kolnes, K.J.; Lee, S.; Eckel, J.; Schürmann, A. The myokine decorin is regulated by contraction and involved in muscle hypertrophy. Biochem. Biophys. Res. Commun. 2014, 450, 1089–1094. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Tian, Y.; Valenzuela, P.L.; Huang, C.; Zhao, J.; Yin, S.; Lucia, A. Myokine response to high-intensity interval vs. resistance exercise: An individual approach. Front. Physiol. 2018, 9, 416035. [Google Scholar] [CrossRef] [PubMed]
- Haghighi, A.H.; Hajinia, M.; Askari, R.; Abbasian, S.; Goldfied, G. Effect of high-intensity interval training and high-intensity resistance training on irisin and fibroblast growth factor 21 in men with overweight and obesity. Can. J. Physiol. Pharmacol. 2022, 100, 937–944. [Google Scholar] [CrossRef] [PubMed]
- Vega, S.R.; Knicker, A.; Hollmann, W.; Bloch, W.; Strüder, H.K. Effect of resistance exercise on serum levels of growth factors in humans. Horm. Metab. Res. 2010, 42, 982–986. [Google Scholar] [CrossRef] [PubMed]
- Bazgir, B.; Salesi, M.; Koushki, M.; Amirghofran, Z. Effects of eccentric and concentric emphasized resistance exercise on IL-15 serum levels and its relation to inflammatory markers in athletes and non-athletes. Asian J. Sports Med. 2015, 6, e27980. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-J.; So, B.; Choi, M.; Kang, D.; Song, W. Resistance exercise training increases the expression of irisin concomitant with improvement of muscle function in aging mice and humans. Exp. Gerontol. 2015, 70, 11–17. [Google Scholar] [CrossRef]
- Lantis, D.J.; Farrell III, J.W.; Cantrell, G.S.; Larson, R.D. Eight weeks of high-volume resistance training improves onset of blood lactate in trained individuals. J. Strength Cond. Res. 2017, 31, 2176–2182. [Google Scholar] [CrossRef]
- Deng, H.; Zhang, W.; Ruan, D.; Chen, D.; Xu, X.; He, X.; Chen, C.; Li, L. The Combination of Aerobic and Resistance Exercise Induces Weight Loss via the PGC-1α/Irisin/UCP-1 Pathway. J. Biomater. Tissue Eng. 2019, 9, 1388–1394. [Google Scholar] [CrossRef]
- Gavin, T.; Drew, J.; Kubik, C.; Pofahl, W.; Hickner, R. Acute resistance exercise increases skeletal muscle angiogenic growth factor expression. Acta Physiol. 2007, 191, 139–146. [Google Scholar] [CrossRef]
- García-Hermoso, A.; Ramírez-Vélez, R.; Díez, J.; González, A.; Izquierdo, M. Exercise training-induced changes in exerkine concentrations may be relevant to the metabolic control of type 2 diabetes mellitus patients: A systematic review and meta-analysis of randomized controlled trials. J. Sport Health Sci. 2023, 12, 147–157. [Google Scholar] [CrossRef]
- Rodríguez-Gutiérrez, E.; Torres-Costoso, A.; Pascual-Morena, C.; Pozuelo-Carrascosa, D.P.; Garrido-Miguel, M.; Martínez-Vizcaíno, V. Effects of resistance exercise on neuroprotective factors in middle and late life: A systematic review and meta-analysis. Aging Dis. 2023, 14, 1264. [Google Scholar] [CrossRef] [PubMed]
- Reeve, A.; Simcox, E.; Turnbull, D. Ageing and Parkinson’s disease: Why is advancing age the biggest risk factor? Ageing Res. Rev. 2014, 14, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, S.; Reisberg, B.; Zaudig, M.; Petersen, R.C.; Ritchie, K.; Broich, K.; Belleville, S.; Brodaty, H.; Bennett, D.; Chertkow, H. Mild cognitive impairment. Lancet 2006, 367, 1262–1270. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.L.; Ukropec, J.; Ukropcová, B.; Pai, M.C. An acute bout of aerobic or strength exercise specifically modifies circulating exerkine levels and neurocognitive functions in elderly individuals with mild cognitive impairment. NeuroImage Clin. 2018, 17, 272–284. [Google Scholar] [CrossRef] [PubMed]
- Kettinen, J.; Tikkanen, H.; Hiltunen, M.; Murray, A.; Horn, N.; Taylor, W.R.; Venojärvi, M. Cognitive and biomarker responses in healthy older adults to a 18-hole golf round and different walking types: A randomised cross-over study. BMJ Open Sport Exerc. Med. 2023, 9, e001629. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Sayer, A.A. Sarcopenia. Lancet 2019, 393, 2636–2646. [Google Scholar] [CrossRef] [PubMed]
- Peball, M.; Mahlknecht, P.; Werkmann, M.; Marini, K.; Murr, F.; Herzmann, H.; Stockner, H.; de Marzi, R.; Heim, B.; Djamshidian, A. Prevalence and associated factors of sarcopenia and frailty in Parkinson’s disease: A cross-sectional study. Gerontology 2019, 65, 216–228. [Google Scholar] [CrossRef]
- Kwon, J.H.; Moon, K.M.; Min, K.-W. Exercise-induced myokines can explain the importance of physical activity in the elderly: An overview. Healthcare 2020, 8, 378. [Google Scholar] [CrossRef]
- Piccirillo, R. Exercise-induced myokines with therapeutic potential for muscle wasting. Front. Physiol. 2019, 10, 444796. [Google Scholar] [CrossRef]
- Graham, Z.A.; Lavin, K.M.; O’Bryan, S.M.; Thalacker-Mercer, A.E.; Buford, T.W.; Ford, K.M.; Broderick, T.J.; Bamman, M.M. Mechanisms of exercise as a preventative measure to muscle wasting. Am. J. Physiol.-Cell Physiol. 2021, 321, C40–C57. [Google Scholar] [CrossRef]
- Ellingson, L.D.; Zaman, A.; Stegemöller, E.L. Sedentary Behavior and Quality of Life in Individuals With Parkinson’s Disease. Neurorehabil. Neural Repair 2019, 33, 595–601. [Google Scholar] [CrossRef] [PubMed]
- MacNeil, L.G.; Tarnopolsky, M.A.; Crane, J.D. Acute, exercise-induced alterations in cytokines and chemokines in the blood distinguish physically active and sedentary aging. J. Gerontol. Ser. A 2021, 76, 811–818. [Google Scholar] [CrossRef] [PubMed]
- Cirillo, J.; Lavender, A.P.; Ridding, M.C.; Semmler, J.G. Motor cortex plasticity induced by paired associative stimulation is enhanced in physically active individuals. J. Physiol. 2009, 587, 5831–5842. [Google Scholar] [CrossRef]
- McDonnell, M.N.; Buckley, J.D.; Opie, G.M.; Ridding, M.C.; Semmler, J.G. A single bout of aerobic exercise promotes motor cortical neuroplasticity. J. Appl. Physiol. (1985) 2013, 114, 1174–1182. [Google Scholar] [CrossRef] [PubMed]
- Steib, S.; Wanner, P.; Adler, W.; Winkler, J.; Klucken, J.; Pfeifer, K. A Single Bout of Aerobic Exercise Improves Motor Skill Consolidation in Parkinson’s Disease. Front. Aging Neurosci. 2018, 10, 328. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.; Raymick, J.; Imam, S. Neuroprotective and therapeutic strategies against Parkinson’s disease: Recent perspectives. Int. J. Mol. Sci. 2016, 17, 904. [Google Scholar] [CrossRef]
- Faden, A.I.; Stoica, B. Neuroprotection: Challenges and opportunities. Arch. Neurol. 2007, 64, 794–800. [Google Scholar] [CrossRef]
- Nay, K.; Smiles, W.J.; Kaiser, J.; McAloon, L.M.; Loh, K.; Galic, S.; Oakhill, J.S.; Gundlach, A.L.; Scott, J.W. Molecular Mechanisms Underlying the Beneficial Effects of Exercise on Brain Function and Neurological Disorders. Int. J. Mol. Sci. 2021, 22, 4052. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, B.K. Physical activity and muscle-brain crosstalk. Nat. Rev. Endocrinol. 2019, 15, 383–392. [Google Scholar] [CrossRef]
- Vajda, F.J. Neuroprotection and neurodegenerative disease. J. Clin. Neurosci. 2002, 9, 4–8. [Google Scholar] [CrossRef]
- Murphy, R.M.; Watt, M.J.; Febbraio, M.A. Metabolic communication during exercise. Nat. Metab. 2020, 2, 805–816. [Google Scholar] [CrossRef] [PubMed]
- Walzik, D.; Wences Chirino, T.Y.; Zimmer, P.; Joisten, N. Molecular insights of exercise therapy in disease prevention and treatment. Signal Transduct. Target. Ther. 2024, 9, 138. [Google Scholar] [CrossRef] [PubMed]
- Reddy, I.; Yadav, Y.; Dey, C.S. Cellular and molecular regulation of exercise—A neuronal perspective. Cell. Mol. Neurobiol. 2023, 43, 1551–1571. [Google Scholar] [CrossRef] [PubMed]
- Ryan, G.B.; Majno, G. Acute inflammation. A review. Am. J. Pathol. 1977, 86, 183. [Google Scholar] [PubMed]
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W. Chronic inflammation in the etiology of disease across the life span. Nat. Med. 2019, 25, 1822–1832. [Google Scholar] [CrossRef]
- Abbas, A.K.; Lichtman, A.H.; Pillai, S. Basic Immunology E-Book: Functions and Disorders of the Immune System; Elsevier Health Sciences: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Müller-Nedebock, A.C.; Dekker, M.C.; Farrer, M.J.; Hattori, N.; Lim, S.-Y.; Mellick, G.D.; Rektorová, I.; Salama, M.; Schuh, A.F.; Stoessl, A.J. Different pieces of the same puzzle: A multifaceted perspective on the complex biological basis of Parkinson’s disease. npj Park. Dis. 2023, 9, 110. [Google Scholar] [CrossRef]
- Hawley, J.A.; Hargreaves, M.; Joyner, M.J.; Zierath, J.R. Integrative biology of exercise. Cell 2014, 159, 738–749. [Google Scholar] [CrossRef]
- Neufer, P.D.; Bamman, M.M.; Muoio, D.M.; Bouchard, C.; Cooper, D.M.; Goodpaster, B.H.; Booth, F.W.; Kohrt, W.M.; Gerszten, R.E.; Mattson, M.P. Understanding the cellular and molecular mechanisms of physical activity-induced health benefits. Cell Metab. 2015, 22, 4–11. [Google Scholar] [CrossRef]
- Hood, D.A.; Memme, J.M.; Oliveira, A.N.; Triolo, M. Maintenance of skeletal muscle mitochondria in health, exercise, and aging. Annu. Rev. Physiol. 2019, 81, 19–41. [Google Scholar] [CrossRef]
- Finck, B.N.; Kelly, D.P. PGC-1 coactivators: Inducible regulators of energy metabolism in health and disease. J. Clin. Investig. 2006, 116, 615–622. [Google Scholar] [CrossRef]
- Spiering, B.A.; Kraemer, W.J.; Anderson, J.M.; Armstrong, L.E.; Nindl, B.C.; Volek, J.S.; Maresh, C.M. Resistance exercise biology: Manipulation of resistance exercise programme variables determines the responses of cellular and molecular signalling pathways. Sports Med. 2008, 38, 527–540. [Google Scholar] [CrossRef] [PubMed]
- Schoenfeld, B.J. The mechanisms of muscle hypertrophy and their application to resistance training. J. Strength Cond. Res. 2010, 24, 2857–2872. [Google Scholar] [CrossRef] [PubMed]
- Damas, F.; Libardi, C.A.; Ugrinowitsch, C. The development of skeletal muscle hypertrophy through resistance training: The role of muscle damage and muscle protein synthesis. Eur. J. Appl. Physiol. 2018, 118, 485–500. [Google Scholar] [CrossRef] [PubMed]
- McGee, S.L.; Hargreaves, M. Exercise adaptations: Molecular mechanisms and potential targets for therapeutic benefit. Nat. Rev. Endocrinol. 2020, 16, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Farup, J.; Sørensen, H.; Kjølhede, T. Similar changes in muscle fiber phenotype with differentiated consequences for rate of force development: Endurance versus resistance training. Hum. Mov. Sci. 2014, 34, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Hoppeler, H.; Baum, O.; Lurman, G.; Mueller, M. Molecular mechanisms of muscle plasticity with exercise. Compr. Physiol. 2011, 1, 1383–1412. [Google Scholar]
- Dyer, A.H.; Vahdatpour, C.; Sanfeliu, A.; Tropea, D. The role of Insulin-Like Growth Factor 1 (IGF-1) in brain development, maturation and neuroplasticity. Neuroscience 2016, 325, 89–99. [Google Scholar] [CrossRef]
- Chen, J.; Zhou, R.; Feng, Y.; Cheng, L. Molecular mechanisms of exercise contributing to tissue regeneration. Signal Transduct. Target. Ther. 2022, 7, 383. [Google Scholar] [CrossRef]
- Hortobágyi, T.; Vetrovsky, T.; Balbim, G.M.; Silva, N.C.B.S.; Manca, A.; Deriu, F.; Kolmos, M.; Kruuse, C.; Liu-Ambrose, T.; Radák, Z. The impact of aerobic and resistance training intensity on markers of neuroplasticity in health and disease. Ageing Res. Rev. 2022, 80, 101698. [Google Scholar] [CrossRef]
- Grazioli, E.; Tranchita, E.; Borriello, G.; Cerulli, C.; Minganti, C.; Parisi, A. The effects of concurrent resistance and aerobic exercise training on functional status in patients with multiple sclerosis. Curr. Sports Med. Rep. 2019, 18, 452–457. [Google Scholar] [CrossRef]
- Lee, J. Effects of aerobic and resistance exercise interventions on cognitive and physiologic adaptations for older adults with mild cognitive impairment: A systematic review and meta-analysis of randomized control trials. Int. J. Environ. Res. Public Health 2020, 17, 9216. [Google Scholar] [CrossRef] [PubMed]
- Krumpolec, P.; Vallova, S.; Slobodova, L.; Tirpakova, V.; Vajda, M.; Schon, M.; Klepochova, R.; Janakova, Z.; Straka, I.; Sutovsky, S. Aerobic-strength exercise improves metabolism and clinical state in Parkinson’s disease patients. Front. Neurol. 2017, 8, 698. [Google Scholar] [CrossRef] [PubMed]
- Caminiti, G.; Iellamo, F.; Mancuso, A.; Cerrito, A.; Montano, M.; Manzi, V.; Volterrani, M. Effects of 12 weeks of aerobic versus combined aerobic plus resistance exercise training on short-term blood pressure variability in patients with hypertension. J. Appl. Physiol. 2021, 130, 1085–1092. [Google Scholar] [CrossRef] [PubMed]
- D’hooge, R.; Hellinckx, T.; Van Laethem, C.; Stegen, S.; De Schepper, J.; Van Aken, S.; Dewolf, D.; Calders, P. Influence of combined aerobic and resistance training on metabolic control, cardiovascular fitness and quality of life in adolescents with type 1 diabetes: A randomized controlled trial. Clin. Rehabil. 2011, 25, 349–359. [Google Scholar] [CrossRef] [PubMed]
- Villareal, D.T.; Aguirre, L.; Gurney, A.B.; Waters, D.L.; Sinacore, D.R.; Colombo, E.; Armamento-Villareal, R.; Qualls, C. Aerobic or resistance exercise, or both, in dieting obese older adults. N. Engl. J. Med. 2017, 376, 1943–1955. [Google Scholar] [CrossRef] [PubMed]
- Pouresmaeili-Babaki, E.; Esmaeili-Mahani, S.; Abbasnejad, M.; Ravan, H. Protective effect of neuropeptide apelin-13 on 6-hydroxydopamine-induced neurotoxicity in SH-SY5Y dopaminergic cells: Involvement of its antioxidant and antiapoptotic properties. Rejuvenation Res. 2018, 21, 162–167. [Google Scholar] [CrossRef]
- Zhu, J.; Dou, S.; Jiang, Y.; Bai, B.; Chen, J.; Wang, C.; Cheng, B. Apelin-36 exerts the cytoprotective effect against MPP+-induced cytotoxicity in SH-SY5Y cells through PI3K/Akt/mTOR autophagy pathway. Life Sci. 2019, 224, 95–108. [Google Scholar] [CrossRef]
- Zhu, J.; Gao, W.; Shan, X.; Wang, C.; Wang, H.; Shao, Z.; Dou, S.; Jiang, Y.; Wang, C.; Cheng, B. Apelin-36 mediates neuroprotective effects by regulating oxidative stress, autophagy and apoptosis in MPTP-induced Parkinson’s disease model mice. Brain Res. 2020, 1726, 146493. [Google Scholar] [CrossRef]
- Chen, P.; Wang, Y.; Chen, L.; Song, N.; Xie, J. Apelin-13 protects dopaminergic neurons against rotenone—Induced neurotoxicity through the AMPK/mTOR/ULK-1 mediated autophagy activation. Int. J. Mol. Sci. 2020, 21, 8376. [Google Scholar] [CrossRef]
- Chen, Y.; Shen, J.; Qi, G.; Zha, Q.; Zhang, C.; Yao, W.; Gao, X.; Chen, S. Potential therapeutic role of fibroblast growth factor 21 in neurodegeneration: Evidence for ameliorating parkinsonism via silent information regulator 2 homolog 1 and implication for gene therapy. Neuropharmacology 2020, 181, 108335. [Google Scholar] [CrossRef]
- Fang, X.; Ma, J.; Mu, D.; Li, B.; Lian, B.; Sun, C. FGF21 protects dopaminergic neurons in Parkinson’s disease models via repression of neuroinflammation. Neurotox. Res. 2020, 37, 616–627. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Li, E.; Park, S. Insulin-like growth factor-1 inhibits 6-hydroxydopamine-mediated endoplasmic reticulum stress-induced apoptosis via regulation of heme oxygenase-1 and Nrf2 expression in PC12 cells. Int. J. Neurosci. 2012, 122, 641–649. [Google Scholar] [CrossRef] [PubMed]
- Quesada, A.; Lee, B.Y.; Micevych, P.E. PI3 kinase/Akt activation mediates estrogen and IGF-1 nigral DA neuronal neuroprotection against a unilateral rat model of Parkinson’s disease. Dev. Neurobiol. 2008, 68, 632–644. [Google Scholar] [CrossRef] [PubMed]
- Novelli, G.; Calcaterra, G.; Casciani, F.; Pecorelli, S.; Mehta, J.L. ‘Exerkines’: A Comprehensive Term for the Factors Produced in Response to Exercise. Biomedicines 2024, 12, 1975. [Google Scholar] [CrossRef] [PubMed]
- Mazur-Bialy, A.I.; Pocheć, E. The time-course of antioxidant irisin activity: Role of the Nrf2/HO-1/HMGB1 axis. Antioxidants 2021, 10, 88. [Google Scholar] [CrossRef]
- Wang, Y.; Dong, Z.; Zhang, Z.; Wang, Y.; Yang, K.; Li, X. Postconditioning with irisin attenuates lung ischemia/reperfusion injury by suppressing ferroptosis via induction of the Nrf2/HO-1 signal axis. Oxidative Med. Cell. Longev. 2022, 2022, 9911167. [Google Scholar] [CrossRef]
- Luo, J.; Tang, C.; Chen, X.; Ren, Z.; Qu, H.; Chen, R.; Tong, Z. Impacts of aerobic exercise on depression-like behaviors in chronic unpredictable mild stress mice and related factors in the AMPK/PGC-1α pathway. Int. J. Environ. Res. Public Health 2020, 17, 2042. [Google Scholar] [CrossRef]
- Burtscher, J.; Millet, G.P.; Place, N.; Kayser, B.; Zanou, N. The muscle-brain axis and neurodegenerative diseases: The key role of mitochondria in exercise-induced neuroprotection. Int. J. Mol. Sci. 2021, 22, 6479. [Google Scholar] [CrossRef]
- Heo, J.; Noble, E.E.; Call, J.A. The role of exerkines on brain mitochondria: A mini-review. J. Appl. Physiol. 2023, 134, 28–35. [Google Scholar] [CrossRef]
- Cefis, M.; Chaney, R.; Wirtz, J.; Méloux, A.; Quirié, A.; Leger, C.; Prigent-Tessier, A.; Garnier, P. Molecular mechanisms underlying physical exercise-induced brain BDNF overproduction. Front. Mol. Neurosci. 2023, 16, 1275924. [Google Scholar] [CrossRef]
- Palasz, E.; Wysocka, A.; Gasiorowska, A.; Chalimoniuk, M.; Niewiadomski, W.; Niewiadomska, G. BDNF as a promising therapeutic agent in Parkinson’s disease. Int. J. Mol. Sci. 2020, 21, 1170. [Google Scholar] [CrossRef] [PubMed]
- Maass, A.; Düzel, S.; Brigadski, T.; Goerke, M.; Becke, A.; Sobieray, U.; Neumann, K.; Lövdén, M.; Lindenberger, U.; Bäckman, L. Relationships of peripheral IGF-1, VEGF and BDNF levels to exercise-related changes in memory, hippocampal perfusion and volumes in older adults. Neuroimage 2016, 131, 142–154. [Google Scholar] [CrossRef] [PubMed]
- Jurcau, A.; Andronie-Cioara, F.L.; Nistor-Cseppento, D.C.; Pascalau, N.; Rus, M.; Vasca, E.; Jurcau, M.C. The involvement of neuroinflammation in the onset and progression of Parkinson’s disease. Int. J. Mol. Sci. 2023, 24, 14582. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.M.; Doss, H.M.; Kim, K.S. Multifaceted physiological roles of adiponectin in inflammation and diseases. Int. J. Mol. Sci. 2020, 21, 1219. [Google Scholar] [CrossRef] [PubMed]
- Tilg, H.; Moschen, A.R. Role of adiponectin and PBEF/visfatin as regulators of inflammation: Involvement in obesity-associated diseases. Clin. Sci. 2008, 114, 275–288. [Google Scholar] [CrossRef]
- Luo, H.; Xiang, Y.; Qu, X.; Liu, H.; Liu, C.; Li, G.; Han, L.; Qin, X. Apelin-13 suppresses neuroinflammation against cognitive deficit in a streptozotocin-induced rat model of Alzheimer’s disease through activation of BDNF-TrkB signaling pathway. Front. Pharmacol. 2019, 10, 395. [Google Scholar] [CrossRef] [PubMed]
- Shippy, D.C.; Wilhelm, C.; Viharkumar, P.A.; Raife, T.J.; Ulland, T.K. β-Hydroxybutyrate inhibits inflammasome activation to attenuate Alzheimer’s disease pathology. J. Neuroinflammation 2020, 17, 280. [Google Scholar] [CrossRef] [PubMed]
- Quartarone, A.; Ghilardi, M.; Boller, F. Defining neuroplasticity. In Neuroplasticity: From Bench to Bedside; Elsevier Science: Amsterdam, The Netherlands, 2022; Volume 3. [Google Scholar]
- Vints, W.A.; Levin, O.; Fujiyama, H.; Verbunt, J.; Masiulis, N. Exerkines and long-term synaptic potentiation: Mechanisms of exercise-induced neuroplasticity. Front. Neuroendocrinol. 2022, 66, 100993. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xu, E.; Musich, P.R.; Lin, F. Mitochondrial dysfunction in neurodegenerative diseases and the potential countermeasure. CNS Neurosci. Ther. 2019, 25, 816–824. [Google Scholar] [CrossRef]
- Cheng, C.-F.; Ku, H.-C.; Lin, H. PGC-1α as a pivotal factor in lipid and metabolic regulation. Int. J. Mol. Sci. 2018, 19, 3447. [Google Scholar] [CrossRef]
- Santos, S.F.; De Oliveira, H.L.; Yamada, E.S.; Neves, B.C.; Pereira Jr, A. The gut and Parkinson’s disease—A bidirectional pathway. Front. Neurol. 2019, 10, 574. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, J.C.C.; Oliveira, L.F.; Noleto, F.M.; Gusmão, C.T.P.; de Castro Brito, G.A.; de Barros Viana, G.S. Gut-microbiome-brain axis: The crosstalk between the vagus nerve, alpha-synuclein and the brain in Parkinson’s disease. Neural Regen. Res. 2023, 18, 2611–2614. [Google Scholar] [CrossRef] [PubMed]
- Liddle, R.A. Parkinson’s disease from the gut. Brain Res. 2018, 1693, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Elfil, M.; Kamel, S.; Kandil, M.; Koo, B.B.; Schaefer, S.M. Implications of the gut microbiome in Parkinson’s disease. Mov. Disord. 2020, 35, 921–933. [Google Scholar] [CrossRef] [PubMed]
- Imdad, S.; Lim, W.; Kim, J.-H.; Kang, C. Intertwined relationship of mitochondrial metabolism, gut microbiome and exercise potential. Int. J. Mol. Sci. 2022, 23, 2679. [Google Scholar] [CrossRef]
- Blume, G.R.; Royes, L.F.F. Peripheral to brain and hippocampus crosstalk induced by exercise mediates cognitive and structural hippocampal adaptations. Life Sci. 2024, 352, 122799. [Google Scholar] [CrossRef]
- Rojas-Valverde, D.; Bonilla, D.A.; Gómez-Miranda, L.M.; Calleja-Núñez, J.J.; Arias, N.; Martínez-Guardado, I. Examining the Interaction between Exercise, Gut Microbiota, and neurodegeneration: Future research directions. Biomedicines 2023, 11, 2267. [Google Scholar] [CrossRef]
- Ellis, T.; Rochester, L. Mobilizing Parkinson’s Disease: The Future of Exercise. J. Park. Dis. 2018, 8, S95–S100. [Google Scholar] [CrossRef]
- Petzinger, G.M.; Fisher, B.E.; Van Leeuwen, J.E.; Vukovic, M.; Akopian, G.; Meshul, C.K.; Holschneider, D.P.; Nacca, A.; Walsh, J.P.; Jakowec, M.W. Enhancing neuroplasticity in the basal ganglia: The role of exercise in Parkinson’s disease. Mov. Disord. 2010, 25, S141–S145. [Google Scholar] [CrossRef]
- Pedersen, B.K. Which type of exercise keeps you young? Curr. Opin. Clin. Nutr. Metab. Care 2019, 22, 167–173. [Google Scholar] [CrossRef]
- Campos, C.; Rocha, N.B.F.; Lattari, E.; Paes, F.; Nardi, A.E.; Machado, S. Exercise-induced neuroprotective effects on neurodegenerative diseases: The key role of trophic factors. Expert Rev. Neurother. 2016, 16, 723–734. [Google Scholar] [CrossRef] [PubMed]
- Cruise, K.; Bucks, R.; Loftus, A.; Newton, R.; Pegoraro, R.; Thomas, M. Exercise and Parkinson’s: Benefits for cognition and quality of life. Acta Neurol. Scand. 2011, 123, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, A.; Barbirato, D.; Araujo, N.; Martins, J.V.; Cavalcanti, J.L.S.; Santos, T.M.; Coutinho, E.S.; Laks, J.; Deslandes, A.C. Comparison of strength training, aerobic training, and additional physical therapy as supplementary treatments for Parkinson’s disease: Pilot study. Clin. Interv. Aging 2015, 10, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Zhao, P.; Guo, X.; Wang, J.; Wang, R. Effectiveness of aerobic and resistance training on the motor symptoms in Parkinson’s disease: Systematic review and network meta-analysis. Front. Aging Neurosci. 2022, 14, 935176. [Google Scholar] [CrossRef] [PubMed]
- Fernández del Olmo, M.Á.; Sánchez, J.A.; Morenill, L.; Gómez Varela, J.; Fernández-Lago, H.; Bello, O.; Santos García, D. Aerobic and resistance exercises in Parkinson’s disease: A narrative review. Eur. J. Hum. Mov. 2018, 41, 149–174. [Google Scholar]
- Roeder, L.; Costello, J.T.; Smith, S.S.; Stewart, I.B.; Kerr, G.K. Effects of resistance training on measures of muscular strength in people with Parkinson’s disease: A systematic review and meta-analysis. PLoS ONE 2015, 10, e0132135. [Google Scholar] [CrossRef]
- Brienesse, L.A.; Emerson, M.N. Effects of resistance training for people with Parkinson’s disease: A systematic review. J. Am. Med. Dir. Assoc. 2013, 14, 236–241. [Google Scholar] [CrossRef]
- Pinho, R.A.; Aguiar, A.S., Jr.; Radak, Z. Effects of Resistance Exercise on Cerebral Redox Regulation and Cognition: An Interplay Between Muscle and Brain. Antioxidants 2019, 8, 529. [Google Scholar] [CrossRef]
- Hirsch, M.A.; Farley, B.G. Exercise and neuroplasticity in persons living with Parkinson’s disease. Eur. J. Phys. Rehabil. Med. 2009, 45, 215–229. [Google Scholar]
- Crotty, G.F.; Schwarzschild, M.A. Chasing protection in Parkinson’s disease: Does exercise reduce risk and progression? Front. Aging Neurosci. 2020, 12, 186. [Google Scholar] [CrossRef]
- McFarthing, K.; Buff, S.; Rafaloff, G.; Fiske, B.; Mursaleen, L.; Fuest, R.; Wyse, R.K.; Stott, S.R. Parkinson’s disease drug therapies in the clinical trial pipeline: 2023 update. J. Park. Dis. 2023, 13, 427–439. [Google Scholar] [CrossRef] [PubMed]
- Sanford, J.A.; Nogiec, C.D.; Lindholm, M.E.; Adkins, J.N.; Amar, D.; Dasari, S.; Drugan, J.K.; Fernández, F.M.; Radom-Aizik, S.; Schenk, S. Molecular transducers of physical activity consortium (MoTrPAC): Mapping the dynamic responses to exercise. Cell 2020, 181, 1464–1474. [Google Scholar] [CrossRef] [PubMed]
- Ahlskog, J.E. Aerobic exercise: Evidence for a direct brain effect to slow parkinson disease progression. Mayo Clin. Proc. 2018, 93, 360–372. [Google Scholar] [CrossRef] [PubMed]
- Lam, F.M.; Huang, M.-Z.; Liao, L.-R.; Chung, R.C.; Kwok, T.C.; Pang, M.Y. Physical exercise improves strength, balance, mobility, and endurance in people with cognitive impairment and dementia: A systematic review. J. Physiother. 2018, 64, 4–15. [Google Scholar] [CrossRef] [PubMed]
- McKenzie, R.T. Exercise in Education and Medicine; Saunders: Philadelphia, PA, USA, 1909; 604p. [Google Scholar]

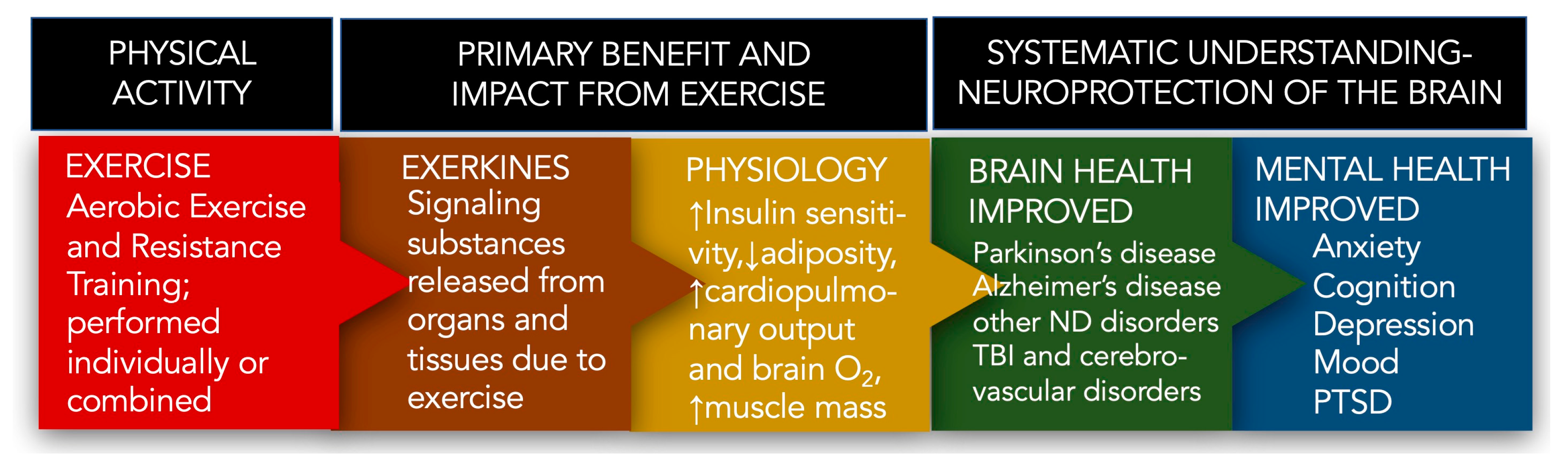
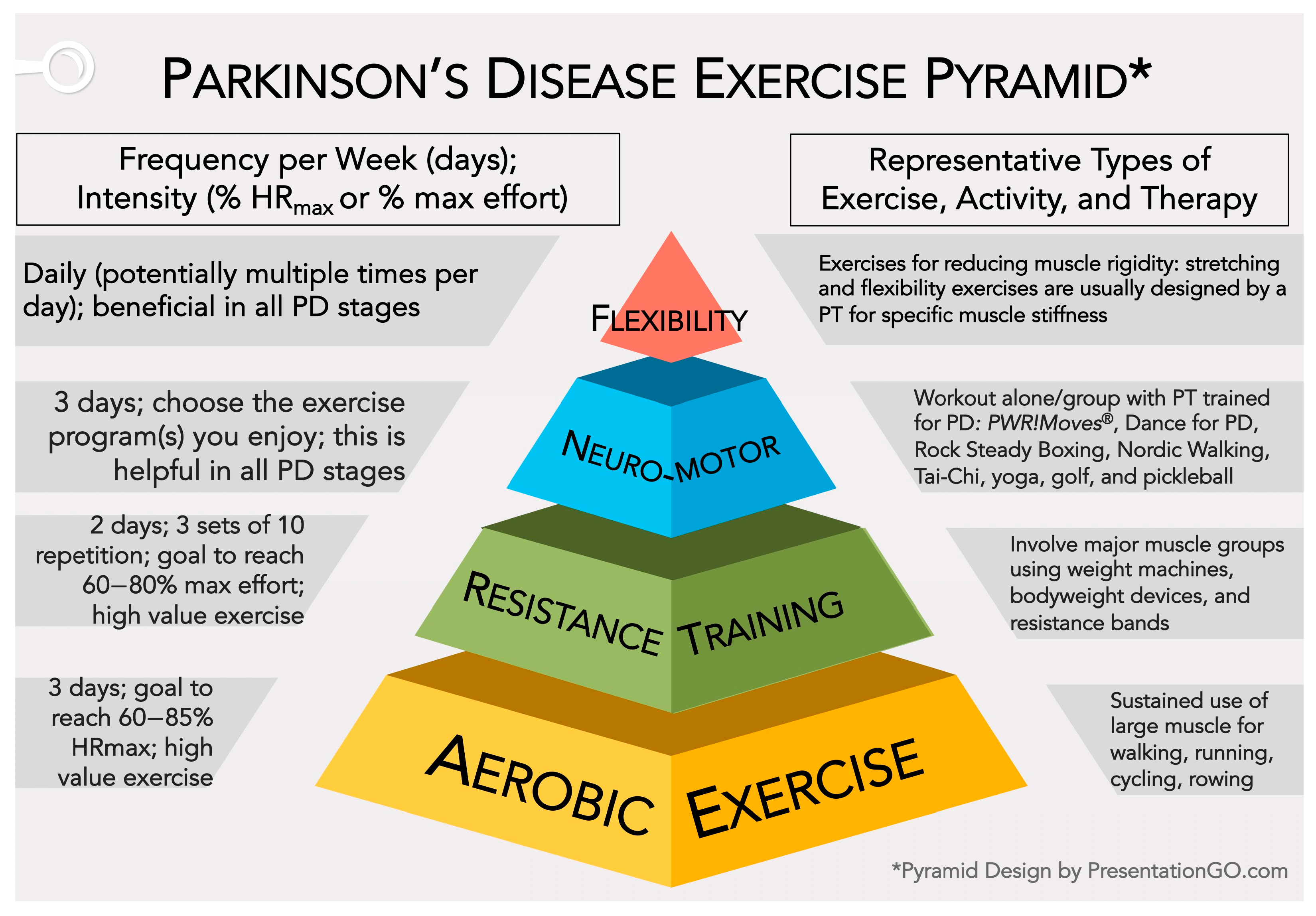
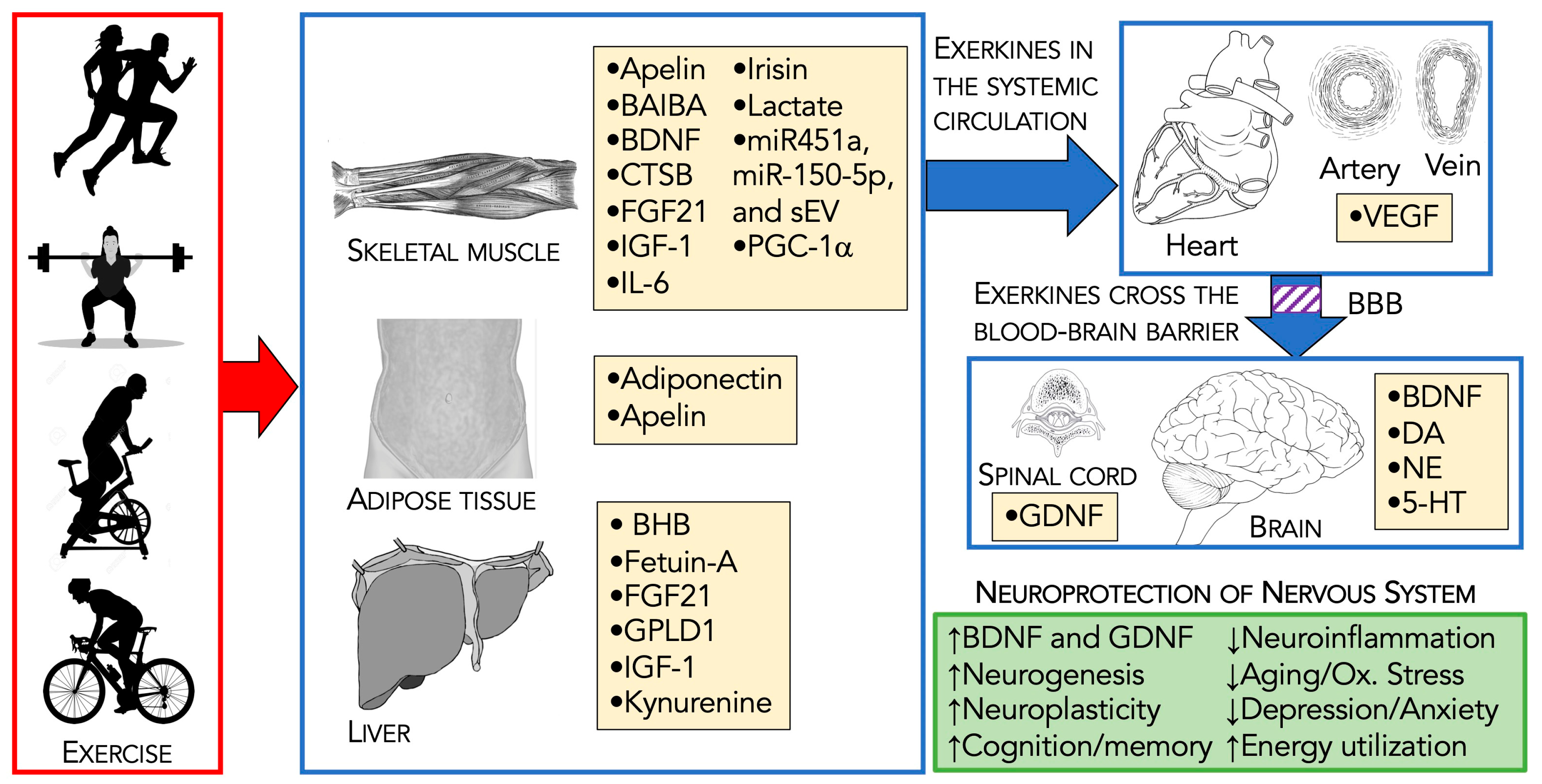
| Individual Profile | Aerobic Exercise | Resistance Training | Neuromotor Rehabilitation Programs | Flexibility/Stretching Exercises |
|---|---|---|---|---|
| Eighty-year-old male with advanced PD, ambulates with a rollator and requires caregiver support. He experiences some “off times” in which his medication becomes less effective. He is highly motivated with a great family and friend network. | Rides a NuStep or recumbent bike for 40 min 3×/week. Target HR of 60–70% HRmax | Participated in a seated strengthening program with use of resistance bands for upper body and supervision of a caregiver 2×/week. Completed during “on time” of medication cycle. | Currently receiving PT once every two weeks for maintenance PT. Also receives restorative PT periodically throughout the year. Goal of working on weight shifts with his rollator alongside a trained family member 3×/week. | Participates in daily stretches in his bed before getting up in the morning. Goal of taking a Dance for PD class online 1×/week. |
| Retired 70-year-old female with short term memory loss who enjoys staying active so she can spend time with her children and grandchildren | Walks 1–2 miles every day (when it is not raining). Target RPE for walks is 6/10. | Goes to the gym 2×/week and uses resistance machines after participation in exercise classes. | Receives restorative PT approximately 1–2×/year. Goal of taking 1–2 classes at her gym each week. (Varies between PWR!Moves, tai chi and Dance for PD) Sometimes her daughter will join her. | Participates in 10 min of home stretches as instructed by PT 5×/week. |
| Sixty-five-year-old female who was diagnosed one year ago. She has lived a lifetime of being active and is very willing to try to explore exercise options. She is still working and will retire in 1–2 years. | Walks on a treadmill 3×-week with goal of 75% max HR and plays tennis 2×/month | Works out in her home gym using free weights 2×/week | Participates in benchmark assessments 2×/year and restorative PT as needed Participates in a mix of the following activities 1–2×/week: tennis, tai chi, golf, PWR!Moves classes | Incorporates 15 min of stretching as a cool down after her resistance workouts, 2×/week |
| Recently diagnosed retired 76-year-old male with high blood pressure (controlled with medication) who is relatively sedentary, resistant to begin exercising but willing to give it a chance. He checks his BP daily. | Walks his family dog 3×/week for 20 min. He is working towards a goal of walking for 25 min. | Goal of participating in Rocksteady boxing 1×/week, Goal of completing body weight resistance exercises 2×/week during the commercial breaks of a television show he watches every day | Referred to PT to help establish an exercise program and address balance. PT will also instruct on modifications as needed to improve safety with exercise in individuals with high blood pressure. | Goal of incorporating 10 min of stretches after he gets up in the morning Goal of completing PWR!Moves seated mobility exercises 3×/week during the commercial breaks of a television show he watches every day |
| Retired 70-year-old male originally diagnosed at age 59 who experiences right side stiffness, right arm tremor, controlled with Carbidopa/Levodopa, NeuPro patch; follows an integrated approach to treatment. | Rides his Peloton bike for 45 min with goal of 80% HRmax 3×/week; 3–5 mile power walk 1×/week | 30–45 min weightlifting on major muscle groups 2×/week | LSVT BIG, and regularly works with a PT for any sports-related injury | Daily stretching, and 2× week aim for PWR!Moves or RockSteady Boxing. Participates in golf 2–3×/week. |
| Forty-one-year-old female who was diagnosed at age 39. She has two children ages 10 and 12 and is still working. She was previously active but not a formal exerciser. Over the past two years has worked with her healthcare team to start an exercise program that emphasizes aerobic components. | She initially used the couch to 5K program to begin running. Now participates in a running group and runs 3–5 miles 2–3×/week with target HRmax of 80%, also walks 20–30 min 2–3×/week. (She will often walk during her children’s soccer practice.) | Goes rock climbing with her son 2×/month. Goal of participating in 30 min of strength training 2×/week. She uses an online fitness membership to guide her workouts. | She worked with a PT to initiate an exercise program and participates in benchmark testing 2×/year. Returns to PT as needed to adjust exercises. Goal of participating in yoga 2×/week in addition to running. | Goal of completing a 15 min yoga video 2×/week. Goes rock climbing with her son 2×/month. |
| Name | Source Tissue(s) | Target Tissue(s) | Example(s) of Biological Action | Reference(s) |
|---|---|---|---|---|
| Adiponectin | WAT | Brain, heart, liver, muscle | Anti-inflammatory, glucose and fatty acid metabolism | [195,196,197,198] |
| Apelin | Skeletal muscle, WAT | Brain, bone, skeletal muscle | Anti-apoptotic | [48,199] |
| BAIBA | Skeletal muscle | Many sites | Metabolic regulator, anti-inflammatory, anti-oxidative | [200,201,202] |
| BHB | Liver | Many sites | Improves fuel utilization, anti-inflammatory | [203,204,205] |
| BDNF | Brain, skeletal muscle | Brain | Enhances synaptic plasticity, and cell growth | [206,207,208,209] |
| CTSB | Skeletal muscle | Brain | Neurogenesis, memory | [210,211] |
| Fetuin-A | Liver | Liver, brain | Neuroprotective, anti-inflammatory | [212,213,214] |
| FGF21 | Many tissues, liver, WAT | Many tissues, heart, bone, gut, brain, WAT | Improves fuel utilization (glucose, lipid) | [215,216,217] |
| GDNF | Spinal cord | Brain | Maintenance of spinal motor neurons and midbrain dopaminergic neurons | [218,219,220] |
| GPLD1 | Liver | Brain | Improves neurogenesis and cognition | [221,222] |
| IGF-1 | Liver, skeletal muscle, and other tissues | Brain, many sites | A mediator of brain health following exercise including neurogenesis and cognitive improvement | [223,224,225] |
| IL-6 | Skeletal muscle | Many sites | Energy sensor, anti-inflammatory in brain | [226,227,228] |
| Irisin (FNDC5) | Skeletal muscle | WAP, bone, β-cells, brain | Promotes neuronal health | [229,230,231] |
| Lactate | Skeletal muscle | Many tissues, including CNS | Brain fuel source | [232,233,234] |
| Lac-Phe | Epithelial cells in gut and kidney | Many tissues, including CNS | Suppresses feeding and obesity | [235,236,237] |
| mIR451a/mIR-150-5p/sEV | Skeletal muscle and other tissues | Many tissues including CNS | Protects against depression (miR-451a) and anxiety (miR-150-5p) | [238,239,240] |
| Monoamine neurotrans-mitter (DA, NE, 5-HT) | Brain | Brain | DA—Motor control, learning, executive function; NE—stress resistance memory, cognition; 5-HT—relieve anxiety, stress protection, cognition | [241,242,243] |
| PGC-1α/ Kynurenine | Skeletal muscle, liver, and other tissues | Skeletal muscle, and brain | PGC-1α activates kynurenine aminotransferase, switching the ratio of kynurenine (neurotoxic) to kynurenic acid (neuroprotective) | [244,245,246] |
| VEGF | Skeletal muscle and other tissues | Brain endothelium and other tissues | Promotes angiogenesis and exercise-induced neurogenesis | [247,248,249] |
| Aerobic Exercise [Reference(s)] | Resistance Training [Reference(s)] |
|---|---|
| Apelin ↑ [253,337] BAIBA ↑ [338] BDNF ↑ [339,340] CTSB ↑ [341] FGF21 ↑ [342] Fractalkine ↑ [343] IGF-1 ↑ [285,344] IL-6 ↑ [345,346] IL-15 ↑ [347] Irisin ↑ [229] Lactate ↑ [348] LIF ↑ [349] MCP-1 ↑ [343] Musclin ↑ [350] Myonectin ↑ [351] Myostatin ↓ [352] PGC-1α ↑ [353,354] SDF1 ↑ [355] SPARC ↑ [356] RANTES ↑ [216] VEGF ↑ [357] | Angiopoietin-like 4 ↑ [358] BDNF ↑ [359] BMP7 ↑ [360] CTSB ↑ [361] Decorin ↑ [362] FGF21 ↑ [363,364] IGF-1 ↑ [283,365] IL-6 ↑ [346] IL-15 ↑ [366] Irisin ↑ [364,367] Lactate ↑ [368] Myostatin ↓ [352] PGC-1α ↑ [369] VEGF ↑ [370] |
| Myokine | Experimental Model | Mechanism of Action | Physiological Function | Reference |
|---|---|---|---|---|
| Apelin-13 | SH-ST5Y cells + MPP+ + apelin-13 | ↑ ERK1/2 phosphorylation ↓ ER stress | ↓ Cell death | [418] |
| Apelin-36 | SH-ST5Y cells + MPP+ + apelin-36 | ↑ PI3K/Akt/mTOR autophagy | ↑ α-Synuclein clearance | [419] |
| Apelin-36 | Mice + MPTP + apelin-36 | ↑ GSH and SOD ↓ Caspase-3 | ↓ Anti-oxidative stress | [420] |
| Apelin-13 | SH-ST5Y cells + 6-OHDA + apelin-13 | ↑ PI3K ↓ Caspase 3 ↓ Cytochrome c | ↓ Cell death | [421] |
| FGF21 | Mice + MPTP + FGF21 SH-ST5Y cells + MPTP + FGF21 | ↑ SIRT1-autophagy | ↑ α-Synuclein clearance | [422] |
| FGF21 | Mice injected with MPTP + FGF21 SH-ST5Y cells + MPTP + FGF21 | ↑ BCL2/Bax ↓ Caspase 3 cleavage and JNK phosphorylation | ↓ Cell death | [423] |
| FGF21 | Primary dopaminergic neurons + MPP+ + FGF21 | ↓ IL-1, IL-12, IFN-γ, and TNF-α ↓ Astrocyte and microglia activation | ↑ Anti-inflammation | [423] |
| IGF-1 | PC12 cells + 6-OHDA + IGF-1 | ↓ Caspase-3 activation, and PARP cleavage | ↓ Cell death | [424] |
| IGF-1 | Rats + 6-OHDA + IGF-1 | ↑ PI3K/Akt | ↓ Cell death | [425] |
| IGF-1 | Rats + 6-OHDA + IGF-1 | ↑ ERK 1/2/CREB ↑ Akt/GSK3α/β/ β-catenin | ↓ Cell death | [287] |
| IGF-1 | PC12 cells + 6-OHDA + IGF-1 | ↑ HO-1 | ↓ Anti-oxidative stress | [424] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mitchell, A.K.; Bliss, R.R.; Church, F.C. Exercise, Neuroprotective Exerkines, and Parkinson’s Disease: A Narrative Review. Biomolecules 2024, 14, 1241. https://doi.org/10.3390/biom14101241
Mitchell AK, Bliss RR, Church FC. Exercise, Neuroprotective Exerkines, and Parkinson’s Disease: A Narrative Review. Biomolecules. 2024; 14(10):1241. https://doi.org/10.3390/biom14101241
Chicago/Turabian StyleMitchell, Alexandra K., Rebecca R. Bliss, and Frank C. Church. 2024. "Exercise, Neuroprotective Exerkines, and Parkinson’s Disease: A Narrative Review" Biomolecules 14, no. 10: 1241. https://doi.org/10.3390/biom14101241
APA StyleMitchell, A. K., Bliss, R. R., & Church, F. C. (2024). Exercise, Neuroprotective Exerkines, and Parkinson’s Disease: A Narrative Review. Biomolecules, 14(10), 1241. https://doi.org/10.3390/biom14101241








