Metalloproteinases as Biomarkers and Sociomarkers in Human Health and Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Search Strategy
2.3. Inclusion and Exclusion Criteria
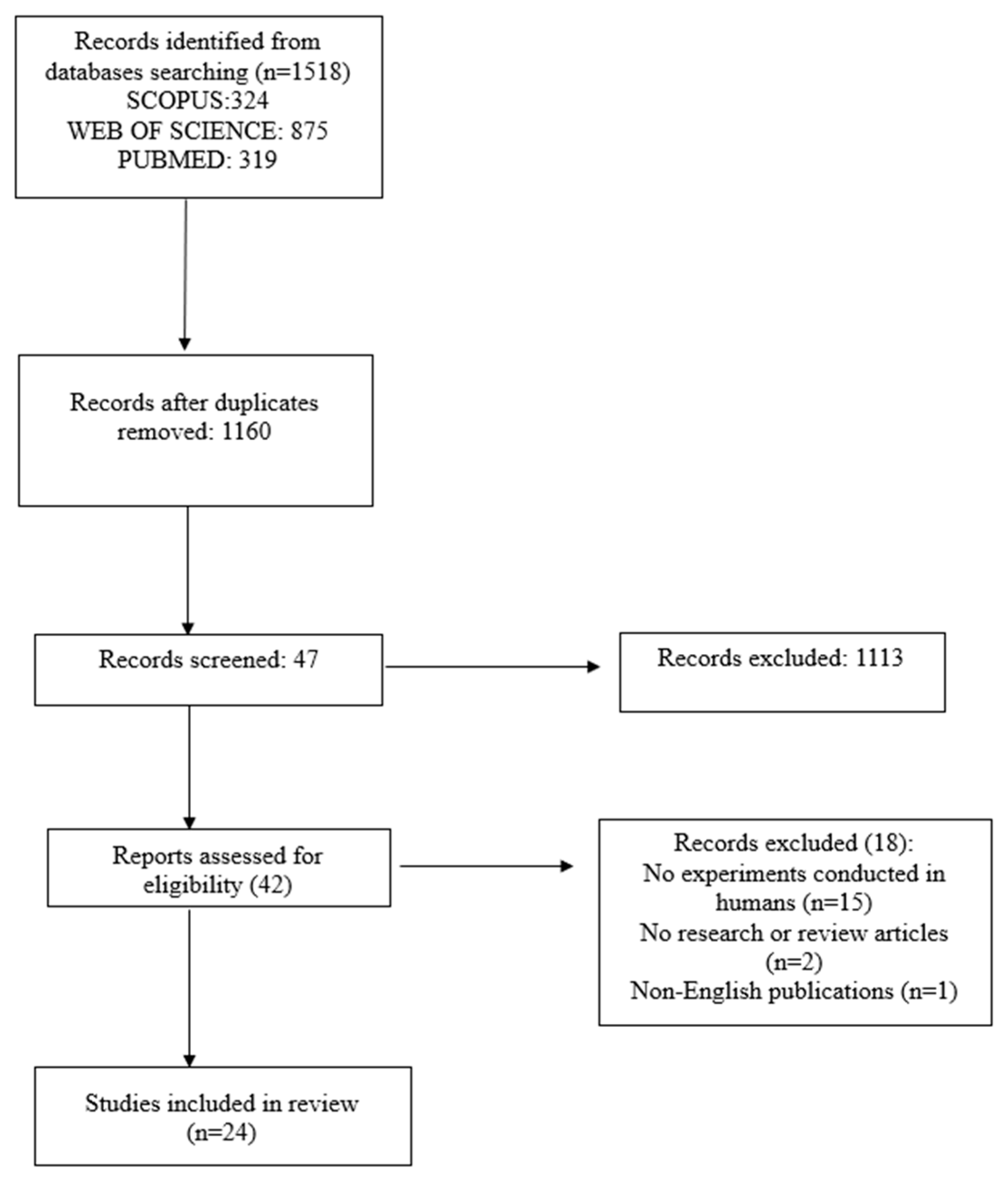
2.4. Study Selection
2.5. Data Extraction and Synthesis
3. Results
3.1. Selected Studies
3.2. General Characteristics of the Included Studies
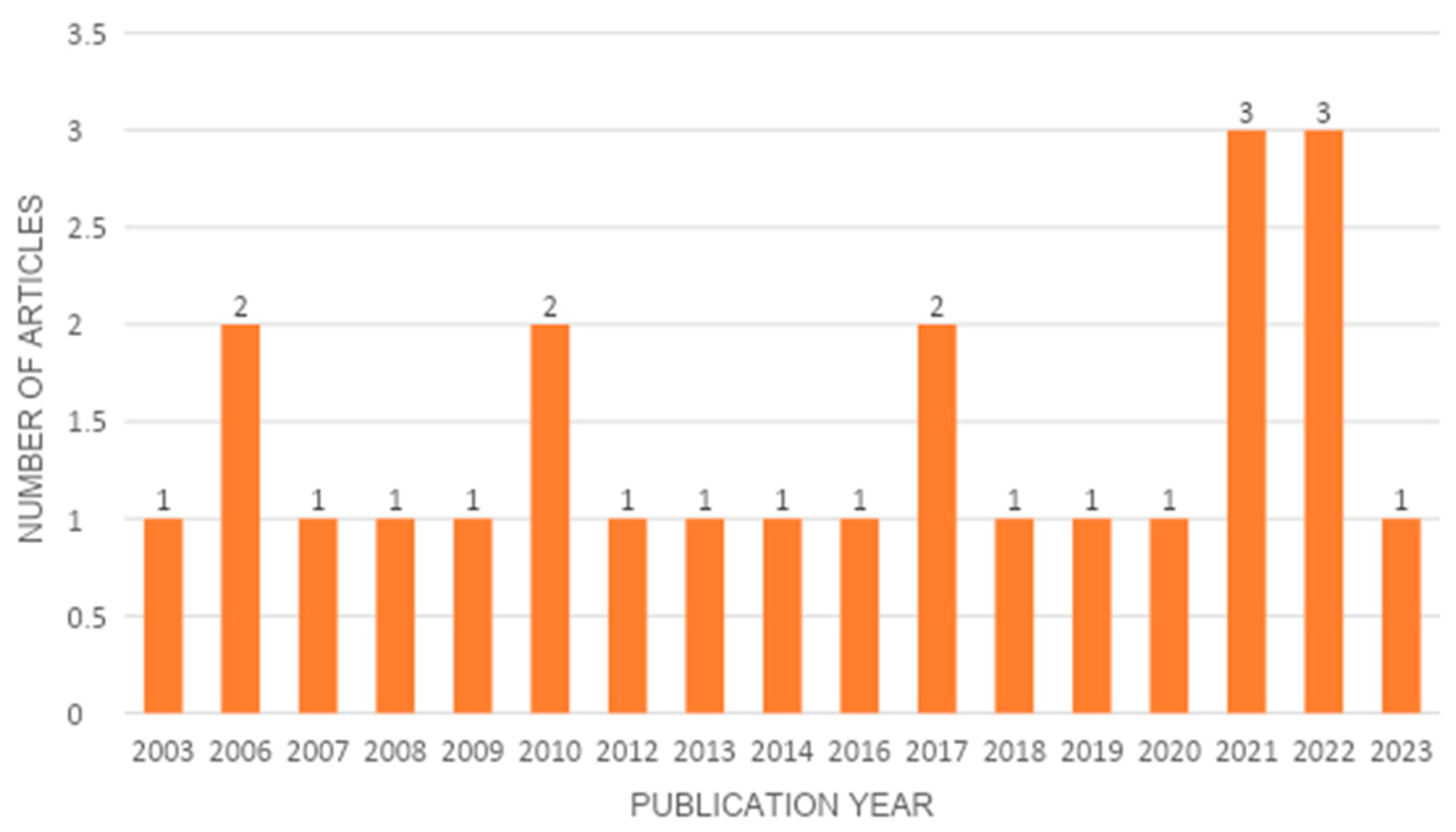
3.3. Temporal Extension of the Included Studies
3.4. Distribution of the Journals of the Included Studies and Their Research Areas
3.5. Geospatial Distribution of Included Studies
3.6. Matrix Metalloproteinases: Neuropsychiatric Diseases and Psychosocial Implications
3.7. Matrix Metalloproteinases: Cardiovascular Diseases and Psychosocial Implications
3.8. Matrix Metalloproteinases: Cancer and Psychosocial Implications
3.9. Matrix Metalloproteinases: Rheumatoid Arthritis and Psychosocial Implications
3.10. Matrix Metalloproteinases: Wound Healing, Periodontal Disease, and Psychosocial Implications
3.11. How MMPs Relate to Social Issues
4. Discussion
4.1. The Inflammatory Function of Matrix Metalloproteinases in Human Diseases
4.2. Matrix Metalloproteinases and Psychosocial Implications in Chronic and Complex Diseases
4.3. Matrix Metalloproteinases, Psychosocial Implications, and the Paradigm of Complexity
4.4. Strengths and Limitations of the Research
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Fortelny, N.; Cox, J.H.; Kappelhoff, R.; Starr, A.E.; Lange, P.F.; Pavlidis, P.; Overall, C.M. Network analyses reveal pervasive functional regulation between proteases in the human protease web. PLoS Biol. 2014, 12, e1001869. [Google Scholar] [CrossRef]
- MEROPS the Peptidase Database. Available online: http://merops.sanger.ac.uk (accessed on 9 October 2023).
- Puente, X.S.; Sanchez, L.M.; Overall, C.M.; Lopez-Otın, C. Human and mouse proteases: A comparative genomic approach. Nat. Rev. Genet. 2003, 4, 544–558. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Main, K.; Wang, H.; Julien, O.; Dufour, A. Biochemical tools for tracking proteolysis. J. Proteome Res. 2021, 20, 5264–5279. [Google Scholar] [CrossRef] [PubMed]
- Sternlicht, M.D.; Werb, Z. How matrix metalloproteinases regulate cell behavior. Annu. Rev. Cell Dev. Biol. 2021, 17, 463–516. [Google Scholar] [CrossRef]
- Sela-Passwell, N.; Kikkeri, R.; Dym, O.; Rozenberg, H.; Margalit, R.; Arad-Yellin, R.; Eisenstein, M.; Brenner, O.; Shoham, T.; Danon, T.; et al. Antibodies targeting the catalytic zinc complex of activated matrix metalloproteinases show therapeutic potential. Nat. Med. 2011, 18, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Tokito, A.; Jougasaki, M. Matrix metalloproteinases in non-neoplastic disorders. Int. J. Mol. Sci. 2016, 17, 1178. [Google Scholar] [CrossRef] [PubMed]
- Maskos, K. Crystal structures of MMPs in complex with physiological and pharmacological inhibitors. Biochimie 2005, 87, 249–263. [Google Scholar] [CrossRef]
- Gomis-Rüth, F.X. Structural aspects of the metzincin clan of metalloendopeptidases. Mol. Biotechnol. 2003, 24, 157–202. [Google Scholar] [CrossRef]
- Sterchi, E.E.; Stöcker, W.; Bond, J.S. Meprins, membrane-bound and secreted astacin metalloproteinases. Mol. Asp. Med. 2008, 29, 309–328. [Google Scholar] [CrossRef]
- Woessner, J.F., Jr. Catabolism of collagen and non-collagen protein in the rat uterus during post-partum involution. Biochem. J. 1962, 83, 304–314. [Google Scholar] [CrossRef]
- Gross, J.; Lapiere, C.M. Collagenolytic activity in amphibian tissues: A tissue culture assay. Proc. Natl. Acad. Sci. USA 1962, 48, 1014–1022. [Google Scholar] [CrossRef] [PubMed]
- Nagai, Y.; Lapiere, C.M.; Gross, J. Tadpole collagenase. Preparation and purification. Biochemistry 1966, 5, 3123–3130. [Google Scholar] [CrossRef] [PubMed]
- Collier, I.E.; Wilhelm, S.M.; Eisen, A.Z.; Marmer, B.L.; Grant, G.A.; Seltzer, J.L.; Kronberger, A.; He, C.S.; Bauer, E.A.; Goldberg, G.I. H-ras oncogene-transformed human bronchial epithelial cells (TBE-1) secrete a single metalloprotease capable of degrading basement membrane collagen. J. Biol. Chem. 1988, 263, 6579–6587. [Google Scholar] [CrossRef] [PubMed]
- Galloway, W.A.; Murphy, G.; Sandy, J.D.; Gavrilovic, J.; Cawston, T.E.; Reynolds, J.J. Purification and characterization of a rabbit bone metalloproteinase that degrades proteoglycan and other connective-tissue components. Biochem. J. 1983, 209, 741–752. [Google Scholar] [CrossRef] [PubMed]
- Chin, J.R.; Murphy, G.; Werb, Z. Stromelysin, a connective tissue-degrading metalloendopeptidase secreted by stimulated rabbit synovial fibroblasts in parallel with collagenase. Biosynthesis, isolation, characterization, and substrates. J. Biol. Chem. 1985, 260, 12367–12376. [Google Scholar] [CrossRef] [PubMed]
- de Almeida, L.G.N.; Thode, H.; Eslambolchi, Y.; Chopra, S.; Young, D.; Gill, S.; Devel, L.; Dufour, A. Matrix Metalloproteinases: From Molecular Mechanisms to Physiology, Pathophysiology, and Pharmacology. Pharmacol. Rev. 2022, 74, 712–768. [Google Scholar] [CrossRef]
- Nagase, H.; Woessner, J.F. Matrix metalloproteinases. J. Biol. Chem. 1999, 274, 21491–21494. [Google Scholar] [CrossRef]
- Bode, W.; Gomis-Rüth, F.X.; Stocker, W. Astacins, serralysins, snake venom and matrix metalloproteinases exhibit identical zinc-binding environments (HEXXHXXGXXH and Met-turn) and topologies and should be grouped into a common family, the ‘metzincins’. FEBS Lett. 1993, 331, 134–140. [Google Scholar] [CrossRef]
- Nagase, H.; Visse, R.; Murphy, G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc. Res. 2006, 69, 562–573. [Google Scholar] [CrossRef]
- Dufour, A.; Overall, C.M. Subtracting Matrix Out of the Equation: New Key Roles of Matrix Metalloproteinases in Innate Immunity and Disease. In Matrix Metalloproteinase Biology, 1st ed.; Sagi, I., Gaffney, J.P., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2015; pp. 131–152. [Google Scholar]
- Hynes, R.O. The extracellular matrix: Not just pretty fibrils. Science 2009, 326, 1216–1219. [Google Scholar] [CrossRef]
- Hynes, R.O.; Naba, A. Overview of the matrisome–an inventory of extracellular matrix constituents and functions. Cold Spring Harb. Perspect. Biol. 2012, 4, a004903. [Google Scholar] [CrossRef]
- Bonnans, C.; Chou, J.; Werb, Z. Remodelling the extracellular matrix in development and disease. Nat. Rev. Mol. Cell Biol. 2014, 15, 786–801. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.; Takai, K.; Weaver, V.M.; Werb, Z. Extracellular matrix degradation and remodeling in development and disease. Cold Spring Harb. Perspect. Biol. 2011, 3, a005058. [Google Scholar] [CrossRef]
- Cox, T.R. The matrix in cancer. Nat. Rev. Cancer 2021, 21, 217–238. [Google Scholar] [CrossRef] [PubMed]
- Brassart-Pasco, S.; Brezillon, S.; Brassart, B.; Ramont, L.; Oudart, J.-B.; Monboisse, J.C. Tumor microenvironment: Extracellular matrix alterations influence tumor progression. Front. Oncol. 2020, 10, 397. [Google Scholar] [CrossRef] [PubMed]
- Martin, D.R.; Santamaria, S.; Koch, C.D.; Ahnstrom, J.; Apte, S.S. Identification of novel ADAMTS1, ADAMTS4 and ADAMTS5 cleavage sites in versican using a labelfree quantitative proteomics approach. J. Proteom. 2021, 249, 104358. [Google Scholar] [CrossRef] [PubMed]
- Patterton, D.; Hayes, W.P.; Shi, Y.-B. Transcriptional activation of the matrix metalloproteinase gene stromelysin-3 coincides with thyroid hormone-induced cell death during frog metamorphosis. Dev. Biol. 1995, 167, 252–262. [Google Scholar] [CrossRef]
- Ishizuya-Oka, A.; Li, Q.; Amano, T.; Damjanovski, S.; Ueda, S.; Shi, Y.-B. Requirement for matrix metalloproteinase stromelysin-3 in cell migration and apoptosis during tissue remodeling in Xenopus laevis. J. Cell Biol. 2000, 150, 1177–1188. [Google Scholar] [CrossRef]
- Amano, T.; Kwak, O.; Fu, L.; Marshak, A.; Shi, Y.-B. The matrix metalloproteinase stromelysin-3 cleaves laminin receptor at two distinct sites between the transmembrane domain and laminin binding sequence within the extracellular domain. Cell Res. 2005, 15, 150–159. [Google Scholar] [CrossRef]
- Schultz, G.S.; Wysocki, A. Interactions between extracellular matrix and growth factors in wound healing. Wound Repair Regen. 2009, 17, 153–162. [Google Scholar] [CrossRef]
- Park, J.E.; Keller, G.A.; Ferrara, N. The vascular endothelial growth factor (VEGF) isoforms: Differential deposition into the subepithelial extracellular matrix and bioactivity of extracellular matrix-bound VEGF. Mol. Biol. Cell 1993, 4, 1317–1326. [Google Scholar] [CrossRef]
- Bergers, G.; Brekken, R.; McMahon, G.; Vu, T.H.; Itoh, T.; Tamaki, K.; Tanzawa, K.; Thorpe, P.; Itohara, S.; Werb, Z.; et al. Matrix metalloproteinase-9 triggers the angiogenic switch during carcinogenesis. Nat. Cell Biol. 2000, 2, 737–744. [Google Scholar] [CrossRef]
- Dufour, A.; Overall, C.M. Missing the target: Matrix metalloproteinase antitargets in inflammation and cancer. Trends Pharmacol. Sci. 2013, 34, 233–242. [Google Scholar] [CrossRef]
- Chopra, S.; Overall, C.M.; Dufour, A. Matrix metalloproteinases in the CNS: Interferons get nervous. Cell. Mol. Life Sci. 2019, 76, 3083–3095. [Google Scholar] [CrossRef]
- Young, D.; Das, N.; Anowai, A.; Dufour, A. Matrix metalloproteases as influencers of the cells’ social media. Int. J. Mol. Sci. 2019, 20, 3847. [Google Scholar] [CrossRef]
- McQuibban, G.A.; Gong, J.H.; Tam, E.M.; McCulloch, C.A.; Clark-Lewis, I.; Overall, C.M. Inflammation dampened by gelatinase A cleavage of monocyte chemoattractant protein-3. Science 2000, 289, 1202–1206. [Google Scholar] [CrossRef]
- Van Den Steen, P.E.; Wuyts, A.; Husson, S.J.; Proost, P.; Van Damme, J.; Opdenakker, G. Gelatinase B/MMP-9 and neutrophil collagenase/MMP-8 process the chemokines human GCP-2/CXCL6, ENA-78/CXCL5 and mouse GCP-2/LIX and modulate their physiological activities. Eur. J. Biochem. 2003, 270, 3739–3749. [Google Scholar] [CrossRef] [PubMed]
- Dean, R.A.; Cox, J.H.; Bellac, C.L.; Doucet, A.; Starr, A.E.; Overall, C.M. Macrophagespecific metalloelastase (MMP-12) truncates and inactivates ELR1 CXC chemokines and generates CCL2, -7, -8, and -13 antagonists: Potential role of the macrophage in terminating polymorphonuclear leukocyte influx. Blood 2008, 112, 3455–3464. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Schulze, C.J.; Suarez-Pinzon, W.L.; Dyck, J.R.B.; Sawicki, G.; Schulz, R. Intracellular action of matrix metalloproteinase-2 accounts for acute myocardial ischemia and reperfusion injury. Circulation 2002, 106, 1543–1549. [Google Scholar] [CrossRef] [PubMed]
- Si-Tayeb, K.; Monvoisin, A.; Mazzocco, C.; Lepreux, S.; Decossas, M.; Cubel, G.; Taras, D.; Blanc, J.-F.; Robinson, D.R.; Rosenbaum, J. Matrix metalloproteinase 3 is present in the cell nucleus and is involved in apoptosis. Am. J. Pathol. 2006, 169, 1390–1401. [Google Scholar] [CrossRef] [PubMed]
- Itoh, Y.; Takamura, A.; Ito, N.; Maru, Y.; Sato, H.; Suenaga, N.; Aoki, T.; Seiki, M. Homophilic complex formation of MT1-MMP facilitates proMMP-2 activation on the cell surface and promotes tumor cell invasion. EMBO J. 2001, 20, 4782–4793. [Google Scholar] [CrossRef] [PubMed]
- Dean, R.A.; Overall, C.M. Proteomics discovery of metalloproteinase substrates in the cellular context by iTRAQTM labeling reveals a diverse MMP-2 substrate degradome. Mol. Cell. Proteom. 2007, 6, 611–623. [Google Scholar] [CrossRef] [PubMed]
- Dean, R.A.; Butler, G.S.; Hamma-Kourbali, Y.; Delbé, J.; Brigstock, D.R.; Courty, J.; Overall, C.M. Identification of candidate angiogenic inhibitors processed by matrix metalloproteinase 2 (MMP-2) in cell-based proteomic screens: Disruption of vascular endothelial growth factor (VEGF)/heparin affin regulatory peptide (pleiotrophin) and VEGF/Connective tissue growth factor angiogenic inhibitory complexes by MMP-2 proteolysis. Mol. Cell. Biol. 2007, 27, 8454–8465. [Google Scholar] [CrossRef]
- Löffek, S.; Schilling, S.; Franzke, C.-W. Biological role of matrix metalloproteinases: A critical balance. Eur. Respir. J. 2011, 38, 191–208. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, D.; Morrison, C.J.; Overall, C.M. Matrix metalloproteinases: What do they not do? New substrates and biological roles identified by murine models and proteomics. BBA—Mol. Cell. Res. 2010, 1803, 39–54. [Google Scholar] [CrossRef] [PubMed]
- Busceti, M.T.; Grande, R.; Amato, B.; Gasbarro, V.; Buffone, G.; Amato, M.; Gallelli, L.; Serra, R.; de Franciscis, S. Pulmonary embolism, metalloproteinsases and neutrophil gelatinase associated lipocalin. Acta Phlebol. 2013, 14, 115–121. [Google Scholar]
- Tayebjee, M.H.; Lip, G.Y.; MacFadyen, R.J. Matrix metalloproteinases in coronary artery disease: Clinical and therapeutic implications and pathological significance. Curr. Med. Chem. 2005, 12, 917–925. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Van den Steen, P.E.; Sang, Q.X.; Opdenakker, G. Matrix metalloproteinase inhibitors as therapy for inflammatory and vascular diseases. Nat. Rev. Drug Discov. 2007, 6, 480–498. [Google Scholar] [CrossRef]
- Murphy, G.; Nagase, H. Reappraising metalloproteinases in rheumatoid arthritis and osteoarthritis: Destruction or repair? Nat. Clin. Pract. Rheumatol. 2008, 4, 128–135. [Google Scholar] [CrossRef]
- Yong, V.W. Metalloproteinases: Mediators of pathology and regeneration in the CNS. Nat. Rev. Neurosci. 2005, 6, 931–944. [Google Scholar] [CrossRef]
- Overall, C.M.; Lopez-Otin, C. Strategies for MMP inhibition in cancer: Innovations for the post-trial era. Nat. Rev. Cancer 2002, 2, 657–672. [Google Scholar] [CrossRef] [PubMed]
- Overall, C.M. Dilating the degradome: Matrix metalloproteinase 2 (MMP-2) cuts to the heart of the matter. Biochem. J. 2004, 383, e5–e7. [Google Scholar] [CrossRef] [PubMed]
- Balbin, M.; Fueyo, A.; Tester, A.M.; Pendas, A.M.; Pitiot, A.S.; Astudillo, A.; Overall, C.M.; Shapiro, S.D.; Lopez-Otin, C. Loss of collagenase-2 confers increased skin tumor susceptibility to male mice. Nat. Genet. 2003, 35, 252–257. [Google Scholar] [CrossRef]
- Egeblad, M.; Werb, Z. New functions for the matrix metalloproteinases in cancer progression. Nat. Rev. Cancer 2002, 2, 161–174. [Google Scholar] [CrossRef] [PubMed]
- Overall, C.M.; Kleifeld, O. Towards third generation matrix metalloproteinase inhibitors for cancer therapy. Br. J. Cancer 2006, 94, 941–946. [Google Scholar] [CrossRef] [PubMed]
- Leppert, D.; Leib, S.L.; Grygar, C.; Miller, K.M.; Schaad, U.B.; Hollander, G.A. Matrix metalloproteinase (MMP)-8 and MMP-9 in cerebrospinal fluid during bacterial meningitis: Association with blood-brain barrier damage and neurological sequelae. Clin. Infect. Dis. 2000, 31, 80–84. [Google Scholar] [CrossRef] [PubMed]
- Yong, V.W.; Power, C.; Forsyth, P.; Edwards, D.R. Metalloproteinases in biology and pathology of the nervous system. Nat. Rev. Neurosci. 2001, 2, 502–511. [Google Scholar] [CrossRef]
- Meli, D.N.; Christen, S.; Leib, S.L. Matrix metalloproteinase-9 in pneumococcal meningitis: Activation via an oxidative pathway. J. Infect. Dis. 2003, 187, 1411–1415. [Google Scholar] [CrossRef]
- Cox, J.H.; Starr, A.E.; Kappelhoff, R.; Yan, R.; Roberts, C.R.; Overall, C.M. Matrix metalloproteinase 8 deficiency in mice exacerbates inflammatory arthritis through delayed neutrophil apoptosis and reduced caspase 11 expression. Arthritis Rheum. 2010, 62, 3645–3655. [Google Scholar] [CrossRef]
- Bellac, C.L.; Dufour, A.; Krisinger, M.J.; Loonchanta, A.; Starr, A.E.; Auf dem Keller, U.; Lange, P.F.; Goebeler, V.; Kappelhoff, R.; Butler, G.S.; et al. Macrophage matrix metalloproteinase-12 dampens inflammation and neutrophil influx in arthritis. Cell Rep. 2014, 9, 618–632. [Google Scholar] [CrossRef]
- Dufour, A. Degradomics of matrix metalloproteinases in inflammatory diseases. Front. Biosci. 2015, 7, 150–167. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.L.; George, S.J.; Newby, A.C.; Jackson, C.L. Divergent effects of matrix metalloproteinases 3, 7, 9, and 12 on atherosclerotic plaque stability in mouse brachiocephalic arteries. Proc. Natl. Acad. Sci. USA 2005, 102, 15575–15580. [Google Scholar] [CrossRef] [PubMed]
- Costa, D.; Ielapi, N.; Minici, R.; Bevacqua, E.; Ciranni, S.; Cristodoro, L.; Torcia, G.; Di Taranto, M.D.; Bracale, U.M.; Andreucci, M.; et al. Metalloproteinases between History, Health, Disease, and the Complex Dimension of Social Determinants of Health. J. Vasc. Dis. 2023, 2, 282–298. [Google Scholar] [CrossRef]
- Marks, J. Dossier: Le groupe des Dix, des précurseurs de l’interdisciplinarité–Biology and complexity: Edgar Morin and Henri Atlan. Nat. Sci. Soc. 2019, 27, 159–168. [Google Scholar] [CrossRef]
- Coveney, P.V. Self-Organization and Complexity: A New Age for Theory, Computation and Experiment. Philos. Trans. Math. Phys. Eng. Sci. 2003, 361, 1057–1079. [Google Scholar] [CrossRef] [PubMed]
- Morin, E. Le Paradigme Perdue: La Nature Humane; Éditions du Seuil: Paris, France, 1973; p. 129. [Google Scholar]
- Prigogine, I.; Stengers, I. La Nouvelle Alliance. Métamorphose de la Science; Gallimard: Paris, France, 1979; pp. 485–488. [Google Scholar]
- Morin, E. Journal de Californie; Seuil: Paris, France, 1970; pp. 42–43. [Google Scholar]
- Morin, E. Restricted complexity, general complexity. In Worldviews, Science and Us: Philosophy and Complexity; Gershenson, C., Aerts, D., Edmonds, B., Eds.; World Scientific: Singapore, 2007; pp. 5–29. [Google Scholar]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Arksey, H.; O’Malley, L. Scoping studies: Towards a methodological framework. Int. J. Soc. Res. Methodol. 2005, 8, 19–32. [Google Scholar] [CrossRef]
- Haddaway, N.R.; Collins, A.M.; Coughlin, D.; Kirk, S. The Role of Google Scholar in Evidence Reviews and Its Ap-plicability to Grey Literature Searching. PLoS ONE 2015, 10, e0138237. [Google Scholar] [CrossRef]
- Samson, D.; Schoelles, K.M. Medical tests guidance (2) developing the topic and structuring systematic reviews of medical tests: Utility of PICOTS, analytic frameworks, decision trees, and other frameworks. J. Gen. Intern. Med. 2012, 27, 11–19. [Google Scholar] [CrossRef]
- Rahimi, S.; Sayad, A.; Moslemi, E.; Ghafouri-Fard, S.; Taheri, M. Blood Assessment of the Expression Levels of Matrix Metalloproteinase 9 (MMP9) and Its Natural Inhibitor, TIMP1 Genes in Iranian Schizophrenic Patients. Metab. Brain Dis. 2017, 32, 1537–1542. [Google Scholar] [CrossRef]
- Li, H.; Sheng, Z.; Khan, S.; Zhang, R.; Liu, Y.; Zhang, Y.; Yong, V.W.; Xue, M. Matrix Metalloproteinase-9 as an Important Contributor to the Pathophysiology of Depression. Front. Neurol. 2022, 13, 861843. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, M.W.; Michel, T.M. Matrix Metalloproteinases in Autism Spectrum Disorders. J. Mol. Psychiatry 2013, 1, 16. [Google Scholar] [CrossRef] [PubMed]
- Razak, K.A.; Binder, D.K.; Ethell, I.M. Neural Correlates of Auditory Hypersensitivity in Fragile X Syndrome. Front. Psychiatry 2021, 12, 720752. [Google Scholar] [CrossRef] [PubMed]
- Fernandez Machulsky, N.; Gagliardi, J.; Fabre, B.; Miksztowicz, V.; Lombardo, M.; García Escudero, A.; Gigena, G.; Blanco, F.; Gelpi, R.J.; Schreier, L.; et al. Matrix Metalloproteinases and Psychosocial Factors in Acute Coronary Syndrome Patients. Psychoneuroendocrinology 2016, 63, 102–108. [Google Scholar] [CrossRef]
- Garvin, P.; Nilsson, L.; Carstensen, J.; Jonasson, L.; Kristenson, M. Plasma Levels of Matrix Metalloproteinase-9 Are Independently Associated with Psychosocial Factors in a Middle-Aged Normal Population. Psychosom. Med. 2009, 71, 292–300. [Google Scholar] [CrossRef] [PubMed]
- Jönsson, S.; Lundberg, A.K.; Jonasson, L. Overexpression of MMP-9 and Its Inhibitors in Blood Mononuclear Cells after Myocardial Infarction—Is It Associated with Depressive Symptomatology? PLoS ONE 2014, 9, e0105572. [Google Scholar] [CrossRef] [PubMed]
- Shafi, B.H.; Bøttcher, M.; Ejupi, A.; Jensen, G.; Osler, M.; Lange, T.; Prescott, E. Socioeconomic Disparity in Cardiovascular Disease: Possible Biological Pathways Based on a Proteomic Approach. Atherosclerosis 2022, 352, 62–68. [Google Scholar] [CrossRef]
- Lutgendorf, S.K.; Lamkin, D.M.; Jennings, N.B.; Arevalo JM, G.; Penedo, F.; DeGeest, K.; Langley, R.R.; Lucci, J.A.; Cole, S.W.; Lubaroff, D.M.; et al. Biobehavioral Influences on Matrix Metalloproteinase Expression in Ovarian Carcinoma. Clin. Cancer Res. 2008, 14, 6839–6846. [Google Scholar] [CrossRef]
- Thaker, P.H.; Lutgendorf, S.K.; Sood, A.K. The Neuroendocrine Impact of Chronic Stress on Cancer. Cell Cycle 2007, 6, 430–433. [Google Scholar] [CrossRef]
- Sobhani, M.E.; Molla Md, A.W.; Rahman, M.S. Review on Biomolecular Basis of the Role of Psychological Stress in the Development and Progression of Cancer. Memo 2010, 3, 136–141. [Google Scholar] [CrossRef]
- Eckerling, A.; Ricon-Becker, I.; Sorski, L.; Sandbank, E.; Ben-Eliyahu, S. Stress and cancer: Mechanisms, significance and future directions. Nat. Rev. Cancer. 2021, 21, 767–785. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Avila, G.; Sommer, B.; Garcia-Hernandez, A.A.; Ramos, C.; Delgado, J.; Vazquez, L.; Gonzalez, R.A.; Sandoval, C.; Flores-Soto, E. Matrix Metalloproteinases and Stress Hormones in Lung Cancer Progression. J. Oncol. 2022, 2022, 5349691. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Misra, S.; Li, M.; Su, J.; Chong, L.P.; McCuske, M.; Williams, J.; Xu, W.; Ghoraie, L.S.; Sutherland, D.R.; et al. Inflammatory Biomarkers, Hematopoietic Stem Cells, and Symptoms in Breast Cancer Patients Undergoing Adjuvant Radiation Therapy. JNCI Cancer Spectr. 2020, 4, pkaa037. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.-D.; Dougados, M.; Goupille, P.; Cantagrel, A.; Meyer, O.; Sibilia, J.; Daurès, J.-P.; Combe, B. Health Assessment Questionnaire Score Is the Best Predictor of 5-Year Quality of Life in Early Rheumatoid Arthritis. J. Rheumatol. 2006, 33, 1936–1941. [Google Scholar] [PubMed]
- Vendrusculo-Fangel, L.M.; Fangel, R.; Vieira de Sousa Neto, I.; Nobrega, O.T.; Dos Reis FJ, J.; Durigan JL, Q.; de Cassia Marqueti, R. Structural Equation Modelling Provides Insights to Understand the Construct of Chronic Pain in Women with Rheumatoid Arthritis. Mod. Rheumatol. 2022, 32, 554–564. [Google Scholar] [CrossRef] [PubMed]
- Durez, P.; Nzeusseu Toukap, A.; Lauwerys, B.R.; Manicourt, D.H.; Verschueren, P.; Westhovens, R.; Devogelaer, J.P.; Houssiau, F.A. A randomised comparative study of the short term clinical and biological effects of intravenous pulse methylprednisolone and infliximab in patients with active rheumatoid arthritis despite methotrexate treatment. Ann. Rheum. Dis. 2004, 63, 1069–1074. [Google Scholar] [CrossRef] [PubMed]
- Murakami, M.; Nishimoto, N. Inflammatory cytokines in rheumatoid arthritis. Clin. Calcium. 2012, 22, 1737–1746. [Google Scholar]
- Vedhara, K.; Miles, J.N.V.; Wetherell, M.A.; Dawe, K.; Searle, A.; Tallon, D.; Cullum, N.; Day, A.; Dayan, C.; Drake, N.; et al. Coping Style and Depression Influence the Healing of Diabetic Foot Ulcers: Observational and Mechanistic Evidence. Diabetologia 2010, 53, 1590–1598. [Google Scholar] [CrossRef]
- Kallis, P.J.; Friedman, A.J. Collagen Powder in Wound Healing. J. Drugs Dermatol. 2018, 17, 403–408. [Google Scholar] [PubMed]
- Lobmann, R.; Zemlin, C.; Motzkau, M.; Reschke, K.; Lehnert, H. Expression of matrix metalloproteinases and growth factors in diabetic foot wounds treated with a protease absorbent dressing. J. Diabetes Complicat. 2006, 20, 329–335. [Google Scholar] [CrossRef]
- Schmidt, J.; Strecker, P.; Kreuz, M.; Löffler, M.; Kiess, W.; Hirsch, C.; Thiery, J.; Baber, R.; Bae, Y.J.; Kratzsch, J.; et al. Stress-Related Hormones in Association with Periodontal Condition in Adolescents-Results of the Epidemiologic LIFE Child Study. Clin. Oral. Investig. 2019, 23, 1793–1802. [Google Scholar] [CrossRef]
- Jung, S.; Gavriiloglou, M.; Séverac, F.; Haumesser, L.; Sayeh, A.; Chatelus, E.; Martin, T.; Huck, O. Influence of systemic sclerosis on periodontal health: A case-control study. J. Clin. Periodontol. 2023, 50, 1348–1359. [Google Scholar] [CrossRef]
- Noack, B.; Kipping, T.; Tervahartiala, T.; Sorsa, T.; Hoffmann, T.; Lorenz, K. Association between serum and oral matrix metalloproteinase-8 levels and periodontal health status. J. Periodontal Res. 2017, 52, 824–831. [Google Scholar] [CrossRef]
- Chaves Filho, A.J.M.; Mottin, M.; Lós, D.B.; Andrade, C.H.; Macedo, D.S. The tetrapartite synapse in neuropsychiatric disorders: Matrix metalloproteinases (MMPs) as promising targets for treatment and rational drug design. Biochimie 2022, 201, 79–99. [Google Scholar] [CrossRef] [PubMed]
- Van Lint, P.; Libert, C. Chemokine cytokine processing by matrix metalloproteinases its effect on leukocyte migration inflammation. J. Leukoc. Biol. 2007, 82, 1375–1381. [Google Scholar] [CrossRef] [PubMed]
- Bitanihirwe, B.K.Y.; Woo, T.U.W. A conceptualized model linking matrix metalloproteinase-9 to schizophrenia pathogenesis. Schizophr. Res. 2020, 218, 28–35. [Google Scholar] [CrossRef]
- Dwir, D.; Giangreco, B.; Xin, L.; Tenenbaum, L.; Cabungcal, J.H.; Steullet, P.; Goupil, A.; Cleusix, M.; Jenni, R.; Chtarto, A.; et al. MMP9/RAGE pathway overactivation mediates redox dysregulation and neuroinflammation, leading to inhibitory/excitatory imbalance: A reverse translation study in schizophrenia patients. Mol. Psychiatr. 2020, 25, 2889–2904. [Google Scholar] [CrossRef]
- Koskinen, M.K.; Mourik, Y.; van Smit, A.B.; Riga, D.; Spijker, S. From stress to depression: Development of extracellular matrix-dependent cognitive impairment following social stress. Sci. Rep. 2020, 10, 17308. [Google Scholar] [CrossRef]
- Domenici, E.; Willé, D.R.; Tozzi, F.; Prokopenko, I.; Miller, S.; McKeown, A.; Brittain, C.; Rujescu, D.; Giegling, I.; Truck, C.W.; et al. Plasma protein biomarkers for depression and schizophrenia by multi analyte profiling of case-control collections. PLoS ONE. 2010, 5, e9166. [Google Scholar] [CrossRef] [PubMed]
- Bobi’nska, K.; Szemraj, J.; Czarny, P.; Gałecki, P. Role of MMP-2, MMP-7, MMP-9 and TIMP-2 in the development of recurrent depressive disorder. J. Affect. Disord. 2016, 205, 119–129. [Google Scholar] [CrossRef]
- Chandrasekaran, V.; Subramanian, K.; Kattimani, S.; Nandheesha, H.; Sarkar, S.; Penchilaiya, V. Relationship between matrix metalloproteinase-9 and lifetime history of suicidal behavior in remitted patients with bipolar I disorder: A cross-sectional pilot study. Indian J. Psychol. Med. 2020, 42, 200–202. [Google Scholar] [CrossRef] [PubMed]
- Hagerman, R.J.; Berry-Kravis, E.; Hazlett, H.C.; Bailey, D.B., Jr.; Moine, H.; Kooy, R.F.; Tassone, F.; Gantois, I.; Sonenberg, N.; Mandel, J.L.; et al. Fragile X syndrome. Nat. Rev. Dis. Primers 2017, 3, 17065. [Google Scholar] [CrossRef] [PubMed]
- Shigemori, Y.; Katayama, Y.; Mori, T.; Maeda, T.; Kawamata, T. Matrix metalloproteinase-9 is associated with blood-brain barrier opening and brain edema formation after cortical contusion in rats. Acta Neurochir. 2006, 96, 130–133. [Google Scholar]
- Lee, I.T.; Lin, C.C.; Wu, Y.C.; Yang, C.M. TNF-alpha induces matrix metalloproteinase-9 expression in A549 cells: Role of TNFR1/TRAF2/PKC-alpha-dependent signaling pathways. J. Cell. Physiol. 2010, 224, 454–464. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.L. Matrix metalloproteinases: Influence on smooth muscle cells and atherosclerotic plaque stability. Expert Rev. Cardiovasc. Ther. 2007, 5, 265–282. [Google Scholar] [CrossRef]
- Galis, Z.S.; Sukhova, G.K.; Lark, M.W.; Libby, P. Increased expression of matrix metalloproteinases and matrix degrading activity in vulnerable regions of human atherosclerotic plaques. J. Clin. Investig. 1994, 94, 2493–2503. [Google Scholar] [CrossRef]
- Yabluchanskiy, A.; Ma, Y.; Iyer, R.P.; Hall, M.E.; Lindsey, M.L. Matrix metalloproteinase-9: Many shades of function in cardiovascular disease. Physiology 2013, 28, 391–403. [Google Scholar] [CrossRef] [PubMed]
- Noji, Y.; Kajinami, K.; Kawashiri, M.A.; Todo, Y.; Horita, T.; Nohara, A.; Higashikata, T.; Inazu, A.; Koizumi, J.; Takegoshi, T.; et al. Circulating matrix metalloproteinases and their inhibitors in premature coronary atherosclerosis. Clin. Chem. Lab. Med. 2001, 39, 380–384. [Google Scholar] [CrossRef]
- Inokubo, Y.; Hanada, H.; Ishizaka, H.; Fukushi, T.; Kamada, T.; Okumura, K. Plasma levels of matrix metalloproteinase- 9 and tissue inhibitor of metalloproteinase-1 are increased in the coronary circulation in patients with acute coronary syndrome. Am. Heart J. 2001, 141, 211–217. [Google Scholar] [CrossRef]
- Landskron, G.; De la Fuente, M.; Thuwajit, P.; Thuwajit, C.; Hermoso, M.A. Chronic Inflammation and Cytokines in the Tumor Microenvironment. J. Immunol. Res. 2014, 2014, 149185. [Google Scholar] [CrossRef]
- Walker, C.; Mojares, E.; del Río Hernández, A. Role of Extracellular Matrix in Development and Cancer Progression. Int. J. Mol. Sci. 2018, 19, 3028. [Google Scholar] [CrossRef] [PubMed]
- Al-Alem, L.; Curry, T.E., Jr. Ovarian cancer: Involvement of the matrix metalloproteinases. Reproduction 2015, 150, R55–R64. [Google Scholar] [CrossRef] [PubMed]
- Merchant, N.; Nagaraju, G.P.; Rajitha, B.; Lammata, S.; Jella, K.K.; Buchwald, Z.S.; Lakka, S.S.; Ali, A.N. Matrix metalloproteinases: Their functional role in lung cancer. Carcinogenesis 2017, 38, 766–780. [Google Scholar] [CrossRef] [PubMed]
- Radisky, E.S.; Radisky, D.C. Matrix metalloproteinases as breast cancer drivers and therapeutic targets. Front. Biosci. 2015, 20, 1144–1163. [Google Scholar] [CrossRef] [PubMed]
- Mittal, R.; Patel, A.P.; Debs, L.H.; Nguyen, D.; Patel, K.; Grati, M.; Mittal, J.; Yan, D.; Chapagain, P.; Liu, X.Z. Intricate Functions of Matrix Metalloproteinases in Physiological and Pathological Conditions. J. Cell. Physiol. 2016, 231, 2599–2621. [Google Scholar] [CrossRef] [PubMed]
- Bian, Y.; Xiang, Z.; Wang, Y.; Ren, Q.; Chen, G.; Xiang, B.; Wang, J.; Zhang, C.; Pei, S.; Guo, S.; et al. Immunomodulatory Roles of Metalloproteinases in Rheumatoid Arthritis. Front. Pharmacol. 2023, 14, 1285455. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.T.; Wang, J.Y. Specialized surface protrusions of invasive cells, invadopodia and lamellipodia, have differential MT1-MMP, MMP-2, and TIMP-2 localization. Ann. N. Y. Acad. Sci. 1999, 878, 361–371. [Google Scholar] [CrossRef]
- Fu, K.; Zheng, X.; Chen, Y.; Wu, L.; Yang, Z.; Chen, X.; Song, W. Role of Matrix Metalloproteinases in Diabetic Foot Ulcers: Potential Therapeutic Targets. Front. Pharmacol. 2022, 13, 1050630. [Google Scholar] [CrossRef]
- Kandhwal, M.; Behl, T.; Singh, S.; Sharma, N.; Arora, S.; Bhatia, S.; Al-Harrasi, A.; Sachdeva, M.; Bungau, S. Role of matrix metalloproteinase in wound healing. Am. J. Transl. Res. 2022, 14, 4391–4405. [Google Scholar]
- Darby, I.A.; Laverdet, B.; Bonté, F.; Desmoulière, A. Fibroblasts and myofibroblasts in wound healing. Clin. Cosmet. Investig. Dermatol. 2014, 7, 301–311. [Google Scholar] [CrossRef]
- Zhang, W.-Q.; Tang, W.; Hu, S.-Q.; Fu, X.-L.; Wu, H.; Shen, W.-Q.; Chen, H.-L. Effect of matrix metalloproteinases on the healing of diabetic foot ulcer: A systematic review. J. Tissue Viability 2023, 32, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Luchian, I.; Goriuc, A.; Sandu, D.; Covasa, M. The Role of Matrix Metalloproteinases (MMP-8, MMP-9, MMP-13) in Periodontal and Peri-Implant Pathological Processes. Int. J. Mol. Sci. 2022, 23, 1806. [Google Scholar] [CrossRef] [PubMed]
- Verstappen, J.; Von den Hoff, J.W. Tissue Inhibitors of Metalloproteinases (TIMPs): Their Biological Functions and Involvement in Oral Disease. J. Dent. Res. 2006, 85, 1074–1084. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Yue, Y.; Tian, Y.; Li, J.; Wang, M.; Liang, H.; Liao, P.; Loo, W.T.Y.; Cheung, M.N.B.; Chow, L.W.C. Association of Matrix Metalloproteinase (MMP)-1, 3, 9, Interleukin (IL)-2, 8 and Cyclooxygenase (COX)-2 Gene Polymorphisms with Chronic Periodontitis in a Chinese Population. Cytokine 2012, 60, 552–560. [Google Scholar] [CrossRef] [PubMed]
- Niitsu, T.; Ishima, T.; Yoshida, T.; Hashimoto, T.; Matsuzawa, D.; Shirayama, Y.; Nakazato, M.; Shimizu, E.; Hashimoto, K.; Iyo, M. A positive correlation between serum levels of mature brain-derived neurotrophic factor and negative symptoms in schizophrenia. Psychiatry Res. 2014, 215, 268–273. [Google Scholar] [CrossRef] [PubMed]
- WHO. Depression Fact Sheet; World Health Organization: Geneva, Switzerland, 2015; Available online: http://www.who.int/mediacentre/factsheets/fs369/en/ (accessed on 11 November 2023).
- Gao, J.; Yi, H.; Tang, X.; Feng, X.; Yu, M.; Sha, W.; Wang, X.; Zhang, X.; Zhang, X. DNA Methylation and Gene Expression of Matrix Metalloproteinase 9 Gene in Deficit and Non-deficit Schizophrenia. Front. Genet. 2020, 11, 823. [Google Scholar] [CrossRef]
- Feigenson, K.A.; Kusnecov, A.W.; Silverstein, S.M. Inflammation and the two hit hypothesis of schizophrenia. Neurosci. Biobehav. Rev. 2014, 38, 72. [Google Scholar] [CrossRef] [PubMed]
- Reinhard, S.M.; Razak, K.; Ethell, I.M. A delicate balance: Role of MMP-9 in brain development and pathophysiology of neurodevelopmental disorders. Front. Cell Neurosci. 2015, 9, 280. [Google Scholar] [CrossRef]
- Lord, J.R.; Mashayekhi, F.; Salehi, Z. How matrix metalloproteinase (MMP)- 9(rs3918242) polymorphism affects MMP-9 serum concentration and associates with autism spectrum disorders: A case-control study in Iranian population. Dev. Psychopathol. 2021, 1, e7. [Google Scholar] [CrossRef]
- Abbeduto, L.; McDuffie, A.; Thurman, A.J. The fragile X syndrome-autism comorbidity: What do we really know? Front. Genet. 2014, 5, 355. [Google Scholar] [CrossRef]
- World Health Organization. Depression and Other Common Mental Disorders: Global Health Estimates; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Yoshida, T.; Ishikawa, M.; Niitsu, T.; Nakazato, M.; Watanabe, H.; Shiraishi, T.; Shiina, A.; Hashimoto, T.; Kanahara, N.; Hasegawa, T.; et al. Correction: Decreased serum levels of mature brain-derived neurotrophic factor (BDNF), but not its precursor proBDNF, in patients with major depressive disorder. PLoS ONE 2013, 8. [Google Scholar] [CrossRef]
- Vogelzangs, N.; de Jonge, P.; Smit, J.H.; Bahn, S.; Penninx, B.W. Cytokine production capacity in depression and anxiety. Transl. Psychiatry 2016, 6, e825. [Google Scholar] [CrossRef] [PubMed]
- van der Kooij, M.A.; Fantin, M.; Rejmak, E.; Grosse, J.; Zanoletti, O.; Fournier, C.; Ganguly, K.; Kalita, K.; Kaczmarek, L.; Sandi, C. Role for MMP-9 in stress-induced downregulation of nectin-3 in hippocampal CA1 and associated behavioural alterations. Nat. Commun. 2014, 5, 4995. [Google Scholar] [CrossRef] [PubMed]
- Peterson, P.N. JAHA Spotlight on Psychosocial Factors and Cardiovascular Disease. J. Am. Heart Assoc. 2020, 9, e017112. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, A.K.; Jönsson, S.; Stenmark, J.; Kristenson, M.; Jonasson, L. Stress-induced release of matrix metalloproteinase-9 in patients with coronary artery disease: The possible influence of cortisol. Psychoneuroendocrinology 2016, 73, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Szymanowski, A.; Nijm, J.; Kristenson, M.; Jonasson, L. Elevated Levels of Circulating Matrix Metalloproteinase-9 Are Associated with a Dysregulated Cortisol Rhythm—A Case-Control Study of Coronary Artery Disease. Psychoneuroendocrinology 2011, 36, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Hassan, A.A. Psychological Affection in Rheumatoid Arthritis Patients in Relation to Disease Activity. Medicine 2019, 98, e15373. [Google Scholar] [CrossRef] [PubMed]
- Lwin, M.N.; Serhal, L.; Holroyd, C.; Edwards, C.J. Rheumatoid Arthritis: The Impact of Mental Health on Disease: A Narrative Review. Rheumatol. Ther. 2020, 7, 457–471. [Google Scholar] [CrossRef]
- Liu, Y.; Tian, S.; Ning, B.; Huang, T.; Li, Y.; Wei, Y. Stress and cancer: The mechanisms of immune dysregulation and management. Front. Immunol. 2022, 13, 1032294. [Google Scholar] [CrossRef]
- Dai, S.; Mo, Y.; Wang, Y.; Xiang, B.; Liao, Q.; Zhou, M.; Li, X.; Li, Y.; Xiong, W.; Li, G.; et al. Chronic Stress Promotes Cancer Development. Front. Oncol. 2020, 10, 1492. [Google Scholar] [CrossRef]
- Gonzalez-Avila, G.; Delgado, J.; Mendoza-Posada, D.A.; Sommer, B.; Ramos, C.; Aquino-Galvez, A.; Camacho, C. Differences in plasma MMPs and TIMPs protein expression and chemotherapy response in patients with tobacco- or wood-smoke-induced lung cancer. Respiration 2013, 85, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Barre, P.V.; Padmaja, G.; Rana, S.; Tiamongla. Stress and Quality of Life in Cancer Patients: Medical and Psychological Intervention. Indian J. Psychol. Med. 2018, 40, 232–238. [Google Scholar] [CrossRef]
- Ball, H.; Moore, S.; Leary, A. A systematic literature review comparing the psychological care needs of patients with mesothelioma and advanced lung cancer. Eur. J. Oncol. Nurs. 2016, 25, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Niedzwiedz, C.L.; Knifton, L.; Robb, K.A.; Katikireddi, S.V.; Smith, D.J. Depression and anxiety among people living with and beyond cancer: A growing clinical and research priority. BMC Cancer 2019, 19, 943. [Google Scholar] [CrossRef] [PubMed]
- Ayuk, S.M.; Abrahamse, H.; Houreld, N.N. The Role of Matrix Metalloproteinases in Diabetic Wound Healing in relation to Photobiomodulation. J. Diabetes Res. 2016, 2016, 2897656. [Google Scholar] [CrossRef] [PubMed]
- Jones, J. Stress responses, pressure ulcer development and adaptation. Br. J. Nurs. 2003, 12 (Suppl. S11), S17–S18, S20, S22. [Google Scholar] [CrossRef]
- Charalambous, C.; Vassilopoulos, A.; Koulouri, A.; Eleni, S.; Popi, S.; Antonis, F.; Pitsilidou, M.; Roupa, Z. The Impact of Stress on Pressure Ulcer Wound Healing Process and on the Psychophysiological Environment of the Individual Suffering from them. Med. Arch. 2018, 72, 362–366. [Google Scholar] [CrossRef]
- Proctor, G.B. The physiology of salivary secretion. Periodontol. 2000 2016, 70, 11–25. [Google Scholar] [CrossRef]
- Sorsa, T.; Tervahartiala, T.; Leppilahti, J.; Hernandez, M.; Gamonal, J.; Tuomainen, A.M.; Lauhio, A.; Pussinen, P.J.; Mäntylä, P. Collagenase-2 (MMP-8) as a point-of-care biomarker in periodontitis and cardiovascular diseases. Therapeutic response to non-antimicrobial properties of tetracyclines. Pharmacol. Res. 2011, 63, 108–113. [Google Scholar] [CrossRef]
- Goyal, S.; Gupta, G.; Thomas, B.; Bhat, K.M.; Bhat, G.S. Stress and periodontal disease: The link and logic!! Ind. Psychiatry J. 2013, 22, 4–11. [Google Scholar] [CrossRef]
- Schoultz, M.; Beattie, M.; Gorely, T.; Leung, J. Assessment of causal link between psychological factors and symptom exacerbation in inflammatory bowel disease: A systematic review utilising Bradford Hill criteria and meta-analysis of prospective cohort studies. Syst. Rev. 2020, 9, 169. [Google Scholar] [CrossRef]
- Joyce, B.; Hou, L. Telomere Length: The Intersection of Sociology, Molecular Biology, and Human Disease. EBioMedicine 2016, 11, 27–28. [Google Scholar] [CrossRef]
- Carroll, A.; Stokes, D.; Darley, A. Use of complexity theory in health and social care: A scoping review protocol. BMJ Open 2021, 11, e047633. [Google Scholar] [CrossRef]
- Cabral, M.d.F.C.T.; Viana, A.L.; Gontijo, D.T. Use of the complexity paradigm in the field of health: Scope review. Escola Anna Nery 2020, 24, e20190235. [Google Scholar]
- Fajardo-Ortiz, G.; Fernández-Ortega, M.Á.; Ortiz-Montalvo, A.; Olivares-Santos, R.A. The dimension of the paradigm of complexity in health systems. Cir. Cir. 2015, 83, 81–86. [Google Scholar] [CrossRef]
- Institute of Medicine (US) Committee on Health and Behavior: Research, Practice, and Policy. Health and Behavior: The Interplay of Biological, Behavioral, and Societal Influences; Social Risk Factors; National Academies Press: Washington, DC, USA, 2001. Available online: https://www.ncbi.nlm.nih.gov/books/NBK43750/ (accessed on 11 November 2023).
- Serra, R.; Ielapi, N.; Barbetta, A.; Andreucci, M.; de Franciscis, S. Novel biomarkers for cardiovascular risk. Biomark. Med. 2018, 12, 1015–1024. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.H.; Di, Y.P. Matrix metallopeptidase-gene signature predicts stage I lung adenocarcinoma survival outcome. Int. J. Mol. Sci. 2023, 24, 2382. [Google Scholar] [CrossRef] [PubMed]
- Shin, E.K.; Mahajan, R.; Akbilgic, O.; Shaban-Nejad, A. Sociomarkers and biomarkers: Predictive modeling in identifying pediatric asthma patients at risk of hospital revisits. NPJ Digit. Med. 2018, 1, 50. [Google Scholar] [CrossRef] [PubMed]
- Ielapi, N.; Andreucci, M.; Licastro, N.; Faga, T.; Grande, R.; Buffone, G.; Mellace, S.; Sapienza, P.; Serra, R. Precision Medicine and precision nursing: The Era of biomarkers and precision Health. Int. J. Gen. Med. 2020, 13, 1705–1711. [Google Scholar] [CrossRef]
- Institute of Medicine (US) Committee on Capitalizing on Social Science and Behavioral Research to Improve the Public’s Health; Smedley, B.D.; Syme, S.L. (Eds.) Promoting Health: Intervention Strategies from Social and Behavioral Research; PAPER CONTRIBUTION F, Behavioral and Social Science Contributions to the Health of Adults in the United States; National Academies Press: Washington, DC, USA, 2000. Available online: https://www.ncbi.nlm.nih.gov/books/NBK222825/ (accessed on 11 November 2023).

| Keywords | Scopus | Web of Science | PubMed |
|---|---|---|---|
| Metalloproteinase * and social * | 324 | 875 | 319 |
| Metalloproteinase * and social aspect * | 40 | 15 | 43 |
| Metalloproteinase * and social determinant * of health | 56 | 209 | 8450 |
| Metalloproteinase * and psychosocial factor * | 38 | 26 | 909 |
| Parameter | Inclusion Criteria | Exclusion Criteria |
|---|---|---|
| Patient | Human patients | Non-human patients |
| Intervention | To analyze the relationship between MMPs, disease, and psychosocial aspects | To analyze only aspects of MMPs (biological, medical, etc.) without the relationship with disease and psychosocial impacts |
| Comparator | None | None |
| Outcomes | To underline the interconnection between MMPs and the psychosocial sphere with certain results. | Models, articles, or tools not focused on the complex relationship between MMPs, diseases, and psychosocial aspects |
| Timeframe | Unrestricted (Final extraction: October 2023) | Unrestricted (Final extraction: October 2023) |
| Study Type | Research articles and review articles published in academic journals | Book chapters, book reviews, vignette studies, supplements, study protocols, commentaries, guidelines, editorials, book, meeting abstract, letter to editors |
| Language | English | Non-English |
| Authors | Title | Year | Country | Methods/Study Design | Population | Study Content |
|---|---|---|---|---|---|---|
| Rahimi S. et al. [76] | Blood evaluation of the expression levels of matrix metalloproteinase 9 (MMP9) and its natural inhibitor, TIMP1 genes in Iranian schizophrenic patients | 2017 | Iran | Experimental study | 50 patients with schizophrenia and 50 healthy controls | The MMP9/TIMP1 expression ratio of MMP9/TIP1 is significantly higher in schizophrenic patients compared with healthy subjects |
| Li H. et al. [77] | Matrix metalloprotein ase-9 as an important contributor to the pathophysiology of depression | 2022 | China | Systematic review | None | To review the literature characterizing the negative effect of MMP-9 on depression |
| Abdallah M.W. et al. [78] | Matrix metalloproteinases in autism spectrum disorders | 2013 | Germany | Systematic review | None | To focus on the mechanisms through which MMPs can contribute to autism spectrum disorder |
| Razak K.A. et al. [79] | Neural Correlates of Auditory Hypersensitivity in Fragile X Syndrome | 2021 | USA | Systematic review | None | To examine the etiopathological mechanisms of the auditory system in Fragile X Syndrome, a genetic factor linked to behavior associated with autism spectrum disorders |
| Fernandez Machulsky N. et al. [80] | Matrix metalloproteinases and psychosocial factors in patients with acute coronary syndrome patients | 2016 | Argentina | Prospective study | 76 patients after angioplasty | To examine the relationships between hostility, perceived stress, and social support and their impact on MMP-2 and MMP-9 activity, pro-inflammatory cytokines, and hemodynamic factors in patients diagnosed with acute coronary syndrome |
| Garvin P. et al. [81] | Plasma levels of matrix metalloproteinase-9 are independently associated with psychosocial factors in a middle-aged normal population | 2009 | Sweden | Prospective study | 402 participants from a normal population | To examine the potential correlation between psychosocial factors and circulating levels of MMP-9 while considering comorbidities and traditional risk factors for cardiovascular disease |
| Jonsson S. et al. [82] | Overexpression of MMP-9 and its inhibitors in blood mononuclear cells after myocardial infarction—Is It Associated with Depressive Symptomatology? | 2014 | Sweden | Observational study | 57 patients after myocardial infarction and 41 healthy controls | To examine the potential link between levels of MMP-9 and depressive symptoms in a middle-aged normal population from Sweden following a myocardial infarction |
| Shafi B.H. et al. [83] | Socioeconomic disparity in cardiovascular disease: Possible biological pathways based on a proteomic approach | 2022 | Denmark | Experimental study | 1142 participants derived from the Copenhagen Heart Study | To analyze the social inequality in cardiovascular health by linking proteomic factors to socioeconomic position (SEP) in patients with cardiovascular disease |
| Lutgendorf S.K. et al. [84] | Biobehavioral Influences on Matrix Metalloproteinase Expression in Ovarian Carcinoma | 2008 | USA | Experimental study | 56 ovarian cancer patients | To focus on the link between behavioral distress and higher levels of MMPs produced by ovarian cancer |
| Thaker P.H. et al. [85] | The neuroendocrine impact of chronic stress on cancer | 2007 | USA | Systematic review | None | Neuroendocrine influence on cancer, how ovarian cancer progression results from the stress response, and the mechanisms by which β-adrenergic receptors promote tumor growth on ovarian cancer cells |
| Sobhani M.E. et al. [86] | A review on biomolecular basis of the role of psychological stress in cancer development and progres-sion of cancer | 2010 | Bangladesh | Systematic review | None | Review on the role of psychological stress and immune response in the progression of cancer |
| Eckerling A. et al. [87] | Stress and cancer: mechanisms, significance and future directions | 2021 | Israel | Systematic review | None | To describe the mechanisms that determine the correlation between cancer and stress |
| Gonzalez-Avila et al. [88] | Matrix Metalloproteinases and Stress Hormones in Lung Cancer Progression | 2022 | Mexico | Observational study | 104 patients with lung adenocarcinoma | To determine blood levels of MMPs and stress hormones in lung adenocarcinoma patients |
| Shi W. et al. [89] | Inflammatory Biomarkers, Hematopoietic Stem Cells, and symptoms in Breast Cancer Patients Undergoing Adjuvant Radiation Therapy | 2020 | USA | Longitudinal study | 147 patients with a diagnosis of breast cancer | Evaluation of psychological symptoms, cytokines, and MMPs in patients with breast cancer |
| Cohen J.D. et al. [90] | Health assessment questionnaire score is the best predictor of 5-year quality of life in early rheumatoid arthritis | 2006 | France | Prospective study | 191 patients followed over 5 years | The baseline health assessment questionnaire score proved to be the most accurate predictor of the 5-year quality of life in patients with early rheumatoid arthritis |
| Vendrusculo-Fangel L.M. et al. [91] | Structural equation modeling provides insights into understanding the construct of chronic pain in women with rheumatoid arthritis | 2021 | Brazil | Observational and cross-sectional study | 42 women with rheumatoid arthritis (RA) and 42 women without RA | To describe the relationships between RA disease and biopsychosocial aspects: acceptance of pain and quality of life in women with RA |
| Durez P. et al. [92] | A randomised comparative study of the short-term clinical and biological effects of intravenous pulse methylprednisolone and infliximab in patients with active rheumatoid arthritis despite methotrexate treatment | 2003 | Belgium | Randomized comparative study | 27 patients with severe RA | Comparison of treatment with methylprednisolone and infliximab for patients with rheumatoid arthritis to improve their quality of life |
| Murakami M. et al. [93] | Inflammatory cytokines in rheumatoid arthritis | 2012 | Japan | Review | None | Analysis of cytokines and MMPs involved in the progression of rheumatoid arthritis |
| Vedhara K. et al. [94] | Coping style and depression influence the healing of diabetic foot ulcers: observational and mechanistic evidence | 2010 | UK | Prospective and observational study | 93 patients with diabetic foot ulcer | In order to comprehend the impact of psychological distress on the healing process of diabetic foot ulcers, it is essential to analyze the biological mechanisms that influence the healing process |
| Kellis P.J. et al. [95] | Collagen Powder in Wound Healing | 2018 | Washington | Review | None | To discuss the use of collagen in the healing process of chronic wounds, which represents a problem in their management due to high costs, low quality of life, and significant morbidity and mortality |
| Lobmann R. et al. [96] | Expression of matrix metalloproteinases and growth factors in diabetic foot wounds treated with a protease absorbent dressing | 2006 | Germany | Observational study | 33 patients with chronic diabetic foot ulcer | To analyze the effects of a protease inhibitor in chronic diabetic foot ulcer |
| Schmidt J. et al. [97] | Stress-related hormones in association with periodontal disease in adolescents results of the epidemiologic LIFE Child study | 2019 | Germany | Observational study | 498 adolescents with early signs of periodontal disease | To examine the correlation between stress-related hormone levels, inflammatory markers, and early indicators of periodontal disease in children and adolescents. |
| Jung S. [98] | Influence of systemic sclerosis on periodontal health: A case-control study | 2023 | France | case–control study | 39 patients and 30 controls | Evaluation of oral health and quality of life in patients with systemic sclerosis |
| Noack B. et al. [99] | Association between serum and oral matrix metalloproteinase-8 levels and periodontal health status | 2017 | Germany | Cross-sectional study | 59 subjects: 19 healthy controls, 20 with gingivitis, and 20 with periodontal disease | Correlation between serum aMMP-9, oral MMP-8, and periodontal health |
| Name of Journal | Number of Articles | Research Areas |
|---|---|---|
| ATHEROSCLEROSIS | 1 | Cardiovascular system and cardiology |
| CELL CYCLE | 1 | Cell biology |
| CLINICAL CANCER RESEARCH | 1 | Oncology |
| CLINICAL ORAL INVESTIGATIONS | 1 | Dentistry, oral surgery, and medicine |
| DIABETOLOGIA | 1 | Endocrinology and metabolism |
| FRONTIERS IN NEUROLOGY | 1 | Neuroscience and neurology |
| FRONTIERS IN PSYCHIATRY | 1 | Psychiatry |
| JOURNAL OF MOLECULAR PSYCHIATRY | 1 | Psychiatry and neuropsychiatry |
| MAGAZINE OF EUROPEAN MEDICAL ONCOLOGY | 1 | Oncology |
| METABOLIC BRAIN DISEASE | 1 | Endocrinology and metabolism; neuroscience and neurology |
| MODERN RHEUMATOLOGY | 1 | Rheumatology |
| PLOS ONE | 1 | Cardiology |
| PSYCHONEUROENDOCRINOLOGY | 1 | Endocrinology and metabolism; neuroscience & neurology |
| PSYCHOSOMATIC MEDICINE | 1 | Psychiatry and psychology |
| THE JOURNAL OF RHEUMATOLOGY | 1 | Rheumatology |
| NATURE REVIEW CANCER | 1 | Oncology |
| JOURNAL OF ONCOLOGY | 1 | Oncology |
| JNCI CANCER SPECTRUM | 1 | Oncology and cancer |
| ANNALS OF RHEUMATIC DISEASE | 1 | Rheumatology |
| CLINICAL CALCIUM | 1 | Rheumatology and osteoporosis |
| JOURNAL OF DRUGS IN DERMATOLOGY | 1 | Dermatology and wound healing |
| JOURNAL OF DIABETES AND ITS COMPLICATIONS | 1 | Diabetes, wound healing |
| JOURNAL OF CLINICAL PERIODONTOLOGY | 1 | Periodontal disease |
| JOURNAL OF PERIODONTAL DISEASE | 1 | Periodontal disease |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costa, D.; Scalise, E.; Ielapi, N.; Bracale, U.M.; Andreucci, M.; Serra, R. Metalloproteinases as Biomarkers and Sociomarkers in Human Health and Disease. Biomolecules 2024, 14, 96. https://doi.org/10.3390/biom14010096
Costa D, Scalise E, Ielapi N, Bracale UM, Andreucci M, Serra R. Metalloproteinases as Biomarkers and Sociomarkers in Human Health and Disease. Biomolecules. 2024; 14(1):96. https://doi.org/10.3390/biom14010096
Chicago/Turabian StyleCosta, Davide, Enrica Scalise, Nicola Ielapi, Umberto Marcello Bracale, Michele Andreucci, and Raffaele Serra. 2024. "Metalloproteinases as Biomarkers and Sociomarkers in Human Health and Disease" Biomolecules 14, no. 1: 96. https://doi.org/10.3390/biom14010096
APA StyleCosta, D., Scalise, E., Ielapi, N., Bracale, U. M., Andreucci, M., & Serra, R. (2024). Metalloproteinases as Biomarkers and Sociomarkers in Human Health and Disease. Biomolecules, 14(1), 96. https://doi.org/10.3390/biom14010096








