Multipurpose Iron-Chelating Ligands Inspired by Bioavailable Molecules
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Synthesis
2.3. Solution Equilibrium Studies
2.4. Cyclic Voltammetry Studies
3. Results
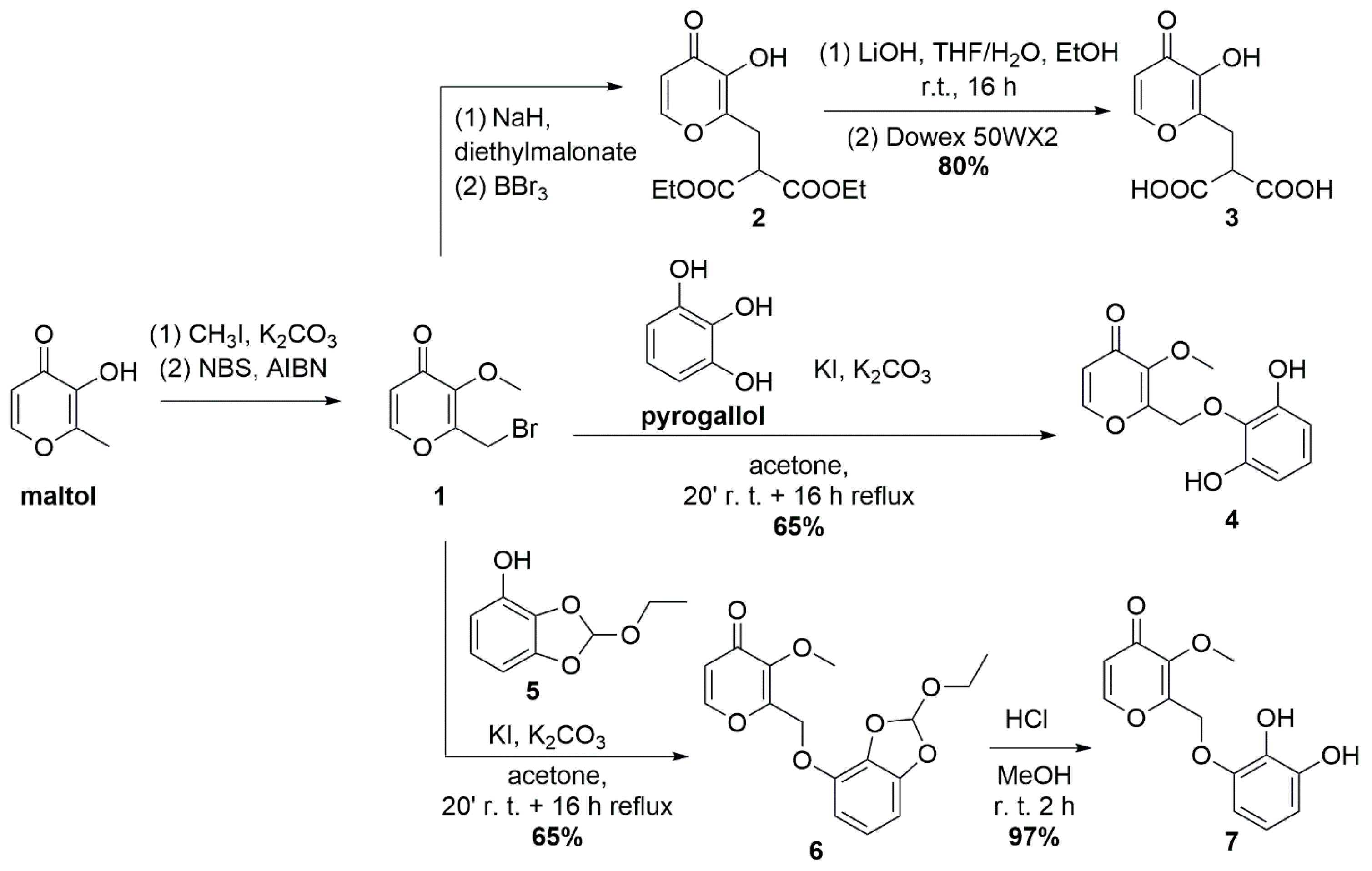
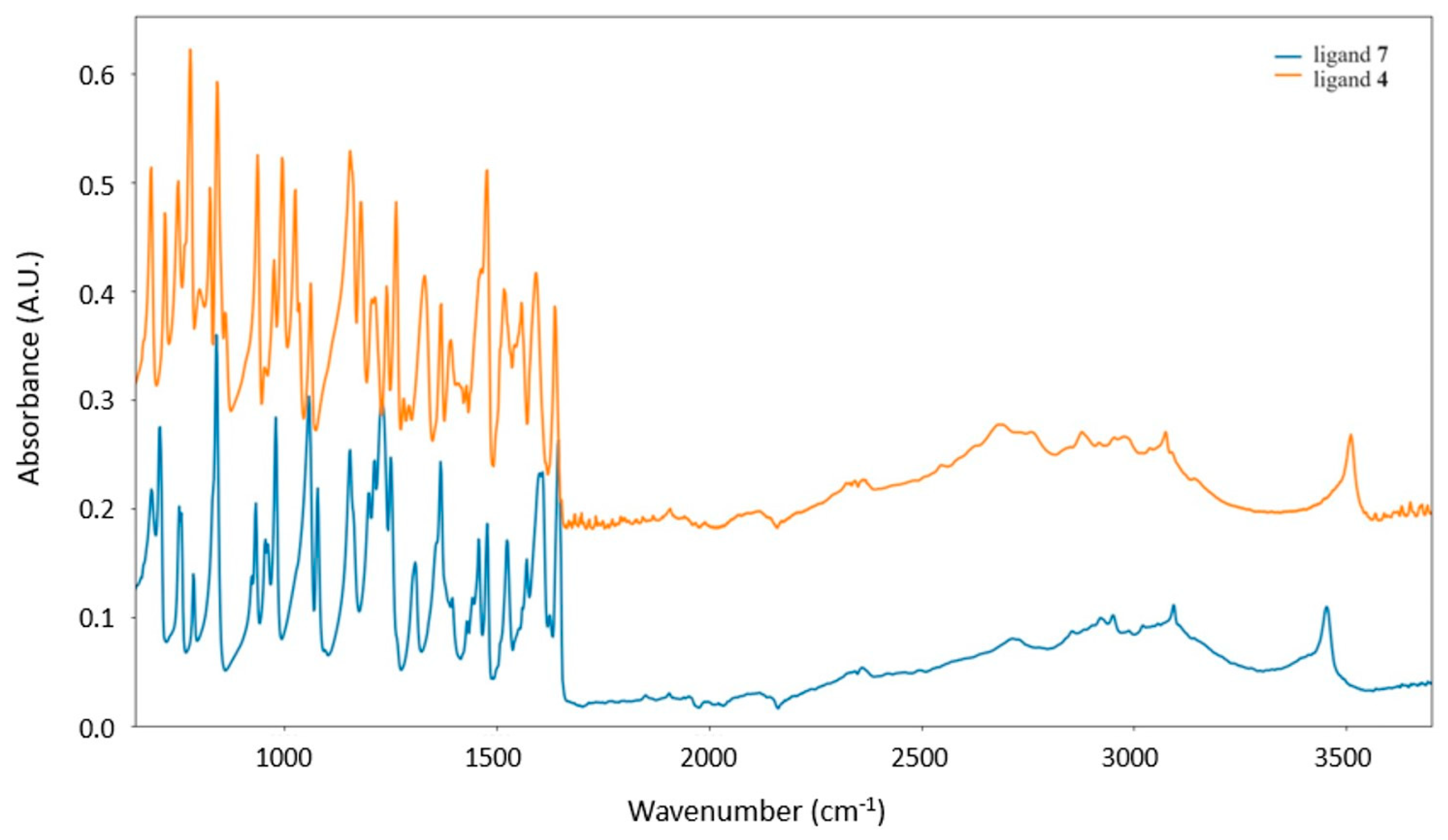
3.1. Synthesis and ATR-Characterization
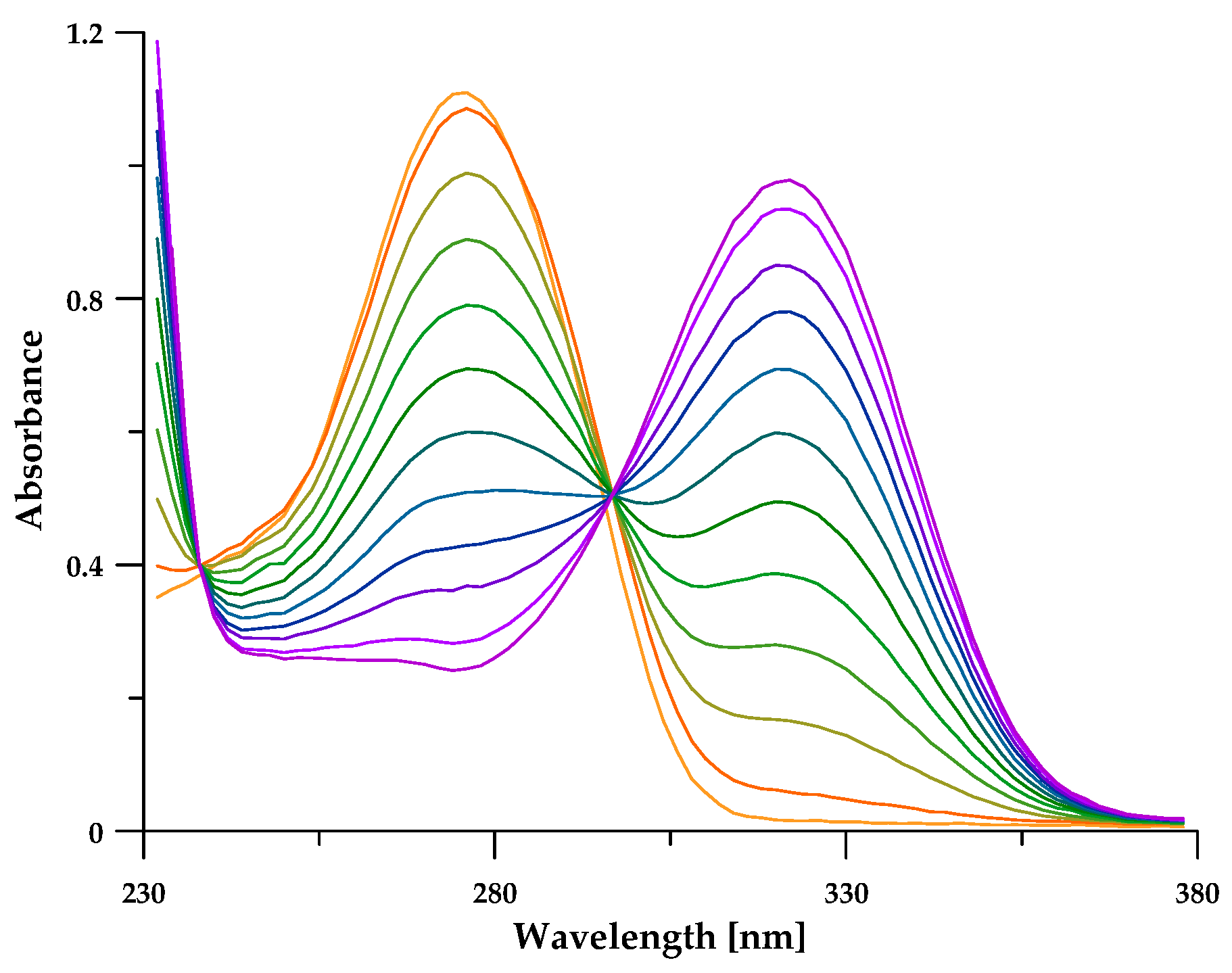
3.2. Protonation Equilibria
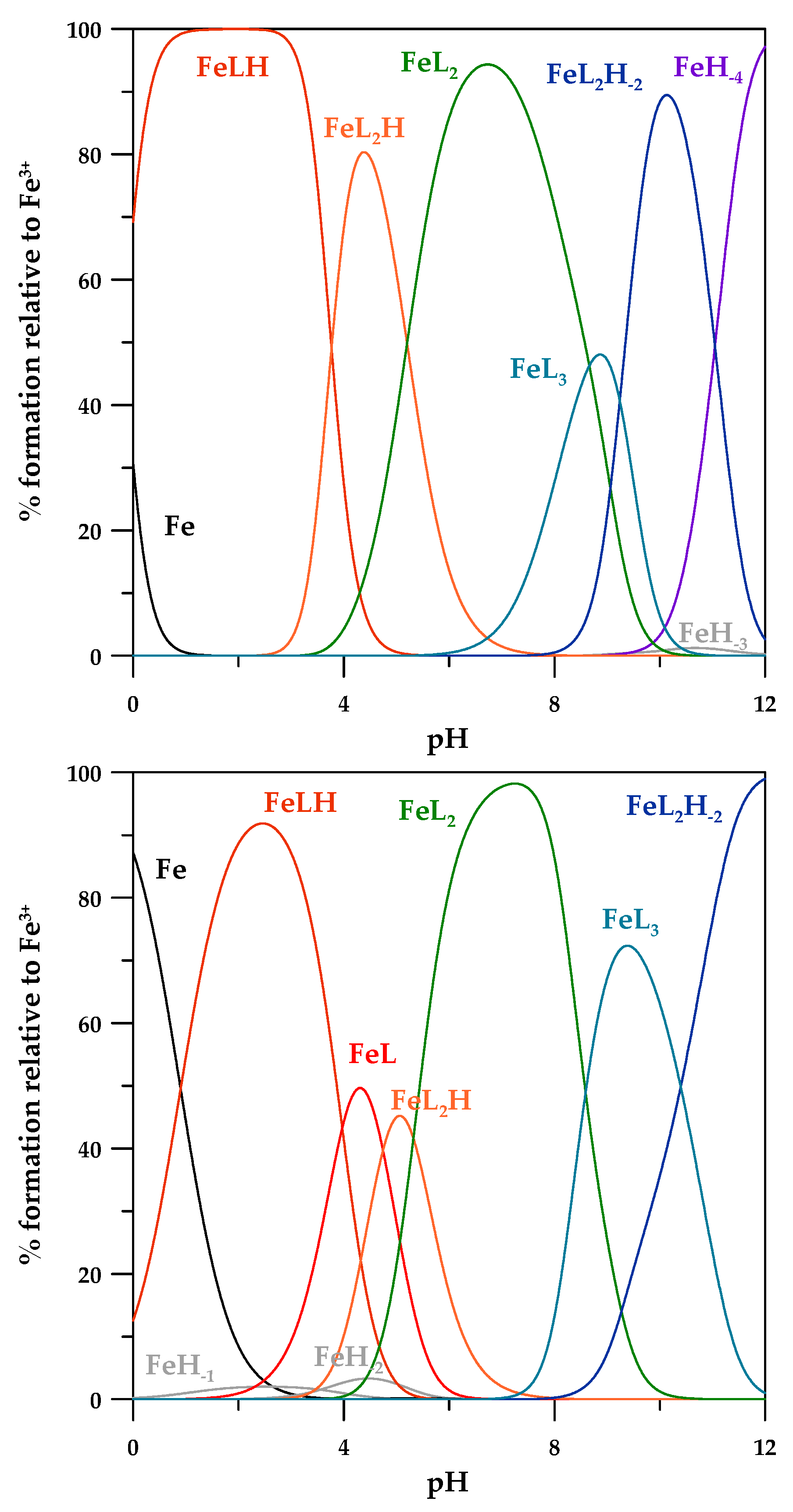
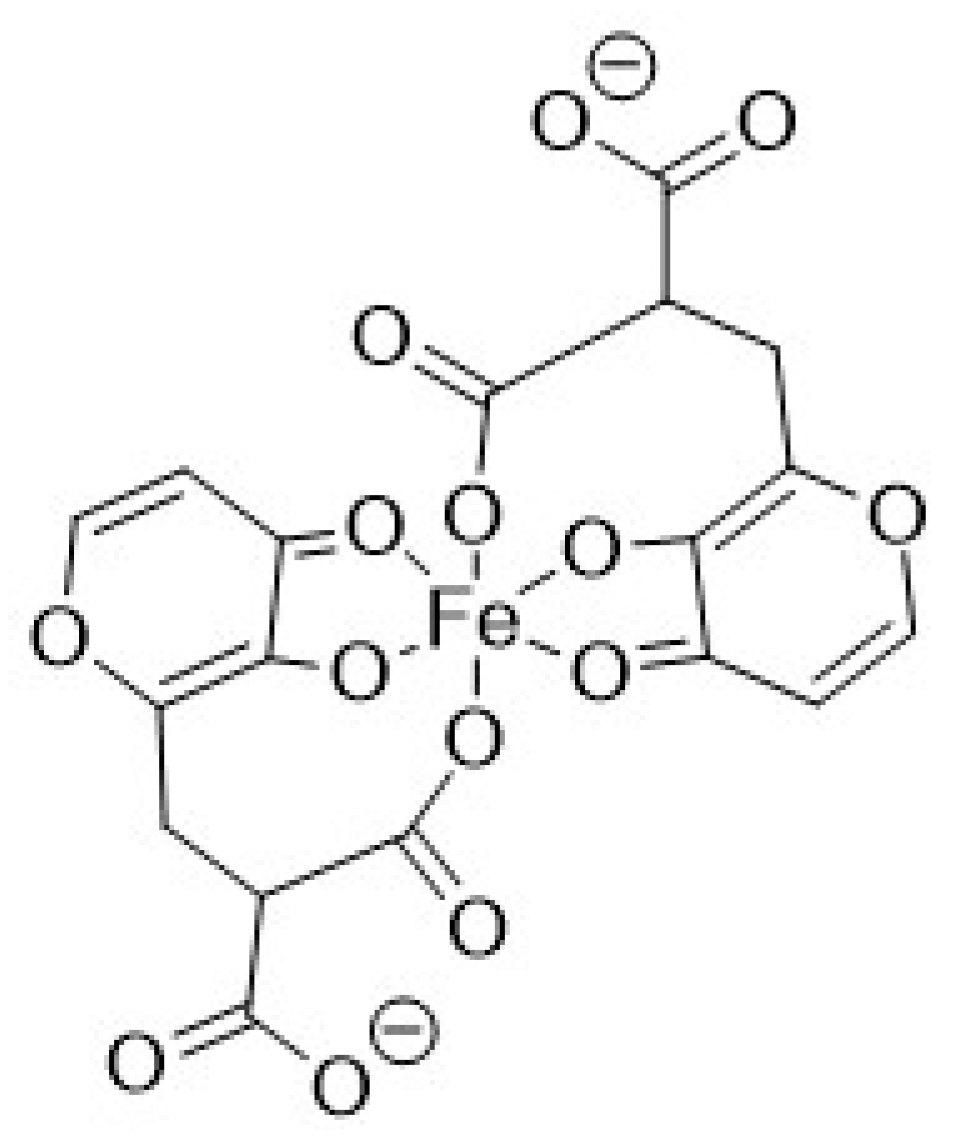
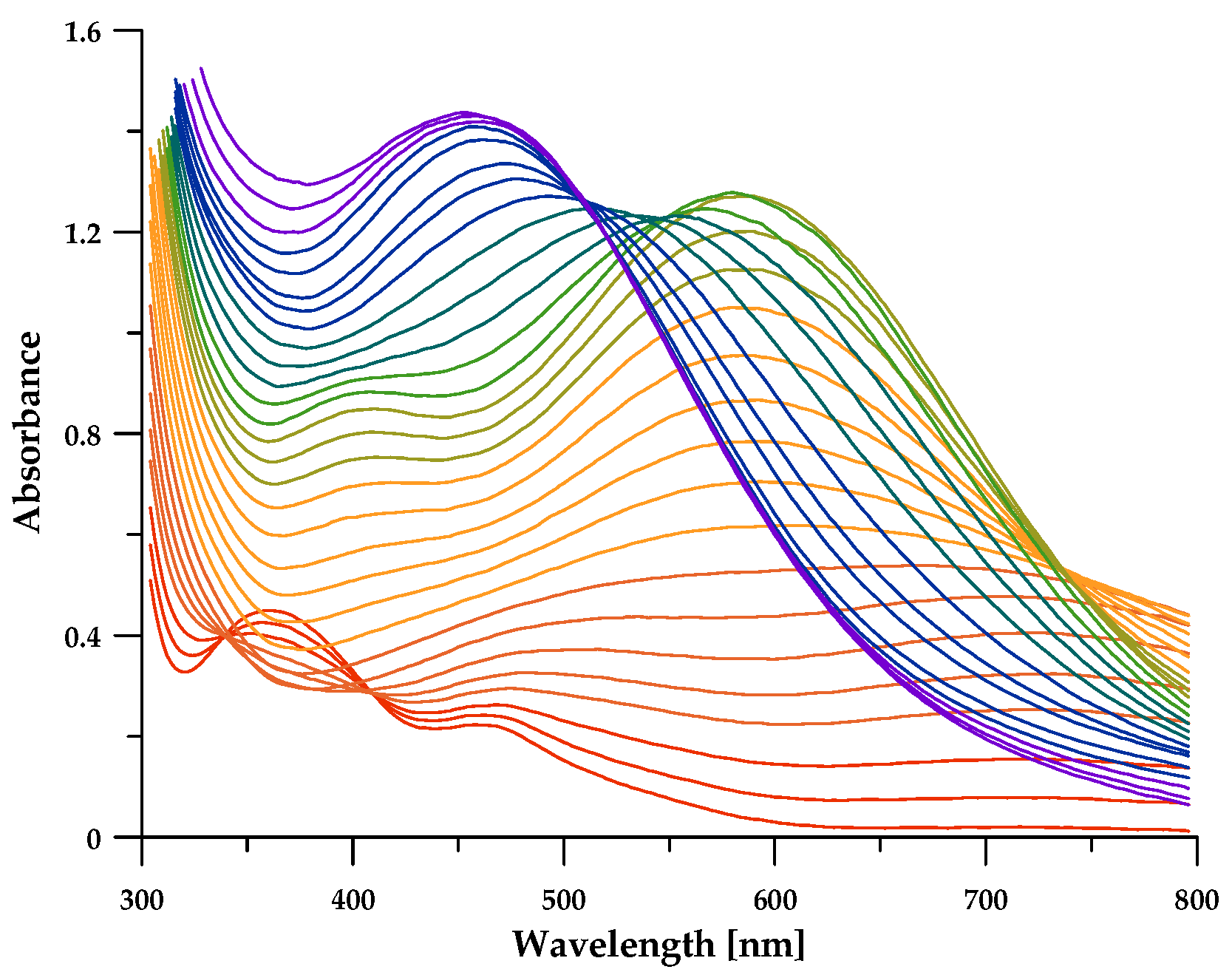
3.3. Fe3+ Complex Formation Equilibria
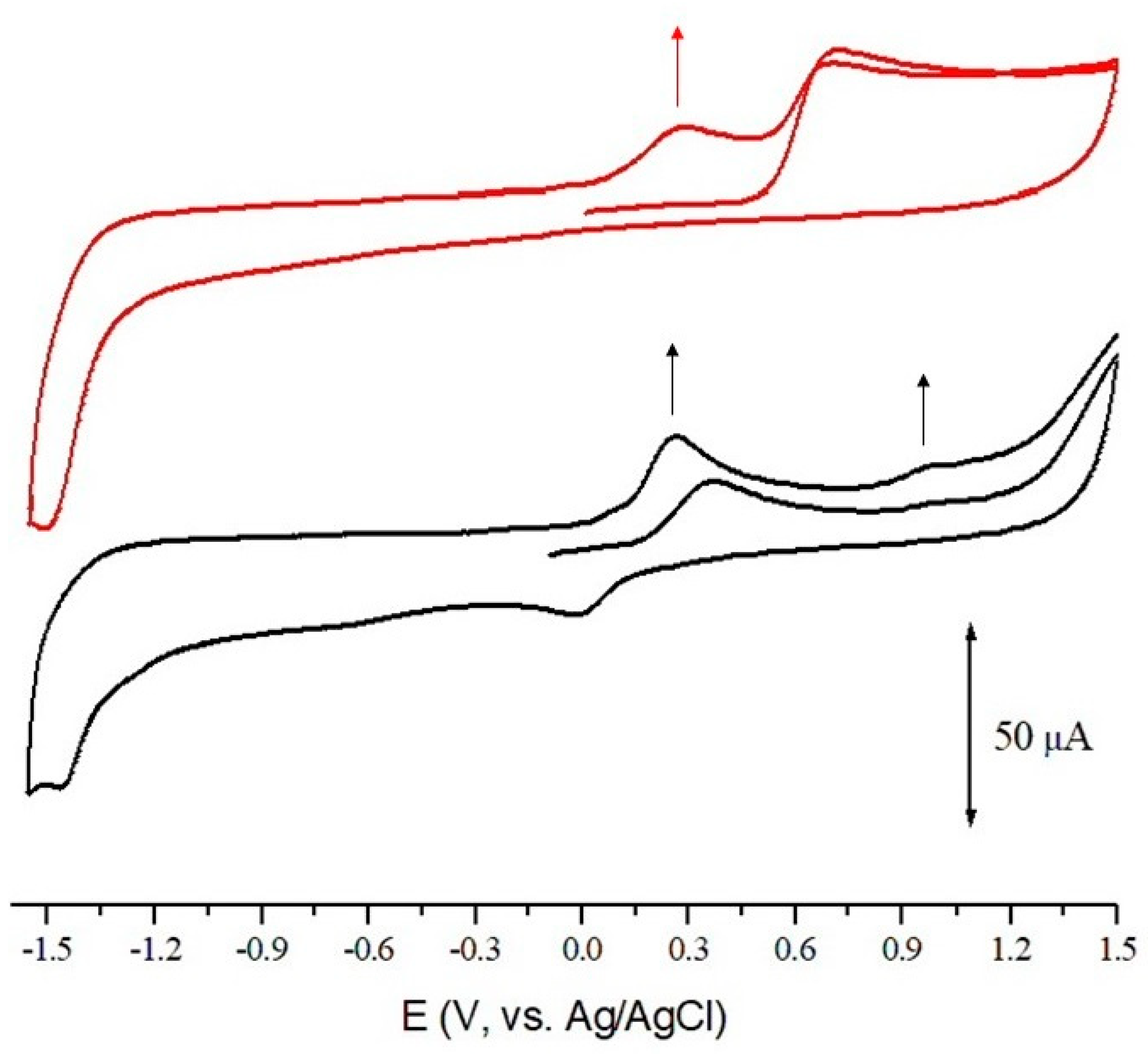
3.4. Cyclic Voltammetry
3.5. Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dixon, S.J.; Stockwell, B.R. The role of iron and reactive oxygen species in cell death. Nat. Chem. Biol. 2014, 10, 9–17. [Google Scholar] [CrossRef]
- Cairo, G.; Bernuzzi, F.; Recalcati, S. A precious metal: Iron, an essential nutrient for all cells. Genes Nutr. 2006, 1, 25–39. [Google Scholar] [CrossRef]
- Gozzelino, R.; Arosio, P. Iron Homeostasis in Health and Disease. Int. J. Mol. Sci. 2016, 17, 130. [Google Scholar] [CrossRef] [PubMed]
- Kontoghiorghes, G.J.; Pattichi, K.; Hadjigavriel, M.; Kolnagou, A. Transfusional iron overload and chelation therapy with deferoxamine and deferiprone (L1). Transfus. Sci. 2000, 23, 211–223. [Google Scholar] [CrossRef] [PubMed]
- Nurchi, V.M.; Crisponi, G.; Lachowicz, J.I.; Medici, S.; Peana, M.; Zoroddu, M.A. Chemical features of in use and in progress chelators for iron overload. J. Trace Elements Med. Biol. 2016, 38, 10–18. [Google Scholar] [CrossRef]
- Nurchi, V.M.; de Guadalupe Jaraquemada-Pelaez, M.; Crisponi, G.; Lachowicz, J.I.; Cappai, R.; Gano, L.; Santos, M.A.; Melchior, A.; Tolazzi, M.; Peana, M.; et al. A new tripodal kojic acid derivative for iron sequestration: Synthesis, protonation, complex formation studies with Fe3+, Al3+, Cu2+ and Zn2+, and in vivo bioassays. J. Inorg. Biochem. 2019, 193, 152–165. [Google Scholar] [CrossRef]
- Cappai, R.; Chand, K.; Lachowicz, J.I.; Chaves, S.; Gano, L.; Crisponi, G.; Nurchi, V.M.; Peana, M.; Zoroddu, M.A.; Santos, M.A. A new tripodal-3-hydroxy-4-pyridinone for iron and aluminium sequestration: Synthesis, complexation and in vivo studies. N. J. Chem. 2018, 42, 8050–8061. [Google Scholar] [CrossRef]
- Nurchi, V.M.; Cappai, R.; Crisponi, G.; Sanna, G.; Alberti, G.; Biesuz, R.; Gama, S. Chelating Agents in Soil Remediation: A New Method for a Pragmatic Choice of the Right Chelator. Front. Chem. 2020, 8, 597400. [Google Scholar] [CrossRef]
- Nurchi, V.M.; Cappai, R.; Spano, N.; Sanna, G. A friendly complexing agent for spectrophotometric determination of total iron. Molecules 2021, 26, 3071. [Google Scholar] [CrossRef]
- Lachowicz, J.I.; Nurchi, V.M.; Fanni, D.; Gerosa, C.; Peana, M.; Zoroddu, M.A. Nutritional Iron Deficiency: The Role of Oral Iron Supplementation. Curr. Med. Chem. 2014, 21, 3775–3784. [Google Scholar] [CrossRef]
- Raymond, K.N.; Dertz, E.A.; Kim, S.S. Enterobactin: An archetype for microbial iron transport. Proc. Natl. Acad. Sci. USA 2003, 100, 3584–3588. [Google Scholar] [CrossRef] [PubMed]
- Neilands, J.B. Siderophores: Structure and Function of Microbial Iron Transport Compounds. J. Biol. Chem. 1995, 270, 26723–26726. [Google Scholar] [CrossRef] [PubMed]
- Hider, R.C.; Mohd-Nor, A.R.; Silver, J.; Morrison, I.E.G.; Rees, L.V.C. Model compounds for microbial iron-transport compounds. Part 1. Solution chemistry and Mössbauer study of iron(II) and iron(III) complexes from phenolic and catecholic systems. J. Chem. Soc. Dalton Trans. 1981, 609–622. [Google Scholar] [CrossRef]
- Kontoghiorghes, G. Deferiprone and Iron–Maltol: Forty Years since Their Discovery and Insights into Their Drug Design, Development, Clinical Use and Future Prospects. Int. J. Mol. Sci. 2023, 24, 4970. [Google Scholar] [CrossRef]
- Kandioller, W.; Kurzwernhart, A.; Hanif, M.; Meier, S.M.; Henke, H.; Keppler, B.K.; Hartinger, C.G. Pyrone derivatives and metals: From natural products to metal-based drugs. J. Organomet. Chem. 2011, 696, 999–1010. [Google Scholar] [CrossRef]
- Thompson, K.H.; Barta, C.A.; Orving, C. Metal complexes of maltol and close analogues in medicinal inorganic chemistry. Chem. Soc. Rev. 2006, 35, 545–556. [Google Scholar] [CrossRef]
- Lai, D.; Brotz-Oesterhelt, H.; Muller, W.; Wray, V.; Proksch, P. Bioactive polyketides and alkaloids from Penicillium citrinum, a fungal endophyte isolated from Ocimum tenuiflorum. Fitoterapia 2013, 91, 100–106. [Google Scholar] [CrossRef]
- Fusi, S.; Di Florio, G.; Margiotta, N.; Barbanente, A.; Cini, E.; Finetti, F.; Paradisi, L.; Trabalzini, L.; Fabrizi de Biani, F.; Corsini, M. Synthesis, characterization, electrochemistry and in vitro cytotoxicity of a new “Triazole-Maltol” ligand and its platinum(II) complex. Inorganica Chim. Acta 2022, 532, 120756. [Google Scholar] [CrossRef]
- Ehrlich, M.; Carell, T. Total Syntheses and Biological Evaluation of 3-O-Methylfunicone and Its Derivatives Prepared by TMPZnCl·LiCl-Mediated Halogenation and Carbonylative Stille Cross-Coupling. Eur. J. Org. Chem. 2013, 2013, 77–83. [Google Scholar] [CrossRef]
- Gran, G. Determination of the equivalence point in potentiometric acid-base titrations. Analyst 1952, 77, 661–671. [Google Scholar] [CrossRef]
- Gans, P.; Sabatini, A.; Vacca, A. Investigation of equilibria in solution. Determination of equilibrium constants with the HYPERQUAD suite of programs. Talanta 1996, 43, 1739–1753. [Google Scholar] [CrossRef] [PubMed]
- Gans, P.; Sabatini, A.; Vacca, A. To improve accuracy of the calculated pKa values. Ann. Chim. 1999, 89, 45–49. [Google Scholar]
- Baes, C.F.; Mesmer, R.E. The Hydrolysis of Cations; John Wiley & Sons: New York, NY, USA, 1976. [Google Scholar]
- Kostelnik, T.I.; Scheiber, H.; Cappai, R.; Choudhary, N.; Lindheimer, F.; De Guadalupe Jaraquemada-Peláez, M.; Orvig, C. Phosphonate Chelators for Medicinal Metal Ions. Inorg. Chem. 2021, 60, 5343–5361. [Google Scholar] [CrossRef]
- Migliorini, F.; Cini, E.; Dreassi, E.; Finetti, F.; Ievoli, G.; Macrì, G.; Petricci, E.; Rango, E.; Trabalzini, L.; Taddei, M. A pH-responsive crosslinker platform for antibody-drug conjugate (ADC) targeting delivery. Chem. Commun. 2022, 58, 10532–10535. [Google Scholar] [CrossRef] [PubMed]
- Fusi, S.; Frosini, M.; Biagi, M.; Zór, K.; Rindzevicius, T.; Baratto, M.C.; De Vico, L.; Corsini, M. Iron(III) complexing ability of new ligands based on natural γ-pyrone maltol. Polyhedron 2020, 187, 114650. [Google Scholar] [CrossRef]
- Zborowski, K.; Grybos, R.; Proniewicz, L.M. Structure modification of maltol (3-hydroxy-2-methyl-4H-pyran-4-one) upon cation and anion formation studied by vibrational spectroscopy and quantum-mechanical calculations. Vib. Spectrosc. 2007, 43, 344–350. [Google Scholar] [CrossRef]
- Mohammed-Ziegler, I.; Billes, F. Vibrational spectroscopic calculations on pyrogallol and gallic acid. J. Mol. Struct.-Theochem. 2002, 618, 259–265. [Google Scholar] [CrossRef]
- Williams, D.H.; Fleming, I. Spectroscopic Methods in Organic Chemistry; McGraw-Hill Book Company: Maidenhead, UK, 1966. [Google Scholar]
- Parajon-Costa, B.S.; Baran, E.J. Vibrational spectra of bis(maltolato)zinc(II), an interesting insulin mimetic agent. Spectrochim. Acta A 2013, 113, 337–339. [Google Scholar] [CrossRef]
- Ostacoli, G.; Vanni, A.; Roletto, E. Complex formation between alkyl-substituted malonic acids and bivalent metal ions in aqueous solutions. Ric. Sci. 1968, 38, 318–321. [Google Scholar]
- Amico, P.; Bonomo, R.P.; Cali, R.; Cucinotta, V.; Daniele, P.G.; Ostacoli, G.; Rizzarelli, E. Ligand-ligand Interactions in Metal Complexes: Thermodynamic and Spectroscopic Investigation of Simple and Mixed Copper(II) and Zinc(II) Substituted-malonate Complexes with 2,2’-bipyridyl in Aqueous Solution. Inorg. Chem. 1989, 28, 3555–3561. [Google Scholar] [CrossRef]
- DeRobertis, A.; DeStefano, C.; Foti, C. Medium effects on the protonation of carboxylic acids at different temperatures. J. Chem. Eng. Data 1999, 44, 262–270. [Google Scholar] [CrossRef]
- Petrola, R. Spectrophotometric study on the equilibria of pyromeconic acid derivatives with proton in aqueous solution. Finn. Chem. Lett. 1985, 12, 207. [Google Scholar]
- Chiaccherini, E.; Bartušek, M. Complex of maltol with uranyl and aluminium. Collect. Czechoslov. Chem. Commun. 1969, 34, 530–536. [Google Scholar] [CrossRef]
- Herrero-Martínez, J.M.; Sanmartin, M.; Rosés, M.; Bosch, E.; Ràfols, C. Determination of dissociation constant of flavonoids by capillary electrophoresis. Electrophoresis 2005, 10, 1886–1895. [Google Scholar] [CrossRef] [PubMed]
- Sigel, H.; Huber, P.R.; Griesser, R.; Bernhard, P. Ternary Complexes in Solution. XV.1 Mixed-Ligand Copper(II) Complexes with 2,2 -Bipyridyl or 1,10-Phenanthroline and Pyrocatecholate or Derivatives Thereof. Inorg. Chem. 1973, 12, 1198–1200. [Google Scholar] [CrossRef]
- Swain, C.G.; Lupton, E.C. Field and Resonance Components of Substituent Effects. J. Am. Chem. Soc. 1968, 90, 4328–4337. [Google Scholar] [CrossRef]
- Alderighi, L.; Gans, P.; Ienco, A.; Peters, D.; Sabatini, A.; Vacca, A. Hyperquad simulation and speciation (HySS): A utility program for the investigation of equilibria involving soluble and partially soluble species. Coord. Chem. Rev. 1999, 184, 311–318. [Google Scholar] [CrossRef]
- Stefanović, A.; Havel, J.; Sommel, L. On the reaction of iron(III). Collect. Czechoslov. Chem. Commun. 1968, 33, 4198–4214. [Google Scholar] [CrossRef]
- Salvadó, V.; Ribas, X.; Zelano, V.; Ostacoli, G.; Valiente, M. The chemistry of iron in biosystems-III. Complex formation between FeIII and malonic acid in aqueous solutions. Polyhedron 1989, 8, 813–818. [Google Scholar] [CrossRef]
- Nurchi, V.M.; Pivetta, T.; Lachowicz, J.I.; Crisponi, G. Effect of substituents on complex stability aimed at designing new iron(III) and aluminum(III) chelators. J. Inorg. Biochem. 2009, 103, 227–236. [Google Scholar] [CrossRef]
- Barham, A.S.; Kennedy, B.M.; Cunnane, V.J.; Daous, M.A. The Electrochemical polymerisation of 1,2 dihydroxybenzene and 2-hydroxybenzyl alcohol prepared in different solutions media. Electrochim. Acta 2014, 147, 19–24. [Google Scholar] [CrossRef]
- Marczewska, B.; Przegaliński, M. Poly(catechol) electroactive film and its electrochemical properties. Synth. Met. 2013, 182, 33–39. [Google Scholar] [CrossRef]
- Ball, V. Electrodeposition of pyrogallol versus pyrocatechol using cyclic voltammetry and chronoamperometry. J. Electroanal. Chem. 2022, 909, 116142. [Google Scholar] [CrossRef]
- Crisponi, G.; Remelli, M. Iron chelating agents for the treatment of iron overload. Coord. Chem. Rev. 2008, 252, 1225–1240. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 1997, 23, 3–26. [Google Scholar] [CrossRef]
| 3 | 4 | 7 | |||||
|---|---|---|---|---|---|---|---|
| species | log β | log K | species | log β | log K | log β | log K |
| [LH]2− | 8.999 (1) | 8.999 | [LH]− | 11.55 (2) | 11.55 | 11.30 (6) | 11.30 |
| [LH2]− | 13.87 (4) | 4.87 | LH2 | 20.46 (2) | 8.91 | 20.25 (3) | 8.95 |
| LH3 | 16.70 (5) | 2.83 | |||||
| 3 | 7 | ||||
|---|---|---|---|---|---|
| Species | log β | pK | Species | log β | pK |
| [FeLH]+ | 20.39 (1) | [FeLH]2+ | 22.44 (1) | 3.97 | |
| [FeL2H]2− | 30.33 (1) | 5.20 | [FeL]+ | 18.47 (2) | |
| [FeL2]3− | 25.13 (1) | 9.06 | FeL2H | 37.29 (1) | 5.32 |
| [FeL2H-2]5− | 7.01 (2) | [FeL2]− | 31.97 (1) | 9.27 | |
| [FeL3]6− | 29.60 (3) | [FeL2H-2]3− | 13.43 (2) | ||
| [FeL3]3− | 39.03 (1) | ||||
| pFe3+ | 17.72 | 16.86 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cini, E.; Crisponi, G.; Fantasia, A.; Cappai, R.; Siciliano, S.; Florio, G.D.; Nurchi, V.M.; Corsini, M. Multipurpose Iron-Chelating Ligands Inspired by Bioavailable Molecules. Biomolecules 2024, 14, 92. https://doi.org/10.3390/biom14010092
Cini E, Crisponi G, Fantasia A, Cappai R, Siciliano S, Florio GD, Nurchi VM, Corsini M. Multipurpose Iron-Chelating Ligands Inspired by Bioavailable Molecules. Biomolecules. 2024; 14(1):92. https://doi.org/10.3390/biom14010092
Chicago/Turabian StyleCini, Elena, Guido Crisponi, Alessandra Fantasia, Rosita Cappai, Sofia Siciliano, Giuseppe Di Florio, Valeria M. Nurchi, and Maddalena Corsini. 2024. "Multipurpose Iron-Chelating Ligands Inspired by Bioavailable Molecules" Biomolecules 14, no. 1: 92. https://doi.org/10.3390/biom14010092
APA StyleCini, E., Crisponi, G., Fantasia, A., Cappai, R., Siciliano, S., Florio, G. D., Nurchi, V. M., & Corsini, M. (2024). Multipurpose Iron-Chelating Ligands Inspired by Bioavailable Molecules. Biomolecules, 14(1), 92. https://doi.org/10.3390/biom14010092










