Abstract
The increase in presynaptic striatal dopamine is the main dopaminergic abnormality in schizophrenia (SCZ). SCZ is primarily treated by modulating the activity of monoamine systems, with a focus on dopamine and serotonin receptors. Glial cell line-derived neurotrophic factor (GDNF) is a strong dopaminergic factor, that recently was shown to correlate with SCZ in human CSF and in striatal tissue. A 2-3-fold increase in GDNF in the brain was sufficient to induce SCZ-like dopaminergic and behavioural changes in mice. Here, we analysed the effect of acute, chronic, and embryonic methamphetamine, a drug known to enhance the risk of psychosis, on Gdnf and its receptors, Gfra1 and Ret, as well as on monoamine metabolism-related gene expression in the mouse brain. We found that acute methamphetamine application increases Gdnf expression in the striatum and chronic methamphetamine decreases the striatal expression of GDNF receptors Gfra1 and Ret. Both chronic and acute methamphetamine treatment upregulated the expression of genes related to dopamine and serotonin metabolism in the striatum, prefrontal cortex, and substantia nigra. Our results suggest a potential mechanism as to how methamphetamine elicits individual psychosis risk in young adults—variation in initial striatal GDNF induction and subsequent GFRα1 and RET downregulation may determine individual susceptibility to psychosis. Our results may guide future experiments and precision medicine development for methamphetamine-induced psychosis using GDNF/GFRa1/RET antagonists.
Keywords:
schizophrenia; dopamine; GDNF; GFRa1; RET; monoamines; serotonin; psychosis; methamphetamine 1. Introduction
Schizophrenia (SCZ) is a debilitating psychiatric disorder with remarkable interindividual variation. SCZ has many hypotheses surrounding its pathophysiology, including theories relating to altered GABA-ergic and glutamatergic systems, inflammation, and oxidative stress. However, the molecular drivers of the disease remain relatively poorly understood. The most consistent and scientifically supported hypothesis describes altered brain dopaminergic function as a driver and/or contributor to SCZ [1,2]. This hypothesis is supported by multiple independent lines of evidence. A serendipitous discovery in the early 1950s ′s revealed that dopamine (DA) receptor 2 (D2R) blocking drugs can reverse psychosis, whereas DA-enhancing drugs, like methamphetamine, enhance the disease and increase psychosis risk [3,4,5]. In line with this, one of the more robust abnormalities in schizophrenia is an increase in striatal dopamine activity. Brain imaging studies, using SPECT and PET, reveal increased striatal DA synthesis and/or release as a consistent, but non-ubiquitous, feature in individuals with SCZ and individuals with increased risk for SCZ [6,7,8]. More recently, it was shown that about half of first-episode patients (FEP) display enhanced striatal dopamine metabolism and that only those patients with elevated striatal dopamine synthesis respond to treatment with antipsychotics [9], which notably all target the D2R [7,8,10].
The fact that only about half of individuals with SCZ respond to anti-psychotics and that there are no single causal gene mutations for SCZ, collectively imply that a one-size-fits-all explanation for SCZ does not exist. Hence, to create precision medicine in the future, targeting enhanced striatal dopamine function in SCZ, we first need to understand the mechanisms driving the increased striatal dopamine and altered monoamine metabolism in SCZ [1,2]. This information can then be used to define disease subsets, which may eventually lead to the development of subgroup-specific precision medicine.
We have recently made progress in this direction. GDNF is a strong DA neuron function-enhancing factor. We have found that GDNF protein levels in the CSF positively correlate with disease severity in FEP SCZ patients [2]. Furthermore, we found that GDNF mRNA levels are increased in the post-mortem striatum of SCZ patients and that a similar increase in GDNF expression in mice is sufficient to trigger an SCZ-like increase in striatal dopamine, in dopaminergic system function, gene expression, neurophysiology, and animal behavior [2].
The striatum is a key site of dopamine metabolism in the brain. GDNF is expressed specifically in the parvalbumin-expressing interneurons of the striatum, which regulate striatal output [11,12]. Previously, we found that striatal GDNF levels regulate amphetamine-induced DA release, and DA transporter reversal time, in striatal dopamine axons [13] and that endogenous GDNF levels regulate striatal DA release and reuptake [14]. Furthermore, it is well established that excess ectopically applied GDNF strongly stimulates striatal dopaminergic function when GDNF is delivered into Parkinson’s disease models, where striatal dopamine fibers degenerate [15,16,17,18,19,20,21].
Methamphetamine strongly enhances striatal DA metabolism and induces psychosis in some individuals, with the highest prevalence in adolescence [5,22,23,24,25]. Methamphetamine is often used to replicate schizophrenia-like symptoms in animals [26,27,28,29,30,31]. However, mechanisms related to methamphetamine-induced susceptibility to psychosis, and why some individuals are more susceptible than others, have remained unknown. Here, we performed an exploratory study to analyze if, and how, methamphetamine modulates spatiotemporal gene expression of Gdnf, and its receptors Gfrα1 and Ret, in the brain in acute, chronic, and embryonic regimens.
We found that acute methamphetamine induces striatal Gdnf mRNA expression, whereas chronic methamphetamine induces downregulation of striatal GDNF receptors Gfrα1 and Ret mRNA expression levels. This result suggests a possible mechanism for individual differences in psychosis induction—individuals with a greater acute striatal GDNF induction and a lower GFRα1 and RET downregulation upon chronic drug application are likely the most susceptible to psychosis. Methamphetamine also upregulated the expression of the majority of dopamine and serotonin metabolism-related genes in the prefrontal cortex (PFC), striatum, and substantia nigra (SN), supporting the monoamine hypotheses of SCZ (Figure 1).
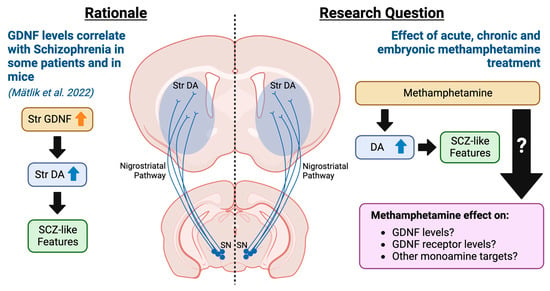
Figure 1.
Rationale and research questions of the current exploratory study. Previously, we showed that GDNF, a potent enhancer of nigrostriatal dopamine, is elevated in a sub-set of SCZ patients and that a similar increase in GDNF in mice is sufficient to drive disease [2]. Here, we investigate how methamphetamine, known to increase the risk of FEP, modulates GDNF, its receptors, and monoamine function-related gene expression after acute, chronic, and embryonic methamphetamine exposure.
2. Materials and Methods
2.1. Animals
Animal experiments were conducted according to the 3R principles of the European Union Directive 2010/63/EU governing the care and use of experimental animals. Experiments were also conducted according to local laws and regulations (Finnish Act on the Protection of Animals Used for Scientific or Educational Purposes (491/2013), Government Decree on the Protection of Animals Used for Scientific or Educational Purposes (564/2013)). The protocols were authorized by the National Animal Experiment Board of Finland (ESAVI/12046/04.10.07/2017—Osahanke 1). Mice were maintained in a 129Ola/ICR/C57bl6 mixed genetic background. In order to replicate a similar genetic background used in Mätlik et al. [2], mice were maintained in a mixed 129Ola/ICR/C57bl6 background where a 2-to-4-fold midgestational upregulation of GDNF resulted in a spectrum of SCZ-like features from neurophysiology to animal behaviour. Mice were group-housed with free food and water access under a 12 h light-dark cycle and room temperature of 21 ± 1 °C. Bedding (aspen chips, Tapvei, Harjumaa, Estonia) and nest material (Tapvei, Harjumaa, Estonia) were changed weekly. Researchers were blinded to mouse genotypes during tissue collection and processing. Male mice were used for acute and chronic methamphetamine experiments and female dams were used for embryonic methamphetamine experiments.
2.2. Methamphetamine Injections
To assess changes in mRNA levels in response to methamphetamine, wild-type (WT) adolescent (1 month old) male mice were treated with methamphetamine following either an acute or chronic dosing regimen [32] (Figure 2). For acute methamphetamine (aMETH) treatment, mice were given IP injections of escalating doses of methamphetamine, diluted in 0.9% saline, over three days (1 mg/kg on day one, 2 mg/kg on day two, 4 mg/kg on day three). For chronic methamphetamine treatment (cMETH), mice were given IP injections of escalating doses of methamphetamine diluted in 0.9% saline for three weeks. Escalating doses were used to replicate methamphetamine abuse patterns observed in humans, where humans normally use lower doses of methamphetamine initially before gradually increasing the dose [33,34,35]. In week one, mice received 1 mg/kg methamphetamine once daily for five days. In week two, mice received 2 mg/kg methamphetamine twice daily for five days. In week three, mice received 4 mg/kg methamphetamine twice daily for five days. The two-day rest period between the five days of methamphetamine dosing was used in an attempt to replicate a temporary cessation of the drug intake in humans associated with psychostimulant withdrawal [35,36]. Similar dosing regimens have been used successfully in previous studies [34,37]. Wild-type mice injected only with saline were used as controls for both regimens. Mice were sacrificed by cervical dislocation and decapitation and dissected approximately 1–2 h post-final injection for acute regimens and 24 h post-final injection for chronic regimens. Brains were removed, placed into a chilled cutting block on ice, and quickly dissected according to the areas of interest and immediately frozen on dry ice and stored at −80 °C. To measure changes in mRNA levels in embryos, pregnant dams were injected IP with 1 mg/kg methamphetamine diluted in 0.9% saline solution once daily for two days (E11–E12; Figure 2). Saline-injected wild-type mice were used as controls. The pregnant dams were sacrificed 1–2 h post-final injection and the embryos (E12.5) were dissected on ice immediately. All tissues were snap-frozen on dry ice and stored at −80 °C until mRNA analysis.
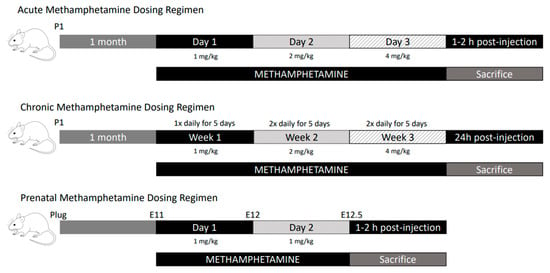
Figure 2.
Schematic drawing of methamphetamine dosing regimens.
2.3. Reverse Transcription and Quantitative PCR
RNA was isolated using a Trizol reagent (Thermo Fischer Scientific, Vantaa, Finland). 400 ng of total RNA per sample was treated with RNase-free DNase I (Thermo Fischer Scientific, Vantaa, Finland). DNase I was inactivated with 50 mM EDTA at 65 °C for 10 min. The reverse transcription reaction was performed using random hexamer primers and RevertAid Reverse Transcriptase (Thermo Fischer Scientific, Vantaa, Finland). Complementary DNA (cDNA) was diluted 1:10 and stored at 20 °C until used for qPCR.
Quantitative PCR was performed using LightCycler 480 SYBER Green I Master (Roche, Espoo, Finland) and 100 μM primers, diluted 1:20, in 384-well plates with 10 μL total volume. All samples were analysed in triplicates or duplicates. Each reaction contained two negative controls (water and minus-reverse transcription). A combination of B-Actin, Ribosomal Protein S6, Beta-2-Microglobulin, and Gapdh were used as housekeepers for normalisation. Primer sequences are provided in Table 1.

Table 1.
Primers used for RT-qPCR.
2.4. Statistical Analysis
All results are presented as the mean ± SEM. Normality was first assessed in all data sets to ensure normal distributions, and outliers were removed justified by Grubbs’ test. To then assess nominal significance, a Welch’s t-test was performed to compare specific differences due to methamphetamine treatment between mRNA expression levels of specific genes. However, as this is subject to false positives and false negatives, to simultaneously limit errors in comparing variance both across genes and between treatments, we performed a two-way ANOVA followed by the Holm-Sidak correction for multiple comparisons.
Statistical analysis was performed using GraphPad Prism 8.4.2. Quantitative PCR data was analysed as previously described [38] using the geometric mean of reference genes for normalisation. The statistical significance level was set at p < 0.05.
3. Results
3.1. Acute Methamphetamine Application Induces a 2-Fold Increase in Striatal Gdnf Expression
A recent study revealed that a 2–3-fold increase in endogenous GDNF expression in the adult striatum and the embryonic brain induces dopaminergic changes similar to that seen in schizophrenia [2]. Methamphetamine, on the other hand, induces psychosis with incomplete penetrance [22]. Here, we investigated the effect of acute methamphetamine application (Figure 2) on the mRNA expression of Gdnf and its receptors Gfrα1 and Ret, as well as on the expression of a range of marker genes covering dopaminergic and serotonergic systems, as selected from gene data sets from GeneOntology (GO:0001963, GO:0006837, GO:0035860) [39,40] in the striatum, prefrontal cortex and substantia nigra. Since parvalbumin (Pvalb) expressing GABA-ergic interneurons are found to be responsible for the majority of GDNF produced in the rodent striatum [11,12], we also assessed Pvalb mRNA levels, which may provide further insight into the signalling capacity of GDNF in response to methamphetamine. Our results show that an acute methamphetamine treatment induces about a 2-fold increase in Gdnf mRNA in the striatum (p < 0.05 *, p < 0.001 ###, Figure 3B). However, acute methamphetamine treatment had no effect on the mRNA levels of Gfra1, Ret, and Pvalb in the striatum, prefrontal cortex, and substantia nigra (Figure 3).
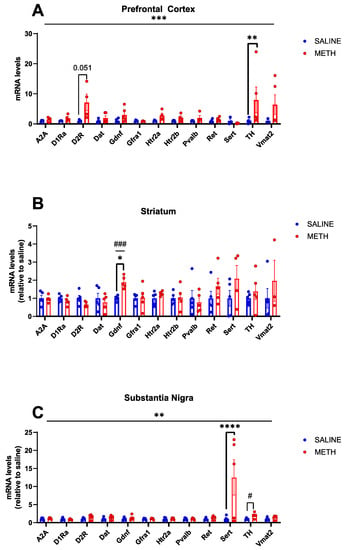
Figure 3.
Effects of acute methamphetamine treatment on mRNA levels in WT adolescent mice, as relative to saline controls. (A) In the PFC, the overall effect of treatment is significant (p < 0.001 ***), and a significant increase in TH mRNA levels is observed in response to acute methamphetamine treatment (p < 0.01 **) and a near significant increase in D2R mRNA levels in response to acute methamphetamine (p = 0.051). (B) In the STR, there is a significant increase in Gdnf mRNA levels in response to acute methamphetamine (p < 0.05 *, p < 0.001 ###). (C) In the SN, there is an overall significant effect of the treatment (p < 0.01 **) and a significant increase in SERT mRNA levels in response to acute methamphetamine treatment (p < 0.0001 ****), and a significant increase in TH is observed when comparing saline vs. methamphetamine mRNA levels with a Student’s t-test (p < 0.05 #). N = 4–5 mice per group. * Significance measured by two-way ANOVA with Holm-Sidak post hoc test. # Significance measured by Student’s t-test.
3.2. Acute Methamphetamine Treatment Enhances Expression of Genes Related to Dopaminergic and Serotonergic System Function
The dopamine hypothesis of schizophrenia theorizes that hyperactivity of dopamine 2 receptor (D2R) neurotransmission in subcortical and limbic regions of the brain may contribute to positive symptoms of schizophrenia, potentially in part due to an increase in D2R density [41,42,43,44,45]. Negative and cognitive symptoms of schizophrenia on the other hand are believed to be associated, at least in part, with cortical hypofunctionality of the dopamine 1 receptor [46,47]. We observed a trend toward increased D2R mRNA levels in response to acute methamphetamine treatment in the prefrontal cortex (p = 0.051, Figure 3A). No changes were observed in D1Ra mRNA levels in the mouse brain in response to acute methamphetamine (Figure 3).
Tyrosine hydroxylase (TH) is the rate-limiting enzyme in the production of dopamine [48]. Post-mortem analysis in SCZ patients yielded inconsistent results, with some studies showing increased tyrosine hydroxylase levels in the substantia nigra [49], while others find no difference [50,51]. We found that acute methamphetamine treatment induces a statistically significant increase in TH mRNA expression in the prefrontal cortex (p < 0.01 **, Figure 3A).
Polymorphisms in the serotonin transporter gene have been linked to treatment-resistant schizophrenia [52] and changes in SERT expression in brain tissue from individuals with schizophrenia have been documented [53]. We found that acute methamphetamine application increases Sert mRNA expression in the substantia nigra (p < 0.0001 ***, Figure 3C), with no changes observed in the striatum or prefrontal cortex.
Taken together, our results show that an acute methamphetamine regimen enhances the expression of key metabolic genes in both dopaminergic and serotonergic systems.
3.3. Chronic Methamphetamine Treatment Upregulates Ret Expression in the Substantia Nigra but Downregulates Gfra1 and Ret mRNA Levels in the Striatum
To compare the effects of chronic methamphetamine treatment with acute methamphetamine treatment, we next used a chronic regimen with 1–2 daily methamphetamine injections for three weeks (Figure 2) and analysed the same set of genes in the same brain regions. We found that Ret mRNA expression is significantly increased in the substantia nigra in response to chronic methamphetamine treatment (p < 0.05 *, Figure 4C). Furthermore, we found a significant reduction in both Gfra1 and Ret mRNA levels in the striatum (p < 0.05 *, Figure 4B) and we found that chronic methamphetamine nonsignificantly enhances the expression of Pvalb, which marks Gdnf expressing interneurons [11] in the striatum (p = 0.07, Figure 4B).
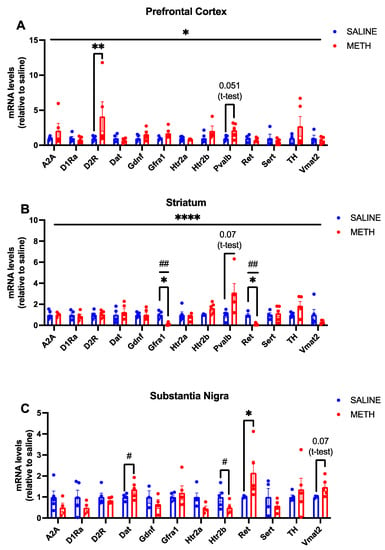
Figure 4.
Effects of chronic methamphetamine treatment on mRNA levels in WT adolescent mice. (A) When results were expressed as relative to the saline control, there was a significant effect of treatment (p < 0.05 *) but no significant interaction was observed. Chronic methamphetamine treatment significantly increased D2R mRNA levels (p < 0.01 **), and a trend towards increased Pvalb mRNA levels was observed (p = 0.051). (B) In the striatum, an overall significant interaction by ANOVA between gene and treatment is observed (p < 0.0001 ****). A significant reduction in Gfra1 and Ret mRNA levels is also observed (p < 0.05 *; t-test result p < 0.01 ##) and a trend towards an increase in Pvalb mRNA levels is detected in response to chronic methamphetamine treatment (p = 0.07). (C) In the SN, there is an overall significant interaction between the genes and treatment (p < 0.05 *) and an overall significant difference in genes (p < 0.05 *). A significant increase in Dat (p < 0.05 #) and Ret (p < 0.05 *) mRNA levels was observed and a trend towards increased Vmat2 mRNA levels was found (p = 0.07) in response to chronic methamphetamine treatment. N = 4–5 mice per group. * Significance measured by two-way ANOVA with Holm-Sidak post hoc test. # Significance measured by Student’s t-test.
3.4. Chronic Methamphetamine Maintain Elevated D2R Expression in the Prefrontal Cortex and Induces DAT Expression in the Substantia Nigra
Somewhat surprisingly, we found that despite the initial boost in SERT and TH encoding mRNA expression observed post-acute methamphetamine application, an increase in their expression is no longer observed upon chronic treatment. However, increased D2R expression in the PFC was observed in both acute and chronic treatments (Figure 3A and Figure 4A). We also found that chronic methamphetamine treatment increases DAT expression in the substantia nigra (Figure 4). Serotonergic markers were largely unchanged post-chronic methamphetamine treatment, except for a significant reduction in Htr2b mRNA in the substantia nigra (p < 0.05 #, Figure 4C).
3.5. The Effect of Acute E11–E12.5 Methamphetamine Application on GDNF and Monoamine Systems-Related Gene Expression in the Brain
Schizophrenia has been associated with complications in pregnancy, including prenatal exposure to influenza, prenatal nutritional deprivation, and rhesus incompatibility [54,55,56,57]. A recently published study suggests that an increase in GDNF expression in the CNS, at around midgestation (E12.5), results in schizophrenia-like changes in dopamine metabolism and animal behaviour. Therefore, we analysed whether acute methamphetamine exposure during E11-E12 (Figure 2) could induce Gdnf expression at E12.5 in the whole brain. We analysed Gdnf induction during pregnancy since midgestational upregulation of GDNF induced a range of SCZ-like features in mice [2]. Thus, we explored whether methamphetamine usage during pregnancy induces a Gdnf peak in the embryonal brain as a possible risk factor for SCZ later in life.
We also analysed the effect of methamphetamine on the expression of the above set of monoamine metabolism-related genes. We found that acute methamphetamine induces D1R and adenosine receptor Adora2a (A2A) encoding mRNA expression in the embryonic brain at E12.5 (Figure 5). However, acute methamphetamine treatment has no effect on the expression of Gdnf and its receptors at this age, nor did we find any changes in the monoamine metabolism-related gene expression that were altered in adolescent mice upon acute and chronic methamphetamine exposure at that age.
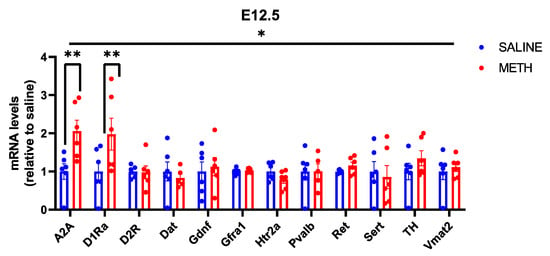
Figure 5.
Effects of methamphetamine treatment on mRNA levels in E12.5 mice, when expressed as relative to the saline control. There is a significant effect of both gene (p < 0.05 *) and treatment (p < 0.05 *), as well as a significant interaction between gene and treatment (p < 0.05 *). The significant increase in A2A mRNA levels observed in response to methamphetamine was maintained (p < 0.01 **) and a significant increase in D1Ra was detected in response to methamphetamine treatment (p < 0.01 **). N = 6 mice per group. * Significance measured by two-way ANOVA with Holm-Sidak post hoc test.
4. Discussion
A presynaptic increase in striatal dopamine is the main dopaminergic anomaly in schizophrenia [6,7,8]. GDNF is specifically expressed in the striatum in parvalbumin-expressing interneurons [11,12], which regulate striatal output [58]. In the striatum, GDNF receptor RET is exclusively expressed in dopamine axons. These axons project to the striatum from the substantia nigra, where the cell bodies of dopamine neurons are located [15] (Figure 6). GDNF strongly enhances the expression of its receptors, Gfrα1 and Ret, as shown by others [59,60] and by us, in various brain regions, including the substantia nigra [2,14]. Importantly, GDNF is one of the strongest factors known for enhancing dopamine neuron metabolism and function [2,61,62,63] and GDNF elicits its dopamine-enhancing effects on dopamine neurons via GFRα1 and RET receptors [64,65]. However, the role of the GDNF system in striatal hyperdopaminergia observed in schizophrenia has remained obscure. There are several reasons for this.
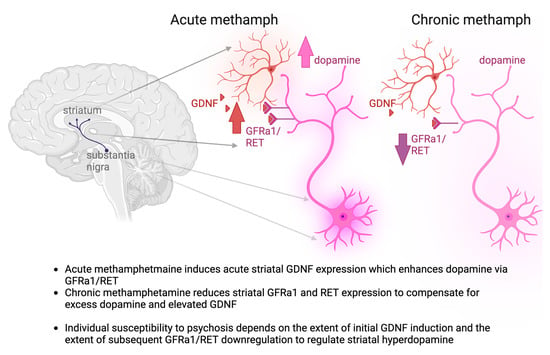
Figure 6.
Summary of our findings and the proposed mechanism of individual susceptibility to psychosis. Red neuron—GDNF producing striatal interneuron, red hue—secreted GDNF; violet neuron—dopamine neuron of the substantia nigra, violet hue—secreted dopamine.
First, it is currently unknown how GDNF levels in serum and CSF reflect GDNF levels in the brain. This, combined with a well-known lack of high-affinity antibodies that exclusively recognize endogenous GDNF, has complicated the drawing of a firm conclusion on GDNF biology in schizophrenia. Reflecting this, several studies report contradicting results in patients, with some claiming no change in GDNF serum levels [66,67,68], while other studies report decreased GDNF [69,70], with one of the aforementioned studies finding a correlation between increased GDNF and cognitive and attentional deficits in schizophrenia [68]. Our previous analysis using a hyper-sensitive endogenous GDNF protein detection system, available at Olink Ltd., Uppsala, Sweden, showed that GDNF levels correlate with schizophrenia in the CSF and that GDNF mRNA levels are increased in the striatum in schizophrenia patients [2]. Importantly, in mice, we established that a similar increase in GDNF results in schizophrenia-like molecular, neurophysiological, and behavioral changes, with increased presynaptic dopamine levels and release [2]. In our previous work, we therefore demonstrated that a 2- to 4-fold increase of GDNF from its endogenous locus both during midgestation and adulthood is sufficient to trigger various SCZ-like features in mice [2]. Methamphetamine, on the other hand, is well known to increase the risk of psychosis in humans [22,71] and has been used to model SCZ in animals [32,34,37,72]. Mice given the dosing regimen we have selected have been shown to exhibit greater amphetamine-induced locomotion indicating schizophrenia-like striatal hyperdopaminergia, and schizophrenia-like changes to the brain proteome as well as alterations in paired-pulse inhibition in mice with mutations related to schizophrenia susceptibility. [32,34,37,72]. Hence, here we performed an exploratory study to see if and how methamphetamine, applied at the doses and application regimen used in the above studies to induce SCZ-like behavior in animals, induces and modulates the expression of genes related to the GDNF system. As multiple studies reveal that Gdnf mRNA levels correlate with GDNF protein levels in the mouse brain [2,14,73,74], in this current exploratory study, we decided to measure Gdnf mRNA levels. Additionally, previous studies have demonstrated that Gfra1 and Ret mRNA levels also correlate with protein levels in mice [75,76,77,78].
Here, our analysis of the acute and chronic effects of methamphetamine, a known risk enhancer for psychosis, gave further insight into how GDNF expression may relate to susceptibility to schizophrenia. We show that, in mice, acute methamphetamine treatment induces about a 2-fold increase in striatal Gdnf expression. The chronic methamphetamine regimen, on the other hand, induces a reduction in Ret and Gfra1 encoding mRNA expression in the striatum. This is important, as the observed dual effect suggests a mechanism as to how methamphetamine induces psychosis with incomplete penetrance—individuals with higher GDNF induction and less prominent GFRa1/RET downregulation are more likely to develop psychosis. This, when supported by further analysis in methamphetamine-induced psychosis patients, suggests that temporary inhibition of GDNF signaling may serve as a future precision medicine for the treatment of methamphetamine-induced psychosis patients with elevated GDNF responses. This could be implemented using transient treatment with already available RET inhibitors Selpercatinib and Pralsetinib, which do reach the brain [79,80,81]. Selpercatinib and Pralsetinib are recently developed drugs, used to treat specific types of cancer [79,80,81]. Or this could be implemented using another yet-to-be-developed drug targeting, for example, GDNF-GFRa1 interactions. However, in the absence of data on RET phosphorylation and direct demonstration of reduced RET activity, this hypothesis, at this point, remains speculative. Nevertheless, this potential mechanism can guide future studies towards a novel avenue for psychopharmacological development of SCZ treatments for a subset of patients. Importantly, GDNF is well known to enhance striatal DA [2,14,82], and striatal hyperdopaminergia is strongly linked to SCZ [1,2,8,9]. Our results reveal a correlation between methamphetamine and GDNF induction, suggesting mechanistically that potentially methamphetamine could predispose one to or increase one’s risk of developing SCZ via GDNF upregulation-inducing STR hyperdopaminergia.
Furthermore, our analysis suggests that RET mRNA axonal localization is an actively controlled process, which is regulated by methamphetamine application. Upon chronic methamphetamine application, we observe an increase in Ret mRNA expression in the substantia nigra, with a simultaneous decrease in Ret mRNA expression in the striatum (Figure 4). Since Ret is only expressed in dopamine neurons and their axonal projections to the striatum [83] are the main source of striatal Ret mRNA [84], the most likely explanation is the existence of an actively controlled soma-to-axon Ret mRNA transportation mechanism which is modulated by methamphetamine. Future analysis of Ret mRNA localization would be useful for supporting this hypothesis.
Methamphetamine use among pregnant women is increasing, and substance abuse during pregnancy is associated with an increased risk of neurodevelopmental disorders [85]. However, drug-dependent mothers may use multiple drugs with various pharmacological properties, making it hard to determine the effect of a single drug on embryonic development. As we have already shown that endogenously increasing Gdnf developmentally at midgestational age E11–E12 resulted in a schizophrenia-like phenotype in adult mice, we here investigated whether methamphetamine administration during the same midgestational age may result in a similar Gdnf induction, potentially underlying the developmental difficulties associated with drug use during pregnancy. Although we observed an increase in A2a and Drd1a expression, we did not observe any changes in either Gdnf expression or the expression of its receptors Gfra1 and Ret, however. Thus, methamphetamine may not induce changes to GDNF or its receptors during pregnancy, although future studies at different time points and dosages could examine this potential lack of effect in more detail.
Taken together, our results suggest that acute methamphetamine induces an acute increase in striatal Gdnf expression, which in turn may enhance Ret expression in dopamine cell bodies in the substantia nigra, as reported earlier [15,84,86]. It is well established that, due to its functional importance, the dopamine system is strictly regulated by various regulatory feedback loops [87]. Thus, it is perhaps not surprising that here we provide evidence for yet another mechanism—spatial control over Ret mRNA abundance in the dopamine cell bodies and axons. Since methamphetamine and GDNF both act as strong stimulants of dopamine function, and GDNF stimulates Ret mRNA expression, dampening striatal dopamine function via suppression of axonal Ret mRNA abundance in the striatum is an intuitive mechanism to reduce striatal hyperdopaminergic function. This is also well in line with our previous results demonstrating dampened dopamine function after an adult-onset 50% reduction in striatal Gdnf expression [13]. Further experiments to delineate molecular mechanisms through which Ret mRNA axonal abundance is regulated could also reveal novel drug targets for treating methamphetamine-induced psychosis in the future. Additionally, further reinforcement of this hypothesis via histological analysis of synaptic changes in nigrostriatal dopamine neurons, as well as further behavioral analysis of these animals, is an important topic of future study. A summary of our findings and the proposed mechanism of individual susceptibility to psychosis stemming from our research is summarized in Figure 6. Briefly, acute methamphetamine treatment, in addition to direct release of dopamine, induces the expression of dopamine system enhancer GDNF, whereas chronic methamphetamine reduces the expression of GDNF receptors, likely as a compensatory mechanism.
Author Contributions
Conceptualization, L.C., D.R.G., A.M.-R. and J.-O.A.; methodology, D.R.G.; validation, L.C., D.R.G. and A.M.-R.; formal analysis, L.C., D.R.G. and J.-O.A.; investigation, L.C., D.R.G. and A.M.-R.; resources, J.-O.A.; data curation, L.C. and J.-O.A.; writing—original draft preparation, L.C.; writing—review and editing, D.R.G. and J.-O.A.; visualization, J.-O.A.; supervision, J.-O.A.; project administration, J.-O.A.; funding acquisition, J.-O.A. All authors have read and agreed to the published version of the manuscript.
Funding
J.-O.A., L.C. and A.M. were supported by the Academy of Finland (grants no. 297727 and 350678), Sigrid Juselius Foundation, ERA-NET NEURON grant nr 352077, Center of Innovative Medicine (CIMED), Hjärnfonden, Swedish Research Council (grants no. 2019-01578 and 2022-01093), Helsinki Institute of Life Science, and by European Research Council (ERC, grant no. 724922). D.R.G. was supported by the University of Helsinki Doctoral Programme Brain and Mind. Open access funding provided by University of Helsinki.
Institutional Review Board Statement
The animal study protocol was approved by the Institutional Review Board (or Ethics Committee) of the University of Helsinki (ESAVI/12046/04.10.07/2017—Osahanke 1).
Informed Consent Statement
Not applicable.
Data Availability Statement
All data related to this manuscript are available upon request.
Acknowledgments
The authors would like to thank Esa Korpi for kindly providing the methamphetamine used in the study.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| A2A | Adenosine receptor 2A |
| CSF | Cerebrospinal fluid |
| DA | Dopamine |
| DAT | Dopamine reuptake transporter |
| D1Ra | Dopamine 1 receptor |
| D2R | Dopamine 2 receptor |
| FEP | First-episode psychosis |
| GAPDH | Glyceraldehyde 3-phosphate dehydrogenase |
| GDNF | Glial cell line-derived neurotrophic factor |
| GFRa1 | GDNF family receptor alpha 1 |
| HTR2a | 5-hydroxytryptamine receptor 2a |
| HTR2b | 5-hydroxytryptamine receptor 2b |
| aMETH | acute methamphetamine |
| cMETH | chronic methamphetamine |
| PFC | Prefrontal cortex |
| Pvalb | Parvalbumin |
| RET | Rearranged during transfection receptor tyrosine kinase |
| RPS6 | Ribosomal protein subunit 61 |
| SCZ | Schizophrenia |
| SERT | 5-hydroxytryptamine reuptake transporter |
| SPECT | Single-photon emission computed tomography |
| SN | Substantia Nigra |
| TH | Tyrosine hydroxylase |
| Vmat2 | Vesicular monoamine transporter 2 |
| WT | Wild-type |
References
- Stępnicki, P.; Kondej, M.; Kaczor, A.A. Current Concepts and Treatments of Schizophrenia. Molecules 2018, 23, 2087. [Google Scholar] [CrossRef] [PubMed]
- Mätlik, K.; Garton, D.R.; Montaño-Rodríguez, A.R.; Olfat, S.; Eren, F.; Casserly, L.; Damdimopoulos, A.; Panhelainen, A.; Porokuokka, L.L.; Kopra, J.J.; et al. Elevated endogenous GDNF induces altered dopamine signalling in mice and correlates with clinical severity in schizophrenia. Mol. Psychiatry 2022, 27, 3247–3261. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.K.; Lin, S.K.; Sham, P.C.; Ball, D.; Loh, E.W.; Hsiao, C.C.; Chiang, Y.L.; Ree, S.C.; Lee, C.H.; Murray, R.M. Pre-morbid characteristics and co-morbidity of methamphetamine users with and without psychosis. Psychol. Med. 2003, 33, 1407–1414. [Google Scholar] [CrossRef] [PubMed]
- McKetin, R.; McLaren, J.; Lubman, D.I.; Hides, L. The prevalence of psychotic symptoms among methamphetamine users. Addiction 2006, 101, 1473–1478. [Google Scholar] [CrossRef] [PubMed]
- McKetin, R.; Lubman, D.I.; Baker, A.L.; Dawe, S.; Ali, R.L. Dose-Related Psychotic Symptoms in Chronic Methamphetamine Users: Evidence From a Prospective Longitudinal Study. JAMA Psychiatry 2013, 70, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Kesby, J.P.; Eyles, D.W.; McGrath, J.J.; Scott, J.G. Dopamine, psychosis and schizophrenia: The widening gap between basic and clinical neuroscience. Transl. Psychiatry 2018, 8, 30. [Google Scholar] [CrossRef] [PubMed]
- Howes, O.D.; Kambeitz, J.; Kim, E.; Stahl, D.; Slifstein, M.; Abi-Dargham, A.; Kapur, S. The nature of dopamine dysfunction in schizophrenia and what this means for treatment. Arch. Gen. Psychiatry 2012, 69, 776–786. [Google Scholar] [CrossRef]
- Howes, O.D.; Kapur, S. The dopamine hypothesis of schizophrenia: Version III--the final common pathway. Schizophr. Bull. 2009, 35, 549–562. [Google Scholar] [CrossRef]
- Jauhar, S.; Veronese, M.; Nour, M.M.; Rogdaki, M.; Hathway, P.; Turkheimer, F.E.; Stone, J.; Egerton, A.; McGuire, P.; Kapur, S.; et al. Determinants of treatment response in first-episode psychosis: An 18F-DOPA PET study. Mol. Psychiatry 2019, 24, 1502–1512. [Google Scholar] [CrossRef]
- Masri, B.; Salahpour, A.; Didriksen, M.; Ghisi, V.; Beaulieu, J.M.; Gainetdinov, R.R.; Caron, M.G. Antagonism of dopamine D2 receptor/beta-arrestin 2 interaction is a common property of clinically effective antipsychotics. Proc. Natl. Acad. Sci. USA 2008, 105, 13656–13661. [Google Scholar] [CrossRef]
- Hidalgo-Figueroa, M.; Bonilla, S.; Gutiérrez, F.; Pascual, A.; López-Barneo, J. GDNF Is Predominantly Expressed in the PV+ Neostriatal Interneuronal Ensemble in Normal Mouse and after Injury of the Nigrostriatal Pathway. J. Neurosci. 2012, 32, 864–872. [Google Scholar] [CrossRef] [PubMed]
- D’Anglemont de Tassigny, X.; Pascual, A.; López-Barneo, J. GDNF-based therapies, GDNF-producing interneurons, and trophic support of the dopaminergic nigrostriatal pathway. Implications for Parkinson’s disease. Front. Neuroanat. 2015, 9, 10. [Google Scholar] [PubMed]
- Kopra, J.J.; Panhelainen, A.; Af Bjerkén, S.; Porokuokka, L.L.; Varendi, K.; Olfat, S.; Montonen, H.; Piepponen, T.P.; Saarma, M.; Andressoo, J.-O. Dampened Amphetamine-Stimulated Behavior and Altered Dopamine Transporter Function in the Absence of Brain GDNF. J. Neurosci. Off. J. Soc. Neurosci. 2017, 37, 1581–1590. [Google Scholar] [CrossRef]
- Kumar, A.; Kopra, J.; Varendi, K.; Porokuokka, L.L.; Panhelainen, A.; Kuure, S.; Marshall, P.; Karalija, N.; Härma, M.-A.; Vilenius, C.; et al. GDNF Overexpression from the Native Locus Reveals its Role in the Nigrostriatal Dopaminergic System Function. PLoS Genet. 2015, 11, e1005710. [Google Scholar] [CrossRef] [PubMed]
- Ibáñez, C.F.; Andressoo, J.-O. Biology of GDNF and its receptors—Relevance for disorders of the central nervous system. Neurobiol. Dis. 2017, 97, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Tomac, A.; Lindqvist, E.; Lin, L.F.H.; Ögren, S.O.; Young, D.; Hoffer, B.J.; Olson, L. Protection and repair of the nigrostriatal dopaminergic system by GDNF in vivo. Nature 1995, 373, 335–339. [Google Scholar] [CrossRef]
- Gash, D.M.; Zhang, Z.; Ovadia, A.; Cass, W.A.; Yi, A.; Simmerman, L.; Russell, D.; Martin, D.; Lapchak, P.A.; Collins, F.; et al. Functional recovery in parkinsonian monkeys treated with GDNF. Nature 1996, 380, 252–255. [Google Scholar] [CrossRef]
- Love, S.; Plaha, P.; Patel, N.K.; Hotton, G.R.; Brooks, D.J.; Gill, S.S. Glial cell line–derived neurotrophic factor induces neuronal sprouting in human brain. Nat. Med. 2005, 11, 703–704. [Google Scholar] [CrossRef]
- Kirik, D.; Rosenblad, C.; Björklund, A.; Mandel, R.J. Long-Term rAAV-Mediated Gene Transfer of GDNF in the Rat Parkinson’s Model: Intrastriatal But Not Intranigral Transduction Promotes Functional Regeneration in the Lesioned Nigrostriatal System. J. Neurosci. 2000, 20, 4686–4700. [Google Scholar] [CrossRef]
- Moriarty, N.; Gantner, C.W.; Hunt, C.P.J.; Ermine, C.M.; Frausin, S.; Viventi, S.; Ovchinnikov, D.A.; Kirik, D.; Parish, C.L.; Thompson, L.H. A combined cell and gene therapy approach for homotopic reconstruction of midbrain dopamine pathways using human pluripotent stem cells. Cell Stem Cell 2022, 29, 434–448.e5. [Google Scholar] [CrossRef]
- Kordower, J.H.; Emborg, M.E.; Bloch, J.; Ma, S.Y.; Chu, Y.; Leventhal, L.; McBride, J.; Chen, E.Y.; Palfi, S.; Roitberg, B.Z.; et al. Neurodegeneration prevented by lentiviral vector delivery of GDNF in primate models of Parkinson’s disease. Science 2000, 290, 767–773. [Google Scholar] [CrossRef]
- Glasner-Edwards, S.; Mooney, L.J. Methamphetamine Psychosis: Epidemiology and Management. CNS Drugs 2014, 28, 1115–1126. [Google Scholar] [CrossRef]
- Wearne, T.A.; Cornish, J.L. A Comparison of Methamphetamine-Induced Psychosis and Schizophrenia: A Review of Positive, Negative, and Cognitive Symptomatology. Front. Psychiatry 2018, 9, 491. [Google Scholar] [CrossRef]
- O’Dell, S.J.; Weihmuller, F.B.; Marshall, J.F. Multiple methamphetamine injections induce marked increases in extracellular striatal dopamine which correlate with subsequent neurotoxicity. Brain Res. 1991, 564, 256–260. [Google Scholar] [CrossRef]
- Stephans, S.E.; Yamamoto, B.K. Methamphetamine-induced neurotoxicity: Roles for glutamate and dopamine efflux. Synapse 1994, 17, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, K.; Kanzaki, A.; Tsuchida, K.; Ujike, H. Methamphetamine-induced behavioral sensitization and its implications for relapse of schizophrenia. Schizophr. Res. 1994, 12, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Archer, T.; Kostrzewa, R.M. Neuroteratology and Animal Modeling of Brain Disorders. In Neurotoxin Modeling of Brain Disorders—Life-Long Outcomes in Behavioral Teratology; Kostrzewa, R.M., Archer, T., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 1–40. [Google Scholar]
- Jones, C.A.; Watson, D.J.G.; Fone, K.C.F. Animal models of schizophrenia. Br. J. Pharmacol. 2011, 164, 1162–1194. [Google Scholar] [CrossRef]
- Machiyama, Y. Chronic methamphetamine intoxication model of schizophrenia in animals. Schizophr. Bull. 1992, 18, 107–113. [Google Scholar] [CrossRef]
- Oka, M.; Ito, K.; Koga, M.; Kusumi, I. Changes in subunit composition of NMDA receptors in animal models of schizophrenia by repeated administration of methamphetamine. Prog. Neuro Psychopharmacol. Biol. Psychiatry 2020, 103, 109984. [Google Scholar] [CrossRef]
- Shin, E.-J.; Dang, D.-K.; Tran, T.-V.; Tran, H.-Q.; Jeong, J.H.; Nah, S.-Y.; Jang, C.-G.; Yamada, K.; Nabeshima, T.; Kim, H.-C. Current understanding of methamphetamine-associated dopaminergic neurodegeneration and psychotoxic behaviors. Arch. Pharmacal Res. 2017, 40, 403–428. [Google Scholar]
- Greening, D.W.; Notaras, M.; Chen, M.; Xu, R.; Smith, J.D.; Cheng, L.; Simpson, R.J.; Hill, A.F.; van den Buuse, M. Chronic methamphetamine interacts with BDNF Val66Met to remodel psychosis pathways in the mesocorticolimbic proteome. Mol. Psychiatry 2021, 26, 4431–4447. [Google Scholar] [CrossRef] [PubMed]
- Kramer, J.C.; Fischman, V.S.; Littlefield, D.C. Amphetamine Abuse: Pattern and Effects of High Doses Taken Intravenously. JAMA 1967, 201, 305–309. [Google Scholar] [CrossRef] [PubMed]
- Manning, E.E.; van den Buuse, M. BDNF deficiency and young-adult methamphetamine induce sex-specific effects on prepulse inhibition regulation. Front. Cell. Neurosci. 2013, 7, 92. [Google Scholar] [CrossRef]
- Paulson, P.E.; Camp, D.M.; Robinson, T.E. Time course of transient behavioral depression and persistent behavioral sensitization in relation to regional brain monoamine concentrations during amphetamine withdrawal in rats. Psychopharmacology 1991, 103, 480–492. [Google Scholar] [CrossRef] [PubMed]
- Pogorelov, V.; Nomura, J.; Kim, J.; Kannan, G.; Yang, C.; Taniguchi, Y.; Abazyan, B.; Valentine, H.; Krasnova, I.N.; Kamiya, A.; et al. Mutant DISC1 affects methamphetamine-induced sensitization and conditioned place preference: A comorbidity model. Neuropharmacology 2012, 62, 1242–1251. [Google Scholar] [CrossRef] [PubMed]
- Manning, E.E.; Halberstadt, A.L.; van den Buuse, M. BDNF-Deficient Mice Show Reduced Psychosis-Related Behaviors Following Chronic Methamphetamine. Int. J. Neuropsychopharmacol. 2016, 19, pyv116. [Google Scholar] [CrossRef]
- Varendi, K.; Kumar, A.; Härma, M.-A.; Andressoo, J.-O. miR-1, miR-10b, miR-155, and miR-191 are novel regulators of BDNF. Cell. Mol. Life Sci. 2014, 71, 4443–4456. [Google Scholar] [CrossRef]
- Ashburner, M.; Ball, C.A.; Blake, J.A.; Botstein, D.; Butler, H.; Cherry, J.M.; Davis, A.P.; Dolinski, K.; Dwight, S.S.; Eppig, J.T.; et al. Gene Ontology: Tool for the unification of biology. Nat. Genet. 2000, 25, 25–29. [Google Scholar] [CrossRef]
- The Gene Ontology, C.; Carbon, S.; Douglass, E.; Good, B.M.; Unni, D.R.; Harris, N.L.; Mungall, C.J.; Basu, S.; Chisholm, R.L.; Dodson, R.J.; et al. The Gene Ontology resource: Enriching a GOld mine. Nucleic Acids Res. 2021, 49, D325–D334. [Google Scholar] [CrossRef]
- Lee, T.; Seeman, P. Brain dopamine receptors in schizophrenia. In Biological Markers in Psychiatry and Neurology; Usdin, E., Hanin, I., Eds.; Pergamon: Oxford, UK, 1982; pp. 219–226. [Google Scholar]
- Hirvonen, J.; van Erp, T.G.M.; Huttunen, J.; Aalto, S.; Någren, K.; Huttunen, M.; Lönnqvist, J.; Kaprio, J.; Hietala, J.; Cannon, T.D. Increased Caudate Dopamine D2 Receptor Availability as a Genetic Marker for Schizophrenia. Arch. Gen. Psychiatry 2005, 62, 371–378. [Google Scholar] [CrossRef][Green Version]
- Seeman, P.; Kapur, S. Schizophrenia: More dopamine, more D2 receptors. Proc. Natl. Acad. Sci. USA 2000, 97, 7673–7675. [Google Scholar] [CrossRef]
- Brisch, R.; Saniotis, A.; Wolf, R.; Bielau, H.; Bernstein, H.-G.; Steiner, J.; Bogerts, B.; Braun, K.; Jankowski, Z.; Kumaratilake, J.; et al. The Role of Dopamine in Schizophrenia from a Neurobiological and Evolutionary Perspective: Old Fashioned, but Still in Vogue. Front. Psychiatry 2014, 5, 47. [Google Scholar]
- Brunelin, J.; Fecteau, S.; Suaud-Chagny, M.-F. Abnormal Striatal Dopamine Transmission in Schizophrenia. Curr. Med. Chem. 2013, 20, 397–404. [Google Scholar] [PubMed]
- Toda, M.; Abi-Dargham, A. Dopamine hypothesis of schizophrenia: Making sense of it all. Curr. Psychiatry Rep. 2007, 9, 329–336. [Google Scholar] [PubMed]
- Okubo, Y.; Suhara, T.; Suzuki, K.; Kobayashi, K.; Inoue, O.; Terasaki, O.; Someya, Y.; Sassa, T.; Sudo, Y.; Matsushima, E.; et al. Decreased prefrontal dopamine D1 receptors in schizophrenia revealed by PET. Nature 1997, 385, 634–636. [Google Scholar] [CrossRef] [PubMed]
- Daubner, S.C.; Le, T.; Wang, S. Tyrosine Hydroxylase and Regulation of Dopamine Synthesis. Arch. Biochem. Biophys. 2011, 508, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Mueller, H.T.; Haroutunian, V.; Davis, K.L.; Meador-Woodruff, J.H. Expression of the ionotropic glutamate receptor subunits and NMDA receptor-associated intracellular proteins in the substantia nigra in schizophrenia. Brain Res. Mol. Brain Res. 2004, 121, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Ichinose, H.; Ohye, T.; Fujita, K.; Pantucek, F.; Lange, K.; Riederer, P.; Nagatsu, T. Quantification of mRNA of tyrosine hydroxylase and aromatic L-amino acid decarboxylase in the substantia nigra in Parkinson’s disease and schizophrenia. Journal of Neural Transmission. Park. Dis. Dement. Sect. 1994, 8, 149–158. [Google Scholar] [CrossRef]
- Purves-Tyson, T.D.; Owens, S.J.; Rothmond, D.A.; Halliday, G.M.; Double, K.L.; Stevens, J.; McCrossin, T.; Shannon Weickert, C. Putative presynaptic dopamine dysregulation in schizophrenia is supported by molecular evidence from post-mortem human midbrain. Transl. Psychiatry 2017, 7, e1003. [Google Scholar] [CrossRef]
- Bilic, P.; Jukic, V.; Vilibic, M.; Savic, A.; Bozina, N. Treatment-resistant schizophrenia and DAT and SERT polymorphisms. Gene 2014, 543, 125–132. [Google Scholar] [CrossRef]
- Dean, B.; Hayes, W.; Opeskin, K.; Naylor, L.; Pavey, G.; Hill, C.; Keks, N.; Copolov, D.L. Serotonin2 receptors and the serotonin transporter in the schizophrenic brain. Behav. Brain Res. 1996, 73, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Cannon, M.; Murray, R.M. Neonatal origins of schizophrenia. Arch. Dis. Child. 1998, 78, 1–3. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jenkins, T. Perinatal complications and schizophrenia: Involvement of the immune system. Front. Neurosci. 2013, 7, 110. [Google Scholar] [CrossRef]
- Aguilar-Valles, A.; Rodrigue, B.; Matta-Camacho, E. Maternal Immune Activation and the Development of Dopaminergic Neurotransmission of the Offspring: Relevance for Schizophrenia and Other Psychoses. Front. Psychiatry 2020, 11, 852. [Google Scholar] [CrossRef]
- Choudhury, Z.; Lennox, B. Maternal Immune Activation and Schizophrenia—Evidence for an Immune Priming Disorder. Front. Psychiatry 2021, 12, 585742. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Holley, S.M.; Shobe, J.L.; Chong, N.C.; Cepeda, C.; Levine, M.S.; Masmanidis, S.C. Parvalbumin Interneurons Modulate Striatal Output and Enhance Performance during Associative Learning. Neuron 2017, 93, 1451–1463.e4. [Google Scholar] [CrossRef]
- Ola, R.; Jakobson, M.; Kvist, J.; Perälä, N.; Kuure, S.; Braunewell, K.-H.; Bridgewater, D.; Rosenblum, N.D.; Chilov, D.; Immonen, T.; et al. The GDNF target Vsnl1 marks the ureteric tip. J. Am. Soc. Nephrol. 2011, 22, 274–284. [Google Scholar] [CrossRef]
- Lu, B.C.; Cebrian, C.; Chi, X.; Kuure, S.; Kuo, R.; Bates, C.M.; Arber, S.; Hassell, J.; MacNeil, L.; Hoshi, M.; et al. Etv4 and Etv5 are required downstream of GDNF and Ret for kidney branching morphogenesis. Nat. Genet. 2009, 41, 1295–1302. [Google Scholar] [CrossRef]
- Bowenkamp, K.E.; Hoffman, A.F.; Gerhardt, G.A.; Henry, M.A.; Biddle, P.T.; Hoffer, B.J.; Granholm, A.-C.E. Glial cell line-derived neurotrophic factor supports survival of injured midbrain dopaminergic neurons. J. Comp. Neurol. 1995, 355, 479–489. [Google Scholar] [CrossRef]
- Lin, L.-F.H.; Doherty, D.H.; Lile, J.D.; Bektesh, S.; Collins, F. GDNF: A Glial Cell Line-Derived Neurotrophic Factor for Midbrain Dopaminergic Neurons. Science 1993, 260, 1130–1132. [Google Scholar] [CrossRef]
- Hoffer, B.J.; Hoffman, A.; Bowenkamp, K.; Huettl, P.; Hudson, J.; Martin, D.; Lin, L.-F.H.; Gerhardt, G.A. Glial cell line-derived neurotrophic factor reverses toxin-induced injury to midbrain dopaminergic neurons in vivo. Neurosci. Lett. 1994, 182, 107–111. [Google Scholar] [CrossRef]
- Taraviras, S.; Marcos-Gutierrez, C.V.; Durbec, P.; Jani, H.; Grigoriou, M.; Sukumaran, M.; Wang, L.C.; Hynes, M.; Raisman, G.; Pachnis, V. Signalling by the RET receptor tyrosine kinase and its role in the development of the mammalian enteric nervous system. Development 1999, 126, 2785–2797. [Google Scholar] [CrossRef] [PubMed]
- Drinkut, A.; Tillack, K.; Meka, D.P.; Schulz, J.B.; Kügler, S.; Kramer, E.R. Ret is essential to mediate GDNF’s neuroprotective and neuroregenerative effect in a Parkinson disease mouse model. Cell Death Dis. 2016, 7, e2359. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Kunugi, H.; Nanko, S. Glial cell line-derived neurotrophic factor (GDNF) gene and schizophrenia: Polymorphism screening and association analysis. Psychiatry Res. 2001, 104, 11–17. [Google Scholar] [CrossRef]
- Skibinska, M.; Kapelski, P.; Pawlak, J.; Rajewska-Rager, A.; Dmitrzak-Weglarz, M.; Szczepankiewicz, A.; Czerski, P.; Twarowska-Hauser, J. Glial Cell Line-Derived Neurotrophic Factor (GDNF) serum level in women with schizophrenia and depression, correlation with clinical and metabolic parameters. Psychiatry Res. 2017, 256, 396–402. [Google Scholar] [CrossRef] [PubMed]
- Niitsu, T.; Shirayama, Y.; Matsuzawa, D.; Shimizu, E.; Hashimoto, K.; Iyo, M. Association between serum levels of glial cell-line derived neurotrophic factor and attention deficits in schizophrenia. Neurosci. Lett. 2014, 575, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.-S.; Chu, C.-L.; Wu, C.-C.; Lu, T. Serum nerve growth factor beta, brain- and glial-derived neurotrophic factor levels and psychopathology in unmedicated patients with schizophrenia. J. Chin. Med. Assoc. 2018, 81, 577–581. [Google Scholar] [CrossRef] [PubMed]
- Tunca, Z.; Akdede, B.K.; Özerdem, A.; Alkın, T.; Polat, S.; Ceylan, D.; Bayın, M.; Kocuk, N.C.; Şimşek, S.; Resmi, H.; et al. Diverse Glial Cell Line-Derived Neurotrophic Factor (GDNF) Support Between Mania and Schizophrenia: A Comparative Study in Four Major Psychiatric Disorders. Eur. Psychiatry 2015, 30, 198–204. [Google Scholar] [CrossRef]
- Grant, K.M.; LeVan, T.D.; Wells, S.M.; Li, M.; Stoltenberg, S.F.; Gendelman, H.E.; Carlo, G.; Bevins, R.A. Methamphetamine-associated psychosis. J. Neuroimmune Pharmacol. 2012, 7, 113–139. [Google Scholar] [CrossRef]
- Van den Buuse, M. Modeling the positive symptoms of schizophrenia in genetically modified mice: Pharmacology and methodology aspects. Schizophr. Bull. 2010, 36, 246–270. [Google Scholar] [CrossRef]
- Kopra, J.; Vilenius, C.; Grealish, S.; Härma, M.-A.; Varendi, K.; Lindholm, J.; Castrén, E.; Võikar, V.; Björklund, A.; Piepponen, T.P.; et al. GDNF is not required for catecholaminergic neuron survival in vivo. Nat. Neurosci. 2015, 18, 319–322. [Google Scholar] [CrossRef] [PubMed]
- Olfat, S.; Mätlik, K.; Kopra, J.J.; Garton, D.R.; Iivanainen, V.H.; Bhattacharya, D.; Jakobsson, J.; Piepponen, T.P.; Andressoo, J.-O. Increased Physiological GDNF Levels Have No Effect on Dopamine Neuron Protection and Restoration in a Proteasome Inhibition Mouse Model of Parkinson’s Disease. eNeuro 2023, 10. [Google Scholar] [CrossRef] [PubMed]
- Bonafina, A.; Trinchero, M.F.; Ríos, A.S.; Bekinschtein, P.; Schinder, A.F.; Paratcha, G.; Ledda, F. GDNF and GFRα1 Are Required for Proper Integration of Adult-Born Hippocampal Neurons. Cell Rep. 2019, 29, 4308–4319.e4. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Jiang, J.; Hofmann, M.C.; Dym, M. Gfra1 silencing in mouse spermatogonial stem cells results in their differentiation via the inactivation of RET tyrosine kinase. Biol. Reprod. 2007, 77, 723–733. [Google Scholar] [CrossRef]
- Pozas, E.; Ibáñez, C.F. GDNF and GFRalpha1 promote differentiation and tangential migration of cortical GABAergic neurons. Neuron 2005, 45, 701–713. [Google Scholar] [CrossRef]
- Kimura, T.; Yoshimoto, K.; Tanaka, C.; Ohkura, T.; Iwahana, H.; Miyauchi, A.; Sano, T.; Itakura, M. Obvious mRNA and protein expression but absence of mutations of the RET proto-oncogene in parathyroid tumors. Eur. J. Endocrinol. 1996, 134, 314–319. [Google Scholar] [CrossRef]
- Drilon, A.; Oxnard, G.R.; Tan, D.S.W.; Loong, H.H.F.; Johnson, M.; Gainor, J.; McCoach, C.E.; Gautschi, O.; Besse, B.; Cho, B.C.; et al. Efficacy of Selpercatinib in RET Fusion–Positive Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2020, 383, 813–824. [Google Scholar] [CrossRef]
- Nguyen, L.; Monestime, S. Pralsetinib: Treatment of metastatic RET fusion–positive non–small cell lung cancer. Am. J. Health-Syst. Pharm. 2022, 79, 527–533. [Google Scholar] [CrossRef]
- Syed, Y.Y. Pralsetinib: A Review in Advanced RET Fusion-Positive NSCLC. Drugs 2022, 82, 811–816. [Google Scholar] [CrossRef]
- Burke, R.E. GDNF as a candidate striatal target-derived neurotrophic factor for the development of substantia nigra dopamine neurons. J. Neural Transm. 2006, 70, 41–45. [Google Scholar]
- Trupp, M.; Arenas, E.; Fainzilber, M.; Nilsson, A.-S.; Sieber, B.-A.; Grigoriou, M.; Kilkenny, C.; Salazar-Grueso, E.; Pachnis, V.; Arumäe, U.; et al. Functional receptor for GDNF encoded by the c-ret proto-oncogene. Nature 1996, 381, 785–789. [Google Scholar] [CrossRef]
- Meka, D.P.; Müller-Rischart, A.K.; Nidadavolu, P.; Mohammadi, B.; Motori, E.; Ponna, S.K.; Aboutalebi, H.; Bassal, M.; Annamneedi, A.; Finckh, B.; et al. Parkin cooperates with GDNF/RET signaling to prevent dopaminergic neuron degeneration. J. Clin. Investig. 2015, 125, 1873–1885. [Google Scholar] [CrossRef] [PubMed]
- Etemadi-Aleagha, A.; Akhgari, M. Psychotropic drug abuse in pregnancy and its impact on child neurodevelopment: A review. World J. Clin. Pediatr. 2022, 11, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Airaksinen, M.S.; Saarma, M. The GDNF family: Signalling, biological functions and therapeutic value. Nat. Rev. Neurosci. 2002, 3, 383–394. [Google Scholar] [CrossRef] [PubMed]
- Grace, A.A.; Gomes, F.V. The Circuitry of Dopamine System Regulation and its Disruption in Schizophrenia: Insights Into Treatment and Prevention. Schizophr. Bull. 2019, 45, 148–157. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).