Metformin Prevents NDEA-Induced Memory Impairments Associated with Attenuating Beta-Amyloid, Tumor Necrosis Factor-Alpha, and Interleukin-6 Levels in the Hippocampus of Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Drugs
2.2. Animals
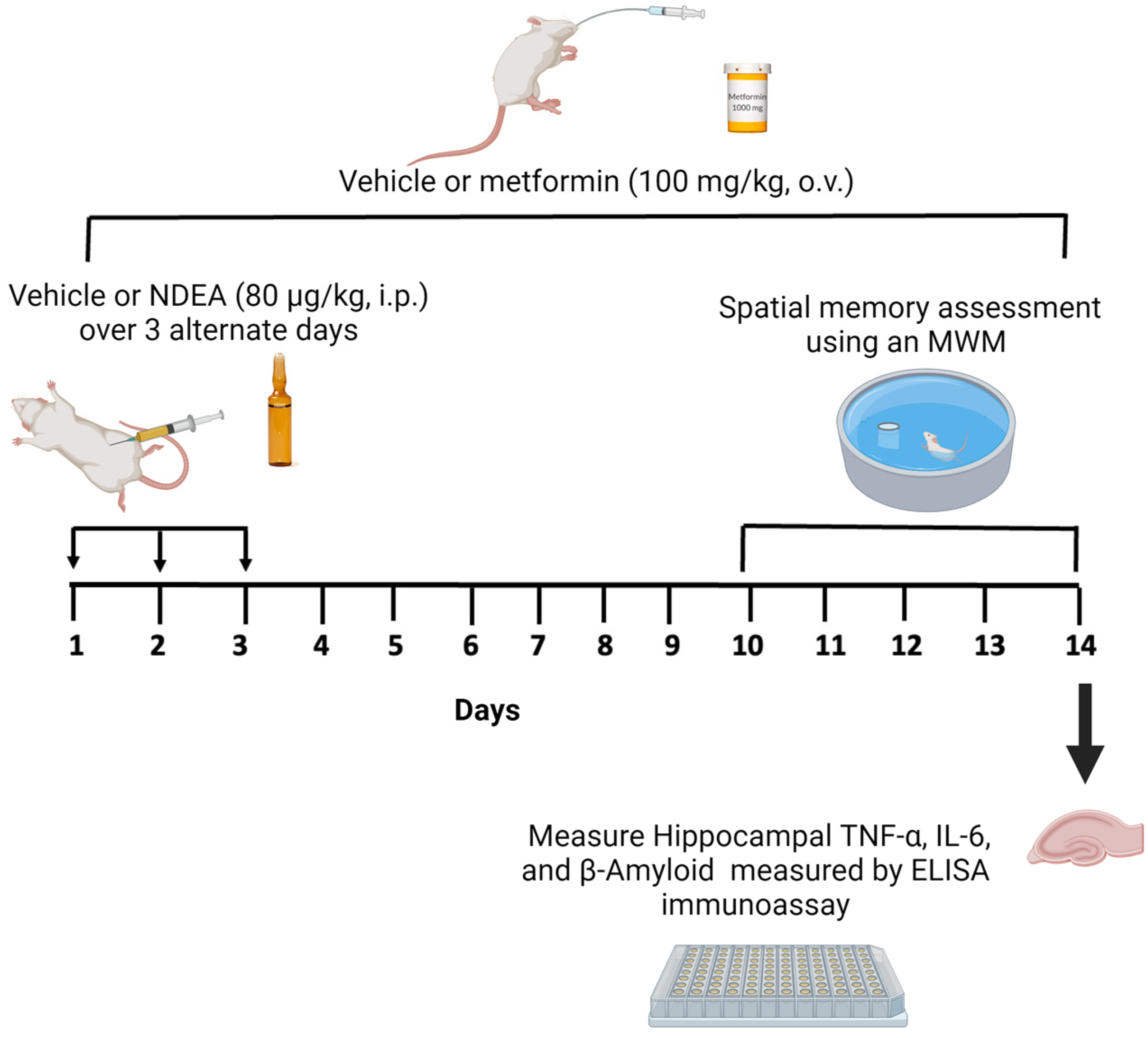
2.3. Experimental Design
2.4. Morris Water Maze Test
2.5. Brain Tissue Preparation
2.6. Enzyme-Linked Immunosorbent Assay (ELISA)
2.7. Statistical Analysis
3. Results
3.1. NDEA Impairs Spatial Memory as Measured by the Morris Water Maze Test
3.2. Metformin Treatment Rescues Spatial Memory in NDEA Rats
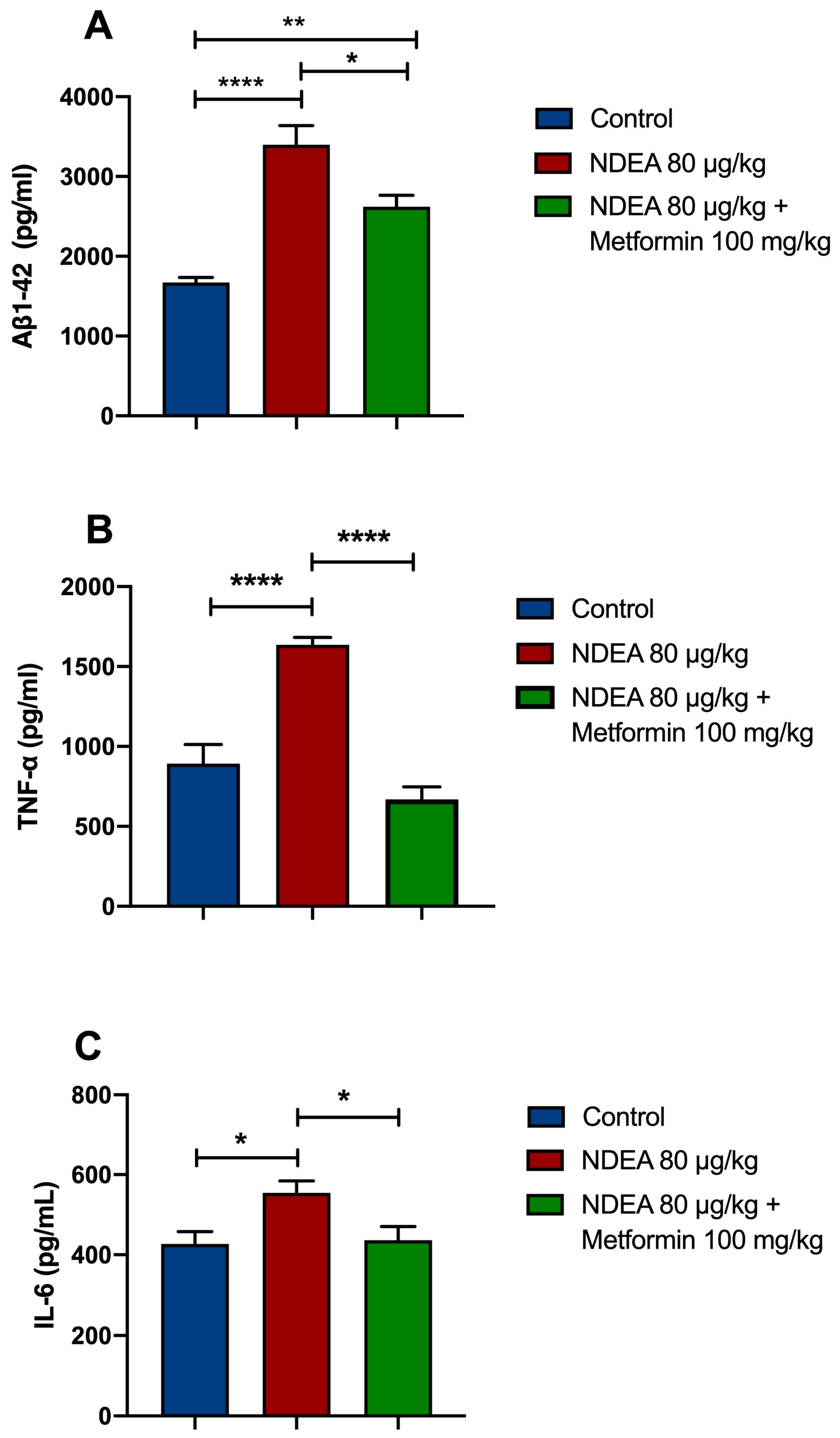
3.3. Metformin Reduces Brain Aβ1-42 Levels in NDEA Rats
3.4. Metformin Reduces Proinflammatory Cytokine Levels
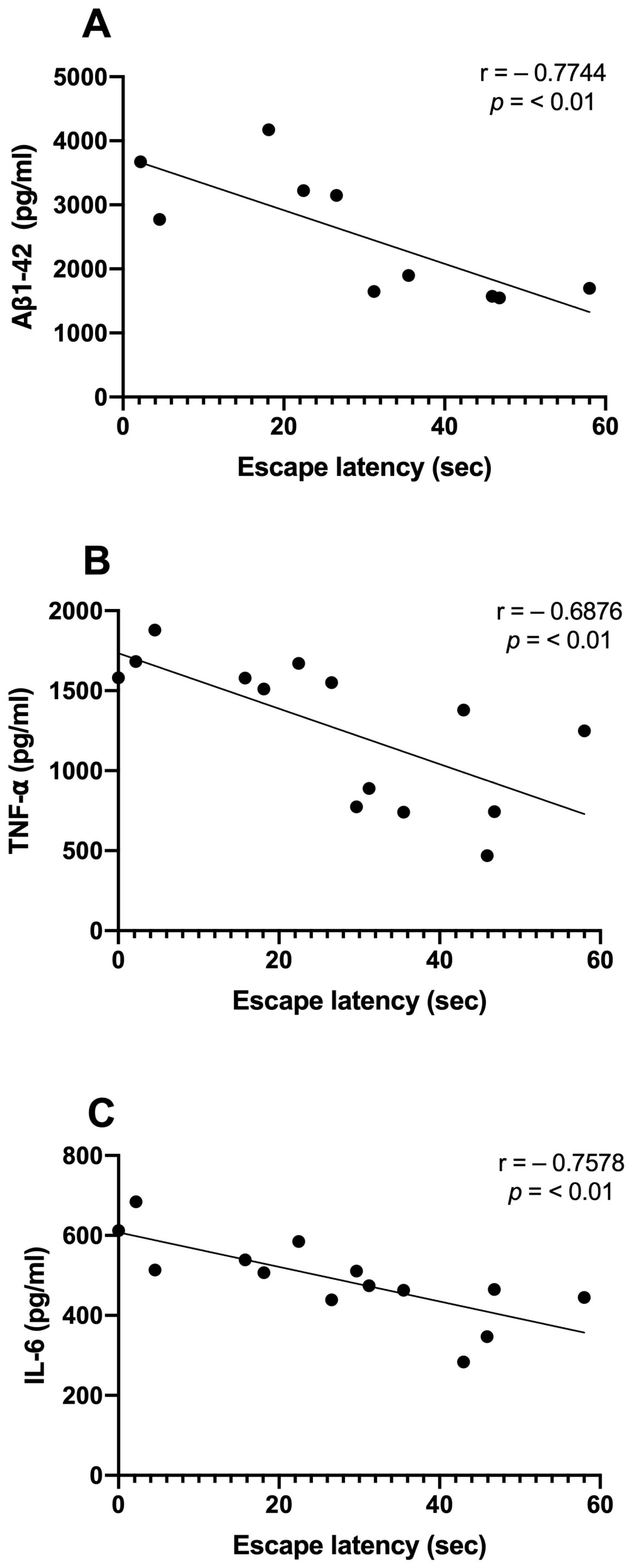
3.5. Correlations between Aβ, Inflammatory Markers, and Memory Consolidation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lijinsky, W. N-Nitroso compounds in the diet. Mutat. Res. Genet. Toxicol. Environ. Mutagen 1999, 443, 129–138. [Google Scholar] [CrossRef]
- Gushgari, A.J.; Halden, R.U. Critical review of major sources of human exposure to N-nitrosamines. Chemosphere 2018, 210, 1124–1136. [Google Scholar] [CrossRef]
- Hotchkiss, J.H. Preformed N-nitroso compounds in foods and beverages. Cancer Surv. 1989, 8, 295–321. [Google Scholar]
- Park, J.-E.; Seo, J.-E.; Lee, J.-Y.; Kwon, H. Distribution of seven N-nitrosamines in food. Toxicol. Res. 2015, 31, 279–288. [Google Scholar] [CrossRef]
- Brunnemann, K.D.; Hoffmann, D. Analytical stu7dies on tobacco-specific N-nitrosamines in tobacco and tobacco smoke. Crit. Rev. Toxicol. 1991, 21, 235–240. [Google Scholar] [CrossRef]
- Xia, Y.; McGuffey, J.E.; Bhattacharyya, S.; Sellergren, B.; Yilmaz, E.; Wang, L.; Bernert, J.T. Analysis of the tobacco-specific nitrosamine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol in urine by extraction on a molecularly imprinted polymer column and liquid chromatography/atmospheric pressure ionization tandem mass spectrometry. Anal. Chem. 2005, 77, 7639–7645. [Google Scholar] [CrossRef]
- Wang, L.-H.; Hsia, H.-C.; Wang, C.-C. Simultaneous determination of five volatile and non-volatile N-nitrosamines in biological fluids and cosmetic products by liquid chromatography with photodiode array detection. J. Liq. Chromatogr. Relat. 2006, 29, 1737–1751. [Google Scholar] [CrossRef]
- Cal, D.H.S. A Brief History of NDMA Findings in Drinking Water Supplies; California Department of Health Services: Sacramento, CA, USA, 2002.
- FDA Updates and Press Announcements on Angiotensin II Receptor Blocker (ARB) Recalls (Valsartan, Losartan, and Irbesartan); Drug Safety and Availability; U.S. Food and Drug Administration: Silver Spring, MD, USA, 2019.
- Li, K.; Ricker, K.; Tsai, F.C.; Hsieh, C.J.; Osborne, G.; Sun, M.; Marder, M.E.; Elmore, S.; Schmitz, R.; Sandy, M.S. Estimated cancer risks associated with nitrosamine contamination in commonly used medications. Int. J. Environ. Res. Public Health 2021, 18, 9465. [Google Scholar] [CrossRef]
- Vermeer, I.T.M.; Pachen, D.M.F.A.; Dalliga, J.W.; Kleinjans, J.C.S.; Van Maanen, J.M.S. Volatile N-nitrosamine formation after intake of nitrate at the ADl Level in combination with an aminerich diet. Environ. Health Perspect. 1998, 106, 459–462. [Google Scholar] [CrossRef]
- Kolb, E.; Haug, M.; Janzowski, C.; Vetter, A.; Eisenbrand, G. Potential nitrosamine formation and its prevention during biological denitrification of red beet juice. Food Chem. Toxicol. 1997, 35, 219–224. [Google Scholar] [CrossRef]
- Kumar, A.; Sunita, P.; Pattanayak, S.P. Silibinin inhibits the hepatocellular carcinoma in NDEA-induced rodent carcinogenesis model: An evaluation through biochemical and bio-structural parameters. J. Cancer Sci. Ther. 2015, 7, 206–215. [Google Scholar]
- Afzal, M.; Kazmi, I.; Gupta, G.; Rahman, M.; Kimothi, V.; Anwar, F. Preventive effect of Metformin against N-nitrosodiethylamine-initiated hepatocellular carcinoma in rats. Saudi Pharm. J. 2012, 20, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Hamid, N.M.; Nazmy, M.H.; Abdel-Ghany, M.I.; Nazmy, W.H. Cytokines as important playmakers of experimental hepatocarcinogenesis confounded by diabetes. Ann. Hepatol. 2012, 11, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Tong, M.; Neusner, A.; Longato, L.; Lawton, M.; Wands, J.R.; de la Monte, S.M. Nitrosamine exposure causes insulin resistance diseases: Relevance to type 2 diabetes mellitus, non-alcoholic steatohepatitis, and Alzheimer’s disease. J. Alzheimers Dis. 2009, 17, 827–844. [Google Scholar] [PubMed]
- de la Monte, S.M.; Tong, M. Mechanisms of nitrosamine-mediated neurodegeneration: Potential relevance to sporadic Alzheimer’s disease. J. Alzheimers Dis. 2009, 17, 817–825. [Google Scholar] [CrossRef]
- Askarova, S.; Umbayev, B.; Masoud, A.-R.; Kaiyrlykyzy, A.; Safarova, Y.; Tsoy, A.; Olzhayev, F.; Kushugulova, A. The links between the gut microbiome, aging, modern lifestyle and Alzheimer’s disease. Front. Cell Infect. Microbiol. 2020, 10, 104. [Google Scholar] [CrossRef]
- Chen, G.F.; Xu, T.H.; Yan, Y.; Zhou, Y.R.; Jiang, Y.; Melcher, K.; Xu, H.E. Amyloid beta: Structure, biology and structure-based therapeutic development. Acta Pharmacol. Sin. 2017, 38, 1205–1235. [Google Scholar] [CrossRef]
- Vadukul, D.M.; Maina, M.; Franklin, H.; Nardecchia, A.; Serpell, L.C.; Marshall, K.E. Internalisation and toxicity of amyloid-β 1-42 are influenced by its conformation and assembly state rather than size. FEBS Lett. 2020, 594, 3490–3503. [Google Scholar] [CrossRef]
- Brunnström, H.R.; Englund, E.M. Cause of death in patients with dementia disorders. Eur. J. Neurol. 2009, 16, 488–492. [Google Scholar] [CrossRef]
- Chai, A.B.; Leung, G.K.F.; Callaghan, R.; Gelissen, I.C. P-Glycoprotein: A Role in the Export of Amyloid-B in Alzheimer’s Disease? FEBS J. 2020, 287, 612–625. [Google Scholar] [CrossRef]
- Folch, J.; Ettcheto, M.; Busquets, O.; Sánchez-López, E.; Castro-Torres, R.D.; Verdaguer, E.; Manzine, P.R.; Poor, S.R.; García, M.L.; Olloquequi, J.; et al. The implication of the brain insulin receptor in late onset Alzheimer’s disease dementia. Pharmaceuticals 2018, 11, 11. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-G. Cognitive Dysfunctions in Individuals with Diabetes Mellitus. Yeungnam Univ. J. Med. 2019, 36, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Biessels, G.J.; Reagan, L.P. Hippocampal insulin resistance and cognitive dysfunction. Nat. Rev. Neurosci. 2015, 16, 660–671. [Google Scholar] [CrossRef]
- Boucher, J.; Kleinridders, A.; Kahn, C.R. Insulin receptor signaling in normal and insulin-resistant states. Cold Spring Harb. Perspect. Biol. 2014, 6, a009191. [Google Scholar] [CrossRef] [PubMed]
- Bassil, F.; Fernagut, P.-O.; Bezard, E.; Meissner, W.G. Insulin, IGF-1 and GLP-1 signaling in neurodegenerative disorders: Targets for disease modification? Prog. Neurobiol. 2014, 118, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Ransohoff, R.M.; Brown, M.A. Innate immunity in the central nervous system. J. Clin. Investig. 2012, 122, 1164–1171. [Google Scholar] [CrossRef]
- Bhatia, V.; Sharma, S. Role of mitochondrial dysfunction, oxidative stress and autophagy in progression of Alzheimer’s disease. J. Neurol. Sci. 2021, 421, 117253. [Google Scholar] [CrossRef]
- Holmes, C. Review: Systemic inflammation and Alzheimer’s disease: Systemic inflammation and AD. Neuropathol. Appl. Neurobiol. 2013, 39, 51–68. [Google Scholar] [CrossRef]
- Fakhoury, M. Microglia and astrocytes in Alzheimer’s disease: Implications for therapy. Curr. Neuropharmacol. 2018, 16, 508–518. [Google Scholar] [CrossRef]
- Agostinho, P.; Cunha, R.A.; Oliveira, C. Neuroinflammation, oxidative stress and the pathogenesis of Alzheimer’s disease. Curr. Pharm. Des. 2010, 16, 2766–2778. [Google Scholar] [CrossRef]
- Brietzke, S.A. Oral antihyperglycemic treatment options for type 2 diabetes mellitus. Med. Clin. N. Am. 2015, 99, 87–106. [Google Scholar] [CrossRef] [PubMed]
- Flory, J.; Lipska, K. Metformin in 2019. JAMA 2019, 321, 1926. [Google Scholar] [CrossRef]
- Kaneto, H.; Kimura, T.; Obata, A.; Shimoda, M.; Kaku, K. Multifaceted mechanisms of action of metformin which have been unraveled one after another in the long history. Int. J. Mol. Sci. 2021, 22, 2596. [Google Scholar] [CrossRef]
- Zhou, G.; Myers, R.; Li, Y.; Chen, Y.; Shen, X.; Fenyk-Melody, J.; Wu, M.; Ventre, J.; Doebber, T.; Fujii, N.; et al. Role of AMP-activated protein kinase in mechanism of metformin action. J. Clin. Investig. 2001, 108, 1167–1174. [Google Scholar] [CrossRef] [PubMed]
- Kelly, B.; Tannahill, G.M.; Murphy, M.P.; Neill, L.A. Metformin inhibits the production of reactive oxygen species from NADH: Ubiquinone oxidoreductase to limit induction of interleukin-1β (IL-1β) and boosts interleukin-10 (IL-10) in lipopolysaccharide (LPS)-activated macrophages. J. Biol. Chem. 2015, 290, 20348–20359. [Google Scholar] [CrossRef] [PubMed]
- Bharath, L.P.; Nikolajczyk, B.S. The intersection of metformin and inflammation. Am. J. Physiol. Cell Physiol. 2021, 320, C873–C879. [Google Scholar] [CrossRef] [PubMed]
- Soberanes, S.; Misharin, A.V.; Jairaman, A.; Morales-Nebreda, L.; McQuattie-Pimentel, A.C.; Cho, T.; Hamanaka, R.B.; Meliton, A.Y.; Reyfman, P.A.; Walter, J.M.; et al. Metformin targets mitochondrial electron transport to reduce air-pollution-induced thrombosis. Cell Metab. 2019, 29, 335–347.e5. [Google Scholar] [CrossRef]
- Ali, D.-E.; Shah, M.; Ali, A.; Malik, M.O.; Rehman, F.; Badshah, H.; Ehtesham, E.; Vitale, S.G. Treatment with metformin and combination of metformin plus pioglitazone on serum levels of IL-6 and IL-8 in polycystic ovary syndrome: A randomized clinical trial. Horm. Metab. Res. 2019, 51, 714–722. [Google Scholar] [CrossRef]
- Victor, V.M.; Rovira-Llopis, S.; Bañuls, C.; Diaz-Morales, N.; Castelló, R.; Falcón, R.; Gómez, M.; Rocha, M.; Hernández-Mijares, A. Effects of metformin on mitochondrial function of leukocytes from polycystic ovary syndrome patients with insulin resistance. Eur. J. Endocrinol. 2015, 173, 683–691. [Google Scholar] [CrossRef]
- Ye, J.; Zhu, N.; Sun, R.; Liao, W.; Fan, S.; Shi, F.; Lin, H.; Jiang, S.; Ying, Y. Metformin inhibits chemokine expression through the AMPK/NF-κB signaling pathway. J. Interferon Cytokine Res. 2018, 38, 363–369. [Google Scholar] [CrossRef]
- Gönül, T.; Soydas, G. NF-κB as the mediator of metformin’s effect on ageing and ageing-related diseases. Clin. Exp. Pharmacol. Physiol. 2019, 46, 413–422. [Google Scholar]
- Moiseeva, O.; Deschênes-Simard, X.; St-Germain, E.; Igelmann, S.; Huot, G.; Cadar, A.E.; Bourdeau, V.; Pollak, M.N.; Ferbeyre, G. Metformin inhibits the senescence associated secretory phenotype by interfering with IKK/NF-κB activation. Aging Cell 2013, 12, 489–498. [Google Scholar] [CrossRef] [PubMed]
- Femminella, G.D.; Bencivenga, L.; Petraglia, L.; Visaggi, L.; Gioia, L.; Grieco, F.V.; de Lucia, C.; Komici, K.; Corbi, G.; Edison, P.; et al. Antidiabetic drugs in Alzheimer’s disease: Mechanisms of action and future perspectives. J. Diabetes Res. 2017, 2017, 7420796. [Google Scholar] [CrossRef]
- Zhong, K.L.; Chen, F.; Hong, H.; Ke, X.; Lv, Y.G.; Tang, S.S.; Zhu, Y.B. New views and possibilities of antidiabetic drugs in treating and/or preventing mild cognitive impairment and Alzheimer’s Disease. Metab. Brain Dis. 2018, 33, 1009–1018. [Google Scholar] [CrossRef]
- Madhu, L.N.; Kodali, M.; Shetty, A.K. Promise of metformin for preventing age-related cognitive dysfunction. Neural Regen Res. 2022, 17, 503–507. [Google Scholar] [CrossRef]
- de la Monte, S.M.; Tong, M.; Lawton, M.; Longato, L. Nitrosamine exposure exacerbates high fat diet-mediated type 2 diabetes mellitus, non-alcoholic steatohepatitis, and neurodegeneration with cognitive impairment. Mol. Neurodegener. 2009, 4, 54. [Google Scholar] [CrossRef] [PubMed]
- Morris, R. Developments of a water-maze procedure for studying spatial learning in the rat. J. Neurosci. Methods 1984, 11, 47–60. [Google Scholar] [CrossRef] [PubMed]
- Morris Spatial localization does not require the presence of local cues. Learn. Motiv. 1981, 12, 239–260. [CrossRef]
- Lissner, L.J.; Wartchow, K.M.; Toniazzo, A.P.; Gonçalves, C.-A.; Rodrigues, L. Object recognition and Morris water maze to detect cognitive impairment from mild hippocampal damage in rats: A reflection based on the literature and experience. Pharmacol. Biochem. Behav. 2021, 210, 173273. [Google Scholar] [CrossRef]
- Dubois, B.; Feldman, H.H.; Jacova, C.; Hampel, H.; Molinuevo, J.L.; Blennow, K.; DeKosky, S.T.; Gauthier, S.; Selkoe, D.; Bateman, R.; et al. Advancing research diagnostic criteria for Alzheimer’s disease: The IWG-2 criteria. Lancet Neurol. 2014, 13, 614–629. [Google Scholar] [CrossRef]
- Montinel, T.J.; Phelps, C.H.; Beach, T.G. National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease: A practical approach. Acta Neuropathol. 2012, 123, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Bachstetter, A.D.; Van Eldik, L.J.; Schmitt, F.A.; Neltner, J.H.; Ighodaro, E.T.; Webster, S.J.; Patel, E.; Abner, E.L.; Kryscio, R.J.; Nelson, P.T. Disease-related microglia heterogeneity in the hippocampus of Alzheimer’s disease, dementia with Lewy bodies, and hippocampal sclerosis of aging. Acta Neuropathol. Commun. 2015, 3, 32. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Hussain, M.D.; Yan, L.-J. Microglia, neuroinflammation, and beta-amyloid protein in Alzheimer’s disease. Int. J. Neurosci. 2014, 124, 307–321. [Google Scholar] [CrossRef]
- Cacquevel, M.; Lebeurrier, N.; Chéenne; Vivien, D. Cytokines in neuroinflammation and Alzheimer’s disease. Curr. Drug Targets 2004, 5, 529–534. [Google Scholar] [CrossRef] [PubMed]
- Mrak, R.E.; Griffin, W.S.T. Glia and their cytokines in progression of neurodegeneration. Neurobiol. Aging 2005, 26, 349–354. [Google Scholar] [CrossRef]
- Von Bernhardi, R.; Tichauer, J.E.; Eugenín, J. Aging dependent changes of microglial cells and their relevance for neurodegenerative disorders. J. Neurochem. 2010, 112, 1099–1114. [Google Scholar] [CrossRef]
- Zhang, B.; Gaiteri, C.; Bodea, L.-G.; Wang, Z.; McElwee, J.; Podtelezhnikov, A.A.; Zhang, C.; Xie, T.; Tran, L.; Dobrin, R.; et al. Integrated systems approach identifies genetic nodes and networks in late-onset Alzheimer’s disease. Cell 2013, 153, 707–720. [Google Scholar] [CrossRef]
- Serrano-Pozo, A.; Mielke, M.L.; Gómez-Isla, T.; Betensky, R.A.; Growdon, J.H.; Frosch, M.P.; Hyman, B.T. Reactive glia not only associates with plaques but also parallels tangles in Alzheimer’s disease. Am. J. Pathol. 2011, 179, 1373–1384. [Google Scholar] [CrossRef]
- Ismaiel, A.A.; Espinosa-Oliva, A.M.; Santiago, M.; García-Quintanilla, A.; Oliva-Martín, M.J.; Herrera, A.J.; Venero, J.L.; de Pablos, R.M. Metformin, besides exhibiting strong in vivo anti-inflammatory properties, increases mptp-induced damage to the nigrostriatal dopaminergic system. Toxicol. Appl. Pharmacol. 2016, 298, 19–30. [Google Scholar] [CrossRef]
- Ou, Z.; Kong, X.; Sun, X.; He, X.; Zhang, L.; Gong, Z.; Huang, J.; Xu, B.; Long, D.; Li, J.; et al. Metformin treatment prevents amyloid plaque deposition and memory impairment in APP/PS1 mice. Brain Behav. Immun. 2018, 69, 351–363. [Google Scholar] [CrossRef]
- Lu, X.-Y.; Huang, S.; Chen, Q.-B.; Zhang, D.; Li, W.; Ao, R.; Leung, F.C.-Y.; Zhang, Z.; Huang, J.; Tang, Y.; et al. Metformin ameliorates Aβ pathology by insulin-degrading enzyme in a transgenic mouse model of Alzheimer’s disease. Oxid. Med. Cell Longev. 2020, 2020, 2315106. [Google Scholar] [CrossRef]
- Oliveira, W.H.; Nunes, A.K.; França, M.E.R.; Santos, L.A.; Lós, D.B.; Rocha, S.W.; Barbosa, K.P.; Rodrigues, G.B.; Peixoto, C.A. Effects of metformin on inflammation and short-term memory in streptozotocin-induced diabetic mice. Brain Res. 2016, 1644, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Kristófi, R.; Eriksson, J.W. Metformin as an anti-inflammatory agent: A short review. J. Endocrinol. 2021, 251, R11–R22. [Google Scholar] [CrossRef] [PubMed]
- Slomovitz, B.M.; Coleman, R.L. The PI3K/AKT/mTOR pathway as a therapeutic target in endometrial cancer. Clin. Cancer Res. 2012, 18, 5856–5864. [Google Scholar] [CrossRef] [PubMed]
- Rotermund, C.; Machetanz, G.; Fitzgerald, J.C. The therapeutic potential of metformin in neurodegenerative diseases. Front. Endocrinol. 2018, 9, 400. [Google Scholar] [CrossRef]
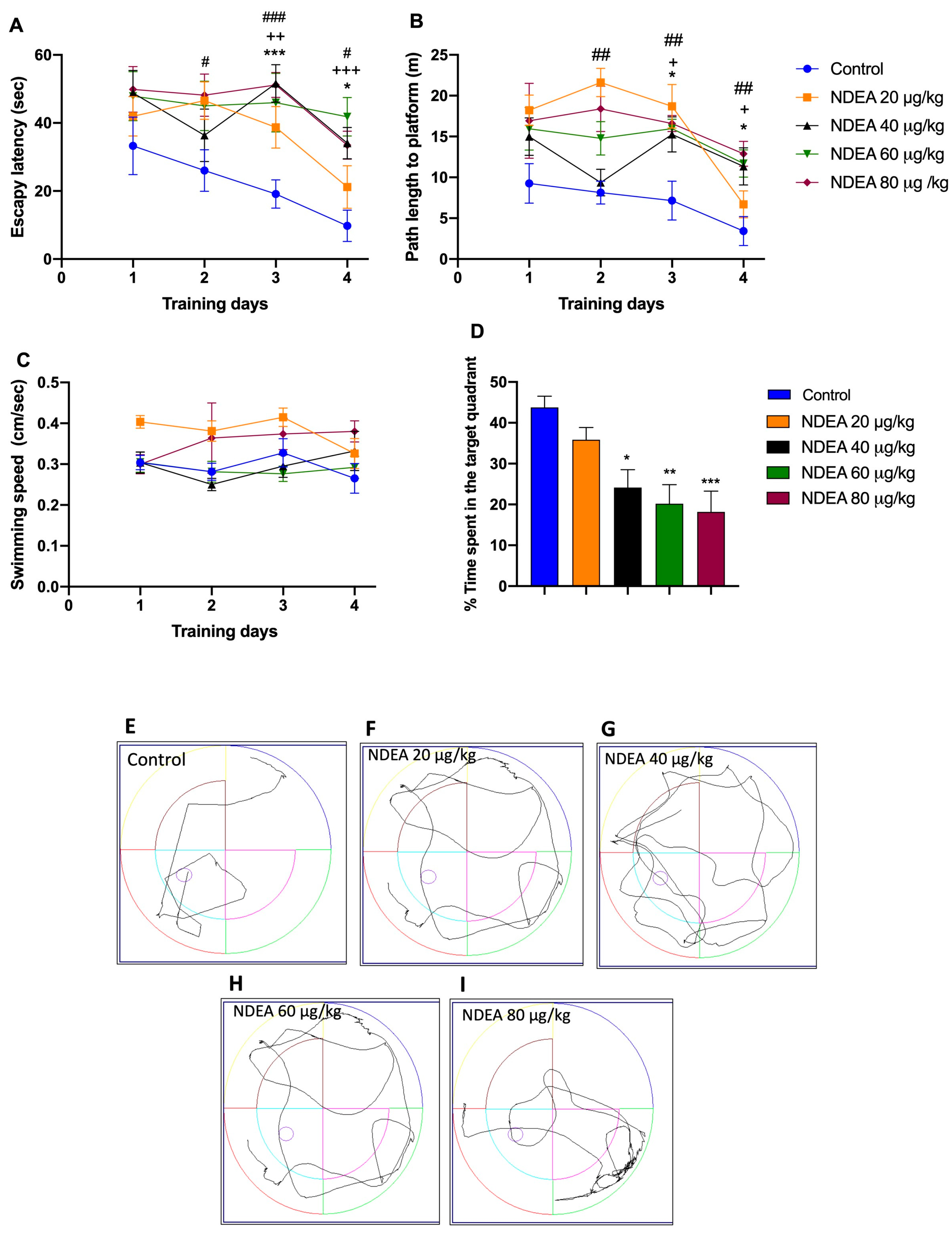
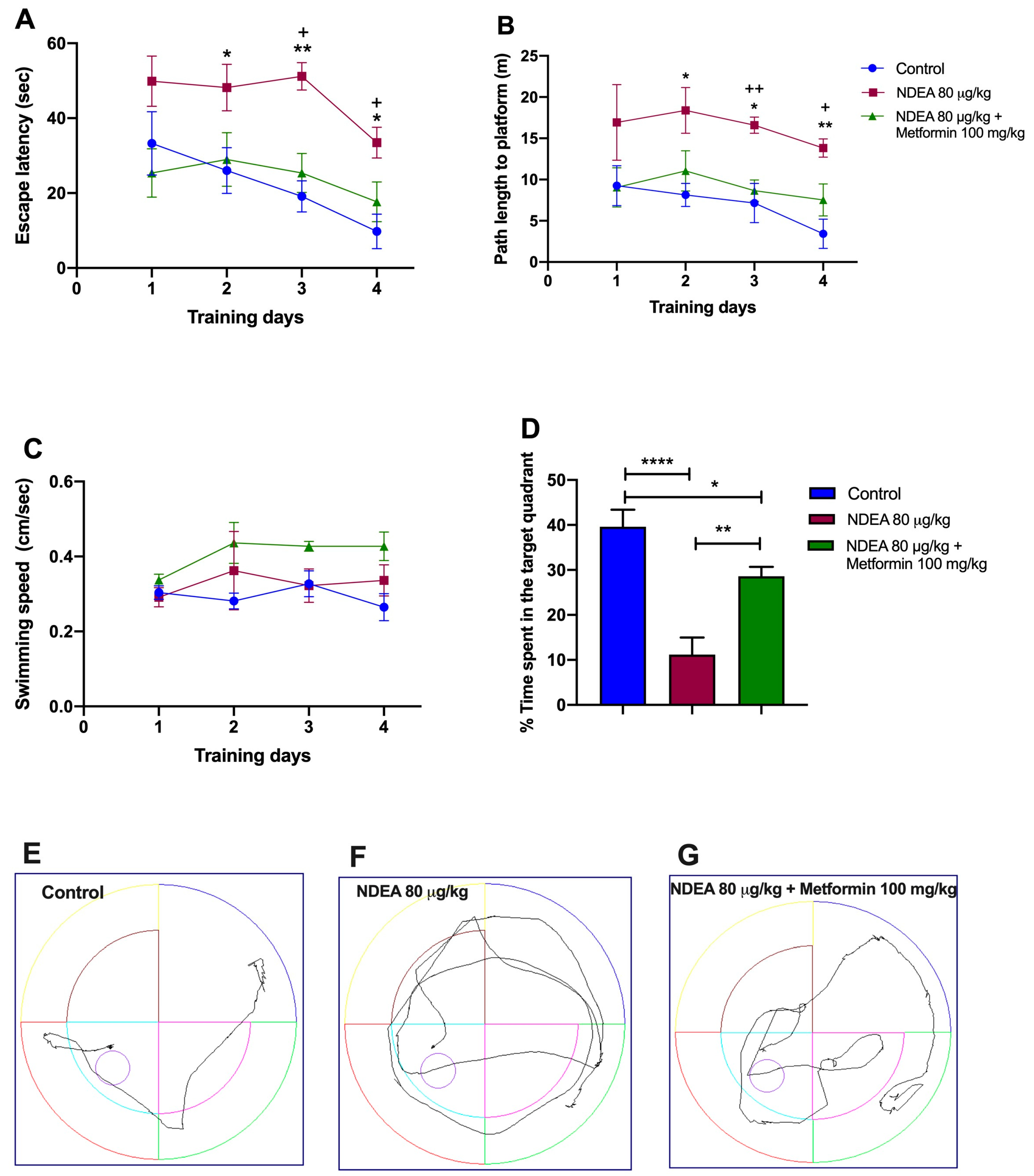

| Training | Escape Latency | Traveled Distance | ||
|---|---|---|---|---|
| Day 1 | p-Values | p-Values | ||
| Control vs. NDEA 20 μg/kg | ns | >0.9999 | * | =0.0124 |
| Control vs. NDEA 40 μg/kg | ns | =0.2291 | ns | =0.1749 |
| Control vs. NDEA 60 μg/kg | ns | =0.2955 | ns | =0.0880 |
| Control vs. NDEA 80 μg/kg | ns | =0.1726 | * | =0.0396 |
| Day 2 | ||||
| Control vs. NDEA 20 μg/kg | ns | =0.0518 | **** | <0.0001 |
| Control vs. NDEA 40 μg/kg | ns | =0.8223 | ns | =0.982 |
| Control vs. NDEA 60 μg/kg | ns | =0.0850 | ns | =0.0904 |
| Control vs. NDEA 80 μg/kg | * | =0.0303 | ** | =0.0033 |
| Day 3 | ||||
| Control vs. NDEA 20 μg/kg | ns | =0.0703 | *** | =0.0008 |
| Control vs. NDEA 40 μg/kg | *** | =0.0005 | * | =0.0275 |
| Control vs. NDEA 60 μg/kg | ** | =0.0054 | * | =0.0143 |
| Control vs. NDEA 80 μg/kg | *** | =0.0006 | ** | =0.0078 |
| Day 4 | ||||
| Control vs. NDEA 20 μg/kg | ns | =0.6468 | ns | =0.6443 |
| Control vs. NDEA 40 μg/kg | * | =0.014 | * | =0.0322 |
| Control vs. NDEA 60 μg/kg | **** | =0.0006 | * | =0.0237 |
| Control vs. NDEA 80 μg/kg | * | =0.0172 | ** | =0.0076 |
| Training | Escape Latency | Traveled Distance | ||
|---|---|---|---|---|
| Day 1 | p-Values | p-Values | ||
| Control vs. NDEA 80 μg/kg | ns | =0.4845 | ns | =0.3419 |
| Control vs. NDEA 80 μg/kg + Metformin 100 mg/kg | ns | =0.7811 | ns | =0.9987 |
| NDEA 80 μg/kg vs. NDEA 80 μg/kg + Metformin 100 mg/kg | * | =0.0384 | ns | =0.4276 |
| Day 2 | ||||
| Control vs. NDEA 80 μg/kg | * | =0.0379 | * | =0.0352 |
| Control vs. NDEA 80 μg/kg + Metformin 100 mg/kg | ns | =0.9576 | ns | =0.5966 |
| NDEA 80 μg/kg vs. NDEA 80 μg/kg + Metformin mg/kg | ns | =0.1327 | ns | =0.1438 |
| Day 3 | ||||
| Control vs. NDEA 80 μg/kg | ** | =0.0083 | * | =0.0128 |
| Control vs. NDEA 80 μg/kg + Metformin 100 mg/kg | ns | =0.5468 | ns | =0.8058 |
| NDEA 80 μg/kg vs. NDEA 80 μg/kg + Metformin mg/kg | * | =0.0176 | ** | =0.0042 |
| Day 4 | ||||
| Control vs. NDEA 80 μg/kg | * | =0.0249 | ** | =0.0063 |
| Control vs. NDEA 80 μg/kg + Metformin mg/kg | ns | =0.2969 | * | =0.0207 |
| NDEA 80 μg/kg vs. NDEA 80 μg/kg+ Metformin 100 mg/kg | * | =0.0274 | * | =0.0483 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ponce-Lopez, T.; González Álvarez Tostado, J.A.; Dias, F.; Montiel Maltez, K.H. Metformin Prevents NDEA-Induced Memory Impairments Associated with Attenuating Beta-Amyloid, Tumor Necrosis Factor-Alpha, and Interleukin-6 Levels in the Hippocampus of Rats. Biomolecules 2023, 13, 1289. https://doi.org/10.3390/biom13091289
Ponce-Lopez T, González Álvarez Tostado JA, Dias F, Montiel Maltez KH. Metformin Prevents NDEA-Induced Memory Impairments Associated with Attenuating Beta-Amyloid, Tumor Necrosis Factor-Alpha, and Interleukin-6 Levels in the Hippocampus of Rats. Biomolecules. 2023; 13(9):1289. https://doi.org/10.3390/biom13091289
Chicago/Turabian StylePonce-Lopez, Teresa, José Antonio González Álvarez Tostado, Fernando Dias, and Keren Happuck Montiel Maltez. 2023. "Metformin Prevents NDEA-Induced Memory Impairments Associated with Attenuating Beta-Amyloid, Tumor Necrosis Factor-Alpha, and Interleukin-6 Levels in the Hippocampus of Rats" Biomolecules 13, no. 9: 1289. https://doi.org/10.3390/biom13091289
APA StylePonce-Lopez, T., González Álvarez Tostado, J. A., Dias, F., & Montiel Maltez, K. H. (2023). Metformin Prevents NDEA-Induced Memory Impairments Associated with Attenuating Beta-Amyloid, Tumor Necrosis Factor-Alpha, and Interleukin-6 Levels in the Hippocampus of Rats. Biomolecules, 13(9), 1289. https://doi.org/10.3390/biom13091289




