Cantharidin-Based Verbenone Derivatives as a Novel Insecticide against Plutella xylostella: Design, Synthesis, Insecticidal Activity Evaluation, and 3D QSAR Study
Abstract
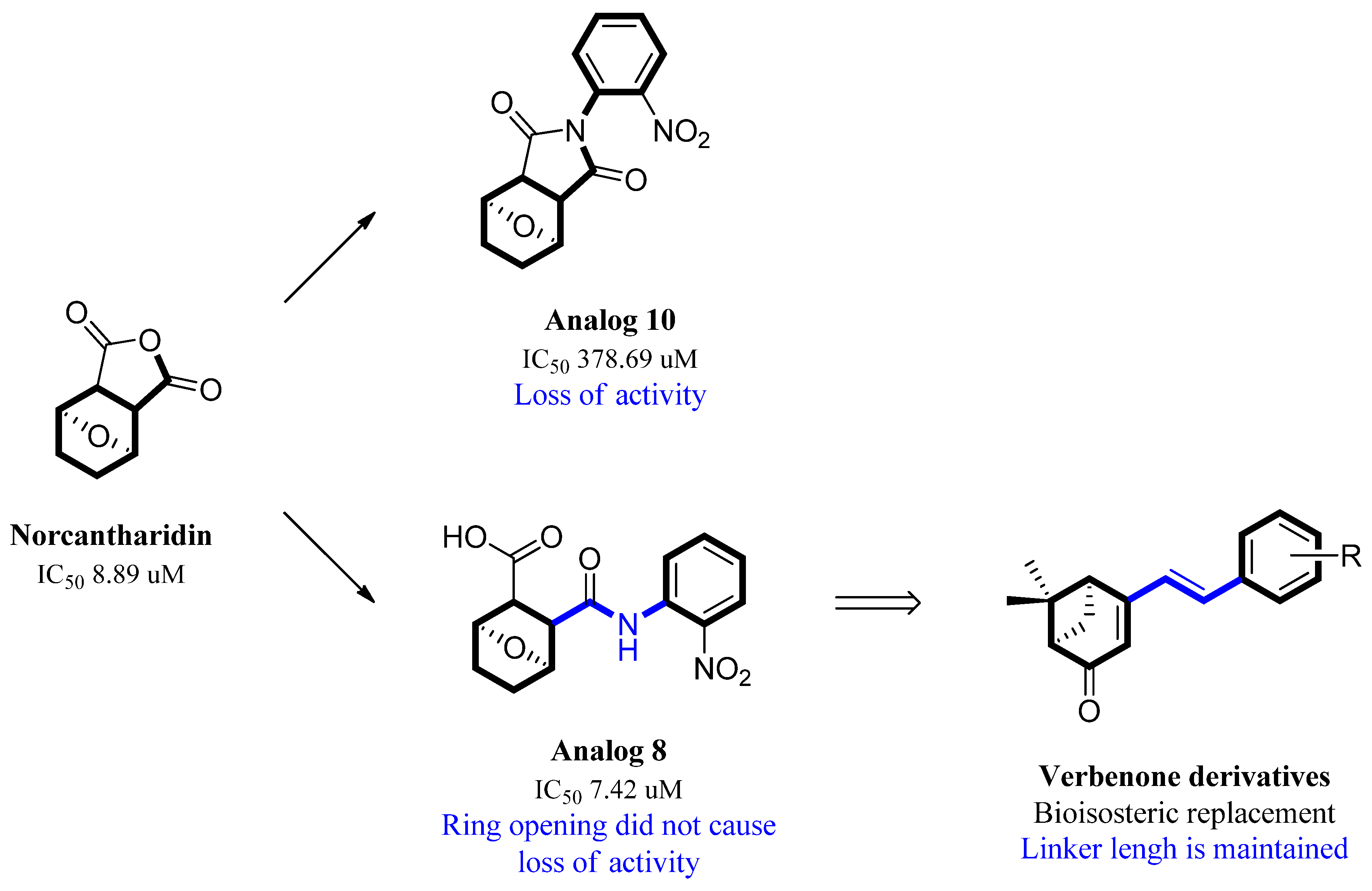
:1. Introduction
2. Results and Discussion
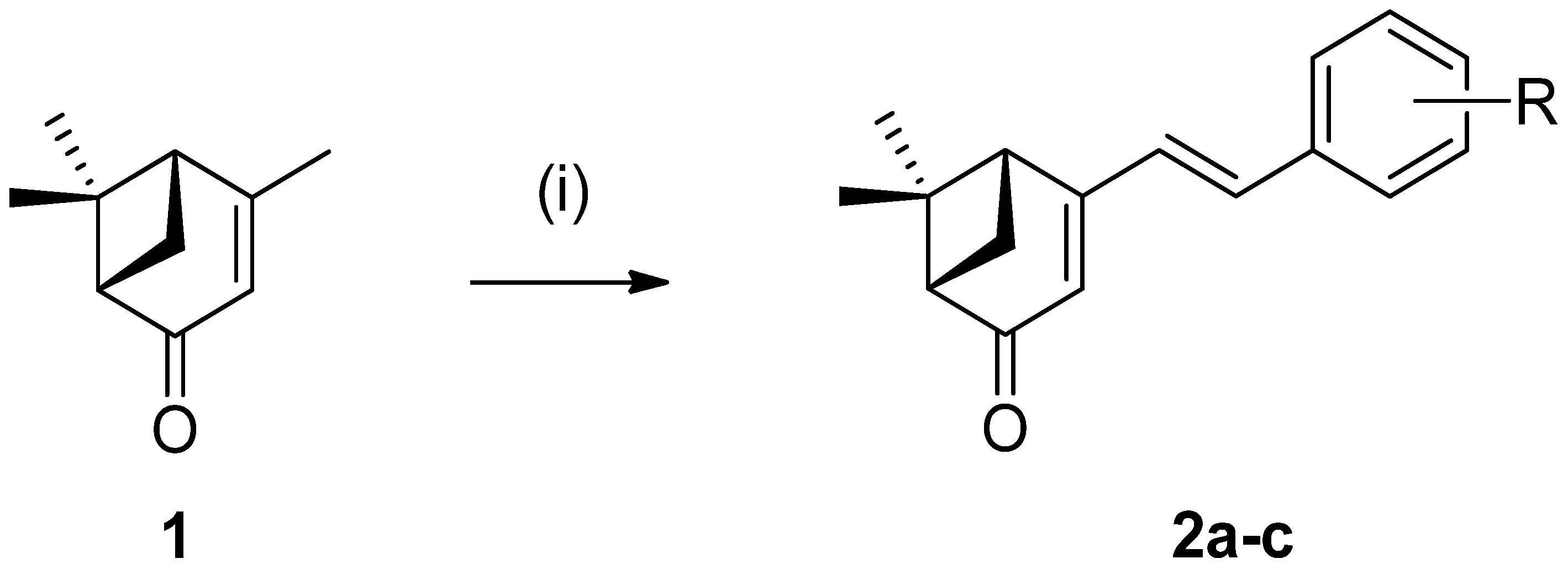
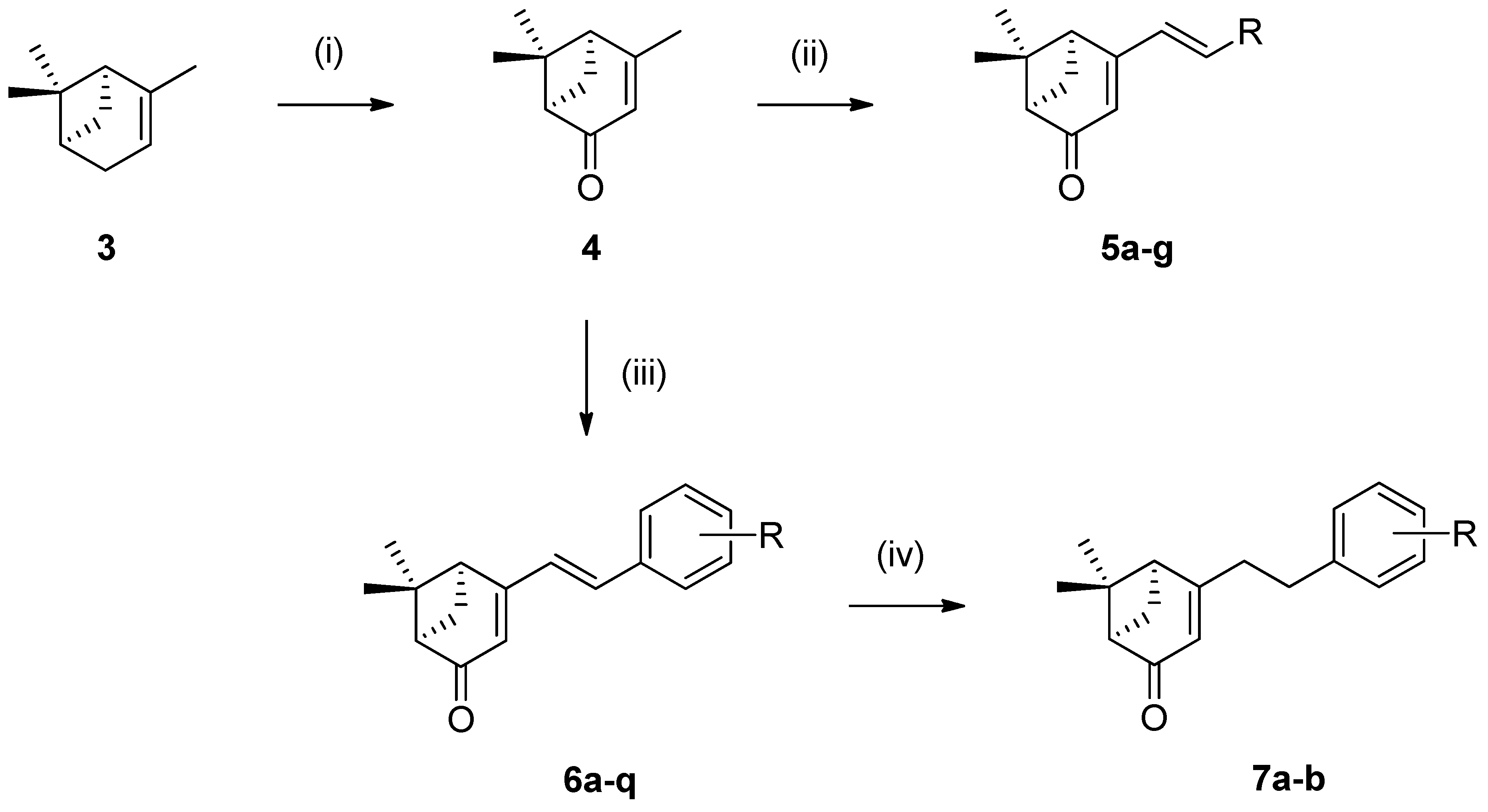
2.1. Chemistry
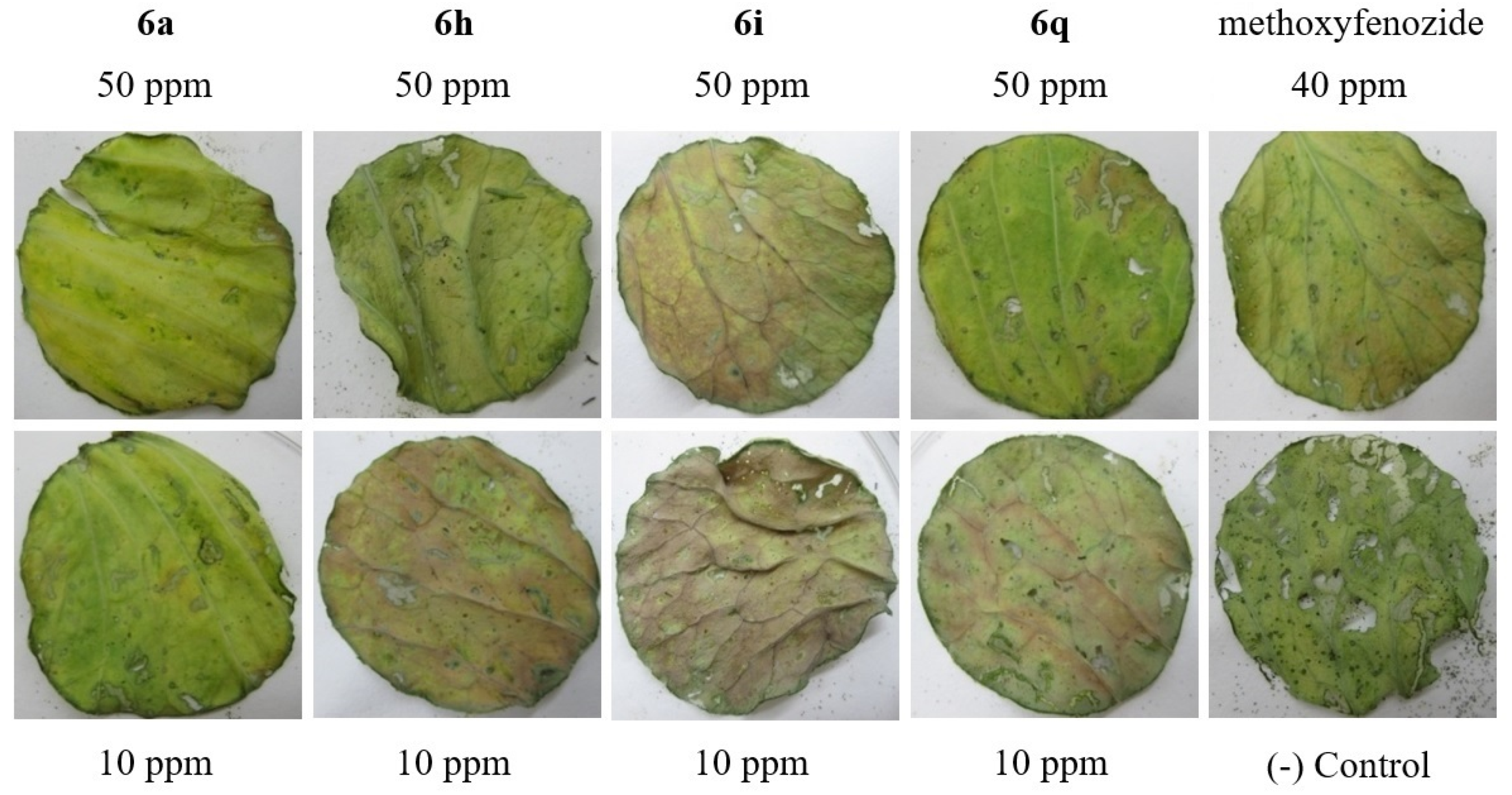
2.2. Biological Activity
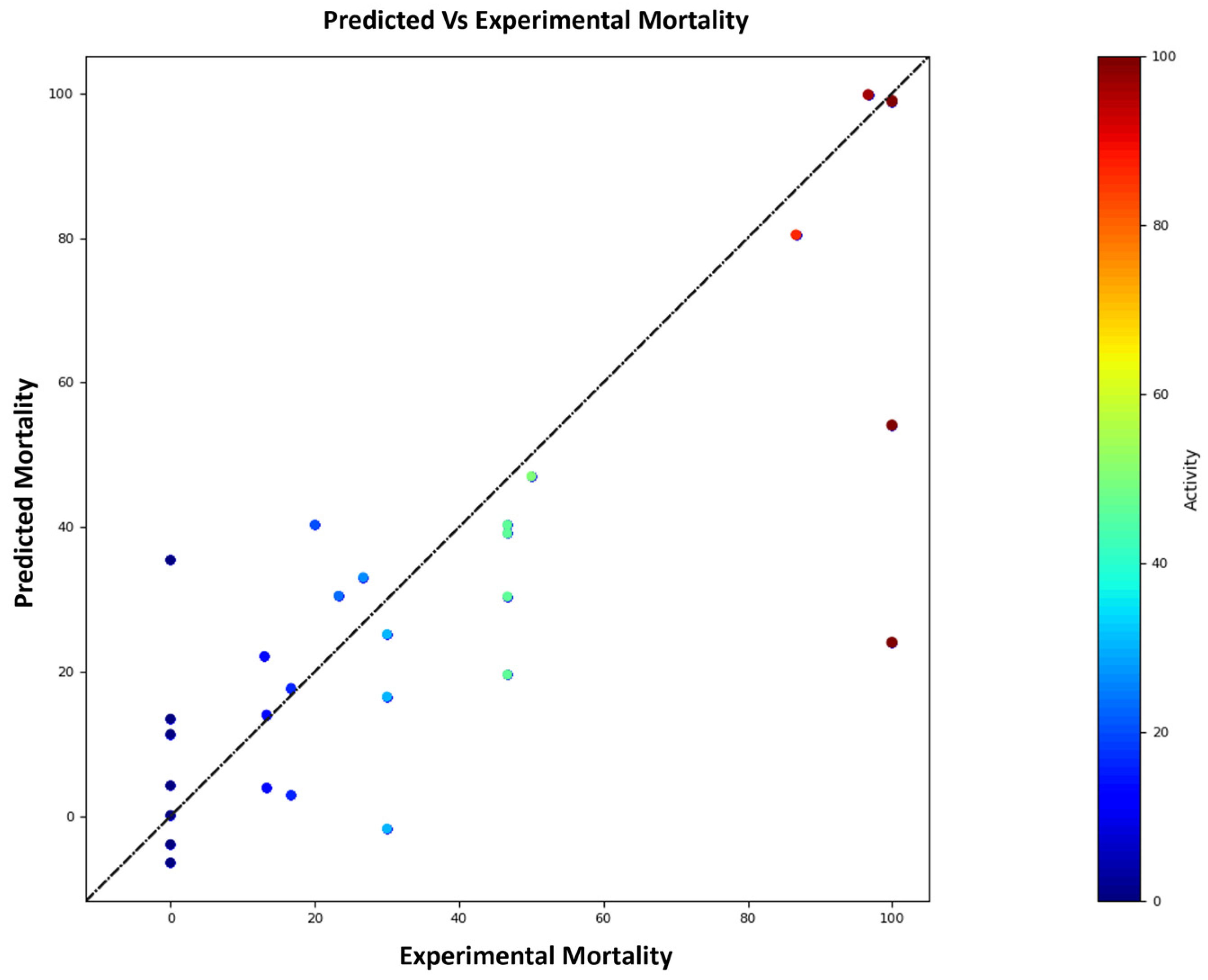
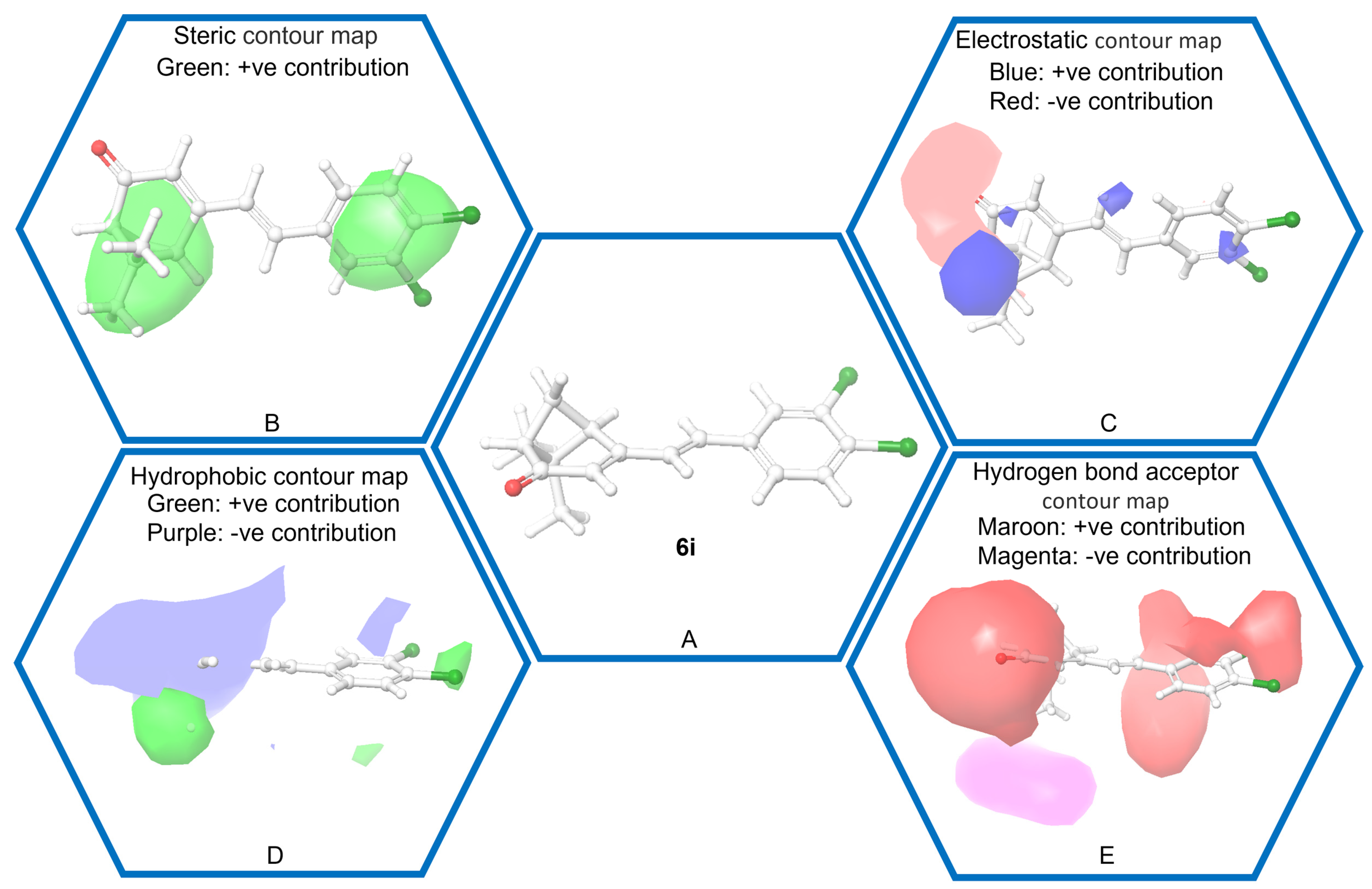
2.3. 3D QSAR
3. Materials and Methods
3.1. Equipment and Materials
3.2. General Procedure for the Synthesis of Verbenone Derivatives
3.2.1. (1S,5R)-6,6-dimethyl-4-((E)-2-(pyridin-2-yl)vinyl)bicyclo[3.1.1]hept-3-en-2-one (2a)
3.2.2. (1S,5R)-6,6-dimethyl-4-((E)-2-(pyridin-3-yl)vinyl)bicyclo[3.1.1]hept-3-en-2-one (2b)
3.2.3. (1S,5R)-6,6-dimethyl-4-((E)-2-(pyridin-4-yil)vinyl)bicyclo[3.1.1]hept-3-en-2-one (2c)
3.2.4. (1S,5R)-4,6,6-trimethylbicyclo[3.1.1]hept-3-en-2-one (4) [20]
3.2.5. (1S,5R)-6,6-dimethyl-4-((E)-2-(pyridin-2-yl)vinyl)bicyclo[3.1.1]hept-3-en-2-one (5a)
3.2.6. (1S,5R)-6,6-dimethyl-4-((E)-2-(pyridin-3-yl)vinyl)bicyclo[3.1.1]hept-3-en-2-one (5b)
3.2.7. (1S,5R)-6,6-dimethyl-4-((E)-2-(pyridin-4-yl)vinyl)bicyclo[3.1.1]hept-3-en-2-one (5c)
3.2.8. (1S,5R)-6,6-dimethyl-4-((E)-2-(thiophen-2-yl)vinyl)bicyclo[3.1.1]hept-3-en-2-one (5d)
3.2.9. (1S,5R)-6,6-dimethyl-4-((E)-2-(3-methylthiophen-2-yl)vinyl)bicyclo[3.1.1]hept-3-en-2-one (5e)
3.2.10. (1S,5R)-6,6-dimethyl-4-((E)-2-(4-methylthiophen-2-yl)vinyl)bicyclo[3.1.1]hept-3-en-2-one (5f)
3.2.11. (1S,5R)-6,6-dimethyl-4-((E)-2-(5-methylthiophen-2-yl)vinyl)bicyclo[3.1.1]hept-3-en-2-one (5g)
3.2.12. (1S,5R)-6,6-dimethyl-4-((E)-styryl)bicyclo[3.1.1]hept-3-en-2-one (6a)
3.2.13. (1S,5R)-4-(2-fluorostyryl)-6,6-dimethylbicyclo[3.1.1]hept-3-en-2-one (6b)
3.2.14. (1S,5R)-4-(3-fluorostyryl)-6,6-dimethylbicyclo[3.1.1]hept-3-en-2-one (6c)
3.2.15. (1S,5R)-4-(4-fluorostyryl)-6,6-dimethylbicyclo[3.1.1]hept-3-en-2-one (6d)
3.2.16. (1S,5R)-4-(2,4-difluorostyryl)-6,6-dimethylbicyclo[3.1.1]hept-3-en-2-one (6e)
3.2.17. (1S,5R)-4-(3,4-difluorostyryl)-6,6-dimethylbicyclo[3.1.1]hept-3-en-2-one (6f)
3.2.18. (1S,5R)-4-(3,5-difluorostyryl)-6,6-dimethylbicyclo[3.1.1]hept-3-en-2-one (6g)
3.2.19. (1S,5R)-4-(2,4-dichlorostyryl)-6,6-dimethylbicyclo[3.1.1]hept-3-en-2-one (6h)
3.2.20. (1S,5R)-4-(3,4-dichlorostyryl)-6,6-dimethylbicyclo[3.1.1]hept-3-en-2-one (6i)
3.2.21. (1S,5R)-4-(3,5-dichlorostyryl)-6,6-dimethylbicyclo[3.1.1]hept-3-en-2-one (6j)
3.2.22. (1S,5R)-6,6-dimethyl-4-(2-(trifluoromethoxy)styryl)bicyclo[3.1.1]hept-3-en-2-one (6k)
3.2.23. (1S,5R)-6,6-dimethyl-4-(3-(trifluoromethoxy)styryl)bicyclo[3.1.1]hept-3-en-2-one (6l)
3.2.24. (1S,5R)-6,6-dimethyl-4-(4-(trifluoromethoxy)styryl)bicyclo[3.1.1]hept-3-en-2-one (6m)
3.2.25. (1S,5R)-6,6-dimethyl-4-(2-methylstyryl)bicyclo[3.1.1]hept-3-en-2-one (6n)
3.2.26. (1S,5R)-6,6-dimethyl-4-(3-methylstyryl)bicyclo[3.1.1]hept-3-en-2-one (6o)
3.2.27. (1S,5R)-6,6-dimethyl-4-(4-methylstyryl)bicyclo[3.1.1]hept-3-en-2-one (6p)
3.2.28. (1S,5R)-4-(4-ethylstyryl)-6,6-dimethylbicyclo[3.1.1]hept-3-en-2-one (6q)
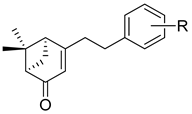
3.2.29. (1S,5R)-6,6-dimethyl-4-phenethylbicyclo[3.1.1]hept-3-en-2-one (7a)
3.2.30. (1S,5R)-4-(4-ethylphenethyl)-6,6-dimethylbicyclo[3.1.1]hept-3-en-2-one (7b)
3.3. Evaluation of Insecticidal Activity against P. xylostella
3.4. QSAR Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| DBM | Diamondback moth |
| DDT | Dichlorodiphenyltrichloroethane |
| PP1 | Protein phosphatase 1 |
| PP2A | Protein phosphatase 2A |
| rPxPP5 | Recombinant Plutella xylostella protein serine/threonine phosphatase gene 5 |
| MeOH | Methanol |
| NaOMe | Sodium methoxide |
| TFA | Trifluoroacetic acid |
| Pd/C | Palladium on carbon |
| 1H NMR | Proton nuclear magnetic resonance |
| 13C NMR | Carbon-13 nuclear magnetic resonance |
| HRMS | High-resolution mass spectrometry |
| HPLC | High-performance liquid chromatography |
References
- Sarfraz, M.; Keddie, A.B.; Dosdall, L.M. Biological control of the diamondback moth, Plutella xylostella: A review. Biocontrol Sci. Tech. 2005, 15, 763–789. [Google Scholar] [CrossRef]
- Attique, M.N.; Khaliq, A.; Sayyed, A.H. Could resistance to insecticides in Plutella xylostella (Lep., Plutellidae) be overcome by insecticide mixtures? J. Appl. Entomol. 2006, 130, 122–127. [Google Scholar] [CrossRef]
- Sidhu, A. Understanding the Diamondback Moth (Plutella xylostella) Performance in Plant Alternative Cropping Systems, Brock University. 2017. Available online: http://hdl.handle.net/10464/12955 (accessed on 29 June 2023).
- Kumar, J.; Ramlal, A.; Mallick, D.; Mishra, V. An overview of some biopesticides and their importance in plant protection for commercial acceptance. Plants 2021, 10, 1185. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, Y. Cantharidin impedes the activity of protein serine/threonine phosphatase in Plutella xylostella. Mol. Biosyst. 2014, 10, 240–250. [Google Scholar]
- Ye, L.; Jia, Y.; Ji, K.E.; Sanders, A.J.; Xue, K.; Ji, J.; Mason, M.D.; Jiang, W.G. Traditional Chinese medicine in the prevention and treatment of cancer and cancer metastasis. Oncol. Lett. 2015, 10, 1240–1250. [Google Scholar] [CrossRef]
- Khan, R.A.; Rashid, M.; Naveed, M. Cantharidin: A chemical precursor for the development of novel bioinsecticides. Bulg. Chem. Commun. 2022, 54, 19–28. [Google Scholar]
- Wang, G.; Dong, J.; Deng, L. Overview of cantharidin and its analogues. Curr. Med. Chem. 2018, 25, 2034–2044. [Google Scholar] [CrossRef]
- Tresch, S.; Schmotz, J.; Grossmann, K. Probing mode of action in plant cell cycle by the herbicide endothall, a protein phosphatase inhibitor. Pestic. Biochem. Physiol. 2011, 99, 86–95. [Google Scholar] [CrossRef]
- Mumby, M.C.; Walter, G. Protein serine/threonine phosphatases: Structure, regulation, and functions in cell growth. Physiol. Rev. 1993, 73, 673–699. [Google Scholar] [CrossRef]
- Khan, R.A.; Liu, J.; Zhang, Y. Catalytic inactivation of alkaline phosphatase by cantharidin, an inhibitor of protein phosphatase. RSC Adv. 2014, 91, 49987–49994. [Google Scholar] [CrossRef]
- Yadav, D.K.; Saloni, S.P.; Misra, S.; Singh, H.; Mancera, R.L.; Kumar, S. Studies of the benzopyran class of selective COX-2 inhibitors using 3D-QSAR and molecular docking. Arch. Pharm. Res. 2018, 41, 1178–1189. [Google Scholar] [CrossRef]
- José, L.V.; Juliana, A.M.; Alexander, F.; Julio, C. Structural requirements of n-alpha-mercaptoacetyl dipeptide (namdp) inhibitors of pseudomonas Aeruginosa Virulence factor LasB: 3D-QSAR, molecular docking, and interaction fingerprint studies. Int. J. Mol. Sci. 2019, 20, 6133. [Google Scholar]
- Varpe, B.D.; Jadhav, S.B.; Chatale, B.C.; Mali, A.S.; Jadhav, S.Y.; Kulkarni, A.A. 3D-QSAR and Pharmacophore modeling of 3, 5-disubstituted indole derivatives as Pim kinase inhibitors. Struct. Chem. 2020, 31, 1675–1690. [Google Scholar] [CrossRef]
- Balasubramanian, P.K.; Balupuri, A.; Kang, H.Y.; Cho, S.J. Receptor-guided 3D-QSAR studies, molecular dynamics simulation and free energy calculations of Btk kinase inhibitors. BMC Syst. Biol. 2017, 11, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Fan, N.; Zhang, S.; Sheng, T.; Zhao, L.; Liu, Z.; Liu, J.; Wang, X. Docking field-based QSAR and pharmacophore studies on the substituted pyrimidine derivatives targeting HIV-1 reverse transcriptase. Chem. Biol. Drug Des. 2018, 91, 398–407. [Google Scholar] [CrossRef] [PubMed]
- Kumari, A.; Kaur, M.; Bahia, M.S.; Silakari, O. 3D-QSAR analysis of anilinoquinoline inhibitors of colony stimulating factor-1 kinase (cFMS): Implementation of field-based molecular alignment. Med. Chem. Res. 2013, 22, 5167–5183. [Google Scholar] [CrossRef]
- Kamaria, P.; Kawathekar, N. Ligand-based 3D-QSAR analysis and virtual screening in exploration of new scaffolds as Plasmodium falciparum glutathione reductase inhibitors. Med. Chem. Res. 2014, 23, 25–33. [Google Scholar] [CrossRef]
- Osman, E.A.; Abdalla, M.A.; Abdelraheem, M.O.; Ali, M.F.; Osman, S.A.; Tanir, Y.M.; Alzain, A.A. Design of novel coumarins as potent Mcl-1 inhibitors for cancer treatment guided by 3D-QSAR, molecular docking and molecular dynamics. Inform. Med. Unlocked 2021, 26, 100765. [Google Scholar] [CrossRef]
- Islam, S.; Paul, S.; Roy, A.S.; Banerjee, S.; Ghosh, K.; Dey, R.C.; Santra, S.C. Catalytic activity of an iron (III) Schiff base complex bound in a polymer resin. Transit. Met. Chem. 2013, 38, 675–682. [Google Scholar] [CrossRef]
- Zhang, C.; Tian, Q.; Li, Y. Design, synthesis, and insecticidal activity evaluation of piperine derivatives. Front. Chem. 2022, 10, 973630. [Google Scholar] [CrossRef]
- Zhao, Z.; Xu, Q.; Chen, W.; Wang, S.; Yang, Q.; Dong, Y.; Zhang, J. Rational Design, Synthesis, and Biological Investigations of N-Methylcarbamoylguanidinyl Azamacrolides as a Novel Chitinase Inhibitor. J. Agric. Food. Chem. 2022, 70, 4889–4898. [Google Scholar] [CrossRef] [PubMed]
 | ||||
|---|---|---|---|---|
| Entry | Compound | (R)/(S) | R | Mortality (% ± SD) a |
| 1 | 2a |  | 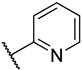 | 0.0 ± 0.0 |
| 2 | 2b |  |  | 0.0 ± 0.0 |
| 3 | 2c |  | 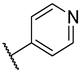 | 30.0 ± 10.0 |
| 4 | 5a |  | 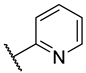 | 13.3 ± 5.8 |
| 5 | 5b |  | 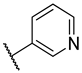 | 46.7 ± 5.8 |
| 6 | 5c | 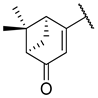 |  | 50.0 ± 0.0 |
| 7 | 5d |  | 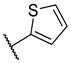 | 23.3 ± 5.8 |
| 8 | 5e | 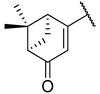 | 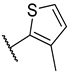 | 46.7 ± 5.8 |
| 9 | 5f | 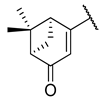 |  | 26.7 ± 15.3 |
| 10 | 5g | 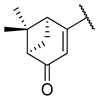 |  | 46.7 ± 5.8 |
 | |||
|---|---|---|---|
| Entry | Compound | R | Mortality (% ± SD) a |
| 1 | 6a | Phenyl | 100 ± 0.0 |
| 2 | 6b | 2-F-Ph | 13.3 ± 5.8 |
| 3 | 6c | 3-F-Ph | 16.7 ± 5.8 |
| 4 | 6d | 4-F-Ph | 30.0 ± 17.3 |
| 5 | 6e | 2,4-F2-Ph | 0.0 ± 0.0 |
| 6 | 6f | 3,4-F2-Ph | 0.0 ± 0.0 |
| 7 | 6g | 3,5-F2-Ph | 86.7 ± 5.8 |
| 8 | 6h | 2,4-Cl2-Ph | 100 ± 0.0 |
| 9 | 6i | 3,4-Cl2-Ph | 100 ± 0.0 |
| 10 | 6j | 3,5-Cl2-Ph | 0.0 ± 0.0 |
| 11 | 6k | 2-OCF3-Ph | 96.7 ± 5.8 |
| 12 | 6l | 3-OCF3-Ph | 30.0 ± 10.0 |
| 13 | 6m | 4-OCF3-Ph | 46.7 ± 11.5 |
| 14 | 6n | 2-Me-Ph | 16.7 ± 5.8 |
| 15 | 6o | 3-Me-Ph | 20.0 ± 10.0 |
| 16 | 6p | 4-Me-Ph | 13.3 ± 5.8 |
| 17 | 6q | 4-Et-Ph | 100 ± 0.0 |
 | |||
|---|---|---|---|
| Entry | Compound | R | Mortality (% ± SD) a |
| 1 | 7a | Phenyl | 0.0 ± 0.0 |
| 2 | 7b | 4-Et-Ph | 0.0 ± 0.0 |
| Compound | Conc. (mg/L) | Mortality after Incubation (% ± SD) | |||
|---|---|---|---|---|---|
| 24 h | 48 h | 72 h | 96 h | ||
| 6a | 100 | 0.0 ± 0.0 | 43.3 ± 5.8 | 93.3 ± 11.5 | 100.0 ± 0.0 |
| 50 | 0.0 ± 0.0 | 33.3 ± 5.8 | 53.3 ± 11.5 | 100.0 ± 0.0 | |
| 10 | 0.0 ± 0.0 | 23.3 ± 5.8 | 26.7 ± 5.8 | 53.3 ± 11.5 | |
| 6h | 100 | 0.0 ± 0.0 | 40.0 ± 10.0 | 93.3 ± 5.8 | 100.0 ± 0.0 |
| 50 | 0.0 ± 0.0 | 23.3 ± 5.8 | 23.3 ± 5.8 | 50.0 ± 0.0 | |
| 10 | 0.0 ± 0.0 | 13.3 ± 5.8 | 16.7 ± 5.8 | 36.7 ± 5.8 | |
| 6i | 100 | 0.0 ± 0.0 | 36.7 ± 5.8 | 90.0 ± 10.0 | 100.0 ± 0.0 |
| 50 | 0.0 ± 0.0 | 20.0 ± 0.0 | 23.3 ± 5.8 | 50.0 ± 0.0 | |
| 10 | 0.0 ± 0.0 | 20.0 ± 10.0 | 20.0 ± 10.0 | 33.3 ± 5.8 | |
| 6q | 100 | 0.0 ± 0.0 | 43.3 ± 5.8 | 93.3 ± 5.8 | 100.0 ± 0.0 |
| 50 | 0.0 ± 0.0 | 36.7 ± 5.8 | 36.7 ± 5.8 | 56.7 ± 5.8 | |
| 10 | 0.0 ± 0.0 | 23.3 ± 5.8 | 26.7 ± 5.8 | 43.3 ± 5.8 | |
| methoxyfenozide | 40 | 0.0 ± 0.0 | 33.3 ± 5.8 | 73.3 ± 5.8 | 96.7 ± 5.8 |
| (-) control | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | |
| Compound | Experimental Mortality (%) | Predicted Mortality (%) | Prediction Error (%) |
|---|---|---|---|
| 2a | 0.00 | −6.4 | −6.40 |
| 2b | 0.00 | 11.37 | 11.37 |
| 2c | 30.00 | 16.54 | −13.49 |
| 5a | 13.00 | 22.17 | 9.17 |
| 5c | 50.00 | 47.03 | −2.97 |
| 5b | 46.70 | 30.41 | −16.29 |
| 5d | 23.30 | 30.52 | 7.22 |
| 5e | 46.70 | 39.14 | −7.56 |
| 5f | 26.70 | 33.08 | 6.38 |
| 5g | 46.70 | 19.61 | −27.09 |
| 6a | 100.00 | 24.05 | −75.95 |
| 6b | 13.30 | 14.03 | 0.73 |
| 6c | 16.70 | 2.93 | −13.77 |
| 6d | 30.00 | 25.17 | −4.83 |
| 6e | 0.00 | 13.51 | 13.51 |
| 6f | 0.00 | 4.25 | 4.25 |
| 6g | 86.70 | 80.46 | −6.24 |
| 6h | 100.00 | 98.89 | −1.11 |
| 6i | 100.00 | 99.02 | −0.98 |
| 6j | 0.00 | −3.86 | −3.86 |
| 6k | 96.70 | 99.83 | 3.14 |
| 6l | 30.00 | 30.81 | 0.81 |
| 6m | 46.70 | 40.31 | −6.39 |
| 6n | 16.70 | 17.72 | 1.025 |
| 6o | 20.00 | 40.33 | 20.33 |
| 6p | 13.30 | 19.99 | 6.69 |
| 6q | 100.00 | 54.11 | −45.89 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, K.; Nada, H.; Kim, M.; Park, H.; Lee, K.; Seo, D.; Lee, K.; Choi, Y. Cantharidin-Based Verbenone Derivatives as a Novel Insecticide against Plutella xylostella: Design, Synthesis, Insecticidal Activity Evaluation, and 3D QSAR Study. Biomolecules 2023, 13, 1272. https://doi.org/10.3390/biom13081272
Lee K, Nada H, Kim M, Park H, Lee K, Seo D, Lee K, Choi Y. Cantharidin-Based Verbenone Derivatives as a Novel Insecticide against Plutella xylostella: Design, Synthesis, Insecticidal Activity Evaluation, and 3D QSAR Study. Biomolecules. 2023; 13(8):1272. https://doi.org/10.3390/biom13081272
Chicago/Turabian StyleLee, Kwanshik, Hossam Nada, Minkyoung Kim, Hyejun Park, Kiho Lee, Dongho Seo, Kyeong Lee, and Yongseok Choi. 2023. "Cantharidin-Based Verbenone Derivatives as a Novel Insecticide against Plutella xylostella: Design, Synthesis, Insecticidal Activity Evaluation, and 3D QSAR Study" Biomolecules 13, no. 8: 1272. https://doi.org/10.3390/biom13081272
APA StyleLee, K., Nada, H., Kim, M., Park, H., Lee, K., Seo, D., Lee, K., & Choi, Y. (2023). Cantharidin-Based Verbenone Derivatives as a Novel Insecticide against Plutella xylostella: Design, Synthesis, Insecticidal Activity Evaluation, and 3D QSAR Study. Biomolecules, 13(8), 1272. https://doi.org/10.3390/biom13081272







