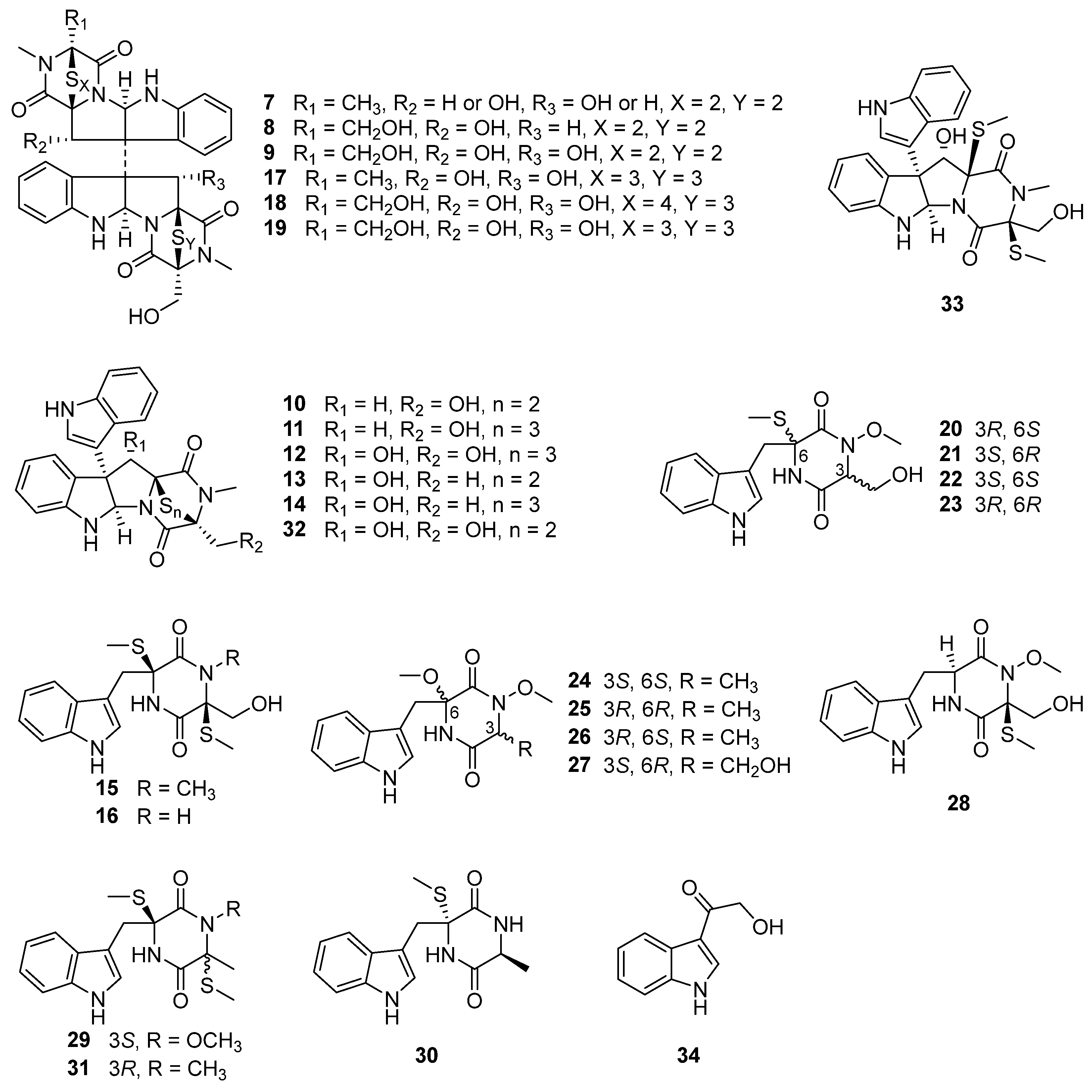Genus Acrostalagmus: A Prolific Producer of Natural Products
Abstract
1. Introduction
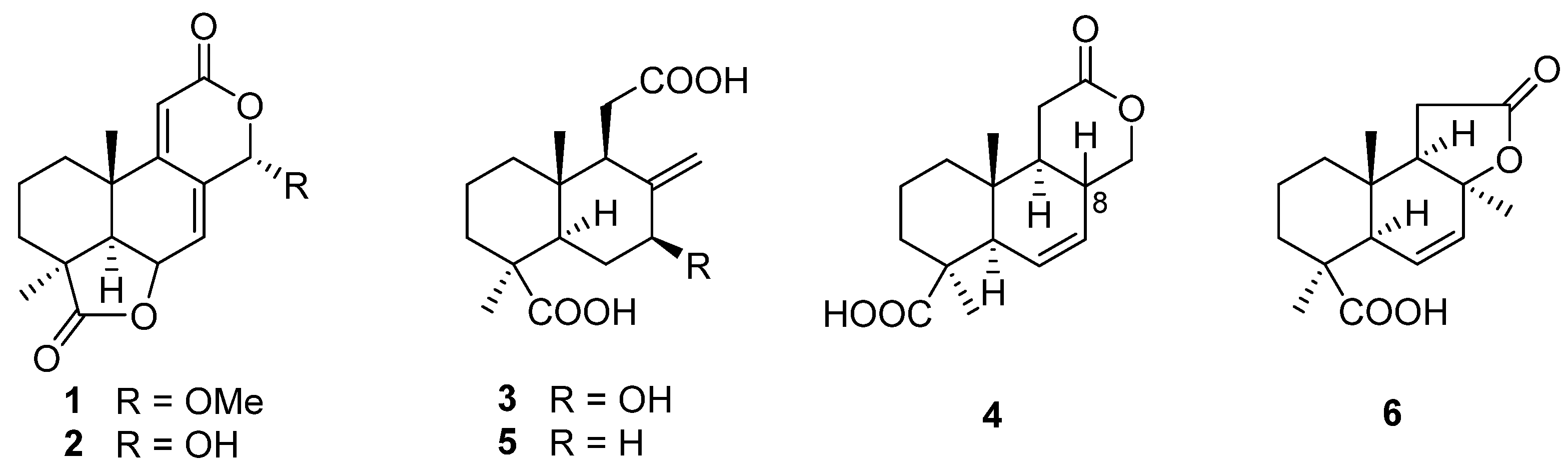
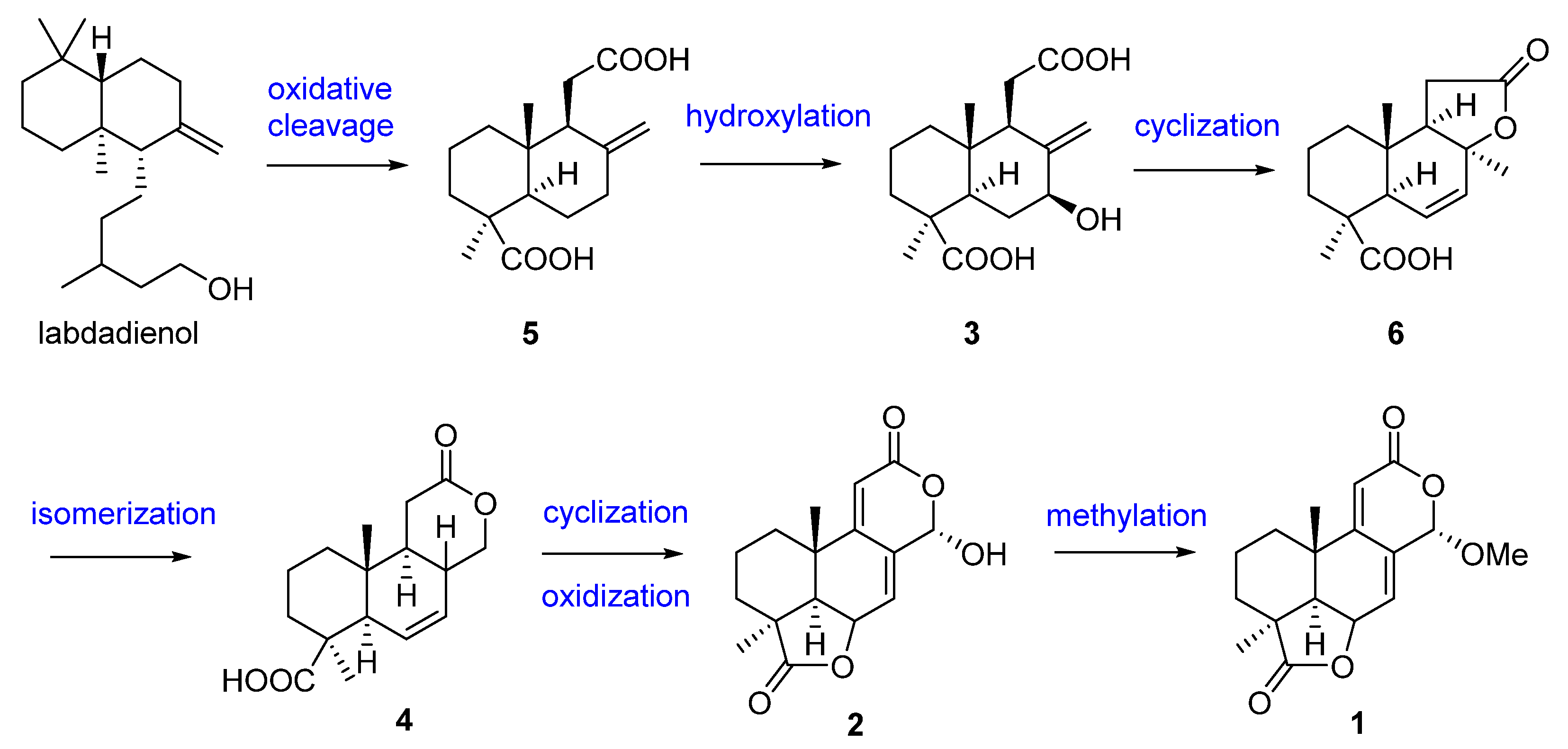
2. Terpenoids
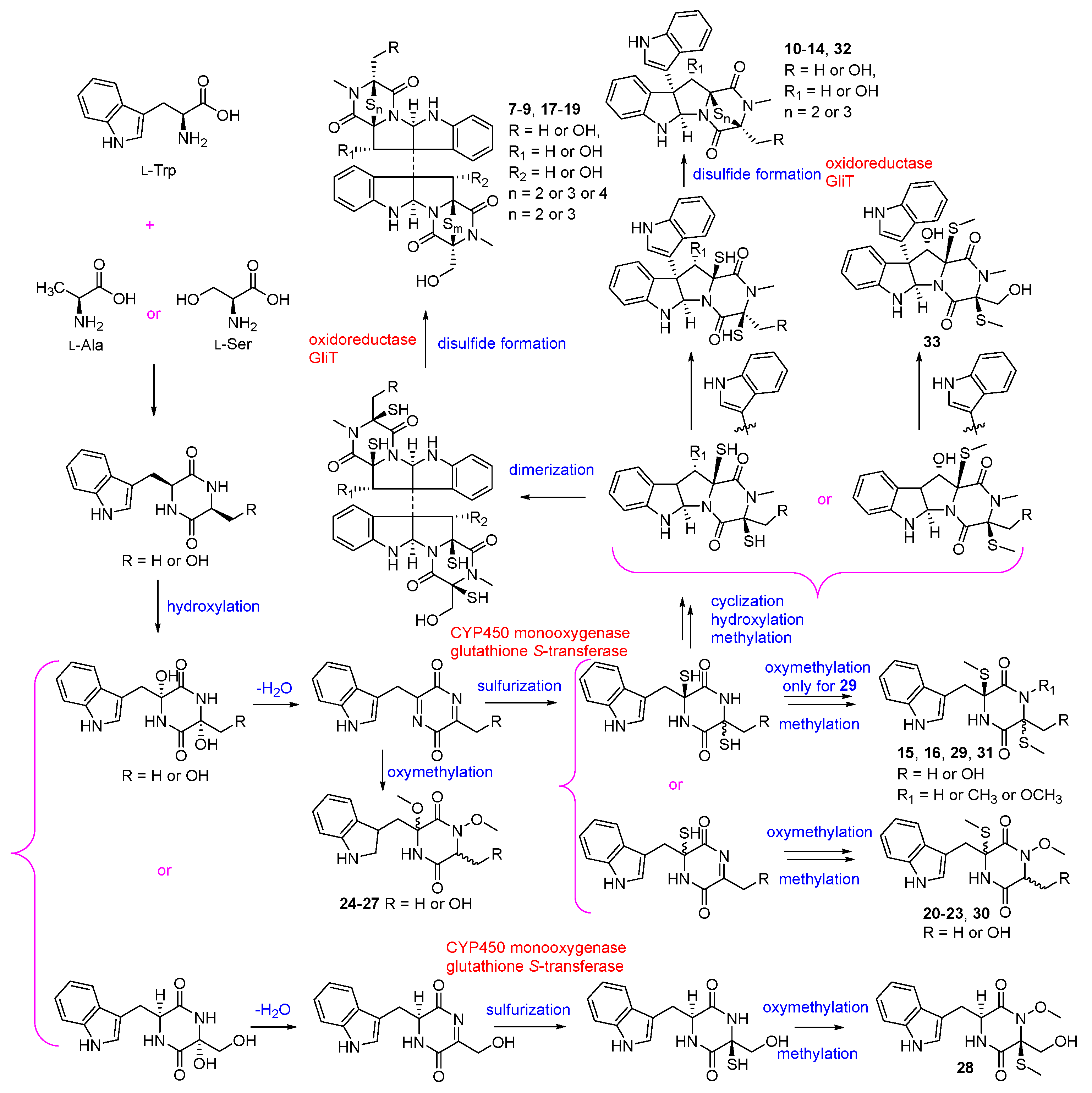
3. Alkaloids
4. Others
4.1. Cyclic Dipeptides
4.2. Pyranone Derivatives
4.3. Paulownin and Benzene Derivatives
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- He, S.; Jin, X.; Wang, S. Antagonistic activity of Acrostalagmus luteo-albus against plant pathogenic fungi. J. Gansu Agric. Univ. 2010, 45, 60–65. [Google Scholar]
- Bondarenko, S.A.; Georgieva, M.L.; Bilanenko, E.N. Alkalitolerant micromycetes in acidic and neutral soils of the temperate zone. Microbiology 2016, 85, 737–744. [Google Scholar] [CrossRef]
- Bondarenko, S.A.; Ianutsevich, E.A.; Sinitsyna, N.A.; Georgieva, M.L.; Bilanenko, E.N.; Tereshina, B.M. Dynamics of the cytosol soluble carbohydrates and membrane lipids in response to ambient pH in alkaliphilic and alkalitolerant fungi. Microbiology 2018, 87, 21–32. [Google Scholar] [CrossRef]
- Rojas, N.L.; Cavalitto, S.F.; Cabello, M.; Hours, R.A.; Voget, C.E. Alkaline polysaccharidases produced in solid state cultures by alkalophilic fungi isolated from Argentina. J. Pure Appl. Microbiol. 2008, 2, 1–10. [Google Scholar]
- Kavitha, P.G.; Sudha, A.; Devi, P.A.; Kumaran, K. A comparative study on forest soil microbial diversity and biomass in nilgiri biosphere of Southern India. Int. J. Curr. Microbiol. Appl. Sci. 2020, 9, 3701–3715. [Google Scholar] [CrossRef]
- Monoson, H.L.; Conway, T.D.; Nelson, R.E. Four endoparasitic nematode destroying fungi isolated from sand ridge state forest soil. Mycopathologia 1975, 57, 59–62. [Google Scholar] [CrossRef]
- Nguyen, M.T.H.D.; Thomas, T. Diversity, host-specificity and stability of sponge-associated fungal communities of co-occurring sponges. PeerJ 2018, 6, e4965. [Google Scholar] [CrossRef] [PubMed]
- Youssef, F.S.; Simal-Gandara, J. Comprehensive overview on the chemistry and biological activities of selected alkaloid producing marine-derived fungi as a valuable reservoir of drug entities. Biomedicines 2021, 9, 485. [Google Scholar] [CrossRef]
- Shi, T.; Li, X.-Q.; Wang, Z.-M.; Yu, Y.-Y.; Dai, J.-J.; Shi, D.-Y.; Zheng, L. Bioactivity-guided screening of antimicrobial secondary metabolites from Antarctic cultivable fungus Acrostalagmus luteoalbus CH-6 combined with molecular networking. Mar. Drugs 2022, 20, 334. [Google Scholar] [CrossRef]
- Amatayakul, T. Synthesis of fibrinolysin by fungi. Ohio J. Sci. 1955, 55, 343–353. [Google Scholar]
- Artigues, M.; Davet, P. β-(1 → 3)-glucanase and chitinase activities in some fungi in relation to their antisclerotic activity towards Corticium rolfsii in sterile soil. Soil Biol. Biochem. 1984, 16, 527–528. [Google Scholar] [CrossRef]
- Rojas, N.L.; Voget, C.E.; Hours, R.A.; Cavalitto, S.F. Purification and characterization of a novel alkaline α-L-rhamnosidase produced by Acrostalagmus luteoalbus. J. Ind. Microbiol. Biotechnol. 2011, 38, 1515–1522. [Google Scholar] [CrossRef]
- Soprunov, F.F.; Galiulina, Z.A. Predatory hyphomycetes from Turkmenistan soils. Mikrobiologiya 1951, 20, 489–499. [Google Scholar]
- Jackson, R.M. Some aspects of soil fungistasis. J. Gen. Microbiol. 1958, 19, 390–401. [Google Scholar] [CrossRef]
- Jensen, H.L. Carbon nutrition of some microorganisms decomposing halogen-substituted aliphatic acids. Acta Agric. Scand. 1963, 13, 404–412. [Google Scholar] [CrossRef]
- Huang, Z.; Wang, F.; Tian, X.; Li, J.; Zhang, S. Identification and activities of fungal strain 00457 isolated from the deep-sea sediment of northern south china sea. Shengwu Jishu Tongbao 2012, 10, 199–204. [Google Scholar]
- Khalmuratovalt, I.; Choilt, D.-H.; Yoon, H.-J.; Yoon, T.-W.; Kim, J.-G. Diversity and plant growth promotion of fungal endophytes in five halophytes from the buan salt marsh. J. Microbiol. Biotechnol. 2021, 31, 408–418. [Google Scholar] [CrossRef] [PubMed]
- Andersson, A.M.A.; Salo, J.; Mikkola, R.; Kurnitski, J.; Salonen, H.; Marik, T.; Kredics, L. Melinacidin-producing Acrostalagmus luteoalbus, a major constituent of mixed mycobiota contaminating insulation material in an outdoor wall. Pathogens 2021, 10, 843. [Google Scholar] [CrossRef]
- Ellestad, G.A.; Evans, R.H., Jr.; Kunstmann, M.P. Structure of a C17 antifungal terpenoid from an unidentified Acrostalagmus species. J. Am. Chem. Soc. 1969, 91, 2134–2136. [Google Scholar] [CrossRef]
- Ellestad, G.A.; Evans, R.H., Jr.; Kunstmann, M.P.; Lancaster, J.E.; Morton, G.O. Structure and chemistry of antibiotic LL-Z1271α, an antifungal carbon-17 terpene. J. Am. Chem. Soc. 1970, 92, 5483–5489. [Google Scholar] [CrossRef] [PubMed]
- Ellestad, G.A.; Evans, R.H., Jr.; Kunstmann, M.P. LL-Z1271β [C16H24O5], an additional C16 terpenoid metabolite from an Acrostalagmus species. Tetrahedron Lett. 1971, 12, 497–500. [Google Scholar] [CrossRef]
- Sato, M.; Ruo, T.-I.; Hayashi, T.; Kakisawa, H.; Miyaki, T.; Yamamoto, H.; Fujisawa, K. Structure of C16-terpenes from Acrostalagmus. Tetrahedron Lett. 1974, 15, 2183–2186. [Google Scholar] [CrossRef]
- Rusman, Y.; Wilson, M.B.; Williams, J.M.; Held, B.W.; Blanchette, R.A.; Anderson, B.N.; Lupfer, C.R.; Salomon, C.E. Antifungal norditerpene oidiolactones from the fungus Oidiodendron truncatum, a potential biocontrol agent for White-Nose Syndrome in bats. J. Nat. Prod. 2020, 83, 344–353. [Google Scholar] [CrossRef]
- Pettit, G.R.; Tan, R.; Herald, D.L.; Hamblin, J.; Pettit, R.K. Antineoplastic agents. 488. isolation and structure of Yukonin from a yukon territory fungus. J. Nat. Prod. 2003, 66, 276–278. [Google Scholar] [CrossRef]
- Deng, C.; Huang, C.; Wu, Q.; Pang, J.; Lin, Y. A new sesquiterpene from the mangrove endophytic fungus Aspergillus terreus (No. GX7-3B). Nat. Prod. Res. 2013, 27, 1882–1887. [Google Scholar] [CrossRef]
- Kakisawa, H.; Sato, M.; Ruo, T.-i.; Hayashi, T. Biosynthesis of a C16-terpenoid lactone, a plant growth regulator. J. Chem. Soc. Chem. Commun. 1973, 20, 802–803. [Google Scholar] [CrossRef]
- Barrero, A.F.; Sánchez, J.F.; Elmerabet, J.; Jiménez-González, D.; Macías, F.A.; Simonet, A.M. Enantiospecific syntheses of the potent bioactives nagilactone F and the mould metabolite LL-Z1271α an evaluation of their allelopathic potential. Tetrahedron 1999, 55, 7289–7304. [Google Scholar] [CrossRef]
- Dinarello, C.A. Inflammatory cytokines: Interleukin-1 and tumor necrosis factor as effector molecules in autoimmune diseases. Curr. Opin. Immunol. 1991, 3, 941–948. [Google Scholar] [CrossRef]
- Ichikawa, K.; Inagaki, T.; Kachi-Tonai, H.; Kojima, Y.; Nakamura, T.-a.; Nishida, H.; Ueno, Y.; Binding, P.; Gabel, C.A.; Lucas, V. LL-Z1271α: An interleukin-1β production inhibitor. Biochem. Biophys. Res. Commun. 2001, 286, 697–700. [Google Scholar] [CrossRef]
- Ichikawa, K.; Hirai, H.; Ishiguro, M.; Kambara, T.; Kato, Y.; Kim, Y.J.; Kojima, Y.; Matsunaga, Y.; Nishida, H.; Shiomi, Y. Cytokine production inhibitors produced by a fungus, Oidiodendron griseum. J. Antibiot. 2001, 54, 697–702. [Google Scholar] [CrossRef][Green Version]
- Argoudelis, A.D.; Reusser, F. Melinacidins, a new family of antibiotics. J. Antibiot. 1971, 24, 383. [Google Scholar] [CrossRef]
- Reusser, F. Mode of action of melinacidin, an inhibitor of nicotinic acid biosynthesis. J. Bacteriol. 1968, 96, 1285. [Google Scholar] [CrossRef]
- Argoudelis, A.D. Melinacidins II, III, and IV. New 3,6-epidithiadiketopiperazine antibiotics. J. Antibiot. 1972, 25, 171. [Google Scholar] [CrossRef]
- Argoudelis, A.D.; Mizsak, S.A. Melinacidins II, III and IV structural studies. J. Antibiot. 1977, 30, 468. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Blunt, J.W.; Cole, A.L.J.; Cannon, J.F.; Robinson, W.T.; Munro, M.H.G. Two novel cytotoxic cyclodepsipeptides from a mycoparasitic Cladobotryum sp. J. Org. Chem. 2003, 68, 2002–2005. [Google Scholar] [CrossRef] [PubMed]
- Ebead, G.A.; Overy, D.P.; Berrué, F.; Kerr, R.G. Westerdykella reniformis sp. nov., producing the antibiotic metabolites melinacidin IV and chetracin B. IMA Fungus 2012, 3, 189–201. [Google Scholar] [CrossRef]
- Wang, F.-Z.; Huang, Z.; Shi, X.-F.; Chen, Y.-C.; Zhang, W.-M.; Tian, X.-P.; Li, J.; Zhang, S. Cytotoxic indole diketopiperazines from the deep sea-derived fungus Acrostalagmus luteoalbus SCSIO F457. Bioorg. Med. Chem. Lett. 2012, 22, 7265–7267. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Wang, Y.; Yu, R.; Feng, Y.; Wang, L.; Che, Q.; Gu, Q.; Li, D.; Li, J.; Zhu, T. Chetracins E and F, cytotoxic epipolythiodioxopiperazines from the marine-derived fungus Acrostalagmus luteoalbus HDN13-530. RSC Adv. 2018, 8, 53–58. [Google Scholar] [CrossRef]
- Cao, J.; Li, X.-M.; Meng, L.-H.; Konuklugil, B.; Li, X.; Li, H.-L.; Wang, B.-G. Isolation and characterization of three pairs of indolediketopiperazine enantiomers containing infrequent N-methoxy substitution from the marine algal-derived endophytic fungus Acrostalagmus luteoalbus TK-43. Bioorg. Chem. 2019, 90, 103030. [Google Scholar] [CrossRef]
- Cao, J.; Li, X.-M.; Li, X.; Li, H.-L.; Konuklugil, B.; Wang, B.-G. Uncommon N-methoxyindolediketopiperazines from Acrostalagmus luteoalbus, a marine algal isolate of endophytic fungus. Chin. J. Chem. 2021, 39, 2808–2814. [Google Scholar] [CrossRef]
- Chen, X.-Y.; Zhong, W.-M.; Zeng, Q.; Wang, F.-Z. A preliminary study on the chemical diversity of the deep-sea derived fungus Acrostalagmus luteoalbus SCSIO F457 based on OSMAC strategy. Zhongguo Haiyang Yaowu 2020, 39, 11–19. [Google Scholar]
- Adams, T.C.; Payette, J.N.; Cheah, J.H.; Movassaghi, M. Concise total synthesis of (+)-luteoalbusins A and B. Org. Lett. 2015, 17, 4268–4271. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Blunt, J.W.; Cole, A.L.J.; Munro, M.H.G. Novel cytotoxic thiodiketopiperazine derivatives from a Tilachlidium sp. J. Nat. Prod. 2004, 67, 2090–2092. [Google Scholar] [CrossRef]
- Dong, J.-Y.; He, H.-P.; Shen, Y.-M.; Zhang, K.-Q. Nematicidal epipolysulfanyldioxopiperazines from Gliocladium roseum. J. Nat. Prod. 2005, 68, 1510–1513. [Google Scholar] [CrossRef]
- Fujimoto, H.; Sumino, M.; Okuyama, E.; Ishibashi, M. Immunomodulatory constituents from an ascomycete, Chaetomium seminudum. J. Nat. Prod. 2004, 67, 98–102. [Google Scholar] [CrossRef] [PubMed]
- Marmouzi, I.; El Abbes Faouzi, M.; Saidi, N.; Cherrah, Y.; Rehberg, N.; Ebada, S.S.; Ebrahim, W.; Kalscheuer, R.; Proksch, P. Bioactive secondary metabolites from Chaetomium globosum, an endophyte from the Moroccan plant Avena sativa. Chem. Nat. Compd. 2017, 53, 1208–1211. [Google Scholar] [CrossRef]
- Zhai, Y.-J.; Huo, G.-M.; Zhang, Q.; Li, D.; Wang, D.-C.; Qi, J.-Z.; Han, W.-B.; Gao, J.-M. Phaeosphaones: Tyrosinase inhibitory thiodiketopiperazines from an endophytic Phaeosphaeria fuckelii. J. Nat. Prod. 2020, 83, 1592–1597. [Google Scholar] [CrossRef]
- Li, L.; Li, D.; Luan, Y.; Gu, Q.; Zhu, T. Cytotoxic metabolites from the Antarctic psychrophilic fungus Oidiodendron truncatum. J. Nat. Prod. 2012, 75, 920–927. [Google Scholar] [CrossRef]
- Wei, W.; Jiang, N.; Mei, Y.N.; Chu, Y.L.; Ge, H.M.; Song, Y.C.; Ng, S.W.; Tan, R.X. An antibacterial metabolite from Lasiodiplodia pseudotheobromae F2. Phytochemistry 2014, 100, 103–109. [Google Scholar] [CrossRef]
- Arora, P.; Wani, Z.A.; Nalli, Y.; Ali, A.; Riyaz-Ul-Hassan, S. Antimicrobial potential of thiodiketopiperazine derivatives produced by Phoma sp., an endophyte of Glycyrrhiza glabra Linn. Microb. Ecology 2016, 72, 802–812. [Google Scholar] [CrossRef]
- Scharf, D.H.; Remme, N.; Habel, A.; Chankhamjon, P.; Scherlach, K.; Heinekamp, T.; Hortschansky, P.; Brakhage, A.A.; Hertweck, C. A Dedicated glutathione S-transferase mediates carbon-sulfur bond formation in gliotoxin biosynthesis. J. Am. Chem. Soc. 2011, 133, 12322–12325. [Google Scholar] [CrossRef]
- Zhao, P.; Xue, Y.; Li, J.; Li, X.; Zu, X.; Zhao, Z.; Quan, C.; Gao, W.; Feng, S. Non-lipopeptide fungi-derived peptide antibiotics developed since 2000. Biotechnol. Lett. 2019, 41, 651–673. [Google Scholar] [CrossRef] [PubMed]
- Scharf, D.H.; Heinekamp, T.; Remme, N.; Hortschansky, P.; Brakhage, A.A.; Hertweck, C. Biosynthesis and function of gliotoxin in Aspergillus fumigatus. Appl. Microbiol. Biotechnol. 2012, 93, 467–472. [Google Scholar] [CrossRef]
- Kim, J.; Movassaghi, M. Biogenetically-inspired total synthesis of epidithiodiketopiperazines and related alkaloids. Acc. Chem. Res. 2015, 48, 1159–1171. [Google Scholar] [CrossRef]
- Scharf, D.H.; Remme, N.; Heinekamp, T.; Hortschansky, P.; Brakhage, A.A.; Hertweck, C. Transannular disulfide formation in gliotoxin biosynthesis and its role in self-resistance of the human pathogen Aspergillus fumigatus. J. Am. Chem. Soc. 2010, 132, 10136–10141. [Google Scholar] [CrossRef]
- Sun, S.; Dai, X.; Sun, J.; Bu, X.; Weng, C.; Li, H.; Zhu, H. A diketopiperazine factor from Rheinheimera aquimaris QSI02 exhibits anti-quorum sensing activity. Sci. Rep. 2016, 6, 39637. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Sun, S.; Li, H.; Sun, J.; Liu, A.; Zhou, W. Preparation, structural identification and application of quorum sensing inhibitor. CN105130963, 9 December 2015. [Google Scholar]
- Lu, X.; Zhang, M.; Qiu, Y.; Liu, X.; Wang, C.; Chen, J.; Zhang, H.; Wei, B.; Yu, Y.; Ying, Y.; et al. α-Glucosidase inhibitors from two mangrove-derived actinomycetes. Molecules 2023, 28, 3822. [Google Scholar] [CrossRef]
- Zhao, D.; Cao, F.; Guo, X.-J.; Zhang, Y.-R.; Kang, Z.; Zhu, H.-J. Antibacterial indole alkaloids and anthraquinones from a sewage-derived fungus Eurotium sp. Chem. Nat. Compd. 2018, 54, 399–401. [Google Scholar] [CrossRef]
- Meng, L.-H.; Du, F.-Y.; Li, X.-M.; Pedpradab, P.; Xu, G.-M.; Wang, B.-G. Rubrumazines A-C, indolediketopiperazines of the isoechinulin class from Eurotium rubrum MA-150, a fungus obtained from marine mangrove-derived rhizospheric soil. J. Nat. Prod. 2015, 78, 909–913. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.-Z.; Pu, X.; Luo, G.; Zhao, L.-X.; Xu, L.-H.; Li, W.-J.; Luo, Y. Isolation and characterization of new p-terphenyls with antifungal, antibacterial, and antioxidant activities from halophilic actinomycete Nocardiopsis gilva YIM 90087. J. Agric. Food Chem. 2013, 61, 3006–3012. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Jiang, W.; Miao, L.; Jin, C.; Bao, W. Anti-diatom compounds from marine bacterium Pseudomonas putida. Weishengwu Xuebao 2013, 53, 825–831. [Google Scholar]
- Ortiz-Castro, R.; Diaz-Perez, C.; Martinez-Trujillo, M.; del Rio, R.E.; Campos-Garcia, J.; Lopez-Bucio, J. Transkingdom signaling based on bacterial cyclodipeptides with auxin activity in plants. Proc. Natl. Acad. Sci. USA 2011, 108, 7253. [Google Scholar] [CrossRef]
- Wang, G.; Dai, S.; Chen, M.; Wu, H.; Xie, L.; Luo, X.; Li, X. Two diketopiperazine cyclo(Pro-Phe) isomers from marine bacterium Bacillus subtilis sp. 13-2. Chem. Nat. Compd. 2010, 46, 583–585. [Google Scholar] [CrossRef]
- Rhee, K.-H. Cyclic dipeptides exhibit synergistic, broad spectrum antimicrobial effects and have anti-mutagenic properties. Int. J. Antimicrob. Agents 2004, 24, 423–427. [Google Scholar] [CrossRef]
- Kwak, M.-K.; Liu, R.; Kim, M.-K.; Moon, D.; Kim, A.H.; Song, S.-H.; Kang, S.-O. Cyclic dipeptides from lactic acid bacteria inhibit the proliferation of pathogenic fungi. J. Microbiol. 2014, 52, 64–70. [Google Scholar] [CrossRef]
- Kumar, N.; Mohandas, C.; Nambisan, B.; Kumar, D.R.S.; Lankalapalli, R.S. Isolation of proline-based cyclic dipeptides from Bacillus sp. N strain associated with rhabitid entomopathogenic nematode and its antimicrobial properties. World J. Microbiol. Biotechnol. 2013, 29, 355–364. [Google Scholar] [CrossRef]
- Alshaibani, M.M.; MohamadZin, N.; Jalil, J.; Sidik, N.M.; Ahmad, S.J.; Karna, N.; Edrada-Ebel, R. Isolation, purification, and characterization of five active diketopiperazine derivatives from endophytic Streptornyces SUK 25 with antimicrobial and cytotoxic activities. J. Microbiol. Biotechnol. 2017, 27, 1249–1256. [Google Scholar] [CrossRef]
- Brauns, S.C.; Milne, P.; Naude, R.; van de Venter, M. Selected cyclic dipeptides inhibit cancer cell growth and induce apoptosis in HT-29 colon cancer cells. Anticancer Res. 2004, 24, 1713–1719. [Google Scholar]
- Brauns, S.C.; Dealtry, G.; Milne, P.; Naude, R.; Van De Venter, M. Caspase-3 activation and induction of PARP cleavage by cyclic dipeptide cyclo(Phe-Pro) in HT-29 cells. Anticancer Res. 2005, 25, 4197–4202. [Google Scholar]
- Rhee, K.-H. Inhibition of DNA topoisomerase I by cyclo(L-prolyl-L-phenylalanyl) isolated from Streptomyces sp. AMLK-335. J. Microbiol. Biotechnol. 2002, 12, 1013–1016. [Google Scholar]
- Li, X.; Dobretsov, S.; Xu, Y.; Xiao, X.; Hungi, O.S.; Qian, P.-Y. Antifouling diketopiperazines produced by a deep-sea bacterium, Streptomyces fungicidicus. Biofouling 2006, 22, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Qi, S.-H.; Xu, Y.; Gao, J.; Qian, P.-Y.; Zhang, S. Antibacterial and antilarval compounds from marine bacterium Pseudomonas rhizosphaerae. Ann. Microbiol. 2009, 59, 229–233. [Google Scholar] [CrossRef]
- Takaya, Y.; Furukawa, T.; Miura, S.; Akutagawa, T.; Hotta, Y.; Ishikawa, N.; Niwa, M. Antioxidant constituents in distillation residue of awamori spirits. J. Agric. Food Chem. 2007, 55, 75–79. [Google Scholar] [CrossRef]
- Lin, W.-X.; Xie, C.-L.; Zhou, M.; Xia, M.-L.; Zhou, T.-T.; Chen, H.-F.; Yang, X.-W.; Yang, Q. Chemical constituents from the deep sea-derived Streptomyces xiamenensis MCCC 1A01570 and their effects on RXRα transcriptional regulation. Nat. Prod. Res. 2020, 34, 1461–1464. [Google Scholar] [CrossRef]
- Yang, B.; Huang, J.; Lin, X.; Zhang, Y.; Tao, H.; Liu, Y. A new diketopiperazine from the marine sponge Callyspongia species. Rec. Nat. Prod. 2016, 10, 117–121. [Google Scholar]
- Hou, Y.; Sun, S.; Wu, L.; Wang, X.; Li, T.; Zhang, M.; Wang, J.; Wang, L. Calcium sensitizers isolated from the edible pine mushroom, Tricholoma matsutake (S. Ito & Imai) Sing. Z. Naturforsch. C J. Biosci. 2013, 68, 113–117. [Google Scholar]
- Kang, H.; Ku, S.-K.; Choi, H.; Bae, J.-S. Three diketopiperazines from marine-derived bacteria inhibit LPS-induced endothelial inflammatory responses. Bioorg. Med. Chem. Lett. 2016, 26, 1873–1876. [Google Scholar] [CrossRef]
- Jung, B.; Ku, S.-K.; Gao, M.; Kim, K.-M.; Han, M.-S.; Choi, H.; Bae, J.-S. Suppressive effects of three diketopiperazines from marine-derived bacteria on TGFBIp-mediated septic responses in human endothelial cells and mice. Arch. Pharmacal Res. 2016, 39, 843–854. [Google Scholar] [CrossRef]
- Maglangit, F.; Kyeremeh, K.; Deng, H. Deletion of the accramycin pathway-specific regulatory gene accJ activates the production of unrelated polyketide metabolites. Nat. Prod. Res. 2022, 37, 2753–2758. [Google Scholar] [CrossRef]
- Fujimoto, H.; Sumino, M.; Nagano, J.; Natori, H.; Okuyama, E.; Yamazaki, M. Immunomodulatory constituents from three Ascomycetes, Gelasinospora heterospora, G. multiforis, and G. longispora. Chem. Pharm. Bull. 1999, 47, 71–76. [Google Scholar] [CrossRef]
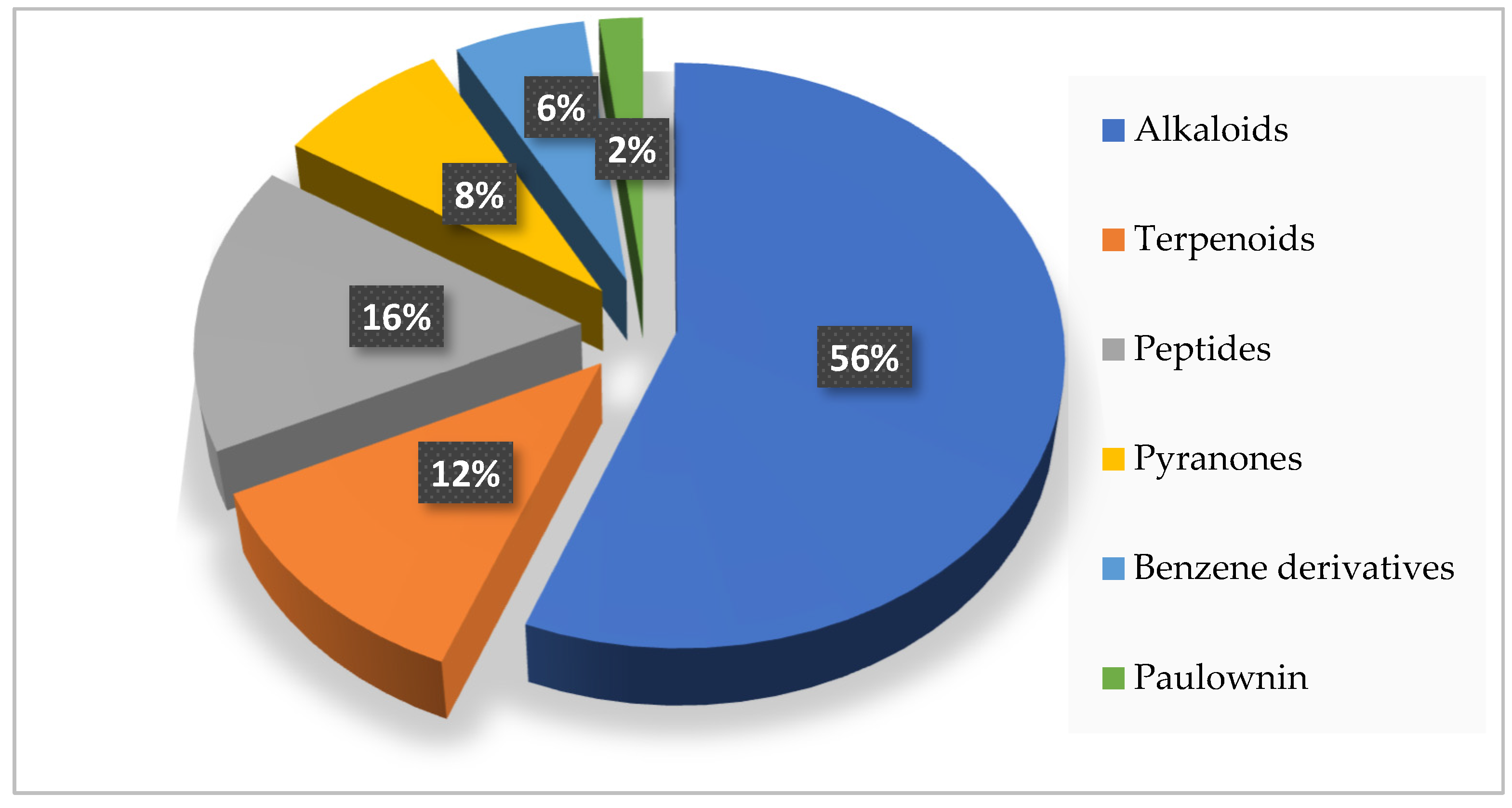

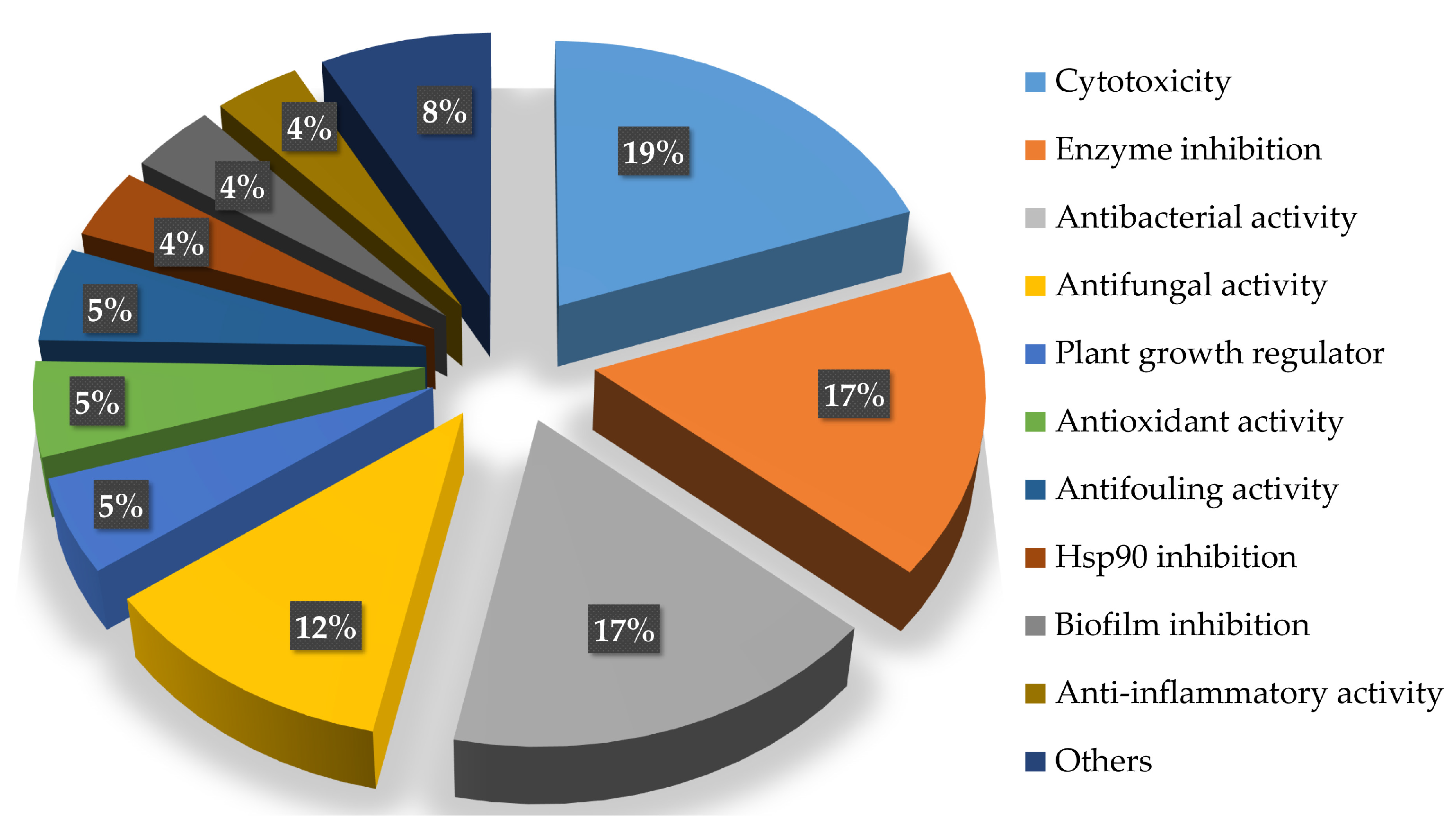
| Types | Compounds | Sources | Distribution | Years | Refs. |
|---|---|---|---|---|---|
| Terpenoids | 1, 2 | Acrostalagmus sp. NRRL-3481 | 1969 | [19] | |
| 3 | 1971 | [21] | |||
| 4–6 | 1974 | [22] | |||
| Alkaloids | 7–9 | A. cinnabarinus var. melinacidinus | 1972 | [33] | |
| 10–16 | Deep-sea sediment-derived fungus A. luteoalbus SCSIO F457 (GenBank No. MN860118) | South China Sea | 2012 | [37] | |
| 17–19 | Soil derived fungus A. luteoalbus HDN13-530 (GenBank No. KP969081) | Liaodong Bay, China | 2017 | [38] | |
| 20–25 | Marine green alga Codium fragile derived endophytic fungus A. luteoalbus TK-43 (GenBank No. MH836621) | Sinop, Turkey | 2019 | [39] | |
| 26–34 | 2021 | [40] | |||
| Peptides | 35–37 | Deep-sea sediment-derived fungus A. luteoalbus SCSIO F457 (GenBank No. MN860118) | South China Sea | 2012 | [37] |
| 38–42 | 2020 | [41] | |||
| Pyranone derivatives | 43 | Deep-sea sediment-derived fungus A. luteoalbus SCSIO F457 (GenBank No. MN860118) | South China Sea | 2020 | [41] |
| 44–46 | Antarctic soil derived fungus A. luteoalbus CH-6 (Genbank No. MT367202.1) | Fields Peninsula, Antarctica | 2022 | [9] | |
| Paulownin | 47 | Deep-sea sediment-derived fungus A. luteoalbus SCSIO F457 (GenBank No. MN860118) | South China Sea | 2020 | [41] |
| Benzene derivatives | 48–50 | Deep-sea sediment-derived fungus A. luteoalbus SCSIO F457 (GenBank No. MN860118) | South China Sea | 2020 | [41] |
| Cell Lines | Compounds | Values (IC50) | Values of Positive Controls (IC50) | Pros and Cons |
|---|---|---|---|---|
| P388 | 1 (μg/mL) | 4.1 | Pros: Strong and broad spectrum cytotoxicity [23]. | |
| BXPC-3 | 0.36 | |||
| MCF-7 | 0.33 | |||
| SF268 | 0.24 | |||
| NCI-H460 | 0.24 | |||
| KM20L | 0.21 | |||
| DU-145 | 0.14 | |||
| HL-60 | 3 (μM) | 0.60 | 0.71 | Pro: Strong cytotoxicity with the same level as the positive control [25]. |
| P388 | 9/12 (μM) | 0.05/0.25 | Pro: Potent cytotoxicity against murine P388 leukemia cells [35,43]. | |
| SF-268 | 10/11/12/ 13/14 (μM) | 0.46 ± 0.05/0.59 ± 0.03/1.04 ± 0.03/ 0.73 ± 0.05/2.49 ± 0.07 | 4.76 ± 0.27 | Pros: Compounds 10–14 exhibited potent cytotoxicity, and 10 and 11 showed stronger cytotoxicity against all four tested cancer cell lines than that of the positive control cisplatin [37]. |
| MCF-7 | 0.23 ± 0.03/0.25 ± 0.00/0.91 ± 0.03/ 0.23 ± 0.03/0.65 ± 0.07 | 3.99 ± 0.13 | ||
| NCI-H460 | 1.15 ± 0.03/1.31 ± 0.12/5.60 ± 0.58/ 6.57 ± 0.81/17.78 ± 0.27 | 2.91 ± 0.18 | ||
| HepG-2 | 0.91 ± 0.03/1.29 ± 0.16/3.52 ± 0.74/ 0.53 ± 0.04/2.03 ± 0.07 | 2.45 ± 0.07 | ||
| A549 | 10/11 (μM) | 2.33 ± 0.59/0.91 ± 0.29 | Pro: Prominent cytotoxic activities [42]. | |
| HeLa | 1.00 ± 0.24/0.52 ± 0.15 | |||
| HCT116 | 1.22 ± 1.02/0.58 ± 0.38 | |||
| L5178Y | 15/16 (μM) | 0.26/0.82 | 4.3 | Pro: Potent cytotoxic activities against murine lymphoma L5178Y cell line, which are more potent than that of the positive control kahalalide F [46]. |
| A549 | 17/18/19 (μM) | 0.4/1.9/0.7 | 0.2 | Pro: Extensive cytotoxicity, 17 showed stronger activity to H1975 than that of positive drug doxorubicin hydrochloride [38] |
| HCT116 | 0.4/2.1/0.3 | 0.2 | ||
| K562 | 0.4/1.9/1 | 0.2 | ||
| H1975 | 0.2/3.6/0.8 | 0.8 | ||
| HL-60 | 1.9/1.9/1.5 | 0.02 | ||
| HCT-8 | 19 (μM) | 0.49 ± 0.09 | Pro: Significant cytotoxicity against a panel of cancer cell lines [48] | |
| Bel-7402 | 0.38 ± 0.03 | |||
| BGC-823 | 0.70 ± 0.04 | |||
| A2780 | 0.58 ± 0.03 | |||
| HeLa | 38 (mM) | 2.92 ± 1.55 | Con: Weak activity [69]. | |
| HT-29 | 4.04 ± 1.15 | |||
| MCF-7 | 6.53 ± 1.26 | |||
| ECA-109 | 42 (inhibition rate at 20 µM) | 44% | Con: Weak activity [75]. | |
| Hela-S3 | 52% | |||
| PANC-1 | 55% |
| Strains | Compounds | Values (MIC) | Values of Positive Controls (MIC) | Pros and Cons |
|---|---|---|---|---|
| Cryptococcus neoformans ATCC 90112 | 1 (μg/mL) | 2 | Pro: Strong activity against fungus C. neoformans caused infection in human [23,24]. | |
| Candida albicans ATCC 90028 | 8 | |||
| Pseudogymnoascus destructans ATCC MYA 4855 | 15 | |||
| methicillin-resistant Staphylococcus aureus (MRSA) | 9 (μg/mL) | 0.7 | 1.4 | Pros: Strong antibacterial activity to MRSA, the activity was double of the positive control [36]. |
| vancomycin-resistant Enterococcus faecium (VRE) | 22 | 2.4 | ||
| S. aureus ATCC29213 | 12/32 (μM) | 3.8 ± 0.40/5.8 ± 0.45 | 0.362 ± 0.09 | Pros: Broad-spectrum antimicrobial activity; Strong activity against MRSA compared with positive control. Con: Moderate or weak antimicrobial activity to some of the test strains [50]. |
| MRSA | 8.4 ± 1.01/5.6 ± 0.99 | 9.33 ± 2.6 | ||
| Bacillus cereus IIIM25 | 9.2 ± 0.77/9.9 ± 0.81 | 0.12 ± 0.009 | ||
| Klebsiella pneumoniae ATCC75388 | 19.1 ± 1.1/4.5 ± 0.77 | 0.015 ± 0.0006 | ||
| Bacillus thuringiensis MTCC 809 | 14.8 ± 0.28/19 ± 0.84 | 0.003 ± 0.001 | ||
| Yersinia enterocolitica MTCC840 | 38 ± 1.7/65.3 ± 1.6 | 3.5 ± 0.202 | ||
| Erwinia herbicola MTCC3609 | 15.4 ± 2.7/14.2 ± 1.4 | 0.006 ± 0.0009 | ||
| Shigella dysenteriae NCTC 11311 | 82.3 ± 1.3/– | 0.006 ± 0.0003 | ||
| Lactococcus lactis MTCC440 | 28.7 ± 1.7/39.4 ± 1.1 | 0.006 ± 0.001 | ||
| S. epidermidis MTCC35 | 22.6 ± 2.2/23.4 ± 1.5 | 0.06 ± 0.006 | ||
| Alcaligenes faecalis MTCC126 | –/– | 1.2 ± 0.06 | ||
| S. warneri MTCC4436 | 5.05 ± 0.4/7.5 ± 0.4 | 2.4 ± 0.105 | ||
| Pseudomonas fluorescens MTCC103 | 18.4 ± 0.3/26.1 ± 2.7 | 0.151 ± 0.051 | ||
| Xanthobacter flavus MTCC 132 | 98.3 ± 1.1/– | 2.3 ± 0.021 | ||
| S. pyogenes MTCC442 | 1.8 ± 0.2/3.1 ± 0.15 | 0.015 ± 0.0006 | ||
| Shigella boydii NCTC9357 | 31.5 ± 1.2/26.7 ± 0.9 | 1.12 ± 0.063 | ||
| Clostridium pasteurianum MTCC116 | 92.3 ± 0.4/54.0 ± 0.5 | 0.015 ± 0.003 | ||
| Salmonella typhimurium MTCC98 | –/86.2 ± 1.9 | 0.015 ± 0.003 | ||
| C. albicans MTCC4748 | –/35.8 ± 1.4 | 1.5 ± 0.022 | ||
| C. albicans | 10/13/32 (μM) | 12.5/25/6.25 | 6.25 | Pro: Compound 32 showed broad-spectrum antimicrobial activity. Con: Weak activity [9]. |
| Aeromonas salmonicida | 12.5/50/3.125 | 6.25 | ||
| Photobacterium halotolerans | -/-/25 | 0.195 | ||
| Pseudomonas fulva | -/-/25 | 1.56 | ||
| S. aureus | -/-/25 | 3.125 | ||
| Escherichia coli | 16/30/32 (μM) | -/-/8 | 12 | Pros: Compound 32 showed broad-spectrum antimicrobial activity, and the activity is significant and comparable to that of the positive control; compounds 16 and 30 displayed specific remarkable antibacterial activities toward Ed. ictaluri [40]. |
| Edwardsiella tarda | -/-/2 | 2 | ||
| Ed. ictaluri | 5/3/2 | 2 | ||
| Aeromonas hydrophila | -/-/4 | 3 | ||
| Micrococcus luteus | -/-/33 | 3 | ||
| Pseudomonas aeruginosa | -/-/8 | 6 | ||
| Vibrio alginolyticus | -/-/8 | 2 | ||
| V. anguillarum | -/-/2 | 3 | ||
| V. harveyi | -/-/4 | 3 | ||
| V. parahemolyticus | -/-/2 | 12 | ||
| V. vulnificus | -/-/33 | 3 | ||
| Fusarium solani | 23 (μg/mL) | 32 | Pro: 23 exhibited specific antifungal activity toward F. solani [39]. | |
| Veillonella parvula | 31 (μg/mL) | 0.25 | 0.12 | Pro: 31 exhibited strong antibacterial activity, comparable or even more significant than that of positive control [49]. |
| Actinomyces israelii | 32 | 8 | ||
| Streptococcus sp. | 0.12 | 0.25 | ||
| Bacteroides vulgatus | 0.12 | 0.5 | ||
| Peptostreptococcus sp. | 0.12 | 0.5 | ||
| E. coli | 35 (mg/mL) | 6.4 | Con: Weak activity [56,57]. | |
| Chromobacterium violaceum CV026 | 3.2 | |||
| Pseudomonas aeruginosa PA01 | 6.4 | |||
| S. aureus | 3.2 | |||
| C. albicans 00147 | 6.4 | |||
| B.cereus | 36 (μM) | 1.56 | 0.78 | Con: Medium activity [59]. |
| Proteus vulgaris | 3.13 | 0.20 | ||
| Enterococcus faecium (K-99-38) | 38 and cyclo(L-Leu-L-Pro)/38 (μg/mL) | 1/64 | 64 | Pro: Combination of 38 and cyclo(L-Leu-L-Pro) displayed prominent antimicrobial activity, much stronger than those of positive controls [64,65]. |
| E. faecalis (K-99-17) | 0.5/16 | 128 | ||
| E. faecalis (K-99-258) | 0.25/32 | >256 | ||
| E. faecalis (K-01-312) | 2/16 | 128 | ||
| E. faecium (K-01-511) | 0.5/32 | 128 | ||
| E. col | 0.5/64 | 32 | ||
| B. subtilis | 1/128 | 64 | ||
| Micrococcus luteus | 0.25/64 | 32 | ||
| S. faecalis | 2/>256 | 64 | ||
| P. aeruginosa | 1/64 | 12.5 | ||
| S. aureus | 0.5/256 | 25 | ||
| Penicillin resistant S. aureus | 4/256 | 64 | ||
| C. albicans | 0.25/64 | 32 | ||
| C. glabrata | 4/256 | 16 | ||
| C. tropicalis | 0.5/32 | 128 | ||
| Amphotericin B resistant C. tropicalis | 0.5/64 | 16 | ||
| Cryptococcus neoformans | 0.25/32 | 16 | ||
| Amphotericin B resistant C. neoformans | 2/>256 | 32 | ||
| Ganoderma plantarum | 38/39/40 (mM) | 6.8/8.2/8.2 | Con: Weak activity [66]. | |
| Candida sp. | 7.0 | |||
| B. subtilis MTCC2756 | 38/39 (µg/mL) | 16/64 | 5 | Pro: Demonstrated prominent activities against agriculturally important fungi, much higher than the commercial fungicide bavistin [67] |
| S. aureus MTCC902 | 16/32 | 5 | ||
| E. coli MTCC2622 | 8/32 | 5 | ||
| P. aeruginosa MTCC2642 | 32/- | 10 | ||
| Aspergillus flavus MTCC183 | 128/32 | 100 | ||
| C. albicans MTCC277 | 64/32 | 50 | ||
| Fusarium oxysporum MTCC284 | 4/8 | 25 | ||
| Rhizoctonia solani MTCC4634 | 4/8 | 25 | ||
| Pencillium expansum MTCC2006 | 2/4 | 50 | ||
| MRSA 43300 (inhibition zone) | 40 (mm) | 15 | 22 | Con: Medium activity [68]. |
| S. aureus ATCC 25923 | 43 (µg/mL) | 25 | Con: Medium activity [80]. | |
| Enterococcus faecalis ATCC 29212 | 12.5 | |||
| E. faecium K59–68 | 12.5 |
| Bioactivities | Cells/Stains/Enzyme | Compounds | Values | Values of Positive Controls | Pros and Cons |
|---|---|---|---|---|---|
| Plant growth regulator, inhibition of the germination and growth development at 10−4 M (%) | Avena coleoptile | 1 | Pro: Significant inhibitory activity, and more active than the commercial herbicide LOGRAN® [26,27]. | ||
| Allium cepa | >80% | 65% | |||
| Hordeum vulgare | >80% | <60% | |||
| Lactuca sativa | >80% | <60% | |||
| Plant growth regulator | Auxin signaling and plant growth promotion | 38–40 | Pro: Established a significant function for DKPs mediating transkingdom signaling between prokaryote and eukaryote [63]. | ||
| Anti-inflammatory activity (IC50, μM) | IL-1β | 1/2 | 0.049/69 | Pro: Compound 1 showed potent inhibitory activity to the production of IL-1β. Con: Compound 2 showed weak activity [28,29,30]. | |
| TNF-α | 3.0/11 | ||||
| Leucine uptake | 11/120 | ||||
| Inhibition the LPS-induced migration, adhesion, and hyperpermeability of leukocytes | 42 | Pro: Potential candidate for therapy of the different vascular inflammatory diseases [78,79] | |||
| Suppress TGFBIp-mediated and CLP-induced septic responses | |||||
| Nematicidal activity (ED50, µg/mL) | Caenorhabditis elegans | 13/14 | 200/200 | Con: Weak activities [44]. | |
| Panagrellus redivivus | 250/250 | ||||
| Biofilm inhibition at MIC values (%) | S.aureus | 12/32 | 70.3%/ 68.8% | Pros: Strong activities [50], 35 displayed stronger biofilm inhibition than that of positive control azithromycin [56,57]. | |
| S. pyogenes | 60.75%/ 86.4% | ||||
| Pseudomonas aeruginosa PA01 | 35 (1/32 MIC) | 59.9% | 53.9% | ||
| Immunosuppressive activity, IC50 value on Con A-(T-cells)-induced or LPS-induced proliferations of mouse splenic lymphocytes (µg/mL) | Con A-(T-cells)-induced | 15/46 | 24/0.9 | 2.7 | Pro: Compound 46 showed significant immunosuppressive activity and stronger than that of positive control azathioprine [81]. Con: Weak activity of 15 [45]. |
| LPS-induced | 46 | 1.2 | 2.7 | ||
| Enzyme inhibition (IC50, µM) | Mushroom tyrosinase | 15 | 31.7 ± 0.2 | 40.4 ± 0.1 | Pro: Stronger than the inhibition of the positive control kojic acid [47]. |
| AChE | 20 and 21 | 9.5 | 0.14 | Pro: Compounds 35 and 38 with stronger enzyme inhibition than their positive control acarbose [58,71]. Con: Medium or weak activity [39,40]. | |
| 20/21 | 2.3/13.8 | ||||
| 22 and 23 | 60.7 | ||||
| 22/23 | 78.8/49.2 | ||||
| 24 and 25 | 130.5 | ||||
| 24/25 | 160.6/ 121.7 | ||||
| 26/27/ 28/29 | 18.9/32/ 8.4/32 | ||||
| α-Glucosidase | 35 | 164.5 ± 15.5 | 422.3 ± 8.44 | ||
| Topoisomerase I | 38 | 13 | 17 | ||
| Hsp90 inhibition at the concentration of 0.5 μM | H1975 cells | 17/18/19 | Reduce the expressions of Akt, EGFR, and the active forms of Akt, EGFR, Erk, and Stat3 (Hsp90 client oncoproteins) [38]. | ||
| Anti-quorum sensing activity (0.2 mg/mL) | Inhibiting the production of violacein in Chromobacterium violaceum CV026 | 35 | 67% | 80% in 0.05 mg/mL | Pro: Strong activity [56,57]. |
| Reduction in elastase activity | 40% | 49% in 0.05 mg/mL | |||
| Brine shrimp lethality (LD50, μM) | 36 | 25.5 | 19.4 | Con: Medium activity [60]. | |
| Antifouling activity | Anti-diatom attachment activity | 36 (50 µg/mL) | 85% | Pro: Strong activities of 36 and 42 [62,76]. Con: Weak activities of 38 and 40 [72,73]. | |
| Balanus amphitrite (EC50) | 38/40 (mM) | 0.28/0.10 | |||
| Cyprid larvae of the barnacle (LC50) | 42 (μg/mL) | 3.5 | |||
| Antioxidant activity, DPPH free radical scavenging | ABTS+• scavenging capacity at 2 mg/mL | 36 | 54.6 ± 0.6% | 79.1 ± 4.3% at 0.16 mg/mL | Con: Medium or weak activities [41,61,74]. |
| OH• inhibition at 2.5 μM | 38/40 | 64.9%/54.1% | |||
| IC50 (μg/mL) | 48 | 240.05 | 16.87 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, T.; Wang, H.; Li, Y.-J.; Wang, Y.-F.; Pan, Q.; Wang, B.; Shang, E.-L. Genus Acrostalagmus: A Prolific Producer of Natural Products. Biomolecules 2023, 13, 1191. https://doi.org/10.3390/biom13081191
Shi T, Wang H, Li Y-J, Wang Y-F, Pan Q, Wang B, Shang E-L. Genus Acrostalagmus: A Prolific Producer of Natural Products. Biomolecules. 2023; 13(8):1191. https://doi.org/10.3390/biom13081191
Chicago/Turabian StyleShi, Ting, Han Wang, Yan-Jing Li, Yi-Fei Wang, Qun Pan, Bo Wang, and Er-Lei Shang. 2023. "Genus Acrostalagmus: A Prolific Producer of Natural Products" Biomolecules 13, no. 8: 1191. https://doi.org/10.3390/biom13081191
APA StyleShi, T., Wang, H., Li, Y.-J., Wang, Y.-F., Pan, Q., Wang, B., & Shang, E.-L. (2023). Genus Acrostalagmus: A Prolific Producer of Natural Products. Biomolecules, 13(8), 1191. https://doi.org/10.3390/biom13081191